Abstract
Epithelial morphogenesis is critical during development and wound healing, and alterations in this program contribute to neoplasia. Met, the hepatocyte growth factor (HGF) receptor, promotes a morphogenic program in epithelial cell lines in matrix cultures. Previous studies have identified Gab1, the major phosphorylated protein following Met activation, as important for the morphogenic response. Gab1 is a docking protein that couples the Met receptor with multiple signaling proteins, including phosphatidylinositol-3 kinase, phospholipase Cγ, the adapter protein Crk, and the tyrosine specific phosphatase SHP-2. HGF induces sustained phosphorylation of Gab1 and sustained activation of extracellular signal-regulated kinase (Erk) in epithelial Madin-Darby canine kidney cells. In contrast, epidermal growth factor fails to promote a morphogenic program and induces transient Gab1 phosphorylation and Erk activation. To elucidate the Gab1-dependent signals required for epithelial morphogenesis, we undertook a structure-function approach and demonstrate that association of Gab1 with the tyrosine phosphatase SHP-2 is required for sustained Erk activation and for epithelial morphogenesis downstream from the Met receptor. Epithelial cells expressing a Gab1 mutant protein unable to recruit SHP-2 elicit a transient activation of Erk in response to HGF. Moreover, SHP-2 catalytic activity is required, since the expression of a catalytically inactive SHP-2 mutant, C/S, abrogates sustained activation of Erk and epithelial morphogenesis by the Met receptor. These data identify SHP-2 as a positive modulator of Erk activity and epithelial morphogenesis downstream from the Met receptor.
Epithelial morphogenesis plays an important role during development and wound healing, and understanding the mechanisms that regulate this function will provide insights into how alterations in this morphogenic program lead to neoplasia. Hepatocyte growth factor (HGF) is a mesenchymally derived factor that has been implicated in epithelial morphogenesis in addition to multiple other biological functions (15, 25, 30, 42, 67, 72, 80). In vitro, HGF promotes epithelial cell growth and survival as well as epithelial-mesenchymal transition, where it stimulates the dissociation and dispersal of colonies of epithelial cells and the acquisition of a fibroblastic morphology resulting in increased cellular motility and invasiveness (25, 42, 74, 80, 82). HGF triggers a morphogenic program in epithelial cell lines when cultured in a collagen matrix (41, 65, 74). Increasing evidence demonstrates an important in vivo role for HGF during the development of the liver and placenta, as well as in the development and innervation of skeletal muscle, the ductal growth of mammary explants, and directing the growth of axonal cones (9, 37, 60, 70, 77). Importantly, the receptor for HGF, the Met tyrosine kinase, is shown to be deregulated through either gene amplification, overexpression, or activating point mutations in a number of human cancers, suggesting that the Met receptor may play an important role in human tumorigenesis (8, 24, 36, 57, 61).
To characterize signaling pathways downstream from the Met receptor involved in epithelial morphogenesis, we and others have used receptor chimeras to demonstrate that the Met receptor cytoplasmic domain is sufficient for the biological responses attributed to HGF and that Met tyrosine kinase activity is required for these responses (29, 74, 83). Two tyrosine residues within the carboxyl terminus of Met (Y1349 and Y1356), which are highly conserved between the other members of the Met receptor tyrosine kinase gene family, Sea and Ron, are crucial for cell scatter and epithelial morphogenesis in Madin-Darby canine kidney (MDCK) epithelial cells (29, 48, 58, 74, 83). Y1356 forms a multisubstrate-binding site, coupling the Met receptor with the Grb2 and Shc adapter proteins, as well as the Cbl proto-oncogene and the Grb2-associated binder 1 (Gab1) (10–12, 44, 51, 73).
Gab1 was initially identified in a library screen as a Grb2 binding protein and belongs to a family of docking proteins, including closely related proteins Gab2 and Daughter of Sevenless (DOS) and the more remotely related insulin receptor substrate 1 (IRS-1), IRS-2, IRS-3, downstream of kinases (Dok), and fibroblast growth factor (FGF) receptor substrate 2 (FRS2) (6, 16, 18, 21, 54, 75, 78, 81). These proteins lack enzymatic activities. Following activation of tyrosine kinase and cytokine receptors, they become phosphorylated on tyrosine residues, providing binding sites for multiple proteins involved in signal transduction. In this manner they act to potentiate and diversify the signals downstream from receptors by virtue of their ability to assemble multiprotein complexes. Furthermore, these proteins contain, in the amino terminus, a domain that allows for membrane recruitment. Many docking proteins contain a pleckstrin homology (PH) domain, as is the case for Gab1, and their association with phospholipids results in the targeting of proteins to membrane subdomains (reviewed in references 17, 34, 35, 39, and 62). Other docking proteins, like FRS2, are targeted to the membrane via a myristoylation signal (31). In addition, several of these proteins contain a phosphotyrosine binding (PTB) domain that promotes association with specific receptor tyrosine kinases. Gab1 lacks a known PTB domain, and instead, its association with the Met or epidermal growth factor (EGF) receptor may be both direct and indirect and requires a proline-rich domain defined as the Met binding domain and the association of these receptors with the Grb2 adapter protein (44, 55, 73).
Murine Gab1 contains 18 tyrosine residues, some of which, if phosphorylated, provide potential binding sites for SH2 or PTB domain-containing proteins. We have previously demonstrated that Gab1 is highly phosphorylated following stimulation of epithelial cells with HGF and couples with the p85 subunit of phosphatidylinositol-3 kinase (PI3K) and associated kinase activity, phospholipase Cγ (PLCγ1), and the tyrosine phosphatase SHP-2 (38, 44). MDCK cells expressing Met receptor mutants with decreased ability to recruit Gab1 fail to form branching tubules upon Met activation. Importantly, overexpression of Gab1 rescues the tubulogenesis defect of these mutants (38). To investigate the mechanism through which Gab1 mediates this function, we undertook a structure-function analysis of Gab1 and demonstrated that the Gab1 PH domain is essential to target Gab1 to sites of cell-cell contact in the vicinity of the Met receptor in epithelial cells and is also essential for the ability of Gab1 to promote epithelial morphogenesis downstream from the Met receptor (38).
Gab1 is phosphorylated downstream from multiple receptors, including the EGF, insulin, and TrkA receptors, as well as members of the cytokine family interleukin-3 (IL-3), IL-6, alpha and gamma interferon receptors, and T- and B-cell antigen receptors (21–23, 45, 68). Insight into the mechanism through which Gab1 could modulate distinct biological signals has revealed that Gab1 is phosphorylated with distinct kinetics. Gab1 is phosphorylated for a prolonged period of time (>60 min) downstream from the Met receptor, where it promotes branching morphogenesis of MDCK epithelial cells, whereas Gab1 is transiently phosphorylated (15 min) in response to EGF, which fails to induce a morphogenic program (38). Furthermore, while extracellular signal-regulated kinase (Erk) activity is sustained in response to HGF and parallels Gab1 phosphorylation, EGF-stimulated Erk activity is transient (28). Overexpression of Gab1 potentiates Erk activation downstream from the EGF receptor (55), suggesting that Gab1 provides a link from receptor tyrosine kinases to Erk activation. However, it remained unclear if Gab1 provided a link from the Met receptor tyrosine kinase to Erk activation and if Gab1 was required for sustained Erk activity.
The tyrosine specific phosphatase SHP-2 is recruited to Gab1 following HGF stimulation. However, its role in the regulation of Met-mediated signaling pathways and biological activities has remained undefined. Accumulating evidence implicates SHP-2 as a positive regulator of Erk activity downstream from receptor tyrosine kinases (4, 5, 7, 43, 47, 69). In Drosophila, genetic studies place the homologue of SHP-2, Corkscrew, as a positive regulator of Erk activity in a pathway downstream from receptor tyrosine kinases (18, 54), and in Xenopus oocytes, SHP-2 activity was required for full Erk activation in response to FGF (47). Moreover, fibroblasts derived from SHP-2 exon 3−/− mice have decreased ability to activate Erk in response to EGF and platelet-derived growth factor (PDGF) (53, 63). SHP-2 contains two tandem SH2 domains followed by a phosphatase domain. Following growth factor stimulation, SHP-2 is recruited through its SH2 domain directly to the EGF or PDGF receptors or indirectly via a docking protein, FRS2, to the FGF receptor and becomes phosphorylated on tyrosine residues (26, 31, 43, 65, 66). Both tyrosine phosphorylation of SHP-2 and binding of its SH2 domains to tyrosine phosphorylated peptides enhance its catalytic activity (32, 49, 71), possibly through the release of negative regulatory constraints on the phosphatase domain mediated by the SH2 domains of SHP-2 (3, 20).
In this paper we describe our investigation of the contribution of Gab1-associated SHP-2 to Met biological activity. We show that the interaction of Gab1 with SHP-2 is necessary for epithelial morphogenesis and for the sustained activation of Erk by the Met receptor. Importantly, the expression of a catalytically inactive SHP-2 protein (C/S) abrogates both epithelial tubulogenesis and sustained Erk activation, demonstrating that SHP-2 phosphatase activity is required for its function downstream from the Met receptor tyrosine kinase.
MATERIALS AND METHODS
Cell culture and DNA transfections.
MDCK cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS). The generation of MDCK cell lines expressing the wild-type colony-stimulating factor (CSF)-Met receptor and mutants thereof by retroviral infection has been previously described (12, 82). For the generation of stable cell lines expressing wild-type and mutant hemagglutinin (HA)-tagged Gab1, the Gab1 cDNA was cloned into the pCDNA1.1+ vector and was cotransfected with a PLXSH vector, which confers resistance to hygromycin, by the calcium phosphate method as described elsewhere (76). Cell lines were selected in hygromycin (300 μg/ml). For transient transfection assays, 293T cells were seeded at 106/100-mm-diameter petri dish and were transfected 24 h later with 2 μg of plasmid DNA encoding wild-type Gab1 without or with CSF-Met cDNA following the calcium phosphate precipitation method. Sixteen hours later, cells were washed twice in DMEM lacking FBS and were cultured for 24 h in medium containing 10% FBS, following which the cells were serum starved in 0.02% FBS overnight and then harvested.
Antibodies and reagents.
