Abstract
Colistin is a last-resort antimicrobial agent for treating carbapenem-resistant Acinetobacter baumannii infections. The activation of PmrAB by several environmental signals induces colistin resistance in Gram-negative bacteria. This study investigated the molecular mechanisms of colistin resistance in A. baumannii under acidic conditions using wild-type (WT) A. baumannii 17978, ΔpmrA and ΔpmrB mutants, and pmrA-complemented strains. The pmrA or pmrB deletion did not affect the growth of A. baumannii under acidic or aerobic conditions. A. baumannii under acidic (pH 5.5) and high-iron (1 mM) conditions showed 32- and 8-fold increases in the minimum inhibitory concentrations (MICs) of colistin, respectively. The ΔpmrA and ΔpmrB mutants at pH 5.5 showed a significant decrease in colistin MICs compared to the WT strain at pH 5.5. No difference in colistin MICs was observed between WT and mutant strains under high-iron conditions. The pmrCAB expression significantly increased in the WT strain at pH 5.5 compared to the WT strain at pH 7.0. The pmrC expression significantly decreased in two mutant strains at pH 5.5 compared to the WT strain at pH 5.5. The PmrA protein was expressed in the ΔpmrA strain carrying ppmrA_FLAG plasmids at pH 5.5 but not at pH 7.0. Lipid A modification by the addition of phosphoethanolamine was observed in the WT strain at pH 5.5. In conclusion, this study demonstrated that A. baumannii under acidic conditions induces colistin resistance via the activation of pmrCAB operon and subsequent lipid A modification.
Keywords: PmrAB, Acinetobacter baumannii, lipopolysaccharide, lipid A, colistin
1. Introduction
Acinetobacter baumannii is a Gram-negative, non-fermenting pathogen that causes various nosocomial infections, especially in severely ill patients admitted to intensive care units. This microorganism is a low-virulent pathogen but poses a global threat to human health through antimicrobial resistance [1,2]. The World Health Organization declared carbapenem-resistant A. baumannii as the first priority pathogen for which the development of new antimicrobial agents is urgently needed [3]. Due to the prevalence of multidrug-resistant (MDR) and carbapenem-resistant A. baumannii strains, colistin is one of the last therapeutic options to treat MDR A. baumannii infections [4]. However, colistin-resistant A. baumannii has been increasingly reported worldwide [5,6]. The complete loss of lipopolysaccharide (LPS) by mutations in enzymes involved in the lipid A biosynthetic pathway (lpxA, lpxC, and lpxD) and LPS modification by mutations in pmrAB are the two main mechanisms of colistin resistance in A. baumannii [7,8,9].
Bacteria have several signaling mechanisms for adapting to various environmental conditions. The two-component system (TCS) is an important mediator of signal transduction by which bacteria sense and respond to environmental stimuli. TCS plays a central role in bacterial adaptation responses that control antimicrobial resistance, cell-to-cell communication, pathogenesis, and bacterial survival [10,11]. Genome analysis of clinical A. baumannii isolates revealed approximately 20 genes encoding TCS [12]. Of the well-characterized proteins of TCS in A. baumannii, PmrAB is associated with resistance to polymyxins by regulating genes involved with lipid A modification [13]. PmrAB consists of a sensor kinase, PmrB, and a response regulator, PmrA, encoded by the pmrCAB operon [14]. The pmrAB activation leads to the upregulation of pmrC (also called eptA), naxD, and arnBCADTEF-pmrE operon (also called pmrHFIJKLM-ugd), encoding phosphoethanolamine transferase, N-acetylgalactosamine deacetylase (GalNAc), and 4-amino-4-deoxy-L-arabinose (L-Ara4N) transferase, respectively [12,13,14]. PhoPQ TCS activates pmrAB indirectly through pmrD, but the PhoPQ and L-Ara4N biosynthetic pathways are absent in A. baumannii [14,15]. PmrAB is activated by several environmental signals such as acidic pH, high iron, or low magnesium levels, leading to the induction of resistance to polymyxins through lipid A modification by pmrC expression in Gram-negative bacteria, including Salmonella enterica, Escherichia coli, and Klebsiella pneumoniae [16,17,18,19]. Adams et al. [13] showed that colistin susceptibility decreased in A. baumannii under low pH or iron supplementation. However, little is known about the induction of colistin resistance associated with the pmrCAB operon in A. baumannii exposed to different environmental factors. As A. baumannii has to adapt to and survive in harsh environments during infection, it is important to understand the regulatory mechanisms of colistin resistance in A. baumannii exposed to various environmental conditions. This study investigated the molecular mechanisms of colistin resistance by activating the pmrCAB operon in A. baumannii under acidic conditions.
2. Results
2.1. Construction of ΔpmrA and ΔpmrB Mutants and pmrA-Complemented Strains
To investigate whether the induction of colistin resistance in A. baumannii exposed to different environmental conditions was mediated by PmrAB TCS, ΔpmrA (HJ2751D) and ΔpmrB (HJ2750D) mutant strains were constructed using the wild-type (WT) A. baumannii ATCC 17978 (Supplementary Figure S1A,B). The pmrA-complemented strain (HJ2751C) was constructed by introducing the pWH1266 plasmid carrying the ompA promoter, pmrA gene, and T1 terminator into the HJ2751D strain (Supplementary Figure S1C). The ompA promoter was inserted in the pWH1266 plasmid for high pmrA gene expression. The deletion and complementation of genes were confirmed by polymerase chain reaction (PCR) analysis. The expected amplicon sizes of 3444, 2775, 2115, and 1422 bp were observed in WT, HJ2751D, HJ2750D, and HJ2751C strains, respectively (Supplementary Figure S1D).
