Abstract
The dysregulation of cellular apoptosis pathways has emerged as a critical early event associated with the development of many types of human cancers. Numerous viral and cellular oncogenes, aside from their inherent transforming properties, are known to induce programmed cell death, consistent with the hypothesis that genetic defects are required to support tumor survival. Here, we report that nuclear expression of the CREB-binding protein (CBP)/p300-binding domain of the human T-cell lymphotropic virus type 1 (HTLV-1) transactivator, Tax, triggers an apoptotic death-inducing signal during short-term clonal analyses, as well as in transient cell death assays. Coexpression of the antiapoptotic factor Bcl-2 increased serum stimulation; incubation with the chemical caspase inhibitor z-Val-Ala-dl-Asp fluoromethylketone antagonized Tax-induced cell death. The CBP/p300-binding defective Tax mutants K88A and V89A exhibited markedly reduced cytotoxic effects compared to the wild-type Tax protein. Importantly, nuclear expression of the minimal CBP/p300-binding peptide of Tax induced apoptosis in the absence of Tax-dependent transcriptional activities, while its K88A counterpart did not cause cell death. Further, Tax-mediated apoptosis was effectively prevented by ectopic expression of the p300 coactivator. We also report that activation of the NF-κB transcription pathway by Tax, under growth arrest conditions, results in apoptosis that occurs independent of direct Tax coactivator effects. Our results allude to a novel pivotal role for the transcriptional coactivator p300 in determining cell fate and raise the possibility that dysregulated coactivator usage may pose an early barrier to transformation that must be selectively overcome as a prerequisite for the initiation of neoplasia.
Apoptosis is an active physiological process that plays an essential role during tissue development and in the elimination of virus-infected or potentially cancerous cells. Accumulating evidence indicates that imbalances occurring between cellular death-inducing and proliferation pathways significantly contribute to oncogenesis (45, 46, 52). The mechanisms by which transforming viruses cooperate with cellular factors to promote neoplasia provide paradigm examples of this phenomenon, as certain transforming viruses are reported to cause programmed cell death under various conditions. The human T-cell lymphotropic virus type 1 (HTLV-1) has been linked to the development of adult T-cell leukemia–lymphoma (ATLL) as well as a neurodegenerative disorder known as HTLV-1-associated myelopathy–tropical spastic paraparesis (HAM/TSP) (15, 37, 42). The viral transactivator, Tax, is thought to play an essential role during the initial stages of CD4+ T-cell immortalization by HTLV-1. However, persistent infection of lymphocytes in vivo is usually correlated with reduced Tax expression. Of related importance, immortalization of peripheral blood mononuclear cells by HTLV-1 in vitro is strictly dependent on interleukin-2 (IL-2) and could reflect IL-2-induced increases in intracellular levels of the antiapoptotic factors Bcl-2 and Bcl-XL (33). Somatic mutations are believed to select for IL-2 independence corresponding with increases in detectable Tax protein. Significantly, numerous studies have shown that persistent Tax expression is associated with apoptosis in nonlymphoid and lymphoid-derived cell lines (11, 12, 18, 28, 31, 36, 58). In this respect, Tax resembles other cellular and viral oncogene products, such as c-Myc, c-Jun, adenovirus E1A 12S protein, polyomavirus T antigen, and human papillomavirus E7 protein, that possess both transforming and apoptosis-inducing properties (1, 38, 39, 57). Tax has also been shown to affect various cell cycle modulators and therefore is similar to certain regulators of cellular proliferation, including E2F, pRB, p53, and cyclin D, which are known to function as potent inducers of cellular death (1, 5, 8, 39, 41).
