Abstract
Agricultural crops are subject to a variety of biotic and abiotic stresses that adversely affect growth and reduce the yield of crop plantss. Traditional crop stress management approaches are not capable of fulfilling the food demand of the human population which is projected to reach 10 billion by 2050. Nanobiotechnology is the application of nanotechnology in biological fields and has emerged as a sustainable approach to enhancing agricultural productivity by alleviating various plant stresses. This article reviews innovations in nanobiotechnology and its role in promoting plant growth and enhancing plant resistance/tolerance against biotic and abiotic stresses and the underlying mechanisms. Nanoparticles, synthesized through various approaches (physical, chemical and biological), induce plant resistance against these stresses by strengthening the physical barriers, improving plant photosynthesis and activating plant defense mechanisms. The nanoparticles can also upregulate the expression of stress-related genes by increasing anti-stress compounds and activating the expression of defense-related genes. The unique physico-chemical characteristics of nanoparticles enhance biochemical activity and effectiveness to cause diverse impacts on plants. Molecular mechanisms of nanobiotechnology-induced tolerance to abiotic and biotic stresses have also been highlighted. Further research is needed on efficient synthesis methods, optimization of nanoparticle dosages, application techniques and integration with other technologies, and a better understanding of their fate in agricultural systems.
Keywords: Nanobiotechnology, Plant stresses, Abiotic factors, Biotic factors, Insect pests, Plant diseases, Nanoparticles
Introduction
Crop production has been stagnant during the last decades while food demand is increasing sharply due to ever increasing human population [1]. It has been reported that almost 800 million people are chronically hungry and 2 billion suffer micronutrient deficiencies while 653 million people would still be undernourished in 2030 [2]. Therefore, food security will remain a huge challenge as the world’s human population will reach around 10 billion in 2050 [3]. The global losses of one-third of food produced have been estimated by FAO (Food and Agriculture Organization) in 2011 which amounts to about 1.3 billion tons per year [4]. The pre-harvest crop losses reported are around 35% due to different factors (diseases, animal pests, weeds, abiotic stresses) which account for 1051.5 Mt (million tonnes). In addition, the losses during harvesting and storage are about 690 Mt [5].
Both biotic and abiotic stresses cause significant crop yield losses. However, the global crop yield losses due to biotic stresses vary among major crops and regions [6]. Insect pests cause 15–20% yield losses in principal food and cash crops [7]. Similarly, the global estimates of yield losses due to pathogenic disease range from 11 to 30% [6, 8, 9]. Abiotic stresses (drought, water logging, temperatures, salinity, heavy metals and mineral toxicity, etc.) lead to morphological, physiological, biochemical, and molecular changes in plants that adversely affect their growth, development, and productivity [10]. They can cause significant losses (50–70%) in growth and yield [11–15]. The overproduction of reactive oxygen species (ROS) is one of the major reasons for crop losses caused by abiotic stresses [16]. The global models have predicted an increase in CO2 levels from 400 to 800 ppm [17, 18]. Moreover, more than 45% of arable lands are endangered by drought [19, 20], above 27% of the global area is under aridity, and most crop species are sensitive to salt stress (1.0–1.8 dS m−1). These abiotic stresses can cause 10 to 50% yield loss [21, 22]. In addition, heavy metal (Cr, Cd, As, Pb, Cu, Hg) pollution negatively affects seed germination, photosynthesis, respiration, and transpiration and ultimately reduces growth, yield as well as yield quality [23, 24].
The use of synthetic chemicals had been the main focus to mitigate the effects of abiotic and biotic stresses in crop plants. The estimated increase in pesticide use was up to 3.5 Mt in 2020 [25] with an estimated value of US$ 103.5 billion. The global pesticide market is predicted to reach more than US$ 107.5 to 184 billion in 2023 to 2033 respectively with a steady growth of 5.5% (https://www.persistencemarketresearch.com/market-research/pesticides-market.asp: data retrieved on 12-02-2023). The facts about health hazards and impacts on non-target organisms of pesticides revealed a 35% decrease in soil respiration, a 90% water pollution of agricultural lands, a 70% decline in insect biomass, a 50% decline in farmland birds, a 30% decline in the honey bee population, a 42% reduction in species richness in Europe, Australia and Americas. In addition, a 25–30% increase in cancer and mental health risks and a 50% risk of leukemia, lymphoma and brain cancer is linked to pesticide exposure to children [26]. Moreover, the combined impacts of different stresses increase the complexity of plant responses. Thus, a second green revolution is needed to fulfill the food demand of the human population. Therefore, this is required to find some alternative solutions focusing on environmental sustainability and human health.
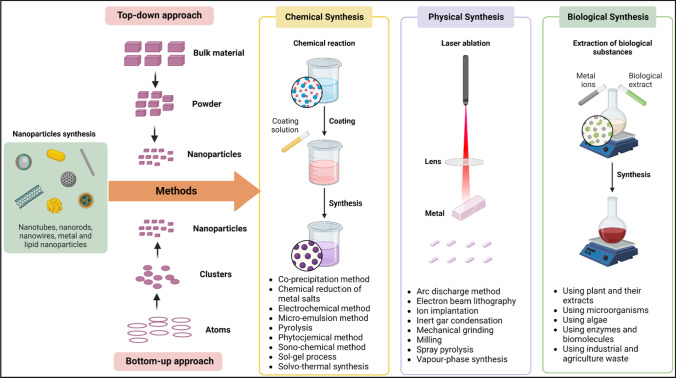
Nanotechnology as a “Key Enabling Technology’’ [27] has the potential to serve as a key alternative to achieve the goal of sustainable agriculture [28]. Nanoparticles are synthesized through physical, chemical and biological approaches (Fig. 1) by using metal or metal oxide [29]. Metal-based nano- insecticides, pesticides, and insect repellant formulations show significant potential against plant pathogens and insect pests [30]. However, the biological synthesis (green synthesis) of nanoparticles using plants or plant extracts, entomopathogens and other biomaterial has potential benefits over other approaches. Plants or plant extracts contain enzymes, sugars, and phytochemicals like flavonoids, latex, phenolics, terpenoids, alcohols, amines and cofactors, etc. which act as reducing and stabilizing agents during the synthesis of metal nanoparticles. This helps to prepare not only the most promising and eco-friendly nanoparticles with well-defined sizes and shapes but also prevent environmental contamination [31–33]. Therefore, the present review encapsulates the innovations in nanobiotechnology applications in agriculture especially focusing on the potential of biosynthesized nanoparticles to mitigate abiotic and biotic stresses in crop production.
Fig. 1.
Nanoparticles synthesis with topdown and bottom up approaches showing the chemical, physical and biological synthesis methods [34]
Nanobiotechnology for abiotic stress tolerance
Abiotic stresses significantly affect plant growth and cause a substantial reduction in crop yield (by about 50%) [11, 13–15]. These stresses disturb the plant metabolism lead causing a significant reduction in plant growth, development, and yield formation [35]. In addition to other effects, the overproduction of reactive oxygen species (ROS) is one of the major reasons for crop losses caused by abiotic stresses [16, 36]. The application of nutrients, osmoprotectants and stress signaling compounds has been effective in scavenging the ROS and improving the tolerance against abiotic stresses [13, 15, 37]. However, the application of these materials in nano-sizes can be more effective. For example, increasing evidence indicates that delivery of the above-mentioned substances as nanomaterials improves plant tolerance against heat [38], drought [39], salinity [40] and trace metal [41] stresses. Nanomaterials have high surface energy and a high surface/volume ratio that helps improve their biochemical activity, reactivity and effectiveness when delivered to plants [28]. In plant defense, nanomaterials not only shield against ROS but also act as oxidative stress inducers at the same time [42]. The latter stimulates the plant antioxidant defense system. Novel properties of nanoparticles (NPs) exhibit their potential not only for crop management but also to deal with abiotic stresses. Several metals including silver (Ag), copper (Cu), gold (Au), iron (Fe), titanium (Ti) and zinc (Zn) and their oxides have been used to produce NPs. These NPs have been recently used for the green synthesis of NPs using plants and their extracts, micro-organisms and membranes, and DNA of viruses including diatoms. Green-synthesized NPs widely used in agriculture include AgNPs [43, 44].
In the following lines, the role of nanobiotechnology in improving tolerance against different abiotic stresses has been discussed.
Drought stress
Drought is one of the major abiotic stresses with devastating effects on growth and productivity of crop plants [13, 45]. The US weather disaster analysis from 1980 to 2012 revealed that drought and heat stresses caused extensive agricultural losses of around $200 billion in which drought alone caused $50 billion worth of damage [46]. Drought exerts several morphological, physiological, biochemical and molecular responses in crop plants for adaptation to drought. Some adaptive mechanisms include the activation of the defence system, reducing leaf area, osmotic balance, hormonal homeostasis, expression of stress genes and shortening of plant life cycle [45]. However, only a few studies report the role of green synthesized NPs in the alleviation of drought stress. For instance, the application of green synthesized AgNPs increased antimicrobial activity against Escherichia coli and Staphylococcus aureus under drought stress in Tephrosia apollinea [47]. The improved response was observed with a decrease in membrane damage and an increase in hydrogen peroxide (H2O2) contents in the roots of T. apollinea under incremental drought. Likely, Ag-synthesized green NPs improved the seed germination (89.5%), germination rate (6.88) and seedling biomass in lentil under drought stress. This improvement was associated with the maintainance of tissue water balance [48]. Thus, green synthesized NPs can be used to reduce the detrimental effects of drought, however, further investigation of associated physiological, biochemical and molecular mechanisms is needed. Future studies on other metals NPs may be extended to explore their biological roles under drought.
Salt stress
More than 20% of cultivated lands are affected salt stress. Salt stress affects the plant growth through salinity-induced osmotic stress, specific ion toxicity and mineral imbalance. The application of NPs improves plant growth, modulates carbohydrate and protein synthesis, and enhances the activities of antioxidant enzymes such as catalase (CAT), guaiacol peroxidase (POX), ascorbate peroxidase (APX) under salt stress and thus helps reducing the impact of salt stress by ROS detoxification and hormonal regulation [49]. In another study, the foliage applied Se and Cu nanofertilizer reduced the impact of saline water and significantly improved the tomato growth, nevertheless, soil properties were negatively affected due to the application of saline water [50].
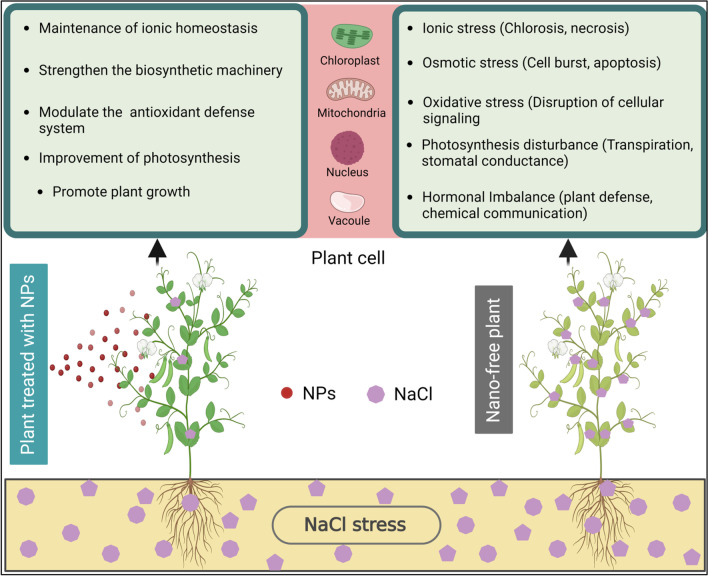
Under salt stress, Na+ and Cl− uptake increases that causes oxidative damage and restrict the K+ and Ca2+ uptake [51]. The Application of NPs restrict Na+ and Cl− entry to plants under salinity. The application of ZnO-NPs improved the uptake of K+ and Ca2+ while reducing Na+ and Cl− accumulation [52]. The NPs of iron (Fe), cesium (Cs) and cobalt (Co) play a supportive role for catalase enzyme, whereas, copper (Cu), iron (Fe), caesium (Cs) and manganese (Mn) do the same role for peroxidase (POD) enzyme [53]. The application of silver NPs at low concentrations improved antioxidant enzymatic activity and improved plant growth under salt stress conditions [54]. Likewise, the application of TiO2-NPs improved salt tolerance in Dracocephalum moldavica through the activation of antioxidant defense system that helped reduce the salinity-induced oxidative damages and improve plant growth under salt stress [55]. The use of NPs can help improve plant salt tolerance through the activation of antioxidant system, maintenance of tissue water status and ion homeostasis, etc. (Fig. 2). However, further research is desired to improve the efficiency of NPs for field-scale use to improve plant salt tolerance.
Fig. 2.
Schematic mechanisms in plant cell associated with NPs application alleviating salt stress [56]
Thermal stresses
Temperature extremes, high and low, have a strong impact on plant growth, development and yield formation [37, 57]. High temperature stress or heat stress is the rise in temperature beyond a critical threshold for a period of time sufficient to cause irreversible damage to plant growth, development, and yield [13, 58]. Heat stress induces the overproduction of ROS causing oxidative damage to plant biological membranes and other vital molecules [59, 60] and causing a significant reduction in photosynthetic pigments and carbon assimilation in wheat and chickpea [60, 61]. Low temperature may cause chilling (0–15 °C) or freezing (< 0 °C) stresses. The chilling stress causes injuries without ice crystal formation whereas the freezing stress damage plant tissues by forming ice crystals [37]. Low temperature stresses affect plant growth due to photoinhibition, ROS-induced oxidative damages, reduction in the nutrient update, activities of various enzymes and carbon assimilation [13].
The use of nanomaterials has been quite effective in improving the plant tolerance to heat stress (Table 1). For example, Iqbal et al. [62] reported significant improvement in plant biomass and leaf area in wheat under heat stress by exogenous application of AgNPs synthesized with moringa extract. The green-synthesized AgNPs balanced the tissue water content status and improved chlorophyll content under heat stress compared to the control in wheat crop [62]. The application of moringa extract-synthesized AgNPs improved the tolerance against heat stress through the activation of antioxidant defense system [62] as AgNPs application modulates the activation of antioxidant genes (MeCu/ZnSOD and MeAPX2) under thermal stresses in Arabidopsis thaliana [63].
Table 1.
Role of different nanoparticles in improving tolerance against thermal stresses in different plant species
| Nanoparticles (NP) | Plant species | Effects | Reference |
|---|---|---|---|
| Ag NP | Wheat | Increased the root (5 to 5.4%) and shoot length (22.2 to 26.1%), fresh (1.3 to 2%) and dry (0.36 to 0.60%) weight, and leaf area (18.3 to 33.8%) under heat stress compared to control | [62] |
| Wheat | Balanced relative water content (RWC) and improved chlorophyll content under heat stress compared to control | [64] | |
| CeO2 NP | Maize | Enhanced activities of catalase (2000 to 3000%) and peroxidase (162 to 1400%) compared with control, and upregulated HSP70 expression under heat stress | [65] |
| Ce NP | Arabidopsis | Reduced (52%) the leaf reactive species levels, and Increased the quantum yield of photosystem II (19%), the carbon assimilation rate (67%) and the Rubisco carboxylation rate (61%) compared with control under cold and heat stresses | [38] |
| MWCNTs (multi-walled carbon nanotubes) | Tomato | Upregulated the expression of various stress-related genes including HSP90 under heat stress | [66] |
| Na2SeO4 NP | Tomato | Improved root volume (33 to 60%) and leaf chlorophyll (19 to 18%) contents compared with control under heat and cold stresses | [67] |
| Se NP | Sorghum | Stimulated the antioxidant defense system by enhancing activities of antioxidant enzymes including superoxide dismutase (150%), catalase (120%) and peroxidase (40%) compared with control under heat stress | [68] |
| Se NP | Chrysanthemum | Improved plant biomass through the activation of antioxidant enzymes (peroxidase and catalase) | [69] |
| SiO2 NP | Wheatgrass | Overcame seed dormancy, and improved seed germination (28%) and seedling weight (49%) compared with control under cold stress | [70] |
| TiO2 NP | Tomato | Increased photosynthetic efficiency under heat stress compared with control | [71] |
| Chickpea | Enhanced activities of antioxidative enzymes (superoxide dismutase, catalase, ascorbate peroxidase, glutathione peroxidase, guaiacol peroxidase, polyphenol oxidase, lipoxygenase, allenoxide synthase) and chlorophyll contents (1–50%), and decreased H2O2 content (27 to 31% and electrolyte leakage (7 to 25%) compared with control under cold stress | [72] | |
| Chickpea | Increased the Rubisco activity (7%), decreased the H2O2 contents (29 to 34%) compared with control under cold stress | [73] | |
| Chickpea | Decrease the rate of electrolyte leakage (14 to 32%) and increased the expression of cold tolerance genes compared with control under cold stress | [74] | |
| Chitosan NP | Banana plants (Musa acuminata var. Baxi) | Decreased the lipid peroxidation (33%), reactive oxygen species (H2O2, •OH, and O2•− by 33, 33, 40, and 48%, respectively), and increased the fresh (14%) and dry weights (41%), and antioxidant activities (peroxidase (and superoxide dismutase by 8 and 17%, respectively), and oxidative damages under cold stress | [75] |
Selenium (Se) is known for its vital role in the plant antioxidant defense system. Application SeNPs, at low concentration, stimulated the antioxidant defense system by enhancing the activities of antioxidant enzymes, and improved the photosynthetic pigments and plant growth of tomato [67] and grain sorghum [68]. The application of SeNPs, green-synthesized with a bacterial strain Lactobacillus casei, improved the tolerance against heat stress in chrysanthemum through the activation of the antioxidant defense system [69]. The application of titanium dioxide (TiO2) NPs also contributed to heat tolerance in tomato by regulating the stomatal oscillation [71].
Heat shock proteins (HSPs) are molecular chaperones expressed in plant exposure to heat stress [76]. These HSPs induce heat tolerance in plant species by stabilizing the protein structure [77]. The application of nanomaterials has been very effective in inducing heat stress through the expression of HSPs (Table 1). For instance, Zhao et al. [65] reported that the application of cerium oxide (CeO2) NPs improved maize growth under heat stress due to the upregulation of HSP70. In another study, the application of multi-walled carbon nanotubes (MWCNTs) improved tomato growth under heat stress through the expression of various stress-related genes including HSP90 [66].
The use of nanomaterials has been found effective to induce cold tolerance in different plant species. For example, SeNPs application improved the photosynthetic pigments, activated the plant antioxidant defense system and increased tomato plant growth under cold stress [67]. Likewise, the application of titanium oxide (TiO2) NPs improved the cold tolerance in chickpea through a significant increase in the activities of antioxidant enzymes (superoxide dismutase, catalase, ascorbate peroxidase, glutathione peroxidase, guaiacol peroxidase, polyphenol oxidase, lipoxygenase, allenoxide synthase), chlorophyll contents, and a significant reduction in ROS and oxidative damage compared with control under cold stress [78] The application of TiO2NPs also induced the expression of cold tolerance genes [74].
Plant photosynthesis, the key physiological process responsible for food production for all, is sensitive to cold stress [16]. In addition to ROS-induced oxidative damages, cold stress causes a significant reduction in photosynthetic pigments and activity of key photosynthetic enzymes including Rubisco carbon resulting in a decrease in photosynthetic rate [13, 79]. However, the application of nanomaterials has been found to reduce the ROS-induced damage in the thylakoid membrane [80, 81] and improves the light absorption capacity of chloroplast [82], electron transport rate and the activity and efficiency of Rubisco [73, 83, 84]. The application of TiO2 NPs in chickpea caused a significant increase in the activities of antioxidant enzymes (superoxide dismutase, catalase, ascorbate peroxidase, glutathione peroxidase, guaiacol peroxidase, polyphenol oxidase, lipoxygenase, allenoxide synthase), chlorophyll contents [78] and expression of genes controlling the chlorophyll-binding protein and Rubisco [73] under cold stress and increased plant cold tolerance (Table 1).
Toxic metals stress
Soil contamination by toxic metals has been recognized as an important threat to plant development, soil ecosystem, and human health. Metals are grouped into two categories including (i) essential metals and (ii) non-essential metals. Essential metals are required to support plant development as micronutrients. These include cobalt (Co), copper (Cu), iron (Fe), manganese (Mn), molybdenum (Mo), nickel (Ni), selenium (Se), and zinc (Zn). Nevertheless these essential metals becomes toxic to plants if their concentration increases the threshold levels [85]. On the other hand, non-essential metals like arsenic (As), cadmium (Cd), chromium (Cr), lead (Pb), mercury (Hg), and silver (Ag),do not have any biological role in plantsand can have detrimental impacts even at low concentration [86].
Toxic metals stress affect plant metabolism by disturbing the protein structure, hampering functional groups of vital molecules, and causing oxidative damage to biological membranes [87–89].
The use of various nanomaterials with microbes simultaneously or sequentially, can impede the effects of toxic metals in plants. For example, the inoculation of arbuscular mycorrhizal fungi along with nanoscale zero-valent iron (nZVI) even in lower amounts (100 mg kg–1) improved the immobilization of Cd reducing their uptake in sweet sorghum in acidic polluted soil [90]. Similarly, the use of polyvinylpyrrolidone-coated iron oxide (Fe2O3) nanoparticles along with Gram-negative bacterium Halomonas sp. completely removed Cd and Pb while shortening the remediation time. On the other hand, nanoparticles alone removed about 66% of Cd and 82% of Pb as compared to 84% of Cd and 81% of Pb removal by bacterium alone [91].
