ABSTRACT
BACKGROUND:
Subdural empyemas (SDEs) are rare intracranial infections mostly secondary to sinusitis. Incidence of SDEs is 5–25%. Interhemispheric SDEs are even rarer, which makes their diagnosis and treatment difficult. Aggressive surgical interventions and wide-spectrum antibiotics are needed for treatment. In this retrospective clinical study, we intended to evaluate the results of surgical management supported by antibiotics in patients with interhemispheric SDE.
METHODS:
Clinical and radiological features, medical and surgical management and outcomes of 12 patients treated for interhemispheric SDE have been evaluated.
RESULTS:
12 patients were treated for interhemispheric SDE between 2005 and 2019. Ten (84%) were male, two (16%) were female. Mean age was 19 (7–38). Most common complaint was headache (100%). Five patients were diagnosed with frontal sinusitis prior SDE. Initially, three patients (27%) underwent burr hole aspiration and ten patients (83%) underwent craniotomy. In one patient both were done in the same session. Six patients were reoperated (50%). Weekly magnetic resonance imaging and blood tests were used for follow-up. All patients received antibiotics for at least 6 weeks. There was no mortality. Mean follow-up period was 10 months.
CONCLUSION:
Interhemispheric SDEs are rare, challenging intracranial infections that have been related to high morbidity and mortality rates in the past. Both antibiotics and surgical interventions play role in treatment. Careful choice of surgical approach and repeated surgeries if necessary, accompanied by appropriate antibiotic regimen, leads to good prognosis reducing morbidity and mortality.
Keywords: Interhemispheric, intracranial infection, parasagittal craniotomy, sinusitis, subdural empyema
INTRODUCTION
Subdural empyemas (SDEs) are rare intracranial infections that are located between the dura mater and arachnoid mater causing mass effect, seizures, focal neurological deficits, coma, and death. Incidence of SDE is reported from 5 to 25% of all intracranial infections.[1] SDEs, not only cause mass effect but also trigger inflammatory responses and result in encephalitis, cortical vein thrombosis, vasospasm and hydrocephalus, necessitating emergency intervention.[2] Interhemispheric or infratentorial SDEs are even more uncommon and surgical approaches and treatment strategies may be controversial and challenging.[3] Mostly they occur as an extension of frontal sinusitis however, cases that are secondary to thrombophlebitis are also known.[4] Management includes surgical evacuation of the pus, treatment of increased intracranial pressure and antibiotics. Surgical procedures include burr hole aspiration and craniotomy.[5] Even though prognosis improves with use of better and wider spectrum antibiotics in recent years, high mortality rates (around 10%) are still reported.[6]
MATERIALS AND METHODS
Records of 12 patients treated for interhemispheric SDE between 2005 and 2019 were reviewed retrospectively. Patients’ clinical features, possible source of infection, radiological imaging studies, surgical intervention (burr hole or craniotomy or both), antimicrobial therapy and outcome are evaluated.
Informed Consent
Institutional review board approved our study. All patients or relatives signed informed consents.
Surgical Technique
According to location, patient status and surgeon’s choice, only burr-holes or a craniotomy was used for drainage. Location of burr holes was planned for optimal access and drainage of infected material. If needed, a parasagittal craniotomy extending to the other side over sagittal sinus was made (Fig. 1a). Dissection of the interhemispheric fissure allowed us to evacuate the empyema and irrigate the cavity (Fig. 1b and c). Cranial magnetic resonance imaging (MRI) and biochemical markers were evaluated every week during treatment (Fig. 2). Normalized serum markers and sufficient regression of contrast enhancement in neuroradiological imaging’s under neuroradiologist approvals led us to terminate inpatient treatment.
Figure 1.
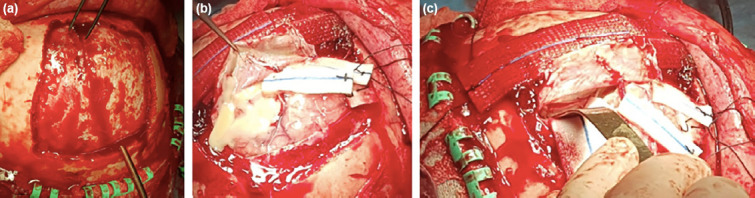
(a) Parasagittal craniotomy extending to contralateral side. Burr holes are placed on either side of the superior sagittal sinus. (b) Sinus-based opening of the dura and dissection of the interhemispheric fissure helped us to evacuate the empyema. (c) Leyla retractor was used when necessary to reach interhemispheric space.
Figure 2.
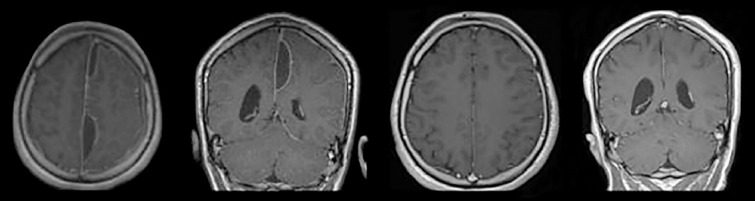
Pre-operative and post-operative MRIs of a patient with multiloculated interhemispheric subdural empyema treated with craniotomy.
