Abstract
The yeast mitochondrial DNA group II introns aI1 and aI2 are retroelements that insert site specifically into intronless alleles by a process called homing. Here, we used patterns of flanking marker coconversion in crosses with wild-type and mutant aI2 introns to distinguish three coexisting homing pathways: two that were reverse transcriptase (RT) dependent (retrohoming) and one that was RT independent. All three pathways are initiated by cleavage of the recipient DNA target site by the intron-encoded endonuclease, with the sense strand cleaved by partial or complete reverse splicing, and the antisense strand cleaved by the intron-encoded protein. The major retrohoming pathway in standard crosses leads to insertion of the intron with unidirectional coconversion of upstream exon sequences. This pattern of coconversion suggests that the major retrohoming pathway is initiated by target DNA-primed reverse transcription of the reverse-spliced intron RNA and completed by double-strand break repair (DSBR) recombination with the donor allele. The RT-independent pathway leads to insertion of the intron with bidirectional coconversion and presumably occurs by a conventional DSBR recombination mechanism initiated by cleavage of the recipient DNA target site by the intron-encoded endonuclease, as for group I intron homing. Finally, some mutant DNA target sites shift up to 43% of retrohoming to another pathway not previously detected for aI2 in which there is no coconversion of flanking exon sequences. This new pathway presumably involves synthesis of a full-length cDNA copy of the inserted intron RNA, with completion by a repair process independent of homologous recombination, as found for the Lactococcus lactis Ll.LtrB intron. Our results show that group II intron mobility can occur by multiple pathways, the ratios of which depend on the characteristics of both the intron and the DNA target site. This remarkable flexibility enables group II introns to use different recombination and repair enzymes in different host cells.
Two self-splicing group II introns of the COXI gene of yeast mitochondrial DNA (mtDNA), aI1 and aI2, are mobile elements (reviewed in reference 8). In crosses between yeast strains carrying or lacking one or both of those introns, the introns insert site specifically in the intronless allele by a process known as homing (4, 10, 14). In wild-type crosses, most homing events occur by retrohoming, which depends on activities of both the intron-encoded reverse transcriptase (RT) protein and the intron RNA. The protein first promotes splicing of the intron by facilitating formation of the catalytically active structure of the intron RNA, and it then remains associated with the excised intron RNA lariat to form a ribonucleoprotein (RNP) particle that has RT and site-specific DNA endonuclease activities (7, 22, 23). Retrohoming is initiated by this RNP endonuclease cleaving the DNA target site lacking the intron. The intron RNA catalyzes its own insertion into the sense strand by reverse splicing, and then the Zn domain of the intron-encoded protein cleaves the antisense strand at a site 10 nucleotides (nt) downstream in the 3′ exon. The cleaved site serves as the primer for first-strand cDNA synthesis in a reaction known as target DNA-primed reverse transcription (TPRT).
In vitro, the aI2 RNP particles cleave the sense strand of a standard substrate primarily by a partial reverse splicing reaction that joins the intron RNA lariat to the 3′ exon (22). The aI1 enzyme is similar in most respects, except that it is much more efficient for full reverse splicing (∼50%), which inserts the linear intron RNA between the two DNA exons (21). Subsequent studies showed that the proportion of full reverse splicing for aI2 is increased by certain DNA target site mutations (5). Group II intron homing and reverse splicing depend on three specific pairings between the intron RNA and the DNA target site (IBS1-EBS1, IBS2-EBS2, and δ-δ′), and consequently the target site specificity can be altered by compensating changes in the target site and the donor intron RNA (4, 5, 12). The protein component of the endonuclease recognizes the upstream portion of the DNA target (positions −21 to −13) and contributes to DNA unwinding enabling the intron RNA to base pair to the DNA target between positions −12 and +1 for the reverse splicing reaction. Additional interactions between the protein and the 3′ exon region of the DNA target site (+1 to +10) are required for antisense strand cleavage (5).
In vivo homing tolerates sequence changes at some sites in the target exons (4, 10, 14). Both strain-specific polymorphisms and site-directed changes have been used as flanking markers in crosses to reveal that intron insertion is associated with coconversion of flanking exon sequences. For aI2 homing, there is very efficient coconversion of markers for ca. 100 bp upstream but much less coconversion farther upstream and also less coconversion downstream. The initial explanation for this asymmetric coconversion was that the template for cDNA synthesis in retrohoming might be a pre-mRNA containing aI2 (14, 23). Using RNP particles from certain mutant strains, the aI2 RT was shown to utilize pre-mRNA as a template, initiating in the 3′ exon at roughly the same position as in TPRT reactions (7, 24).
Mutations of the YADD motif of the aI2 RT domain abolish RT activity but inhibit aI2 mobility only partially, indicating that an active RT-independent homing pathway also exists (14). This RT-independent pathway is inhibited by mutations in the Zn (endonuclease) domain, indicating that it requires this function of the intron-encoded protein. We subsequently showed that RT-deficient mutants still have the aI2-encoded endonuclease activity and that the level of residual homing activity correlates with the level of endonuclease activity (22). Thus, RT-independent group II intron homing appears to occur by a DNA-level pathway that is initiated by the intron-encoded endonuclease and completed by the very active double-strand break repair (DSBR) recombination system of yeast mitochondria (9). The excised intron RNA is essential for this pathway because it is crucial for DNA cleavage, but the intron RNA is not used as a template for cDNA synthesis.
Detailed studies of flanking marker coconversion associated with aI1 homing better defined the two main homing pathways in standard crosses (4). Most aI1 retrohoming occurs with efficient unidirectional coconversion upstream. Because aI1 retrohoming occurs without downstream coconversion, even in the part of that exon that is copied by the RT (E2+1 to E2+10), the cleaved recipient DNA target site with its inserted aI1 RNA must be the initial template for cDNA synthesis. In order to account for the efficient coconversion of upstream markers, we proposed that aI1 retrohoming is a hybrid mechanism initiated by TPRT leading to the synthesis of a partial or complete cDNA of the intron, which can then invade a donor allele to initiate DSBR recombination. Homing of aI1 was limited to RT-independent events by a mutation in the RT domain, and in that cross both upstream and downstream markers were coconverted, the pattern expected for conventional DSBR recombination events in this system. In wild-type aI1 crosses, about 80% of the recombinant progeny have unidirectional upstream coconversion, indicative of retrohoming, and 20% have bidirectional coconversion, indicative of RT-independent events.
Recent studies of homing of a group II intron, Ll.LtrB, from the gram-positive bacterium Lactococcus lactis, both in Escherichia coli and in L. lactis (3, 13) revealed similarities to the yeast system but also several striking differences. Efficient homing by Ll.LtrB is limited to retrohoming events that are independent of the host recA system. Also, Ll.LtrB is inserted without any coconversion of flanking markers, and it was suggested that its retrohoming depends on synthesis of full-length cDNAs.
The previously analyzed yeast aI2 crosses had useful 5′ flanking markers but lacked markers in the first 10 bp of the 3′ exon that are needed to identify the template for initial cDNA synthesis (see Fig. 1) (14). In this study we constructed DNA target sites with additional markers and used them to analyze aI2 homing. In addition, we took advantage of the earlier observation (5) that some mutations of the aI2 target site activate full reverse splicing by wild-type aI2 RNP particles. Thus, it was possible to compare crosses in which either partial or full reverse splicing might predominate. One mutant target site that increases both the extent of reverse splicing and the proportion of full reverse splicing in vitro strongly activates a retrohoming pathway, previously undetected for aI2, in which the intron is inserted without coconversion of flanking exons, resembling the main pathway used by the Lactococcus intron. Our results indicate that the choice of a retrohoming pathway for aI2 is influenced strongly by the characteristics of both the DNA target site and the intron, as well as by the DNA recombination and repair enzymes present in mitochondria.
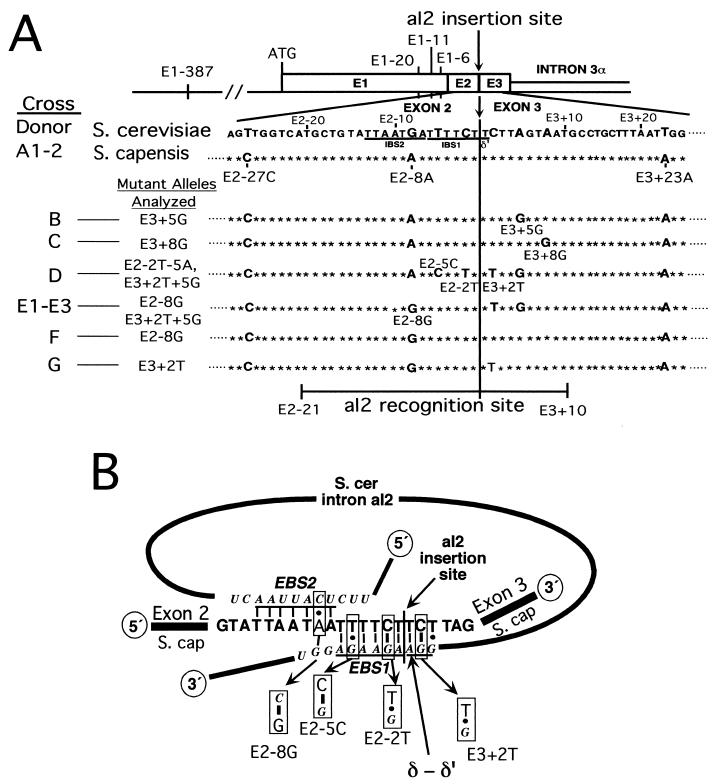
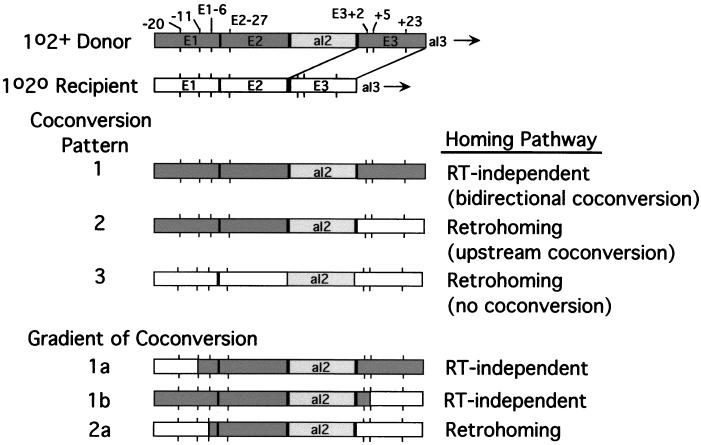
FIG. 1.
