ABSTRACT
BACKGROUND:
In brachial plexus birth palsy (BPBP), botulinum toxin may be utilized to prevent glenohumeral dysplasia and to maintain the stable growth of the glenohumeral joint. Repeated injections may cause muscular atrophy and their functional effects are uncertain. The aim of this study was to compare the microstructure and the function of the muscles that received two injections before transfer with the muscles that were not injected.
METHODS:
BPBP patients that were operated between January 2013 and December 2015 were included in the study. Latissimus dorsi and teres major muscles were transferred to humerus in standard fashion. Patients were divided in two groups according to botulinum toxin status. Group 1 was toxin negative whereas Group 2 was toxin positive. For each patient, mean latissimus dorsi myocyte thickness (LDMT) was measured with electron microscopy and pre-operative and post-operative active shoulder abduction, flexion, external and internal rotation, and Mallet scores were evaluated with goniometry.
RESULTS:
Fourteen patients (seven patients per group) were evaluated. Five patients were female whereas nine were male. Mean LDMT was not affected significantly (p>0.05). The operation improved shoulder abduction, flexion, and external rotation significantly (p<0.05), independent of the toxin status. The internal rotation decreased significantly only in Group 2 (p<0.05). The Mallet score increased in both groups, but it was not significant (p>0.05), independent of the toxin status.
CONCLUSION:
Botulinum toxin that was applied twice prevented glenohumeral dysplasia and it did not cause permanent latissimus dorsi muscle atropy and function loss in late period. It augmented upper extremity functions by alleviating internal rotation contracture.
Keywords: Botulinum toxin type A, brachial plexus birth palsy, electron microscopy, internal rotation contracture, tendon transfer
INTRODUCTION
Brachial plexus birth palsy (BPBP) is a serious traumatic complication that may be caused by the tractional forces of labor.[1–3] With a prevalence of 2.9/1000 in live births, the clinical manifestations of BPBP varies according to the number of the affected nerves and the severity of the traumatic injury.[3–5] Although prompt physical therapy and surveillance enables total recovery in most of the patients, permanent joint stiffness and severe anatomic and functional disorders persist in 10% of the patients.[1,6,7]
Cocontraction which is the simultaneous contraction of agonist and antagonist mucles may be present in the recovery phase of BPBP and it may avoid the performance of a successful physical therapy.[8] In BPBP, the contraction of the latissimus dorsi and the teres major muscles may be weakened temporarily by botulinum toxin injections to facilitate the physical therapy and to maintain the anatomic growth of the shoulder and the elbow joints.[8,9] However, recurring botulinum toxin injections may cause muscular atrophy and the clinical effects of these injections on the future tendon transfers of the injected muscles are uncertain.[10]
BPBP patients who do not respond to conservative physical therapy may be referred for early nerve surgery (6–12 months of age) or late reconstructive surgery (2 years of age).[1,2] As a late reconstructive procedure, the latissimus dorsi and the teres major muscle tendons may be transferred to the major tubercle of the humerus to restore the shoulder abduction and external rotation.[1,2]
In this study, the electron microscopic structure and the clinical function of the latissimus dorsi and teres major muscles that were injected with botulinum toxin 2 times before the tendon transfer were compared with the same muscles that were not injected with the toxin. The aim of this study was to demonstrate the structural and functional effects of recurring botulinum toxin therapy on transferable muscles.
MATERIALS AND METHODS
The study was evaluated and approved by the Local Clinical Studies Ethical Committee and informed consent was obtained from the guardians of every patient.
The BPBP patients who required latissimus dorsi and teres major tendon transfers for the restoration of shoulder abduction and external rotation between January 2013 and December 2015 were included in this study. All the patients were under the active surveillance of the specialized hand surgery board since their first postnatal month and all the interventions were done by the same surgical team.
