Abstract
Cyclooxygenase 2 (COX-2) inhibits nerve growth factor (NGF) withdrawal apoptosis in differentiated PC12 cells. The inhibition of apoptosis by COX-2 was concomitant with prevention of caspase 3 activation. To understand how COX-2 prevents apoptosis, we used cDNA expression arrays to determine whether COX-2 regulates differential expression of apoptosis-related genes. The expression of dynein light chain (DLC) (also known as protein inhibitor of neuronal nitric oxide synthase [PIN]) was significantly stimulated in PC12 cells overexpressing COX-2. The COX-2-dependent stimulation of DLC expression was, at least in part, mediated by prostaglandin E2. Overexpression of DLC also inhibited NGF withdrawal apoptosis in differentiated PC12 cells. Stimulation of DLC expression resulted in an increased association of DLC/PIN with neuronal nitric oxide synthase (nNOS), thereby reducing nNOS activity. Furthermore, nNOS expression and activity were significantly increased in differentiated PC12 cells after NGF withdrawal. This increased nNOS activity as well as increased nNOS dimer after NGF withdrawal were inhibited by COX-2 or DLC/PIN overexpression. An nNOS inhibitor or a membrane-permeable superoxide dismutase (SOD) mimetic protected differentiated PC12 cells from NGF withdrawal apoptosis. In contrast, NO donors induced apoptosis in differentiated PC12 cells and potentiated apoptosis induced by NGF withdrawal. The protective effects of COX-2 on apoptosis induced by NGF withdrawal were also overcome by NO donors. These findings suggest that COX-2 promotes cell survival by a mechanism linking increased expression of prosurvival genes coupled to inhibition of NO- and superoxide-mediated apoptosis.
Prostaglandins have been shown to mediate inflammatory responses as well as to regulate a number of signal transduction pathways that modulate cellular adhesion, growth, and differentiation. Cyclooxygenase (COX) is the key enzyme in the production of prostaglandins. The isoform COX-1 is constitutively expressed in most tissues, whereas the expression of isoform COX-2 is induced by growth factors, tumor promoters, cytokines (10, 47), and vasoactive peptides such as endothelin 1 (27). In addition to involvement in the inflammatory responses, COX-2 and its products, especially prostaglandin E2 (PGE2), have been reported to be important in inhibition of apoptosis (46, 55). Apoptosis, or programmed cell death, is a normal physiological process which occurs during embryonic development as well as in maintenance of tissue homeostasis. Inappropriate induction of apoptosis has been associated with organ injury, whereas a failure to undergo apoptosis may cause abnormal cell overgrowth and malignancy (20).
Previous studies in rat intestinal epithelial cells have shown that COX-2 overexpression leads to a number of effects that could be associated with tumorigenesis: increased adhesion to extracellular matrix proteins, inhibition of butyrate-induced apoptosis, decreased expression of both E-cadherin and transforming growth factor β2 receptor, and stimulation of Bcl-2 protein expression (55). The model systems involving coculture of endothelial cells with colon carcinoma cells showed that COX-2-expressing cells produce high level of angiogenic factors, which stimulate endothelial tube formation in the coculture model (56). Indeed, the level of COX-2 protein has been reported to increase dramatically in human colorectal adenocarcinomas (11), in colorectal tumors (33, 44), in adenomas taken from APC mutant mice (39), and in intestinal tumors from carcinogen-treated rats (9). High levels of constitutive COX-2 expression are also detected in the human colon cancer cell line HCA-7. Treatment of HCA-7 cells with SC-58125, a highly selective COX-2 inhibitor, results in inhibition of growth and increase of apoptotic cells, which is reversed by PGE2 stimulation (46). In addition, overexpression of COX-2 in RAW 264.7 macrophages inhibits apoptosis (57). Inhibition of COX-2 activity by SC-58236 or downregulation of COX-2 protein by antisense expression in medullary interstitial cells causes apoptosis (18). Therefore, these data suggest that COX-2 may function as a survival factor and protect cells from apoptosis. To further explore the mechanisms of antiapoptotic effects of COX-2, we established a PC12 pheochromocytoma cell line stably transfected with a rat COX-2 cDNA or vector alone under the control of an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter (lacSwitch promoter) (37). PC12 cells have been commonly used as a cell culture model for studies of neuronal development and functions. In particular, PC12 cells are also a convenient alternative to cultured neurons for studying the trophic and differentiative actions of nerve growth factor (NGF) since PC12 cells can be induced by NGF to differentiate to acquire many characteristics of mature sympathetic neurons, including extended long branching neurites (52). Moreover, differentiated PC12 cells undergo pronounced and well-characterized apoptosis upon NGF withdrawal that resembles apoptosis in cultured sympathetic neurons (59). We have demonstrated that COX-2 overexpression inhibits the apoptosis by NGF withdrawal of differentiated PC12 cells (37).
The aim of this study was to determine a possible downstream mediator(s) of COX-2 in antiapoptotic signaling. The cDNA probes generated from PC12 cells overexpressing COX-2 (PCXII cells) or mock-transfected cells (PC-MT cells) were used for expression array screening using the Atlas human cDNA expression array (Clontech). The screening showed an enhanced expression of the cytoplasmic dynein light chain (DLC) (also known as protein inhibitor of neuronal nitric oxide synthase [PIN]) gene. DLC protein expression was elevated not only in PCXII cells but also in PC12 or human mesangial cells infected with adenovirus encoding COX-2. Furthermore, PC12 cells overexpressing DLC (PC-DLC cells) were more resistant than parental (PC-Off) cells to apoptosis induced by NGF withdrawal. Coimmunoprecipitation assays showed increased association of DLC protein with neuronal nitric oxide synthase (nNOS) in PCXII or PC-DLC cells, which decreased nNOS activity. We also observed that nNOS expression and activity were further elevated in differentiated PC12 cells after NGF withdrawal. However, this increased nNOS activity was inhibited by COX-2 or DLC overexpression. An nNOS inhibitor as well as a membrane-permeable superoxide dismutase (SOD) mimetic prevented differentiated PC12 cell death induced by NGF withdrawal. NO donors induced apoptosis in differentiated PC-MT cells and reversed the protective effects of COX-2 on apoptosis induced by NGF withdrawal, partially due to activation of caspase 3. Taken together, our results provide a new molecular mechanism underlying the protective role of COX-2 in differentiated PC12 cell death in response to NGF withdrawal.
MATERIALS AND METHODS
Cell culture.
The PCXII and PC-MT cell lines were constructed using the lacSwitch expression system (Stratagene) as described previously (37). Both PC12 cell lines were cultured in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated horse serum, 5% fetal bovine serum, 2 mM glutamine, penicillin (100 U/ml), streptomycin (100 U/ml), Geneticin (G418; 0.8 mg/ml; Gibco-BRL), and hygromycin B (0.08 mg/ml; Gibco-BRL) in culture plates coated with rat tail collagen (Becton Dickson).
Human mesangial cells were provided by Jean-Daniel Sraer and cultured as described elsewhere (49). For PGE2 treatment, human mesangial cells were serum starved for 48 h in RPMI medium containing 0.1% fetal bovine serum, and PC12 cells were serum starved for 17 h in F-12K medium containing 1% horse serum. Cells were then treated with PGE2 for 6 h.
Hybridization of cDNA expression array.
PCXII or PC-MT cells were grown in regular serum medium in the presence of IPTG (2.5 mM) for 17 h on collagen-coated culture plates. mRNA was isolated from two 100-mm-diameter plates of each cell line, using a QuickPrep Micro mRNA purification kit (Amersham Pharmacia Biotech). 32P-labeled cDNA probes were prepared and hybridized to the Atlas human cDNA expression array (Clontech) as instructed by the manufacturer. Membranes were exposed to Kodak BioMax MS X-ray film with a BioMax MS intensifying screen at −70°C for 24 h.