Sequences of recipient alleles and base-pairing interactions with the intron RNA. (A) Diagram of the aI2 target site and sequence differences among recipient strains. The diagram shows the region of the COXI gene containing the target site for aI2 homing. The exon sequence containing the aI2 target site of the S. cerevisiae donor strain is shown with sequence differences between it and the 1o2o Scap recipient allele containing COXI exons 1 to 3 derived from S. capensis indicated as larger boldface letters. Nucleotides are numbered according to their distance from the aI2 insertion site, which is between E2−1 and E3+1 (arrow and boldface vertical line). Other recipient alleles analyzed are derivatives of 1o2o Scap and are shown with nucleotide differences from the S. cerevisiae donor. Relevant sequence differences between donor and recipient COXI alleles outside of the region shown are indicated above the diagram. E1−387 is a small insertion containing a HpaII site that is present in the donor but not in the recipient allele. As indicated at the bottom, the recognition site for aI2 homing extends from E2−21 through E3+10 (5). (B) Effects of mutations on base pairing between the intron RNA and DNA target site. The diagram illustrates the known interactions between nucleotides of aI2 RNA and the sense strand of the aI2 target site. The conserved pairings IBS2-EBS2, IBS1-EBS1, and δ-δ′, spanning 7, 6, and 3 bp, respectively, are shown for the cross 1o2+ × 1o2o Scap. Certain mutations alter one or another of those pairings, as shown. E2−8G improves the EBS2-IBS2 pairing, changing a C-A pair to C-G. Mutations E2−2T and E2−5C exchange G-T and G-C base pairs in IBS1-EBS1. E3+2T destabilizes the extended δ-δ′ interaction of the original donor-recipient pair.
MATERIALS AND METHODS
Yeast strains and strain constructions.
The COXI alleles of donor and recipient strains are denoted by a convention (4, 14) in which a superscript “+” indicates the wild-type intron, a superscript “o” indicates that the intron is absent, and other superscripts denote specific alleles. Three previously described aI2 donor alleles, 1o2+ (previously called 1o2+t), 1o2YAAA, and 1o2P714T were used, each in the nuclear background of strain ID41-6/161 (MATa ade1 lys1) (see reference 14). Donor mtDNAs have six introns in the COXI gene (see Fig. 2A) and five introns in the COB gene (14).
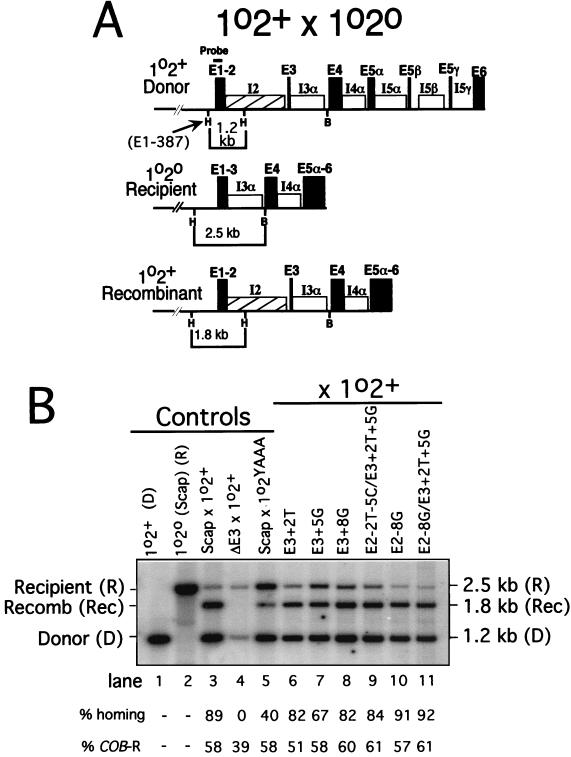
FIG. 2.
Analysis of aI2 homing in crosses. (A) Diagram of the cross between strains with the 1o2+ and 1o2o COXI alleles. The donor (1o2+) and recipient (1o2o) COXI alleles and the major 1o2+ recombinant allele resulting from aI2 homing are diagrammed. Relevant restriction sites and the sizes of restriction fragments that are diagnostic for each parent and the major recombinant COXI allele are indicated. Not all progeny with the 1.8-kb allele have the same 3′ COXI features shown in the diagram; some also have introns aI5α, β, and γ due to aI5α homing and associated coconversion of aI5β and 5γ (16). The probe used hybridizes with the indicated part of exon 1. H, HpaII site; B, BamHI site. (B) Outputs of COXI alleles in crosses. Crosses between the indicated donor and recipient strains noted were carried out as described in Materials and Methods, and the output of COXI alleles was measured using the HpaII-BamHI digest diagrammed in panel A. Lanes 1 and 2 show the parental donor and recipient alleles, respectively. Lanes 3 to 10 show results of crosses between the pairs of strains indicated above each lane. Each lane contains DNA from mixed progeny of tens of thousands of matings so that the observed ratio of alleles reflects the extent of homing. Recipient strain ΔE3 is deleted for exon 3 (E3+1 through E3+35) and is a negative control in which the wild-type donor strain has no residual homing activity. Donor strain 1o2YAAA (lane 5) has missense mutations inactivating the aI2 RT activity. Sequence differences among the recipient strains in lanes 6 to 10 are defined in Fig. 1A. This gel is a representative outcome of these crosses, each of which was carried out at least in duplicate with analysis of two or more blots of each DNA sample. The same DNA samples were also scored for the output of alleles of the COB gene (as in reference 14), and the percentage of progeny with the recipient COB allele (COB-R) is indicated below the lanes. The percent COB-R alleles was used to calculate the percent homing, which is defined as the percentage of recipient COXI alleles that acquired aI2 by homing (see Materials and Methods for details about this calculation). The values shown below the lanes are for this gel, and mean values for each cross are provided in Table 3, column 1. A minor band present in some of the samples is due to cross-hybridization with other sequences in the DNA sample (compare lane 2 with lane 11).
All 1o2o recipient strains have the nuclear background of strain GRF18 (MATα leu2 his3). The original COXI recipient allele from strain 1o2o Scap (also called GII-0 in reference 14) contains COXI exons 1, 2, and 3 derived from Saccharomyces capensis. Sequence differences between the 1o2o Scap allele and the Saccharomyces cerevisiae donor allele are summarized in Fig. 1 and at the top of Table 1. New derivatives of the recipient COXI allele (the six shown in Fig. 1A plus ΔE3) were constructed by site directed mutagenesis of plasmid pJVM161 (4). In the ΔE3 recipient allele positions E3+1 through E3+35 are deleted. Each new allele was placed in mitochondria by biolistic transformation and transferred to 1o2o Scap mtDNA by recombination as described elsewhere (4).
TABLE 1.
Analysis of crosses in which homing is associated with coconversion of flanking exon sequencesa
| Cross | Donor allele | Recipient allele | Line no. | No. recb | Exon 1 markers
|
Exon 2 markers
|
Exon 3 markers
|
Type event | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E1−387 [Hpa+, Hpa−] | E1−20 [T, A] | E1−11 [G, A] | E1−6 [T, A] | E2−27 [T, C] | E2−8 [G, A] | E2−5 [T, (C)] | E2−2 [C, (T)] | E3+2 [C, (T)] | E3+5 [A, (G)] | E3+8 [A, (G)] | E3+23 [T, A] | ||||||
| A1 | 1o2+, wild-type | 1o2o, Scap | 1 | 2 | D | D | D | D | D | D | * | * | * | * | * | R | RNA |
| 2 | 5 | R | D | D | D | D | D | * | * | * | * | * | R | RNA | |||
| 3 | 2 | D | D | D | D | D | D | * | * | * | * | * | D | DNA | |||
| 4 | 2 | R | D | D | D | D | D | * | * | * | * | * | D | DNA | |||
| A2 | 1o2YAAA, RT mutant | 1o2o, Scap | 5 | 2 | D | D | D | D | D | D | * | * | * | * | * | D | DNA |
| 6 | 7 | R | D | D | D | D | D | * | * | * | * | * | D | DNA | |||
| 7 | 1 | R | R | R | R | R | D | * | * | * | * | * | R | DNA | |||
| B | 1o2+, wild-type | 1o2o, E3+5G | 8 | 3 | D | D | D | D | D | D | * | * | * | R | * | R | RNA |
| 9 | 6 | R | D | D | D | D | D | * | * | * | R | * | R | RNA | |||
| 10 | 3 | D | D | D | D | D | D | * | * | * | D | * | D | DNA | |||
| 11 | 8 | R | D | D | D | D | D | * | * | * | D | * | D | DNA | |||
| C | 1o2+, wild-type | 1o2o, E3+8G | 12 | 6 | R | D | D | D | D | D | * | * | * | * | R | R | RNA |
| 13 | 2 | R | R | D | D | D | D | * | * | * | * | R | R | RNA | |||
| 14 | 2 | D | D | D | D | D | D | * | * | * | * | D | D | DNA | |||
| 15 | 7 | R | D | D | D | D | D | * | * | * | * | D | D | DNA | |||
| 16 | 1 | R | R | R | R | R | R | * | * | * | * | D | D | DNA | |||
| 17 | 1 | R | D | D | D | D | D | * | * | * | * | D | R | DNA | |||
| D | 1o2+, wild-type | 1o2o, E2−2T−5C E3+2T+5G | 18 | 4 | D | D | D | D | D | D | D | D | R | R | * | R | RNA |
| 19 | 14 | R | D | D | D | D | D | D | D | R | R | * | R | RNA | |||
| 20 | 1 | R | R | D | D | D | D | D | D | R | R | * | R | RNA | |||
| 21 | 2 | R | R | R | R | D | D | D | D | R | R | * | R | RNA | |||
| 22 | 10 | D | D | D | D | D | D | D | D | D | D | * | D | DNA | |||
| 23 | 7 | R | D | D | D | D | D | D | D | D | D | * | D | DNA | |||
| 24 | 1 | R | R | D | D | D | D | D | D | D | D | * | D | DNA | |||
| 25 | 1 | R | D | D | D | D | D | D | D | D | D | * | R | DNA | |||
Recombinant progeny were isolated from each cross as described in Materials and Methods. For each recombinant strain the exons flanking the aI2 insertion site were sequenced so that all of the flanking markers present in each cross were scored (see also Fig. 1). The nucleotide present at each site in donor and recipient strains is shown in brackets above each column as follows: [donor, recipient]. Alleles in parentheses are present only in some of the recipient strains, as indicated. Asterisks indicate that the donor and recipient strains in that cross have the same nucleotide. For each cross, results are organized according to the type of event that likely led to each coconversion pattern. Coconversion tracts are indicated in boldface type. In the type event column, “RNA” denotes the products of retrohoming, while “DNA” denotes the products of RT-independent homing. The rationale for these assignments is presented in the text. Figure 5 illustrates some of these patterns schematically. D, donor allele (intron insertion was accompanied by coconversion at that site); R, recipient allele (intron insertion was not accompanied by coconversion at that site).
