Abstract
Obesity is considered the primary environmental factor associated with morbidity and severity of wide-ranging inflammatory disorders. Molecular mechanism linking high fat or cholesterol diet to imbalances in immune responses, beyond the increased production of generic inflammatory factors, is just beginning to emerge. Diet cholesterol byproducts are now known to regulate function and migration of diverse immune cell subsets in tissues. The hydroxylated metabolites of cholesterol oxysterols as central regulators of immune cell positioning in lymphoid and mucocutaneous tissues is the focus of this review. Dedicated immunocyte cell surface receptors sense spatially distributed oxysterol tissue depots to tune cell metabolism and function, to achieve the “right place at the right time” axiom of efficient tissue immunity.
Dysregulation of lipid metabolism, in particular elevated cholesterol levels in obesity, is invariably associated with chronic diseases of overt inflammation, including atherosclerosis, diabetes, dementia, psoriasis and gut dysbiosis. Commensurate with the clinical importance, cholesterol biosynthesis and cholesterol homeostasis have been the focus of intense investigation that gave rise to several classes of drugs to treat and prevent cardiovascular diseases by controlling serum cholesterol levels (1). Immune system-specific requirements for cholesterol are well established, although most studies have focused on specific cell types and only the most prominent genes of cholesterol biosynthesis, resulting in disconnect from integrative physiology. Moreover, the biology of cholesterol-derived metabolites in shaping tissue immune responses has been largely uncharacterized, despite the recognition of the bidirectional crosstalk between cholesterol homeostasis and immune system as a major determinant in the pathogenesis of metabolic diseases (2, 3).
Cholesterol is insoluble in water, and its transport into and within the body requires association with various chaperons and carrier proteins that are subsequently sensed by dedicated receptors. However, enzymatic addition of hydroxyl group(s) can reduce cholesterol hydrophobicity. Oxysterols are generated by cholesterol oxidation, involving enzymes with specificity for carbons at selected positions of the sterol ring. These hydrophilic byproducts can be more easily transported in aqueous environment, making them ideal as intercellular cues. Oxysterols have multifaceted effects on immune cells (Table 1), depending on their ability to be sensed by intracellular or surface receptors (4). Expression of certain oxysterol-generating enzymes are tissue-specific (5), and single cell RNAseq studies have begun to identity hematopoietic and non-hematopoietic cells that participate in the establishment of oxysterol depots(6–9). However, a tissue map of oxysterol network (complex oxysterol receptor expression patterns and unresolved oxysterol transport dynamics) remains poorly charted, hindering efforts to determine impacts of oxysterol on immune responses during infections and in steady versus diseased states. Recent advances in the mode by which the oxysterols 25-hydroxycholesterol (25-HC), 27-HC and their dihydroxy metabolites 7α25-HC and 7α,27-HC impact immune responses in tissues and lymphocyte development are beginning to reveal higher resolution molecular circuits linking cholesterol and inflammatory immune responses, and is the focus of this review. Emphasis here will be on those oxysterols with verified function in tissues to coordinate immune responses and readers are referred to other insightful reviews on cholesterol metabolism and immune system for a larger context (4, 10–12).
Table 1.
Cells producing oxysterols and cells responding to oxysterols
| Source | Oxysterol | Target cells | Effect | Mechanism | in vitro | in vivo |
|---|---|---|---|---|---|---|
|
| ||||||
| Stromal cells (SC) | 7α,25-HC | B/T cells, DCs, ILC3, eosinophils | Migration | GPR813 ligand | Yes | Yes |
| SC | 7α,27-HC | DC | Migration | GPR813 ligand | Yes | Yes |
| SC | 7α,27-HC | CD4 T cells | IL-17 production | RORγt ligand | Yes | Young animals only |
| Unknown | 7β,27-HC | CD4 T cells | IL-17 production | RORγt ligand | Yes | Young animals only |
| Macrophages (Mph) |
25-HC | Mph | Inflammasome inhibition |
AIM2 | Yes | Yes |
| Mph | 25-HC | Mph | Inflammatory cytokine release |
Not LXR | Yes | Yes |
| Mph | 25-HC | Mph | Antiviral | Membrane cholesterol/ Viral components |
Yes | Yes |
| Follicular DC | 25-HC | B cells | Altered PC differentiation | SREBP2 | Yes | Yes |
| Mph | 27-HC | Mph | Inflammatory cytokine release |
Estrogen receptor α | Yes | No |
| Tumor | 22-HC | Myeloid | Migration | CXCR2 | Yes | Exogenous 22-HC |
Oxysterol sensing inside the cells.
The enzymes involved in the generation of oxysterols are intracellular proteins that reside in either the endoplasmic reticulum (ER) or the mitochondria(5) (Fig. 1); their distinct location inside the cells suggests that active systems able to transport cholesterol metabolites must exist, but little is known beside the possible involvement of Oxysterol binding proteins(13), and Aster proteins (14).
Figure. 1. Oxysterol production and sensing.
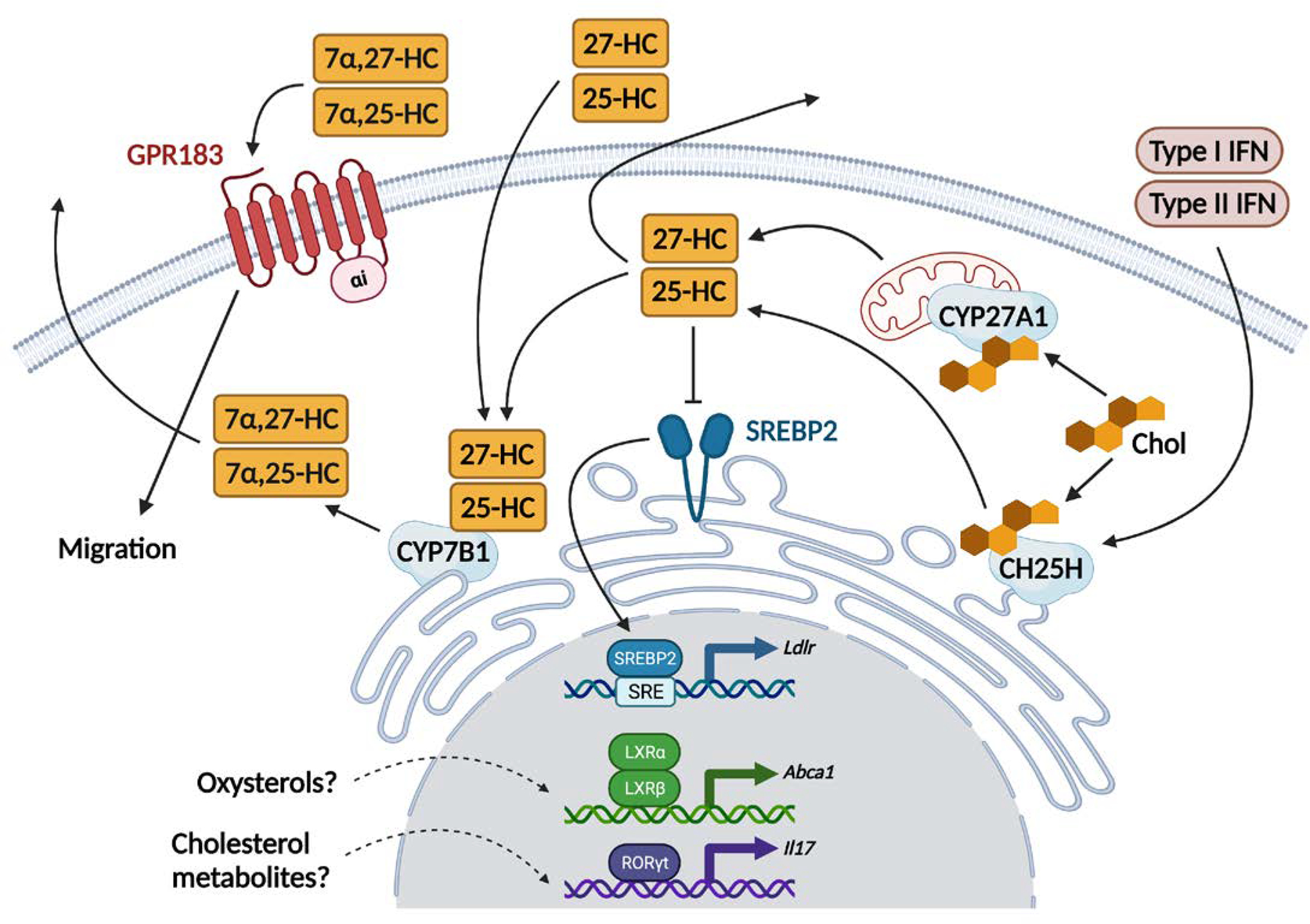
Cholesterol (Chol) derived from the diet or produced intracellularly can be metabolized to generate immune modulating oxysterols. First, the endoplasmic reticulum (ER) resident enzyme cholesterol 25-hydroxylase (CH25H) adds a hydroxyl group at position 25 of cholesterol to synthetize 25-hydroxycholesterol (25-HC). Then in the ER the cytochrome P450 7B1 (CYP7B1) mediates the hydroxylation at the 7α position of 25-HC to generate 7α,25-dihydroxycholesterol (7α,25-HC). GPR183, a G-protein coupled receptor known to mediate migration of several immune cells in tissues, is the receptor for 7α,25-HC. CYP7B1 also produces a second, less potent GPR183 ligand, the oxysterol 7α,27-dihydroxycholesterol (7α,27-HC) converting 27-HC generated from cholesterol by the mitochondrial enzyme sterol 26-hydroxylase (CYP27A1). Type I and Type II Interferons (IFNs) induced by viruses and bacteria drive the expression of CH25H. 25-HC restrains the activation of the sterol response element binding protein 2 (SREBP2, expressed in both lymphocytes and myeloid cells) directly in the ER and prevents SREBP2 translocation to the Golgi (not depicted), leading to eventual deficits in the transcription of genes involved in cholesterol metabolism. Generation and sensing of oxysterols can be uncoupled such that oxysterols produced in trans can engage surface receptors or internalized and transported to ER and nucleus. In vitro experiments have suggested that oxysterols can bind to the nuclear receptors LXR (α and β) and RORγt (expressed in T cells and ILCs only). However, in vivo data supporting such interactions are sparse.
Sterol response element-binding proteins (SREBPs).
