Abstract
Simple Summary
Mycoplasma gallisepticum causes respiratory disease in chickens. This study used RNA-seq to investigate the immune response of chicken embryos and chicks to infection. Weight loss and immune damage were observed in infected chickens of both ages. Transcriptome analysis revealed differentially expressed genes related to innate immunity and inflammation, with toll-like receptors and cytokine pathways playing a dominant role in the immune response. The immune response was stronger in embryos than in chicks. These findings provide valuable insights for developing disease control strategies.
Abstract
Mycoplasma gallisepticum (MG) is a major cause of chronic respiratory diseases in chickens, with both horizontal and vertical transmission modes and varying degrees of impact on different ages. The innate immune response is crucial in resisting MG infection. Therefore, this study aimed to investigate the innate immune response of chicken embryos and newly hatched chicks to MG infection using comparative RNA-seq analysis. We found that MG infection caused weight loss and immune damage in both chicken embryos and chicks. Transcriptome sequencing analysis revealed that infected chicken embryos had a stronger immune response than chicks, as evidenced by the higher number of differentially expressed genes associated with innate immunity and inflammation. Toll-like receptor and cytokine-mediated pathways were the primary immune response pathways in both embryos and chicks. Furthermore, TLR7 signaling may play an essential role in the innate immune response to MG infection. Overall, this study sheds light on the development of innate immunity to MG infection in chickens and can help in devising disease control strategies.
Keywords: Mycoplasma gallisepticum, immune response, chicken embryo, chick, RNA-seq
1. Introduction
Mycoplasma gallisepticum (MG) is the major cause of chronic respiratory disease (CRD) in chickens. Infection with MG leads to slow growth, poor feed conversion, and reduced hatchability in chickens, thus bringing huge economic losses to the poultry industry [1,2]. Notably, MG can not only spread horizontally through the environment but also vertically to the next generations, which is a primary consideration for international trade [3]. Vaccination and antibiotics are currently effective methods to control MG infection. However, the available vaccines and antibiotics all have limitations due to high strain antigen variability, drug residues, and the emergence of resistant strains [1,4]. A thorough understanding of the molecular mechanism of the host immune system against MG infection is necessary to explore new prevention and treatment strategies for MG.
Transcriptome sequencing has great advantages in providing the host immune response to microbial infections. As shown in Table 1, there is growing evidence from transcriptome sequencing that MG infection is characterized by vigorous inflammatory responses and dysregulated immune responses in the respiratory tract of chickens. Recent findings not only reaffirm these results but also suggest that the immune protection provided by MG vaccine strains was achieved in part by balancing cytokines and chemokines production and TLRs expressions in chickens [5]. Due to high reproducibility and well-known biology and physiology, the chicken embryo is an important model for studying the development of innate immunity and vaccine efficacy [6,7]. Increasingly, studies have shown that the in ovo administration of immune modulators can enhance the innate immunity of chickens in adulthood [8,9]. However, the regulatory pathways and molecular mechanisms underlying the immune response induced by MG in chicken embryos have been rarely explored.
Hence, this study aimed to explore the genes and pathways underlying the MG-induced pathological and immune events in newly hatched chicks and chicken embryos’ lungs. Alveolar epithelial cells (AECII) are important functional and structural cells in the alveoli. They can differentiate into AECI cells and secrete cytokines and chemokines, which are essential for maintaining the normal function of lung tissue and regulating the lung immune balance [10]. Therefore, we also verified the transcriptome sequencing results in primary AECII cells. Results reveal the transcriptional responses of chicks and chicken embryos’ lungs in defense against MG and detail the involved immune-related genes and pathways in the transcriptome. Understanding the differences in the immune response to MG infection between embryonic chickens and chicks will help better develop disease control strategies.
Table 1.
Summary of transcriptome expression in response to MG infection in chicken tracheal and lung tissues.
| Animal | Sample | Groups | Pathways | Reference |
|---|---|---|---|---|
| 5-week SPF female chicken |
Tracheal epithelial cells | CG vs. MG Rlow, Rhigh, Rlow LAMP, and Rhigh LAMP |
Up: TLRs, TNF-α/NF-kB, adipogenesis, senescence and autophagy, MAPK, apoptosis, EGFR1, Type II interferon | [11] |
| 5-week SPF chicken |
1, 3, 5, and 7 days after challenge Tracheal |
CG vs. MG Rlow |
Up: apoptosis, TLRs, MAPK, JAK-SAT, NKC mediated cytotoxicity, CCRI, intestinal immune network for IgA production | [12] |
| 5-week SPF chicken |
1 and 2 days after infection Tracheal lumen cell |
CG vs. MG Rlow, GT5, and Mg7 |
Up: CCRI, MAPK, NOD receptor, JAK-SAT, TLRs | [13] |
| 5-week SPF chicken |
6 days after infection Tracheal rings |
CG/H3N8 vs. MGRlow/H3N8 |
Up: phagosome, cell adhesion molecules, intestinal immune network for IgA production, JAK/STAT, TLRs Down: cardiac muscle contraction, purine metabolism, oxidative phosphorylation |
[14] |
| 10d chick | 3 days after infection Lung tissue |
CG vs. MG Rlow/E. coli O78 |
Up: CCRI, phagosome, IL-17, NF-κB, cell adhesion molecules | [15] |
| 70-week or 4-week SPF chicken |
Two weeks after challenge Tracheal tissues |
CG vs. MG Asp3A |
Up: DNA replication, cell cycle, CCRI, TLRs, phagosome, Down: formation and motor movement of the cilia, adherens junctions, tight junctions |
[5] |
| 3-week SPF chicken |
15 days after infection Tracheal |
CG vs. MG-HY |
Up: cell adhesion molecules, phagosome, MAPK, calcium, and PPAR Down: Tight, ECM-receptor interaction, focal, AMPK, p53, Rap1, regulation of actin |
[16] |
2. Materials and Methods
2.1. Ethics in Animal Experimentation
Our experimental protocols for animals were authorized by the Ethical Committee on Animal Research at Huazhong Agricultural University (HZAUCH-2020-0003) and met the International Guiding Principles for Biomedical Research Involving Animals as issued by the Council for the International Organizations of Medical Sciences.
2.2. Mycoplasma gallisepticum Strain and Culture
The HS strain of MG used in the study is a pathogenic strain that was isolated from a local chicken farm in Hubei province in 1988. The culture medium was FM-4 medium supplemented with 12% inactivated porcine serum, 1% phenol red, and penicillin sodium (10,000 IU/mL), as previously described [17]. MG was inoculated in FM-4 medium and cultured at 37 °C with 5% CO2 until the medium color changed from red to orange. Hemagglutination and color-changing unit assays were used to determine the virulence of MG and the concentration of MG per milliliter of medium.
2.3. Cell Culture and Treatment
Primary chicken alveolar type II cells (AEC-II) were obtained from 13.5–15 embryonic day (ED) chickens. The method of isolation and preparation for AEC-II has been described previously [18]. In this study, AEC-II cells were seeded at a density of 1 × 106 into six-well plates and cultivated at 37 °C with 5% CO2. When the cell confluence reached 60–70%, the MG group cells were treated with 200 uL of MG (1 × 109 CCU/mL), and the control group cells were treated with the same volume of PBS. After 6 h of infection, the total RNA of treated cells was collected for further study.
2.4. Experimental Infection of Chicks and Chicken Embryos
The methods and procedures for establishing the MG-infected specific-pathogen-free (SPF) chicken embryo model have been described previously [19]. Briefly, 300 1-day-old SPF fertilized eggs were purchased from Merial Vital Laboratory Animal Technology (Beijing, China) and incubated to 9 days of age in biochemical incubators with a relative humidity of 55–60% and temperature of 37.8 ± 0.1 °C. On the ninth day of incubation, 180 embryos were treated with 800 μL of MG-HS at 1 × 109 CCU/mL through the allantoic cavity injection. The others were injected with the same volume of saline as controls. Three to 11 days post-infection, 12 embryonic eggs were randomly selected from each group every day for body weight measurement and tissue isolation. To determine the immune organ index, fresh spleen tissue, bursa of Fabricius tissue, and thymus tissue were weighed immediately.
The methods and procedures for establishing the MG-infected chick model have been described previously [2]. Briefly, 50 one-day-old SPF fertilized eggs were purchased from Merial Vital Laboratory Animal Technology (Beijing, China) and incubated until hatching in a sterilized biochemical incubator with a relative humidity of 55–60% and temperature of 37.8 ± 0.1 °C. Thirty-five newborn chicks were reared for 10 days in a separate sterile brood room. At 10 days old, 35 chicks were randomly divided into 2 groups with 3 replicates and 5 chicks each replicate. The chicks in the infected group were treated with 1 mL MG-HS at 1 × 109 CCU/mL through the left air sacs, nose, and eye. The others were injected with the same volume of saline as controls. At 5 days post-infection, the chicks in the infected group all showed clinical symptoms of rales, mouth breathing, coughing, and sneezing and were humanely euthanized for body weight detection and tissue collection. Similarly, to determine the immune organ index, fresh spleen tissue, bursa of Fabricius tissue, and thymus tissue were weighed immediately. All isolated tissue was immediately stored in liquid nitrogen for further experiments.