Several recent reports have demonstrated that HTLV-1 Tax recruits the transcriptional coactivators CREB-binding protein (CBP) and its synologue p300, in order to drive constitutive, signal-independent long terminal repeat (LTR) transactivation (16, 20, 21, 27). Numerous factors have been shown to interact with CBP/p300 in an often mutually exclusive manner (13, 17, 24), an observation that has led to the suggestion that rate-limiting nuclear CBP/p300 may arbitrate between antagonistic signals (23, 25, 44, 48). Indeed, perturbation of CBP/p300 functions has been associated with both excessive cellular death (degenerative disorders) and proliferative diseases (cancer). Heterozygous allelic mutations of CBP in humans have been linked to the genetic disorder Rubenstein-Taybi syndrome, which is frequently associated with mental retardation and developmental abnormalities (40). Moreover, homozygous p300 knockout mice were reported to exhibit high degrees of embryonic lethality as well as profound neuronal developmental defects, illustrating the importance of CBP/p300 for the maintenance of cellular homeostasis (60).
ATLL and HAM/TSP have their etiologies in uncontrolled cellular proliferation and excessive cell death, respectively. While direct and/or indirect perturbation of CBP/p300 activities by Tax might significantly contribute to the concerted dysregulation of growth and proliferative pathways, it also represents an attractive candidate mechanism by which Tax expression might induce apoptosis. In this study, we investigated the molecular mechanisms underlying Tax-mediated cell death. Our results demonstrate that nuclear expression of the CBP/p300-binding domain of Tax induces apoptosis in HeLa cells under growth arrest conditions. The Tax mutants K88A and V89A, defective for CBP/p300 interactions, exhibited markedly reduced cytotoxic effects compared to the wild-type Tax protein. Nuclear expression of the minimal coactivator-binding peptide of Tax caused programmed cell death in the absence of transactivation. Consistent with these observations, Tax-mediated apoptosis was efficiently prevented by ectopic expression of p300. We have also observed that NF-κB transcriptional activation by Tax results in significant levels of apoptosis, occurring independent of direct Tax coactivator effects. These findings suggest that limiting nuclear coactivator concentrations may pose an early barrier to oncogenic transformation which must be selectively overcome as a prerequisite for the establishment of neoplasia.
MATERIALS AND METHODS
Cell culture.
HeLa cells obtained from the American Type Culture Collection were cultured in Dulbecco's modified Eagle's medium (DMEM; Life Technologies, Inc.), supplemented with 10% fetal bovine serum (FBS), penicillin (100 U), and streptomycin sulfate (100 μg/ml). HeLa clones that transiently expressed various Tax-derived mutant proteins were generated by electroporation using a BTX Electro Cell Manipulator (set at 250 V, 800 μF, and 13 ohms). After 2 weeks of selection in DMEM containing 10% FBS and puromycin (2 μg/ml), several clones were isolated for each Tax mutant, expanded in puromycin-free medium, and analyzed.
Plasmids and transfections.
The retrovirus-based and cytomegalovirus (CMV)-driven wild-type and mutant Tax expression plasmids, as well as the CMV-p300 expression construct, have been previously reported (14, 21, 34, 49). Green fluorescent protein (GFP)-nuclear localization signal (NLS)-Tax peptide fusion expression vectors NLS-Tax76–95-GFP and NLS-K88A76–95-GFP were generated by annealing the oligonucleotides 3′-ACTCACTAACCGCCCCATTCCTGGAACTCCCAGAATCTCCAAGAGAAGGCAAAGAAAAACCCGTA-5′ plus 3′-ATACGGGTTTTTCTTTGCCTTCTCTTGGAGATTCTGGGAGTTCCAGGAATGGGGCGGTTAGTGAG-5′ (NLS-Tax76–95) and 3′-ACTCACTAACCGCCCCATTCCTGGCGCTCCCAGAATCTCCAAGAGAAGGCAAAGAAAAACCCGTA-5′ plus 3′-ATACGGGTTTTTCTTTGCCTTCTCTTGGAGATTCTGGGAGCGCCAGGAATGGGGCGGTTAGTGAG-5′ (NLS-K88A76–95), followed by ligation into the pcDNA3.1/CT-GFP-TOPO cloning plasmid (Invitrogen, Inc.). Clones were checked for orientation by KpnI/NheI digestion and visually for expression of GFP in transfected HeLa cells. The β-galactosidase reporter plasmid pCMV.SPORT-βgal was purchased from Life Technologies. Transient DNA transfections of HeLa cells were performed using the Lipofectamine reagent (Life Technologies) in accordance with the manufacturer's instructions. HTLV-1 LTR– and NF-κB–luciferase reporter plasmids (HTLV-LTR-Luc and NF-κB-Luc, respectively) as well as the dominant mutant IκBα S32/34A have been previously reported (32).
