Abstract
Accumulating evidence suggests that α-synuclein plays a role in the pathophysiology of Alzheimer’s disease (AD). This study examined whether α-synuclein level in cerebrospinal fluid (CSF) was associated with cognitive functioning among older adults. We also explored whether this relationship was mediated by proinflammatory cytokines TNF-α and IL-6, along with sIL-6R and vascular endothelial growth factor (VEGF). Using a cross-sectional Alzheimer’s Disease Neuroimaging Initiative (ADNI; N = 148) sample, we examined the relationship between α-synuclein and participants’ performance on Mini-Mental State Examination (MMSE) and Alzheimer’s Disease Assessment Scale Cognitive Subscale (ADAS-Cog 13) at baseline. Mediation analyses were utilized, adjusting for age, education, APOEe4, and Geriatric Depression Scale scores. All biological markers were measured in CSF. Participants in the current sample were 58.3% males, 41.7% females, and Caucasian (95.5%); their average education and age were 15.5 (standard deviation [SD] = 2.97) and 74.4 (SD = 7.51) years, respectively. Higher accumulation of α-synuclein was associated with poorer MMSE scores (β = −0.41, standard error [SE] = 1.54, p < .001). This relationship appeared to be mediated by VEGF (β = 0.27, SE = 2.15, p = .025) and IL-6r (β = 0.22, SE = 1.66, p < .026). In addition, α-synuclein was associated with poorer performance on the ADAS-Cog 13 (β = 0.34, p = .005) and mediated by VEGF (β = −0.19, SE = 4.13, p = .025) after adjusting for age, education, APOEe4, and depressive symptoms. α-Synuclein may serve as an additional biomarker for determining poor cognitive functioning. VEGF and IL-6 soluble receptors were significant mediators of the relationship between α-synuclein and cognitive functioning. If confirmed in prospective analyses, these findings can further inform the pathologic cascade and early diagnosis of AD.
Keywords: Alzheimer’s disease, Biomarker, Cognitive decline, Immune response, α-Synuclein
Alzheimer’s disease (AD) affects roughly 5.8 million persons in the United States (1). AD is a progressive neurodegenerative disease that causes significant deterioration in cognition with impairment in memory and executive functioning and behavioral/personality changes. Early AD diagnosis can be challenging given that the disease begins roughly 20 years prior to symptom presentation (1). Risk factors for AD include advanced age (65 years or older), genetics (APOEɛ4), family history of the disease, cardiovascular disease, diabetes mellitus, socioeconomic status, and lifestyle factors such as excessive alcohol consumption and lack of physical activity (1–3). Mild cognitive impairment (MCI), an intermediate stage of AD, is classified as subjective or objective cognitive impairment in one or more domains, including attention, language, memory, executive function, and visuospatial skills (4). About 15%–20% of older adults in the United States (age 65 and older) present with MCI symptoms, and approximately 32% of MCI patients develop AD within 5 years (5).
For the past few decades, the dominant hypothesis for the pathogenesis of AD has been amyloid-beta (Aβ) plaques and intraneuronal tangles of hyperphosphorylated tau protein. These biomarkers have been used for predicting cognitive decline. In addition to Aβ plaques and tangles, up to 60% of patients with AD show evidence of dementia with Lewy body (DLB) pathology (6). Lewy bodies are considered abnormal aggregation of the protein α-synuclein in neurons (6), and DLB can occur when these clumps develop in the cortex. CSF concentration of α-synuclein has been linked to CSF AD biomarkers, inversely with Aβ peptide and positively with tau protein (7). α-Synuclein increases tau phosphorylation and plays an important role in the regulation of microtubules by binding to tubulin (8). Further findings from humans as well as transgenic mice revealed that elevated level of soluble α-synuclein is related to alterations in presynaptic protein vesicles (9). Multiple studies have reported increased CSF levels of α-synuclein among patients with AD and MCI compared to healthy adults (10–12). Even in the absence of DLB pathology, α-synuclein level among patients with AD is higher than in healthy adults (11). An examination of a cross-sectional sample of participants carrying Autosomal Dominant Alzheimer’s Disease mutations from the Dominantly Inherited Alzheimer Network suggested that CSF α-synuclein levels were positively correlated to the estimated years from disease onset (12). These findings suggest α-synuclein is another amyloid-like protein that contributes to the early development of AD (9). Therefore, further investigation is needed to understand whether α-synuclein is associated with AD-related cognitive decline in older adults.
α-Synuclein is secreted by cells and is present in biological fluids such as CSF. It is expressed in the central nervous system (CNS), with its highest concentration in the thalamus, neocortex, cerebellum, striatum, and hippocampus (13). In humans, α-synuclein is a natively unfolded protein with 140 amino acids and consists of 3 distinct parts: amphipathic N-terminus (membrane binding), NAC (nonamyloid component and aggregation-prone), and acidic C-terminus, which is unstructured and decreases aggregation (9). The N-terminal amphipathic region is capable of binding to lipids and causes the unfolded α-synuclein structure to change to an α-helix structure (9). In vitro and in vivo data show that an increased concentration of α-synuclein increases the tendency of the protein to aggregate (14,15). Misfolded α-synuclein forms oligomers and plays a leading role in neurodegenerative diseases such as Parkinson’s disease (PD), DLB, multiple system atrophy, neurodegeneration with brain iron accumulation type I, and Lewy body variant of AD (16).