RESULTS
12 patients were treated for interhemispheric SDE. Ten (84%) were male, two (16%) were female. Mean age was 19 (7–38). Headache was present in all patients. Nine patients had fever (75%), seven had nausea and vomiting (63%), four had cognitive decline (36%), and five had various degrees of hemiparesis (42%). One patient had Wernicke dysphasia and two patients had seizures.
All patients were treated with β-lactam antibiotics before neuroradiological imaging due to sinusitis, preceptal cellulitis, or fever of unknown origin. They underwent MRI and were referred to our clinic after SDE was shown.
Five patients were diagnosed with frontal sinusitis prior to admission. Three of them had undergone endoscopic sinus surgery (FESS) for the frontal sinusitis and one had only received antibiotics. For the fifth patient, sinus drainage was performed at the same session with craniotomy. Remaining patients were initially treated for fever of unknown origin and were diagnosed after neurological deterioration. They were also diagnosed with sinusitis and drainage was performed for these patients by ear-nose-throat (ENT) surgeons. Mean duration of symptoms until SDE diagnosis was 21 days (4–45).
All patients underwent surgery, either craniotomy or burr-hole aspiration. The choice between burr hole and craniotomy was made by the surgeon according to exact location and size of empyema and clinical status of patient. Resistant empyemas, cases with more edema and worse neurological status led us to choose craniotomy to achieve more decompression. Drain was left in the epidural space in eight patients, if postoperative hemorrhage was suspected, no drains were placed in the interhemispheric area. There were three burr-hole aspiration procedure and ten craniotomies. One patient had both. For this one patient, burr hole aspiration was found to be insufficient perioperatively, which led surgeons to perform craniotomy in the same session. Six patients needed second evacuation for recurrence (50%). Decision for reoperation was made according to at least one of the following factors: Reaccumulation of infective material in control MRI’s, clinical deterioration, and progressive worsening of laboratory markers. Four patients underwent craniotomy, and two patients underwent burr hole aspirations for second intervention. All six of these patients had craniotomies in their previous surgeries (Table 1).
Table 1.
Patient characteristics
| Patient No | Age | Sex | Complaint | Sinusitis | First surgery | Second surgery | Bacteriology | Antibiotic regimen | Antibiotherapy duration (weeks) | Previous antibiotherapy |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38 | M | H/A, motor deficit | + | BH | None | Sterile | V + M + Met | 7 | + |
| 2 | 14 | M | H/A, fever | + | BH | None | Sterile | V + M + Met | 8 | + |
| 3 | 34 | M | H/A, dysphasia | + | Craniotomy | None | Fusobacterium | V + Pip + Cl + A | 10 | + |
| 4 | 18 | M | H/A, motor deficit | + | Craniotomy | None | Sterile | V + M + Met | 8 | + |
| 5 | 7 | M | H/A, N/V, fever | + | Craniotomy | Craniotomy | Sterile | V + C + Met | 7 | + |
| 6 | 11 | M | H/A, N/V, AMS, fever, seizure | + | Craniotomy | Craniotomy | NHS | V + M + Met | 8 | + |
| 7 | 29 | M | H/A, N/V, AMS, motor deficit, fever | + | Craniotomy | Craniotomy | Sterile | V + C + Met | 9 | + |
| 8 | 17 | M | H/A, N/V, fever | + | Craniotomy | BH | Sterile | V + M + Met | 8 | + |
| 9 | 14 | F | H/A, N/V, AMS, fever | + | Craniotomy | BH | NHS | V + C + Met | 9 | + |
| 10 | 20 | M | H/A, N/V, AMS, motor deficit, fever | + | Craniotomy | None | Sterile | V + M + Met | 10 | + |
| 11 | 10 | F | H/A, N/V, fever, seizure | + | Both | None | NHS | V + C + Met | 10 | + |
| 12 | 12 | M | H/A, fever, motor deficit | + | Craniotomy | Craniotomy | Sterile | V + M | 8 | + |
M: Male; F: Female; H/A: Headache; N/V: Nausea and vomiting; NHS: Nonhemolytic streptococcus; AMS: Altered mental status; BH: Burrhole aspiration; V: Vancomycin; M: Meropenem; Met: Metronidazole; Pip: Piperacillin/tazobactam; Cl: Clindamycin; A: Amphotericin B; C: Ceftriaxone.
Microbiological studies were done for all patients. Culture results revealed nonhemolytic streptococcus in three patients and Fusobacterium in one patient. Cultures of the remaining eight patients ended up sterile.
All patients were treated with wide spectrum intravenous antibiotics for at least 6 weeks (6–10, mean: 7.5 weeks). Most commonly used combination of antibiotics was vancomycin, meropenem and metronidazole (6/12, 50%). Four patients received a combination therapy of vancomycin, ceftriaxone, and metronidazole, while one patient received only vancomycin and meropenem. One patient, who was found to be infected by Fusobacterium, was treated with vancomycin, piperacillin/tazobactam, clindamycin, and additional amphotericin B. Antibiotic regimens were determined on patients’ microbiological studies under infectious disease consultation.