25-HC, the product of the enzyme cholesterol 25-HC hydroxylase (CH25H), was initially identified as a sterol able to suppress cholesterol biosynthesis by preventing activation and nuclear translocation of SREBP transcription factors (15). SREBPs regulate the expression of enzymes in the cholesterol biosynthetic pathway, including 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) and the low-density lipoprotein (LDL) receptor (16), which is responsible for cholesterol uptake. With high cholesterol and oxysterol concentration, SREBPs are retained in the ER by the multi-transmembrane SREBP cleavage-activating protein (SCAP), which binds the ER-resident insulin-induced gene (INSIG). Cholesterol itself can control SREBP activation by binding a sterol-sensing domain in SCAP, while 25-HC suppresses SREBP by binding INSIG. Reduced sterol levels induce SCAP detachment from INSIG through a conformational change (17–19). SCAP then escorts SREBPs into the Golgi, where proteases cleave SREBPs and activate them as transcription factors. Three SREBP proteins, SREBP1a, SREBP1c, and SREBP2, encoded by the genes Srebf1 and Srebf2, exist. Although structurally similar, they have different tissue expression patterns, display preferences for transcription of lipogenic or cholesterologenic gene programming, and are distinctly regulated by cholesterol and oxysterols. These features suggest that variations in individual SREBP function might underpin vastly different tissue immune responses impacted by cholesterol.
Nuclear liver X receptor (LXR).
LXRα and LXRβ are members of the nuclear hormone receptor family of transcription factors that control lipid homeostasis(20). LXRα is ubiquitously expressed, while LXRβ expression is higher in cells and tissues that are metabolically active (21). Oxysterols and other cholesterol metabolites were reported to activate LXRs (22), mainly from in vitro experiments (23) or in the liver (24). While deficiency of one or both LXRs impacts myeloid cells, lymphocytes, and stromal cells (25–27), no enzymatic deficiency in sterol intermediates from the cholesterol or cholesterol biosynthetic pathway have been shown to phenocopy the absence of LXRs. For example, while 25-HC has been implicated as a LXR agonist (28), macrophages that lack Ch25h show no alteration in LXR-dependent gene transcription (29). This suggests that LXR activation in distinct cells might be context dependent, with multiple different activators being generated in different local tissue niches.
Retinoic acid receptor related orphan receptor γ, T isoform (RORγt).
Oxysterols (22(R)-HC, 25-HC, 27-HC and 7β-27-HC), and cholesterol biosynthetic intermediates have been described as potential ligands for RORγt (30–33), an orphan nuclear receptor that is critical for lymphoid tissue organogenesis and the development and function of Type 3 cytokine (IL-17, IL-22) secreting lymphocytes (T3L, which can also produce GM-CSF and Amphiregulin. Human T3L is further characterized as IL-26 producers). However, mice and cells lacking specific cholesterol metabolites or unable to generate cholesterol biosynthetic intermediates failed to completely recapitulate RORγt deficiency (29, 34, 35), again raising the possibility that multiple agonists exist in vivo that regulate RORγt function.
Oxysterols and oxysterol byproducts as secondary messengers outside the cells.
Immune cell access to tissues has been largely described as a function of chemokine G-protein coupled receptors (GPCRs) that drives the cell migration in response to a spatial chemokine gradient(36), radiating from chemokine-producing cell(s), allowing directional migration of responding cells toward higher chemokine concentration locales. While this mode of action dovetails well with the need of immunocyte to move from blood into tissues and lymphoid organs(37–39), GPCRs that respond to signals other than proteins to mediate tissue dynamics within discrete sub-anatomical zones exist (40–44), suggesting that diverse enzymatic products are needed for efficient tissue zonation. While CXCR5 is critical for B cell access to B cell follicles, the 7α,25-HC and 7α,27-HC receptor GPR183 was initially identified as critical for a targeted migration of naïve B cells toward the outer follicle (45, 46), fine-tuning their positioning in the lymphoid organs.
The oxysterols 7α,25-HC and 7α,27-HC are synthetized from cholesterol by the action of CH25H and CYP27A1 that generate 25-HC and 27-HC, respectively, followed by the enzymatic activity of CYP7B1, which places a hydroxyl group at the 7α position. Genetic deletion of these enzymes revealed that both 7α,25-HC and 7α,27-HC drive migration of adaptive and innate immune cells in lymph node and spleen via GPR183 (47–55). The oxysterol-degrading enzyme HSD3B7, which generates bile acids (BA), has been shown to be essential for establishing the oxysterol gradient in vivo that allows for directional Gαi-dependent migration (56). While GPR183 is widely expressed by immune cells (B and T cells, dendritic cells (DCs), eosinophils and innate lymphoid cells-3 (ILC3)) in human and rodent secondary lymphoid organs (8, 9, 57), anatomically discrete expression of oxysterol enzymes is predicted to direct distinct cells to specific tissue niches (58). Moreover, magnitude of GPR183 responses to 7α,25-HC and 7α,27-HC seems to be cell type specific, with B and T cell migration mostly dependent on CH25H, while DC migration requires both CH25H and CYP27A1 byproducts (59). We recently showed that increased dietary cholesterol enhanced 25-HC production in intestinal lymphoid organs (60). Coupled with the central role of 25-HC in the regulation of intracellular cholesterol metabolism (15) and its dependency on innate immune system cues (61–63), it is tempting to speculate that GPR183 represents a stereotypical surface receptor that integrates anatomical, metabolic, and immunological cues to shape immunocyte tissue migration.
The immune cell migration in response to GPR183 ligands differs from migration in response to classic chemokine gradient in two ways. First, since oxysterol concentration in tissue is balanced by spatially defined pattern of enzymes that generate and degrade oxysterol intermediates, GPR183 equipped cells can reach discrete tissue depots of the ligand(s). This process might facilitate migration into survival or differentiation niches where cell-displayed or low-diffusible molecules are present. Second, modulation of GPR183 ligands in tissue might be extremely rapid as oxysterol concentration is mainly dependent on substrate abundance and enzymatic kinetics, without necessarily requiring de novo transcription and translation. While chemokine receptor and GPR183-dependent migrations are not mutally exclusive, and are likely to be integrated for immune cell localization, fine tuned regulation of GPR183+ cell migration and GPR183 ligand production might be more prominent at muco-cutaneous barriers that are routinely exposed to fluctuation of metabolites, including lipids.
Oxysterols as BA precursors.
The generation of BA is the major mechanism of cholesterol catabolism as it transforms insoluble cholesterol to water-soluble byproducts that can be easily excreted from the body (64). Moreover, BA has emerged as a critical regulator of Th17 and FOXP3+ regulatory T cell (Treg) generation by interacting with RORγt (65–67). BA synthesis from cholesterol requires extensive enzymatic modifications that give rise to several oxysterols during intermediate reactions (68). Enzymes that catalyze 7α-hydroxylation of cholesterol (CYP7A1) or sterol precursors (CYP7B1) are required for the maintenance of the BA pool, and genetic deficiency in both mice and humans impacts BA and cholesterol metabolism (69–71). CYP27A1 and HSD3B7 are also involved in BA production (72, 73); the relative importance of each of these enzymes in the generation of BA that control Th17 and Treg differentiation in the gut is currently unknown.
Oxysterol function in tissues
Spleen
GPR183 ligands were initially identified in spleen (74, 75) as regulators of B cell positioning(45, 46, 76) and have been extensively reviewed elsewhere(4). Additional work has established that in addition to B cells, GPR183 also controls positioning and function of dendritic cells (77–79) and CD4 T cells (80, 81). GPR183 is intrinsically required in splenic dendritic cells for homeostasis and particulate antigen capture in the marginal zone bridging channel; and for effective antigen recognition and Tfh differentiation in CD4 T cells. Generation of GPR183 ligands that act on locally dispersed immune cells is dependent on discrete patterns of expression of enzymes in stromal cells (56, 79, 82) that allow GPR183 ligand gradient to be simultaneously generated in distinct anatomical locales. Whether splenic GPR183 ligand concentration, and associated GPR183-dependent immune processes, are regulated by additional cues such as infection, diet, or developmental stage-associated factors remains to be investigated.
Liver
Oxysterol and bile acid syntheses are prominent features of the liver. Genetic evidence exists for oxysterols (24-HC, 25-HC, 27-HC) as regulators of hepatic LXR activity (24). Despite the longstanding investigation of LXR modulation in bone marrow derived macrophages (BMDMs), data on Kupffer cells or monocyte- recruited macrophages are limited and variable in interpretations, with some suggesting a role for LXR as a negative regulator of macrophage homeostasis and innate responses (83), while others have concluded that LXRα agonism dampened hepatic inflammation and fibrosis by reducing the activation of hepatic stellate cell and Kupffer cell activation (84, 85).
Hepatic oxysterols control cholesterol biosynthetic gene expression. Mice with hepatocyte-specific deficiency of SREBP2 exhibit reduced LXR activity, suggesting that the cholesterol biosynthesis pathway generates an unknown LXR ligand(s) in the liver (86). Nonalcoholic fatty liver disease (NAFLD), the most common cause of chronic liver disease that can progress to nonalcoholic steatohepatitis (NASH), has been suggested to involve cholesterol overload (87). NASH is characterized by chronic inflammation and immune cell infiltration in the liver (88) and patients show increased in 7-hydroxylated oxysterols compared to healthy individuals. Mice lacking GPR183, CH25H, and CYP7B1 were indistinguishable from controls in a high-fat diet model of NASH (89), and an involvement of the GPR183-7α,25-HC axis in NASH patients has not been established.
Intestine
Oxysterol generation and uptake from diet, as well as oxysterol immunomodulatory activity have been prominently studied in the gut. We recently showed that 25-HC production in the Peyer’s patches (PPs) (90), secondary lymphoid organs that are only present in the small intestine in both human and mice, is modulated by dietary cholesterol and impacts the generation of antigen specific IgA during germinal center reaction(60). While GPR183 ligand is easily detectable in PPs and controls follicular B cell positioning (45, 76), the effect of 25-HC on IgA plasma cells requires SREBP2, but not GPR183 expression on B cells.
A single nucleotide polymorphism (SNP) in GPR183 has been linked to increased risk for ulcerative colitis and Crohn’s disease (91, 92). In patients with GPR183 SNP, IBD susceptibility correlates with increased GPR183 expression on Th17 cells (92). Conversely, mice lacking GPR183 showed reduced overall inflammation (85, 86) in some, but not all, colitis models (93, 94). In the colon, GPR183 controls tissue positioning of ILC3, a process linked to Ch25h expression in stromal cells (94). Animals lacking GPR183 fail to form colonic lymphoid clusters and show blunted response to enteric bacterial infection (95). The discrepancy between intestinal immune cells controlled by GPR183 in mice and humans can be explained by the restricted specificity of murine Th17 cells to the gut commensal Segmented filamentous bacteria (96, 97), and the dominant role of murine ILC3 in maintaining the intestinal barrier function and tissue homeostasis(98, 99). In contrast, positioning and function of lymphoid tissue inducer cells (LTi), embryonically derived ILC3 that are required for normal PP and mesenteric lymph node (mLN) development, are not dependent on GPR183 despite their ability to respond to 7α,25-HC in vitro (94). Whether this difference arises from embryo-specific oxysterol function, production and/or sensing, or whether the embryonic hematopoietic system is uniquely insensitive to cholesterol metabolites is unknown.