Antibodies raised in a rabbit against a C-terminal peptide of human Met were used (56). Antibodies raised in a rabbit against full-length murine Gab1 were generated. Anti-p85 was kindly provided by T. Pawson, Mount Sinai Hospital, University of Toronto, Toronto, Ontario, Canada. Antiphosphotyrosine (4G10) was obtained from Upstate Biotechnology Incorporated, Lake Placid, N.Y. Anti-HA (HA.11) was purchased from BABCO, Richmond, Calif., and anti-SOSn, anti-Grb2, and anti-AKT (sc-1618) were purchased from Santa Cruz Biotechnology, Inc. Anti-Shc was kindly provided by J. Bergeron, McGill University, Montreal, Quebec, Canada, and anti-SHP-2 was kindly provided by G.-S. Feng, Indiana University School of Medicine, Indianapolis, Ind. Anti-phospho-Akt and anti-phospho-Erk were purchased from New England Biolabs, and total Erk (p44Erk1 and p42Erk2) antibody was a gift from J. Blenis, Harvard Medical School, Boston, Mass. prCMV vector encoding wild-type SHP-2 was kindly provided by S. Ali, McGill University, and CEP4 vector encoding the SHP-2 C/S mutant was kindly provided by A. Saltiel, Parke Davis/Warner-Lambert Co., Ann Arbor, Mich. (40).
Site-directed mutagenesis.
Site-directed mutagenesis within Gab1 was performed using the Chameleon double-stranded site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. The mutagenesis primers were the following: for Y637F, ACAAACAAGTCGAATTCCTGGATTTAGAC, and for Y659F, GGCAGACGAGAGGGTCGACTTCGTTGTGGTGGACC. The mutated sequences are underlined.
HGF stimulation of MDCK cell lines expressing wild-type and mutant ΔSHP-2 Gab1.
Cells were seeded at 106 per 100-mm-diameter dish. Twenty-four hours later cells were washed once with DMEM and then starved overnight in 10 ml of DMEM containing 0.02% FBS. HGF or CSF was added at 100 U/ml in 2 ml for the indicated times. Cells were immediately lysed in 1 ml of lysis buffer (50 mM HEPES [pH 7.4], 150 mM NaCl, 10% glycerol, 0.5% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 μg each of leupeptin and aprotinin/ml, 1 mM Na3VO4).
Immunoprecipitations and Western blotting.
MDCK cell lysates (2 mg of total protein) or 293T cell lysates (50 μg) were incubated with the indicated antibodies for 1 h at 4°C with gentle rotation. Twenty microliters of a 50% slurry of either protein A or protein G-Sepharose was added for an additional hour to collect immune complexes. For the quantitative coimmunoprecipitations of Gab1 with SHP-2, 500 μg of proteins was subjected to immunoprecipitation with anti-HA, anti-SHP-2, or anti-Shc as indicated, followed by either protein A or protein G-Sepharose. The supernatant from this first immunoprecipitation was sequentially subjected to an incubation with beads alone and then an immunoprecipitation with the same antibodies. The resulting supernatant was then again subjected to protein A- or G-Sepharose beads alone, followed by a final immunoprecipitation with anti-HA and protein G-Sepharose. Following three washes in lysis buffer, the proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane. The membranes were blocked for 1 h with 3% bovine serum albumin in TBST (10 mM Tris-HCl [pH 7.4], 2.5 mM EDTA, 150 mM NaCl, 0.1% Tween 20) and then with primary antibody (1:1,000) for an additional hour. Following five washes in TBST, the proteins were revealed with secondary anti-mouse antibody (Jackson ImmunoResearch Laboratories, Inc.) or protein A (Amersham) conjugated to horseradish peroxidase. The proteins were visualized with an ECL detection system (Amersham). For the determination of Erk and Akt phosphorylation, 50 μg of total cellular proteins was resolved on an SDS–10% PAGE gel, transferred to a nitrocellulose membrane, and immunoblotted with an antibody specific for the activated form of Erk1 and Erk2 or Akt.
GST association assays.
Glutathione S-transferase (GST) fusion proteins were immobilized on glutathione-Sepharose beads, incubated with 500 μg of cell lysates from MDCK cells overexpressing wild-type Gab1, and stimulated or not with 100 U of HGF/ml or 50 μg of 293T cells transiently expressing CSF-Met and Gab1, as indicated in the figures. After 2 h on a rotator at 4°C, bound proteins were washed three times with lysis buffer, boiled in Laemmli buffer, resolved by SDS-PAGE, and revealed following Western blotting as described above.
Collagen assays.
The ability of MDCK cells to form branching tubules was assayed as previously described (82). Briefly, 5 × 103 cells were resuspended in 500 μl of collagen solution (Vitrogen 100 from Cohesion, Palo Alto, Calif.) prepared following the manufacturer's instructions and were layered over 350 μl of the collagen solution in a 24-well plate. Cells were maintained in Liebowitz medium (GIBCO) containing 5% FBS and were allowed to form cysts for 5 to 7 days. For stimulations, 5 U of HGF (kindly provided by G. Vande Woude, Grand Falls, Mich.) per ml or 5 U of rhCSF-1 (kindly provided by the Genetics Institute, Boston, Mass.) per ml was added to the Liebowitz medium containing 5% FBS. Tubules were apparent by light microscopy 5 to 10 days after the addition of growth factors. The medium was changed every 5 days, and photographs were taken at day 14 using Kodak TMY400 films at a magnification of ×10. For quantitation of the morphogenic response, 60 colonies in each of six independent cultures were scored for the ability to form branching tubules, and the results were plotted as the average number of cysts able to undergo tubulogenesis/culture/100.
Immunofluorescence.
MDCK cells overexpressing wild-type Gab1 or the Gab1 mutants were plated on glass coverslips (Bellco Glass Inc.) in a 24-well dish (Nunc) for the indicated times in DMEM containing 10% FBS. Cells were stimulated with 50 U of HGF per ml for 15 min where indicated. Cells were fixed in 2% paraformaldehyde in phosphate-buffered saline (PBS) for 30 min at room temperature, washed twice in PBS, and incubated for 10 min in PBS containing 50 mM ammonium chloride. Following one additional wash in PBS, cells were treated with PBS containing 0.1% Triton X-100 and 5% FBS (buffer A) for 10 min at room temperature. Anti-HA was diluted (1:300) in buffer A, and after three washes in the same buffer, CY3-conjugated anti-mouse antibody (1:2,000) was added for 10 min, followed by three washes in buffer A. The glass coverslips were mounted onto slides in Immunofluore medium (ICN) and visualized using a Nikon Labophot-2 epifluorescence microscope at a magnification of ×60. Photographs were taken using Kodak TMZ3200 film. Where indicated, cells were visualized by a Bio-Rad confocal microscope at a magnification of ×63.
RESULTS
Activation of the Met receptor tyrosine kinase results in the association of Gab1 with SHP-2 and SHP-2 tyrosine phosphorylation.
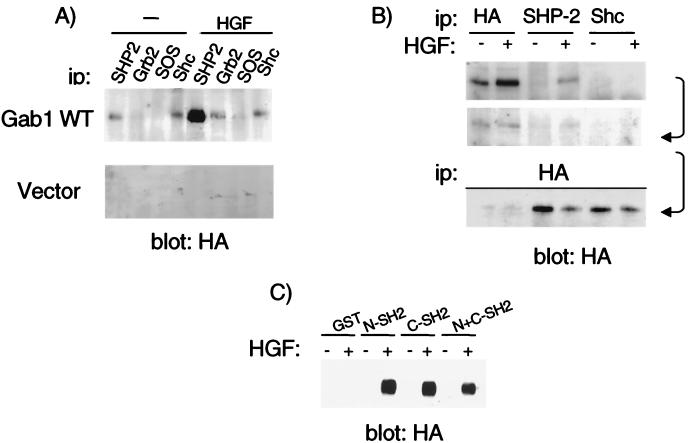
It has been shown previously that the tyrosine phosphatase SHP-2 can be recruited to the Met receptor tyrosine kinase (10); however, the functional significance of this interaction in epithelial morphogenesis had not been determined. SHP-2 is predominantly recruited to the Met receptor through the docking protein Gab1. MDCK epithelial cells stably expressing HA epitope-tagged Gab1 were stimulated with HGF. Lysates were subjected to immunoprecipitation with antibodies specific for the Grb2 or Shc adapter protein SOS or SHP-2, followed by Western blotting with anti-HA, which revealed that Gab1 was highly associated with SHP-2 (Fig. 1A). This was further supported by quantitative coimmunoprecipitation of Gab1 with SHP-2, where up to 50% of Gab1 was depleted from HGF-stimulated cells, compared with unstimulated cells (Fig. 1B). In GST pull-down experiments using lysates from unstimulated or HGF-stimulated MDCK cells expressing HA-Gab1, both the individual N-SH2 and C-SH2 domains of SHP-2 associated with Gab1 (Fig. 1C). Together, these results suggest that either SHP-2 SH2 domain can associate with Gab1 and that this interaction is phosphotyrosine dependent since both Met activation and Gab1 phosphorylation are required.
FIG. 1.
SHP-2 association with Gab1 is tyrosine phosphorylation dependent. (A) MDCK cells stably transfected with vector or constructs that express HA-Gab1 were stimulated for 15 min with 100 U of HGF/ml. SHP-2, Grb2, SOS, and Shc were immunoprecipitated with specific antibodies. Proteins resolved by SDS-PAGE were transferred to a nitrocellulose membrane and immunoblotted with anti-HA. (B) Lysates from HA-Gab1-expressing cells, stimulated or not with 100 U of HGF/ml, were subjected to immunoprecipitation as indicated. The resulting supernatants were subjected to a second round of immunoprecipitation with the indicated antibodies, followed by a third round of immunoprecipitation with anti-HA. (C) Lysates from HA-Gab1-expressing MDCK cells, stimulated or not with 100 U of HGF/ml, were subjected to a pull-down assay using GST-SHP-2 SH2 domain fusion proteins. Proteins were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and immunoblotted with anti-HA. ip, immunoprecipitate; WT, wild type.