No. rec, number of recombinant progeny analyzed for flanking markers.
Flanking markers in exons 2 and 3 are named according to their sequence and location on the sense strand relative to the aI2 insertion site (5). For example, E3+23 defines the nucleotide in exon 3 that is 23 bp downstream from the aI2 insertion site; E3+23A is the allele in all recipient strains used here, and E3+23T is the allele in all donor strains (see Tables 1 and 2). Because markers in exon 2 are upstream from the aI2 insertion site, they are designated E2−n (e.g., E2−8). Sites in exon 1 are named relative to the aI1 insertion site (e.g., E1−6) (see Fig. 1 and Tables 1 and 2). When a strain contains several markers in a given exon (e.g., E3+2T and E3+5G) we use the shorthand form E3+2T+5G.
TABLE 2.
Analysis of crosses using recipient strains that support retrohoming without coconversion of flanking exon markersa
| Cross | Donor allele | Recipient allele | Line no. | No. rec | Exon 1 markers
|
Exon 2 markers
|
Exon 3 markers
|
Type event | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E1–387 [Hpa+, Hpa−] | E1–20 [T, A] | E1−11 [G, A] | E1−6 [T, A] | E2−27 [T, C] | E2−8 [G, A] | E2−5 [T, (C)] | E2−2 [C, (T)] | E3+2 [C, (T)] | E3+5 [A, (G)] | E3+8 [A, (G)] | E3+23 [T, A] | ||||||
| E1 | 1o2+, wild-type | 1o2o, E2−8G E3+2T+5G | 1 | 1 | D | D | D | D | D | * | * | * | R | R | * | R | RNA |
| 2 | 10 | R | D | D | D | D | * | * | * | R | R | * | R | RNA | |||
| 3 | 1 | R | R | R | D | D | * | * | * | R | R | * | R | RNA | |||
| 4 | 9 | R | R | R | R | R | * | * | * | R | R | * | R | RNA | |||
| 5 | 5 | D | D | D | D | D | * | * | * | D | D | * | D | DNA | |||
| 6 | 19 | R | D | D | D | D | * | * | * | D | D | * | D | DNA | |||
| 7 | 1 | R | R | D | D | D | * | * | * | D | D | * | D | DNA | |||
| 8 | 1 | D | D | D | D | D | * | * | * | D | D | * | R | DNA | |||
| E2 | 1o2YAAA, RT mutant | 1o2o, E2−8G E3+2T+5G | 9 | 2 | D | D | D | D | D | * | * | * | D | D | * | D | DNA |
| 10 | 8 | R | D | D | D | D | * | * | * | D | D | * | D | DNA | |||
| 11 | 2 | R | D | D | D | D | * | * | * | D | D | * | R | DNA | |||
| E3 | 1o2P714T, endo mutant | 1o2o, E2−8G E3+2T+5G | 12 | 6 | R | D | D | D | D | * | * | * | R | R | * | R | RNA |
| 13 | 6 | R | R | R | R | R | * | * | * | R | R | * | R | RNA | |||
| 14 | 2 | R | D | D | D | D | * | * | * | D | D | * | D | DNA | |||
| F | 1o2+, wild-type | 1o2o, E2−8G | 15 | 1 | D | D | D | D | D | * | * | * | * | * | * | R | RNA |
| 16 | 11 | R | D | D | D | D | * | * | * | * | * | * | R | RNA | |||
| 17 | 2 | R | R | D | D | D | * | * | * | * | * | * | R | RNA | |||
| 18 | 2 | R | R | R | R | R | * | * | * | * | * | * | R | RNA | |||
| 19 | 5 | D | D | D | D | D | * | * | * | * | * | * | D | DNA | |||
| 20 | 7 | R | D | D | D | D | * | * | * | * | * | * | D | DNA | |||
| G | 1o2+, wild-type | 1o2o, E3+2T | 21 | 1 | D | D | D | * | D | D | * | * | R | * | * | R | RNA |
| 22 | 9 | R | D | D | * | D | D | * | * | R | * | * | R | RNA | |||
| 23 | 1 | R | R | R | * | R | D | * | * | R | * | * | R | RNA | |||
| 24 | 7 | R | R | R | * | R | R | * | * | R | * | * | R | RNA | |||
| 25 | 7 | D | D | D | * | D | D | * | * | D | * | * | D | DNA | |||
| 26 | 10 | R | D | D | * | D | D | * | * | D | * | * | D | DNA | |||
| 27 | 2 | R | R | R | * | R | R | * | * | D | * | * | D | DNA | |||
| 28 | 1 | R | D | D | * | D | D | * | * | D | * | * | R | DNA | |||
Data for crosses E1 to G were obtained and are presented as described in Table 1, footnotes a and b. The parental strains used in each cross are indicated in the second and third columns, and the flanking marker patterns observed are summarized.
Genetic methods.
Cell cultures and crosses were carried out at 30°C using standard media (14). All recombinants analyzed were obtained by a multistep screen of progeny colonies from crosses as described previously (4). Three parallel colony blots were used to identify progeny that resulted from homing (i.e., those that have the inserted aI2 together with the 3′ end of the recipient COXI gene plus the recipient COB allele). Southern hybridizations were used to confirm that each isolate contains a recombinant COXI gene. Each strain was also scored on a DNA blot for the polymorphic HpaII site 5′ of the COXI coding sequence (E1−387). RNA was extracted from a culture of each strain, and a cDNA was made by RT PCR using primers in COXI exons 1 and 4. The cDNA was then sequenced to score that strain for flanking markers (4).
Oligonucleotide probes used to screen colony blots were as follows: aI2, oligonucleotide aI2-S-4384, containing nt 4384 to 4412 of the COXI gene; aI5γ, oligonucleotide Snab-AS, complementary to nt 9024 to 9041 of the COXI gene; and bI1, oligonucleotide bI1-S-748, containing nt 748 to 769 of bI1. The primer for reverse transcription of COXI mRNA is M2-AS, which is complementary to COXI nt 6851 to 6869 (exon 4). The primer for amplification of the sense strand of COXI cDNA is JME1-S containing nt −6 to +15 of the COXI exon 1; that oligonucleotide was biotinylated at its 5′ end so that the sense strand could be purified using magnetic beads. The primer for sequencing the resulting amplified cDNA is oligonucleotide E4-AS, which is complementary to nt 6730 to 6751 of exon 4. All COXI and COB gene coordinates are as defined elsewhere (1, 18).
Analysis of homing in crosses.
Homing of aI2 in crosses was analyzed by the restriction fragment length polymorphism (RFLP) procedures used previously (14). Briefly, mixed diploid progeny from tens of thousands of matings were grown for about 20 generations following mating, and mtDNA was isolated from a 200-ml culture by lysis of spheroplasts followed by banding in bisbenzimide CsCl gradients. The purified mtDNA was desalted, digested with HpaII plus BamHI, and analyzed in a 1% agarose gel. Gels were blotted to Hybond-N nylon membranes (Amersham) and hybridized with a 5′ end-labeled oligonucleotide probe complementary to sequences in COXI exon 1 to determine the proportion of progeny with parental and nonparental COXI alleles. A blot of HincII-digested mtDNA was hybridized with a COB intron 4 probe to determine the ratio of parental mtDNAs. The resulting signals were quantitated with a PhosphorImager (Molecular Dynamics) using ImageQuant software. Each cross was carried out at least in duplicate, and each DNA sample was analyzed at least twice and some were analyzed many times.
The parameter “percent homing” for each cross measures the percentage of recipient COXI alleles that acquired aI2 by homing (14). For crosses with >20% homing, the value was calculated using the following formula: percent homing = [(COB-R − COXI-R)/COB-R] × 100, where COB-R is the fraction of progeny with the recipient COB allele and COXI-R is the fraction of progeny with the recipient COXI allele. In the crosses shown in lanes 4 and 5 of Fig. 4C, where homing is <20%, the measured fraction of progeny with the recombinant COXI allele (REC) was used in place of (COB-R − COXI-R) in the above formula. That approximation is more accurate than using the measured COXI-R in those cases where COXI-R is nearly the same value as COB-R but could underestimate the level of homing by as much as 20% due to the small proportion of homing products that score as donor-like due to coconversion of the far-upstream marker E1−387.
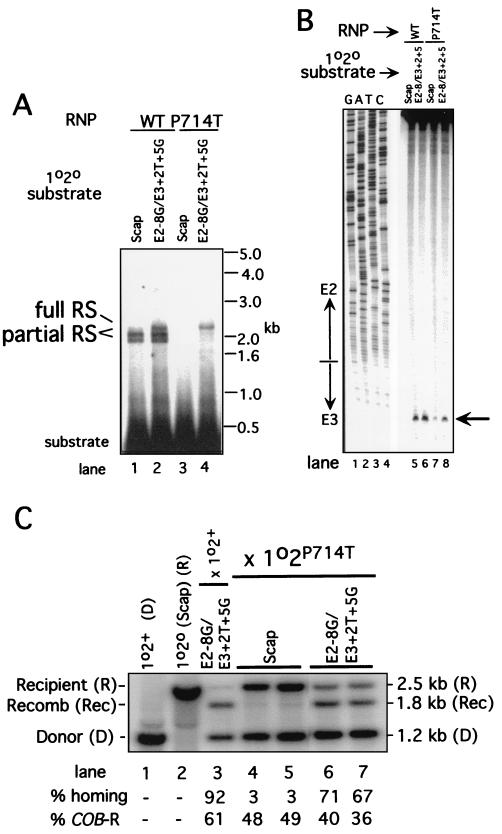
FIG. 4.
Analysis of effects of the Zn domain mutation P714T on reverse splicing, antisense strand cleavage, and homing. (A) Reverse splicing. Internally labeled DNA substrates were prepared from the recipient strains shown above each lane and incubated with RNP particles from strain 1o2+ (WT) and 1o2P714T (P714T), as described in Fig. 3A. Size standards are shown on the right, and the substrate and products of reverse splicing are identified on the left. The levels of products were quantitated, and the values are reported in the text. (B) Antisense strand cleavage. DNA substrates from the recipient alleles shown above the lanes were made by PCR with a 5′-end label on the antisense strand, and antisense strand cleavage reactions were carried out with RNP particles from the wild-type (WT) and P714T strains as in Fig. 3B. The amounts of cleavage product were quantitated, and the values are reported in the text. (C) Homing in 1o2P714T × 1o2o crosses. Crosses between the Zn domain mutant P714T donor strain and the indicated recipient strains were carried out and analyzed as in Fig. 2B. The RFLP alleles are as diagrammed in Fig. 2A. Lanes 1 and 2 contain DNA from the parental strains, and lane 3 contains DNA from the control cross between 1o2+ × 1o2o E2−8G E3+2T+5G (cross E1, see Table 2). Data for cross E3 are shown in lanes 6 and 7. The parameters “% COB-R” and “% homing” are as defined in Fig. 2 and in Materials and Methods.