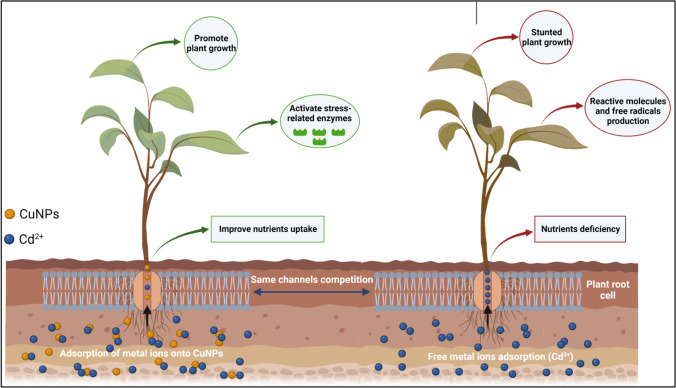
Nanomaterials can also improve the phytoextraction efficiency for soil remediation. For example, Lolium perenne plants in the presence of nZVI (100 mg kg–1) showed the highest accumulation of Pb (1175 μg per pot). However, higher doses of nZVI (2000 mg kg–1) reduced the uptake of Pb (to 832 μg per pot) by causing oxidative stress to plants [92]. Nanobiotechnology can also rely on the use of biologically synthesized materials to combat toxic metal stress in soils [93, 94]. For example, biogenic copper nanoparticles developed by involving a copper-resistant bacterium (Shigella flexneri SNT22) decreased Cd uptake (by 50%) from contaminated soil to wheat plants [94]. This treatment also improved the shoot dry weight (by 28%), plant length (by 44%), and the contents of nitrogen (41%), and phosphorus (58%). It has been proposed that biogenic copper nanoparticles adsorb Cd on its surface and prevent its uptake by plants [94]. Moreover, these nanoparticles hinder metal translocation into plants by competing with metal for membrane entry channels. After they enter into the plant body, these nanoparticles activate many defense-related enzymes reducing the metal translocation to plants (Fig. 3).
Fig. 3.
The role of biogenic copper nanoparticles (CuNPs) in reducing the translocation of Cd from soil to plants and facilitating the plant to activate defense system to combat Cd-stress. This figure is reproduced with permission from [94]
Despite the potential of nanobiotechnology in alleviating metal stress, there exist limited studies in this emerging field. The integration of nanotechnology and bioremediation can combine the benefits of both strategies and can facilitate the development of sustainable remediation technology. However, the biogeochemistry of contaminated soils can have a significant effect on the efficiency and interactions of nanoparticles with pollutants and microbes [95]. This needs to be explored for field application with an emphasis on the fate and environmental impacts of nanomaterials.
Organic pollutants stress
Organic pollutants (OPs) are ubiquitous in all compartments of the environment. They are very diverse in nature and are classified into more than 20 different classes [96]. The OPs are classified as industrial chemicals, pesticides, and pharmaceuticals. Polycyclic aromatic hydrocarbons, organochlorine pesticides, polychlorinated dichlorodiphenyltrichloroethane, polybrominated diphenyl ethers, and hexachloro-cyclohexane, are among the most common classes of OPs [97]. These compounds are very toxic; some are mutagenic and carcinogenic even at low concentrations. They are highly stable and may persist in the environment for many decades due to their recalcitrant and hydrophobic nature. These can be absorbed in the food chain and threaten human health, and even at higher concentrations, can cause phytotoxicity. A higher concentration of OPs affects the germination and growth of plants mainly by affecting physiological processes including activities ofkey enzymes, carbon assimilation and several other metabolic events. Like other abiotic stresses, POPs also induce oxidative stress through stimulation of ROS, [98], which leads to lipid peroxidation (LPO) that in turn damage DNA/RNA, and their membranes.
Phytoremediation involves the use of plants for de-contamination of OPs and is a very cost-effective and environment-friendly approach; however, it doesn’t work at higher concentrations of OPs in the environment mainly due to their toxicity in plants. The application of nanomaterials would be effective in reducing the toxicity of OPs to plants. In a study on the effect of Ni/Fe bimetallic-nanoparticles on polybrominated diphenyl ethers (PBDEs) toxicity in Chinese cabbage, nanomaterials significantly decreased the phytotoxicity of PBDEs. This was inferred that the coupling of NMs and bioremediation could reduce the toxicities of soil contaminants and NMs in the plants simultaneously [99]. In another study, the effect of carbon nanotubes (CNTs), on the uptake of four persistent organochlorine insecticides, by lettuce was evaluated. The application of CNTs dramatically decreased the uptake of chlordane components and p,p'-DDE by lettuce seedlings. The reduced uptake by plants was probably due to the higher sorption capacity of CNTs for OPS [100]. The use of Ag NPs has also been found effective in decreasing the uptake and accumulation of p,p′-DDE in Zucchini and soybean [Glycine max (L.) [101]. Similarly, the use of multiwalled carbon nanotubes decreased the pyrene and 1-methylpyrene concentrations in roots and shoots, and reduced their translocation in maize [102].
Some NPs such as ZnO, carbon nanotubes, nZVI particles, graphene quantum dots, Ag, etc. could improve plant growth under OPs stress conditions through different mechanisms. For example, ZnO and graphene quantum dots might serve as nanofertilizer [103]. Praveen et al. [104] reported that the application of ZnO NPs would increase tolerance against Fe2O3 stress in Indian mustard by altering enzyme activities. In addition, NPs may increase growth by improving the uptake and translocation of water and nutrients and by stimulating the soil microbial community.
Biosynthesized nanomaterials have also shown promising results in the degradation of several organic pollutants from the environment. For instance, biosynthesized PdNPs were many folds effective, catalyst powder of commercial palladium, in the dehalogenation of several toxic congeners of polychlorinated biphenyl’s (PCBs) in water and sediments [105]. Likewise, five times more catalytic dehalogenation of a flame retardants tris-(chloroisopropyl)-phosphate (TCPP) was noted with the palletized cells of Desulfovibrio desulfuricans compared to the chemically reduced palladium powder [106]. Another study reported that the surface application of biosynthesized PdNPs on a graphite cathode caused a significant increase in the rates of trichloroethylene (TCE) dehalogenation and diatrizoate deiodination [107]. Although several studies have shown the promising results of biosynthesized nanoparticles on the overall dissipation of OPs from the environment, no studies are found on the ameliorative impact of biosynthesized NPs on the phytotoxicity of OPs.
Hypoxia and anoxia stresses
Due to climate change, plants experience many environmental stresses including submerged and waterlogged conditions [108]. Both stresses cause variable availability of oxygen (O2) ranging from normal O2 level (normoxia) to hypoxia (partial O2 deficiency) and anoxia (total O2 deficiency) because O2 diffusion is slower in water than in air [15]. Flooding stress/hypoxia conditions inhibit respiration, seed germination, and plant growth [109]. Under O2 deficiency (hypoxia and anoxia stresses), the phosphorelation (ATPs generation) significantly reduces. The accumulation of lactate and ethanol, produced during oxygen-deficient respiration, becomes toxic to plants beyond a threshold nd results in cytosolic acidification [110]. Hypoxia-anoxia stresses in plants reduce green pigments, gaseous exchange at stomata, and photosynthetic activity. In addition, hypoxia stress causes oxidative damage by accelerating lipid peroxidation due to the hyperproduction of reactive oxygen species (ROS) in leaf and root tissues [111].
To enhance hypoxia and anoxia tolerance in plants, several molecular and breeding techniques along with natural selection are being used [112]. The exploration and exploitation of novel strategies (nanobiotechnology) complementing existing conventional approaches have become very crucial for sustainable plant growth [112]. The development of green-(bio) nanotechnology by exploiting biological routes helps in minimizing hazards of abiotic stresses in plants including hypoxia/anoxia stresses [113]. In this regard, silicon nanoparticles (SiNPs) -treated plants combat hypoxia stress more efficiently compared with conventional Si by improving antioxidant activities, osmoprotectant accumulation, and micronutrient regulation [111]. Almutairi [114] reviewed that AgNPs and aluminum oxide nanoparticles (Al2O3NPs) decreased the adverse effects of flooding stress. Similarly, Mustafa et al. [115] used Al2O3NPs, ZnONPs, and AgNPs in soybeans to alleviate the abiotic stress of flooding. Proteome analysis revealed that most of the protein expressions related to energy metabolism were changed under flooding stress and this change was decreased with the usage of Al2O3NPs.
Mustafa and Komatsu [116] indicated that the influence of the size of NPs in anoxia tolerance was more prominent in soybean, rather than the quantity and types. Nevertheless, nanomaterials reduce flooding stress (hypoxia and anoxia stresses) and enhance plant growth by hindering ethylene biosynthesis in Arabidopsis [117]. Further studies may be initiated to explore the potential of nanomaterial application in improving plant tolerance against hypoxia and anoxia stresses.
Nanobiotechnology and tolerance against biotic stresses
The state of a plant in which living organisms (viruses, bacteria, fungi, nematodes, insects, arachnids, and weeds) disrupt their normal metabolic (growth, vigor, and productivity) activities is known as biotic stress. Biotic stresses are responsible to cause significant economic yield losses in major crops like wheat (28.2%), rice (37.4%), maize (31.2%), potatoes (40.3%), soybeans (26.3%), and cotton crops (28.8%) [118]. The weeds alone causes the highest (32%) yield loss followed by animal pests (18%), fungi and bacteria (15%) and viruses (3%) [8]. Thus, a number of pests are responsible for causing infections and ultimately inciting biotic stress to host plants. Iqbal et al. [111] reported that insects and mites impair plants by piercing and sucking the cell sap or chewing their parts. In addition, insects also act as vectors and carriers for different viral and bacterial diseases. Fungi can kill host cells by toxin secretions (necrotrophic) or feed on living host cells (biotrophic). Nematodes cause nutrient deficiency, stunted growth, and wilting by feeding on host plants. Plant pathogenic bacterial infection symptoms include galls and overgrowths, wilts, leaf spots, specks and blights. Similarly, viruses cause damage resulting in chlorosis and stunting the growth of host plants [111]. Plants defend themselves against biotic stresses through their immune system, physical barriers (cuticles, wax, and trichomes) and chemical compounds [119, 120]. Pesticides (herbicides, insecticides, fungicides, etc.) are used as a major component for the management of pests causing biotic stresses [121, 122]. The facts about health hazards and impacts on non-target organisms of pesticides are linked with serious effects on micro and macro flora as well as fauna including negative impacts on human health [26, 123, 124]. In this regard, the use of nanobiotechnology has been proven very effective for the management of the aforementioned issues. Therefore, the potential of nanobiotechnology to counter biotic stress has been highlighted and discussed below.
Insect pest management
In developing countries, pre-harvest and post-harvest grain crop losses ranging from 15 to 100% and 10–60% [125]. Insects belong to the phylum Arthropoda and 20% of global annual crop losses, valued at over US$ 470 billion are attributed to arthropod pests [126]. In addition to direct damage, insect pests can also transmit or facilitate pathogens (viruses, bacteria, fungi) to cause diseases in plants [127]. The damages reported [128] in major crops due to insect pests are 25%, 5–10%, 30%, 35%, 20%and 50% in rice, wheat, pulses, oilseeds, sugarcane and cotton respectively. The intensive use of pesticides has led to the development of resistance, resurgence and replacement of insect pest species. The resistant insects and mite species had risen to more than 700 [129]. These facts highlight the need for alternate control tactics for the sustainable management of insect pests. The implications of biosynthesized nanoparticles against insect pests have been discussed in this section.
Entomopathogenic bacteria with nanoparticles
Bacteria are ubiquitous in nature and have developed a variety of interactions with insects. They have evolved an array of tactics to invade the host insects, and overcome their immune responses to infect and kill them. Entomopathogenic bacteria (EPB) are generally recognized as low-risk substances than conventional synthetic pesticides. The application of EPB alone for pest management has some limitations i.e., (i) ingestion of spores in the host body to cause infection, (ii) solar radiation, (iii) leaf temperature, (iv) vapor pressure, (v) host resistance and can affect the pathogenicity/virulence of these pathogens [130, 131]. Several studies highlight the compatibility and synergistic effects of EPB and nanoparticles for insect pest management [132]. The bioefficacy of EPB-based nanobiopesticides with different biocompatible chemical elements like silver (Ag), zinc oxide (ZnO), copper oxide (CuO, Cu2O), gold (Au), etc., have been proven effective (Table 2).
Table 2.
The influence of nanoparticles on the motality of target insect pests
| Type of EPB | Metal | Target Pest | Technical Name | Order/Family | Efficacy | Reference |
|---|---|---|---|---|---|---|
| Entomopathogenic Bacteria + Nanoparticles of different metals | ||||||
| Bacillus thuringiensis | Silver (Ag) | Cabbage looper | Trichoplusia ni (Hübner) | Lepidoptera/Noctuidae | Cabbage looper showed dose dependent mortality (40–100%) | [133] |
| Black cutworm | Agrotis ipsilon (Hufnagel) | Lepidoptera/Noctuidae | Significant mortality (22–83%) of black cutworm was recorded | |||
| Red palm weevil |
Rhynchophorus ferrugineus (Olivier) |
Coleoptera/Curculionidae | Significant larval (85%) and adult (75%) mortality | [134] | ||
| Zinc (Zn) | House Fly | Musca domestica | Diptera/Muscidae | Significantredution in LC10, LC20, LC50 and LC90 values of 4.17, 6.11, 12.73 and 38.90 μg/g of larval diet than control | [135] | |
| Pulse beetle | Callosobruchus maculatus | Coleoptera/Chrysomelidae | Caused 100% mortality at 25 μg/mL | [136] | ||
| Ag | Pink Bollworm | Pectinophora gosypiella | Lepidoptera/Gelechiidae | Reduced (31.2%) female fertility, prevented the adult emergence and stopped the life cycle | [137] | |
| Bacillus subtilis | Ag | Red palm weevil |
Rhynchophorus ferrugineus (Olivier) |
Coleoptera/Curculionidae | Significant larval (77%) and adult (67%) mortality | [134] |
| Bacillus megaterium | Dengue vector/ malarial |
Cx. quinquefasciatus Ae. Aegypti |
Diptera/Culicidae | Mortality decreases as compared to individual compound | [138] | |
| Xenorhabdus ssp | Copper (Cu) | Armyworm | Spodoptera litura | Lepidoptera/Noctuidae | 80% mortality was observed at 100 µl/mL of biosynthesized CuNPs | [139] |
| Xenorhabdus nematophila NP-1 strain | Ag | Armyworm | Spodoptera litura | Lepidoptera/Noctuidae | Significantly highest mortality (90%) after 48 h in 100 µg/mL concentration | [140] |
| Entomopathogenic Fungi + Nanoparticles of different metals | ||||||
| Beauveria bassiana | Ag | Dengue vector mosquito | Aedes aegypti | Diptera/Culicidae | The LC50 and LC 90 values were 0.79 and 1.09 with respect to the Ae.aegypti treated with B.bassiana, silver nanoparticles. The highest percentage mortality was found 83.3% | [138] |
| Mustard aphid | Lipaphis erysimi Kalt | Hemiptera/Aphididae | Silver NPs showed the maximum mortality (60–90%) | [141] | ||
| Mustard aphid | Lipaphis erysimi Kalt | Hemiptera/Aphididae | Isolates B4 and B13 showed the maximum mortality (60.088%) | |||
| House fly | Musca domestica | Diptera/Muscidae | Significant mortility of 1st (95–100%), 2nd (70–100%) and 3rd (60–100%) instar larvae of pest | [142] | ||
| Whitefly | Bemisia tabaci | Hemiptera/Aleyrodidae | Green AgNPs of B. bassiana JAU2 gave better insecticidal activity causing 8–97% mortality at different concentrations | [143] | ||
| Zn | Greenhouse whitefly | Trialeurodes vaporariorum | Aleyrodidae/Hemiptera | Mortality rates obtained with ZnO NPs and fungi at the highest concentration were 91.6% and 88.8%, respectively | [144] | |
| Metarhizium anisopliae | Ag | Anopheles culicifacies | Diptera/Culicidae | 50% mortality of Anopheles culicifacies by using silver nanoparticles at 32.8 ppm (I), 39.8 ppm (II), 45.9 ppm (III), 51.9 (IV), and 60.0 ppm (pupa) | [145] | |
| Red palm weevil |
Rhynchophorus ferrugineus (Olivier) |
Coleoptera/Curculionidae | M. anisopliae mediated silver nanoparticles caused highest % mortality (90%), (95%) and (77%) against eggs, larvae and adults of R. ferrugineus | [134] | ||
| Trichoderma viride | Titanium (Ti) | American Bollworm | Helicoverpa armigera | Lepidoptera/Noctuidae | TDNPs exhibited highest mortality rate on first (100%), second (100%) and third (92.34%), instar larvae of H. armigera at 100 ppm | [146] |
| M. anisopliae | Ti | Wax moth | Galleria mellonella | Lepidoptera/Pyralidae | Prodcued highest mortality percentage (82%) | [147] |
| B. bassiana;M. anisopliae; Verticillium lecanii; | Ag | Tortoise beetle, | Cassida vittata | Coleoptera/Chrysomelidae | 3 EPFs caued 47–95% mortality rates within 7 days of exposure | [148] |
| Isaria fumosorosea | zero-valent iron (ZVI) | Whitefly | Bemisia tabaci | Homoptera/Aleyrodidae | Mortality increased with increasing concentrations with highest mortality being at 90.12% for 100 ppm | [149] |
| Sixteen isolates of B. bassiana (13); M. anisopliae (2); I. fumosorosea (1) | Ag | Mealworm | Tenebrio molitor | Coleoptera/Tenebrionidae | B. bassian isolated showed14-94% mortality, M. anisopliae exhibited 78–86% mortality and I. fumosorosea caused 10% mortality | [150] |
| B. bassiana; M. anisopliae; I. fumosorosea | Diamondback moth | Plutella xylostella | Lepidoptera/Plutellidae | The CL50 value of 0.691 mg/mL was determined at 72-h for the 2nd instar larvae of the P. xylostella, causing 78% of cumulative mortality rate | [151] | |
| M. Rileyi | Armyworm | Spodoptera litura | Lepidoptera/Noctuidae | Maximum larval mortality was 80% and 78.75% in laboratory and field conditions | [152] | |
| Plant Extracts + Nanoparticles of different metals | ||||||
| Annona muricata | Ag | Mosquitos | Aedes aegypti | Diptera/Culicidae | AgNps exhibited 100% mortality at 48 h observation | [153] |
| Euphorbia prostrata | Rice weevil | Sitophilus oryzae | Coleoptera/Curculionidae | 71–97% mortality at 50–250 mg/kg concentrations of synthesized silver nanoparticles after 14 days of exposure | [154] | |
| Origanum majorana* | Spotted bollworm | Earias insulana | Lepidoptera/Noctuidae | More than 60% reduction of Earias insulana infestation | [155] | |
| Hypnea musciformis | Diamondback moth | Plutella xylostella | Lepidoptera/Plutellidae | LC50 from 24.5 to 38.23 ppm for L1 and pupae of P. xylostella | [156] | |
|
Ficus religiosa and Ficus benghalensis |
Gram caterpillar | Helicoverpa armigera | Lepidoptera/Noctuidae | Significantly reduced both larval weight and survival rate of H. armigera | [157] | |
| Myriostachya wightiana | Flour beetle | Tribolium castaneum | Coleoptera/Tenebrionidae | Moderate significant efficacy against target pests. After 24 h of exposure, at highest concentration (150 µg) biogenic silver treatment was found to be comparatively toxic and killed 55.2% of T. castaneum, 52.8 ± 0.24% of R. dominica and 47.4 ± 0.16 of S. oryzae insects after 24 h | [158] | |
| Lesser grain borer | Rhyzopertha dominica (F.) | Coleoptera/Bostrichidae | ||||
| Rice weevil | Sitophilus oryzae (L.) | Coleoptera/Curculionidae | ||||
| Datura stramonium and Syzygium aromaticum | khapra beetle | Trogoderma granarium | Coleoptera/Dermestidae | Significanlty highest control 67.89% with biosynthesized nanoparticles | [159] | |
| Ocimum tenuiflorum | American bollworm | Helicoverpa armigera | Lepidoptera/Noctuidae | 50% mortality caused at 0.25% contration of biosynthesized AgNPs causes mortality | [160] | |
| Vernonia anthelmintica (L.) Willd | Armyworm | Spodoptera litura | Lepidoptera/Noctuidae | 86.90% and 89.83% antifeedant activity and larvicidal activity of (LC50) 56.42 μg/mL and 63.65 μg/mL against S. litura and H. armigera respectively | [161] | |
| American bollworm | Helicoverpa armigera | Lepidoptera/Noctuidae | ||||
| Borago officinalis | Cotton leafworm | Spodoptera littoralis | Lepidoptera/Noctuidae | LC50 values of the crude extract, and synthesized AgNPs were 22.6 and 0.33 mg/g respectively | [162] | |
| pomegranate and watermelon peels extracts | Cotton leafworm | Spodoptera littoralis | Lepidoptera/Noctuidae | additive effect and synergism recorded in results | [163] | |
| Glochidion eriocarpum | Termite | Odontotermes sp | Isoptera/Termitidae | Strong repellent (80.97%) and antifeedant activity | [164] | |
| Ocimum basilicum | Tobacco cutworm | Spodoptera litura | Lepidoptera/Noctuidae | ObAgNPs were most effective as compared to the selected synthetic chemicals | [165] | |
| Nerium oleander | Common green bottle fly | Lucilia sericata M | Diptera/Calliphoridae | 100% mortality of treated larvae at 50 ppm | [166] | |
| Dicrocephala integrifolia | American bollworm | Helicoverpa armigera | Lepidoptera/Noctuidae | Significant Larval mortality and pupal mortality was 68.04% and 72.11% respectively | [161] | |
| Armyworm | Spodoptera litura | Lepidoptera/Noctuidae | Significant Larval mortality and pupal mortality was 69.76% and 74.30% respectively | |||
| Fusarium pallidoroseum | White grubs | Holotrichia sp | Coleoptera/Scarabaeidae | Lethal dosage (LD50) was significant than control | [167] | |
| Avicennia marina | Silver (Ag) + Lead (Pb) | Rice weevil | Sitophilus oryzae | Coleoptera/Curculionidae | Aroud 90–100% mortality at 25–100 mg/mL concentration | [168] |
| Sargassum wightii | Zn | American bollworm | Helicoverpa armigera | Lepidoptera/Noctuidae | LC50 from 12.278 (larva I) to 20.798 ppm (pupa) and also reduced longevity and fecundity | [169] |
| Pongamia pinnata | Pulse beetle | Callosobruchus maculatus | Coleoptera/Chrysomelidae | 100% mortality at 25 μg/mL with LC50 to be 10.85 μg/mL | [170] | |
| Spinach leaves | Rice moth | Corcyra cephalonica (S.) | Lepidoptera/Pyralidae | Increase in larval mortality, pupal mortality and adult deformity | [171] | |
| Adhathoda vasica and Asafoetida | American bollworm | Helicoverpa armigera | Lepidoptera/Noctuidae | Asafoetida based zinc nanoparticles were More than 80% 2nd instar larval moratliy | [172] | |
| Eucalyptus globulus L | Lesser grain borer | Rhyzopertha dominica (F.) | Coleoptera/Bostrichidae | LC50 for leaf extract of E. globulus and ZnONPs were 1043.06 and 202.11 ppm respectively | [173] | |
| Zingiber officinale | Armyworm | Spodoptera litura | Lepidoptera/Noctuidae |
The 3rd instar larvae of S. litura and adults of M. euphorbiae showed 100% mortality 500 ppm |
[174] | |
| Potato aphid | Macrosiphum euphorbiae | Hemiptera/Aphididae | ||||
| Punica granatum | Cu | Green peach aphid | Myzus persicae | Hemiptera/Aphididae | There was 40–86% mortality at different concentrations (250–8000 μg/mL) | [175] |
|
Blumea balsamifera LINN |
Fruit Fly | Bactrocera dorsalis (HENDEL) | Diptera/Tephritidae | Mortality rates ranged from 25–100% within only 12 h exposure | [176] | |
| Tulasi leaves | Rice moth | Corcyra cephalonica (S.) | Lepidoptera/Pyralidae | Increase in larval mortality, pupal mortality and adult deformity | [171] | |
| Grewia asiatica L | Termite | Heterotermes indicola | Coleoptera/Rhinotermitidae | Significant mortality at 100 ppm | [177] | |
| Rice hust | Silica (SiO2) | Rice moth | Corcyra cephalonica (S.) | Lepidoptera/Pyralidae | Increase in larval mortality, pupal mortality and adult deformity | [171] |
| Entomopathogenic Nematodes + Nanoparticles of Different metals | ||||||
|
Steinernema feltiae EPNs |
Silver (Ag) Gold (Au) Copper (Cu) |
Lesser mealworm | Alphitobius diaperinus | Coleoptera/Tenebrionidae | Nematodes and nanoparticles caused a high mortality and the extensity of infection in host larvae, from 12 to 100% and from 8 to 83% respectively | [178] |
| Steinernema carpocapsae | ZnO, TiO2 and Fe3O4 | Wax moth | Galleria mellonella | Lepidoptera/Pyralidae | Survival rate of nematodes decreased with increased concentrations, with no difference between NPs. But both have the significant mortality on wax moth. Nanoparticles had less influence on survival of infective juveniles | [179] |
The Bacillus thuringiensis kurstaki mediated silver nanoparticles (Btk-AgNPs) application against cabbage looper (Trichoplusia ni Hübner) and black cutworm (Agrotis ipsilon Hufnagel) demonstrated to be significantly more virulent toward larvae of T. ni than to A. ipsilon [133]. The marine pathogen Shewanella alga is known to produce a strong neurotoxin (tetrodotoxin). Shewanella alga-mediated AgNPs significantly increased the mortality of 3rd instar white grub beetle, Lepidiota mansueta Burmeister (Coleoptera: Scarabaeidae) in all concentrations used [180]. The larvicidal toxicity of Bt-AgNPs was significantly higher than control against 3rd instar larvae of Aedes aegypti [138]. Similarly, Soni and Prakash [181] reported that Listeria monocytogenes, Bacillus subtilius and Streptomyces anulatus-mediated AgNPs revealed significantly more larval and pupal toxicity against Culex quinquefasciatus than Anopheles stephensi. In contrast, An. stephensi were found more susceptible than Culex quinquefasciatus at the adult stage. The Bacillus megaterium-mediated AgNPs by using the extracellular method were found to show higher insecticidal efficacy against Culex quinquefasciatus and Aedes aegypti [138]. Several studies revealed more promising results than control treatments but the focus of research is quite limited (Table 2). Further studies are needed to utilize the potential of EPB-mediated nanobiopesticides for the management of economic insect pests of various crops.