All patients were discharged with Glasgow Outcome Scale 5 out of 5 and oral antibiotics were continued at least 2 months. 3-months-interval neuroimagings were done in outpatient clinic. Mean follow-up period was 10 months (6–18 months) (Table 1). All patients’ neurological deficits improved following treatment, except for one patient who was discharged with minor motor deficit.
Illustrative Cases
Patient 1
A 34-year-old male patient admitted to the ENT unit of another hospital for chronic sinusitis causing headache and an endoscopic sinus surgery was made. Since his complaints did not resolve postoperatively and a Wernicke dysphasia came up in postoperative day 1, cranial MRI was done. He was diagnosed with the left temporal and interhemispheric SDE in addition to empyema in frontal sinuses (Fig. 3a and b). He was referred to our hospital and an emergency craniotomy was done for left temporal SDE immediately. Since the underlying reason for Wernicke dysphasia was tought to be temporal SDE, we only tried to evacuate this one and try medical therapy for interhemispheric SDE. Culture results yielded Fusobacterium as the infectious agent. His antibiotics regimen was adjusted as penicillin G, metronidazole, and moxifloxacin. 2 days after his surgery, ENT team performed an endoscopic drainage to evacuate the empyema in the frontal sinus. However, as control MRI after 1 week revealed the interhemispheric SDE to be enlarged in spite of medical treatment, a second craniotomy over the midline was performed. He continued to get IV antibiotics for 2 more weeks and was discharged home without any deficit on oral antibiotics. Follow-up MRIs of the patient showed no residual SDE in an outpatient clinic appointment 2 months after discharge (Fig. 3c and d).
Figure 3.
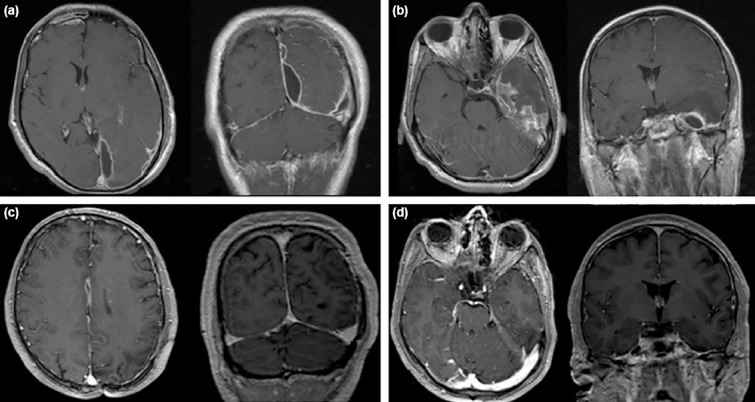
(a) First MRI of the patient when he became symptomatic showing interhemispheric SDE. (b) Initial MRI of the patient showing temporal SDE. (c and d) Follow-up MRI of the patient 2 months after discharge, without residual empyema can be seen both in interhemispheric space and temporal lobe.
Patient 2
A 12-year-old male patient was evaluated for headache and mild fever and prescribed wide spectrum antibiotics for fever of unknown origin. On the 10th day of his treatment swelling of his right eye occurred and he was hospitalized with the diagnosis of preceptal cellulitis. On his follow-up he developed left sided hemiparesis and cranial MRI was made (Fig. 4a). He was diagnosed with SDE with accompanying sinusitis. As his hemiparesis was worsening, he had emergency craniotomy. ENT team also joined the surgery for FESS procedure. Postoperatively his motor deficit improved, and immediate post-operative control MRI showed minimal pus (Fig. 4b). His microbiological studies remained sterile. He needed a second craniotomy due to recollection of infective tissue in his post-operative 1-week MRI, more posteriorly this time (Fig. 4c). He was discharged home 8 weeks after second surgery with follow-up MRIs showing no residual collection (Fig. 4d).
Figure 4.
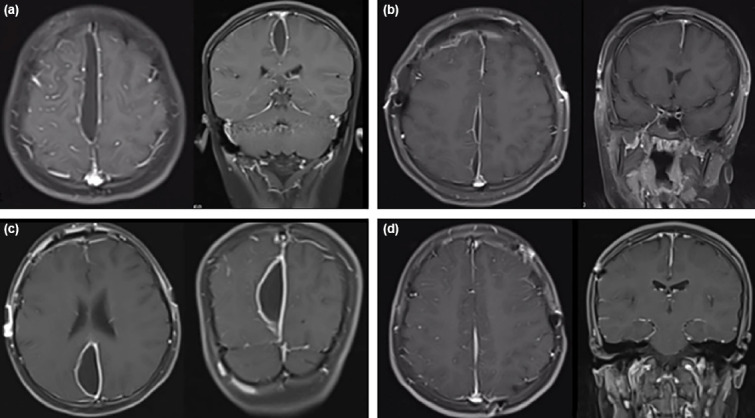
(a) Initial MRI of the patient, after motor deficit developed. (b) First post-operative MRI immediately after surgery of the patient revealed no further pus collections. (c) Post-operative 1 week MRI showing enlargement of the collection more posteriorly. (d) Control MRI in post-operative 6th month showed no residual collection.