Reverse splicing and DNA endonuclease assays.
DNA substrates for reverse splicing and DNA endonuclease assays were 240-bp double-stranded DNAs generated by PCR. First, a region extending from exon 1 to intron 3 of the COXI gene was amplified directly from recipient yeast cells by an initial PCR using primers yt1 (nt 1 to 23 of COXI exon 1) and aI3 (complementary to nt 5694 to 5722 of aI3). The initial PCR product was extracted with phenol-chloroform-isoamyl alcohol, ethanol precipitated, purified in a 2% agarose gel, and then used as template for a second PCR (25 cycles) with the same primers in the presence of [α-32P]dTTP to obtain internally labeled substrate or with 5′-end-labeled primer aI3 to obtain substrate labeled at the 5′ end of the antisense strand. The conditions for PCR and labeling were as described elsewhere (22, 23). The final PCR product was purified in a 2% agarose gel for reverse splicing assays or in a nondenaturing 6% polyacrylamide gel for DNA endonuclease assays.
For reverse splicing and DNA endonuclease assays, 32P-labeled DNA substrate (∼150,000 cpm) was incubated with mtRNP particles (0.025 optical density at 260 nm unit) for 20 min at 37°C, as described elsewhere (22, 23). Reverse splicing products were denatured with glyoxal and analyzed in a 1% agarose gel, and endonuclease products were analyzed in a denaturing 6% polyacrylamide gel. The gels were dried and quantitated with a PhosphorImager.
RESULTS
Flanking marker coconversion associated with aI2 homing.
Our initial analysis of coconversion of exon markers flanking group II intron insertion sites was in a cross between a donor strain carrying both aI1 and aI2 (1+2+) and a recipient strain lacking both introns (1o2o Scap) (14). The COXI exons of that recipient strain originated in an isolate of S. capensis and differ in sequence from those of the S. cerevisiae donor strain at seven positions shown in Fig. 1A (E1−387, E1−20, E1−11, E1−6, E2−27, E2−8, and E3+23). In nearly all of the intron homing events in that cross both aI1 and aI2 were inserted into the recipient allele. All markers were scored in five individual recombinants, and E1−387 and E2−27 were also analyzed as RFLPs in mtDNA isolated from a large sample of progeny cells. The homing events occurred with essentially quantitative coconversion of the proximal upstream markers, E1−20 through E2−8, but only ∼20% coconversion at the farthest upstream marker, E1−387, and ∼60% at the sole downstream marker, E3+23. Subsequent experiments showed that one of the point mutations in exon 1 of the recipient strain blocks the independent mobility of aI1 (4) so that the efficient homing of aI1 in those crosses resulted solely from coconversion in aI2-initiated events.
To obtain control data for this study, we analyzed coconversion of flanking markers in the cross 1o2+ × 1o2o Scap (Tables 1 and 3, cross A1), which uses a donor strain that contains aI2 but not aI1 together with the same recipient strain used in the original study. As reported previously (14), ∼89% of the recipient alleles acquire aI2 in this cross (Fig. 2B, lane 3). Most aI2 insertions occur without coconversion of the HpaII site present at E1−387 of the donor strain, forming a nonparental allele that gives a 1.8-kb HpaII fragment in a HpaII-BamHI digest (Fig. 2A). The remaining aI2 insertions occur with coconversion of that HpaII site present at E1−387 of the donor strain and are recovered as an excess of donor-like alleles (1.2-kb HpaII-BamHI fragment) (see reference 14). The negative control cross, in which aI2 homing was inactivated by deleting the 35-bp exon 3 of the recipient strain (Fig. 2B, lane 4), shows that the appearance of the recombinant allele and the excess of donor alleles observed in the other crosses depend on an active target site. Eleven recombinant progeny from cross A1 were scored for the seven flanking markers, and the results are summarized in Table 1. Coconversion of markers at E1−20, E1−11, E1−6, E2−27, and E2−8 was 100% (11 of 11). There was much less coconversion at the most distal markers E1−387 and E3+23, ∼36% (4 of 11) for each. This outcome is basically the same as was obtained in the 1+2+ × 1o2o cross, where both aI1 and aI2 are inserted together most of the time.
TABLE 3.
Summary of homing events analyzed in crosses A1 to Ga
| Cross | Donor allele | Recipient allele | % Homing ± SD | No. of progeny scored | % Retrohoming | % Retrohoming (no coconversion) |
|---|---|---|---|---|---|---|
| A1 | 1o2+, wt | 1o2o, Scap | 89 ± 4 | 11 | 64 | 0 |
| A2 | 1o2YAAA, no RT | 1o2o, Scap | 39 ± 11 | 10 | 0 | 0 |
| B | 1o2+, wt | 1o2o, E3+5G | 74 ± 14 | 20 | 45 | 0 |
| C | 1o2+, wt | 1o2o, E3+8G | 81 ± 6 | 19 | 42 | 0 |
| D | 1o2+, wt | 1o2o, E2−2T−5C E3+2T+5G | 84 ± 6 | 40 | 52 | 0 |
| E1 | 1o2+, wt | 1o2o, E2−8G E3+2T+5G | 93 ± 4 | 47 | 45 | 43 |
| E2 | 1o2YAAA, no RT | 1o2o, E2−8G E3+2T+5G | 52 ± 6 | 12 | 0 | 0 |
| E3 | 1o2P714T, no endo | 1o2o, E2−8G E3+2T+5G | 69 ± 3 | 14 | 86 | 50 |
| F | 1o2+, wt | 1o2o, E2−8G | 94 ± 3 | 28 | 57 | 13 |
| G | 1o2+, wt | 1o2o, E3+2T | 70 ± 9 | 38 | 47 | 39 |
The percent homing column sums up the data for all experiments in Tables 1 and 2. The number of progeny scored column summarizes the number of progeny scored for flanking marker patterns from each cross. The percent retrohoming column reports the percentage of events identified as resulting from retrohoming, as discussed in the text. The percent retrohoming (no coconversion) column gives the percentage of retrohoming with no coconversion. wt, wild type; endo, endonuclease.
Homing by an RT-deficient aI2 donor strain occurs with nearly quantitative coconversion in the downstream exon.
Next, coconversion was measured in the cross 1o2YAAA × 1o2o Scap, reported earlier to carry out some RT-independent homing of aI2 (14). This donor strain lacks RT activity due to a mutation of the YADD motif of the aI2 RT domain but still gives ∼40% conversion of 1o2o alleles to 1o2+ alleles (Fig. 2B, lane 5). Flanking markers were scored from 10 recombinants from that cross, and the results are summarized in Table 1 (cross A2). These data show that homing without RT activity occurs with coconversion of nearby sites in the upstream exons but much less coconversion at E1−387 farther upstream (2 of 10 events). It is striking that coconversion of the downstream marker, E3+23, is substantially more efficient (∼90%) than in the control cross A1 (∼36%). One of the recombinants from the RT-deficient cross (line 7) is donor-like at E2−8, the closest marker to the aI2 insertion site, but recipient-like at E2−27 and the other markers farther upstream. This recombinant likely resulted from repair of the cleaved target site with little gapping prior to strand invasion. This aI2 cross yielded essentially the same outcome as the analogous aI1 cross in which it was concluded that RT-independent homing events are initiated by the intron endonuclease and completed by DSBR recombination (4).
Both major coconversion patterns in the RT-deficient cross (Table 1, lines 5 and 6) were present as minor recombinant types in the wild-type aI2 cross (Table 1, lines 3 and 4). The simplest interpretation is that intron homing in the wild-type cross is a mixture of retrohoming and RT-independent events in a 64/36 ratio. According to this interpretation, the E3+23 marker (or other downstream markers; see below) is the key to determining whether a given recombinant is the product of one pathway or the other. Coconversion at E3+23 indicates an RT-independent, or DNA level, event whereas no coconversion there indicates an RT-dependent (retrohoming) event. Although it is formally possible that coconversion at E3+23 could occur in an RT-dependent event by using a resected antisense strand as a primer for reverse transcription of donor pre-mRNA, findings presented here and elsewhere indicate that the reverse-spliced intron RNA and not the pre-mRNA is by far the predominant template for cDNA synthesis (see the next section and Discussion). Furthermore, it is unlikely that an antisense strand resected beyond position E3+23 would remain in position to function as a primer for RT bound to the DNA target site.
Identification of the initial template for cDNA synthesis in aI2 retrohoming events.
To investigate whether the pre-mRNA or inserted intron RNA is the initial template for cDNA synthesis in aI2 retrohoming, it was necessary to modify the target site of the recipient strain so that coconversion could be evaluated in the key interval from E3+1 to E3+10. Guo et al. (5) analyzed point mutations of an S. cerevisiae aI2 target site in vitro and reported that mutations at E3+5 and E3+8 (Fig. 1A) do not greatly affect reverse splicing or antisense strand cleavage. Mutations at a few other sites inhibit antisense strand cleavage, and several greatly increase the proportion of full reverse splicing (see below).
The mutations E3+5G and E3+8G were tested for effects on reverse splicing and antisense strand cleavage activities. These DNA substrates were prepared with a longer 5′ exon than previously (5, 22) to better separate products of full and partial reverse splicing. The results of reverse splicing and DNA endonuclease assays using RNP particles from strain 1o2+ with several DNA substrates are shown in Fig. 3A. Both the E3+5G (lane 2) and E3+8G (lane 3) substrates are somewhat more efficient for reverse splicing than the control substrate (lane 1) (ratios of 1.7 ± 0.5 and 2.0 ± 0.5; see the legend to Fig. 3). In both cases the proportion of full reverse splicing remains quite low: 5 to 10% for both mutant substrates and the wild-type control (Scap). The E3+5G substrate is about as active as the control for antisense-strand cleavage, while the E3+8G substrate is inhibited about 60% (Fig. 3B, lanes 5 to 7).