2.2. Effects of pmrAB on the Growth of A. baumannii
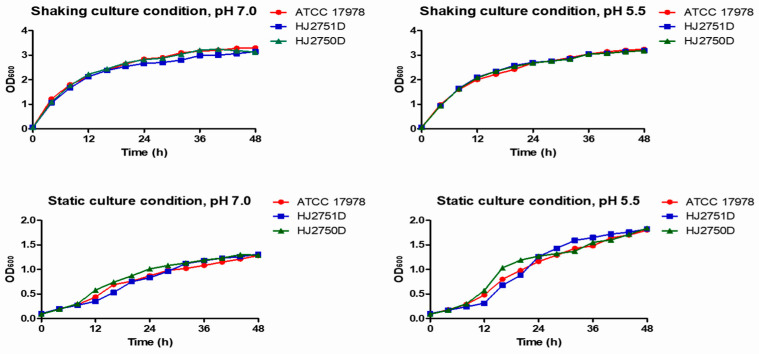
To investigate whether pmrAB could affect the growth of A. baumannii, bacterial growth was monitored in WT, HJ2751D, and HJ2750D strains under static or shaking conditions. Bacterial strains were grown in lysogeny broth (LB), where the initial pH of the culture medium was 7.0 and 5.5 separately. Here, pH 7.0 was selected as a control for acidic pH. No significant difference in bacterial growth was observed among WT, HJ2751D, and HJ2750D strains at pH 7.0 or 5.5 (Figure 1). These results suggest that PmrAB does not affect the growth of A. baumannii under acidic pH or aerobic conditions.
Figure 1.
Growth of A. baumannii strains under different culture conditions. A. baumannii ATCC 17978, HJ2751D, and HJ2750D strains were grown in LB with the initial pH of 7.0 or 5.5 under shaking or static conditions. Bacterial growth was measured at the indicated time points. Data are representative of two experiments with similar results.
2.3. Induction of Colistin Resistance in A. baumannii under Acidic or High-Iron Conditions
To determine the effect of pH on the induction of colistin resistance in A. baumannii, four A. baumannii strains (WT, HJ2751D, HJ2750D, and HJ2751C) were grown in cation-adjusted Mueller–Hinton broth (CAMHB) of different pH levels (pH 7.0, 6.5, 5.5, and 4.5). The minimum inhibitory concentrations (MICs) of colistin were 1 μg/mL in WT, HJ2751D, and HJ2750D strains at pH 7.0 and 6.5, whereas the WT strain grown at pH 5.5 showed a dramatic increase in colistin MIC (32 μg/mL) (Table 1). However, colistin MICs in HJ2751D and HJ2750D at pH 5.5 increased only 2-fold compared to those in strains at pH 7.0. No growth was observed in A. baumannii strains grown at pH 4.5. The pmrA complementation in the HJ2751D strain restored the colistin MIC (256 μg/mL) at pH 5.5. Next, to evaluate the effects of iron on the induction of colistin resistance, A. baumannii strains were grown in CAMHB supplemented with 100 μM, 500 μM, and 1 mM ferric chloride. With increasing ferric chloride concentrations, colistin MICs of the WT strain at pH 7.0 increased from 1 to 8 μg/mL. However, colistin MICs were not different among WT, HJ2751D, HJ2750D, and HJ2751C strains at 500 μM or 1 mM ferric chloride. These results suggest that A. baumannii induces colistin resistance under acidic or high-iron conditions. PmrAB is responsible for the induction of colistin resistance in A. baumannii under acidic conditions, but colistin resistance in A. baumannii under high-iron conditions is independent of PmrAB.
Table 1.
Effects of pmrAB on the colistin susceptibility of A. baumannii strains under different culture conditions.
| Culture Conditions | Colistin MIC (μg/mL) | |||||
|---|---|---|---|---|---|---|
| ATCC 17978 | HJ2751D | HJ2750D | HJ2751C | |||
| CAMHB * | pH | pH 7.0 | 1 | 1 | 1 | 1 |
| pH 6.5 | 1 | 1 | 1 | 1 | ||
| pH 5.5 | 32 | 2 | 2 | 256 | ||
| pH 4.5 | - | - | - | - | ||
| CAMHB pH 7.0 |
Fe3+ | 100 μM FeCl3 | 1 | 2 | 2 | 2 |
| 500 μM FeCl3 | 4 | 4 | 4 | 4 | ||
| 1 mM FeCl3 | 8 | 8 | 8 | 8 | ||
| CAMHB pH 5.5 |
Fe3+ | 1 mM FeCl3 | 16 | 16 | 16 | 256 |
* CAMHB, cation-adjusted Mueller–Hinton broth.