Accumulation of α-synuclein oligomers can cause neuronal cell death by disrupting the integrity of cellular membranes (17). Microglial cells are activated and produce inflammatory mediators when foreign chemicals or dead cells are present in the CNS. Overexpression of α-synuclein in microglial cells results in the activation and production of proinflammatory factors (18). Altogether, α-synuclein’s role in synaptic plasticity is well known; its ability to produce β-sheet structures, its role in aggregation of Aβ and tau protein under certain conditions, combined with its tendency to activate an inflammatory response, makes this protein an intriguing biomarker.
Research suggests the pathology of inflammatory mechanisms affects the progression of cognitive decline (19,20). In past research with animal models, investigators have observed upregulated gene expression of IL-1, IL-6, and TNF-α after injecting α-synuclein (21). Proinflammatory cytokines such as IL-6 have been associated with amyloid fibrils depositions (22). Soluble IL-6 receptor (sIL-6R) involved in IL-6 trans-signaling has also been associated with the progression of AD (23). Additionally, astrocytes and neurons respond better to IL-6/sIL-6R complex for trans-signaling but not to classic IL-6 signaling. In young mice, IL-6 trans-signaling seems to be the main mechanism for the neurogenesis disruption that is mediated by IL-6 (24). Given the role of IL-6 trans-signaling in AD pathology, investigating the relationship between α-synuclein with IL-6 and sIL-6R would provide a better understanding of how these biological markers may contribute to cognitive decline.
Another key cytokine associated with α-synuclein in neurodegeneration is vascular endothelial growth factor (VEGF), which is known to play a role in promoting anti-inflammatory responses and neurogenesis (25). In vitro studies have shown that a higher concentration of VEGF is linked to promoting neuronal apoptosis, loss of microvessels (26), and neurodegenerative diseases such as AD and DLB (26,27). Moreover, in DLB, VEGF is inversely associated with the accumulation of α-synuclein. In PD, VEGF serves as a neuroprotective agent to prevent dopaminergic neuron apoptosis (28). VEGF and α-synuclein are known factors in neurodegenerative diseases like DLB and PD (6,9,12,26–28). Given VEGF’s role in learning and memory, it was necessary to investigate VEGF function in the relationship between α-synuclein and cognitive functioning in older adults.
There is also a difference in the prevalence of AD among women and men. About two third of individuals diagnosed with AD and other dementias are women, approximately 3.5 million compared to 2.1 million men, respectively (1). It is important to identify biomarkers that provide a further understanding of why such sex differences exist. For example, estrogen is linked to cognitive functioning by providing neuro-protection in major brain structures relevant to memory, such as the hippocampus (29). However, research on estrogen as a protective factor has been inconsistent (30,31). Therefore, examining additional biomarkers such as α-synuclein in other dementia-related disorders may help explain sex differences. α-Synuclein has been identified as the main biomarker in PD and DLB. The relationship between α-synuclein and sex has also been observed in DLB patients (32). Although DLB prevalence and CSF α-synuclein levels appear to be higher in men, women are more likely to experience hallucinations and a shorter duration of memory complaints before clinical diagnosis, suggesting a faster progression (32). Similarly, in PD, α-synuclein levels appear to be higher in men. Even though the onset of clinical and cognitive presentation appears later in women, the progression occurs faster (33). Therefore, sex differences in the context of the relationship between α-synuclein and cognitive impairment should be further studied.
Given the aging U.S. population, it is important to investigate potential biomarkers for cognitive decline. We hypothesized that there would be: (a) a positive relationship between α-synuclein and proinflammatory cytokines IL-6 and TNF-α, and additional immune markers, VEGF and sIL-6R. (b) α-Synuclein would predict cognitive functioning in older adults through an immune response. (c) Sex will operate as a moderator in the relationship between α-synuclein and cognitive functioning.
Method
Data used in our analysis were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public–private partnership led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to assess whether serial magnetic resonance imaging, positron emission tomography, other biological markers, clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. In ADNI-1, participants between the ages of 55–90 were enrolled in 57 sites in the United States and Canada. Each site PI ensures the study protocol is approved by the local Institutional Review Board. About 51% of participants enrolled in ADNI-1 agreed to provide CSF samples at baseline. For the current study, a total of 295 nonrandomly sampled participants from the ADNI-1 cohort had their α-synuclein and immune markers measured in CSF. As shown in Table 1, participants in the current sample were 58% males, Caucasian (95.5%), average education of 15.5 (standard deviation [SD] = 2.97) years, with an average age of 74.4 (SD = 7.51). In terms of cognitive performance, the average Mini-Mental State Examination (MMSE) score was 26.63 (SD = 2.57), and the average Alzheimer’s Disease Assessment Scale Cognitive Subscale (ADAS-Cog 13) was 18.64 (SD = 8.85). The majority of participants had MCI (43%), 28% of the participants were diagnosed with AD, and 29% were cognitively normal.
