Abstract
The transcription factor c-Jun is critically involved in the regulation of proliferation and differentiation as well as cellular transformation induced by oncogenic Ras. The signal transduction pathways that couple Ras activation to c-Jun phosphorylation are still partially elusive. Here we show that an activated version of the Ras effector Rlf, a guanine nucleotide exchange factor (GEF) of the small GTPase Ral, can induce the phosphorylation of serines 63 and 73 of c-Jun. In addition, we show that growth factor-induced, Ras-mediated phosphorylation of c-Jun is abolished by inhibitory mutants of the RalGEF-Ral pathway. These results suggest that the RalGEF-Ral pathway plays a major role in Ras-dependent c-Jun phosphorylation. Ral-dependent regulation of c-Jun phosphorylation includes JNK, a still elusive JNKK, and possibly Src.
The Ral guanine nucleotide exchange factors (RalGEFs) activate the Ras-like small GTPases RalA and RalB by the exchange of GDP for GTP (12). Three of the known RalGEFs, RalGDS, Rgl, and Rlf, interact with and can be activated by oncogenic Ras (2). Epidermal growth factor (EGF) and insulin-induced Ral activation is dependent on Ras activation in mouse fibroblasts, implying that RalGEFs also function as Ras effector molecules in growth factor signaling (43). The function of the Ral signaling pathway is still unclear, but it has been implicated in cellular transformation induced by oncogenic Ras (24, 41) as well as in the regulation of Ras-mediated cell growth and differentiation (14, 33, 39).
Recent results show that signal transduction from Ras to Ral plays a role in the transcriptional regulation of insulin-responsive genes by activating a pathway that controls phosphorylation of the fork head transcription factor AFX (22). This Ras- and Ral-dependent regulation of AFX may be involved in progression through the G1 phase of the cell cycle (26), providing at least one mechanism for the effects of the Ras-Ral signaling pathway on cellular behavior. In addition, activation of RalGEFs results in promoter activation of the growth-regulatory c-fos, collagenase, and ANF genes (13, 28, 30, 42). Together, these studies suggest that RalGEFs are important mediators of Ras-dependent regulation of genes involved in cell growth and differentiation. They also point to an important role of RalGEFs in mediating the cellular responses to insulin.
The use of mutants of active Ras that specifically activate either RalGEFs, phosphatidylinositol 3-kinase (PI-3K), or Raf1 kinase has demonstrated that, at least in fibroblasts, signaling through the Ras-Ral pathway is distinct from the pathways emerging from the Ras effectors PI-3K and Raf1, respectively (32, 40). Although a number of Ral binding proteins have been identified (12), the signal transduction pathways downstream of RalGTP and the role of Ral binding proteins in mediating transcriptional regulation have not been resolved. However, the studies mentioned above suggest that RalGEFs may activate a pathway that specifically leads to the activation of one or more protein kinases. In addition, it was recently shown that c-Src is involved in signaling events downstream from Ral (15).
Previously we found that differentiation of F9 embryonic carcinoma cells toward primitive endoderm cells can be comparably induced by ectopic expression of either the transcription factor c-Jun, active Rlf, or oncogenic Ras (39). The differentiation induced by oncogenic Ras, but not by c-Jun, was dependent on Ral signaling (39). This observation suggested to us that Ras and Ral acted upstream in a signaling cascade, which activates c-Jun. In addition, we had observed that an active mutant of Rlf induces the activation of a reporter construct regulated by AP1, a c-Jun-containing transcription factor. Together, these observations prompted us to investigate the role of Ral signaling in the regulation of c-Jun.
c-Jun is essential for normal mouse development, fibroblast proliferation (18, 20), and cellular transformation induced by oncogenic Ras (19). In response to different extracellular stimuli, including growth factors, cytokines, and cellular stress inducers, Jun NH2-terminal kinase (JNK) is activated and phosphorylates c-Jun at two regulatory sites within its transcriptional activation domain, serine 63 and serine 73 (10). Phosphorylation of these sites increases the transactivating potential of c-Jun and is required for proper regulation of apoptosis and proliferation in fibroblasts (1, 21, 35). A number of studies indicate that the small GTPase Rac and other Rho family members play a role in transmitting signals from oncogenic Ras to c-Jun through a pathway involving a Jun kinase kinase (JNKK-1, also known as SEK1 and MKK4) and JNK (16). Here we identified a pathway induced by activation of the small GTPase Ral that regulates the phosphorylation of the transcription factor c-Jun. We show that insulin-induced NH2-terminal phosphorylation of c-Jun requires activation of Ras and Ral. The signal transduction pathway involves JNK activation and probably c-Src. This pathway is likely to play a role in RalGEF-dependent regulation of proliferation, differentiation, and oncogenesis.
MATERIALS AND METHODS
Plasmids.
The Arg-328-to-Glu mutation in pMT2-HA-Rlf-CAAX (42) was generated by using oligonucleotides carrying the desired point mutation, PCR amplification using Pfu heat-stable polymerase, and digestion of nonmutated plasmid DNA with DpnI. Escherichia coli DH5 bacteria were transformed with the reaction mixture, plasmid DNA positive for the mutation was isolated, and the Rlf-CAAX open reading frame was entirely controlled by bidirectional DNA sequencing. pSVE-RasV12, pMT2HA-Ral, pMT2HA-RalA-N28 (42), and pMT2-HA-RalGDS-RBD (31) have been described previously. Mammalian expression vectors encoding HA-p54-JNK and Myc-Cdc42 have been described previously (9, 36). pRK5-Myc-RalBP-ΔGAP, which encodes RalBP with its N-terminal GAP domain deleted, and pSG5-RalB-N28 were a kind gift of Jacques H. Camonis, Institut Curie, INSERM, Paris, France. pCD20 is an expression vector for the extracellular region of CD20.
Antibodies and Western blotting.