Cloning of cytoplasmic DLC cDNA.
Total RNA was isolated from IPTG-stimulated PCXII cells by using TRIzol reagent (Gibco-BRL). The rat DLC cDNA was amplified by reverse transcription-PCR (Amersham Pharmacia Biotech) using primers corresponding to the rat DLC gene (GenBank accession no. R47168). The 5′ primer (5′-ACTGACATATGTGCGACCGGAAGGCGG-3′) contained an NdeI site, and the 3′ primer (5′-TCAGTAGGATCCTTAACCAGATTTGAACAGAAG-3′) contained a BamHI site. The amplified DLC cDNA was subcloned into the NdeI-BamHI sites of pKoz/M-Flag, a plasmid containing a Kozak sequence and an N-terminal Flag tag (R. Jakobi, unpublished data), and sequenced to confirm the identity.
Establishment of PC-DLC cells.
PC12 cells were obtained from the American Type Tissue Collection (Manassas, Va.) and grown in F-12K medium supplemented with 15% heat-inactivated horse serum and 2.5% fetal bovine serum in rat tail collagen-coated culture plates. The retroviral gene delivery and expression system RevTet-Off (Clontech) was used to establish PC12 cells expressing the rat DLC cDNA. The vector pRevTet-Off encodes the tetracycline transcriptional activator, which binds to the tetracycline response element and activates transcription in the absence of tetracycline or doxycycline. Addition of either antibiotic at 10 μg/ml inhibits expression of DLC. pRevTRE is a response vector in which DLC cDNA was cloned with a Kozak sequence and an N-terminal Flag tag downstream of the tetracycline response element. To create the parental cell line PC-Off, the packaging cell line (Phoenix Eco) (23) was transfected with the regulator vector (pRevTet-Off), and the resulting virus-containing supernatant was used to infect PC12 cells. Stable PC12 cell populations expressing the transcriptional activator were selected in the presence of G418 (0.75 mg/ml; Gibco-BRL) and served as parental cells as well as control cells for establishing stable cells expressing DLC (PC-DLC cells). Phoenix Eco cells were then transfected with pRevTRE, which contained DLC cDNA, and the supernatant was used to infect PC-Off cells. Stable cell populations (PC-DLC cells) were selected in the presence of hygromycin B (0.3 mg/ml).
Induction of differentiated PC12 cells.
PC12 cells were differentiated for 7 to 9 days in medium containing 1% horse serum and 50 ng of NGF (Becton Dickson) per ml. To obtain the maximal expression of COX-2, IPTG (2.5 mM) was added into the differentiation medium for PC-MT or PCXII cells.
Adenovirus COX-2 gene transfer.
The recombinant adenovirus vector AdCOX-2, expressing COX-2, was constructed from replication-deficient adenovirus type 5 (Ad5) with deletions in the E1 and E3 genes, Addl327, and a plasmid containing Ad5 sequences from 22 to 5790 with a deletion of the E1 region from bp 342 to 3523, a polycloning site under control of the cytomegalovirus promoter, the COX-2 cDNA, and the simian virus 40 polyadenylation signal. Human mesangial or PC12 cells were incubated with AdCOX-2 or an Ad5-based β-galactosidase expression vector (AdLacZ) (at a multiplicity of infection [MOI] of 25 for human mesangial cells or MOI 50 for PC12 cells) for 1 h, followed by addition of complete medium. At 24 h after infection, cells were lysed and subjected to Western blot analysis or stained for 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) as described previously (15).
Immunoprecipitation; Western and Northern blot analyses.
Cells were washed and lysed in lysis buffer as described previously (15). The cleared cell lysates (200 μg) were incubated with nNOS antibody (R20; 4 μg; Santa Cruz Biotechnology) overnight at 4°C, followed by incubation with protein A-Sepharose (Amersham Pharmacia Biotech) for 1 h. The immunoprecipitates were washed three times with 1 ml of lysis buffer and boiled in Laemmli buffer for 5 min at 95°C.
Immunoprecipitates or cell lysates (30 to 40 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Micron Separation Inc.), and immunodetection was carried out as described previously (15). nNOS monoclonal antibody for Western blotting was from Transduction Laboratories, and antibodies for COX-2 (N20) and caspase 3 (H227) were from Santa Cruz.
For Northern blot analysis, total RNA was isolated from PCXII or PC-MT cells by using TRIzol reagent (Gibco-BRL). RNA samples (10 μg) were then separated by electrophoresis on 1.2% agarose gels containing formamide and transferred to a Hybond-N+ nylon membrane (Amersham Pharmacia Biotech). The membrane was hybridized with 32P-labeled full-length rat COX-2 cDNA, full-length DLC cDNA, or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) cDNA in ExpressHyb solution (Clontech).
Apoptosis.
Differentiated PC12 cells were induced for apoptosis by NGF withdrawal as described previously (59). NGF withdrawal was carried out by washing the cells once with NGF-free medium followed by incubation in NGF-free medium containing rabbit neutralizing antibody to 2.5s NGF (Sigma) at a 1:500 dilution for 17 h. The NO donor 2-2′-(hydroxynitrosohydrazino)bisethanamine (DETA-NONOate; 200 μM; Cayman) or S-nitro-N-acetylpenicillamine (SNAP; 200 μM; Cayman) or the nNOS inhibitor N5-(1-imino-3-buteny)-l-orthine (l-VNIO; 10 μM; gift from Owen Griffith, Medical College of Wisconsin) (2) or manganese III 4,4′,4",4′"(21H,2H-porphine-5,10,15,20-tetrayl)tetrakis (benzoic acid; better known as manganese TBAT; 200 μM; Alexis) was added to the cells as indicated. Cells washed once in NGF-free medium and then incubated in NGF-containing medium were used as controls for apoptotic cells. Cells were fixed with a mixture of acetone and methanol (1:1 ratio) for 20 min at −20°C, and the nuclei were stained with Hoechst 33258 (25 μg/ml; Sigma) for 15 min at room temperature and observed under fluorescence microscopy using a 4′,6-diamidino-2-phenylindole (DAPI) filter. Fragmented or condensed nuclei were scored as apoptotic.
To visualize oligonucleosomal fragmentation, DNA was extracted from control or apoptotic cells with phenol-chloroform-isoamyl alcohol as described previously (50) and analyzed on a 2% agarose gel.
nNOS activity assay.
nNOS activity was measured using the conversion of [3H]arginine to [3H]citrulline by a modification (21) of the method described by Kiedrowski et al. (28). PC12 cells were cultured at a density of 200,000 cells per well on collagen-coated six-well plates with or without NGF treatment, and so nNOS activity was measured within the linear range. Cells were preincubated with [3H]arginine (3 μCi) for 5 min at 37°C. After removal of [3H]arginine, cells were washed and then scraped in 1 ml of 0.3 mM HClO4, the lysate was centrifuged, and the supernatant was neutralized with K2CO3. An aliquot of the supernatant was counted for 3H to assess [3H]arginine uptake. A second aliquot of the supernatant was placed onto columns containing Dowex AG 50W-X8 cation-exchange resin (sodium form; Bio-Rad). The flowthrough and eluate following the addition of 2 ml of water were combined, and counts per minute was determined. Studies using [14C]citrulline and [3H]arginine standards demonstrate that the columns retain over 98% of added arginine and less than 7% of added citrulline. Each treatment was measured in triplicate, and each experiment was repeated three times; means ± standard deviations (SD) are reported.
RESULTS
Inhibition of caspase 3 activation by COX-2 overexpression.