Table 1.
Participant’s Demographic Characteristics
| N | AD | LMCI | CN | |
|---|---|---|---|---|
| Female | 83 | 26 | 32 | 25 |
| Male | 116 | 29 | 54 | 33 |
| Racial category | ||||
| White | 190 | 55 | 81 | 54 |
| Black | 6 | 2 | 4 | |
| Asian | 3 | 3 | ||
| Ethnicity | ||||
| Non-Hispanic | 197 | |||
| Hispanic | 2 | |||
| Total | 199 | 55 | 86 | 58 |
Note: CN = cognitively normal, LMCI = Late MCI.
Biomarkers
Baseline CSF samples were obtained in the morning after an overnight fast. CSF samples were collected from all newly enrolled participants at baseline and every 2 years thereafter. All immune markers (VEGF, IL-6, sIL-6R, and TNF-α) examined in this study were collected as part of the Biomarkers Consortium CSF Proteomics MRM data set. Lumbar puncture was performed with a 20- or 24-gauge spinal needle as previously described (34); (see also ADNI procedures manual http://www.adni-info.org/ and https://adni.loni.usc.edu/wp-content/uploads/2010/09/ADNI_GeneralProceduresManual.pdf). Samples were normalized across plates using CSF standard values. The interplate coefficient of variation (CV) was less than 10% for TNF and IL-6. Test–retest validation for 16 random participant samples was performed to ensure reliability. Analytes were excluded during ADNI’s quality control (QC) if the mean percentage difference was greater than 35%, if the test–retest sample was less than 7, if the absolute percentage difference was greater than 60%, or if the intercept differed significantly from zero. VEGF’s average CV was 18% and passed all QC procedures. More details are available on the ADNI website (https://adni.loni.usc.edu/wp-content/uploads/2012/01/2011Dec28-Biomarkers-Consortium-Data-Primer-FINAL1.pdf). α-Synuclein was measured by Luminex assays as previously described (35). The α-synuclein Luminex assay applied in the ADNI cohort confirmed low day-to-day as well as plate-to-plate signal variability (<5% with >80 plates analyzed), with high signal reproducibility and linear performance in the low pg range (the lower limit of quantitation [LLOQ] is 9.0 pg/mL). Accuracy for the assay, as determined by the recovery of spiked α-synuclein, was about 93% (36). In addition, as α-synuclein is abundant in red blood cells and the release of this protein from lysed red blood cells in CSF may confound the interpretation of α-synuclein levels, hemoglobin (Hgb) was also measured in CSF samples to assess CSF contamination by red blood cells (36,37). The current study only includes CSF samples with less than 200 ng/mL hemoglobin, which is the cutoff value to avoid red blood cell contamination (37).
Cognitive Functioning
The Mini-Mental State Examination
Mini-Mental State Examination is a widely used, dependable, and validated method of screening for cognitive impairment and mental status (38). This screening tool is generally used to determine if someone needs further diagnostic workup, including comprehensive neuropsychological testing (39). The scores on the MMSE range from 0 to 30, with scores of 25 or higher considered normal. Generally, scores of less than 10 indicate severe impairment, and scores between 23 and 26 suggest MCI. The scores are also adjusted based on the education level.
ADAS-COG 13
The ADAS-Cog is widely used to assess the effectiveness of antidementia interventions. ADAS-Cog was initially designed to examine the severity of cognitive and noncognitive dysfunction from mild to severe AD (40). The assessment takes about 45 minutes, and scores range from 0 to 85 in the extended version (ADAS-Cog 13) and 0–70 in the standard version (ADAS-Cog 11) by adding the number of errors on each task. A higher score on ADAS-Cog indicates worse performance. The ADAS consists of 2 subscales that measure noncognitive and cognitive function. The noncognitive portion measures depressive symptoms, attention, agitation, psychosis, and tremors. The cognitive subscale examines word recall, naming objects, fingers command constructional praxis, ideational praxis, orientation, word recognition, and language (40).
Geriatric Depression Scale
The Geriatric Depression Scale (GDS) was designed to assess depression among the older population. The self-report scale consists of 30 yes/no questions with higher scores indicating severe depressive symptoms. The scale also has a condensed version called (15 questions) short form (41).