The known GPR183-ILC3 axis in the gut impacts colon (94) and mLN (95), but it is unclear how cholesterol or oxysterols are disseminated throughout the gut from the site of cholesterol absorption, which is restricted to the proximal portion of the small intestine (100, 101). Cholesterol uptake from the diet is mediated by Niemann-Pick C1-Like 1 protein (NPC1L1) that is exclusively expressed on intestinal epithelial cells (IECs) (100, 101)These cells incorporate cholesterol and other lipids in chylomicrons, lipoprotein vesicles that assure delivery into lymphatics and eventually into the circulation (102, 103). Thus, one attractive hypothesis is that dietary cholesterol absorption regulates local oxysterol concentration in the gut by providing circulating cholesterol for subsequent enzymatic conversion, possibly by local stromal cells (95, 104). Additionally, diet-derived, IEC-packaged cholesterol might calibrate immune responses directly in the lamina propria of the duodenum that are propagated throughout the gut. Experimental approaches which combine conditional genetic deletion, dietary modulation, and pharmacological intervention will be required to tease apart the spatial generation and effector function of oxysterols in tissues.
Lung
Cholesterol is an integral component of the pulmonary surfactant (105) and modulation of cholesterol bioavailability impacts the function of pulmonary air-liquid interface (106). More than 80% of the lung cholesterol is derived from the plasma, making it particularly sensitive to dietary lipid intake, while the remaining cholesterol is synthetized by lung-resident cells (107). The lung is one of the organs with the highest amounts of Ch25h transcripts at steady state. At three days after birth in mice, fetal-origin alveolar macrophages (AM) abundantly express Ch25h (108). 25-HC can mediate either amplification or resolution of lung inflammation (109–112). It also has a direct effect on viral entry into airway epithelial cells in both mouse and human upon infection with influenza viruses (113) and might amplify the response to other RNA viruses (111, 114), possibly by alteration of cholesterol-enriched cytomembrane. Similar to 25-HC, 27-HC is also expressed at high levels in the lung (115), is modulated during lung diseases (116, 117), and mediates antiviral effects by sequestration of viral particles in late endosome (118).
For lymphocytes, evidence for the role of cholesterol in shaping early life pulmonary innate and innate-like lymphocyte responses is just beginning to emerge. Lung innate-like T3L (iT3L, Tγδ17, MAIT17 and NKT17) express GPR183. They are able to colonize the newborn lungs (119, 120) and rapidly respond to pulmonary pathogens (121, 122). Tγδ17 cells originate from the thymus (123, 124) and comprise two distinct subsets: fetal Vγ4 and neonatal Vγ2 (TCRγ nomenclature of Garman and Raulet (125)) expressing cells that populate all mucocutaneus barrier tissues. Neonatal lung Tγδ17 cells are required for optimal response to flu virus during early life (126) and their maintenance in the lung depends on GPR183 (unpublished). Recently, it has been shown that embryonic macrophages allow for the expansion of invariant NKT cells that populate the barrier tissues, including the lung and skin (127). Cross-regulation of AM and early life dominant lung resident innate-like lymphocytes involving cholesterol byproducts may account for the noted age-associated differences in pulmonary immune responses. Focused studies on oxysterol network in the lung are warranted to test this possibility.
Brain
Oxysterol metabolism in the brain has been long considered to be controlled primarily by de novo cholesterol synthesis (128). CYP46A1 regulates cholesterol levels in the brain by converting it into 24-HC (129). Polymorphisms in CYP46A1 are associated with increased risk of Alzheimer’s Disease (AD), but whether 24-HC can influence immune cells during the disease initiation or progression is largely unknown. GPR183 ligand is present in the brain (48), but little is known about its regulation. CYP27A1 required for 27-HC production is not expressed in the brain under homeostatic conditions. However, 27-HC can cross the blood-brain barrier and enter the brain (130), where it undergoes enzymatic conversion before export into the circulation (131). Mutations in CYP27A1 leads to Cerebrotendinous Xanthomatosis (132), with gut specific symptoms due to defective BA generation, and brain degeneration due to accumulation of cholesterol and cholestanol (133). CYP7B1 that converts 25-HC into 7α,25-HC is expressed in the brain (134), and CYP7B1 deficiency is responsible for Spastic Paraplegia Type 5 (135), a neurodegenerative disorder driven by the accumulation of neurotoxic level of oxysterols. Mice deficient in Cyp7b1 also show increased 25-HC amounts in the brain (136). Ch25h expression is not observed in healthy microglial cells, a primary candidate for 25-HC production in utero and during neonatal window (137). However, amounts of Ch25h transcripts increase with age, possibly due to the emergence of IFN-responsive microglia (137) and it is rapidly upregulated during inflammatory insults, including in AD and Experimental Autoimmune Encephalomyelitis, a mouse model of multiple sclerosis (138). For the latter, Th17 cells are pathogenic (29) and GPR183 can enhance trafficking of encephalitic CD4 T cells (139, 140). In mice, fetal-derived, commensal-independent, GPR183+ Tγδ17 cells (141) infiltrate the meninges after birth, with life-long persistence (142, 143). They have been implicated in anxiety-like behavior, in line with the critical impact of maternal IL-17 in fetal cortical brain developmental abnormalities leading to autism-like symptoms(144, 145). Involvement of oxysterols in immunocyte-mediated brain inflammation is plausible given the well-established link between neurons and tissue T3L, especially Tγδ17 cells in mucocutaneous tissues (146), and is an active area of investigation.
Skin
Dermal Vγ2+ Tγδ17 cells are essential for assuring skin barrier homeostasis by fortifying epithelial cells after birth in response to commensal bacteria, although their development, unlike that of fetal Tγδ17 cells, is not wholly dependent on microbiota (147). We have recently discovered that neonatal Tγδ17 cell positioning and maintenance in the murine dermis require GPR183. Moreover, in the Imiquimod (TLR7 agonist) induced, neonatal Tγδ17 cell-dependent psoriasis model, genetic and diet modulated GPR183 ligand availability dominantly specifies psoriatic responses. Interfollicular epidermal (IFE) cells, basal keratinocytes located at the dermal-epidermal border, express high levels of CH25H, and neonatal Tγδ17 cells are localized at the border. The expression pattern of cholesterol processing enzymes is likely conserved in the skin of mice and humans (148), although in the latter fibroblasts may play a more prominent role in oxysterol generation.
Other skin resident lymphoid cells of early life have intimate relationship with keratinocytes. Majority of Treg express GPR183 and they colonize the neonatal skin to mediate tolerance to commensal bacteria. In addition, Treg localize in the hair follicle bulge to regulate epithelial stem cell differentiation (149) Type 2 cytokine (IL-4, 5, 13) producing innate lymphoid cells-2 (ILC2), which are seeded in the skin during fetal development as precursors, function within the upper hair follicle. They control sebaceous gland (SG) function by regulating commensal bacteria (150). Sebocytes, specialized epithelial cells that secrete a complex mixture of lipids (sebum) including cholesterol, express the oxysterol sensors LXR and SREBP. The relationship between SG, SG-associated ILC, cholesterol metabolites and immune cell function is unknown.
GPR183 expressing immunocytes are confined to the dermis at steady state, but the domain of oxysterol impact is likely widespread, especially during skin damage. GPR183 ligand is made from 25-HC, which also dampens SREBP2 activity (60, 151). In the skin, genetic ablation of SREBP2 in macrophages leads to enhanced wound healing, by promoting epithelialization, angiogenesis, and myofibroblast-induced wound contraction(152). Moreover, 25-HC has been shown to mediate protection against bacterial pore-forming toxins in the skin, via IFN-dependent cholesterol metabolism reprograming in myeloid cells (153, 154). Thus, it is possible that alteration of 25-HC and other cholesterol metabolite bioavailability in the skin, possibly via dietary cholesterol, modulates the balance between inflammatory and reparative responses.
Thymus
Arguably the strongest evidence to date of the importance of oxysterol sensing by T cells is the observation that there exists a thymic epithelial niche of GPR183 ligand production and that neonatal Tγδ17 cells must sense oxysterols for proper maturation and homing to the skin and lung (Frascoli et al, 2022 submitted). Cholesterol processing enzymes, in particular Ch25h, but excluding the BA-generating Hsd3b7, are prominently expressed in medullary thymic epithelial cells (mTEC), which are also the source of key chemokines such as CCL21, required for normal αβ T cell selection. Ch25h+ mTECs are distinct from Aire+ mTECs that mediate negative selection of tissue antigen specific αβ T cells and for perinatal Treg cell generation. The oxysterol thymic niche discovered in mice is remarkably conserved in the human thymus (8), and given that the sole function of the thymus is to generate fit and useful T cells, such an evolutionary conservation supports the functional primacy of oxysterol sensing in some thymic-derived GPR183+ cells. In mice, neonatal thymic Tγδ17 cell maturation for export is independent of commensals, and perhaps T cell receptor signaling (123, 155). That cholesterol metabolites may be the central arbiter of postnatal Tγδ17 thymic selection presages that GPR183+ T3L effector function is calibrated by cholesterol and oxysterol bioavailability in tissues. Human Vδ2+ T cells that are the focus of cancer immunotherapy clinical trials recognize isopentenyl pyrophosphate produced by the mevalonate pathway that generates de novo cholesterol. Future studies will need to tackle the overriding question of how and why sensing of bioavailable cholesterol and cholesterol metabolites by immunocytes is intimately intertwined into the regulatory circuits that control their function.
Conclusions
In a dozen year since the first report of immunocyte regulation by oxysterols it has become apparent that lymphocyte migration and function in tissues is finely tuned by lipid processing stromal and myeloid cells. Conversion of cholesterol into immune modulatory lipids is a multistep cell relay system that is likely to involve diverse sensory cells that monitor tissue fitness and environmental perturbations. As a major component of the relay GPR183 has garnered interest as the prototypic oxysterol-dependent cell surface modulator of T3L in mucocutaneous tissues. Detailed parsing of diet-derived cholesterol regulation of T3L should lead to definitive molecular insights into the correlative link between diet and human lymphocyte-driven tissue inflammatory diseases. Progress in this area will require basic mapping of human oxysterol regulatory circuits in mucocutaneus tissues. Much is unknown in the transport of oxysterols in and out of the cells and the full understanding of how diet and inflammatory cues modulate oxysterol bioactivity will require not only the complete charting of the pathway generating immunodulatory lipids in tissues but also the cellular processes that construct and sustain these lipid depots, in health and disease.
Figure. 2. Tissue functions of oxysterols.