2.4. High Expression of pmrCAB Operon and Production of PmrA in A. baumannii under Acidic Conditions
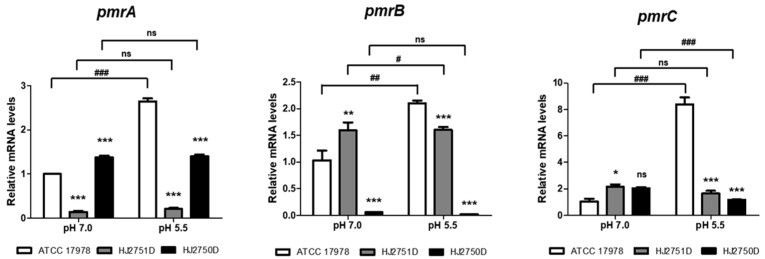
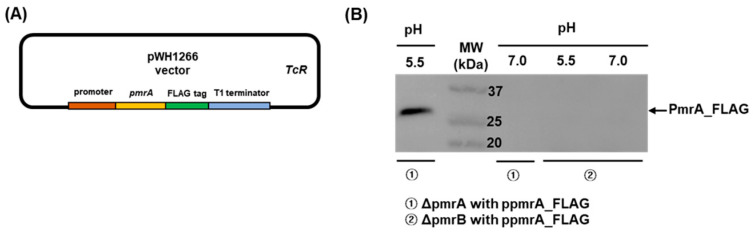
To investigate whether colistin resistance in A. baumannii under acidic conditions was directly mediated by PmrAB TCS, quantitative real-time PCR (qPCR) was performed to determine the expression of the pmrCAB operon in WT, HJ2751D, and HJ2750D strains grown at pH 7.0 and 5.5 individually. The WT strain at pH 5.5 exhibited a 2.6-fold increase in pmrA expression, a 2.1-fold increase in pmrB, and an 8.3-fold increase in pmrC compared to the WT strain at pH 7.0 (Figure 2). The pmrA and pmrB expressions increased in HJ2750D (ΔpmrB mutant) and HJ2751D (ΔpmrA mutant) strains at pH 7.0 or 5.5, respectively. The pmrC expression significantly increased in the HJ2751D strain at pH 7.0 compared to that in the WT strain at pH 7.0, but the colistin MICs of the two strains were the same (1 μg/mL). The pmrA, pmrB, and pmrC expressions in the HJ2750D and HJ2751D mutant strains at pH 5.5 significantly decreased compared to those in the WT strain at pH 5.5. Next, to determine whether the production of response regulator PmrA increased in A. baumannii under acidic conditions due to high pmrA expression, western blot analysis was performed in HJ2751D and HJ2750D strains carrying ppmrA_FLAG plasmids. The ppmrA_FLAG plasmids carried the C-terminal FLAG-tagged pmrA with its promoter in the pWH1266 vector (Figure 3A). PmrA-FLAG-tagged proteins were only detected in the HJ2751D strain carrying ppmrA_FLAG plasmids at pH 5.5 but not at pH 7.0 (Figure 3B). However, they were not detected in the HJ2750D strain carrying ppmrA_FLAG plasmids at pH 5.5 or 7.0. These results suggest that pmrAB is upregulated in A. baumannii under acidic conditions, which induces pmrC upregulation.
Figure 2.
The expression of pmrCAB in A. baumannii strains cultured under different pH conditions. WT ATCC 17978, HJ2751D, and HJ2750D strains were grown in LB at pH 7.0 or 5.5 until OD600 reached 0.25. The expressions of pmrA, pmrB, and pmrC were determined by qPCR. The data are presented as mean ± SD of three independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001 compared to the WT strain. # p < 0.05, ## p < 0.01, ### p < 0.001 compared to bacteria grown at pH 7.0. ns, no statistically significant difference.
Figure 3.
The production of PmrA protein in the ΔpmrA mutant strain carrying ppmrA_FLAG plasmids in response to acidic conditions. (A) Schematic diagram of the construction of ppmrA_FLAG plasmids. (B) Western blot analysis of bacterial extracts prepared from ΔpmrA or ΔpmrB mutant strains carrying ppmrA_FLAG plasmids. Bacteria were grown in LB at pH 7.0 or 5.5. Equal amounts of bacterial proteins were run in 10% SDS-PAGE gel and PmrA-FLAG proteins were detected using a FLAG Epitope Tag monoclonal antibody.
