ABSTRACT
Objective
This study examined the effects of oral and topical (PR Lotion; Momentous) sodium bicarbonate (NaHCO3) during a battery of team sport-specific exercise tests.
Method
In a block randomized, crossover, double-blind, placebo-controlled design, 14 recreationally trained male team sport athletes performed a familiarization visit and three experimental trials receiving: (i) 0.3 g·kg−1 body mass (BM) NaHCO3 in capsules + placebo lotion (SB-ORAL), (ii) placebo capsules +0.9036 g·kg−1 BM PR Lotion (SB-LOTION), or (iii) placebo capsules + placebo lotion (PLA). Supplements were given ~120 min prior to the team sport-specific exercise tests: countermovement jumps (CMJ), 8 × 25 m repeated sprints and Yo-Yo Intermittent Recovery Level 2 (Yo-Yo IR2). Blood acid–base balance (pH, bicarbonate) and electrolytes (sodium, potassium) were measured throughout. Rating of perceived exertion (RPE) was recorded after each sprint and post-Yo-Yo IR2.
Results
Distance covered during the Yo-Yo IR2 was 21% greater for SB-ORAL compared with PLA (+94 m; p = 0.009, d = 0.64) whereas performance was only 7% greater for SB-LOTION compared with PLA (480 ± 122 vs. 449 ± 110 m; p = 0.084). Total completion time for the 8 × 25 m repeated sprint test was 1.9% faster for SB-ORAL compared with PLA (−0.61 s; p = 0.020, d = 0.38) and 2.0% faster for SB-LOTION compared with PLA (−0.64 s; p = 0.036, d = 0.34). CMJ performance was similar between treatments (p > 0.05). Blood acid–base balance and electrolytes were significantly improved for SB-ORAL compared with PLA, but no differences were observed for SB-LOTION. Compared to PLA, RPE was lower for SB-LOTION after the fifth (p = 0.036), sixth (p = 0.012), and eighth (p = 0.040) sprints and for SB-ORAL after the sixth (p = 0.039) sprint.
Conclusions
Oral NaHCO3 improved 8 × 25 m repeated sprint (~2%) and Yo-Yo IR2 performance (21%). Similar improvements in repeated sprint times were observed for topical NaHCO3 (~2%), but no significant benefits were reported for Yo-Yo IR2 distance or blood acid–base balance compared to PLA. These findings suggest that PR Lotion might not be an effective delivery system for transporting NaHCO3 molecules across the skin and into systematic circulation, therefore further research is needed to elucidate the physiological mechanisms responsible for the ergogenic effects of PR Lotion.
KEYWORDS: Supplements, alkalosis, team sports, Yo-Yo intermittent recovery test level 2, repeated sprint ability
1. Introduction
Athletes competing in field-based team sports repeatedly perform high-intensity efforts (i.e. sprints, jumps) interspersed with short rest periods [1]. Repeated sprint ability (RSA) is an important determinant of success in team sports but declines throughout competitive matches [2]. Substantial anaerobic energy demand from completing maximal sprint efforts leads to the accumulation of hydrogen ions (H+) within muscles [3], which may cause an intramuscular acidosis. While the deleterious effects of declining pH during exercise are debated [4], a cellular acidosis is suggested to inhibit energy production via anaerobic glycolysis and limit action potentials required for muscle contractions [3,5,6]. These biochemical changes contribute toward skeletal muscle fatigue [7,8], therefore nutritional strategies that restore acid–base balance could prove beneficial during team sport exercise.
Sodium bicarbonate (NaHCO3) is an extracellular buffering aid that has been extensively researched [9]. Ingestion of 0.3 g·kg−1 body mass (BM) NaHCO3 in fluid or capsules 90–120 min pre-exercise raises the concentration of blood bicarbonate (HCO3−) by ~5.0–6.0 mmol·L−1, which elevates the pH gradient between intracellular and extracellular compartments, subsequently upregulating the lactate-H+ co-transporter to remove H+ from muscles [10]. Additionally, ingesting NaHCO3 increases the amount of sodium (Na+) in the blood [11,12], which may lead to improvements in hydration status [13]. Considering that NaHCO3 ingestion may attenuate losses of intramuscular potassium (K+) during exercise [12], these changes in electrolytes could also result in upregulation of Na+/K+-ATPase activity to limit muscle depolarization and sustain excitation–contraction coupling [8].
An established method for evaluating the ergogenic effect of NaHCO3 on team sport-specific exercise performance is the Yo-Yo Intermittent Recovery Test Level 2 (Yo-Yo IR2) [14]. NaHCO3 ingestion has improved distance covered during the Yo-Yo IR2 [15,16]. Similar results have been reported in team sport athletes for the effect of NaHCO3 on RSA (3 sets, 6 × 20 m) [17]. Interestingly, others have shown no improvements for RSA in rugby [18] and soccer [19] players after 0.3 g·kg−1 BM NaHCO3. It is possible that gastrointestinal (GI) discomfort commonly associated with NaHCO3 ingestion prevented these participants from improving their performance [18,20]. Orally ingested NaHCO3 dissociates into Na+ and HCO3− upon reaching the stomach, where the HCO3− neutralizes gastric acid, which generates excessive carbon dioxide production that can induce belching and vomiting [21]. These large amounts of exogenous Na+ may also aggravate intestinal mucosa and prompt osmotic fluctuations that can cause diarrhea [18]. Many athletes are deterred from using NaHCO3 due to these GI side effects, the poor palatability of fluid beverages and/or the high number of capsules (~35 for a 90 kg athlete) required to achieve an ergogenic dose [22]. NaHCO3 supplementation strategies that bypass the GI tract could therefore provide a favorable alternative to oral ingestion [23].
One novel approach is PR Lotion (Momentous, Park City, Utah), which is a topically applied muscle cream that is purported to transport NaHCO3 molecules across the skin via a transdermal drug delivery system [24]. PR Lotion adopts an innovative formulation that encapsulates NaHCO3 molecules within fatty acid salts that fluidize the outermost layer of skin and modulate tight junctions in the epidermis, allowing NaHCO3 molecules to be absorbed into the bloodstream [25]. McKay et al. [26] reported that PR Lotion did not significantly improve average power during 3 × 30 s Wingate cycling tests compared to a placebo (p = 0.108). However, they also found no differences in time-to-exhaustion (TTE) cycling performance between oral NaHCO3 and PR Lotion (363 ± 80 vs. 349 ± 119 s, p = 0.697). Given their lack of a placebo group during the TTE cycling task, it is difficult to conclude whether there was an ergogenic effect of PR Lotion. Furthermore, it is not yet known whether PR Lotion alters the concentration of electrolytes and hydration status to a similar degree as oral NaHCO3 ingestion. Additional research comparing oral and topical NaHCO3 is needed before conclusions can be drawn regarding the efficacy of PR Lotion. Therefore, the aim of this study was to examine the effects of oral and topical NaHCO3 (PR Lotion) during a battery of team sport-specific exercise tests. Our hypothesis was that oral and topical NaHCO3 would improve team sport-specific exercise performance (countermovement jumps [CMJ], 8 × 25 m repeated sprints, Yo-Yo IR2) compared with a placebo.
2. Materials and methods
2.1. Study design
A block randomized, double-blind, placebo-controlled, crossover design was used for this study. Participants were randomly allocated to receive each of the three nutritional supplements (oral NaHCO3, topical NaHCO3 and placebo) in a counterbalanced order using an online sequence generator (www.randomization.com).
2.2. Participants
Our sample size calculation conducted on G*Power (version 3.1.9.4) revealed that 15 participants were needed to achieve statistical power (β = 0.80; α=0.05). This assumed that repeated measures analysis of variances (ANOVA; within-factors) would be used to analyze performance outcomes, with an expected medium effect size (ηp2 = 0.06). Correlation between repeated measures was estimated from reliability data for our performance outcomes [27]. To account for 10–20% drop out rates, 18 participants were recruited; however, three withdrew due to injury and one because of time constraints. Therefore, 14 recreationally trained male team sport athletes (body mass: 81.9 ± 10.1 kg; stature: 182.0 ± 5.4 cm; age: 26.5 ± 5.8 years, sporting background: 3 × hockey, 7 × soccer, 2 × basketball, 1 × rugby, 1 × cricket) completed the study. Participants received a £25 gift voucher and free supplements after taking part. Eligibility criteria stated that participants performed ≥2 training sessions per week of their sport. They were excluded if they had: (i) previously used NaHCO3, (ii) an intolerance to cornflour, and (iii) a medical condition that impacts high-intensity exercise. Ethical approval was obtained from the Institutional Ethics Committee (ER42014516). Participants completed a health questionnaire and provided written informed consent before commencing the study.