DISCUSSION
SDEs are rare intracranial infections that are usually secondary to sinusitis in spite of continuously developing antibiotic regimes.[7] They need to be managed as a neurosurgical emergency, because patients’ neurological status may worsen rapidly due to ischemic and inflammatory changes, thrombosis, and edema underneath the empyema. Previously published case reports are summarized in Table 2.[1,2,5,7–28]
Table 2.
Patient characteristics summary of previously published case reports
| Author | Age | Sex | Complaint | Origin of infection | Surgery type | Bacteriology | Antibiotic regimen | Duration of antibiotics(weeks) |
|---|---|---|---|---|---|---|---|---|
| Baerlocher[9] | 7 | M | Fever, H/A, motor deficit, seizure | Otitis media | Craniotomy | Staphylococcus intermedius | NA | 4 |
| Rasazza[10] | 14 | M | Fever, H/A, motor deficit | Sinusitis | None | NA | Ampicillin, chloramphenicol | 6 |
| 11 | M | Fever, H/A | Sinusitis | Craniotomy | Staphlycoccus | Ampicillin | 6 | |
| Luken and Whelan[11] | 15 | F | Fever, H/A, motor deficit | Sinusitis | Craniotomy | Staphylococcus epidermidis | Methicillin, chloramphenicol | 4 |
| Borovich[12] | 16 | F | H/A, motor deficit, fever, seizure, AMS | Sinusitis | Craniotomy | Strept. B.hemolyticus, | Chloramphenicol, bacitracin | 4 |
| Bacteroides, Bacillus fusiformis | ||||||||
| Leys[13] | 29 | M | Fever, motor deficit, aphasia, | Sinusitis | Craniotomy | Sterile | Ampicillin, sisomicine, | 6 |
| seizure, AMS | trimethoprim-sulfamethoxazole | |||||||
| 19 | F | Fever, motor deficit, seizure | Sinusitis | None | NA | Ampicillin, sisomicine, | 6 | |
| trimethoprim-sulfamethoxazole | ||||||||
| 24 | F | Fever, aphasia, seizure, AMS | Sinusitis | None | NA | Ampicillin, sisomicine, pristinamycine | 16 | |
| Mauser[14] | 21 | M | Fever, H/A, aphasia, seizure, AMS | Sinusitis | None | NA | Ampicillin, chloramphenicol, | 6 |
| flucloxacillin | ||||||||
| Kagawa[15] | 22 | M | H/A, N/V, motor deficit, AMS | Sinusitis | Craniotomy | Sterile | NA | 4 |
| Lalkaka[16] | 16 | M | Fever, N/V, H/A, seizure, AMS | NA | None | NA | NA | NA |
| Dill[17] | 35 | M | Fever, H/A, aphasia, seizure | Sinusitis | Burrhole | Sterile | Nafcillin, chloramphenicol | 4 |
| 41 | M | Fever, H/A, motor deficit, aphasia | Sinusitis | Craniotomy | Bacteroides fragilis | Penicillin, metronidazole | 4 | |
| 23 | M | Fever, H/A, motor deficit, seizure, AMS | Sinusitis | Craniotomy | Sterile | Penicillin, metronidazole,ceftriaxone | 4 | |
| Mitsuoka[18] | 14 | M | Fever, H/A, motor deficit, seizure, AMS | Sinusitis | Craniotomy | Streptococcus | Cefotaxime, amikacin sulfate | NA |
| Inamasu[19] | 25 | M | Fever, H/A, motor deficit, seizure, AMS | Sinusitis | Craniotomy | Staphylococcus intermedius | Cefotaxim | NA |
| Stephanov[8] | 56 | M | Fever, H/A, motor deficit, AMS | Meningoencephalitis | Burrhole | Sterile | NA | 4 |
| Kawano[20] | 29 | M | Fever, H/A, motor deficit, AMS | Sinusitis | Craniotomy | NA | NA | NA |
| Klein[21] | 0.4 | M | Fever | Otitis media | Craniotomy | Pneumococcus | Amoxicillin/clavulanic acid | NA |
| 7 | M | Fever, seizure, motor deficit, AMS | Sinusitis | Craniotomy | Streptococcus intermedius | Cefaclor, claritromycine, | NA | |
| amoxicillin, amikacin | ||||||||
| Salunke[22] | 12 | M | Fever, H/A, N/V, motor deficit, seizure | NA | Craniotomy | Sterile | NA | 6 |
| 24 | M | Fever, H/A, N/V, motor deficit, seizure | NA | Craniotomy | Nonhemolytic streptococci | NA | 6 | |
| 5 | M | Fever, H/A, N/V | NA | Craniotomy | Sterile | NA | 6 | |
| Ramates[23] | 12 | M | H/A, motor deficit | Sinusitis | Craniotomy | NA | NA | 12 |
| 8 | F | Fever, H/A, N/V | Sinusitis | Craniotomy | Staphylococcus intermedius | NA | 12 | |
| Kazemi[24] | 16 | M | Fever, N/V, motor deficit, seizure | Sinusitis | Craniotomy | Sterile | NA | NA |
| Saravu[25] | 39 | M | Fever, motor deficit, seizure | Disseminated melioidosis | None | B.pseudomallei | Ceftazidime, cotrimoxazole, | 8 |
| doxycycline | ||||||||
| Sammartino[5] | 13 | M | Fever, H/A, seizure, AMS | Sinusitis | Burrhole | Streptococcus intermedius | Ceftriaxone | 4 |
| Yüksel[26] | 17 | F | Motor deficit, seizure | Sinusitis | Craniotomy | Sterile | Ceftriaxone, metronidazole, | 3 |
| vancomycin | ||||||||
| Arifianto[7] | 17 | M | Fever, H/A, motor deficit, seizure, AMS | NA | Craniotomy | Staphylococcus epidermidis | Ceftriaxone, metronidazole, | NA |
| gentamycin | ||||||||
| Akhaddar[27] | 14 | F | NA | NA | Craniotomy | NA | NA | NA |
| Shen[2] | 13 | F | Fever, motor deficit, AMS | Sinusitis | None | NA | NA | 2 days |
| Prieto[1] | 21 | F | Fever, H/A, motor deficit, seizure | Sinusitis | Craniotomy | Sterile | Ceftriaxone, vancomycin | 10 |
| Kapu[28] | 15 | M | HA, N/V, seizure, motor deficit | Subclinical sinusitis/mastoiditis | Craniotomy | Streptococci | NA | 6 |
| 12 | F | HA, N/V, seizure | Subclinical sinusitis/mastoiditis | Craniotomy | Sterile | NA | 6 | |
| 22 | M | HA, fever, seizure, motor deficit | Subclinical sinusitis/mastoiditis | Craniotomy | Sterile | NA | 6 |
M: Male; F: Female; H/A: Headache; N/V: Nausea and vomiting; AMS: Altered mental status; BH: Burrhole aspiration; NA: Not available.
In the literature, most patients are reported to be young adult males.[28] In our series, 84% of the patients are male and mean age is 19, consistent with the literature. Seven patients were in the pediatric age group (58%).
Spread of infection into the intracranial space occurs through different ways such as septic thrombophlebitis and direct extension due to close proximity of frontal sinuses. In our series, only five patients had previously known sinusitis, but our imaging studies revealed sinusitis of the remaining patients as well. Similarly, in the case series of Kapu et al.,[28] three patients could not be diagnosed with neither sinusitis nor mastoditis; furthermore there were no history of meningitis and trauma, which lead them to attribute the origin of the infection to be subclinical sinusitis, which was the case with our seven patient who could not be diagnosed with sinusitis prior the diagnosis of SDE.
Most common pathogens causing SDE are known to be anaerobes, aerobic Streptococci, Staphylococci, Haemophilus influenza, Streptococcus pneumoniae, and other gram-negative bacilli. Most common pathogens that specifically cause SDE secondary to sinusitis are Streptococcus milleri group. In our series, only in four patients, pathogens could be isolated. Most common pathogen isolated was nonhemolytic streptococcus. In patients with known sinusitis, 66% of the cultures were sterile. This is a little above the rate of 7–53% reported by other studies, but it can be related to the fact that patients were already on antibiotics when they were admitted.[22]
In one of our patients, Fusobacterium was identified as a causative agent, a rare one for SDE. Fusobacterium is an anaerobic, Gram-negative pathogen which causes Lemierre’s disease, described as postanginal sepsis and thrombophlebitis of internal jugular vein. Even though there are some reported cases of Fusobacterium causing intracranial abscess, meningoencephalitis and internal carotid artery aneurysms; SDEs are extremely rare. In a recent case report of Haddad, they report a 2-year-old female patient who had Fusobacterium tonsillitis complicated by SDE.[29] In all case reports published in English language, patients were diagnosed with tonsillitis before SDE. But our patient did not have any oral or peritonsillary infection previously.
Neurological manifestations of SDEs may mimic other intracranial infections and are not pathognomonic. Headache is one of the most common complaints among patients, which was similar in our series, followed by focal neurologic deficits, altered mental status, nausea, and vomiting and seizures. Falx syndrome, which is characterized by convulsions starting in the lower extremity and then progressing to generalized seizures sparing the face, can be seen among these patients.[30] In our series, only two patients had seizures on admission. Four patients of ours were neurologically intact. All of them complained of headache, while two had additional nausea and vomiting and three had high fever along headache.
Diagnostic tools include neuroradiological imaging studies and laboratory studies. Computerized tomography (CT) with contrast can visualize an infectious intracranial event very well but MRI is the gold standard. However, there are some groups which supports CT as first-line choice since it is easier to assess and faster than MRI.[7] All of our patients underwent MRI before treatment. However, few patients also underwent CT scan with contrast either at their first admission to hospital before diagnosis or to use as navigation tool when endoscopic procedures are planned.
White cell count, neutrophile percentage and infection markers such as erythrocyte sedimentation rate and C-reactive protein may be elevated. Blood culture may not always be diagnostic.[1] All of our patients were screened with these parameters weekly along with MRI. These parameters not only showed the progress of the infection but also helped us to screen systemic changes of the patients who were under multidrug antibiotics.
Cerebrospinal fluid (CSF) analysis may show elevated white cell count, elevated protein levels, and decreased glucose levels as in many intracranial infections. However, because of high intracranial pressure, lumbar puncture is usually avoided. We did not use it in any of our patients either.
Treatment of SDEs is still controversial. Evacuation of the pus is recommended for good prognosis, but conservative treatment with antibiotics is still a modality that some groups prefer for small collections.[14] There are studies which showed unusual development of meningeal arteries into the SDE, which are believed to transport antibiotics and to be the reason why sample cultures are very frequently sterile.[11,17] On contrary, it is known that surgical evacuation within 72 h of the diagnosis reduces the disability rate from 70% to 10%.[5] Furthermore, in case of failed medical therapy and worsening neurological status, rapid surgery is lifesaving.[31]
Neurosurgical intervention not only reduces the amount of pus but also helps to decompress the elevated intracranial pressure and to identify to organism to further reshape the antibiotic regimen. Burr hole aspiration and craniotomy are the main procedures that are being widely used.[3] Choice of the procedure usually depends on multiple factors such as size and location of empyema, patient’s clinical status, and surgeon’s preference. In our series, three out of thirteen procedures were burr hole aspiration, and the remaining, craniotomies.
Burr hole aspiration is an effective method to evacuate SDEs. However, since interhemispheric empyemas are usually multiloculated, large craniotomies may be required as well as second surgeries. In addition, since interhemispheric space is a narrow area which is difficult to reach and SDEs cause further adhesions, craniotomies take advantage over burr holes. Another disadvantage of burr holes is that they can lead to further damage of the friable cortex.[31]
To perform less invasive procedures but also to increase the evacuation amount of the pus, Sammartino et al.[5] performed endoscope assisted burr hole aspiration and published their results. In our series, three patients underwent burr hole aspiration and ten patients underwent craniotomy, one patient needed craniotomy in the same session when burr hole aspiration failed. Recollection on control MRI was the main indication for a second operation even in patients with decreasing laboratory signs of infection. All six patients (50%), who required second surgeries, had craniotomies as first surgery. Out of these six patients, four underwent craniotomy and two underwent burr hole aspiration. This was not consisted with the literature data, which shows initial surgeries of patients that underwent second surgery is usually burr hole aspiration. We believe, burr hole aspiration for the second intervention worked for our patients because previous craniotomies were helpful to reduce the majority of the pus and ongoing antibiotics ease aspiration. Furthermore, in these cases, we observed a change of pus location after first craniotomy and therefore, instead of performing a new craniotomy, we used burr holes.
Large studies confirmed lower mortality rates in craniotomy-performed patients.[32] This is likely explained by better and faster decrease in intracranial pressure and better exploration of the narrow interhemispheric space. Nevertheless, there are also some studies that showed no difference in outcome and mortality rates between craniotomy and burr hole aspiration.[3] In our series, burr hole aspirations group did not require further surgical intervention whereas six patients of craniotomy group needed second surgery for recurrence (6/10, 60%). There was no mortality in our series. All patients’ neurological examination, except one that had minor motor deficit at the time of discharge, was normal.
All patients received wide spectrum antibiotics combined with surgical treatment. Most common antibiotic combination was vancomycin, meropenem and metronidazole (50%). Furthermore, combination of other antibiotics that can pass blood-brain barrier, such as ceftriaxone, was used (4/12). Antibiotics regimens were consistent with literature. As summarized in Table 2 antibiotic regimens evolved over time from beta lactam antibiotics and chloramphenicol, which is hazardous, to broad spectrum antibiotics with lower side effects and higher penetration to CSF. One patient with Fusobacterium infection received additional antifungal medications. Medical therapy regimens were decided on patients’ microbiological studies. Case-specific medical therapy was formed by infectious disease specialists. Overall intravenous antibiotics duration was decided on patient’s clinical status, neuroradiological, and biochemical improvement. All patients received intravenous antibiotics for at least 6 weeks (6–10, mean: 7.5 weeks).
Conclusion
Interhemispheric SDEs are rare, life threatening infections mostly secondary to sinusitis. Treatment modalities include antibiotics and surgical evacuation. Surgery is helpful to reduce the amount of the pus, to identify the pathogen and to decrease the intracranial pressure when necessary. Burr hole aspiration and craniotomy are two mainstream surgical methods. Even though they are both shown to reduce mortality, case specific choice must be made. And in necessary cases, repeat surgeries should be considered.
Footnotes
Ethics Committee Approval: This study was approved by the Bakırköy Dr. Sadi Konuk Training and Research Hospital Clinical Research Ethics Committee (Date: 03.01.2022, Decision No: 2022-01).
Peer-review: Externally peer-reviewed.
Authorship Contributions: Concept: D.G.G., D.D., İ.D., S.B., T.C.Ü., E.Ö., G.A., H.M.Ö., A.A., M.K., A.S.; Design: D.G.G., D.D., İ.D., S.B., T.C.Ü., E.Ö., G.A., H.M.Ö., A.A., M.K., A.S.; Supervision: G.G., A.A., A.Ç.; Resource: G.G., A.A., A.Ç.; Materials: M.K., H.Ö.; Data: G.A., S.B.; Analysis: İ.D., T.C.Ü., E.Ö.; Literature search: İ.D., T.C.Ü., E.Ö.; Writing: G.G., D.D.; Critical revision: G.G., A.A., A.Ç.
Conflict of Interest: None declared.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Prieto R, Ortega C. Parafalcine subdural empyema:The unresolved controversy over the need for surgical treatment. Surg Neurol Int. 2019;10:203. doi: 10.25259/SNI_392_2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen YY, Cheng ZJ, Chai JY, Dai TM, Luo Y, Guan YQ, et al. Interhemispheric subdural empyema secondary to sinusitis in an adolescent girl. Chin Med J (Engl) 2018;131:2989–90. doi: 10.4103/0366-6999.247213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bok AP, Peter JC. Subdural empyema:Burr holes or craniotomy?A retrospective computerized tomography-era analysis of treatment in 90 cases. J Neurosurg. 1993;78:574–8. doi: 10.3171/jns.1993.78.4.0574. [DOI] [PubMed] [Google Scholar]
- 4.Benevides GN, Salgado GA, Jr, Ferreira CR, Felipe-Silva A, Gilio AE. Bacterial sinusitis and its frightening complications:Subdural empyema and Lemierre syndrome. Autopsy Case Rep. 2015;5:19–26. doi: 10.4322/acr.2015.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sammartino F, Feletti A, Fiorindi A, Mazzucco GM, Longatti P. Aspiration of parafalcine empyemas with flexible scope. Childs Nerv Syst. 2016;32:1123–9. doi: 10.1007/s00381-016-3082-6. [DOI] [PubMed] [Google Scholar]
- 6.Niklewski F, Petridis AK, Al Hourani J, Blaeser K, Ntoulias G, Bitter A, et al. Pediatric parafalcine empyemas. J Surg Case Rep. 2013;2013:rjt067. doi: 10.1093/jscr/rjt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arifianto MR, Ma'ruf AZ, Ibrahim A, Bajamal AH. Interhemispheric and Infratentorial subdural empyema with preseptal cellulitis as complications of sinusitis:A case report. Pediatr Neurosurg. 2018;53:128–33. doi: 10.1159/000481512. [DOI] [PubMed] [Google Scholar]
- 8.Stephanov S, Sidani AH, Amacker JJ. Interhemispheric subdural empyema--case report. Swiss Surg. 2001;7:229–32. doi: 10.1024/1023-9332.7.5.229. [DOI] [PubMed] [Google Scholar]
- 9.Baerlocher K, Arregger G, Benini A, Gaspar B, Valavanis A, Schubiger O. Subdural interhemispheric empyema in a 7-year-old boy. Helv Paediatr Acta. 1979;34:583–8. [PubMed] [Google Scholar]
- 10.Rosazza A, de Tribolet N, Deonna T. Nonsurgical treatment of interhemispheric subdural empyemas. Helv Paediatr Acta. 1979;34:577–81. [PubMed] [Google Scholar]
- 11.Luken MG, 3rd, Whelan MA. Recent diagnostic experience with subdural empyema. J Neurosurg. 1980;52:764–71. doi: 10.3171/jns.1980.52.6.0764. [DOI] [PubMed] [Google Scholar]
- 12.Borovich B, Braun J, Honigman S, Joachims HZ, Peyser E. Supratentorial and parafalcial subdural empyema diagnosed by computerized tomography. Case report. J Neurosurg. 1981;54:105–7. doi: 10.3171/jns.1981.54.1.0105. [DOI] [PubMed] [Google Scholar]
- 13.Leys D, Destee A, Petit H, Warot P. Management of subdural intracranial empyemas should not always require surgery. J Neurol Neurosurg Psychiatry. 1986;49:635–9. doi: 10.1136/jnnp.49.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mauser HW, Ravijst RA, Elderson A, van Gijn J, Tulleken CA. Nonsurgical treatment of subdural empyema. Case report. J Neurosurg. 1985;63:128–30. doi: 10.3171/jns.1985.63.1.0128. [DOI] [PubMed] [Google Scholar]
- 15.Kagawa R, Shima T, Matsumura S, Okada Y, Nishida M, Yamada T, et al. Primary interhemispheric subdural abscess:Report of a case. No Shinkei Geka. 1989;17:647–52. [PubMed] [Google Scholar]
- 16.Lalkaka JA, Parikh JM, Nath AR, Meisheri YV, Vengsarkar US, Deshpande DV. Interhemispheric empyema. An unusual form of subdural empyema. J Assoc Physicians India. 1989;37:394–6. [PubMed] [Google Scholar]
- 17.Dill SR, Cobbs CG, McDonald CK. Subdural empyema:Analysis of 32 cases and review. Clin Infect Dis. 1995;20:372–86. doi: 10.1093/clinids/20.2.372. [DOI] [PubMed] [Google Scholar]
- 18.Mitsuoka H, Tsunoda A, Mori K, Tajima A, Maeda M. Hypertrophic anterior falx artery associated with interhemispheric subdural empyema--case report. Neurol Med Chir (Tokyo) 1995;35:830–2. doi: 10.2176/nmc.35.830. [DOI] [PubMed] [Google Scholar]
- 19.Inamasu J, Horiguchi T, Saito R, Nakamura Y, Ichikizaki K, Takahashi K, et al. Interhemispheric subdural empyema in a young man. Am J Emerg Med. 2001;19:602–3. doi: 10.1053/ajem.2001.28041. [DOI] [PubMed] [Google Scholar]
- 20.Kawano H, Yonemura K, Misumi Y, Hashimoto Y, Hirano T, Uchino M. A case of interhemispheric subdural empyema with sinusitis diagnosed by diffusion-weighted MRI. Rinsho Shinkeigaku. 2005;45:449–52. [PubMed] [Google Scholar]
- 21.Klein O, Freppel S, Schuhmacher H, Pinelli C, Auque J, Marchal JC. Subdural empyema in children:Therapeutic strategy Five cases. Neurochirurgie. 2006;52:111–8. doi: 10.1016/s0028-3770(06)71205-x. [DOI] [PubMed] [Google Scholar]
- 22.Salunke PS, Malik V, Kovai P, Mukherjee KK. Falcotentorial subdural empyema:Analysis of 10 cases. Acta Neurochir (Wien) 2011;153:164–9. doi: 10.1007/s00701-010-0695-5. discussion 170. [DOI] [PubMed] [Google Scholar]
- 23.Ratamess NA. Announcement:New JSCR article format. J. Strength Cond Res. 2019;33:1179. doi: 10.1519/JSC.0000000000003174. [DOI] [PubMed] [Google Scholar]
- 24.Kazemi KA, Pishjoo M, Safdari Z. Interhemispheric subdural empyema in 16 years old boy A case report. Int J Med Investig. 2015;4:407–9. [Google Scholar]
- 25.Saravu K, Kadavigere R, Shastry AB, Pai R, Mukhopadhyay C. Neurologic melioidosis presented as encephalomyelitis and subdural collection in two male labourers in India. J Infect Dev Ctries. 2015;9:1289–93. doi: 10.3855/jidc.6586. [DOI] [PubMed] [Google Scholar]
- 26.Yüksel MO, Gürbüz MS, Karaarslan N, Caliskan T. Rapidly progressing interhemispheric subdural empyema showing a three-fold increase in size within 12 hours:Case report. Surg Neurol Int. 2016;7(Suppl 37):S872–5. doi: 10.4103/2152-7806.194495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akhaddar A. Atlas of Infections in Neurosurgery and Spinal Surgery. Berlin, Germany: Springer; 2017. Available from: https://www.atithibooks.com/product/atlas-of-infections-in-neurosurgery-and-spinal-surgery-2017-by-akhaddar-publisher-springer . [Google Scholar]
- 28.Kapu R, Pande A, Vasudevan MC, Ramamurthi R. Primary interhemispheric subdural empyemas:A report of three cases and review of literature. Indian J Neurosurg. 2013;2:66–70. [Google Scholar]
- 29.Haddad N, Morris T, Dhillon R, Gibbon F. Unusual neurological presentation of Fusobacterium necrophorum disease. BMJ Case Rep. 2016;2016:bcr2015210710. doi: 10.1136/bcr-2015-210710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.List CF. Diagnosis and treatment of acute subdural empyema. Neurology. 1955;5:663–70. doi: 10.1212/wnl.5.9.663. [DOI] [PubMed] [Google Scholar]
- 31.Yilmaz N, Kiymaz N, Yilmaz C, Bay A, Yuca SA, Mumcu C, et al. Surgical treatment outcome of subdural empyema:A clinical study. Pediatr Neurosurg. 2006;42:293–8. doi: 10.1159/000094065. [DOI] [PubMed] [Google Scholar]
- 32.Nathoo N, Nadvi SS, Gouws E, van Dellen JR. Craniotomy improves outcomes for cranial subdural empyemas:Computed tomography-era experience with 699 patients. Neurosurgery. 2001;49:872–7. doi: 10.1097/00006123-200110000-00017. discussion 877–8. [DOI] [PubMed] [Google Scholar]