To detect phosphorylation of endogenous c-Jun and glutathione S-transferase (GST)–c-Jun1–79, we used polyclonal anti-phosphoserine-73 c-Jun antibody and polyclonal anti-phosphoserine-63 c-Jun antibody (New England Biolabs). The anti-phosphoserine-63 c-Jun antibody is specific for phosphorylated c-Jun, and the anti-phosphoserine-73 might additionally recognize phosphorylation of serine 73 of JunD. However, in JunD immunoprecipitates we found hardly any increases in JunD phosphorylation at time points when c-Jun was phosphorylated. In addition, JunD runs at a slightly different position from c-Jun on our gels (data not shown). The c-Jun antibody used was rabbit polyclonal H79 (Santa Cruz) or rabbit polyclonal anti-c-Jun Ab1 (Oncogene Science). ATF2 phosphorylation and total ATF2 were detected using an anti-ATF2 phosphothreonine-71 polyclonal antibody or polyclonal anti-ATF2 (New England Biolabs). Polyclonal anti-phospho-JNK was from Promega. This antibody recognizes various phosphorylated proteins including phosphorylated extracellular signal-related kinase (ERK). Therefore, increases in JNK phosphorylation were measured in JNK1 immunoprecipitates using mouse monoclonal anti-JNK1 F3 (Santa Cruz), which is specific for JNK1 and recognizes no other mitogen-activated protein kinases. Anti-phospho-ERK and anti-phospho-SEK were from New England Biolabs. Goat anti-Ral-B was from Santa Cruz. The anti-Ras antibody was from Transduction Laboratories. Western blots were blocked for 3 h at room temperature in phosphate-buffered saline (PBS) containing 0.5% Tween 20, 5% nonfat dry milk, 2% bovine serum albumin and 200 μM sodium vanadate. The Western blots were incubated overnight with the indicated antibodies in PBS containing 0.5% Tween 20, 2% bovine serum albumin, 200 μM sodium vanadate, using the dilutions recommended by the manufacturers.
Isolation of transfected cells by MACS.
A14 cells (NIH 3T3 cells expressing human insulin receptors) and HEK-293 cells were cultured in 100-mm-diameter dishes containing Dulbecco's modified Eagle's medium–10% fetal calf serum–0.05% glutamine. When necessary, cells were transfected by the calcium phosphate method and isolated 40 h after transfection. For growth factor studies, cells were washed once with serum-free medium and serum starved for at least 16 h. Stimulation with insulin (1 μM) or EGF (20 ng/ml) was carried out for the times indicated. For isolation of transfected cells, the cells were cotransfected with pCD20 and isolated on magnetic cell sorting (MACS) separation columns type MS+ as specified by the manufacturer (Miltenyi Biotec, Bergisch Gladbach, Germany). In brief, the cells were washed with ice-cold 5 mM EDTA in PBS after stimulation and left on ice for 5 min. Then they were scraped in ice-cold 5 mM EDTA in PBS, isolated by centrifugation, washed with ice-cold wash buffer (1% fetal calf serum in PBS), and incubated with a monoclonal anti-CD20 antibody (DAKO). After being washed with 10 ml of wash buffer, the cells were incubated with the anti-mouse antibody coupled to iron beads. Finally, the cells were isolated by magnetic force on a separating column after being washed with wash buffer. The isolated transfected cells were lysed (in 0.5% Triton X-100–50 mM HEPES–100 mM NaCl–2 mM sodium vanadate–10 mM NaF), protein levels were equalized, and total cellular proteins were solubilized in Laemmli sample buffer. The samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), immunoblotted onto polyvinylidene difluoride, and probed with the antibodies indicated in the figure legends. In some experiments the fold induction was determined from the scanned images by using the NIH Image 1.62 program.
Expression of RasN17 by recombinant vaccinia virus.
Subconfluent serum-starved A14 cells were infected with 20 PFU of recombinant vaccinia virus (His6-RasN17 in pEAGPT vector) or the vector viral growth factor-minus strain of vaccinia virus (11). After 60 min, the medium was replaced and the cells were maintained in the absence of serum for 8 h. The cells were stimulated with insulin (1 μM) for the indicated times and lysed in sample buffer prior to Western analysis.
Analysis of endogenous Ral activation.
Ral activation was determined using GST-RalBD essentially as described previously (43). In brief, bacterially produced GST-RalBD (15 μg per sample) was precoupled to glutathione-agarose beads (10 μl/sample) and washed in Ral buffer (15% glycerol, 50 mM Tris [pH 7.4], 1% NP-40, 200 mM NaCl, 5 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride, 1 μM leupeptin, 0.1 μM aprotinin, 10 μg of soybean trypsin inhibitor per ml). The cells were lysed in Ral buffer, and cleared lysates were split and used for the determination of Ral activity or for the analysis of protein expression. Samples were separated by SDS-PAGE (12.5% polyacrylamide), immunoblotted, and probed with either monoclonal anti-Ral (Transduction Laboratories), or monoclonal anti-HA (12CA5) antibody.
Immunoprecipitation and in vitro kinase assays.
A14 cells were lysed in kinase lysis buffer (10% glycerol, 50 mM HEPES [pH 7.4], 0.5% Triton X-100, 200 mM NaCl, 2.5 mM EDTA, 2.5 mM EGTA, 10 mM NaF, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 1 μM leupeptin, 0.1 μM aprotinin, 10 μg of soybean trypsin inhibitor per ml), and endogenous JNK was immunoprecipitated using either 10 μg of GST–Jun1–79 per μl precoupled to glutathionine beads or monoclonal anti-JNK1 p46 antibody F3 (Santa Cruz) precoupled to protein G-Sepharose beads. The beads were washed twice with kinase lysis buffer and twice with kinase reaction buffer (50 mM HEPES [pH 7.4], 15 mM MgCl2, 200 μM sodium vanadate). For kinase reactions, the beads were incubated in kinase buffer (containing 100 μM ATP or 5 μM ATP and 10 μCi of [γ-32P]ATP per reaction) at 25°C for 30 min, taken up in sample buffer, and analyzed by SDS-PAGE followed by either Western blotting with phosphospecific anti-c-Jun or autoradiography.
RESULTS
Ras-dependent c-Jun phosphorylation by insulin.
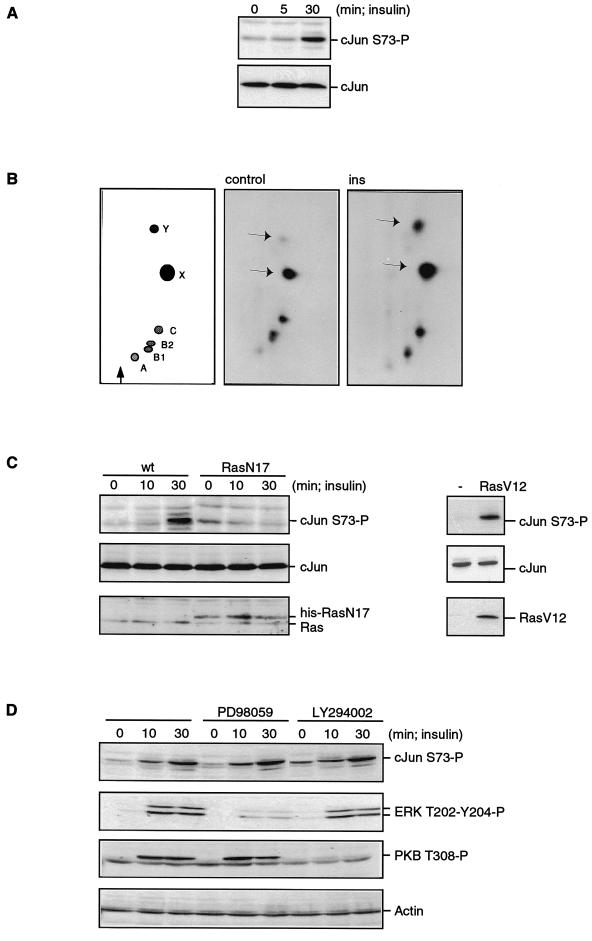
To analyze the phosphorylation of c-Jun in response to endogenous Ras activation, A14 cells were treated for various times with insulin, which induces a strong and sustained increase in RasGTP levels (5). c-Jun NH2-terminal phosphorylation, as monitored by Western blotting using an anti-c-Jun phosphoserine-73 polyclonal antibody, was strongly increased after 30 min of insulin treatment (Fig. 1A) and remained elevated for at least 1 h (data not shown). We also measured NH2-terminal phosphorylation by tryptic peptide mapping of c-Jun immunoprecipitated from A14 cells that were labeled with [32P]orthophosphate. Figure 1B shows that both the c-Jun peptides X and Y, which contain serine 73 and serine 63, respectively (3), were strongly phosphorylated in response to insulin.