The patients were classified in two groups according to their botulinum toxin injection status. Group 1 consisted of patients who did not receive any injection to their latissimus dorsi and teres major muscles before the tendon transfer surgeries. Group 2 consisted of patients who received two rounds of botulinum toxin injections to their latissimus dorsi and teres major muscles before the tendon transfer surgeries. In fact, botulinum toxin injections were performed to overcome the cocontraction of the muscles and to avoid glenohumeral dysplasia.
In Group 2, botulinum toxin (Botox®, Allergan, Inc., Irvine, California, USA) was injected intramuscularly, close to the motor endplates of latissimus dorsi, teres major, and subscapularis muscles. Twenty units of botulinum toxin were applied through two puncture sites for each muscle and this process was performed 2 times before the tendon transfer surgery.
Standard tendon transfer was performed in both groups. Latissimus dorsi and teres major tendons were detached from the intertubercular sulcus of humerus to be reinserted to the tuberculum majus of the same bone. The glenohumeral joint was immobilized in a cast for 8 weeks and each patient was evaluated at the in-house physiotherapy unit. Partial strengthening exercises were commenced at the 8th post-operative week and stretching exercises were performed at the 3rd post-operative month.
During the operation, latissimus dorsi mucle was dissected and a biopsy specimen of 2×2 centimeters was obtained 2 cm caudal to the motor end plate of the muscle. This standardized biopsy technique was performed in each patient. The tissue biopsies were fixated in a 2.5% glutaraldehyde solution and they were transferred to the histology and embryology department with a cold chain transport principle. The tissues were manipulated with osmium tetroxide and ethanol solutions and they were embedded in epoxy rosin. The epoxy rosin blocks were sectioned as 80 nanometers slices and ten tissue sections were prepared for each patient. Transmission electron microscopy was performed for each tissue section and myocyte thickness which may be affected by muscle atrophy was measured for all the myocytes. All the tissue sections and measurements were photographed (Fig. 1). Mean latissimus dorsi myocyte thickness (LDMT) values were calculated for each patient and each group.
Figure 1.
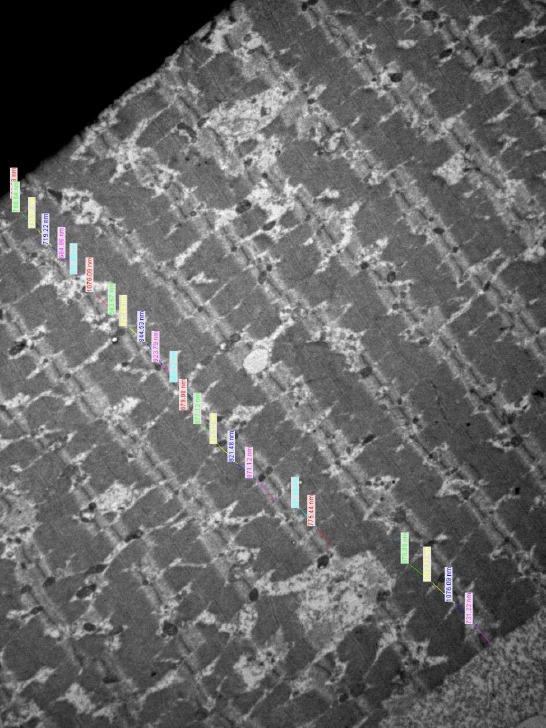
The electron microscopic structure of the latissimus dorsi muscle of a patient from Group 1 is demonstrated (80 nanometers; epoxy rosin preparation). Latissimus dorsi myocyte thickness (LDMT) was measured for each myocyte and the mean value was calculated for each patient.
Figure 2.
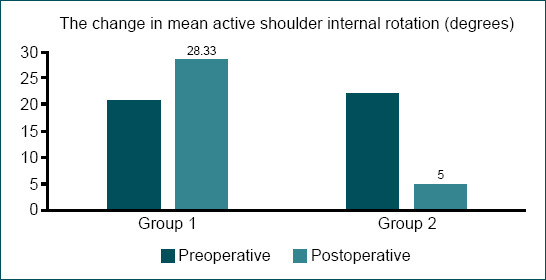
The coloumn graph compares the pre-operative and post-operative mean values of active internal rotation between the groups.