2.5. Total RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
The total RNA from the lungs, spleen, bursal, and thymus was collected by TRIzol reagent (Invitrogen, Waltham, MS, USA) according to the manufacturer’s instructions. The RNA integrity and quality were monitored on 1% agarose gels and assessed using the RNA Nanodrop2000 (Thermo Scientific, CA, USA) machine. Then, 1 μg total RNA was reverse transcribed into cDNA using the first strand cDNA synthesis kit (TaKaRa, Tokyo, Japan). Subsequently, RT-qPCR was used to determine the relative mRNA expression of specific genes using TransStart Top Green qPCR SuperMix (YEASEN, Shanghai, China). The relative expression levels of genes were normalized to GAPDH. All DNA primer sequences are shown in Table 2.
Table 2.
DNA sequence of primers.
| Name | Primers | Accession No. |
|---|---|---|
| TLR1B-F | GCCGTTGCCTTCTAAAAGAGG | NM_001081709.4 |
| TLR1B-R | CCAGGACACTCTGACAGGGA | NM_001081709.4 |
| TLR2A-F | AAATCAGCGGGATGCACCTA | NM_001396827.1 |
| TLR2A-R | TTGGCATCGGATCACAGGTC | NM_001396827.1 |
| TLR7-F | TCTTTCAGAGGTGGCTGCAC | XM_046908011.1 |
| TLR7-R | GATCCCTCTGGGGACTTCCT | XM_046908011.1 |
| TLR4 -F | ATCTTTCAAGGTGCCACATC | NM_001030693.2 |
| TLR4 -R | GGATATGCTTGTTTCCACCA | NM_001030693.2 |
| TLR3-F | CTGCGGAATCTGACTGTCCT | NM_001011691.4 |
| TLR3-R | TCCTCCTGGGTTTGCACATTT | NM_001011691.4 |
| TLR5-F | CACCTGTGCCAAAGGACACT | NM_001398059.1 |
| TLR5-R | AAACGTTGCAAACCCGCAAA | NM_001398059.1 |
| TLR15-F | GGCTGTGGTATGTGAGAATG | NM_001398238.1 |
| TLR15-R | ATCGTGCTCGCTGTATGA | NM_001398238.1 |
| TLR21-F | CAGGGTATGCAGCTGTGTCA | NM_001030558.3 |
| TLR21-R | CAGGGTATGCAGCTGTGTCA | NM_001030558.3 |
| CSF3-F | ACCACGACTTCCAGCTCTTC | NM_205279.2 |
| CSF3-R | CTGGAAGGTGTCACACACGG | NM_205279.2 |
| CSF3R-F | TCCTCACACTCCCTCTTTGC | NM_001030898.2 |
| CSF3R-R | ATGGGATGGTGCCTTCTGC | NM_001030898.2 |
| TNFRSF6B-F | AGTGCCTCTACTGCAACGTC | XM_417434.6 |
| TNFRSF6B-R | CAGAGGGGGAACCCAACTTC | XM_417434.6 |
| IL-1β-F | TGCCTGCAGAAGAAGCCTCG | NM_204524.1 |
| IL-1β-R | CTCCGCAGCAGTTTGGTCAT | NM_204524.1 |
| CCL4-F | AGCGTAGGAACTCCACTCTC | XM_015295666.2 |
| CCL4-R | CCTTCTTTGTGATGAGATGATGGC | XM_015295666.2 |
| CXCL13L2-F | CAACTGCCTTCATTCCCTTGC | NM_001348656.1 |
| CXCL13L2-R | GCAGCTTTCTTCTTCAGCTTCG | NM_001348656.1 |
| CXCL13-F | TGTCAAAGTGACTGCCCAGA | NM_001348657.1 |
| CXCL13-R | CCTCTTCAGGGTGAGGATGATCT | NM_001348657.1 |
| IL20RA-F | CGGCGCACACAGGAATGTT | XM_015284185.2 |
| IL20RA-R | GCTCTTCATGGTCAGCAGTCT | XM_015284185.2 |
| IL8L1-F | ATGTGAACCTCACCCCTAGC | NM_205018.1 |
| IL8L1-R | TGAATGGCGTTGTCTCCCAC | NM_205018.1 |
| IL13RA2-F | GGAGGTCCAGAGTGAATGGC | NM_001048078.1 |
| IL13RA2-R | AGCATGCAGCCCATGTTTAC | NM_001048078.1 |
| IL1R2-F | ATTTCCGCTGTGCCTTGAGT | XM_015277810.2 |
| IL1R2-R | CAGTAGGAGTTGTTCCTGTGCT | XM_015277810.2 |
| MMP7-F | CAGAGCCTTCCGTTTCCAGT | NM_001006278.1 |
| MMP7-R | GGGATTCCACATCTGGGCTG | NM_001006278.1 |
| OLFM4-F | AGAAGACTACCAGCCAGCAC | NM_001040463.2 |
| OLFM4-R | AAAGGTGGTATCCGGCAAGT | NM_001040463.2 |
| AVD-F | CCCACCTTTGGCTTCACTG | NM_205320.2 |
| AVD-R | ACCTCCTTCCCGTTCCTGT | NM_205320.2 |
| IL8L2-F | TTCAGCTGCTCTGTCGCAA | NM_205498.2 |
| IL8L2-R | GCACACCTCTCTTCCATCCTT | NM_205498.2 |
| S100A9-F | CCACTTCTGTGAGGACCACC | NM_001305151.2 |
| S100A9-R | GGCAGCAAAACCATCAAACCT | NM_001305151.2 |
2.6. Illumina Sequencing
Magnetic beads containing Oligo(dT) were first used to enrich mRNA from total RNA. Then mRNA was used as a template to synthesize single-stranded cDNA and then double-stranded cDNA. The cDNA was then purified and subjected to end repair, A-tailed, and ligated with sequencing adapters, and then AMPure XP beads were used for fragment size selection. Finally, PCR amplification was performed, and the PCR products were purified to obtain the final cDNA library. Qubit 2.0 was used for preliminary quantification. The Illumina high-throughput sequencing platform NovaSeq 6000 (Illumina, CA, USA) was used for sequencing to generate 150 bp paired-end reads.
2.7. Transcriptome Data Analysis
The low-quality and adaptor sequences were removed from the raw data using the Sequence CLEAN program to obtain clean data (Fastq files). After quality control with fastaQC (version 0.11.7), the sequences were mapped to the Gallus gallus genome assembly with Hisat2 (Gallus genome Version 5.0.1 NCBI) [20]. DESeq2 was used to determine DEGs according to default criteria consisting of a |log2 Fold Change (FC)| > 1.5 and an adjust-p value < 0.05. Functional analysis of DEGs was performed using the Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway, and Reactome pathway databases. All data represent the average change in gene expression from three independent replicates. In this study, GO and pathway terms enriched at an FDR < 0.05 were reported and considered significant.
2.8. Histopathological Evaluation of Trachea, Lungs, and Immune Organs in Birds
The trachea, lung, spleen, bursa of Fabricius, and thymus of 3 birds of each group were histopathologically evaluated using hematoxylin-eosin (H&E) staining, which has been described previously [21].
2.9. Statistical Analysis
IBM SPSS Statistics 19 software (Armonk, NY, USA) was used to analyze the data. The differences between groups were analyzed by one-way ANOVA using Duncan’s multiple-range test. The results are presented as the mean ± SD. Each experiment group has at least three samples. A value of p < 0.05 was statistically significant.
3. Results
3.1. MG Infection Leads to Reduced Body Weight and Dysregulated Immune Organs Index in Chicken Embryos and Newly Hatched Chicks
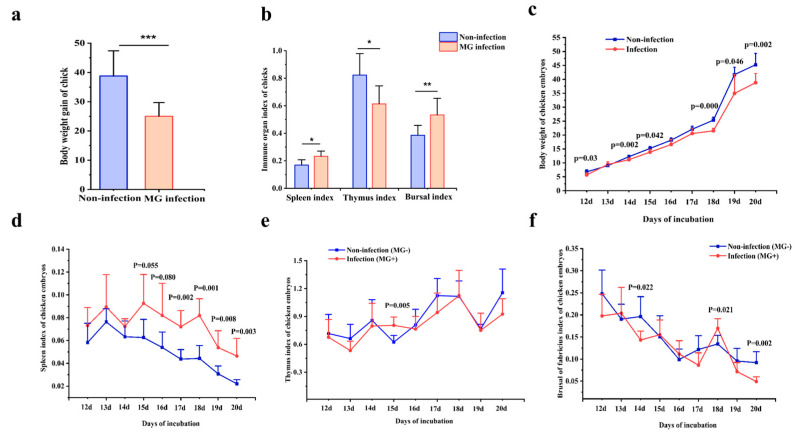
To evaluate the effects of MG infection on the chickens’ growth and immunity, the body weight and immune organ weights of two birds were weighed. As shown in Figure 1a, the body weight of infected chicken embryos at 3, 5, 6, 9, 10, and 11 day(s)post-infection (equivalent to 12, 14, 15, 18, 19, and 20 day(s) of hatching) was decreased significantly relative to the control group. As shown in Figure 1b, MG infection resulted in an obvious reduction in the weight gain of newly hatched chicks compared with the non-infection group. What’s more, the spleen index of chicken embryos at 7, 8-, 9-, 10-, and 11-day post-infection (Figure 1c), the thymus index of chicken embryos at 6 d post-infection (Figure 1d), and the bursal index at 9 days post-infection were remarkably elevated in the infection group, while the bursal index at 5- and 11-day(s) post-infection were decreased in the infection group relative to the control group (Figure 1e). In addition, the spleen and bursal indexes of infected chicks were significantly elevated relative to the control group, while the thymus index of infected chicks was reduced (Figure 1f). Taken together, MG infection reduces body weight in chickens and embryos and leads to abnormal immune organ indexes in chicks and chicken embryos.
Figure 1.