FIG. 1.
Insulin treatment induces phosphorylation of c-Jun in a Ras-dependent manner. (A) Insulin treatment induces NH2-terminal phosphorylation of c-Jun. Cell extracts from subconfluent, serum-starved A14 cells were treated with insulin (1 μM) for the indicated times and analyzed by Western blotting using anti-c-Jun phosphoserine-73 (upper panel) or anti-c-Jun (lower panel). (B) Insulin induces phosphorylation of both serine 63 and serine 73. c-Jun was immunoprecipitated from orthophosphate-labelled serum-starved A14 cells left untreated (middle panel, control) or treated with insulin (1 μM) for 60 min (right panel, ins). Phosphorylated c-Jun was processed for tryptic peptide mapping as described previously (22). The left panel shows the relative position of the tryptic c-Jun phosphopeptides. Phosphopeptides X and Y, indicated by the arrows, contain the phosphorylation sites serine 73 and serine 63, respectively (3). (C) RasN17 blocks insulin-induced c-Jun serine 73 phosphorylation. In the left panel, A14 cells were infected with 20 PFU of vector or His6-RasN17 vaccinia virus. After insulin treatment for the indicated times, the cells were lysed in sample buffer and analyzed as in panel A. The lower panel shows expression of the His6-RasN17 protein together with endogenous Ras. In the right panel, cells were transfected with either empty vector (−) or RasV12, lysed in sample buffer, and analyzed as in panel A. Expression of RasV12 was detected by using the anti-Ras antibody. wt, wild type. (D) Pretreatment of A14 cells for 15 min with the MEK inhibitor PD98059 (40 μM) or the PI-3K inhibitor LY294002 (10 μM) blocks insulin-induced ERK phosphorylation and PKB phosphorylation but not c-Jun phosphorylation. Phosphorylation was monitored using phosphospecific antibodies as indicated.
To determine the role of Ras in the observed increment in c-Jun phosphorylation, A14 cells were infected with either a wild-type vaccinia virus or vaccinia virus expressing dominant negative Ras, RasN17. Previously, we have shown that RasN17 inhibits insulin-induced Ras activation completely (4, 11). Ectopic expression of RasN17 completely blocked insulin-induced c-Jun phosphorylation of serine 73 (Fig. 1C) and serine 63 (data not shown). This demonstrated that the pathway leading to c-Jun NH2-terminal phosphorylation in response to insulin required activation of Ras. In addition, transfection of an active mutant of Ras (RasV12) resulted in a substantial induction of c-Jun phosphorylation compared to that in vector-transfected cells (Fig. 1C), supporting the data on an insulin-induced Ras-mediated pathway leading to c-Jun phosphorylation.
To investigate the role of the Raf-MEK-ERK pathway in the observed c-Jun phosphorylation, we pretreated A14 cells with the MEK inhibitor PD98059 prior to insulin stimulation. However, insulin-induced ERK phosphorylation, but not insulin-induced c-Jun phosphorylation, was inhibited (Fig. 1D).
Previously we showed that RasN17 does not inhibit PI-3K or protein kinase B (PKB) activation induced by insulin, demonstrating that PI-3K is not a Ras effector in insulin signaling in A14 cells (6). Indeed, the PI-3K inhibitor LY294002 did not block insulin-induced NH2-terminal phosphorylation of c-Jun whereas PKB phosphorylation was abolished (Fig. 1D). These results show that the pathway downstream from Ras leading to c-Jun phosphorylation does not involve the MEK-ERK pathway or the PI-3K–PKB pathway.
RalGEF-induced c-Jun phosphorylation.
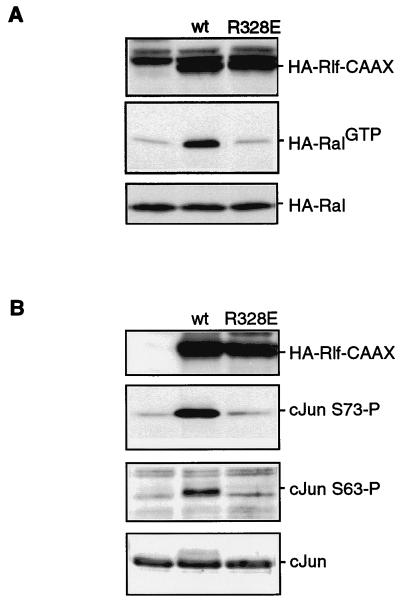
Next, we tested whether insulin-induced c-Jun NH2-terminal phosphorylation could be downstream of a RalGEF. Therefore we used a HA-Rlf-CAAX construct, in which the Ras binding domain of Rlf has been replaced by a CAAX membrane localization motif, resulting in a constitutively active RalGEF (42). As a control, we used the same construct carrying an inactivating arginine-to-glutamate mutation in the catalytic domain, Rlf-R328E-CAAX. Figure 2A shows that the HA-Rlf-R328E-CAAX construct indeed does not activate coexpressed HA-Ral.
FIG. 2.