For both groups, goniometric measurements of the shoulder joint were performed 1 day before and 2 years after the tendon transfer surgery. Active shoulder abduction, flexion, external rotation, internal rotation degrees, and Mallet scores were recorded for each patient.
The following descriptive statistical techniques were utilized while evaluating the data of this study: mean, standard deviation, median, frequency, ratio, and minimum and maximum values. Furthermore, Mann–Whitney U-test was performed for two group comparisons of numerical data that did not show a normal distribution. The statistical significance value was accepted as p<0.05. All statistical evaluations were performed with Number Cruncher Statistical System 2007 software (Kaysville, Utah, USA).
RESULTS
Fourteen patients were operated between January 2012 and December 2015. Five patients were female and nine patients were male. The mean age of the patients was 8 years (6–11) and the mean follow-up period of the patients was 27 months (25–31).
In Group 1, nine tissue sections (4–13) were prepared for the biopsy specimen and 21 myocytes (17–24) were measured per section. Mean LDMT was 914.71 nanometers (821.28–1045.84). In Group 2, 11 tissue sections (1–20) were prepared for the biopsy specimen and 19 myocytes (13–24) were measured per section. Mean LDMT was 996.20 nanometers (821.38–1324.99). There was not a significant difference between the number of measured tissue sections and both groups were comparable. The difference of mean LDMT was not significant between the groups (p>0.05) and botulinum toxin that was injected 2 times before the operation did not affect the microstructure of the transferred muscle.
The pre-operative and post-operative mean active shoulder abduction, flexion, external and internal rotation degrees, and Mallet scores are demonstrated in Table 1.
Table 1.
The preroperative and postoperative mean active shoulder abduction, flexion, external and internal rotations and Mallet scores of the groups are demonstrated
| Group 1 Preoperative | Group 1 Postoperative | Group 2 Preoperative | Group 2 Postoperative | |
|---|---|---|---|---|
| Mean active shoulder abduction (Degrees) | 60.83±26.16 | 115.00±34.93 | 45.71±31.42 | 129.29±51.19 |
| Mean active shoulder flexion (Degrees) | 57.50±26.03 | 135.00±25.30 | 48.57±32.37 | 116.43±47.50 |
| Mean active shoulder external rotation (Degrees) | 1.67±4.08 | 67.50±22.75 | 0 | 59.29±16.18 |
| Mean active shoulder ınternal rotation (Degrees) | 20.83±6.65 | 28.33±26.20 | 22.14±16.80 | 5.00±6.45 |
| Mean Mallet Score | 11.67±1.75 | 15.00±3.10 | 11.14±1.57 | 14.43±2.57 |
After the operation, the active shoulder abduction increased by an average of 54.17±57.92 degrees in Group 1 (p=0.028), whereas the increase was 67.86±48.64 degrees (p=0.018) in Group 2. There was not a statistically significant difference between these increases; thus, they were not affected by the botulinum toxin status (p=0.391).
After the operation, the active shoulder flexion increased by an average of 77.50±44.80 degrees in Group 1 (p=0.028), whereas the increase was 83.57±43.85° (p=0.018) in Group 2. There was not a statistically significant difference between these increases; thus, they were not affected by the botulinum toxin status (p=0.566).
After the operation, the active shoulder external rotation increased by an average of 65.83±24.58 degrees in Group 1 (p=0.037), whereas the increase was 59.29±16.18 degrees (p=0.018) in Group 2. There was not a statistically significant difference between these increases; thus, they were not affected by the botulinum toxin status (p=0.566).
After the operation, the increase in active shoulder internal rotation was not statistically significant in Group 1 (p=0.752). However, this function decreased by 17.14±19.12 degrees in Group 2 and this difference was statistically significant (p=0.048). Active shoulder internal rotation was affected significantly by botulinum toxin status (p=0.022).
After the operation, Mallet scores increased in both groups. However, the increases were not significant statistically (p=0.115 for Group 1 and p=0.061 for Group 2) and they were not affected by the botulinum toxin status (p=0.566).