Transient apoptosis assays.
Transient cell death assays were performed as previously reported (7, 29, 30). The Tax expression vectors to be analyzed were cotransfected with plasmid pCMV.SPORT-βgal in serum-free medium, using the Lipofectamine reagent; 6 h posttransfection, the medium was replaced with DMEM–10% FBS, and reaction mixtures were incubated overnight. On the following day, the medium was replaced with DMEM–1% FBS, and the cells were incubated for an additional 48 h. Nonadherent cells were removed by washing the monolayers three times with phosphate-buffered saline (PBS). The remaining adherent cells were fixed with 0.2% glutaraldehyde–1% formaldehyde in PBS for 5 min at room temperature, washed twice with PBS, and stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) reagent as previously described (48). In this assay, the expression of a death-inducing gene results in a significant decrease in the observed number of β-galactosidase-expressing cells. Therefore, the percentages of cytotoxicity reported for Tax-expressing vectors inversely correlates with the number of β-galactosidase-expressing cells. The percentage of cytotoxicity reported for each construct is derived from the average number of blue cells counted within 10 visual fields at a magnification of ×400 from at least three independent experiments.
Flow cytometry.
HeLa vector control (N2)-transfected (HeLa-N2) and HeLa Tax-expressing (HeLa-Tax) clones were serum starved; both adherent and non adherent cells were collected by centrifugation, washed with PBS–10 mM HEPES (pH 7.3), and fixed in 70% ethanol–PBS. The cells were then washed twice and incubated for 45 min at 37°C in a propidium iodide solution (69 μM propidium iodide in 38 mM sodium citrate buffer containing 5 μg of RNase/ml); cellular DNA contents were determined by fluorescence-activated cell sorting (FACS) analyses (Beckman-Coulter Epics Elite flow cytometer). Data curves were fitted using the MODFIT LT software package (Berity Software House, Inc., Topsham, Maine) in order to calculate the percentage of cells containing subgenomic DNA contents reflective of apoptosis.
Oligonucleosomal DNA fragmentation.
HeLa-N2, HeLa-Tax M47, and HeLa-NLS-ΔN81 clones were serum starved, and both adherent and nonadherent cells were collected by centrifugation. Cells were lysed in Tris (10 mM [pH 8.0])-EDTA (1 mM)-sodium dodecyl sulfate (SDS; 0.5%); RNase (20 μg/ml) was added, and reaction mixtures were incubated for 1 h at 37°C. Proteinase K (100 μg/ml) was added, and samples were incubated overnight at 56°C. Proteins were extracted by phenol-chloroform, and DNA was precipitated overnight at −80°C; 10 μg of DNA from each clone was analyzed in a 2% agarose gel.
Western blot analyses.
Total cellular proteins were extracted in radioimmunoprecipitation assay buffer (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.5% [vol/vol] NP-40, 0.1% deoxycholate, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 μg of aprotinin/ml, 2 μg of leupeptin/ml); protein concentrations were determined using the Bradford microassay. Fifty micrograms of protein for each sample were resolved through an SDS–12.5% polyacrylamide gel and transferred onto nitrocellulose membranes (Schleicher & Schuell, Inc.). Nonspecific sites were blocked by incubation at 4°C for 2 h in PBS containing 3% (wt/vol) bovine serum albumin and 0.5% (vol/vol) Tween 20. Tax proteins were detected with a rabbit polyclonal antibody (Tax-C; diluted 1:5,000 in BLOTTO buffer [50 mM Tris-HCl {pH 8.0}, 2 mM CaCl2, 80 mM NaCl, 0.2% {vol/vol} NP-40, 0.02% {wt/vol} sodium azide, 5% {wt/vol} nonfat dry milk]) or with a monoclonal antibody against Tax (diluted 1:20 in BLOTTO buffer). The p300 transcriptional coactivator was detected using a rabbit polyclonal antibody against recombinant human p300 (Santa Cruz Biotechnology, Inc.). Following incubation with the primary antibodies, the blots were washed and incubated for 1 h at 4°C with appropriate horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) and developed using a chemiluminescent substrate (SuperSignal; Pierce, Inc.).