The tyrosine phosphatase SHP-2 has been demonstrated to be recruited to and phosphorylated by several receptor tyrosine kinases (for a review, see references 43 and 66). Thus, we determined whether SHP-2 could be phosphorylated as a consequence of Met activation and whether Met and/or Gab1 could act as physiological substrates for SHP-2. Overexpression of Met in 293T cells results in ligand-independent Met activation (38). In the presence of activated Met, immunoprecipitation of cotransfected SHP-2 proteins (Fig. 2A, lane 2) revealed an increase in the level of tyrosine phosphorylation of SHP-2 compared to control cells (Fig. 2A, compare lanes 2 and 4). Furthermore, the increase in the tyrosine phosphorylation of cotransfected SHP-2 was enhanced in cells expressing a catalytically inactive mutant SHP-2 C/S protein (Fig. 2A, lane 3). Importantly, in the presence of activated Met, endogenous SHP-2 coprecipitated with two phosphoproteins: p120 and p150 (Fig. 2A, lane 1). To investigate whether p150 and p120 corresponded to Met and Gab1, respectively, SHP-2 immunoprecipitates were immunoblotted with either Met or Gab1 antibodies identifying these proteins as Met and Gab1 (Fig. 2A). Notably, Gab1 is present in endogenous SHP-2 or Met immunoprecipitates, suggesting that Gab1 may act as an intermediate to recruit SHP-2 to Met (Fig. 2A). HA-Gab1 was highly phosphorylated on tyrosine residues in cells coexpressing Met (Fig. 2B, lane 1). However, coexpression of SHP-2 resulted in a prominent decrease in Gab1 phosphorylation that was restored in cells coexpressing catalytically inactive C/S SHP-2 (Fig. 2B, lanes 2 and 3). The level of tyrosine phosphorylation of Met was also diminished in cells coexpressing wild-type SHP-2, in contrast to cells expressing the C/S SHP-2 mutant. Taken together, these results suggest that binding of SHP-2 to Gab1 provides a mechanism through which SHP-2 can be recruited to the Met receptor. Moreover, both the Met receptor and Gab1 could function as physiological substrates for SHP-2 in cells where SHP-2 is overexpressed.
FIG. 2.
Gab1 can function as a substrate for SHP-2 phosphatase activity. (A) 293T cells were transiently transfected with vectors encoding CSF-Met together with either wild-type (WT) SHP-2 or a catalytically inactive SHP-2 C/S mutant. Lysates were subjected to immunoprecipitation with anti-SHP-2 and Western blotting with anti-PY, anti-Met, or anti-Gab1 as indicated. (B) 293T cells were transfected with vectors encoding CSF-Met, together with either wild-type SHP-2 or the catalytically inactive SHP-2 C/S mutant, and Gab1. Gab1, Met, and SHP-2 were immunoprecipitated and immunoblotted, as indicated, with specific antibodies. ip, immunoprecipitate.
Gab1-SHP-2 binding mutant fails to rescue branching tubulogenesis downstream from Met receptor mutants.
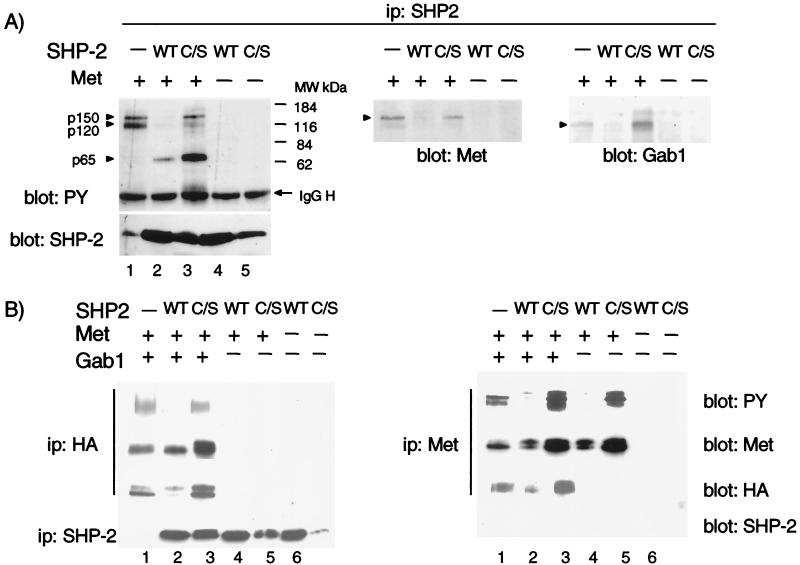
Recruitment of Gab1 requires two tyrosine residues in the C terminus of the Met receptor (Y1349 and Y1356). Structure-function studies using chimeric CSF-Met receptor mutants have revealed that receptor mutants impaired in their association with Gab1 (CSF-Met Y1356F and N1358H) were unable to induce branching tubules (12, 44, 82). Overexpression of Gab1 in these cell lines rescued the ability of CSF-Met mutants to promote branching tubulogenesis in response to CSF-1 (38). Since Gab1 is highly associated with SHP-2 following Met activation, we determined the biological function of this interaction by performing site-directed mutagenesis and studying the biological consequence of loss of SHP-2 association with Gab1 on epithelial tubulogenesis. Gab1 contains two tyrosine residues with a putative binding capacity for the SHP-2 SH2 domains. Tyrosine 637 or tyrosine 659 was mutated to phenylalanine alone or in combination, and the ability of these mutants to associate with SHP-2 was assessed. Met was coexpressed transiently with wild-type Gab1 or Gab1 ΔSHP-2 mutants in 293T cells (Fig. 3A). While all Gab1 variant proteins were expressed at similar levels and were phosphorylated when coexpressed with Met, SHP-2 was immunoprecipitated only with wild-type Gab1 or the Y659F Gab1 mutant but not with the Y637F or the Y637/659F mutants (Fig. 3A). These data identify Y637 as crucial for the interaction of Gab1 with SHP-2, and the Gab1-Y637F (Gab1 ΔSHP-2) mutant protein was used in all subsequent analyses.
FIG. 3.
Gab1 ΔSHP-2 mutant fails to rescue branching tubulogenesis downstream from a Met receptor mutant. (A) 293T cells were transfected with vectors encoding Gab1 mutants at Y637F and/or Y659F in the absence or presence of CSF-Met. Lysates were subjected to immunoprecipitation with anti-HA followed by Western blotting with either anti-SHP-2, anti-PY or anti-HA. (B) MDCK cells expressing the CSF-Met receptor mutant N1358H were stably transfected with vectors encoding wild-type (WT) the Gab1 or Gab1 ΔSHP-2 mutant. Proteins from cell lysates were immunoprecipitated with anti-HA and resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and blotted with anti-HA. (C) Cells expressing wild-type Gab1 (clone 3) and cells expressing the Gab1 ΔSHP-2 mutant protein (clone 5) were grown in collagen for 5 days, during which time they formed cysts. RhCSF-1 or HGF, both at 5 U/ml, were added, and 14 days later branching tubules were visualized at a magnification of ×10. (D) Quantitation of the tubulogenic response following stimulation with HGF and CSF was performed as described in Materials and Methods. The responses are plotted as the percentage of cysts that have undergone branching tubulogenesis. The values were derived from three independent experiments. ip, immunoprecipitate.
MDCK cells expressing CSF-Met mutant proteins that are unable to support branching tubules (Y1356F and N1358H CSF-Met) were stably transfected with expression vectors encoding Gab1 ΔSHP-2. Five clones of each cell line, with similar levels of HA-Gab1 expression, were selected and assayed for branching tubulogenesis. While similar results were obtained with the two Met mutant-expressing cell lines, data are shown only for the N1358H cell lines. The level of expression of Gab1 ΔSHP-2 in the selected cell lines was similar to that observed in wild-type HA-Gab1-expressing cells (Fig. 3B); the tubulogenic response is shown for one representative clone (Fig. 3C, clone 5), and the quantitation of this response is shown for two clones (Fig. 3D). Cell lines expressing CSF-Met mutants N1358H (Fig. 3C) or Y1356F (data not shown) formed cysts when grown in a collagen matrix (12). Stimulation of the CSF-Met receptor with CSF did not induce branching tubulogenesis in these cells (Fig. 3C and reference 12). However, as previously shown, overexpression of wild-type Gab1 rescued the tubulogenic response (Fig. 3 and reference 38). Importantly, although cell lines expressing the Gab1 ΔSHP-2 proteins could form cysts in a collagen matrix, in all cell lines tested, branching tubules failed to develop following activation of the CSF-Met receptor (0% with the Gab1 ΔSHP-2 mutant compared to 48% for cells expressing wild-type Gab1; Fig. 3C and D). Interestingly, the expression of Gab1 ΔSHP-2 reduced the ability of two of these lines (one is shown, clone 5) to form tubules in response to stimulation of the endogenous Met receptor with HGF. These results suggest not only that the SHP-2 binding site within the Gab1 C terminus is essential for rescue of tubulogenesis downstream from CSF-Met mutants but also that the expression of this mutant protein per se can detectably inhibit the formation of branching tubules induced through the wild-type endogenous Met receptor.
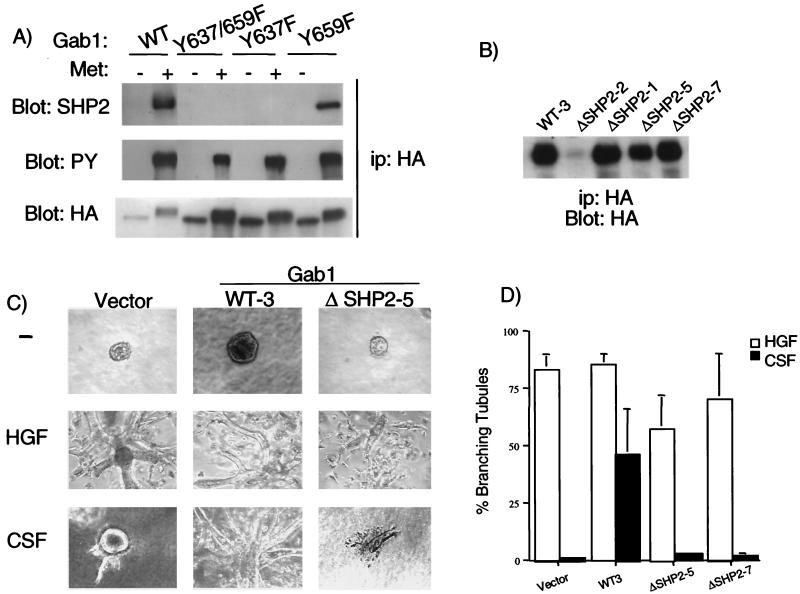
To investigate this possibility, we generated MDCK cells expressing either wild-type Gab1 or Gab1 ΔSHP-2 mutant proteins and compared their abilities to form tubules in response to HGF. The results are shown for two cell clones expressing Gab1 ΔSHP-2 (clone 6B and clone 8B). While the levels of expression of Gab1 in the different experimental groups were similar (Fig. 4A), the expression of Gab1 ΔSHP-2 proteins inhibited the ability of HGF to induce the formation of tubules to 8 and 19% (Fig. 4B and C). These results suggest that Gab1 ΔSHP-2 can act dominantly to interfere with the formation of branching tubules following Met receptor activation.