Effect of MG infection on body weight and immune organ index in chickens. (a) Body weight gains of chicks before and after MG infection. (b) Organ indexes for spleen, thymus, and bursa in chicks. (c) body weight of chicken embryos after 3–11 days of MG infection; spleen, thymus, and bursal indexes of chicken embryos after 3–11 days of MG infection (d–f). The data are represented as mean ± SD. * p < 0.05; ** p < 0.01; *** p < 0.001, n = 6.
3.2. MG-Induced Histopathological Damage in Chicks and Chicken Embryos
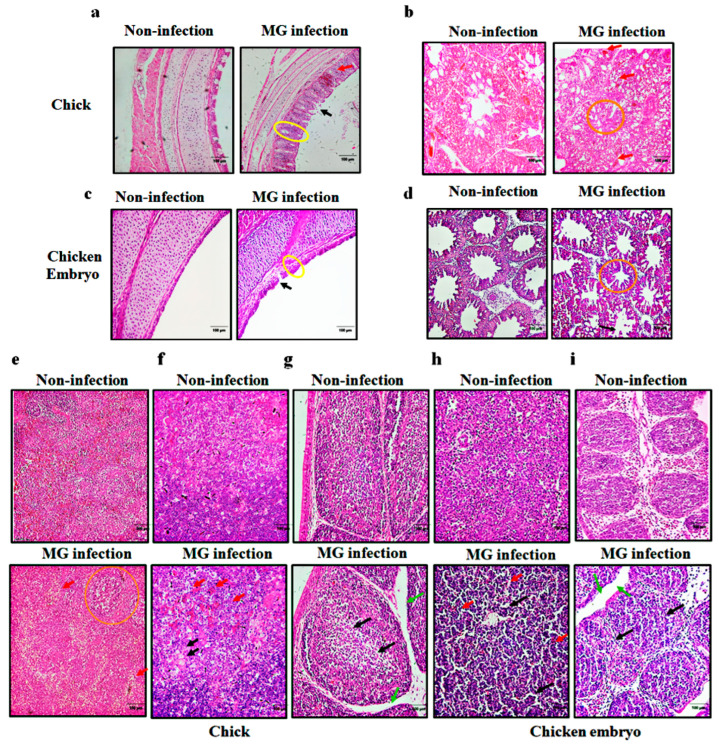
Next, we further investigated the histopathological effects of MG infection on the respiratory tract and immune organs of two birds. As shown in Figure 2a,b, no pathological changes were found in the lungs and trachea of chicken embryos in the non-infection group. In contrast, after MG infection, the trachea in chicken embryos showed inflammatory infiltration and increased mucosal tracheal thickness (Figure 2a), and the lungs showed narrowed alveolar space and cell shedding (Figure 2b). As shown in Figure 2c,d, the lung and trachea in the non-infection group showed normal and intact structure. However, MG infection resulted in significant lymphocytes infiltration, increased trachea mucosa thickness, and shedding of ciliated epithelial cells in chick trachea (Figure 2c) and narrowed alveolar space and severe inflammatory lesions in chick lung tissue (Figure 2d).
Figure 2.
Effect of MG infection on histopathology of trachea, lung, and immune organs in chicks and chicken embryos. Histopathological changes of trachea and lungs in chicken embryos (a,b) and chicks (c,d). Increased tracheal mucosa thickness (yellow circles); inflammatory cell infiltration (red arrows); an increase in the alveolar space (orange circle); shedding of cilia or epithelial cells (black arrows). (e–i) histopathological changes of spleen and bursa of Fabricius in chicken embryos (e,f) and newly hatched chicks (g–i). Enlarged splenic nodule (orange circle); lymphocyte reduction (black arrows); inflammatory cell infiltration (red arrows); interstitial edema (green arrows). (Magnification: ×20; scale bar: 100 μm; n = 3).
Furthermore, obvious pathological changes were also found in the immune organs of infected chicks and chicken embryos. As indicated in Figure 2e,f, non-infected embryonic spleen and bursa showed normal morphology, while infected embryonic spleen and bursa showed lymphocyte reduction and interstitial edema. Similar results were also observed in chicks. As shown in Figure 2g–i, the non-infected chick spleen, thymus, and bursa showed normal and complete structure without any histopathological changes. In contrast, infected chick spleens showed enlarged splenic nodules, inflammatory factor infiltration, lymphocyte reduction, and irregular arrangement of cells (Figure 2g); infected chick thymus and bursa of Fabricius all exhibited inflammatory cell infiltration and lymphocytes reduction (Figure 2h,i). In brief, MG infection induced immune damage in the respiratory tract and immune organs of both chicks and chicken embryos.
3.3. Transcriptome Sequencing Analysis of Chicks and Chicken Embryos Lung Tissues
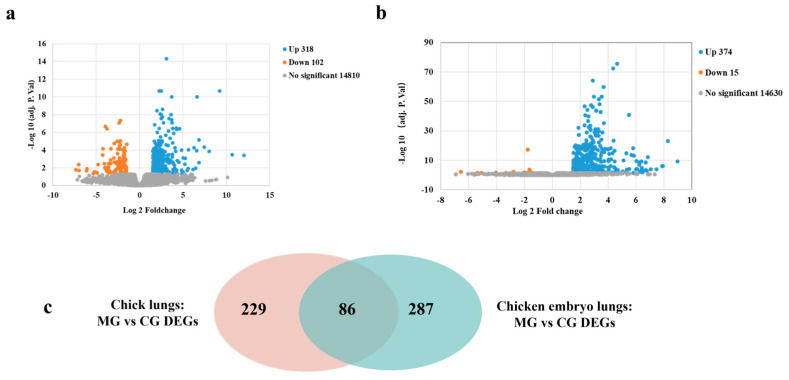
Illumine deep sequencing was applied to assess the global transcripts from the lungs of chicks and chicken embryos with MG infection. At an adjust-p value < 0.05, 389 DEGs were found in the lungs of infected chicken embryos compared to the control group, with much more upregulated genes (374/389, 96.14%) than downregulated genes (15/374, 3.86%) (Figure 3a). The top 30 upregulated and all 15 down-regulated genes in the lungs of infected chicken embryos were listed in Tables S1 and S2, respectively. Meanwhile, 417 DEGs were found in the lungs of infected chicks compared to the control group, among which the up-regulated genes (315/417 75.53%) were more than the down-regulated genes (102/417, 24.46%) (Figure 3b). The top 30 upregulated and downregulated genes in the lungs of infected chicks were listed in Tables S3 and S4, respectively. Through the Venn diagram, we found that there were 287 chicken embryo-specific DEGs, 229 chick-specific DEGs, and 86 shared DEGs (Figure 3c). Of the overlapped DEGs, all 86 DEGs were up-regulated in MG-infected chick and chicken embryo lungs. The top 30 overlapped up-regulated genes are listed in Table 3.
Figure 3.
Global DEGs identified between the MG infection group and control group in chicks and chicken embryos. Volcano plots of DEGs between the two groups in chicken embryos (a) and chicks (b). Blue dots indicate significantly up-regulated genes (Log2 FC > 1.5 and adjusted-p value < 0.05); orange dots indicate significantly down-regulated genes (Log2 FC < 1.5 and adjusted-p value < 0.05); gray dots indicate insignificant DEGs (adjusted-p value > 0.05). (c) Venn diagram of identified DEGs that are identical or specific between chicks and chick embryos with MG infection.
Table 3.
Top 30 common upregulated genes in the lung tissues of MG-infected chicken embryos and newly hatched chicks.
