Abstract
Purpose
We aimed to evaluate whether high flow nasal cannula (HFNC) is an effective and safe method for adult patients with acute hypercapnic respiratory failure (AHRF).
Methods
We searched the Cochrane Library, Embase, and PubMed databases from inception to August 2022 to obtain randomized controlled trials (RCTs) that compared HFNC with conventional oxygen treatment (COT) or non-invasive ventilation (NIV) in patients with AHRF, and then performed a meta-analysis.
Results
A total of ten parallel RCTs with 1265 individuals were identified. Of them, two studies compared HFNC with COT and eight studies compared HFNC with NIV. In terms of intubation rate, mortality, and arterial blood gas (ABG) improvement, HFNC showed comparable effects to NIV and COT. However, HFNC was more comfortable (mean difference [MD] −1.87, 95% confidence interval [CI] =−2.59, −1.15, P <0.00001, I2 =0%) and resulted in fewer adverse events (odds ratio [OR] 0.12, 95% CI=0.06, 0.28, P<0.00001, I2 = 0%), compared with NIV. In comparison to NIV, HFNC could significantly lower heart rate (HR) (MD −4.66, 95% CI=−6.82, −2.50, P <0.0001, I2 =0%), respiratory rate (RR) (MD −1.17, 95% CI=−2.03, −0.31, P =0.008, I2 =0%), and hospital stay length (MD −0.80, 95% CI=−1.44, −0.16, P =0.01, I2 =0%). NIV showed a decreased frequency in the treatment crossover rate, compared with HFNC in patients with pH<7.30 (OR 5.78, 95% CI=1.50, 22.31, P = 0.01, I2: not applicable). Contrary to COT, HFNC could considerably reduce the need for NIV (OR 0.57, 95% CI=0.35, 0.91, P=0.02, I2=0%).
Conclusion
HFNC was effective and safe in patients with AHRF. However, in patients with pH <7.30, HFNC may result in a higher incidence of treatment crossover, compared with NIV. Compared to COT, HFNC may decrease the need for NIV in patients with compensated hypercapnia.
Keywords: high-flow nasal cannula, non-invasive ventilation, conventional oxygen treatment, hypercapnic respiratory failure
Introduction
Acute hypercapnic respiratory failure (AHRF), which is commonly defined as arterial partial pressure of carbon dioxide (PaCO2) ≥ 45 mmHg and frequently accompanied by reduced levels of arterial partial pressure of oxygen (PaO2), can occur in a variety of etiologies, mainly in chronic respiratory diseases, such as exacerbation of chronic obstructive pulmonary disease, cystic fibrosis, thoracic deformities, as well as other conditions, such as neuromuscular disease.1,2 Respiratory support is important for these patients, and aims at facilitating alveolar ventilation, maintaining adequate oxygenation, relieving respiratory muscle fatigue, and finally improving survival. Non-invasive ventilation (NIV), which can enable positive pressure ventilation, has been the main method of treatment for patients with hypercapnia for the past 20 years.3 NIV is preferable over invasive ventilation as the initial mode of ventilation to treat acute respiratory failure in hospitalized AECOPD patients, as given in the 2023 Global Initiative for Chronic Obstructive Lung Disease (GOLD) report.4 The European Respiratory Society/American Thoracic Society (ERS/ATS) Guidelines also strongly advise its use for acidotic individuals with a pH of 7.25 to 7.35.3
Unfortunately, NIV is inapplicable to some patients who have poor tolerance to a tight mask. Furthermore, it can cause a relatively high incidence of adverse events, such as claustrophobia, nasofacial skin breakdown, eye irritation, gastric, and intestinal flatulence, which in some cases may lead to aspiration, which is a serious incident with a poor prognosis.5 Additionally, NIV is not recommended for patients who have hypercapnia but no acidosis because it does not outperform conventional oxygen treatment (COT), in terms of intubation or mortality.4 In these situations, additional modalities, including COT and high flow nasal cannula (HFNC), can be used in selected patients to avoid unnecessary invasive ventilation. However, there is currently no established alternative for these patients with hypercapnia who are ineligible for NIV.
HFNC is a non-invasive delivery system that can provide patients with a warmed and humidified air-oxygen mixture.6 It normally consists of an air/oxygen blender, humidifier, one heated tube, and nasal cannula. It is increasingly been used for patients with respiratory failure for a variety of underlying diseases due to its excellent level of patient comfort and other important physiological advantages, such as the delivery of a predictable and constant inspired oxygen fraction (FiO2) (as high as 100%) and provision of airflows as high as 60 L/min.6–8
Physiological research has shown that HFNC can produce a modest level of positive end-expiratory pressure (PEEP), which is flow-dependent and affected by whether the mouth is closed or not.9 A study conducted on ten adult volunteers in Australia revealed that HFNC can result in positive expiratory pressure, and that the expiratory pharyngeal pressures (EPP) increased from 0.8 to 7.4 cmH2O with the mouth closed, as the flow rates increased from 0 to 60 L/min.10
In addition, the persistent high-flow gas produced by HFNC can effectively wash out carbon dioxide from an anatomically dead space, preventing carbon dioxide rebreathing and as a result, lowering the breathing effort.
Due to the above-mentioned physiological benefits, HFNC seems able to decrease the partial pressure of carbon dioxide (PaCO2) and reverse respiratory acidosis. It has been used to successfully manage patients with hypercapnic respiratory failure, particularly those who were unable to tolerate NIV.11,12 Several clinical studies have shown that HFNC is comparable to NIV in terms of intubation and mortality in AHRF patients.13,14 It should be mentioned that the German S3 guidelines indicates that for patients with moderate hypercapnia, HFNC appears to be non-inferior to NIV.15
Recently, several systematic reviews and meta-analyses compared HFNC with NIV in patients with hypercapnia have found that the main consistent finding is that HFNC showed no difference in endotracheal intubation and mortality, compared with NIV. It is noteworthy that some of these meta-analyses only included researches that were published in Chinese-language journals,16,17 others18,19 included cohort studies, while the most recent meta-analysis20 included non-randomized controlled trial (RCT)14 and cross-over RCTs21,22 that had a very short duration of 30 minutes and were aimed at evaluating the pathophysiological effects.
Furthermore, to the best of our knowledge, until recently, no meta-analyses have performed subgroup analyses based on the severity of acidosis to determine the population that would benefit most from HFNC.
Therefore, we conducted a meta-analysis of RCTs on adult patients with acute hypercapnic respiratory failure to evaluate whether HFNC is an effective and safe method of treatment and whether it has advantages over COT or whether it can be used as an alternative strategy for NIV in this population.
Materials and Methods
We conducted this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.23 The detailed PRISMA Checklist of this meta-analysis is shown in Supplementary Table 1.
Criteria for Inclusion and Exclusion
Relevant studies that compared HFNC with COT or NIV in patients with acute hypercapnic respiratory failure were included. The inclusion criteria were as follows: (1) Population: adult patients (age>18 years) with acute hypercapnic respiratory failure (PaCO2 > 45 mmHg); (2) Intervention and comparisons: Comparison of HFNC therapy with COT or NIV; (3) Type of study: parallel group RCTs; (4) Outcomes: intubation rate, treatment crossover rate, mortality, vital signs (heart rate [HR], mean arterial pressure [MAP], respiratory rate [RR]), arterial blood gases (ABGs), length of intensive care unit (ICU) stay and hospital stay, comfort score, dyspnea score, and adverse events. The exclusion criteria were: (1) non-RCTs. To prevent potential cross-over effects of different therapies, we also excluded cross-over RCTs; (2) studies in which HFNC was used to evaluate only pathophysiological mechanisms; (3) studies in which the HFNC flow rate was less than 20 L/min or the treatment time was under an hour; (4) studies that did not report on any outcomes of interest; (6) conference abstracts without full-text manuscripts.
Search Strategy
Using relevant keywords and MeSH terms, a systematic search approach was applied. Additionally, relevant references listed in each of the studies included were manually reviewed to identify any other potentially acceptable studies.