2.3. Procedures
Participants were instructed to avoid strenuous exercise and alcohol for 24 h prior to sessions. They attended the indoor sports hall on four separate occasions. The first visit was a familiarization session to habituate participants to the team sport-specific exercise tests. During the remaining three sessions, participants completed exercise tests after receiving either oral NaHCO3, topical NaHCO3 or a placebo. These sessions were separated by 5–7 days to ensure appropriate recovery and washout of treatments. Testing was conducted at the same time of day to control for the confounding effect of circadian rhythms on exercise performance [28]. Participants were asked to wear the same footwear for each session and to replicate their diet 24 h prior to each visit.
2.4. Team sport-specific exercise tests
Participants completed a 10-min warm up of jogging, 3 × 10 m sprints, agility drills, stretches, and 3 × 20 m runs at 50%, 70%, and 90% of perceived maximum. After 5 min, they performed a first CMJ using an optimal measurement system (Optojump Next, Microgate, USA). Participants completed one practice and three maximal jumps separated by 1 min. After 3 min, they performed an 8 × 25 m repeated sprint test. Timing gates (Brower timing systems, Draper, Utah, USA) were placed at 0 and 25 m to record sprint times. Following each sprint, participants jogged back to the start line, with sprints departing every 25 s until the protocol had been repeated eight times. Participants received 2 min of recovery and then conducted a second CMJ test. After a further 5 min, they performed the Yo-Yo IR2, which required them to repeatedly carry out 2 × 20 m shuttle runs at increasing speeds dictated by audio signals [14]. Each stage was separated by 10 s recovery where participants jogged around a cone positioned 5 m behind the start line. Test termination was classified as two failures to reach the finish line before the signal, at which point distance covered during the Yo-Yo IR2 was recorded. After 5 min recovery, participants performed a final CMJ test.
2.5. Supplementation protocol
During experimental trials, participants performed the team sport-specific exercise tests after receiving: (i) NaHCO3 in capsules + placebo lotion (SB-ORAL), (ii) placebo capsules + PR Lotion (SB-LOTION), and (iii) placebo capsules + placebo lotion (PLA). Supplements were prepared by a laboratory technician not involved with the study. Oral NaHCO3 was given as a 0.3 g·kg−1 BM dose in size 0 vegetarian capsules (Your Supplements, Stockport, UK). An equal number of capsules (31 ± 4) containing cornflour was used as a placebo for SB-LOTION and PLA. Cornflour is an inert substance that effectively blinds NaHCO3 [22]. Capsules were filled using a capsule filling device (ALL-IN Capsule, USA) and contained either ~0.8 g NaHCO3 (Health Leads Ltd, UK) or ~0.4 g cornflour (Sainsbury’s, UK). These were checked for weight and administered as three equal doses with 7 mL·kg−1 BM water at 15-min intervals across a 30-min period [29] commencing 135 min before the team sport-specific exercise tests. Participants applied PR Lotion to their legs and lower back as a 0.9036 g·kg−1 BM dose during the final 15 min of this 30-min supplementation period. PR Lotion was administered 120 min prior to the team sport-specific exercise tests as evidence suggests peak changes in muscle/blood pH occur ~120 min after applying PR Lotion [30]. PR Lotion is ~33% NaHCO3; therefore, a 0.9036 g·kg−1 BM dose was chosen to theoretically match the amount of NaHCO3 given during SB-ORAL and SB-LOTION. The placebo lotion was matched for all ingredients except NaHCO3 and both lotions were provided in plastic tubs. Supplements were given alongside a carbohydrate-rich meal (1.5 g·kg−1 BM; biscuits, wholegrain cereal bars, cornflakes with milk) to standardize food consumed prior to testing and minimize GI discomfort after NaHCO3 ingestion [29].
2.6. Experimental trials
Baseline urine samples were analyzed for color using an 8-point Likert scale [31] and osmolality using an osmometer (Vitech Scientific, Partridge Green, UK). Capillary blood samples (95 μL) were analyzed for acid–base balance (pH, HCO3), electrolytes (Na+, K+), hemoglobin, and hematocrit using a blood gas analyzer (i-STAT Alinity, Abbott, USA). Plasma volume was estimated from hemoglobin and hematocrit concentration [32]. Additional 20 μL blood samples were analyzed for lactate using a Biosen C-Line (EKF Diagnostics, Cardiff, UK). Visual analog scales (VAS) for eight GI side effects were completed to quantify aggregate GI discomfort [11,33].
Supplements were administered across a 30-min window. Participants then completed VAS and treatment assignment questionnaires that asked them to select which treatment they believed had been given (“oral NaHCO3,” “topical NaHCO3,” “placebo,” “unsure”) and explain their reasons [22]. Participants rested for a further 85 min, before blood and urine samples were taken pre-warm-up. They also repeated VAS and treatment assignment questionnaires and rated on 1–5 Likert type scales how much they expected the supplement they thought had been given to improve performance (“1” = no expectations, “5” = extremely high expectations).
After the 10-min warm-up, blood samples were repeated. Participants commenced the team sport-specific exercise tests 120 min after applying PR Lotion (~105 min after the final set of capsules). Additional blood samples were analyzed for lactate after CMJ tests and the repeated sprints. Rating of perceived exertion (RPE; 6–20 Borg scale) was recorded after each sprint and post-Yo-Yo IR2. Blood sampling was repeated pre- and post-Yo-Yo IR2. Post-exercise urine samples, treatment assignment questionnaires, and VAS were completed after the final CMJ test. An overview of experimental procedures is shown in Figure 1.
Figure 1.
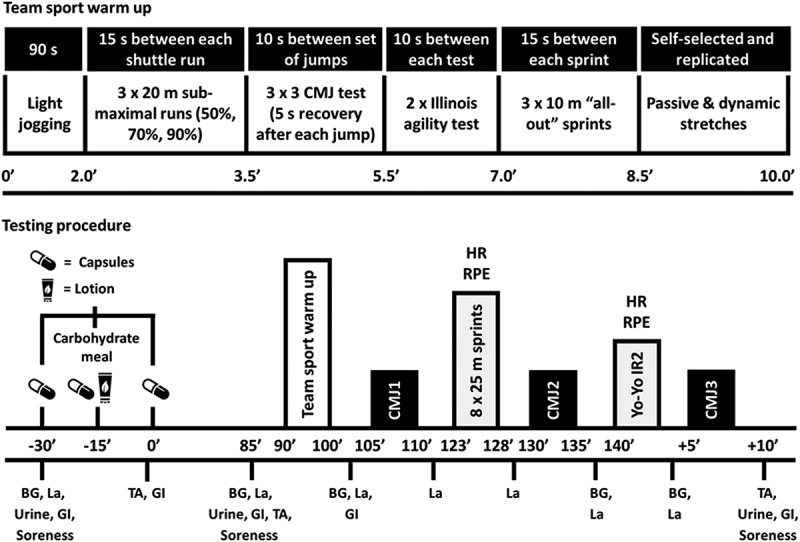
Experimental schematic showing timings (minutes) and procedures for warm up and battery of team sport-specific exercise tests. BG = blood gas (pH, bicarbonate, hemoglobin, hematocrit, sodium, potassium), La = blood lactate, GI = gastrointestinal discomfort questionnaire, TA = treatment assignment questionnaire.
2.7. Statistical analysis
Statistical analyses were performed using SPSS 26.0 for Windows (IBM, Chicago, IL) and Exploratory Software for Confidence Intervals (ESCI, https://thenewstatistics.com/itns/esci/). Grouped data and standardized residuals were assessed for normality using Shapiro–Wilks tests. Homogeneity of variance/sphericity were analyzed using Mauchly tests and any violations were corrected via Greenhouse–Geisser adjustments. Two-way repeated measures ANOVA were used to determine treatment * time interactions for sprint completion times, CMJ performance, blood metabolites, urine osmolality and RPE during the repeated sprint test. All other normally distributed outcome measures were assessed using one-way repeated measures ANOVA. When significant effects were found, post hoc pairwise comparisons were made using Bonferroni correction factors. Effect sizes were calculated using SPSS for ηp2 and ESCI for Cohen’s d. These were interpreted using the classifications of 0.01, 0.06, and 0.14 as small, medium, and large effect sizes for ηp2 and 0.2, 0.5, and 0.8 as small, moderate, and large effect sizes for Cohen’s d [34]. Friedman tests were used to assess treatment effects for non-normally distributed data (GI discomfort, urine color, expectations), with Chi-square (χ2) reported as the test statistic. When significant effects were found, post hoc pairwise comparisons were conducted, with median and Z values presented. Treatment assignment ratings (“correct,” “incorrect”) were analyzed using 2 × 2 χ2 tests to determine blinding efficacy. Mean differences and 95% CI are reported for treatment comparisons. Data are presented as mean ± SD (unless stated) and statistical significance was set at p ≤0.05.