2.5. Lipid A Modification in A. baumannii under Acidic Conditions
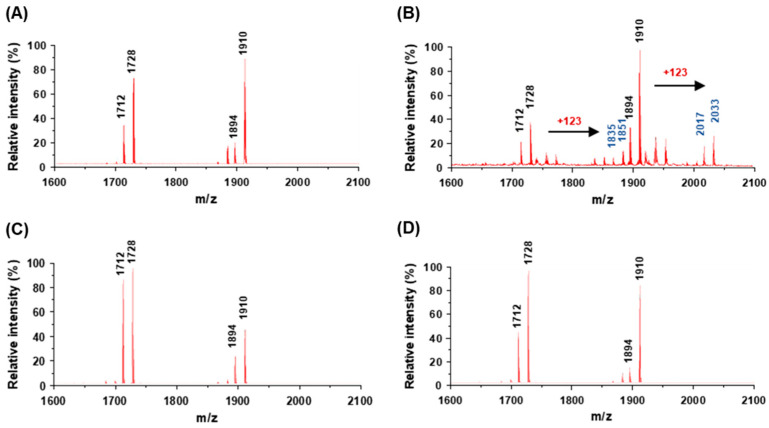
To determine whether A. baumannii under acidic conditions modified lipid A of LPS, matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) was performed. Lipid A of LPS was extracted from the WT strain grown at pH 7.0 or 5.5. Four major ion peaks, m/z 1712, 1728, 1894, and 1910, were observed in lipid A extracted from the WT strain at pH 7.0 (Figure 4A). The lipid A structure at m/z 1728 is a species of hexa-acylated, bis-phosphorylated lipid A with two phosphates and two 2-amino-2-deoxyglucose residues with three 12:0 (3-OH), two 14:0 (3-OH), and one 12:0 [20]. The lipid A structure at m/z 1910 is a species of hepta-acylated, bis-phosphorylated lipid A with two phosphates and two 2-amino-2-deoxyglucose residues with three 12:0 (3-OH), two 14:0 (3-OH), and two 12:0. The ion peaks with m/z 1712 and 1894 are hexa- and hepta-acylated bis-phosphorylated lipid A, where the hydroxyl group is eliminated (m/z −16) with substitution of one 14:0 (3-OH) with one 14:0. Lipid A from the WT strain at pH 5.5 showed four additional ion peaks with m/z 1835, 1851, 2017, and 2033 (Figure 4B). These additional ion peaks represented a shift of m/z +123 from the four main peaks, corresponding to the addition of a single phosphoethanolamine residue [9]. However, lipid A from the HJ2750D and HJ2751D mutant strains showed the same ion peaks as the WT strain (Figure 4C,D). These results suggest that A. baumannii under acidic conditions modifies lipid A by the addition of phosphoethanolamine, resulting in the induction of colistin resistance.
Figure 4.
MALDI/TOF MS analysis of lipid A isolated from A. baumannii strains grown under different pH conditions. (A) A. baumannii ATCC 17978 strain grown at pH 7.0. (B) A. baumannii ATCC 17978 strain grown at pH 5.5. (C) ΔpmrA mutant (HJ2751D) strain grown at pH 5.5. (D) ΔpmrB mutant (HJ2750D) strain grown at pH 5.5. Lipid A isolated from A. baumannii ATCC 17978 grown at pH 7.0 showed the prototype lipid A with m/z 1712, 1728, 1894, and 1910 in the spectrum. A. baumannii ATCC 17978 grown at pH 5.5 showed four additional peaks of a shift of m/z +123 from four main peaks, corresponding to the addition of a single phosphoethanolamine residue.
3. Discussion
This study investigated colistin resistance linked to PmrAB TCS in A. baumannii in response to acidic or high-iron conditions. Colistin resistance in A. baumannii under acidic conditions was induced by the upregulation of the pmrCAB operon and subsequent lipid A modification by the addition of phosphoethanolamine. Colistin resistance was also induced in A. baumannii under high-iron conditions, but it was independent of PmrAB.
Colistin is a last-resort drug for treating carbapenem-resistant A. baumannii infections [4]. The antibacterial activity of colistin occurs through the displacement of divalent cations from the phosphate groups of LPS [21]. The electrostatic interaction between the positively charged colistin and negatively charged phosphate groups of LPS stabilizes the complex. The insertion of colistin into the outer membrane induces membrane disruption and the leakage of intracellular contents, leading to bacterial death [22]. Colistin resistance is primarily mediated by the modification of lipid A-head groups to reduce the electrostatic interaction of colistin with LPS. The addition of positively charged residues, such as L-Ara4N and phosphoethanolamine, to lipid A decreases the net negative charge of lipid A to 0 and −1.5 to −1 on the bacterial surface, respectively [23]. LPS modification by PhoPQ or PmrAB activation through mutations or environmental signals leads to the overexpression of genes associated with lipid A modification [24]. Beceiro et al. [25] demonstrated that the development of colistin resistance in A. baumannii requires point mutations in pmrB, upregulation of pmrAB, and expression of pmrC, leading to the addition of phosphoethanolamine to lipid A. In addition, PmrAB TCS is activated by various environmental signals such as acidic pH, high iron levels, and low magnesium concentrations in Gram-negative bacteria [26,27,28]. Low magnesium conditions in A. baumannii slightly upregulate pmrA expression and lipid A modification by the addition of ion peaks with m/z 2034 in MALDI-TOF MS [25], suggesting that PmrAB is activated by low magnesium conditions in A. baumannii.
In this study, ferric chloride supplementation increased the colistin MICs against the WT A. baumannii strain in a dose-dependent manner. No difference in the colistin MICs was observed between WT and ΔpmrA or ΔpmrB mutant strains, suggesting no association of colistin resistance with PmrAB in A. baumannii under high-iron conditions. However, A. baumannii grown at pH 5.5 increased the colistin MIC (32-fold) compared to bacteria grown at pH 7.0. This result was consistent with the previous study that showed an increase in the colistin MIC of the A. baumannii ATCC 17978 strain grown at pH 5.5 [13]. This study demonstrated that the WT strain at pH 5.5 exhibited pmrB, pmrA, and pmrC upregulation compared to the WT strain at pH 7.0. However, the previous study did not show a significant difference in pmrA expression between A. baumannii at pH 7.7 and 5.5, although pmrA expression in A. baumannii at pH 5.5 slightly increased compared to that at pH 7.7 [13]. The difference between the two studies was medium pH and bacterial number for RNA extraction; bacteria were grown to an optical density at 600 nm (OD600) of 0.25 and 0.5 in this study and the previous study, respectively. This study demonstrated the production of PmrA proteins in the ΔpmrA mutant carrying pmrA at pH 5.5, but they were not detected in the same bacteria grown at pH 7.0 or the ΔpmrB mutant carrying pmrA at pH 7.0 or 5.5. These results suggest that acidic conditions induce the production of a response regulator, PmrA, and that a sensor kinase, PmrB, is essential to express PmrA proteins. Furthermore, the WT strain at pH 5.5 showed a significant increase in the pmrC expression compared to the WT strain at pH 7.0. A. baumannii ATCC 17978 grown at pH 5.5 induced the enzymatic activity of phosphoethanolamine transferase to modify lipid A, but the addition of phosphoethanolamine to lipid A was not observed in the ΔpmrA or ΔpmrB mutant strains at pH 5.5. PmrAB TCS also regulates naxD [29]. NaxD deacetylates N-acetylgalactosamine to galactosamine. This conversion step is required for the addition of galactosamine to lipid A. However, lipid A modification by the addition of galactosamine (m/z 2071) was not observed in the WT strain grown at pH 5.5.