Two reviewers (CPX and QMW) independently searched the PubMed, Embase, and Cochrane Library from inception until 16 August 2022, no language or publication restrictions were applied. The detailed search strategy followed is shown in Supplementary Table 2.
Selection of Studies
Two reviewers (CPX and QMW) screened the titles and abstracts of all citations. The full text manuscripts of all potentially eligible studies were independently reviewed based on the inclusion and exclusion criteria. Any disagreements were resolved through discussion with a third researcher (WG) to reach a consensus.
Data Extraction
Using a pre-designed data extraction form, two reviewers (CPX and QMW) independently extracted the data. The information included author identification, publication year, country, setting, study design, participant information, sample size, baseline arterial potential of hydrogen (pH) and PaCO2, baseline Acute Physiology, and Chronic Health Evaluation II (APACHE II) score, characteristics of interventions, measurement time points, and outcomes. Disagreements were resolved through discussion with a third researcher (WG) to reach a consensus. If necessary, we also checked the Supplemental materials of the included studies for more detailed information.
Quality Assessment
The quality of each trial included was assessed by two independent reviewers (CPX and QMW), using the Cochrane Risk of Bias tool. The risk of bias included seven different domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Based on the method of the trials, each potential source of bias was judged as high, low, or unclear. Two reviewers made judgments independently, and disagreements were resolved through discussion with a third reviewer (GW) to reach an agreement.
Statistical Analysis
We used Cochrane systematic review software Review Manager to perform all statistical analyses. Continuous variables are presented as mean ± standard deviation (SD), while dichotomous variables are presented as frequencies or percentages. The statistical heterogeneity of the studies included was assessed using a chi-square test; statistical heterogeneity was defined as a P < 0.1 and I2 > 50%, in which case a random-effect model was employed. Conversely, a fixed-effect model was used. For dichotomous variables, the results of the statistical analysis were reported as odds ratio (OR) with a 95% confidence interval (CI), while for continuous variables, results were reported as mean difference (MD) with a 95% CI. A P value of <0.05 was considered to indicate statistical significance. Sensitivity analysis was conducted by removing trials with a high concern of bias. To assess the possibility of publication bias, funnel plots with Egger’s test and Begg’s test were used.
Subgroup Analysis
First, to assess the potential effects of different respiratory support methods on the clinical outcomes, we conducted a subgroup analysis based on the different interventions of the control group (COT or NIV). Second, in the comparison between HFNC and NIV, we performed a subgroup analysis based on the condition of the patient (post-extubation vs non-post-extubation) to explore whether there was a difference in the treatment effect. Additionally, we performed another subgroup analysis in this group based on the degree of acidosis to investigate potential effects on outcomes.
Results
Literature Identification and Selection
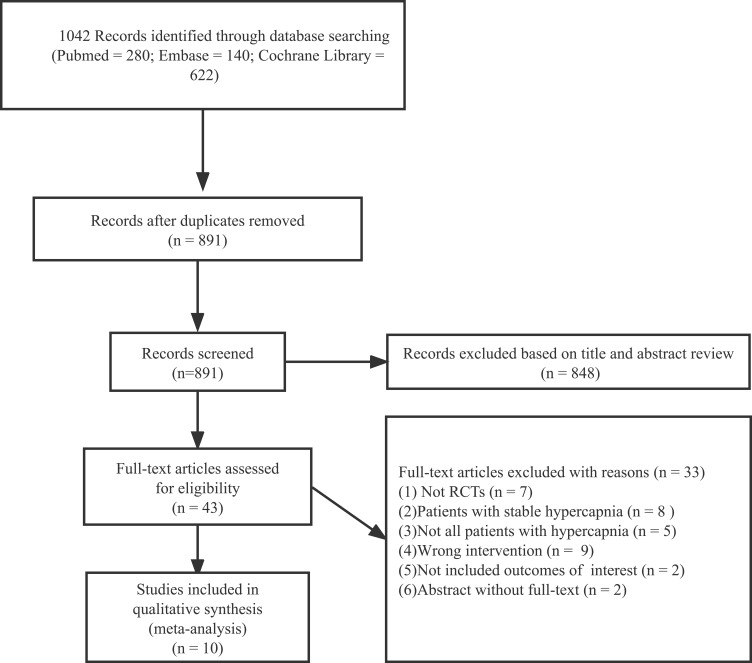
The initial literature search identified 1042 citations (PubMed, n= 280; Embase, n = 140; Cochrane Library, n= 622). After removing duplicates, we examined the remaining 891 articles based on their titles and abstracts, and 839 articles were secondarily excluded based on the eligibility criteria. The full text of the remaining 43 articles were screened, and finally, ten parallel RCTs that met our inclusion criteria were identified.13,24–32 The flow diagram of the selection of studies is shown in Figure 1.
Figure 1.
Flow chart of study selection and exclusion.
Abbreviations: RCTs, randomized controlled trials; n, number.
Characteristics of the Trials Included
Ten parallel RCTs with 1265 patients were included, eight13,24–29,32 were published in English-language journals and two30,31 were published in Chinese-language journals, the publication year ranged from 2019 to 2022. Of the ten studies included, half were multicenter studies. Two studies were conducted at emergency departments (EDs),24,25 five were conducted at intensive care units (ICUs),26,27,29–31 while two studies were conducted in the respiratory unit,28,32 whereas another study was conducted in either the ED, ICU, or respiratory units.13 The longest measurement time points ranged from 4 hours to 120 hours. HFNC was compared with NIV in eight studies13,24–27,29–31 and with COT in two studies.28,32 In the eight studies that compared HFNC with NIV, 615 of the enrolled patients had a mean age of 69.4±10.6 years, while 58% were males. The baseline data showed that the mean pH was 7.29±0.08, the mean PaCO2 was 65.7±15.8 mmHg, and the mean APACHE II score was 20.8±8.3. In this group, two studies included mixed populations with a hypercapnic, while the other six studies only included patients with AECOPD, which three of these studies focusing on post-extubation in AECOPD patients. Both the two studies that compared HFNC with COT included patients with AECOPD, 650 of the enrolled patients had a mean age of 68.8±7.5 years, and 74% were males. The mean baseline pH was 7.39±0.03, the mean PaCO2 was 52.6±7.1 mmHg, and the mean APACHE II score was 12.56±6.03. The characteristics of the trials included are presented in Table 1.
Table 1.