Coimmunoprecipitations.
HeLa cells were transfected with 2 μg of CMV–wild-type Tax (CMV-Tax) or CMV-mutant Tax expression construct. Cells were harvested by scraping, washed three times with PBS, and lysed in radioimmunoprecipitation assay buffer containing 50 ng of each of the protease inhibitors pepstatin, leupeptin, chymostatin, bestatin, and antipain dihydrochloride (Boehringer Mannheim Corp.) per ml. Lysates were precleared by incubation with 20 μl of protein G-agarose (Gibco/BRL, Life Technologies, Inc.) and 5 μl of nonspecific antiserum for 30 min at 4°C, followed by centrifugation at 1,200 rpm for 5 min. Rabbit polyclonal anti-human p300 antibody (7.5 μl; Santa Cruz Biotechnology) was added to each sample, and binding reaction mixtures were incubated at 4°C for 1 h; 60 μl of protein G-agarose was then added, and the samples were incubated overnight. Immunocomplexes were washed three times with 500 μl of lysis buffer and resuspended in SDS-polyacrylamide gel electrophoresis loading buffer. Samples were resolved through an SDS–12.5% polyacrylamide gel and transferred to nitrocellulose membranes; an anti-Tax monoclonal antibody was used to detect wild-type or mutant Tax proteins immunocomplexed with p300.
Immunofluorescence and confocal microscopy.
HeLa cells plated on culture slides (Nalge Nunc International) were transfected with CMV-Tax, CMV-K88A, CMV-V89A, CMV-L90A, or an empty CMV vector as a control. Alternatively, cells were transfected with a constant concentration of CMV-Tax or empty vector in the presence of increasing concentrations of a CMV-driven p300 expression vector. Six hours posttransfection, the medium was replaced with DMEM–1% FBS and reaction mixtures were incubated for an additional 24 h. The serum-starved cells were washed twice with PBS, fixed, and blocked for 1 h at room temperature in 3% (wt/vol) bovine serum albumin–0.5% (vol/vol) Tween 20 in PBS. Slides were incubated for 2 h with an anti-Tax monoclonal antibody, then incubated for 1 h in rhodamine red-conjugated donkey anti-mouse antibody (Jackson ImmunoResearch Laboratories, Inc.), and stained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI; 2 μg/ml; Molecular Probes, Inc., Eugene, Oreg.). Nuclear fragmentation (pyknosis), characteristic of apoptosis, was easily identifiable by atypical DAPI-staining nuclei in Tax-expressing cells. The procedure used for the detection of truncated Tax mutant proteins by immunofluorescence microscopy has been previously reported (34). GFP and GFP-NLS-Tax peptide fusions were observed, and relative intensities were quantified in transfected HeLa cells under high-serum (20% FBS) conditions by quantitative confocal microscopy at a magnification of ×4,000 using a Leica TCS spectrophotometric confocal microscope equipped with krypton and argon lasers and controlled by a Windows NT-based workstation with Leica TCS quantitative software.
RESULTS
Persistent NF-κB activation enhances HTLV-1 Tax-induced apoptotic effects.