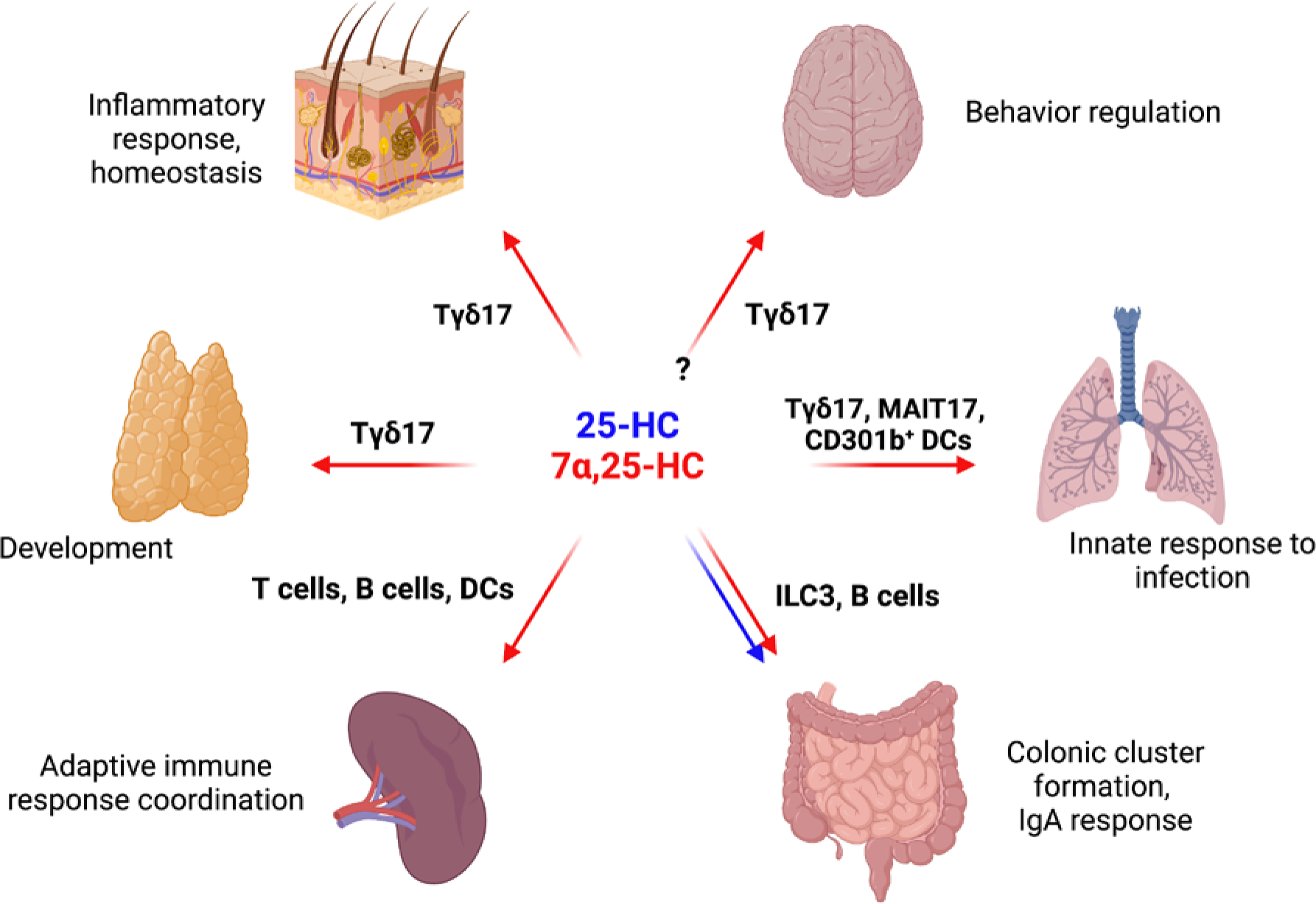
Overview of oxysterol acitivities in different tissues. Red and blue arrows represent defined function of 7α,25-HC and 25-HC in tissues, respectively. In the skin, IL-17 production by neonatal GPR183+ Tγδ17 cells is dependent on 7α,25-HC. Basal keratinocytes express CH25H that synthetize 25-HC, and characterization of the immune or non-immune cells expressing CYP7B1 responsible for the terminal production of GPR183 ligand in the skin is in progress. Tγδ17 cell maturation in the thymus is controlled by Ch25h-expressing medullary thymic epithelial cells (mTEC). Additional thymocyte subsets regulated by oxysterol depots have only been cursorily surveyed. In the spleen, CD4 T cells, follicular B cells and dendritic cells rely on GPR183 to position in discrete sub-anatomical locations (outer T cell zone, outer B follicle, and bridging channel, respectively) to assure efficient antigen capture, antigen presentation, and T and B cell activation. Ch25h is expressed by splenic stromal cells, in particular by marginal reticular cells, interfollicular reticular cells and high endothelial cells, while Cyp7b1 expression appears more broadly distributed. In the gut, Peyer’s patch follicular dendritic cells produce 25-HC to restrain SREBP2 in germinal center B cells and permit the differentiation of IgA-secreting plasma cells. In the colonic lamina propria, fibroblastic stromal cells provide a local source of 7α,25-HC to guide ILC3 migration and colonic lymphoid cluster formation. Lung alveolar macrophages are noted for their capacity to produce high amounts of CH25H, and their role in regulating GPR183+ IL-17/22 producing innate-like T cells and CD301b+ DCs (and other myeloid cells) is just beginning to be explored. Brain Tγδ17 cells that regulate anxiety-like behaviors express GPR183, but whether oxysterols are involved, and if so, the source(s) of the GPR183 ligand, remain to be determined.
Acknowledgements
We thank members of the labs for discussion and Dr. Jonathan Kipnis (Wash U.) for sharing unpublished data.
REFERENCES
- 1.Goldstein JL, and Brown MS. 2015. A Century of Cholesterol and Coronaries: From Plaques to Genes to Statins. Cell 161: 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanneganti T-D, and Dixit VD. 2012. Immunological complications of obesity. Nat Immunol 13: 707–712. [DOI] [PubMed] [Google Scholar]
- 3.Zmora N, Bashiardes S, Levy M, and Elinav E. 2017. The Role of the Immune System in Metabolic Health and Disease. Cell Metab 25: 506–521. [DOI] [PubMed] [Google Scholar]
- 4.Cyster JG, Dang EV, Reboldi A, and Yi T. 2014. 25-Hydroxycholesterols in innate and adaptive immunity. Nature Publishing Group 14: 731–743. [DOI] [PubMed] [Google Scholar]
- 5.Russell DW 2003. The Enzymes, Regulation, and Genetics of Bile Acid Synthesis. Annu. Rev. Biochem. 72: 137–174. [DOI] [PubMed] [Google Scholar]
- 6.Rodda LB, Bannard O, Ludewig B, Nagasawa T, and Cyster JG. 2015. Phenotypic and Morphological Properties of Germinal Center Dark Zone Cxcl12-Expressing Reticular Cells. J Immunol 195: 4781–4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart BJ, Ferdinand JR, Young MD, Mitchell TJ, Loudon KW, Riding AM, Richoz N, Frazer GL, Staniforth JUL, Braga FAV, Botting RA, Popescu D-M, Vento-Tormo R, Stephenson E, Cagan A, Farndon SJ, Polanski K, Efremova M, Green K, Velasco-Herrera MDC, Guzzo C, Collord G, Mamanova L, Aho T, Armitage JN, Riddick ACP, Mushtaq I, Farrell S, Rampling D, Nicholson J, Filby A, Burge J, Lisgo S, Lindsay S, Bajenoff M, Warren AY, Stewart GD, Sebire N, Coleman N, Haniffa M, Teichmann SA, Behjati S, and Clatworthy MR. 2019. Spatiotemporal immune zonation of the human kidney. Science 365: 1461–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park J-E, Botting RA, Conde CD, Popescu D-M, Lavaert M, Kunz DJ, Goh I, Stephenson E, Ragazzini R, Tuck E, Wilbrey-Clark A, Roberts K, Kedlian VR, Ferdinand JR, He X, Webb S, Maunder D, Vandamme N, Mahbubani KT, Polanski K, Mamanova L, Bolt L, Crossland D, de Rita F, Fuller A, Filby A, Reynolds G, Dixon D, Saeb-Parsy K, Lisgo S, Henderson D, Vento-Tormo R, Bayraktar OA, Barker RA, Meyer KB, Saeys Y, Bonfanti P, Behjati S, Clatworthy MR, Taghon T, Haniffa M, and Teichmann SA. 2020. A cell atlas of human thymic development defines T cell repertoire formation. Science 367: eaay3224–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elmentaite R, Kumasaka N, Roberts K, Fleming A, Dann E, King HW, Kleshchevnikov V, Dabrowska M, Pritchard S, Bolt L, Vieira SF, Mamanova L, Huang N, Perrone F, Kai’En IG, Lisgo SN, Katan M, Leonard S, Oliver TRW, Hook CE, Nayak K, Campos LS, Conde CD, Stephenson E, Engelbert J, Botting RA, Polanski K, van Dongen S, Patel M, Morgan MD, Marioni JC, Bayraktar OA, Meyer KB, He X, Barker RA, Uhlig HH, Mahbubani KT, Saeb-Parsy K, Zilbauer M, Clatworthy MR, Haniffa M, James KR, and Teichmann SA. 2021. Cells of the human intestinal tract mapped across space and time. Nature 597: 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kidani Y, and Bensinger SJ. 2017. ScienceDirect Reviewing the impact of lipid synthetic flux on Th17 function. Current Opinion in Immunology 46: 121–126. [DOI] [PubMed] [Google Scholar]
- 11.Fessler MB 2016. The Intracellular Cholesterol Landscape: Dynamic Integrator of the Immune Response. Trends in Immunology 37: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spann NJ, and Glass CK. 2013. Sterols and oxysterols in immune cell function. Nat Immunol 14: 893–900. [DOI] [PubMed] [Google Scholar]
- 13.Raychaudhuri S, and Prinz WA. 2010. The Diverse Functions of Oxysterol-Binding Proteins. Annu Rev Cell Dev Bi 26: 157–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandhu J, Li S, Fairall L, Pfisterer SG, Gurnett JE, Xiao X, Weston TA, Vashi D, Ferrari A, Orozco JL, Hartman CL, Strugatsky D, Lee SD, He C, Hong C, Jiang H, Bentolila LA, Gatta AT, Levine TP, Ferng A, Lee R, Ford DA, Young SG, Ikonen E, Schwabe JWR, and Tontonoz P. 2018. Aster Proteins Facilitate Nonvesicular Plasma Membrane to ER Cholesterol Transport in Mammalian Cells. Cell 175: 514–529.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein JL, DeBose-Boyd RA, and Brown MS. 2006. Protein Sensors for Membrane Sterols. Cell 124: 35–46. [DOI] [PubMed] [Google Scholar]
- 16.Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, and Goldstein JL. 2003. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc National Acad Sci 100: 12027–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams CM, Reitz J, Brabander JKD, Feramisco JD, Li L, Brown MS, and Goldstein JL. 2004. Cholesterol and 25-Hydroxycholesterol Inhibit Activation of SREBPs by Different Mechanisms, Both Involving SCAP and Insigs*. J Biol Chem 279: 52772–52780. [DOI] [PubMed] [Google Scholar]
- 18.Kober DL, Radhakrishnan A, Goldstein JL, Brown MS, Clark LD, Bai X, and Rosenbaum DM. 2021. Scap structures highlight key role for rotation of intertwined luminal loops in cholesterol sensing. Cell 184: 3689–3701.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan R, Cao P, Song W, Qian H, Du X, Coates HW, Zhao X, Li Y, Gao S, Gong X, Liu X, Sui J, Lei J, Yang H, Brown AJ, Zhou Q, Yan C, and Yan N. 2021. A structure of human Scap bound to Insig-2 suggests how their interaction is regulated by sterols. Science 371: eabb2224. [DOI] [PubMed] [Google Scholar]
- 20.Wang B, and Tontonoz P. 2018. Liver X receptors in lipid signalling and membrane homeostasis. Nat Rev Endocrinol 14: 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang B, and Tontonoz P. 2018. Liver X receptors in lipid signalling and membrane homeostasis. Nat Rev Endocrinol 14: 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janowski BA, Grogan MJ, Jones SA, Wisely GB, Kliewer SA, Corey EJ, and Mangelsdorf DJ. 1999. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. P Natl Acad Sci Usa 96: 266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janowski BA, Willy PJ, Devi TR, Falck JR, and Mangelsdorf DJ. 1996. An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature 383: 728–731. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Chen G, Head DL, Mangelsdorf DJ, and Russell DW. 2007. Enzymatic Reduction of Oxysterols Impairs LXR Signaling in Cultured Cells and the Livers of Mice. Cell Metabolism 5: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan CT, Fenn AM, Harder NK, Mindur JE, McAlpine CS, Patel J, Valet C, Rattik S, Iwamoto Y, He S, Anzai A, Kahles F, Poller WC, Janssen H, Wong LP, Fernández-Hernando C, Koolbergen DR, van der Laan AM, Yvan-Charvet L, Sadreyev RI, Nahrendorf M, Westerterp M, Tall AR, Gustafsson J-Å, and Swirski FK. 2020. Liver X receptors are required for thymic resilience and T cell output. Journal of Experimental Medicine 217: 245–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.A-Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Díaz M, Gallardo G, de Galarreta CR, Salazar J, Lopez F, Edwards P, Parks J, Andujar M, Tontonoz P, and Castrillo A. 2009. Apoptotic Cells Promote Their Own Clearance and Immune Tolerance through Activation of the Nuclear Receptor LXR. Immunity 31: 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong C, Kidani Y, A-Gonzalez N, Phung T, Ito A, Rong X, Ericson K, Mikkola H, Beaven SW, Miller LS, Shao W-H, Cohen PL, Castrillo A, Tontonoz P, and Bensinger SJ. 2012. Coordinate regulation of neutrophil homeostasis by liver X receptors in mice. J. Clin. Invest. 122: 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janowski BA, Willy PJ, Devi TR, Falck JR, and Mangelsdorf DJ. 1996. An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature 383: 728–731. [DOI] [PubMed] [Google Scholar]