Statistical Analyses
All data were analyzed using R-Studio. Data were screened for errors, outliers, normality distributions, skewness, homogeneity of variance, and multicollinearity. IL-6 and TNF-α scores were significantly skewed; thus, they were normalized using log transformation. In this study, we controlled for the most cited covariates that are associated with cognitive performance, including age, education, depressive symptoms (GDS), and APOE status (1,41). APOE genotyping was described in http://www.adni-info.org in detail. The APOE gene is polymorphic with the following 3 major isoforms: APOE ε2, APOE ε3, and APOE ε4. This study controlled for APOE status (carrier of 0, 1, or 2 APOE ε4 alleles); APOE ε4 carriers were coded as 0, 1, and 2, respectively.
For hypothesis 1, we used the Lavaan model syntax to specify a regression model. In our model, we had 1 exogenous variable (α-synuclein), 2 proinflammatory markers (TNF-α and IL-6), and 2 immune markers (VEGF and sIL-6R) for their critical roles in neurogenesis and trans-signaling in AD progression. Indicators of model fit included comparative fit index (CFI), Tucker-Lewis index (TLI), root mean square error of approximation (RMSEA), and standardized root mean squared residual (SRMR). Generally, CFI and TLI greater than 0.95, and RMSEA and SRMR less than 0.08 indicate adequate fit. The final sample size was 199 and the estimation of model fit suggested that the model fit the data satisfactorily, p < .01. CFI = 1, TLI = 1; RMSEA = 0.00; SRMR = 0.00.
For hypothesis 2, we used Hayes’ mediation model 4 to specify mediation models to examine whether α-synuclein could predict cognitive functioning through immune markers VEGF, sIL-6R, and TNF-α. MMSE and ADAS-Cog 13 scores were used as proxies for cognitive functioning. These mediation models included GDS score, age, APOEɛ4, and education as covariates. Hayes (42) applies the principles of ordinary least squares regression to estimate direct, indirect, and conditional effects. The new edition of process (42) has included R function, making it possible to use process models in RStudio.
Results
Hypothesis 1
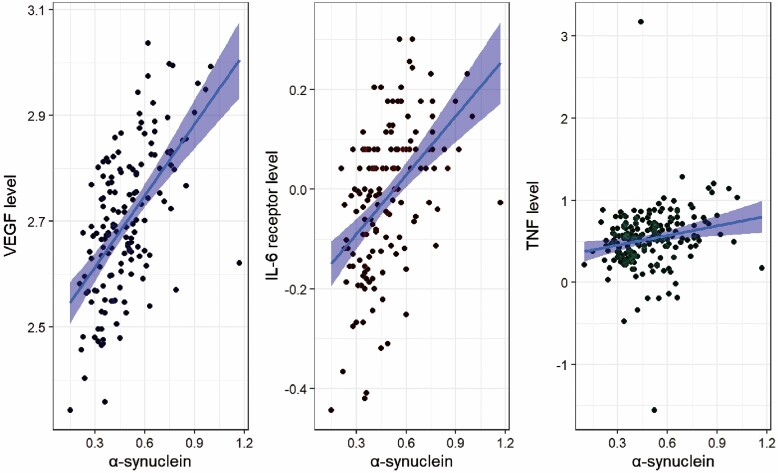
The first hypothesis proposed that there would be a positive association between α-synuclein and VEGF, IL-6, sIL-6R, and TNF-α. This was partially supported (see Figure 1). α-synuclein predicted TNF-α (β = 0.195, standard error [SE] = 0.12, p < .01), sIL-6R (β = 0.5, SE = 0.06, p < .001), and VEGF level (β = 0.6, SE = 0.07, p < .001). Higher α-synuclein was associated with higher TNF-α, sIL-6R, and VEGF levels. Surprisingly, α-synuclein did not predict IL-6 (p = .06). In the predicted direction, as demonstrated in Table 2, α-synuclein explained 24.6% of the variance in sIL-6R, 3.8% of the variance in TNF-α, and 35% of the variance in VEGF.
Figure 1.
Accumulation of α-synuclein had a positive relationship with VEGF (β = 0.6, p < .001), TNF-α (β = 0.2, p < .01), and sIL-6R Levels (β = 0.5, p < .001). VEGF = vascular endothelial growth factor.
Table 2.
Standardized Regression Coefficients of α-Synuclein on Immune Markers
| Variable | β | p | SE | R 2 | N = 199 |
|---|---|---|---|---|---|
| IL-6 | 0.119 | .066 | 0.211 | 0.014 | |
| sIL-6r | 0.496 | .000 | 0.063 | 0.246 | |
| TNF-α | 0.195 | .001 | 0.122 | 0.038 | |
| VEGF | 0.592 | .000 | 0.07 | 0.350 |
Notes: Path analysis showed that the model fit was appropriate (CFI = 1, TLI = 1; RMSEA = 0.00; SRMR = 0.00). α-Synuclein significantly predicted sIL-6r, VEGF, and TNF-α. R2 is for each inflammatory marker’s regression coefficient that contributed to the model. SE = standard error; VEGF = vascular endothelial growth factor.