3. Results
3.1. Team sport-specific exercise performance
There was an effect of treatment on Yo-Yo IR2 performance (p = 0.001, ηp2 = 0.414; Figure 2). Total distance covered was 21% greater for SB-ORAL compared with PLA (+94 m; 95% CI: 23, 166; p = 0.009). Cohen’s d revealed a medium effect of SB-ORAL (d = 0.64; 95% CI: 0.21, 1.05). Although not statistically significant, the total distance covered was 13% greater for SB-ORAL compared with SB-LOTION (+63 m; 95% CI: −8, 134; p = 0.089; d = 0.41) and 7% greater for SB-LOTION compared with PLA (+31 m; 95% CI: −3, 66; p = 0.084; d = 0.27).
Figure 2.
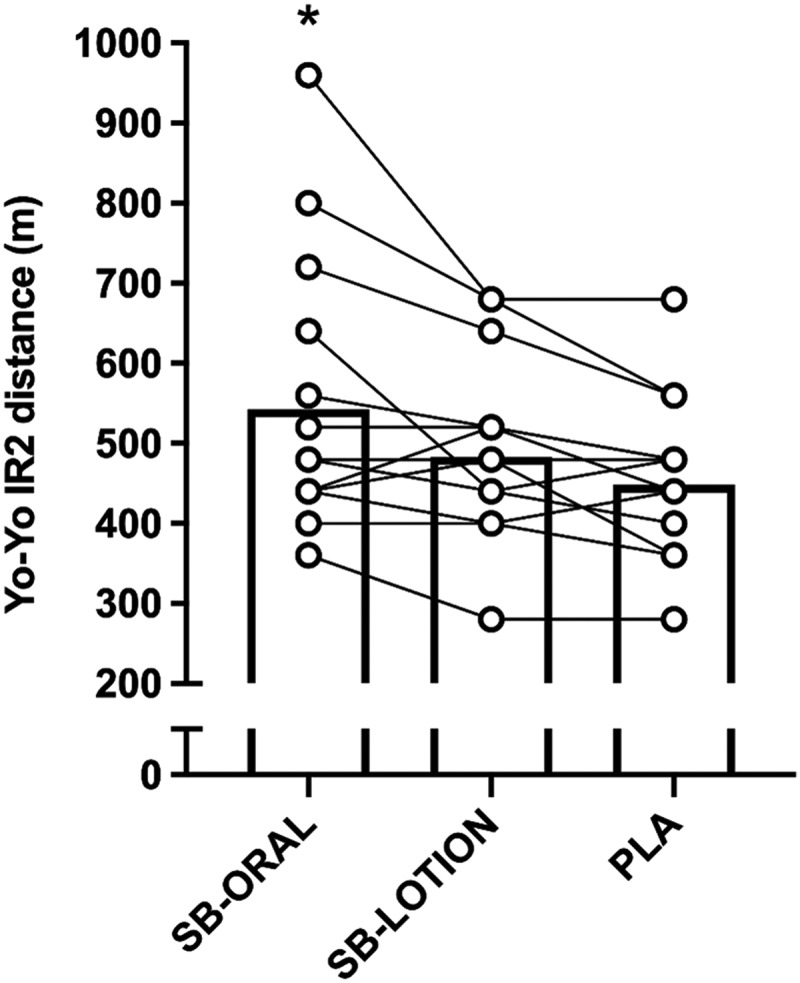
Total distance covered during the Yo-Yo IR2 test. Bars represent mean values. Individual treatment differences depicted by symbol/line. SB-ORAL = oral sodium bicarbonate, SB-LOTION = topical sodium bicarbonate, PLA = placebo; * greater than PLA (p < 0.05). .
There was no significant treatment * time interaction for individual sprint times during the repeated sprint test (p = 0.490, ηp2 = 0.069) but there was an effect of treatment (p = 0.024, ηp2 = 0.250). Significant differences in completion times for individual sprints are shown in Table 1. Cohen’s d revealed medium effects of SB-ORAL during the fifth (d = 0.59, 95% CI: 0.19, 0.97), sixth (d = 0.52, 95% CI: 0.14, 0.87), and eighth (d = 0.61, 95% CI: 0.10, 1.10) sprints. Cohen’s d revealed a medium effect of SB-LOTION during the eighth (d = 0.56, 95% CI: 0.14, 0.97) and small effects during the fifth (d = 0.47, 95% CI: 0.09, 0.83) and sixth (d = 0.49, 95% CI: 0.14, 0.83) sprints. Completion times for individual sprints were similar for SB-ORAL and SB-LOTION (all p > 0.05; Table 1).
Table 1.
Completion times (s) for individual sprints during the 8 × 25 m repeated sprint test.
| Sprint 1 | Sprint 2 | Sprint 3 | Sprint 4 | Sprint 5 | Sprint 6 | Sprint 7 | Sprint 8 | |
|---|---|---|---|---|---|---|---|---|
| SB-ORAL | 4.05 ± 0.26 | 4.10 ± 0.24 | 4.11 ± 0.21 | 4.14 ± 0.21 | 4.09 ± 0.17 * | 4.12 ± 0.23 * | 4.14 ± 0.22 | 4.12 ± 0.21 * |
| SB-LOTION | 4.03 ± 0.26 | 4.07 ± 0.31 | 4.14 ± 0.29 | 4.11 ± 0.29 | 4.10 ± 0.25 * | 4.12 ± 0.25 * | 4.15 ± 0.28 | 4.13 ± 0.21 * |
| PLA | 4.06 ± 0.26 | 4.13 ± 0.24 | 4.18 ± 0.24 | 4.20 ± 0.20 | 4.20 ± 0.21 | 4.23 ± 0.21 | 4.23 ± 0.21 | 4.23 ± 0.15 |
Values are presented as mean ± SD. SB-ORAL = oral sodium bicarbonate, SB-LOTION = topical sodium bicarbonate, PLA = placebo; * faster than PLA (p < 0.05).
There were significant effects of treatment on average (p = 0.025, ηp2 = 0.247) and total (p = 0.024, ηp2 = 0.250) but not fastest (p = 0.227, ηp2 = 0.180) sprint times or decrement score (p = 0.091, ηp2 = 0.169). The average sprint time was 1.8% faster for SB-ORAL compared with PLA (−0.08 s; 95% CI: −0.14, −0.01; p = 0.023) and 1.9% faster for SB-LOTION compared with PLA (−0.08 s; 95% CI: −0.15, −0.01; p = 0.036). Cohen’s d revealed small effects for SB-ORAL (d = 0.38, 95% CI: 0.11, 0.65) and SB-LOTION (d = 0.34, 95% CI: 0.08, 0.60). The total sprint time was 1.8% faster for SB-ORAL compared with PLA (−0.61 s; 95% CI: −1.13, −0.09; p = 0.020) and 2.0% faster for SB-LOTION compared with PLA (−0.64 s; 95% CI: −1.24, −0.04; p = 0.036). Cohen’s d revealed small effects for SB-ORAL (d = 0.38, 95% CI: 0.11, 0.65) and SB-LOTION (d = 0.34, 95% CI: 0.08, 0.60). The average and total sprint times were similar for SB-ORAL and SB-LOTION (both p > 0.05; Figure 3a,b).
Figure 3.
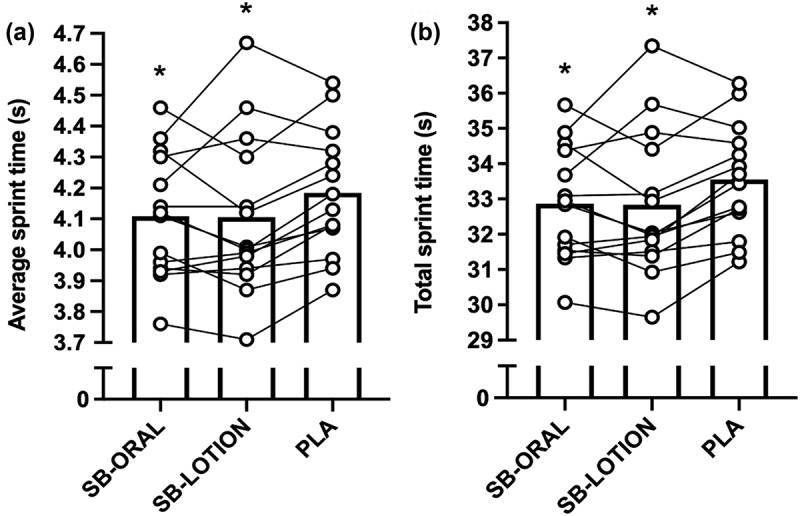
(a, b) 8 × 25 m repeated sprint test average times (a) and total times (b). Bars represent mean values. Individual treatment differences depicted by symbol/line. SB-ORAL = oral sodium bicarbonate, SB-LOTION = topical sodium bicarbonate, PLA = placebo; * faster than PLA (p < 0.05).
There were no significant treatment * time interactions for average (p = 0.922,ηp2 = 0.017) or maximum (p = 0.815, ηp2 = 0.029) CMJ height. There were also no significant treatment effects for average (p = 0.607, ηp2 = 0.038) or maximum (p = 0.746, ηp2 = 0.022) CMJ height.
3.2. Blood acid–base balance and electrolytes
There were significant treatment * time interactions for blood pH (p < 0.001, ηp2 = 0.333) and HCO3− (p < 0.001, ηp2 = 0.516). Blood pH and HCO3− were elevated for SB-ORAL compared with SB-LOTION pre-warm-up (+0.05 au., +5.1 mmol·L−1; both p < 0.001), pretest (+0.06 au., +5.3 mmol·L−1; p = 0.001, p < 0.001), pre-Yo-Yo IR2 (+0.06 au., +5.3 mmol·L−1; both p < 0.001) and post-Yo-Yo IR2 (+0.04 au., +2.4 mmol·L−1; p = 0.019, p = 0.007). Blood pH and HCO3− were elevated for SB-ORAL compared with PLA pre-warm-up (+0.06 au., +5.3 mmol·L−1; both p < 0.001), pretest (+0.08 au., +5.8 mmol·L−1; both p < 0.001), pre-Yo-Yo IR2 (+0.09 au., +6.5 mmol·L−1; both p < 0.001) and post-Yo-Yo IR2 (+0.07 au., +3.2 mmol·L−1; p = 0.003, p < 0.001). No significant differences in blood pH and HCO3− were observed for SB-LOTION compared with PLA pre-warm-up (+0.01 au., +0.2 mmol·L−1; p = 0.969, p = 1.000), pretest (+0.017 au., +0.6 mmol·L−1; p = 0.421, p = 1.000), pre-Yo-Yo IR2 (+0.021 au., +1.1 mmol·L−1; p = 0.527, p = 0.449) or post-Yo-Yo IR2 (+0.03 au., +0.9 mmol·L−1; p = 0.178, p = 0.745) (Figure 4a, b).
Figure 4.
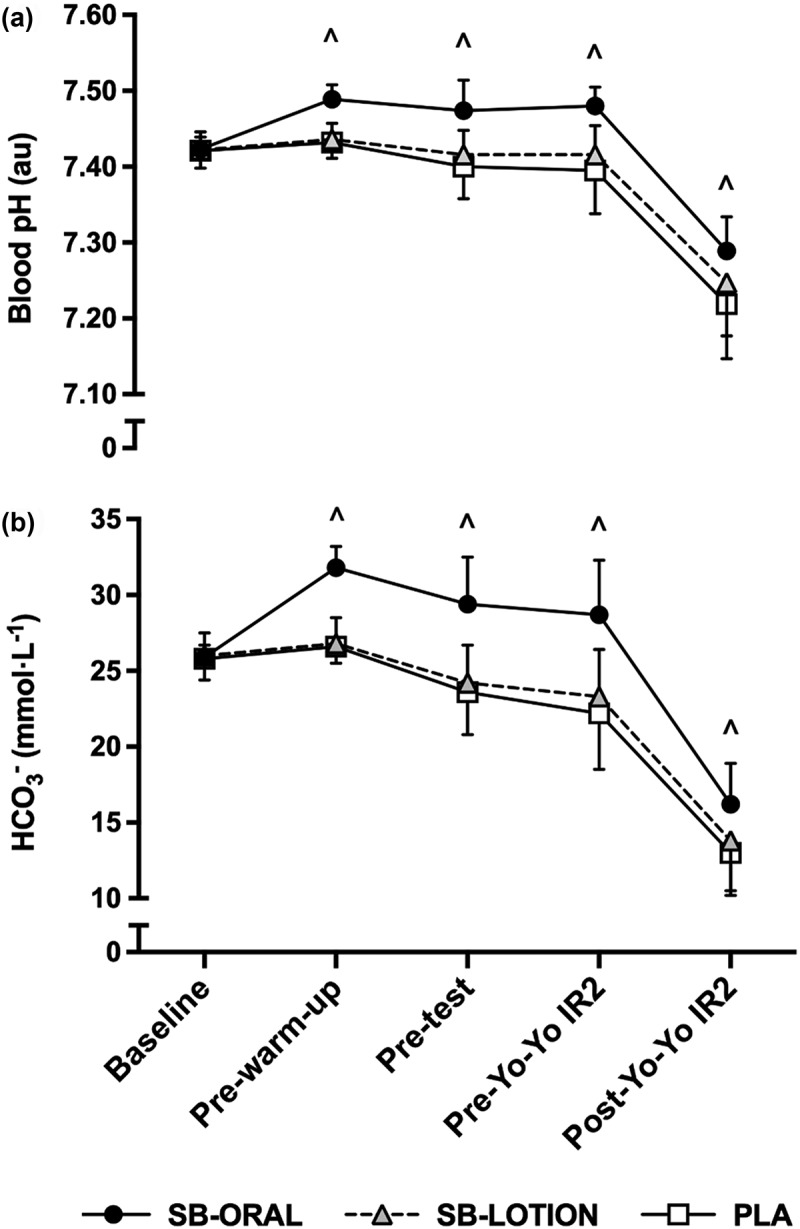
(a, b) Mean ± SD responses for blood acid–base balance (a, pH; b, bicarbonate). Bars represent mean values. Some SD error bars were removed for clarity. SB-ORAL = oral sodium bicarbonate, SB-LOTION = topical sodium bicarbonate, PLA = placebo; ^ elevated for SB-ORAL compared with SB-LOTION and PLA (p < 0.05).
There was a significant treatment * time interaction for blood lactate (p = 0.001, ηp2 = 0.280) but not Na+ (p = 0.244, ηp2 = 0.092) or K+ (p = 0.166, ηp2 = 0.103). There were significant treatment effects for Na+ (p < 0.001, ηp2 = 0.466) and K+ (p = 0.030, ηp2 = 0.237). Post Yo-Yo IR2 blood lactate was higher for SB-ORAL compared with SB-LOTION (+2.65 mmol·L−1; p = 0.009) and PLA (+2.42 mmol·L−1; p = 0.026) but was similar between SB-LOTION and PLA (−0.23 mmol·L−1; p = 1.000). Blood Na+ was elevated for SB-ORAL compared with SB-LOTION and PLA pre-warm up (+1 mmol·L−1, +2 mmol·L−1; p = 0.036, p < 0.001) and compared with PLA pretest (+2 mmol·L−1; p = 0.011), pre-Yo-Yo IR2 (+3 mmol·L−1; p = 0.008) and post-Yo-Yo IR2 (+2 mmol·L−1; p = 0.016). Pre-warm-up blood K+ was lower for SB-ORAL compared with SB-LOTION (−0.4 mmol·L−1; p = 0.019) and PLA (−0.4 mmol·L−1; p = 0.003). No significant differences in blood Na+ and K+ were observed for SB-LOTION compared with PLA pre-warm-up (+1 mmol·L−1, −0.1 mmol·L−1; p = 0.449, p = 1.000), pretest (+1 mmol·L−1, −0.04 mmol·L−1; p = 0.647, p = 1.000), pre-Yo-Yo IR2 (+1 mmol·L−1, −0.2 mmol·L−1; p = 0.804, p = 0.711) or post-Yo-Yo IR2 (+1 mmol·L−1, −0.3 mmol·L−1; p = 1.000, p = 0.458) (Table 2).
Table 2.
Blood lactate and electrolyte response throughout the battery of team sport-specific exercise tests.
| Baseline | Pre-warm-up | Pre-test | Pre-sprints | Post-sprints | Pre-Yo-Yo IR2 | Post-Yo-Yo IR2 | |
|---|---|---|---|---|---|---|---|
| SB-ORAL | |||||||
| BLa− (mmol·L−1) | 1.16 ± 0.15 | 1.32 ± 0.33 | 2.63 ± 0.81 | 2.35 ± 0.46 | 7.13 ± 1.78 | 4.09 ± 1.93 | 13.87 ± 2.90 ^ |
| Na+ (mmol·L−1) | 135 ± 2 | 137 ± 2 * | 137 ± 2 * | - | - | 138 ± 3 * | 139 ± 2 * |
| K+ (mmol·L−1) | 4.4 ± 0.5 | 3.9 ± 0.3 * | 4.0 ± 0.5 | - | - | 3.9 ± 0.4 | 3.8 ± 0.4 |
| SB-LOTION | |||||||
| BLa− (mmol·L−1) | 1.21 ± 0.28 | 1.36 ± 0.45 | 2.71 ± 1.06 | 2.41 ± 0.74 | 7.02 ± 2.43 | 3.82 ± 2.36 | 11.22 ± 2.48 |
| Na+ (mmol·L−1) | 135 ± 2 | 136 ± 2 | 136 ± 2 | - | - | 136 ± 3 | 137 ± 3 |
| K+ (mmol·L−1) | 4.3 ± 0.2 | 4.2 ± 0.4 | 4.2 ± 0.4 | - | - | 4.0 ± 0.4 | 3.9 ± 0.3 |
| PLA | |||||||
| BLa− (mmol·L−1) | 1.15 ± 0.28 | 1.31 ± 0.24 | 3.08 ± 1.68 | 2.55 ± 1.28 | 6.01 ± 2.45 | 4.20 ± 2.42 | 11.45 ± 2.55 |
| Na+ (mmol·L−1) | 134 ± 1 | 135 ± 2 | 135 ± 1 | - | - | 135 ± 2 | 136 ± 2 |
| K+ (mmol·L−1) | 4.3 ± 0.4 | 4.3 ± 0.3 | 4.2 ± 0.4 | - | - | 4.3 ± 0.7 | 4.2 ± 0.8 |
Values are presented as mean ±SD. Blood electrolytes were not measured pre- or post-sprints. SB-ORAL = oral sodium bicarbonate, SB-LOTION = topical sodium bicarbonate, PLA = placebo; * difference compared with PLA, ^ difference compared with SB-LOTION and PLA (p < 0.05).
3.3. Hydration status
There were significant effects of treatment on changes in plasma volume from baseline to pre-warm-up (p < 0.001, ηp2 = 0.457), pretest (p = 0.002, ηp2 = 0.392), pre-Yo-Yo IR2 (p = 0.003, ηp2 = 0.363), and post-Yo-Yo IR2 (p = 0.003, ηp2 = 0.356). Changes in plasma volume from baseline to pre-warm-up were greater for SB-ORAL compared with SB-LOTION (+7.0%; p = 0.030) and PLA (+9.3%; p = 0.004). Plasma volume expansion from baseline was also elevated for SB-ORAL compared with PLA pretest (+8.9%; p = 0.006) and pre-Yo-Yo (+7.6%; p = 0.015). The overall decline in plasma volume from baseline to post-Yo-Yo IR2 was attenuated for SB-ORAL compared with PLA (−7.7%; p = 0.006) but was similar for SB-LOTION compared with PLA (−2.4%; p = 0.765; Figure 5).
Figure 5.
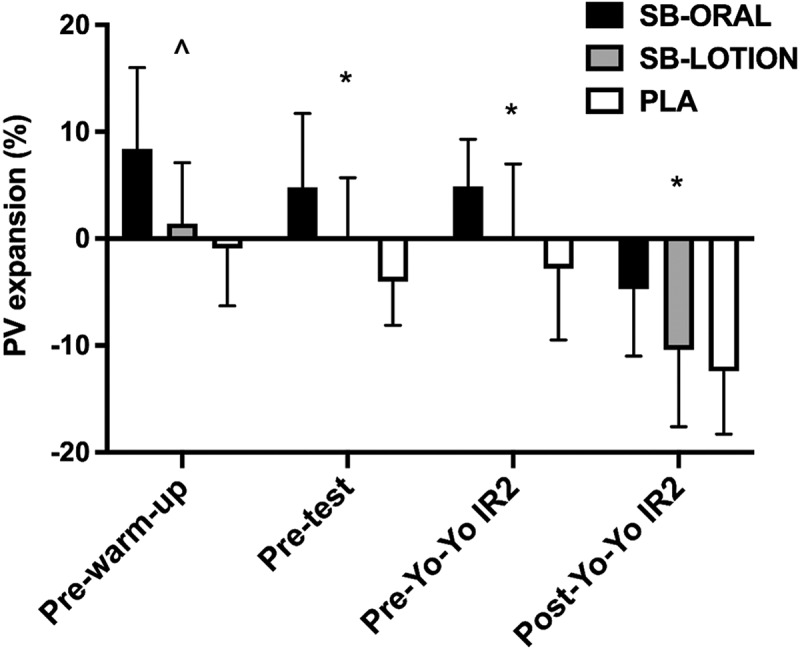
Mean ± SD for changes in plasma volume (PV) from baseline. Bars represent mean values. Some SD error bars were removed for clarity. SB-ORAL = oral sodium bicarbonate, SB-LOTION = topical sodium bicarbonate, PLA = placebo; ^ SB-ORAL higher than SB-LOTION and PLA, * SB-ORAL higher than PLA (p < 0.05).
There was a significant treatment * time interaction for urine osmolality (p = 0.042, ηp2 = 0.171). Post-exercise urine osmolality was higher for SB-ORAL compared with PLA (+224 mOsmols·kgH2O; p = 0.015) but was not statistically different for SB-ORAL compared with SB-LOTION (+148 mOsmols·kgH2O; p = 0.131) or SB-LOTION compared with PLA (500 ± 197 vs. 424 ± 226 mOsmols·kgH2O; p = 0.918). Urine color was similar between treatments at baseline (χ2(2) = 0.140, p = 0.933) and pre-exercise (χ2(2) = 4.512, p = 0.105) but there was a significant treatment effect post-exercise (χ2(2) = 7.538, p = 0.002). Urine color was significantly darker post-exercise for SB-ORAL compared with PLA (median: 4.0 vs. 3.0; Z = 0.857, p = 0.023) but was not significantly different for SB-LOTION (median: 3.5) compared with SB-ORAL (p = 0.156) and PLA (p = 0.395).
3.4. Rating of perceived exertion
There was no significant treatment * time interaction for RPE during the repeated sprint test (p = 0.098, ηp2 = 0.142) but there was a significant treatment effect (p = 0.015, ηp2 = 0.278). RPE was lower for SB-LOTION compared with PLA after the fifth (−0.9 au.; p = 0.036), sixth (−1.1 au.; p = 0.012), and eighth (−1.4 au.; p = 0.040) sprints and for SB-ORAL compared with PLA after the 6th (−0.9 au.; p = 0.039) sprint. Post-Yo-Yo IR2 RPE was similar between treatments (all p > 0.05; Table 3).
Table 3.
RPE (au.) after each of the 8 × 25 m repeated sprints and post-Yo-Yo IR2.
| Sprint 1 | Sprint 2 | Sprint 3 | Sprint 4 | Sprint 5 | Sprint 6 | Sprint 7 | Sprint 8 | Yo-Yo IR2 | |
|---|---|---|---|---|---|---|---|---|---|
| SB-ORAL | 10.7 ± 3.0 | 11.3 ± 2.8 | 12.5 ± 2.8 | 13.4 ± 2.6 | 14.0 ± 2.4 | 14.9 ± 2.2 * | 15.5 ± 2.1 | 16.6 ± 2.0 | 19.0 ± 1.1 |
| SB-LOTION | 10.5 ± 3.1 | 11.7 ± 2.8 | 12.4 ± 2.8 | 13.1 ± 2.7 | 13.9 ± 2.3 * | 14.6 ± 2.0 * | 15.7 ± 1.9 | 16.1 ± 2.2 * | 19.2 ± 0.8 |
| PLA | 10.8 ± 3.2 | 11.6 ± 3.0 | 12.7 ± 2.6 | 14.1 ± 2.3 | 14.8 ± 2.0 | 15.8 ± 1.6 | 16.4 ± 1.5 | 17.5 ± 1.2 | 19.1 ± 0.9 |
Values are presented as mean ± SD. RPE values were measured using the 6–20 Borg scale. SB-ORAL = oral sodium bicarbonate, SB-LOTION = topical sodium bicarbonate, PLA = placebo; * lower than PLA (p < 0.05).
3.5. Gastrointestinal discomfort, blinding, and expectations
GI side effects were reported by eight participants (57%) for SB-ORAL, six participants (43%) for SB-LOTION and seven participants (50%) for PLA. Aggregate GI discomfort scores were not significantly different between treatments at baseline (χ2(2) = 0.095, p = 0.953), post-consumption (χ2(2) = 4.414, p = 0.110), pre-warm-up (χ2(2) = 3.161, p = 0.206) and post-exercise (χ2(2) = 3.935, p = 0.140). Blinding was protected for SB-ORAL and PLA as the highest number of participants able to correctly identify treatments at each time-point was less than 50% expected by chance alone (SB-ORAL: 34%; PLA: 14%). There were no significant differences in the number of correct and incorrect treatment assignment ratings for SB-ORAL and PLA (p > 0.05). SB-LOTION was identified by 50% participants at each time point, with the number of correct ratings significantly greater than for PLA pre-exercise (χ2(2) = 8.137, p = 0.017). Participants suggested “thicker texture” (5/7) and/or “a strong cooling effect on application” (6/7) as their reasons for being able to identify SB-LOTION. There was an effect of treatment on expectations (χ2(2) = 18.184, p < 0.001). Expectations were higher for SB-LOTION (median: 3.0) compared with SB-ORAL (median: 1.0; Z = 0.583, p = 0.008) and PLA (median: 1.0; Z = 0.488, p = 0.025) but similar for SB-ORAL compared with PLA (p = 0.663).
4. Discussion
The aim of this study was to examine the effects of oral and topical NaHCO3 during a battery of team sport-specific exercise tests. Topical NaHCO3 (0.9036 g·kg−1 BM PR Lotion) improved 8 × 25 m repeated sprint performance by ~2% but had no significant effect on blood acid–base balance or electrolytes. Improvements in Yo-Yo IR2 and repeated sprint performance for oral NaHCO3 were likely due to elevated extracellular buffering, increased glycolytic flux and sustained excitation-contraction coupling. We propose that the faster repeated sprint times for PR Lotion might be attributed to an interaction between NaHCO3 molecules and menthol that intensified the localized cooling sensation, in turn reducing participants’ perception of effort and allowing them to exert greater effort during exercise. Further research is required to replicate our findings for PR Lotion and elucidate the physiological mechanisms responsible for ergogenic effects.
Yo-Yo IR2 performance improved by 21% for SB-ORAL compared to PLA, which represented a moderate effect and is congruent with evidence from a meta-analysis [35]. In contrast, SB-LOTION showed a small but statistically insignificant effect (7% greater total distance) compared to PLA. This is comparable to findings from McKay et al. [26] that demonstrated small, non-significant effects of PR Lotion during 3 × 30 s Wingate cycling tests and suggests that oral NaHCO3 remains the most effective supplementation strategy for improving Yo-Yo IR2 performance. There were no improvements in CMJ performance for SB-ORAL or SB-LOTION, supporting some previous NaHCO3 studies [19] but not others [36]. Discrepancies between studies could relate to differences in athlete cohort; we recruited a variety of team sport athletes meaning that there was a large variability in CMJ performance that may have decreased the likelihood of observing any treatment effects. Interestingly, SB-ORAL and SB-LOTION improved 8 × 25 m repeated sprint performance (total and average times) by ~2%. These results add to equivocal previous findings for the effect of NaHCO3 on RSA in team sport athletes [17–19] and provide evidence for the performance enhancing effects of PR Lotion during this exercise task. It is possible that we found improvements in repeated sprint performance for oral NaHCO3 because of the absence of GI discomfort [20], as team sport athletes often experience severe side-effects [18]. They might be at greater risk of suffering GI discomfort than other athletes (i.e. cyclists) as their higher body mass requires larger absolute NaHCO3 doses to achieve ergogenic benefits. PR Lotion could offer an alternative strategy for improving RSA in team sport athletes, but the effect on Yo-Yo IR2 and CMJ performance remains unclear. Since this is the first study to show ergogenic effects of PR Lotion, it is important future work replicates our findings and investigates whether performance benefits exist for other exercise modalities.
Pre-exercise changes in blood buffering capacity for SB-ORAL typically achieved the minimum ergogenic threshold (i.e. HCO3− >5.0 mmol·L−1 for 71% participants) [9] and blood acid–base balance was elevated compared to PLA throughout. Our improvements in repeated sprint and Yo-Yo IR2 performance for SB-ORAL were likely attributed to greater HCO3− buffering, which increased H+ efflux from the muscle and protected against declining intramuscular pH [10]. This is supported by significantly higher blood lactate post-Yo-Yo IR2 for SB-ORAL, suggesting that NaHCO3 ingestion may have upregulated glycolytic flux by preventing the inhibition of glycolytic enzymes such as glycogen phosphorylase and phosphofructokinase-1 [5,37]. While blood lactate is only an indirect measure of glycolytic flux, our ~2.5 mmol·L−1 difference was comparable to studies reporting greater Yo-Yo IR2 performance for NaHCO3 [15,16]. Additionally, ergogenic effects of SB-ORAL could be explained by altered electrolyte concentration. SB-ORAL significantly increased blood Na+ and reduced blood K+ concentrations compared to PLA pre-warm-up, which agrees with previous findings [11,12]. In theory, these changes may lead to upregulation of Na+/K+-ATPase activity that would increase excitation-coupling contraction and sustain force generating capacity of muscles [8,12]. Since exercise-induced losses in intramuscular K+ are one of the causes of depressed muscle excitability [8], attenuating reductions in intracellular K+ might be crucial for the ergogenic effects for NaHCO3. Interestingly, blood K+ was not significantly lower post-Yo-Yo IR2 for SB-ORAL compared to PLA. Participants covered greater distance for SB-ORAL, therefore we cannot disregard that a significant effect would have been shown if total work had been matched between conditions. It is also important to note that our results only reveal changes occurring within extracellular compartments, and not whether NaHCO3 altered electrolyte concentrations within contracting muscles.
No significant effects of SB-LOTION were observed for blood acid–base balance or electrolytes. The small, non-significant effect of SB-LOTION on blood lactate after the repeated sprint test (+1.01 mmol·L−1) may have contributed to performance benefits, whereas post-Yo-Yo IR2 blood pH was slightly elevated (+0.03 au.) for SB-LOTION compared with PLA despite participants covering more distance. Although not practically feasible, it is possible that a greater dose of PR Lotion would have increased the magnitude of any effects. Overall, our results add to findings by McKay et al. [26] and suggest that topical application of 0.9036 g·kg−1 BM PR Lotion is not able to effectively deliver NaHCO3 molecules into systematic circulation. Whilst we were unable to trace the absorption of NaHCO3 molecules from PR Lotion across the skin, it is logical that their negative lipophilicity (partition coefficient, −0.82) somewhat restricts their ability to penetrate lipid bilayers in the stratum corneum [38]. Transdermal drug delivery is also only effective when small doses (mg per day) are required [25]. If PR Lotion does allow NaHCO3 molecules to penetrate the stratum corneum, given the large doses of NaHCO3 ingested orally (~20–30 g), it is unclear whether enough NaHCO3 from PR Lotion could reach the bloodstream to elicit ergogenic changes in HCO3− [9]. As such, we suggest an alternative mechanism was responsible for faster repeated sprint times during SB-LOTION.
One explanation could be a localized cooling sensation from menthol (~0.5%) in PR Lotion [39]. Menthol is a cyclic terpene alcohol that may improve exercise performance by inducing a “cooling” effect to the skin via stimulation of the membrane-bound ion channel transient receptor potential melastatin 8 [39]. PR Lotion contains proportionately less menthol than other topical menthol formulations [40,41], but a similar absolute amount might have been given as we administered considerably more PR Lotion (~0.4 g menthol; assuming average dose for SB-LOTION was ~74 g) than researchers have used when investigating menthol gels (i.e. 2 mL of ~3.5% menthol Biofreeze® gel [41]). We propose that NaHCO3 molecules in PR Lotion interacted with menthol to form a protective layer over the skin that intensified menthols’ “cooling” sensation. Support for this can be drawn from the 43% participants who reported a “strong cooling” effect for SB-LOTION, whereas participants did not suggest a “cooling” sensation for SB-ORAL or PLA despite our placebo lotion also containing ~0.5% menthol. Furthermore, RPE was significantly lower for SB-LOTION compared with PLA during the repeated sprint test, which agrees with previous findings for topically applied menthol [40]. Reductions in RPE after NaHCO3 indicate ergogenic benefits might be explained by centrally mediated mechanisms [15,42]. Traditionally, this has been underpinned by the deleterious effects of H+ on the force generating capabilities of muscles [7], whereby declining pH causes localized pain [42,43]. Ingesting NaHCO3 likely leads to peripheral alterations (i.e. fewer H+ in the muscle) that modulate activation of group III and IV muscle afferents, in turn reducing negative feedback from muscles and sustaining drive to motor neurons [43,44]. We propose that the “cooling” effect from menthol in PR Lotion induced a similar centrally acting mechanism that attenuated localized muscle pain [45], meaning participants’ perception of muscle discomfort was lower than their actual degree of muscle fatigue [41], which allowed them to exert greater effort during the repeated sprint test. Future work should attempt to further elucidate potential physiological mechanisms responsible for ergogenic benefits of PR Lotion.