- 29.Reboldi A, Dang EV, McDonald JG, Liang G, Russell DW, and Cyster JG. 2014. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science 345: 679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin L, Martynowski D, Zheng S, Wada T, Xie W, and Li Y. 2010. Structural Basis for Hydroxycholesterols as Natural Ligands of Orphan Nuclear Receptor RORγ. Mol Endocrinol 24: 923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huh JR, Leung MWL, Huang P, Ryan DA, Krout MR, Malapaka RRV, Chow J, Manel N, Ciofani M, Kim SV, Cuesta A, Santori FR, Lafaille JJ, Xu HE, Gin DY, Rastinejad F, and Littman DR. 2011. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature 472: 486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soroosh P, Wu J, Xue X, Song J, Sutton SW, Sablad M, Yu J, Nelen MI, Liu X, Castro G, Luna R, Crawford S, Banie H, Dandridge RA, Deng X, Bittner A, Kuei C, Tootoonchi M, Rozenkrants N, Herman K, Gao J, Yang XV, Sachen K, Ngo K, Fung-Leung W-P, Nguyen S, de Leon-Tabaldo A, Blevitt J, Zhang Y, Cummings MD, Rao T, Mani NS, Liu C, McKinnon M, Milla ME, Fourie AM, and Sun S. 2014. Oxysterols are agonist ligands of RORγt and drive Th17 cell differentiation. Proc National Acad Sci 111: 12163–12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santori FR, Huang P, van de Pavert SA, Douglass EF, Leaver DJ, Haubrich BA, Keber R, Lorbek G, Konijn T, Rosales BN, Rozman D, Horvat S, Rahier A, Mebius RE, Rastinejad F, Nes WD, and Littman DR. 2015. Identification of Natural RORγ Ligands that Regulate the Development of Lymphoid Cells. Cell Metab 21: 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santori FR, Huang P, van de Pavert SA, Jr EFD, Leaver DJ, Haubrich BA, Keber R, Lorbek G, Konijn T, Rosales BN, Rozman D, Horvat S, Rahier A, Mebius RE, Rastinejad F, Nes WD, and Littman DR. 2015. Identification of Natural RORγ Ligands that Regulate the Development of Lymphoid Cells. Cell Metabolism 21: 286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soroosh P, Wu J, Xue X, Song J, Sutton SW, Sablad M, Yu J, Nelen MI, Liu X, Castro G, Luna R, Crawford S, Banie H, Dandridge RA, Deng X, Bittner A, Kuei C, Tootoonchi M, Rozenkrants N, Herman K, Gao J, Yang XV, Sachen K, Ngo K, Fung-Leung W-P, Nguyen S, de Leon-Tabaldo A, Blevitt J, Zhang Y, Cummings MD, Rao T, Mani NS, Liu C, McKinnon M, Milla ME, Fourie AM, and Sun S. 2014. Oxysterols are agonist ligands of RORγt and drive Th17 cell differentiation. Proc National Acad Sci 111: 12163–12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rot A, and von Andrian UH. 2004. Chemokines in Innate and Adaptive Host Defense: Basic Chemokinese Grammar for Immune Cells. Annu Rev Immunol 22: 891–928. [DOI] [PubMed] [Google Scholar]
- 37.Förster R, Mattis AE, Kremmer E, Wolf E, Brem G, and Lipp M. 1996. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87: 1037–1047. [DOI] [PubMed] [Google Scholar]
- 38.Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, Wolf E, and Lipp M. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99: 23–33. [DOI] [PubMed] [Google Scholar]
- 39.Okada T, Ngo VN, Ekland EH, Förster R, Lipp M, Littman DR, and Cyster JG. 2002. Chemokine Requirements for B Cell Entry to Lymph Nodes and Peyer’s Patches. Journal of Experimental Medicine 196: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Sumida H, and Cyster JG. 2014. GPR18 is required for a normal CD8αα intestinal intraepithelial lymphocyte compartment. J Exp Medicine 211: 2351–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sumida H, Lu E, Chen H, Yang Q, Mackie K, and Cyster JG. 2017. GPR55 regulates intraepithelial lymphocyte migration dynamics and susceptibility to intestinal damage. Sci Immunol 2: eaao1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu E, Wolfreys FD, Muppidi JR, Xu Y, and Cyster JG. 2019. S-Geranylgeranyl-l-glutathione is a ligand for human B cell-confinement receptor P2RY8. Nature 567: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muppidi JR, Lu E, and Cyster JG. 2015. The G protein–coupled receptor P2RY8 and follicular dendritic cells promote germinal center confinement of B cells, whereas S1PR3 can contribute to their dissemination. Journal of Experimental Medicine 212: 2213–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green JA, Suzuki K, Cho B, Willison LD, Palmer D, Allen CDC, Schmidt TH, Xu Y, Proia RL, Coughlin SR, and Cyster JG. 2011. The sphingosine 1-phosphate receptor S1P2 maintains the homeostasis of germinal center B cells and promotes niche confinement. Nat Immunol 12: 672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pereira JP, Kelly LM, Xu Y, and Cyster JG. 2009. EBI2 mediates B cell segregation between the outer and centre follicle. Nature 460: 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gatto D, Paus D, Basten A, Mackay CR, and Brink R. 2009. Guidance of B Cells by the Orphan G Protein-Coupled Receptor EBI2 Shapes Humoral Immune Responses. Immunity 31: 259–269. [DOI] [PubMed] [Google Scholar]
- 47.Cyster JG, Dang EV, Reboldi A, and Yi T. 2014. 25-Hydroxycholesterols in innate and adaptive immunity. Nature Reviews Immunology 14: 731–743. [DOI] [PubMed] [Google Scholar]
- 48.Kelly LM, Pereira JP, Yi T, Xu Y, and Cyster JG. 2011. EBI2 guides serial movements of activated B cells and ligand activity is detectable in lymphoid and nonlymphoid tissues. Journal of immunology (Baltimore, Md. : 1950) 187: 3026–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pereira JP, Kelly LM, Xu Y, and Cyster JG. 2009. EBI2 mediates B cell segregation between the outer and centre follicle. Nature 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hannedouche S, Zhang J, Yi T, Shen W, Nguyen D, Pereira JP, Guerini D, Baumgarten BU, Roggo S, Wen B, Knochenmuss R, Noël S, Gessier F, Kelly LM, Vanek M, Laurent S, Preuss I, Miault C, Christen I, Karuna R, Li W, Koo D-I, Suply T, Schmedt C, Peters EC, Falchetto R, Katopodis A, Spanka C, Roy M-O, Detheux M, Chen YA, Schultz PG, Cho CY, Seuwen K, Cyster JG, and Sailer AW. 2011. Oxysterols direct immune cell migration via EBI2. Nature 475: 524–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gatto D, Paus D, Basten A, Mackay CR, and Brink R. 2009. Guidance of B Cells by the Orphan G Protein-Coupled Receptor EBI2 Shapes Humoral Immune Responses. Immunity 31: 259–269. [DOI] [PubMed] [Google Scholar]
- 52.Yi T, Wang X, Kelly LM, An J, Xu Y, Sailer AW, Gustafsson J-A, Russell DW, and Cyster JG. 2012. Oxysterol Gradient Generation by Lymphoid Stromal Cells Guides Activated B Cell Movement during Humoral Responses. Immunity 37: 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, Lu E, Yi T, and Cyster JG. 2019. EBI2 augments Tfh cell fate by promoting interaction with IL-2-quenching dendritic cells. Nature 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gatto D, Wood K, Caminschi I, Murphy-Durland D, Schofield P, Christ D, Karupiah G, and Brink R. 2013. The chemotactic receptor EBI2 regulates the homeostasis, localization and immunological function of splenic dendritic cells. Nature Immunology 14: 446–453. [DOI] [PubMed] [Google Scholar]
- 55.Yi T, and Cyster JG. 2013. EBI2-mediated bridging channel positioning supports splenic dendritic cell homeostasis and particulate antigen capture. Elife 2: e00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yi T, Wang X, Kelly LM, An J, Xu Y, Sailer AW, Gustafsson J-Å, Russell DW, and Cyster JG. 2012. Oxysterol Gradient Generation by Lymphoid Stromal Cells Guides Activated B Cell Movement during Humoral Responses. Immunity 37: 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clottu AS, Mathias A, Sailer AW, Schluep M, Seebach JD, Pasquier RD, and Pot C. 2017. EBI2 Expression and Function: Robust in Memory Lymphocytes and Increased by Natalizumab in Multiple Sclerosis. CellReports 18: 213–224. [DOI] [PubMed] [Google Scholar]
- 58.Baptista AP, Gola A, Huang Y, Milanez-Almeida P, Torabi-Parizi P, Jr JFU, Shapiro VS, Gerner MY, and Germain RN. 2019. The Chemoattractant Receptor Ebi2 Drives Intranodal Naive CD4+ T Cell Peripheralization to Promote Effective Adaptive Immunity. Immunity 50: 1188–1201.e6. [DOI] [PubMed] [Google Scholar]