Ral guanine nucleotide exchange activity induces phosphorylation of endogenous c-Jun. (A) Ral activation by Rlf-CAAX is abolished by the R328E mutation in Rlf-CAAX. A14 cells were cotransfected with HA-Ral and HA-Rlf-CAAX or HA-Rlf-R328E-CAAX. The cells were lysed in Ral buffer, and HA-RalGTP levels were monitored after anti-HA (12CA5) Western blotting of affinity-isolated RalGTP as described in Materials and Methods (middle panel). The bottom panel shows the expression of HA-Ral in total lysates. The top panel shows equal expression of the Rlf-CAAX proteins. wt, wild type. (B) RalGEF-induced NH2-terminal phosphorylation of endogenous c-Jun. A14 cells were cotransfected with pCD20 and either pMT2HA, pMT2HA-Rlf-CAAX, or pMT2HA-Rlf-R328E-CAAX as indicated. At 40 h after transfection, transfected cells were isolated by MACS as described in Materials and Methods. Isolated cells were lysed, protein levels were equalized, and samples were taken up in sample buffer and analyzed for c-Jun phosphorylation on serine 73 (second panel), serine 63 (third panel) or c-Jun protein expression (bottom panel). The top panel is a blot of the same samples using anti-HA (12CA5) to show equal expression of HA-Rlf-CAAX and HA-Rlf-R328E-CAAX. wt, wild type.
To analyze increases in NH2-terminal phosphorylation of endogenous c-Jun, we isolated transfected A14 cells by MACS. Phosphorylation of c-Jun was subsequently monitored by Western blotting of the extracts of the transfected cells by using anti-c-Jun phosphoserine-73 or anti-c-Jun phosphoserine-63 antibodies. Figure 2B shows that c-Jun is strongly phosphorylated on both serine 73 and serine 63 in HA-Rlf-CAAX-expressing but not in HA-Rlf-R328E-CAAX-expressing cells. No increase in the c-Jun protein level was observed 40 h after transfection (Fig. 2B). These results demonstrate that activation of Ral exchange activity can induce increased c-Jun NH2-terminal phosphorylation.
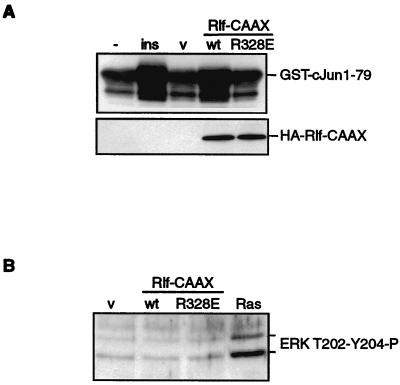
We asked whether the observed increase in c-Jun phosphorylation resulted from increased JNK activity. Therefore, JNK was pulled down from the lysates of transfected cells by using GST–Jun1–79 and the kinase activity was assayed in vitro. Figure 3A shows that insulin and Rlf-CAAX induced JNK activity to a similar extent. As a control, Fig. 3B shows that endogenous ERK phosphorylation was elevated in A14 cells transiently expressing oncogenic Ras but not in cells that expressed HA-Rlf-CAAX or HA-Rlf-R328E-CAAX (Fig. 3B). We conclude from these experiments that activation of a pathway involving an elevation of RalGTP levels is sufficient to induce increased JNK activity.
FIG. 3.
Insulin and Ral guanine nucleotide exchange activity induce activation of JNK. (A) Activation of JNK activity by insulin and Rlf-CAAX. A14 cells were either transfected with pCD20 and treated with insulin (1 μM) for 30 min (left two lanes) or cotransfected with pCD20 and either pMT2HA (v), pMT2HA-RlfCAAX (wt), or pMT2HA-Rlf-R328E-CAAX (R328E) as indicated (right three lanes). Transfected cells were isolated by MACS. Endogenous JNK was isolated by using GST–c-Jun1–79 precoupled to glutathione beads. JNK activity using GST–c-Jun1–79 as a substrate was assayed directly by adding ATP. Phosphorylation of GST–c-Jun1–79 was monitored by Western blotting using anti-c-Jun phosphoserine 73. (B) Rlf-CAAX does not induce ERK phosphorylation. A14 cells were cotransfected with pCD20 and either pMT2HA (v), pMT2HA-Rlf-CAAX (wt), pMT2HA-Rlf-R328E-CAAX (R328E), or pMT2HA-RasV12 (Ras) as indicated and transfected cells were isolated by magnetic cell sorting. Phosphorylation of ERK was detected by Western blotting using anti-ERK phosphothreonine-202 phosphotyrosine-204 polyclonal antibody. The positions of phosphorylated ERK1 and ERK2 are indicated.
Ral-dependent c-Jun phosphorylation.
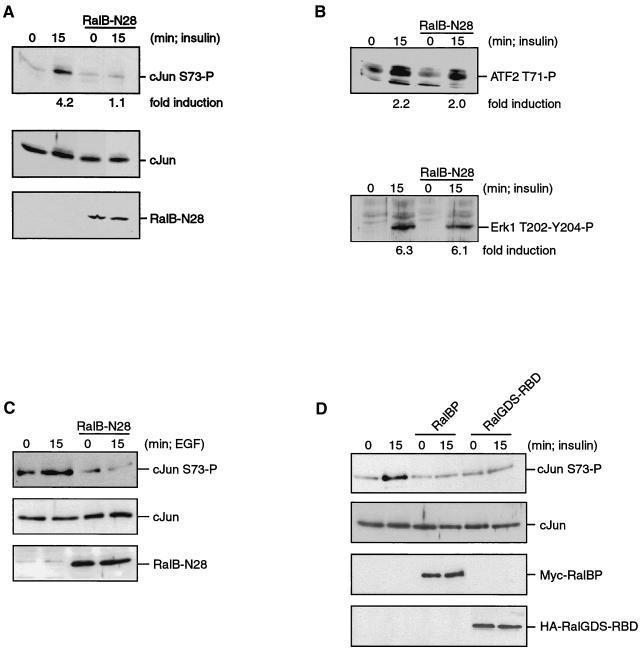
To investigate if the activation of Ral is also involved in transmitting signals from growth factor receptors to c-Jun phosphorylation, we transfected A14 cells with a construct encoding a dominant negative form of Ral, RalB-N28. RalB-N28 strongly inhibited Rlf activity in vitro (unpublished data). Figure 4A shows that c-Jun serine 73 phosphorylation is induced after 15 min of insulin treatment in A14 cells transiently transfected with the control vector. However, expression of RalB-N28 completely blocked c-Jun phosphorylation by insulin. The same inhibition was observed when HA-RalA-N28 was used as a dominant negative construct (data not shown). ATF2 is a transcriptional factor which is regulated by overlapping pathways as the Jun kinases (38). However, as shown in Fig. 4B, insulin-induced threonine 71 phosphorylation of ATF2 is not blocked by RalB-N28. Also, insulin-induced phosphorylation of ERK1 and ERK2 was not inhibited by RalB-N28 (Fig. 4B, lower panel). These results show the specificity of the observed inhibition of c-Jun phosphorylation.
FIG. 4.