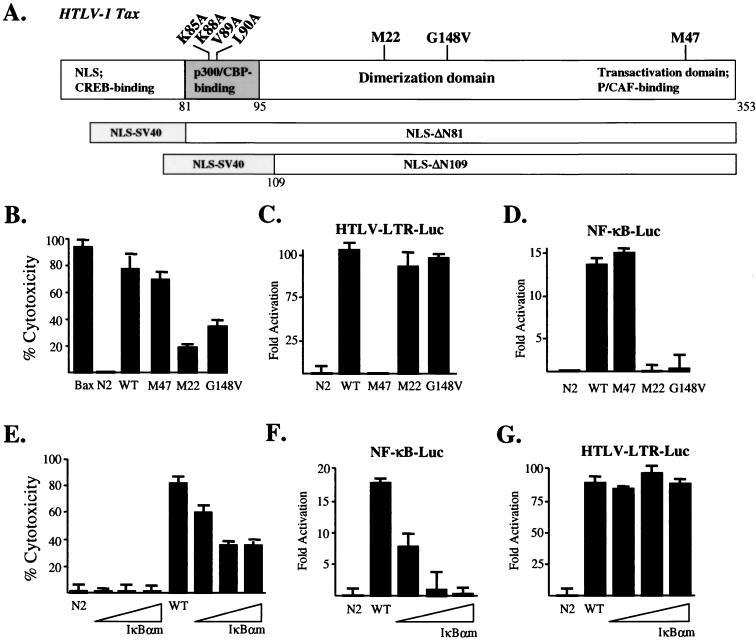
Like other cellular and viral oncogene products, the HTLV-1 Tax has been shown to induce programmed cell death in various cell types, including fibroblasts and Jurkat lymphocytes, in response to growth arrest signals such as γ irradiation, serum deprivation, or genotoxic agents (11, 12, 18, 28, 31, 36, 58). While Tax-mediated apoptosis may contribute to neurodegenerative conditions observed in HAM/TSP patients, the molecular basis underlying Tax death-inducing effects remains poorly understood. Thus, the apoptotic potentials of wild-type Tax and mutants M47, M22, and G148V were analyzed using a transient apoptosis assay referred to elsewhere as the blue-death assay (Fig. 1A; references 7, 29, and 30). This system is based on the fact that concomitant expression of a death-inducing gene, together with a CMV-lacZ reporter gene, will yield a significant reduction in the number of β-galactosidase-expressing cells observed as a measure of cell death. Hence, the degree of cytotoxicity is inversely correlated with the number of β-galactosidase-expressing cells. Consistent with reports by others, M47 activated the NF-κB transcription pathway but remained defective for transactivation via CREB/ATF-1 factors (Fig. 1C and D). The M22 and G148V mutants were still able to transactivate through CREB/ATF-1 signaling but were unable to stimulate NF-κB activation (Fig. 1C and D). Interestingly, both M47 and wild-type Tax proteins reproducibly yielded higher cytoxicities compared to mutants M22 and G148V, suggesting that under cell stress conditions, persistent activation of NF-κB may cause a further increase in the intrinsic toxicity of the HTLV-1 transactivator (Fig. 1B). Previous studies have demonstrated that M47 and G148V differentially interact with the transcriptional coactivators CBP/p300 (4). To assess whether differential coactivator utilization, as opposed to NF-κB transactivation, was involved in Tax-mediated cell death, a CMV-driven Tax expression construct was cotransfected in the presence of increasing concentrations of IκBα S32/34, a potent inhibitor of NF-κB (Fig. 1F). Surprisingly, Tax-induced cell death was partially inhibited in the presence of increasing amounts of IκBα S32/34, indicating that NF-κB transactivation may be involved in promoting Tax-mediated apoptosis (Fig. 1E). This effect was not due to general alterations in Tax transcriptional activities, as the ability of Tax to transactivate the HTLV-1 LTR, in either the absence or presence of IκBα S32/34, was uncompromised (Fig. 1G). Indeed, the fact that M22 and G148V mutants retained some degrees of cytotoxicity suggested that in addition to NF-κB transactivation, other Tax-associated functions are involved in promoting the apoptotic phenotypes observed.
FIG. 1.