- 59.Lu E, Dang EV, McDonald JG, and Cyster JG. 2017. Distinct oxysterol requirements for positioning naïve and activated dendritic cells in the spleen. Science Immunology 2: eaal5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trindade BC, Ceglia S, Berthelette A, Raso F, Howley K, Muppidi JR, and Reboldi A. 2021. The cholesterol metabolite 25-hydroxycholesterol restrains the transcriptional regulator SREBP2 and limits intestinal IgA plasma cell differentiation. Immunity 54: 2273–2287.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu S-Y, Aliyari R, Chikere K, Li G, Marsden MD, Smith JK, Pernet O, Guo H, Nusbaum R, Zack JA, Freiberg AN, Su L, Lee B, and Cheng G. 2012. Interferon-Inducible Cholesterol-25-Hydroxylase Broadly Inhibits Viral Entry by Production of 25-Hydroxycholesterol. Immunity 38: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blanc M, Hsieh WY, Robertson KA, Kropp KA, Forster T, Shui G, Lacaze P, Watterson S, Griffiths SJ, Spann NJ, Meljon A, Talbot S, Krishnan K, Covey DF, Wenk MR, Craigon M, Ruzsics Z, Haas J, Angulo A, Griffiths WJ, Glass CK, Wang Y, and Ghazal P. 2012. The Transcription Factor STAT-1 Couples Macrophage Synthesis of 25-Hydroxycholesterol to the Interferon Antiviral Response. Immunity 38: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park K, and Scott AL. 2010. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons. Journal of Leukocyte Biology 88: 1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Molinaro A, Wahlström A, and Marschall H-U. 2018. Role of Bile Acids in Metabolic Control. Trends in Endocrinology & Metabolism 29: 31–41. [DOI] [PubMed] [Google Scholar]
- 65.Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, Ha S, Nelson BN, Kelly SP, Wu L, Zheng Y, Longman RS, Rastinejad F, Devlin AS, Krout MR, Fischbach MA, Littman DR, and Huh JR. 2020. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 576: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, Geva-Zatorsky N, Jupp R, Mathis D, Benoist C, and Kasper DL. 2019. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 577: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paik D, Yao L, Zhang Y, Bae S, D’Agostino GD, Zhang M, Kim E, Franzosa EA, Avila-Pacheco J, Bisanz JE, Rakowski CK, Vlamakis H, Xavier RJ, Turnbaugh PJ, Longman RS, Krout MR, Clish CB, Rastinejad F, Huttenhower C, Huh JR, and Devlin AS. 2022. Human gut bacteria produce ΤΗ17-modulating bile acid metabolites. Nature 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Russell DW 2003. THE ENZYMES, REGULATION, AND GENETICS OF BILE ACID SYNTHESIS. Annu Rev Biochem 72: 137–174. [DOI] [PubMed] [Google Scholar]
- 69.Ishibashi S, Schwarz M, Frykman PK, Herz J, and Russell DW. 1996. Disruption of Cholesterol 7α-Hydroxylase Gene in Mice I. POSTNATAL LETHALITY REVERSED BY BILE ACID AND VITAMIN SUPPLEMENTATION*. J Biol Chem 271: 18017–18023. [DOI] [PubMed] [Google Scholar]
- 70.Pullinger CR, Eng C, Salen G, Shefer S, Batta AK, Erickson SK, Verhagen A, Rivera CR, Mulvihill SJ, Malloy MJ, and Kane JP. 2002. Human cholesterol 7α-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest 110: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Setchell KD, Schwarz M, O’Connell NC, Lund EG, Davis DL, Lathe R, Thompson HR, Tyson RW, Sokol RJ, and Russell DW. 1998. Identification of a new inborn error in bile acid synthesis: mutation of the oxysterol 7alpha-hydroxylase gene causes severe neonatal liver disease. J Clin Invest 102: 1690–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosen H, Reshef A, Maeda N, Lippoldt A, Shpizen S, Triger L, Eggertsen G, Björkhem I, and Leitersdorf E. 1998. Markedly Reduced Bile Acid Synthesis but Maintained Levels of Cholesterol and Vitamin D Metabolites in Mice with Disrupted Sterol 27-Hydroxylase Gene*. J Biol Chem 273: 14805–14812. [DOI] [PubMed] [Google Scholar]
- 73.Shea HC, Head DD, Setchell KDR, and Russell DW. 2007. Analysis of HSD3B7 knockout mice reveals that a 3α-hydroxyl stereochemistry is required for bile acid function. Proc National Acad Sci 104: 11526–11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hannedouche S, Zhang J, Yi T, Shen W, Nguyen D, Pereira JP, Guerini D, Baumgarten BU, Roggo S, Wen B, Knochenmuss R, Noël S, Gessier F, Kelly LM, Vanek M, Laurent S, Preuss I, Miault C, Christen I, Karuna R, Li W, Koo D-I, Suply T, Schmedt C, Peters EC, Falchetto R, Katopodis A, Spanka C, Roy M-O, Detheux M, Chen YA, Schultz PG, Cho CY, Seuwen K, Cyster JG, and Sailer AW. 2011. Oxysterols direct immune cell migration via EBI2. Nature 475: 524–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu C, Yang XV, Wu J, Kuei C, Mani NS, Zhang L, Yu J, Sutton SW, Qin N, Banie H, Karlsson L, Sun S, and Lovenberg TW. 2011. Oxysterols direct B-cell migration through EBI2. Nature 475: 519–523. [DOI] [PubMed] [Google Scholar]
- 76.Kelly LM, Pereira JP, Yi T, Xu Y, and Cyster JG. 2011. EBI2 Guides Serial Movements of Activated B Cells and Ligand Activity Is Detectable in Lymphoid and Nonlymphoid Tissues. J Immunol 187: 3026–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yi T, and Cyster JG. 2013. EBI2-mediated bridging channel positioning supports splenic dendritic cell homeostasis and particulate antigen capture. eLife 2: 309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gatto D, Wood K, Caminschi I, Murphy-Durland D, Schofield P, Christ D, Karupiah G, and Brink R. 2013. The chemotactic receptor EBI2 regulates the homeostasis, localization and immunological function of splenic dendritic cells. Nat Immunol 14: 446–453. [DOI] [PubMed] [Google Scholar]
- 79.Lu E, Dang EV, McDonald JG, and Cyster JG. 2017. Distinct oxysterol requirements for positioning naïve and activated dendritic cells in the spleen. Sci. Immunol. 2: eaal5237–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li J, Lu E, Yi T, and Cyster JG. 2016. EBI2 augments Tfh cell fate by promoting interaction with IL-2-quenching dendritic cells. Nature Publishing Group 533: 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baptista AP, Gola A, Huang Y, Milanez-Almeida P, Torabi-Parizi P, Jr JFU., Shapiro VS, Gerner MY, and Germain RN. 2019. The Chemoattractant Receptor Ebi2 Drives Intranodal Naive CD4+ T Cell Peripheralization to Promote Effective Adaptive Immunity. Immunity 50: 1188–1201.e6. [DOI] [PubMed] [Google Scholar]
- 82.Rodda LB, Lu E, Bennett ML, Sokol CL, Wang X, Luther SA, Barres BA, Luster AD, Ye CJ, and Cyster JG. 2018. Single-Cell RNA Sequencing of Lymph Node Stromal Cells Reveals Niche-Associated Heterogeneity. Immunity 48: 1014–1028.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Endo-Umeda K, Nakashima H, Komine-Aizawa S, Umeda N, Seki S, and Makishima M. 2018. Liver X receptors regulate hepatic F4/80+CD11b+ Kupffer cells/macrophages and innate immune responses in mice. Sci Rep-uk 8: 9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beaven SW, Wroblewski K, Wang J, Hong C, Bensinger S, Tsukamoto H, and Tontonoz P. 2011. Liver X Receptor Signaling Is a Determinant of Stellate Cell Activation and Susceptibility to Fibrotic Liver Disease. Gastroenterology 140: 1052–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang YY, Dahle MK, Ågren J, Myhre AE, Reinholt FP, Foster SJ, Collins JL, Thiemermann C, Aasen AO, and Wang JE. 2006. activation of the liver x receptor protects against hepatic injury in endotoxemia by suppressing kupffer cell activation. Shock 25: 141–146. [DOI] [PubMed] [Google Scholar]
- 86.Rong S, Cortés VA, Rashid S, Anderson NN, McDonald JG, Liang G, Moon Y-A, Hammer RE, and Horton JD. 2017. Expression of SREBP-1c Requires SREBP-2-mediated Generation of a Sterol Ligand for LXR in Livers of Mice. Elife 6: e25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Horn CL, Morales AL., Savard C, Farrell GC, and Ioannou GN. 2022. Role of Cholesterol-Associated Steatohepatitis in the Development of NASH. Hepatology Commun 6: 12–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koo S-Y, Park E-J, and Lee C-W. 2020. Immunological distinctions between nonalcoholic steatohepatitis and hepatocellular carcinoma. Exp Mol Medicine 52: 1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raselli T, Hearn T, Wyss A, Atrott K, Peter A, Frey-Wagner I, Spalinger MR, Maggio EM, Sailer AW, Schmitt J, Schreiner P, Moncsek A, Mertens J, Scharl M, Griffiths WJ, Bueter M, Geier A, Rogler G, Wang Y, and Misselwitz B. 2019. Elevated oxysterol levels in human and mouse livers reflect nonalcoholic steatohepatitis. J Lipid Res 60: 1270–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reboldi A, and Cyster JG. 2016. Peyer’s patches: organizing B-cell responses at the intestinal frontier. Immunological Reviews 271: 230–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D’Amato M, Jong DD, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, Vos MD, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, and Mathew CG. 2012. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491: 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruiz F, Wyss A, Rossel J, Sulz MC, Brand S, Moncsek A, Mertens JC, Roth R, Clottu AS, Burri E, Juillerat P, Biedermann L, Greuter T, Rogler G, Pot C, Misselwitz B, and Group SICS. 2021. A single nucleotide polymorphism in the gene for GPR183 increases its surface expression on blood lymphocytes of patients with inflammatory bowel disease. Brit J Pharmacol 178: 3157–3175. [DOI] [PubMed] [Google Scholar]
- 93.Wyss A, Raselli T, Perkins N, Ruiz F, Schmelczer G, Klinke G, Moncsek A, Roth R, Spalinger MR, Hering L, Atrott K, Lang S, Frey-Wagner I, Mertens JC, Scharl M, Sailer AW, Pabst O, Hersberger M, Pot C, Rogler G, and Misselwitz B. 2019. The EBI2-oxysterol axis promotes the development of intestinal lymphoid structures and colitis. Mucosal Immunol 12: 733–745. [DOI] [PubMed] [Google Scholar]