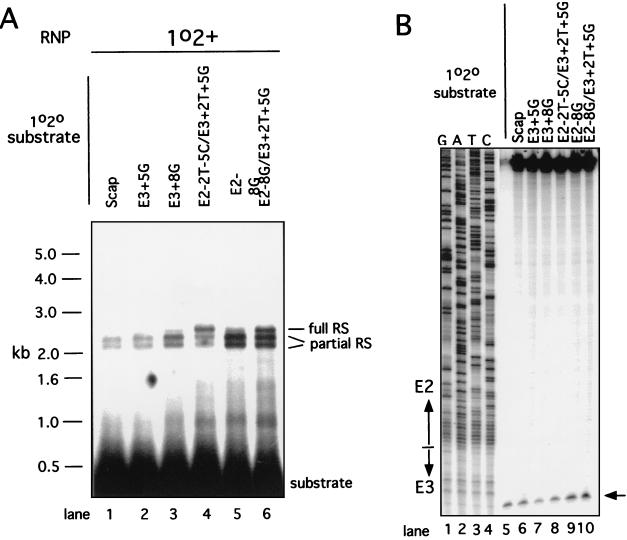
FIG. 3.
Assays of reverse splicing and antisense strand cleavage activities of RNP particles from strain 1o2+. (A) Reverse splicing activity with different DNA substrates. Internally labeled DNA substrates were prepared from the recipient strains shown above each lane and incubated with RNP particles from strain 1o2+, as described in Materials and Methods. Each lane contains the same number of counts of each substrate and the same amount of RNP particles. Size standards are shown to the left, and the substrate and the products of reverse splicing (RS) are identified to the right. The levels of reverse splicing products on this gel and four replicates were quantitated, and the mean values are reported in the text. (B) Antisense strand cleavage activity with different DNA substrates. DNA substrates from the indicated recipient alleles were made by PCR with a 5′-end label on the antisense strand, and antisense strand cleavage reactions were carried out with RNP particles from strain 1o2+. The antisense strand is cleaved between nucleotides E3+10 and E3+11. The amount of cleavage product was quantitated on this gel and one repeat, and the values are reported in the text.
The E3+5G and E3+8G mutations were transformed into mtDNA, and the resulting recipient strains were analyzed in crosses with the standard 1o2+ donor strain (Fig. 2B, lanes 7 and 8). Each of those alleles is somewhat less efficient for aI2 homing than is the parental allele (74 and 81% homing versus 89% in the control; see Table 3 for the average of all experiments). Individual progeny resulting from aI2 insertion were isolated from each cross and scored for flanking markers, and the resulting data are summarized in Table 1, crosses B (E3+5G) and C (E3+8G).
In both crosses, about half of the homing events show coconversion at upstream markers but have both downstream markers from the recipient strain (E3+5G or E3+8G plus E3+23A) (Table 1, cross B, lines 8 and 9, and cross C, lines 12 and 13). As explained above, this pattern is expected to result from retrohoming in which initial cDNA synthesis uses the 5′ overhang of the recipient exon 3 DNA and not pre-mRNA as template. Most of the remaining recombinants show coconversion of both upstream and downstream markers typical of RT-independent homing (compare Table 1, lines 10 and 11 and lines 14 to 16 with lines 5 to 7). Among these, the recombinants in lines 10 and 11 (cross B) and lines 14 and 15 (cross C) differ only at the distal upstream marker, E1−387, with only a minority being coconverted there.
Cross C yielded four recombinants with coconversion patterns different from those observed in the previous crosses. The recombinants of line 13 show no coconversion downstream but are coconverted upstream only through the E1−11 marker; they presumably result from the major retrohoming pathway, with resolution upstream between E1−11 and E1−20. The recombinant of line 16 is coconverted at both sites in exon 3 but is not coconverted at any upstream site. This pattern likely results from an RT-independent homing event in which there was resolution between E2−1 and E2−8. Finally, the recombinant in line 17 retains the recipient marker at E3+23 but has donor markers at E3+8 and at upstream sites through E1−20. This is the pattern predicted to result from retrohoming events in which the pre-mRNA was the template for cDNA synthesis; however, it could also have resulted from an RT-independent event in which 3′ resolution occurred between E3+8 and E3+23. This same pattern is detected in a single event in three other crosses using this same donor strain analyzed below (Table 1, cross D, line 25; Table 2, cross E1, line 8; and Table 2, cross G, line 28). Even if all four such events resulted from retrohoming using pre-mRNA as a template, that mechanism would account for no more than a minor fraction of aI2 retrohoming.
Retrohoming of aI2 with efficient full reverse splicing.
Our studies show that the standard alleles of aI1 and aI2 are comparably efficient for homing (4, 14). However, unlike aI1, which supports efficient full reverse splicing (4, 20, 21), >90% of the reaction of aI2 RNP particles with the 1o2o Scap DNA substrate is partial reverse splicing (22). Detailed analysis of the aI2 target site (5) showed that RNP particles from the wild-type aI2 donor strain can carry out efficient full reverse splicing, but only when a suitable variant DNA target site is used. Specifically, variants of the S. cerevisiae target site with point mutations at E3+1 and E3+2 activated a high proportion of full reverse splicing.
Crosses A1, B, and C of this study employed recipient alleles that support little (<10%) full reverse splicing, and the data of Table 1 indicate that each cross has about 50% retrohoming events (see Table 3). Published aI1 homing experiments, in which the proportion of full reverse splicing is at least 50%, have a higher proportion of retrohoming events (70 and 90% in two different crosses) (4). Thus, we wondered whether the nature of the reverse splicing reaction influences the ratio of retrohoming and DNA level events. In this and the next sections we analyzed aI2 homing in crosses using recipient strains with target sites that support elevated levels of full reverse splicing and also have flanking markers that permit coconversion patterns to be scored.
The recipient strain E2−2T−5C/E3+2T+5G was constructed some years ago for another purpose (15). It has four silent mutations flanking the aI2 insertion site, two in exon 2 (E2−2T and E2−5C) that make compensating changes in the IBS1-EBS1 pairing (see Fig. 1) and two in exon 3 (E3+2T and E3+5G). As shown in Fig. 3A, lane 4, this mutant target site has slightly higher overall reverse splicing activity than the Scap target site (1.4 ± 0.7-fold in five experiments) and also supports an elevated proportion of full reverse splicing (28 to 49%). The level of antisense strand cleavage is about the same as with the Scap target site (Fig. 3B, lanes 8 and 5, respectively). Significantly, this was the first recipient allele available for aI2 that supports a high proportion of full reverse splicing similar to that obtained for aI1 (4, 21).
The E2−2T−5C/E3+2T+5G recipient strain supports about 84% aI2 homing in crosses with the standard donor strain (Fig. 2, lane 9, and Table 3, column 1). Coconversion patterns were analyzed for 40 recombinant progeny from this cross, and the findings are summarized in Table 1, cross D (lines 18 to 25). Nearly all of these progeny have one of the coconversion patterns already discussed as resulting from retrohoming (lines 18 to 21) or RT-independent homing (lines 22 to 24). The recombinant in line 25 resembles the one in line 17, discussed above, in being coconverted at upstream markers and proximal downstream markers (E3+2 and E3+5) but not at E3+23. Overall, the percentage of retrohoming (∼52%) is about the same as in the previous crosses (Table 3, column 3). This sample of recombinant progeny is large enough to provide clear evidence for a gradient of upstream coconversion in both retrohoming and RT-independent homing events.
These data show that products of aI2 homing with a DNA target site that supports 28 to 49% full reverse splicing in vitro are surprisingly similar to those in crosses A1, B, and C with target sites that support <10% full reverse splicing. These crosses have similar overall levels of homing, and about half of the homing events have coconversion patterns indicative of retrohoming, while the remainder have coconversion patterns like the progeny of the RT-independent cross (cross A2) (Table 3, columns 1 and 3). Thus, a DNA target site that increases the proportion of full reverse splicing in vitro does not, by itself, alter the ratio of retrohoming to RT-independent homing or the manner in which retrohoming events are completed.
A new recipient strain activates retrohoming without coconversion.
We next constructed another recipient allele (E2−8G E3+2T+5G) that also elevates the extent of full reverse splicing but gives a different outcome in crosses. All target sites used in crosses A through D have the E2−8A allele, derived from the 1o2o Scap COXI gene. That allele introduces an A-C mismatch into the 7-bp IBS2-EBS2 pairing with the donor intron RNA (Fig. 1B). A derivative of the 1o2o Scap target site was made with E2−8G to restore a G-C pair, and it was evaluated as an endonuclease substrate. As shown in Fig. 3A, lane 5, the E2−8G mutation increased the extent of reverse splicing relative to the control substrate ca. fourfold (3.9 ± 0.2 compared with lane 1), but the proportion of full reverse splicing events remained low (12% ± 2%). This mutation also elevated the level of antisense-strand cleavage ∼30% (Fig. 3B, lane 9). Next, the E2−8G mutation was combined with the E3+2T mutation, which stimulates full reverse splicing (5), and the E3+5G allele (analyzed above), and the resulting triple mutant was analyzed in vitro. The E2−8G E3+2T+5G target site supports ∼5 times more reverse splicing than does the Scap substrate and gives a higher proportion of full reverse splicing (24% ± 4%), though not as much as the allele used in cross D (compare lanes 4 and 6 of Fig. 3A). The triple-mutant substrate also supports 1.5- to 2.1-fold more antisense strand cleavage than does the Scap substrate (Fig. 3B, lane 10).
These new alleles were transformed into mtDNA, and their activity in aI2 homing was analyzed in crosses. As shown in Fig. 2B, E2−8G (lane 10) and E2−8G E3+2T+5G (lane 11) are both very efficient homing sites (∼90% homing; Table 3, column 1). Recombinant progeny were first analyzed from the cross with the E2−8G E3+2T+5G recipient, and the resulting coconversion patterns are summarized in Table 2, cross E1 (lines 1 to 8). Based on the fraction of progeny without coconversion in exon 3, this cross has about 45% retrohoming events (lines 1 to 4), and the rest have patterns expected for RT-independent homing (lines 5 to 8).
Of the 21 progeny attributed to retrohoming, 12 have patterns similar to those in the other crosses, with coconversion upstream but not downstream of the inserted intron (lines 1 to 3). The remaining nine progeny summarized in Table 2, line 4, have a new pattern of flanking markers not observed in any of the previous aI2 crosses: the intron was inserted with no coconversion upstream or downstream. That pattern was observed at a much lower frequency in one aI1 cross (4), where it was interpreted as resulting from retrohoming with resolution before the first 5′ marker. This pattern can also be explained by postulating a retrohoming pathway analogous to that for the Lactococcus Ll.LtrB intron, in which a full-length cDNA is made and events are completed without switching to DSBR recombination. Evidently, some feature of this mutant target site activates this retrohoming pathway, so that it now accounts for a substantial fraction (∼43%) of the retrohoming events.
Lack of coconversion with the E2−8G E3+2T+5G substrate is not due to modification of the DSBR pathway.