In conclusion, this study demonstrated that A. baumannii upregulates pmrCAB expression and modifies lipid A under acidic conditions by the addition of phosphoethanolamine, leading to colistin resistance. Colistin resistance is also induced by high iron levels, but this phenomenon is independent of PmrAB in A. baumannii. This study provides insights into the molecular mechanisms of colistin resistance in A. baumannii in harsh environmental conditions such as acidic pH. As PmrAB also regulates virulence determinants responsible for pathogenicity [30,31,32], the complex regulatory network of A. baumannii in response to different environmental factors should be further investigated.
4. Materials and Methods
4.1. Bacterial Strains, Plasmids, and Growth Media
The bacterial strains and plasmids used in this study are listed in Table 2. Bacteria were cultured in LB (Bioshop, Burlington, ON, Canada) or LB agar plates. Bacterial culture media were adjusted to a desired pH with HCl (Duksan Pure Chemicals, Ansan, Republic of Korea) or NaOH (Duksan Pure Chemicals). MHB (Difco, Detroit, MI, USA) was used to determine the susceptibility of colistin (Sigma-Aldrich, St. Louis, MO, USA). Kanamycin (50 μg/mL), chloramphenicol (20 μg/mL), or tetracycline (10 μg/mL) were added to the growth medium to maintain plasmids in bacteria and select the mutant or complementary colonies.
Table 2.
Bacterial strains and plasmids used in this study.
| Bacteria/Plasmids | Relevant Characteristics * | Reference of Source |
|---|---|---|
| Bacterial strains | ||
| A. baumannii | ||
| ATCC 17978 | Wild-type strain | ATCC |
| HJ2751D | ∆pmrA of A. baumannii ATCC 17978 | This study |
| HJ2750D | ∆pmrB of A. baumannii ATCC 17978 | This study |
| HJ2751C | pmrA rescued in ∆pmrA with pWH1266 | This study |
| E. coli | ||
| DH5α pir (SY327 λ pir) | supE44 ΔlacU169 (Φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 λpir (phage lysogen); plasmid replication | Laboratory collection |
| sm10λ pir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu Km λpir π-requiring plasmids; conjugal donor | Laboratory collection |
| Plasmids | ||
| pOH4 | pHKD01 with the ompA coding region of A. baumannii ATCC 17978 under the control of its native promoter with nptI; KmR | Laboratory collection |
| pDM4 | Suicide vector, ori R6K; CmR; sacB | GenBank accession no. KC795686 |
| pWH1266 | Shuttle-vector with Acinetobacter and E. coli origin used for cloning vehicle; ampR; tetR | Laboratory collection |
| pHJ2751D | pDM4 with ∆pmrA::nptI; CmR, KmR | This study |
| pHJ2750D | pDM4 with ∆pmrB::nptI; CmR, KmR | This study |
| pHJ2751C | pWH1266 carrying pmrA with the ompA promoter and T1 terminator; TetR | This study |
| ppmrA_FLAG | pWH1266 carrying the FLGA-tagged pmrA coding region under the control of its native promoter with t1 terminator; TetR | This study |
* CmR, chloramphenicol resistant; KmR, kanamycin resistant; AmpR, ampicillin resistant; TetR, tetracycline resistant.
4.2. Construction of ΔpmrA and ΔpmrB Mutants and pmrA-Complemented Strains
ΔA1S_2751 (pmrA) and ΔA1S_2750 (pmrB) mutant strains were constructed using the WT A. baumannii ATCC 17978 by the markerless gene deletion method as previously described [33]. pmrA complementation in the HJ2751D strain was conducted using an overlap extension PCR. The specific PCR primers used to construct the mutant and complemented strains are listed in Supplementary Table S1. The construction of mutant and complemented strains is presented in Supplementary Figure S1 and Supplementary Materials and Methods in detail.
4.3. Construction of ppmrA_FLAG Plasmids
The pmrA coding region with a FLAG epitope sequence just before the stop codon, its promoter, and T1 terminator was amplified using primer sets of EcoRI_pmrAB_promoter (F)/Promoter_2751 (R), Promoter_2751 (F)/2751_FLAG (R), and FLAG_T1 (F)/T1_PstI (R), respectively (Supplementary Table S1). Three PCR products were assembled by an overlap extension PCR using EcoRI_pmrAB_promoter (F)/T1_PstI (R) primers. The PCR fragments were digested with EcoRI and PstI and introduced into the pWH1266 plasmids to construct ppmrA_FLAG plasmids.
