Abstract
Simple Summary
It has been widely reported that seaweed has numerous bioactive molecules that have been examined for health and growth-promoting effects. The current study was conducted to analyze the effects of various levels (starting from 0.25% to 1.25%) of brown seaweed and green seaweed on blood plasma antioxidant enzyme activities, hepatic antioxidant genes expression, blood plasma lipid profile, breast meat quality, and chemical composition in broiler chickens. The result showed that different levels of brown seaweed and green seaweed had no significant effects on broiler blood plasma catalase, superoxide dismutase, and glutathione peroxidase enzyme activities. In contrast, the hepatic superoxide dismutase 1 gene mRNA expression was significantly higher for birds fed 0.50% and 0.75% brown seaweed. Meanwhile, the total cholesterol and high-density lipoprotein levels were higher in blood plasma for birds fed 0.75 and 1% brown seaweed compared to the negative and positive control groups. The findings showed that different levels of brown seaweed and green seaweed had significantly higher breast meat crude protein content. The current research findings are useful for further studies to investigate the mechanisms and components responsible for affecting the hepatic antioxidant gene expression and blood plasma profile.
Abstract
The study was designed to analyze the effects of brown seaweed (BS) and green seaweed (GS) on blood plasma antioxidant enzyme activities, hepatic antioxidant genes expression, blood plasma lipid profile, breast meat quality, and chemical composition in broiler chickens. The dietary treatment groups contained basal diet [negative control (NC)], basal diet + vitamin E (100 mg/kg feed) [positive control (PC)], basal diet + 0.25, 0.50, 0.75, 1, and 1.25% BS and GS supplements separately. The findings showed that both BS and GS exhibited remarkable antioxidant activity. In contrast, the maximum antioxidant activity was recorded by BS (55.19%), which was significantly higher than the GS (25.74%). Results showed that various levels of BS and GS had no significant effects on broiler blood plasma catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) enzyme activities. The hepatic superoxide dismutase 1 (SOD1) gene mRNA expression was significantly higher for birds fed 0.50% and 0.75% BS. Regarding the plasma lipid profile, the total cholesterol (TC) and high-density lipoprotein (HDL) levels were higher (p < 0.05) for birds fed 0.75 and 1% BS compared to the negative and positive control groups. The findings showed that different levels of BS and GS had significantly higher breast meat crude protein (CP) content.
Keywords: antioxidant enzyme activity, antioxidant gene expression, broiler chickens, blood plasma, brown seaweed, green seaweed, meat composition, meat quality
1. Introduction
Seaweed is a composition of macroalgae of various sizes, colors, and compositions. It has no specifically marked stems, flowers, and leaves [1], and it absorbs light through a wide variety of pigments for photosynthesis. Chlorophylls, carotenoids (carotenes and xanthophylls), and phycobiliproteins are the main photosynthetic pigments in macroalgae [2]. The pigments in the seaweed can be divided into three main groups: brown seaweed (Phaeophyceae), green seaweed (Chlorophyceae), and red seaweed (Rhodophyceae) [3,4].
The poultry industry significantly contributes to agri-food value chains. Therefore, seaweed has the potential to be utilized in animal feeds as a rich source of essential nutrients. A study conducted by Azizi et al. [5] reported that brown seaweed (BS) and green seaweed (GS) contained 59.8 and 55.88% CP, 1.28 and 0.30% ether extract (EE), 5.78 and 5.19% crude fiber (CF), 29.19 and 34.68% carbohydrates, and 9.7 and 9.14% ash contents, respectively. Regarding the mineral contents, BS and GS contained 0.14 and 0.13% Ca, 0.18 and 0.14% Na, 2.96 and 2.20% K, 0.73 and 0.55% Mg (10.73 and 8.54 mg). An amount of 100 g−1 Zn (3.21 and 2.63 mg) was also observed. An amount of 100 g−1 Cu (14.67 and 11.73 mg) was also observed. An amount of 100 g−1 Fe and 13.34 and 11.14 mg. An amount of 100 g−1 Mn contents, respectively, were also observed. They [5] also reported the amino acids composition of seaweed and stated that BS and GS have 13.66 and 11.34 ng/mg lysine, 4.76 and 2.84 ng/mg leucine, 1.53 and 2.40 ng/mg phenylalanine, 24.24 and 8.41 ng/mg threonine, 9.41 and 8.32 ng/mg arginine, 4.18 and 25.41 ng/mg glycine, 4.05 and 2.57 ng/mg aspartic acid, 12.34 and 5.70 ng1/mg glutamic acid, 10.13 and 8.40 ng/mg serine, and 3.77 and 3.30 ng/mg alanine contents, respectively.
Furthermore, seaweed is a fascinating natural source of biologically active compounds used as medicinal components [6]. Among the other bioactive compounds in seaweed, polysaccharides have been proven to have various beneficial properties, such as antioxidant, anticoagulant, anti-inflammatory, antiviral activities, and anticarcinogenic [7].
Numerous free radical species can damage organic molecules, including carbohydrates, lipids, and proteins. It also damages DNA in different mechanisms, such as disrupting DNA duplication, interfering with DNA maintenance, breaking open the molecule, or altering the structure by reacting with the DNA bases [8]. Meanwhile, lipid oxidation is a major cause of food deterioration that may affect the color, flavor, texture, and nutritional value of poultry meat [9]. Therefore, natural antioxidants have been of high interest in recent years. Seaweed contains dietary antioxidants that can be used as medicinal and preventative agents [6,10,11].
Phenolic compounds of seaweed that have more than one hydroxyl group are considered effective primary antioxidants. The phenolic compounds’ antioxidant property is due to their capability to donate an H atom to free radicals and create relatively unreactive phenoxyl radicals [12]. Furthermore, sulphated polysaccharides, such as ulvan and fucoidan, also have substantial antioxidant activities [11]. The antioxidant mechanism of sulphated polysaccharides could be due to the hydrogen of polysaccharides, which combines with free radicals and forms a stable radical to cut off the radical chain reaction [13,14]. A study conducted by Airanthi et al. [15] determined that fucoxanthin and phenolics were responsible for the high antioxidant activities tested. The quest for alternative natural antioxidants has been studied because of the adverse effects of synthetic antioxidants on organisms [15,16]. The presence of additional antioxidant content, such as β-carotene, vitamin C, and α-tocopherol could contribute to brown, green, and red seaweed antioxidant efficacy. Natural tocopherol, ascorbic acid, and caffeic acid work as primary antioxidants, but they are typically less efficient than synthetic ones [12]. Polyphenolic compounds, sulfated polysaccharides, and carotenoids are the most significant antioxidants derived from different types of seaweed [12]. The current study was conducted to examine the effects of different levels of BS and GS on antioxidant status, blood plasma profile, and meat quality in broiler chickens.
2. Materials and Methods
2.1. Birds, Diets, and Experimental Design
This study was conducted in the Poultry Unit, Department of Animal Science, Universiti Putra Malaysia (UPM), following the guidelines approved by the Institutional Animal Care and Use Committee (IACUC) (UPM/IACUC/AUP-R093/2019).
A total of 504 day-old male broiler chickens (Cobb 500) were used for the experiment. The birds were labelled, weighed, and randomly allocated into twelve treatment groups. Each group had six replicates, with seven birds per replicate. The raising conditions followed commercial recommendations for Cobb 500. Birds were raised in a closed house with a penning cage system [120 × 120 cm (length × width)]. The house temperature was set at 32 ± 1 °C on day 1, and it was gradually reduced to around 24 ± 1 °C by the end of the trial. Birds were vaccinated against Newcastle and infectious bronchitis diseases (ND-IB) and infectious bursal disease (IBD) by eye drop at 7 and 21 days. The experimental dietary allocated groups were as follows: NC = negative control (basal diet), PC = positive control (basal diet + vitamin E, 100 mg/kg feed), BS 0.25 = basal diet + 0.25% BS, BS 0.50 = basal diet + 0.50% BS, BS 0.75 = basal diet + 0.75% BS, BS 1 = basal diet + 1% BS, BS 1.25 = basal diet + 1.25% BS, GS 0.25 = basal diet + 0.25% GS, GS 0.50 = basal diet + 0.50% GS, GS 0.75 = basal diet + 0.75% GS, GS 1 = basal diet + 1% GS, and GS 1.25 = basal diet + 1.25% GS. The starter period (Table 1) and finisher period (Table 2) diets were offered from days 0–21 and 22–42, respectively. The diets were formulated based on the broiler nutrient requirements (NRC, 1994) using the FeedLIVE software (FeedLIVE 1.60, Mueang Nonthaburi, Thailand).
Table 1.