Entomopathogenic fungi with nanoparticles
Entomopathogenic fungi (EPF) are natural inhabitants of the soil and are mostly isolated from insect cadavers [182]. The EPF consists of over 100 genera and > 700 species [183, 184]. They provide a direct adaptive response through different mechanisms (adhesion and recognition of host surface), specialized infectious structures (penetrant tubes or appressoria), enzymes (lipase/esterases, catalases, cytochrome P450s, proteases, and chitinases), and secondary metabolites [185]. Currently, there has been a resurgence of interest in EPF use due to increasing insecticide resistance and environmental concerns over pesticide use [123, 186]. Several insect pests of different crops can be managed by EPF [187–189]. The most common fungal infection in fresh water, soil surfaces, and aerospaces environments are Metarhizum, Beauveria, Nomurea rileyi, Verticillumlecanii, and Hirsutella. The EPF can also produce broad-spectrum secondary metabolites and physical as well as biological alterations to manage insect pests [190].
The formation of nanoparticles utilizing fungus is known as myco-nanotechnology. The nanotechnology integration with EPF for entomotoxicity can enhance their effectiveness many folds (Table 2). The literature revealed the higher pesticidal efficacy of Chitosan Nanoparticle Coated Fungal Metabolite (CNPCFM) than Uncoated Fungal Metabolite (UFM) and Fungal Spores (FS). Chinnaperuma et al. [146] reported a significant reduction of detoxifying enzymes of Helicoverpa armigera due to Trichoderma viride-mediated biosynthesis of titanium dioxide nanoparticles (TDNPs) and showed significant mortality of 1st instar (100%), 2nd instar (100%) and 3rd instar (92.34%) larvae of H. armigera. Similarly, Sitophilus oryzae infestation was effectively managed in storage bags treated with nano-based formuations of B. bassiana and M. anisopliae [191]. The use of the cell filtration method to prepare B. bassiana mediated AgNPs showed maximum (60.09%) mortality of Lipaphis erysimi [141]. The significant reduction in fecundity of females and malformed development of adults was also reported in potato tuber moth (Phthorimaea operculella) when treated with the nano-based formulation of fungus Metarhizium rileyi [191].]. The resistance development can also be managed with nano-based EPF formulations. The combination of mycosynthesised TiNPs and M. anisopliae revealed synergistic interaction against Galleria mellonella larvae with a synergistic factor (SF) of 1.6 for LC50 and 4.2 for LC90 [147]. It was concluded that EPF can be effectively employed for the reduction of increased insect resistance to entomopathogenic fungi. Therefore, nanotechnology integration with EPFs can enhance their efficacy many folds as compared to NPs or EPFs alone.
Bonatnicals with nanoparticles
Many plants have biocidal properties and are being used against insect pests due to their efficacy, biodegradability, varied modes of action and low toxicity to non-target organisms [192]. Therefore, many botanical pesticides are used mainly for insect pest management [193–198]. Many studies have listed the plant species with known and yet to be exploited pesticidal properties [199, 200]. The commercially available botanical pesticides sources include Tanacetum cinerariifolium (pyrethrum), Azadirachta indica (neem), Schoenocaulon officinale (sabadilla), Nicotiana tabacum (tobacco) and Ryania speciose (ryania) [201]. Overall, extract from the leaves, flowers and twigs of many plants can be used as an insecticide [202].
Several promising outcomes of plant extracts integration with nanoparticles of different metals [203–205] are reported. The plant extracts act as capping and reducing agents and convert metals into nanoparticles with the aid of alkaloids, phenolic acids, polyphenols, proteins and terpenoids. The AgNPs and neem extract showed no toxicity against Cu. quinquefasciatus and A. aegypti during individual application of neem extract and AgNO3 in aqueous formulations. The neem-mediated AgNPs revealed 0.047 mg/L and 0.006 mg/L LC50 values against Cu. quinquefasciatus and A. aegypti [206]. Similar outcomes were reported by [170] in a study of Pongamia pinnata leaf extract mediated ZnONPs against pulse beetle. They reported significant variations in hatchability, larval time, pupal period and fertility including 100% mortality of pulse beetle. Similarly, characterization and entomotoxicity of Hypnea musciformis mediated AgNPs demonstrated effective management of mosquitos and diamondback moth (Plutella xylostella L.) and environment friendly nature [156].
Entomopathogenic nematodes with nanoparticles
The phylum Nematoda consists of around 1 million species and only 27,000 species have been described till now [207]. Entomopathogenic nematodes (EPNs) belong to the families Steinemernatidae and Heterorhabditidae. They parasitize soil inhibiting pest insects and kill them due to the associated mutualistic bacteria (Xenorhabdus, Photorhabdus, Heterorhabditis) [208, 209]. EPNs cause infection in individuals of a number of insect orders e.g., Coleoptera, Dictyoptera, Lepidoptera, Hemiptera, and Orthoptera [210].
The EPNs and nanotechnology integration is quite promising and can produce effective control of insect pests (Table 2). However, protocols should be developed to optimize the efficacy of EPNs and nanoparticles as EPNs efficacy is dependent on nanoparticle concentrations and exposure time [211]. For instance, the reproduction rates were enhanced with two concentrations assayed (500 and 1500 ppm) while a little variation was recorded in pathogenicity. Similar results have also been reported in other studies on the mortality of Steinernema feltiae (Owinema biopreparation) and Heterorhabditis bacteriophora (Nematop biopreparation) EPNs [212, 213]. The Au nanoparticles also produced similar results of mortality when exposed to Steinernema feltiae from Owinema biopreparation of nanoparticles [213]. The mortality increased with increased concentrations of Au nanoparticles. Kucharska et al. [214] used copper (Cu) nanoparticles and also showed S. feltiae mortality as well as its ability to control Alphitobius diaperinus depending on the exposure time and nano-Cu concentrations. The combined application of EPNs with nanoparticles of different metals (Ag, Au, or Cu) to control lesser mealworm (Alphitobius diaperinus) revealed variations of host growth stages sensitivity and susceptibility to S. feltiae and H. bacteriophora. In addition, the negative effect of AuNPs was also recorded on Alphitobius diaperinus adults infected by S. feltiae (Owinema) [178]. Furthermore, there are also reports of toxicity of nanoparticles (silica, titanium oxide, ZnO, Al2O3, silver, and Fe2O3) on Caenorhabditis elegans [215–217]. Further research is needed to improve the integration of nanoparticles with EPNs.
Nanobiotechnology and Management of Plant Diseases
Phytopathogens (fungi, bacteria, mollicutes, nematodes, viruses) causes enormous losses to human society by damaging their food production, economic growth, sustainable agriculture, environmental resilience and natural landscape. In this regard, bacterial diseases are the most damaging and economically significant pathogens invading various agricultural crops. The wide host range, survival capability and sustainable latent infection make bacteria a challenging pathogen to control [218]. Likewise, more than 19,000 fungi are reported to involve in causing diseases in crop plants globally. Additionally, the fungal spores are freely disseminated by wind currents, water, soil, insects, and other invertebrates, which make the whole crop to be invaded [219]. Nevertheless, there is not a single crop that has been free of plant parasitic nematodes (PPNs) infection. They pose a substantial yield loss (~ 173 billion $), annually [220]. Crop rotation is a routine practice to manage PPNs, but the polyphagous characteristics make this tactic unworkable [221]. In contrast, plant viruses have been revealed to cause ~ 50% of total crop losses which is a great threat to worldwide food security [222]. According to one of the surveys, nearly more than 900 plant virus species are responsible to infect over 700 crop species [223]. They are very challenging due to the fact that they are distributed by insect vectors. The virus management relies fundamentally upon (i) immunization, and (ii) prophylaxis measures. Several strategies including chemicals are unable to offer ultimate control [224]. Therefore, a prodigious opportunity prevails to utilize the applications of nanotechnology for the sustainable management of plant disease epidemics.
Management of Bacterial Diseases
Bacteria are single-celled prokaryotic organisms exhibiting symbiotic, parasitic, and saprophytic natures. Various factors such as size, density, the shape of NPs, as well as bacterial motility and specificity (gram + ve and − ve) influence the efficacy of nanoparticles [225]. According to previous research work, different concentrations of biosynthesised AgNPs such as 10, 20, 30, and 40 ppm respectively were applied against Citrus reticulata suffering from canker disease at different time intervals. The AgNPs at a concentration of 30 ppm were the most effective concentration to produce the resistance in Citrus reticulata against canker disease [226]. The direct application of AgNPs eradicates the bacteria responsible for Huanglongbing disease (Candidatus Liberibacter asiaticus) on sick trees and reduced 80–90% of bacterial titre [227]. Bacterial leaf blight (BLB) caused by Xanthomonas oryzae pv. oryzae (Xoo) were effectively treated with biologically synthesized AgNPs from Bacillus cereus strain SZT1 and it was found to be effective weapon for BLB management. The AgNPs significantly increased the plant biomass with a decreased cellular concentration of ROS and increased concentration of antioxidant enzyme activity in the pot-treated plants [228]. Similarly, AuNPs synthesized from biogenic materials exhibited eco-friendly and strong antibacterial properties [229]. Gram-positive and gram-negative bacteria were effectively inhibited by the plant based gold nanoparticles. Furthermore, it has been also reported that biogenic ZnONPs have a much stronger antibacterial impact than chemically generated nanoparticles [230, 231]. Plant extracts such as O. basilicum T. pratenese, C. fistula and others have been used for green synthesis of ZnNPs [232]. The antibacterial effect of ZnONPs synthesized by Olea europaea on Xoo strain GZ 0003 had an inhibition zone of 2.2 cm at 16.0 µg mL−1 that was significantly different from zinc oxide nanoparticles synthesized by Lycopersicon esculentum and Matricaria chamomilla. The biofilm formation, swimming motility, bacterial cell membrane and bacterial growth of Xoo strain GZ 0003 were significantly affected by ZnONPs [233]. Biosynthesized CuNPs provide significant results because of the antimicrobial ability of these nanoparticles against the bacterial blight of rice [234] and it CuNPs also proved to be less harmful to the environment [235]. The increasing concentrations (50, 100, and 200 ppm) of CuNPs suppressed the bacterial growth by approximately 61%, 64% and 77% compared to the control [236]. Overall, the biologically synthesized nanoparticles have great potential to counter bacterial disease in plants (Table 3).
Table 3.
The influence of nanoparticles on plant pathogenic bacteria
| NPs | Source | Targeted pathogen | Order/ Family | Effects | References |
|---|---|---|---|---|---|
| Ag | Cannabis sativa extracts | Pseudomonas syringae pv. tomato | Pseudomonadales/Pseudomonadaceae | Silver nanoparticles containing 90% lower silver content compared to the un-dialyzed silver salt (Ag-UD) exhibited at least 20% more inhibition | [237] |
| AgNO3 and NaBH4 | Ralstonia solanacearum | Burkholderiales/Burkholderiaceae | Silver nanoparticles formed in EPS1 solution exhibited a concentration-dependent inhibition of bacteria. Silver nanoparticles at 0.8 mg/mL have been shown to have antibacterial activity but a very low cytotoxicity on the RAW264.7 murine macrophage cells | [238] | |
| Moringa oleifera | Xanthomonas axonopodis pv citri | Xanthomonadales/Xanthomonadaceae | Biosynthesised AgNPs at different concentrations (10, 20, 30, and 40 ppm) were exogenously applied on the already infected plants (canker) of Citrus reticulata at different day intervals. The AgNPs at a concentration of 30 ppm was found to be the most effective concentration against citrus canker | [226] | |
| Bacillus cereus SZT1cereus SZT1 | Xanthomonas oryzae pv. oryzae | Xanthomonadales/Xanthomonadaceae | Silver nanoparticles showed substantial antibacterial potency (24.21 ± 1.01 mm) for Xanthomonas oryzae pv. Oryzae | [228] | |
| polyvinylpyrrolidone with metallic silver | Candidatus liberobacter | Hyphomicrobiales/Rhizobiaceae | The AgNPs were applied by foliar sprinkling and trunk injection of 93 diseased trees with remarkable results. Both methods produce an 80–90% decrease of bacterial titre | [227] | |
| Aqueous extract of strawberry waste | Ralstonia solanacearum | Burkholderiales/Burkholderiaceae | A strong inhibition zone was found around the paper disc dipped in 100 µg/mL AgNPs, placed in NA media inoculated with R. solanacearum and the inhibition zone was absent around the control disc | [239] | |
| Dioscorea bulbifera | Bacillus sp. | Bacillales/Bacillaceae | Inhibition zone ranged from 6.00 ± 0.41 to 11.00 ± 0.87 mm was observed at a concentration of 100 ppm | [240] | |
| Enterobacter cloacae | Enterobacterales/Enterobacteriaceae | ||||
| Penicillium simplicissimum, Aspergillus niger, and Fusarium oxysporum | Pectobacterium carotovorum | Enterobacterales/Enterobacteriaceae | Inhibition zone was found up to 15.3 mm at a concentration of 100 ppm | [241] | |
| Larrea tridentata | Clavibacter michiganensis | Micrococcales/Microbacteriaceae | The disease incidence did not exceed 20%, reduced disease severity by 36%, inhibition of bacterial growth in the tissue (up to 95%) | [242] | |
| Eucalyptus globulus | Xanthomonas citri pv. Citri | Xanthomonadales/Xanthomonadaceae | AgNPs and CuNPs in combination showed maximum growth inhibition (21.06 mm) followed by AgNPs (18.26 mm) and CuNPs (15.27 mm) | [243] | |
| Au | Olea europaea fruit extract, Acacia nilotica and husk extract | Pseudomonas spp | Pseudomonadales/Pseudomonadaceae | AuNPs expressed moderate antibacterial activity and inhibition zone up to 8 mm was found | [244] |
| Phoma sp. | Xanthomonas oryzae pv. Oryzae | Xanthomonadales/Xanthomonadaceae | Inhibition rate for sclerotia formation was (15, 33, 74 and 93% at concentrations (10, 20, 40 and 80) μg/mL of AuNPs respectively | [245] | |
| Zn | Morus alba plant leaf extract | Xanthomonas axonopodis pv. Malvacearum | Xanthomonadales/Xanthomonadaceae | These NPs was found to be very effective in controlling the bacterial spread in comparison to streptomycin that was used as control | [246] |
| Sigma-Aldrich, Steinheim | P. syringae | Pseudomonadales/Pseudomonadaceae |
An inhibition zone of 0.72 mm was observed at concentration of 0.10 mg/mL ZnO NP discs on plates inoculated with Pectobacterium carotovorum |
[247] | |
| Matricaria chamomilla L., Olea europaea and Lycopersicon esculentum M | Xanthomonas oryzae pv. Oryzae | Xanthomonadales/Xanthomonadaceae | ZnONPs synthesized by Olea europaeahad the highest inhibition zone of 2.2 cm at concentration of 16 mg/mL | [233] | |
| Green tomato extract | Xanthomonas oryzae pv. oryzae | Xanthomonadales/Xanthomonadaceae | Zinc oxide nanoparticles powder at the concentration of 4.0, 8.0, and 16 μg/mL expressed an inhibitory zone of 2.4, 2.6, and 2.9 cm, compared with that of 1.4, 1.5, and 1.8 cm from bulk zinc oxide, respectively | [248] | |
| Matricaria chamomilla | Ralstonia solanacearum | Burkholderiales/Burkholderiaceae | At concentration of 18 µg/mL, Zinc oxide nanoparticles showed the highest inhibition area of 22.3 mm | [249] | |
| Bacillus cereus RNT6 | Burkholderia glumae | Burkholderiales/Burkholderiaceae | At 50 µg/mL concentration, pathogen growth was reduced by 71.2% | [250] | |
| B. gladioli | |||||
| Withania coagulans | Ralstonia solanacearum | Burkholderiales/Burkholderiaceae | Highest inhibitory area of 16.2 mm was exhibited at highest concentration (80 μg/mL) of ZnONPs + leaf extract | [251] | |
| Cu | Shell copper and Multivalent copper | Xanthomonas perforans | Xanthomonadales/Xanthomonadaceae | Cu-composites significantly decrease disease severity, using 80% less metallic copper in comparison with Cu-mancozeb in field evaluation (P < 0.05) | [252] |
| Datura Innoxia | Xanthomonas oryzae pv. Oryzae | Xanthomonadales/Xanthomonadaceae | CuNPs exhibited effective antibacterial potency against Xanthomonas oryzae pv. oryzae with mean inhibition zone of approximately 18 mm | [234] | |
| Carica papaya | Ralstonia solanacearum | Burkholderiales/Burkholderiaceae | After an initial incubation (12 h), NPs posed no effect on biofilm. Maximum inhibition (35% and 37%) was observed at dosage of 125 and 250 μg/mL at 24 h and 12% and 38% reduction in biofilms at 72 h respectively | [253] | |
| Eucalyptus globulus | Xanthomonas citri pv. Citri | Xanthomonadales/Xanthomonadaceae | AgNPs + CuNPs exhibited maximum inhibitory area of 21.06 mm followed by AgNPs 18.26 mm and CuNPs 15.27 mm | [243] |
Management of fungal diseases
There are approximately 1.5 million fungal species that are saprophytic and parasitic in nature responsible for ~ 70 to 80% of crop losses equal to 200 billion Euros [254]. Myco-nanotechnology presents a greener alternative to chemically generated nanoparticles (Table 4) because of their broad applicability in disease detection and control [255]. Green synthesis of CuNPs utilizing Citron juice (Citrus medica) demonstrated repressing effects against the F. graminearum, Fusarium culmorum, and F. oxysporum. The F. oxysporum was shown the most susceptible to CuNPs when compared with F. graminearum and F. oxysporum [256]. The clove (S. aromaticum) bud extract containing CuNPs demonstrated significant antifungal ability against Aspergillus niger, Aspergillus flavus, and Penicillium spp [257]. Similarly, in vitro application of CuNPs against phytopathogens such as Alternaria alternata, and Curvularia lunata, Alternaia alternata, Phoma destructiva, Phytophthora cinnamon, Fusarium oxysporum, Fusarium solani, and Penecillium digitatum showed fungal growth inhibition at 20 and 60 µg mL−1 [258]. An antifungal nanocomposite based on biosynthesized CuONPs was made that has the potential to increase banana roots and seedling growth and also protects them from fungal diseases [259]. The biosynthesis of AuNPs through fresh fruit extract of P. serrulate proved more effective against the Aspergillus flavus, Didymella bryoniae, and Fusarium oxysporum compared to traditional fungicides [260]. The uses of Melia azedarach leaf extract for the green synthesis of AgNPs against Verticillium dahliae in eggplants (Solanum melongena L.) both in vitro and in vivo conditions significantly decreased the growth of Verticillium dahlia compared with controls [261]. Trichoderma spp could produce metal NPs, particularly Ag which is an effective controlling agent against F. oxysporium f. sp. ciceri [262, 263]. In addition, AgNPs synthesized by Trichoderma spp (Trichoderma viride, Trichoderma hamatum, Trichoderma harzianum and Trichoderma koningii) [264] have been used as an antifungal to control four Fusarium spp (F. solani, F. semitectum, F. oxysporum, and F. roseum) which are considered serious soil-borne fungi. The study proved significant inhibitory effects against all four pathogenic Fusarium species [265].