Growth factor-induced phosphorylation of endogenous c-Jun requires Ral signaling. A14 or HEK-293 cells were transiently transfected with either empty vector or a construct expressing either RalB-N28, RalBP-ΔGAP, or RalGDS-RBD as indicated. After 24 h, the cells were serum starved for 16 h and then stimulated with insulin (1 μM) or EGF (20 ng/ml) as indicated, and transfected cells were isolated by MACS. (A) RalB-N28 blocks insulin-induced c-Jun phosphorylation. A14 cells transiently expressing empty pSG5 or pSG5-RalB-N28 as indicated were treated with insulin for the times indicated, and transfected cells were isolated. Samples were analyzed for c-Jun phosphorylation on serine 73 (top panel), c-Jun protein expression (middle panel) or RalB-protein expression (bottom panel). The numbers below the panels indicate fold induction compared to the unstimulated cells. (B) RalB-N28 does not block insulin-induced ATF2 or ERK phosphorylation. Equal amounts of whole-cell extracts isolated in panel A were analyzed for increases in ATF2 phosphorylation and for phosphorylation of ERK. ATF2 phosphorylation was detected by Western blotting using an anti-ATF2 phosphothreonine-71 polyclonal antibody (upper panel). Phosphorylation of ERK was monitored by Western blotting using anti-ERK phosphothreonine-202 phosphotyrosine-204 polyclonal antibody (lower panel). Fold induction is indicated below the panels. (C) RalB-N28 blocks EGF-induced c-Jun phosphorylation in HEK-293 cells. HEK-293 cells transiently expressing empty pSG5 or pSG5-RalB-N28 as indicated were treated with EGF for the times indicated, and transfected cells were isolated. Samples were analyzed for c-Jun phosphorylation on serine 73 (top panel), c-Jun protein expression (middle panel), or RalB-protein expression (bottom panel). (D) RalBP-ΔGAP and RalGDS-RBD block insulin-induced phosphorylation of c-Jun. A14 cells transiently expressing empty pMT2HA, pRK5-MYC-RalBP-ΔGAP, or pMT2HA-RalGDS-RBD as indicated were treated with insulin for the times indicated, and transfected cells were isolated. Isolated samples were analyzed for c-Jun phosphorylation on serine 73 (top panel), c-Jun protein expression (second panel), Myc-RalBP-ΔGAP expression (third panel), or HA-RalGDS-RBD expression (bottom panel).
To investigate whether Ral is involved in the phosphorylation of c-Jun in other cell types and induced by other growth factors, we analyzed the involvement of Ral in EGF-induced c-Jun phosphorylation in HEK-293 cells. We observed that in these cells the moderate but consistent EGF-induced c-Jun phosphorylation was completely abolished by HA-RalB-N28 (Fig. 4C), showing that the involvement of Ral in c-Jun phosphorylation is not cell type or growth factor specific.
To further support the involvement of Ral in insulin-mediated c-Jun phosphorylation, we have blocked the Ras-Ral signaling pathway at the level of Ral. To that end, RalBP-ΔGAP, which is RalBP with its N-terminal GAP domain deleted, was expressed. RalBP binds tightly to Ral-GTP and thus is predicted to interfere in the Ral-effector interaction. As shown in Fig. 4D, expression of RalBP-ΔGAP completely abrogates the insulin-induced phosphorylation of c-Jun. Furthermore, expression of the Ras binding domain of RalGDS, which specifically blocks signaling downstream of Ras via its effector region, blocks insulin-induced phosphorylation of c-Jun to the same extent as RalBP-ΔGAP does (Fig. 4D). Together, these results demonstrate that activation of the Ral pathway is required for c-Jun NH2-terminal phosphorylation in response to insulin.
Activation of the JNKK pathway by Ral signaling.
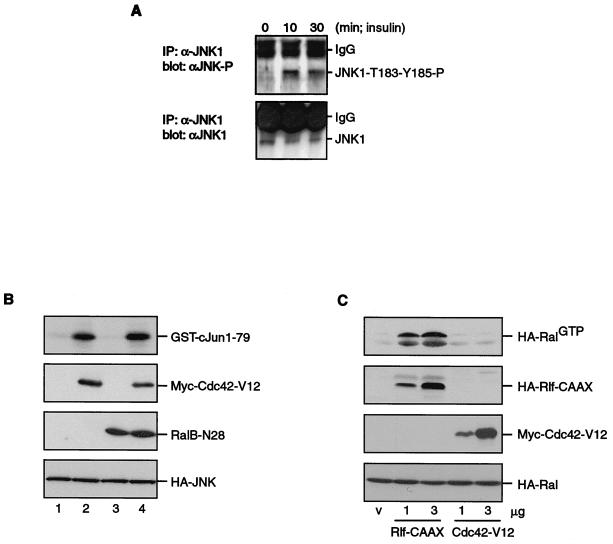
Since the insulin-induced c-Jun phosphorylation was completely inhibited by dominant negative Ral, we investigated the effects of insulin on well-known upstream regulators of c-Jun phosphorylation to find targets of Ral signaling. To investigate whether the RalGEF-induced activation of JNK activity was the result of activation of any JNKK activity, we tested whether insulin could induce phosphorylation of JNK1. JNK1 was immunoprecipitated using the monoclonal F3 antibody, and phosphorylation was detected on Western blots using an anti-JNK phosphothreonine-183 and phosphotyrosine-185 antibody. Figure 5A shows that insulin induced a clear increase in JNK1 phosphorylation on these JNKK sites.
FIG. 5.
Regulation of the JNK pathway by Ral signaling. (A) Insulin-induced JNK1 phosphorylation at the JNKK-1 sites. Serum-starved, subconfluent A14 cells were treated with insulin for the indicated times and lysed. JNK was immunoprecipitated (IP) using 10 μg of monoclonal anti-JNK1, precoupled to protein G-Sepharose, for 3 h at 4°C. After extensive washing with lysis buffer, 90% of the protein samples were analyzed by Western blotting using anti-JNK phosphothreonine-183 phosphotyrosine-185 (upper panel). Total JNK1 was detected using the remaining 10% of the samples (lower panel). Ig, immunoglobulin G. (B) Cdc42-V12 induces JNK activity independently of Ral. A14 cells were transfected with either empty vector (lane 1), Myc-Cdc42-V12 (lane 2), both empty vector and pSG5-RalB-N28 (lane 3), or Myc-Cdc42-V12 and pSG5-RalB-N28 (lane 4), together with HA-p54-JNK. HA-JNK was immunoprecipitated from cellular lysates, and kinase activity was assayed in vitro using GST–c-Jun1–79 as a substrate. GST-Jun phosphorylation was monitored by autoradiography after SDS-PAGE of kinase assays (top panel). Myc-Cdc42-V12, RalB-N28, and HA-JNK expression, identified by Western blotting, are shown in the lower panels. (C) Cdc42-V12 does not activate Ral. A14 cells were cotransfected with HA-Ral and HA-Rlf-CAAX or Myc-Cdc42-V12. Cells were lysed in Ral buffer, and HA-RalGTP levels were monitored after anti-HA (12CA5) Western blotting of affinity-isolated RalGTP as described in Materials and Methods (top panel). The other panels show the expression of HA-Rlf-CAAX, Myc-Cdc42-V12, and HA-Ral in total lysates.