Hypothesis 2
Because the relationship between α-synuclein and IL-6 was insignificant in the first hypothesis, we did not include IL-6 in the mediation model for the second hypothesis. The second hypothesis proposed that there would be a negative relationship between α-synuclein and cognitive performance, and this relationship would be explained by inflammatory markers TNF-α, VEGF, and sIL-6R. We controlled for age, education, APOE4, and GDS score in both mediation models. As predicted, α-synuclein was negatively related to the MMSE score (β = −0.42, SE = 1.05, p < .001) and positively related to ADAS-Cog 13 score (β = 0.32, SE = 5.67, p < .01), indicating that higher levels of α-synuclein are associated with poorer cognitive performance. The relationship between α-synuclein and MMSE score remained significant after adding mediators and was indirectly mediated by VEGF (β = 0.18, SE = 1.09, p < .05) and sIL-6R (β = 0.12, SE = 0.74, p < .05). Similarly, the relationship between α-synuclein and ADAS-Cog 13 remained significant after adding mediators and was indirectly mediated via VEGF (β = −0.19, SE = 4.13, p < .05). Among covariates, APOE4 predicted ADAS-Cog 13 (β = 0.23, SE = 1.13, p < .05) and MMSE (β = −0.28, SE = 0.25, p < .001). GDS predicted ADAS-Cog 13 (β = 0.18, SE = 0.57, p < .05). Education predicted MMSE (β = 0.13, SE = 0.06, p < .05). Results are presented in Table 3.
Table 3.
The Standardized and Unstandardized Total, Direct and Indirect Effect of α-Synuclein on MMSE and ADAS-Cog 13
| B | β | SE | 95% CI | N | |
|---|---|---|---|---|---|
| MMSE | N = 148 | ||||
| sIL-6 receptor | 1.63* | 0.12 | 0.74 | 0.36 to 3.27 | |
| VEGF | 2.49* | 0.18 | 1.09 | 0.54 to 4.82 | |
| TNF-α | −0.4 | −0.03 | 0.23 | −0.96 to −0.03 | |
| Direct effect | −5.92*** | −0.42 | 1.5 | −9.1 to −3.29 | |
| Total effect | −2.2 | −0.16 | 1.05 | −4.28 to −0.2 | |
| ADAS-Cog 13 | N = 149 | ||||
| sIL-6 receptor | −2.14 | −0.04 | 2.34 | −7.55 to 2.01 | |
| VEGF | −9.28* | −0.19 | 4.13 | −17.9 to −2.05 | |
| TNF-α | 1 | 0.02 | 0.81 | −0.014 to 3.06 | |
| Direct effect | 15.89** | 0.32 | 5.67 | 6.24 to 28.11 | |
| Total effect | 5.47 | 0.11 | 4.02 | −2.21 to 13.55 |
Notes: *p < .05; **p < .01; ***p < .001. MMSE = Mini-Mental State Examination; ADAS-Cog = Alzheimer’s Disease Assessment Scale Cognitive Subscale; VEGF = vascular endothelial growth factor.
Following Hayes’ moderation analysis of model 15, we examined whether sex moderated the relationship between α-synuclein and mediators as well as the relationship between cognitive functioning and α-synuclein. Male participants were coded as 1, and female participants were coded as 0. Results indicated that the relationship between α-synuclein and ADAS-Cog 13 did not depend on sex (B = 9.92, 95% confidence interval [CI]: 1.86 to 20.44). In addition, the indirect effect did not depend on sex via sIL-6R (B = −4.86, 95% CI: −10.16 to −1.24) and VEGF (B = −10.79, 95% CI: −21.25 to −4.72). The same pattern was observed with MMSE scores. Results indicated that the direct effect of α-synuclein and MMSE did not depend on the sex of the participants (B = 2.27, 95% CI: 0.99 to 3.9). The indirect effect also did not depend on the sex of participants via sIL-6R (B = −4.34, 95% CI: −7.08 to −2.205) nor VEGF (B = 3.43, 95% CI: 1.647 to 6.004).
Discussion
AD is a heterogeneous disease that involves multiple pathogenic pathways and features cognitive decline as one of the key clinical manifestations. Detection of sensitive biomarkers of cognitive dysfunction is essential in studying the progression of AD and can aid in the development of new interventions, lessen disease burden, and the cost and time of clinical trials. This study examined the association between α-synuclein accumulation and the level of cognitive functioning through elevated levels of immune biomarkers. First, we hypothesized that there would be a positive association between α-synuclein with VEGF, soluble IL-6 receptor, and proinflammatory cytokines IL-6, and TNF-α. This was partially supported except for IL-6. This study demonstrated that α-synuclein accumulation is strongly associated with VEGF, soluble IL-6 receptor, and TNF-α. As a proinflammatory cytokine, IL-6 is essential in acute inflammatory phase response. Although IL-6 and α-synuclein were not significantly associated in the current study, this relationship has been observed in animal studies21. Evidence from blood and CSF samples of PD patients has shown elevated levels of proinflammatory cytokines such as IL-6 and TNF-α (21). There are 2 pathways that IL-6 can signal: classic and trans-signaling. In classic signaling, IL-6 binds to the cell membrane IL-6 receptor (IL-6R). For trans-signaling, IL-6 binds to the soluble form of IL-6R receptor (sIL-6R), and the latter promotes an indirect inflammatory response to IL-6. The complex sIL-6R (IL-6/sIL-6R) can bind to the glycoprotein 130 (gp130) present in most cell membranes. The ternary complex promotes IL-6 trans-signaling (24). Elevated levels of soluble IL-6 receptors can impair proper IL-6 responsiveness via trans-signaling. This result is consistent with previous findings suggesting that IL-6 trans-signaling has a key role in the development of neurodegenerative disease in mice (24). Moreover, the disruption in IL-6 trans-signaling has been associated with stronger astrocyte activation which can further increase neuroinflammation (24).