Ingredient composition of the starter period (day 1–22) diet.
| Ingredients (%) | Dietary Treatments 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | BS 0.25 | BS 0.50 | BS 0.75 | BS 1 | BS 1.25 | GS 0.25 | GS 0.50 | GS 0.75 | GS 1 | GS 1.25 | |
| Corn | 46.0 | 46.0 | 46.0 | 46.0 | 46.0 | 46.0 | 46.0 | 46.0 | 46.0 | 46.0 | 46.0 | 46.0 |
| Soybean meal | 40.0 | 40.0 | 39.8 | 39.5 | 39.3 | 39.0 | 38.8 | 39.8 | 39.5 | 39.3 | 39.0 | 38.8 |
| Wheat pollard | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Palm oil | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| L-Lysine 2 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| DL-Methionine 3 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| DCP 4 | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 |
| Calcium carbonate | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| Choline chloride | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Mineral mix 5 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Vitamin mix 6 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Antioxidants | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Toxin binder | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Seaweed | - | - | 0.25 | 0.50 | 0.75 | 1.00 | 1.25 | 0.25 | 0.50 | 0.75 | 1.00 | 1.25 |
| Vitamin E | - | 0.01 | - | - | - | - | - | - | - | - | - | - |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Calculated analysis 7 | ||||||||||||
| ME (kcal/kg) 8 | 3040.2 | 3039.9 | 3041.0 | 3041.9 | 3042.7 | 3043.6 | 3044.5 | 3040.7 | 3041.3 | 3041.9 | 3042.5 | 3043.0 |
| Protein | 21.95 | 21.95 | 21.94 | 21.91 | 21.90 | 21.89 | 21.87 | 21.93 | 21.90 | 21.87 | 21.85 | 21.82 |
| Fat | 5.98 | 5.98 | 5.98 | 5.98 | 5.98 | 5.98 | 5.98 | 5.98 | 5.98 | 5.98 | 5.97 | 5.97 |
| Fiber | 4.34 | 4.34 | 4.33 | 4.31 | 4.31 | 4.29 | 4.28 | 4.32 | 4.31 | 4.30 | 4.29 | 4.28 |
| Calcium | 0.83 | 0.83 | 0.83 | 0.83 | 0.83 | 0.83 | 0.83 | 0.83 | 0.83 | 0.83 | 0.83 | 0.83 |
| Total phosphorous | 1.01 | 1.01 | 1.01 | 1.01 | 1.01 | 1.00 | 1.00 | 1.01 | 1.01 | 1.00 | 1.00 | 1.00 |
| Available phosphorus | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
1 Dietary treatments: NC (negative control) = basal diet, PC (positive control) = basal diet + vitamin E (100 mg/kg feed), BS 0.25 = basal diet + 0.25% brown seaweed, BS 0.50 = basal diet + 0.50% brown seaweed, BS 0.75 = basal diet + 0.75% brown seaweed, BS 1 = basal diet + 1% brown seaweed, BS 1.25 = basal diet + 1.25% brown seaweed, GS 0.25 = basal diet + 0.25% green seaweed, GS 0.50 = basal diet + 0.50% green seaweed, GS 0.75 = basal diet + 0.75% green seaweed, GS 1 = basal diet + 1% green seaweed, GS 1.25 = basal diet + 1.25% green seaweed. 2 L-Lysine 78.8% (minimum). 3 DL-Methionine 99%. 4 Dicalcium phosphate. 5 Mineral mix [provided per Kg of the product (mineral mix)]: Selenium 0.20 g; iron 80.0 g; manganese 100.0 g; zinc 80.0 g; copper 15.0 g; potassium 4.0 g; sodium 1.50 g; iodine 1.0 g and cobalt 0.25 g. 6 Vitamin premix [provided per Kg of the product (vitamin premix)]: Vitamin A 35.0 MIU; vitamin D3 9.0 MIU; vitamin E 90.0 g; vitamin K3 6.0 g; vitamin B1 7.0 g; vitamin b2 22.0 g; vitamin B6 12.0 g; vitamin B12 0.070 g; pantothenic acid 35.0 g; nicotinic acid 120.0 g; folic acid 3.0 g; biotin 300.000 mg; phytase 25,000.0 FTU cobalamin 0.05 mg; thiamine 1.43 mg; riboflavin 3.44 mg; folic acid 0.56 mg; biotin 0.05 mg; pantothenic acid 6.46 mg; niacin 40.17 mg and pyridoxine 2.29 mg. 7 The diets were formulated using FeedLIVE software. 8 Metabolizable energy.
Table 2.
Ingredient composition of the finisher period (day 22–42) diet.
| Ingredients (%) | Dietary Treatments 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | BS 0.25 | BS 0.50 | BS 0.75 | BS 1 | BS 1.25 | GS 0.25 | GS 0.50 | GS 0.75 | GS 1 | GS 1.25 | |
| Corn | 52.0 | 52.0 | 52.0 | 52.0 | 52.0 | 52.0 | 52.0 | 52.0 | 52.0 | 52.0 | 52.0 | 52.0 |
| Soybean meal | 32.0 | 32.0 | 31.8 | 31.5 | 31.3 | 31.0 | 30.8 | 31.8 | 31.5 | 31.3 | 31.0 | 30.8 |
| Wheat pollard | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| Palm oil | 5.10 | 5.10 | 5.10 | 5.10 | 5.10 | 5.10 | 5.10 | 5.10 | 5.10 | 5.10 | 5.10 | 5.10 |
| L-Lysine 2 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| DL-Methionine 3 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| DCP 4 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 |
| Calcium carbonate | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Choline chloride | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Mineral mix 5 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Vitamin mix 6 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Antioxidants | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Toxin binder | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Seaweed | - | - | 0.25 | 0.50 | 0.75 | 1.00 | 1.25 | 0.25 | 0.50 | 0.75 | 1.00 | 1.25 |
| Vitamin E | - | 0.01 | - | - | - | - | - | - | - | - | - | - |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Calculated analysis 7 | ||||||||||||
| ME (kcal/kg) 8 | 3149.8 | 3149.5 | 3150.7 | 3151.5 | 3152.4 | 3153.3 | 3154.1 | 3150.4 | 3150.9 | 3151.6 | 3152.1 | 3152.7 |
| Protein | 19.06 | 19.06 | 19.05 | 19.03 | 19.01 | 19.00 | 18.98 | 19.04 | 19.01 | 18.98 | 18.96 | 18.93 |
| Fat | 7.19 | 7.19 | 7.19 | 7.19 | 7.19 | 7.19 | 7.19 | 7.19 | 7.19 | 7.18 | 7.18 | 7.18 |
| Fiber | 4.00 | 4.00 | 3.99 | 3.98 | 3.97 | 3.96 | 3.95 | 3.99 | 3.98 | 3.97 | 3.96 | 3.94 |
| Calcium | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 |
| Total phosphorous | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 |
| Available phosphorus | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 |
1 Dietary treatments: NC (negative control) = basal diet, PC (positive control) = basal diet + vitamin E (100 mg/kg feed), BS 0.25 = basal diet + 0.25% brown seaweed, BS 0.50 = basal diet + 0.50% brown seaweed, BS 0.75 = basal diet + 0.75% brown seaweed, BS 1 = basal diet + 1% brown seaweed, BS 1.25 = basal diet + 1.25% brown seaweed, GS 0.25 = basal diet + 0.25% green seaweed, GS 0.50 = basal diet + 0.50% green seaweed, GS 0.75 = basal diet + 0.75% green seaweed, GS 1 = basal diet + 1% green seaweed, GS 1.25 = basal diet + 1.25% green seaweed. 2 L-Lysine (minimum) 78.8%. 3 DL-Methionine 99%. 4 Dicalcium phosphate. 5 Mineral mix [provided per Kg of the product (mineral mix)]: Selenium 0.20 g; iron 80.0 g; manganese 100.0 g; zinc 80.0 g; copper 15.0 g; potassium 4.0 g; sodium 1.50 g; iodine 1.0 g and cobalt 0.25 g. 6 Vitamin premix [provided per Kg of the product (Vitamin premix)]: Vitamin A 35.0 MIU; vitamin D3 9.0 MIU; vitamin E 90.0 g; vitamin K3 6.0 g; vitamin B1 7.0 g; vitamin b2 22.0 g; vitamin B6 12.0 g; vitamin B12 0.070 g; pantothenic acid 35.0 g; nicotinic acid 120.0 g; folic acid 3.0 g; biotin 300.000 mg; phytase 25,000.0 FTU cobalamin 0.05 mg; thiamine 1.43 mg; riboflavin 3.44 mg; folic acid 0.56 mg; biotin 0.05 mg; pantothenic acid 6.46 mg; n7iacin 40.17 mg and pyridoxine 2.29 mg. 7 The diets were formulated using FeedLIVE software. 8 Metabolizable energy.
2.2. Sample Collection
Dried seaweed samples were ground as a fine powder and used to determine antioxidant capacity. The seaweed was obtained from Promise Earth (M) Sdn. Bhd., Selangor, Malaysia.
At the end of the feeding trial, six chickens from each treatment were randomly selected and euthanized (cervical dislocation) for the sampling. Blood samples were collected into the blood collection tubes containing anticoagulant sodium heparin during the neck cutting and were stored in ice. The blood samples were then centrifuged at 3500× g for 15 min at 4 °C to harvest the plasma. The plasma was kept for enzymes activities determination and lipid profile analysis. Parts of the liver were collected, immediately frozen in liquid nitrogen, and stored at −80 °C for the gene expression analysis. In addition, approximately 30–40 g of fresh meat samples from the pectoralis major muscle were collected and immediately stored at 4 °C for meat quality traits and meat proximate composition determination.