The Characteristics of Included Studies
| Study | Setting | Study Design | Patients | HFNC Group | Control Group | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Arterial pH and PaCO2 (mmHg), Mean ± SD | Baseline APACHE II, Mean ± SD | Intervention (Mean ± SD) | Baseline Arterial pH and PaCO2 (mmHg), Mean ± SD | Baseline APACHE II, Mean ± SD | Intervention | |||||
| Cortegiani A, 2020 | ED, ICU, or respiratory unit | Multi-center | AECOPD, N=79 | pH, 7.30±0.03; PaCO2, 73.7±12.8 | SAPS II (mean ± SD), 30±9 | Flow rate, 50.0±11.1 L/min; FiO2, 0.36 ±0.10; Temperature, 37.0±2.2°C | pH, 7.29±0.03; PaCO2, 72.0±13.0 | SAPS II (mean ± SD), 33±10 | NIV: PSV mode, PS (cmH2O), 12.0±2.9; PEEP (cmH2O), 6.0±1.1; FiO2, 0.30 ±0.10 | Intubation rate, mortality, treatment crossover rate, pH, PaCO2, PaO2/FiO2, RR, hospital stay, dyspnea score, discomfort score |
| Li C, 2019 | ICU | Single-center | AECOPD, N=168 | pH, 7.25±0.08; PaCO2, 72.1±16.3 | NR | Flow rate, 30–35L/min; Temperature, 37.0°C | pH, 7.27±0.09; PaCO2, 72.9±16.4 | NR | IPAP was set at 10 cmH2O and PEEP was set at 5 cm H2O at beginning and gradually increased after the patient adapted | pH, PaCO2, PaO2, hospital stay, adverse events |
| Wang J, 2019 | RICU | Single-center | AECOPD, N=63 | pH, 7.24±0.02; PaCO2, 66.8±3.9 | 19.0±2.6 | NR | pH, 7.24±0.02; PaCO2, 68.3±3.1 | 19.8±2.3 | NR | Intubation rate, mortality, ICU and hospital lengths, adverse events |
| Doshi PB, 2020 | ED | Multi-center | Patients with AHRF, N=65 | pH, 7.33±0.12; PaCO2, 56.0±17.7 | 31.0±4.4 | Initial flow rate was 35 L/min, FiO2 was adjusted to maintain oxygen saturation above 88% | pH, 7.32±0.09; PaCO2, 64.6±31.8 | 29.0±5.9 | NIV, initial settings: IPAP, 10–20 cmH2O; EPAP, 5–10 cmH2O; FiO2 was adjusted to maintain oxygen saturation above 88% | Intubation rate, treatment crossover rate, pH, PaO2, PaCO2, RR, HR, ICU and hospital stay, Modified Borg score |
| Papachatzakis Y, 2020 | ED | Single-center | Patients with AHRF, N=40 | pH, 7.40±0.10; PaCO2, 60.4±9.9 | 21.6±8.9 | Initial flow rate was 35 L/min, titrating flow upward if tolerated to 45–50 L/min, in order to maintain SaO2 >90% | pH, 7.40±0.10; PaCO2, 56.8±9.7 | 19.3±6.1 | NIV, S/T mode, expiratory and inspiratory pressures were gradually increased to the maximum tolerated to maintain SaO2 >90% | Mortality, pH, PaO2, PaCO2, RR, HR, hospital stay, comfort score, dyspnea score |
| Tan D, 2020 | ICU | Multi-center | Post-extubation AECOPD, N=86 | pH, 7.48±0.06; PaCO2, 50.5±7.2 | 14±5.7 | Initial flow rate was 50 L/min, temperature was 37.0°C, FiO2, 0.32±0.074 | pH, 7.45±0.06; PaCO2, 53.0±9.2 | 13±3.8 | NIV, initial EPAP was set at 4 cmH2O, IPAP was set at 8 cmH2O and gradually increased to achieve a satisfactory tidal volume with acceptable tolerance, FiO2 (mean ± SD), 0.35±0.07 | Intubation rate, mortality, treatment crossover rate, pH, PaO2, PaCO2, HR, RR, ICU and hospital lengths, adverse events, comfort score, dyspnea score |
| Jing G, 2019 | RICU | Single-center | Post-extubation AECOPD, N=42 | pH, 7.46±0.04; PaCO2, 53.2±6.7 | 11.8±3.1 | Flow rate, 52.0±6.3L/min; FiO2, 0.40±0.10; temperature, 37.0°C | pH, 7.44±0.06; PaCO2, 53.7±8.6 | 10.4±2.5 | NIV, IPAP (mean ± SD), 11.4±2.0 cmH2O; PEEP, 4.6±0.5 (cmH2O); FiO2 was adjusted to maintain SpO2 88–92% | Intubation rate, 28-day mortality, pH, PaCO2, PaO2/FiO2, RR, HR, comfort score, ICU stay, adverse events |
| Li X, 2020 | Respiratory Unit | Multi-center | AECOPD with compensated AHRF, N=320 | pH, 7.38±0.03; PaCO2, 54.9±7.1 | 15.8±6.5 | Flow rate, 33.4 ± 5.6 L/min; FiO2, 0.28 ± 0.01; temperature, 33.8 ± 4.1°C | pH, 7.39±0.04; PaCO2, 54.2±6.0 | 14.7±6.0 | COT, oxygen flow was set to achieve SpO2 at 90% to 93% | Intubation rate, mortality, treatment failure rate, pH, PaO2, PaCO2, RR, length of hospitalization, comfort score |
| Xia J, 2022 | Respiratory Unit | Multi-center | AECOPD with mild hypercapnia, N=330 | pH, 7.40±0.03; PaCO2, 50.4±6.6 | 10.0±4.4 | Flow rate, 35.0±11.1 L/min; FiO2, 0.33±0 0.07; temperature, 34.0±2.2 °C | pH, 7.40±0.04; PaCO2, 51.7±7.7 | 10.0±4.8 | COT, oxygen flow (mean ± SD), 2.0±0.7 L/min | Intubation rate, mortality, treatment failure rate, pH, PaCO2, PaO2, SpO2, RR, HR, hospital stay |
| Yu Z, 2019 | RICU | Single-center | Post-extubation AECOPD, N=72 | pH, 7.26±0.03; PaCO2, 73.5±6.9 | 28.6±2.8 | Flow rate, 30–60L/min; FiO2, 0.30–0.80; temperature, 37.0°C | pH, 7.26±0.03; PaCO2, 73.5±6.2 | 28.5±3.4 | NIV: S/T mode; IPAP (cmH2O), 10–14; PEEP (cmH2O), 4–6; FiO2, 0.30–0.80 | Reintubation rate, 28-day mortality, pH, PaO2, PaCO2, PaO2/FiO2, RR, HR, ICU stay, adverse events |
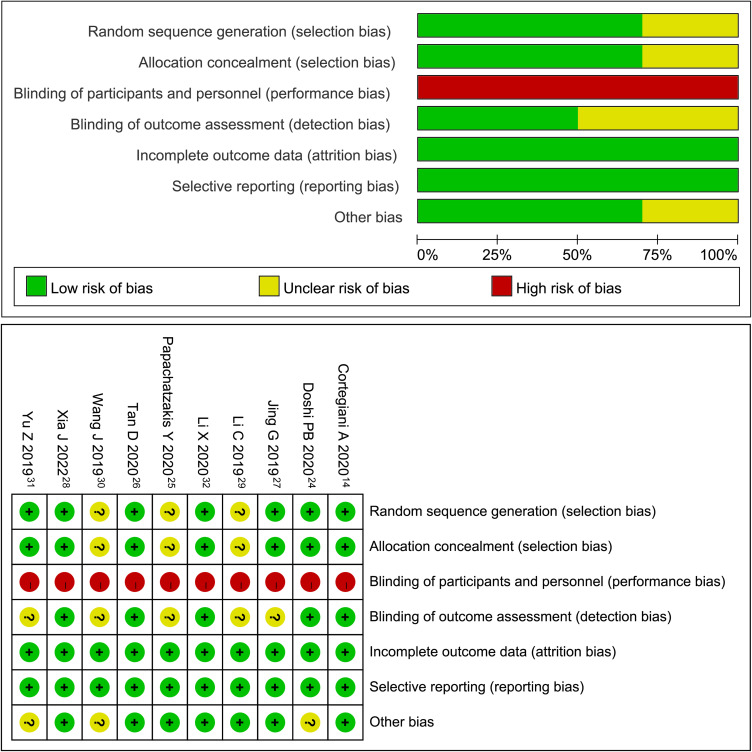
Risk of Bias
Three studies were found to contain unclear risks in random sequence generation and allocation concealment as they failed to describe the specific methods used.25,29,30 All studies included were found to have a high risk of blinding participants and personnel bias due to natural differences between the three interventions. Since blinding of the outcome assessment was not described in half of the studies included,25,27,29–31 the risk associated with this domain was found to have unclear risks. There was no bias in reporting selection and incomplete outcome data in all of the trials included. Three trials were found to have an unclear risk of other biases as they did not provide funding information.24,30,31 The detailed quality assessment is presented in Supplementary Table 3 and Figure 2.
Figure 2.
Results of risk assessment of bias using Cochrane risk of bias tool.