The second aim of the study posited that α-synuclein can positively predict the level of cognitive functioning in ADNI participants through inflammatory biomarkers. This was partially supported. VEGF, IL-6 receptor, and TNF-α did mediate the relationship between α-synuclein accumulation and cognitive functioning using MMSE. Among all investigated mediators, only VEGF was a significant mediator in the relationship between α-synuclein and ADAS-Cog 13 scores. VEGF can affect both neuronal and vascular cells (25), and this study observed that as a neuroprotective cytokine, it may be a key factor in neurodegeneration. The direct relationship between α-synuclein and the level of cognitive functioning was also observed. The associations between ADAS-Cog 13 and immune markers VEGF and soluble IL-6 receptors were surprisingly negative. Higher levels of soluble IL-6 receptor and VEGF were associated with better performance in ADAS-Cog 13. Conversely, poorer performance on the MMSE was associated with higher levels of proinflammatory cytokines IL-6 and TNF-α. This unexpected direction of the relationship between VEGF and ADAS-Cog 13 can be partly explained by VEGF’s vital role in neurogenesis and may still have a protective role in the specific brain regions assessed by ADAS-Cog 13 measure. However, the same relationship was not observed with MMSE as a global cognitive measure. The current findings also suggest a positive relationship between sIL-6R and MMSE scores. This was contrary to our expectation but is consistent with a longitudinal study by Metti et al. (43) finding that older women with an elevated level of sIL-6R were less likely to develop dementia over a 16-year period. Similarly, in another study, sIL-6R measured in CSF was significantly decreased among patients with AD compared to cognitively healthy controls (44).
Given the fact that more women are diagnosed with AD or other dementia when compared with men, this study used moderated mediation analysis to evaluate whether the magnitude of mediation effect of α-synuclein on the level of cognitive functioning depended on the sex of participants. Results showed that the indirect and direct effect of α-synuclein on cognitive functioning was not contingent on sex. Admittedly, the sex differences in the relationship between α-synuclein and cognitive decline may be present in a longitudinal examination rather than cross-sectional. The sex differences in AD prevalence can be partially explained by the fact that women live longer than men considering that age is a major risk factor for AD. In addition, women have an increased rate of other risk factors such as depression, lower education, physical inactivity, and becoming a caregiver (45). Our findings suggest that identifying individual biomarkers that interact with sex may not fully represent the complexity of the sex differences that exist.
To minimize the effects of confounding variables, several considerations were made in this study. Because there was blood contamination in CSF α-synuclein, all cases with a significant amount of Hgb were excluded from the study. Important advances in knowledge during the past 15 years include the growing understanding of biological mechanisms that may lead to AD neuropathology. The current study examined immune markers, α-synuclein, and cognitive assessments at one point in time. Additional analyses may inform the long-term effect of α-synuclein accumulation and neuroinflammation on cognitive decline. Another limitation of this study is the use of measures to assess global cognitive functioning. Future studies should utilize measures that assess individual cognitive domains, particularly areas vulnerable to AD pathology, such as memory. Finally, given the small sample size, we did not include additional covariates known to impact cognitive functioning. Future research should include other factors not introduced in this study known to influence global cognition.
Furthermore, the current study used data from ADNI, a multisite, multistudy program, and was unable to control for any measurement errors that occurred at the research site. Despite these limitations, the current study established a positive relationship between α-synuclein and immune markers and the mediating role of the immune system in the relationship between α-synuclein and cognitive functioning. The present study provided a unique perspective by using α-synuclein as a potential biomarker to assess 2 neurocognitive measures. These results extended our knowledge about additional biomarkers to aid in understanding AD’s development over time. Further longitudinal studies should examine the concentration of these biomarkers and their role in the progression of MCI to AD over time.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI; National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Contributor Information
Sanaz Dabiri, Department of Psychology, Howard University, Washington, District of Columbia, Washington, DC, USA.
Mara I Ramírez Ruiz, Department of Psychology, Howard University, Washington, District of Columbia, Washington, DC, USA.
Girardin Jean-Louis, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, Miami, Florida, USA.