FIG. 4.
Overexpression of a Gab1 ΔSHP-2 inhibits branching tubulogenesis downstream from the endogenous Met receptor. (A) Proteins from lysates of MDCK cells expressing wild-type (WT) Gab1 (clone 9) and MDCK cells expressing Gab1 ΔSHP-2 mutant proteins (clones 6B and 8B) were subjected to immunoprecipitation and Western blotting with anti-HA. (B) Quantitation of the tubulogenic response was performed as described in Materials and Methods. The responses are plotted as the percentage of cysts that have undergone branching tubulogenesis. The values are derived from three independent experiments. (C) Cells were grown in collagen for 5 days, during which they formed cysts. HGF (5 U/ml) was added, and 14 days later branching tubules were visualized at a magnification of ×10. ip, immunoprecipitate.
Loss of SHP-2 binding does not affect Gab1 cellular localization or interaction with the Met receptor.
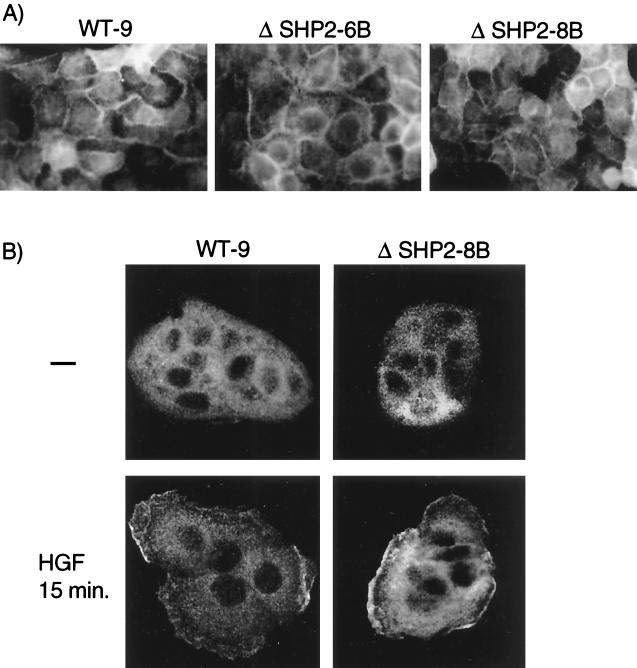
We and colleagues have previously shown that cellular localization of Gab1 to cell-cell junctions in the vicinity of the Met receptor correlated with the ability of Gab1 to promote branching tubulogenesis (38). Gab1 proteins that lack the entire PH domain or have mutations in the conserved phospholipid binding site within the PH domain fail to rescue branching tubulogenesis and fail to localize to sites of cell-cell contact in 10% serum (38, 39). To establish if the inability of the Gab1 ΔSHP-2 protein to rescue tubulogenesis reflected an altered cellular localization, the HA-tagged Gab1 ΔSHP-2 proteins stably expressed in MDCK epithelial cells were labeled by indirect immunofluorescence using anti-HA followed by CY3-conjugated anti-mouse antibody as a secondary antibody. Both wild-type Gab1 and Gab1 ΔSHP-2 proteins localized to sites of cell-cell contact, indicating that the inability to bind SHP-2 did not affect Gab1 localization in colonies of MDCK cells grown in 10% FBS (Fig. 5A). Moreover, we have previously demonstrated that in single cells or small MDCK cell colonies prior to the formation of phosphatidylinositol 3,4,5-trisphosphate-rich cell-cell junctions, Gab1 was predominantly present in the cytoplasm and, upon stimulation with HGF, relocalized to the membrane. We therefore determined whether the interaction of Gab1 with SHP-2 was a prerequisite for the HGF-mediated recruitment of Gab1 to the membrane. As shown in Fig. 5B, recruitment of Gab1 to the membrane was independent of the association of Gab1 with SHP-2.
FIG. 5.
The cellular localization of Gab1 is not altered in the absence of a Gab1-SHP-2 interaction. (A) MDCK cells (104 cells) expressing either wild-type (WT) Gab1 or Gab1 ΔSHP-2 mutant proteins were grown for 72 h on glass coverslips in DMEM containing 10% FBS. Cells were fixed in 2% paraformaldehyde and were subjected to indirect immunofluorescence using anti-HA, followed by CY3-conjugated anti-mouse antibody. Photographs were taken at a magnification of ×60. (B) MDCK cells (104 cells) expressing either wild-type Gab1 or Gab1 ΔSHP-2 mutant proteins were grown overnight on glass coverslips in DMEM containing 10% FBS. Cells were stimulated with 50 U of HGF/ml prior to fixation and indirect immunofluorescence with anti-HA, followed by CY3-conjugated anti-mouse antibody. Results were visualized by confocal microscopy at a magnification of ×63.
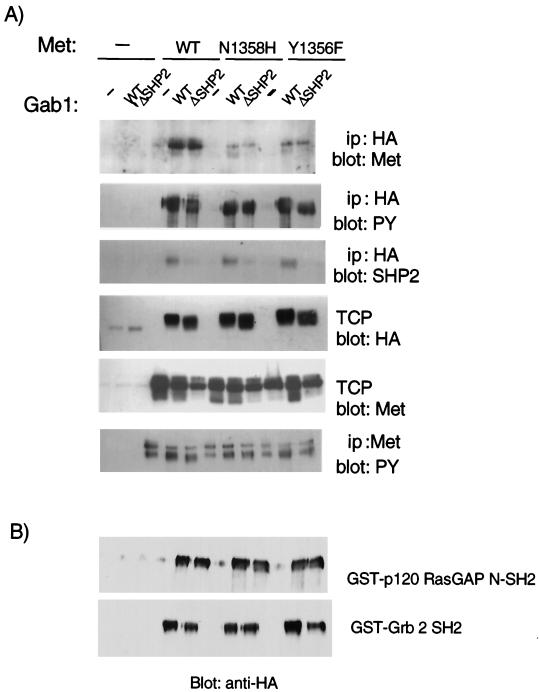
Furthermore, to establish whether the failure of the Gab1 ΔSHP-2 protein to rescue tubulogenesis in CSF-Met mutant-expressing cells resulted from the failure of this mutant protein to be recruited to the Met receptor, the ability of Gab1 ΔSHP-2 to coimmunoprecipitate with wild type or with N1358H or Y1356F CSF-Met mutants was investigated. In transient transfections, wild-type CSF-Met coimmunoprecipitated with the Gab1 ΔSHP-2 mutant proteins as efficiently as with wild-type Gab1 (Fig. 6A). The N1358H and the Y1356F CSF-Met receptor mutants, as described previously (38, 44), associated less efficiently with Gab1 than did the wild-type CSF-Met receptor. Importantly, the Gab1 ΔSHP-2 mutant was comparable to wild-type Gab1 in the efficiency with which it coimmunoprecipitated with either the N1358H or Y1356F Met mutant (Fig. 6A). Similar levels of the various CSF-Met mutant proteins and similar levels of Gab1 proteins were expressed in the different experimental groups, although fewer Gab1 proteins were expressed in the absence of CSF-Met cotransfection (Fig. 6A). Thus, neither inappropriate cellular localization nor defective recruitment to the various CSF-Met receptors could account for the inability of Gab1 ΔSHP-2 to promote branching tubulogenesis downstream from the Met receptor. Interestingly, the Gab1 ΔSHP-2 mutant had a faster electrophoretic mobility compared to wild-type Gab1, suggesting that the phosphorylation of Gab1 ΔSHP-2 was decreased (Fig. 6A).
FIG. 6.
The association of wild-type Gab1 and Gab1 ΔSHP-2 with Met and with p120 RasGAP and Grb2 fusion proteins. (A) 293T cells were transfected with vectors encoding wild type (WT), N1358H, or Y1356F CSF-Met receptors together with either wild-type Gab1 or the Gab1 ΔSHP-2 mutant. Lysates were subjected to immunoprecipitation with anti-HA and blotting with anti-Met, anti-PY, or anti-SHP-2 and immunoprecipitation with anti-Met, followed by blotting with anti-PY. Fifty micrograms of total cellular proteins (TCP) was resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and blotted with anti-HA or anti-Met. (B) Lysates from A were subjected to a pull-down experiment using GST-p120 RasGAP N-SH2 and GST-Grb2 SH2 domain fusion proteins. Associated proteins were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and immunoblotted with anti-HA. ip, immunoprecipitate.
Kinetics of HGF-stimulated Erk activation are altered in cells expressing Gab1 ΔSHP-2.