- 94.Emgård J, Kammoun H, García-Cassani B, Chesné J, Parigi SM, Jacob J-M, Cheng H-W, Evren E, Das S, Czarnewski P, Sleiers N, Melo-Gonzalez F, Kvedaraite E, Svensson M, Scandella E, Hepworth MR, Huber S, Ludewig B, Peduto L, Villablanca EJ, Veiga-Fernandes H, Pereira JP, Flavell RA, and Willinger T. 2018. Oxysterol Sensing through the Receptor GPR183 Promotes the Lymphoid-Tissue-Inducing Function of Innate Lymphoid Cells and Colonic Inflammation. Immunity 48: 120–132.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chu C, Moriyama S, Li Z, Zhou L, Flamar A-L, Klose CSN, Moeller JB, Putzel GG, Withers DR, Sonnenberg GF, and Artis D. 2018. Anti-microbial Functions of Group 3 Innate Lymphoid Cells in Gut-Associated Lymphoid Tissues Are Regulated by G-Protein-Coupled Receptor 183. CellReports 23: 3750–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, Alonzo F, Ng C, Chen A, Lin X, Sczesnak A, Liao J-J, Torres VJ, Jenkins MK, Lafaille JJ, and Littman DR. 2014. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature 510: 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG, Laufer TM, Ignatowicz L, and Ivanov II. 2014. Segmented Filamentous Bacteria Antigens Presented by Intestinal Dendritic Cells Drive Mucosal Th17 Cell Differentiation. Immunity 40: 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sonnenberg GF, and Artis D. 2012. Innate Lymphoid Cell Interactions with Microbiota: Implications for Intestinal Health and Disease. Immunity 37: 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, Tardif MR, Sathaliyawala T, Kubota M, Farber DL, Collman RG, Shaked A, Fouser LA, Weiner DB, Tessier PA, Friedman JR, Kiyono H, Bushman FD, Chang K-M, and Artis D. 2012. Innate Lymphoid Cells Promote Anatomical Containment of Lymphoid-Resident Commensal Bacteria. Science 336: 1321–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Altmann SW, Davis HR, Zhu L, Yao X, Hoos LM, Tetzloff G, Iyer SPN, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, and Graziano MP. 2004. Niemann-Pick C1 Like 1 Protein Is Critical for Intestinal Cholesterol Absorption. Science 303: 1201–1204. [DOI] [PubMed] [Google Scholar]
- 101.H. R. D. Jr., Zhu L, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SPN, Lam M-H, Lund EG, Detmers PA, Graziano MP, and Altmann SW. 2004. Niemann-Pick C1 Like 1 (NPC1L1) Is the Intestinal Phytosterol and Cholesterol Transporter and a Key Modulator of Whole-body Cholesterol Homeostasis. J. Biol. Chem. 279: 33586–33592. [DOI] [PubMed] [Google Scholar]
- 102.Mansbach CM, and Siddiqi SA. 2010. The Biogenesis of Chylomicrons. Annu. Rev. Physiol. 72: 315–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Randolph GJ, and Miller NE. 2014. Lymphatic transport of high-density lipoproteins and chylomicrons. J. Clin. Invest. 124: 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Emgård J, Kammoun H, García-Cassani B, Chesné J, Parigi SM, Jacob J-M, Cheng H-W, Evren E, Das S, Czarnewski P, Sleiers N, Melo-Gonzalez F, Kvedaraite E, Svensson M, Scandella E, Hepworth MR, Huber S, Ludewig B, Peduto L, Villablanca EJ, Veiga-Fernandes H, Pereira JP, Flavell RA, and Willinger T. 2018. Oxysterol Sensing through the Receptor GPR183 Promotes the Lymphoid-Tissue-Inducing Function of Innate Lymphoid Cells and Colonic Inflammation. Immunity 48: 120–132.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fessler MB, and Summer RS. 2016. Surfactant Lipids at the Host–Environment Interface. Metabolic Sensors, Suppressors, and Effectors of Inflammatory Lung Disease. Am J Resp Cell Mol 54: 624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vockeroth D, Gunasekara L, Amrein M, Possmayer F, Lewis JF, and Veldhuizen RAW. 2010. Role of cholesterol in the biophysical dysfunction of surfactant in ventilator-induced lung injury. Am J Physiol-lung C 298: L117–L125. [DOI] [PubMed] [Google Scholar]
- 107.Turley SD, Andersen JM, and Dietschy JM. 1981. Rates of sterol synthesis and uptake in the major organs of the rat in vivo. J Lipid Res 22: 551–69. [PubMed] [Google Scholar]
- 108.Yu X, Buttgereit A, Lelios I, Utz SG, Cansever D, Becher B, and Greter M. 2017. The Cytokine TGF-β Promotes the Development and Homeostasis of Alveolar Macrophages. Immunity 47: 903–912.e4. [DOI] [PubMed] [Google Scholar]
- 109.Bottemanne P, Paquot A, Ameraoui H, Guillemot-Legris O, Alhouayek M, and Muccioli GG. 2021. 25-Hydroxycholesterol metabolism is altered by lung inflammation, and its local administration modulates lung inflammation in mice. Faseb J 35: e21514. [DOI] [PubMed] [Google Scholar]
- 110.Madenspacher JH, Morrell ED, Gowdy KM, McDonald JG, Thompson BM, Muse GW, Martinez J, Thomas SY, Mikacenic C, Nick JA, Abraham E, Garantziotis S, Stapleton RD, Meacham JM, Thomassen MJ, Janssen WJ, Cook DN, Wurfel MM, and Fessler MB. 2020. Cholesterol-25-hydroxylase promotes efferocytosis and resolution of lung inflammation. Jci Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gold ES, Diercks AH, Podolsky I, Podyminogin RL, Askovich PS, Treuting PM, and Aderem A. 2014. 25-Hydroxycholesterol acts as an amplifier of inflammatory signaling. Proceedings of the National Academy of Sciences 111: 10666–10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.SUGIURA H, KOARAI A, ICHIKAWA T, MINAKATA Y, MATSUNAGA K, HIRANO T, AKAMATSU K, YANAGISAWA S, FURUSAWA M, UNO Y, YAMASAKI M, SATOMI Y, and ICHINOSE M. 2012. Increased 25-hydroxycholesterol concentrations in the lungs of patients with chronic obstructive pulmonary disease. Respirology 17: 533–540. [DOI] [PubMed] [Google Scholar]
- 113.Liu S-Y, Aliyari R, Chikere K, Li G, Marsden MD, Smith JK, Pernet O, Guo H, Nusbaum R, Zack JA, Freiberg AN, Su L, Lee B, and Cheng G. 2013. Interferon-Inducible Cholesterol-25-Hydroxylase Broadly Inhibits Viral Entry by Production of 25-Hydroxycholesterol. Immunity 38: 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koarai A, Yanagisawa S, Sugiura H, Ichikawa T, Kikuchi T, Furukawa K, Akamatsu K, Hirano T, Nakanishi M, Matsunaga K, Minakata Y, and Ichinose M. 2012. 25-hydroxycholesterol enhances cytokine release and toll-like receptor 3 response in airway epithelial cells. Respir Res 13: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Andersson S, Davis DL, Dahlbäck H, Jörnvall H, and Russell DW. 1989. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biological Chem 264: 8222–9. [PubMed] [Google Scholar]
- 116.Babiker A, Andersson O, Lindblom D, van der Linden J, Wiklund B, Lütjohann D, Diczfalusy U, and Björkhem I. 1999. Elimination of cholesterol as cholestenoic acid in human lung by sterol 27-hydroxylase: evidence that most of this steroid in the circulation is of pulmonary origin. J Lipid Res 40: 1417–25. [PubMed] [Google Scholar]
- 117.Kikuchi T, Sugiura H, Koarai A, Ichikawa T, Minakata Y, Matsunaga K, Nakanishi M, Hirano T, Akamatsu K, Yanagisawa S, Furukawa K, Kawabata H, and Ichinose M. 2012. Increase of 27-Hydroxycholesterol in the Airways of Patients With COPD Possible Role of 27-Hydroxycholesterol in Tissue Fibrosis. Chest 142: 329–337. [DOI] [PubMed] [Google Scholar]
- 118.Civra A, Cagno V, Donalisio M, Biasi F, Leonarduzzi G, Poli G, and Lembo D. 2014. Inhibition of pathogenic non-enveloped viruses by 25-hydroxycholesterol and 27-hydroxycholesterol. Sci Rep-uk 4: 7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Oherle K, Acker E, Bonfield M, Wang T, Gray J, Lang I, Bridges J, Lewkowich I, Xu Y, Ahlfeld S, Zacharias W, Alenghat T, and Deshmukh H. 2020. Insulin-like Growth Factor 1 Supports a Pulmonary Niche that Promotes Type 3 Innate Lymphoid Cell Development in Newborn Lungs. Immunity 52: 716–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leeansyah E, Loh L, Nixon DF, and Sandberg JK. 2014. Acquisition of innate-like microbial reactivity in mucosal tissues during human fetal MAIT-cell development. Nat Commun 5: 3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maele LV, Carnoy C, Cayet D, Ivanov S, Porte R, Deruy E, Chabalgoity JA, Renauld J-C, Eberl G, Benecke AG, Trottein F, Faveeuw C, and Sirard J-C. 2014. Activation of Type 3 Innate Lymphoid Cells and Interleukin 22 Secretion in the Lungs During Streptococcus pneumoniae Infection. J Infect Dis 210: 493–503. [DOI] [PubMed] [Google Scholar]
- 122.Guo XJ, Dash P, Crawford JC, Allen EK, Zamora AE, Boyd DF, Duan S, Bajracharya R, Awad WA, Apiwattanakul N, Vogel P, Kanneganti T-D, and Thomas PG. 2018. Lung γδ T Cells Mediate Protective Responses during Neonatal Influenza Infection that Are Associated with Type 2 Immunity. Immunity 49: 531–544.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Spidale NA, Frascoli M, and Kang J. 2019. γδTCR-independent origin of neonatal γδ T cells prewired for IL-17 production. Curr Opin Immunol 58: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haas JD, Ravens S, Düber S, Sandrock I, Oberdörfer L, Kashani E, Chennupati V, Föhse L, Naumann R, Weiss S, Krueger A, Förster R, and Prinz I. 2012. Development of Interleukin-17-Producing γδ T Cells Is Restricted to a Functional Embryonic Wave. Immunity 37: 48–59. [DOI] [PubMed] [Google Scholar]
- 125.Garman RD, Doherty PJ, and Raulet DH. 1986. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell 45: 733–742. [DOI] [PubMed] [Google Scholar]