Oyonumo E Ntekim, Department of Graduate Nutritional Sciences, Howard University, Washington, District of Columbia, Washington, DC, USA.
Thomas O Obisesan, Division of Geriatrics, Department of Medicine, Howard University Hospital, Washington, District of Columbia, Washington, DC, USA.
Alfonso L Campbell, Jr, Department of Psychology, Howard University, Washington, District of Columbia, Washington, DC, USA.
Denée T Mwendwa, Department of Psychology, Howard University, Washington, District of Columbia, Washington, DC, USA.
Funding
None declared.
Conflict of Interest
None declared.
References
- 1. Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15(3):321–387. doi: 10.1016/j.jalz.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 2. Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11(2):111–128. doi: 10.31887/DCNS.2009.11.2/cqiu [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silva MVF, Loures CMG, Alves LCV, de Souza LC, Borges KBG, Carvalho MDG. Alzheimer’s disease: risk factors and potentially protective measures. J Biomed Sci. 2019;26(1):33. doi: 10.1186/s12929-019-0524-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 5. Ward A, Tardiff S, Dye C, Arrighi HM. Rate of conversion from prodromal Alzheimer’s disease to Alzheimer’s dementia: a systematic review of the literature. Dement Geriatr Cogn Dis Extra. 2013;3(1):320–332. doi: 10.1159/000354370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lippa CF, Fujiwara H, Mann DM, et al. Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol. 1998;153(5):1365–1370. doi: 10.1016/s0002-9440(10)65722-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Irwin DJ, White MT, Toledo JB, et al. Neuropathologic substrates of Parkinson’s disease dementia. Ann Neurol. 2012;72(4):587–598. doi: 10.1002/ana.23659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee HJ, Khoshaghideh F, Lee S, Lee SJ. Impairment of microtubule-dependent trafficking by overexpression of α-synuclein. Eur J Neurosci. 2006a;24:3153–3162. doi: 10.1111/j.1460-9568.2006.05210.x [DOI] [PubMed] [Google Scholar]
- 9. Larson ME, Sherman MA, Greimel S, et al. Soluble α-synuclein is a novel modulator of Alzheimer’s disease pathophysiology. J Neurosci. 2012;32(30):10253–10266. doi: 10.1523/JNEUROSCI.0581-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Korff A, Liu C, Ginghina C, Shi M, Zhang J; Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s Disease Neuroimaging Initiative. α-Synuclein in cerebrospinal fluid of Alzheimer’s disease and mild cognitive impairment. J Alzheimers Dis. 2013;36(4):679–688. doi: 10.3233/JAD-130458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Slaets S, Vanmechelen E, Le Bastard N, et al. Increased CSF α-synuclein levels in Alzheimer’s disease: correlation with tau levels. Alzheimers Dement. 2014;10(suppl 5):S290–S298. doi: 10.1016/j.jalz.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 12. Bender AR, Raz N. Age-related differences in memory and executive functions in healthy APOE ɛ4 carriers: the contribution of individual differences in prefrontal volumes and systolic blood pressure. Neuropsychologia. 2012;50(5):704–714. doi: 10.1016/j.neuropsychologia.2011.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iwai A, Masliah E, Yoshimoto M, et al. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14(2):467–475. doi: 10.1016/0896-6273(95)90302-x [DOI] [PubMed] [Google Scholar]
- 14. Peng C, Gathagan RJ, Lee VM. Distinct α-synuclein strains and implications for heterogeneity among α-Synucleinopathies. Neurobiol Dis. 2018;109(Pt B):209–218. doi: 10.1016/j.nbd.2017.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uversky VN. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem. 2007;103(1):17–37. doi: 10.1111/j.1471-4159.2007.04764.x [DOI] [PubMed] [Google Scholar]
- 16. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166 [DOI] [PubMed] [Google Scholar]
- 17. Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142 [DOI] [PubMed] [Google Scholar]
- 18. Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol Aging. 2008;29(11):1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parbo P, Ismail R, Hansen KV, et al. Brain inflammation accompanies amyloid in the majority of mild cognitive impairment cases due to Alzheimer’s disease. Brain. 2017;140(7):2002–2011. doi: 10.1093/brain/awx120 [DOI] [PubMed] [Google Scholar]
- 20. Lee KS, Chung JH, Choi TK, Suh SY, Oh BH, Hong CH. Peripheral cytokines and chemokines in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2009;28(4):281–287. doi: 10.1159/000245156 [DOI] [PubMed] [Google Scholar]
- 21. Wilms H, Rosenstiel P, Romero-Ramos M, et al. Suppression of MAP kinases inhibits microglial activation and attenuates neuronal cell death induced by alpha-synuclein protofibrils. Int J Immunopathol Pharmacol. 2009;22(4):897–909. doi: 10.1177/039463200902200405 [DOI] [PubMed] [Google Scholar]