Another novel finding from this study was the positive effect of oral but not topical NaHCO3 on hydration status, with the changes in plasma volume for SB-ORAL compared with PLA (~8%) similar to previous results [13,46]. Post-exercise urine osmolality and color were also significantly different for SB-ORAL compared with PLA. Ingestion of water alone is relatively ineffective as a hyperhydration strategy, as most fluid is lost via urine [47]. This may explain why lower urine osmolality and lighter urine color were observed for PLA. Adding substances such as NaHCO3 with a high osmotic load enhances fluid retention by increasing plasma osmolality and volume [46,47]. Interestingly, differences between supplementation strategies and our participants’ high body masses meant that the amount of Na+ consumed during SB-ORAL (~6.5 g; proportion of Na+ in NaHCO3 was 27%, assuming 1 mol NaHCO3 is 84 g·mol−1) was greater than previous NaHCO3 studies [13,46] and more than the ~4 g given for traditional hyperhydration aids [47]. Despite the high amount of Na+ consumed, we believe that NaHCO3 can be safely incorporated into hyperhydration strategies of team sport athletes (i.e. American football, soccer) as they can lose ~4–6 g of Na+ per hour during training [48,49]. Our results also reinforce that NaHCO3 ingestion might be an effective strategy for improving fluid retention and exercise performance during challenging thermal conditions [50]. Additional research is needed to determine the reproducibility of changes in hydration status after NaHCO3 supplementation.
There are methodological limitations that need to be considered when interpreting our results that should be addressed in the future. First, plasma volume changes from our study were based on indirect measures (e.g. hemoglobin and hematocrit). This approach has been used for estimating changes in plasma volume during maximal exercise [13,31,46], but future research investigating the effect of NaHCO3 on plasma volume expansion should aim to use direct evaluation techniques [51]. Second, the intensified cooling sensation from menthol in PR Lotion contributed toward 50% participants identifying SB-LOTION, which may explain why participants reported significantly higher expectations of positive outcomes for SB-LOTION. Interestingly, however, 71% participants unable to identify SB-LOTION still improved their repeated sprint performance, and therefore it is difficult to conclude that the ergogenic effects of PR Lotion can be attributed solely to greater expectations. Further work is required to determine the most efficacious strategy for blinding PR Lotion during randomized placebo-controlled trials examining sports performance.
5. Conclusions
Topical NaHCO3 (0.9036 g·kg−1 BM PR Lotion) improved 8 × 25 m repeated sprint times by ~2% but had no significant effect on Yo-Yo IR2 or CMJ performance compared to a placebo. Oral NaHCO3 improved Yo-Yo IR2 and repeated sprint performance, which can likely be explained by elevated HCO3− buffering, increased glycolytic flux and sustained excitation–contraction coupling. PR Lotion had no significant effect on blood acid–base balance, suggesting that it did not effectively transport NaHCO3 across the skin into systematic circulation. Therefore, we propose that ~2% improvements in repeated sprint times for PR Lotion can be attributed to an interaction between NaHCO3 molecules and menthol that intensified the cooling sensation, subsequently reducing participants’ perception of effort and allowing them to exert greater physical effort during the 8 × 25 m repeated sprint test. In conclusion, PR Lotion appears to improve RSA in team sport athletes, but further research is required to elucidate the physiological mechanisms responsible for performance benefits.
Acknowledgments
We gratefully acknowledge the commitment of all participants and would like to thank our laboratory technicians for preparing supplements.
Funding Statement
Momentous are funding a PhD studentship at Sheffield Hallam University (for which this study is one output) but no additional funding was received to assist with the preparation of this manuscript.
Authors’ contributions
Conceptualisation and methodology: W.H.G., L.A.G., A.L and M.K.R. Formal analysis: W.H.G., A.L. and M.K.R. Investigation: W.H.G., K.C. and J.G. Project administration: W.H.G. Resources: W.H.G., A.L and M.K.R. Writing – Original Draft: W.H.G, A.L. and M.K.R; Writing – Review and Editing: W.H.G., K.C., J.G., L.A.G., A.L and M.K.R.
Disclosure statement
Momentous provided the lotions used in this study free of charge but had no influence over the study design or in preparation of the manuscript. The results of this study do not constitute endorsement of the product by the authors or the journal.
Data availability statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request https://shura.shu.ac.uk/.
Ethics approval and consent to participate
The study protocol was approved by the Institutional Ethics Committee (ER42014516) according to the 1964 Declaration of Helsinki and later amendments. All participants provided written informed consent prior to their inclusion in the study.
Publication statement
This manuscript has not been published elsewhere and has not been submitted simultaneously for publication elsewhere.
References
- 1.Spencer, M, Bishop, D, Dawson, B, et al. Physiological and metabolic responses of repeated-sprint activities: specific to field-based team sports. Sports Med. 2005;35(12):1025–449. DOI: 10.2165/00007256-200535120-00003 [DOI] [PubMed] [Google Scholar]
- 2.Bradley, PS, Di Mascio, M, Peart, D, et al. High-intensity activity profiles of elite soccer players at different performance levels. J Strength Cond Res. 2010;24(9):2343–2351. DOI: 10.1519/JSC.0b013e3181aeb1b3 [DOI] [PubMed] [Google Scholar]
- 3.Spriet, L, Lindinger, M, McKelvie, R, et al. Muscle glycogenolysis and H+ concentration during maximal intermittent cycling. J Appl Physiol. 1989;66(1):8–13. 1985. DOI: 10.1152/jappl.1989.66.1.8. [DOI] [PubMed] [Google Scholar]
- 4.Westerblad, H. Acidosis is not a significant cause of skeletal muscle fatigue. Med Sci Sports Exercise. 2016;48(11):2339–2342. [DOI] [PubMed] [Google Scholar]
- 5.Messonnier, L, Kristensen, M, Juel, C, et al. Importance of pH regulation and lactate/H+ transport capacity for work production during supramaximal exercise in humans. J Appl Physiol. 2007;102(5):1936–1944. DOI: 10.1152/japplphysiol.00691.2006 [DOI] [PubMed] [Google Scholar]
- 6.Debold, EP, Beck, SE, Warshaw, DM. Effect of low pH on single skeletal muscle myosin mechanics and kinetics. Am J Physiol-Cell Physiol. 2008;295(1):C173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitts, R. The role of acidosis in fatigue: pro perspective. Med Sci Sports Exercise. 2016;48(11):2335–2338. [DOI] [PubMed] [Google Scholar]
- 8.Cairns, SP, Lindinger, MI. Do multiple ionic interactions contribute to skeletal muscle fatigue? J Physiol. 2008;586(17):4039–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr, AJ, Hopkins, WG, Gore, CJ. Effects of acute alkalosis and acidosis on performance. Sports Med. 2011;41(10):801–814. [DOI] [PubMed] [Google Scholar]
- 10.Bishop, D, Edge, J, Davis, C, et al. Induced metabolic alkalosis affects muscle metabolism and repeated-sprint ability. Med Sci Sports Exerc. 2004;36(5):807–813. [DOI] [PubMed] [Google Scholar]
- 11.Gurton, WH, Faulkner, SH, James, RM. Effect of warm-up and sodium bicarbonate ingestion on 4-km cycling time-trial performance. Int J Sports Physiol Perform. 2021;16(11):1573–1579. [DOI] [PubMed] [Google Scholar]
- 12.Sostaric, SM, Skinner, SL, Brown, MJ, et al. Alkalosis increases muscle K+ release, but lowers plasma [K+] and delays fatigue during dynamic forearm exercise. J Physiol. 2006;570(1):185–205. DOI: 10.1113/jphysiol.2005.094615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegler, JC, Carr, AJ, Jardine, WT, et al. The hyperhydration potential of sodium bicarbonate and sodium citrate. Int J Sport Nutr Exerc Metab. 2021;32(2):74–81. DOI: 10.1123/ijsnem.2021-0179 [DOI] [PubMed] [Google Scholar]
- 14.Bangsbo, J, Iaia, FM, Krustrup, P. The Yo-Yo intermittent recovery test. Sports Med. 2008;38(1):37–51. [DOI] [PubMed] [Google Scholar]
- 15.Krustrup, P, Ermidis, G, Mohr, M. Sodium bicarbonate intake improves high-intensity intermittent exercise performance in trained young men. J Int Soc Sports Nutr. 2015;12(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marriott, M, Krustrup, P, Mohr, M. Ergogenic effects of caffeine and sodium bicarbonate supplementation on intermittent exercise performance preceded by intense arm cranking exercise. J Int Soc Sports Nutr. 2015;12(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ducker, KJ, Dawson, B, Wallman, KE. Effect of beta alanine and sodium bicarbonate supplementation on repeated-sprint performance. J Strength Cond Res. 2013;27(12):3450–3460. [DOI] [PubMed] [Google Scholar]
- 18.Cameron, SL, McLay-Cooke, RT, Brown, RC, et al. Increased blood pH but not performance with sodium bicarbonate supplementation in elite rugby union players. Int J Sport Nutr Exerc Metab. 2010;20(4):307–321. DOI: 10.1123/ijsnem.20.4.307 [DOI] [PubMed] [Google Scholar]
- 19.dos Santos Guimarães, R, de Morais Junior, AC, Schincaglia, RMS, et al. Sodium bicarbonate supplementation does not improve running anaerobic sprint test performance in semiprofessional adolescent soccer players. Int J Sport Nutr Exerc Metab. 2020;30(5):330–337. DOI: 10.1123/ijsnem.2020-0031 [DOI] [PubMed] [Google Scholar]
- 20.Saunders, B, Sale, C, Harris, RC, et al. Sodium bicarbonate and high-intensity-cycling capacity: variability in responses. Int J Sports Physiol Perform. 2014;9(4):627–632. DOI: 10.1123/ijspp.2013-0295 [DOI] [PubMed] [Google Scholar]
- 21.Turnberg, LA, Fordtran, JS, Carter, NW, et al. Mechanism of bicarbonate absorption and its relationship to sodium transport in the human jejunum. J Clin Investig. 1970;49:548–556. American Society for Clinical Investigation. DOI: 10.1172/JCI106265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurton, WH, Matta, GG, Gough, LA, et al. Efficacy of sodium bicarbonate ingestion strategies for protecting blinding. Eur J Appl Physiol. 2022;122(12):2555–2563. DOI: 10.1007/s00421-022-05031-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Oliveira, LF, Saunders, B, Artioli, GG. Is bypassing the stomach a means to optimize sodium bicarbonate supplementation? A case study with a postbariatric surgery individual. Int J Sport Nutr Exerc Metab. 2018;28(6):660–663. [DOI] [PubMed] [Google Scholar]
- 24.Prausnitz, MR, Langer, R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prausnitz, M, Mitragotri, S, Langer, R, et al. Langer RCurrent status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3(2):115–124. Nat Rev Drug Discov 3:115-124. DOI: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 26.Mckay, A, Peeling, P, Binnie, M, et al. Topical sodium bicarbonate: no improvement in blood buffering capacity or exercise performance. Int J Sports Physiol Perform. 2019;15(7):1–7. DOI: 10.1123/ijspp.2019-0345 [DOI] [PubMed] [Google Scholar]
- 27.Ranchordas, MK, Pratt, H, Parsons, M, et al. Effect of caffeinated gum on a battery of rugby-specific tests in trained university-standard male rugby union players. J Int Soc Sports Nutr. 2019;16(1):17. DOI: 10.1186/s12970-019-0286-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reilly, T. Human circadian rhythms and exercise. Crit Rev Biomed Eng. 1990;18(3):165–180. [PubMed] [Google Scholar]
- 29.Carr, A, Slater, G, Gore, C, et al. Effect of Sodium Bicarbonate on [HCO3−], pH, and gastrointestinal symptoms. Int J Sport Nutr Exerc Metab. 2011;21(3):189–194. DOI: 10.1123/ijsnem.21.3.189 [DOI] [PubMed] [Google Scholar]
- 30.Gibson, BM, Needham, KW, Kaiser, BW, et al. Transcutaneous delivery of sodium bicarbonate increases intramuscular pH. Front Physiol. 2023;14:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong, LE, Maresh, CM, Castellani, JW, et al. Urinary indices of hydration status. Int J Sport Nutr Exerc Metab. 1994;4(3):265–279. DOI: 10.1123/ijsn.4.3.265 [DOI] [PubMed] [Google Scholar]
- 32.Dill, DB, Costill, DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37(2):247–248. [DOI] [PubMed] [Google Scholar]
- 33.Gurton, WH, Gough, LA, Sparks, SA, et al. Sodium bicarbonate ingestion improves time-to-exhaustion cycling performance and alters estimated energy system contribution: a dose-response investigation. Front Nutr. 2020;7. doi: 10.3389/fnut.2020.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen, J. Statistical power analysis for the behavioral sciences. 2nd ed. New York: Routledge; 1988. [Google Scholar]
- 35.Grgic, J, Garofolini, A, Pickering, C, et al. Isolated effects of caffeine and sodium bicarbonate ingestion on performance in the Yo-Yo test: a systematic review and meta-analysis. J Sci Med Sport. 2020;23(1):41–47. DOI: 10.1016/j.jsams.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 36.Delextrat, A, Mackessy, S, Arceo-Rendon, L, et al. Effects of three-day serial sodium bicarbonate loading on performance and physiological parameters during a simulated basketball test in female university players. Int J Sport Nutr Exerc Metab. 2018;28(5):547–552. DOI: 10.1123/ijsnem.2017-0353 [DOI] [PubMed] [Google Scholar]
- 37.Hollidge-Horvat, MG, Parolin, ML, Wong, D, et al. Effect of induced metabolic alkalosis on human skeletal muscle metabolism during exercise. Am J Physiol Endocrinol Metab. 2000;278(2):E316–329. DOI: 10.1152/ajpendo.2000.278.2.E316 [DOI] [PubMed] [Google Scholar]
- 38.Comer, J, Tam, K. Lipophilicity profiles: theory and measurement. Pharmacokinet Optim Drug Res. John Wiley & Sons, Ltd; 2001. p. 275–304. DOI: 10.1002/9783906390437.ch17 [DOI] [Google Scholar]
- 39.Jeffries, O, Waldron, M. The effects of menthol on exercise performance and thermal sensation: a meta-analysis. J Sci Med Sport. 2019;22(6):707–715. [DOI] [PubMed] [Google Scholar]
- 40.Barwood, MJ, Corbett, J, Thomas, K, et al. Relieving thermal discomfort: effects of sprayed L-menthol on perception, performance, and time trial cycling in the heat. Scand J Med Sci Sports. 2015;25:211–218. [DOI] [PubMed] [Google Scholar]
- 41.Johar, P, Grover, V, Topp, R, et al. A comparison of topical menthol to ice on pain, evoked tetanic and voluntary force during delayed onset muscle soreness. Int J Sports Phys Ther. 2012;7(3):314–322. [PMC free article] [PubMed] [Google Scholar]
- 42.Swank, A, Robertson, RJ. Effect of induced alkalosis on perception of exertion during intermittent exercise. J Appl Physiol. 1989;67(5):1862–1867. [DOI] [PubMed] [Google Scholar]
- 43.Robertson, RJ, Nixon, PA, Caspersen, CJ, et al. Abatement of exertional perceptions following dynamic exercise: physiological mediators. Med Sci Sports Exercise. 1992;24(3):346–353. DOI: 10.1249/00005768-199203000-00011 [DOI] [PubMed] [Google Scholar]
- 44.Amann, M, Sidhu, SK, Weavil, JC, et al. Autonomic responses to exercise: group III/IV muscle afferents and fatigue. Auton Neurosci. 2015;188:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan, R, Tian, Y, Gao, R, et al. Central mechanisms of menthol-induced analgesia. J Pharmacol Exp Ther. 2012;343(3):661–672. DOI: 10.1124/jpet.112.196717 [DOI] [PubMed] [Google Scholar]
- 46.Kupcis, PD, Slater, GJ, Pruscino, CL, et al. Influence of sodium bicarbonate on performance and hydration in lightweight rowing. Int J Sports Physiol Perform. 2012;7(1):11–18. DOI: 10.1123/ijspp.7.1.11 [DOI] [PubMed] [Google Scholar]
- 47.Goulet, EDB, De La Flore, A, Savoie, FA, et al. Salt + glycerol-induced hyperhydration enhances fluid retention more than salt- or glycerol-induced hyperhydration. Int J Sport Nutr Exerc Metab. 2018;28(3):246–252. DOI: 10.1123/ijsnem.2017-0310 [DOI] [PubMed] [Google Scholar]
- 48.Godek, SF, Peduzzi, C, Burkholder, R, et al. Sweat rates, sweat sodium concentrations, and sodium losses in 3 groups of professional football players. J Athl Train. 2010;45(4):364–371. DOI: 10.4085/1062-6050-45.4.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maughan, RJ, Merson, SJ, Broad, NP, et al. Fluid and electrolyte intake and loss in elite soccer players during training. Int J Sport Nutr Exerc Metab. 2004;14(3):333–346. DOI: 10.1123/ijsnem.14.3.333 [DOI] [PubMed] [Google Scholar]
- 50.Gough, LA, Williams, JJ, Newbury, JW, et al. The effects of sodium bicarbonate supplementation at individual time-to-peak blood bicarbonate on 4-km cycling time trial performance in the heat. Eur J Sport Sci. 2021;22(12):1856–1864. DOI: 10.1080/17461391.2021.1998644 [DOI] [PubMed] [Google Scholar]
- 51.Fudim, M, Miller, WL. Calculated estimates of plasma volume in patients with chronic heart failure—comparison with measured volumes. J Card Fail. 2018;24(9):553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request https://shura.shu.ac.uk/.


