Abstract
Physiological function fluctuates across 24 h due to ongoing daily patterns of behaviors and environmental changes, including the sleep/wake, rest/activity, light/dark, and daily temperature cycles. The internal circadian system prepares the body for these anticipated behavioral and environmental changes, helping to orchestrate optimal cardiovascular and metabolic responses to these daily changes. In addition, circadian disruption, caused principally by exposure to artificial light at night (e.g., as occurs with night-shift work), increases the risk for both cardiovascular and metabolic morbidity and mortality. Regular exercise is a countermeasure against cardiovascular and metabolic risk, and recent findings suggest that the cardiovascular benefits on blood pressure and autonomic control are greater with evening exercise compared to morning exercise. Moreover, exercise can also reset the timing of the circadian system, which raises the possibility that appropriate timing of exercise could be used to counteract circadian disruption. This article introduces the overall functional relevance of the human circadian system and presents the evidence surrounding the concepts that the time of day that exercise is performed can modulate the cardiovascular and metabolic benefits. Further work is needed to establish exercise as a tool to appropriately reset the circadian system following circadian misalignment to preserve cardiovascular and metabolic health.
Introduction
The internal circadian system enables humans to adapt to the predictable changes in the environment and the anticipated behaviors that occur on a daily basis, including daily changes in temperature, wake/sleep, activity/rest, and the feeding/fasting cycles (35). The circadian system consists of central and peripheral molecular clocks in all nucleated cells. The central neural clock located in the bilateral suprachiasmatic nucleus (SCN) of the hypothalamus is mainly synchronized by the light/dark cycle via the retinohypothalamic tract (38). This central pacemaker synchronizes the peripheral clocks across the body via numerous inputs, including influences from the nervous system, the endocrine system, and changes in body temperature (118). This circadian system thereby causes approximately 24 h oscillations in cardiovascular (CV) physiology, and glucose homeostasis, such as the increase in cardiac sympathovagal balance and skeletal muscle insulin sensitivity that typically occurs across the morning transition from sleep to wakefulness, changes in posture, and exercise (114, 131, 134). Since mustering optimal physiological responses to rapidly changing behaviors can take time (e.g., maximal release of cortisol can take tens of minutes), the circadian modulation of the body’s physiological state improves the physiological readiness compared to a state if the body simply responded to the changing environment or behaviors via negative feedback control. Perhaps, the best example of the circadian system preparing for anticipated behavior is the increase in circulating cortisol, heart rate (HR), and blood pressure (BP) before the end of sleep in preparation for awakening (134). Along these lines, it is not surprising therefore that another common behavior (i.e., exercise) conducted at different times of the circadian cycle produces different CV responses (123), and it is conceivable that these different responses may provide different degrees of CV benefits in the long term. Indeed, different research groups have consistently found greater CV benefits when exercise is conducted in the evening compared to other times of the day. One of the objectives of this article will be to discuss if there is a best time of day to exercise for optimal CV benefits and consider whether this is related to the circadian system. Furthermore, considering metabolism, increased glycolytic activity during exercise occurs when exercise is performed in the early active phase of mice, with enhanced carbohydrate metabolism when exercise is performed during the habitual rest phase of mice (112). This daily pattern of metabolic responses to exercise in mice suggests a potential time of day influence of exercise on glucose homeostasis in humans, which we also discuss in the present article.
While the central circadian system provides physiological stability in the face of anticipated daily environmental and behavioral changes, this stability comes at the cost of inability to change quickly, and the central SCN pacemaker can only be reset to a new time by small amounts of up to approximately 1h each day based on strong light input (35, 117). This resultant sluggishness of entrainment of the internal central pacemaker to the external clock (i.e., timing of environmental or behavioral changes) is best illustrated by the “jet lag” that can last many days following trans-meridian jet travel (54, 137). These disruptions of the “normal” circadian patterns also commonly occur in the nearly ubiquitous “24/7 lifestyle” of modern society. Importantly, such chronic circadian disruptions are associated with increased CV and metabolic risk (54, 107, 108, 127, 132, 133, 137). Indeed, systematic reviews of longitudinal studies have concluded that regular night-shift workers have a higher risk of developing hypertension (77), diabetes and obesity (101), and coronary heart disease (127). Multiday experimental studies conducted under stringent environmental and behavioral control have uncovered some of the central circadian mechanisms that may underlie this increased CV and metabolic risk. These mechanisms have been studied in healthy individuals who usually sleep at night (87, 113) as well as in regular nightshift workers (88). Disruption of the circadian system, such as internal circadian desynchronization, can occur when the central circadian pacemaker and varied peripheral circadian clocks respond by altering their phases differently during environmental or behavioral exposures (90). As a countermeasure to resynchronize these varied rhythms, exercise has been suggested to influence peripheral clocks as well as the central clock (3) and forms the basis behind the concept that timed exercise can be used as a clinical tool to appropriately reset the circadian system following circadian misalignment to preserve CV health.
In this article, we have indicated those studies that mention only times of day comparisons (termed diurnal studies, which do not distinguish day/night patterns based on habitual timing of behaviors from underlying endogenous circadian effects). While there can be wide individual variations among subjects in the time relative to internal circadian phase of common daily behaviors such as sleep [e.g., the habitual circadian “phase angle of entrainment” for sleep (61)], extrapolation of average results of any such diurnal studies to internal circadian time would require that the subjects between studies had a similar average phase angle of entrainment. Such common average circadian rhythm synchronization would be more likely if studies excluded shift work and subjects had similar habitual sleep times, and excluded people with extreme late or early chronotypes, as in many of the studies discussed here (7–9, 11–13, 25, 103). Nonetheless, this remains an assumption as the phase angle of entrainment was not measured in those studies. Additionally, we also discuss those relatively rare studies that do examine the underlying endogenous circadian variations (termed circadian studies, which show rhythms in CV physiology that persist under constant behavioral and environmental conditions, or where there are variations in CV responses to identical exercise bouts across the day and night wherein these changes in responsivity are caused by effects of the endogenous circadian system). This article introduces the overall functional relevance of the human circadian system and presents the evidence surrounding the concepts that (i) the time of day that exercise is performed can modulate CV and metabolic benefits and (ii) the possibility of using exercise as a tool to appropriately reset the circadian system following circadian misalignment to preserve CV health.
Chronobiology of Exercise
In this article, “exercise” is considered as a structured and programmed physical activity with a goal of greater well-being and improvement or maintenance of physical capacities, while “physical activity” is considered as any nonprogrammed movement, such as cleaning the house or movements performed during work (19).
The influence of exercise on synchronizing biological rhythms, involving changes to the molecular clock machinery, and the influence of biological rhythms on physiological responses promoted by exercise (i.e., during, recovery postexercise, or chronic adaptations from exercise training) are both considered as chronobiological aspects of exercise. Exercise can be used as a tool to synchronize disrupted circadian rhythms due to its phase-shifting properties observed on body temperature (30), melatonin (140), and thyroid-stimulating hormone (130). In addition, studies have reported changes in the molecular clock in skeletal muscle after exercise in mice (112), and in humans after both resistance (141) and aerobic exercise (27). For example, exercise affects the gene expression of HIF-1a (95), PGC1a (70), and AMPK (68) that are associated with the molecular clock. Indeed, it is well established by recent systematic reviews and meta-analyses that there is a diurnal variation in many physical capacities, such as strength and power (65, 105), plus the ability to perform repeated sprints (102), which display their peaks between afternoon and evening, while the time of day for peak endurance performance is still uncertain (65). Presently, the best time to perform exercise to achieve a better CV and metabolic function is not well understood. In this article, we have attempted to distill available information to approach these gaps in knowledge.
Cardiovascular Diurnal Variation and Exercise
Almost all physiological markers of the CV system oscillate on a 24-h pattern. For instance, BP and HR are higher during the day than the night (26, 81, 135). The SCN coordinates circadian rhythms of the CV system directly by multisynaptic projections to the nucleus of the solitary tract (58), heart (116), blood vessels (2), and adrenal cortex (15), in addition to the inputs to the peripheral clocks in the heart and the vasculature (110, 142). These diurnal variations open windows of cellular and tissue repair and preparatory substrate storage during the anticipated daily quiescent periods (usually at night in humans) and for optimal substrate usage during the anticipated active periods during the day. The existence of daily variations in maximal double product (the product of systolic BP and HR) and in the acute CV responses to a single session of exercise at different times of day suggests that both the exercise peak performance and the long-term benefits of exercise training may vary based on the phase of the internal circadian clock at which exercise is performed (or the time of day, for studies that do not estimate internal circadian time). The first concept regarding athletic performance has been reviewed recently (102), and the current article considers the potential beneficial effects of timed exercise on CV health. Recent studies have shown that evening exercise is more beneficial to CV health compared to morning exercise. These results were observed after one single session of exercise (8, 13, 25, 56, 57, 93) and as a chronic effect after a training program (11, 12, 126). Thus, determination of the best time to exercise while taking advantage of a possible window of opportunity for optimal CV effects may bring important advances to the field. Moreover, as noted above, exercise itself can also affect the timing of peripheral as well as central clocks (3, 17, 18, 83, 140).
In healthy individuals, a sudden large increase in double product occurs immediately after awakening from sleep and standing, with the greatest CV responses around 9 to 10AM, followed by a nadir in the afternoon around 3PM and a second peak around 7PM (100). This daily variation is in part due to increased sympathetic nervous system activity in the morning (caused by the combination of the internal circadian system and the ongoing behaviors), evidenced by the increased plasma levels of cortisol, norepinephrine, and epinephrine (16), which can lead to increased systemic vascular resistance (39). This increase in BP in the morning is facilitated by the lower baroreflex sensitivity across this period (119, 125). Circadian laboratory studies have determined that cardiac parasympathetic modulation, plasma levels of cortisol, and circulating catecholamines exhibit internal circadian rhythms independent of daily behaviors (e.g., sleep and physical activity), and that these circadian rhythms can interact with the behavioral responses, causing different responses to identical stressful behaviors at different circadian times of day (51, 114). Similarly, cardiac sympathetic and parasympathetic tones are also affected by sleep (6, 16, 117), which can modulate responses to awakening from sleep and standing, and these responses could depend on the prevailing circadian phase.
In parallel to these daily variations in autonomic regulation, vascular endothelial function, evaluated by flow-mediated dilation or reactive hyperemia techniques, is also diminished in the morning according to a diurnal study, a formal circadian study, and in a protocol that separated sleep at night from the inactivity that normally accompanies sleep across the night (92, 121, 122). Overall, these studies suggested that the morning decrease in vasodilatory capacity is primarily caused by the internal circadian system rather than sleep or the inactivity that accompanies sleep across the night. The morning decrease in vasodilatory capacity was also not associated with nocturnal plasma levels of endothelin-1 (122), a potent vasoconstrictor produced by the vascular endothelium. However, according to a diurnal study, levels of another important vasoconstrictor, angiotensin II, are higher in the morning than in the evening (22). Conversely, the serum levels of nitric oxide, a vasodilator largely produced by endothelial tissue, are lower in the morning than in the evening (59). The balance between vasoconstrictor-vasodilator effects along with the increased prothrombotic milieu observed in the morning due to increased platelet aggregability (86) and plasminogen activator inhibition 1 (PAI-1) (115) could partly explain the propensity toward an increased risk of adverse CV events in the morning (75, 89). In the early evening, these patterns of CV variations are mostly reversed, culminating in reduced double product and lower CV risk due to interactions between effects from the internal circadian system, the environment, and ongoing evening behaviors (16, 55, 115, 121).