(A) Schematic diagram of HTLV-1 Tax indicating the relative positions of point mutations and functional domains. The ΔN81 and ΔN109 truncations are shown fused to the NLS of the SV40 large T antigen. (B) Cytotoxicity induced by the wild-type Tax (WT) and Tax mutants was quantified by transient cell death assays in HeLa cells as described in Materials and Methods. Transactivation phenotypes for each Tax vector were confirmed by cotransfection of HeLa cells with the HTLV-LTR-Luc (1 μg) (C) or NF-κB-Luc (1 μg) (D) reporter construct with various Tax expression plasmids (2 μg). CMV-Renilla (0.1 μg) was added to control for transfection efficiencies. (E) The empty N2 vector or a wild-type Tax expression construct was cotransfected together with increasing amounts (0.05, 0.1, and 0.15 μg) of the IκBα S32/34A plasmid in transient cytotoxicity assays. Total amounts of transfected DNAs were held constant by adding the RcCMV vector; CMV-Renilla (0.1 μg) was added to control for transfection efficiencies. Effects of IκBα S32/34A expression on Tax-mediated transactivation was assayed by cotransfecting HeLa cells with the NF-κB-Luc (1 μg) (F) or HTLV-LTR-Luc (1 μg) (G) reporter plasmid with a wild-type Tax expression construct (2 μg). Error bars represent standard deviations between experiments.
Tax induces apoptosis during short-term clonal analyses in HeLa cells.
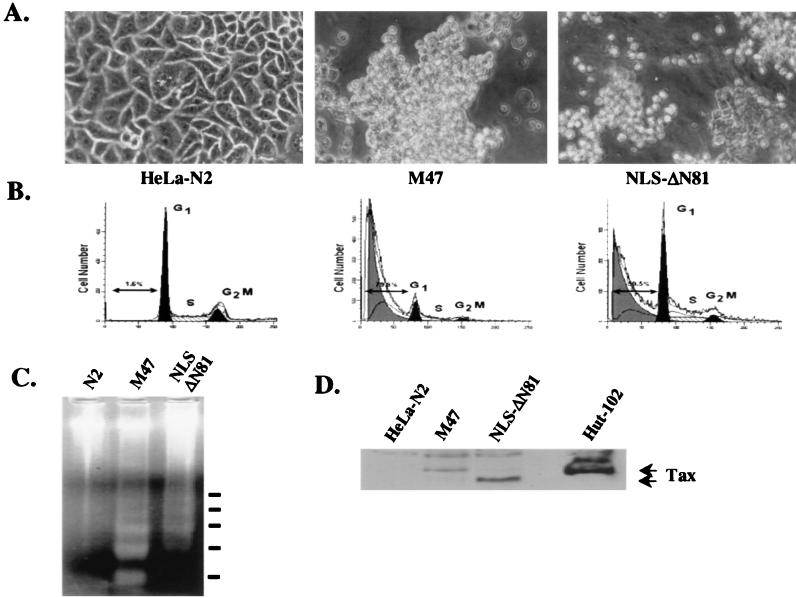
We have previously described several HeLa clones, expressing the cytoplasmic, Tax-derived truncations ΔN109 and ΔN81, devoid of cytopathic effects (Fig. 1A; reference 34). While a wild-type Tax-expressing HeLa clone could not be derived, several clones that expressed the mutants M47 and NLS-ΔN81 were obtained. Under high-density culture conditions, these cells exhibited altered morphologies resembling those previously reported to be associated with apoptosis (Fig. 2A). In the presence of a growth-arresting signal such as serum deprivation, M47 and NLS-ΔN81-expressing HeLa clones displayed a loss of surface adherence properties and tended to round up and cluster, appearing in a focal plane separate from that occupied by HeLa-N2 control cells cultured under identical conditions (Fig. 2A). When the DNA contents of these clones were analyzed by flow cytometry, both M47- and NLS-ΔN81-expressing HeLa clones presented clear sub-G1 peak populations, displaying approximately 80 and 50% apoptotic fractions, respectively (Fig. 2B). By contrast, the HeLa-N2 cells exhibited a normal cell cycle progression profile. Prominent oligonucleosomal DNA fragmentation, a unique feature of apoptotic cell death, was detected in the M47- and, to a lesser extent, NLS-ΔN81-expressing HeLa clones but not in the N2 vector control-containing cells (Fig. 2C). Expression of the M47 and NLS-ΔN81 mutant proteins in HeLa clones was confirmed by Western blot analysis; the HTLV-1 transformed cell line Hut-102 was included for comparison (Fig. 2D).