Outcomes
Intubation Rate
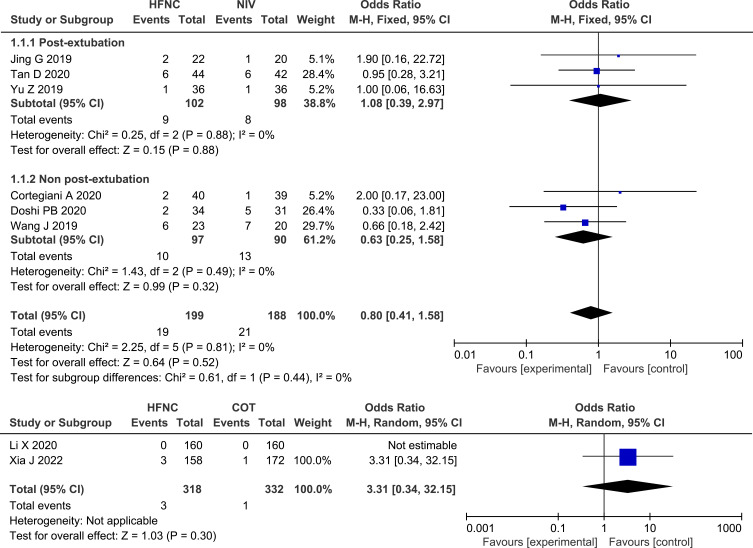
Six of the studies13,24,26,27,30,31 that compared HFNC with NIV reported on the intubation rate and no significant difference was found (OR 0.80, 95% CI=0.41,1.58, P = 0.52, I2 = 0%, n=387). The subgroup analysis showed that there was no significant difference in the intubation rate between HFNC and NIV, in either post-extubation patients or non-post-extubation patients. Both studies28,32 that compared HFNC with COT reported on the intubation rate, and a similar result was found (OR 3.31, 95% CI=0.34, 32.15, P = 0.39, heterogeneity: not applicable, n=650). Forest plot of intubation rate is shown in Figure 3.
Figure 3.
Forest plot comparing intubation rate between HFNC and control groups.
Abbreviations: HFNC, high flow nasal cannula; NIV, non-invasive ventilation; COT, conventional oxygen therapy; blue squares, the odds ratio value for an individual study result; black diamonds, the overall effect size for study results of the group; M–H, Mantel–Haenszel method; CI, confidence interval.
Mortality
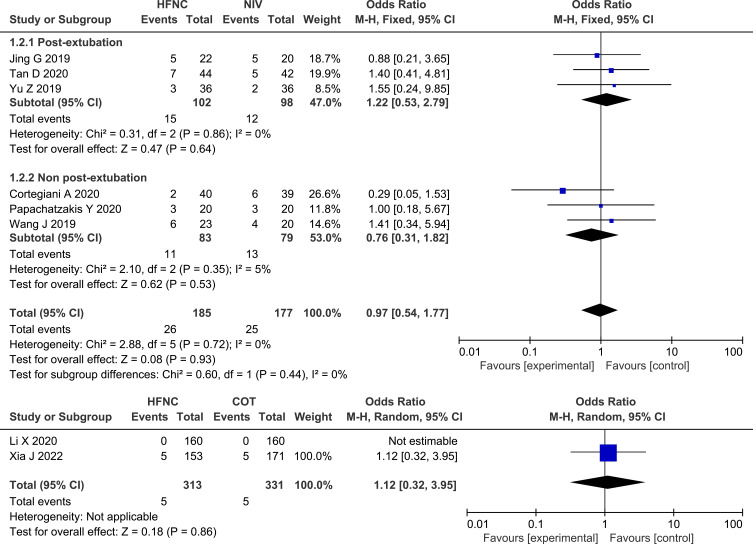
Mortality was reported on in eight of the studies,13,25–28,30–32 and the pooled data showed no significant difference between HFNC and NIV (OR 0.97, 95% CI=0.54, 1.77, P = 0.93, I2 = 0%, n=362) or between HFNC and COT (OR 1.12, 95% CI=0.32, 3.95, P = 0.86, heterogeneity: not applicable, n=650). Similarly, for patients who were between post-extubation and non-post-extubation, the results were not statistically significant. Forest plot of mortality is shown in Figure 4.
Figure 4.
Forest plot comparing mortality between HFNC and control groups.
Abbreviations: HFNC, high flow nasal cannula; NIV, non-invasive ventilation; COT, conventional oxygen therapy; blue squares, the odds ratio value for an individual study result; black diamonds, the overall effect size for study results of the group; M–H, Mantel–Haenszel method; CI, confidence interval.
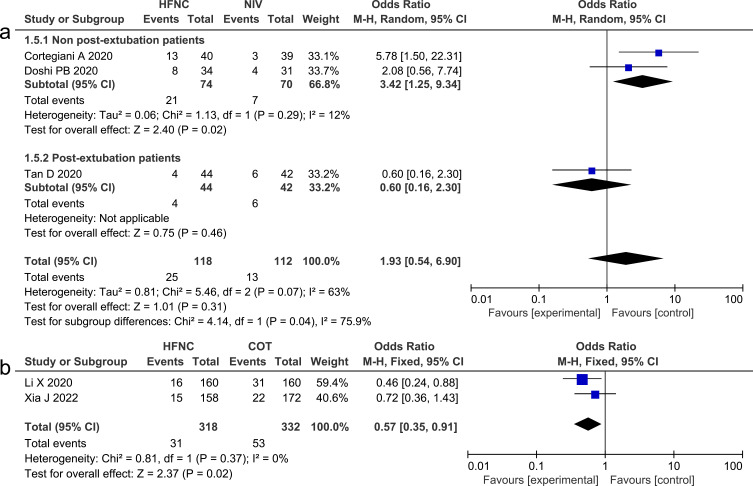
Treatment Crossover Rate
We analyzed the treatment crossover rate, which is defined as switching to the other intervention (changing from HFNC to NIV or from NIV to HFNC), in studies that compared HFNC with NIV. This outcome was reported on in three studies,13,24,26 and the pooled data revealed that there was no significant difference (OR 1.93, 95% CI=0.54, 6.90, P =0.31, I2=63%, n=230). However, the subgroup analysis revealed that NIV could significantly lower the frequency of treatment crossover, compared with HFNC, in non-post-extubation patients (OR 3.42, 95% CI=1.25, 9.34, P = 0.02, I2=12%, n=144). We investigated the need for NIV in studies that compared HFNC with COT and found that HFNC could significantly reduce the need for NIV, compared with COT (OR 0.57, 95% CI=0.35, 0.91, P=0.02, I2= 0%, n=650).28,32 Forest plots of treatment crossover rate and the need for NIV are presented in Figure 5.
Figure 5.
(a) Forest plot comparing treatment crossover rate between HFNC and NIV; (b) Forest plot comparing the need for NIV between HFNC and COT.
Abbreviations: HFNC, high flow nasal cannula; NIV, non-invasive ventilation; COT, conventional oxygen therapy; blue squares, the odds ratio value for an individual study result; black diamonds, the overall effect size for study results of the group; M–H, Mantel–Haenszel method; CI, confidence interval.
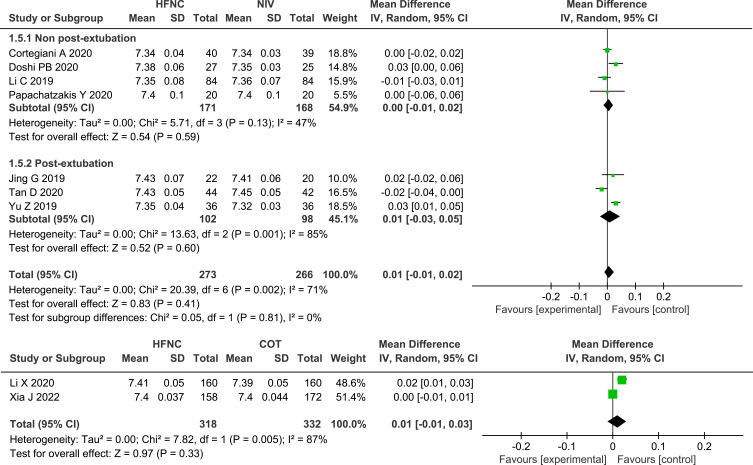
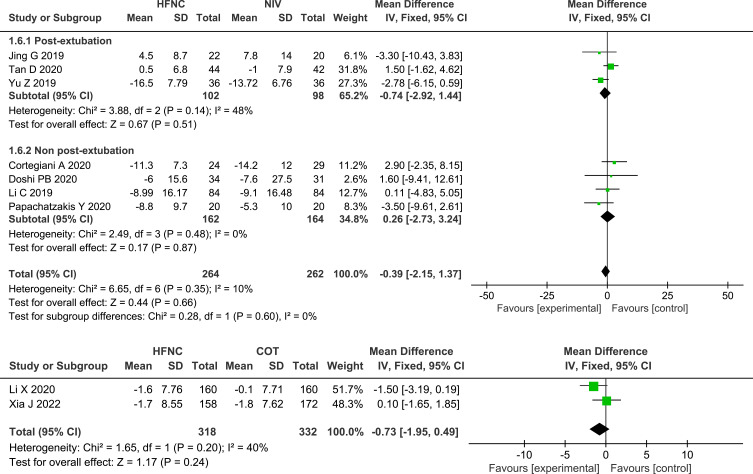
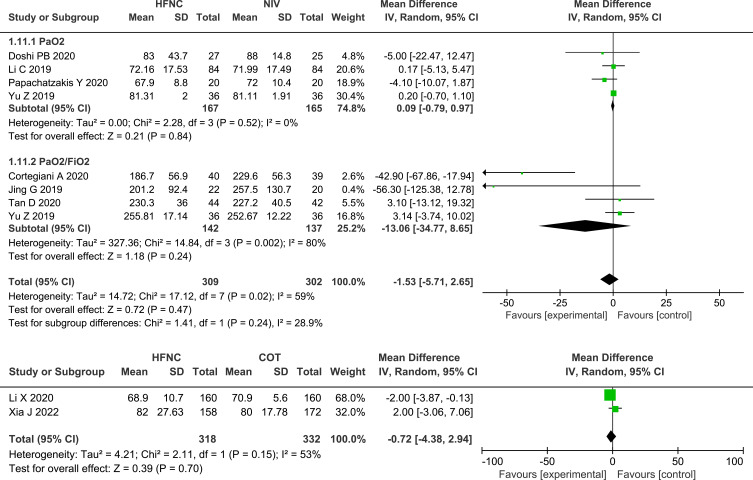
ABGs
Seven of the studies13,24–27,29,31 that compared HFNC with NIV and both studies28,32 that compared HFNC with COT measured the change in pH, and no significant differences were found, either between HFNC and NIV (MD 0.01, 95% CI=−0.01, 0.02, I2 = 71%, P = 0.41, n=539) or between HFNC and COT (MD 0.01, 95% CI=−0.01, 0.03, P = 0.33, I2 = 87%, n=650). Nine studies13,24–29,31,32 assessed the change in PaO2 or PaO2/FiO2, and no significant differences were found between HFNC and NIV (MD −1.53, 95% CI=−5.71, 2.65, I2 = 59%, P = 0.47, n=611) or between HFNC with COT (MD −0.72, 95% CI=−4.38, 2.94, P = 0.70, I2 = 53%, n=650). Compared with NIV or COT, HFNC showed a similar effect in the decrease in PaCO2 (HFNC VS NIV: MD −0.39, 95% CI=−2.15, 1.37, I2 = 10%, P = 0.66, n=526; HFNC VS COT: MD −0.73, 95% CI=−1.95, 0.49, P =0.24, I2 =40%, n=650). Likewise, the change in pH, oxygenation, and PaCO2 between post-extubation patients and non-post-extubation patients did not show any significant differences, as shown in the subgroup analysis. Forest plots of pH, PaCO2 and oxygenation are shown in Figures 6–8.
Figure 6.
Forest plot comparing pH between HFNC and control groups.
Abbreviations: pH, potential of hydrogen; HFNC, high flow nasal cannula; NIV, non-invasive ventilation; COT, conventional oxygen therapy; green squares, the mean difference value for an individual study result; black diamonds, the overall effect size for study results of the group; IV, Inverse variance method; CI, confidence interval; SD, standard deviation.
Figure 7.
Forest plot comparing PaCO2 between HFNC and control groups.
Abbreviations: HFNC, high flow nasal cannula; NIV, non-invasive ventilation; COT, conventional oxygen therapy; PaCO2, arterial partial pressure of carbon dioxide; green squares, the mean difference value for an individual study result; black diamonds, the overall effect size for study results of the group; IV, Inverse variance method; CI, confidence interval; SD, standard deviation.
Figure 8.
Forest plot comparing oxygenation between HFNC and control groups.
Abbreviations: HFNC, high flow nasal cannula; NIV, non-invasive ventilation; COT, conventional oxygen therapy; PaO2, partial arterial oxygen pressure; FiO2, the fraction of inspired oxygen; green squares, the mean difference value for an individual study result; black diamonds, the overall effect size for study results of the group; IV, Inverse variance method; CI, confidence interval; SD, standard deviation.
Vital Signs
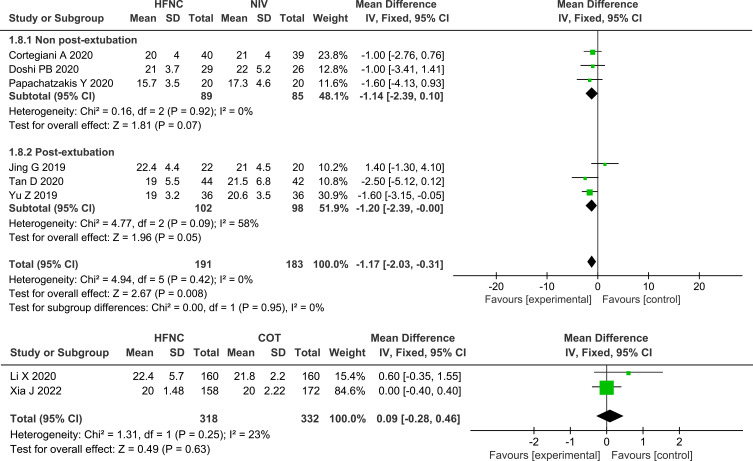
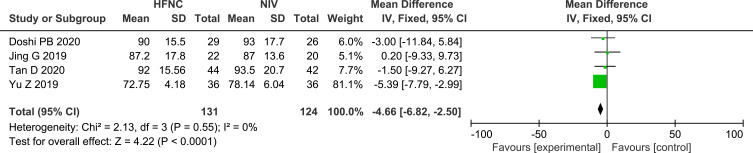
Four studies reported on HR,24,26,27,31 and eight studies reported on RR,13,24–28,31,32 the pooled data revealed that, compared with NIV, HFNC could significantly reduce both HR (MD −4.66, 95% CI=−6.82,-2.50, P <0.0001, I2 =0%, n=255) and RR (MD −1.17, 95% CI=−2.03,-0.31, P =0.008, I2 =0%, n=374), whereas the subgroup analysis between post-extubated patients and non-post-extubated patients revealed that there were no significant differences in the reduction of RR between HFNC and NIV. There were no significant differences in the reduction of RR between the two studies that compared HFNC with COT (MD 0.09, 95% CI=−0.28, 0.46, P =0.63, I2 =23%, n=650). Forest plots of RR and HR are shown in Figures 9 and 10.
Figure 9.
Forest plot comparing RR between HFNC and control groups.
Abbreviations: HFNC, high flow nasal cannula; NIV, non-invasive ventilation; COT, conventional oxygen therapy; RR, respiratory rate; green squares, the mean difference value for an individual study result; black diamonds, the overall effect size for study results of the group; IV, Inverse variance method; CI, confidence interval; SD, standard deviation.
Figure 10.
Forest plot comparing HR between HFNC and NIV.
Abbreviations: HFNC, high flow nasal cannula; NIV, non-invasive ventilation; HR, heart rate; green squares, the mean difference value for an individual study result; black diamonds, the overall effect size for study results of the group; IV, Inverse variance method; CI, confidence interval; SD, standard deviation.
Length of Stay
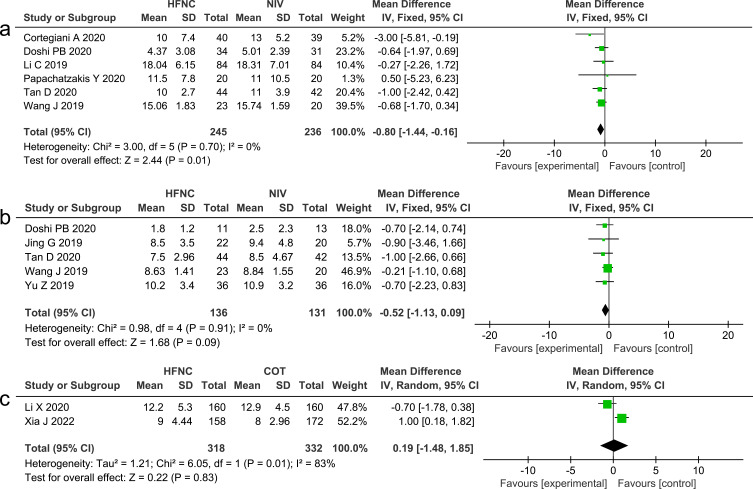
Five studies24,26,27,30,31 that compared HFNC with NIV reported on the length of ICU stay, and the results were not statistically significant (MD −0.52, 95% CI=−1.13, 0.09, P =0.09, I2 =0%, n=267). Eight studies13,24–26,28–30,32 measured the length of hospital stay, the pooled dates showed that HFNC could significantly reduce the duration of hospital stay, compared with NIV (MD −0.80, 95% CI=−1.44, −0.16, P =0.01, I2 =0%, n=481) but did not significantly reduce the duration of hospital stay, compared with COT (MD 0.19, 95% CI=−1.48, 1.85, P =0.83, I2 =83%, n=650). Forest plots of length of stay are shown in Figure 11.
Figure 11.
(a) Forest plot comparing hospital stay between HFNC and NIV; (b) Forest plot comparing ICU stay between HFNC and NIV; (c) Forest plot comparing hospital stay between HFNC and COT.
Abbreviations: HFNC, high flow nasal cannula; NIV, non-invasive ventilation; COT, conventional oxygen therapy; ICU, intensive care unit; green squares, the mean difference value for an individual study result; black diamonds, the overall effect size for study results of the group; IV, Inverse variance method; CI, confidence interval; SD, standard deviation.
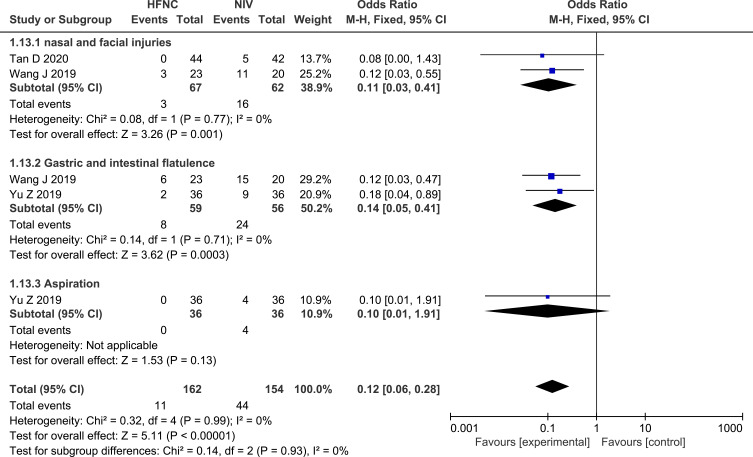
Adverse Events
Among the eight studies that compared HFNC with NIV, two26,30 reported on the incidence of nasal facial skin breakdown, two30,31 reported on the incidence of gastric and intestinal flatulence, while one31 reported on the incidence of aspiration. The pooled analysis demonstrated that the application of HFNC led to a significant decrease in the incidence of adverse events, compared with NIV (OR 0.12, 95% CI=0.06,0.28, P<0.00001, I2 = 0%). Forest plot of adverse events is shown in Figure 12.
Figure 12.
Forest plot comparing adverse events between HFNC and NIV.
Abbreviations: HFNC, high flow nasal cannula; NIV, non-invasive ventilation; blue squares, the odds ratio value for an individual study result; black diamonds, the overall effect size for study results of the group; M–H, Mantel–Haenszel method; CI, confidence interval.
Comfort Score and Dyspnea Score
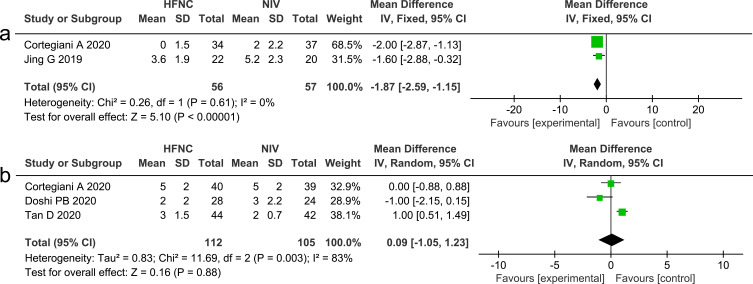
The comfort score of the patient was assessed using a modified 10 cm visual analog scale, in which 0 or 1 meant very comfortable and 10 meant very uncomfortable Two studies13,27 that measured comfort score between HFNC and NIV showed that HFNC could reduce patient discomfort (MD −1.87, 95% CI=−2.59, −1.15, P <0.00001, I2 =0%, n=113). Dyspnea was evaluated using the Borg Score, while three studies13,24,26 that compared HFNC with NIV reported on dyspnea score, and the results showed no significant differences between the two interventions (MD 0.09, 95% CI=−1.05, 1.23, P =0.88, I2 =83%, n=217). Forest plots of comfort score and dyspnea score are presented in Figure 13.
Figure 13.
(a) Forest plot comparing comfort score between HFNC and NIV; (b) Forest plot comparing dyspnea score between HFNC and NIV.
Abbreviations: HFNC, high flow nasal cannula; NIV, non-invasive ventilation; green squares, the mean difference value for an individual study result; black diamonds, the overall effect size for study results of the group; IV, Inverse variance method; CI, confidence interval; SD, standard deviation.
Subgroup Analysis
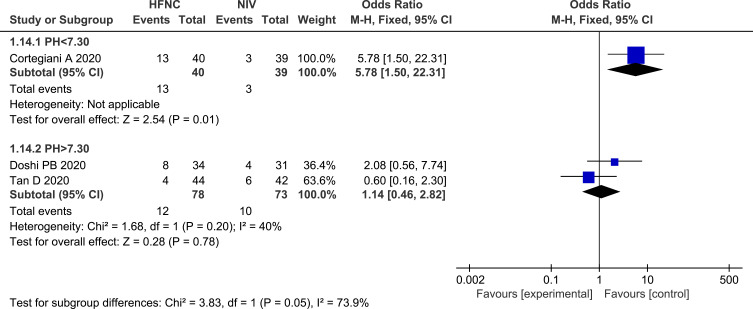
The subgroup analysis between patients with pH <7.30 and patients with PH>7.30 showed that NIV could reduce the incidence of treatment crossover, compared with HFNC in patients with pH <7.30 (OR 5.78, 95% CI=1.50, 22.31, P = 0.01, I2: not applicable, n=79). Forest plot of this subgroup analysis is shown in Figure 14. However, other outcomes, including intubation rate, majority, change in ABGs, and length of stay, were not significantly different in this subgroup analysis.
Figure 14.
Forest plot and subgroup analyses comparing treatment crossover rate between pH<7.30 and pH>7.30.
Abbreviations: HFNC, high flow nasal cannula; NIV, non-invasive ventilation; pH, potential of hydrogen; blue squares, the odds ratio value for an individual study result; black diamonds, the overall effect size for study results of the group; M–H, Mantel–Haenszel method; CI, confidence interval.
Sensitivity Analyses and Publication Bias
The quality assessment showed that one study was considered to have concerns of bias.30 We removed this study and performed a sensitivity analysis, and the results of the analyses were unchanged. The small number of trials included prevented us from performing statistical analysis to assess publication bias.