DISCUSSION
Various methods were defined for detecting muscular atrophy.[11–15] Gutmann et al.[11] resected and weighed specific muscles to find their atrophy status; however, such a method was not feasible for this clinical study. Furthermore, electrophysiologic techniques were performed as indirect measurements of muscle atrophy.[15] Ma et al.[15] injected botulinum toxin into the gastroknemius muscle of the rats and they measured motor evoked action potentials for 12 months. The electrophysiologic values showed a severe decrease at the seconde week and they returned to normal levels at the 6th month.[15] This technique was not preferred for this study because electrophysiology is not a direct predictor of muscle atrophy.
After the development of electron microscopy, microstructures of the myocytes and myofibrils were defined.[16] Denervated skeletal muscles were examined with electron microscopy and a decrease in myocyte thickness was defined as the basic sign of late term muscular atrophy.[12,13] Borodic et al.[14] injected the longissimus dorsi muscles of the rabbits with different doses of botulinum toxin and they evaluated the electron microscopic changes in myocyte thickness 5 weeks after the injection. They demonstrated the inverse proportion between the myocyte thickness and the toxin dose.[14] LDMT was measured with transmission electron microscopy and the presence of atrophy was questioned in this study.
Duchen et al.[17] injected botulinum toxin locally to the gastroknemius and soleus muscles of the rats and they evaluated the acute and chronic changes in electron microscopy. Although the microstructures of the muscles were impaired at the acute phase, myocyte regeneration began at the chronic phase (4–6th weeks).[17] These findings supported the regeneration potential of skeletal muscles after botulinum toxin injections. In this study, a significant difference was not found between the groups and botulinum toxin that was injected twice did not cause any permanent atrophy on the microstructure of the latissimus dorsi muscle at the late term.
Fortuna et al.[18] performed repetitive intramuscular (gastroknemius) botulinum toxin injections in a rabbit model and they demonstrated local and systemic atrophy at skeletal muscles after three injections. In another study, Fortuna et al. stimulated the muscles of the rabbits with electric signals after botulinum toxin injections and the stimulation had protective effects over muscle atrophy.[19] These findings were not contradicting with the results of this study. In fact, BPBP patients received less than three botulinum toxin injections and all patients were following a strict physical therapy regimen after the injections.
Magermans et al.[19] defined the shoulder functions that were integral in daily life activities and abduction and flexion were the dominant ones. In BPBP, these dominant functions benefit from the transfer of the latissimus dorsi and teres major tendons to the major tubercle of the humerus.[1,2] Waters et al.[20] demonstrated a significant increase in all shoulder functions and Mallet scores after this transfer. The results of this study were concordant with the literature and the patients had a statistically significant increase in shoulder abduction, flexion, and external rotation after the transfers. Although there was an increase in the Mallet scores of both groups, it was not significant and it may be caused by the relatively low number of the patients of this study.
According to botulinum toxin status, the patients did not show any significant difference in shoulder abduction, flexion, external rotation, and Mallet scores. These findings are comforting for BPBP patients who are prone to glenohumeral dysplasia; in fact, safe injections of botulinum toxin may be performed twice for avoiding this pathologic process. While protecting the shoulder joint, it may be possible to transfer the tendons of the injected muscles in the future.
Price et al.[21] performed botulinum toxin injections of pectoralis major muscles of BPBP patients before the transfer of the latissimus dorsi and teres major tendons. They demonstrated a decrease in internal rotation contracture and a permanent benefit on the external rotation deficit.[21] Such permanent effects of botulinum toxin therapy were evaluated by Currà et al.[22] According to their hypothesis, the toxin causes neuromuscular and peripheral changes which send different stimuli to the central nervous system, altering its excitability potential.[22] The results of these two studies are concordant with the most important finding of the present study which was the decrease in internal rotation in Group 2. This decrease may be attributed to the peripheral and central effects of the botulinum toxin injections on the internal rotation contractures. Furthermore, it may not be regarded as a functional loss for the patients because internal rotation of the shoulder may be compansated by shoulder abduction and flexion.[19]
As the strengths of this study, the groups were statistically comparable in all aspects, the follow-up period was long and the results were confirmed by dual methodology with electron microscopy and goniometry. However, the relatively low number of patients was a weakness and future studies may be performed with more patients.