Exercise and Time of Day
Regular exercise at any time of day is beneficial for the CV system and overall health (47). Available evidence in limited cohorts, including in people with hypertension, suggests that exercise-based adaptations for CV health may be best achieved when exercise is performed in the late afternoon and evening (8, 11–13, 25, 56, 57, 93, 103, 126). All of these diurnal studies comparing different times of day have conducted aerobic exercise (i.e., cycle ergometer, walking, and running), and most of them at moderate intensity [i.e., between 50% and 70% of or between anaerobic threshold and respiratory compensation point (e.g., ventilatory threshold)], while two evaluated BP and HR recovery responses after maximal cardiopulmonary exercise tests (7, 11). In line with these data, although not proven, it seems prudent to suggest that in people with existing chronic disease or increased risk for adverse CV events, strenuous exercise should be avoided in the morning to reduce the risk of these events (4, 114). Indeed, a circadian study demonstrated that parasympathetic withdrawal and circulating catecholamines had their peak during exercise at 9AM (114).
Diurnal variation in CV responses to exercise has been studied for decades, with most recent interest on the acute benefits of exercise in decreasing double product, mainly via the reduction in BP after exercise compared with BP values before exercise. This phenomenon, called postexercise hypotension (PEH) (10, 62), is clinically relevant since a single bout of exercise can reduce BP for up to 24h (10, 62). There is unequivocal evidence from diurnal studies demonstrating that the magnitude of PEH is greater in the evening than in the morning. In people with normotension, evening exercise promoted greater reduction than morning exercise of approximately 10mmHg for systolic BP and approximately 6mmHg for mean BP (56, 57). Participants with prehypertension presented greater reduction after evening than morning exercise of approximately 3mmHg for systolic BP (assessed in the laboratory) and approximately 4mmHg for asleep systolic BP (assessed from ambulatory BP monitoring) (13, 25). In people with hypertension, evening exercise provided greater decrease than morning exercise by approximately 4mmHg for both systolic and mean BP (assessed in the laboratory) but only for patients receiving angiotensin receptor blocker (ARB) antihypertensive medication (8, 93). Individuals with hypertension and a nocturnal non-dipper profile (i.e., hypertensive patients in whom the average asleep BP does not decrease by 10% compared to the average awake BP) had a greater reduction in asleep systolic BP after evening than morning exercise of approximately 5mmHg (8, 93). All these findings were reinforced by a circadian study in people with normotension (103) where the fastest recovery rate for systolic BP postexercise occurred in the biological late afternoon (corresponding to ~5PM), while slowest recovery occurred in the biological morning (corresponding to 8:30AM) (103) (Table 1). In these studies, normotension or prehypertension were determined by office measurements of BP based on standard guidelines (7, 9, 13, 56, 57, 103), and in the two studies patients with hypertension had prior diagnoses from their primary care physicians (8, 93). Despite the similarities of findings, the aforementioned studies had some differences that are worth considering. For example, one study was conducted with untreated patients after discontinuing antihypertensive medication under their physicians’ approval (93), while the other investigated medicated patients with hypertension (8). Surprisingly, the patients with hypertension who received ARB had greater PEH in the evening than in the morning, but this did not occur in those receiving angiotensin converting enzyme inhibitor (ACEi) (8). These results suggest that the renin-angiotensin-aldosterone system may play a role in the diurnal pattern of PEH. ACEi but not ARB increases the plasma levels of angiotensin 1–7 and bradykinin, two vasodilatory substances (91) that are released during exercise and stay increased after exercise in parallel to PEH (84). Thus, the chronically higher levels of angiotensin 1–7 and bradykinin in hypertensive patients receiving ACEi might blunt its greater increase after evening exercise. However, this exploratory study did not control the time of day that medications were taken, and this essential aspect should be investigated in the future. Few studies have compared the effects of morning versus evening exercise on BP across the subsequent sleep period. The average asleep BP is reduced only after evening exercise (13), mainly in non-dipper hypertensives (93). This result is clinically relevant since the non-dipper pattern of BP presents a higher risk to target organ damage and CV events than the dipper profile, independent of the mean BP level (48, 49). This relative nocturnal drop in BP with evening exercise slightly increased the overall amplitude of the 24-h BP profile, assessed by cosinor analysis (13), thus trending more toward the healthier dipping BP pattern. Although environmental and behavioral influences are likely involved in this greater PEH after evening exercise, a recent study demonstrated that the internal circadian system is also involved (103). Variation in ambulatory BP postexercise results may be related to the sample sizes involved in the reported studies. In particular, Park and colleagues only had nine subjects classified as dippers and five as non-dippers based on the control ambulatory BP monitoring sessions (93). Ambulatory BP after exercise had excellent reproducibility in 18 patients with hypertension, but the authors did not find any decrease in ambulatory BP postexercise, suggesting a small effect size for these variables (69). Negative findings in small studies can be a concern (i.e., potentially false negative), such that future investigations should base the sample size on power analyses to determine clinically meaningful differences in ambulatory BP responses to exercise at different times of the day.
Table 1.
Summary of Acute and Chronic Studies Comparing Cardiovascular Outcomes at Different Times of the Day
| Author | Mean age |
n(sex) | Clinical condition |
Interventions | Type of exercise | Exercise protocol | Main findings | Secondary findings |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Acute exercise studies | ||||||||
| de Brito et al. | 32 | 16(males) | Pre-HT | ME (9 AM) | Cycle ergometer | 45 min/50%VO2peak | ↓SBP both times of the day | ↓SBP after ME was due to |
| (25) | MC (9 AM) | – | At rest | ↓Cardiac output | ||||
| EE (6 PM) | Cycle ergometer | 45 min/50%VO2peak | ↓SBP greater in EE than ME | ↓Systemic vascular | ||||
| EC (6 PM) | – | At rest | resistance↓SBP after EE was due to | |||||
| Brito et al. (7) | 33 | 10(males) | Pre-HT | ME (8–10 AM) EE (6–8 PM) |
Cycle ergometer Cycle ergometer |
CPET CPET |
Parasympathetic reactivation is blunted in the EE than ME | – |
| Brito et al. | 33 | 13 (males) | Pre-HT | ME (9 AM) | Cycle ergometer | 45 min/50%VO2peak | ↓Asleep SBP occurred only after EE | ↓MESOR SBP occurred only after EE |
| (13) | MC (9 AM) | – | At rest | |||||
| EE (6 PM) | Cycle ergometer | 45 min/50%VO2peak | ||||||
| EC (6 PM) | – | At rest | ||||||
| Brito et al. (9) | 25 | 5 (females) 5(males) |
Healthy | ME (8:30 AM) EE (6 PM) |
Single-leg ergometer Single-leg ergometer |
60min/60%peak power 60min/60%peak power |
↑Leg blood flow similar between ME and EE |
↑Vascular conductance similar between ME and EE |
| Brito et al. (8) | 49 | 15 (males) | HT | ARB-ME (7–9 AM) | Cycle ergometer | CPET | ↓SBP ARB was greater after EE | ↓SBP occurred in both groups and sessions |
| ARB-EE (8–10 PM) | Cycle ergometer | CPET | ||||||
| 50 | 14(males) | HT | ACEi-ME (7–9 AM) | Cycle ergometer | CPET | ↓SBP ACEi was the same between ME and EE | ||
| ACEi-EE (8–10 PM) | Cycle ergometer | CPET | ||||||
| Jones et al. | 26 | 12 (males) | Healthy | ME (8 AM) | Cycle ergometer | 30min/70% VO2peak | ↓MBP was greater after EE | – |
| (55) | EE (4 PM) | Cycle ergometer | 30min/70% VO2peak | |||||
| Jones et al. | 31 | 6(males) | Healthy | 4 AM | Cycle ergometer | 30min/60% VO2peak | ↓MBP in all afternoon exercise sessions | – |
| (56) | 6 AM | Cycle ergometer | 30min/60% VO2peak | |||||
| 8 AM | Cycle ergometer | 30min/60% VO2peak | ||||||
| 10 AM | Cycle ergometer | 30min/60% VO2peak | ||||||
| 4 PM | Cycle ergometer | 30min/60% VO2peak | ||||||
| 6 PM | Cycle ergometer | 30min/60% VO2peak | ||||||
| 8 PM | Cycle ergometer | 30min/60% VO2peak | ||||||
| 10 PM | Cycle ergometer | 30min/60% VO2peak | ||||||
| Park et al. (93) | 58 | 3 (females) 2 (males) |
HT | Non-dipper ME | Treadmill | 30min/50% VO2peak | ↓Asleep SBP was greater after EE in non-dipper than dipper HT patients | |
| Non-dipper MC | – | At rest | ↓Awake SBP was similar between groups after ME | |||||
| Non-dipper EE | Treadmill | 30min/50% VO2peak | ||||||
| Non-dipper EC | – | At rest | ||||||
| 56 | 3(females) | HT | Dipper ME | Treadmill | 30min/50% VO2peak | |||
| 6(males) | Dipper MC | – | At rest | |||||
| Dipper EE | Treadmill | 30min/50% VO2peak | ||||||
| Dipper EC | – | At rest | ||||||
| Qian et al. (103) |
26 | 6(females) 6(males) |
Healthy | Forced desynchrony protocol 2 AM 6 AM |
Cycle ergometer | 15 min/60%HRmax | ↓SBP presented circadian variation with the greatest decrease at 4:30 PM | DBP and HR recovery did no present circadian variation |
| 10 AM | ||||||||
| 2 PM | ||||||||
| 6 PM | ||||||||
| 10 PM | ||||||||
| Chronic exercise studies | ||||||||
| Brito et al. (12) |
51 | 15(males) | HT | MT (7–10 AM) | Cycle ergometer | 3×/w to 10 weeks 45 min/moderate |
↓Clinic SBP and ↓ambulatory DBP occurred only after ET ↓Systemic vascular resistance and vasomotor sympathetic modulation occurred only after ET |
↓HR, sympathovagal balance, and t cardiac baroreflex sensitivity occurred after MT and ET, but cardiac baroreflex sensitivity was greater after ET |
| 49 | 15(males) | ET (6–9 PM) | Cycle ergometer | 3×/w to 10 weeks 45 min/moderate |
||||
| 50 | 20(males) | C (10 at 7–10 AM/ 10 at 6–9 PM) |
Stretching | 3×/w to 10 weeks 30 min |
||||
| Brito et al. (11) |
51 | 15(males) | HT | MT (7–10 AM) | Cycle ergometer | 3×/w to 10 weeks 45 min/moderate |
↑HR recovery occurred only after ET | – |
| 49 | 15(males) | ET (6–9 PM) | Cycle ergometer | 3×/w to 10 weeks 45 min/moderate |
||||
| 50 | 19(males) | C (10 at 7–10 AM/ 9 at 6–9 PM) |
Stretching | 3×/w to 10 weeks 30 min |
||||
SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; Pre-HT, prehypertensives; HT, hypertensives; VO2peak, maximal oxygen uptake; HR, heart rate; ME, morning exercise session; EE, evening exercise session; MC, morning control session; EC, evening control session; MT, morning training; ET, evening training; C, control; ARB, angiotensin receptor blocker; ACEi, angiotensin-converting enzyme inhibitor; –, no data.