Both genetic and biochemical approaches support a role for mammalian SHP-2, as well as the Drosophila and Xenopus homologues, in modulating the Erk pathway downstream from several receptor tyrosine kinases, including the insulin, EGF, and FGF receptors (1, 63, 69). Moreover, Erk activity is essential for branching morphogenesis (28). We therefore analyzed whether the failure of cells expressing the Gab1 ΔSHP-2 mutant to undergo branching tubulogenesis correlated with an alteration in the prolonged activation of Erk typical of Met receptor stimulation. While similar results were obtained for cells expressing the N1358H mutant of the CSF-Met receptor or parental MDCK cells expressing either wild-type Gab1 or Gab1 ΔSHP-2 (data not shown), results are shown for MDCK cells stimulated with HGF (Fig. 7). Proteins from total cellular extracts were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with an antibody that specifically recognizes the activated form of Erk (phosphorylated at Thr202 and Tyr204). Phosphorylation of Erk was observed, which was maintained for 180 min in response to HGF (Fig. 7A). Importantly, in cell lines expressing Gab1 ΔSHP-2, Erk activation was transient (15 min), while the total level of Erk was equivalent to that in control cells (Fig. 7A). This occurs despite similar levels of Gab1 expression and kinetics in the induction of tyrosine phosphorylation of the Gab1 ΔSHP-2 mutant (Fig. 7B). Importantly, similar association of the wild type and Gab1 ΔSHP-2 with the p85 subunit of Pl3K was observed (Fig. 7B). In addition, HGF-mediated activation of protein kinase B/Akt, a downstream effector of Pl3K, was not altered in cells expressing Gab1 ΔSHP-2 when compared to cells expressing wild-type Gab1 (Fig. 7C). Hence, the specific reduction in the duration of Erk activity was consistent with the loss of SHP-2 binding to Gab1.
FIG. 7.
Activation of Erk is altered in cells expressing Gab1 ΔSHP-2 mutant proteins. (A) Cell lines expressing either wild-type (WT) Gab1 (clone 9) or a Gab1 ΔSHP-2 mutant (clone 8B) were stimulated with 100 U of HGF/ml for the indicated times at 37°C. Fifty micrograms of total cellular proteins was resolved by SDS–10% PAGE, transferred to a nitrocellulose membrane, and blotted with anti-phospho-Erk. Blots were stripped and reprobed with anti-total Erk. (B) Lysates were subjected to immunoprecipitation with anti-HA followed by Western blotting with anti-PY, anti-SHP-2, anti-p85 or anti-HA as indicated. (C) Fifty micrograms of total cellular proteins was resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and blotted with anti-phospho-AKT. Blots were stripped and reprobed with anti-total AKT. ip, immunoprecipitate.
The Erk pathway has been shown to be positively regulated by Grb2/SOS and negatively regulated through recruitment of p120 Ras GTPase-activating protein (p120 RasGAP). Importantly, the Drosophila SHP-2 homologue, Csw, positively regulates the Erk pathway by dephosphorylating a tyrosine residue in the Torso receptor tyrosine kinase, which binds to p120 RasGAP (7). We therefore determined whether the mechanism through which Gab1 ΔSHP-2 alters Erk activation involved modifications in the interaction of Gab1 with the regulators of Erk activity: p120 rasGAP and Grb2. The analysis was performed for 293T cells expressing the CSF-Met receptor and mutants thereof, together with either wild-type Gab1 or Gab1 ΔSHP-2 as in Fig. 6B. Lysates from these cells were subjected to GST pull-down assays using either GST-Grb2-SH2 or GST-p120 RasGAP-SH2 domain fusion protein. Associated Gab1 was revealed following Western blotting with anti-HA. No significant differences in the ability of Gab1 ΔSHP-2 to associate with the p120 rasGAP-N-SH2 domain was detected (Fig. 6B). However, a modest decrease in the ability of the Grb2-SH2 domain to associate with Gab1 ΔSHP-2 was consistently observed.
Overexpression of a catalytically inactive SHP-2 C/S mutant protein inhibits tubulogenesis and sustained Erk activation in response to HGF.
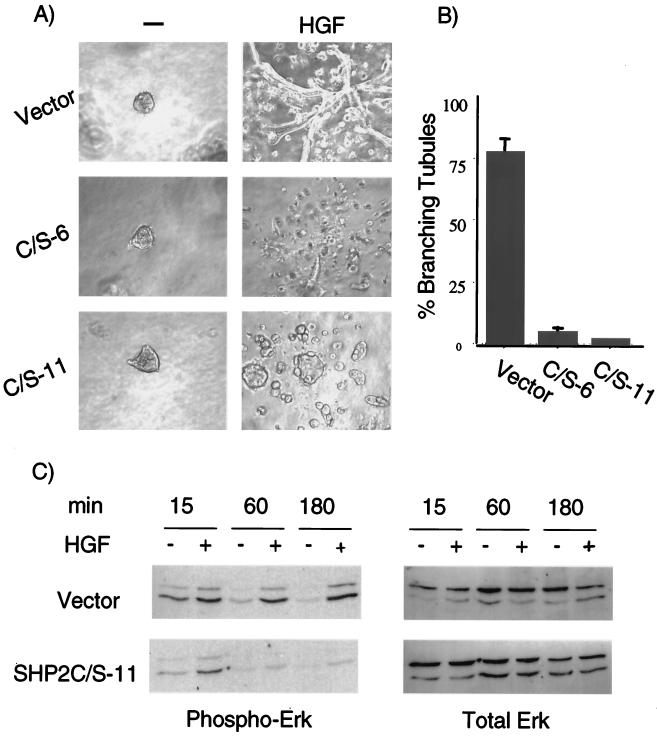
The interaction of Gab1 with SHP-2 was essential for the ability of Gab1 to rescue branching tubulogenesis downstream from the Met receptor (Fig. 3). However, this interaction was also critical for efficient induction of branching tubulogenesis through the endogenous wild-type Met receptor (Fig. 3 and 4), suggesting that overexpression of the Gab1 ΔSHP-2 mutant functioned as an inhibitory protein for tubulogenesis downstream from Met. To establish whether the mechanism involved in this inhibitory effect was dependent on the recruitment to Gab1 of a catalytically active SHP-2 protein, we generated five stable lines of MDCK cells expressing the catalytically inactive SHP-2 C/S protein and assayed HGF-mediated tubulogenesis. While the assays performed with the five clones yielded similar results, branching tubulogenesis and quantitation of this response are shown for two clones (Fig. 8A and B). Cell lines expressing the vector and the C/S SHP-2 mutants formed cysts when grown in a three-dimensional collagen matrix. Following stimulation with HGF, cells expressing the C/S SHP-2 mutant formed a distinct irregularly shaped cell mass and no organized branching tubules, suggesting that the phosphatase activity of SHP-2 was critical for epithelial tubulogenesis.
FIG. 8.
Catalytic activity of SHP-2 is required for branching tubulogenesis and sustained Erk activation induced by HGF. MDCK cells stably expressing SHP-2 C/S were generated (clones C/S-6 and -11). (A) Cells were grown in collagen for 5 days, during which they formed cysts. HGF (5 U/ml) was added, and 14 days later branching tubules were visualized at a magnification of ×10. (B) Quantitation of the tubulogenic response following stimulation with HGF was performed as described in Materials and Methods. The responses are plotted as the percentage of cysts that have undergone branching tubulogenesis. The values are derived from three independent experiments. (C) MDCK cells expressing vector control or SHP-2 C/S mutant proteins were stimulated with 100 U of HGF/ml for the indicated time. Fifty micrograms of total cellular proteins was resolved on an SDS–10% PAGE gel, transferred to a nitrocellulose membrane, and blotted for anti-phospho-Erk. Gels were stripped and reprobed with anti-total Erk.
We determined whether the kinetics of HGF-mediated Erk activation were altered in MDCK cells expressing the catalytically inactive C/S SHP-2 mutant. Stimulation of cells expressing the vector alone or wild-type Gab1 with HGF resulted in a marked increase in the activation of Erk that was sustained up to 3 h following stimulation (Fig. 8C). In contrast, the stimulation of cells expressing the C/S catalytically inactive SHP-2 mutant protein showed transient Erk activation, as did cells expressing the Gab1 ΔSHP-2 mutant. Thus, both the inability of Gab1 to bind SHP-2 and the overexpression of an SHP-2 catalytically inactive mutant acted to decrease the duration of Erk activation induced by HGF, suggesting that recruitment of enzymatically competent SHP-2 to the Met receptor through Gab1 was necessary for sustained Erk activation and for efficient branching tubulogenesis.
DISCUSSION
The Met receptor tyrosine kinase regulates the dispersal of epithelial sheets in culture and promotes the inherent morphogenic program of epithelia when grown in a collagen matrix. However, it is unclear how the Met receptor orchestrates the signaling pathways leading to its pleiotropic biological activities. We and colleagues have previously demonstrated that the multisubstrate binding protein Gab1 is required for the initiation of the morphogenic program (38). In the absence of any catalytic activity, Gab1 functions as a docking protein that, when phosphorylated by Met or other receptors, recruits multiple signaling proteins (21, 38). Gab1 acts to recruit Pl3K downstream from the Met, EGF, and TrkA receptors (21, 22, 38). This interaction has been shown to be essential for the survival of the neuronal PC12 cell line following stimulation of nerve growth factor (22) but is not required for the induction of the morphogenic program by Gab1 (38). While other proteins including PLCγ1, Crk, and SHP-2 also associate with Gab1, the contribution of these to the biological activities of the Met receptor is unknown (14, 38). We therefore undertook a structure-function approach to determine the contribution of SHP-2 in Met-mediated branching tubulogenesis. We show in this paper that both the interaction of Gab1 with SHP-2 and SHP-2 phosphatase activity are essential for epithelial tubulogenesis downstream from the Met receptor tyrosine kinase. Moreover, the recruitment of SHP-2 to Gab1 is required for the sustained activation of the Erk pathway observed following HGF stimulation of MDCK cells.