The pathway leading to activation of JNK has been described to involve JNKK-1 (also known as SEK1 or MKK4) (21). We found that insulin only very weakly induced phosphorylation of JNKK-1 at threonine 223, an activating phosphorylation site. This phosphorylation is at least 10-fold lower than after anisomycin (10 μM) treatment or osmotic shock (0.5 M NaCl) (data not shown). We also could hardly detect insulin-induced increases in overall HA–JNKK-1 phosphorylation in immunoprecipitates from 32P-labeled A14 cells after transfection of an HA–JNKK-1 construct (data not shown). This suggested either that JNKK-1 is not involved in Ral-induced c-Jun phosphorylation or that Ral regulates JNKK-1 activity independently of phosphorylation. Therefore, the JNKK responsible for JNK phosphorylation remains elusive.
The small GTPases Rac and Cdc42 have previously been implicated in transmitting signals from oncogenic Ras to JNK and may also play a role in the regulation of c-Jun phosphorylation in response to growth factors (7, 8, 27). However, we did not observe a clear inhibition of insulin-induced c-Jun serine 73 phosphorylation by overexpression of the dominant negative RacN17 (data not shown). In addition, although expression of the constitutively active RacV12 induced lamellipodia in A14 cells (36), we never observed JNK activation in A14 cells (data not shown). In contrast, ectopic expression of an activated mutant of Cdc42, Myc-Cdc42-V12, which activates JNKK-1 (8), resulted in an increase in JNK activity (Fig. 5B). Importantly, this Cdc42-V12-mediated increase in activity of JNK was not inhibited by cotransfection of Ral-N28 (Fig. 5B). In addition, Cdc42-V12 was incapable of activating Ral, as shown in Fig. 5C. Both these experiments show that Cdc42 and Ral induce JNK activation through different pathways.
In conclusion, our data indicate that insulin-induced c-Jun phosphorylation is dependent on activation of Ras and Ral but independent of Rac and Cdc42 and at least involves the activation of a still elusive JNKK and subsequent JNK phosphorylation and activation.
A PP1-sensitive Ral effector involved in JNK activation.
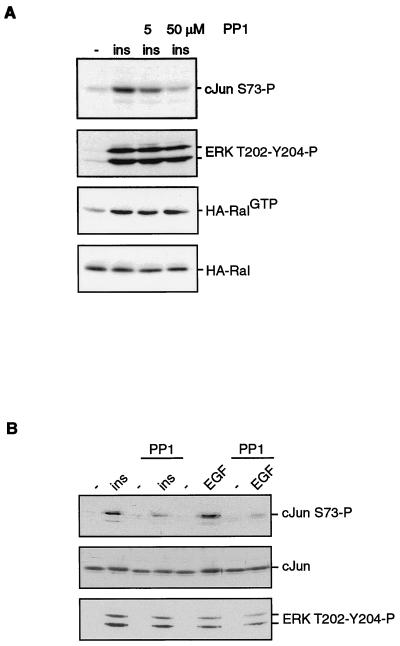
Recently, a role for the Src tyrosine kinase as a target of Ral signaling has been suggested (15). Therefore, we tested whether c-Src was involved in the insulin-mediated c-Jun phosphorylation. Interestingly, the Src-specific kinase inhibitor PP1 (17) could significantly inhibit the insulin-induced increases in c-Jun NH2-terminal phosphorylation. This inhibition could already be seen at a very low concentration of PP1 and was even more dramatic when cells were pretreated with the concentration normally used for this inhibitor (Fig. 6A). This c-Src kinase dependency of c-Jun phosphorylation was not restricted to insulin stimulation since EGF-mediated phosphorylation of c-Jun was also found to be sensitive to PP1 (Fig. 6B). Longer exposure of Fig. 6B shows that the basal level of c-Jun phosphorylation was not affected by PP1 (data not shown). Furthermore, PP1 did not influence insulin- or EGF-induced phosphorylation of ERK1 and ERK2 (Fig. 6) or Ral activation (Fig. 6A and data not shown). This suggest that the PP1 target, presumably c-Src (15), functions downstream from Ras and Ral but upstream from JNK.
FIG. 6.
Insulin-induced c-Jun phosphorylation is sensitive to the Src kinase inhibitor PP1. (A) Serum-starved A14 cells were treated with 5 or 50 μM PP1 before being stimulated with 1 μM insulin for 15 min, as indicated. The cells were lysed in Ral buffer in the presence of PP1. Half of the sample was taken up in sample buffer and analyzed for c-Jun phosphorylation (top panel) or ERK phosphorylation (second panel). The second half of the lysate was used to isolate RalGTP, as described in the legend to Fig. 2A, using monoclonal anti-RalA (third panel). The bottom panel shows equal protein levels by detection of the RalA protein in whole extracts. (B) Serum-starved A14 cells were treated with 50 μM PP1 before being stimulated with 1 μM insulin or 20 ng of EGF per ml for 15 min, as indicated. The cells were lysed in sample buffer and analyzed for c-Jun phosphorylation (top panel), c-Jun protein levels (middle panel), or ERK phosphorylation (bottom panel).
DISCUSSION
The function of the ubiquitously expressed small GTPases RalA and RalB, which are more than 90% identical, has just recently begun to emerge. Activation of Ral can contribute to the specific biological effects induced by active Ras, such as proliferation and differentiation, depending on the cell type. For example, we recently found that Ral signaling was essential for differentiation of F9 embryonic carcinoma cells induced by Ras but not for differentiation induced by c-Jun (39). This is epistatic evidence that Ral may have a function in a pathway that leads to c-Jun activation. Additionally, Ral and c-Jun are both targets for oncogenic Ras in cellular transformation. Therefore, we investigated whether Ras-induced c-Jun activation is mediated by Ral in A14 fibroblasts. In these cells, Ral activation is completely dependent on the activation of Ras (43). We observed that insulin-induced phosphorylation of both serine 63 and serine 73 of c-Jun is also dependent on Ras activation. We show that an active RalGEF, but not a catalytically inactive RalGEF, induced c-Jun NH2-terminal phosphorylation similarly to the induction by insulin treatment. Importantly, c-Jun NH2-terminal phosphorylation in response to insulin was completely dependent on Ral activation. This pathway involves activation and phosphorylation of JNK-1 and the activation of a JNKK.