To exclude the possibility that the E2−8G E3+2T+5G target site simply limits the extent of coconversion via the DSBR recombination pathway, we carried out an additional cross using this recipient allele with a donor strain that lacks RT activity (cross E2). As summarized in Table 2, lines 9 to 11, all of the homing events in that cross occur with efficient bidirectional coconversion, as in cross A2 involving the RT-deficient donor strain with the standard 1o2o Scap target site. The majority of homing events show coconversion at all markers, except for the most distal upstream marker (E1−387). Two events are coconverted at all markers (line 9), and two are coconverted at all markers except E1−387 and E3+23 (line 11). These findings indicate that the retrohoming without coconversion observed in cross E1 does not reflect modification of the DSBR pathway by the E2−8G E3+2T+5G target site.
Both E2−8G and E3+2T contribute to aI2 retrohoming without coconversion.
To determine which changes in the E2−8G E3+2T+5G target site induce this alternative mode of retrohoming, we analyzed coconversion patterns in crosses using recipient strains, each carrying one of the mutations present in the E2−8G E3+2T+5G strain. Cross B, presented above, using a recipient strain with E3+5G, had no progeny with this new coconversion pattern (Table 1, lines 8 to 11). The E2−8G recipient strain was analyzed in cross F (Table 2, lines 15 to 20), and the E3+2T recipient strain was analyzed in cross G (Table 2, lines 21 to 28). E3+2T and E3+5G support somewhat less homing than does E2−8G (Table 3, column 1), though all three crosses have similar proportions of retrohoming (Table 3, column 3). The E2−8G allele supports ∼13% retrohoming without coconversion (Table 2, line 18), while E3+2T supports ∼39% (line 24). We conclude that both E2−8G, which increases the overall level of reverse splicing, and E3+2T, which increases the proportion of full reverse splicing, contribute to the activation of retrohoming without coconversion. The recipient strain used in the previous cross D (E2−2T−5C E3+2T+5G) also contained E3+2T and had an elevated level of full reverse splicing; however, it did not give detectable retrohoming without coconversion, presumably due to interference by one or both of the markers, E2−2T and E2−5C, that are unique to that cross.
An endonuclease mutation favors RT-dependent pathways over RT-independent ones.
The mutation P714T in the Zn domain of the aI2 protein strongly inhibits both reverse splicing and antisense-strand cleavage activity with the 1o2o Scap DNA substrate and eliminates detectable homing with that recipient strain (14, 22, 23). Significantly, P714T is the only Zn domain mutation in our collection that retains high RT activity, which is activated even in the absence of the normal TPRT primer (7, 24). We analyzed reverse splicing and antisense strand cleavage activity of RNP particles from strain 1o2P714T using the 1o2o Scap and E2−8G E3+2T+5G DNA substrates. As shown in Fig. 4A, RNP particles from the mutant strain have a very low level of reverse splicing activity with the 1o2o Scap substrate and a higher level of activity with the E2−8G E3+2T+5G substrate (∼10% of the control reaction using wild-type RNP particles; compare lanes 2 and 4 of Fig. 4A). It is striking that nearly all of the reaction with the latter substrate is full reverse splicing. The P714T RNP particles have ∼8% of the control level of antisense strand cleavage activity with the 1o2o Scap substrate and a somewhat higher level (20%) with the E2−8G E3+2T+5G substrate (Fig. 4B, lanes 7 and 8).
As shown in Fig. 4C, the P714T donor strain has a very low level of homing with the 1o2o Scap recipient (∼3%) and a much higher level of homing in the cross with the E2−8G E3+2T+5G recipient (∼70%). Crosses with the other recipient strains demonstrate that the higher level of homing shown here is mainly due to the mutation at E2−8G with a small contribution from E3+2T (not shown). Flanking marker coconversion patterns were analyzed in 14 progeny from the cross 1o2P714T × 1o2o E2−8G E3+2T+5G and the results are summarized as cross E3 in Table 2. Strikingly, this cross has the highest proportion of retrohoming events in this study (∼86% versus ∼50% in the other crosses), divided equally between events with and without upstream coconversion (Table 2, lines 12 and 13). Only 2 of the 14 events scored have the coconversion pattern predicted for RT-independent homing (line 14). This cross shows that a combination of donor and recipient alleles can make retrohoming without coconversion more prominent by reducing the fraction of homing intermediates channeled through the RT-independent pathway. This outcome may result from the combined effects of the greatly increased proportion of full reverse splicing, the decreased rate of antisense strand cleavage, and the activation of RT activity.
DISCUSSION
This study provides genetic and molecular data that define three major pathways of site-specific homing of the group II intron aI2 of yeast mtDNA (Fig. 5 and 6). All three pathways are initiated by cleavage of the recipient DNA target by the intron endonuclease, with the sense strand cleaved by reverse splicing and the antisense strand cleaved by the endonuclease (Zn) domain of the intron-encoded protein (Fig. 6). In the retrohoming pathways, the fully or partially reverse spliced intron is used as the template for cDNA synthesis, whereas in the RT-independent pathway the double-strand break at the target site leads to insertion of an intron copy by DSBR recombination as in group I intron homing. Although all of the strains used here are wild type for mitochondrial recombination, some retrohoming events occur without coconversion of flanking exon sequences and are presumably completed by a repair mechanism independent of the homologous recombination system (Fig. 6). We find that the choice and relative levels of the different homing pathways are strongly influenced by specific nucleotides of the target site and by mutations of the intron-encoded protein.
FIG. 5.
Coconversion patterns associated with aI2 homing. The first two lines are diagrams of the aI2 donor and recipient alleles used in the crosses analyzed here. These crosses yield three major coconversion patterns in various proportions as discussed in the text. Pattern 1, in which there is efficient coconversion of markers in both 5′ and 3′ exons, results from the main pathway for RT-independent homing in which cleavage of the DNA target site is followed by DSBR (see also Fig. 6d). Pattern 2, in which there is efficient coconversion of markers in the 5′ exon but no coconversion in the 3′ exon, results from the major retrohoming pathway (Fig. 6b and c). Pattern 3, in which there is no coconversion associated with aI2 insertion, results from retrohoming events that are activated by the E2−8G and E3+2T mutations of the recipient strain (Fig. 6a). Also shown are three minor coconversion patterns that reveal a gradient of coconversion. Patterns 1a and 1b result from RT-independent homing events, while pattern 2a results from retrohoming events.
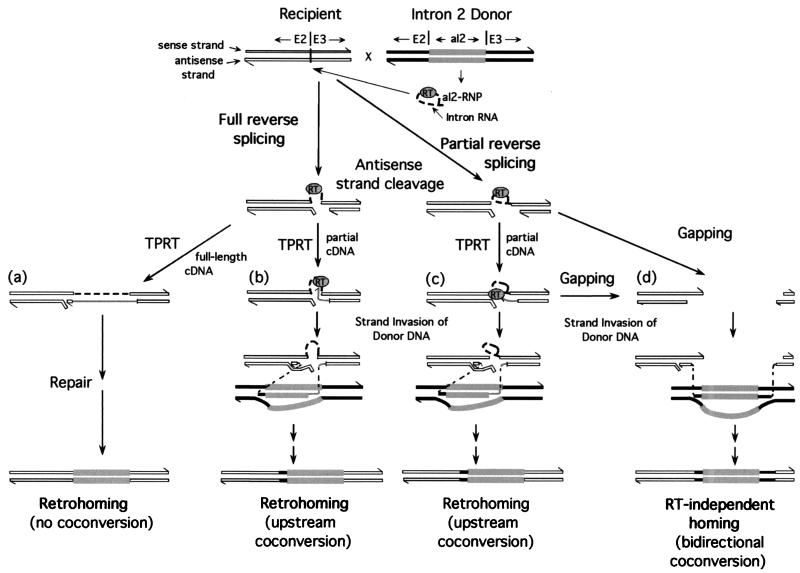
FIG. 6.
Different pathways used for aI2 homing. The diagram summarizes features of the aI2 homing pathways analyzed in this study. The donor and recipient alleles are shown in the first line. Sense and antisense strands of the donor and recipient DNAs are identified, and the strand polarity is indicated by half arrowheads at the 3′ ends. White strands indicate recipient DNA exons, black strands indicate donor DNA exons, and thick gray strands indicate intron DNA. The donor strain synthesizes aI2 RNP particles containing excised intron RNA (thick dashed lines) and the intron-encoded RT protein. Homing is initiated by partial or full reverse splicing into the aI2 DNA target site. The next line shows the products of partial and full reverse splicing, which are intermediates in all of these homing pathways. Each intermediate can potentially be reverse transcribed by the intron-encoded RT to yield partial or full-length cDNAs (gray lines). In pathway a, a full-length cDNA synthesized from fully reverse spliced intron RNA leads to retrohoming via a repair process that does not appear to involve recombination. This pathway results in insertion of the intron into the recipient DNA with no coconversion of flanking exon sequences, as indicated at the bottom of the figure. In pathways b and c, incomplete cDNAs synthesized from fully or partially reverse spliced intron RNA complete retrohoming by using the mitochondrial DSBR recombination system are shown. The figure illustrates strand invasion of the donor DNA by the cDNA and completion of the intron DNA synthesis using the donor DNA as template with copying beginning in the intron and extending into the 5′ exon, followed by strand exchange back to the recipient DNA. Subsequent steps, including removal of the intron RNA and synthesis of the opposite DNA strand, are not shown. These events result in insertion of the intron with unidirectional coconversion of upstream exon sequences. In the RT-independent pathway d, a cleaved DNA target site containing partially reverse-spliced intron RNA leads to homing via gapping and strand invasion of the donor mtDNA. The same outcome could result from a cleaved DNA target site that initially contains a fully reverse spliced intron RNA (not shown, for simplicity). The RT-independent pathway results in insertion of the intron with coconversion of both upstream and downstream exon sequences, as in group I intron homing.
Products of RT-independent homing have a distinctive coconversion pattern.
Crosses A2 and E2, in which the donor strain lacks RT activity, define a surprisingly efficient RT-independent homing pathway for aI2. This pathway is initiated by cleavage of the intronless recipient allele by the aI2 endonuclease, which remains active in the RT-deficient mutant (22), and leads to aI2 insertion with bidirectional coconversion of flanking markers (Fig. 5, pattern 1), as expected for a conventional DSBR recombination mechanism (see also reference 3). The coconversion of markers downstream from the antisense strand cleavage site presumably results from gapping of the cleaved target site before strand invasion of the donor mtDNA (Fig. 6d).