Conclusion
Repetitive botulinum toxin injections may be performed on latissimus dorsi and teres major muscles during the non-operative treatment of BPBP patients. The muscles of these tendons may be transferred safely in future reconstructive surgeries because their microstructures and clinical functions are similar to non-injected muscles.
Footnotes
Ethics Committee Approval: This study was approved by the İstanbul University İstanbul Foculty of Medicine Clinical Research Ethics Committee (Date: 31.05.2016, Decision No: 2016/668).
Peer-review: Externally peer-reviewed.
Authorship Contributions: Concept: H.Ö.B., E.K., A.A., S.Ö., B.E.A., S.S.; Design: H.Ö.B., E.K., A.A., S.Ö., B.E.A., S.S.; Supervision: H.Ö.B., E.K., A.A., S.Ö., B.E.A., S.S.; Resource: E.K., H.Ö.B., A.A.; Materials: E.K., H.Ö.B., A.A.; Data: E.K., S.Ö., Ö.B., A.A.; Analysis: E.K., B.E.A., S.S.; Literature search: E.K., Ö.B., S.S., S.Ö.; Writing: E.K., Ö.B., A.A., B.E.A.; Critical revision: H.Ö.B., E.K., A.A., S.Ö., B.E.A., S.S.
Conflict of Interest: None declared.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Özkan T, Aydin A, Önel D, Özkan S. Dogumsal brakiyal pleksus felcinde omuz abdüksiyon ve eksternal rotasyonunun rekonstrüksiyonu. Acta Orthop Traumatol Turc. 2004;38:161–9. [PubMed] [Google Scholar]
- 2.Öztürk K, Bülbül M, Demir BB, Büyükkurt CD, Ayanoğlu S, Esenyel CZ. Doğumsal brakiyal pleksus felcinde omuz abdüksiyon ve dışrotasyonunun latissimus dorsi-teres majör tendon transferi ile düzeltilmesi. Acta Orthop Traumatol Turc. 2010;44:186–93. doi: 10.3944/AOTT.2010.2332. [DOI] [PubMed] [Google Scholar]
- 3.Hoeksma AF, Wolf H, Oei SL. Obstetrical brachial plexus injuries:Incidence, natural course and shoulder contracture. Clin Rehabil. 2000;14:523–6. doi: 10.1191/0269215500cr341oa. [DOI] [PubMed] [Google Scholar]
- 4.Socolovsky M, Costales JR, Paez MD, Nizzo G, Valbuena S, Varone E. Obstetric brachial plexus palsy:Reviewing the literature comparing the results of primary versus secondary surgery. Childs Nerv Syst. 2016;32:415–25. doi: 10.1007/s00381-015-2971-4. [DOI] [PubMed] [Google Scholar]
- 5.Lagerkvist AL, Johansson U, Johansson A, Bager B, Uvebrant P. Obstetric brachial plexus palsy:A prospective, population-based study of incidence, recovery, and residual impairment at 18 months of age. Dev Med Child Neurol. 2010;52:529–34. doi: 10.1111/j.1469-8749.2009.03479.x. [DOI] [PubMed] [Google Scholar]
- 6.Brown KL. Review of obstetrical palsies Nonoperative treatment. Clin Plast Surg. 1984;11:181–7. [PubMed] [Google Scholar]
- 7.Hoffer MM. The shoulder in neonatal brachial palsy. Clin Orthop Relat Res. 1999;368:101–4. [PubMed] [Google Scholar]
- 8.Heise CO, Gonçalves LR, Barbosa ER, Gherpelli JL. Botulinum toxin for treatment of cocontractions related to obstetrical brachial plexopathy. Arq Neuropsiquiatr. 2005;63:588–91. doi: 10.1590/s0004-282x2005000400006. [DOI] [PubMed] [Google Scholar]
- 9.Arad E, Stephens D, Curtis CG, Clarke HM. Botulinum toxin for the treatment of motor imbalance in obstetrical brachial plexus palsy. Plast Reconstr Surg. 2013;131:1307–15. doi: 10.1097/PRS.0b013e31828bd487. [DOI] [PubMed] [Google Scholar]