- 22. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kauwe JS, Bailey MH, Ridge PG, et al. Genome-wide association study of CSF levels of 59 Alzheimer’s disease candidate proteins: significant associations with proteins involved in amyloid processing and inflammation. PLoS Genet. 2014;10(10):e1004758. doi: 10.1371/journal.pgen.1004758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Campbell IL, Erta M, Lim SL, et al. Trans-signaling is a dominant mechanism for the pathogenic actions of interleukin-6 in the brain. J Neurosci. 2014;34(7):2503–2513. doi: 10.1523/JNEUROSCI.2830-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Caballero B, Sherman SJ, Falk T. Insights into the mechanisms involved in protective effects of VEGF-B in dopaminergic neurons. Parkinsons Dis. 2017;2017:1–13. doi: 10.1155/2017/4263795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miners S, Moulding H, de Silva R, Love S. Reduced vascular endothelial growth factor and capillary density in the occipital cortex in dementia with Lewy bodies. Brain Patholgy. 2014;24(4):334–343. doi: 10.1111/bpa.12130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tang H, Mao X, Xie L, Greenberg DA, Jin K. Expression level of vascular endothelial growth factor in hippocampus is associated with cognitive impairment in patients with Alzheimer’s disease. Neurobiol Aging. 2013;34(5):1412–1415. doi: 10.1016/j.neurobiolaging.2012.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yasuhara T, Shingo T, Kobayashi K, et al. Neuroprotective effects of vascular endothelial growth factor (VEGF) upon dopaminergic neurons in a rat model of Parkinson’s disease. Eur J Neurosci. 2004;19(6):1494–1504. doi: 10.1111/j.1460-9568.2004.03254.x [DOI] [PubMed] [Google Scholar]
- 29. Ali SA, Begum T, Reza F. Hormonal influences on cognitive function. Malays J Med Sci. 2018;25(4):31–41. doi: 10.21315/mjms2018.25.4.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jacobs EG, Weiss BK, Makris N, et al. Impact of sex and menopausal status on episodic memory circuitry in early midlife. J Neurosci. 2016;36(39):10163–10173. doi: 10.1523/JNEUROSCI.0951-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Asthana S. Estrogen and cognition: the story so far. J Gerontol A Biol Sci Med Sci. 2003;58(4):M322–M323. doi: 10.1093/gerona/58.4.M322 [DOI] [PubMed] [Google Scholar]
- 32. van de Beek M, Babapour Mofrad R, van Steenoven I, et al. Sex-specific associations with cerebrospinal fluid biomarkers in dementia with Lewy bodies. Alzheimers Res Ther. 2020;12:44. doi: 10.1186/s13195-020-00610-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Piscopo P, Bellenghi M, Manzini V, et al. A sex perspective in neurodegenerative diseases: microRNAs as possible peripheral biomarkers. Int J Mol Sci. 2021;22(9):4423. Published 2021 Apr 23. doi: 10.3390/ijms22094423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. ; Alzheimer's Disease Neuroimaging Initiative. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–413. doi: 10.1002/ana.21610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lindersson E, Beedholm R, Hojrup P, Moos T, Gai W, Hendil KB, Jensen PH. Proteasomal inhibition by alpha-synuclein filaments and oligomers. J Biol Chem. 2004;279(13):12924–12934. doi: 10.1074/jbc.M306390200. [PubMed: 14711827] [DOI] [PubMed] [Google Scholar]
- 36. Hong Z, Shi M, Chung KA, et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain. 2010;133(Pt 3):713–726. doi: 10.1093/brain/awq008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kang JH, Irwin DJ, Chen-Plotkin AS, et al. Association of cerebrospinal fluid β-amyloid 1-42, T-tau, P-tau181, and α-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol. 2013;70(10):1277–1287. doi: 10.1001/jamaneurol.2013.3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 39. Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration Norms and Commentary. 3rd ed. Oxford University Press; 2006. [Google Scholar]
- 40. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141(11):1356–1364. doi: 10.1176/ajp.141.11.1356 [DOI] [PubMed] [Google Scholar]
- 41. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. doi: 10.1300/J018v05n01_09 [DOI] [Google Scholar]
- 42. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. 3rd ed. The Guilford Press, 2022. [Google Scholar]
- 43. Metti AL, Yaffe K, Boudreau RM, et al. Change in inflammatory markers and cognitive status in the oldest–old women from the Study of Osteoporotic Fractures. J Am Geriatr Soc. 2014;62(4):662–666. doi: 10.1111/jgs.12739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hampel H, Schoen D, Schwarz MJ, et al. Interleukin-6 is not altered in cerebrospinal fluid of first-degree relatives and patients with Alzheimer’s disease. Neurosci Lett. 1997;228(3):143–146. doi: 10.1016/s0304-3940(97)00379-0 [DOI] [PubMed] [Google Scholar]
- 45. Nebel RA, Aggarwal NT, Barnes LL, et al. Understanding the impact of sex and gender in Alzheimer’s disease: a call to action. Alzheimers Dement. 2018;14(9):1171–1183. doi: 10.1016/j.jalz.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]