2.3. Antioxidant Property of Brown and Green Seaweed
2.3.1. Preparation of Seaweed Extract
The seaweed methanolic extract was prepared, as described by [17], with a minor modification. A 60 mg dried seaweed sample was weighed in a 1.5 mL tube, and 0.5 mL of methanol was added. The sample was sonicated for 20 min at 30 °C and centrifuged at 16,000× g for 5 min. The supernatants were collected in separated 1.5 mL tubes, and the residue was re-extracted two times under the same conditions.
2.3.2. Antioxidant Activity Assay
The 2,2-Diphenyl-1-picryl-hydrazyl (DPPH) assay was conducted to determine the antioxidant activity of seaweed. The assay was performed according to the 96-well plate scheme, as described by [17]. Briefly, 10 µL from the early prepared sample extract was arranged in the 96-well plate, and 100 µL of DPPH solution (761 µM DPPH in 80% methanol) was added to each well. The mixture was incubated for 2 h in a dark chamber at room temperature. The absorbance was measured at 515 nm using a microplate spectrophotometer (Thermo Scientific™ Multiskan™ GO Microplate Spectrophotometer, Waltham, MA, USA). The equation below was used to calculate the percentage of DPPH free radical scavenging activity [18].
| (1) |
where, A0 = absorbance of the DPPH alone, AS = absorbance of the sample.
2.4. Plasma Antioxidant Enzymes Activities
The plasma CAT, SOD, and GPx enzyme activities were measured using commercial kits, based on the quantitative colorimetric determination method.
2.4.1. Catalase Activity
The CAT activity was measured using the EnzyChromTM catalase assay kit (ECAT-100, BioAssay Systems, Hayward, CA, USA). The test depends on the degradation of H2O2 using redox dye. Following the manufacturer’s instructions, all kit components were equilibrated to room temperature and briefly centrifuged all tubes before opening. A 10 µL of the samples, assay buffer as sample blank, and reconstituted positive control solution (catalase) as positive control were loaded into wells of the 96-well plate. Afterward, 50 µM H2O2 substrate was prepared, and 90 µL of the reagent was added to the samples, sample blank, and positive control wells. The microplate was shaken to mix and incubated at room temperature for 30 min. A four-point standard curve was prepared by serial concentration, mixing 400 µM H2O2 reagent with dH2O. Next, 10 µL of the standard was transferred to separate wells of the plate, and 90 µL assay buffers were added to the standards. At the end of 30 min of incubation, the detection reagent (100 µL) was added and incubated for 10 min at room temperature. Lastly, the optical density was read at 570 nm using a microplate reader (Multiskan TM GO, Thermo Scientific TM, Waltham, MA, USA), and the CAT activity was calculated from the standard curve.
2.4.2. Superoxide Dismutase Activity
EnzyChromTM Superoxide Dismutase Assay Kit (ESOD-100, BioAssay Systems, Hayward, CA, USA) was used to determine plasma SOD activity. The assay provides superoxide through a xanthin oxidase catalyzed reaction, and superoxide reacts with a specific dye (WST-1) to form a colored product. Based on the protocol supplied by the manufacturer, all reagents were prepared, and 20 µL samples were loaded into a 96-well plate. A 3 U/mL SOD enzyme standard was made, serially diluted, and 20 µL of each diluent was transferred to separated wells. The working reagent was prepared by mixing assay buffer, xanthine, and WST-1 dye. A 160 µL working reagent was transferred to each well and mixed. In the last step, 20 µL diluted xanthine oxidase enzyme (1:20 in diluent) was added to each assay well and mixed. The assay well plate was read at 450 nm immediately, and, then, after 60 min, dark incubation at room temperature was performed using a microplate reader (Multiskan GO, Thermo Scientific, Waltham, MA, USA). The standard curve was used to calculate the SOD concentration in the samples.
2.4.3. Glutathione Peroxidase Activity
Plasma GPx activity was determined using EnzyChromTM Glutathione Peroxidase Assay Kit (EGPx-100, BioAssay Systems, Hayward, CA, USA). The assay is based on the direct measures of NADPH consumption in the enzyme-coupled reactions. According to the manufacturer’s protocol, reagents were prepared/reconstituted, and standards were prepared by mixing 6 mM NADPH with H2O. A 10 µL of each diluted standard was loaded to separated wells of a 96-well plate, followed by adding 190 µL assay buffers to each standard well. A 10 µL of each sample was transferred into wells, and background control wells were prepared by adding a 10 µL assay buffer to each well. The working reagent was prepared by mixing assay buffer, glutathione, NADPH, and glutathione reductase enzyme. A 90 µL working reagent was added to the sample and control wells and mixed. In the last step, 100 µL diluted peroxide solution was added to the sample and control wells and mixed. The optical density was read immediately, and, after 4 min at 340 nm, a microplate reader (Multiskan GO, Thermo Scientific, USA) was used. The standard curve was used to calculate the GPx activity of samples.
2.5. Hepatic Antioxidant Genes Expression
The RNA was extracted from the liver tissue following the manufacturer’s instructions of the NucleoSpin® RNA Plus kit (MACHEREY-NAGEL, Allentown, PA, USA). The gDNA was removed through the lysate filtration using a NucleoSpin® gDNA Removal Column (MACHEREY-NAGEL, Allentown, USA). Based on the instructions of the manufacturer, the RNA was purified using a NucleoSpin® RNA Plus Column (MACHEREY-NAGEL, Allentown, USA). The ultraviolet-visible spectroscopy was used to determine the concentration and purity of RNA by using a spectrophotometer (Multiskan GO, Thermo Scientific, USA). The RNA was then converted into complementary DNA (cDNA) using a cDNA synthesis kit (Biotechrabbit, Hennigsdorf, Germany), following the manufacturer’s instructions.
Real-time PCR was conducted using a LightCycler® 480 qPCR system (Roche Molecular Systems, Indianapolis, IN, USA). The Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene [19]. A qPCR master mix (20 µL) was prepared using a CAPITALTM qPCR Green Mix, 4× (Biotechrabbit, Hennigsdorf, Germany). The master mix contained 5 µL of SYBR Green Master Mix, 1 µL of each 200 nm forward and reverse primers, 1 µL of template cDNA, and 12 µL of RNase-free water.
The qPCR cycling condition was programmed as follows. The initial denaturation temperature was 95 °C for 2.5 min, followed by 45 cycles of denaturation at 95 °C for 15 sec and annealing for 30 sec at 60 °C. The final extension for melt analysis was based on the instrument instruction, LightCycler® 480 qPCR system (Roche Molecular Systems, Indianapolis, IN, USA). In order to confirm the specificity of the amplification, a melting curve analysis was performed at the end of the amplification cycle. The relative gene expression was quantified following the Livak and Schmittgen [20] recommendation, based on housekeeping gene amplification. The targeted gene primer sequences are presented in Table 3.
Table 3.
The primer sequences of the housekeeping and target genes.
| Target Gene | Primer Sequence 5′-3′ | bp | Accession No. | |
|---|---|---|---|---|
| SOD1 | F-CACTGCATCATTGGCCGTACCA | R-GCTTGCACACGGAAGAGCAAGT | 224 | NM_205064.1 |
| GPX1 | F-GCTGTTCGCCTTCCTGAGAG | R-GTTCCAGGAGACGTCGTTGC | 118 | NM_001277853.2 |
| CAT | F-TGGCGGTAGGAGTCTGGTCT | R-GTCCCGTCCGTCAGCCATTT | 139 | NM_001031215.2 |
| GAPDH | F-CTGGCAAAGTCCAAGTGGTG | R-AGCACCACCCTTCAGATGAG | 275 | NM_204305.1 |
F = Forward, R: Reverse. bp (base pair) = Product size. SOD1 = Superoxide dismutase 1, GPX1 = Glutathione peroxidase 1, CAT = Catalase, GAPDH = Glyceraldehyde-3-phosphate dehydrogenase.
2.6. Plasma Lipid Profile
Plasma was separated by centrifugation at 3500× g at 4 °C for 15 min [21]. The plasma was harvested in the 1.5 mL tube and stored at −80 °C until analysis. The analysis of plasma TC, triglycerides (TG), HDL, and low-density lipoprotein (LDL) were determined on a Dimension® Xpand® Plus Integrated Chemistry System (Siemens Healthcare Diagnostics, Deerfield, IL, USA). In addition, the very-low-density lipoprotein (VLDL) concentration was calculated by dividing plasma TG by 5 [22].
2.7. Proximate Analysis of Breast Meat
Proximate analysis was performed for feed and digesta as described in AOAC (AOAC, 1995). Briefly, the dry moisture content was determined by drying the samples at 105 °C for 24 h. The ash contents were determined by the combustion of samples at 550 °C in a muffle furnace for 6 h. The Kjeldahl method determined the CP content using Foss Digestor™ System (Foss Analytical, Denmark) for sample digestion and Foss Kjeltec 2300 (Foss Analytical, Denmark) for distillation and titration. The EE content was determined using the Soxtec system (Tecator, Sweden).
2.8. Meat Quality Determination
2.8.1. Meat pH
A pH meter (Mettler Toledo, AG 8603, Switzerland) was used to measure the pH of breast muscle. Approximately 0.5 g of breast meat was weighed, crushed, and homogenized for around 20 sec in 10 mL ice-cold distilled water using the homogenizer (Wiggen Hauser® D-500, Germany), and the pH of each sample was recorded.
2.8.2. Meat Color
The meat samples were removed from the packaging and allowed to bloom in the air for 20 min before color measurement. Meat color measurement was conducted using a Color Flex Spectrophotometer (Hunter Lab Reston, VA, USA), using the International Commission on Illumination (CIE) color values. The instrument was calibrated at a 400–700 nm wavelength to express the meat color data. Three measurements were taken at three separate locations for each sample. The color for each sample was expressed according to CIE values for L* (lightness), a* (redness), and b* (yellowness).
2.8.3. Water Holding Capacity of Meat
The water holding capacity (WHC) was determined in terms of drip and cooking losses, as described by Honikel [23].
Around 30 g of fresh meat sample was weighed (initial weight) and vacuum-packed to determine the drip loss. The sample was kept in the chiller at 4 °C for 24 h and seven days. The samples were then patted dry using a paper towel and weighed again (final weight). The calculation was performed as follows:
| (2) |
The breast meat sample was kept in a chiller at 4 °C for 24 h and seven days for the cooking loss determination. The sample was weighed and recorded (W1) and transferred into a preheated water bath set at 80 °C for 20 min. After cooling, the sample was patted dry using a paper towel without squeezing, reweighed, and recorded (W2). The cooking loss was calculated based on the following equation:
| (3) |
where, W1= initial sample weight before cooking, W2= sample weight after cooking.
2.8.4. Meat Tenderness or Shear Force
The tenderness or shear force examination is based on the mechanical force (kg) required to shear the cooked meat’s muscle fibers. The previously cooked samples for cooking loss determination (at 80 °C for 20 min) were used to measure meat tenderness. The samples were cut as; 1 cm width, 1 cm thickness, and 2 cm length, matching the muscle fibers’ direction [24]. The method was applied according to the procedures described by [25]. The sample was sheared perpendicularly to the muscle fibers using TA.HD plus® texture analyzer (Stable Micro System, Surrey, UK). The analyzer was equipped with a Volodkevitch blade set. The apparatus was standardized at 15 mm return distance for height, 5 kg for weight, and the blade speed was set at 10 mm/sec. The shear force values are noted as the average of all samples value for each sample.
2.9. Statistical Analysis
The general linear model (GLM), by one-way ANOVA of the statistical analysis system (SAS), was used for the statistical analysis. Duncan’s multiple range test was used to compare the significant difference between the treatment groups at p < 0.05. The orthogonal polynomial contrast of SAS was used to determine the linear and quadratic effects of dietary increasing brown and green seaweed inclusion levels. The statistical model for the experiment was Yijk = µ + Tij + Eijk. Whereas, Yijk = dependent variable, µ = general mean, Tij = effect of dietary treatment, and Eijk = experimental error.
3. Results
3.1. Antioxidant Property of Brown and Green Seaweed
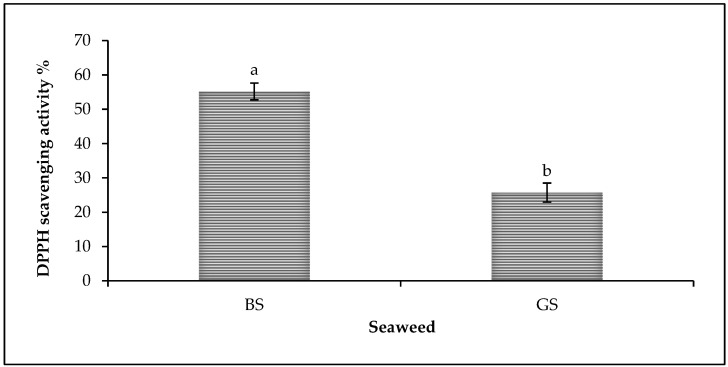
The result of the DPPH scavenging potential of seaweed is presented in Figure 1. The findings showed that both BS and GS exhibited remarkable antioxidant activity. Whereas, the maximum antioxidant activity was recorded by BS (55.19%), which was also significantly higher (p < 0.05) than the GS (25.74%).
Figure 1.
DPPH radical scavenging activities percentage of seaweed. BS = brown seaweed, GS = green seaweed. a,b Different letters on standard error bars indicate a significant difference between seaweed types (p < 0.05). Data are shown as means and standard error (n = 6).
3.2. Plasma Antioxidant Enzymes Activities
The effects of seaweed on broiler chickens’ blood plasma antioxidant enzyme activities are presented in Table 4. The results showed that various brown and green seaweed supplementations had no significant effects on blood plasma CAT, SOD, or GPx enzyme activities.
Table 4.
Effects of different brown and green seaweed levels on plasma antioxidant enzyme activities in broiler chickens.
| Enzymes 1 | Dietary Treatments 2 | SEM 3 | p-Values | Contrast p-Values 4 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | BS 0.25 | BS 0.50 | BS 0.75 | BS 1 | BS 1.25 | GS 0.25 | GS 0.50 | GS 0.75 | GS 1 | GS 1.25 | Line. | Quad. | |||
| CAT (U/L) | 4.11 | 3.71 | 3.73 | 3.73 | 3.65 | 3.84 | 4.16 | 3.46 | 3.97 | 3.96 | 3.92 | 4.00 | 0.21 | 0.6222 | 0.3072 | 0.1506 |
| SOD (U/mL) | 3.10 | 3.11 | 3.14 | 3.08 | 3.11 | 3.09 | 3.15 | 3.04 | 3.10 | 3.12 | 3.14 | 3.11 | 0.04 | 0.9395 | 0.4899 | 0.8876 |
| GPx (U/L) | 0.48 | 0.48 | 0.51 | 0.46 | 0.49 | 0.47 | 0.43 | 0.51 | 0.44 | 0.46 | 0.46 | 0.47 | 0.03 | 0.6493 | 0.5120 | 0.6833 |
1 Enzymes: CAT = catalase. One unit is the amount of CAT that decomposes 1 µmole of H2O2 per min at pH 7.0 and room temperature; SOD = superoxide dismutase. One unit is the amount of SOD that catalyze 1 µmole of superoxide into O2 and H2O2 per min under the condition of the assay; GPx = glutathione peroxidase. One unit is the amount of GPX that produces 1 µmole of glutathione disulfide per min at pH 7.6 and room temperature. 2 Dietary treatments: NC (negative control) = basal diet, PC (positive control) = basal diet + vitamin E (100 mg/kg feed), BS 0.25 = basal diet + 0.25% brown seaweed, BS 0.50 = basal diet + 0.50% brown seaweed, BS 0.75 = basal diet + 0.75% brown seaweed, BS 1 = basal diet + 1% brown seaweed, BS 1.25 = basal diet + 1.25% brown seaweed, GS 0.25 = basal diet + 0.25% green seaweed, GS 0.50 = basal diet + 0.50% green seaweed, GS 0.75 = basal diet + 0.75% green seaweed, GS 1 = basal diet + 1% green seaweed, GS 1.25 = basal diet + 1.25% green seaweed. 3 SEM = standard error of means. 4 Contrast p-Values = orthogonal polynomial contrasts of dietary increasing brown and green seaweed inclusion levels (0.0 to 1.25%).
3.3. Hepatic Antioxidant Genes Expression
The mRNA expression of the hepatic SOD1, GPX1, and CAT genes of broiler chickens fed various levels of brown and green seaweed are presented in Table 5. The result showed that the hepatic SOD1 mRNA expression was higher (p < 0.05) for birds fed 0.50% and 0.75% BS than in the NC and PC groups. On the other hand, no difference (p > 0.05) was observed in the SOD1 mRNA expression for different GS groups compared to the control groups. Meanwhile, brown and green seaweed had no effects (p > 0.05) on the hepatic GPX1 and CAT genes’ mRNA expression.
Table 5.
Effects of brown and green seaweed on SOD, GPX1, and CAT mRNA expression in broiler chickens.
| Parameters 1 (mRNA Fold Change) |
Dietary Treatments 2 | SEM 3 | p-Values | Contrast p-Values 4 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | BS 0.25 | BS 0.50 | BS 0.75 | BS 1 | BS 1.25 | GS 0.25 | GS 0.50 | GS 0.75 | GS 1 | GS 1.25 | Line. | Quad. | |||
| SOD1 | 1 c | 1.053 c | 1.13 b,c | 1.517 a,b | 1.604 a | 0.798 c | 1.174 a,b,c | 0.935 c | 1.208 a,b,c | 1.176 a,b,c | 0.992 c | 1.211 a,b,c | 0.049 | 0.0193 | 0.5515 | 0.0597 |
| GPX1 | 1 | 1.038 | 1.096 | 0.763 | 1.020 | 0.979 | 0.972 | 0.834 | 0.643 | 1.013 | 0.779 | 0.912 | 0.046 | 0.4557 | 0.4080 | 0.3860 |
| CAT | 1 | 1.002 | 1.301 | 1.050 | 1.007 | 1.034 | 0.793 | 1.029 | 0.847 | 1.074 | 1.064 | 0.858 | 0.041 | 0.7925 | 0.2322 | 0.5524 |
a,b,c Means with different superscripts in the same row indicates significant difference (p < 0.05). 1 Parameters: SOD1 = superoxide dismutase 1, GPX1 = glutathione peroxidase 1, CAT = catalase. 2 Dietary treatments: NC (negative control) = basal diet, PC (positive control) = basal diet + vitamin E (100 mg/kg feed), BS 0.25 = basal diet + 0.25% brown seaweed, BS 0.50 = basal diet + 0.50% brown seaweed, BS 0.75 = basal diet + 0.75% brown seaweed, BS 1 = basal diet + 1% brown seaweed, BS 1.25 = basal diet + 1.25% brown seaweed, GS 0.25 = basal diet + 0.25% green seaweed, GS 0.50 = basal diet + 0.50% green seaweed, GS 0.75 = basal diet + 0.75% green seaweed, GS 1 = basal diet + 1% green seaweed, GS 1.25 = basal diet + 1.25% green seaweed. 3 SEM = standard error of means. 4 Contrast p-values = orthogonal polynomial contrasts of dietary increasing brown and green seaweed inclusion levels (0.0 to 1.25%).
3.4. Plasma Lipid Profile
The effects of BS and GS on blood plasma TC, TG, HDL, LDL, and VLD of broiler chicken are presented in Table 6. Various brown and green seaweed levels had no effects (p > 0.05) on plasma TG, LDL, and VLDL levels. However, the TC and HDL levels were significantly higher (p < 0.05) for birds fed 0.75 and 1% BS compared to the NC groups. No significant difference was observed in plasma TC and HDL for birds fed GS supplemented feed compared to the NC and PC groups.
Table 6.
Effects of brown and green seaweed on broiler plasma lipid profile.
| Parameters 1 (mmol/L) |
Dietary Treatments 2 | SEM 3 | p-Values | Contrast p-Values 4 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | BS 0.25 | BS 0.50 | BS 0.75 | BS 1 | BS 1.25 | GS 0.25 | GS 0.50 | GS 0.75 | GS 1 | GS 1.25 | Line. | Quad. | |||
| TC | 2.43 c | 2.76 b,c | 2.9 b,c | 2.95 b,c | 4.0 a | 3.5 a,b | 2.98 b,c | 3.05 b,c | 3.28 a,b,c | 3.18 a,b,c | 2.9 b,c | 2.6 b,c | 0.26 | 0.0287 | 0.0182 | 0.1473 |
| TG | 0.31 | 0.29 | 0.3 | 0.34 | 0.37 | 0.39 | 0.42 | 0.54 | 0.41 | 0.29 | 0.45 | 0.48 | 0.07 | 0.3979 | 0.0555 | 0.6822 |
| HDL | 1.85 c | 2.68 b,c | 2.35 b,c | 2.45 b,c | 3.68 a | 2.74 b | 2.44 b,c | 2.5 b,c | 2.64 b,c | 2.68 b,c | 2.42 b,c | 2.19 b,c | 0.22 | 0.0067 | 0.0110 | 0.0124 |
| LDL | 0.57 | 0.65 | 0.57 | 0.58 | 0.82 | 0.57 | 0.58 | 0.58 | 0.75 | 0.6 | 0.51 | 0.53 | 0.09 | 0.5385 | 0.1541 | 0.5914 |
| VLDL | 0.06 | 0.07 | 0.08 | 0.08 | 0.11 | 0.08 | 0.06 | 0.09 | 0.1 | 0.06 | 0.07 | 0.07 | 0.01 | 0.3979 | 0.0566 | 0.6360 |
a,b,c Means with different superscripts in the same row indicate a significant difference (p < 0.05). 1 Parameters: TC = total cholesterol, TG = triglyceride, HDL = high-density lipoprotein, LDL = low-density lipoprotein, VLDL = very low-density lipoprotein. 2 Dietary treatments: NC (negative control) = basal diet, PC (positive control) = basal diet + vitamin E (100 mg/kg feed), BS 0.25 = basal diet + 0.25% brown seaweed, BS 0.50 = basal diet + 0.50% brown seaweed, BS 0.75 = basal diet + 0.75% brown seaweed, BS 1 = basal diet + 1% brown seaweed, BS 1.25 = basal diet + 1.25% brown seaweed, GS 0.25 = basal diet + 0.25% green seaweed, GS 0.50 = basal diet + 0.50% green seaweed, GS 0.75 = basal diet + 0.75% green seaweed, GS 1 = basal diet + 1% green seaweed, GS 1.25 = basal diet + 1.25% green seaweed. 3 SEM = standard error of means. 4 Contrast p-Values = orthogonal polynomial contrasts of dietary increasing brown and green seaweed inclusion levels (0.0 to 1.25%).
3.5. Proximate Analysis of Breast Meat
The chemical composition of breast meat in broiler chickens fed seaweed is presented in Table 7. Birds fed 0.25 and 0.50% BS and 0.25, 0.75 and 1% GS had higher (p < 0.05) breast meat CP content compared to the NC birds. In contrast, the EE content of breast meat was significantly lower for birds fed 0.50% BS and 0.75 and 1.25% GS. No differences (p > 0.05) were observed in the moisture and ash contents of the breast meat among the dietary treatments.
Table 7.
Effects of brown and green seaweed on broiler breast meat proximate composition.
| Nutrients 1 (%) |
Dietary Treatments 2 | Contrast p-Values 4 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | BS 0.25 | BS 0.50 | BS 0.75 | BS 1 | BS 1.25 | GS 0.25 | GS 0.50 | GS 0.75 | GS 1 | GS 1.25 | SEM 3 | p-Values | Line. | Quad. | |
| Moisture | 74.43 | 73.03 | 74.25 | 74.03 | 74.18 | 74.38 | 74.17 | 73.5 | 73.56 | 74.71 | 74.04 | 74.1 | 0.40 | 0.3168 | 0.0755 | 0.6850 |
| CP | 23.04 c | 23.85 b,c | 24.51 a,b | 24.69 a,b | 23.93 a,b,c | 23.79 b,c | 24.29 a,b,c | 24.33 a,b | 25.14 a | 24.04 a,b,c | 24.75 a,b | 23.71 b,c | 0.38 | 0.0404 | 0.0218 | 0.4461 |
| EE | 2.23 a | 2.19 a,b | 1.99 a,b,c | 1.88 b,c | 1.93 a,b,c | 1.92 a,b,c | 2.11 a,b | 2.05 a,b | 1.96 a,b,c | 1.70 c | 2.05 a,b | 1.87 b,c | 0.09 | 0.0258 | 0.7959 | 0.0619 |
| Ash | 1.79 | 1.89 | 1.68 | 1.66 | 1.75 | 1.67 | 1.76 | 1.78 | 1.74 | 1.67 | 1.7 | 1.64 | 0.07 | 0.4587 | 0.5104 | 0.2256 |
a,b,c Means with different superscripts in the same row indicates significant difference (p < 0.05). 1 Nutrients: CP = crude protein, EE (ether extract). 2 Dietary treatments: NC (negative control) = basal diet, PC (positive control) = basal diet + vitamin E (100 mg/kg feed), BS 0.25 = basal diet + 0.25% brown seaweed, BS 0.50 = basal diet + 0.50% brown seaweed, BS 0.75 = basal diet + 0.75% brown seaweed, BS 1 = basal diet + 1% brown seaweed, BS 1.25 = basal diet + 1.25% brown seaweed, GS 0.25 = basal diet + 0.25% green seaweed, GS 0.50 = basal diet + 0.50% green seaweed, GS 0.75 = basal diet + 0.75% green seaweed, GS 1 = basal diet + 1% green seaweed, GS 1.25 = basal diet + 1.25% green seaweed. 3 SEM = standard error of means. 4 Contrast p-Values = orthogonal polynomial contrasts of dietary increasing brown and green seaweed inclusion levels (0.0 to 1.25%).
3.6. Meat Quality
The effects of BS and GS on breast meat color, pH, drip loss, cooking loss, and shear force after days one and seven of storage are presented in Table 8.
Table 8.
Effects of brown and green seaweed on broiler breast meat quality.
| Parameters | Dietary Treatments 1 | SEM 2 | p-Values | Contrast p-Values 3 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | BS 0.25 | BS 0.50 | BS 0.75 | BS 1 | BS 1.25 | GS 0.25 | GS 0.50 | GS 0.75 | GS 1 | GS 1.25 | Line. | Quad. | |||
| Day 1 | ||||||||||||||||
| pH | 6.02 | 5.90 | 5.78 | 5.87 | 6.03 | 5.87 | 6.05 | 5.94 | 5.79 | 5.97 | 5.83 | 5.83 | 0.07 | 0.2827 | 0.7491 | 0.9708 |
| Drip loss% | 1.80 | 1.55 | 1.72 | 1.70 | 1.44 | 1.72 | 1.38 | 1.46 | 1.39 | 1.07 | 1.23 | 1.56 | 0.23 | 0.6304 | 0.9737 | 0.9700 |
| Cooking loss% | 28.8 | 27.21 | 27.98 | 25.88 | 23.62 | 25.56 | 23.55 | 21.02 | 26.2 | 22.28 | 23.04 | 25.94 | 28.8 | 0.0939 | 0.2273 | 0.1439 |
| Shear force (g) | 978.3 | 811.5 | 1112.9 | 832.9 | 913.2 | 971.2 | 788.7 | 869.3 | 1058.6 | 1016.6 | 869.9 | 978.8 | 88.8 | 0.1726 | 0.2048 | 0.1535 |
| Colour 4 | ||||||||||||||||
| L* | 57.47 a | 50.93 b,c | 47.15 c,d | 44.06 d | 50.65 b,c | 50 b,c | 50.25 b,c | 48.59 b,c,d | 50.48 b,c | 50.63 b,c | 49.49 b,c,d | 53.43 a,b | 1.73 | 0.0018 | 0.8265 | 0.0002 |
| a* | 4.75 | 5.73 | 6.54 | 6.72 | 5.76 | 6.74 | 5.60 | 5.80 | 6.08 | 6.72 | 7.80 | 6.77 | 0.94 | 0.7647 | 0.0745 | 0.5778 |
| b* | 17.43 b,c | 20.45 a,b | 18.74 a,b,c | 20.35 a,b | 15.98 c | 19.48 a,b | 20.46 a,b | 19.57 a,b | 20.99 a,b | 20.12 a,b | 21.66 a | 20.73 a,b | 1.07 | 0.0390 | 0.0227 | 0.2827 |
| Day 7 | ||||||||||||||||
| pH | 6.07 | 5.97 | 6.04 | 6.13 | 6.18 | 6.09 | 5.89 | 5.84 | 5.95 | 5.87 | 5.96 | 5.95 | 0.07 | 0.0968 | 0.5520 | 0.4604 |
| Drip loss% | 3.53 a | 3.27 | 3.33 a,b | 3.05 a,b | 2.18 b | 3.25 a,b | 2.65 a,b | 3.16 b | 2.38 a,b | 3.31 a,b | 3.38 ab | 3.5 a | 0.39 | 0.3103 | 0.0128 | 0.9328 |
| Cooking loss% | 25.45 a | 19.47 | 22.22 a,b | 23.81 a | 22.13 a,b | 23.43 a | 18.17 b | 18.83 b | 20.59 a,b | 24.04 a,b | 19.83 a,b | 22.63 a,b | 1.91 | 0.4316 | 0.0206 | 0.7182 |
| Shear force (g) | 1148.9 | 1152.1 | 1101.6 | 1137.3 | 1226.8 | 1008.2 | 945.2 | 1133.2 | 995.2 | 820.1 | 898.2 | 1067.9 | 105.3 | 0.3495 | 0.5628 | 0.8429 |
| Color | ||||||||||||||||
| L* | 55.01 | 47.79 | 51.22 | 52.82 | 52.95 | 51.52 | 50.25 | 50.24 | 50.76 | 51.43 | 50.33 | 50.89 | 1.51 | 0.2781 | 0.1628 | 0.4581 |
| a* | 6.28 | 6.73 | 7.19 | 6.20 | 7.15 | 7.09 | 7.00 | 7.58 | 7.58 | 6.57 | 7.14 | 6.57 | 0.69 | 0.9400 | 0.1152 | 0.8437 |
| b* | 20.61 | 18.11 | 20.79 | 20.83 | 20.29 | 21.67 | 22.21 | 21 | 21.25 | 20.18 | 19.92 | 19.43 | 0.81 | 0.1433 | 0.0571 | 0.7256 |
a,b,c,d Means with different superscripts in the same row indicates significant difference (p < 0.05). 1 Dietary treatments: NC (negative control) = basal diet, PC (positive control) = basal diet + vitamin E (100 mg/kg feed), BS 0.25 = basal diet + 0.25% brown seaweed, BS 0.50 = basal diet + 0.50% brown seaweed, BS 0.75 = basal diet + 0.75% brown seaweed, BS 1 = basal diet + 1% brown seaweed, BS 1.25 = basal diet + 1.25% brown seaweed, GS 0.25 = basal diet + 0.25% green seaweed, GS 0.50 = basal diet + 0.50% green seaweed, GS 0.75 = basal diet + 0.75% green seaweed, GS 1 = basal diet + 1% green seaweed, GS 1.25 = basal diet + 1.25% green seaweed. 2 SEM = standard error of means. 3 Contrast p-Values = orthogonal polynomial contrasts of dietary increasing brown and green seaweed inclusion levels (0.0 to 1.25%). 4 Color: L* = lightness, a* = redness, b* = yellowness.
The BS and GS had no significant effects on breast meat pH value, shear force, cooking loss, and drip loss percentage after days one and seven of storage. The cooking loss for the 1.25% BS and 0.25% GS groups and drip loss for the 0.75% BS and 1.25% GS were significantly decreased linearly and quadratically compared to the NC group after day seven. Chickens fed 0.25%, 0.50%, 1%, and 1.25% BS and GS had significantly lower meat lightness than the NC group. In addition, the day one meat color yellowness was lower (p < 0.05) for chickens fed 0.75% BS than the NC group. The color yellowness was higher (p < 0.05) for chickens fed 1% GS than the NC group. There was no significant difference in breast meat color parameters after seven days among the dietary treatments.
4. Discussion
4.1. Antioxidant Properties of Brown and Green Seaweed
DPPH is a simple, rapid, and inexpensive antioxidant capacity measurement technique. This assay evaluates the overall antioxidant capacity by determining the ability of compounds to act as free radical scavengers or H atoms donors [26]. This study showed that both BS and GS show prominent antioxidant activity. A series of earlier research findings have reported that seaweed is a source of natural antioxidants. The presence of numerous biologically active compounds, such as sulfated polysaccharides, carotenoids, β-carotene, vitamin C, and α- tocopherol, could contribute to the antioxidant efficiency of seaweed [13,14,27].
The result of the current study also showed that the BS recorded the maximum antioxidant activity. Fucoidan is the main polysaccharide present in BS that can reach up to 20 to 200 g kg−1 of BS dry weight [28]. Research has shown that fucoidan polysaccharides showed considerable antioxidant activity [27]. Furthermore, the fucoxanthin carotenoid is another voluble biological compound of BS that can reach up to 5000 mg/kg of algal mass [1,29]. It is reported that fucoxanthin has exhibited excellent antioxidant properties that can protect cells against oxidation-induced damage [12,15]. Moreover, vitamin E is one of the most important fat-soluble vitamins of seaweed with a strong antioxidant capability [12,30,31], whereas it had shown that the vitamin E content of BS is higher than the GS [31]. Additionally, the phenolic compounds of seaweed are also effective primary antioxidants due to their capability to donate their H atoms to the free radicals to make them stable [12].
4.2. Plasma Antioxidant Enzymes Activities
Numerous free radicals, including hydrogen peroxide, superoxide anion, singlet oxygen, hydroxyl radical, peroxyl radicals, alkoxy radicals, and reactive nitrogen species, can damage mitochondria, DNA, and almost all organic molecules, such as carbohydrates, lipids, and proteins [8].
Antioxidants consist of enzymatic and non-enzymatic antioxidants. The main enzymatic antioxidants are SOD, CAT, and GPx [26]. CAT, SOD, and GPx are the main antioxidant enzymes in chicken plasma that transform free radicals into non-radical and non-toxic products. CAT enzyme catalysis is the decomposition of hydrogen peroxide (H2O2) to water and O2. SOD enzyme catalyzes the dismutation of superoxide into O2 and H2O2. In contrast, the GPX prevents the peroxidation of cellular membranes lipid by removing free peroxide in the cell [32,33]. Natural antioxidants have been of great in interest in recent years. Seaweed contains dietary antioxidants that can be used as medicinal and preventative agents [6,10,11]. Numerous in vitro studies have revealed that seaweed has reactive oxygen species (ROS) scavenging properties [34]. The current study showed that different levels of brown and green seaweed had no significant effects on birds’ plasma CAT, SOD, and GPx enzyme activities. Consistent with the present study, Ramirez-Higuera et al. [35] showed that administration of BS Lessonia trabeculata and GS Ulva linza at 400 mg/kg−1 body weight in Wistar rats did not significantly affect the CAT, SOD, and GPx enzymes activities in liver tissues. Similarly, Maheswari et al. [36] reported a 2.5% inclusion of a red and brown seaweed blend (Kappaphycus alvarezii, Gracilaria salicornia and Turbinaria conoides) (1:1:1) in lactating Murrah buffaloes’ diet did not affect their plasma SOD enzyme activity. Whereas, the results obtained by Gumus et al. [16] have stated that 100 and 200 mg/kg inclusion levels of BS fucoxanthin extract in broiler chickens increased the CAT and SOD activities in the liver, breast, and drumstick tissues. This result indicates that the antioxidant effects of fucoxanthin extract may be higher compared to the whole seaweed.
4.3. Hepatic Antioxidant Genes Expression
Previous research has indicated that seaweed bioactive compounds can act as antioxidants. Thereby, seaweed may protect cells and tissues from the adverse effects of free radicals and singlet oxygen [37,38]. The current study showed that the hepatic SOD1 mRNA expression was higher for birds fed 0.50% and 0.75% BS than in the NC and PC groups. This finding can be linked to the result from this study—that, due to the DPPH scavenging potential, BS recorded significantly higher antioxidant activity than GS. The findings reported by Gumus et al. [16] showed that 100 and 200 mg/kg of fucoxanthin extract in broiler diets increased the SOD1 level in the liver, breast, and drumstick tissues. Fucoxanthin carotenoid is a voluble biological compound of BS that can reach up to 5000 mg/kg of algal mass [1,29]. It was reported that fucoxanthin exhibited excellent antioxidant properties that protect cells against oxidation-induced damage [12,15].