Differences in the degree of PEH across the day could be caused by variation in more than one underlying mechanisms. For instance, while PEH in the morning is due principally to a reduction in cardiac output, PEH in the evening is more related to a decrease in systemic vascular resistance (25, 56). In these studies, reduction in stroke volume after morning exercise is not adequately compensated for an increase in HR that was lower at this time of the day compared to the evening (25, 56), reflecting a smaller increase in the cardiac sympathovagal balance in the morning (25). This might be explained by differences in baroreflex sensitivity which is decreased postexercise (41, 46). The baroreflex sensitivity is already low in the morning even without exercise (119), and exercise might promote still lower values at this time of the day that would explain the diminished cardiac sympathovagal balance. The vasodilation produced during aerobic exercise in the morning is not enough to keep systemic vascular resistance below the preexercise values during the recovery period postexercise at this time of the day (25, 56, 57). The inadequate reduction in systemic vascular resistance is possibly a mechanism underlying impaired vascular function in the morning (121) (Figure 1) and potentially governed through the SCN and peripheral circadian clocks (94).
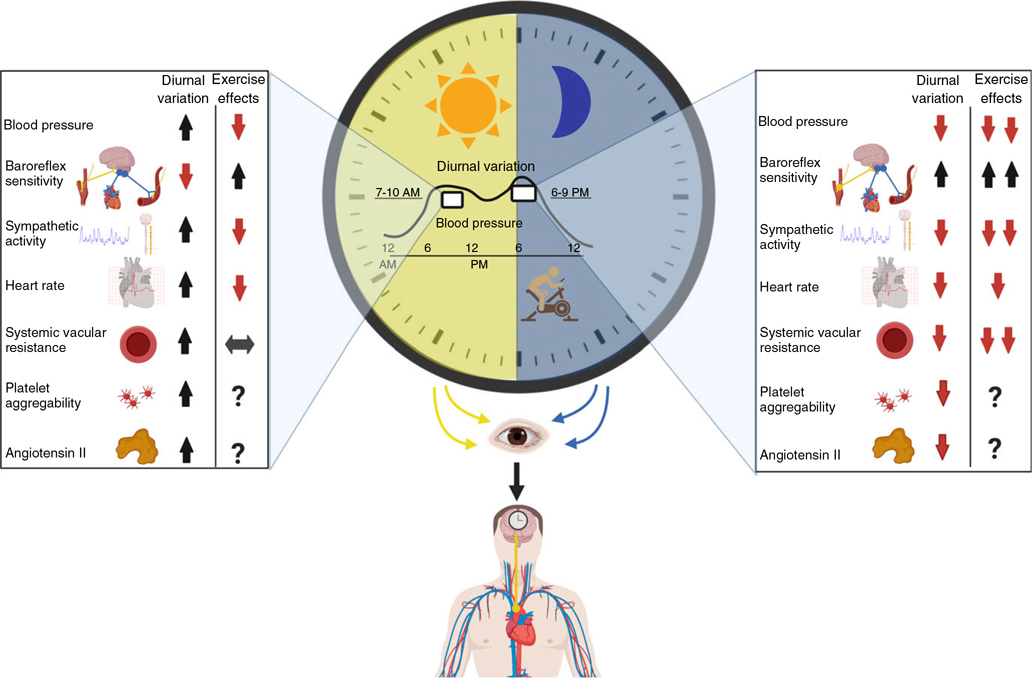
Figure 1. Diurnal variations in baseline cardiovascular parameters and their responses to exercise.
Typical responses during the morning are shown on the left, and those in the evening on the right. Arrows indicate the pattern of a variable at the time of day for diurnal variation, and it indicates the chronic effect of exercise:  (increase);
(increase);  (decrease);
(decrease);  (no changes);
(no changes);  (greater increase)
(greater increase)  (lower decrease); ? (no data in the literature). Environmental light primarily synchronizes the central circadian clock (SCN), which drives the diurnal variation in cardiovascular regulation. Daily changes in cardiovascular regulation lead to increased cardiovascular workload in the morning, while these patterns change, leading to lower cardiovascular workload in the early evening. Exercise in the early evening (6–9 PM) appears relatively advantageous in terms of reduced cardiovascular workload, as evidenced by a greater postexercise decrease in blood pressure (8, 13, 25, 56, 57, 93, 103) and systemic vascular resistance (25, 56, 57) at this time of the day. Additionally, a recent randomized controlled trial demonstrated the superiority of aerobic training conducted in the evening than in the morning for improvements in the subsequent clinic and ambulatory blood pressure, cardiac baroreflex sensitivity, and sympathetic vasomotor modulation in treated hypertensive patients (men) (12). This result suggests that evening exercising may promote greater benefits compared to morning exercise. Created with biorender.com.
(lower decrease); ? (no data in the literature). Environmental light primarily synchronizes the central circadian clock (SCN), which drives the diurnal variation in cardiovascular regulation. Daily changes in cardiovascular regulation lead to increased cardiovascular workload in the morning, while these patterns change, leading to lower cardiovascular workload in the early evening. Exercise in the early evening (6–9 PM) appears relatively advantageous in terms of reduced cardiovascular workload, as evidenced by a greater postexercise decrease in blood pressure (8, 13, 25, 56, 57, 93, 103) and systemic vascular resistance (25, 56, 57) at this time of the day. Additionally, a recent randomized controlled trial demonstrated the superiority of aerobic training conducted in the evening than in the morning for improvements in the subsequent clinic and ambulatory blood pressure, cardiac baroreflex sensitivity, and sympathetic vasomotor modulation in treated hypertensive patients (men) (12). This result suggests that evening exercising may promote greater benefits compared to morning exercise. Created with biorender.com.
After evening exercise, the vasodilation promoted by exercise is sustained during recovery, likely due to reduced systemic vascular resistance (25, 56). Indeed, the greatest postexercise vasodilation across the daytime occurs between 3 and 7PM (data were collected from 9AM through 10:30PM but not overnight) (60). Sustained vasodilation postexercise is strongly dependent on histaminergic mechanisms in young recreationally active men and women (5). However, the diurnal changes in the degree of vasodilation postexercise do not seem to be associated with histaminergic signaling (9). Instead, it is possible that the greater decrease in systemic vascular resistance after evening exercise is mainly due to the postexercise decrease in muscular sympathetic nervous activity. Taken together, these differences between CV mechanisms postexercise in the morning and evening might explain the greater BP reduction after evening exercise. The BP reduction after a single session of exercise has a positive and strong correlation with chronic effect to decrease BP, which has been observed in healthy subjects (124), patients with prehypertension and those with hypertension (28, 44, 71, 85), and patients with coronary heart disease (63). Some authors propose that the summation of acute effects of exercise on BP (e.g., PEH) leads to chronic exercise lowering of BP (10, 24, 72, 109). Thus, it is possible that repetition of greater hypotensive responses after acute exercise sessions in the evening may generate better chronic control of BP.
Another vital consideration is the chronic hypotensive effect of exercise training due to a decrease in systemic vascular resistance in the presence of maintained cardiac output (21), which could lead to evening exercise promoting better CV adaptations overall (Figure 1). This theory was tested in two recent randomized-control trials in which middle-aged men with hypertension performed 10 weeks of moderate aerobic exercise training. In one study, a more pronounced BP reduction was observed after performing the aerobic training in the evening (−8mmHg) than in the morning (−4mmHg) (12). The same study discovered that 60% of participants had a reduction in BP greater than the minimal detectable change (−4.7mmHg) after evening exercise training, while only 20% crossed this minimal detectable change cutoff point after morning exercise training (12). Moreover, only evening training promoted a decrease in 24-h (−3mmHg) and asleep (−3mmHg) diastolic BP (12). The other randomized-control trial reported a greater improvement in HR recovery after a maximal cardiopulmonary exercise test when aerobic training was performed in the evening (+8bpm) than in the morning (+4bpm) (11). CV benefits of exercise, such as HR decrease, lower sympathovagal balance, and increased cardiac baroreflex sensitivity, were also observed after morning exercise training. However, evening exercise training conferred superior benefits for cardiac baroreflex sensitivity, vasomotor sympathetic modulation (12), and cardiac autonomic recovery (11). It is also important to consider the possibility that a longer period of aerobic exercise training (i.e., 6 months or longer) could promote similar results for CV adaptations between morning and evening training. Another important aspect for acute BP responses is that morning exercise blunts the expected BP increase in the morning (25), although its chronic benefits are still unknown. Finally, the efficacy of evening exercise to influence the CV system needs to be studied in larger cohorts for improved generalizability.
Diurnal Variation in Glucose Metabolism and Exercise
Glycemic regulation and insulin sensitivity have a well-established diurnal variation with a peak around 2AM (128). Insulin responsiveness, circulatory glucoregulatory hormones, and oxidation substrates are the main factors behind the circadian variation in glucose homeostasis (131). Regarding specific tissues, skeletal muscle has a less-efficient glucose uptake in the evening, associated with an impairment of insulin responsiveness (131). Insulin secretion from the pancreas is also attenuated at this time of the day (111). In addition, the capacity of the white adipose tissue and skeletal muscle to store fatty acid postprandially has a diurnal peak in the evening (79). Such variations in insulin signaling and intracellular substrates across the 24-h period can underlie daily variations in lipid storage into tissues, including skeletal muscle (34). On the other hand, intramyocellular glucose uptake of human skeletal muscle is partially determined by mitochondrial oxidative capacity, which presents its peak activity in the evening (129). Of note, peripheral circadian clocks also play a role in glucose homeostasis. Animal knockout models for CLOCK and BMAL-1 in pancreatic cells decreased the production of insulin (78). Additionally, muscle-targeted deletion of BMAL-1 dampened muscular glucose uptake, promoting glycemic imbalance (42). While circadian disruption can lead to increased body weight (54, 107, 108, 137), exercise is a useful tool to control glucose homeostases, regardless of the time of day. Recent studies with animals suggest beneficial effects not only by stimulating Glucose Transporter 4 (GLUT4) but also due to insulin-stimulated intramyocellular glucose disposal (67, 74).