Discussion
The main findings of our study were that HFNC had a similar efficacy to NIV in terms of intubation rate, mortality, treatment failure, and ABG improvement in adult patients with acute hypercapnic respiratory failure. However, HFNC was more comfortable for the patients and had a lower rate of complications. When comparing HFNC to COT in patients with acute compensated hypercapnic respiratory failure, both treatments could achieve similar changes in ABGs and vital signs and had a comparable intubation rate and mortality, but HFNC could significantly reduce the need for NIV.
In our study, similar tracheal intubation and mortality rates were observed between HFNC and NIV, and between HFNC and COT, and this result is in accordance with previously published meta-analyses.18–20 It is worthy to note that the subgroup analysis revealed that in the non-post-extubation group, patients receiving HFNC appeared to show crossover to NIV more frequently than patients receiving NIV due to ventilate failure. However, among post-extubation patients, treatment crossover rates between the two therapies were comparable. This was mainly due to the fact that patients in the non-post-extubation group had more severe acidosis and a higher PaCO2 at baseline (mean pH, 7.30±0.07; mean PaCO2, 67.1±20.5mmHg) than patients in the post-extubation group (mean pH, 7.46±0.06; mean PaCO2, 51.7±8.2mmHg). It has been recognized that HFNC is an open ventilation system that neither effectively pushes nor pulls gas, so it cannot provide sufficient ventilation for severity patients.8 Our study indicated that HFNC treatment was more likely to fail in patients with severe acidosis and higher PaCO2. On the other hand, NIV has been demonstrated to actively improve the inspiratory tidal volume (VT), and it can effectively increase pH and decrease PaCO2, which ultimately can help avoid intubation and invasive ventilation. Therefore, NIV is considered as the cornerstone of respiratory support for managing patients with hypercapnic respiratory acidosis.3,33 Furthermore, Cortegiani13 found that patients for whom HFNC failed as first therapy and then received NIV needed a longer duration of NIV than those who received NIV at first, even though the intubation rate did not increase. Given this, it is very important to identity predictors for HFNC failure. Li performed a propensity score analysis, which confirmed that a PaCO2 > 59 mmHg after HFNC treatment for 24 h was an independent risk factor for HFNC failure.32 However, there is a lack of sufficient evidence to confirm the most appropriate degree of acidosis for HFNC application, and further severity-related studies are required to determine the population that will benefit the most from HFNC. This study also demonstrated that HFNC can reduce the need for NIV in patients with compensated hypercapnic respiratory failure, compared with COT, suggesting that HFNC may be the first-line oxygen strategy in patients with mild hypercapnia.
In contrast to the results of a prior meta-analyses,18 our investigation showed that HFNC significantly reduced RR, compared with NIV. Several physiological studies have indicated that the use of HFNC can improve horacio-abdominal synchrony, decrease the rapid shallow breathing index (RSBI), reduce inspiratory effort, and finally reduce the I:E ratio and respiratory rate.8,11,34 In a recent physiological study that included 15 patients with cystic fibrosis pulmonary exacerbations, Michael et al confirmed the positive effect of HFNC in decreasing the RR by 3 breaths/min, compared with NIV.21
Another important finding of this study is that HFNC could provide coordinative efficacy, compared with NIV in terms of gas exchange, including decreasing PaCO2 and improving oxygenation, in patients with acute hypercapnia. This result is in accordance with the results of previous meta-analyses.18,20 It is well known that HFNC can maintain stable oxygenation in patients with respiratory failure, making it is possible to explain the observed improvement in oxygenation. However, the result that HFNC can reduce carbon dioxide suggests that despite having an open system, it can still provide ventilatory support for patients with acute hypercapnia. The achieved ventilatory benefit provided by HFNC can most likely be explained through several mechanisms, including the decrease in anatomical dead space and generation of PEEP.
The high flow rate produced by HFNC can continuously wash out carbon dioxide from the anatomical dead space, which subsequently preventing carbon dioxide rebreathing, and effectively decreases PaCO2.9 Moreover, HFNC can generate a modest degree of PEEP, which is flow-dependent, with an average range of 1.5–7 cm- H2O with the mouth closed.10,35 Since positive expiratory pressure may help counterbalance the effects of intrinsic PEEP (PEEPi),5 it can reduce airway resistance, which subsequently reduces the work needed for breathing, and finally achieves improvement in alveolar ventilation. In an interventional clinical study performed on 67 patients hospitalized due to COPD, Jens et al found that HFNC could increase VT by a degree of 93 mL, compared to spontaneous breathing, but no significant differences were observed between different flows.35
Similarly, in two of the included studies that compared HFNC with COT in patients with acute compensated hypercapnic respiratory failure, we found a similar impact upon oxygenation improvement and CO2 clearance between the two interventions, indicating that HFNC exerted a similar effect on gas exchange in this population, compared with COT, despite other benefits of HFNC observed in prognosis.
According to our study, HFNC was generally well tolerated by patients with hypercapnia, and was more comfortable for these patients than NIV and COT, and resulted in a lower rate of complications, compared with NIV. It has been recognized that NIV requires a tight mask, which often causes discomfort and claustrophobia for some patients, increasing the risk of treatment failure.1,33 It should be noted that in GOLD 2023, one of the indications of invasive mechanical ventilation in patients with AECOPD was an inability to tolerate NIV or NIV failure.36 According to Doshi et al24 and Tang,26 treatment intolerance of NIV was significantly higher than that of HFNC, and poor tolerance accounted for about 50% of NIV failure. In contrast, HFNC is an open system that can deliver a well-humidified air stream to patients, and can heat and humidify the gas to avoid nasal and oral dryness caused by conventional oxygen, while it can also avoid nasofacial skin breakdown and gastric and intestinal flatulence caused by NIV.6,7 Therefore, better tolerance by patients contributes to a lower level of treatment interruption in patients treated by HFNC, which subsequently leads to a lower level of treatment failure. Furthermore, for patients for whom NIV failed secondary to tolerance, 75% of patients were successfully treated with HFNC.24 Therefore, HFNC is a potential alternative strategy to NIV that can prevent the need for intubation and invasive ventilation in patients who are intolerant or have contraindications to NIV.
However, our study has certain limitations. First, due to the nature of the interventions, blinding was not possible in all of the studies included, which would have caused bias. Moreover, some of the studies included contained a certain degree of bias due to random sequence generation, allocation concealment, and blinding of outcome assessment, which resulted in low quality. Second, population selection, control intervention, parameter setting, and application time differed between studies, which may have led to a certain level of heterogeneity. Considering these limitations, we conducted several subgroup analyses based on intervention (NIV vs COT), population (post-extubation vs non-post-extubation patients), and severity of acidosis (pH >7.30 vs pH <7.30) to explore whether these differences had an impact on treatment effect. Third, the samples were relatively small in most of the studies included, and as a result, the statistical power of these results may be not be strong enough to confirm our conclusions.
Conclusion
In conclusion, HFNC may have similar efficacy to NIV and COT in terms of intubation rate, mortality, and ABG improvement in adult patients with acute hypercapnic respiratory failure. However, patients with pH<7.30 seemed to experience HFNC failure more frequently, compared with NIV. Otherwise, HFNC may decrease the need for NIV compared to COT in patients with compensated hypercapnia. Large-scale and multicenter RCT trials are needed to clarify the most appropriate population for the application of HFNC for the treatment of acute hypercapnic respiratory failure.
Acknowledgments
We would like to thank TopEdit (www.topeditsci.com) for providing the linguistic assistance during the preparation of this manuscript.
Funding Statement
No funding was received for this study.
Data Sharing Statement
All data supporting the findings of this study are available in the article and its Supplementary Files.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
No conflict of interest existed in this study.
References
- 1.Comellini V, Pacilli AMG, Nava S. Benefits of non‐invasive ventilation in acute hypercapnic respiratory failure. Respirology. 2019;24(4):308–317. doi: 10.1111/resp.13469 [DOI] [PubMed] [Google Scholar]
- 2.Roussos C, Koutsoukou A. Respiratory failure. Eur Respir J. 2003;22(Supplement47):3s–14s. doi: 10.1183/09031936.03.00038503 [DOI] [PubMed] [Google Scholar]
- 3.Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50(2):1602426. doi: 10.1183/13993003.02426-2016 [DOI] [PubMed] [Google Scholar]
- 4.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease.