Our previous studies have shown that overexpression of COX-2 in differentiated PC12 cells results in inhibition of programmed cell death induced by NGF withdrawal compared to mock-transfected cells (37). Caspase 3 has been implicated as an indicator of apoptosis since it was discovered as a key protease in the execution phase of apoptosis (53). The activated caspase 3 comprises a 17-kDa large subunit containing the active site and a small subunit of approximately 10 kDa (53). In PC-MT cells, activation of caspase 3 was detected as early as 3 h after NGF withdrawal and reached maximal levels at 6 h, as shown by the presence of the p17 caspase 3 cleavage product (Fig. 1). The activation of caspase 3 was transient since the p17 cleavage product disappeared between 6 and 17 h of apoptosis (Fig. 1). Little activation of caspase 3 was observed in PCXII cells (Fig. 1), indicating that COX-2 either acts at the level of pro-caspase 3 or disrupts the upstream signal that leads to activation of caspase 3 activity.
FIG. 1.
COX-2 overexpression suppressed activation of caspase 3 after NGF withdrawal. PC-MT and PCXII cells were differentiated by NGF for 7 days in the presence of IPTG (2.5 mM), followed by NGF withdrawal for 0, 3, 6, 17, and 24 h. Cell lysates (40 μg) were separated by SDS–8 to 15% PAGE, and activation of caspase 3 was analyzed by Western blotting using caspase 3 antibody.
COX-2 overexpression stimulates the expression of DLC/PIN.
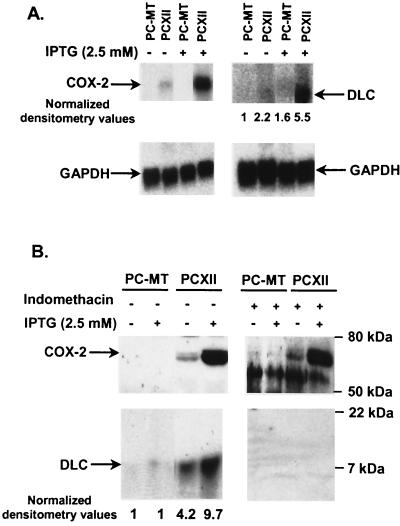
To identify the differential expression of genes involved in antiapoptotic effects mediated by COX-2, Atlas human cDNA expression arrays (Clontech) were screened with 32P-labeled cDNA generated from mRNA from PC-MT or PCXII cells. Under the high-stringency conditions used, only six genes were found to be expressed differentially between PC-MT and PCXII cells. A difference of more than threefold was considered significant. Signals were normalized to signals from housekeeping genes (data not shown). Expression of cytoplasmic DLC displayed the most change and was sixfold greater in PCXII cells than in PC-MT cells. DLC has been shown to be important for cellular viability (8, 16); hence, we evaluated the correlation between stimulation of DLC expression by COX-2 and COX-2-dependent antiapoptotic effects in differentiated PC12 cells. DLC was initially identified as a component of microtubule-associated motor complex with a molecular mass of 8 kDa (30), which is also known as PIN (25). To confirm the results of cDNA expression array screening, RNA and cell lysates from PC-MT and PCXII cells were analyzed by Northern and Western blot analyses, respectively. The levels of DLC mRNA as well as DLC protein were significantly higher in PCXII cells than in PC-MT cells (Fig. 2). Furthermore, pretreatment of PCXII cells with the nonselective COX inhibitor indomethacin blocked the stimulation of DLC expression without affecting COX-2 expression (Fig. 2B). Thus, stimulation of DLC expression requires the enzymatic activity of COX-2.
FIG. 2.
COX-2 overexpression in PC12 cells stimulated DLC expression. (A) Northern blot analysis of RNA from PC-MT and PCXII cells untreated or treated with IPTG for 17 h. Equal loading of RNA samples (10 μg) was confirmed by reprobing with GAPDH cDNA. Arrows indicate the positions of COX-2 and DLC. (B) Western blot analysis of cell lysates from PC-MT and PCXII cells untreated or treated with IPTG for 17 h. For indomethacin treatment, PC-MT and PCXII cells were pretreated with 10 μM indomethacin for 24 h and then incubated with or without IPTG for an additional 17 h. Cell lysates (30 μg) were resolved by SDS–8 to 20% PAGE, transferred onto a nitrocellulose membrane (0.22-μm pore size), and analyzed by Western blotting using COX-2 or DLC antibody. The densitometric values of the signals are normalized such that the zero time point is defined as 1.
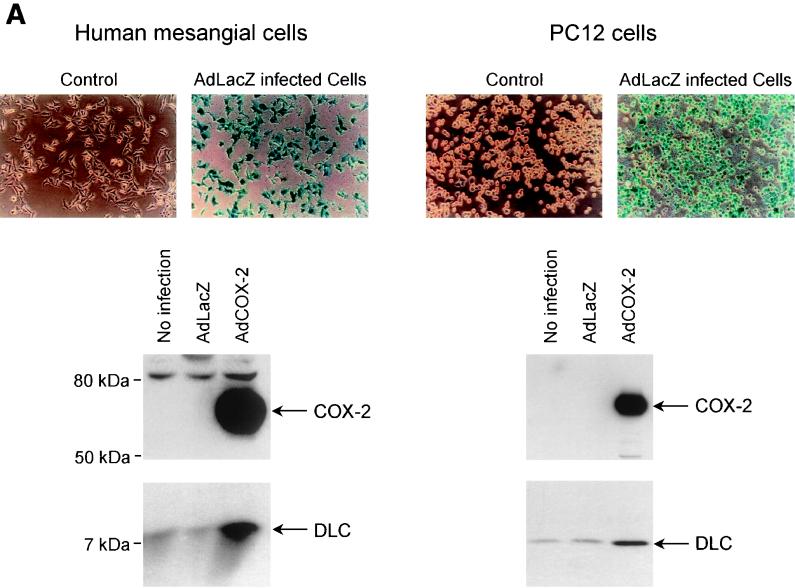
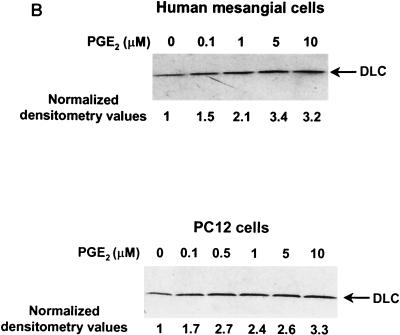
Next, the construct AdCOX-2 was used to transfect PC12 cells and human mesangial cells to determine whether transient expression of COX-2 also stimulates DLC expression. Mesangial cells, a prominent cell type in the glomerulus, are involved in the regulation of glomerular hemodynamics and glomerulonephritis (7, 26). The transgenic adenovirus construct AdLacZ was used to determine the MOI in order to obtain 100% transfection efficiency (Fig. 3A). COX-2 expression in AdCOX-2-transfected cells was confirmed by Western blotting (Fig. 3A). The level of DLC protein in AdLacZ-transfected cells was the same as that in nontransfected cells and was significantly increased in AdCOX-2-transfected cells (Fig. 3A). Treatment of PC12 and human mesangial cells with PGE2 increased DLC expression (Fig. 3B). In PC12 cells, DLC expression started to be increased at a PGE2 concentration of 1 nM and was evident at PGE2 concentrations of 5 to 25 nM (data not shown). These results suggest that stimulation of DLC expression in PC12 cells and in human mesangial cells by COX-2 overexpression is, at least in part, mediated by the COX-2 product, PGE2.
FIG. 3.