SHP-2 is one of the signaling proteins recruited to Gab1 following Met activation, yet the role of SHP-2 biological activity in epithelial cell morphogenesis and signaling downstream from the Met receptor has not been addressed. To investigate the role of this interaction in Met-mediated signaling pathways involved in epithelial tubulogenesis, we identified the SHP-2 binding site on Gab1 (Y637) that is phosphorylated following Met activation (Fig. 3) and show that mutation of this site results in Gab1 proteins unable to recruit SHP-2. Although overexpression of SHP-2 results in dephosphorylation of Gab1 (Fig. 2), mutating the SHP-2 binding site does not enhance Gab1 phosphorylation in stable cell lines in response to Met activations. Instead, the level of phosphorylation of the Gab1 ΔSHP-2 mutant protein is lower than that observed in wild-type Gab1 (Fig. 7). This is consistent with the observation that the SHP-2 binding site (Y637) in the Gab1 carboxy terminus is the predominant site of phosphorylation of Gab1 in vitro by a recombinant EGF receptor kinase domain (33). Importantly, the failure to recruit SHP-2 results in the inability of Gab1 to rescue tubulogenesis in MDCK cells that express mutant CSF-Met receptors, implying, for the first time, a role for SHP-2 in the morphogenic response of epithelial cells. In addition, the overexpression of the Gab1 ΔSHP-2 mutant abrogates tubulogenesis downstream from the endogenous Met receptor (Fig. 3 and 4), suggesting that overexpressed Gab1 ΔSHP-2 proteins function as dominant inhibitory proteins by competing with endogenous Gab1 proteins. Importantly, the overexpression of a catalytically inactive SHP-2 C/S mutant protein inhibits tubulogenesis by the Met receptor in the presence of wild-type Gab1, implicating SHP-2 catalytic activity in the morphogenic program (Fig. 8).
The ability of HGF to promote branching tubulogenesis correlates with the sustained phosphorylation of Gab1 and the prolonged activation of signaling pathways such as Erk (28, 38). The inhibition of MEK with a pharmacological agent, PD98059, inhibits tubulogenesis following HGF stimulation in MDCK cells (28), suggesting that the tubulogenic response in MDCK cells requires Erk activation. However, it remained to be determined how the Met receptor regulated sustained Erk activity. We have shown that the interaction of Gab1 with SHP-2 is required for the sustained Erk activity in response to HGF (180 min), whereas epithelial cells that express Gab1 proteins which fail to associate with SHP-2 display transient activation of Erk (15 min; Fig. 7). Importantly, we show that the kinetics of phosphorylation of the Gab1 ΔSHP-2 mutant protein is similar to that observed with wild-type Gab1 (Fig. 7). Moreover, the Gab1 ΔSHP-2 protein associated with the p85 subunit of Pl3K to a similar extent as wild-type Gab1, and activation of Akt is observed in cells expressing Gab1 ΔSHP-2 or wild-type Gab1 proteins with similar kinetics (Fig. 7). Hence, mutation of the SHP-2 binding site of Gab1 did not affect the coupling of Gab1 with the Pl3K signaling pathway, yet resulted in attenuation of the Erk pathway downstream from Met.
While this paper was in preparation, a role for Gab1 in the activation of Erk downstream from the EGF receptor was suggested using SHP-2 exon 3−/− fibroblasts (64). However, a Gab1 ΔSHP2 mutant protein was not evaluated (64). Since mutation of the SHP-2 binding site within the Gab2 multisubstrate docking protein did not abrogate Erk activation stimulated through the IL-3 receptor (16), this raised the possibility that Gab1-SHP-2 interactions were not required for Erk activity. Our data provide the first direct evidence that the recruitment of SHP-2 to Gab1 is important for Erk activation. This supports data from Drosophila indicating that the interaction of corkscrew with DOS, a docking protein related to Gab1, is required for positive regulation of Erk (1). Moreover, in a manner similar to that of Gab1, mutation of the SHP-2 binding site on FRS2 compromises activation of Erk and neurite outgrowth in PC12 cells following stimulation with FGF (16), implying an important role for docking proteins in modulating Erk activity dependent on SHP-2.
A positive role for SHP-2 as a regulator of the Erk pathway has been proposed based on the observation that SHP-2 is phosphorylated on tyrosine residues in response to PDGF. This provides a binding site for the Grb2 adapter protein and hence the ability to form a SHP-2/Grb2/SOS complex with potential to activate the Ras pathway (5). However, the overexpression of a SHP-2 mutant that fails to bind the Grb2 adapter protein has not been shown to alter Erk activation downstream from several receptor tyrosine kinases, suggesting that this is unlikely to be a significant mechanism through which SHP-2 can regulate Erk activity (4, 69). However, since SHP-2 is phosphorylated following activation of the Met receptor (Fig. 2), and reduced Grb2 is recruited to the Gab1 ΔSHP2 mutant, we cannot exclude the possibility that SHP-2 links Met at least in part to Grb2/SOS/Ras.
The phosphatase activity of SHP-2 is required for Erk activation both in mammalian systems and in lower organisms, such as Drosophila and Xenopus embryos, suggesting that the dephosphorylation of proteins by SHP-2 may be critical for the onset of signaling pathways leading to Erk activation (1, 2, 4, 69). Consistent with these observations, we show that the overexpression of a catalytically inactive SHP-2 mutant abrogates sustained Erk activation downstream from the Met receptor tyrosine kinase (Fig. 8). Importantly, the overexpression of the catalytically inactive SHP-2 C/S mutant also inhibits branching tubulogenesis, indicating that the phosphatase activity of SHP-2 is required for the ability of Met-Gab1 to induce branching tubulogenesis in epithelial cells (Fig. 8).
The Drosophila homologue of SHP-2, Csw, dephosphorylates a tyrosine residue on the Torso receptor tyrosine kinase that binds to p120 Ras GAP, thus uncoupling a negative regulator of Ras from the Torso receptor (7). The dephosphorylation of the Gab1-related docking protein DOS by Csw has also been implicated in linking the receptor tyrosine kinase Sevenless to the Ras pathway (18, 19). Similarly, in cells overexpressing SHP-2, Gab1 can also act as a substrate for SHP-2 following phosphorylation by the Met receptor (Fig. 2) and following activation of the EGF receptor (64). This supports the observation that in response to IL-6, Gab1 is an in vitro substrate for GST fusion protein containing the SHP-2 phosphatase domain (45). Since we have shown that Gab1 can associate with the SH2 domain of p120 RasGAP, it is possible that SHP-2 dephosphorylates tyrosine residues important for the interaction of p120 RasGAP with Gab1. However, either in transient overexpression assays (Fig. 6) or in stable epithelial cell lines expressing Gab1 ΔSHP-2 mutant proteins (not shown), the ability of Gab1 to associate with a p120 RasGAP SH2 domain fusion protein is not altered. Thus, under these conditions, the binding site for p120 RasGAP on Gab1 is not dephosphorylated by SHP-2.
We have shown that either the failure to recruit the SHP-2 phosphatase to Gab1 or the overexpression of a catalytically inactive SHP-2 phosphatase results in improper epithelial organization in response to Met activation. This is consistent with studies of SHP-2 exon 3−/− mutant mice, where gastrulation is interrupted due to inappropriate mesodermal cell migration and organization (59). Moreover, expression of a dominant negative SHP-2 mutant inhibits elongation of Xenopus animal caps in response to FGF, a process that involves the reorganization of existing cells (69). Epithelial morphogenesis requires both cell proliferation and the remodeling of epithelial junctions (50). MEK-Erk activity is necessary for the breakdown of adherens junctions in response to HGF (52). Thus, the transient activation of Erk in cells expressing the Gab1 ΔSHP-2 or SHP-2 C/S mutant may be insufficient for the remodeling of adherens junctions and for the cell proliferation required for epithelial morphogenesis. Both Gab1 and the Met receptor are localized to the basolateral membranes of polarized epithelial cells in the vicinity of adherens junctions, and this localization of Gab1 is important for its ability to rescue branching tubulogenesis in cells expressing Met receptor mutants (38). In a manner similar to that of wild-type Gab1, the Gab1 ΔSHP-2 protein is localized to sites of cell-cell contact (38), demonstrating that association with SHP-2 is not essential for Gab1 subcellular localization (Fig. 5). However, the inability of SHP-2 to be recruited to Gab1 may itself result in the failure of SHP-2 to colocalize with Gab1 in the proximity of critical membrane-associated substrates.
Fibroblasts isolated from SHP-2 exon 3−/− mice have increased numbers of focal adhesions and actin aggregation at the cell periphery, suggesting that SHP-2 could also play a role in the regulation of cell spreading and migration on extracellular matrix (ECM) (46, 79). A class of adhesion proteins, the signal-regulatory proteins, including SHP substrate 1 and the distantly related PECAM and PIR-B/p91A proteins, are SHP-2 binding proteins and may serve as substrates for SHP-2 (13, 27). A role for SHP-2 in regulating SHP substrate 1 function and integrin signaling has been proposed, where the catalytic activity of SHP-2 is required for Erk activation downstream from cell-ECM interactions (46). Hence SHP-2 may modify cell-ECM-dependent Erk signals required for branching morphogenesis. The determination of substrates dephosphorylated by SHP-2 that modify epithelial tubulogenesis downstream from the Met receptor will provide a better understanding of how these processes are normally controlled and which events lead to loss of epithelial organization during tumorigenesis.
ACKNOWLEDGMENTS
This research was supported by an operating grant from the National Cancer Institute of Canada with funds from the Canadian Cancer Society (to M.P.), an American Cancer Society grant, and National Institutes of Health grants NS34514 and CA69495 (to A.J.W.), with a fellowship from the Medical Research Council (to C.M.) and a fellowship from the Ministerio de Educacion y Ciencia of Spain (to M.H.-M.). M.P. is a Scientist of the Medical Research Council of Canada.
We are grateful to G. F. Vande Woude for HGF, the Genetics Institute for recombinant CSF-1, T. Pawson for anti-p85, G. S. Feng for anti-SHP2, S. Ali for the vector encoding wild-type SHP-2, A. Saltiel for the vector encoding SHP-2 C/S, S. Sadekova for help in confocal microscopy, and members of the Park laboratory for helpful comments.