4.4. Antimicrobial Susceptibility Testing
The MICs of colistin were determined by the broth microdilution method according to the guidelines of the Clinical Laboratory Standards Institute [34]. E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as quality control strains. Colistin susceptibility testing was performed in 96-well plates containing CAMHB supplemented with different concentrations of colistin. MICs were defined as the lowest colistin concentration that inhibits the visible growth of bacteria.
4.5. Bacterial Growth Curve
A. baumannii strains were grown in LB overnight at 37 °C and diluted to an OD600 of 1.0. The bacterial suspension was diluted to 1:20 in fresh LB, where the initial pH was 7.0 or 5.5, and cultured under shaking or static conditions for 48 h. Bacteria were sampled at 4 h intervals, and OD600 was measured using a spectrophotometer (Biochrom, Cambridge, UK). The bacterial growth assay was performed in two independent experiments.
4.6. Western Blot Analysis
The FLAG-tagged PmrA protein was detected by western blot analysis as previously described with some modifications [35]. Bacteria were grown in LB overnight at 37 °C. Cultured bacteria were inoculated into a fresh LB with pH 7.0 or 5.5, where the initial OD600 was 0.02, and cultured at 37 °C with shaking conditions. Bacteria were harvested at an OD600 of 0.25 and resuspended in a B-PER (Thermo Scientific, Waltham, MA, USA) containing an ethylenediaminetetraacetic acid-free protease inhibitor cocktail (GenDEPOT, Barker, TX, USA). After incubation for 15 min at room temperature, cell debris was removed by centrifugation. The protein concentration was determined using the Pierce™ BCA Protein Assay Kit (Thermo Scientific). Equal amounts of cell lysates were suspended in sodium dodecyl sulfate (SDS) sample buffer (250 mM Tris HCl (pH 6.8), 8% SDS, 0.02% bromophenol blue, glycerol, and β-mercaptoethanol), and the samples were run on a 10% polyacrylamide gel. The proteins were transferred onto a polyvinylidene fluoride membrane by electroblotting. The membrane was blocked with 5% skim milk in Tris-buffered saline with 0.05% Tween-20 (TBST) for 2 h. Membranes were incubated with a FLAG epitope-tagged (DYKDDDDK) monoclonal antibody (Thermo Scientific) at a dilution of 1:500 in TBST with 5% skim milk at 4 °C overnight. After washing with TBST, membranes were incubated with a horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibody (AbFrontier, Seoul, Republic of Korea) at a dilution of 1:2000 in TBST with 5% skim milk for 2 h. Chemiluminescent detection was performed using the SuperSignal West Femto (Thermo Scientific). Blotting images were captured using Fusion FX6 Edge (Vilber-Lourmat, Marne-la-Vallée, France).
4.7. Isolation of Lipid A and MALDI-TOF MS Analysis
A. baumannii strains were grown in LB overnight at 37 °C, and cultured bacteria were inoculated into a fresh LB with pH 7.0 or 5.5, where the initial OD600 was 0.02. Bacteria were grown under shaking conditions until OD600 reached 0.25. The bacterial cultures (100 mL) were centrifuged at 13,000 rpm for 10 min. LPS was extracted using the LPS extraction kit (iNtRON, Seongnam, Republic of Korea) according to the manufacturer’s instructions. Briefly, bacterial cells were lysed by the lysis buffer, and the samples were vortexed vigorously. After adding chloroform to cell lysates, the samples were vortexed vigorously and incubated for 5 min. The mixture was centrifugated at 13,000 rpm for 10 min at 4 °C, and the purification buffer was added to the supernatants. The mixtures were incubated for 30 min at −20 °C, centrifuged at 13,000 rpm for 15 min at 4 °C, and removed from the upper layer to obtain LPS pellets. LPS pellets were washed with 70% ethanol and centrifuged at 13,000 rpm for 3 min at 4 °C. The upper layer was discarded, and LPS pellets were dried using a speed vacuum. Lipid A was isolated from LPS by mild-acid hydrolysis [36]. LPS pellets were dissolved in 1% acetic acid and hydrolyzed by boiling at 100 °C for 2 h. The samples were incubated with chloroform and methanol, and the phases were separated by centrifugation at 8000 rpm for 5 min at 15 °C. The lower phase (chloroform layer) was extracted and dried in the air. The dried lipid A was solubilized in chloroform/methanol (4:1, v/v). α-cyano-4-hydroxycinnamic acid (10 mg/mL) in TA50 (50% acetonitrile/0.1% trifluoroacetic acid) solution was used as a matrix. MALDI plates were spotted with the sample and matrix solution at a 1:1 (v/v) ratio, and lipid A structural spectra were acquired using reflector mode on the Autoflex max MALDI-TOF/TOF MS (Bruker Daltonics, Bremen, Germany) [37].
4.8. RNA Isolation and qPCR
A. baumannii strains were grown in LB overnight at 37 °C and inoculated into a fresh LB with pH 7.0 or 5.5, where the initial OD600 was 0.02. Bacteria were grown under shaking conditions until the OD600 reached 0.25. Total RNA was isolated using the RNeasy Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Complementary DNA was synthesized by reverse transcription of 2 μg total RNA as a template using a TOPscript™ cDNA Synthesis Kit (Enzynomics, Daejeon, Republic of Korea). Quantification of gene transcripts was performed by real-time PCR using TOPreal™ qPCR 2X PreMIX (SYBR Green with high ROX; Enzynomics) using the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Gene expression was normalized to 16S rRNA levels, and fold change was calculated by the ΔΔCt method [38]. The primers used in this study are listed in Supplementary Table S2. Gene expression assays were performed in three independent experiments.