- 126.Guo XJ, Dash P, Crawford JC, Allen EK, Zamora AE, Boyd DF, Duan S, Bajracharya R, Awad WA, Apiwattanakul N, Vogel P, Kanneganti T-D, and Thomas PG. 2018. Lung γδ T Cells Mediate Protective Responses during Neonatal Influenza Infection that Are Associated with Type 2 Immunity. Immunity 49: 531–544.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gensollen T, Lin X, Zhang T, Pyzik M, See P, Glickman JN, Ginhoux F, Waldor M, Salmi M, Rantakari P, and Blumberg RS. 2021. Embryonic macrophages function during early life to determine invariant natural killer T cell levels at barrier surfaces. Nat Immunol 22: 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.BJÖRKHEM I 2006. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med 260: 493–508. [DOI] [PubMed] [Google Scholar]
- 129.Lund EG, Xie C, Kotti T, Turley SD, Dietschy JM, and Russell DW. 2003. Knockout of the Cholesterol 24-Hydroxylase Gene in Mice Reveals a Brain-specific Mechanism of Cholesterol Turnover*. J Biol Chem 278: 22980–22988. [DOI] [PubMed] [Google Scholar]
- 130.Iuliano L, Crick PJ, Zerbinati C, Tritapepe L, Abdel-Khalik J, Poirot M, Wang Y, and Griffiths WJ. 2015. Cholesterol metabolites exported from human brain. Steroids 99: 189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meaney S, Heverin M, Panzenboeck U, Ekström L, Axelsson M, Andersson U, Diczfalusy U, Pikuleva I, Wahren J, Sattler W, and Björkhem I. 2007. Novel route for elimination of brain oxysterols across the blood-brain barrier: conversion into 7α-hydroxy-3-oxo-4-cholestenoic acid. J Lipid Res 48: 944–951. [DOI] [PubMed] [Google Scholar]
- 132.Cali JJ, Hsieh CL, Francke U, and Russell DW. 1991. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J Biological Chem 266: 7779–83. [PMC free article] [PubMed] [Google Scholar]
- 133.Skrede S, Björkhem I, Buchmann MS, Hopen G, and Fausa O. 1985. A novel pathway for biosynthesis of cholestanol with 7 alpha-hydroxylated C27-steroids as intermediates, and its importance for the accumulation of cholestanol in cerebrotendinous xanthomatosis. J Clin Invest 75: 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu Z, Martin KO, Javitt NB, and Chiang JY. 1999. Structure and functions of human oxysterol 7alpha-hydroxylase cDNAs and gene CYP7B1. J Lipid Res 40: 2195–203. [PubMed] [Google Scholar]
- 135.Schöls L, Rattay TW, Martus P, Meisner C, Baets J, Fischer I, Jägle C, Fraidakis MJ, Martinuzzi A, Saute JA, Scarlato M, Antenora A, Stendel C, Höflinger P, Lourenco CM, Abreu L, Smets K, Paucar M, Deconinck T, Bis DM, Wiethoff S, Bauer P, Arnoldi A, Marques W, Jardim LB, Hauser S, Criscuolo C, Filla A, Züchner S, Bassi MT, Klopstock T, Jonghe PD, Björkhem I, and Schüle R. 2017. Hereditary spastic paraplegia type 5: natural history, biomarkers and a randomized controlled trial. Brain 140: 3112–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meljon A, Crick PJ, Yutuc E, Yau JL, Seckl JR, Theofilopoulos S, Arenas E, Wang Y, and Griffiths WJ. 2019. Mining for Oxysterols in Cyp7b1−/− Mouse Brain and Plasma: Relevance to Spastic Paraplegia Type 5. Biomol 9: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, Walker AJ, Gergits F, Segel M, Nemesh J, Marsh SE, Saunders A, Macosko E, Ginhoux F, Chen J, Franklin RJM, Piao X, McCarroll SA, and Stevens B. 2019. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 50: 253–271.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Haimon Z, Volaski A, Orthgiess J, Boura-Halfon S, Varol D, Shemer A, Yona S, Zuckerman B, David E, Chappell-Maor L, Bechmann I, Gericke M, Ulitsky I, and Jung S. 2018. Re-evaluating microglia expression profiles using RiboTag and cell isolation strategies. Nat Immunol 19: 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wanke F, Moos S, Croxford AL, Heinen AP, Gräf S, Kalt B, Tischner D, Zhang J, Christen I, Bruttger J, Yogev N, Tang Y, Zayoud M, Israel N, Karram K, Reißig S, Lacher SM, Reichhold C, Mufazalov IA, Ben-Nun A, Kuhlmann T, Wettschureck N, Sailer AW, Rajewsky K, Casola S, Waisman A, and Kurschus FC. 2017. EBI2 Is Highly Expressed in Multiple Sclerosis Lesions and Promotes Early CNS Migration of Encephalitogenic CD4 T Cells. Cell Reports 18: 1270–1284. [DOI] [PubMed] [Google Scholar]
- 140.Chalmin F, Rochemont V, Lippens C, Clottu A, Sailer AW, Merkler D, Hugues S, and Pot C. 2015. Oxysterols regulate encephalitogenic CD4+ T cell trafficking during central nervous system autoimmunity. J Autoimmun 56: 45–55. [DOI] [PubMed] [Google Scholar]
- 141.Lima K. A. de, Rustenhoven J, Mesquita SD, Wall M, Salvador AF, Smirnov I, Cebinelli GM, Mamuladze T, Baker W, Papadopoulos Z, Lopes MB, Cao WS, Xie XS, Herz J, and Kipnis J. 2020. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat Immunol 21: 1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ribeiro M, Brigas HC, Temido-Ferreira M, Pousinha PA, Regen T, Santa C, Coelho JE, Marques-Morgado I, Valente CA, Omenetti S, Stockinger B, Waisman A, Manadas B, Lopes LV, Silva-Santos B, and Ribot JC. 2019. Meningeal γδ T cell–derived IL-17 controls synaptic plasticity and short-term memory. Science Immunology 4: eaay5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lima K. A. de, Rustenhoven J, Mesquita SD, Wall M, Salvador AF, Smirnov I, Cebinelli GM, Mamuladze T, Baker W, Papadopoulos Z, Lopes MB, Cao WS, Xie XS, Herz J, and Kipnis J. 2020. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nature Immunology 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, and Huh JR. 2016. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351: 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Reed MD, Yim YS, Wimmer RD, Kim H, Ryu C, Welch GM, Andina M, King HO, Waisman A, Halassa MM, Huh JR, and Choi GB. 2020. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature 577: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, Paust S, Wood JN, and von Andrian UH. 2014. Nociceptive sensory neurons drive interleukin-23- mediated psoriasiform skin inflammation. Nature 510: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Spidale NA, Malhotra N, Frascoli M, Sylvia K, Miu B, Freeman C, Stadinski BD, Huseby E, and Kang J. 2020. Neonatal-derived IL-17 producing dermal γδ T cells are required to prevent spontaneous atopic dermatitis. eLife 9: 596–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Reynolds G, Vegh P, Fletcher J, Poyner EFM, Stephenson E, Goh I, Botting RA, Huang N, Olabi B, Dubois A, Dixon D, Green K, Maunder D, Engelbert J, Efremova M, Polanski K, Jardine L, Jones C, Ness T, Horsfall D, McGrath J, Carey C, Popescu D-M, Webb S, Wang X-N, Sayer B, Park J-E, Negri VA, Belokhvostova D, Lynch MD, McDonald D, Filby A, Hagai T, Meyer KB, Husain A, Coxhead J, Vento-Tormo R, Behjati S, Lisgo S, Villani A-C, Bacardit J, Jones PH, O’Toole EA, Ogg GS, Rajan N, Reynolds NJ, Teichmann SA, Watt FM, and Haniffa M. 2021. Developmental cell programs are co-opted in inflammatory skin disease. Science 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong H-A, Lai K, Ahn R, Corbin K, Lowe MM, Scharschmidt TC, Taravati K, Tan MR, Ricardo-Gonzalez RR, Nosbaum A, Bertolini M, Liao W, Nestle FO, Paus R, Cotsarelis G, Abbas AK, and Rosenblum MD. 2017. Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell 169: 1119–1123.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kobayashi T, Voisin B, Kim DY, Kennedy EA, Jo J-H, Shih H-Y, Truong A, Doebel T, Sakamoto K, Cui C-Y, Schlessinger D, Moro K, Nakae S, Horiuchi K, Zhu J, Leonard WJ, Kong HH, and Nagao K. 2019. Homeostatic Control of Sebaceous Glands by Innate Lymphoid Cells Regulates Commensal Bacteria Equilibrium. Cell 176: 982–997.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brown MS, and Goldstein JL. 1997. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89: 331–340. [DOI] [PubMed] [Google Scholar]
- 152.Kusnadi A, Park SH, Yuan R, Pannellini T, Giannopoulou E, Oliver D, Lu T, Park-Min K-H, and Ivashkiv LB. 2019. The Cytokine TNF Promotes Transcription Factor SREBP Activity and Binding to Inflammatory Genes to Activate Macrophages and Limit Tissue Repair. Immunity 51: 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhou QD, Chi X, Lee MS, Hsieh WY, Mkrtchyan JJ, Feng A-C, He C, York AG, Bui VL, Kronenberger EB, Ferrari A, Xiao X, Daly AE, Tarling EJ, Damoiseaux R, Scumpia PO, Smale ST, Williams KJ, Tontonoz P, and Bensinger SJ. 2020. Interferon-mediated reprogramming of membrane cholesterol to evade bacterial toxins. Nat Immunol 21: 746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Abrams ME, Johnson KA, Perelman SS, Zhang L-S, Endapally S, Mar KB, Thompson BM, McDonald JG, Schoggins JW, Radhakrishnan A, and Alto NM. 2020. Oxysterols provide innate immunity to bacterial infection by mobilizing cell surface accessible cholesterol. Nature Microbiology 5: 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Spidale NA, Sylvia K, Narayan K, Miu B, Frascoli M, Melichar HJ, Zhihao W, Kisielow J, Palin A, Serwold T, Love P, Kobayashi M, Yoshimoto M, Jain N, and Kang J. 2018. Interleukin-17-Producing γδ T Cells Originate from SOX13+ Progenitors that Are Independent of γδTCR Signaling. Immunity 49: 857–872.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]