| Gene Name | Chicken Embryos CG vs. MG |
Newly Hatched Chicks CG vs. MG |
||
|---|---|---|---|---|
| Log2 FC | p Value-Adj | Log2 FC | p Value-Adj | |
| LOC770026 | 7.871279 | 5.93 × 10−7 | 5.628906 | 9.02 × 10−5 |
| IL4I1 | 8.292192 | 9.01 × 10−24 | 4.34217 | 0.001511 |
| IL8L2 | 8.973782 | 4.22 × 10−10 | 2.677474 | 0.000107 |
| AVD | 8.112968 | 1.10 × 10−229 | 3.530443 | 0.001406 |
| CXCL13 | 4.050071 | 2.39 × 10−4 | 7.463658 | 4.76 × 10−5 |
| IL8L1 | 6.075514 | 5.63 × 10−5 | 4.837175 | 0.01823 |
| CXCL13L2 | 5.491891 | 1.62 × 10−41 | 5.034946 | 0.000766 |
| CLEC5A | 6.426947 | 1.13 × 10−5 | 3.781138 | 0.000599 |
| DCSTAMP | 5.106985 | 8.03 × 10−11 | 4.602756 | 3.74 × 10−7 |
| CD72L1 | 5.84905 | 7.68 × 10−14 | 3.81117 | 1.56 × 10−5 |
| MLKL | 6.022089 | 2.49 × 10−10 | 3.513237 | 0.002472 |
| CD72 | 4.639555 | 5.09 × 10−3 | 4.272585 | 4.67 × 10−7 |
| MRGPRH | 4.359756 | 4.33 × 10−73 | 4.054451 | 0.010629 |
| S100A9 | 4.645942 | 2.61 × 10−76 | 3.256855 | 0.001827 |
| LOC419276 | 4.625564 | 3.41 × 10−3 | 2.765353 | 0.025887 |
| MX1 | 4.17664 | 5.01 × 10−19 | 2.5511 | 0.00012 |
| LOC101748032 | 3.504725 | 4.38 × 10−14 | 3.160072 | 0.001962 |
| LPAR5 | 3.489633 | 0.023681 | 2.833963 | 0.014503 |
| CCL5 | 3.601561 | 9.63 × 10−7 | 2.597259 | 0.018252 |
| CYBB | 3.470943 | 1.64 × 10−43 | 2.539925 | 0.005234 |
3.4. GO Analysis of the DEGs in Chicken Embryos and Chicks after MG Infection
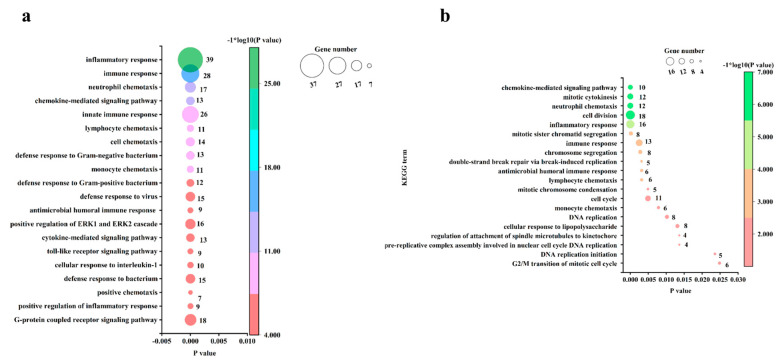
GO analysis of DEGs enabled the genes to be categorized based on enriched cellular components (CCs), molecular functions (MFs), and biological processes (BPs). At an FDR of <0.05, 374 upregulated genes in infected chicken embryo lungs were assigned to 3, 14, and 53 GOs for CCs, MFs, and BPs, respectively. The enriched CCs and MFs for upregulated genes are shown in Figure S1, and the top 20 significant enriched GOs for upregulated genes under BPs are shown in Figure 4a. The top three CCs enriched with upregulated genes were mainly associated with extracellular space, the external side of the plasma membrane, and the integral component of the membrane (Figure S1); the top three MFs enriched with upregulated genes were mainly associated with chemokine activity, CCR6 chemokine receptor binding, and CCR chemokine receptor binding (Figure S1), and the top 3 BPs enriched with upregulated genes were mainly associated with inflammatory response, immune response, neutrophil chemotaxis (Figure 4a). At an FDR < 0.05, 315 upregulated genes in infected chick lungs were assigned to 7, 10, and 21 GOs for CCs, MFs, and BPs, respectively, while downregulated genes were assigned to 3, 0, and 0 GOs for CCs, MFs, and BPs, respectively. The enriched CCs and MFs for up-and down-regulated genes are shown in Figure S2, and the top 21 enriched BPs for up-regulated genes are shown in Figure 4b. The top three CCs enriched with upregulated genes were mainly associated with chromosome, MCM complex, and nucleoplasm (Figure S2), the top 3 MFs enriched with upregulated genes were associated with ATP-dependent microtubule motor activity, transmembrane signaling receptor activity, and ATP binding (Figure S2), and the top three BPs enriched with upregulated genes were involved in inflammatory response, cell division, and neutrophil chemotaxis (Figure 4b). The CCs enriched with downregulated genes were associated with myelin sheath, haptoglobin–hemoglobin complex, and hemoglobin complex. Taken together, the co-activation of inflammatory response, immune response, and neutrophil chemotaxis in MG-infected chicken embryos and chick lung tissue suggests that these biological processes play a dominant role in the response of chickens to MG infection.
Figure 4.
The most significant enriched BPs based on FDR. (a) BPs enriched with up-regulated genes in MG-infected chicken embryo lungs compared with the control group. (b) BPs enriched with upregulated genes in MG-infected chick lungs compared with the control group. BP terms enriched at an FDR < 0.05 were listed.
In order to further explore the similarities and differences in immune responses between chicken embryos and chick lungs after MG infection, we calculated the number of DEGs involved in immune processes. We found that 75.84% of DEGs (295/389) in infected chick embryo lung tissue were associated with immune responses, which was significantly higher than that of 13.19% (55/417 DEGs) in chicks, indicating that the immune response of MG-infected chicken embryos was stronger than that of chicks.
3.5. Pathway Analysis of the DEGs in Chicks and Chicken Embryos after MG Infection
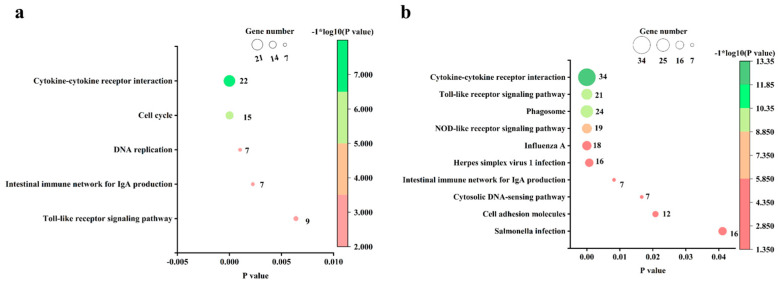
At an FDR of <0.05, there are 10 KEGG pathways enriched in the upregulated genes in infected chicken embryo lungs relative to the control group, but no KEGG pathway was enriched in the downregulated genes. The most significantly enriched pathway was the cytokine–cytokine receptor interaction (CCRI) pathway, followed by the toll-like receptor (TLR) pathway, phagosome, and NOD-like receptor, influenza A, herpes simplex virus 1 infection, intestinal immune network for IgA production, cytosolic DNA-sensing pathway, cell adhesion molecules, and salmonella infection signaling pathways (Figure 5a). At an FDR < 0.05, there were 5 KEGG pathways enriched in the upregulated genes in infected newly hatched chicks’ lungs, while no KEGG pathways were enriched with downregulated genes. The most significantly enriched pathway was also the CCRI pathway, followed by cell cycle, DNA replication, intestinal immune network for IgA production, and TLR signaling pathway (Figure 5b). The summary diagram based on GO and KEGG enrichment items is shown in Figure 6 and Figure 7.
Figure 5.
The significantly enriched KEGG pathways based on FDR. (a) KEGG pathways enriched in upregulated genes in MG-infected chicken embryo lungs compared to the control group. (b) KEGG pathways enriched in upregulated genes in MG-infected chick lungs compared to the control group. KEGG terms enriched at an FDR < 0.05 were listed.
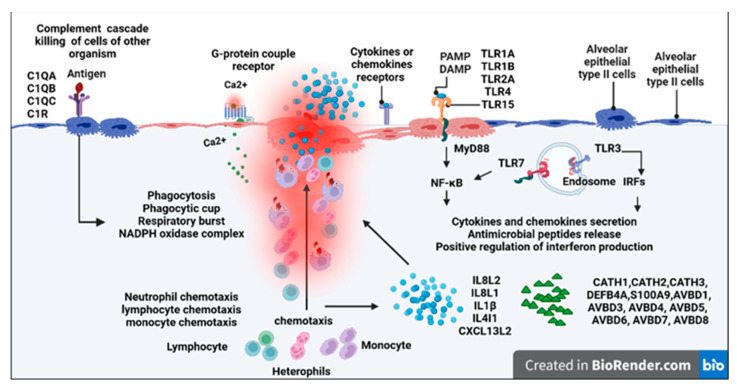
Figure 6.
Chicken embryo lungs responses to MG infection. The text indicates genes, gene ontologies, protein classes, and pathways enriched with upregulated genes. Created with BioRender.com (accessed on 1 February 2023).
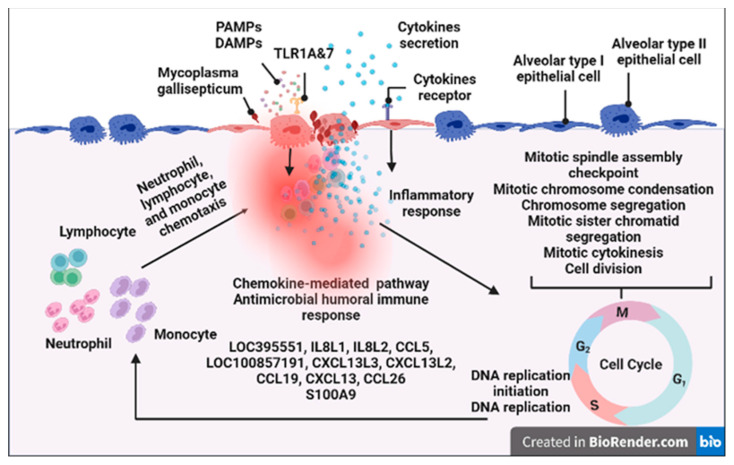
Figure 7.
Newly hatched chick lungs responses to MG infection. The text indicates genes, gene ontologies, protein classes, and pathways enriched with upregulated genes. Created with BioRender.com (accessed on 1 February 2023).