The JNKK activated by RalGTP may be JNKK-1/SEK1/MKK4, which can function as a target of activated Rac and Cdc42 in various cell lines (8). However, in A14 cells we could not detect a clear Ral-dependent effect on JNKK-1 threonine 223 phosphorylation that is typical for this pathway. Since we did observe an increase in JNK phosphorylation in response to insulin, this may suggest that a protein kinase other than JNKK-1 functions as a target of Ral signaling. Alternatively, Ral may regulate JNKK-1 activity independent of phosphorylation. The latter model would be consistent with the observation that nonmuscle filamin (also known as ABP-280) is both a Ral GTP effector molecule and a JNKK-1 binding module (25, 29) and therefore may serve as a Ral-dependent scaffold protein for JNKK-1.
A number of proteins that can form a complex with Ral have been described which could be involved in the effects described in this paper. For instance, RalBP (also known as RIP or RLIP) contains GTPase activity for Rac and Cdc42 in vitro (12). As such, RalBP may be involved in inhibiting Cdc42 signaling in a Ral-dependent manner. Perhaps RalBP allows a negative feedback between Ral and Cdc42-induced signaling toward c-Jun phosphorylation and may be important for the downregulation of JNK activity by Ral that is necessary for proper differentiation in Drosophila (33). PLD1, another protein that forms a complex with Ral, plays an important role in EGF-induced cellular transformation but does not regulate JNK activity (24). Recently, it was shown that the tyrosine kinase c-Src functions downstream from Ral (15). Indeed, we observed that the Src tyrosine kinase inhibitor PP1 blocks insulin-induced c-Jun phosphorylation, suggesting that Src (or a Src family member) might be involved in the signaling from Ral to c-Jun. Although we cannot exclude the possibility that the PP1-sensitive Ral target is distinct from Src or related tyrosine kinases, PP1 has been described as a highly specific inhibitor of this family of kinases (17, 34). The way Ral activates Src and the way Src activates JNK are currently unclear. Interestingly, a physiological link between Src and JNK also exists in Drosophila; i.e., activation of the JNK homolog Basket (Bsk) functions downstream of Src in epidermal closure (37).
JNK activation has also been implicated in signaling in response to cellular stresses and the subsequent onset of apoptosis (1). However, Ral is not activated in response to cellular stresses (data not shown). In addition, we did not find any effect of dominant negative Cdc42-N17 on insulin-mediated c-Jun phosphorylation, excluding a prominent role for Cdc42 in the insulin-Ral pathway leading to phosphorylation of c-Jun. It is clear that the effects of c-Jun on cellular responses will depend strongly on the cell type and cellular environment (23). Importantly, c-Jun is required for Ras-induced transformation and proliferation of rodent fibroblasts and plays a determinant role in the regulation of normal mammalian development (23). Our results can provide an explanation for the overlapping effects of Ral signaling and activation of c-Jun, indicating that regulation of c-Jun can at least in part explain the biological effects of Ral signaling. c-Jun is not the only transcription factor that is under control of the Ras-Ral pathway. For example, the fork head transcription factor AFX is regulated by phosphorylation in response to Ral signaling and insulin treatment (22). Phosphorylation of AFX plays an important role in Ras-dependent cell cycle control and transformation (26).
In conclusion, in this paper we describe a signal transduction pathway that couples growth factor signaling to the phosphorylation of c-Jun. This pathway involves Ral, presumably Src, and a still elusive JNKK. Ral-mediated phosphorylation of c-Jun and AFX, transcription factors that critically determine cellular growth responses, firmly establishes that the Ral pathway plays an important role in transcriptional regulation downstream of Ras.
ACKNOWLEDGMENTS
N.D.D.R. and R.M.F.W. contributed equally to this work.
We thank Paul Coffer for assistance and reagents and Jacques Camonis for the RalB-Asn28 and RalBP-ΔGAP.
This work was supported by the Dutch Cancer Society.
REFERENCES
- 1.Behrens A, Sibilia M, Wagner E F. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999;21:326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- 2.Bos J L. All in the family? New insights and questions regarding interconnectivity of Ras, Rap1 and Ral. EMBO J. 1998;17:6776–6782. doi: 10.1093/emboj/17.23.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle W J, Smeal T, Defize L H, Angel P, Woodgett J R, Karin M, Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991;64:573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- 4.Burgering B M T, de Vries-Smits A M, Medema R H, van Weeren P C, Tertoolen L G, Bos J L. Epidermal growth factor induces phosphorylation of extracellular signal-regulated kinase 2 via multiple pathways. Mol Cell Biol. 1993;13:7248–7256. doi: 10.1128/mcb.13.12.7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgering B M T, Medema R H, Maassen J A, van de Wetering M L, van der Eb A J, McCormick F, Bos J L. Insulin stimulation of gene expression mediated by p21ras activation. EMBO J. 1991;10:1103–1109. doi: 10.1002/j.1460-2075.1991.tb08050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgering B M T, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 7.Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 8.Coso O A, Teramoto H, Simonds W F, Gutkind J S. Signaling from G protein-coupled receptors to c-Jun kinase involves beta gamma subunits of heterotrimeric G proteins acting on a Ras and Rac1-dependent pathway. J Biol Chem. 1996;271:3963–3966. doi: 10.1074/jbc.271.8.3963. [DOI] [PubMed] [Google Scholar]
- 9.de Groot R P, van Dijk T B, Caldenhoven E, Coffer P J, Raaijmakers J A, Lammers J W J, Koenderman L. Activation of 12-O-tetradecanoylphorbol-13-acetate response element- and dyad symmetry element-dependent transcription by interleukin-5 is mediated by Jun N-terminal kinase/stress-activated protein kinase kinases. J Biol Chem. 1997;272:2319–2325. doi: 10.1074/jbc.272.4.2319. [DOI] [PubMed] [Google Scholar]
- 10.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 11.de Vries-Smits A M, Burgering B M T, Leevers S J, Marshall C J, Bos J L. Involvement of p21ras in activation of extracellular signal-regulated kinase 2. Nature. 1992;357:602–604. doi: 10.1038/357602a0. [DOI] [PubMed] [Google Scholar]
- 12.Feig L A, Urano T, Cantor S. Evidence for a Ras/Ral signaling cascade. Trends Biochem Sci. 1996;21:438–441. doi: 10.1016/s0968-0004(96)10058-x. [DOI] [PubMed] [Google Scholar]