- 5.Nava S, Navalesi P, Gregoretti C. Interfaces and humidification for noninvasive mechanical ventilation. Respir Care. 2009;54(1):71–84. [PubMed] [Google Scholar]
- 6.Nishimura M. High-flow Nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016;61(4):529–541. doi: 10.4187/respcare.04577 [DOI] [PubMed] [Google Scholar]
- 7.Oczkowski S, Ergan B, Bos L, et al. ERS clinical practice guidelines: high-flow nasal cannula in acute respiratory failure. Eur Respir J. 2022;59(4):2101574. doi: 10.1183/13993003.01574-2021 [DOI] [PubMed] [Google Scholar]
- 8.Bräunlich J, Beyer D, Mai D, Hammerschmidt S, Seyfarth H, Wirtz H. Effects of nasal high flow on ventilation in volunteers, COPD and idiopathic pulmonary fibrosis patients. Respiration. 2013;85(4):319–325. doi: 10.1159/000342027 [DOI] [PubMed] [Google Scholar]
- 9.Bräunlich J, Köhler M, Wirtz H. Nasal highflow improves ventilation in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care. 2007;20(4):126–131. doi: 10.1016/j.aucc.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 11.Pisani L, Fasano L, Corcione N, et al. Change in pulmonary mechanics and the effect on breathing pattern of high flow oxygen therapy in stable hypercapnic COPD. Thorax. 2017;72(4):373–375. doi: 10.1136/thoraxjnl-2016-209673 [DOI] [PubMed] [Google Scholar]
- 12.Bae S, Han M, Kim C, et al. High-flow nasal cannula oxygen therapy can be effective for patients in acute hypoxemic respiratory failure with hypercapnia: a retrospective, propensity score-matched cohort study. J Korean Med Sci. 2020;35(10). doi: 10.3346/jkms.2020.35.e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortegiani A, Longhini F, Madotto F, et al. High flow nasal therapy versus noninvasive ventilation as initial ventilatory strategy in COPD exacerbation: a multicenter non-inferiority randomized trial. Crit Care. 2020;24(1). doi: 10.1186/s13054-020-03409-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee MK, Choi J, Park B, et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. 2018;12(6):2046–2056. doi: 10.1111/crj.12772 [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb J, Capetian P, Hamsen U, et al. German S3 guideline: oxygen therapy in the acute care of adult patients. Respiration. 2022;101(2):214–252. doi: 10.1159/000520294 [DOI] [PubMed] [Google Scholar]
- 16.Xu Z, Zhu L, Zhan J, Liu L. The efficacy and safety of high-flow nasal cannula therapy in patients with COPD and type II respiratory failure: a meta-analysis and systematic review. Eur J Med Res. 2021;26(1). doi: 10.1186/s40001-021-00587-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu W, Zhu M, Xia L, et al. Transnasal high-flow oxygen therapy versus noninvasive positive pressure ventilation in the treatment of COPD with type II respiratory failure: a meta-analysis. Comput Math Methods Med. 2022;2022:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Huang Y, Lei W, Zhang W, Huang J, Trisolini R. High-flow nasal cannula in hypercapnic respiratory failure: a systematic review and meta-analysis. Can Respir J. 2020;2020:1–13. doi: 10.1155/2020/7406457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alnajada AA, Blackwood B, Mobrad A, Akhtar A, Pavlov I, Shyamsundar M. High flow nasal oxygen for acute type two respiratory failure: a systematic review. F1000Research. 2021;10:482. doi: 10.12688/f1000research.52885.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ovtcharenko N, Ho E, Alhazzani W, et al. High-flow nasal cannula versus non-invasive ventilation for acute hypercapnic respiratory failure in adults: a systematic review and meta-analysis of randomized trials. Crit Care. 2022;26(1). doi: 10.1186/s13054-022-04218-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sklar MC, Dres M, Rittayamai N, et al. High-flow nasal oxygen versus noninvasive ventilation in adult patients with cystic fibrosis: a randomized crossover physiological study. Ann Intensive Care. 2018;8(1). doi: 10.1186/s13613-018-0432-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rezaei A, Fakharian A, Ghorbani F, Idani E, Abedini A, Jamaati H. Comparison of high‐flow oxygenation with noninvasive ventilation in COPD exacerbation: a crossover clinical trial. Clin Respir J. 2021;15(4):420–429. doi: 10.1111/crj.13315 [DOI] [PubMed] [Google Scholar]
- 23.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. doi: 10.1371/journal.pmed.1003583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doshi PB, Whittle JS, Dungan G, et al. The ventilatory effect of high velocity nasal insufflation compared to non-invasive positive-pressure ventilation in the treatment of hypercapneic respiratory failure: a subgroup analysis. Heart Lung. 2020;49(5):610–615. doi: 10.1016/j.hrtlng.2020.03.008 [DOI] [PubMed] [Google Scholar]
- 25.Papachatzakis Y, Nikolaidis PT, Kontogiannis S, Trakada G. High-flow oxygen through nasal cannula vs non-invasive ventilation in hypercapnic respiratory failure: a randomized clinical trial. Int J Environ Res Public Health. 2020;17(16):5994. doi: 10.3390/ijerph17165994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan D, Walline JH, Ling B, et al. High-flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease patients after extubation: a multicenter, randomized controlled trial. Crit Care. 2020;24(1). doi: 10.1186/s13054-020-03214-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jing G, Li J, Hao D, et al. Comparison of high flow nasal cannula with noninvasive ventilation in chronic obstructive pulmonary disease patients with hypercapnia in preventing postextubation respiratory failure: a pilot randomized controlled trial. Res Nurs Health. 2019;42(3):217–225. doi: 10.1002/nur.21942 [DOI] [PubMed] [Google Scholar]
- 28.Xia J, Gu S, Lei W, et al. High-flow nasal cannula versus conventional oxygen therapy in acute COPD exacerbation with mild hypercapnia: a multicenter randomized controlled trial. Crit Care. 2022;26(1). doi: 10.1186/s13054-022-03973-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cong L, Zhou L, Liu H, Wang J. Outcomes of high-flow nasal cannula versus non-invasive positive pressure ventilation for patients with acute exacerbations of chronic obstructive pulmonary disease. Int J Clin Exp Med. 2019;12(8):10863–10867. [Google Scholar]
- 30.Wang JJ, Li Q. Randomized controlled study of HFNC and NPPV in the treatment of AECOPD combined with type II respiratory failure. Chin J Integr Med. 2019;2019:1–15. [Google Scholar]
- 31.Yu Z. Efficacy and safety of humidified high flow nasal cannula in chronic obstructive pulmonary disease complicated with type 2 respiratory failure patients after extubation: a randomized controlled trial. Acad J Second Mil Med Univ. 2019;2019:989–994. [Google Scholar]
- 32.Li X, Tang X, Wang R, et al. High-flow nasal cannula for chronic obstructive pulmonary disease with acute compensated hypercapnic respiratory failure: a randomized, controlled trial. Int J Chron Obstruct Pulmon Dis. 2020;15:3051–3061. doi: 10.2147/COPD.S283020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frat JP, Coudroy R, Thille AW. Non‐invasive ventilation or high‐flow oxygen therapy: when to choose one over the other? Respirology. 2019;24(8):724–731. doi: 10.1111/resp.13435 [DOI] [PubMed] [Google Scholar]
- 34.Fraser JF, Spooner AJ, Dunster KR, Anstey CM, Corley A. Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: a randomised crossover trial. Thorax. 2016;71(8):759–761. doi: 10.1136/thoraxjnl-2015-207962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bräunlich J, Dellweg D, Bastian A, et al. Nasal high-flow versus noninvasive ventilation in patients with chronic hypercapnic COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:1411–1421. doi: 10.2147/COPD.S206111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venkatesan P. GOLD COPD report: 2023 update. Lancet Respira Med. 2023;11(1):18. doi: 10.1016/S2213-2600(22)00494-5 [DOI] [PubMed] [Google Scholar]