4.4. Plasma Lipid Profile
The result showed that different brown and green seaweed levels did not affect plasma TG, LDL, and VLD levels. Meanwhile, no significant effects were observed on plasma TC and HDL for birds fed all levels of GS and 0.25, 0.50, and 1.25% BS. Nevertheless, 0.75 and 1% BS had higher TC and HDL levels. These findings are consistent with those of Choi et al. [39], who reported that the inclusion of 0.5% BS had no effects on plasma TG, TC, and HDL levels in broiler chickens. The results contradict the findings from Kumar [40] that include 1, 2, 3, and 4% BS Sargassum in the broiler diet-decreased plasma TC level, while increasing the TG level, compared to the control birds. These results for plasma lipid profile are also inconsistent with earlier studies on rats, which found that various brown, green, and red seaweed levels decreased animals’ plasma TC, TG, and LDL levels [27,41,42]. The differences in the animal systems, seaweed species, and basal feeds can explain these contradictory results.
4.5. Proximate Analysis of Breast Meat
The results demonstrated that 0.25 and 0.50% BS and 0.25, 0.75, and 1% GS had improved the CP content of broiler breast meat. This improvement in CP may be due to the high protein and amino acid contents of seaweed. The amino acid value of seaweed proteins is higher than vegetables and cereals [27]. Moreover, our results showed that 0.50% BS and 0.75 and 1.25% GS reduced breast meat’s EE content. The biological compounds present in seaweed may affect lipid metabolism [43]. It has been determined that brown, green, and red seaweed showed cholesterol-lowering activities [6]. Polysaccharides of seaweed absorb substances, including cholesterol, and they remove them from the digestive tract [27].
4.6. Meat Quality
For the meat industry, meat quality is a term used to describe overall meat characteristics [44]. Apart from the health consequences, the meat quality obtained from broiler chicken is also an essential parameter. Lipid oxidation can negatively affect broiler meat’s color, flavor, texture, and nutritional value [9]. Besides adversely affecting meat quality, lipid peroxidation also constitutes a health risk in the event of the consumption of meat that has undergone lipid peroxidation [16]. Thus, incorporating natural dietary antioxidants can be more effective and practical in controlling lipid-oxidation-related products and providing nutritious and healthy products to consumers [9,16]. The current study results showed that different levels of BS and GS had no significant effects on various meat quality indicators, such as pH value, cooking loss, and drip loss after days one and seven of storage compared to the NC group. Whereas, the chickens fed different BS and GS feed levels had lower lightness than the NC group birds meat on day one. There were no significant differences in breast meat color lightness, redness, and yellowness after seven days of storage among dietary treatments.
Little published research is available to show the effect of seaweed on meat quality in broiler chickens. However, an earlier report showed that 100 g/kg fucoxanthin supplements from BS had no effects on broiler meat color’s lightness and redness parameters on the first day of storage, while the yellowness was significantly increased. Simultaneously, the fucoxanthin did not affect the pH value of birds’ meat [16]. In another study, Abudabos et al. [45] reported that the inclusion of 1 and 3% of GS in broiler chicken feeding had no effect on broiler breast meat color parameters.
5. Conclusions
BS and GS exhibited remarkable antioxidant activity. Brown and green seaweed up to 1.25% inclusion levels had no significant effects on blood plasma antioxidant enzyme activities. In contrast, the hepatic SOD1 gene mRNA expression was increased for birds fed 0.50% and 0.75% of BS. At the same time, BS and GS had no effects on blood plasma TG, LDL, and VLDL levels in broiler chickens. The EE content of breast meat was decreased for birds fed 0.50% BS and 0.75 and 1.25% GS-containing feed. Meanwhile, birds fed 0.25 and 0.50% BS, as well as 0.25, 0.75, and 1% GS, had increased CP content. In contrast, no differences were observed among the dietary treatments for the breast meat pH, shear force, cooking loss, and drip loss percentage after days one and seven of storage. The current research findings are useful for further studies investigating the potential antioxidant activity within the blood and several tissues. Discoveries from this research are also helpful for further studies to focus on the effects of seaweed on broiler meat amino acids and lipid profiles.
Acknowledgments
The authors are grateful to MOSTI, Malaysia, for supporting this project. We are thankful to Promise Earth (M) Sdn. Bhd. for providing us with the seaweed.
Author Contributions
Conceptualization, M.N.A., T.C.L., H.L.F. and H.A.; investigation, M.N.A., T.C.L., H.L.F., H.A., W.I.I. and D.Y.; resources, M.N.A., T.C.L. and H.L.F.; data curation, M.N.A., T.C.L., H.L.F., W.I.I. and D.Y.; writing—original draft preparation, M.N.A., T.C.L. and H.L.F.; writing—review and editing, M.N.A., T.C.L., H.L.F. and W.I.I.; supervision, T.C.L., H.L.F. and H.A.; funding acquisition, T.C.L. and H.L.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted following the guidelines approved by the Institutional Animal Care and Use Committee of the UPM (UPM/IACUC/AUP-R093/2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The funding for the seaweed study was obtained from the Ministry of Science, Technology, and Innovation (MOSTI) of Malaysia (RD1019E1335).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Corino C., Modina S.C., Di Giancamillo A., Chiapparini S., Rossi R. Seaweeds in pig nutrition. Animals. 2019;9:1126. doi: 10.3390/ani9121126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verma P., Kumar M., Mishra G., Sahoo D. Multivariate analysis of fatty acid and biochemical constitutes of seaweeds to characterize their potential as bioresource for biofuel and fine chemicals. Bioresour. Technol. 2017;226:132–144. doi: 10.1016/j.biortech.2016.11.044. [DOI] [PubMed] [Google Scholar]
- 3.Belghit I., Rasinger J.D., Heesch S., Biancarosa I., Liland N., Torstensen B., Waagbø R., Lock E.J., Bruckner C.G. In-Depth metabolic profiling of marine macroalgae confirms strong biochemical differences between brown, red and green algae. Algal Res. 2017;26:240–249. doi: 10.1016/j.algal.2017.08.001. [DOI] [Google Scholar]
- 4.El-Deek A.A., Al-Harthi M.A., Abdalla A.A., Elbanoby M.M. The use of brown algae meal in finisher broiler. Egyt. Poult. Sci. J. 2011;31:767–781. [Google Scholar]
- 5.Azizi M.N., Loh T.C., Foo H.L., Akit H., Izuddin W.I., Shazali N., Teik Chung E.L., Samsudin A.A. Chemical compositions of brown and green seaweed, and effects on nutrient digestibility in broiler chickens. Animals. 2021;11:2147. doi: 10.3390/ani11072147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matanjun P., Mohamed S., Mustapha N.M., Muhammad K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J. Appl. Phycol. 2009;21:75–80. doi: 10.1007/s10811-008-9326-4. [DOI] [Google Scholar]
- 7.Tanna B., Mishra A. Nutraceutical Potential of Seaweed Polysaccharides: Structure, Bioactivity, Safety, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019;18:817–831. doi: 10.1111/1541-4337.12441. [DOI] [PubMed] [Google Scholar]
- 8.Gupta D. Methods for Determination of Antioxidant Capacity: A Review. Int. J. Pharm. Sci. Res. 2015;6:546–566. doi: 10.13040/IJPSR.0975-8232.6(2).546-66. [DOI] [Google Scholar]
- 9.Abdulla N.R., Loh T.C., Akit H., Sazili A.Q., Foo H.L., Mohamad R., Abdul Rahim R., Ebrahimi M., Sabow A.B. Fatty acid profile, cholesterol and oxidative status in broiler chicken breast muscle fed different dietary oil sources and calcium levels. S. Afr. J. Anim. Sci. 2015;45:153–163. doi: 10.4314/sajas.v45i2.6. [DOI] [Google Scholar]
- 10.Rao P.V.S., Periyasamy C., Kumar K.S., Annabattula S.R., Anantharaman P. Seaweeds: Distribution, Production and Uses. In: Noor M.M., Bhatnagar S.K., Sinha S.K., editors. Bioprospecting of Algae. Society for Plant Research India; Greater Noida, India: 2018. pp. 59–67. [Google Scholar]
- 11.Hayes M. Marine Bioactive Compounds: Sources, Characterrizaiton and Application. Library of Congress; Dublin, Ireland: 2012. [Google Scholar]