Stimulation of DLC expression by transient expression of COX-2 using adenovirus gene delivery as well as by PGE2 treatment. (A) Human glomerular mesangial and PC12 cells were mock infected or infected with AdLacZ or AdCOX-2. As assessed by X-Gal staining, AdLacZ infected ∼100% of cells. Cell lysates (40 μg) were analyzed by Western blotting using anti-COX-2 or anti-DLC/PIN antibody. (B) Human mesangial cells were treated with increasing concentrations of PGE2, and control cells were treated with vehicle (dimethyl sulfoxide) for 6 h. Cell lysates (20 μg) from human mesangial and PC12 cells were separated by SDS–4 to 20% PAGE and analyzed by Western blotting using DLC/PIN antibody. Experiments were repeated three times with similar results.
Overexpression of DLC inhibits apoptosis in differentiated PC12 cells induced by NGF withdrawal.
In Drosophila melanogaster, a total loss-of-function DLC mutant results in degeneration during embryogenesis, and dying cells showed morphological changes characteristic of apoptosis (8). These data suggested that DLC plays a role in the inhibition of apoptosis. We established the PC-DLC cell line (see Materials and Methods) to examine whether DLC is involved in inhibition of apoptosis induced by NGF withdrawal. The expression of DLC in PC12 cells was characterized by Western blot analysis (Fig. 4A). The level of DLC can be downregulated by addition of doxycycline or tetracycline, but basal levels may still exist (Fig. 4A, lanes 3 and 4). Therefore, the parental (PC-Off) cells were used for comparison with PC-DLC cells in the following experiments. As analyzed by Hoechst staining, approximately 30% of differentiated PC-Off cells were apoptotic at 17 h after NGF withdrawal, whereas only a few apoptotic PC-DLC cells were observed (Fig. 4B). Fragmentation of genomic DNA was observed at 17 h after NGF withdrawal in PC-Off cells but was not observed in PC-DLC cells (Fig. 4C). In PC-Off cells, the earliest appearance of active caspase 3 as determined by the cleavage product p17 was detected 2 h after NGF withdrawal; the level of p17 was maximal at 6 h and then decreased at 19 h (Fig. 4D). In contrast, very little caspase 3 p17 was detected in PC-DLC cells throughout the time course. Therefore, DLC overexpression also blocked apoptosis by NGF withdrawal in PC12 cells, indicating that inhibition of apoptosis by COX-2 is, at least in part, mediated by DLC/PIN.
FIG. 4.
Effects of DLC overexpression on differentiated PC12 cell apoptosis induced by NGF withdrawal. (A) Western blot analysis of PC12 cells stably expressing DLC. Lanes 1 and 2, PC-Off (parental) cells treated without (lane 1) or with (lane 2) doxycycline (10 μg/ml) for 48 h; lanes 3 and 4, PC-DLC cells stimulated without (lane 3) or with (lane 4) doxycycline (10 μg/ml) for 48 h. (B) Prevention of PC12 cell apoptosis induced by NGF withdrawal by DLC overexpression as determined by Hoechst staining. PC-Off and PC-DLC cells were differentiated by NGF for 7 days, followed by treatment with or without NGF withdrawal for 17 h. Data shown are the averages of three independent experiments, and error limits are within 3%. (C) Prevention of PC12 cell apoptosis induced by NGF withdrawal by DLC overexpression as determined by DNA fragmentation. Soluble cytoplasmic DNA from differentiated PC-Off or PC-DLC cells with (+) or without (−) NGF withdrawal for 17 h was analyzed by agarose gel electrophoresis. Some undegraded RNA was also strongly stained with ethidium bromide at the bottom of the gel. (D) Inhibition of caspase 3 activation induced by NGF withdrawal by DLC overexpression. Differentiated PC-Off or PC-DLC cells were incubated with or without anti-NGF antibody. At indicated time points, cells lysates (40 μg) were analyzed by Western blotting for caspase 3.
Association of DLC with nNOS results in reduction of nNOS activity.
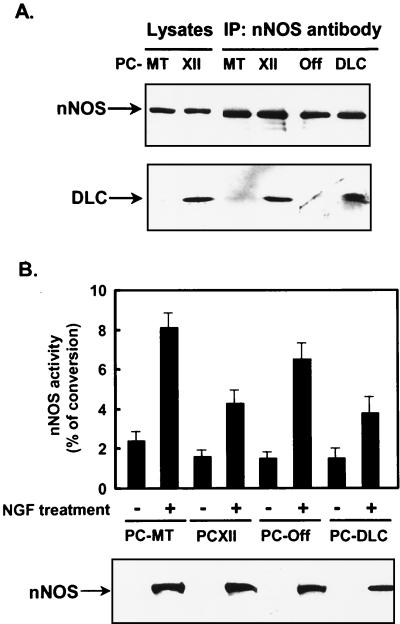
DLC interacts with nNOS, resulting in inhibition of nNOS activity (25). PC12 cells were differentiated by NGF for 7 days to fully induce nNOS expression (40, 45). Although equal amounts of nNOS were immunoprecipitated with nNOS antibody from all differentiated PC12 cell lines, more DLC was coimmunoprecipitated with nNOS from PCXII or PC-DLC cells than from PC-MT or PC-Off cells, respectively (Fig. 5A). These data were consistent with a previous report that the complex of DLC and nNOS is detected in rat cerebellum extracts by coimmunoprecipitation (25). nNOS activity was then examined in all cell lines with or without NGF stimulation for 7 days. Very little nNOS activity as well as nNOS protein was detected in all cell lines without NGF treatment (Fig. 5B), consistent with previous studies (40, 45). After NGF treatment for 7 days, the expression of nNOS in all cell lines was induced to similar extents, while nNOS activity was significantly increased only in PC-MT or PC-Off cells, not in PCXII or PC-DLC cells (Fig. 5B). Neither endothelial nor inducible NOS protein was detected in these lysates by Western blotting using a specific antibody (data not shown), consistent with the report from Sheehy et al. (45). These results suggest that the stimulation of DLC expression led to an increase of association with nNOS and thus decreased nNOS activity in differentiated PC12 cells.
FIG. 5.
Association of DLC with nNOS in differentiated PC12 cells reduced nNOS activity. (A) PC-MT, PCXII, PC-Off, and PC-DLC cells were treated with NGF for 7 days; IPTG was added to PC12-MT and PCXII cells. Cell lysates (200 μg) were immunoprecipitated with nNOS antibody. Lysates (40 μg) and immunoprecipitates were analyzed by Western blotting using nNOS (top) or DLC (bottom) antibody. (B) nNOS activity was assayed in PC-MT, PCXII, PC-Off, and PC-DLC cells treated with or without NGF for 7 d; IPTG was added to PC-MT and PCXII cells. The activity of nNOS is presented as the percentage of conversion of [3H]arginine to [3H]citrulline. Values are means ± SD of three independent experiments. In the parallel experiments, cell lysates were analyzed for nNOS expression by Western blotting using nNOS antibody.
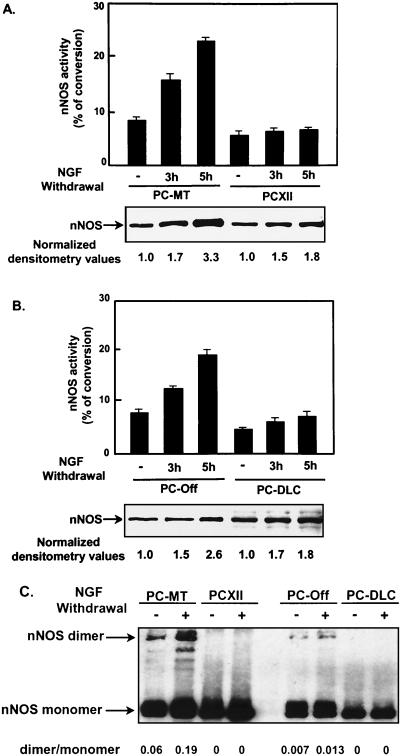
NGF withdrawal further increases nNOS expression and activity.