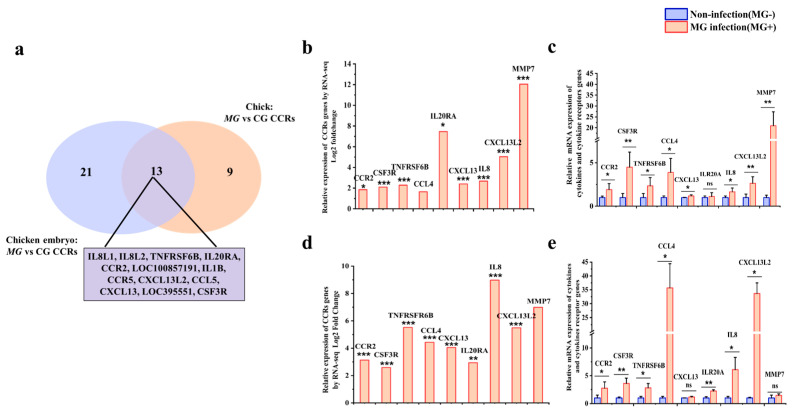
3.6. Validation of CCRs in Both Chicks and Chicken Embryos after MG Infection
As the CCRI pathway was the most enriched pathway in both infected chicken embryos and newly hatched chicks, we further analyzed DEGs enriched in this pathway. According to the KEGG pathway analysis, there are 34 and 22 DEGs enriched in the CCRI pathways in infected chicken embryos and chick lung tissues, respectively (Figure 8a). To validate the sequencing result, eight common DEGs, including CCR2, CSF3R, TNFRSF6B, CCL4, IL20RA, IL8, CXCL13, and CXCL13L2 and one added chick-unique gene MMP7, were selected for further analysis. Transcriptome sequencing results showed that these eight CCRI genes were upregulated by 4.64 times in chicken embryo lung tissue (Figure 8b) and 3.18 times on average in that of chicks after MG infection (Figure 8d). The RT-qPCR results showed that, except for the CXCL13 and MMP7, all shared seven genes were upregulated by about 12.4-fold on average in chicken embryo lungs after infection (Figure 8c), while in MG-infected chick lung tissue, all eight genes were upregulated by about 5.4-fold on average, except for IL20RA (Figure 8e). The results of RT-qPCR were basically consistent with the sequencing results except for the different multiples of gene expression changes, which confirmed the reliability of the sequencing results. In addition, the results of sequencing and RT-qPCR showed that the number and multiple of upregulated CCRI genes in chicken embryos after MG infection were higher than those in chicks.
Figure 8.
Effects of MG infection on CCRs genes expressions in chicken embryos and chicks. (a) Venn diagram of DEGs in the CCRI pathway in infected chicken embryo and chick lung tissue. (b) The mRNA expression level of CCRs and MMP7 in lungs of chicken embryos after MG infection by RNA-seq. (c) The mRNA expression level of CCRs and MMP7 in lungs of chicken embryos after MG infection by RT-qPCR. (d) The mRNA expression level of CCRs and MMP7 in lungs of chicks after MG infection by RNA-seq. (e) The mRNA expression level of CCRs and MMP7 in lungs of chicks after MG infection by RT-qPCR. GAPDH was used as a control. The data were represented as the mean ± SD. n ≥ 4. * p < 0.05; ** p < 0.01, *** p < 0.001.
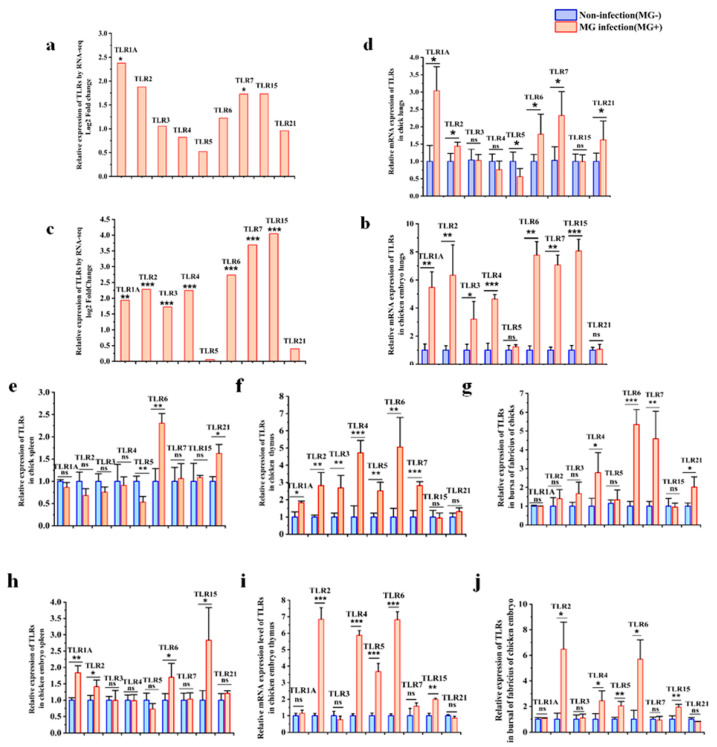
3.7. Validation of TLRs in Both Chick and Chicken Embryo after MG Infection
Since DEGs were significantly enriched in the TLRs pathway in the lung tissues of chicks and chick embryos infected with MG, we further analyzed all avian TLRs in the lungs and immune organs. The RNA-seq results showed that all avian TLRs, except for TLR5 and TLR21, were upregulated by 4.79-fold on average in infected chicken embryos’ lungs compared to the control group (Figure 9a), while only TLR1A and TLR7 were upregulated by about 2.05-fold on average in infected chick lungs compared to the control group (Figure 9c). RT-qPCR results showed that the expressions of all avian TLRs, except for TLR5 and TLR21, were evidently increased by about 6-fold on average in infected embryonic lungs (Figure 9b). However, in infected chick lungs, TLR1A, 2, 6, 7, and 21 expressions were significantly increased by 1.75-fold on average, TLR5 expression was decreased by about 0.5-fold, and for TLR3, four expressions showed no significant change (Figure 9d). These results were generally consistent with the sequencing results, indicating the reliability of the sequencing data.
Figure 9.
Effects of MG infection on TLRs gene expressions in chicken embryos and chicks. (a) The mRNA expression level of TLRs in lungs of chicken embryos after MG infection by RNA-seq. (b) The mRNA expression level of TLRs in lungs of chicken embryos after MG infection by RT-qPCR. (c) The mRNA expression level of TLRs in lungs of chicks after MG infection by RNA-seq. (d) The mRNA expression level of TLRs in lungs of chicks after MG infection by RT-qPCR. The mRNA expression level of TLRs in the spleen (e), thymus(f), and bursa of Fabricius (g) of chicken embryos after MG infection by RT-qPCR. The mRNA expression level of TLRs in the spleen (h), thymus (i), and bursa of Fabricius (j) of chicks after MG infection by RT-qPCR. GAPDH was used as a control. The data were represented as the mean ± SD. n ≥ 4. * p < 0.05; ** p < 0.01, *** p < 0.001.
To further understand the roles of TLRs in the host immune system, we further examined the expressions of these TLRs in immune organs after MG infection. The results in chicken embryos showed that TLRs were expressed differently in different tissues, and the overall expression of TLRs in the spleen was less changed than that in the lungs, thymus, and bursa of Fabricius. In the infected embryonic spleens, TLR1A, 2, 6, and 15 were significantly upregulated, while TLR3, 4, 5, 7, and 21 had no significant changes (Figure 9e). However, in the infected embryonic thymus, TLR2, 4, 5, 6, and 15 were highly expressed, while TLR1A, 3, 7, and 21 had no significant changes (Figure 9f). In infected embryonic bursa of Fabricius, TLR2, 4, 5, 6, and 15 were highly elevated relative to the control group, while TLR1A, 3, 7, and 21 had no significant changes (Figure 9g).
The results in chicks also showed that the overall expression of TLRs in the spleen was less changed than that in the lungs, thymus, and bursa of Fabricius after MG infection. In infected chick spleen, except for the upregulation of TLR6 and TLR21 and downregulation of TLR5, the expression of most TLRs did not change significantly (Figure 9h). However, in infected chick thymus, all the TLRs except TLR15 and TLR21 were upregulated relative to the control group (Figure 9i). In infected chick bursa, only TLR4, 6, 7, and 21 were highly expressed, but TLR1A, 15, 5, 2, and 3 had no significant changes compared to the control group (Figure 9j).
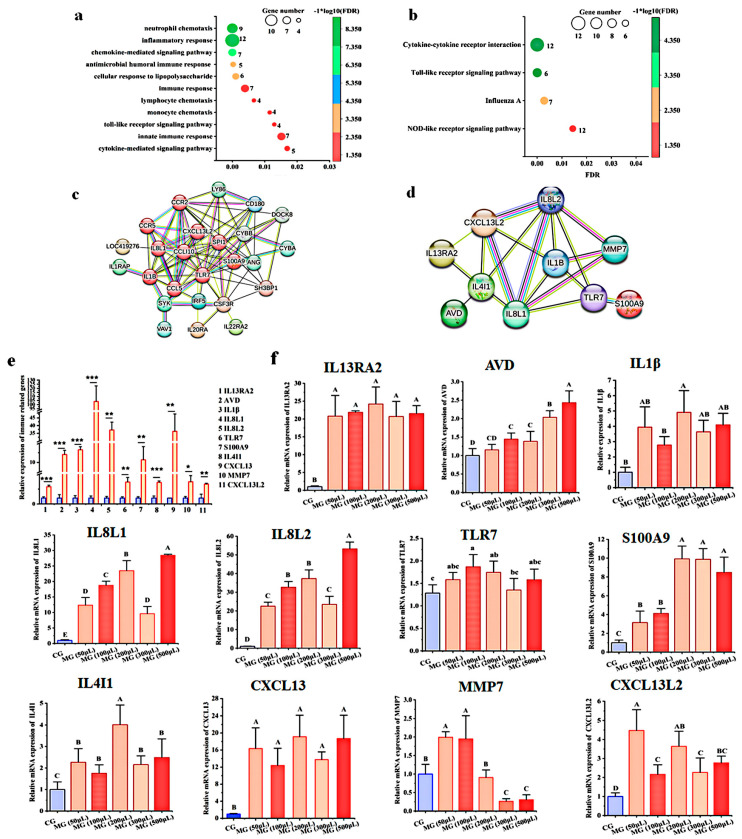
3.8. Analysis of Protein Interaction Network
To find out the core immune genes in regulating the immune response to MG infection, we first analyzed the 86 DEGs shared by infected chicken embryos and newly hatched chicks through GO and pathway analysis. At an FDR of <0.05, these DEGs were assigned to 0, 3, and 13 GOs for CCs, MFs, and BPs, respectively. Consistently, the three MFs were chemokine activity, CXCR chemokine receptor binding, and transmembrane signaling receptor activity, and the top five BPs enriched with upregulated genes were neutrophil chemotaxis, inflammatory response, chemokine-mediated signaling pathway, and antimicrobial humoral immune response (Figure 10a). KEGG pathway analysis showed that activation of the CCRI and TLRs signaling pathways was conserved in the immune response of chick embryos and chicks to MG infection (Figure 10b). The similarities and differences in immune responses of chicks and chicken embryos to MG infection are outlined in Table 4.