REFERENCES
- 1.Allard J D, Chang H C, Herbst R, McNeill H, Simon M A. The SH2-containing tyrosine phosphatase corkscrew is required during signaling by sevenless, Ras and Raf. Development. 1996;122:1137–1146. doi: 10.1242/dev.122.4.1137. [DOI] [PubMed] [Google Scholar]
- 2.Allard J D, Herbst R, Carroll P M, Simon M A. Mutational analysis of the SRC homology 2 domain protein-tyrosine phosphatase Corkscrew. J Biol Chem. 1998;273:13129–13135. doi: 10.1074/jbc.273.21.13129. [DOI] [PubMed] [Google Scholar]
- 3.Barford D, Neel B G. Revealing mechanisms for SH2 domain-mediated regulation of the protein tyrosine phosphatase SHP-2. Structure. 1998;6:249–254. doi: 10.1016/s0969-2126(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 4.Bennett A M, Hausdorff S F, O'Reilly A M, Freeman R M J, Neel B G. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett A M, Tang T L, Sugimoto S, Walsh C T, Neel B G. Protein tyrosine phosphatase SHPTP2 couples platelet-derived growth factor receptor β to Ras. Proc Natl Acad Sci USA. 1994;91:7335–7339. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpino N, Wisniewski D, Strife A, Marshak D, Kobayashi R, Stillman B, Clarkson B. p62(dok): a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell. 1997;88:197–204. doi: 10.1016/s0092-8674(00)81840-1. [DOI] [PubMed] [Google Scholar]
- 7.Cleghon V, Feldmann P, Ghigilone C, Copeland T D, Perrimon N, Hughes D A, Morrison D K. Opposing actions of CSW and RasGAP modulate the strength of Torso RTK signaling in the Drosophila terminal pathway. Mol Cell. 1998;2:719–727. doi: 10.1016/s1097-2765(00)80287-7. [DOI] [PubMed] [Google Scholar]
- 8.Di Renzo M F, Narsimhan R P, Olivero M, Bretti S, Giordano S, Medico E, Gaglia P, Zara P, Comoglio P M. Expression of the Met/HGF receptor in normal and neoplastic human tissues. Oncogene. 1991;6:1997–2003. [PubMed] [Google Scholar]
- 9.Ebens A, Brose K, Leonardo E D, Hanson M G J, Bladt F, Birchmeier C, Barres B A, Tessler-Lavigne M. Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron. 1996;17:1157–1172. doi: 10.1016/s0896-6273(00)80247-0. [DOI] [PubMed] [Google Scholar]
- 10.Fixman E D, Fournier T M, Kamikura D M, Naujokas M A, Park M. Pathways downstream of Shc and Grb2 are required for cell transformation by the Tpr-Met oncoprotein. J Biol Chem. 1996;271:13116–13122. doi: 10.1074/jbc.271.22.13116. [DOI] [PubMed] [Google Scholar]
- 11.Fixman E D, Holgado-Madruga M, Nguyen L, Kamikura D M, Fournier T M, Wong A J, Park M. Efficient cellular transformation by the Met oncoprotein requires a functional Grb2 binding site and correlates with phosphorylation of the Grb2-associated proteins Cbl and Gab1. J Biol Chem. 1997;272:20167–20172. doi: 10.1074/jbc.272.32.20167. [DOI] [PubMed] [Google Scholar]
- 12.Fournier T M, Kamikura D, Teng K, Park M. Branching tubulogenesis, but not scatter of Madin-Darby canine kidney cells, requires a functional Grb2 binding site in the Met receptor tyrosine kinase. J Biol Chem. 1996;271:22211–22217. doi: 10.1074/jbc.271.36.22211. [DOI] [PubMed] [Google Scholar]
- 13.Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada T, Kasuga M. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16:6887–6899. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Guzman M, Dolfi F, Zeh K, Vuori K. Met-induced JNK activation is mediated by the adapter protein Crk and correlates with the Gab1-Crk signaling complex formation. Oncogene. 1999;18:7775–7786. doi: 10.1038/sj.onc.1203198. [DOI] [PubMed] [Google Scholar]
- 15.Gherardi E, Gray J, Stoker M, Perryman M, Furlong R. Purification of scatter factor, a fibroblast-derived basic protein that modulates epithelial interactions and movement. Proc Natl Acad Sci USA. 1989;86:5844–5848. doi: 10.1073/pnas.86.15.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu H, Pratt J C, Burakoff S J, Neel B G. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol Cell. 1998;6:729–740. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- 17.Harlan J E, Hajduk P J, Yoon H S, Fesik S W. Pleckstrin homology domains bind to phosphatidylinositol 4,5-bisphosphate. Nature. 1994;371:168–170. doi: 10.1038/371168a0. [DOI] [PubMed] [Google Scholar]
- 18.Herbst R, Caroll P M, Allard J D, Schilling J, Raabe T, Simon M A. Daughter of sevenless is a substrate of the phosphotyrosine phosphatase Corkscrew and functions during sevenless signaling. Cell. 1996;85:899–909. doi: 10.1016/s0092-8674(00)81273-8. [DOI] [PubMed] [Google Scholar]
- 19.Herbst R, Zhang X, Qin J, Simon M A. Recruitment of the protein phosphatase CSW by DOS is an essential step during signaling by the sevenless receptor tyrosine kinase. EMBO J. 1999;18:6950–6961. doi: 10.1093/emboj/18.24.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hof P, Pluskey S, Dhe-Paganon S, Eck M J, Shoelson S E. Crystal structure of the SH2 domain phosphatase SHP-2. Cell. 1998;98:441–450. doi: 10.1016/s0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 21.Holgado-Madruga M, Emlet D R, Moscatello D K, Godwin A K, Wong A J. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 22.Holgado-Madruga M, Moscatello D K, Emlet D R, Dieterich R, Wong A J. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc Natl Acad Sci USA. 1997;94:12419–12424. doi: 10.1073/pnas.94.23.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingham R J, Holgado-Madruga M, Siu C, Wong A J, Gold M R. The Gab1 protein is a docking site for multiple proteins involved in signaling by the B cell antigen receptor. J Biol Chem. 1998;273:30630–30637. doi: 10.1074/jbc.273.46.30630. [DOI] [PubMed] [Google Scholar]
- 24.Jeffers M, Koochekpour S, Fiscella M, Sathyanarayana B K, Vande Woude G F. Signaling requirements for oncogenic forms of the Met tyrosine kinase receptor. Oncogene. 1998;17:2691–2700. doi: 10.1038/sj.onc.1202209. [DOI] [PubMed] [Google Scholar]
- 25.Kan M, Zhang G H, Zarnegar R, Michalopoulos G, Myoken Y, McKeehan W L, Stevens J I. Hepatocyte growth factor/hepatopoietin A stimulates the growth of rat kidney proximal tubule epithelial cells (RPTE), rat nonparenchymal liver cells, human melanoma cells, mouse keratinocytes and stimulates anchorage-independent growth of SV-40 transformed RPTE. Biochem Biophys Res Commun. 1991;174:331–337. doi: 10.1016/0006-291x(91)90524-b. [DOI] [PubMed] [Google Scholar]
- 26.Kazlauskas A, Feng G-S, Pawson T, Vallus M. The 64-kDa protein that associates with the platelet-derived growth factor receptor β subunit via Tyr-1009 is the SH2-containing phosphotyrosine phosphatase Syp. Proc Natl Acad Sci USA. 1993;90:6939–6942. doi: 10.1073/pnas.90.15.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signaling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 28.Khwaja A, Lehmann K, Marte B, Downward J. Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J Biol Chem. 1998;273:18793–18801. doi: 10.1074/jbc.273.30.18793. [DOI] [PubMed] [Google Scholar]
- 29.Komada M, Kitamura N. The cell dissociation and motility triggered by scatter factor/hepatocyte growth factor are mediated through the cytoplasmic domain of the c-Met receptor. Oncogene. 1993;8:2381–2390. [PubMed] [Google Scholar]
- 30.Komada M, Miyazawa K, Ishii T, Kitamura N. Characterization of hepatocyte-growth-factor receptors on Meth A cells. Eur J Biochem. 1991;204:857–864. doi: 10.1111/j.1432-1033.1992.tb16705.x. [DOI] [PubMed] [Google Scholar]
- 31.Kouhara H, Hadari Y R, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell. 1997;89:693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- 32.Lechleider R J, Sugimoto S, Bennett A M, Kashishian A S, Cooper J A, Shoelson S E, Walsh C T, Neel B G. Activation of the SH2-containing phosphotyrosine phosphatase SH-PTP2 by its binding site, phosphotyrosine 1009, on the human platelet-derived growth factor receptor b. J Biol Chem. 1993;268:21478–21481. [PubMed] [Google Scholar]
- 33.Lehr S, Kotzka J, Herkner A, Klein E, Siethoff C, Knebel B, Noelle V, Brüning J C, Klein H W, Meyer H E, Krone W, Müller-Wieland D. Identification of tyrosine phosphorylation sites in human Gab-1 protein by EGF receptor kinase in vitro. Biochemistry. 1999;38:151–159. doi: 10.1021/bi9818265. [DOI] [PubMed] [Google Scholar]
- 34.Lemmon M A, Falasca M, Ferguson K M, Schlessinger J. Regulatory recruitment of signaling molecules to the cell membrane by pleckstrin homology domains. Trends Cell Biol. 1997;7:237–242. doi: 10.1016/S0962-8924(97)01065-9. [DOI] [PubMed] [Google Scholar]
- 35.Lemmon M A, Ferguson K M, Schlessinger J. PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]
- 36.Liu C, Park M, Tsao S. Over-expression of met protooncogene but not epidermal growth factor receptor or c-erb2 in primary human colorectal carcinomas. Oncogene. 1992;7:181–185. [PubMed] [Google Scholar]
- 37.Maina F, Hilton M C, Ponzetto C, Davies A M, Klein R. Met receptor signaling is required for sensory nerve development and HGF promotes axonal growth and survival of sensory neurones. Genes Dev. 1997;11:3341–3350. doi: 10.1101/gad.11.24.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maroun C R, Holgado-Madruga M, Royal I, Naujokas M A, Fournier T M, Wong A J, Park M. The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the Met receptor tyrosine kinase. Mol Cell Biol. 1999;19:1784–1799. doi: 10.1128/mcb.19.3.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maroun C R, Moscatello D K, Naujokas M A, Holgado-Madruga M, Wong A J, Park M. A conserved inositol phospholipid binding site within the pleckstrin homology domain of the Gab1 docking protein is required for epithelial morphogenesis. J Biol Chem. 1999;274:31719–31726. doi: 10.1074/jbc.274.44.31719. [DOI] [PubMed] [Google Scholar]