4.9. Statistical Analysis
Data were analyzed using GraphPad Prism 5.0 (San Diego, CA, USA). Averages and standard deviations of the mean were calculated from at least three independent experiments. Data from different experimental groups were analyzed using one-way ANOVA with Dunnett’s post hoc analysis or Student’s t-test. p < 0.05 was considered statistically significant.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12050813/s1, Construction of ΔpmrA and ΔpmrB mutant strains in A. baumannii ATCC 17978, Construction of pmrA-complemented A. baumannii strain; Table S1: Oligonucleotide primers used in this study, Table S2: Primers used in qPCR, Supplementary Figure S1: Construction of the ΔpmrA, ΔpmrB, and pmrA-complemented strains.
Author Contributions
Conceptualization, S.-Y.K. (Seo-Yeon Ko), M.S. and J.-C.L.; methodology, S.-Y.K. (Seo-Yeon Ko), N.K., S.-Y.P. and S.-Y.K. (Seong-Yeop Kim). validation, S.-Y.K. (Seo-Yeon Ko); formal analysis, S.-Y.K. (Seo-Yeon Ko), M.S. and J.-C.L.; investigation, S.-Y.K. (Seo-Yeon Ko), N.K., S.-Y.P. and S.-Y.K. (Seong-Yeop Kim); writing—original draft preparation, S.-Y.K. (Seo-Yeon Ko); writing—review and editing, S.-Y.K. (Seo-Yeon Ko), M.S. and J.-C.L.; funding acquisition, J.-C.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by a grant from the National Research Foundation of Republic of Korea [grant No. NRF-2020R1A2B5B01002228].
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Howard A., O’Donoghue M., Feeney A., Sleator R.D. Acinetobacter baumannii: An emerging opportunistic pathogen. Virulence. 2012;3:243–250. doi: 10.4161/viru.19700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antunes L., Visca P., Towner K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014;71:292–301. doi: 10.1111/2049-632X.12125. [DOI] [PubMed] [Google Scholar]
- 3.Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 4.Falagas M.E., Kasiakou S.K. Colistin: The revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 2005;40:1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- 5.Rice L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008;197:1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 6.Manchanda V., Sanchaita S., Singh N. Multidrug resistant acinetobacter. J. Glob. Infect. Dis. 2010;2:291–304. doi: 10.4103/0974-777X.68538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olaitan A.O., Morand S., Rolain J.M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014;5:643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moffatt J.H., Harper M., Harrison P., Hale J.D., Vinogradov E., Seemann T., Henry R., Crane B., St Michael F., Cox A.D., et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother. 2010;54:4971–4977. doi: 10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelletier M.R., Casella L.G., Jones J.W., Adams M.D., Zurawski D.V., Hazlett K.R.O., Doi Y., Ernst R.K. Unique structural modifications are present in the lipopolysaccharide from colistin-resistant strains of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013;57:4831–4840. doi: 10.1128/AAC.00865-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stock A.M., Robinson V.L., Goudreau P.N. Two-component signal transduction. Annu. Rev. Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 11.Tiwari S., Jamal S.B., Hassan S.S., Carvalho P.V.S.D., Almeida S., Barh D., Ghosh P., Silva A., Castro T.L.P., Azevedo V. Two-component signal transduction systems of pathogenic bacteria as targets for antimicrobial therapy: An overview. Front. Microbiol. 2017;8:1878. doi: 10.3389/fmicb.2017.01878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Silva P.M., Kumar A. Signal transduction proteins in Acinetobacter baumannii: Role in antibiotic resistance, virulence, and potential as drug targets. Front. Microbiol. 2019;10:49. doi: 10.3389/fmicb.2019.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams M.D., Nickel G.C., Bajaksouzian S., Lavender H., Murthy A.R., Jacobs M.R., Bonomo R.A. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob. Agents Chemother. 2009;53:3628–3634. doi: 10.1128/AAC.00284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunn J.S., Ryan S.S., Van Velkinburgh J.C., Ernst R.K., Miller S.I. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar typhimurium. Infect. Immun. 2000;68:6139–6146. doi: 10.1128/IAI.68.11.6139-6146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X., Ribeiro A.A., Guan Z., Raetz C.R.H. Identification of undecaprenyl phosphate-β-d-galactosamine in Francisella novicida and its function in lipid A modification. Biochemistry. 2009;48:1162–1172. doi: 10.1021/bi802211k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishino K., Hsu F.-F., Turk J., Cromie M.J., Wösten M.M.S.M., Groisman E.A. Identification of the lipopolysaccharide modifications controlled by the Salmonella PmrA/PmrB system mediating resistance to Fe(III) and Al(III) Mol. Microbiol. 2006;61:645–654. doi: 10.1111/j.1365-2958.2006.05273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin E.J., Herrera C.M., Crofts A.A., Trent M.S. PmrD is required for modifications to Escherichia coli endotoxin that promote antimicrobial resistance. Antimicrob. Agents Chemother. 