In crosses with wild-type donor strains, retrohoming and RT-independent homing occur concurrently. Importantly, the activity of the RT-independent pathway for aI2 (36 to 50% homing in crosses with the RT-deficient donor strains) is sufficient to account for all of the events involving bidirectional coconversion in crosses with wild-type donor strains (36 to 58%) (Table 3). By contrast, crosses with RT-deficient mutants of aI1 gave about 46% homing, whereas only about 20% of the progeny of wild-type donor crosses showed bidirectional coconversion (4). This situation likely reflects that following antisense strand cleavage there is competition among factors that favor the retrohoming or RT-independent pathways (Fig. 6). For example, retrohoming requires the retention of the inserted intron RNA in the cleaved target site long enough for initial cDNA synthesis to occur, while RT-independent homing may be favored by removal of the RNA to facilitate end resection and strand invasion. Also, resection of the 3′ end of the antisense strand by a 3′ exonuclease (Fig. 6) may divert intermediates away from retrohoming by placing the primer for cDNA synthesis in a position where it is less accessible to the RT. The preferential use of the retrohoming pathway in wild-type donor aI1 crosses may reflect that the coupling between antisense strand cleavage and that initiation of reverse transcription is tighter for aI1 than for aI2.
The major retrohoming pathway for aI2 uses reverse spliced intron RNA as a template for cDNA synthesis.
Initially, we suggested that the pre-mRNA may be the initial template for cDNA synthesis in aI2 retrohoming (14). The subsequent discovery that intron RNA inserts into the DNA target site by reverse splicing did not exclude that hypothesis but supported a scenario in which the template for TPRT is the reverse spliced intron RNA (21, 22). As shown previously for aI1 (4), these models can be distinguished by analyzing coconversion patterns in the 3′ exon, particularly in the region +1 to +10, which could be copied from either the donor pre-mRNA or the 5′ overhang of the sense strand in the cleaved recipient DNA. A key feature of the present study was the development of new markers between E3+1 and E3+10 so that these two hypotheses could be distinguished for aI2.
Among 81 progeny attributed to retrohoming in six suitably marked crosses using the wild-type (1o2+) donor strain (crosses B, C, D, E1, and G), 77 retain the recipient markers at all positions in the 3′ exon, including the key region between E3+1 and E3+10. This finding shows that by far the major retrohoming pathway for aI2 involves the RT copying the 5′ overhang of the sense strand of the cleaved DNA target site (Fig. 6a to c). This conclusion is also supported by in vitro evidence that the reverse spliced intron is the predominant template for cDNA synthesis in TPRT reactions of both aI1 and aI2 (20, 24).
Only 5% of the inferred retrohoming products (4 of 81) have the coconversion pattern expected to result from use of pre-mRNA as the initial template (donor markers between E3+1 and E3+10, recipient marker at E3+23; Table 1, lines 17 and 25, and Table 2, lines 8 and 28). This same pattern was obtained at an even higher frequency in crosses using the RT-deficient donor strain (10% of events in cross A2; 17% of events in cross E2) and was also evident in a cross with an RT-deficient aI1 donor strain (21%) (4). Clearly, this pattern can also result from a subset of RT-independent events in which downstream gapping does not extend as far as E3+23.
Of the 77 retrohoming events that have no downstream coconversion, 79% are coconverted for some or all of the upstream markers. Thus, the major retrohoming pathway inserts aI2 with no coconversion downstream but very efficient coconversion upstream. As proposed first for aI1, the efficient coconversion of upstream markers is explained by the hypothesis that most aI2 retrohoming events are completed by DSBR recombination (see Fig. 6b and c). In that case, retrohoming does not require synthesis of a full-length cDNA of the inserted intron. Pausing of cDNA synthesis at sites within the intron may be followed by the incomplete cDNA initiating strand invasion of a donor mtDNA (Fig. 6b and c). Target sites in which the intron is inserted by partial reverse splicing may be inefficient templates for cDNAs longer than 17 nt, due to the strong stop at the lariat branch point. Pausing there may sometimes be followed by template switching, either to an excised intron RNA or to an aI2-containing pre-mRNA, leading to synthesis of longer cDNAs that initiate gap repair (not shown in Fig. 6). A full-length cDNA copy of the reverse spliced intron could also initiate gap repair. If there is any read-through of lariat branchpoints during cDNA synthesis, it appears to be highly accurate because nine products of retrohoming from crosses B and C, where there is little full reverse splicing in vitro, were found to have the wild-type intron sequence at and around the branch nucleotide (L. Liu and P. S. Perlman, unpublished).
A coconversion gradient supports a role for recombination in group II intron homing.
In the present work, we analyzed enough progeny to obtain clear evidence for a gradient of coconversion upstream of the intron insertion site in both retrohoming and RT-independent events (see Fig. 5, patterns 1a, 1b, and 2a). Summing up all of the crosses using the wild-type donor strain, 23% of the progeny (47 of 203) were coconverted at the most upstream marker, E1−387 (see Fig. 1), and 124 of the remaining 156 were coconverted at the next closest site, E1−20. Assuming that this pattern arises during completion of events by DSBR, then ∼62% of events resolve between E1−20 and E1−387, while ∼23% resolve upstream of E1−387. Fourteen progeny (∼7%) result from resolution in the 56 bp between the aI2 insertion site and E1−20. This gradient of coconversion supports the inference that recombination is a key factor in generating the upstream coconversion in both the major retrohoming pathway and the RT-independent pathway.
Of the 127 RT-independent homing events scored in all of these crosses, 124 (98%) are coconverted at the nearest upstream marker and 121 (95%) are coconverted at the most distant exon 3 marker, E3+23. Evidently when downstream gapping of cleaved target sites occurs, it progresses beyond E3+23 most of the time. These data show clearly that coconversion is bidirectional in the RT-independent homing pathway. The two progeny of cross E2 that are coconverted at E3 sites proximal to the intron but have the recipient allele at E3+23 (Table 2, line 11) indicate a gradient of coconversion downstream from the intron insertion site.
Insertion of mobile group I introns into target sites in yeast mtDNA occurs by DSBR and is associated with efficient coconversion of flanking markers (8). In yeast mitochondria, coconversion has been most thoroughly characterized for the ω intron of the large subunit rRNA gene (6). A point mutation 54 nt downstream from the ω intron is coconverted ∼99% of the time compared to ∼95% for the E3+23 site in aI2 RT-independent events. A point mutation 736 bp upstream from the ω intron is coconverted about 40% of the time compared to 23% coconversion of the E1−387 site, located 423 bp 5′ of the aI2 insertion site. These data indicate that the coconversion gradient upstream is somewhat steeper for aI2 than for ω. It is not clear, however, whether this difference is caused by the different mobile introns or flanking DNA sequences.
Target site mutations activate a retrohoming pathway in which there is no coconversion.
Cross E1, involving the recipient strain E2−8G E3+2T+5G, gave the unexpected finding that target site mutations can activate a retrohoming pathway, previously undetected for this intron, in which insertion of the intron occurs without any coconversion of flanking exon markers (Fig. 5, pattern 3, and Fig. 6a). Analysis of crosses B, F, and G, each using a recipient strain carrying just one of the above mutations, showed that this pathway is activated by a combination of the E2−8G and E3+2T mutations, which together lead to an increased level of full reverse splicing. The E2−8G mutation fixes a non-Watson-Crick pairing in IBS2-EBS2 (Fig. 1B) and results in a fourfold increase in the level of reverse splicing but no increase in the proportion of full reverse splicing (Fig. 3A). By itself, this mutation only weakly supports retrohoming without coconversion (13%). The other mutation, E3+2T, has little effect on the level of reverse spliced products but increases the proportion of full reverse splicing (see reference 5). This mutation, by itself, activates the new retrohoming pathway to nearly the same extent as does the triple mutant target site used in cross E1 (39 versus 43%). The E3+2T mutation may weaken RNA or protein interactions with the 3′ exon that are required for antisense strand cleavage and thus improve the extent of the second step of reverse splicing (5). As noted previously, the wild-type E3+2C allele potentially extends the δ-δ′ interaction between the intron and the 3′ exon boundary (see Fig. 1B), and that extra base pair is disrupted by the E3+2T mutation.
An even more dramatic change in the ratio of homing pathways was observed in a cross between the donor strain with the P714T mutation of the Zn domain and recipient strain E2−8G E3+2T+5G (cross E3). While the levels of reverse splicing and antisense strand cleavage activities were reduced from wild-type control levels by the P714T mutation about 10-fold and 5-fold, respectively, and the level of homing with this target site was reduced to about 70%, the fraction of retrohoming was increased from ∼50 to 86%. In this cross, 43% of all homing events occurred without coconversion. Our finding that RNP particles from that mutant strain yield over 90% full reverse splicing in vitro (Fig. 4A, lane 4) strongly supports our inference that full reverse splicing favors retrohoming by that pathway. Other experiments show that the no-coconversion pathway also accounts for the previously described rare recombinants in the cross 1+2+ × 1o2o, where aI2 inserts independently of aI1 (reference 14 and unpublished data).
Our biochemical and genetic data suggest that retrohoming without coconversion involves synthesis of a full-length cDNA copy of the inserted intron RNA with homing events completed by a repair mechanism that is independent of the mitochondrial DSBR system (Fig. 6a). An intact antisense strand could be restored by nuclease trimming of excess nucleotides, followed by ligation of the cDNA to the resected DNA strand. Removal of the inserted RNA by an mtRNase H would form a gap in the sense strand that could be filled in by a DNA polymerase activity and the event completed by ligation. This scenario is the same as that deduced for the major retrohoming pathway used by the L. lactis Ll.LtrB intron (3).
In principle, retrohoming without coconversion of flanking exon sequences could also result from a pathway in which the intron RNA reverse splices into an mRNA transcribed from recipient mtDNA to yield a recombinant pre-mRNA, which is then reverse transcribed and incorporated into mtDNA by homologous recombination. That pathway was proposed previously to account for the low level of ectopic transposition of aI1 (17) and also appears to be used for transposition of the Ll.LtrB intron to ectopic sites in L. lactis (2). Retrohoming via reverse splicing into RNA is expected to require the RT activity of the aI2 protein but not its DNA endonuclease activity. In that case, the P714T mutation, which increases RT activity but inhibits endonuclease activity, should increase the proportion of retrohoming events without coconversion and decrease the proportion that occur by TPRT with upstream coconversion. The finding that the P714T donor strain does not alter the proportion of retrohoming events occurring with or without upstream coconversion argues that both pathways are initiated by reverse splicing into DNA followed by TPRT. While recombination with cDNAs probably plays a role in intron deletion events in yeast mitochondria (11), the frequency of such events is very low relative to retrohoming. More direct tests of the possible role of reverse splicing into RNA as a step in retrohoming or ectopic transposition of yeast mitochondrial introns are under way.
Factors that affect the choice of retrohoming pathway.