Although nNOS expression and activity were stimulated by NGF, we found that nNOS activity was further increased in both PC-MT and PC-Off cells after NGF withdrawal (Fig. 6). The nNOS activity in PCXII or PC-DLC cells remained unchanged after NGF withdrawal. Western blot analysis showed that nNOS protein was also significantly increased in all PC12 cell lines as early as 3 h after NGF withdrawal and up to two- to threefold at 6 h (Fig. 6). The levels of nNOS then declined at 17 h after NGF withdrawal (data not shown). In PCXII and PC-DLC cells, nNOS protein was increased after NGF withdrawal but the activity remained unchanged, possibly due to association of DLC with nNOS. Dimerization of nNOS (∼320 kDa) is essential for its activity and can be analyzed by low-temperature SDS-PAGE in the presence of tetrahydrobiopterin and arginine (31). nNOS dimerization was increased two- to threefold in PC-MT and PC-Off cells at 6 h after induction of apoptosis compared to differentiated cells. Dimerization was not detected in PCXII or PC-DLC cells either at the differentiated stage or after NGF withdrawal (Fig. 6C). These results suggest that nNOS activity plays an important role in apoptosis in differentiated PC12 cells induced by NGF withdrawal and that overexpression of either COX-2 or DLC could prevent this increased nNOS activity.
FIG. 6.
NGF withdrawal from differentiated PC12 cells further stimulated nNOS expression and activity, and nNOS activity was inhibited by COX-2 or DLC overexpression. (A and B) PC-MT, PCXII, PC-Off, and PC-DLC cells were treated with NGF for 7 days; IPTG was added to PC-MT and PCXII cells. At 0, 3, and 5 h after NGF withdrawal, nNOS expression was analyzed by Western blotting, and activity was measured as described in Materials and Methods. Values are means ± SD of three experiments. (C) Cell lysates from differentiated PC-MT, PCXII, PC-Off, or PC-DLC cells incubated with (+) or without (−) anti-NGF antibody for 6 h were analyzed for nNOS dimerization by SDS-PAGE under low-temperature conditions as described previously (31), followed by Western blot analysis using nNOS antibody. The dimer/monomer ratio was determined by densitometrically scanning of both nNOS bands on autoradiogram.
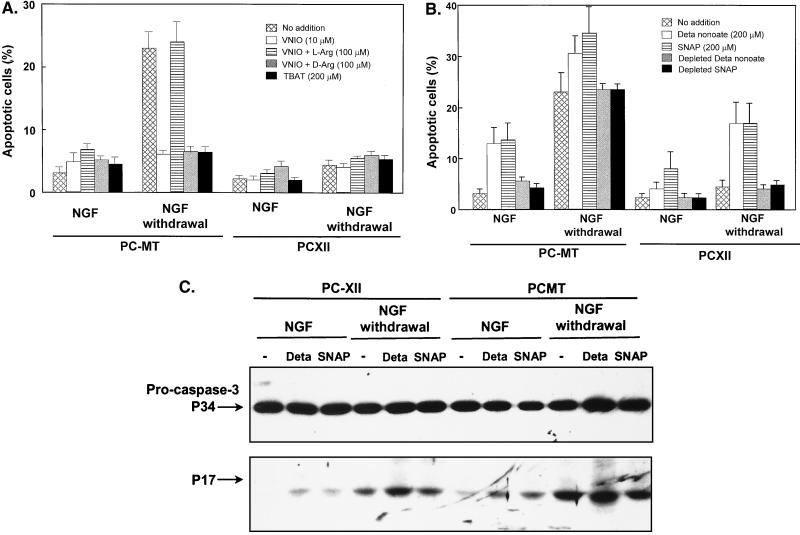
NO donors induce apoptosis, while NOS inhibitor or SOD mimetic manganese TBAT prevents apoptosis.
l-VNIO has been recently shown to be a potent and selective inhibitor for nNOS (2). Treatment with 10 μM l-VNIO prevented apoptosis in PC-MT cells and had no effect on PCXII cells (Fig. 7A). Similar results have been obtained using a nonselective NOS inhibitor, NG-nitro-l-arginine methyl ester (l-NAME; 10 μM) (data not shown). The protection from apoptosis by l-VNIO could be overcome by the NOS substrate l-arginine (100 μM) but not d-arginine, suggesting that l-VNIO is a stereospecific inhibitor of nNOS. A membrane-permeable scavenger of superoxide and peroxynitrite, manganese TBAT (14, 51), also increased survival of PC-MT cells after NGF withdrawal (Fig. 7A), suggesting that an intracellular increase in superoxide was required to induce cell death. In contrast, NO donors DETA-NONOate and SNAP caused differentiated PC-MT cells to undergo apoptosis but to a lesser extent compared to NGF withdrawal, while NO donors had no effect on PCXII cells (Fig. 7B). Furthermore, NO donors potentiated the apoptosis in PC-MT cells induced by NGF withdrawal and also reversed the protective effects of COX-2 on apoptosis. The depleted NO donors had no effect on apoptosis. Caspase 3 was also activated in differentiated PC-MT cells by both DETA-NONOate and SNAP but to a lesser extent compared to NGF withdrawal. DETA-NONOate but not SNAP further increased the activated caspase 3 in differentiated PC-MT cells after NGF withdrawal (Fig. 7C). Both NO donors had no effect on caspase 3 activation in differentiated PCXII cells, but DETA-NONOate potentiated caspase 3 activation in the same cells after NGF withdrawal (Fig. 7C). These results suggested that NO is necessary but not sufficient to induce differentiated PC12 cell apoptosis. Superoxide or possibly peroxynitrite (a strong oxidant formed by the reaction of NO and superoxide) may also play an important role in induction of apoptosis in differentiated PC12 cells by NGF withdrawal.
FIG. 7.
Effects of NO donors, nNOS inhibitor, or manganese TBAT on apoptosis in differentiated PC12 cells. (A) Differentiated PC-MT and PCXII cells were treated with or without nNOS inhibitor as indicated in the presence of NGF (25 ng/ml) for 24 h or anti-NGF antibody for 17 h. Nuclei were stained with Hoechst 33528. Cells containing condensed or fragmented nuclei were scored as apoptotic. (B) Differentiated PC-MT and PCXII cells were treated with or without NO donor as indicated in the presence of NGF (25 ng/ml) for 24 h or anti-NGF antibody (1:1,000 dilution) for 17 h. For depletion, the NO donors were incubated at room temperature in serum-free medium for 4 days to completely liberate NO. Values are means ± SD of four experiments. (C) Differentiated PC-MT or PCXII cells, treated with or without NO donor (200 μM DETA-NONOate [Deta] or SNAP) in the presence of NGF (25 ng/ml) or anti-NGF antibody (1:500 dilution) for 6 h. Cell lysates (40 μg) were separated by SDS–8 to 15% PAGE, and activation of caspase 3 was analyzed by Western blotting using caspase 3 antibody.
DISCUSSION
The significance of the antiapoptotic properties of COX-2 have been emphasized because of the contribution of COX-2 to the progression of various proliferative diseases. The enhancement of glomerular COX-2 mRNA level has been shown in animal models of proliferative glomerulonephritis (5, 22). The progression of proliferative glomerulonephritis is accompanied by intensive proliferation of glomerular mesangial cells, which is usually attenuated by programmed cell death (apoptosis). The constitutive expression of COX-2 has been identified in a majority of colon cancer specimens (11, 33, 58). Overexpression of COX-2 in intestinal epithelial cells leads to inhibition of apoptosis and correlates with induction of Bcl-2 expression (55). Our previous data showed that induction of apoptosis in differentiated PC12 cells by NGF withdrawal was blocked by overexpression of COX-2 and that this inhibitory effect was concomitant with inhibition of caspase 3 activation (37). Therefore, it appears that stimulation of COX-2 expression interferes with a program of self-regulated destruction of undesirable cells and thus can contribute to uncontrolled cell growth. Although the capacity of COX-2 to prevent apoptosis is documented in a number of systems, the mechanism of antiapoptotic effect of COX-2 overexpression remains unknown. The data presented here provide evidence for the molecular mechanisms of the antiapoptotic effect of COX-2. The results suggest a crucial role of COX-2 in regulation of nNOS activity in the prevention of apoptosis, which we postulate is dependent on upregulation of DLC expression.
In this study, we demonstrated that COX-2 overexpression resulted in a significant induction of DLC expression. This stimulation of DLC expression by COX-2 required the enzymatic activity of COX-2, as indomethacin inhibited and PGE2 stimulated DLC expression. Previously, overexpression of COX-2 in rat intestinal epithelial cells has been shown to induce Bcl-2 expression (55). However, Bcl-2 protein was not detectable in our system with or without COX-2 overexpression, suggesting that the induction of Bcl-2 is cell specific (Y.-W. E. Chang et al., unpublished data). DLC has been identified as a component of cellular motor complex with a molecular mass of 8 kDa (4, 30). Partial loss-of-function mutations of DLC in Drosophila lead to morphogenetic defects in bristle and wing development, female sterility, and disruption of sensory axon trajectories, while total loss-of-function mutations induces apoptosis and embryonic lethality (8). The mRNA levels of DLC are rapidly induced by global ischemia in pyrimidal neurons of the hippocampal CA3 region and granule cells of the dentate gyrus, which are shown to be resistant to ischemic damage (16). A new member of Bcl-2 family, Bim, has been demonstrated to provoke apoptosis (38), and the association of Bim with DLC causes sequestration of Bim to the microtubule-associated dynein motor complex, thereby presumably delaying the access of Bim to antiapoptotic Bcl-2 family members in cells exposed to a death stimulus (43). Therefore, these studies suggest that DLC/PIN plays an important role in regulation of apoptosis. Here, we showed that overexpression of DLC significantly inhibited apoptosis as well as activation of caspase 3 in differentiated PC12 cells induced by NGF withdrawal. Therefore, our data provide direct evidence that DLC can be an antiapoptotic protein and function as a downstream mediator of COX-2 in antiapoptotic signaling.
DLC binds to nNOS, resulting in destabilization of nNOS dimer and thereby inhibiting nNOS activity (25). In PCXII or PC-DLC cells, DLC associated with nNOS, thus preventing the increase of nNOS activity in response to NGF treatment. Moreover, NOS activity in differentiated PC-MT and PC-Off cells was found to be rapidly stimulated further after NGF withdrawal and as a result of increased nNOS expression. However, nNOS activity remained unchanged in PCXII PC-DLC cells after NGF withdrawal due to association of DLC with nNOS. Our results suggested that increased nNOS activity is important for differentiated PC12 cell apoptosis induced by NGF withdrawal. In agreement with this hypothesis, we demonstrated that NOS inhibitors reduced PC12 cell apoptosis, NO donors increased apoptosis of PC12 cells, and NO donors reversed the protective effects of COX-2 on apoptosis. Further evidence was provided by increasing activation of caspase 3 by NO donors. However, our data show that NO is required but not sufficient enough to induce apoptosis. nNOS has been shown to produce NO and superoxide in vitro (41, 42). We found that superoxide and peroxynitrite played a significant role in induction of apoptosis, as shown by treatment with manganese TBAT, a membrane-permeable SOD mimetic (14, 51), protecting differentiated PC12 cells from apoptosis induced by NGF withdrawal. Thus, NO and superoxide are required for apoptosis in differentiated PC12 cells induced by NGF withdrawal.
Motor neuron apoptosis induced by trophic factor withdrawal involves both increased NO production after induction of nNOS expression and augmented intracellular production of superoxide, in which NO reacts with superoxide to produce peroxynitrite (13). Peroxynitrite, a stronger and more toxic oxidant than NO and superoxide, has been shown to stimulate apoptosis of PC12 cells (12, 48, 54). Therefore, we concluded that NGF withdrawal from differentiated PC12 cells increases peroxynitrite, which contributes to apoptosis of PC12 cells.
NO, a potentially oxidant radical, can function as either a pro- or an antiapoptotic agent (29). NO is physiologically produced by NOS in various cellular types including endothelial cells and neurons, and it acts as a pleiotropic messenger molecule regulating blood flow and cellular signaling (29). However, many studies suggested that NO is associated with several neuropathological processes and triggers apoptosis in different cell types (29, 35). Induction of ischemia causes activation of nNOS, resulting in excess production of NO (32, 36) and allowing it to react with superoxide to form peroxynitrite, which is associated with cell death (36). Addition of NO donors induces apoptosis in macrophages (1), astrocytes (24), cerebellar granule cells (34), PC12 cells (19), and human neuroblastoma cells (6). Recently, DLC has been implicated to function as an endogenous regulator of nNOS by showing association with nNOS in vivo (25). In addition, its expression level is nearly parallel with that of nNOS in different brain regions (17). Furthermore, DLC levels rapidly increase in brain regions that are resistant to ischemic damage, suggesting that induction of DLC expression following global ischemia counteracts the rise of nNOS activity, thereby protecting neurons from excess NO-induced damage (16). Also, DLC functions as an antiapoptosis factor contributing to resistance to kainic acid-induced apoptosis in the rat hippocampus (3). Our present study showed that stimulation of DLC expression could inhibit programmed cell death by preventing the increase of nNOS activity. Therefore, an increase of DLC expression may be used as an endogenous mechanism to counteract the elevation of nNOS activity and protect neurons from excess NO-induced damage.
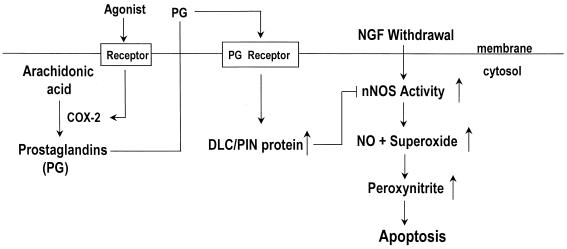
In summary, the model for the mechanism by which COX-2 inhibits apoptosis by NGF withdrawal in PC12 cells is that DLC functions as a downstream mediator of COX-2 (Fig. 8). COX-2 stimulates DLC expression and leads to increased association of DLC with nNOS. This prevents elevation of nNOS activity, thereby inhibiting apoptosis of PC12 cells. The data presented here provide initial evidence for a mechanism linking COX-2 with regulation of nNOS and inhibition of apoptosis.
FIG. 8.
Model for regulation of apoptosis by COX-2. Removal of NGF from differentiated PC12 cells elevates nNOS expression and activity, thereby leading to production of excess NO and superoxide, which contributes to cell death by producing peroxynitrite. Stimulation of COX-2 expression by agonists is concomitant with increased production of PGE2. PGE2 is released from the cells and can stimulate the prostaglandin (PG) receptors on PC12 cells, leading to an increase in DLC expression. Stimulation of DLC expression results in an increase in association of DLC with nNOS, causing inactivation of nNOS and thus reducing production of NO and superoxide. This may prevent PC12 cells apoptosis induced by NGF withdrawal.
ACKNOWLEDGMENTS
We thank Cecilia J. Hillard (Medical College of Wisconsin) for her help with the nNOS activity assay, Owen Griffith (Medical College of Wisconsin) for his generous gift of l-VNIO, and Samie R. Jaffrey (John Hopkins University) for his generous gift of DLC (PIN) polyclonal antibody. We are grateful to Bradley Miller for excellent technical assistance.
This work was supported by National Institutes of Health research grants DK 41684 to A.S., HL 22563 to M.J.D., and ACS-IRG 170 to R.J.
REFERENCES
- 1.Albina J E, Cui S, Mateo R B, Reichner J S. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993;150:5080–5085. [PubMed] [Google Scholar]
- 2.Babu B R, Griffith O W. N5-(1-Imino-3-butenyl)-l-ornithine. A neuronal isoform selective mechanism-based inactivation of nitric oxide synthase. J Biol Chem. 1998;273:8882–8889. doi: 10.1074/jbc.273.15.8882. [DOI] [PubMed] [Google Scholar]
- 3.Becker A J, Gillardon F, Blumcke I, Langendorfer D, Beck H, Wiestler O D. Differential regulation of apoptosis-related genes in resistant and vulnerable subfields of the rat epileptic hippocampus. Mol Brain Res. 1999;67:172–176. doi: 10.1016/s0169-328x(99)00060-1. [DOI] [PubMed] [Google Scholar]
- 4.Benashski S E, Harrison A, Patel-King R S, King S M. Dimerization of highly conserved light chain shared by dynein and myosin V. J Biol Chem. 1997;272:20929–20935. doi: 10.1074/jbc.272.33.20929. [DOI] [PubMed] [Google Scholar]
- 5.Chammugam P, Feng L, Liou S, Jang B C, Boudreau M, Yu G, Le J H, Kwon H J, Beppu T, Yoshida M, Xia Y, Wilson C B, Hwang D. Radicicol, a protein tyrosine kinase inhibitor, suppresses the expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide and in experimental glomerulonephritis. J Biol Chem. 1995;270:5418–5426. doi: 10.1074/jbc.270.10.5418. [DOI] [PubMed] [Google Scholar]
- 6.Ciriolo M R, De Martino A, Lafavia E, Rossi L, Carri M T, Rotilio G. Cu-Zn-superoxide dismutase-dependent apoptosis induced by nitric oxide in neuronal cells. J Biol Chem. 2000;275:5065–5072. doi: 10.1074/jbc.275.7.5065. [DOI] [PubMed] [Google Scholar]
- 7.Davies M. The mesangial cell: a tissue culture view. Kidney Int. 1994;45:320–327. doi: 10.1038/ki.1994.41. [DOI] [PubMed] [Google Scholar]
- 8.Dick T, Ray K, Salz H K, Chia W. Cytoplasmic dynein (ddlc1) mutations cause morphogenetic defects and apoptotic cell death in Drosophila melanogaster. Mol Cell Biol. 1996;16:1966–1977. doi: 10.1128/mcb.16.5.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DuBois R N, Radhika A, Reddy B S, Entingh A J. Increased cyclooxygenase-2 levels in carcinogen-induced rat colonic tumors. Gastroenterology. 1996;110:1259–1262. doi: 10.1053/gast.1996.v110.pm8613017. [DOI] [PubMed] [Google Scholar]
- 10.DuBois R N, Abramson S B, Crofford L, Gupta R A, Simon L S, van de Puter L B A, Lipsky P E. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 11.Eberhart C E, Coffey R J, Radhika A, Giardiello F M, Ferrenbach S, DuBois R N. Up-regulation of cyclooxygenase-2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 12.Estevez A G, Radi R, Barbeito L, Shin J T, Thompson J A, Beckman J S. Peroxynitrite-induced cytotoxity in PC12 cells: evidence for an apoptotic mechanism differentially modulated by neutrophic factors. J Neurochem. 1995;65:1543–1550. doi: 10.1046/j.1471-4159.1995.65041543.x. [DOI] [PubMed] [Google Scholar]
- 13.Estevez A G, Spear N, Manuel S M, Radi R, Henderson C E, Barbeito L, Beckman J S. Nitric oxide and superoxide contribute to motor neuron apoptosis induced by trophic factor deprivation. J Neurosci. 1998;18:923–931. doi: 10.1523/JNEUROSCI.18-03-00923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faulkner K M, Liochev S I, Fridovich I. Stable Mn(III)porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J Biol Chem. 1994;269:23471–23476. [PubMed] [Google Scholar]
- 15.Foschi M, Chari S, Dunn M J, Sorokin A. Biphasic activation of P21ras by endothelin-1 sequentially activates the ERK cascade and phosphatidylinositol 3-kinase. EMBO J. 1997;16:6439–6451. doi: 10.1093/emboj/16.21.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillardon F, Krep H, Brinker G, Lenz C, Bottiger B, Hossmann K-A. Induction of protein inhibitor of neuronal nitric oxide synthase/cytoplasmic dynein light chain following cerebral ischemia. Neuroscience. 1998;84:81–88. doi: 10.1016/s0306-4522(97)00479-x. [DOI] [PubMed] [Google Scholar]
- 17.Greenwood M T, Guo Y, Kumar U, Beausejous S, Hussain S N. Distribution of protein inhibitor of neuronal nitric oxide synthase in rat brain. Biochem Biophys Res Commun. 1997;238:617–621. doi: 10.1006/bbrc.1997.7361. [DOI] [PubMed] [Google Scholar]
- 18.Hao C-M, Komhoff M, Guan Y, Redha R, Breyer M D. Selective targeting of cyclooxygenase-2 reveals its role in renal medullary interstitial cell survival. Am J Physiol. 1999;277:F352–359. doi: 10.1152/ajprenal.1999.277.3.F352. [DOI] [PubMed] [Google Scholar]
- 19.Heneka M T, Loschmann P-A, Gleichmann M, Weller M, Schulz J B, Wullner U, Klockgether T. Induction of nitric oxide synthase and nitric oxide-mediated apoptosis in neuronal PC12 cells after stimulation with tumor necrosis factor-a/lipopolysaccharide. J Neurochem. 1998;71:88–94. doi: 10.1046/j.1471-4159.1998.71010088.x. [DOI] [PubMed] [Google Scholar]
- 20.Hengartner M. Apoptosis. Death by crowd control. Science. 1998;281:1298–1299. doi: 10.1126/science.281.5381.1298. [DOI] [PubMed] [Google Scholar]
- 21.Hillard C J, Muthian S, Kearn C S. Effects of CB1 cannabinoid receptor activation on cerebellar granule cell nitric oxide synthase activity. FEBS Lett. 1999;459:277–281. doi: 10.1016/s0014-5793(99)01253-3. [DOI] [PubMed] [Google Scholar]
- 22.Hirose S, Yamamoto T, Feng L, Yaoita E, Kawasaki K, Goto S, Fujinaka H, Wilson C B, Arakawa M, Kihara I. Expression and localization of cyclooxygenase isoforms and cytosolic phospholipase A2 in anti-Thy-1 glomerulonephritis. J Am Soc Nephrol. 1998;9:408–416. doi: 10.1681/ASN.V93408. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann A, Nolan G P, Blau H M. Rapid retroviral delivery of tetracycline-inducible genes in a single autoregulatory cassette. Proc Natl Acad Sci USA. 1996;93:5185–5190. doi: 10.1073/pnas.93.11.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu J, van Eldik L J. S100b induced apoptotic cell death in cultured astrocytes via a nitric oxide-dependent pathway. Biochim Biophys Acta. 1996;1313:239–245. doi: 10.1016/0167-4889(96)00095-x. [DOI] [PubMed] [Google Scholar]
- 25.Jaffrey S R, Snyder S H. PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science. 1996;274:774–777. doi: 10.1126/science.274.5288.774. [DOI] [PubMed] [Google Scholar]
- 26.Johnson R J, Floege J, Yoshimura A, Iida H, Couser W G, Alpers C E. The activated mesangial cell: a glomerular “myofibroblast?”. J Am Soc Nephrol. 1992;2:S190–S197. doi: 10.1681/ASN.V210s190. [DOI] [PubMed] [Google Scholar]
- 27.Kester M, Coroneos E, Thomas P J, Dunn M J. Endothelin stimulates prostaglandin endoperoxide synthase-2 mRNA expression and protein synthesis through a tyrosine kinase-signaling pathway in rat mesangial cells. J Biol Chem. 1994;269:22574–22580. [PubMed] [Google Scholar]
- 28.Kiedrowski L, Costa E, Wroblewski J T. Glutamate receptor agonists stimulate nitric oxide synthase in primary cultures of cerebellar granule cells. J Neurochem. 1992;58:335–341. doi: 10.1111/j.1471-4159.1992.tb09315.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y M, Bombeck C A, Billiar T R. Nitric oxide as a bifunctional regulator of apoptosis. Circ Res. 1999;84:253–256. doi: 10.1161/01.res.84.3.253. [DOI] [PubMed] [Google Scholar]
- 30.King S M, Barbarese E, Dillman III J F, Patel-King R S, Carson J H, Pfister K K. Brain cytoplasmic and flagellar outer arm dyneins share a highly conserved Mr 8,000 light chain. J Biol Chem. 1996;271:19358–19366. doi: 10.1074/jbc.271.32.19358. [DOI] [PubMed] [Google Scholar]
- 31.Klatt P, Schmidt K, Lehner D, Glatter O, Bachinger H P, Mayer B. Structural analysis of porcine brain nitric oxide synthase reveals a role for tetrahydrobiopterin and l-arginine in the formation of an SDS-resistant dimer. EMBO J. 1995;14:3687–3695. doi: 10.1002/j.1460-2075.1995.tb00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuppusamy P, Ohnishi S T, Numagami Y, Ohnishi T, Zweier J L. Three-dimensional imaging of nitric oxide production in the rat brain subjected to ischemia-hypoxia. J Cerebr Blood Flow Metab. 1995;15:899–903. doi: 10.1038/jcbfm.1995.114. [DOI] [PubMed] [Google Scholar]
- 33.Kutchera W, Jones D A, Matsunami N, Groden J, McIntyre T M, Zimmerman G A, White R L, Prescott S M. Prostaglandin H synthase-2 is expressed abnormally in human colon cancer: evidence for a transcriptional effect. Proc Natl Acad Sci USA. 1996;93:4816–4820. doi: 10.1073/pnas.93.10.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leist M, Volbracht C, Kuhnle S, Fava E, Ferrando-May E, Nicotera P. Caspase-mediated apoptosis in neuronal excitotoxicity triggered by nitric oxide. Mol Med. 1997;3:750–764. [PMC free article] [PubMed] [Google Scholar]
- 35.Leist M, Nicotera P. Apoptosis, excitotoxicity, and neuropathology. Exp Cell Res. 1998;239:183–201. doi: 10.1006/excr.1997.4026. [DOI] [PubMed] [Google Scholar]
- 36.Liasson M J, Huang Z, Ferrante R J, Sasamata M, Molliver M E, Snyder S H. Neuronal nitric oxide synthase activation and peroxynitrite formation in ischemic stroke linked to neuronal damage. J Neurosci. 1999;19:5910–5918. doi: 10.1523/JNEUROSCI.19-14-05910.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGinty A, Chang Y W E, Sorokin A, Bokemeyer D, Dunn M J. Cyclooxygenase-2 expression inhibits trophic withdrawal apoptosis in nerve growth factor differentiated PC12 cells. J Biol Chem. 2000;275:12095–12010. doi: 10.1074/jbc.275.16.12095. [DOI] [PubMed] [Google Scholar]
- 38.O'Connor L, Strasser A, O'Reilly L A, Hausmann G, Adams J M, Cory S, Huang D C. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oshima M, Dinchuk J E, Kargman S L, Oshima H, Hancock B, Kwong E, Trzaskos J M, Evans J F, Taketo M M. Suppression of intestinal polyposis in APC716 knockout mice by inhibition of prostaglandin endoperoxide synthase-2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 40.Peunova N, Enikolopov G. Nitric oxide triggers a switch to growth arrest during differentiation of neuronal cells. Nature. 1995;375:68–73. doi: 10.1038/375068a0. [DOI] [PubMed] [Google Scholar]
- 41.Pou S, Pou W S, Bredt D S, Snyder S H, Rosen G M. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 42.Pou S, Keaton L, Surichamom W, Rosen G M. Mechanism of superoxide generation by neuronal nitric-oxide synthase. J Biol Chem. 1999;274:9573–9580. doi: 10.1074/jbc.274.14.9573. [DOI] [PubMed] [Google Scholar]
- 43.Puthalakath H, Huang D C, O'Reilly L A, King S M, Strasser A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- 44.Sano H, Kawahito Y, Wilder R L, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 45.Sheehy A M, Phung Y T, Riemer R K, Black S M. Growth factor induction of nitric oxide synthase in rat pheochromocytoma cells. Brain Res Mol Brain Res. 1997;52:71–77. doi: 10.1016/s0169-328x(97)00224-6. [DOI] [PubMed] [Google Scholar]
- 46.Sheng H, Shao J, Morrow J D, Beauchamp R D, DuBois R N. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 47.Smith W L, Garavito R M, DeWitt D L. Prostaglandin endoperoxide H synthases (cyclooxygenase)-1 and -2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 48.Spear N, Estevez A G, Barbeito L, Beckman J S, Johnson G V W. Nerve growth factor protects PC12 cells against peroxynitrite-induced apoptosis via a mechanism dependent on phosphatidylinositol-3 kinase. J Neurochem. 1997;69:53–59. doi: 10.1046/j.1471-4159.1997.69010053.x. [DOI] [PubMed] [Google Scholar]
- 49.Sraer J D, Delarue F, Hagege J, Feunteun J, Pinet F, Nguyen G, Rondeau E. Stable cell lines of T-SV-40 immortalized human glomerular mesangial cells. Kidney Int. 1996;49:267–270. doi: 10.1038/ki.1996.38. [DOI] [PubMed] [Google Scholar]
- 50.Strauss W M. Preparation and analysis of DNA. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons Press; 1998. pp. 2.1.1–2.2.3. [Google Scholar]
- 51.Szabo C, Day B J, Salsman A L. Evaluation of the relative contribution of nitric oxide and peroxynitrite to the suppression of mitochondrial respiration in immunostimulated macrophages using a manganese mesoporphyrin superoxide dismutase mimetic and perooxynitrite scavenger. FEBS Lett. 1996;381:82–86. doi: 10.1016/0014-5793(96)00087-7. [DOI] [PubMed] [Google Scholar]
- 52.Teng K K, Greene L A. Culture PC12 cells: a model for neuronal function and differentiation. In: Celis J E, editor. Cell biology: a laboratory handbook. San Diego, Calif: Academic Press; 1994. pp. 218–224. [Google Scholar]
- 53.Thornberry N A, Lazebnik Y. Caspase: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 54.Troy C M, Shelanski M L. Down-regulation of copper/zinc superoxide dismutase cause apoptotic death in PC12 neuronal cells. Proc Natl Acad USA. 1994;91:6384–6387. doi: 10.1073/pnas.91.14.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsujii M, DuBois R N. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 56.Tsujii M, Kawano S S, Tsuji S, Sawaoka H, Hori M, DuBois R N. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 57.von Knethen A, Brune B. Cyclooxygenase-2: an essential regulator of NO-mediated apoptosis. FASEB J. 1997;11:887–895. [PubMed] [Google Scholar]
- 58.Williams C S, Smalley W, DuBois R N. Aspirin use and potential mechanisms for colorectal cancer prevention. J Clin Investig. 1997;100:1325–1329. doi: 10.1172/JCI119651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinase on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]