Figure 10.
Analysis of protein interaction network (a) BPs enriched with shared upregulated genes by MG-infected chicken embryo and chick lungs. (b) KEGG pathways enriched with shared upregulated genes by MG-infected chicken embryo and chick lungs. GO and KEGG pathway terms enriched at an FDR of <0.05 were listed. (c) STRING analysis of the protein interaction network of shared upregulated genes by MG-infected chicken embryo and chick lungs. (d) STRING analysis of the protein interaction network of core immune genes. (e) The mRNA expression levels of core immune genes in MG-infected AEC-II cells. (f) The effect of MG concentration on the mRNA expressions of core immune genes. GAPDH was used as a control. The data were represented as the mean ± SD. n ≥ 4. * p < 0.05; ** p < 0.01; *** p < 0.001. Bars with different capital letters show a significant difference (p < 0.01), and bars with different lowercase letters show a significant difference (p < 0.05).
Table 4.
Similarities and differences of immune damage induced by MG infection on chicks and chicken embryos.
| Feature | Pathological Changes/Enriched GO and KEGG Term | Affected Genes |
|---|---|---|
| Similarities |
|
No |
|
No | |
|
No | |
|
Upregulation of genes for TLR6, TLR7, and TLR1A in lung tissues; up-regulation of genes for TLR6 in spleen, thymus, and bursa of Fabricius; | |
|
Upregulation of genes for IL8L1, IL8L2, TNFRSF6B, IL20RA, CCR2, LOC100857191, IL1B, CCR5, CXCL13L2, CCL5, CXCL13, LOC395551, and CSF3R; | |
|
RNASE6, S100A9, RSFR, and LBP. | |
|
IL1B, HBE1, RNASE6, S100A9, BST1, ADGRG5, RSFR, LBP, and CXCL13L2; | |
|
IL8L1, CSF3R, IL8L2, SYK, CCL5, LOC100857191, CXCL13L2, CXCL13, CCL26; | |
| Differences |
|
Upregulations of genes for MAP3K8, SPP1, TLR4, TLR2A, TLR15, CD88, FOS, CCL4, TLR6, TLR3, LY96, and IRF7 were observed in chicken embryos, but not chicks |
|
Upregulations of genes for CSF3, IL18RAP, CSF2RB, IL16, IL18R1, IL10RA, CCL20, CSF1R, IL18, IL13RA2, TNFRSF4, IL1R2, XCR1, IL2RA, CCL4, CX3CR1, CXCR1, CRCBL, IL2RG, CSF2RA, and LOC101747500 in were observed in chicken embryos, but not in chicks | |
|
Upregulations of AVBD1, 3, 4, 5, 6, 7, and 8 and DEFB4A, CATH1, CATH2, and CATH3 were observed in chicken embryos only |
|
|
Upregulations of C1R, NCF2, BLB1, NCF4, ITGB2, TUBA4AL, TLR1A, TAP1, NCF1C, CYBB, TUB4A, TLR2A, BF1, MMR1L3, LOC771876, MMR1L4, MARCO, DMA, DMB2, LOC420160, ATP6V1G3, ATP6V0D2, TLR4, LOC428421 were observed in chicken embryos only | |
|
Upregulations of FEN1, RFC3, MCM3, MCM4, MCM5, DNA2, MCM2, BUB1B, CDK1, BUB1 were observed in chicks only |
Notes: The similarities and differences between chick and chicken embryos listed in the table were mainly drawn based on GO and KEGG enrichment analysis results.
Protein interaction network analysis through STRING showed that 10 genes, including but not limited to TLR7, S100A9, IL8L1, and IL1B, were in the center of the interaction network, indicating that these genes may play dominant roles in response to MG infection in both chicken embryos and chicks (Figure 10c). By comprehensively analyzing the immune genes significantly associated with MG infection reported by previous studies and the core genes found in this study, we identified 11 immune genes through the Venn diagram, including CXCL13L2, CXCL13, IL8L2, IL8L1, IL4I1, AVD, IL1B, TLR7, S100A9, IL13RA2, and MMP7, which may be the core immune genes regulating MG infection. STRING analysis showed that IL1B, IL8L1, IL8L2, and CXCL13L2 were at the center of network nodes (Figure 10d). Consistently, RT-qPCR results showed that these genes were significantly upregulated in MG-infected AEC-II cells (Figure 10e).
To further explore whether these genes are related to MG loading, we treated AEC-II cells with different concentrations of MG. The results showed that, with the increase of MG infection concentration, the expressions of CXCL13L2, IL1B, TLR7, IL8L2, IL8L1, and IL4I1 were in dynamic changes; AVD and S100A9 were increased in an MG concentration-dependent manner; MMP7 was significantly up-regulated in low MG treatment, and down-regulated with the increase of MG treatment concentration (Figure 10f).
4. Discussion
Many researchers have shown that MG infection is characterized by airway inflammation and dysregulated immunity. The findings of this study not only affirmed this view but also revealed that infected chicken embryos have higher levels of immune response in quantity and quality than infected chicken embryos.
Innate immunity against respiratory pathogens involves physical and immunological mechanisms. MG infection induced immune damage and apoptosis in the respiratory tract and immune organs of chickens [2,21].
The immune system of chickens begins to develop during the embryonic stage and continues to mature after hatching. During the embryonic development of chickens, the first lymphoid organ to develop is the thymus, then the bursa of Fabricius, and finally, the spleen. At the embryonic stage, the yolk sac and the embryonic bursa of Fabricius are the primary sites of immune cell development. As the chicken grows, the bone marrow and thymus become the primary sites of immune cell production. During embryo development, the spleen has a high contribution to lymphopoiesis.; however, it only becomes a secondary lymphoid organ once the egg hatches [7]. The spleen is a peripheral immune organ in birds, while the bone marrow, bursa, and thymus are considered central immune organs [22]. Damage to immune organs during the embryonic stage can lead to immune dysregulation [23,24]. The results of this study showed that, upon MG infection, inflammatory damage not only occurred in the respiratory tract and immune organ tissues of chickens but also in those of chicken embryos, especially embryonic spleen; the spleen in two infected birds exhibited inflammatory factor infiltration and lymphocyte reduction. These results suggest that MG infection not only crosses the epithelial barrier but may also spread to the immune organs of the chicks and chicken embryos.
Respiratory inflammation induced by MG infection was accompanied by activation of immune response involving cytokines production and cell proliferation signal induction [12,15]. It has been reported that immune responses induced by MG were age-related in chickens. Younger birds were found to show more serious clinical symptoms than older birds after MG infection [25]. The transcriptional changes involved in immune response were stronger in younger birds than in relatively older birds [5]. In the present study, we found that the genes upregulated in chicks were mainly involved in cell proliferation and DNA replication, followed by CCRs interaction and TLR pathway, while genes upregulated in chicken embryos were mostly involved in CCRs interaction and TLR pathways, followed by phagocytosis; the transcriptional changes associated with the immune response were less severe in chicks than in chicken embryos. As an important part of the innate immune system, the transcriptional expression of CCRs and TLRs may be the underlying molecular mechanism of MG-induced immune dysregulation.
Since the upregulated genes in MG-infected chicks and chicken embryos were significantly involved in the regulation of CCR interaction, we further investigated the genes in this pathway. Eight overlapped CCR genes, including CSF3R, TNFRSF6B, IL20RA, IL8L2, CXCL13L2, IL1B, CCR2, and CCL4, were selected for further study by RT-qPCR. According to previous studies, these CCRs were immune and chemotactic regulators, which closely participated in MG infection in chickens [5,26,27]. Consistent with the sequencing data, these CCR genes were highly expressed in the lungs of two birds after MG infection, and their expression levels in chicken embryos were higher than those in chicks. Further GO analysis indicates that these CCR genes were involved in neutrophil degranulation and chemotaxis.
TLRs play important roles in inflammation and innate immune response [28,29,30,31]. Given the significant enrichment of TLR signaling in the host response to MG infection, we focused on the differences in TLR expression between infected chicks and chick embryos. Although most avian TLRs, which are localized at membrane or endosomal, have been reported to be associated with MG infection, especially TLR2, 4, 7, and 15 [5,27], only the role of membrane-localized TLR2, 4, and 6 have been experimentally confirmed. Inhibition of TLR2 suppressed inflammatory cytokines genes induced by MG in tracheal epithelial cells [11]. Our group found that in the absence of TLR2, MG could induce inflammation via TLR6 in DF-1 cells [32], or TLR4 could be activated by HMGB1 to trigger MG-induced immune disorders in avian macrophage cells [33]. TLR3, 7, and 21 are localized at endosomal and induced interferon transcription and mixed Th1 and Th2 cytokines responses. However, synergistic TLR21 and TLR3 agonists led to a stronger Th1-biased immune response in chicken monocytes [34]. TLR15 is unique to avian species; upregulation of TLR15 led to activation of the NF-κB pathway and, therefore, pro-inflammatory cytokines production [35,36]. In this study, we found that most TLRs were upregulated in infected chicken embryo lungs but not TLR5 and TLR21. TLR2, 6, and 7 were conservatively upregulated in the lungs of infected chicken embryos and chicks, while TLR3, 4, 5, 15, and 21 showed an opposite expression pattern. Besides, TLR2 and TLR6 were co-upregulated in the immune organs of the two infected birds, except for the chick spleen. Combined with the above findings, TLR2, 6, and 7 may be responsible for MG-induced immune dysregulation in chickens. An interesting finding here was that the spleen in infected two birds exhibited relatively lower TLRs expression than the thymus and bursa in response to MG. This may be related to lymphocytopenia and inflammatory infiltration caused by MG infection in the spleen. Another interesting finding was that TLR3, 7, and 21 were not upregulated simultaneously after MG but in pairs. However, this did not include TLR3 and TLR21. For example, TLR3 and TLR7 were co-upregulated in infected chicken embryo lungs, but not TLR21; in infected chick lungs, TLR7 and TLR21 expressions were increased simultaneously, but not TLR3. These may suggest that the immune response to MG infection is a mixed Th1 and Th2 cytokines response. Taken together, the higher TLR and CCR expression levels in chicken embryos lead to a stronger innate immune response to MG infection in chick embryos than in chicks.
Of interest, MG does not contain LPS, but TLR4 expression was increased in infected embryonic lungs in the present work. Owing to the function of activating cytokines and defensins, MMP7 was considered to induce TLR4 upregulation in MG infection [12]. A recent study confirmed that the S100A9-coded MRP-126 protein could induce TLR4 expression in the presence of the cofactors CD14 and LY98 [37]. In this study, MMP7 expression was found to be increased 20-fold in chicken lungs and was negatively related to MG load in alveolar epithelial cells induced by MG but not in chicken embryonic lungs. However, S100A9 was evidently upregulated in the lungs of two infected birds and was also related to MG load. Hence, we believe that S100A9 may be responsible for TLR4 activation in MG infection, but this needs to be further studied.
Avian AMPs with antibacterial and immunomodulatory activities are important parts of chicken innate and adaptive immune system, which are composed of cathelicidins (CATH) and β-defensins. Unlike most antibiotics, AMPs are less prone to drug resistance due to their unique antibacterial mechanism [38]. It has been reported that the disease-resistant chicken had higher AMPs levels than those in the susceptible chicken [39,40]. In this study, we found nine HDPs, including AVBD1, 4, 5, 6, 7, CATH-1, 2, 3, and DEFB4A, were highly expressed in infected chicken embryo lungs, while only one, AVBD1, was upregulated in infected chick lungs. As in ovo administration has been widely used to improve the immunity of newly hatched chicks [39,41], in ovo administration of AMPs may be a safe and effective way to improve the resistance of poultry to MG infection.
Overall, there are certain similarities and differences in the way chicks and chicken embryos coordinate innate responses, ranging from the quantity and quality of the recognition of pathogen receptors, downstream cytokines, and antimicrobial peptides to the mechanism of action. This difference may be due to differences in physiological structure and immune development between chickens and their embryos. Compared to chicks, the lung tissue development of chicken embryos is incomplete, and their tissue development integrity and gas exchange capabilities are inferior to those of chicks. Similarly, the immune system development of chicken embryos is not as complete as that of nursing chicks [7]. Although the immune system of a newborn chick is also immature, it is still better developed than that of an embryo. This means that when the embryo is faced with MG invasion, the cytokine and natural antimicrobial peptides-mediated defense mechanisms must be fully activated, resulting in a stronger immune response induced by MG in the embryo than in the chick. As many studies have shown that in ovo vaccination can improve the immunity of newborn chicks, the results of this study are beneficial for future exploration and dissection of innate immune signaling pathways, as well as for designing more effective disease control strategies.
5. Conclusions
In conclusion, the immune response of chicken embryos to MG infection was stronger than that of chicken lungs, which was due to the higher number and expression levels of CCRs, TLRs, and AMPs in chicken embryos. Functional expression of TLRs, CCRs, and AMPs in chicken embryos suggests that immune responses can be modulated and enhanced at this development stage to improve the innate or adaptive immunity of chickens in response to MG infection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13101667/s1, Figure S1: The most significant enriched CCs and MFs based on FDR in chicken embryos; Figure S2: The most significant enriched CCs and MFs based on FDR in chicks. Table S1: Top 30 upregulated genes in the lung tissues of MG-infected chicken embryos; Table S2: All 15 down-regulated genes in the lung tissues of MG-infected chicken embryos; Table S3: Top 30 upregulated genes in the lung tissues of MG-infected newly hatched chicks; Table S4: Top 30 downregulated genes in the lung tissues of MG-infected newly hatched chicks.
Author Contributions
M.Z. and R.L. performed the experiments; T.W. and M.Z. analyzed the data; Y.W. and Y.S. performed the total RNA isolation and cDNA preparation and helped write the manuscript; X.P. conceived the study and helped write the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by Ethical Committee on Animal Research at Huazhong Agricultural University (HZAUCH-2020-0003) and met the International Guiding Principles for Biomedical Research Involving Animals as issued by the Council for the International Organizations of Medical Sciences.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the conclusions of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 31972681) and the National Natural Science Foundation of China (Grant No. 32273010).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Elliott K.E.C., Branton S.L., Evans J.D., Peebles E.D. Occurrence of horizontal transmission in layer chickens after administration of an in ovo strain F Mycoplasma gallisepticum vaccine 1, 2, 3. Poult. Sci. 2019;98:4492–4497. doi: 10.3382/ps/pez306. [DOI] [PubMed] [Google Scholar]
- 2.Luo R., Wang Y., Guo Q., Fan C., Jiang G., Wang L., Zou M., Wang T., Sun Y., Peng X. Andrographolide attenuates Mycoplasma gallisepticum-induced inflammation and apoptosis by the JAK/PI3K/AKT signal pathway in the chicken lungs and primary alveolar type II epithelial cells. Int. Immunopharmacol. 2022;109:108819. doi: 10.1016/j.intimp.2022.108819. [DOI] [PubMed] [Google Scholar]
- 3.Alqhtani A.H., Fatemi S.A., Elliott K.E.C., Branton S.L., Evans J.D., Leigh S.A., Gerard P.D., Peebles E.D. Effects of the In Ovo Vaccination of the ts-11 Strain of Mycoplasma gallisepticum in Layer Embryos and Posthatch Chicks. Animals. 2022;12:1120. doi: 10.3390/ani12091120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Z., Wu Y., Zhou Z., Xia X., Gu X., Cai Q., Shen X., Yang H., Ding H. Pharmacokinetic and Pharmacodynamic Integration and Resistance Analysis of Tilmicosin Against Mycoplasma gallisepticum in an In Vitro Dynamic Model. Front. Pharmacol. 2019;10:670. doi: 10.3389/fphar.2019.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulappu Arachchige S.N., Young N.D., Kanci Condello A., Omotainse O.S., Noormohammadi A.H., Wawegama N.K., Browning G.F. Transcriptomic Analysis of Long-Term Protective Immunity Induced by Vaccination With Mycoplasma gallisepticum Strain ts-304. Front. Immunol. 2020;11:628804. doi: 10.3389/fimmu.2020.628804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davison T.F. The immunologists’ debt to the chicken. Br. Poult. Sci. 2003;44:6–21. doi: 10.1080/0007166031000085364. [DOI] [PubMed] [Google Scholar]
- 7.Garcia P., Wang Y., Viallet J., Macek Jilkova Z. The Chicken Embryo Model: A Novel and Relevant Model for Immune-Based Studies. Front. Immunol. 2021;12:791081. doi: 10.3389/fimmu.2021.791081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radomska K.A., Vaezirad M.M., Verstappen K.M., Wosten M.M., Wagenaar J.A., van Putten J.P. Chicken Immune Response after In Ovo Immunization with Chimeric TLR5 Activating Flagellin of Campylobacter jejuni. PLoS ONE. 2016;11:e0164837. doi: 10.1371/journal.pone.0164837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alkie T.N., Yitbarek A., Hodgins D.C., Kulkarni R.R., Taha-Abdelaziz K., Sharif S. Development of innate immunity in chicken embryos and newly hatched chicks: A disease control perspective. Avian Pathol. 2019;48:288–310. doi: 10.1080/03079457.2019.1607966. [DOI] [PubMed] [Google Scholar]
- 10.Weibel E.R. On the Tricks Alveolar Epithelial Cells Play to Make a Good Lung. Am. J. Respir. Crit. Care Med. 2015;191:504–513. doi: 10.1164/rccm.201409-1663OE. [DOI] [PubMed] [Google Scholar]
- 11.Majumder S., Zappulla F., Silbart L.K. Mycoplasma gallisepticum lipid associated membrane proteins up-regulate inflammatory genes in chicken tracheal epithelial cells via TLR-2 ligation through an NF-kappaB dependent pathway. PLoS ONE. 2014;9:e112796. doi: 10.1371/journal.pone.0112796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaudet J., Tulman E.R., Pflaum K., Liao X., Kutish G.F., Szczepanek S.M., Silbart L.K., Geary S.J. Transcriptional Profiling of the Chicken Tracheal Response to Virulent Mycoplasma gallisepticum Strain R(low) Infect. Immun. 2017;85:e00343-17. doi: 10.1128/IAI.00343-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaudet J., Tulman E.R., Pflaum K., Canter J.A., Silbart L.K., Geary S.J. Immunologic Pathways in Protective versus Maladaptive Host Responses to Attenuated and Pathogenic Strains of Mycoplasma gallisepticum. Infect. Immun. 2019;87:e00613-18. doi: 10.1128/IAI.00613-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canter J.A., Tulman E.R., Beaudet J., Lee D.H., May M., Szczepanek S.M., Geary S.J. Transcriptional and Pathological Host Responses to Coinfection with Virulent or Attenuated Mycoplasma gallisepticum and Low-Pathogenic Avian Influenza A Virus in Chickens. Infect. Immun. 2019;88:e00607-19. doi: 10.1128/IAI.00607-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z., Ding L., Bao J., Liu Y., Zhang Q., Wang J., Li R., Ishfaq M., Li J. Co-infection of Mycoplasma gallisepticum and Escherichia coli Triggers Inflammatory Injury Involving the IL-17 Signaling Pathway. Front. Microbiol. 2019;10:2615. doi: 10.3389/fmicb.2019.02615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C., Zhong L., Zhang L., Li W., Liu B., Huang B., Luo Z., Wu Y. Study on the mechanism of Mycoplasma gallisepticum infection on chicken tracheal mucosa injury. Avian Pathol. 2022;51:361–373. doi: 10.1080/03079457.2022.2068997. [DOI] [PubMed] [Google Scholar]
- 17.Zou M., Yang W., Niu L., Sun Y., Luo R., Wang Y., Peng X. Polydatin attenuates Mycoplasma gallisepticum (HS strain)-induced inflammation injury via inhibiting the TLR6/ MyD88/NF-kappaB pathway. Microb. Pathog. 2020;149:104552. doi: 10.1016/j.micpath.2020.104552. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y., Fu Y., Zou M., Sun Y., Yin X., Niu L., Gong Y., Peng X. Analysis of deep sequencing exosome-microRNA expression profile derived from CP-II reveals potential role of gga-miRNA-451 in inflammation. J. Cell. Mol. Med. 2020;24:6178–6190. doi: 10.1111/jcmm.15244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou M., Yang L., Niu L., Zhao Y., Sun Y., Fu Y., Peng X. Baicalin ameliorates Mycoplasma gallisepticum-induced lung inflammation in chicken by inhibiting TLR6-mediated NF-kappaB signalling. Br. Poult. Sci. 2021;62:199–210. doi: 10.1080/00071668.2020.1847251. [DOI] [PubMed] [Google Scholar]
- 20.Kim D., Langmead B., Salzberg S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu F., Luo R., Duan S., Guo Q., Wang L., Jiang G., Fan C., Zou M., Wang T., Wang Y., et al. Evaluation of Glycyrrhizic Acid Therapeutic Effect and Safety in Mycoplasma gallisepticum (HS Strain)-Infected Arbor Acres Broilers. Animals. 2022;12:1285. doi: 10.3390/ani12101285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song B., Tang D., Yan S., Fan H., Li G., Shahid M.S., Mahmood T., Guo Y. Effects of age on immune function in broiler chickens. J. Anim. Sci. Biotechnol. 2021;12:42. doi: 10.1186/s40104-021-00559-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C., Li J., Zhang W., Shah S.W.A., Ishfaq M. Mycoplasma gallisepticum triggers immune damage in the chicken thymus by activating the TLR-2/MyD88/NF-kappaB signaling pathway and NLRP3 inflammasome. Vet. Res. 2020;51:52. doi: 10.1186/s13567-020-00777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishfaq M., Wu Z., Wang J., Li R., Chen C., Li J. Baicalin alleviates Mycoplasma gallisepticum-induced oxidative stress and inflammation via modulating NLRP3 inflammasome-autophagy pathway. Int. Immunopharmacol. 2021;101:108250. doi: 10.1016/j.intimp.2021.108250. [DOI] [PubMed] [Google Scholar]
- 25.Gaunson J.E., Philip C.J., Whithear K.G., Browning G.F. Age related differences in the immune response to vaccination and infection with Mycoplasma gallisepticum. Vaccine. 2006;24:1687–1692. doi: 10.1016/j.vaccine.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 26.Majumder S., Silbart L.K. Interaction of Mycoplasma gallisepticum with Chicken Tracheal Epithelial Cells Contributes to Macrophage Chemotaxis and Activation. Infect. Immun. 2016;84:266–274. doi: 10.1128/IAI.01113-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kulappu Arachchige S.N., Young N.D., Shil P.K., Legione A.R., Kanci Condello A., Browning G.F., Wawegama N.K. Differential Response of the Chicken Trachea to Chronic Infection with Virulent Mycoplasma gallisepticum Strain Ap3AS and Vaxsafe MG (Strain ts-304): A Transcriptional Profile. Infect. Immun. 2020;88:e00053-20. doi: 10.1128/IAI.00053-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao J., Xu R., Geng Y., Xu S., Guo M. Exposure to polystyrene microplastics triggers lung injury via targeting toll-like receptor 2 and activation of the NF-kappaB signal in mice. Environ. Pollut. 2023;320:121068. doi: 10.1016/j.envpol.2023.121068. [DOI] [PubMed] [Google Scholar]
- 29.Cui J., Zhang Y., Liu L., Zhang Q., Xu S., Guo M.Y. Polystyrene microplastics induced inflammation with activating the TLR2 signal by excessive accumulation of ROS in hepatopancreas of carp (Cyprinus carpio) Ecotoxicol. Environ. Saf. 2023;251:114539. doi: 10.1016/j.ecoenv.2023.114539. [DOI] [PubMed] [Google Scholar]
- 30.Ahn M.Y., Yoon H.E., Park J.H., Lee J., Min S.K., Ahn S.G., Yoon J.H. Characterization of NODs and TLRs in innate immune response of human cementoblast cells. Oral Dis. 2013;19:374–380. doi: 10.1111/odi.12012. [DOI] [PubMed] [Google Scholar]
- 31.Kim S., Kaiser P., Borowska D., Vervelde L. Synergistic effect of co-stimulation of membrane and endosomal TLRs on chicken innate immune responses. Vet. Immunol. Immunopathol. 2018;199:15–21. doi: 10.1016/j.vetimm.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Tian W., Zhao C., Hu Q., Sun J., Peng X. Roles of Toll-like receptors 2 and 6 in the inflammatory response to Mycoplasma gallisepticum infection in DF-1 cells and in chicken embryos. Dev. Comp. Immunol. 2016;59:39–47. doi: 10.1016/j.dci.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y., Wang L., Hu F., Zou M., Luo R., Sun Y., Wang T., Guo Q., Peng X. Extracellular HMGB1 as Inflammatory Mediator in the Progression of Mycoplasma gallisepticum Infection. Cells. 2022;11:2817. doi: 10.3390/cells11182817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He H., Genovese K.J., Swaggerty C.L., MacKinnon K.M., Kogut M.H. Co-stimulation with TLR3 and TLR21 ligands synergistically up-regulates Th1-cytokine IFN-gamma and regulatory cytokine IL-10 expression in chicken monocytes. Dev. Comp. Immunol. 2012;36:756–760. doi: 10.1016/j.dci.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Boyd A.C., Peroval M.Y., Hammond J.A., Prickett M.D., Young J.R., Smith A.L. TLR15 is unique to avian and reptilian lineages and recognizes a yeast-derived agonist. J. Immunol. 2012;189:4930–4938. doi: 10.4049/jimmunol.1101790. [DOI] [PubMed] [Google Scholar]
- 36.Neves F., Munoz-Merida A., Machado A.M., Almeida T., Gaigher A., Esteves P.J., Castro L.F.C., Verissimo A. Uncovering a 500 million year old history and evidence of pseudogenization for TLR15. Front. Immunol. 2022;13:1020601. doi: 10.3389/fimmu.2022.1020601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loes A.N., Bridgham J.T., Harms M.J. Coevolution of the Toll-Like Receptor 4 Complex with Calgranulins and Lipopolysaccharide. Front. Immunol. 2018;9:304. doi: 10.3389/fimmu.2018.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y., Tian H., Chen R., Liu Q., Jia K., Hu D.L., Chen H., Ye C., Peng L., Fang R. Synergistic Antimicrobial Effect of Antimicrobial Peptides CATH-1, CATH-3, and PMAP-36 With Erythromycin Against Bacterial Pathogens. Front. Microbiol. 2022;13:953720. doi: 10.3389/fmicb.2022.953720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jang H.J., Monson M., Kaiser M., Lamont S.J. Induction of Chicken Host Defense Peptides within Disease-Resistant and -Susceptible Lines. Genes. 2020;11:1195. doi: 10.3390/genes11101195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Z., Ahmed I., Xu Z., Sun S., Li T., Gu D., Liu Y., Zhang X., Yan S., Hu W., et al. Profiles of expression pattern and tissue distribution of host defense peptides genes in different chicken (Gallus gallus) breeds related to body weight. PLoS ONE. 2020;15:e0238675. doi: 10.1371/journal.pone.0238675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Harten R.M., Tjeerdsma-van Bokhoven J.L.M., de Greeff A., Balhuizen M.D., van Dijk A., Veldhuizen E.J.A., Haagsman H.P., Scheenstra M.R. d-enantiomers of CATH-2 enhance the response of macrophages against Streptococcus suis serotype 2. J. Adv. Res. 2022;36:101–112. doi: 10.1016/j.jare.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the conclusions of this study are available from the corresponding author upon reasonable request.