- 10.Duchen L. Effects of botulinum toxin on the distribution of succinate dehydrogenase and phosphorylase in fast and slow skeletal muscles of the mouse. J Neurol Neurosurg Psychiatr. 1970;33:580–5. doi: 10.1136/jnnp.33.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutmann E. Effect of delay of innervation on recovery of muscle after nerve lesions. J Neurophysiol. 1948;11:279–94. doi: 10.1152/jn.1948.11.4.279. [DOI] [PubMed] [Google Scholar]
- 12.Wechsler W, Hager H. Elektronenmikroskopische Befunde am atrophischen quergestreiften Skelettmuskel der Ratte nach Nervdurchtrennung. Naturwissenschaften. 1960;47:185–6. [Google Scholar]
- 13.Pellegrino C, Franzini C. An electron microscope study of denervation atrophy in red and white skeletal muscle fibers. J Cell Biol. 1963;17:327–49. doi: 10.1083/jcb.17.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borodic GE, Ferrante R, Pearce LB, Smith K. Histologic assessment of dose-related diffusion and muscle fiber response after therapeutic botulinum A toxin injections. Mov Disord. 1994;9:31–9. doi: 10.1002/mds.870090106. [DOI] [PubMed] [Google Scholar]
- 15.Ma J, Elsaidi GA, Smith TL, Walker FO, Tan KH, Martin E, et al. Time course of recovery of juvenile skeletal muscle after botulinum toxin A injection:An animal model study. Am J Phys Med Rehabil. 2004;83:774–80. doi: 10.1097/01.phm.0000137315.17214.93. [DOI] [PubMed] [Google Scholar]
- 16.Huxley H. The double array of filaments in cross-striated muscle. J Biophys Biochem Cyto. 1957;3:631–48. doi: 10.1083/jcb.3.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duchen LW. Changes in the electron microscopic structure of slow and fast skeletal muscle fibres of the mouse after the local injection of botulinum toxin. J Neurol Sci. 1971;14:61–74. doi: 10.1016/0022-510x(71)90130-4. [DOI] [PubMed] [Google Scholar]
- 18.Fortuna R, Vaz MA, Youssef AR, Longino D, Herzog W. Changes in contractile properties of muscles receiving repeat injections of botulinum toxin (Botox) J Biomech. 2011;44:39–44. doi: 10.1016/j.jbiomech.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Magermans D, Chadwick E, Veeger H, Van Der Helm F. Requirements for upper extremity motions during activities of daily living. Clin Biomech (Bristol Avon) 2005;20:591–9. doi: 10.1016/j.clinbiomech.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Waters PM, Bae DS. Effect of tendon transfers and extra-articular soft-tissue balancing on glenohumeral development in brachial plexus birth palsy. J Bone Joint Surg Am. 2005;87:320–5. doi: 10.2106/JBJS.C.01614. [DOI] [PubMed] [Google Scholar]
- 21.Price A, Ditaranto P, Yaylali I, Tidwell M, Grossman J. Botulinum toxin Type A as an adjunct to the surgical treatment of the medial rotation deformity of the shoulder in birth injuries of the brachial plexus. J Bone Joint Surg Br. 2007;89:327–9. doi: 10.1302/0301-620X.89B3.17797. [DOI] [PubMed] [Google Scholar]
- 22.CurràA , Trompetto C, Abbruzzese G, Berardelli A. Central effects of botulinum toxin Type A:Evidence and supposition. Mov Disord. 2004;19:S60–4. doi: 10.1002/mds.20011. [DOI] [PubMed] [Google Scholar]