- 12.Qin Y. In: Bioactive Seaweeds for Food Applications: Natural Ingredients for Healthy Diets. Osborn P., editor. Andre Gerhard Wolff; London, UK: 2018. [Google Scholar]
- 13.Shao P., Chen M., Pei Y., Sun P. In intro antioxidant activities of different sulfated polysaccharides from chlorophytan seaweeds Ulva fasciata. Int. J. Biol. Macromol. 2013;59:295–300. doi: 10.1016/j.ijbiomac.2013.04.048. [DOI] [PubMed] [Google Scholar]
- 14.Hardouin K., Bedoux G., Burlot A.S., Donnay-Moreno C., Bergé J.P., Nyvall-Collén P., Bourgougnon N. Enzyme-assisted extraction (eae) for the production of antiviral and antioxidant extracts from the green seaweed Ulva Armoricana (Ulvales, Ulvophyceae) Algal Res. 2016;16:233–239. doi: 10.1016/j.algal.2016.03.013. [DOI] [Google Scholar]
- 15.Airanthi M.K.W.A., Hosokawa M., Miyashita K. Comparative antioxidant activity of edible Japanese brown seaweeds. J. Food Sci. 2011;76:104–111. doi: 10.1111/j.1750-3841.2010.01915.x. [DOI] [PubMed] [Google Scholar]
- 16.Gumus R., Urcar Gelen S., Koseoglu S., Ozkanlar S., Ceylan Z.G., Imik H. The Effects of fucoxanthin dietary inclusion on the growth performance, antioxidant metabolism and meat quality of broilers. Rev. Bras. Cienc. Avic. 2018;20:487–496. doi: 10.1590/1806-9061-2017-0666. [DOI] [Google Scholar]
- 17.Ji X., Rivers L., Zielinski Z., Xu M., MacDougall E., Stephen J., Zhang S., Wang Y., Chapman R.G., Keddy P., et al. Quantitative analysis of phenolic components and glycoalkaloids from 20 potato clones and in vitro evaluation of antioxidant, cholesterol uptake, and neuroprotective activities. Food Chem. 2012;133:1177–1187. doi: 10.1016/j.foodchem.2011.08.065. [DOI] [Google Scholar]
- 18.Lim S.J., Wan Aida W.M., Maskat M.Y., Mamot S., Ropien J., Mazita Mohd D. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocoll. 2014;42:280–288. doi: 10.1016/j.foodhyd.2014.03.007. [DOI] [Google Scholar]
- 19.Danladi Y., Loh T.C., Foo H.L., Akit H., Tamrin N.A.M., Azizi M.N. Effects of postbiotics and paraprobiotics as replacements for antibiotics on growth performance, carcass characteristics, small intestine histomorphology, immune status and hepatic growth gene expression in broiler chickens. Animals. 2022;12:917. doi: 10.3390/ani12070917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2-δδct method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Humam A.M., Loh T.C., Foo H.L., Samsudin A.A., Mustapha N.M., Zulkifli I., Izuddin W.I. Effects of feeding different postbiotics produced by Lactobacillus Plantarum on growth performance, carcass yield, intestinal morphology, gut microbiota composition, immune status, and growth gene expression in broilers under heat stress. Animals. 2019;9:644. doi: 10.3390/ani9090644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delong D.M., Delong E.R., Wood P.D., Lippel K., Rifkind B.M. A Comparison of methods for the estimation of plasma low- and very low-density lipoprotein cholesterol: The lipid research clinics prevalence study. J. Am. Med. Assoc. 1986;256:2372–2377. doi: 10.1001/jama.1986.03380170088024. [DOI] [PubMed] [Google Scholar]
- 23.Honikel K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998;49:447–457. doi: 10.1016/S0309-1740(98)00034-5. [DOI] [PubMed] [Google Scholar]
- 24.Sazili A.Q., Parr T., Sensky P.L., Jones S.W., Bardsley R.G., Buttery P.J. The relationship between slow and fast myosin heavy chain content, calpastatin and meat tenderness in different ovine skeletal muscles. Meat Sci. 2005;69:17–25. doi: 10.1016/j.meatsci.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Cavitt L.C., Youm G.W., Meullenet J.F., Owens C.M., Xiong R. Prediction of poultry meat tenderness using razor blade shear, allo-kramer shear, and sarcomere length. J. Food Sci. 2004;69:11–15. doi: 10.1111/j.1365-2621.2004.tb17879.x. [DOI] [Google Scholar]
- 26.Al-Hmoud H.A., Ibrahim N.E., El-Hallous E.I. Concentration and the other formulations effects on the drug release rate from a controlled-release matrix. Afr. J. Pharm. Pharmacol. 2014;8:364–371. [Google Scholar]
- 27.Holdt S.L., Kraan S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011;23:543–597. doi: 10.1007/s10811-010-9632-5. [DOI] [Google Scholar]
- 28.Øverland M., Mydland L.T., Skrede A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019;99:13–24. doi: 10.1002/jsfa.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayani S.S., Saravanan S., Bharathiaraja S., Mahendran S. Extraction, partially purification and study on antioxidant property of fucoxanthin from Sargassum cinereum. J. Chem. Pharm. Res. 2016;8:610–616. [Google Scholar]
- 30.Van Den Burg S., Stuiver M., Veenstra F., Bikker P., Contreras A.L., Palstra A., Broeze J., Jansen H., Jak R., Gerritsen A., et al. A Triple P Review of the Feasibility of Sustainable Offshore Seaweed Production in the North Sea. Wageningen; Wageningen, The Netherlands: 2013. [Google Scholar]
- 31.Kim S.K. Handbook of Marine Macroalgae: Biotechnology and Applied Phycology. Wiley-Blackwall publishing; Oxford, UK: 2011. [Google Scholar]
- 32.Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016;7:37. doi: 10.1186/s40104-016-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bacou E., Walk C., Rider S., Litta G., Perez-Calvo E. Dietary Oxidative Distress: A Review of nutritional challenges as models for poultry, swine and fish. Antioxidants. 2021;10:525. doi: 10.3390/antiox10040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latham H. Temperature stress-induced bleaching of the coralline alga Corallina Officinalis: A role for the enzyme bromoperoxidase. Biosci. Horiz. 2008;1:104–113. doi: 10.1093/biohorizons/hzn016. [DOI] [Google Scholar]
- 35.Ramirez-Higuera A., Quevedo-Corona L., Paniagua-Castro N., Chamorro-Ceballos G., Milliar-Garcia A., Jaramillo-Flores M.E. Antioxidant enzymes gene expression and antihypertensive effects of seaweeds Ulva Linza and Lessonia Trabeculata in rats fed a high-fat and high-sucrose diet. J. Appl. Phycol. 2014;26:597–605. doi: 10.1007/s10811-013-0134-0. [DOI] [Google Scholar]
- 36.Maheswari M., Das A., Datta M., Tyagi A.K. Supplementation of tropical seaweed-based formulations improves antioxidant status, immunity and milk production in lactating Murrah buffaloes. J. Appl Phycol. 2021;33:2629–2643. doi: 10.1007/s10811-021-02473-5. [DOI] [Google Scholar]
- 37.Sachindra N.M., Sato E., Maeda H., Hosokawa M., Niwano Y., Kohno M., Miyashita K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007;55:8516–8522. doi: 10.1021/jf071848a. [DOI] [PubMed] [Google Scholar]
- 38.Michalak I., Mahrose K. Seaweeds, intact and processed, as a valuable component of poultry feeds. J. Mar. Sci. Eng. 2020;8:620. doi: 10.3390/jmse8080620. [DOI] [Google Scholar]
- 39.Choi Y.J., Lee S.R., Oh J.W. Effects of dietary fermented seaweed and seaweed fusiforme on growth performance, carcass parameters and immunoglobulin concentration in broiler chicks. Asian-Australas. J. Anim. Sci. 2014;27:862–870. doi: 10.5713/ajas.2014.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar A.K. Effect of Sargassum wightii on growth, carcass and serum qualities of broiler chickens. Open Access J. Vet. Sci. Res. 2018;3:000156. doi: 10.23880/oajvsr-16000156. [DOI] [Google Scholar]
- 41.Matanjun P., Mohamed S., Muhammad K., Mustapha N.M. Comparison of cardiovascular protective effects of tropical seaweeds, Kappaphycus alvarezii, Caulerpa lentillifera, and Sargassum polycystum, on high-cholesterol/high-fat diet in rats. J. Med. Food. 2010;13:792–800. doi: 10.1089/jmf.2008.1212. [DOI] [PubMed] [Google Scholar]
- 42.Dousip A., Matanjun P., Sulaiman M.R., Tan T.S., Ooi Y.B.H., Lim T.P. Effect of seaweed mixture intake on plasma lipid and antioxidant profile of hyperholesterolaemic rats. J. Appl. Phycol. 2014;26:999–1008. doi: 10.1007/s10811-013-0128-y. [DOI] [Google Scholar]
- 43.Tziveleka L.A., Ioannou E., Roussis V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydr. Polym. 2019;218:355–370. doi: 10.1016/j.carbpol.2019.04.074. [DOI] [PubMed] [Google Scholar]
- 44.Mir N.A., Rafiq A., Kumar F., Singh V., Shukla V. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food Sci. Technol. 2017;54:2997–3009. doi: 10.1007/s13197-017-2789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abudabos A.M., Okab A.B., Aljumaah R.S., Samara E.M., Abdoun K.A., Al-Haidary A.A. Nutritional Value of Green Seaweed (Ulva lactuca) for broiler chickens. Ital. J. Anim. Sci. 2013;12:177–181. doi: 10.4081/ijas.2013.e28. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.