Exercise and Time of Day
Exercise has also been demonstrated as a potent time cue and able to modulate clock genes in skeletal muscle (136, 141). These genes are associated with skeletal muscle metabolism mainly via 5′-adenosine mono phosphate-activated protein kinase (AMPK) (67). The insulin-sensitizing effects of exercise act through AMPK mediating GLUT-4 translocation (64). A recent study conducted in mice found a time of day-dependent response at metabolomic and transcriptomic levels in the skeletal muscle after a single session of exercise. That study revealed that glycolytic activity is enriched after exercise in the early active phase of nocturnal mice, while exercise stimulated carbohydrate metabolism in the rest phase of these nocturnal animals (112). Overall, although the timing of exercise affects the metabolic responses in mice, it is too early to suggest an optimal time of day to exercise for glucose homeostasis in humans.
Acute effects of exercise in humans have been mostly studied using pre- or postprandial experiments. Francois and colleagues found that six sets of 1min of exercise at 90% of maximal HR before each main meal blunted postmeal glucose more efficiently than 30min at 60% of maximal HR before dinner (33). In another work, Iwayama and collaborators reported that 60min of moderate continuous exercise at before breakfast was able to increase fat oxidation across the 24h, but this effect was not observed for the same exercise after lunch or dinner (52). The effect of fat oxidation following morning exercise may represent a long-term benefit on insulin sensitivity (34). Additionally, postprandial low-intensity exercise bouts also promoted postprandial glucose mitigation (76). Episodes of hypoglycemia are a serious concern for people with insulin-dependent diabetes. Acutely, afternoon (4PM) sessions of aerobic exercise (60min of moderate intensity) promoted a higher number of hypoglycemic events following 24h of exercise than did morning exercise (7AM) in patients with type I diabetes (36). The lower concentrations of circulating cortisol in the afternoon and across the first part of the night might explain this outcome, according to the authors (36). On other hand, this pattern suggests a greater potential to reduce glucose levels after afternoon exercise in comparison to morning exercise.
These clear metabolic differences in the effects of exercise at different times of day have led to initial studies of the effects of exercise relative to glucose homeostasis and safety in patients with diabetes. Randomized clinical trials show greater improvements in glucose homeostasis after afternoon/evening than morning exercise training in overweight people and patients with type 2 diabetes (73, 82). This greater potential for afternoon exercise was observed after applying high-intensity interval training with 10 sets of 1min exercise at 95% to 120%Wpeak for 5days (82) and separately when combining moderate aerobic exercise (30min at 70%Wmax twice a week) plus resistance training (8 exercises—3 series of 10 repetitions at 60% one-repetition maximal once a week) for 12weeks (73). However, no differences in glucose homeostasis between morning and evening exercise training have been reported after 12weeks of combining moderate aerobic (30min at 60%–70%) and resistance training (3 series of 12 repetitions at 45%–55% of one-repetition maximal) performed three times a week in normoglycemic overweight or patients with type 2 diabetes (120). Perhaps, the differences in experimental protocols, including the type, intensity, and duration of exercise and differences in nutritional control, may explain this variation (Table 2). Indeed, lack of any detectable difference between morning and afternoon/evening exercise training (120) compared to findings of greater effects after evening training (73, 82) might be explained by lower intensity aerobic exercise (30min at 60%–70% versus 30min at 70%Wmax) and resistance exercise (45%–55% one-maximal repetition versus 60% one-maximal repetition) in those studies. In nocturnal mice, exercise at the beginning of the active phase increases the glycolytic activity in the skeletal muscle (112), and the peak of the mitochondrial oxidative capacity also occurs at this phase (129), which may have potentiated differences when intensity was higher (73). Another important aspect may be the control of nutrient intake, for instance, Teo and colleagues encouraged subjects to eat a meal 1h before every exercise training session (120). Finally, few studies have compared pre- versus postprandial exercise in type 2 diabetes patients. Findings suggest that postprandial exercise promotes greater benefits on glucose homeostasis (20, 45, 99). During postprandial exercise, both contraction and insulin-stimulated glucose uptake occur (37), together with a higher insulin glucagon ratio (98) that favors a reduced hepatic glucose output (66). Future studies should investigate postprandial exercise at different times of the day.
Table 2.
Summary of Acute and Chronic Studies Comparing Glucose Homeostasis Outcomes at Different Times of the Day
| Author | Mean age | n(sex) | Clinical condition |
Interventions | Type of exercise | Exercise protocol | Main findings | Secondary findings |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Acute exercise studies | ||||||||
| Francois et al. (33) | 48 | 2(females) 7(males) |
IR and OB | ME Before breakfast |
Treadmill | 30 min/60%HRmax | ↓Postprandial Gl after breakfast but not after lunch or dinner in Fractionated 3 | – |
| Fractionated 3 (ME, Md, and EE) | Treadmill and resistance bands | 6 sets of 1 min/90%HRmax/l min recovery (slow walk) and | ↓ 24 h Gl mean in Fractionated 3 | |||||
| Before breakfast, lunch, and dinner | Resistance-band exercise (arms, back, and core) 60s of as many repetitions as possible | |||||||
| Fractionated 2 (M and EE) |
Treadmill | 6 sets of 1 min/90%HRmax/1 min recovery (slow walk) | ||||||
| Before breakfast and dinner | ||||||||
| Gomez et al. (36) | 32 | 14(females) 18 (males) |
DM1 | ME (7 AM) AE (4 PM) |
Treadmill Treadmill |
60 min (moderate) 60 min (moderate) |
↑ Hypoglycemic events after AE than ME | ↑ Time spent in euglycemia after ME than AE |
| Chronic exercise studies | ||||||||
| Mancilla et al. (73) | 61 | 1 2 (males) | DM2 and OW | MT (8–1OAM) | Cycle ergometer |
2×/w to 12 weeks 30 min/70%Wmax |
↓ Fasting Gl after AT than MT ↑ Peripheral insulin sensitivity after AT than MT |
↑ Insulin-mediated suppression of adipose-tissue lipolysis after AT than MT |
| Resistance | 1 ×/w to 12 weeks | |||||||
| 3 sets/10 rep/60% MVC (leg extension, leg press, chess press, pull down, triceps and biceps curls, abdominal crunches, and horizontal row) | ↑ Performance during maximal exercise test after AT than MT | |||||||
| ↓ Fat mass after AT than MT | ||||||||
| 57 | 20(males) | AT (3–6 PM) | Cycle ergometer |
2×/w to 12 weeks 30 min/70%Wmax |
||||
| Resistance | 1 ×/w to 1 2 weeks | |||||||
| 3 sets/1 0 rep/60% MVC (leg extension, leg press, chess press, pull down, triceps and biceps curls, abdominal crunches, and horizontal row) | ||||||||
| Moholdt et al. (82) | 35 | 8(males) | OW | MT (6:30 AM) | Cycle ergometer |
5 Training sessions (1:1) 10 sets/1 min at 95–120%Wmax by 1 min low intensity |
↓ Nocturnal Gl after ET than C ↓ Fasting Gl only after ET |
↓ Insulin, cholesterol, triacylglycerol, and LDL-C only after ET ↑ Performance during maximal exercise test similar after ET and MT |
| OW | ET (6:30 PM) | Cycle ergometer |
5 Training sessions (1:1) 10 sets/1 min at 95% tol20%Wmax by 1 min low intensity |
|||||
| OW | c(−) | – | no exercise | |||||
| Teo et al. (120) | 57 | 11 (females) 9(males) |
OW and DM2 | MT (8–10 AM) | Treadmill + |
3×/w to 12 weeks 30 min (60%-70%VOpeak) + |
All glycemic and insulin outcomes, fasting or postprandial, improved similarly between MT and ET | Circadian rhythm of skin temperature did not change in any group |
| Resistance | 4 Resistance exercises (3 sets/12–18 rep at 45%–55% 1RM) — leg press, bench press, lateral pull down, and military press |
|||||||
| 51 | 12 (females) | OW and DM2 | ET (5–7 PM) | Treadmill | 3×/w to 12 weeks | |||
| 8(males) | 30 min (60%–70%VOpeak) | |||||||
| + | + | |||||||
| Resistance | 4 Resistance exercises (3 sets/12–18 rep at 45%–55% 1RM) — leg press, bench press, lateral pull down, and military press |
|||||||
| – | –(females) | Only DM2 | MT (8–1OAM) | Treadmill | 3×/w to 12 weeks 30 min (60–70%VOpeak) |
All glycemic and insulin outcomes, fasting or postprandial, improved similarly between MT and ET | Circadian rhythm of the skin temperature did not change in any group | |
| 10(males) | + | + | ||||||
| Resistance | 4 Resistance exercises 4 Resistance exercises (3 sets/12–18 rep at 45%–55% 1RM)—leg press, bench press, lateral pull down, and military press | |||||||
| – | –(females) | Only DM2 | ET (5–7 PM) | Treadmill | 3×/w to 12 weeks | |||
| 10(males) | + | 30 min (60%–70%VOpeak) + |
||||||
| Resistance | 4 Resistance exercises (3 sets/12–18 rep at 45%–55% 1RM) — leg press, bench press, lateral pull down, and military press |
|||||||
Gl, glucose; IR, insulin resistance; OW, overweight; DM2, type 2 diabetes; DM1, type 1 diabetes; VO2peak, maximal oxygen uptake; HR, heart rate; ME, morning exercise session; EE, evening exercise session; Md, midday exercise session; MC, morning control session; EC, evening control session; MT, morning training; ET, evening training; AT, afternoon training; C, control; rep, repetitions; 1RM, one-maximal repetition; MVC, maximal voluntary contractions; W, workload; –, no data.
Exercise Resetting the Circadian Clock: Potential Clinical Tool
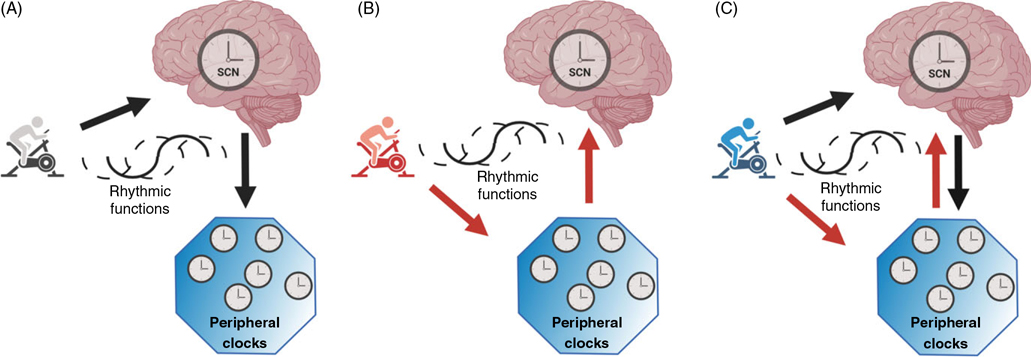
Exercise has emerged as a promising tool to reset the circadian clock in animals (106) and humans (17, 18, 30, 140). The phase-response curves (PRCs) for light and melatonin are the gold standard as chronobiological tools to measure the perturbation in the circadian phase in response to a stimulus since it describes the magnitude and directions (e.g., delay or advance) of the phase shifts after exposure to a zeitgeber, such as bright light, meals, and exercise (53, 140). Indeed, the resetting of the central circadian clock by exercise is influenced by the time of the day. For example, one study found that morning exercise elicited circadian phase delays (the circadian clock shifted to a later time), while evening exercise elicited phase advances (18). This result suggests that evening exercise might be better than morning exercise to resynchronize or minimize any circadian misalignments caused by night-shift work, which often delays the internal circadian rhythms (97, 132). There is still some controversy in this area as there are so few studies, and other studies found phase advances after morning exercise and phase delays after evening exercise (140) or no difference between morning and evening exercises on the circadian clock (30). The reasons for these different responses may lie in different exercise protocols among studies. Although the intensity of exercise was similar across studies (ranging from 60% to 75% at ), the duration of exercise had larger variability: lasting 20min (140), 30min (30), 40min (18), and 60min and 3h (17), and there were also differences in the number of sessions of exercise ranging from a single session (17, 30, 140) to three sessions (18). Future studies are needed to determine how exercise resets the circadian clock after misalignment, whether through peripheral clocks, SCN, or both (Figure 2). Such understanding may help us determine the ideal interventions in terms of intensities, duration, and time of exercise to predictably change the phase of the circadian system. In addition, future randomized controlled trials hopefully will focus on tuning such therapies to minimize harm from circadian disruption in night-shift workers as well as in the more vulnerable people with underlying clinical disorders (e.g., CV disease).
Figure 2. Hypothetical interactions among exercise and the circadian clock.
(A) Exercise influences the neuronal activity in the suprachiasmatic nucleus (SCN), and the central clock synchronizes the peripheral clocks advancing or delaying the rhythmic functions; (B) Exercise influences the peripheral clocks, and they inform the SCN by afferent signaling to resynchronize the central clock advancing or delaying the rhythmic functions; (C) Exercise influences both the SCN and peripheral clocks to resynchronize the clock advancing or delaying their rhythmic functions. Created with biorender.com.
Although not easily perturbed, the neuronal activity of the SCN has been observed to be responsive to numerous stimuli. For instance, infusion of ghrelin (139) and leptin (40) (normally associated with food intake) or infusion of an alpha-adrenergic agonist to increase BP (14) caused changes in the neuronal activity of the SCN in animals. Engelmann and colleagues found an increase in the neuropeptide arginine vasopressin in the SCN during swimming exercise (31), suggesting that exercise can influence the neuronal activity of the SCN (80). The SCN also changes activity during a physiological stress, which could be one of the mechanisms by which exercise resets the phase of the circadian clock.
In humans and mammals, nucleated cells have four families of circadian clock genes [e.g., Clock, Bmal1, Period (Per1, Per2, and Per3), and Cryptochrome (Cry1, Cry2, and Cry3)], which are responsible for clock rhythmicity at the molecular level (118). These clock genes are composed of rhythmic transcriptional and translational feedback that cycles around 24.2h in humans (108). The activity of this molecular clock machinery is strongly associated with metabolic flux in the skeletal muscles (96). Exercise increases muscle metabolism to sustain contractions (43), and it is possible that exercise changes not only the circadian pattern of a physiological variable but also the molecular machinery of the circadian system (32, 141). Indeed, after a single session of three sets of a knee-extension exercise at 80% of a one-maximal repetition in humans, three core clock genes Cry1, Per2, and Bmal1 were upregulated in the skeletal muscle for 6h by up to 1.5-fold (141). In addition, the upregulation in these clock genes by exercise shifts the expression of other diurnal-regulated genes, specifically upregulating the genes involved in signaling (Rrad) for insulin-independent glucose transport in the morning and downregulating the genes involved in glucose metabolism (Gpd2) in the evening (141).
Applied Implications and Future Studies
Most evidence suggests a greater acute benefit of reducing cardiac workload by BP decrease when exercise is conducted in the evening (8, 13, 25, 56, 57, 93, 103). Greater HR decrease is also seen in healthy participants after aerobic training in the evening versus the morning (6PM vs. 7AM) (126). Similar superiority for BP-lowering effects for evening training compared with morning training is seen in people with hypertension when under antihypertensive treatment (12). Defining a better time for exercise may help the treatment in hypertensive patients who are less responsive to treatment, such as those with higher CV risk, resistant hypertension, and non-dipper hypertension. It is worth noting that in people with hypertension, nighttime antihypertensive medication results in improved ambulatory BP control, mainly during sleep, and remarkably reduced major CV events, when compared to morning antihypertensive medication (HYGIA study) (50). This raises the possibility that a combination of evening exercise and bedtime antihypertensive medication might provide even better clinical outcomes, particularly in patients with the perilous non-dipping hypertension. This seems to be a worthwhile basis for a clinical trial. Other promising studies on the role of exercise in chronotherapy could determine: (i) Whether evening exercise is also the best time for CV benefits in people with circadian disruption (e.g., patients with sleep disorders and cardiometabolic diseases); (ii) Whether evening exercise is also the best time for CV benefits in cardiac patients with higher risk (e.g., coronary artery disease and heart failure); (iii) The optimal exercise protocol (e.g., intensity, duration, and frequency) for entraining the circadian clock; and (iv) The mechanisms by which exercise resets the SCN and peripheral clocks. In studying these effects, we propose some recommendations regarding the study design, participant characteristics, and experimental protocols (Table 3).
Table 3.
Recommendations for Chronobiology of Exercise Studies
| Importam | |
|---|---|
|
| |
| Study Design | |
| A priori sample size calculations to ensure sufficient statistical power for primary outcomes | 1 |
| A priori define secondary outcomes | 1 |
| Characteristics of Participants | |
| Assess and describe the clinical condition of participants (e.g., hypertension status, and how assessed, such as office or ambulatory blood pressure monitoring and nocturnal dipping versus non-dipping profile) | 1 |
| Avoid exercise or alcohol for 24 h prior to study | 1 |
| Access habitual wake/sleep timing and duration (e.g., actigraphy, or minimally, a sleep diary) | 1 |
| Report chronotype | 2 |
| Assess circadian phase angle of entrainment (e.g., dim light melatonin onset relative to habitual sleep time) | 3 |
| Experimental Protocol | |
| Report any medications including the time of day medications taken | 1 |
| Control session or control groups conducted at the same time of the day | 1 |
| Report clock time for exercise sessions and assessments | 1 |
| Describe the type of exercise (cycling, rowing, etc.), duration, and frequency of sessions | 1 |
| Standardize meals | 1 |
| Prescribe exercise based on screening tests conducted at | 2 |
| the same time of the day Report environmental light intensity | 3 |
The right column denotes the level of importance for each recommendation: (1) obligatory; (2) recommended; and (3) recommended if feasible.
Limitations
This narrative article presents some strengths and limitations. The main strength is the presentation of consistent results that show a large effect of the time of day on CV and metabolic adaptations to exercise. These repeated observations present a tantalizing route for potentially improving therapy for some CV and metabolic disorders. However, some specifics and certain clinical trials are lacking before clinical recommendations can be made. For example, one major limitation is that it is difficult to distinguish the internal circadian effects (deduced from circadian studies under constant dim light in the laboratory) from any effects related to the daily changes in the environment or behaviors (deduced from diurnal studies in the laboratory or at home). All daily behaviors (e.g., sleep, awakening, altered posture, physical activity, and exercise) cause profound acute CV responses, and these responses may be modulated by the phase of the internal body clock (123). Moreover, there are meaningful differences between people in (i) the period of the internal circadian clock (e.g., ranging from 23:53 to 24:19h) (23); (ii) the habitual time at which people go to sleep relative to external societal clock time (107, 137); and (iii) the usual phase of the internal clock at which people go to sleep (i.e., the phase angle of entrainment) (29). All three of these variations are linked in as much as a shorter circadian period predicts an earlier phase angle of entrainment (138). Thus, ideally, to distinguish circadian effects from behavioral/environmental effects, one would compare the differences in responses to exercise performed at specific times of day, or relative to specific behaviors (time since waking up), versus responses relative to internal clock phase (e.g., time since the onset of melatonin release). Nonetheless, in practice, if we restrict observations to average results and we exclude participants who perform shift work and those with extreme chronotypes, as in many of the studies reported here (7–9, 11–13, 25, 103), then it may be possible to loosely translate the time of day to internal circadian phase—but this remains a practical assumption/limitation unless the phase angles of entrainment of all participants are measured. Secondly, further studies will then be needed in people with different clinical conditions, different chronotypes, and in people who perform night-shift work. Another important point is that exercise can promote beneficial effect at any period of the daytime. Nonetheless, it is conceivable that exercise during the circadian nighttime (i.e., time that most of the people would sleep) could interfere with the normal rest, repair, and growth phases of tissues and cells (1). There is little to no data to determine if exercise across the circadian night still improves the overall health or even has deleterious effects. This concept may be even more critical to consider in people with underlying CV disease (104).
Conclusions
All studies comparing morning versus evening exercise have found that a single session of aerobic exercise conducted in the evening promoted a greater reduction in BP and consequently in the cardiac workload postexercise (8, 13, 25, 56, 57, 93, 103) and across the sleep period (13, 93). This outcome is supported by a lower systemic vascular resistance postexercise in the evening (25, 56, 57). As yet, the mechanisms behind this response are unknown. In terms of chronic exercise, there are only two randomized controlled studies that compare morning versus evening training on CV outcomes. The results for CV responses were also better for evening training compared with morning training (11, 12). Although chronotherapy through exercise is also very promising for glucose homeostasis, the findings are still unclear. Determining the influence of pre- or postprandial exercise on glucose homeostasis at different times of day would be an important next step in this area. With regard to chronic effects, two of three clinical trials suggest that afternoon/evening exercise promotes greater benefits to glucosis homeostasis (73, 82, 120). However, the study that found no differences was the most rigorous concerning supervised meals before each training sessions (120). Finally, exercise may be a promising tool to reset the biological clock in humans (17, 18, 30, 140). Circadian disruption is rife due to our modern 24/7 lifestyle and using evening exercise as a tool to improve clinical outcomes and improve circadian alignment are promising avenues. However, more studies are needed with misaligned populations (e.g., night-shift workers) and with patients under different CV conditions to allow a generalization of findings. Moreover, basic studies are needed to uncover the mechanisms behind the effect of exercise on both the central (i.e., SCN) and peripheral clocks. Thus, research on the chronobiology of exercise is a very promising field.
Didactic Synopsis.
Major teaching points
The circadian system prepares the body for the predictable daily changes in the environment or behaviors, such as the daily temperature, light, and feeding cycles.
Almost all cardiovascular variables and their regulating factors have predictable changes across the day and night. After awakening in the morning, there are concomitant increases in circulating cortisol, sympathovagal balance, heart rate, systemic vascular resistance, blood pressure, and platelet aggregability. This physiological pattern prepares the healthy human body for optimal performance across this morning period.
Circadian disruption, as occurs with chronic night-shift work, increases the risk of many cardiovascular and metabolic diseases.
Aerobic exercise is often recommended as a first option nonpharmacologic therapy for several cardiovascular and metabolic diseases due to its associated benefits on structural and functional cardiorespiratory and metabolic parameters.
The study of postexercise hypotension allows to investigate mechanisms involved in cardiovascular regulation that are strongly related with the chronic effect of exercise training on blood pressure control. Indeed, individuals who present greater reduction in blood pressure after a single session of exercise also present the greater chronic hypotensive effect after a training program.
Acknowledgements
The authors are supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2018/05226-0 and FAPESP 2020/11588-2), NIH grants R35 HL155681-01, KL2 TR002370, and the Oregon Institute of Occupational Health Sciences at Oregon Health & Science University via funds from the Division of Consumer and Business Services of the State of Oregon (ORS 656.630).
References
- 1.Alibhai FJ, Tsimakouridze EV, Reitz CJ, Pyle WG, Martino TA. Consequences of circadian and sleep disturbances for the cardiovascular system. Can J Cardiol 31: 860–872, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD. Vascular disease in mice with a dysfunctional circadian clock. Circulation 119: 1510–1517, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoyama S, Shibata S. Time-of-day-dependent physiological responses to meal and exercise. Front Nutr 7: 18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson G, Jones H, Ainslie PN. Circadian variation in the circulatory responses to exercise: relevance to the morning peaks in strokes and cardiac events. Eur J Appl Physiol 108: 15–29, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett-O’Keefe Z, Kaplon RE, Halliwill JR. Sustained postexercise vasodilatation and histamine receptor activation following small muscle-mass exercise in humans. Exp Physiol 98: 268–277, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Boudreau P, Yeh WH, Dumont GA, Boivin DB. A circadian rhythm in heart rate variability contributes to the increased cardiac sympathovagal response to awakening in the morning. Chronobiol Int 29: 757–768, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Brito L, Pecanha T, Tinucci T, Silva-Junior N, Costa L, Forjaz C. Time of day affects heart rate recovery and variability after maximal exercise in pre-hypertensive men. Chronobiol Int 32: 1385–1390, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Brito LC, Azevedo L, Pecanha T, Fecchio RY, Rezende RA, da Silva GV, Pio-Abreu A, Mion D, Halliwill JR, Forjaz CLM. Effects of ACEi and ARB on post-exercise hypotension induced by exercises conducted at different times of day in hypertensive men. Clin Exp Hypertens 42: 722–727, 2020. [DOI] [PubMed] [Google Scholar]
- 9.Brito LC, Ely MR, Sieck DC, Mangum JE, Larson EA, Minson CT, Forjaz CLM, Halliwill JR. Effect of time of day on sustained postexercise vasodilation following small muscle-mass exercise in humans. Front Physiol 10: 762, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brito LC, Fecchio RY, Pecanha T, Andrade-Lima A, Halliwill JR, Forjaz CLM. Postexercise hypotension as a clinical tool: a “single brick” in the wall. J Am Soc Hypertens 12: e59–e64, 2018. [DOI] [PubMed] [Google Scholar]
- 11.Brito LC, Pecanha T, Fecchio RY, Pio-Abreu A, Silva G, Mion-Junior D, Halliwill JR, Forjaz CLM. Comparison of morning versus evening aerobic-exercise training on heart rate recovery in treated hypertensive men: a randomized controlled trial. Blood Press Monit, 2021. [DOI] [PubMed] [Google Scholar]
- 12.Brito LC, Pecanha T, Fecchio RY, Rezende RA, Sousa P, DAS-J N, Abreu A, Silva G, Mion-Junior D, Halliwill JR, Forjaz CLM. Morning versus evening aerobic training effects on blood pressure in treated hypertension. Med Sci Sports Exerc 51: 653–662, 2019. [DOI] [PubMed] [Google Scholar]
- 13.Brito LC, Rezende RA, Mendes C, Silva-Junior ND, Tinucci T, Cipolla-Neto J, de Moraes Forjaz CL. Separate aftereffects of morning and evening exercise on ambulatory blood pressure in prehypertensive men. J Sports Med Phys Fitness 58: 157–163, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Buijs FN, Cazarez F, Basualdo MC, Scheer FA, Perusquia M, Centurion D, Buijs RM. The suprachiasmatic nucleus is part of a neural feedback circuit adapting blood pressure response. Neuroscience 266: 197–207, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Buijs RM, Wortel J, Van Heerikhuize JJ, Kalsbeek A. Novel environment induced inhibition of corticosterone secretion: physiological evidence for a suprachiasmatic nucleus mediated neuronal hypothalamo-adrenal cortex pathway. Brain Res 758: 229–236, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Burgess HJ, Trinder J, Kim Y, Luke D. Sleep and circadian influences on cardiac autonomic nervous system activity. Am J Physiol 273: H1761–H1768, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Buxton OM, Frank SA, L’Hermite-Baleriaux M, Leproult R, Turek FW, Van Cauter E. Roles of intensity and duration of nocturnal exercise in causing phase delays of human circadian rhythms. Am J Physiol 273: E536–E542, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Buxton OM, Lee CW, L’Hermite-Baleriaux M, Turek FW, Van Cauter E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am J Physiol Regul Integr Comp Physiol 284: R714–R724, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 100: 126–131, 1985. [PMC free article] [PubMed] [Google Scholar]
- 20.Colberg SR, Zarrabi L, Bennington L, Nakave A, Thomas Somma C, Swain DP, Sechrist SR. Postprandial walking is better for lowering the glycemic effect of dinner than pre-dinner exercise in type 2 diabetic individuals. J Am Med Dir Assoc 10: 394–397, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension 46: 667–675, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Cugini P, Lucia P. Circadian rhythm of the renin-angiotensin-aldosterone system: a summary of our research studies. Clin Ter 155: 287–291, 2004. [PubMed] [Google Scholar]
- 23.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284: 2177–2181, 1999. [DOI] [PubMed] [Google Scholar]
- 24.da Nobrega AC. The subacute effects of exercise: concept, characteristics, and clinical implications. Exerc Sport Sci Rev 33: 84–87, 2005. [DOI] [PubMed] [Google Scholar]
- 25.de Brito LC, Rezende RA, da Silva Junior ND, Tinucci T, Casarini DE, Cipolla-Neto J, Forjaz CL. Post-exercise hypotension and its mechanisms differ after morning and evening exercise: a randomized crossover study. PLoS One 10: e0132458, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Degaute JP, van de Borne P, Linkowski P, Van Cauter E. Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men. Hypertension 18: 199–210, 1991. [DOI] [PubMed] [Google Scholar]
- 27.Dickinson JM, D’Lugos AC, Naymik MA, Siniard AL, Wolfe AJ, Curtis DR, Huentelman MJ, Carroll CC. Transcriptome response of human skeletal muscle to divergent exercise stimuli. J Appl Physiol 124 (1529–1540): 2018, 1985. [DOI] [PubMed] [Google Scholar]
- 28.Dos Santos ES, Asano RY, Filho IG, Lopes NL, Panelli P, Nascimento DdaC,CollierSR,PrestesJ.Acuteandchroniccardiovascularresponse to 16 weeks of combined eccentric or traditional resistance and aerobic training in elderly hypertensive women: a randomized controlled trial. J Strength Cond Res 28: 3073–3084, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Duffy JF, Wright KP Jr. Entrainment of the human circadian system by light. J Biol Rhythms 20: 326–338, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Edwards B, Waterhouse J, Atkinson G, Reilly T. Exercise does not necessarily influence the phase of the circadian rhythm in temperature in healthy humans. J Sports Sci 20: 725–732, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Engelmann M, Ebner K, Landgraf R, Wotjak CT. Swim stress triggers the release of vasopressin within the suprachiasmatic nucleus of male rats. Brain Res 792: 343–347, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Ezagouri S, Zwighaft Z, Sobel J, Baillieul S, Doutreleau S, Ladeuix B, Golik M, Verges S, Asher G. Physiological and molecular dissection of daily variance in exercise capacity. Cell Metab 30: 78–91 e74, 2019. [DOI] [PubMed] [Google Scholar]
- 33.Francois ME, Baldi JC, Manning PJ, Lucas SJ, Hawley JA, Williams MJ, Cotter JD. ‘Exercise snacks’ before meals: a novel strategy to improve glycaemic control in individuals with insulin resistance. Diabetologia 57: 1437–1445, 2014. [DOI] [PubMed] [Google Scholar]
- 34.Froy O, Garaulet M. The circadian clock in white and brown adipose tissue: mechanistic, endocrine, and clinical aspects. Endocr Rev 39: 261–273, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev 90: 1063–1102, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Gomez AM, Gomez C, Aschner P, Veloza A, Munoz O, Rubio C, Vallejo S. Effects of performing morning versus afternoon exercise on glycemic control and hypoglycemia frequency in type 1 diabetes patients on sensor-augmented insulin pump therapy. J Diabetes Sci Technol 9: 619–624, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodyear LJ, Chang PY, Sherwood DJ, Dufresne SD, Moller DE. Effects of exercise and insulin on mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Am J Physiol 271: E403–E408, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci 4: 1165, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Grassi G, Bombelli M, Seravalle G, Dell’Oro R, Quarti-Trevano F. Diurnal blood pressure variation and sympathetic activity. Hypertens Res 33: 381–385, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Grosbellet E, Gourmelen S, Pevet P, Criscuolo F, Challet E. Leptin normalizes photic synchronization in male ob/ob mice, via indirect effects on the suprachiasmatic nucleus. Endocrinology 156: 1080–1090, 2015. [DOI] [PubMed] [Google Scholar]
- 41.Halliwill JR, Taylor JA, Hartwig TD, Eckberg DL. Augmented baroreflex heart rate gain after moderate-intensity, dynamic exercise. Am J Physiol 270: R420–R426, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Harfmann BD,Schroder EA,Kachman MT,Hodge BA,Zhang X,Esser KA. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet Muscle 6: 12, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hargreaves M, Spriet LL. Skeletal muscle energy metabolism during exercise. Nat Metab 2: 817–828, 2020. [DOI] [PubMed] [Google Scholar]
- 44.Hecksteden A, Grutters T, Meyer T. Association between postexercise hypotension and long-term training-induced blood pressure reduction: a pilot study. Clin J Sport Med 23: 58–63, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Heden TD, Winn NC, Mari A, Booth FW, Rector RS, Thyfault JP, Kanaley JA. Postdinner resistance exercise improves postprandial risk factors more effectively than predinner resistance exercise in patients with type 2 diabetes. J Appl Physiol 118 (624–634): 2015, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heffernan KS, Collier SR, Kelly EE, Jae SY, Fernhall B. Arterial stiffness and baroreflex sensitivity following bouts of aerobic and resistance exercise. Int J Sports Med 28: 197–203, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Hellsten Y, Nyberg M. Cardiovascular adaptations to exercise training. Compr Physiol 6: 1–32, 2015. [DOI] [PubMed] [Google Scholar]
- 48.Hermida RC, Ayala DE, Fernandez JR, Mojon A. Sleep-time blood pressure: prognostic value and relevance as a therapeutic target for cardiovascular risk reduction. Chronobiol I.nt 30: 68–86, 2013. [DOI] [PubMed] [Google Scholar]
- 49.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Blunted sleep-time relative blood pressure decline increases cardiovascular risk independent of blood pressure level—the “normotensive non-dipper” paradox. Chronobiol Int 30: 87–98, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Hermida RC, Crespo JJ, Dominguez-Sardina M, Otero A, Moya A, Rios MT, Sineiro E, Castineira MC, Callejas PA, Pousa L, Salgado JL, Duran C, Sanchez JJ, Fernandez JR, Mojon A, Ayala DE, Hygia Project I. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur Heart J, 2019. [DOI] [PubMed] [Google Scholar]
- 51.Hu K, Scheer FA, Laker M, Smales C, Shea SA. Endogenous circadian rhythm in vasovagal response to head-up tilt. Circulation 123: 961–970, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iwayama K, Kurihara R, Nabekura Y, Kawabuchi R, Park I, Kobayashi M, Ogata H, Kayaba M, Satoh M, Tokuyama K. Exercise increases 24-h fat oxidation only when it is performed before breakfast. EBioMedicine 2: 2003–2009, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson CH, Elliott JA, Foster R. Entrainment of circadian programs. Chronobiol Int 20: 741–774, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Johnson DA, Reid M, Vu TT, Gallo LC, Daviglus ML, Isasi CR, Redline S, Carnethon M. Associations of sleep duration and social jetlag with cardiometabolic risk factors in the study of Latino youth. Sleep Health, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones H, Atkinson G, Leary A, George K, Murphy M, Waterhouse J. Reactivity of ambulatory blood pressure to physical activity varies with time of day. Hypertension 47: 778–784, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Jones H, Pritchard C, George K, Edwards B, Atkinson G. The acute postexercise response of blood pressure varies with time of day. Eur J Appl Physiol 104: 481–489, 2008. [DOI] [PubMed] [Google Scholar]
- 57.Jones HG K; Edwards B; Atkinson G Effects of time of day on postexercise blood pressure: circadian or sleep-related influences? Chronobiol Int 25: 987–998, 2008. [DOI] [PubMed] [Google Scholar]
- 58.Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. SCN outputs and the hypothalamic balance of life. J Biol Rhythms 21: 458–469, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Kanabrocki EL, George M, Hermida RC, Messmore HL, Ryan MD, Ayala DE, Hoppensteadt DA, Fareed J, Bremner FW, Third JL, Shirazi P, Nemchausky BA. Day-night variations in blood levels of nitric oxide, T-TFPI, and E-selectin. Clin Appl Thromb Hemost 7: 339–345, 2001. [DOI] [PubMed] [Google Scholar]
- 60.Kaneko M, Zechman FW, Smith RE. Circadian variation in human peripheral blood flow levels and exercise responses. J Appl Physiol 25: 109–114, 1968. [DOI] [PubMed] [Google Scholar]
- 61.Kantermann T, Eastman CI. Circadian phase, circadian period and chronotype are reproducible over months. Chronobiol Int 35: 280–288, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kenney MJ, Seals DR. Postexercise hypotension. Key features, mechanisms, and clinical significance. Hypertension 22: 653–664, 1993. [DOI] [PubMed] [Google Scholar]
- 63.Kiviniemi AM, Hautala AJ, Karjalainen JJ, Piira OP, Lepojarvi S, Ukkola O, Huikuri HV, Tulppo MP. Acute post-exercise change in blood pressure and exercise training response in patients with coronary artery disease. Front Physiol 5: 526, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kjobsted R, Hingst JR, Fentz J, Foretz M, Sanz MN, Pehmoller C, Shum M, Marette A, Mounier R, Treebak JT, Wojtaszewski JFP, Viollet B, Lantier L. AMPK in skeletal muscle function and metabolism. FASEB J 32: 1741–1777, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knaier R, Qian J, Roth R, Infanger D, Notter T, Wang W, Cajochen C, Scheer F. Diurnal variation in maximum endurance and maximum strength performance: a systematic review and meta-analysis. Med Sci Sports Exerc 54: 169–180, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kowalski GM, Moore SM, Hamley S, Selathurai A, Bruce CR. The effect of ingested glucose dose on the suppression of endogenous glucose production in humans. Diabetes 66: 2400–2406, 2017. [DOI] [PubMed] [Google Scholar]
- 67.Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326: 437–440, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lassiter DG, Sjogren RJO, Gabriel BM, Krook A, Zierath JR. AMPK activation negatively regulates GDAP1, which influences metabolic processes and circadian gene expression in skeletal muscle. Mol Metab 16: 12–23, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lehmkuhl LA, Park S, Zakutansky D, Jastremski CA, Wallace JP. Reproducibility of postexercise ambulatory blood pressure in Stage I hypertension. J Hum Hypertens 19: 589–595, 2005. [DOI] [PubMed] [Google Scholar]
- 70.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature 447: 477–481, 2007. [DOI] [PubMed] [Google Scholar]
- 71.Liu S, Goodman J, Nolan R, Lacombe S, Thomas SG. Blood pressure responses to acute and chronic exercise are related in prehypertension. Med Sci Sports Exerc 44: 1644–1652, 2012. [DOI] [PubMed] [Google Scholar]
- 72.Luttrell MJ, Halliwill JR. Recovery from exercise: vulnerable state, window of opportunity, or crystal ball? Front Physiol 6: 204, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mancilla R, Brouwers B, Schrauwen-Hinderling VB, Hesselink MKC, Hoeks J, Schrauwen P. Exercise training elicits superior metabolic effects when performed in the afternoon compared to morning in metabolically compromised humans. Physiol Rep 8: e14669, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mancilla R, Krook A, Schrauwen P, Hesselink MKC. Diurnal regulation of peripheral glucose metabolism: potential effects of exercise timing. Obesity (Silver Spring) 28 (Suppl 1): S38–S45, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Manfredini R, Boari B, Smolensky MH, Salmi R, la Cecilia O, Maria Malagoni A, Haus E, Manfredini F. Circadian variation in stroke onset: identical temporal pattern in ischemic and hemorrhagicevents. Chronobiol Int 22: 417–453, 2005. [DOI] [PubMed] [Google Scholar]
- 76.Manohar C, Levine JA, Nandy DK, Saad A, Dalla Man C, McCrady-Spitzer SK, Basu R, Cobelli C, Carter RE, Basu A, Kudva YC. The effect of walking on postprandial glycemic excursion in patients with type1diabetesandhealthypeople.DiabetesCare 35:2493–2499,2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manohar S, Thongprayoon C, Cheungpasitporn W, Mao MA, Herrmann SM. Associations of rotational shift work and night shift status with hypertension: a systematic review and meta-analysis. J Hypertens 35: 1929–1937, 2017. [DOI] [PubMed] [Google Scholar]
- 78.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466: 627–631, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McQuaid SE, Hodson L, Neville MJ, Dennis AL, Cheeseman J, Humphreys SM, Ruge T, Gilbert M, Fielding BA, Frayn KN, Karpe F. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes 60: 47–55, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mihai R, Coculescu M, Wakerley JB, Ingram CD. The effects of [Arg8]vasopressin and [Arg8]vasotocin on the firing rate of suprachiasmatic neurons in vitro. Neuroscience 62: 783–792, 1994. [DOI] [PubMed] [Google Scholar]
- 81.Millar-Craig MW, Bishop CN, Raftery EB. Circadian variation of blood-pressure. Lancet 1: 795–797, 1978. [DOI] [PubMed] [Google Scholar]
- 82.Moholdt T, Parr EB, Devlin BL, Debik J, Giskeodegard G, Hawley JA. The effect of morning vs evening exercise training on glycaemic control and serum metabolites in overweight/obese men: a randomised trial. Diabetologia 64: 2061–2076, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Montaruli A, Roveda E, Calogiuri G, La Torre A, Carandente F. The sportsman readjustment after transcontinental flight: a study on marathon runners. J Sports Med Phys Fitness 49: 372–381, 2009. [PubMed] [Google Scholar]
- 84.Moraes MR, Bacurau RF, Ramalho JD, Reis FC, Casarini DE, Chagas JR, Oliveira V, Higa EM, Abdalla DS, Pesquero JL, Pesquero JB, Araujo RC. Increase in kinins on post-exercise hypotension in normotensive and hypertensive volunteers. Biol Chem 388: 533–540, 2007. [DOI] [PubMed] [Google Scholar]
- 85.Moreira SR, Cucato GG, Terra DF, Ritti-Dias RM. Acute blood pressure changes are related to chronic effects of resistance exercise in medicated hypertensives elderly women. Clin Physiol Funct Imaging 36: 242–248, 2014. [DOI] [PubMed] [Google Scholar]
- 86.Mores N, Martire M, Pistritto G, Volpe AR, Menini E, Folli G, Cardillo C. Platelet alpha 2-adrenoceptors and diurnal changes of platelet aggregability in hypertensive patients. J Hypertens 12: 939–945, 1994. [PubMed] [Google Scholar]
- 87.Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A 113: E1402–E1411, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morris CJ, Purvis TE, Mistretta J, Hu K, Scheer F. Circadian misalignment increases C-reactive protein and blood pressure in chronic shift workers. J Biol Rhythms 32: 154–164, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 313: 1315–1322, 1985. [DOI] [PubMed] [Google Scholar]
- 90.Nicholls SK, Casiraghi LP, Wang W, Weber ET, Harrington ME. Evidenceforinternaldesynchronycausedbycircadianclockresetting.Yale J Biol Med 92: 259–270, 2019. [PMC free article] [PubMed] [Google Scholar]
- 91.Ohmori M, Fujimura A. ACE inhibitors and chronotherapy. Clin Exp Hypertens 27: 179–185, 2005. [PubMed] [Google Scholar]
- 92.Otto ME, Svatikova A, Barretto RB, Santos S, Hoffmann M, Khandheria B, Somers V. Early morning attenuation of endothelial function in healthy humans. Circulation 109: 2507–2510, 2004. [DOI] [PubMed] [Google Scholar]
- 93.Park S, Jastremski CA, Wallace JP. Time of day for exercise on blood pressure reduction in dipping and nondipping hypertension. J Hum Hypertens 19: 597–605, 2005. [DOI] [PubMed] [Google Scholar]
- 94.Paschos GK, FitzGerald GA. Circadian clocks and vascular function. Circ Res 106: 833–841, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peek CB, Levine DC, Cedernaes J, Taguchi A, Kobayashi Y, Tsai SJ, Bonar NA, McNulty MR, Ramsey KM, Bass J. Circadian clock interaction with HIF1alpha mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab 25: 86–92, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perrin L, Loizides-Mangold U, Chanon S, Gobet C, Hulo N, Isenegger L, Weger BD, Migliavacca E, Charpagne A, Betts JA, Walhin JP, Templeman I, Stokes K, Thompson D, Tsintzas K, Robert M, Howald C, Riezman H, Feige JN, Karagounis LG, Johnston JD, Dermitzakis ET, Gachon F, Lefai E, Dibner C. Transcriptomic analyses reveal rhythmic and CLOCK-driven pathways in human skeletal muscle. Elfie 7, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Phillips AJK, Clerx WM, O’Brien CS, Sano A, Barger LK, Picard RW, Lockley SW, Klerman EB, Czeisler CA. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci Rep 7: 3216, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Poirier P, Mawhinney S, Grondin L, Tremblay A, Broderick T, Cleroux J, Catellier C, Tancrede G, Nadeau A. Prior meal enhances the plasma glucose lowering effect of exercise in type 2 diabetes. Med Sci Sports Exerc 33: 1259–1264, 2001. [DOI] [PubMed] [Google Scholar]
- 99.Poirier P, Tremblay A, Catellier C, Tancrede G, Garneau C, Nadeau A. Impact of time interval from the last meal on glucose response to exercise in subjects with type 2 diabetes. J Clin Endocrinol Metab 85: 2860–2864, 2000. [DOI] [PubMed] [Google Scholar]
- 100.Portaluppi FS, M. H. Circadians rhythms and enviromental determinants of blood pressure regulation in normal and hypertensive conditions. In: Press H, editor. Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics. Totowa: Humana Press, 2007, p. 133–156. [Google Scholar]
- 101.Proper KI, van de Langenberg D, Rodenburg W, Vermeulen RCH, van der Beek AJ, van Steeg H, van Kerkhof LWM. The relationship between shift work and metabolic risk factors: a systematic review of longitudinal studies. Am J Prev Med 50: e147–e157, 2016. [DOI] [PubMed] [Google Scholar]
- 102.Pullinger SA, Cocking S, Robertson CM, Tod D, Doran DA, Burniston JG, Varamenti E, Edwards BJ. Time-of-day variation on performance measures in repeated-sprint tests: a systematic review. Chronobiol Int 37: 451–468, 2020. [DOI] [PubMed] [Google Scholar]
- 103.Qian J, Scheer FA, Hu K, Shea SA. The circadian system modulates the rate of recovery of systolic blood pressure after exercise in humans. Sleep 43, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rabinovich-Nikitin I, Lieberman B, Martino TA, Kirshenbaum LA. Circadian-regulated cell death in cardiovascular diseases. Circulation 139: 965–980, 2019. [DOI] [PubMed] [Google Scholar]
- 105.Racinais S. Different effects of heat exposure upon exercise performance in the morning and afternoon. Scand J Med Sci Sports 20 (Suppl 3): 80–89, 2010. [DOI] [PubMed] [Google Scholar]
- 106.Reebs SG, Mrosovsky N. Effects of induced wheel running on the circadian activity rhythms of Syrian hamsters: entrainment and phase response curve. J Biol Rhythms 4: 39–48, 1989. [DOI] [PubMed] [Google Scholar]
- 107.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol 22: 939–943, 2012. [DOI] [PubMed] [Google Scholar]
- 108.Roenneberg T, Merrow M. The circadian clock and human health. Curr Biol 26: R432–R443, 2016. [DOI] [PubMed] [Google Scholar]
- 109.Romero SA, Minson CT, Halliwill JR. The cardiovascular system after exercise. J Appl Physiol 122 (925–932): 2017, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rudic RD, McNamara P, Reilly D, Grosser T, Curtis AM, Price TS, Panda S, Hogenesch JB, FitzGerald GA. Bioinformatic analysis of circadian gene oscillation in mouse aorta. Circulation 112: 2716–2724, 2005. [DOI] [PubMed] [Google Scholar]
- 111.Saad A, Dalla Man C, Nandy DK, Levine JA, Bharucha AE, Rizza RA, Basu R, Carter RE, Cobelli C, Kudva YC, Basu A. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 61: 2691–2700, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sato S,Basse AL,Schonke M,Chen S,Samad M,Altintas A,Laker RC, Dalbram E, Barres R, Baldi P, Treebak JT, Zierath JR, Sassone-Corsi P. Time of exercise specifies the impact on muscle metabolic pathways and systemic energy homeostasis. Cell Metab 30: 92–110 e114, 2019. [DOI] [PubMed] [Google Scholar]
- 113.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 106: 4453–4458, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Scheer FA, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci U S A 107: 20541–20546, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Scheer FA, Shea SA. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood 123: 590–593, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scheer FA, Ter Horst GJ, van Der Vliet J, Buijs RM. Physiological and anatomic evidence for regulation of the heart by suprachiasmatic nucleus in rats. Am J Physiol Heart Circ Physiol 280: H1391–H1399, 2001. [DOI] [PubMed] [Google Scholar]
- 117.Scheer FA, Van Doornen LJ, Buijs RM. Light and diurnal cycle affect autonomic cardiac balance in human; possible role for the biological clock. Auton Neurosci 110: 44–48, 2004. [DOI] [PubMed] [Google Scholar]
- 118.Takahashi JS. Molecular components of the circadian clock in mammals. Diabetes Obes Metab 17 (Suppl 1): 6–11, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Taylor CE, Atkinson G, Willie CK, Jones H, Ainslie PN, Tzeng YC. Diurnal variation in the mechanical and neural components of the baroreflex. Hypertension 58: 51–56, 2011. [DOI] [PubMed] [Google Scholar]
- 120.Teo SYM, Kanaley JA, Guelfi KJ, Marston KJ, Fairchild TJ. The effect of exercise timing on glycemic control: a randomized clinical trial. Med Sci Sports Exerc 52: 323–334, 2020. [DOI] [PubMed] [Google Scholar]
- 121.Thosar SS, Berman AM, Herzig MX, McHill AW, Bowles NP, Swanson CM, Clemons NA, Butler MP, Clemons AA, Emens JS, Shea SA. Circadian rhythm of vascular function in midlife adults. Arterioscler Thromb Vasc Biol 39: 1203–1211, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thosar SS, Berman AM, Herzig MX, Roberts SA, Lasarev MR, Shea SA. Morning impairment in vascular function is unrelated to overnight sleep or the inactivity that accompanies sleep. Am J Physiol Regul Integr Comp Physiol 315: R986–R993, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thosar SS, Butler MP, Shea SA. Role of the circadian system in cardiovascular disease. J Clin Invest 128: 2157–2167, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tibana RA, de Sousa NM, da Cunha Nascimento D, Pereira GB, Thomas SG, Balsamo S, Simoes HG, Prestes J. Correlation between acute and chronic 24-hour blood pressure response to resistance training in adult women. Int J Sports Med 36: 82–89, 2015. [DOI] [PubMed] [Google Scholar]
- 125.Tochikubo O, Kawano Y, Miyajima E, Toshihiro N, Ishii M. Circadian variation of hemodynamics and baroreflex functions in patients with essential hypertension. Hypertens Res 20: 157–166, 1997. [DOI] [PubMed] [Google Scholar]
- 126.Torii J, Shinkai S, Hino S, Kurokawa Y, Tomita N, Hirose M, Watanabe S, Watanabe S, Watanabe T. Effect of time of day on adaptive response to a 4-week aerobic exercise program. J Sports Med Phys Fitness 32: 348–352, 1992. [PubMed] [Google Scholar]
- 127.Torquati L, Mielke GI, Brown WJ, Kolbe-Alexander T. Shift work and the risk of cardiovascular disease. A systematic review and meta-analysis including dose-response relationship. Scand J Work Environ Health 44: 229–238, 2018. [DOI] [PubMed] [Google Scholar]
- 128.Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest 88: 934–942, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van Moorsel D, Hansen J, Havekes B, Scheer F, Jorgensen JA, Hoeks J, Schrauwen-Hinderling VB, Duez H, Lefebvre P, Schaper NC, Hesselink MKC, Staels B, Schrauwen P. Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. MolMetab 5: 635–645, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Van Reeth O, Sturis J, Byrne MM, Blackman JD, L’Hermite-Baleriaux M, Leproult R, Oliner C, Refetoff S, Turek FW, Van Cauter E. Nocturnal exercise phase delays circadian rhythms of melatonin and thyrotropin secretion in normal men. Am J Physiol 266: E964–E974, 1994. [DOI] [PubMed] [Google Scholar]
- 131.Verrillo A, De Teresa A, Martino C, Di Chiara G, Pinto M, Verrillo L, Torello F, Gattoni A. Differential roles of splanchnic and peripheral tissues in determining diurnal fluctuation of glucose tolerance. Am J Physiol 257: E459–E465, 1989. [DOI] [PubMed] [Google Scholar]
- 132.Vetter C. Circadian disruption: What do we actually mean? Eur J Neurosci 51: 531–550, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vetter C, Devore EE, Wegrzyn LR, Massa J, Speizer FE, Kawachi I, Rosner B, Stampfer MJ, Schernhammer ES. Association between rotating night shift work and risk of coronary heart disease among women. JAMA 315: 1726–1734, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Viola AU, Gabel V, Chellappa SL, Schmidt C, Hommes V, Tobaldini E, Montano N, Cajochen C. Dawn simulation light: a potential cardiac events protector. Sleep Med 16: 457–461, 2015. [DOI] [PubMed] [Google Scholar]
- 135.Voutilainen S, Kupari M, Hippelainen M, Karppinen K, Ventila M. Circadian variation of left ventricular diastolic function in healthy people. Heart 75: 35–39, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wolff G, Esser KA. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med Sci Sports Exerc 44: 1663–1670, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wong PM, Hasler BP, Kamarck TW, Muldoon MF, Manuck SB. Social jetlag, chronotype, and cardiometabolic risk. J Clin Endocrinol Metab 100: 4612–4620, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wright KP Jr, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms 20: 168–177, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yi CX, van der Vliet J, Dai J, Yin G, Ru L, Buijs RM. Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology 147: 283–294, 2006. [DOI] [PubMed] [Google Scholar]
- 140.Youngstedt SD, Elliott JA, Kripke DF. Human circadian phase-response curves for exercise. J Physiol 597: 2253–2268, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zambon AC, McDearmon EL, Salomonis N, Vranizan KM, Johansen KL, Adey D, Takahashi JS, Schambelan M, Conklin BR. Time- and exercise-dependent gene regulation in human skeletal muscle. Genome Biol 4: R61, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang L, Prosdocimo DA, Bai X, Fu C, Zhang R, Campbell F, Liao X, Coller J, Jain MK. KLF15 establishes the landscape of diurnal expression in the heart. Cell Rep 13: 2368–2375, 2015. [DOI] [PubMed] [Google Scholar]