- 13.Fuller S J, Finn S G, Downward J, Sugden P H. Stimulation of gene expression in neonatal rat ventricular myocytes by Ras is mediated by Ral guanine nucleotide dissociation stimulator (Ral.GDS) and phosphatidylinositol 3-kinase in addition to Raf. Biochem J. 1998;335:241–246. doi: 10.1042/bj3350241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gille H, Downward J. Multiple ras effector pathways contribute to G(1) cell cycle progression. J Biol Chem. 1999;274:22033–22040. doi: 10.1074/jbc.274.31.22033. [DOI] [PubMed] [Google Scholar]
- 15.Goi T, Shipitsin M, Lu Z, Foster D A, Klinz S G, Feig L A. An EGF receptor/Ral-GTPase signaling cascade regulates c-Src activity and substrate specificity. EMBO J. 2000;19:623–630. doi: 10.1093/emboj/19.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta S, Barrett T, Whitmarsh A J, Cavanagh J, Sluss H K, Derijard B, Davis R J. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- 17.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brissette W H, Weringer E J, Pollok B A, Connelly P A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 18.Hilberg F, Aguzzi A, Howells N, Wagner E F. c-jun is essential for normal mouse development and hepatogenesis. Nature. 1993;365:179–181. doi: 10.1038/365179a0. . (Erratum, 366:368, 1993.) [DOI] [PubMed] [Google Scholar]
- 19.Johnson R, Spiegelman B, Hanahan D, Wisdom R. Cellular transformation and malignancy induced by ras require c-jun. Mol Cell Biol. 1996;16:4504–4511. doi: 10.1128/mcb.16.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson R S, van Lingen B, Papaioannou V E, Spiegelman B M. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993;7:1309–1317. doi: 10.1101/gad.7.7b.1309. [DOI] [PubMed] [Google Scholar]
- 21.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 22.Kops G J P L, de Ruiter N D, De Vries-Smits A M, Powell D R, Bos J L, Burgering B M T. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 23.Leppa S, Bohmann D. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene. 1999;18:6158–6162. doi: 10.1038/sj.onc.1203173. [DOI] [PubMed] [Google Scholar]
- 24.Lu Z, Hornia A, Joseph T, Sukezane T, Frankel P, Zhong M, Bychenok S, Xu L, Feig L A, Foster D A. Phospholipase D and RalA cooperate with the epidermal growth factor receptor to transform 3Y1 rat fibroblasts. Mol Cell Biol. 2000;20:462–467. doi: 10.1128/mcb.20.2.462-467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marti A, Luo Z, Cunningham C, Ohta Y, Hartwig J, Stossel T P, Kyriakis J M, Avruch J. Actin-binding protein-280 binds the stress-activated protein kinase (SAPK) activator SEK-1 and is required for tumor necrosis factor-alpha activation of SAPK in melanoma cells. J Biol Chem. 1997;272:2620–2628. doi: 10.1074/jbc.272.5.2620. [DOI] [PubMed] [Google Scholar]
- 26.Medema R H, Kops G J P L, Bos J L, Burgering B M T. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 27.Minden A, Karin M. Regulation and function of the JNK subgroup of MAP kinases. Biochim Biophys Acta. 1997;1333:F85–F104. doi: 10.1016/s0304-419x(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 28.Murai H, Ikeda M, Kishida S, Ishida O, Okazaki-Kishida M, Matsuura Y, Kikuchi A. Characterization of Ral GDP dissociation stimulator-like (RGL) activities to regulate c-fos promoter and the GDP/GTP exchange of Ral. J Biol Chem. 1997;272:10483–10490. doi: 10.1074/jbc.272.16.10483. [DOI] [PubMed] [Google Scholar]
- 29.Ohta Y, Suzuki N, Nakamura S, Hartwig J H, Stossel T P. The small GTPase RalA targets filamin to induce filopodia. Proc Natl Acad Sci USA. 1999;96:2122–2128. doi: 10.1073/pnas.96.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okazaki M, Kishida S, Hinoi T, Hasegawa T, Tamada M, Kataoka T, Kikuchi A. Synergistic activation of c-fos promoter activity by Raf and Ral GDP dissociation stimulator. Oncogene. 1997;14:515–521. doi: 10.1038/sj.onc.1200860. [DOI] [PubMed] [Google Scholar]
- 31.Reedquist K A, Ross E, Koop E A, Wolthuis R M F, Zwartkruis F J T, van Kooyk Y, Salmon M, Buckley C D, Bos J L. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J Cell Biol. 2000;148:1151–1158. doi: 10.1083/jcb.148.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 33.Sawamoto K, Winge P, Koyama S, Hirota Y, Yamada C, Miyao S, Yoshikawa S, Jin M H, Kikuchi A, Okano H. The Drosophila Ral GTPase regulates developmental cell shape changes through the Jun NH(2)-terminal kinase pathway. J Cell Biol. 1999;146:361–372. doi: 10.1083/jcb.146.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schindler T, Sicheri F, Pico A, Gazit A, Levitzki A, Kuriyan J. Crystal structure of Hck in complex with a Src family-selective tyrosine kinase inhibitor. Mol Cell. 1999;3:639–648. doi: 10.1016/s1097-2765(00)80357-3. [DOI] [PubMed] [Google Scholar]
- 35.Smeal T, Binetruy B, Mercola D, Grover-Bardwick A, Heidecker G, Rapp U R, Karin M. Oncoprotein-mediated signalling cascade stimulates c-Jun activity by phosphorylation of serines 63 and 73. Mol Cell Biol. 1992;12:3507–3513. doi: 10.1128/mcb.12.8.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spaargaren M, Bos J L. Rab5 induces Rac-independent lamellipodia formation and cell migration. Mol Biol Cell. 1999;10:3239–3250. doi: 10.1091/mbc.10.10.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tateno M, Nishida Y, Adachi-Yamada T. Regulation of JNK by Src during Drosophila development. Science. 2000;287:324–327. doi: 10.1126/science.287.5451.324. [DOI] [PubMed] [Google Scholar]
- 38.van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. ATF-2 is preferentially activated by stress-activated protein kinases to meiate c-jun induction in response to genotoxic agents. EMBO J. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verheijen M H, Wolthuis R M, Defize L H, den Hertog J, Bos J L. Interdependent action of RalGEF and Erk in Ras-induced primitive endoderm differentiation of F9 embryonal carcinoma cells. Oncogene. 1999;18:4435–4439. doi: 10.1038/sj.onc.1202834. [DOI] [PubMed] [Google Scholar]
- 40.White M A, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler M H. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 41.Wolthuis R M, Bos J L. Ras caught in another affair: the exchange factors for Ral. Curr Opin Genet Dev. 1999;9:112–117. doi: 10.1016/s0959-437x(99)80016-1. [DOI] [PubMed] [Google Scholar]
- 42.Wolthuis R M, de Ruiter N D, Cool R H, Bos J L. Stimulation of gene induction and cell growth by the Ras effector Rlf. EMBO J. 1997;16:6748–6761. doi: 10.1093/emboj/16.22.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolthuis R M, Zwartkruis F, Moen T C, Bos J L. Ras-dependent activation of the small GTPase Ral. Curr Biol. 1998;8:471–474. doi: 10.1016/s0960-9822(98)70183-6. [DOI] [PubMed] [Google Scholar]