- 40.Milarski K L, Saltiel A R. Expression of catalytically inactive Syp phosphatase in 3T3 cells blocks stimulation of mitogen-activated protein kinase by insulin. J Biol Chem. 1994;33:21239–21243. [PubMed] [Google Scholar]
- 41.Montesano R, Schaller G, Orci L. Induction of epithelial tubular morphogenesis in vitro by fibroblast-derived soluble factors. Cell. 1991;66:697–711. doi: 10.1016/0092-8674(91)90115-f. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura T. Structure and function of hepatocyte growth factor. Prog Growth Factor Res. 1991;3:67–85. doi: 10.1016/0955-2235(91)90014-u. [DOI] [PubMed] [Google Scholar]
- 43.Neel B G, Tonks N K. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen L, Holgado-Madruga M, Maroun C, Fixman E D, Kamikura D, Fournier T, Charest A, Tremblay M L, Wong A J, Park M. Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J Biol Chem. 1997;272:20811–20819. doi: 10.1074/jbc.272.33.20811. [DOI] [PubMed] [Google Scholar]
- 45.Nishida K, Yoshida Y, Itoh M, Fukada T, Ohtani T, Shirogane T, Atsumi T, Takahashi-Tezuka M, Ishihara K H, Hibi M, Hirano T. Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B- cell antigen receptors. Blood. 1999;93:1809–1816. [PubMed] [Google Scholar]
- 46.Oh E-S, Gu H, Saxton T M, Timms J F, Hausdorff S, Frevert E U, Kahn B B, Pawson T, Neel B G, Thomas S M. Regulation of early events in integrin signaling by protein tyrosine phosphatase SHP-2. Mol Cell Biol. 1999;19:3205–3215. doi: 10.1128/mcb.19.4.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Reilly A M, Neel B G. Structural determinants of SHP-2 function and specificity in Xenopus mesoderm induction. Mol Cell Biol. 1998;18:161–177. doi: 10.1128/mcb.18.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park C Y, Hayman M J. The tyrosines in the bidentate motif of the env-sea oncoprotein are essential for cell transformation and are binding sites for Grb2 and the tyrosine phosphatase SHP-2. J Biol Chem. 1999;274:7583–7590. doi: 10.1074/jbc.274.11.7583. [DOI] [PubMed] [Google Scholar]
- 49.Pluskey S, Wandless T J, Walsh C T, Shoelson S E. Potent stimulation of SH-PTP2 phosphatase activity by simultaneous occupancy of both SH2 domains. J Biol Chem. 1995;270:2897–2900. doi: 10.1074/jbc.270.7.2897. [DOI] [PubMed] [Google Scholar]
- 50.Pollack A L, Runyan R B, Mostov K E. Morphogenic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev Biol. 1998;204:64–79. doi: 10.1006/dbio.1998.9091. [DOI] [PubMed] [Google Scholar]
- 51.Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio P M. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 52.Potempa S, Ridley A J. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction assembly. Mol Biol Cell. 1998;9:2185–2200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qu C-K, Yu W-M, Azzarelli B, Feng G-S. Genetic evidence that Shp-2 tyrosine phosphatase is a signal enhancer of the epidermal growth factor receptor in mammals. Proc Natl Acad Sci USA. 1999;96:8528–8533. doi: 10.1073/pnas.96.15.8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raabe T, Riesgo-Escovar J, Liu X, Bausenwein B S, Deak P, Maröy P, Hafen E. DOS, a novel pleckstrin homology domain-containing protein required for signal transduction between sevenless and RAS1 in Drosophila. Cell. 1996;85:911–920. doi: 10.1016/s0092-8674(00)81274-x. [DOI] [PubMed] [Google Scholar]
- 55.Rodrigues G A, Falasca M, Zhang Z, Ong S H, Schlessinger J. A novel feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol Cell Biol. 2000;20:1448–1459. doi: 10.1128/mcb.20.4.1448-1459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodrigues G A, Naujokas M A, Park M. Alternative splicing generates isoforms of the met receptor tyrosine kinase which undergo differential processing. Mol Cell Biol. 1991;11:2962–2970. doi: 10.1128/mcb.11.6.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rong S, Bodescot M, Blair D, Dunn J, Nakamura T, Mizuno K, Park M, Chan A, Aaronson S, Vande Woude G F. Tumorigenicity of the met proto-oncogene and the gene for hepatocyte growth factor. Mol Cell Biol. 1992;12:5152–5158. doi: 10.1128/mcb.12.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ronsin C, Muscatelli F, Mattei M G, Breathnach R. A novel putative receptor protein tyrosine kinase of the met family. Oncogene. 1993;8:1195–1202. [PubMed] [Google Scholar]
- 59.Saxton T M, Henkemeyer M, Gasca S, Shen R, Rossi D J, Shalaby F, Feng G-S, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase SHP-2. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 61.Schmidt L, Duh F M, Chen F, Kishida T, Glenn G, Choyke P, Scherer S W, Zhuang Z P, Lubensky I, Dean M, Allikmets R, Chimambaram A, Bergerheim U R, Feltis J T, Casadevall C, Zamarron A, Bernues M, Richard S, Lips C J, Walther M M, Tsui L C, Geil L, Orcutt M L, Stackhouse T, Lipan J, Slife L, Brauch H, Decker J, Niehans G, Hughson M D, Moch H, Storkel S, Lerman M I, Linehan W M, Zbar B. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 62.Shaw G. The pleckstrin homology domain: an intriguing multifunctional protein module. Bioessays. 1996;18:35–46. doi: 10.1002/bies.950180109. [DOI] [PubMed] [Google Scholar]
- 63.Shi Z-Q, Lu W, Feng G-S. The Shp-2 tyrosine phosphatase has opposite effects in mediating the activation of extracellular signal-regulated and c-Jun NH2-terminal mitogen-activated protein kinases. J Biol Chem. 1998;273:4904–4908. doi: 10.1074/jbc.273.9.4904. [DOI] [PubMed] [Google Scholar]
- 64.Shi Z-Q, Yu D-H, Park M, Marshall M, Feng G-S. Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol Cell Biol. 2000;20:1526–1536. doi: 10.1128/mcb.20.5.1526-1536.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soriano J V, Pepper M S, Nakamura T, Orci L, Montesano R. Hepatocyte growth factor stimulates extensive development of branching duct-like structures by cloned mammary gland epithelial cells. J Cell Sci. 1995;108:413–430. doi: 10.1242/jcs.108.2.413. [DOI] [PubMed] [Google Scholar]
- 66.Stein-Gerlach M, Wallasch C, Ullrich A. SHP-2, SH2-containing protein tyrosine phosphatase-2. Int J Biochem Cell Biol. 1998;30:559–566. doi: 10.1016/s1357-2725(98)00002-8. [DOI] [PubMed] [Google Scholar]
- 67.Stoker M, Gherardi E. Regulation of cell movement: the motogenic cytokines. Biochim Biophys Acta. 1991;1072:81–102. doi: 10.1016/0304-419x(91)90008-9. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi-Tezuka M, Yoshida Y, Fukada T, Ohtani T, Yamanaka Y, Nishida K, Nakajima K, Hibi M, Hirano T. Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol Cell Biol. 1998;18:4109–4117. doi: 10.1128/mcb.18.7.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang T L, Freeman R M J, O'Reilly A M, Neel B G, Sokol S Y. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 70.Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- 71.Vogel W, Ullrich A. Multiple in vivo phosphorylated tyrosine phosphatase SHP-2 engages binding to Grb2 via tyrosine 584. Cell Growth Differ. 1996;7:1589–1597. [PubMed] [Google Scholar]
- 72.Weidner K M, Arakaki N, Hartmann G, Vandekerchkhove J, Weingart S, Rieder H, Fonatsch C, Tsubouchi H, Hishida T, Daikuhara Y, Birchmeier W. Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci USA. 1991;88:7001–7005. doi: 10.1073/pnas.88.16.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weidner K M, Di Cesare S, Sachs M, Brinkman V, Behrens J, Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 74.Weidner K M, Sachs M, Birchmeier W. The Met receptor tyrosine kinase transduces motility, proliferation, and morphogenic signals of scatter factor/hepatocyte growth factor in epithelial cells. J Cell Biol. 1993;121:145–154. doi: 10.1083/jcb.121.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.White M F, Yenush L. The IRS-signaling system: a network of docking proteins that mediate insulin and cytokine action. Curr Opin Genet Dev. 1998;8:112–126. doi: 10.1007/978-3-642-80481-6_8. [DOI] [PubMed] [Google Scholar]
- 76.Wigler M, Pellicer A, Silverstein S, Axel R, Urlaub G, Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci USA. 1979;76:1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang X M, Park M. Expression of the met/hepatocyte growth factor/scatter factor receptor and its ligand during differentiation of murine P19 embryonal carcinoma cells. Dev Biol. 1993;157:308–320. doi: 10.1006/dbio.1993.1137. [DOI] [PubMed] [Google Scholar]
- 78.Yenush L, White M F. The IRS-signalling system during insulin and cytokine action. Bioessays. 1997;19:491–500. doi: 10.1002/bies.950190608. [DOI] [PubMed] [Google Scholar]
- 79.Yu D-H, Qu C-K, Henegariu O, Lu X, Feng G-S. Protein-tyrosine phosphatase SHP-2 regulates cell spreading, migration and focal adhesion. J Biol Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- 80.Zarnegar R, Michalopoulos G. Purification and biological characterization of human hepatopoietin A, a polypeptide growth factor for hepatocytes. Cancer Res. 1989;49:3314–3320. [PubMed] [Google Scholar]
- 81.Zhao C, Yu D H, Shen R, Feng G-S. Gab2, a new pleckstrin homology domain-containing adaptor protein, acts to uncouple signaling from ERK to Elk-1. J Biol Chem. 1999;274:19649–19654. doi: 10.1074/jbc.274.28.19649. [DOI] [PubMed] [Google Scholar]
- 82.Zhu H, Naujokas M A, Fixman E D, Torossian K, Park M. Tyrosine 1356 in the carboxyl-terminal tail of the HGF/SF receptor is essential for the transduction of signals for cell motility and morphogenesis. J Biol Chem. 1994;269:29943–29948. [PubMed] [Google Scholar]
- 83.Zhu H, Naujokas M A, Park M. Receptor chimeras indicate that the Met tyrosine kinase mediates the motility and morphogenic responses of hepatocyte growth/scatter factor. Cell Growth Differ. 1994;5:359–366. [PubMed] [Google Scholar]