Table 4.
The influence of nanoparticles on plant pathogenic fungi
| NPs | Sources | Targeted Fungi | Order/Family | Effects | References |
|---|---|---|---|---|---|
| Ag | M. azedarach | Colletotrichum sp. | Glomerellales/Glomerellaceae |
At 100 mg/ml concentration, Inhibition of radial growth (40.16 ± 2.35) % was observed AgNPs showed a pronounced antifungal potential with EC50 values ranged between 18.4 and 22.8 µg/mL |
[266] |
| Fusarium solani | Hypocreales/Nectriaceae | ||||
| Alternaria alternate | Pleosporales/Pleosporaceae | ||||
| Macrophomina phaseolina | Botryosphaeriales/Botryosphaeriaceae | ||||
| R. solani | Cantharellales/Ceratobasidiaceae | [267] | |||
| B. cinerea | Helotiales and Sclerotiniaceae | ||||
| F. oxysporum | Hypocreales/Nectriaceae | ||||
| Acidovorax oryzae | Burkholderiales/Comamonadaceae | [268] | |||
| Streptomyces griseoplanus SAI-25 | Macrophomina phaseolina | Botryosphaeriales/Botryosphaeriaceae | Highest zone of inhibition (13 mm) was found at concentration of (1000 µg/mL) | [269] | |
| Rhizospheric microflora of chickpea | F. oxysporum | Hypocreales/Nectriaceae | Highest growth inhibition (95%) was found in vitro at dosage of 100 µg/mL | [264] | |
| Trichoderma harzianum | F. oxysporum | Hypocreales/Nectriaceae | 100% growth inhibition of F. oxysporum was observed at 100 ppm | [270] | |
| T. harzianum | Sclerotinia sclerotiorum | Helotiales/Sclerotiniaceae | Sclerotia were formed from the precursor sclerotium at the edges of the control plate with a mean of 116.5 ± 7.7 sclerotia. AgNP-TS and AgNP-T plates exhibited no new sclerotia and reduction in mycelial growth | [271] | |
| T. harzianum | Alternaria alternata | Pleosporales/Pleosporaceae | Mycelial diameter for A. alternata was reduced 18% at 5 ppm, 42% at 10 ppm and 52% at 20 ppm. Mycelial growth of P. oryzae was reduced 22%, 46% and 68% for each respective nanoparticle’s concentration | [272] | |
| Pyricularia oryzae | Burkholderiales/Comamonadaceae | ||||
| Sclerotinia sclerotiorum | Helotiales/Sclerotiniaceae | ||||
| Streptomyces capillispiralis Ca-1, and Streptomyces zaomyceticus Oc-5 | Alternaria alternata | Pleosporales/Pleosporaceae | Highest growth inhibition (75%) was observed at concentration of 2 mM | [273] | |
| Fusarium oxysporum | Hypocreales/Nectriaceae | ||||
| Pythium ultimum | Peronosporales/Pythiaceae | ||||
| Aspergillus niger | Eurotiales/Trichocomaceae | ||||
| Strawberry waste | Fusarium oxysporum | Hypocreales/Nectriaceae | The highest concentration (18 µg ml−1) of NPs exhibited the inhibition zone of 22.3 mm | [249] | |
| Melia azedarach | F. oxysporum | Hypocreales/Nectriaceae | Growth inhibition (79–98%) was observed | [274] | |
| Pseudomonas aeruginosa | Botrytis cinerea | Helotiales/Sclerotiniaceae | At the concentration of 100 ppm, maximum growth inhibition was observed up to 65.36% | [275] | |
| Pilidium concavum | Helotiales/Leotiomycetidae | ||||
| Pestalotia sp | Xylariales/Amphisphaeriaceae | ||||
| T. harzianum Th3 | Aspergillus niger | Eurotiales/Trichocomaceae | Mycelial growth inhibited up to 60–65% | [276] | |
| Sclerotium rolfsii | Atheliales/Atheliaceae | ||||
| Macrophomina phaseolina | Botryosphaeriales/Botryosphaeriaceae | ||||
| Dioscorea bulbifera | F. oxysporum | Hypocreales/Nectriaceae | Highest inhibition (98–100%) was expressed at concentration of 100 ppm | [240] | |
| Colletotrichum gloeosporioides | Incertae sedis/ Glomerellaceae | ||||
| Bacillus subtilis | Cercospora canescens | Capnodiales/Mycosphaerellaceae | The highest mycelial inhibition (94.00 ± 0.5) was observed at dosage of 800 ppm at 96 h | [277] | |
| Bamboo leaf extract | Bipolaris maydis | Pleosporales/Pleosporaceae | Complete inhibition of conidia germination (100%) was detected at concentration of 100 μg/mL | [278] | |
| Exserohilum turcicum | Pleosporales/Pleosporaceae | ||||
| Curvularia lunata | Pleosporales/Pleosporaceae | ||||
| Au | Glechoma hederacea L. extract | Fusarium oxysporum | Hypocreales/Nectriaceae | Zone of inhibition exhibited by AuNPs against tested pathogens ranges from 30 to 66%and 40 to 54% respectively | [279] |
| Aspergillus niger | Eurotiales/Trichocomaceae | [280] | |||
| Annona muricata | Aspergillus flaws | Eurotiales/Trichocomaceae | Zone of inhibition (30—66%) was observed | [279] | |
| Fusarium oxysperium | Hypocreales/Nectriaceae | ||||
| Phoma sp. | Rhizoctonia solani AG1-IA | Cantharellales/Ceratobasidiaceae | Growth inhibition (93%) was found at concentration of 80 μg/mL | [245] | |
| Zn | Aloe vera extract | Alternaria alternata, | Pleosporales/Pleosporaceae | ZnNPs at both concentrations of 2 mg/L and 4 mg/L expressed inhibition zone of 50% and 54% respectively | [281] |
| Aspergillus niger | Eurotiales/Trichocomaceae | ||||
| Botrytis cinerea | Helotiales/Sclerotiniaceae | ||||
| Fusarium oxysporum | Hypocreales/Nectriaceae | ||||
| Alternaria mali | Pleosporales/Pleosporaceae | ||||
| Botryosphaeria dothidea | Botryosphaeriales/Botryosphaeriaceae | [282] | |||
| Diplodia seriata | Botryosphaeriales/Botryosphaeriaceae | ||||
| Scadoxus multiflorus | Aspergillus niger and Aspergillus flavus | Eurotiales/Trichocomaceae | Zinc oxide nanoparticles expressed significant antifungal potency against A. flavus, with 75% inhibition at 500 ppm and 76% inhibition at 1000 ppm, while A. niger resulted in 57% and 63% growth reduction, respectively | [283] | |
| Morus nigra and Grevillea robusta | Cercospora beticola | Capnodiales/Mycosphaerellaceae | Nanoparticles led to activation and recorded high POD value up to 6 min and polyphenoloxidase up to 4 min estimation periods compared to control and expressed as role in defense against CLS disease | [284] | |
| Strawberry Plants | Botrytis cinerea | Helotiales/Sclerotiniaceae | The most effective concentrations were 26 and 42 mg/ml for non-calcinated and calcinated zinc oxide nanoparticles, respectively. Inhibition in the fungal growth enhanced with the increase in the concentration of NPs | [285] | |
| Sargassum vulgare | Aspergillus sp. | Eurotiales/Trichocomaceae | At concentration of 25 μg/mL, 25 mm inhibition zone was found | [286] | |
| Candida sp. | Hyphomicrobiales/Rhizobiaceae | ||||
| Saccharomyces cerevisiae | Saccharomycetales/Saccharomycetaceae | ||||
| Trichoderma harzianum | Alternaria alternata | Pleosporales/Pleosporaceae | Significantly reduced the mycelial growth (20 mm inhibition zone) | [272] | |
| Pyricularia oryzae | Burkholderiales/Comamonadaceae | ||||
| Sclerotinia sclerotiorum | Helotiales/Sclerotiniaceae | ||||
| Seed coat of almond | Rhizoctonia solani | Cantharellales/Ceratobasidiaceae | Reduction of pathogen growth up to 100% | [287] | |
| Cu | Magnolia leaf extract | Botryosphaeria dothidea | Botryosphaeriales/Botryosphaeriaceae | Mycelial growth inhbited up to 22 mm | [288] |
| Diplodia seriata | Botryosphaeriales/Botryosphaeriaceae | ||||
| Colletotrichum gloeosporioides | Glomerellales/Glomerellaceae | ||||
| Colletotrichum lindemuthianum | Glomerellales/Glomerellaceae | ||||
| Drechslera sorghicola | Pleosporales/Pleosporaceae | ||||
| Fusarium oxysporum f.sp. carthami | Hypocreales/Nectriaceae | ||||
| Fusarium oxysporum f.sp. cicero | Hypocreales/Nectriaceae | ||||
| Fusarium oxysporum f.sp. | Hypocreales/ Nectriaceae | ||||
| Citrus sinesis |
Colletotrichum Capsici |
Glomerellales/Glomerellaceae |
Maximum antifungal activity (28.00 ± 081 mm diameter) was observed at dosage of 200 ppm |
[289] | |
| Azadirachta indica | Alternaria mali | Pleosporales/Pleosporaceae | More than 40% of mycelial growth inhibition was found at 1 g/mL of all the fractions | [290] | |
| Diplodia seriata | Botryosphaeriales/Botryosphaeriaceae | ||||
| Botryosphaeria dothidea | Botryosphaeriales/Botryosphaeriaceae | ||||
| Trichoderma harzianum | Alternaria alternata | Pleosporales/Pleosporaceae | Mycelial diameter for A. alternata was reduced 18% at 5 ppm, 42% at 10 ppm and 52% at 20 ppm | [272] | |
| Pyricularia oryzae | Burkholderiales/Comamonadaceae | ||||
| Sclerotinia sclerotiorum | Helotiales/Sclerotiniaceae | ||||
| Aspergillus flavus | Aspergillus niger | Eurotiales/Trichocomaceae | Growth inhibition was reduced by 19% at 6 ppm, 40% at 12 ppm and 55% at 20 ppm | [291] | |
| Fusariumoxy sporum | Hypocreales/ Nectriaceae | ||||
| Alternaria alternata | Pleosporales/Pleosporaceae | ||||
| Kappaphycus alvarezii |
Colletotrichum Capsici |
Glomerellales/Glomerellaceae | Complete growth inhibition observed at concentration of 100 ppm | [292] | |
| Macrophomina phaseolina | Fusarium verticillioides | Hypocreales/ Nectriaceae | 100% growth inhibition observed at concentration of 50 ppm | [293] | |
| Sclerotium rolfsii | Atheliales/Atheliaceae | ||||
| Pseudomonas fluorescens and Trichoderma viride | Phytophthora parasitica | Peronosporales/Peronosporaceae | Maximum percent inhibition ( 74.8%) was observed at 150 mg/L | [294] | |
| Grewia asiatica L |
Aspergillus niger and Aspergillus oryzae |
Eurotiales/Trichocomaceae | Significant inhibition was recorded at 20 mm and 23 mm due to Maximum | [177] |
Management of viral diseases
Nanotechnologies based on biologically synthesize nanoscale materials offer great potential to use as a novel and eco-friendly antiviral therapy for plant disease management (Table 5). For instance, a recent study used ginger and horsemint extracts for biosynthesis of ZnNPs and reported an increase in viral suppression and inhibition [295]. Likewise, Zn and ammonium synthesized NPs from spearmint and plant flavanol extracts showed strong antiviral activity against tomato mosaic virus (TMV). Another work revealed antiviral activity against cucumber mosaic virus (CMV) by using seaweed extract-mediated ZnNPs [296]. The outcome of this study suggested that ZnNPs could serve as a strong antiviral agent due to their promising antiviral characteristics. A similar observation was also witnessed in the work of El-Shazly et al. [297], who synthesized the AgNPs from salicylic acid (SA) and investigated the strong antiviral activity to counter potato virus Y (PVY). It was found that biosynthesized NPs may enter the viral cell and start their antiviral mechanism via interacting with viral genetic material (RNA or DNA) or by preventing the channels that are indispensable for viral reproduction. Different entomopathogenic bacteria were also used to synthesize AgNPs and exploited them for antiviral activity on Tobacco mosaic virus (TMV), Barley yellow mosaic virus (BaYMV), Sunhemp rosette virus (SHRV) and Bean yellow mosaic virus (BYMV) [298–301]. After a post-infection treatment of a certain incubation time as required for each virus, it was observed that all plants showed typical symptoms of TMV, BaYMV, SHRV and BYMV infection. However, plants treated with NPs exhibited negative symptoms of virus infection. Moreover, viral concentration and disease severity were also observed very low in synthesized NPs treated plants (Table 5). Chitosan is another nontoxic biodegradable compound consisting of different monocrotaline and pyrrolizidine alkaloids and has a strong antiviral activity against virus replication and severity of tobacco mosaic virus (TMV) and alfalfa mosaic virus (AMV) [302, 303]. The biologically (plant extract or pathogen based) synthesized NPs are capable of inhibiting plant virus replication and improving host plant growth, however further studies are needed to identify different biological sources for NPs synthesis which are nontoxic to human health.
Table 5.
The influence of nanoparticles on plant viral and nematode disease suppression
| Type of EPB | Metal | Target Virus | Genome | Order/Family | Efficacy | Reference |
|---|---|---|---|---|---|---|
| Virus section | ||||||
| Different plant extracts + Nanoparticles of different metals | ||||||
| Spearmint | ZnNPs | TMV | + ssRNA | Virgaviridae | Strong antiviral activity at a concentration of ca ~ 50 ppm | [304] |
|
Ginger Horsemint |
SiNPs | TYLCV | ssDNA | Geminiviradae | Suppression of TYLV infection at 100 μg/ml of PPE-AuNPs | [295] |
| Plant flavonol | AuNPs | TMV | + ssRNA | Virgaviridae | Enhanced Antiviral activity and limit the traditional chemicals applications when plants treated with Rugby 60% (4 mL/L) | [305] |
|
Salicylic acid Seaweed |
AgNPs ZnNPs |
PVY CMV |
+ ssRNA | PotyviridaeBromoviridae | Decrease viral infection and increase in growth and yield at 7.89 μg/ml (LC50) | [297] |
| Entomopathogenic/bacteria/viruses + Nanoparticles of different metals | ||||||
| Pseudomonas fluorescens | AgNPs | TMV | + ssRNA | Virgaviridae | Antiviral activity at 100 μl AgNPs | [306] |
| Bacteriophage |
AgNPs AuNPs |
BaYMV | + ssRNA | Potyviridae | Enhance resistance against BaYMV 100–120 μl AgNPs/AuNPs | [307] |
| Bacillus thuringiensis | AgNPs | SHRV | + ssRNA | Virgaviridae | Complete viral disease suppression at 20 μg per pot | [308] |
| Bacillus licheniformis | AgNPs | BYMV | + ssRNA | Potyviridae | Antiviral activity at (1: 0.5 v/v) | [300] |
| Chitosan | Dextran nanoparticles (D-NPs | AMV | + ssRNA | Bromoviridae | Strong elicitor for viral disease control at 100 µg/mL | [303] |
| chitosan Schiff | AgNPs | TMV | + ssRNA | Virgaviridae | Inhibit virus replication and severity at 100 µg/ml application | [302] |
| Virus-Capsid | AgNPs | CPMV | + ssRNA | Comoviridae | Virus control at 75 mg/L | [309] |
| Recombinase | AuNPs | TYLCV | ssDNA | Geminiviradae | Highly sensitive in virus detection and diagnosis at 100 µg/mL foliar application | [310] |
| dsRNA-Virus | Chitosan | ToMV | + ssRNA | Virgaviridae | Decrease toxicity at 17 mg/mL | [311] |
| Nematode Section | ||||||
|---|---|---|---|---|---|---|
| Different plant extracts + Nanoparticles of different metals | ||||||
| Type of EPB | Metal | Target Nematode | Family | Order | Efficacy | Reference |
| Pomegranate | AuNPs | C. elegans | Rhabditidae | Rhabditida | Adversely affect the fertility of C. elegans at 100 μg/mL | [312] |
| Bermuda grass | AgNPs | M. incognita | Heteroderidae | Tylenchida | Nematode production was limited at 17 mg/mL | [313] |
| African locust bean | AuNPs | B. xylophilus | Parasitaphelenchidae | Aphelenchida | Inhibited the growth of nematodes after 72 h, the mortality range ranged from 87.00% to 98.50% at 150 μg/mL | [314] |
| Jacob’s coat | AgNPs | M. incognita | Heteroderidae | Tylenchida | Reduction in nematode population and retard movement of larvae at 21.70 µg/mL | [315] |
| Strawberry | AgNPs | M. incognita | Heteroderidae | Tylenchida | Antinematicidal effect at 1–100 μg/L | [239] |
| Ginger | AgNPs | M. incognita | Heteroderidae | Tylenchida | Restrict nematode infection at EC50 = 3.7 μM and improved plant growth | [316] |
| Drumstick tree | AgNPs | M. incognita | Heteroderidae | Tylenchida | Reduce number of nematodes and their eggs at 300 ul and 400 ul concentrations | [317] |
| Cassod tree | AgNPs | M. incognita | Heteroderidae | Tylenchida | Have nematicidal activity and is cost-effective at 7.89 μg/mL (LC50) | [318] |
| Geen Algae | AgNPs | M. javanica | Heteroderidae | Strong nematicides at 4 mL/L | [319] | |
| Neem | AgNPs | H. contortus | Trichostrongylidae | Exhibited potent anthelmintic properties at LC50 (588.54 μg/mL) | [320] | |
| Cnidoscolus aconitifolius | AgNPs | M. incognita | Heteroderidae | Tylenchida | Limit M. incognita number at 5 mL/L and enhance host plant growth | [321] |
| Lemon grass | FeO-NPs | C. elegans | Rhabditidae | Rhabditida | Effectively control the C. elegans population at 1–100 μg/L | [322] |
| Fresh water Cladophora glomerata and marine alga Ulva fasciata | AgNPs | M. incognita | Heteroderidae | Tylenchida | Significant egg hatching and J2 mortality (up to 100%) compared with Nemaphose (40%) at 17 mg/mL | [323] |
| Eucalyptus | NPs | M. incognita | Heteroderidae | Tylenchida | Reduce nematode population at 4–6 mL/L and increased vegetative growth of host plant | [324] |
|
Neem Turmeric |
NPs | M. incognita | Heteroderidae | Tylenchida | Significant mortality of J2-nematode at 70–100 µg/mL | [325] |
| Common mallow | AgNPs | M. javanica | Heteroderidae | Tylenchida | Antinematicidal effect at 100 µg/mL | [326] |
| Pencil cactus | AgNPs | M. incognita | Heteroderidae | Tylenchida | Infestation of M. incognita significantly reduced after exposure at (100 ppm) concentration | [327] |
|
Chinaberry Drumstick tree |
AgNPs | M. incognita | Heteroderidae | Tylenchida | Strong nematicidal activity against M. incognita at 25 μg/mL | [328] |
| Colpomenia sinuosa and Corallina mediterranea | AgNPs | M. incognita | Heteroderidae | Tylenchida | Significant mortality (87.5%) after 12 h and 100% after 24 and 72 with 100 and 200 ppm | [329] |
| Ulva fasciata | ZnNPs + oxamyl | M. incognita | Heteroderidae | Tylenchida | Significant diminution of J2s (82.77%) in soil and galls number (81.87%) in vivo conditions | [330] |
| Orobanche aegyptiaca | Cu | M. incognita | Heteroderidae | Tylenchida | Significantly enhanced nematicidal activity at 50 μg/mL | [331] |
| Entomopathogenic fungus + Nanoparticles of different metals | ||||||
|
Sea lettuce brown algae |
AgNPs | Effectively controlled nematodes at 17 mg/mL | [332] | |||
| Fusarium oxysporum | SiNPs | M. incognita | Heteroderidae | Tylenchida | Limit nematode reproduction, gall formation and egg masses with 100 and 200 ppm | [333] |
| Duddingtonia flagrans | AgNPs | A. caninum | Ancylostomatidae | Showed nematicides activity, penetrate into the cuticle of the larvae and kill the nematode LC50 (25 μg/mL) | [334] | |
| Neuronal Gα- protein | PS-NPs | C. elegans | Rhabditidae | Control neuron response of the organism at 1–100 μg/L | [335] | |
| Virus-Capsid | TMGMV-NPs | M. incognita | Heteroderidae | Tylenchida | control parasitic nematode at EC50 = 13.8 μM | [336] |
| Bacteria + Nanoparticles of different metals | ||||||
| Bacillus licheniformis strain GPI-2 | AuNPs | M. incognita | Heteroderidae | Tylenchida | 100% mortality at EC50 = 3.7 μM | [337] |
Management of nematode diseases
Plant parasitic nematodes (PPNs) are holoparasites which pose a substantial yield loss (~ 173 billion $) annually [220]. Around 4100 species of PPNs have been recognized, and a majority of them are polyphagous [338]. Biologically (plant, fungus, or bacteria) synthesized NPs exhibiting a strong nematicide activity [309]. For instance, AuNPs synthesized by pomegranate peel and African locust bean extracts, effectively inhibit the multiplication and reduced the fertility of Caenorhabditis elegans and Parasitaph elenchidae [255, 312, 339]. Likewise, biologically synthesized AgNPs by using more than 14 plants showed significant mortality of J2 nematode, and antifilarial effects against Meloidogyne incognita, M. javanica, H. contortus, C. elegans and Setaria cervi [313, 318, 320, 323–325, 327, 340–346]. The presence of flavonoids and other phenolic compounds in plant extracts may help improve the efficiency of the NPs in inhibiting nematode populations [347]. The strong effect of AgNPs might be due to the high concentration of secondary metabolites including epi-shyobunol, aromdendrene, α- and t-cadinol, caryophyllene, α-humulone, β-isocomene, and α- and β-selinene) [328]. The aqueous leaf extract of Jacob’s coat and strawberry waste extract and their antinematod activity restricts the movement of M. incognita but have the ability in preventing both, eggs and J2 stages of nematode population [239, 342]. Different polymers and compounds were investigated from various entomopathogenic fungi including Fusarium oxysporum, Duddingtonia flagrans, sea lettuce (Ulva lactuca), and brown algae to synthesize AgNPs, SiNPs, ZeinNPs, AgB and their nematicidal activity was successfully evaluated [333, 334, 348]. The findings verified the successful inhibition and reduction of J2 population of nematodes. The growth of plants was also remarkable. It was witnessed from different research findings that biologically synthesized NPs exhibited strong nematicidal activities (Table 5).