2015;59:2051–2061. doi: 10.1128/AAC.05052-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitrophanov A.Y., Jewett M.W., Hadley T.J., Groisman E.A. Evolution and dynamics of regulatory architectures controlling polymyxin B resistance in enteric bacteria. PLoS Genet. 2008;4:e1000233. doi: 10.1371/journal.pgen.1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng H.-Y., Chen Y.-F., Peng H.-L. Molecular characterization of the PhoPQ-PmrD-PmrAB mediated pathway regulating polymyxin B resistance in Klebsiella pneumoniae CG43. J. Biomed. Sci. 2010;17:60. doi: 10.1186/1423-0127-17-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.March C., Regueiro V., Llobet E., Moranta D., Morey P., Garmendia J., Bengoechea J.A. Dissection of host cell signal transduction during Acinetobacter baumannii-triggered inflammatory response. PLoS ONE. 2010;5:e10033. doi: 10.1371/journal.pone.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clifton L.A., Skoda M.W.A., Brun A.P.L., Ciesielski F., Kuzmenko I., Holt S.A., Lakey J.H. Effect of divalent cation removal on the structure of gram-negative bacterial outer membrane models. Langmuir. 2015;31:404–412. doi: 10.1021/la504407v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velkov T., Thompson P.E., Nation R.L., Li J. Structure-activity relationships of polymyxin antibiotics. J. Med. Chem. 2010;53:1898–1916. doi: 10.1021/jm900999h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Z., Ribeiro A.A., Lin S., Cotter R.J., Miller S.I., Raetz C.R. Lipid A modifications in polymyxin-resistant Salmonella typhimurium: PMRA-dependent 4-amino-4-deoxy-L-arabinose, and phosphoethanolamine incorporation. J. Biol. Chem. 2001;276:43111–43121. doi: 10.1074/jbc.M106960200. [DOI] [PubMed] [Google Scholar]
- 24.Owusu-Anim D., Kwon D.H. Differential role of two-component regulatory systems (phoPQ and pmrAB) in polymyxin B susceptibility of Pseudomonas aeruginosa. Adv. Microbiol. 2012;2:10.4236/aim.2012.21005. doi: 10.4236/aim.2012.21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beceiro A., Llobet E., Aranda J., Bengoechea J.A., Doumith M., Hornsey M., Dhanji H., Chart H., Bou G., Livermore D.M., et al. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob. Agents Chemother. 2011;55:3370–3379. doi: 10.1128/AAC.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wösten M.M., Kox L.F., Chamnongpol S., Soncini F.C., Groisman E.A. A signal transduction system that responds to extracellular iron. Cell. 2000;103:113–125. doi: 10.1016/S0092-8674(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 27.Perez J.C., Groisman E.A. Acid pH activation of the PmrA/PmrB two-component regulatory system of Salmonella enterica. Mol. Microbiol. 2007;63:283–293. doi: 10.1111/j.1365-2958.2006.05512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.García Véscovi E., Soncini F.C., Groisman E.A. Mg2+ as an extracellular signal: Environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/S0092-8674(00)81003-X. [DOI] [PubMed] [Google Scholar]
- 29.Chin C.Y., Gregg K.A., Napier B.A., Ernst R.K., Weiss D.S. A PmrB-regulated deacetylase required for lipid A modification and polymyxin resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2015;59:7911–7914. doi: 10.1128/AAC.00515-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Khodor S., Kalachikov S., Morozova I., Price C.T., Kwaik Y.A. The PmrA/PmrB two-component system of Legionella pneumophila is a global regulator required for intracellular replication within macrophages and protozoa. Infect. Immun. 2009;77:374–386. doi: 10.1128/IAI.01081-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warner D.M., Duval V., Levy S.B. The contribution of PmrAB to the virulence of a clinical isolate of Escherichia coli. Virulence. 2013;4:634–637. doi: 10.4161/viru.25931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.López-Rojas R., Domínguez-Herrera J., McConnell M.J., Docobo-Peréz F., Smani Y., Fernández-Reyes M., Rivas L., Pachón J. Impaired virulence and in vivo fitness of colistin-resistant Acinetobacter baumannii. J. Infect. Dis. 2011;203:545–548. doi: 10.1093/infdis/jiq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh M.H., Lee J.C., Kim J., Choi C.H., Kan K. Simple method for markerless gene deletion in multidrug-resistant Acinetobacter baumannii. Appl. Environ. Microbiol. 2015;81:3357–3368. doi: 10.1128/AEM.03975-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fourth Informational Supplement, M100-S28. Clinical and Laboratory Standards Institute (CLSI); Wayne, PA, USA: 2018. [Google Scholar]
- 35.Kim J.N., Stanhope M.J., Burne R.A. Core-gene-encoded peptide regulating virulence-associated traits in Streptococcus mutants. J. Bacteriol. 2013;195:2912–2920. doi: 10.1128/JB.00189-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamidi E.A., Tirsoaga A., Novikov A., Hussein A., Caroff M. Microextraction of bacterial lipid A: Easy and rapid method for mass spectrometric characterization. J. Lipid Res. 2005;46:1773–1778. doi: 10.1194/jlr.D500014-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Henderson J.C., O’Brien J.P., Brodbelt J.S., Trent M.S. Isolation and chemical characterization of lipid A from gram-negative bacteria. J. Vis. Exp. 2013;79:e50623. doi: 10.3791/50623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials.