Abstract
Neurodegenerative diseases (NDDs), which are chronic and progressive diseases, are a growing health concern. Among the therapeutic methods, stem-cell-based therapy is an attractive approach to NDD treatment owing to stem cells’ characteristics such as their angiogenic ability, anti-inflammatory, paracrine, and anti-apoptotic effects, and homing ability to the damaged brain region. Human bone-marrow-derived mesenchymal stem cells (hBM-MSCs) are attractive NDD therapeutic agents owing to their widespread availability, easy attainability and in vitro manipulation and the lack of ethical issues. Ex vivo hBM-MSC expansion before transplantation is essential because of the low cell numbers in bone marrow aspirates. However, hBM-MSC quality decreases over time after detachment from culture dishes, and the ability of hBM-MSCs to differentiate after detachment from culture dishes remains poorly understood. Conventional analysis of hBM-MSCs characteristics before transplantation into the brain has several limitations. However, omics analyses provide more comprehensive molecular profiling of multifactorial biological systems. Omics and machine learning approaches can handle big data and provide more detailed characterization of hBM-MSCs. Here, we provide a brief review on the application of hBM-MSCs in the treatment of NDDs and an overview of integrated omics analysis of the quality and differentiation ability of hBM-MSCs detached from culture dishes for successful stem cell therapy.
Keywords: neurodegenerative diseases, stem cell therapy, human bone-marrow-derived mesenchymal stem cells, integrated omics, stemness
1. Introduction
Neurodegenerative diseases (NDDs) are caused by the progressive degeneration of the structure and function of neurons and glial cells in the central and peripheral nervous systems [1,2]. NDDs can be classified according to their causes and symptoms [3]. Alzheimer’s disease (AD) and Parkinson’s disease (PD) are among the most common neurodegenerative disorders. AD shows widespread degeneration of several types of neurons, whereas PD shows selective loss of a specific cell population, such as dopaminergic neurons. Amyotrophic lateral sclerosis (ALS), commonly called Lou Gehrig’s disease, shows selective degeneration of the upper and lower motor neurons [4] and has been associated with genetic mutations in the enzyme Cu/Zn superoxide dismutase 1 (SOD1) [5,6]. Huntington’s disease (HD) is a rare genetic disorder caused by a mutation in the huntingtin gene that causes movement abnormalities and cognitive impairment [7]. As the human lifespan increases, the social burden of NDDs also increases [8]. Due to the incurable and debilitating nature of these conditions, there are several limitations in treating NDDs with conventional methods. Therefore, there is a need for new treatment approaches.
Transplanted stem cells exert paracrine effects on damaged neuronal cells and replace lost neurons or glial cells [9,10]. Thus, stem-cell-based approaches are potential therapies in myocardial infarction [11], ischemic diseases [12], spinal cord injury [13], and NDDs, such as AD (NCT01547689 and NCT02833792) [4], PD [14], and multiple system atrophy [15]. Furthermore, HD could be treated with genetically engineered mesenchymal stem cells (MSCs) overexpressing brain-derived neurotrophic factor (BDNF) (NCT01937923) [16]. ALS is another NDD that could be treated using stem cells [4]. There are several MSC types, such as adipose-derived MSCs, umbilical-cord-derived MSCs, tonsil-derived MSCs [17], dental-pulp-derived MSCs (DPSCs) [18], and bone-marrow-derived MSCs (BM-MSCs). Among them, BM-MSCs, first identified in guinea pig bone marrow in 1970 [19], are attractive therapeutic agents because of their widespread availability, easy attainability, and in vitro manipulation and the absence of ethical concerns [20,21,22]. For these reasons, BM-MSCs are extensively used in clinical applications and therefore the biological characteristics and clinical effects of BM-MSCs are far more well established than MSCs obtained from other sources [23]. In addition, the expression level of HLA class I or II is low in BM-MSCs, avoiding activation of allogenic lymphocytes [24], and BM-MSCs are safe from tumorigenicity after transplantation than other stem cells, including induced pluripotent stem cells (iPSCs) and neural stem cells [25,26]. BM-MSCs exhibit homing to injured sites [27,28,29,30], angiogenic ability [31,32], anti-inflammatory effects [33,34], differentiation capability [35,36], anti-apoptotic properties [37,38], and trophic factor secretion [39,40,41,42]. Furthermore, BM-MSCs have been engineered to express tropic factors, such as BDNF [33,41,43], glial-cell-line-derived neurotrophic factor (GDNF) [44,45,46], and nerve growth factor (NGF) [47,48] to improve therapeutic paracrine effects for NDD treatment. Thus, BM-MSC-based cell therapy is a promising approach for treating NDDs [49,50].
Because MSCs can accelerate tissue regeneration, they are excellent candidates for tissue engineering. Vacanti and Langer define tissue engineering as an interdisciplinary field in which engineering and biological sciences are applied together to develop biological substitutes that can restore, maintain, or enhance tissue function [51]. Tissue engineering facilitates autologous MSCs’ transplantation by seeding the patient’s cells onto a biodegradable scaffold that forms a specific tissue that can then be used to repair injuries [52]. Despite all this, MSC-based tissue engineering faces some challenges, such as the low survival rates of MSCs and the uncertainty of MSC differentiation after infusion. To address these shortcomings, biomaterials are used together with MSCs to maintain their viability, serve as a substrate for cell adhesion, induce differentiation into specific target cells, and as a mechanical tool for tissue regeneration. Biomaterials have demonstrated excellent biocompatibility, provide a suitable cellular microenvironment for MSCs, and are effective and easy to administer. As biomaterials are diverse in their physical, chemical, mechanical, and biological properties, they can contribute to tissue regeneration in different types of injuries [53]. Although several biomaterials have been developed, hydrogels, nanofibers, carbon-based nanomaterials, and cell-free scaffolds have emerged as the frontrunners. For example, the 3D structure of hydrogels supports the proliferation of cells while acting as a barrier to harmful factors [54]. Yan et al. investigated collagen–chitosan scaffolds with BM-MSCs as a therapeutic strategy for traumatic brain injury. Collagen scaffolds with human MSCs have been shown to improve spatial learning and sensorimotor functions, while chitosan serves as a neuroprotector. They reported that these scaffolds exhibited low immunogenicity, good biocompatibility, and therapeutic effects such as neurological, behavioural, and cognitive recovery [55].
In clinical applications, the quality of hBM-MSCs should be analyzed after cell detachment from the culture dish [12,15,56], because cell quality is an essential factor for therapeutic effects. However, there are limitations to the traditional methods used to evaluate the quality and freshness of cells prior to transplantation of hBM-MSCs into humans, such as trypan blue staining or fluorescence-activated cell sorting (FACS) [56,57]. Regardless, it is possible to increase the treatment effect by determining the exact cell condition using various big data analyses, including omics and bioinformatic analyses of the quality and differentiation ability of hBM-MSCs over time after cell detachment from the culture dish. In this review, the transcriptome and metabolome, analyzed using microarray and gas chromatography-mass spectrometry (GC-MS), respectively, were integrated to evaluate the condition of hBM-MSCs. Integrated omics analysis of hBM-MSCs showed increases in reactive oxygen species (ROS) production, lipid peroxidation, and cell damage, leading to the loss of cell quality and differentiation ability as the phosphate-buffered saline (PBS) storage time increased (Figure 1).
Figure 1.
Effect of storage time on cell quality and differentiation potential. Before transplantation, human bone-marrow-derived mesenchymal stem cells (hBM-MSCs), which were expanded ex vivo and detached from the culture dish, were analyzed to evaluate their quality and differentiation potential. The transcriptome [58] and metabolome [57] were analyzed using microarray and gas chromatography-mass spectrometry (GC-MS), respectively. By integrating these datasets with in silico prediction, it was found that as the quality and differentiation potential of hBM-MSCs decreased and ROS production, lipid peroxidation, and cell damage increased in phosphate-buffered saline (PBS) over time.
Omics-based approaches are actively applied and developed to reflect diverse and complementary biological phenotypes and elucidate precise molecular mechanisms as the availability of high-throughput data technologies increases in the biological and medical fields [59]. These have been beneficial in identifying biomarkers for the diagnosis of various diseases. Nevertheless, most omics analyses have been limited to a single dataset, which is accompanied by difficulty in reflecting the actual phenotype [60]. In addition, the datasets used for computational analysis have advanced from structured one to big data with various unstructured and semi-structured characteristics, and the relationship between omics data is expected to become more complex [61]. Artificial intelligence (AI) is increasingly essential in big data mining, including the biological and medical fields [62,63]. Among AI, machine learning and deep learning approaches exert tremendous power in processing and modelling vast and diverse omics data [64]. Integrated omics analysis aims to utilize big data, machine learning, and systematic algorithms to obtain patterns between data and make more accurate predictions [65,66]. Convergence with computational science is necessary for this type of analysis [67].
The current review has two sections. In the first section, we provide an overview of studies on the application of hBM-MSCs to the treatment of various NDDs, and in the second section, we provide an integrated omics analysis of the quality and differentiation ability of hBM-MSCs according to PBS storage time for successful stem cell therapy.
2. Application of hBM-MSCs for the Treatment of Various Neurodegenerative Diseases
Neurodegenerative diseases are characterized by the selective dysfunction and progressive loss of neurons, glial cells, and neural networks in the brain and spinal cord. Synaptic dysfunction, neuronal loss, proteasome dysfunction, and the aggregation of misfolded proteins are common NDD features. NDDs affect multiple facets of function in humans, thereby limiting their ability to perform even the most basic tasks [4,68,69].
Stem cells are crucial for the development, growth, and repair of various tissues and organs. In addition, stem cells have provided breakthroughs across all fields of research and medicine owing to their multipotency and self-renewal properties. MSCs have demonstrated numerous neuroprotective effects such as decreased apoptosis, reduced ROS generation, and the promotion of neuronal growth. In particular, hBM-MSC transplantation has been shown to improve clinical outcomes, decrease cerebral atrophy, and enhance patient performance [4,70,71]. In this section, we briefly review the neuroprotective effects of clinically applied hBM-MSCs on some common NDDs.
2.1. AD
AD is an NDD associated with a progressive decline in cognitive and memory functions. The pathological features of AD include the aggregation of amyloid beta peptides (Aβ), forming amyloid plaques, intracellular neurofibrillary tangles, and hyperphosphorylated tau and leading to neuronal death. Continual build-up of Aβ activates microglia, thus accelerating neuronal loss, cognitive decline, tau pathology, and the secretion of proinflammatory cytokines. These factors induce synaptic deficits in the hippocampus, leading to cognitive impairment and memory decline [50,68,71,72]. Conventional AD treatments consist of two types of pharmacological therapies. The first includes the use of cholinesterase inhibitors to relieve physical symptoms by increasing the levels of the neurotransmitter acetylcholine. The second type of therapy uses memantine, a drug that improves symptoms by inhibiting N-methyl-D-aspartate (NMDA) receptors. Although several drugs and natural compounds are available for the treatment of AD, drugs that can prevent or delay the progression of AD are yet to be discovered [72].
Transplanted hBM-MSCs can differentiate into neurons, produce neurotrophic factors such as BDNF and NGF, and inhibit Aβ- and tau-related cell death [71]. Numerous studies have explored the neuroprotective effects of BM-MSCs in AD mouse models. Lee et al. achieved a significant reduction in oxidative stress, improvement in cognitive function, and mitigation of Aβ-induced neuronal injury both in vitro and in vivo after co-culturing BM-MSCs with hippocampal neurons stimulated by Aβ [73]. In addition, hMB-MSC-derived vesicles alleviated cognitive decline, reduced the number of intracellular plaques, decreased chronic inflammation, and delayed AD pathogenesis in a preclinical mouse model [74]. BM-MSC-derived exosomes have also been shown to ameliorate cognitive damage by secreting miRNAs capable of enhancing neuronal plasticity, promoting cell survival and synaptogenesis, and suppressing inflammation [75]. Collectively, these studies highlight the multiple advantages of BM-MSCs as a therapeutic agent for AD.
2.2. PD
PD is the most common synucleinopathy characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the accumulation of α-synuclein in Lewy bodies, causing tremors, bradykinesia, and cognitive dysfunction [76]. Dopamine (DA) is a neurotransmitter that transmits information between the SNpc and other parts of the brain, thereby controlling the body’s voluntary movements. Mitochondrial dysfunction, excessive ROS generation, and impairment of the ubiquitin-proteasome system are involved in DA neuronal degeneration [77]. A typical therapy for PD involves treatment with the DA precursor L-3,4-dihydroxyphenylalanine (L-Dopa), which can produce adverse effects, such as non-responsiveness and abnormal uncontrollable movements or dyskinesia, upon long-term use. Furthermore, this form of therapy focuses only on alleviating symptoms instead of resolving the primary cause of the disease, thereby permitting disease progression [78,79].
MSC-based cell therapies offer diverse options for the treatment of PD. One case in point involves the implication of a defective autophagy system as a plausible cause of PD. MSCs have been reported to display α-synuclein clearance, the regulation of autophagy lysosomal activity, the activation of autophagy signalling and immunomodulatory effects, such as penetrating injured sites, releasing numerous growth factors, and attenuating inflammation [79]. Another study investigating neural-induced hBM-MSCs (NI-hBMSCs) demonstrated increased cell survival, stabilization of α-synuclein monomers, and promotion of neurogenesis after treatment with NI-hBMSCs [80]. Clinical trials in which hBM-MSCs from healthy donors were intravenously administered to patients with PD have shown promising results. The participants in the study exhibited post-infusion changes in motor and non-motor symptoms that lasted until the end of the study period [81]. Based on the results from these and several other studies on patients with PD and animal models, the therapeutic benefits of hBM-MSCs over conventional treatments are evident.
2.3. ALS
ALS is a gradual, fatal, paralytic NDD characterized by the degeneration of the upper and lower motor neurons. ALS causes weakness and atrophy of the muscles of the limbs, chest, neck, and oropharyngeal area and eventually death due to respiratory failure. As there are only two drugs currently approved for ALS treatment, there is an urgent need for different treatment options [82,83]. MSCs are being explored as a treatment option for ALS because they produce and release neurotrophins, which are proteins that induce the survival, development, and function of neurons. Transplantation of hMSCs has also been reported to mitigate neuroinflammation, improve motor execution, and enhance the bioenergetics of recipient cells [84,85,86].
Clinical trials using hBM-MSCs as therapeutic agents for ALS have shown favourable outcomes after hBM-MSC administration. In one open-label phase I trial, TGF-β and IL-10 levels were elevated following hBM-MSCs administration. TGF-β, a growth factor involved in various aspects of neuron development and function, has been found to be reduced in ALS patients and inversely correlated to disease progression [87]. Another open-label study conducted to evaluate the safety and efficacy of autologous hBM-MSCs via intrathecal and intravenous routes in ALS patients demonstrated a temporary decline in ALS progression after a single dose of hBM-MSCs [88]. To maximize the capability of hBM-MSCs to treat ALS, additional studies are needed on the effective delivery of MSCs to patients, the effectiveness of MSCs expressing diverse growth factors, and their clinical significance [89].
2.4. HD
HD is an autosomal dominant neurodegenerative disease caused by the loss of gamma-aminobutyric acidergic (GABAergic) medium spiny neurons in the striatum. This neuronal loss stems from the expansion of the cytosine–adenine–guanine (CAG) repeat within exon 1 of the huntingtin (htt) gene, which leads to the formation of a malfunctioning mutant HTT protein [90]. The clinical manifestations of HD include chorea, psychiatric symptoms, and cognitive impairment. The reduced availability of neurotrophic factors, such as NGF, BDNF, and neurotrophin-3 (NT-3), contributes to neurodegeneration and therefore, these are considered potential therapeutic agents for HD [91].
In a mouse model of HD using transplanted hBM-MSCs, intrastriatally transplanted hBM-MSCs not only successfully survived and differentiated but also reduced motor function impairment, increased neurogenesis, and boosted animal survival and cell differentiation [92]. Given that levels of neurotrophic factors (NTFs) are reduced in patients with HD, NTF-based therapies are potential strategies for the discovery of new treatment options for HD. Since BDNF has a short half-life, which limits effective delivery strategies for NDDs, genetically engineered hBM-MSCs that deliver BDNF (MSC/BDNF) have advantages of delivering BDNF to the striate and MSC-secreted factor supplementation. Pollock et al. reported a significant surge in neurogenesis, an increased lifespan, and decreased spinal atrophy in mice transplanted with MSCs/BDNF [93]. Moreover, hBM-MSCs induced to differentiate into NTF-secreting cells (NTF+) exhibit therapeutic properties and attenuate neurotoxicity [94]. Although the therapeutic ability of hBM-MSCs in the treatment of HD has been established, additional studies to accurately determine the administration time, dose, and frequency of cells, as well as long-term toxicology studies, should be conducted.
3. Analysis of the Quality and Differentiation Ability of hBM-MSCs
To date, many studies on NDDs have shown different features of neurodegeneration, such as cell viability reduction, genetic mutations, gene expression alterations, and cellular function impairment [95,96,97]. To understand the different cellular processes in NDDs, cell health has been evaluated using several methods, including morphological analysis, viability assays, metabolic assays, and gene expression analysis [98,99,100], because cell quality directly affects cell functionality [101,102].
Similarly, because the quality and differentiation potential of stem cells are crucial for their use in NDD therapy, the condition and properties of stem cells should be assessed prior to use [103]. Moreover, BM-MSCs show a loss of stemness when maintained under certain conditions [104,105]. Stolzing et al. reported that the overall hBM-MSC fitness decreased with age, showing an increase in ROS, p21, and p53 levels [104]. These deteriorating features were also observed during in vitro ageing. Geissler et al. showed that progenitor characteristics were lost, and genes related to cell differentiation, focal adhesion organisation, cytoskeleton turnover, and mitochondrial function were downregulated during long-term in vitro expansion of MSCs [105].
Meanwhile, there are some studies suggesting that gender may affect the efficiency of BM-MSC therapy [106,107]. Sammour et al. reported that female BM-MSCs have more therapeutic effects than male BM-MSCs by showing greater pro-angiogenic and anti-inflammatory effects in mice models [106]. In addition, Crisostomo et al. demonstrated that female BM-MSCs showed lower apoptosis, TNF and IL-6 production, and higher VEGF expression upon stress activation than males, due to their inherent resistance to TNFR1 activation [107]. However, one study reported that in vitro mesodermal differential capacity of hBM-MSCs is not highly related to the donor gender [108]. Although donor gender seems to play a role in the therapeutic effects of BM-MSCs, this is still not clearly elucidated and further studies are required to clarify the effect of gender on BM-MSCs.
Even though MSCs have several advantages, including self-regeneration ability, anti-inflammatory and immunomodulatory effects, and multi-lineage differentiation ability [109,110], keeping them fresh and healthy to maintain their beneficial properties should also be considered.
3.1. Optimizing hBM-MSCs While Preserving Cell Quality
As mentioned above, it is imperative to preserve the quality and properties of stem cells, which significantly affect the achievement of many therapeutic cells during ex vivo expansion [56]. Furthermore, before clinical use, MSCs should be isolated and expanded in vitro until they reach the appropriate cell number, owing to the low frequency of 0.001–0.01% of the total mononucleated cells [111]. hBM-MSCs are detached from the culture dish and kept in a largely different environment from the original one, which can diminish their valuable properties [112,113,114]. Hence, maintaining the quality of hBM-MSCs is crucial when using in NDDs. In this section, we summarize several methods of improving the efficiency of BM-MSC therapy while preserving cell stemness.
Donor age is a well-known factor that should be considered during the transplantation process. Many studies have reported that donor age is closely related to negative effects on MSC proliferation and multipotency [115,116,117]. Zaim et al. investigated the effects of donor age and long-term culture on the morphology, characteristics, and capacity of hBM-MSCs to proliferate and differentiate into adipogenic, chondrogenic, osteogenic, and neurogenic lineages [118]. They found that hBM-MSCs gradually reduced their proliferation rate and lost their typical morphology in an age- and passage-dependent manner. In another study, the older donor group showed lower concentrations of colony forming unit fibroblasts (CFU-Fs) and shorter telomere lengths during in vitro expansion than the young donor group, indicating that the ageing of MSCs can reduce their therapeutic characteristics [119].
Moreover, avoiding oxidative stress is effective in maintaining cell freshness. For example, Shin et al. reported that hBM-MSCs trypsinized and maintained in PBS showed a significant loss of freshness and viability over time [57]. This reduction in freshness is accompanied by several phenotypes, such as increased peroxidation of membranes and intracellular vacuoles, in a PBS storage time-dependent manner [58]. In addition, transcriptomic analysis of hBM-MSCs stored in PBS for 12 h predicted increased ROS generation and lipid peroxidation and decreased cell viability [56]. Regarding ROS production, Lee et al. suggested that treatment with N-acetyl-L-cysteine (NAC) and glutathione (GSH) maintained the quality of PBS-stored hBM-MSCs by reducing ROS and lipid peroxidation [56]. Indeed, one study showed that the pre-conditioning of BM-MSCs with NAC led to lower apoptosis and higher survival against oxidative stress by increasing GSH levels and helped bone regeneration after transplantation in rats [120].
Lastly, treatment with growth factors, such as fibroblast growth factor-2 (FGF-2), transforming growth factor-beta (TGF-β), and insulin-like growth factor-1 (IGF-1), also improved the multi-lineage differentiation ability of stem cells [121,122,123]. Nandy et al. reported that FGF-2-treated hBM-MSCs showed the least cell death and the highest upregulation of tyrosine hydroxylase, a dopaminergic neuron marker, compared to other growth-factor-treated cells [121]. Meanwhile, Longobardi et al. reported that the addition of TGF-β and IGF-1 exerted both proliferative and anti-apoptotic actions and even induced the differentiation of BM-MSCs into chondrocytes, showing an increase in the expression of chondrogenic markers in mice [122]. In addition, IGF-1 stimulates osteoblastic differentiation by activating the mTOR signalling pathway and helps maintain bone marrow mass in mice and rats [123]. The effects of substances for improving the efficiency of BM-MSC therapy are summarized in Table 1. Hence, not only the removal of harmful factors such as ROS but also the addition of supplements can be helpful for maintaining hBM-MSC stemness.
Table 1.
Summary of the functions and effects of substances for improving the efficiency of BM-MSC therapy.
| Substances | Differentiation Direction |
Functions and Effects | References |
|---|---|---|---|
| N-acetyl-L-cysteine (NAC) | Osteoblast | Decreased apoptosis Increased survival Increased GSH level Enhanced bone regeneration |
[120] |
| Fibroblast growth factor-2 (FGF-2) | Dopaminergic neurons | Decreased cell death Increased upregulation of tyrosine hydroxylase Increased dopamine release |
[121] |
| Transforming growth factor-beta (TGF-β) | Chondrocyte | Decreased apoptosis Increased cell proliferation Increased chondrogenic condensation and markers |
[122] |
| Insulin-like growth factor-1 (IGF-1) | Chondrocyte | Decreased apoptosis Increased cell proliferation Increased chondrogenic condensation and markers |
[122] |
| Osteoblast | Induction of osteoblastic differentiation | [123] |
3.2. PBS Storage Time Is a Critical Factor for the Differentiation of hBM-MSCs in Gene Expression and Amino Acid Levels
For transplantation, MSCs should be suspended in PBS to prevent inflammatory reactions caused by foreign proteins and serum [124]. To evaluate the effect of storage time on hBM-MSCs before transplantation, transcriptome profiling data of hBM-MSCs stored in PBS for 0, 6, and 12 h were acquired as described in our previous reports [57,58]. The transcriptomic changes in the 6 h- and 12 h-stored groups were compared to the 0 h-stored control groups using Ingenuity Pathway Analysis (IPA) web-based bioinformatics software (Qiagen, CA, USA) and analyzed. Genes that showed >1.5- and <-1.5-fold changes in expression levels were selected and used for transcriptomic analysis. In the 6 h-stored groups, 1466 genes showed such changes in their gene expression levels (806 downregulated and 660 upregulated), whereas in the 12 h-stored groups, 1817 genes showed such changes in their gene expression levels (1145 downregulated and 672 upregulated).
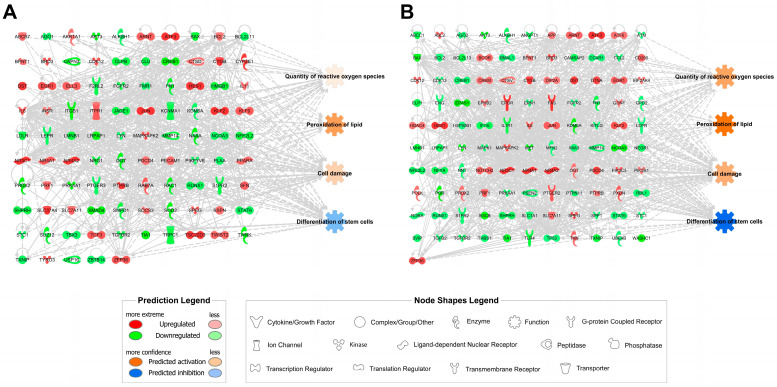
Computational prediction of cellular functions showed that the quantity of ROS, peroxidation of lipid, and cell damage were increased, and that the differentiation of stem cells was inhibited in both 6 h- and 12 h-stored hBM-MSCs compared to the control (Figure 2). Moreover, these transcriptomic networks showed that ROS levels, lipid peroxidation, cellular damage, and differentiation abilities were related. Interestingly, predictions of all these cellular functions were more robustly represented in the 12 h-storage groups than in the 6 h-storage groups (Figure 2B), suggesting that ROS production, lipid peroxidation, cell damage, and stem cell differentiation were adversely affected by increasing the PBS storage time in hBM-MSCs. Detailed fold-change information for each gene in the transcriptomic networks is listed in Table 2 (6 h-stored groups) and Table 3 (12 h-stored groups). Further experimental studies are required to elucidate the differentiation ability of hBM-MSCs.
Figure 2.
Transcriptomic networks with prediction using ingenuity pathway analysis. (A) Transcriptomic network in PBS-stored hBM-MSCs for 6 h; (B) Transcriptomic network in PBS-stored hBM-MSCs for 12 h. The fold change cut-off value used in the analysis for the networks is ±1.5 based on a previous report [58]. Upregulated genes are represented in red while the downregulated genes are in green. Orange and blue indicate the predicted activation and inhibition of cellular functions, respectively. Solid and dotted lines show direct and indirect relationships, respectively.
Table 2.
Ingenuity-pathway-analysis-based transcriptome profiles of the 6 h-stored hBM-MSCs.
| Entrez Gene Name | Symbol | Entrez Gene ID a | Location | Fold Change b |
|---|---|---|---|---|
| ATP binding cassette subfamily B member 7 | ABCB7 | 22 | Cytoplasm | 1.637 |
| argonaute RISC component 1 | AGO1 | 26,523 | Cytoplasm | −1.542 |
| aldo-keto reductase family 1 member A1 | AKR1A1 | 10,327 | Cytoplasm | 1.512 |
| AKT serine/threonine kinase 3 | AKT3 | 10,000 | Cytoplasm | −2.379 |
| alkB homolog 1, histone H2A dioxygenase | ALKBH1 | 8846 | Cytoplasm | −1.503 |
| aryl hydrocarbon receptor nuclear translocator | ARNT | 405 | Nucleus | 1.715 |
| activating transcription factor 3 | ATF3 | 467 | Nucleus | 2.88 |
| BCL2 associated X, apoptosis regulator | BAX | 581 | Cytoplasm | −1.946 |
| BCL2 apoptosis regulator | BCL2 | 596 | Cytoplasm | 1.534 |
| BCL2 like 11 | BCL2L11 | 10,018 | Cytoplasm | −1.579 |
| 3′(2′), 5′-bisphosphate nucleotidase 1 | BPNT1 | 10,380 | Nucleus | 1.564 |
| bromodomain containing 3 | BRD3 | 8019 | Nucleus | 1.855 |
| calpain 3 | CAPN3 | 825 | Cytoplasm | −1.974 |
| cyclin dependent kinase 12 | CDK12 | 51,755 | Nucleus | 1.925 |
| caseinolytic mitochondrial matrix peptidase chaperone subunit B | CLPB | 81,570 | Nucleus | −1.785 |
| Clusterin | CLU | 1191 | Cytoplasm | −1.768 |
| cAMP responsive element binding protein 1 | CREB1 | 1385 | Nucleus | −2.252 |
| cathepsin D | CTSD | 1509 | Cytoplasm | 1.527 |
| Cytoglobin | CYGB | 114,757 | Cytoplasm | 2.368 |
| cytochrome P450 family 2 subfamily E member 1 | CYP2E1 | 1571 | Cytoplasm | 2.095 |
| Dystonin | DST | 667 | Plasma Membrane | 2.126 |
| early growth response 1 | EGR1 | 1958 | Nucleus | 1.915 |
| elongation factor for RNA polymerase II 3 | ELL3 | 80,237 | Nucleus | 1.749 |
| coagulation factor II thrombin receptor like 2 | F2RL2 | 2151 | Plasma Membrane | −1.714 |
| fibroblast growth factor receptor 2 | FGFR2 | 2263 | Plasma Membrane | −1.859 |
| fragile X messenger ribonucleoprotein 1 | FMR1 | 2332 | Cytoplasm | −1.569 |
| fibronectin 1 | FN1 | 2335 | Extracellular Space | −1.951 |
| hes family bHLH transcription factor 1 | HES1 | 3280 | Nucleus | 8.134 |
| high mobility group box 1 | HMGB1 | 3146 | Nucleus | −1.903 |
| interleukin 11 | IL11 | 3589 | Extracellular Space | 1.596 |
| interleukin 6 | IL6 | 3569 | Extracellular Space | 1.784 |
| insulin receptor | INSR | 3643 | Plasma Membrane | 1.561 |
| integrin subunit beta 1 | ITGB1 | 3688 | Plasma Membrane | −2.31 |
| inositol 1,4,5-trisphosphate receptor type 1 | ITPR1 | 3708 | Cytoplasm | 1.775 |
| jade family PHD finger 1 | JADE1 | 79,960 | Nucleus | −1.76 |
| Jun proto-oncogene, AP-1 transcription factor subunit | JUN | 3725 | Nucleus | 2.212 |
| potassium calcium-activated channel subfamily M alpha 1 | KCNMA1 | 3778 | Plasma Membrane | −1.726 |
| lysine demethylase 6A | KDM6A | 7403 | Nucleus | −1.511 |
| KLF transcription factor 2 | KLF2 | 10,365 | Nucleus | 2.261 |
| KLF transcription factor 9 | KLF9 | 687 | Nucleus | 1.803 |
| low density lipoprotein receptor | LDLR | 3949 | Plasma Membrane | −1.783 |
| leptin receptor | LEPR | 3953 | Plasma Membrane | −1.575 |
| lamin B1 | LMNB1 | 4001 | Nucleus | −1.862 |
| LDL receptor related protein associated protein 1 | LRPAP1 | 4043 | Plasma Membrane | −2.12 |
| LYN proto-oncogene, Src family tyrosine kinase | LYN | 4067 | Cytoplasm | −1.541 |
| MAPK activated protein kinase 2 | MAPKAPK2 | 9261 | Nucleus | 1.848 |
| matrix metallopeptidase 14 | MMP14 | 4323 | Extracellular Space | −1.874 |
| N-acylethanolamine acid amidase | NAAA | 27,163 | Cytoplasm | −2.184 |
| nuclear receptor coactivator 3 | NCOA3 | 8202 | Nucleus | −1.572 |
| NFE2 like bZIP transcription factor 2 | NFE2L2 | 4780 | Nucleus | −1.823 |
| nuclear receptor subfamily 3 group C member 1 | NR3C1 | 2908 | Nucleus | 2.214 |
| nuclear receptor subfamily 4 group A member 1 | NR4A1 | 3164 | Nucleus | 1.808 |
| nuclear receptor subfamily 4 group A member 2 | NR4A2 | 4929 | Nucleus | 4.047 |
| neuregulin 1 | NRG1 | 3084 | Plasma Membrane | −1.913 |
| O-linked N-acetylglucosamine (GlcNAc) transferase | OGT | 8473 | Cytoplasm | −2.058 |
| programmed cell death 4 | PDCD4 | 27,250 | Nucleus | 1.554 |
| platelet and endothelial cell adhesion molecule 1 | PECAM1 | 5175 | Plasma Membrane | 1.602 |
| phosphoinositide kinase, FYVE-type zinc finger containing | PIKFYVE | 200,576 | Cytoplasm | −1.529 |
| phospholipase A2 activating protein | PLAA | 9373 | Cytoplasm | −1.507 |
| peroxisome proliferator activated receptor alpha | PPARA | 5465 | Nucleus | 1.646 |
| peroxiredoxin 2 | PRDX2 | 7001 | Cytoplasm | −2.276 |
| perforin 1 | PRF1 | 5551 | Cytoplasm | 1.668 |
| protein kinase AMP-activated catalytic subunit alpha 1 | PRKAA1 | 5562 | Cytoplasm | −2.252 |
| prostaglandin E receptor 3 | PTGER3 | 5733 | Plasma Membrane | −1.539 |
| protein tyrosine phosphatase receptor type B | PTPRB | 5787 | Plasma Membrane | 2.444 |
| RAB7A, member RAS oncogene family | RAB7A | 7879 | Cytoplasm | 1.801 |
| Rac family small GTPase 1 | RAC1 | 5879 | Plasma Membrane | −2.019 |
| RUNX family transcription factor 1 | RUNX1 | 861 | Nucleus | −1.616 |
| sphingosine-1-phosphate receptor 2 | S1PR2 | 9294 | Plasma Membrane | −1.73 |
| Stratifin | SFN | 2810 | Cytoplasm | 1.7 |
| SNF2 histone linker PHD RING helicase | SHPRH | 257,218 | Nucleus | −1.812 |
| solute carrier family 37 member 4 | SLC37A4 | 2542 | Cytoplasm | 3.046 |
| solute carrier family 7 member 11 | SLC7A11 | 23,657 | Plasma Membrane | 1.721 |
| SMAD family member 4 | SMAD4 | 4089 | Nucleus | −2.153 |
| sphingomyelin phosphodiesterase 1 | SMPD1 | 6609 | Cytoplasm | −1.834 |
| suppressor of cytokine signaling 3 | SOCS3 | 9021 | Cytoplasm | 1.628 |
| superoxide dismutase 2 | SOD2 | 6648 | Cytoplasm | −2.242 |
| striated muscle enriched protein kinase | SPEG | 10,290 | Nucleus | 1.771 |
| Sarcospan | SSPN | 8082 | Plasma Membrane | 1.575 |
| signal transducer and activator of transcription 6 | STAT6 | 6778 | Nucleus | −1.571 |
| stanniocalcin 1 | STC1 | 6781 | Extracellular Space | −1.567 |
| SUZ12 polycomb repressive complex 2 subunit | SUZ12 | 23,512 | Nucleus | −2.001 |
| T-box transcription factor 3 | TBX3 | 6926 | Nucleus | −1.533 |
| transcription factor 3 | TCF3 | 6929 | Nucleus | 1.81 |
| transforming growth factor beta receptor 2 | TGFBR2 | 7048 | Plasma Membrane | −1.822 |
| TIA1 cytotoxic granule associated RNA binding protein | TIA1 | 7072 | Nucleus | −2.369 |
| transient receptor potential cation channel subfamily C member 1 | TRPC1 | 7220 | Plasma Membrane | −1.812 |
| TSC22 domain family member 3 | TSC22D3 | 1831 | Nucleus | 2.134 |
| twist family bHLH transcription factor 2 | TWIST2 | 117,581 | Nucleus | 1.652 |
| twinkle mtDNA helicase | TWNK | 56,652 | Cytoplasm | −2.459 |
| thioredoxin interacting protein | TXNIP | 10,628 | Cytoplasm | −1.567 |
| TYRO3 protein tyrosine kinase | TYRO3 | 7301 | Plasma Membrane | 1.905 |
| ubiquitin specific peptidase 16 | USP16 | 10,600 | Cytoplasm | −1.764 |
| zinc finger and BTB domain containing 14 | ZBTB14 | 7541 | Nucleus | −1.55 |
| ZFP36 ring finger protein | ZFP36 | 7538 | Nucleus | 1.827 |
a The Entrez gene ID is the unique integer identifier for humans; b normalized signal fold change in the 6 h-stored group to the corresponding signal of the control group.
Table 3.
Ingenuity-pathway-analysis-based transcriptome profiles of the 12 h-stored hBM-MSCs.
| Entrez Gene Name | Symbol | Entrez Gene ID a | Location | Fold Change b |
|---|---|---|---|---|
| ATP binding cassette subfamily C member 1 | ABCC1 | 4363 | Plasma Membrane | −1.984 |
| ABL proto-oncogene 2, non-receptor tyrosine kinase | ABL2 | 27 | Cytoplasm | 1.744 |
| argonaute RISC catalytic component 2 | AGO2 | 27,161 | Cytoplasm | −1.588 |
| AKT serine/threonine kinase 3 | AKT3 | 10,000 | Cytoplasm | −2.393 |
| alkB homolog 1, histone H2A dioxygenase | ALKBH1 | 8846 | Cytoplasm | −1.83 |
| angiopoietin 1 | ANGPT1 | 284 | Extracellular Space | −1.869 |
| amyloid beta precursor protein | APP | 351 | Plasma Membrane | 1.506 |
| aryl hydrocarbon receptor nuclear translocator | ARNT | 405 | Nucleus | 1.749 |
| activating transcription factor 3 | ATF3 | 467 | Nucleus | 2.535 |
| activating transcription factor 6 | ATF6 | 22,926 | Cytoplasm | 1.56 |
| ATM serine/threonine kinase | ATM | 472 | Nucleus | −1.523 |
| BCL2 associated X, apoptosis regulator | BAX | 581 | Cytoplasm | −2.51 |
| BCL2 apoptosis regulator | BCL2 | 596 | Cytoplasm | −1.646 |
| BCL2 like 13 | BCL2L13 | 23,786 | Cytoplasm | −2.078 |
| BCL6 corepressor | BCOR | 54,880 | Nucleus | 1.656 |
| basic helix-loop-helix ARNT like 1 | BMAL1 | 406 | Nucleus | −1.562 |
| 3′(2′), 5′-bisphosphate nucleotidase 1 | BPNT1 | 10,380 | Nucleus | 1.565 |
| bromodomain containing 3 | BRD3 | 8019 | Nucleus | 2.089 |
| calmodulin regulated spectrin associated protein family member 2 | CAMSAP2 | 23,271 | Cytoplasm | −1.955 |
| cell division cycle and apoptosis regulator 1 | CCAR1 | 55,749 | Nucleus | −1.579 |
| C-C motif chemokine ligand 2 | CCL2 | 6347 | Extracellular Space | −1.655 |
| CD200 molecule | CD200 | 4345 | Plasma Membrane | 1.713 |
| cyclin dependent kinase 12 | CDK12 | 51,755 | Nucleus | 1.849 |
| cyclin dependent kinase 13 | CDK13 | 8621 | Nucleus | −1.555 |
| cAMP responsive element binding protein 1 | CREB1 | 1385 | Nucleus | −1.68 |
| cAMP responsive element modulator | CREM | 1390 | Nucleus | 1.601 |
| cathepsin V | CTSV | 1515 | Cytoplasm | 1.573 |
| cytoglobin | CYGB | 114,757 | Cytoplasm | 2.378 |
| disco interacting protein 2 homolog A | DIP2A | 23,181 | Nucleus | 1.549 |
| dystonin | DST | 667 | Plasma Membrane | 2.201 |
| dystrobrevin alpha | DTNA | 1837 | Plasma Membrane | 2.069 |
| early growth response 1 | EGR1 | 1958 | Nucleus | 1.786 |
| eukaryotic translation initiation factor 2 alpha kinase 4 | EIF2AK4 | 440,275 | Cytoplasm | 1.576 |
| elongator acetyltransferase complex subunit 1 | ELP1 | 8518 | Cytoplasm | −2.024 |
| endoglin | ENG | 2022 | Plasma Membrane | −1.629 |
| endothelial PAS domain protein 1 | EPAS1 | 2034 | Nucleus | −3.025 |
| EPH receptor B2 | EPHB2 | 2048 | Plasma Membrane | 1.591 |
| erythropoietin receptor | EPOR | 2057 | Plasma Membrane | 2.778 |
| endoplasmic reticulum to nucleus signaling 1 | ERN1 | 2081 | Cytoplasm | −1.588 |
| Fas cell surface death receptor | FAS | 355 | Plasma Membrane | 2.388 |
| fibroblast growth factor receptor 2 | FGFR2 | 2263 | Plasma Membrane | −1.515 |
| fibronectin 1 | FN1 | 2335 | Extracellular Space | −2.059 |
| glial cell derived neurotrophic factor | GDNF | 2668 | Extracellular Space | 1.512 |
| glycerol-3-phosphate dehydrogenase 2 | GPD2 | 2820 | Cytoplasm | −1.54 |
| histone deacetylase 4 | HDAC4 | 9759 | Nucleus | 1.563 |
| hes family bHLH transcription factor 1 | HES1 | 3280 | Nucleus | 5.645 |
| heat shock protein 90 beta family member 1 | HSP90B1 | 7184 | Cytoplasm | −1.51 |
| interferon gamma inducible protein 16 | IFI16 | 3428 | Nucleus | −1.892 |
| interleukin 1 receptor type 1 | IL1R1 | 3554 | Plasma Membrane | −2.05 |
| interleukin 6 | IL6 | 3569 | Extracellular Space | 1.777 |
| Jun proto-oncogene, AP-1 transcription factor subunit | JUN | 3725 | Nucleus | 1.827 |
| lysine demethylase 6A | KDM6A | 7403 | Nucleus | −2.947 |
| KIT ligand | KITLG | 4254 | Extracellular Space | −1.654 |
| KLF transcription factor 2 | KLF2 | 10,365 | Nucleus | 1.979 |
| leptin receptor | LEPR | 3953 | Plasma Membrane | −1.507 |
| lamin B1 | LMNB1 | 4001 | Nucleus | −2.167 |
| LDL receptor related protein associated protein 1 | LRPAP1 | 4043 | Plasma Membrane | −1.845 |
| LYN proto-oncogene, Src family tyrosine kinase | LYN | 4067 | Cytoplasm | −2.418 |
| mitogen-activated protein kinase 1 | MAPK1 | 5594 | Cytoplasm | −1.564 |
| MAPK activated protein kinase 2 | MAPKAPK2 | 9261 | Nucleus | 1.95 |
| MET proto-oncogene, receptor tyrosine kinase | MET | 4233 | Plasma Membrane | −2.447 |
| mitofusin 2 | MFN2 | 9927 | Cytoplasm | −1.554 |
| MIA SH3 domain ER export factor 3 | MIA3 | 375,056 | Cytoplasm | −1.621 |
| matrix metallopeptidase 14 | MMP14 | 4323 | Extracellular Space | −1.7 |
| nuclear receptor coactivator 3 | NCOA3 | 8202 | Nucleus | −2.462 |
| neuronal growth regulator 1 | NEGR1 | 257,194 | Plasma Membrane | −1.799 |
| NFE2 like bZIP transcription factor 2 | NFE2L2 | 4780 | Nucleus | −1.85 |
| nuclear transcription factor Y subunit alpha | NFYA | 4800 | Nucleus | −1.914 |
| nicotinamide nucleotide transhydrogenase | NNT | 23,530 | Cytoplasm | −1.592 |
| notch receptor 2 | NOTCH2 | 4853 | Plasma Membrane | 1.741 |
| nuclear receptor subfamily 3 group C member 1 | NR3C1 | 2908 | Nucleus | 2.526 |
| nuclear receptor subfamily 4 group A member 1 | NR4A1 | 3164 | Nucleus | 2.282 |
| nuclear receptor subfamily 4 group A member 2 | NR4A2 | 4929 | Nucleus | 5.217 |
| O-linked N-acetylglucosamine (GlcNAc) transferase | OGT | 8473 | Cytoplasm | 1.511 |
| programmed cell death 4 | PDCD4 | 27,250 | Nucleus | 2.085 |
| phosphatidylinositol 3-kinase catalytic subunit type 3 | PIK3C3 | 5289 | Cytoplasm | 1.887 |
| phosphoinositide-3-kinase regulatory subunit 1 | PIK3R1 | 5295 | Cytoplasm | 1.557 |
| DNA polymerase kappa | POLK | 51,426 | Nucleus | 1.562 |
| cytochrome p450 oxidoreductase | POR | 5447 | Cytoplasm | −2.999 |
| peroxiredoxin 2 | PRDX2 | 7001 | Cytoplasm | 1.62 |
| perforin 1 | PRF1 | 5551 | Cytoplasm | 1.888 |
| protein kinase AMP-activated catalytic subunit alpha 1 | PRKAA1 | 5562 | Cytoplasm | −1.683 |
| presenilin 2 | PSEN2 | 5664 | Cytoplasm | −1.611 |
| prostaglandin E receptor 2 | PTGER2 | 5732 | Plasma Membrane | 1.877 |
| protein tyrosine phosphatase non-receptor type 11 | PTPN11 | 5781 | Cytoplasm | −1.714 |
| protein tyrosine phosphatase receptor type B | PTPRB | 5787 | Plasma Membrane | 1.544 |
| peroxidasin | PXDN | 7837 | Extracellular Space | 1.827 |
| RB transcriptional corepressor like 1 | RBL1 | 5933 | Nucleus | −1.614 |
| RAR related orphan receptor A | RORA | 6095 | Nucleus | −1.609 |
| RUNX family transcription factor 1 | RUNX1 | 861 | Nucleus | −1.563 |
| sphingosine-1-phosphate receptor 2 | S1PR2 | 9294 | Plasma Membrane | −1.619 |
| sarcoglycan beta | SGCB | 6443 | Plasma Membrane | −2.825 |
| SNF2 histone linker PHD RING helicase | SHPRH | 257,218 | Nucleus | −1.917 |
| solute carrier family 1 member 1 | SLC1A1 | 6505 | Plasma Membrane | −1.692 |
| solute carrier family 7 member 11 | SLC7A11 | 23,657 | Plasma Membrane | 1.719 |
| striated muscle enriched protein kinase | SPEG | 10,290 | Nucleus | 1.96 |
| secreted phosphoprotein 1 | SPP1 | 6696 | Extracellular Space | −1.573 |
| signal transducer and activator of transcription 6 | STAT6 | 6778 | Nucleus | −1.638 |
| stanniocalcin 1 | STC1 | 6781 | Extracellular Space | −1.546 |
| small VCP interacting protein | SVIP | 258,010 | Cytoplasm | −1.696 |
| transforming growth factor beta 2 | TGFB2 | 7042 | Extracellular Space | −1.584 |
| transforming growth factor beta receptor 2 | TGFBR2 | 7048 | Plasma Membrane | −1.801 |
| thrombospondin 1 | THBS1 | 7057 | Extracellular Space | −1.66 |
| TIA1 cytotoxic granule associated RNA binding protein | TIA1 | 7072 | Nucleus | −2.305 |
| toll like receptor 4 | TLR4 | 7099 | Plasma Membrane | −2.167 |
| tumor protein p53 | TP53 | 7157 | Nucleus | −1.518 |
| thioredoxin | TXN | 7295 | Cytoplasm | 1.692 |
| thioredoxin interacting protein | TXNIP | 10,628 | Cytoplasm | −2.163 |
| ubiquitination factor E4B | UBE4B | 10,277 | Cytoplasm | −1.591 |
| WASH complex subunit 1 | WASHC1 | 100,287,171 | Cytoplasm | −3.587 |
| ZFP36 ring finger protein | ZFP36 | 7538 | Nucleus | 1.942 |
a The Entrez gene ID is the unique integer identifier for humans; b normalized signal fold change in the 12 h-stored group to the corresponding signal of the control group.
In the omics analysis approaches, each analysis of single omics has advantages and disadvantages. For example, transcriptomics includes tremendous gene expression data, but it is not able to reflect the exact phenotype of the organism [125]. Metabolomics shows the “end products” of biological processes and reflects more exact phenotypes. However, the metabolites that can be analyzed are limited by experimental methods [126]. Hence, an integrated omics approach can compensate for the shortcomings of each omics and provides a more comprehensive vision of complex biological processes. Indeed, using integrated omics approaches, the unexplained delicate toxicity of nanoparticles and particulate matter [65,127,128,129] was analyzed, and the biological meaning of mitochondrial diseases was found in various conditions [130].
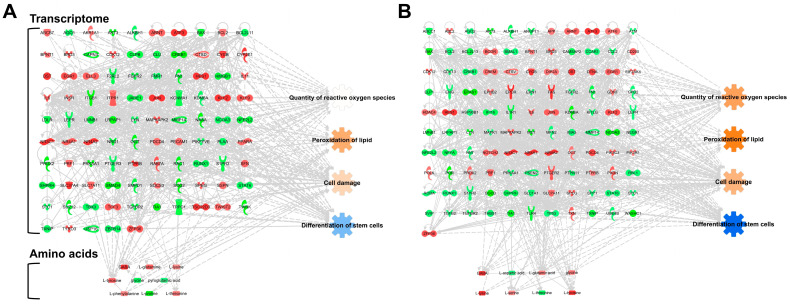
Amino acid profiling omics data using GC-MS provided cellular information in hBM-MSCs over time [57,131], and the amino acid levels at each time point were integrated with transcriptomics to establish metabotranscriptomics which integrates transcriptomics and metabolomics. Fold changes in the levels of amino acids > 1.2 and <−1.2 were used, and nine and eight amino acids were integrated into the IPA networks of hBM-MSCs stored for 6 h and 12 h, respectively. In detail, in the 6 h-stored groups, GABA (2.289, fold change), glycine (−1.624), glutamine (1.702), lysine (1.214), phenylalanine (1.297), proline (−2.255), threonine (1.356), tyrosine (1.685), and pyrrolidonecarboxylic acid (−1.209) were used, and in the 12 h-stored groups, GABA (3.848), glycine (1.458), aspartic acid (−1.438), glutamic acid (1.358), lysine (4.572), serine (1.302), threonine (−1.799), and tyrosine (2.368) were used for analysis. The metabotranscriptomic networks showed a similar tendency to the transcriptomics prediction, maintaining a more robust prediction of cellular functions in the hBM-MSC group stored for 12 h (Figure 3). These transcriptomic and integrated omics analyses suggest that the PBS storage time is a crucial factor for preserving the cell quality and differentiation ability of hBM-MSCs.
Figure 3.
Metabotranscriptomic networks with prediction using ingenuity pathway analysis. (A) Metabotranscriptomic network in PBS-stored hBM-MSCs for 6 h; (B) metabotranscriptomic network in PBS-stored hBM-MSCs for 12 h. The fold change cut-off values used in the analysis for the networks are ±1.5 and ±1.2, respectively. Those used for transcriptome and amino acid analyses are based on a previous report [57]. The figure information is described in the legends of Figure 2.
Although our analyses have been reported previously [58], the data in the current review were analyzed using a new, well-developed bioinformatic program. Notably, the data-based program was curated by a data curator from previously published papers, and the results obtained using artificial intelligence are considered more objective biological information rather than data collected by individuals. Our bioinformatic study showing that the freshness of stem cells decreases over time is consistent with previous reports [56,57,58]. However, the differentiation ability of stem cells after cell detachment from culture dishes is inconsistent with in silico prediction and the osteogenic and adipogenic potentials in the previous report [57]. The discrepancies can be attributed to the experimental differences between omics data analyzed directly at each storage time point and long-term cell culture and frequent exchanges of culture medium for cell differentiation. In conclusion, the freshness and differentiation ability of stem cells in stem cell therapy are closely related to the time after cell detachment from culture dishes. In this regard, this bioinformatic study will be an essential factor to be considered in future stem cell therapies.
4. Conclusions
Herein, we review the application of hBM-MSCs to the treatment of various NDDs and analyze the quality and differentiation ability of hBM-MSCs detached from culture dishes using integrated omics analysis for successful stem cell therapy. Conventional analysis methods have limitations in analyzing the quality and differentiation ability of dissociated hBM-MSCs and cannot comprehensively elucidate the delicate relationships between the cell condition of dissociated hBM-MSCs and therapeutic effects. Multidisciplinary omics and integrated multi-omics approaches provide in-depth and comprehensive information on the quality characteristics and differentiation ability of dissociated hBM-MSCs. Because stemness is a complex process that combines proliferation and self-renewal to generate differentiated cells with identical genotypes, a fragmentary approach is not sufficient to refer to biological changes in the cell. In the future, comprehensive and computational analyses of dissociated hBM-MSCs at the genomic, transcriptomic, small RNAomic, proteomic, phenomic, and metabolomic levels using advanced machine learning algorithms will accelerate studies in the field of stem cell therapy. Thus, these approaches will be helpful in analyzing the condition of dissociated hBM-MSCs and improving their quality and differentiation ability for innovative and successful stem cell therapy.
Author Contributions
Conceptualization and experiment design, S.G.K., N.P.G. and G.L.; writing—original draft preparation, S.G.K., N.P.G., J.S.H., S.P., M.O.K., S.H.L. and G.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Research Foundation (NRF) and the Ministry of Science and ICT (MSIT) in Korea, grant numbers 2020M3E5D9080661 and 2023R1A2C1004585, respectively.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ayeni E.A., Aldossary A.M., Ayejoto D.A., Gbadegesin L.A., Alshehri A.A., Alfassam H.A., Afewerky H.K., Almughem F.A., Bello S.M., Tawfik E.A. Neurodegenerative Diseases: Implications of Environmental and Climatic Influences on Neurotransmitters and Neuronal Hormones Activities. Int. J. Environ. Res. Public Health. 2022;19:12495. doi: 10.3390/ijerph191912495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parsippany N. Understanding Neuromuscular Disease Care. [(accessed on 6 November 2022)]. Available online: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/understanding-neuromuscular-disease-care.pdf.
- 3.Dugger B.N., Dickson D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017;9:a028035. doi: 10.1101/cshperspect.a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahani-Nahayati M., Shariati A., Mahmoodi M., Olegovna Zekiy A., Javidi K., Shamlou S., Mousakhani A., Zamani M., Hassanzadeh A. Stem cell in neurodegenerative disorders; an emerging strategy. Int. J. Dev. Neurosci. 2021;81:291–311. doi: 10.1002/jdn.10101. [DOI] [PubMed] [Google Scholar]
- 5.Basith S., Manavalan B., Lee G. Amyotrophic lateral sclerosis disease-related mutations disrupt the dimerization of superoxide dismutase 1—A comparative molecular dynamics simulation study. Comput. Biol. Med. 2022;151:106319. doi: 10.1016/j.compbiomed.2022.106319. [DOI] [PubMed] [Google Scholar]
- 6.Rosen D.R. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;364:362. doi: 10.1038/364362c0. [DOI] [PubMed] [Google Scholar]
- 7.Mendizabal A., Diaz J.M., Bustamante A.V., Bordelon Y. Health Services in Huntington Disease: A Systematic Literature Review. Neurol. Clin. Pract. 2023;13:e200108. doi: 10.1212/CPJ.0000000000200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feigin V.L., Vos T., Nichols E., Owolabi M.O., Carroll W.M., Dichgans M., Deuschl G., Parmar P., Brainin M., Murray C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020;19:255–265. doi: 10.1016/S1474-4422(19)30411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baraniak P.R., McDevitt T.C. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang C., Yan J., Yao Z., Zhang C., Li X., Mao H.Q. Effects of Mesenchymal Stem Cell-Derived Paracrine Signals and Their Delivery Strategies. Adv. Healthc. Mater. 2021;10:e2001689. doi: 10.1002/adhm.202001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madigan M., Atoui R. Therapeutic Use of Stem Cells for Myocardial Infarction. Bioengineering. 2018;5:28. doi: 10.3390/bioengineering5020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bang O.Y., Lee J.S., Lee P.H., Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 13.Shang Z., Wang M., Zhang B., Wang X., Wanyan P. Clinical translation of stem cell therapy for spinal cord injury still premature: Results from a single-arm meta-analysis based on 62 clinical trials. BMC Med. 2022;20:284. doi: 10.1186/s12916-022-02482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkataramana N.K., Kumar S.K., Balaraju S., Radhakrishnan R.C., Bansal A., Dixit A., Rao D.K., Das M., Jan M., Gupta P.K., et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl. Res. 2010;155:62–70. doi: 10.1016/j.trsl.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Lee P.H., Kim J.W., Bang O.Y., Ahn Y.H., Joo I.S., Huh K. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin. Pharmacol. Ther. 2008;83:723–730. doi: 10.1038/sj.clpt.6100386. [DOI] [PubMed] [Google Scholar]
- 16.Deng P., Torrest A., Pollock K., Dahlenburg H., Annett G., Nolta J.A., Fink K.D. Clinical trial perspective for adult and juvenile Huntington’s disease using genetically-engineered mesenchymal stem cells. Neural. Regen. Res. 2016;11:702–705. doi: 10.4103/1673-5374.182682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H.J., Kim Y.H., Choi D.W., Cho K.A., Park J.W., Shin S.J., Jo I., Woo S.Y., Ryu K.H. Tonsil-derived mesenchymal stem cells enhance allogeneic bone marrow engraftment via collagen IV degradation. Stem. Cell Res. Ther. 2021;12:329. doi: 10.1186/s13287-021-02414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledesma-Martinez E., Mendoza-Nunez V.M., Santiago-Osorio E. Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem. Cells Int. 2016;2016:4709572. doi: 10.1155/2016/4709572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedenstein A.J., Chailakhjan R.K., Lalykina K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y., Holmes C. Tissue Regeneration Capacity of Extracellular Vesicles Isolated From Bone Marrow-Derived and Adipose-Derived Mesenchymal Stromal/Stem Cells. Front. Cell Dev. Biol. 2021;9:648098. doi: 10.3389/fcell.2021.648098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhat S., Viswanathan P., Chandanala S., Prasanna S.J., Seetharam R.N. Expansion and characterization of bone marrow derived human mesenchymal stromal cells in serum-free conditions. Sci. Rep. 2021;11:3403. doi: 10.1038/s41598-021-83088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huo J., Sun S., Geng Z., Sheng W., Chen R., Ma K., Sun X., Fu X. Bone Marrow-Derived Mesenchymal Stem Cells Promoted Cutaneous Wound Healing by Regulating Keratinocyte Migration via beta(2)-Adrenergic Receptor Signaling. Mol. Pharm. 2018;15:2513–2527. doi: 10.1021/acs.molpharmaceut.7b01138. [DOI] [PubMed] [Google Scholar]
- 23.Musial-Wysocka A., Kot M., Majka M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019;28:801–812. doi: 10.1177/0963689719837897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klimczak A., Kozlowska U., Kurpisz M. Muscle Stem/Progenitor Cells and Mesenchymal Stem Cells of Bone Marrow Origin for Skeletal Muscle Regeneration in Muscular Dystrophies. Arch. Immunol. Ther. Exp. 2018;66:341–354. doi: 10.1007/s00005-018-0509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y.H., Wang D.R., Guo Y.C., Liu J.Y., Pan J. The application of bone marrow mesenchymal stem cells and biomaterials in skeletal muscle regeneration. Regen. Ther. 2020;15:285–294. doi: 10.1016/j.reth.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L., Zhang M., Shi L., Yang X., Chen L., Cao N., Lei A., Cao Y. Neural stemness contributes to cell tumorigenicity. Cell Biosci. 2021;11:21. doi: 10.1186/s13578-021-00531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paik M.J., Li W.Y., Ahn Y.H., Lee P.H., Choi S., Kim K.R., Kim Y.M., Bang O.Y., Lee G. The free fatty acid metabolome in cerebral ischemia following human mesenchymal stem cell transplantation in rats. Clin. Chim. Acta. 2009;402:25–30. doi: 10.1016/j.cca.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 28.Rustad K.C., Gurtner G.C. Mesenchymal Stem Cells Home to Sites of Injury and Inflammation. Adv. Wound Care. 2012;1:147–152. doi: 10.1089/wound.2011.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie C., Yang Z., Suo Y., Chen Q., Wei D., Weng X., Gu Z., Wei X. Systemically Infused Mesenchymal Stem Cells Show Different Homing Profiles in Healthy and Tumor Mouse Models. Stem. Cells Transl. Med. 2017;6:1120–1131. doi: 10.1002/sctm.16-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin T.H., Phukan G., Shim J.S., Nguyen D.T., Kim Y., Oh-Lee J.D., Lee H.S., Paik M.J., Lee G. Restoration of Polyamine Metabolic Patterns in In Vivo and In Vitro Model of Ischemic Stroke following Human Mesenchymal Stem Cell Treatment. Stem. Cells Int. 2016;2016:4612531. doi: 10.1155/2016/4612531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang T., Lee Y.W., Rui Y.F., Cheng T.Y., Jiang X.H., Li G. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem. Cell Res. Ther. 2013;4:70. doi: 10.1186/scrt221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saccu G., Menchise V., Gai C., Bertolin M., Ferrari S., Giordano C., Manco M., Dastru W., Tolosano E., Bussolati B., et al. Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Promote Corneal Wound Repair by Regulating Inflammation and Angiogenesis. Cells. 2022;11:3892. doi: 10.3390/cells11233892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almeida A., Lira R., Oliveira M., Martins M., Azevedo Y., Silva K.R., Carvalho S., Cortez E., Stumbo A.C., Carvalho L., et al. Bone marrow-derived mesenchymal stem cells transplantation ameliorates renal injury through anti-fibrotic and anti-inflammatory effects in chronic experimental renovascular disease. Biomed. J. 2022;45:629–641. doi: 10.1016/j.bj.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y.J., Park H.J., Lee G., Bang O.Y., Ahn Y.H., Joe E., Kim H.O., Lee P.H. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia. 2009;57:13–23. doi: 10.1002/glia.20731. [DOI] [PubMed] [Google Scholar]
- 35.Dai W., Hale S.L., Martin B.J., Kuang J.Q., Dow J.S., Wold L.E., Kloner R.A. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: Short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 36.Fan L., Yang K., Yu R., Hui H., Wu W. circ-Iqsec1 induces bone marrow-derived mesenchymal stem cell (BMSC) osteogenic differentiation through the miR-187-3p/Satb2 signaling pathway. Arthritis. Res. Ther. 2022;24:273. doi: 10.1186/s13075-022-02964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y., Jiang Y., Wang Y., Zhao Z., Li T. LINC00473 rescues human bone marrow mesenchymal stem cells from apoptosis induced by dexamethasone through the PEBP1-mediated Akt/Bad/Bcl-2 signaling pathway. Int. J. Mol. Med. 2021;47:171–182. doi: 10.3892/ijmm.2020.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharifi A.M., Ghazanfari R., Tekiyehmaroof N., Sharifi M.A. Investigating the effect of lead acetate on rat bone marrow-derived mesenchymal stem cells toxicity: Role of apoptosis. Toxicol. Mech. Methods. 2011;21:225–230. doi: 10.3109/15376516.2010.543943. [DOI] [PubMed] [Google Scholar]
- 39.Jones J., Jaramillo-Merchan J., Bueno C., Pastor D., Viso-Leon M., Martinez S. Mesenchymal stem cells rescue Purkinje cells and improve motor functions in a mouse model of cerebellar ataxia. Neurobiol. Dis. 2010;40:415–423. doi: 10.1016/j.nbd.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Shim E.K., Lee J.S., Kim D.E., Kim S.K., Jung B.J., Choi E.Y., Kim C.S. Autogenous Mesenchymal Stem Cells from the Vertebral Body Enhance Intervertebral Disc Regeneration via Paracrine Interaction: An in Vitro Pilot Study. Cell Transplant. 2016;25:1819–1832. doi: 10.3727/096368916X691420. [DOI] [PubMed] [Google Scholar]
- 41.Li W.Y., Choi Y.J., Lee P.H., Huh K., Kang Y.M., Kim H.S., Ahn Y.H., Lee G., Bang O.Y. Mesenchymal stem cells for ischemic stroke: Changes in effects after ex vivo culturing. Cell Transplant. 2008;17:1045–1059. doi: 10.3727/096368908786991551. [DOI] [PubMed] [Google Scholar]
- 42.Shi H., Li X., Yang J., Zhao Y., Xue C., Wang Y., He Q., Shen M., Zhang Q., Yang Y., et al. Bone marrow-derived neural crest precursors improve nerve defect repair partially through secreted trophic factors. Stem. Cell Res. Ther. 2019;10:397. doi: 10.1186/s13287-019-1517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheper V., Schwieger J., Hamm A., Lenarz T., Hoffmann A. BDNF-overexpressing human mesenchymal stem cells mediate increased neuronal protection in vitro. J. Neurosci. Res. 2019;97:1414–1429. doi: 10.1002/jnr.24488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park K.W., Eglitis M.A., Mouradian M.M. Protection of nigral neurons by GDNF-engineered marrow cell transplantation. Neurosci. Res. 2001;40:315–323. doi: 10.1016/S0168-0102(01)00242-5. [DOI] [PubMed] [Google Scholar]
- 45.Hoban D.B., Howard L., Dowd E. GDNF-secreting mesenchymal stem cells provide localized neuroprotection in an inflammation-driven rat model of Parkinson’s disease. Neuroscience. 2015;303:402–411. doi: 10.1016/j.neuroscience.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Q., Wu P., Chen F., Zhao Y., Li Y., He X., Huselstein C., Ye Q., Tong Z., Chen Y. Brain Derived Neurotrophic Factor and Glial Cell Line-Derived Neurotrophic Factor-Transfected Bone Mesenchymal Stem Cells for the Repair of Periphery Nerve Injury. Front. Bioeng. Biotechnol. 2020;8:874. doi: 10.3389/fbioe.2020.00874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mortazavi Y., Sheikhsaran F., Khamisipour G.K., Soleimani M., Teimuri A., Shokri S. The Evaluation of Nerve Growth Factor Over Expression on Neural Lineage Specific Genes in Human Mesenchymal Stem Cells. Cell J. 2016;18:189–196. doi: 10.22074/cellj.2016.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li S., Liao X., He Y., Chen R., Zheng W.V., Tang M., Guo X., Chen J., Hu S., Sun J. Exosomes derived from NGF-overexpressing bone marrow mesenchymal stem cell sheet promote spinal cord injury repair in a mouse model. Neurochem. Int. 2022;157:105339. doi: 10.1016/j.neuint.2022.105339. [DOI] [PubMed] [Google Scholar]
- 49.Lindvall O., Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J. Clin. Investig. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrzejewska A., Dabrowska S., Lukomska B., Janowski M. Mesenchymal Stem Cells for Neurological Disorders. Adv. Sci. 2021;8:2002944. doi: 10.1002/advs.202002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vacanti J.P., Langer R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354:S32–S34. doi: 10.1016/S0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 52.Kassem M., Abdallah B.M. Human bone-marrow-derived mesenchymal stem cells: Biological characteristics and potential role in therapy of degenerative diseases. Cell Tissue Res. 2008;331:157–163. doi: 10.1007/s00441-007-0509-0. [DOI] [PubMed] [Google Scholar]
- 53.Li J., Liu Y., Zhang Y., Yao B., Enhejirigala, Li Z., Song W., Wang Y., Duan X., Yuan X., et al. Biophysical and Biochemical Cues of Biomaterials Guide Mesenchymal Stem Cell Behaviors. Front. Cell Dev. Biol. 2021;9:640388. doi: 10.3389/fcell.2021.640388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernández-Serra R., Gallego R., Lozano P., González-Nieto D. Hydrogels for neuroprotection and functional rewiring: A new era for brain engineering. Neural. Regen. Res. 2020;15:783. doi: 10.4103/1673-5374.268891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan F., Li M., Zhang H.-Q., Li G.-L., Hua Y., Shen Y., Ji X.-M., Wu C.-J., An H., Ren M. Collagen-chitosan scaffold impregnated with bone marrow mesenchymal stem cells for treatment of traumatic brain injury. Neural. Regen. Res. 2019;14:1780. doi: 10.4103/1673-5374.257533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee D.Y., Lee S.E., Kwon D.H., Nithiyanandam S., Lee M.H., Hwang J.S., Basith S., Ahn J.H., Shin T.H., Lee G. Strategies to Improve the Quality and Freshness of Human Bone Marrow-Derived Mesenchymal Stem Cells for Neurological Diseases. Stem. Cells Int. 2021;2021:8444599. doi: 10.1155/2021/8444599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin T.H., Lee S., Choi K.R., Lee D.Y., Kim Y., Paik M.J., Seo C., Kang S., Jin M.S., Yoo T.H., et al. Quality and freshness of human bone marrow-derived mesenchymal stem cells decrease over time after trypsinization and storage in phosphate-buffered saline. Sci. Rep. 2017;7:1106. doi: 10.1038/s41598-017-01315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee K.A., Shim W., Paik M.J., Lee S.C., Shin J.Y., Ahn Y.H., Park K., Kim J.H., Choi S., Lee G. Analysis of changes in the viability and gene expression profiles of human mesenchymal stromal cells over time. Cytotherapy. 2009;11:688–697. doi: 10.3109/14653240902974032. [DOI] [PubMed] [Google Scholar]
- 59.Reel P.S., Reel S., Pearson E., Trucco E., Jefferson E. Using machine learning approaches for multi-omics data analysis: A review. Biotechnol. Adv. 2021;49:107739. doi: 10.1016/j.biotechadv.2021.107739. [DOI] [PubMed] [Google Scholar]
- 60.Milward E.A., Shahandeh A., Heidari M., Johnstone D.M., Daneshi N., Hondermarck H. Transcriptomics. Encycl. Cell Biol. 2016;4:160–165. [Google Scholar]
- 61.Kumar K. Integrated benchmarking standard and decision support system for structured, semi structured, unstructured retail data. Wirel. Netw. 2021:1–11. doi: 10.1007/s11276-021-02843-4. [DOI] [Google Scholar]
- 62.Cosgriff C.V., Stone D.J., Weissman G., Pirracchio R., Celi L.A. The clinical artificial intelligence department: A prerequisite for success. BMJ Health Care Inform. 2020;27:e100183. doi: 10.1136/bmjhci-2020-100183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Basith S., Manavalan B., Hwan Shin T., Lee G. Machine intelligence in peptide therapeutics: A next-generation tool for rapid disease screening. Med. Res. Rev. 2020;40:1276–1314. doi: 10.1002/med.21658. [DOI] [PubMed] [Google Scholar]
- 64.Li R., Li L., Xu Y., Yang J. Machine learning meets omics: Applications and perspectives. Brief. Bioinform. 2021;23:bbab460. doi: 10.1093/bib/bbab460. [DOI] [PubMed] [Google Scholar]
- 65.Shin T.H., Manavalan B., Lee D.Y., Basith S., Seo C., Paik M.J., Kim S.W., Seo H., Lee J.Y., Kim J.Y., et al. Silica-coated magnetic-nanoparticle-induced cytotoxicity is reduced in microglia by glutathione and citrate identified using integrated omics. Part Fibre. Toxicol. 2021;18:42. doi: 10.1186/s12989-021-00433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shin T.H., Lee D.Y., Jang Y.E., Kwon D.H., Hwang J.S., Kim S.G., Seo C., Paik M.J., Lee J.Y., Kim J.Y., et al. Reduction in the Migration Activity of Microglia Treated with Silica-Coated Magnetic Nanoparticles and their Recovery Using Citrate. Cells. 2022;11:2393. doi: 10.3390/cells11152393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Picard M., Scott-Boyer M.-P., Bodein A., Périn O., Droit A. Integration strategies of multi-omics data for machine learning analysis. Comput. Struct. Biotechnol. J. 2021;19:3735–3746. doi: 10.1016/j.csbj.2021.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lamptey R.N.L., Chaulagain B., Trivedi R., Gothwal A., Layek B., Singh J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022;23:1851. doi: 10.3390/ijms23031851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramanathan S., Archunan G., Sivakumar M., Tamil Selvan S., Fred A.L., Kumar S., Gulyás B., Padmanabhan P. Theranostic applications of nanoparticles in neurodegenerative disorders. Int. J. Nanomed. 2018;13:5561–5576. doi: 10.2147/IJN.S149022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scopetti M., Santurro A., Gatto V., La Russa R., Manetti F., D′Errico S., Frati P., Fineschi V. Mesenchymal stem cells in neurodegenerative diseases: Opinion review on ethical dilemmas. World J. Stem. Cells. 2020;12:168–177. doi: 10.4252/wjsc.v12.i3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin C., Wang K., Zhang L., Bai L. Stem cell therapy for Alzheimer’s disease: An overview of experimental models and reality. Anim. Model Exp. Med. 2022;5:15–26. doi: 10.1002/ame2.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hernández A.E., García E. Mesenchymal Stem Cell Therapy for Alzheimer’s Disease. Stem. Cells Int. 2021;2021:7834421. doi: 10.1155/2021/7834421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee J.K., Jin H.K., Bae J.S. Bone marrow-derived mesenchymal stem cells attenuate amyloid β-induced memory impairment and apoptosis by inhibiting neuronal cell death. Curr. Alzheimer Res. 2010;7:540–548. doi: 10.2174/156720510792231739. [DOI] [PubMed] [Google Scholar]
- 74.Cone A.S., Yuan X., Sun L., Duke L.C., Vreones M.P., Carrier A.N., Kenyon S.M., Carver S.R., Benthem S.D., Stimmell A.C., et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer’s disease-like phenotypes in a preclinical mouse model. Theranostics. 2021;11:8129–8142. doi: 10.7150/thno.62069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakano M., Kubota K., Kobayashi E., Chikenji T.S., Saito Y., Konari N., Fujimiya M. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA-146a in hippocampus. Sci. Rep. 2020;10:10772. doi: 10.1038/s41598-020-67460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fields C.R., Bengoa-Vergniory N., Wade-Martins R. Targeting alpha-synuclein as a therapy for Parkinson’s disease. Front. Mol. Neurosci. 2019;12:299. doi: 10.3389/fnmol.2019.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Venkatesh K., Sen D. Mesenchymal Stem Cells as a Source of Dopaminergic Neurons: A Potential Cell Based Therapy for Parkinson’s Disease. Curr. Stem. Cell Res. Ther. 2017;12:326–347. doi: 10.2174/1574888X12666161114122059. [DOI] [PubMed] [Google Scholar]
- 78.Blandini F., Cova L., Armentero M.-T., Zennaro E., Levandis G., Bossolasco P., Calzarossa C., Mellone M., Giuseppe B., Deliliers G.L., et al. Transplantation of Undifferentiated Human Mesenchymal Stem Cells Protects against 6-Hydroxydopamine Neurotoxicity in the Rat. Cell Transplant. 2010;19:203–218. doi: 10.3727/096368909X479839. [DOI] [PubMed] [Google Scholar]
- 79.Heris R.M., Shirvaliloo M., Abbaspour-Aghdam S., Hazrati A., Shariati A., Youshanlouei H.R., Niaragh F.J., Valizadeh H., Ahmadi M. The potential use of mesenchymal stem cells and their exosomes in Parkinson’s disease treatment. Stem. Cell Res. Ther. 2022;13:371. doi: 10.1186/s13287-022-03050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramalingam M., Jang S., Jeong H.S. Therapeutic Effects of Conditioned Medium of Neural Differentiated Human Bone Marrow-Derived Stem Cells on Rotenone-Induced Alpha-Synuclein Aggregation and Apoptosis. Stem. Cells Int. 2021;2021:6658271. doi: 10.1155/2021/6658271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schiess M., Suescun J., Doursout M.-F., Adams C., Green C., Saltarrelli J.G., Savitz S., Ellmore T.M. Allogeneic Bone Marrow–Derived Mesenchymal Stem Cell Safety in Idiopathic Parkinson’s Disease. Mov. Dis. 2021;36:1825–1834. doi: 10.1002/mds.28582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Staff N.P., Jones D.T., Singer W. Mesenchymal Stromal Cell Therapies for Neurodegenerative Diseases. Mayo. Clin. Proc. 2019;94:892–905. doi: 10.1016/j.mayocp.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chiò A., Mazzini L., Mora G. Disease-modifying therapies in amyotrophic lateral sclerosis. Neuropharmacology. 2020;167:107986. doi: 10.1016/j.neuropharm.2020.107986. [DOI] [PubMed] [Google Scholar]
- 84.Vercelli A., Mereuta O.M., Garbossa D., Muraca G., Mareschi K., Rustichelli D., Ferrero I., Mazzini L., Madon E., Fagioli F. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2008;31:395–405. doi: 10.1016/j.nbd.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 85.Stavely R., Nurgali K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem. Cells Transl. Med. 2020;9:985–1006. doi: 10.1002/sctm.19-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morata-Tarifa C., Azkona G., Glass J., Mazzini L., Sanchez-Pernaute R. Looking backward to move forward: A meta-analysis of stem cell therapy in amyotrophic lateral sclerosis. NPJ Regen. Med. 2021;6:20. doi: 10.1038/s41536-021-00131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oh K.-W., Moon C., Kim H.Y., Oh S.-i., Park J., Lee J.H., Chang I.Y., Kim K.S., Kim S.H. Phase I Trial of Repeated Intrathecal Autologous Bone Marrow-Derived Mesenchymal Stromal Cells in Amyotrophic Lateral Sclerosis. Stem. Cells Transl. Med. 2015;4:590–597. doi: 10.5966/sctm.2014-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tavakol-Afshari J., Boroumand A.R., Farkhad N.K., Adhami Moghadam A., Sahab-Negah S., Gorji A. Safety and efficacy of bone marrow derived-mesenchymal stem cells transplantation in patients with amyotrophic lateral sclerosis. Regen. Ther. 2021;18:268–274. doi: 10.1016/j.reth.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lewis C.M., Suzuki M. Therapeutic applications of mesenchymal stem cells for amyotrophic lateral sclerosis. Stem. Cell Res. Ther. 2014;5:32. doi: 10.1186/scrt421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi K.-A., Choi Y., Hong S. Stem cell transplantation for Huntington’s diseases. Methods. 2018;133:104–112. doi: 10.1016/j.ymeth.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 91.Alberch J., Pérez-Navarro E., Canals J.M. Neurotrophic factors in Huntington’s disease. Prog. Brain. Res. 2004;146:197–229. doi: 10.1016/s0079-6123(03)46014-7. [DOI] [PubMed] [Google Scholar]
- 92.Lin Y.-T., Chern Y., Shen C.-K.J., Wen H.-L., Chang Y.-C., Li H., Cheng T.-H., Hsieh-Li H.M. Human Mesenchymal Stem Cells Prolong Survival and Ameliorate Motor Deficit through Trophic Support in Huntington’s Disease Mouse Models. PLoS ONE. 2011;6:e22924. doi: 10.1371/journal.pone.0022924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pollock K., Dahlenburg H., Nelson H., Fink K.D., Cary W., Hendrix K., Annett G., Torrest A., Deng P., Gutierrez J., et al. Human Mesenchymal Stem Cells Genetically Engineered to Overexpress Brain-derived Neurotrophic Factor Improve Outcomes in Huntington’s Disease Mouse Models. Mol. Ther. 2016;24:965–977. doi: 10.1038/mt.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sadan O., Shemesh N., Barzilay R., Dadon-Nahum M., Blumenfeld-Katzir T., Assaf Y., Yeshurun M., Djaldetti R., Cohen Y., Melamed E., et al. Mesenchymal stem cells induced to secrete neurotrophic factors attenuate quinolinic acid toxicity: A potential therapy for Huntington’s disease. Exp. Neurol. 2012;234:417–427. doi: 10.1016/j.expneurol.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 95.Chi H., Chang H.-Y., Sang T.-K. Neuronal cell death mechanisms in major neurodegenerative diseases. Int. J. Mol. Sci. 2018;19:3082. doi: 10.3390/ijms19103082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marde V.S., Tiwari P.L., Wankhede N.L., Taksande B.G., Upaganlawar A.B., Umekar M.J., Kale M.B. Neurodegenerative disorders associated with genes of mitochondria. Future J. Pharm. Sci. 2021;7:1–8. doi: 10.1186/s43094-021-00215-5. [DOI] [Google Scholar]
- 97.Wilson D.M., Cookson M.R., Van Den Bosch L., Zetterberg H., Holtzman D.M., Dewachter I. Hallmarks of neurodegenerative diseases. Cell. 2023;186:693–714. doi: 10.1016/j.cell.2022.12.032. [DOI] [PubMed] [Google Scholar]
- 98.Mehta S.R., Tom C.M., Wang Y., Bresee C., Rushton D., Mathkar P.P., Tang J., Mattis V.B. Human Huntington’s disease iPSC-derived cortical neurons display altered transcriptomics, morphology, and maturation. Cell Rep. 2018;25:1081–1096.e6. doi: 10.1016/j.celrep.2018.09.076. [DOI] [PubMed] [Google Scholar]
- 99.Franco-Bocanegra D.K., Gourari Y., McAuley C., Chatelet D.S., Johnston D.A., Nicoll J.A., Boche D. Microglial morphology in Alzheimer’s disease and after Aβ immunotherapy. Sci. Rep. 2021;11:15955. doi: 10.1038/s41598-021-95535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peng J., Peng L., Stevenson F.F., Doctrow S.R., Andersen J.K. Iron and paraquat as synergistic environmental risk factors in sporadic Parkinson’s disease accelerate age-related neurodegeneration. J. Neurosci. 2007;27:6914–6922. doi: 10.1523/JNEUROSCI.1569-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharifi-Rad M., Anil Kumar N.V., Zucca P., Varoni E.M., Dini L., Panzarini E., Rajkovic J., Tsouh Fokou P.V., Azzini E., Peluso I. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020;11:694. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ajoolabady A., Lindholm D., Ren J., Pratico D. ER stress and UPR in Alzheimer’s disease: Mechanisms, pathogenesis, treatments. Cell Death Dis. 2022;13:706. doi: 10.1038/s41419-022-05153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zakrzewski W., Dobrzyński M., Szymonowicz M., Rybak Z. Stem cells: Past, present, and future. Stem. Cell Res. Ther. 2019;10:1–22. doi: 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stolzing A., Jones E., Mcgonagle D., Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 105.Geissler S., Textor M., Kühnisch J., Könnig D., Klein O., Ode A., Pfitzner T., Adjaye J., Kasper G., Duda G.N. Functional comparison of chronological and in vitro aging: Differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells. PLoS ONE. 2012;7:e52700. doi: 10.1371/journal.pone.0052700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sammour I., Somashekar S., Huang J., Batlahally S., Breton M., Valasaki K., Khan A., Wu S., Young K.C. The Effect of Gender on Mesenchymal Stem Cell (MSC) Efficacy in Neonatal Hyperoxia-Induced Lung Injury. PLoS ONE. 2016;11:e0164269. doi: 10.1371/journal.pone.0164269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crisostomo P.R., Wang M., Herring C.M., Markel T.A., Meldrum K.K., Lillemoe K.D., Meldrum D.R. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: Role of the 55 kDa TNF receptor (TNFR1) J. Mol. Cell Cardiol. 2007;42:142–149. doi: 10.1016/j.yjmcc.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Siegel G., Kluba T., Hermanutz-Klein U., Bieback K., Northoff H., Schafer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11:146. doi: 10.1186/1741-7015-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang M., Yuan Q., Xie L. Mesenchymal stem cell-based immunomodulation: Properties and clinical application. Stem. Cells Int. 2018;2018:1–12. doi: 10.1155/2018/3057624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salari V., Mengoni F., Del Gallo F., Bertini G., Fabene P.F. The anti-inflammatory properties of mesenchymal stem cells in epilepsy: Possible treatments and future perspectives. Int. J. Mol. Sci. 2020;21:9683. doi: 10.3390/ijms21249683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crippa S., Santi L., Berti M., De Ponti G., Bernardo M.E. Role of ex vivo expanded mesenchymal stromal cells in determining hematopoietic stem cell transplantation outcome. Front. Cell Dev. Biol. 2021;9:663316. doi: 10.3389/fcell.2021.663316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Binato R., de Souza Fernandez T., Lazzarotto-Silva C., Du Rocher B., Mencalha A., Pizzatti L., Bouzas L., Abdelhay E. Stability of human mesenchymal stem cells during in vitro culture: Considerations for cell therapy. Cell Prolif. 2013;46:10–22. doi: 10.1111/cpr.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shakouri-Motlagh A., O′Connor A.J., Kalionis B., Heath D.E. Improved ex vivo expansion of mesenchymal stem cells on solubilized acellular fetal membranes. J. Biomed. Mater Res. A. 2019;107:232–242. doi: 10.1002/jbm.a.36557. [DOI] [PubMed] [Google Scholar]
- 114.Li X.-Y., Ding J., Zheng Z.-H., Li X.-Y., Wu Z.-B., Zhu P. Long-term culture in vitro impairs the immunosuppressive activity of mesenchymal stem cells on T cells. Mol. Med. Rep. 2012;6:1183–1189. doi: 10.3892/mmr.2012.1039. [DOI] [PubMed] [Google Scholar]
- 115.Bonab M.M., Alimoghaddam K., Talebian F., Ghaffari S.H., Ghavamzadeh A., Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:1–7. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kretlow J.D., Jin Y.-Q., Liu W., Zhang W.J., Hong T.-H., Zhou G., Baggett L.S., Mikos A.G., Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:1–13. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stenderup K., Justesen J., Clausen C., Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 118.Zaim M., Karaman S., Cetin G., Isik S. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann. Hematol. 2012;91:1175–1186. doi: 10.1007/s00277-012-1438-x. [DOI] [PubMed] [Google Scholar]
- 119.Baxter M.A., Wynn R.F., Jowitt S.N., Wraith J.E., Fairbairn L.J., Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem. Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 120.Watanabe J., Yamada M., Niibe K., Zhang M., Kondo T., Ishibashi M., Egusa H. Preconditioning of bone marrow-derived mesenchymal stem cells with N-acetyl-L-cysteine enhances bone regeneration via reinforced resistance to oxidative stress. Biomaterials. 2018;185:25–38. doi: 10.1016/j.biomaterials.2018.08.055. [DOI] [PubMed] [Google Scholar]
- 121.Nandy S.B., Mohanty S., Singh M., Behari M., Airan B. Fibroblast Growth Factor-2 alone as an efficient inducer for differentiation of human bone marrow mesenchymal stem cells into dopaminergic neurons. J. Biomed. Sci. 2014;21:1–10. doi: 10.1186/s12929-014-0083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Longobardi L., O’Rear L., Aakula S., Johnstone B., Shimer K., Chytil A., Horton W.A., Moses H.L., Spagnoli A. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-β signaling. J. Bone. Miner. Res. 2006;21:626–636. doi: 10.1359/jbmr.051213. [DOI] [PubMed] [Google Scholar]
- 123.Xian L., Wu X., Pang L., Lou M., Rosen C.J., Qiu T., Crane J., Frassica F., Zhang L., Rodriguez J.P. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med. 2012;18:1095–1101. doi: 10.1038/nm.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heng B.C., Cowan C.M., Basu S. Temperature and calcium ions affect aggregation of mesenchymal stem cells in phosphate buffered saline. Cytotechnology. 2008;58:69–75. doi: 10.1007/s10616-008-9174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Karahalil B. Overview of Systems Biology and Omics Technologies. Curr. Med. Chem. 2016;23:4221–4230. doi: 10.2174/0929867323666160926150617. [DOI] [PubMed] [Google Scholar]
- 126.Johnson C.H., Gonzalez F.J. Challenges and opportunities of metabolomics. J. Cell Physiol. 2012;227:2975–2981. doi: 10.1002/jcp.24002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shin T.H., Nithiyanandam S., Lee D.Y., Kwon D.H., Hwang J.S., Kim S.G., Jang Y.E., Basith S., Park S., Mo J.S., et al. Analysis of Nanotoxicity with Integrated Omics and Mechanobiology. Nanomaterials. 2021;11:2385. doi: 10.3390/nano11092385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shin T.H., Kim S.G., Ji M., Kwon D.H., Hwang J.S., George N.P., Ergando D.S., Park C.B., Paik M.J., Lee G. Diesel-derived PM(2.5) induces impairment of cardiac movement followed by mitochondria dysfunction in cardiomyocytes. Front. Endocrinol. 2022;13:999475. doi: 10.3389/fendo.2022.999475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shim W., Paik M.J., Nguyen D.T., Lee J.K., Lee Y., Kim J.H., Shin E.H., Kang J.S., Jung H.S., Choi S., et al. Analysis of changes in gene expression and metabolic profiles induced by silica-coated magnetic nanoparticles. ACS Nano. 2012;6:7665–7680. doi: 10.1021/nn301113f. [DOI] [PubMed] [Google Scholar]
- 130.Khan S., Ince-Dunn G., Suomalainen A., Elo L.L. Integrative omics approaches provide biological and clinical insights: Examples from mitochondrial diseases. J. Clin. Investig. 2020;130:20–28. doi: 10.1172/JCI129202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Paik M.J., Cho I.S., Mook-Jung I., Lee G., Kim K.R. Altered free amino acid levels in brain cortex tissues of mice with Alzheimer’s disease as their N(O,S)-ethoxycarbonyl/tert-butyldimethylsilyl derivatives. BMB Rep. 2008;41:23–28. doi: 10.5483/BMBRep.2008.41.1.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings are included within the article.