Challenges/risks of nano biotechnology
Nanobiotechnology could be an important driver for the imminent agri-tech revolutions, especially in the face of climate change and increasing populations which make the existing agricultural practices unsustainable. Therefore, it is needful to explore more about nanomaterials and their characteristics as they behave totally differently than in bulk form. There are concerns that some materials could be toxic at the nanoscale because of their significant, still mysterious, hazardous properties related to their unique physico-chemical characteristics. This can pose risks for a wide range of manufacturers, formulators, handlers, applicators and also the consumers. Therefore, nanotechnology causes heterogeneous effects [349]. Toxic effects on non-target organisms upon contact i.e., nanoparticles can come in direct contact with humans and can cause unfavorable or undesirable toxic effects on humans. The nanoparticles can reach various human body parts to exert ill effects and may disrupt cellular pathways, enzymatic actions and functions of different organs. The disposal of nanomaterials might form a new class of non-biodegradable pollutants in the environment. Nanomaterials can enhance environmental pollution by increasing water, soil and air contamination including health hazards. Studies on nanotoxicity in agriculture are limited but reveal potential risks to plants, beneficial microbes, animals, and even humans. Health issues of workers during different activities (production, packaging, formulations, loading, unloading, or transport) of nanomaterials. The hazardous effects of nanoparticles on non-target organisms include dermal absorption of nanoparticles, translocation to go deep into lungs and brain through inhalation and crossing the brain barriers respectively, environmental concerns due to resilience and reactive potential of some nanomaterials, and lack of knowledge to estimate environmental exposure can pose a number of risks to different stakeholders including human beings.
The possible risks associated with nanomaterials lead to the challenges of basic as well as applied nanobiotechnology in different sectors including crop production. The major possible challenges [349] are:
-
(i)
Mass production of nano-based products with standard quality at an economical cost.
-
(ii)
Availability of nanomaterials in ready to use product with proper particle size, surface chemistry, etc.
-
(iii)
The establishment of a customized nanomaterial production system to fulfill local needs.
-
(iv)
Environmental and human health safety and protection during the use and disposal of nanomaterials.
-
(v)
The challenge to overcome the gap between basic and applied nano-based research.
-
(vi)
The cost of production, intensive risks, and technical knowledge gaps are also considered as major concern/challenge in nanobiotechnology applications.
-
(vii)
The problems faced by regulatory institutions and the lack of inter-institution coordination are the main challenges in the current situations of applied nanobiotechnology.
Therefore, governmental and workforce efforts based on sound scientific research, and technological advancements should be focused to meet the described challenges and associated risks. This would provide the necessary information to devise appropriate guidelines for comprehensive risk management and applications of nanobiotechnology for sustainable crop production.
Conclusion/future perspectives
The biologically synthesized nanoparticles provide promising solutions against biotic (insect pests, plant diseases) and abiotic (drought, salinity, thermal stresses, toxic metals, organic pollutants) stress factors. Nonetheless, nanobiotechnology application in the agriculture sector is at its nascent stage to counter biotic and abiotic stress factors. Remarkable work has been done regarding biosynthesized nano-based formulations but most of the work has been done in vitro conditions while in vivo applications are lacking. Therefore, only a few green nanotechnology-based products are available in the market may be due to production cost, a major hindrance along with other environmental issues towards wider marketing which could be overcome by promoting green nanoformulations. that the industrial scale manufacturing of green nanomaterials, not yet been widely started, can help with affordable prices, and is safer due to little chemical usage and low energy requirements in nanobiotechnology. The biologically synthesized nanoparticles also have some limitations regarding their stability and degradability in the environment which need to be addressed through innovative techniques of application and integration with other molecules. Therefore, it is important to understand plant–nanoparticle interaction and optimization of size, concentration and compatibility of NPs with biological systems before practical applications in the fields to reduce the degradability and negative impact on the natural environment and crops as well. Conclusively, nanobiotechnology requires comprehensive basic and advanced research on fabrication, characterization, standardization, biodegradability and also possible uptake and translocation of nanoparticles by plant systems for sustainable crop production and protection from biotic and abiotic stress factors.
Author contributions
ANand MF conceptulize and edited the article, HR, MU, AW, MS, MSU, SA and MA wrote their respective parts. AN and MF finalized the article and upgraded the figures and tables. All authors read and approved the final manuscript.
Funding
Not Applicable.
Availability of data and materials
All data generated or analyzed in this study are included in this article. The authors also declare that they have no conflict of interest.
Code availability
No codes were used in the manuscript.
Declarations
Ethics approval and consent to participate
We have no disclosure of potential conflicts of interest, no human participants and/or animals.
Consent for publication
Yes.
Competing interests
The authors declare that they have no competing interests.
Footnotes
The original online version of this article was revised to correct co-author Muhammad Shafiq Shahid’s affiliation.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/21/2023
A Correction to this paper has been published: 10.1186/s11671-023-03876-8
Contributor Information
Ahmad Nawaz, Email: ahmad.nawaz1793@uaf.edu.pk.
Muhammad Farooq, Email: farooqcp@squ.edu.om.
References
- 1.Khan M, et al. Agro-nanotechnology as an emerging field: a novel sustainable approach for improving plant growth by reducing biotic stress. Appl Sci. 2021;11(5):2282. [Google Scholar]
- 2.FAO F, Agriculture Organization of the United NationsThe Future of Food and Agriculture. Trends and Challenges, FAO, Rome, 2017.
- 3.Ashraf J, Javed A. Food security and environmental degradation: Do institutional quality and human capital make a difference? J Environ Manage. 2023;331:117330. doi: 10.1016/j.jenvman.2023.117330. [DOI] [PubMed] [Google Scholar]
- 4.Gustavsson J, et al. Global food losses and food waste. 2011, FAO Rome.
- 5.Mesterházy Á, Oláh J, Popp J. Losses in the grain supply chain: causes and solutions. Sustainability. 2020;12(6):2342. [Google Scholar]
- 6.Savary S, Willocquet L, Pethybridge S, Esker P, McRoberts N, Nelson A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019;3:430–439. doi: 10.1038/s41559-018-0793-y. [DOI] [PubMed] [Google Scholar]
- 7.Rathee M, Dalal P. Emerging insect pests in Indian agriculture. Indian J Entomol. 2018;80(2):267–281. [Google Scholar]
- 8.Oerke E-C, Dehne H-W. Safeguarding production—losses in major crops and the role of crop protection. Crop Prot. 2004;23(4):275–285. [Google Scholar]
- 9.Van Esse HP, Reuber TL, van der Does D. Genetic modification to improve disease resistance in crops. New Phytol. 2020;225(1):70–86. doi: 10.1111/nph.15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkinson NJ, Urwin PE. The interaction of plant biotic and abiotic stresses: from genes to the field. J Exp Bot. 2012;63(10):3523–3543. doi: 10.1093/jxb/ers100. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Vinocur B, Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003;218(1):1–14. doi: 10.1007/s00425-003-1105-5. [DOI] [PubMed] [Google Scholar]
- 12.Acquaah G. Sexual hybridization and wide crosses. Principals of plant genetics and breeding. Oxford, UK: Blackwell Publishing; 2007. pp. 164–180. [Google Scholar]
- 13.Farooq M et al. Plant drought stress: effects, mechanisms and management, in Sustainable agriculture. 2009, Springer. p 153-188
- 14.Savary S, et al. Crop losses due to diseases and their implications for global food production losses and food security. Springer; 2012. pp. 519–537. [Google Scholar]
- 15.Zahra N, et al. Hypoxia and anoxia stress: plant responses and tolerance mechanisms. J Agron Crop Sci. 2021;207(2):249–284. [Google Scholar]
- 16.Farooq MA, et al. Acquiring control: the evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol Biochem. 2019;141:353–369. doi: 10.1016/j.plaphy.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 17.Karl TR, et al. Global climate change impacts in the United States. Cambridge University Press; 2009. [Google Scholar]
- 18.Hofmann DJ, Butler JH, Tans PP. A new look at atmospheric carbon dioxide. Atmos Environ. 2009;43(12):2084–2086. [Google Scholar]
- 19.Bot A, Nachtergaele F, Young A. Land resource potential and constraints at regional and country levels. 2000: Food & Agriculture Org.
- 20.Thornton PK, et al. Climate variability and vulnerability to climate change: a review. Glob Change Biol. 2014;20(11):3313–3328. doi: 10.1111/gcb.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bybordi A. Effects of salinity on yield and component characters in canola (Brassica napus L.) cultivars. Notulae Sci Biol. 2010;2(1):81–83. [Google Scholar]
- 22.Panta S, et al. Halophyte agriculture: success stories. Environ Exp Bot. 2014;107:71–83. [Google Scholar]
- 23.ITPS, F., Status of the world’s soil resources (SWSR)—Main report. Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils, 2015: p. 650.
- 24.Manzoor J, Sharma M, Wani KA. Heavy metals in vegetables and their impact on the nutrient quality of vegetables: a review. J Plant Nutr. 2018;41(13):1744–1763. [Google Scholar]
- 25.Zhang W. Global pesticide use: profile, trend, cost/benefit and more. Proc Int Acad Ecol Environ Sci. 2018;8(1):1. [Google Scholar]
- 26.Ali S, et al. Sustainable agriculture reviews 48. Springer; 2021. Environmental and health effects of pesticide residues; pp. 311–336. [Google Scholar]
- 27.Parisi C, Vigani M, Rodríguez-Cerezo E. Agricultural nanotechnologies: What are the current possibilities? Nano Today. 2015;10(2):124–127. [Google Scholar]
- 28.Usman M, et al. Nanotechnology in agriculture: current status, challenges and future opportunities. Sci Total Environ. 2020;721:137778. doi: 10.1016/j.scitotenv.2020.137778. [DOI] [PubMed] [Google Scholar]
- 29.Singh, A., Scope of nanotechnology in crop science: profit or loss. Res Rev J Botan Sci, 2016.
- 30.Srujana, S., Bhagat, D., Sensors for agricultural pest management using nanotechnology/nanomaterials. Nanotechnol Environ Eng, 2022: p. 1–5.
- 31.Kumar V, Yadav SK. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol Int Res Process Environ Clean Technol. 2009;84(2):151–157. [Google Scholar]
- 32.Sharma VK, Yngard RA, Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Coll Interface Sci. 2009;145(1–2):83–96. doi: 10.1016/j.cis.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Siddiqui MH, et al. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ Toxicol Chem. 2014;33(11):2429–2437. doi: 10.1002/etc.2697. [DOI] [PubMed] [Google Scholar]
- 34.Bin Rashid A. Utilization of nanotechnology and nanomaterials in biodiesel production and property enhancement. J Nanomater, 2023. 2023.
- 35.Nadeem, M., et al., Ameliorative effect of silicic acid and silicates on oxidative, osmotic stress, and specific ion toxicity in spring wheat (Triticum aestivum L.) genotypes. J Soil Sci Plant Nutr, 2022: p. 1–12.
- 36.Mansoor S, et al. Reactive oxygen species in plants: from source to sink. Antioxidants. 2022;11(2):225. doi: 10.3390/antiox11020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arshad MS, et al. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiol Biochem. 2017;115:57–72. doi: 10.1016/j.plaphy.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Wu H, Tito N, Giraldo JP. Anionic cerium oxide nanoparticles protect plant photosynthesis from abiotic stress by scavenging reactive oxygen species. ACS Nano. 2017;11(11):11283–11297. doi: 10.1021/acsnano.7b05723. [DOI] [PubMed] [Google Scholar]
- 39.Zhao L, et al. Nano-biotechnology in agriculture: use of nanomaterials to promote plant growth and stress tolerance. J Agric Food Chem. 2020;68(7):1935–1947. doi: 10.1021/acs.jafc.9b06615. [DOI] [PubMed] [Google Scholar]
- 40.Oliveira HC, et al. Nitric oxide-releasing chitosan nanoparticles alleviate the effects of salt stress in maize plants. Nitric Oxide. 2016;61:10–19. doi: 10.1016/j.niox.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Wang F, Gao S. Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environ Sci Pollut Res. 2015;22(4):2837–2845. doi: 10.1007/s11356-014-3525-0. [DOI] [PubMed] [Google Scholar]
- 42.Sarraf M, et al. Metal/metalloid-based nanomaterials for plant abiotic stress tolerance: an overview of the mechanisms. Plants. 2022;11(3):316. doi: 10.3390/plants11030316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yousaf H, et al. Green synthesis of silver nanoparticles and their applications as an alternative antibacterial and antioxidant agents. Mater Sci Eng C. 2020;112:110901. doi: 10.1016/j.msec.2020.110901. [DOI] [PubMed] [Google Scholar]
- 44.Castro-González CG, et al. Exposure of stevia (Stevia rebaudiana B.) to silver nanoparticles in vitro: transport and accumulation. Sci Rep. 2019;9(1):1–10. doi: 10.1038/s41598-019-46828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta AS, Emmanouil D, Sethi A. Clinical hypnotherapy in grief resolution. Indian Psyciatric Soc. 2020;42(2):193–197. doi: 10.4103/IJPSYM.IJPSYM_476_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki N, et al. Abiotic and biotic stress combinations. New Phytol. 2014;203(1):32–43. doi: 10.1111/nph.12797. [DOI] [PubMed] [Google Scholar]
- 47.Ali MA, et al. Exogenous production of silver nanoparticles by Tephrosia apollinea living plants under drought stress and their antimicrobial activities. Nanomaterials. 2019;9(12):1716. doi: 10.3390/nano9121716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hojjat SS, Ganjali A. The effect of silver nanoparticle on lentil seed germination under drought stress. Int J Farm Allied Sci. 2016;5(3):208–212. [Google Scholar]
- 49.Ghosh M, et al. Effects of ZnO nanoparticles in plants: cytotoxicity, genotoxicity, deregulation of antioxidant defenses, and cell-cycle arrest. Mutat Res/Genet Toxicol Environ Mutagen. 2016;807:25–32. doi: 10.1016/j.mrgentox.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Saffan MM, et al. Sustainable production of tomato plants (Solanum lycopersicum L.) under low-quality irrigation water as affected by bio-nanofertilizers of selenium and copper. Sustainability. 2022;14(6):3236. [Google Scholar]
- 51.Gul M, et al. Potassium-induced decrease in cytosolic Na+ alleviates deleterious effects of salt stress on wheat (Triticum aestivum L.) Plant Biol. 2019;21(5):825–831. doi: 10.1111/plb.12999. [DOI] [PubMed] [Google Scholar]
- 52.Ahmad I, Akhtar MS. Salt stress, microbes, and plant interactions: causes and solution. Springer; 2019. Use of nanoparticles in alleviating salt stress; pp. 199–215. [Google Scholar]
- 53.Rico CM, Peralta-Videa J, Gardea-Torresdey J. Nanotechnology and plant sciences. Springer; 2015. Chemistry, biochemistry of nanoparticles, and their role in antioxidant defense system in plants; pp. 1–17. [Google Scholar]
- 54.Sami F, Siddiqui H, Hayat S. Impact of silver nanoparticles on plant physiology: a critical review. Sustain Agric Rev. 2020;41:111–127. [Google Scholar]
- 55.Gohari G, et al. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci Rep. 2020;10(1):1–14. doi: 10.1038/s41598-020-57794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khalid MF, et al. Nanoparticles: the plant saviour under abiotic stresses. Nanomaterials. 2022;12(21):3915. doi: 10.3390/nano12213915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choudhary M, et al. Heat stress during meiosis has lasting impacts on plant growth and reproduction in wheat (Triticum aestivum L.) Agronomy. 2022;12(5):987. [Google Scholar]
- 58.Haider, S., et al., Analyzing the regulatory role of heat shock transcription factors in plant heat stress tolerance: A brief appraisal. Molecular Biology Reports, 2022: p. 1–15. [DOI] [PubMed]
- 59.Mushtaq Z, et al. Changes in growth, photosynthetic pigments, cell viability, lipid peroxidation and antioxidant defense system in two varieties of chickpea (Cicer arietinum L.) subjected to salinity stress. Phyton. 2022;91(1):149. [Google Scholar]
- 60.Ullah A, et al. Heat stress effects on the reproductive physiology and yield of wheat. J Agron Crop Sci. 2022;208(1):1–17. [Google Scholar]
- 61.Ullah A, et al. Adequate zinc nutrition improves the tolerance against drought and heat stresses in chickpea. Plant Physiol Biochem. 2019;143:11–18. doi: 10.1016/j.plaphy.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 62.Iqbal M, et al. Effect of silver nanoparticles on growth of wheat under heat stress. Iran J Sci Technol Trans A Sci. 2019;43(2):387–395. [Google Scholar]
- 63.Kohan-Baghkheirati E, Geisler-Lee J. Gene expression, protein function and pathways of Arabidopsis thaliana responding to silver nanoparticles in comparison to silver ions, cold, salt, drought, and heat. Nanomaterials. 2015;5(2):436–467. doi: 10.3390/nano5020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iqbal M, et al. Assessment of AgNPs exposure on physiological and biochemical changes and antioxidative defence system in wheat (Triticum aestivum L.) under heat stress. IET Nanobiotechnol. 2019;13(2):230–236. doi: 10.1049/iet-nbt.2018.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao L, et al. Stress response and tolerance of Zea mays to CeO2 nanoparticles: cross talk among H2O2, heat shock protein, and lipid peroxidation. ACS Nano. 2012;6(11):9615–9622. doi: 10.1021/nn302975u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khodakovskaya MV, et al. Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc Natl Acad Sci. 2011;108(3):1028–1033. doi: 10.1073/pnas.1008856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haghighi M, Abolghasemi R, da Silva JAT. Low and high temperature stress affect the growth characteristics of tomato in hydroponic culture with Se and nano-Se amendment. Sci Hortic. 2014;178:231–240. [Google Scholar]
- 68.Djanaguiraman M, et al. High-temperature stress alleviation by selenium nanoparticle treatment in grain sorghum. ACS Omega. 2018;3(3):2479–2491. doi: 10.1021/acsomega.7b01934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seliem MK, Hafez Y, El-Ramady H. Using nano-selenium in reducing the negative effects of high temperature stress on Chrysanthemum morifolium Ramat. J Sustain Agric Sci. 2020;46(3):47–60. [Google Scholar]
- 70.Azimi R et al. Interaction of SiO2 nanoparticles with seed prechilling on germination and early seedling growth of tall wheatgrass (Agropyron elongatum L). Polish J Chem Technol, 2014. 16(3).