In all cases analyzed thus far, homing is initiated by reverse splicing of the intron into the sense strand of the DNA target site, followed by antisense strand cleavage. As diagrammed in Fig. 6, the products of these events, which may contain either fully or partially reverse spliced intron RNA, are then partitioned among different pathways, leading to three different outcomes in terms of coconversion of flanking exon sequences. The two retrohoming pathways result in either unidirectional upstream coconversion (Fig. 6b and c) or no coconversion (Fig. 6a), while the RT-independent pathway results in bidirectional coconversion (Fig. 6d). In both yeast mitochondria and bacteria, the primary retrohoming pathways are initiated by the RT copying the 5′ overhang of the cleaved recipient DNA, followed by synthesis of a cDNA copy of the intron. In yeast mitochondria, this pathway may be completed either by DSBR recombination or by synthesis of a full-length cDNA, insertion of which occurs by an as-yet-uncharacterized repair mechanism. In the bacterial systems, which do not carry out efficient DSBR, the latter retrohoming pathway predominates.
The proportion of full to partial reverse splicing is one factor in the choice of retrohoming pathway but not the only factor. Thus, the aI2 crosses A1, B, and C with DNA target sites that support mostly partial reverse splicing in vitro still use the same major retrohoming pathway as aI1, which has a much higher level of full reverse splicing. Moreover, the aI2 homing site used in cross D (E2−2T−5C/E3+2T+5G) increased the proportion of full reverse splicing of aI2 in vitro to about the same level found for aI1 but did not increase the proportion of retrohoming. Even in crosses with the E2−8G E3+2T+5G recipient strain, where increased full reverse splicing activated retrohoming without coconversion, the fraction of retrohoming was not changed. Together, these findings suggest that in crosses with wild-type aI2 the initial intermediate containing either partially or fully reverse spliced intron is divided in a fixed proportion between the RT-dependent and RT-independent pathways. This division likely reflects a balance among the rate of initiation of cDNA synthesis, dissociation of the RT, degradation of the reverse spliced intron RNA, and DNase-mediated resection of the antisense strand.
Our results indicate that the fixed proportion of the homing intermediate that enters the retrohoming pathway can itself be divided between two pathways in which events are completed with or without coconversion of upstream exon sequences. The pathway with no coconversion is activated by mutations in the DNA target site (E2−8G and E3+2T) that increase the level of full reverse splicing in vitro and thus presumably provide an increased opportunity to synthesize full-length intron cDNAs. Other factors that could influence the synthesis of full-length cDNAs include mutations that affect the processivity of the RT, deoxynucleoside triphosphate concentrations in vivo, the rate of degradation of the RNA template, and the folded structure of the intron RNA. Such factors may account for the finding that the target site used in cross D (E2−2T−5C/E3+2T+5G) also includes the E3+2T mutation and leads to increased overall and full reverse splicing but does not lead to increased retrohoming with no coconversion. This target site contains two mutations immediately upstream of the aI2 insertion site that substitute base pairs in the IBS1-EBS1 interaction. Such substitutions could affect the tertiary structure of the intron or protein binding in a way that makes it more difficult to synthesize a full-length cDNA. In addition, it is possible that the level and proportion of full reverse splicing for some target sites differs in vitro and in vivo.
Finally, the most dramatic variation in the ratio of homing pathways was in cross E3, between the Zn domain mutant P714T and the E2−8G E3+2T+5G recipient. Although the overall level of homing was reduced (from 92 to 72%), presumably due to the decreased level of DNA endonuclease activity, the proportion of retrohoming (86%) was much higher than in any other aI2 cross. This change in partitioning of the intermediate toward retrohoming may be due to the activation of RT activity in this mutant, thus increasing the rate of initiation or extent of cDNA synthesis after antisense strand cleavage (6, 23). The ability to use different homing pathways presumably facilitates the dispersal of group II introns by enabling them to use different recombination and repair enzymes and adapt to different conditions in different host cells.
ACKNOWLEDGMENTS
This research was supported by research grants from the National Institutes of Health (GM31480 to P.S.P. and GM37949 to A.M.L.). Robert Eskes was a fellow of the Robert A. Welch Foundation (grant I-1211) during part of this research, and Michael Chao was an NIH predoctoral trainee (T32-HL07360).
Fahd Nasr constructed several of the recipient alleles used in this study. Steven Zimmerly made several observations that helped to focus this study on the recipient alleles shown.
REFERENCES
- 1.Bonitz S G, Coruzzi G, Thalenfield B E, Tzagoloff A, Macino G. Assembly of the mitochondrial membrane system. Structure and nucleotide sequence of the gene coding for subunit I of cytochrome oxidase. J Biol Chem. 1980;255:11927–11941. [PubMed] [Google Scholar]
- 2.Cousineau B, Lawrence S, Smith D, Belfort M. Retrotransposition of a bacterial group II intron. Nature. 2000;404:1018–1021. doi: 10.1038/35010029. [DOI] [PubMed] [Google Scholar]
- 3.Cousineau B, Smith D, Lawrence-Cavanagh S, Mueller J E, Yang J, Mills D, Manias D, Dunny G, Lambowitz A M, Belfort M. Retrohoming of a bacterial group II intron: mobility via complete reverse splicing, independent of homologous DNA recombination. Cell. 1998;94:451–462. doi: 10.1016/s0092-8674(00)81586-x. [DOI] [PubMed] [Google Scholar]
- 4.Eskes R, Yang J, Lambowitz A, Perlman P. Mobility of yeast mitochondrial group II introns: engineering a new site specificity and retrohoming via full reverse splicing. Cell. 1997;88:865–874. doi: 10.1016/s0092-8674(00)81932-7. [DOI] [PubMed] [Google Scholar]
- 5.Guo H, Zimmerly S, Perlman P S, Lambowitz A M. Group II intron endonucleases use both RNA and protein subunits for recognition of specific sequences in double-stranded DNA. EMBO J. 1997;16:6835–6848. doi: 10.1093/emboj/16.22.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacquier A, Dujon B. An intron-encoded protein is active in a gene conversion process that spreads an intron into a mitochondrial gene. Cell. 1985;41:383–394. doi: 10.1016/s0092-8674(85)80011-8. [DOI] [PubMed] [Google Scholar]
- 7.Kennell J C, Moran J V, Perlman P S, Butow R A, Lambowitz A M. Reverse transcriptase activity associated with maturase-encoding group II introns in yeast mitochondria. Cell. 1993;73:133–146. doi: 10.1016/0092-8674(93)90166-n. [DOI] [PubMed] [Google Scholar]
- 8.Lambowitz A M, Belfort M. Introns as mobile genetic elements. Annu Rev Biochem. 1993;62:587–622. doi: 10.1146/annurev.bi.62.070193.003103. [DOI] [PubMed] [Google Scholar]
- 9.Lambowitz A M, Caprara M G, Zimmerly S, Perlman P S. Group I and group II ribozymes as RNPs: clues to the past and guides to the future. In: Gesteland R, Cech T R, Atkins J, editors. The RNA world. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1999. pp. 451–485. [Google Scholar]
- 10.Lazowska J, Meunier B, Macadre C. Homing of a group II intron in yeast mitochondrial DNA is accompanied by unidirectional co-conversion of upstream-located markers. EMBO J. 1994;13:4963–4972. doi: 10.1002/j.1460-2075.1994.tb06823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levra-Juillet E, Boulet A, Seraphin B, Simon M, Faye G. Mitochondrial introns aI1 and/or aI2 are needed for the in vivo deletion of intervening sequences. Mol Gen Genet. 1989;217:168–171. doi: 10.1007/BF00330957. [DOI] [PubMed] [Google Scholar]
- 12.Matsuura M, Saldanha R, Ma H, Wank H, Yang J, Mohr G, Cavanagh S, Dunny G M, Belfort M, Lambowitz A M. A bacterial group II intron encoding reverse transcriptase, maturase, and DNA endonuclease activities: biochemical demonstration of maturase activity and insertion of new genetic information within the intron. Genes Dev. 1997;11:2910–2924. doi: 10.1101/gad.11.21.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills D A, Manias D A, McKay L L, Dunny G M. Homing of a group II intron from Lactococcus lactis subsp. lactis ML3. J Bacteriol. 1997;179:6107–6111. doi: 10.1128/jb.179.19.6107-6111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moran J V, Zimmerly S, Eskes R, Kennell J C, Lambowitz A M, Butow R A, Perlman P S. Mobile group II introns of yeast mitochondrial DNA are novel site-specific retroelements. Mol Cell Biol. 1995;15:2828–2838. doi: 10.1128/mcb.15.5.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moran J V. Ph.D. dissertation. Dallas: University of Texas Southwestern Medical Center; 1994. [Google Scholar]
- 16.Moran J V, Wernette C M, Mecklenburg K L, Butow R A, Perlman P S. Intron 5α of the COXI gene of yeast mitochondrial DNA is a mobile group I intron. Nucleic Acids Res. 1992;20:4069–4076. doi: 10.1093/nar/20.15.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller M W, Allmaier M, Eskes R, Schweyen R J. Transposition of group-II intron al1 in yeast and invasion of mitochondrial genes at new locations. Nature. 1993;366:174–176. doi: 10.1038/366174a0. [DOI] [PubMed] [Google Scholar]
- 18.Nobrega F, Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence and organization of the cytochrome b gene in Saccharomyces cerevisiae D273–10B. J Biol Chem. 1980;255:9828–9837. [PubMed] [Google Scholar]
- 19.Perlman P S, Mahler H R. Genetics and biogenesis of cytochrome b. Methods Enzymol. 1983;9:347–395. doi: 10.1016/0076-6879(83)97150-1. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Mohr G, Perlman P S, Lambowitz A M. Group II intron mobility in yeast mitochondria: target DNA primed reverse transcription activity of aI1 and reverse splicing into DNA transposition sites in vitro. J Mol Biol. 1998;282:505–523. doi: 10.1006/jmbi.1998.2029. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Zimmerly S, Perlman P S, Lambowitz A M. Efficient integration of an intron RNA into double-stranded DNA by reverse splicing. Nature. 1996;381:332–335. doi: 10.1038/381332a0. [DOI] [PubMed] [Google Scholar]
- 22.Zimmerly S, Guo H, Eskes R, Yang J, Perlman P S, Lambowitz A M. A group II intron RNA is a catalytic component of a DNA endonuclease involved in intron mobility. Cell. 1995;83:529–538. doi: 10.1016/0092-8674(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerly S, Guo H, Perlman P S, Lambowitz A M. Group II intron mobility occurs by target DNA-primed reverse transcription. Cell. 1995;82:545–554. doi: 10.1016/0092-8674(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 24.Zimmerly S, Moran J V, Perlman P S, Lambowitz A M. Group II intron reverse transcriptase in yeast mitochondria. Stabilization and regulation of reverse transcriptase activity by the intron RNA. J Mol Biol. 1999;289:473–490. doi: 10.1006/jmbi.1999.2778. [DOI] [PubMed] [Google Scholar]