- 71.Qi M, Liu Y, Li T. Nano-TiO2 improve the photosynthesis of tomato leaves under mild heat stress. Biol Trace Elem Res. 2013;156(1):323–328. doi: 10.1007/s12011-013-9833-2. [DOI] [PubMed] [Google Scholar]
- 72.Mohammadi R, Maali-Amiri R, Abbasi A. Effect of TiO2 nanoparticles on chickpea response to cold stress. Biol Trace Elem Res. 2013;152(3):403–410. doi: 10.1007/s12011-013-9631-x. [DOI] [PubMed] [Google Scholar]
- 73.Hasanpour H, Maali-Amir R, Zeinali H. Effect of TiO2 nanoparticles on metabolic limitations to photosynthesis under cold in chickpea. Russ J Plant Physiol. 2015;62(6):779–787. [Google Scholar]
- 74.Amini S, et al. cDNA-AFLP analysis of transcripts induced in chickpea plants by TiO2 nanoparticles during cold stress. Plant Physiol Biochem. 2017;111:39–49. doi: 10.1016/j.plaphy.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 75.Wang A, et al. Mechanisms of chitosan nanoparticles in the regulation of cold stress resistance in banana plants. Nanomaterials. 2021;11(10):2670. doi: 10.3390/nano11102670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reinle K, Mogk A, Bukau B. The diverse functions of small heat shock proteins in the proteostasis network. J Mol Biol. 2022;434(1):167157. doi: 10.1016/j.jmb.2021.167157. [DOI] [PubMed] [Google Scholar]
- 77.Shekhawat, K., et al., Beat the heat: Plant-and microbe-mediated strategies for crop thermotolerance. Trends Plant Sci, 2022. [DOI] [PubMed]
- 78.Mohammadi R, Maali-Amiri R, Mantri N. Effect of TiO2 nanoparticles on oxidative damage and antioxidant defense systems in chickpea seedlings during cold stress. Russ J Plant Physiol. 2014;61(6):768–775. [Google Scholar]
- 79.Stahl-Rommel S, et al. Cyclic electron flow (CEF) and ascorbate pathway activity provide constitutive photoprotection for the photopsychrophile, Chlamydomonas sp. UWO 241 (renamed Chlamydomonas priscuii) Photosynth Res. 2022;151(3):235–250. doi: 10.1007/s11120-021-00877-5. [DOI] [PubMed] [Google Scholar]
- 80.Giraldo JP, et al. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat Mater. 2014;13(4):400–408. doi: 10.1038/nmat3890. [DOI] [PubMed] [Google Scholar]
- 81.Ranjan A, et al. Nanobionics in crop production: an emerging approach to modulate plant functionalities. Plants. 2022;11(5):692. doi: 10.3390/plants11050692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ze Y, et al. The regulation of TiO2 nanoparticles on the expression of light-harvesting complex II and photosynthesis of chloroplasts of Arabidopsis thaliana. Biol Trace Elem Res. 2011;143(2):1131–1141. doi: 10.1007/s12011-010-8901-0. [DOI] [PubMed] [Google Scholar]
- 83.Gao F, et al. Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach. Biol Trace Elem Res. 2006;111(1):239–253. doi: 10.1385/BTER:111:1:239. [DOI] [PubMed] [Google Scholar]
- 84.Husen A, Siddiqi KS. Phytosynthesis of nanoparticles: concept, controversy and application. Nanoscale Res Lett. 2014;9(1):1–24. doi: 10.1186/1556-276X-9-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Angulo-Bejarano PI, Puente-Rivera J, Cruz-Ortega R. Metal and metalloid toxicity in plants: an overview on molecular aspects. Plants. 2021;10(4):635. doi: 10.3390/plants10040635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh S, et al. Plant life under changing environment. Elsevier; 2020. Heavy metal stress and plant life: uptake mechanisms, toxicity, and alleviation; pp. 271–287. [Google Scholar]
- 87.Dubey S, et al. Toxicity and detoxification of heavy metals during plant growth and metabolism. Environ Chem Lett. 2018;16(4):1169–1192. [Google Scholar]
- 88.Emamverdian A, et al. Heavy metal stress and some mechanisms of plant defense response. Sci World J. 2015;2015:756120. doi: 10.1155/2015/756120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hamid Y, et al. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci Total Environ. 2019;660:80–96. doi: 10.1016/j.scitotenv.2018.12.419. [DOI] [PubMed] [Google Scholar]
- 90.Cheng P, et al. Contribution of nano-zero-valent iron and arbuscular mycorrhizal fungi to phytoremediation of heavy metal-contaminated soil. Nanomaterials. 2021;11(5):1264. doi: 10.3390/nano11051264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cao X, et al. Improved metal remediation using a combined bacterial and nanoscience approach. Sci Total Environ. 2020;704:135378. doi: 10.1016/j.scitotenv.2019.135378. [DOI] [PubMed] [Google Scholar]
- 92.Huang D, et al. Nanoscale zero-valent iron assisted phytoremediation of Pb in sediment: impacts on metal accumulation and antioxidative system of Lolium perenne. Ecotoxicol Environ Saf. 2018;153:229–237. doi: 10.1016/j.ecoenv.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 93.Ahmed T, et al. Current trends and future prospective in nanoremediation of heavy metals contaminated soils: a way forward towards sustainable agriculture. Ecotoxicol Environ Saf. 2021;227:112888. doi: 10.1016/j.ecoenv.2021.112888. [DOI] [PubMed] [Google Scholar]
- 94.Noman M, et al. Biogenic copper nanoparticles synthesized by using a copper-resistant strain Shigella flexneri SNT22 reduced the translocation of cadmium from soil to wheat plants. J Hazard Mater. 2020;398:123175. doi: 10.1016/j.jhazmat.2020.123175. [DOI] [PubMed] [Google Scholar]
- 95.Cecchin I, et al. Nanobioremediation: integration of nanoparticles and bioremediation for sustainable remediation of chlorinated organic contaminants in soils. Int Biodeterior Biodegrad. 2017;119:419–428. [Google Scholar]
- 96.Geissen V, et al. Emerging pollutants in the environment: a challenge for water resource management. Int Soil Water Conserv Res. 2015;3(1):57–65. [Google Scholar]
- 97.Han D, Currell MJ. Persistent organic pollutants in China's surface water systems. Sci Total Environ. 2017;580:602–625. doi: 10.1016/j.scitotenv.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 98.Zhang LZ, et al. Anti-oxidant response of Cucumis sativus L. to fungicide carbendazim. Pestic Biochem Physiol. 2007;89(1):54–59. [Google Scholar]
- 99.Wu J, et al. Effects of Ni/Fe bimetallic nanoparticles on phytotoxicity and translocation of polybrominated diphenyl ethers in contaminated soil. Chemosphere. 2016;162:235–242. doi: 10.1016/j.chemosphere.2016.07.101. [DOI] [PubMed] [Google Scholar]
- 100.Hamdi H, et al. Impact of non-functionalized and amino-functionalized multiwall carbon nanotubes on pesticide uptake by lettuce (Lactuca sativa L.) Nanotoxicology. 2015;9(2):172–180. doi: 10.3109/17435390.2014.907456. [DOI] [PubMed] [Google Scholar]
- 101.De La Torre-Roche R, et al. Multiwalled carbon nanotubes and C60 fullerenes differentially impact the accumulation of weathered pesticides in four agricultural plants. Environ Sci Technol. 2013;47(21):12539–12547. doi: 10.1021/es4034809. [DOI] [PubMed] [Google Scholar]
- 102.Zhang H, et al. Influence of multiwalled carbon nanotubes and sodium dodecyl benzene sulfonate on bioaccumulation and translocation of pyrene and 1-methylpyrene in maize (Zea mays) seedlings. Environ Pollut. 2017;220:1409–1417. doi: 10.1016/j.envpol.2016.10.093. [DOI] [PubMed] [Google Scholar]
- 103.Chakravarty D, Erande MB, Late DJ. Graphene quantum dots as enhanced plant growth regulators: effects on coriander and garlic plants. J Sci Food Agric. 2015;95(13):2772–2778. doi: 10.1002/jsfa.7106. [DOI] [PubMed] [Google Scholar]
- 104.Praveen A, et al. Iron oxide nanoparticles as nano-adsorbents: a possible way to reduce arsenic phytotoxicity in Indian mustard plant (Brassica juncea L.) J Plant Growth Regul. 2018;37(2):612–624. [Google Scholar]
- 105.De Windt W, Aelterman P, Verstraete W. Bioreductive deposition of palladium (0) nanoparticles on Shewanella oneidensis with catalytic activity towards reductive dechlorination of polychlorinated biphenyls. Environ Microbiol. 2005;7(3):314–325. doi: 10.1111/j.1462-2920.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 106.Deplanche K, et al. Versatility of a new bioinorganic catalyst: palladized cells of Desulfovibrio desulfuricans and application to dehalogenation of flame retardant materials. Environ Technol. 2009;30(7):681–692. doi: 10.1080/09593330902860712. [DOI] [PubMed] [Google Scholar]
- 107.De Gusseme B, et al. Biogenic palladium enhances diatrizoate removal from hospital wastewater in a microbial electrolysis cell. Environ Sci Technol. 2011;45(13):5737–5745. doi: 10.1021/es200702m. [DOI] [PubMed] [Google Scholar]
- 108.Raza A, et al. Impact of climate change on crops adaptation and strategies to tackle its outcome: a review. Plants. 2019;8(2):34. doi: 10.3390/plants8020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Komatsu S, Hiraga S, Yanagawa Y. Proteomics techniques for the development of flood tolerant crops. J Proteome Res. 2012;11(1):68–78. doi: 10.1021/pr2008863. [DOI] [PubMed] [Google Scholar]
- 110.Drew MC. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:223–250. doi: 10.1146/annurev.arplant.48.1.223. [DOI] [PubMed] [Google Scholar]
- 111.Iqbal Z, et al. Plant defense responses to biotic stress and its interplay with fluctuating dark/light conditions. Front Plant Sci. 2021;12:631810. doi: 10.3389/fpls.2021.631810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vartapetian, B., et al., Biotechnological approaches to creation of hypoxia and anoxia tolerant plants. Acta Naturae (aнглoязычнaя вepcия), 2014. 6(2 (21)): 19–30. [PMC free article] [PubMed]
- 113.Bartolucci C, et al. Green nanomaterials fostering agrifood sustainability. TrAC Trends Anal Chem. 2020;125:115840. [Google Scholar]
- 114.Almutairi ZM. Plant molecular defense mechanisms promoted by nanoparticles against environmental stresses. Int J Agric Biol. 2019;21(2):259–270. [Google Scholar]
- 115.Mustafa G, Sakata K, Komatsu S. Proteomic analysis of flooded soybean root exposed to aluminum oxide nanoparticles. J Proteomics. 2015;128:280–297. doi: 10.1016/j.jprot.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 116.Mustafa G, Komatsu S. Insights into the response of soybean mitochondrial proteins to various sizes of aluminum oxide nanoparticles under flooding stress. J Proteome Res. 2016;15(12):4464–4475. doi: 10.1021/acs.jproteome.6b00572. [DOI] [PubMed] [Google Scholar]
- 117.Syu Y-Y, et al. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol Biochem. 2014;83:57–64. doi: 10.1016/j.plaphy.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 118.Oerke E-C. Crop losses to pests. J Agric Sci. 2006;144(1):31–43. [Google Scholar]
- 119.Taiz L, Zeiger E. Secondary metabolites and plant defense. Plant Physiol. 2006;4:315–344. [Google Scholar]
- 120.Saijo Y, Loo EPI. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2020;225(1):87–104. doi: 10.1111/nph.15989. [DOI] [PubMed] [Google Scholar]
- 121.Abhilash P, Singh N. Pesticide use and application: an Indian scenario. J Hazard Mater. 2009;165(1–3):1–12. doi: 10.1016/j.jhazmat.2008.10.061. [DOI] [PubMed] [Google Scholar]
- 122.De A, et al. Targeted delivery of pesticides using biodegradable polymeric nanoparticles. Springer; 2014. [Google Scholar]
- 123.Nawaz A, et al. Cellular impact of combinations of endosulfan, atrazine, and chlorpyrifos on human primary hepatocytes and HepaRG cells after short and chronic exposures. Cell Biol Toxicol. 2014;30(1):17–29. doi: 10.1007/s10565-013-9266-x. [DOI] [PubMed] [Google Scholar]
- 124.Nawaz A, et al. Analysis of food resources, host availability and insecticidal impacts on the fecundity, longevity and parasitism efficiency of Diaertiella rapae (M’intosh) Int J Trop Insect Sci. 2021;41(4):2883–2896. [Google Scholar]
- 125.Mihale, M.J., et al., Use of indigenous knowledge in the management of field and storage pests around Lake Victoria basin in Tanzania. Afr J Environ Sci Technol, 2009. 3(9).
- 126.Pimentel, D. and R. Peshin, Integrated pest management: pesticide problems, Vol. 3. 2014: Springer Science & Business Media.
- 127.Constable F, Bertaccini A. Grapevine yellows diseases and their phytoplasma agents. Springer; 2017. Worldwide distribution and identification of grapevine yellows diseases; pp. 17–46. [Google Scholar]
- 128.Dhaliwal G, Jindal V, Dhawan A. Insect pest problems and crop losses: changing trends. Indian J Ecol. 2010;37(1):1–7. [Google Scholar]
- 129.Gill, H., Garg, H., Pesticides: environmental impacts and management strategies, pesticides-toxic aspects. IntechOpen. 2014.
- 130.Soni N, Prakash S. Efficacy of fungus mediated silver and gold nanoparticles against Aedes aegypti larvae. Parasitol Res. 2012;110(1):175–184. doi: 10.1007/s00436-011-2467-4. [DOI] [PubMed] [Google Scholar]
- 131.Surendran A, Vennison SJ. Occurrence and distribution of mosquitocidal Bacillus sphaericus in soil. Acad J Entomol. 2011;4(1):17–22. [Google Scholar]
- 132.Ruiu L, Satta A, Floris I. Emerging entomopathogenic bacteria for insect pest management. Bull Insectol. 2013;66(2):181–186. [Google Scholar]
- 133.Sayed AM, Kim S, Behle RW. Characterisation of silver nanoparticles synthesised by Bacillus thuringiensis as a nanobiopesticide for insect pest control. Biocontrol Sci Tech. 2017;27(11):1308–1326. [Google Scholar]
- 134.Abdel-Raheem M, Reyad NF, Alghamdi HA. Virulence of nanoparticle preparation of entomopathogenic fungi and entomopathogenic bacteria against red palm weevil Rhynchophorus ferrugineus (Olivier)(Coleoptera: Curculionidae) Rom Biotechnol Lett. 2020;25:1151–1159. [Google Scholar]
- 135.Iqbal H, Fatima A, Khan HAA. ZnO nanoparticles produced in the culture supernatant of Bacillus thuringiensis ser israelensis affect the demographic parameters of Musca domestica using the age-stage, two-sex life table. Pest Manag Sci. 2022;78(4):1640–1648. doi: 10.1002/ps.6783. [DOI] [PubMed] [Google Scholar]
- 136.Malaikozhundan B, et al. Bacillus thuringiensis coated zinc oxide nanoparticle and its biopesticidal effects on the pulse beetle, Callosobruchus maculatus. J Photochem Photobiol, B. 2017;174:306–314. doi: 10.1016/j.jphotobiol.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 137.Gabarty A, et al. Assessment of combining biosynthesized silver nanoparticles using Bacillus thuringiensis and gamma irradiation for controlling Pectinophora gossypiella (saunders)(lepidoptera: Gelechiidae) Int J Radiat Biol. 2021;97(9):1299–1315. doi: 10.1080/09553002.2021.1934747. [DOI] [PubMed] [Google Scholar]
- 138.Banu AN, Balasubramanian C. Extracellular synthesis of silver nanoparticles using Bacillus megaterium against malarial and dengue vector (Diptera: Culicidae) Parasitol Res. 2015;114(11):4069–4079. doi: 10.1007/s00436-015-4635-4. [DOI] [PubMed] [Google Scholar]
- 139.Guru Bharathi B. Lalitha K. Shivakumar MS. Biosynthesis of copper nanoparticles using symbiotic bacterium Xenorhabdus sp, isolated from entomopathogenic nematode and its antimicrobial and insecticidal activity against Spodoptera litura. Inorgan Nano-Metal Chem, 2022: p. 1–13.
- 140.Lalitha K, et al. Biosynthesis and characterization of silver nanoparticles from symbiotic bacteria Xenorhabdus nematophila and testing its insecticidal efficacy on Spodoptera litura larvae. Biometals. 2022;35(4):795–812. doi: 10.1007/s10534-022-00403-7. [DOI] [PubMed] [Google Scholar]
- 141.Kamil D et al., Green synthesis of silver nanoparticles by entomopathogenic fungus Beauveria bassiana and their bioefficacy against mustard aphid (Lipaphis erysimi Kalt.). 2017.
- 142.Mahmood HR. Evaluation of silver nanoparticles biosynthesized by entomopathogenic fungus Entomophthora muscae against larval instars of housefly Musca domestica. Al-Qadisiyah J Pure Sci. 2017;22(3):274–281. [Google Scholar]
- 143.Bhadani RV, et al. Biosynthesis and characterization of extracellular metabolites-based nanoparticles to control the whitefly. Arch Microbiol. 2022;204(6):1–15. doi: 10.1007/s00203-022-02917-7. [DOI] [PubMed] [Google Scholar]
- 144.Khooshe-Bast Z. et al. Insecticidal effects of zinc oxide nanoparticles and Beauveria bassiana TS11 on Trialeurodes vaporariorum (Westwood, 1856)(Hemiptera: Aleyrodidae). Acta Agric Slov, 2016. 107(2): 299–309.
- 145.Amerasan D, et al. Myco-synthesis of silver nanoparticles using Metarhizium anisopliae against the rural malaria vector Anopheles culicifacies Giles (Diptera: Culicidae) J Pest Sci. 2016;89(1):249–256. [Google Scholar]
- 146.Chinnaperumal K, et al. Bio-pesticidal effects of Trichoderma viride formulated titanium dioxide nanoparticle and their physiological and biochemical changes on Helicoverpa armigera (Hub) Pestic Biochem Physiol. 2018;149:26–36. doi: 10.1016/j.pestbp.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 147.Yosri M, Abdel-Aziz MM, Sayed R. Larvicidal potential of irradiated myco-insecticide from Metarhizium anisopliae and larvicidal synergistic effect with its mycosynthesized titanium nanoparticles (TiNPs) J Radiat Res Appl Sci. 2018;11(4):328–334. [Google Scholar]
- 148.Saad AF, Abdel-Raheem M. Silver nano-particles from entomopathogenic fungion the tortoise beetle, Cassida vittata (coleoptera: chrysomelidae).
- 149.Wang T-M, Yang J-T. Visual DNA diagnosis of tomato yellow leaf curl virus with integrated recombinase polymerase amplification and a gold-nanoparticle probe. Sci Rep. 2019;9(1):1–8. doi: 10.1038/s41598-019-51650-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Santos TS, et al. Entomopathogenic fungi biomass production and extracellular biosynthesis of silver nanoparticles for bioinsecticide action. Appl Sci. 2021;11(6):2465. [Google Scholar]
- 151.Santos TS, et al. Entomopathogenic fungi-mediated AgNPs: synthesis and insecticidal effect against Plutella xylostella (Lepidoptera: Plutellidae) Materials. 2022;15(21):7596. doi: 10.3390/ma15217596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jamunarani G et al. Efficacy of biosynthesised silver nanoparticles from entomopathogenic fungi Metarhizium rileyi against spodoptera Litura on cabbage. 2021.
- 153.Santhosh SB, Ragavendran C, Natarajan D. Spectral and HRTEM analyses of Annona muricata leaf extract mediated silver nanoparticles and its Larvicidal efficacy against three mosquito vectors Anopheles stephensi, Culex quinquefasciatus, and Aedes aegypti. J Photochem Photobiol B. 2015;153:184–190. doi: 10.1016/j.jphotobiol.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 154.Zahir AA, et al. Efficacy of plant-mediated synthesized silver nanoparticles against Sitophilus oryzae. J Biopest. 2012;5:95. [Google Scholar]
- 155.Al Shater H, Moustafa HZ, Yousef H. Synthesis, phytochemical screening and toxicity measuring against Earias insulana (Boisd.)(Lepidoptera: Noctuidae) of silver nano particles from Origanum marjorana extract in the field. Egypt Acad J Biol Sci F. Toxicol Pest Control, 2020. 12(1): 175–184.
- 156.Roni M, et al. Characterization and biotoxicity of Hypnea musciformis-synthesized silver nanoparticles as potential eco-friendly control tool against Aedes aegypti and Plutella xylostella. Ecotoxicol Environ Saf. 2015;121:31–38. doi: 10.1016/j.ecoenv.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 157.Kantrao S, et al. Effect of biosynthesized Silver nanoparticles on growth and development of Helicoverpa armigera (Lepidoptera: Noctuidae): Interaction with midgut protease. J Asia-Pac Entomol. 2017;20(2):583–589. [Google Scholar]
- 158.Vadlapudi V, Amanchy R. Phytofabrication of silver nanoparticles using Myriostachya wightiana as a novel bioresource, and evaluation of their biological activities. Braz Arch Biol Technol, 2017. 60.
- 159.Gulzar MU, et al. Impacts of bio synthesized silver-nanoparticles (AgNO3) and plant oils against Trogoderma granarium. GSC Biol Pharm Sci. 2020;10(3):010–015. [Google Scholar]
- 160.Qamar SUR et al. Biosynthesis of silver nanoparticles using Ocimum tenuiflorum extract and its efficacy assessment against Helicoverpa armigera. Int J Pest Manag, 2021: p. 1–9.
- 161.Manimegalai T, et al. Facile synthesis of silver nanoparticles using Vernonia anthelmintica (L.) Willd. and their toxicity against Spodoptera litura (Fab.), Helicoverpa armigera (Hüb.) Aedes aegypti Linn. and Culex quinquefasciatus Say. J Clust Sci. 2022;33(5):2287–2303. [Google Scholar]
- 162.Hazaa M, et al. Biosynthesis of silver nanoparticles using Borago officinslis leaf extract, characterization and larvicidal activity against cotton leaf worm, Spodoptera littoralis (Bosid) Int J Trop Insect Sci. 2021;41(1):145–156. [Google Scholar]
- 163.Saad AM, et al. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J Biol Sci. 2021;28(10):5674–5683. doi: 10.1016/j.sjbs.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mishra S, et al. Interaction mechanism of plant-based nanoarchitectured materials with digestive enzymes of termites as target for pest control: evidence from molecular docking simulation and in vitro studies. J Hazard Mater. 2021;403:123840. doi: 10.1016/j.jhazmat.2020.123840. [DOI] [PubMed] [Google Scholar]
- 165.Jafir M et al. Characterization of Ocimum basilicum synthesized silver nanoparticles and its relative toxicity to some insecticides against tobacco cutworm, Spodoptera litura Feb.(Lepidoptera; Noctuidae). Ecotoxicol Environ Saf, 2021. 218: p. 112278. [DOI] [PubMed]
- 166.Abdel-Meguid AD. Larvicidal and biochechemical effects of methanol leaf extract and biosynthesized silver nanoparticles using Nerium oleander L. (Apocynaceae) against Lucilia sericata M. (Diptera: Calliphoridae) Int J Trop Insect Sci. 2022;42(3):2579–2591. [Google Scholar]
- 167.Shukla G, et al. Synthesis of mycogenic silver nanoparticles by Fusarium pallidoroseum and evaluation of its larvicidal effect against white grubs (Holotrichia sp.) Mater Today Proc. 2022;49:3517–3527. [Google Scholar]
- 168.Sankar MV, Abideen S. Pesticidal effect of green synthesized silver and lead nanoparticles using Avicennia marina against grain storage pest Sitophilus oryzae. Int J Nanomater Biostruct. 2015;5(3):32–39. [Google Scholar]
- 169.Murugan K, et al. Sargassum wightii-synthesized ZnO nanoparticles reduce the fitness and reproduction of the malaria vector Anopheles stephensi and cotton bollworm Helicoverpa armigera. Physiol Mol Plant Pathol. 2018;101:202–213. [Google Scholar]
- 170.Malaikozhundan B, Vinodhini J. Nanopesticidal effects of Pongamia pinnata leaf extract coated zinc oxide nanoparticle against the Pulse beetle, Callosobruchus maculatus. Mater Today Commun. 2018;14:106–115. [Google Scholar]
- 171.Biradar W, et al. Entomotoxic effect of green nanoparticle an alternate strategy for stored grain pest management. Int J Trop Insect Sci. 2021;41(4):2829–2840. [Google Scholar]
- 172.Anees M et al. Biosynthesis, characterization and evaluation of green synthesized zinc nanoparticles against Helicoverpa armigera (Hub.)(Noctuidae: Lepidoptera). 2022. [DOI] [PMC free article] [PubMed]
- 173.Siddique MA, Sagheer M, Sahi ST. Comparative toxic effects of Eucalyptus globulus L. (Myrtales: Myrtaceae) and its green synthesized zinc oxide nanoparticles (ZnONPs) against Rhyzopertha dominica (F.)(Coleoptera: Bostrichidae) Int J Trop Insect Sci. 2022;42(2):1697–1706. [Google Scholar]
- 174.Thakur P et al. Nano-insecticide: synthesis, characterization, and evaluation of insecticidal activity of ZnO NPs against Spodoptera litura and Macrosiphum euphorbiae. Appl Nanosci, 2022: p. 1–16.
- 175.Ghidan AY, Al-Antary TM, Awwad AM. Green synthesis of copper oxide nanoparticles using Punica granatum peels extract: effect on green peach Aphid. Environ Nanotechnol Monit Manag. 2016;6:95–98. [Google Scholar]
- 176.Paragas DS, Cruz KD, Fiegalan ER. Green synthesized copper nanoparticles from Blumea balsamifera Linn. leaves and its biocidal activities against Bactrocera dorsalis (Hendel) Malays J Anal Sci. 2020;24(3):436–448. [Google Scholar]
- 177.Tahir A, et al. Green synthesis, characterization and antibacterial, antifungal, larvicidal and anti-termite activities of copper nanoparticles derived from Grewia asiatica L. Bull Natl Res Centre. 2022;46(1):1–11. [Google Scholar]
- 178.Kucharska K et al. Control of the lesser mealworm Alphitobius diaperinus using entomopathogenic nematodes (EPNs) combined with nanoparticles. Ann Warsaw Univ Life Sci SGGW Anim Sci, 2016. 55.
- 179.Makirita WE, et al. Influence of metal oxides nanoparticles on pathogenicity of steinernema carpocapsae nematodes against lepidopteran Galleria mellonella. J Nanosci Nanotechnol. 2020;20(3):1470–1477. doi: 10.1166/jnn.2020.17329. [DOI] [PubMed] [Google Scholar]
- 180.Yokesh Babu M, et al. Application of biosynthesized silver nanoparticles in agricultural and marine pest control. Curr Nanosci. 2014;10(3):374–381. [Google Scholar]
- 181.Soni N, Prakash S. Microbial synthesis of spherical nanosilver and nanogold for mosquito control. Ann Microbiol. 2014;64(3):1099–1111. [Google Scholar]
- 182.Litwin A, Nowak M, Różalska S. Entomopathogenic fungi: unconventional applications. Rev Environ Sci Bio/Technol. 2020;19(1):23–42. [Google Scholar]
- 183.Pucheta Díaz M, et al. Mecanismo de acción de los hongos entomopatógenos. Interciencia. 2006;31(12):856–860. [Google Scholar]
- 184.Hasan S. Entomopathogenic fungi as potent agents of biological control. IJETR. 2014;2:234–237. [Google Scholar]
- 185.Lacey, L.A., Kaya, H.K., Field manual of techniques in invertebrate pathology: application and evaluation of pathogens for control of insects and other invertebrate pests. 2007: Springer.
- 186.Gill HK, Garg H. Pesticide: environmental impacts and management strategies. Pestic Toxic Asp. 2014;8:187. [Google Scholar]
- 187.Nasir M et al. A review on whitefly control by the use of entomopathogenic fungi. In: Proceedings of the 8th National Conference of Plant Pathology, University of Agriculture, Faisalabad, Pakistan. 2011.
- 188.Nawaz A, et al. In vivo and in vitro assessment of Trichoderma species and Bacillus thuringiensis integration to mitigate insect pests of brinjal (Solanum melongena L.) Egypt J Biolog Pest Control. 2020;30(1):1–7. [Google Scholar]
- 189.Nawaz A, et al. Compatibility and synergistic interactions of fungi, Metarhizium anisopliae, and insecticide combinations against the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae) Sci Rep. 2022;12(1):1–10. doi: 10.1038/s41598-022-08841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hussain A, et al. Entomopathogenic fungi disturbed the larval growth and feeding performance of Ocinara varians (Lepidoptera: Bombycidae) larvae. Insect Sci. 2009;16(6):511–517. [Google Scholar]
- 191.Sabbour M, Solieman N. Usage of nanotechnology of the fungi Nomuraea rileyi against the potato tuber moth Phthorimaea operculella (zeller) under laboratory field and store conditions. Int J Inf Res Rev. 2015;2(09):1131–1136. [Google Scholar]
- 192.Neeraj G, et al. Evaluation of nematicidal activity of ethanolic extracts of medicinal plants to Meloidogyne incognita (kofoid and white) chitwood under lab conditions. Int J Pure Appl Biosci. 2017;5:827–831. [Google Scholar]
- 193.Singh U et al. Role of garlic (Allium sativum L.) in human and plant diseases. 2001. [PubMed]
- 194.Isman MB, Grieneisen ML. Botanical insecticide research: many publications, limited useful data. Trends Plant Sci. 2014;19(3):140–145. doi: 10.1016/j.tplants.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 195.Ali S, et al. Insecticidal activity of turmeric (Curcuma longa) and garlic (Allium sativum) extracts against red flour beetle, Tribolium castaneum: a safe alternative to insecticides in stored commodities. J Entomol Zool Stud. 2014;2(3):201–205. [Google Scholar]
- 196.Mkenda PA, et al. Contact and fumigant toxicity of five pesticidal plants against Callosobruchus maculatus (Coleoptera: Chrysomelidae) in stored cowpea (Vigna unguiculata) Int J Trop Insect Sci. 2015;35(4):172–184. [Google Scholar]
- 197.Stevenson PC, Isman MB, Belmain SR. Pesticidal plants in Africa: a global vision of new biological control products from local uses. Ind Crops Prod. 2017;110:2–9. [Google Scholar]
- 198.Isman MB. Bridging the gap: moving botanical insecticides from the laboratory to the farm. Ind Crops Prod. 2017;110:10–14. [Google Scholar]
- 199.Jawalkar N, Zambare S, Zanke S. Insecticidal property of Datura stramonium L. seed extracts against Sitophilus oryzae L.(Coleoptera: Curculionidae) in stored wheat grains. J Entomol Zool Stud. 2016;4(6):92–96. [Google Scholar]
- 200.Erenso T, Berhe D. Effect of neem leaf and seed powders against adult maize weevil (Sitophilus zeamais Motschulsky) mortality. Int J Agric Res. 2016;11:90–94. [Google Scholar]
- 201.Arnason JT, Sims SR, Scott IM. Natural products from plants as insecticides. Encyclopedia of Life Support Systems (EOLSS), 2012: p. 1–8.
- 202.Isman MB. Botanical insecticides in the twenty-first century—fulfilling their promise? Annu Rev Entomol. 2020;65:233–249. doi: 10.1146/annurev-ento-011019-025010. [DOI] [PubMed] [Google Scholar]
- 203.Kim S-I, et al. Insecticidal activities of aromatic plant extracts and essential oils against Sitophilus oryzae and Callosobruchus chinensis. J Stored Prod Res. 2003;39(3):293–303. [Google Scholar]
- 204.Kuganathan N, Saminathan S, Muttukrishna S. Toxicity of Datura alba leaf extract to aphids and ants. Internet J Toxicol. 2008;5(2):1559–3916. [Google Scholar]
- 205.Ravi R, et al. The potential use of Azolla pinnata as an alternative bio-insecticide. Sci Rep. 2020;10(1):1–8. doi: 10.1038/s41598-020-75054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Poopathi S, et al. Synthesis of silver nanoparticles from Azadirachta indica—a most effective method for mosquito control. Environ Sci Pollut Res. 2015;22(4):2956–2963. doi: 10.1007/s11356-014-3560-x. [DOI] [PubMed] [Google Scholar]
- 207.Quist CW, Smant G, Helder J. Evolution of plant parasitism in the phylum nematoda. Annu Rev Phytopathol. 2015;53(1):289–310. doi: 10.1146/annurev-phyto-080614-120057. [DOI] [PubMed] [Google Scholar]
- 208.De Ley P, Blaxter M. The biology of nematodes. CRC Press; 2002. Systematic position and phylogeny; pp. 1–30. [Google Scholar]
- 209.Boemare N. Interactions between the partners of the entomopathogenic bacterium nematode complexes, Steinernema-Xenorhabdus and Heterorhabditis-Photorhabdus. Nematology. 2002;4(5):601–603. [Google Scholar]
- 210.Sepúlveda-Cano PA, López-Núñez JC, Soto-Giraldo A. Effect of two enthomopathogenic nematodes on Cosmopolites sordidus (Coleoptera: Dryophthoridae) Rev Colomb Entomol. 2008;34(1):62–67. [Google Scholar]
- 211.Taha EH, Abo-Shady NM. Effect of silver nanoparticles on the mortality pathogenicity and reproductivity of entomopathogenic nematodes. Int J Zool Res. 2016;12(3–4):47–50. [Google Scholar]
- 212.Kucharska K, Pezowicz E. The effect of silver nanoparticles on mortality and pathogenicity of entomopathogenic nematodes Heterorhabditis bacteriophora (Poinar, 1976) from Nematop biopreparation. 2009.
- 213.Kucharska K et al. Effect of silver nanoparticles on the mortality and pathogenicity of entomopathogenic nematodes. 2011.
- 214.Kucharska K et al. Nanoparticles of copper and entomopathogenic nematodes Steinernema feltiae (Filipjev, 1934) in reducing the number of the lesser mealworm beetle Alphitobius diaperinus (Panzer, 1797). Annals of Warsaw University of Life Sciences-SGGW. Animal Science, 2014. 53.
- 215.Pluskota A, et al. In Caenorhabditis elegans nanoparticle-bio-interactions become transparent: silica-nanoparticles induce reproductive senescence. PLoS ONE. 2009;4(8):e6622. doi: 10.1371/journal.pone.0006622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang H, Wick RL, Xing B. Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Environ Pollut. 2009;157(4):1171–1177. doi: 10.1016/j.envpol.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 217.Roh J-Y, et al. Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Environ Sci Technol. 2009;43(10):3933–3940. doi: 10.1021/es803477u. [DOI] [PubMed] [Google Scholar]
- 218.Belguzar S et al. Effects of rhizobacteria on tomato bacterial cancer and wilt disease. Feb Fresenius Environ Bull, 2021: p. 1075.
- 219.Cook RJ. Advances in plant health management in the twentieth century. Annu Rev Phytopathol. 2000;38:95. doi: 10.1146/annurev.phyto.38.1.95. [DOI] [PubMed] [Google Scholar]
- 220.Elling AA. Major emerging problems with minor Meloidogyne species. Phytopathology. 2013;103(11):1092–1102. doi: 10.1094/PHYTO-01-13-0019-RVW. [DOI] [PubMed] [Google Scholar]
- 221.Abad P, et al. Root-knot nematode parasitism and host response: molecular basis of a sophisticated interaction. Mol Plant Pathol. 2003;4(4):217–224. doi: 10.1046/j.1364-3703.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- 222.Maksimov IV, et al. Mechanisms of plant tolerance to RNA viruses induced by plant-growth-promoting microorganisms. Plants. 2019;8(12):575. doi: 10.3390/plants8120575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chauhan P, et al. A systematic review of conventional and advanced approaches for the control of plant viruses. J Appl Biol Biotechnol. 2019;7(4):8–8. [Google Scholar]
- 224.Khan M, Jairajpuri M. Nematode infestation in industrial crops-national scenario. Nematode infestations part II: industrial crops, Khan, MR and MS Jairajpuri (Eds.). National Academy of Sciences, India, ISBN-13: 9788190554824, 2010: p. 1–19.
- 225.Tripathy A, et al. Natural and bioinspired nanostructured bactericidal surfaces. Adv Coll Interface Sci. 2017;248:85–104. doi: 10.1016/j.cis.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hussain M, et al. Green synthesis and evaluation of silver nanoparticles for antimicrobial and biochemical profiling in Kinnow (Citrus reticulata L.) to enhance fruit quality and productivity under biotic stress. IET Nanobiotechnol. 2019;13(3):250–256. doi: 10.1049/iet-nbt.2018.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Stephano-Hornedo JL, et al. Argovit™ silver nanoparticles to fight Huanglongbing disease in Mexican limes (Citrus aurantifolia Swingle) RSC Adv. 2020;10(11):6146–6155. doi: 10.1039/c9ra09018e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ahmed T, et al. Silver nanoparticles synthesized by using Bacillus cereus SZT1 ameliorated the damage of bacterial leaf blight pathogen in rice. Pathogens. 2020;9(3):160. doi: 10.3390/pathogens9030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Suganya KU, et al. Blue green alga mediated synthesis of gold nanoparticles and its antibacterial efficacy against Gram positive organisms. Mater Sci Eng C. 2015;47:351–356. doi: 10.1016/j.msec.2014.11.043. [DOI] [PubMed] [Google Scholar]
- 230.Vimala K, et al. Green synthesized doxorubicin loaded zinc oxide nanoparticles regulates the Bax and Bcl-2 expression in breast and colon carcinoma. Process Biochem. 2014;49(1):160–172. [Google Scholar]
- 231.Venkatachalam P, et al. Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: a physiochemical analysis. Plant Physiol Biochem. 2017;110:59–69. doi: 10.1016/j.plaphy.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 232.Dobrucka R, Długaszewska J. Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J Biol Sci. 2016;23(4):517–523. doi: 10.1016/j.sjbs.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ogunyemi SO, et al. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif Cells Nanomed Biotechnol. 2019;47(1):341–352. doi: 10.1080/21691401.2018.1557671. [DOI] [PubMed] [Google Scholar]
- 234.Kala A et al. Green synthesis of copper bionanoparticles to control the bacterial leaf blight disease of rice. Curr Sci, 2016: p. 2011–2014.
- 235.Chung IM, et al. Green synthesis of copper nanoparticles using Eclipta prostrata leaves extract and their antioxidant and cytotoxic activities. Exp Ther Med. 2017;14(1):18–24. doi: 10.3892/etm.2017.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Luong HT, et al. Antibacterial effect of copper nanoparticles produced in a Shewanella-supported non-external circuit bioelectrical system on bacterial plant pathogens. RSC Adv. 2022;12(7):4428–4436. doi: 10.1039/d1ra08187j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Marpu S, et al. Photochemical formation of chitosan-stabilized near-infrared-absorbing silver nanoworms: a “Green” synthetic strategy and activity on Gram-negative pathogenic bacteria. J Colloid Interface Sci. 2017;507:437–452. doi: 10.1016/j.jcis.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 238.Chen X, Yan J-K, Wu J-Y. Characterization and antibacterial activity of silver nanoparticles prepared with a fungal exopolysaccharide in water. Food Hydrocoll. 2016;53:69–74. [Google Scholar]
- 239.Khan M, et al. Antibacterial and antifungal studies of biosynthesized silver nanoparticles against plant parasitic nematode Meloidogyne incognita, plant pathogens Ralstonia solanacearum and Fusarium oxysporum. Molecules. 2021;26(9):2462. doi: 10.3390/molecules26092462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Singh R, et al. Antimicrobial potential of silver nanoparticles biosynthesized using aerial yam bulbils for control of selected phytopathogens. Arch Phytopathol Plant Prot. 2021;54(19–20):2275–2293. [Google Scholar]
- 241.Pineda MEB, Lizarazo Forero LM, Sierra Avila CA. Antibacterial activity of biosynthesized silver nanoparticles (AgNps) against Pectobacterium carotovorum. Braz J Microbiol, 2022: p. 1–12. [DOI] [PMC free article] [PubMed]
- 242.Méndez-Andrade R, et al. Efficacy of biosynthesized silver nanoparticles from Larrea tridentata against Clavibacter michiganensis. J Phytopathol. 2022;170(2):91–99. [Google Scholar]
- 243.Atiq M, et al. Green synthesis of silver and copper nanoparticles from leaves of eucalyptus globulus and assessment of its antibacterial potential towards Xanthomonas citri pv. citri causing citrus canker. Appl Ecol Environ Res. 2022;20(3):2205–2213. [Google Scholar]
- 244.Awad MA, et al. Green synthesis of gold nanoparticles: preparation, characterization, cytotoxicity, and anti-bacterial activities. Mater Lett. 2019;256:126608. [Google Scholar]
- 245.Soltani Nejad M, et al. Evaluation of Phoma sp. biomass as an endophytic fungus for synthesis of extracellular gold nanoparticles with antibacterial and antifungal properties. Molecules. 2022;27(4):1181. doi: 10.3390/molecules27041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Grewal S, et al. Assessment of antibacterial activity of biosynthesised ZnO nanoparticles against Xanthomonas axonopodis pv. malvacearum causing bacterial blight in cotton. J Nanosci Nanotechnol. 2021;21(6):3531–3538. doi: 10.1166/jnn.2021.19010. [DOI] [PubMed] [Google Scholar]
- 247.Siddiqui ZA, et al. Effects of graphene oxide and zinc oxide nanoparticles on growth, chlorophyll, carotenoids, proline contents and diseases of carrot. Sci Hortic. 2019;249:374–382. [Google Scholar]
- 248.Abdallah Y, et al. Bioinspired green synthesis of chitosan and zinc oxide nanoparticles with strong antibacterial activity against rice pathogen Xanthomonas oryzae pv. oryzae. Molecules. 2020;25(20):4795. doi: 10.3390/molecules25204795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Khan RAA, et al. Management of Ralstonia solanacearum in tomato using ZnO nanoparticles synthesized through Matricaria chamomilla. Plant Dis. 2021;105(10):3224–3230. doi: 10.1094/PDIS-08-20-1763-RE. [DOI] [PubMed] [Google Scholar]
- 250.Ahmed T, et al. Bioinspired green synthesis of zinc oxide nanoparticles from a native Bacillus cereus strain RNT6: characterization and antibacterial activity against rice panicle blight pathogens Burkholderia glumae and B. gladioli. Nanomaterials. 2021;11(4):884. doi: 10.3390/nano11040884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Guo Y, et al. Enhanced suppression of soil-borne phytopathogenic bacteria Ralstonia solanacearum in soil and promotion of tomato plant growth by synergetic effect of green synthesized nanoparticles and plant extract. J Appl Microbiol. 2022;132(5):3694–3704. doi: 10.1111/jam.15459. [DOI] [PubMed] [Google Scholar]
- 252.Strayer-Scherer A, et al. Advanced copper composites against copper-tolerant Xanthomonas perforans and tomato bacterial spot. Phytopathology. 2018;108(2):196–205. doi: 10.1094/PHYTO-06-17-0221-R. [DOI] [PubMed] [Google Scholar]
- 253.Chen J, et al. Various antibacterial mechanisms of biosynthesized copper oxide nanoparticles against soilborne Ralstonia solanacearum. RSC Adv. 2019;9(7):3788–3799. doi: 10.1039/c8ra09186b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Moore D, Robson GD, Trinci AP. 21st century guidebook to fungi. Cambridge University Press; 2020. [Google Scholar]
- 255.Patil AG et al. Myco-nanotechnology for sustainable agriculture: challenges and opportunities. Recent trends in mycological research, 2021: p. 457–479.
- 256.Shende S, et al. Green synthesis of copper nanoparticles by Citrus medica Linn.(Idilimbu) juice and its antimicrobial activity. World J Microbiol Biotechnol. 2015;31(6):865–873. doi: 10.1007/s11274-015-1840-3. [DOI] [PubMed] [Google Scholar]
- 257.Rajesh K, et al. Assisted green synthesis of copper nanoparticles using Syzygium aromaticum bud extract: physical, optical and antimicrobial properties. Optik. 2018;154:593–600. [Google Scholar]
- 258.Khamis Y et al. Fungicidal efficacy of chemically-produced copper nanoparticles against Penincillium digitatum and Fusarium solani on citrus fruit. Philippine Agricultural Scientist (Philippines), 2017.
- 259.Hasanin M et al. In vitro improvement and rooting of banana plantlets using antifungal nanocomposite based on myco-synthesized copper oxide nanoparticles and starch. Biomass Conv Biorefinery, 2021: p. 1–11.
- 260.Le Thi Thanh T, Garampalli RH. Green synthesis of gold nanoparticles using cassia alata leaf extract and evaluation of antimicrobial activities. 2018, Springer.
- 261.Jebril S, Jenana RKB, Dridi C. Green synthesis of silver nanoparticles using Melia azedarach leaf extract and their antifungal activities: in vitro and in vivo. Mater Chem Phys. 2020;248:122898. [Google Scholar]
- 262.Gajbhiye M, et al. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed Nanotechnol Biol Med. 2009;5(4):382–386. doi: 10.1016/j.nano.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 263.Savithramma N, et al. Antimicrobial activity of silver nanoparticles synthesized by using medicinal plants. Int J ChemTech Res. 2011;3(3):1394–1402. [Google Scholar]
- 264.Kaur P, et al. Management of wilt disease of chickpea in vivo by silver nanoparticles biosynthesized by rhizospheric microflora of chickpea (Cicer arietinum) J Chem Technol Biotechnol. 2018;93(11):3233–3243. [Google Scholar]
- 265.El-Wakil DA. Antifungal activity of silver nanoparticles by Trichoderma species: synthesis, characterization and biological evaluation. Egypt J Phytopathol. 2020;48(1):71–80. [Google Scholar]
- 266.Bernardo-Mazariegos E, et al. Silver nanoparticles from Justicia spicigera and their antimicrobial potentialities in the biocontrol of foodborne bacteria and phytopathogenic fungi. Rev Argent Microbiol. 2019;51(2):103–109. doi: 10.1016/j.ram.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 267.Almadiy AA, Nenaah GE. Ecofriendly synthesis of silver nanoparticles using potato steroidal alkaloids and their activity against phytopathogenic fungi. Braz Arch Biol Technol, 2018. 61.
- 268.Masum M, et al. Biogenic synthesis of silver nanoparticles using Phyllanthus emblica fruit extract and its inhibitory action against the pathogen Acidovorax oryzae strain RS-2 of rice bacterial brown stripe. Front Microbiol. 2019;10:820. doi: 10.3389/fmicb.2019.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Vijayabharathi R, Sathya A, Gopalakrishnan S. Extracellular biosynthesis of silver nanoparticles using Streptomyces griseoplanus SAI-25 and its antifungal activity against Macrophomina phaseolina, the charcoal rot pathogen of sorghum. Biocatal Agric Biotechnol. 2018;14:166–171. [Google Scholar]
- 270.Al-Abboud MA. Fungal biosynthesis of silver nanoparticles and their role in control of Fusarium wilt of sweet pepper and soil-borne fungi in vitro. Int J Pharmacol. 2018;14(6):773–780. [Google Scholar]
- 271.Guilger-Casagrande M, et al. Biosynthesis of silver nanoparticles employing Trichoderma harzianum with enzymatic stimulation for the control of Sclerotinia sclerotiorum. Sci Rep. 2019;9(1):1–9. doi: 10.1038/s41598-019-50871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Consolo VF, Torres-Nicolini A, Alvarez VA. Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens. Sci Rep. 2020;10(1):1–9. doi: 10.1038/s41598-020-77294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Fouda A, et al. Antimicrobial, antioxidant and larvicidal activities of spherical silver nanoparticles synthesized by endophytic Streptomyces spp. Biol Trace Elem Res. 2020;195(2):707–724. doi: 10.1007/s12011-019-01883-4. [DOI] [PubMed] [Google Scholar]
- 274.Ashraf H, et al. Microwave-assisted green synthesis and characterization of silver nanoparticles using Melia azedarach for the management of Fusarium wilt in tomato. Front Microbiol. 2020;11:238. doi: 10.3389/fmicb.2020.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bayat M, et al. In vitro evaluation of antibacterial and antifungal activity of biogenic silver and copper nanoparticles: the first report of applying biogenic nanoparticles against Pilidium concavum and Pestalotia sp. fungi. Molecules. 2021;26(17):5402. doi: 10.3390/molecules26175402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Raja M, et al. Biosynthesis of silver nanoparticles from Trichoderma harzianum Th3 and its efficacy against root rot complex pathogen in groundnut. Mater Today Proc. 2021;43:3140–3143. [Google Scholar]
- 277.Babar M et al. Morpho-functional characterization, in vitro study, and tripartite interaction assay evaluate the potential of biosynthesized silver nanoparticles to manage Cercospora leaf spot disease in Vigna radiata. J Plant Pathol, 2022: p. 1–11.
- 278.Huang W, Yu H, Lan B. Antifungal activity of biosynthesized silver nanoparticles against three maize leaf phytopathogens. Pak J Bot. 2022;54(1):291–296. [Google Scholar]
- 279.Folorunso A, et al. Biosynthesis, characterization and antimicrobial activity of gold nanoparticles from leaf extracts of Annona muricata. J Nanostructure Chem. 2019;9(2):111–117. [Google Scholar]
- 280.Jayaseelan C, et al. Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity. Ind Crops Prod. 2013;45:423–429. [Google Scholar]
- 281.Jamdagni P, et al. Comparative account of antifungal activity of green and chemically synthesized zinc oxide nanoparticles in combination with agricultural fungicides. Int J Nano Dimens. 2018;9(2):198–208. [Google Scholar]
- 282.Rajiv P, Rajeshwari S, Venckatesh R. Bio-Fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens. Spectrochim Acta Part A Mol Biomol Spectrosc. 2013;112:384–387. doi: 10.1016/j.saa.2013.04.072. [DOI] [PubMed] [Google Scholar]
- 283.Al-Dhabi NA, Valan Arasu M. Environmentally-friendly green approach for the production of zinc oxide nanoparticles and their anti-fungal, ovicidal, and larvicidal properties. Nanomaterials. 2018;8(7):500. doi: 10.3390/nano8070500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Farahat G. Biosynthesis of nano zinc and using of some nanoparticles in reducing of Cercospora leaf spot disease of sugar beet in the field. Environ Biodivers Soil Secur. 2018;2018(2):103–117. [Google Scholar]
- 285.Bayat M, et al. Phytoassisted green synthesis of zinc oxide nanoparticles and its antibacterial and antifungal activity. Res Crops. 2019;20:725–730. [Google Scholar]
- 286.Karkhane M, et al. Antifungal, antioxidant and photocatalytic activities of zinc nanoparticles synthesized by Sargassum vulgare extract. Biocatal Agric Biotechnol. 2020;29:101791. [Google Scholar]
- 287.Khan AU, et al. Screening of biosynthesized zinc oxide nanoparticles for their effect on Daucus carota pathogen and molecular docking. Microsc Res Tech. 2022;85(10):3365–3373. doi: 10.1002/jemt.24191. [DOI] [PubMed] [Google Scholar]
- 288.Shende S, Gaikwad N, Bansod S. Synthesis and evaluation of antimicrobial potential of copper nanoparticle against agriculturally important phytopathogens. Synthesis. 2016;1(4):41–47. [Google Scholar]
- 289.Divte PR, et al. Characterization of biosynthesised copper nanoparticle from Citrus sinesis and in-vitro evaluation against fungal pathogen Colletotrichum capsica. Int J Chem Stud. 2019;7:325–330. [Google Scholar]
- 290.Ahmad H, et al. Enhanced biosynthesis synthesis of copper oxide nanoparticles (CuO-NPs) for their antifungal activity toxicity against major phyto-pathogens of apple orchards. Pharm Res. 2020;37(12):1–12. doi: 10.1007/s11095-020-02966-x. [DOI] [PubMed] [Google Scholar]
- 291.Shende S, et al. Myco-fabrication of copper nanoparticles and its effect on crop pathogenic fungi. IEEE Trans Nanobiosci. 2021;20(2):146–153. doi: 10.1109/TNB.2021.3056100. [DOI] [PubMed] [Google Scholar]
- 292.Style M, Chandrashekhara V, Style A. Potential antifungal activity of biosynthesized copper nanoparticles against colletotrichum capsici in Chilly. 2021.
- 293.Dorjee L et al. Biosynthesis of copper nanoparticles using Macrophomina phaseolina and evaluation of its antifungal activity against Fusarium verticillioides and Sclerotium rolfsii. 2022.
- 294.Sawake MM, et al. Management of Phytophthora parasitica causing gummosis in citrus using biogenic copper oxide nanoparticles. J Appl Microbiol. 2022;132(4):3142–3154. doi: 10.1111/jam.15472. [DOI] [PubMed] [Google Scholar]
- 295.El-Sawy MM, et al. Inhibition of tomato yellow leaf curl virus by Zingiber officinale and Mentha longifolia extracts and silica nanoparticles. Int J Antiviral Antiretrovirol. 2018;1:1–6. [Google Scholar]
- 296.El-Sawy MM et al. Antiviral activity of 2–nitromethyl phenol, zinc nanoparticles and seaweed extract against cucumber mosaic virus (CMV) in Eggplant. J Virol Antivir Res, 2017. 6(2).
- 297.El-Shazly MA, et al. Inhibitory effects of salicylic acid and silver nanoparticles on potato virus Y-infected potato plants in Egypt. Middle East J Agric Res. 2017;6:835–848. [Google Scholar]
- 298.Ahsan T. Biofabrication of silver nanoparticles from Pseudomonas fluorescens to control tobacco mosaic virus. Egypt J Biolog Pest Control. 2020;30(1):1–4. [Google Scholar]
- 299.Aref N et al. Multi-functional effects of gold nano-particles inducing plant virus resistance crops. In: Proceedings of the 5th annual world congress of industrial biotechnology-2012, Xi’an, China. 2012.
- 300.Elbeshehy EK, Elazzazy AM, Aggelis G. Silver nanoparticles synthesis mediated by new isolates of Bacillus spp., nanoparticle characterization and their activity against Bean Yellow Mosaic Virus and human pathogens. Front Microbiol. 2015;6:453. doi: 10.3389/fmicb.2015.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Jain D, Kothari S. Green synthesis of silver nanoparticles and their application in plant virus inhibition. J Mycol Plant Pathol. 2014;44(1):21–24. [Google Scholar]
- 302.Wang Y, et al. Preliminary experiments on nano-silver against tobacco mosaic virus and its mechanism. Tob Sci Technol. 2016;49(1):22–30. [Google Scholar]
- 303.Abdelkhalek A, et al. Chitosan nanoparticles inactivate Alfalfa mosaic virus replication and boost innate immunity in nicotiana glutinosa plants. Plants (Basel, Switzerland) 2021;10(12):2701. doi: 10.3390/plants10122701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Abdelkhalek A, Al-Askar AA. Green synthesized ZnO nanoparticles mediated by mentha spicata extract induce plant systemic resistance against tobacco mosaic virus. Appl Sci, 2020. 10(15).
- 305.Wang J et al. Field application of nanoliposomes delivered quercetin by inhibiting specific hsp70 gene expression against plant virus disease. J Nanobiotechnol, 2022. 20(1). [DOI] [PMC free article] [PubMed]
- 306.Ahsan T. Biofabrication of silver nanoparticles from Pseudomonas fluorescens to control tobacco mosaic virus. Egypt J Biol Pest Control. 2020;30(1):66. [Google Scholar]
- 307.Aref N et al. Multi-functional effects of gold nano-particles inducing plant virus resistance crops. Proceedings of the 5th Annual World Congress of Industrial Biotechnology-2012, 2012.
- 308.Jain D. Green synthesis of silver nanoparticles and their application in plant virus inhibition. J Mycol Plant Pathol. 2014;44:21–24. [Google Scholar]
- 309.Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13(10):2638–2650. [Google Scholar]
- 310.Wang TM, Yang JT. Visual DNA diagnosis of tomato yellow leaf curl virus with integrated recombinase polymerase amplification and a gold-nanoparticle probe. Sci Rep. 2019;9(1):15146. doi: 10.1038/s41598-019-51650-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Petrônio MS, et al. Physicochemical and toxicity investigation of Chitosan-based dsRNA nanocarrier formation. Biointerface Res Appl Chem. 2022;12(4):5266–5279. [Google Scholar]
- 312.Patel M et al. Reproductive toxicity of pomegranate peel extract synthesized gold nanoparticles: a multigeneration study in C. elegans. J Nanomater, 2019. 2019.
- 313.Cromwell W, et al. Nematicidal effects of silver nanoparticles on root-knot nematode in bermudagrass. J Nematol. 2014;46(3):261. [PMC free article] [PubMed] [Google Scholar]
- 314.Davids JS, et al. Biocontrol of bacteria associated with pine wilt nematode, Bursaphelenchus xylophilus by using plant mediated gold nanoparticles. Int J Agric Biol. 2021;26(4):517–526. [Google Scholar]
- 315.Heflish AA, et al. Green biosynthesized silver nanoparticles using Acalypha wilkesiana extract control root-knot nematode. J King Saud Univ Sci. 2021;33(6):101516. [Google Scholar]
- 316.El-Deen AN, El-Deeb BA. Effectiveness of silver nanoparticles against root-knot nematode, Meloidogyne incognita infecting tomato under greenhouse conditions. J Agric Sci. 2018;10(2):148–156. [Google Scholar]
- 317.Mohamed YMA, et al. Charcoal rot and root-knot nematode control on faba bean by photosynthesized colloidal silver nanoparticles using bioactive compounds from Moringa oleifera leaf extract. J Plant Prot Res. 2021;61(4):414–429. [Google Scholar]
- 318.Danish M, et al. Green synthesized silver nanoparticles mitigate biotic stress induced by meloidogyne incognita in Trachyspermum ammi (L.) by improving growth biochemical and antioxidant enzyme activities. ACS Omega. 2021;6(17):11389–11403. doi: 10.1021/acsomega.1c00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Ghareeb RY, et al. Utilization of Cladophora glomerata extract nanoparticles as eco-nematicide and enhancing the defense responses of tomato plants infected by Meloidogyne javanica. Sci Rep. 2020;10(1):19968. doi: 10.1038/s41598-020-77005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Tomar R, Preet S. Evaluation of anthelmintic activity of biologically synthesized silver nanoparticles against the gastrointestinal nematode, Haemonchus contortus. J Helminthol. 2017;91(4):454–461. doi: 10.1017/S0022149X16000444. [DOI] [PubMed] [Google Scholar]
- 321.Fabiyi O, et al. Preparation of bio-nematicidal nanoparticles of eucalyptus officinalis for the control of cyst nematode (Heterodera sacchari) J Anim Plant Sci. 2020;30(5):1172–1177. [Google Scholar]
- 322.Patiño-Ruiz D, et al. Green synthesis of iron oxide nanoparticles using Cymbopogon citratus extract and sodium carbonate salt: nanotoxicological considerations for potential environmental applications. Environ Nanotechnol Monit Manag. 2020;14:100377. [Google Scholar]
- 323.Ghareeb RY, et al. Utilization of Cladophora glomerata extract nanoparticles as eco-nematicide and enhancing the defense responses of tomato plants infected by Meloidogyne javanica. Sci Rep. 2020;10(1):1–15. doi: 10.1038/s41598-020-77005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Fabiyi OA. Sustainable management of Meloidogyne incognita infecting carrot (Daucus carota): green synthesis of silver nanoparticles with Cnidoscolus aconitifolius. Vegetos. 2021;34(2):277–285. [Google Scholar]
- 325.Kalaiselvi D, et al. Nematicidal activity of green synthesized silver nanoparticles using plant extracts against root-knot nematode Meloidogyne incognita. Int J Nemat. 2017;27(1):81–94. [Google Scholar]
- 326.Shekoohi SS, et al. Combined effect of β-aminobutyric acid and silver nanoparticles on eggplants, Solanum melongena, infected with Meloidogyne javanica. Nematology. 2021;50(11):1–16. [Google Scholar]
- 327.Kalaiselvi D, et al. Green synthesis of silver nanoparticles using latex extract of Euphorbia tirucalli: a novel approach for the management of root knot nematode, Meloidogyne incognita. Crop Prot. 2019;117:108–114. [Google Scholar]
- 328.Abbassy MA, et al. Nematicidal activity of silver nanoparticles of botanical products against root-knot nematode, Meloidogyne incognita. Arch Phytopathol Plant Prot. 2017;50(17–18):909–926. [Google Scholar]
- 329.Ghareeb RY, et al. Nematicidal activity of seaweed-synthesized silver nanoparticles and extracts against Meloidogyne incognita on tomato plants. Sci Rep. 2022;12(1):1–16. doi: 10.1038/s41598-022-06600-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.El-Ansary MSM, Hamouda RA, Elshamy MM. Using biosynthesized zinc oxide nanoparticles as a pesticide to alleviate the toxicity on banana infested with parasitic-nematode. Waste Biomass Valoriz., 2022: p. 1–11.
- 331.Akhter G, et al. Antibacterial and nematicidal properties of biosynthesized Cu nanoparticles using extract of holoparasitic plant. SN Appl Sci. 2020;2(7):1–6. [Google Scholar]
- 332.Abdellatif KF, Hamouda RA, El-Ansary MSM. Green nanoparticles engineering on root-knot nematode infecting eggplants and their effect on plant dna modification. Iran J Biotechnol. 2016;14(4):250–259. doi: 10.15171/ijb.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.El-Ashry RM, et al. Biological silicon nanoparticles maximize the efficiency of nematicides against biotic stress induced by Meloidogyne incognita in eggplant. Saudi J Biol Sci. 2022;29(2):920–932. doi: 10.1016/j.sjbs.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Barbosa ACMS, et al. Nematicidal activity of silver nanoparticles from the fungus Duddingtonia flagrans. Int J Nanomed. 2019;14:2341. doi: 10.2147/IJN.S193679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Yang Y, Wu Q, Wang D. Neuronal Gα subunits required for the control of response to polystyrene nanoparticles in the range of μg/L in C. elegans. Ecotoxicol Environ Saf. 2021;225:112732. doi: 10.1016/j.ecoenv.2021.112732. [DOI] [PubMed] [Google Scholar]
- 336.Chariou PL, Steinmetz NF. Delivery of pesticides to plant parasitic nematodes using tobacco mild green mosaic virus as a nanocarrier. ACS Nano. 2017;11(5):4719–4730. doi: 10.1021/acsnano.7b00823. [DOI] [PubMed] [Google Scholar]
- 337.Thakur RK, Dhirta B, Shirkot P. Studies on effect of gold nanoparticles on Meloidogyne incognita and tomato plants growth and development. BioRxiv, 2018: p. 428144.
- 338.Nicol J, et al. Genomics and molecular genetics of plant-nematode interactions. Springer; 2011. Current nematode threats to world agriculture; pp. 21–43. [Google Scholar]
- 339.Davids JS, et al. Biocontrol of bacteria associated with Pine Wilt Nematode, Bursaphelenchus xylophilus by using plant mediated gold nanoparticles. Int J Agric Biol. 2021;26:517–526. [Google Scholar]
- 340.El-Deen A, El-Deeb BA. Effectiveness of silver nanoparticles against root-knot nematode, Meloidogyne incognita infecting tomato under greenhouse conditions. J Agric Sci. 2018;10:148–156. [Google Scholar]
- 341.Oluwatoyin F et al. Preparation of bio-nematicidal nanoparticles of Eucalyptus officinalis for the control of CYST nematode (Heterodera sacchari). JAPS J Animal Plant Sci, 2020. 30(5).
- 342.Heflish AA, et al. Green biosynthesized silver nanoparticles using Acalypha wilkesiana extract control root-knot nematode. J King Saud Univ Sci. 2021;33(6):101516. [Google Scholar]
- 343.Mohamed YMA et al. Charcoal rot and root-knot nematode control on faba bean by photosynthesized colloidal silver nanoparticles using bioactive compounds from Moringa oleifera leaf extract. J Plant Prot Res, 2021. 61(4).
- 344.Patiño-Ruiz D, et al. Green synthesis of iron oxide nanoparticles using Cymbopogon citratus extract and sodium carbonate salt: nanotoxicological considerations for potential environmental applications. Environ Nanotechnol Monit Manag. 2020;14:100377. [Google Scholar]
- 345.Saini P, et al. Evidence of reactive oxygen species (ROS) mediated apoptosis in Setaria cervi induced by green silver nanoparticles from Acacia auriculiformis at a very low dose. Exp Parasitol. 2016;160:39–48. doi: 10.1016/j.exppara.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 346.Shekoohi SS, et al. Combined effect of β-aminobutyric acid and silver nanoparticles on eggplants, Solanum melongena, infected with Meloidogyne javanica. Nematology. 2021;23(9):1077–1092. [Google Scholar]
- 347.Elmer W, White JC. The future of nanotechnology in plant pathology. Ann Rev Phytopathol. 2018;56:111–133. doi: 10.1146/annurev-phyto-080417-050108. [DOI] [PubMed] [Google Scholar]
- 348.Abdellatif KF, Abdelfattah RH, El-Ansary MSM. Green nanoparticles engineering on root-knot nematode infecting eggplants and their effect on plant DNA modification. Iran J Biotechnol. 2016;14(4):250. doi: 10.15171/ijb.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Pathak SO et al. Nanotechnology in agriculture: benefits, risks and challenges.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed in this study are included in this article. The authors also declare that they have no conflict of interest.
No codes were used in the manuscript.