FIG. 2.
(A) Morphological changes accompanying the expression of M47 and NLS-ΔN81 Tax-derived proteins in serum-starved HeLa clones were visualized by light-field microscopy at an original magnification of ×400. The HeLa-N2 clone is included for comparison. Representative phenotypes are shown. (B) FACS analyses of the HeLa-N2 and HeLa-Tax clones were performed following 72 h of serum deprivation (1% FBS). The percentage of apoptosis as measured by the sub-G1 peak is indicated for each population. (C) Oligonucleosomal genomic fragmentation in M47- and NLS-ΔN81-expressing HeLa clones. Ten micrograms of DNA was extracted as described in Materials and Methods and resolved on a 2% agarose gel. HeLa-N2 was included as a control. (D) Expression of the M47 and NLS-ΔN81 mutant proteins in HeLa clones was detected by immunoblot analysis using a Tax monoclonal antibody. The HTLV-1-transformed cell line Hut-102 is shown for comparison.
Nuclear expression of amino acids 81 to 109 of Tax coincides with apoptosis.
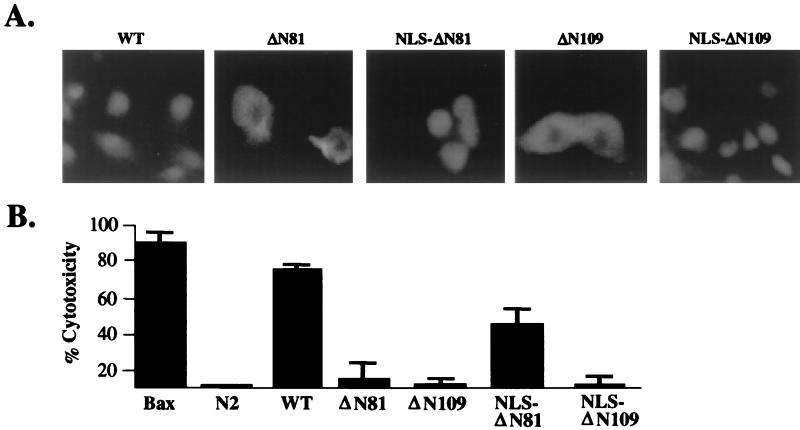
To extend the above-mentioned observations, we next tested the cytotoxic potentials of various Tax-derived truncation mutants, expressed either in the cytoplasm or in the nucleus, in transient cell death assays. Subcellular localizations of the wild-type Tax and mutant proteins were confirmed by immunofluorescence microscopy using a rabbit polyclonal antibody raised against the carboxyl terminus of Tax (Fig. 3A). Expectedly, a control plasmid encoding the proapoptotic gene product Bax was highly apoptotic under conditions used in our assay, resulting in cell death that exceeded 95% relative to the empty vector control (Fig. 3B). Wild-type Tax displayed approximately 80% cytotoxicity, whereas the NLS-ΔN81 construct exhibited reduced (approximately 40%) but significant apoptosis-inducing activity (Fig. 3B). Because expression of the NLS-ΔN109 mutant was not associated with cell death, the apoptotic effects observed using the NLS-ΔN81 construct cannot be attributed to the presence of the simian virus 40 (SV40) large T-antigen-derived NLS. Consistent with our previous results (34), the NLS-ΔN81 construct did not lead to significant activation of the NF-κB pathway (approximately 10% relative to wild-type Tax [unpublished data]); in addition, this mutant was unable to stimulate transcription through CREB/ATF-1 and serum response factor (SRF) pathways. In contrast to the NLS-ΔN81, its cytoplasmic counterpart, ΔN81, was not cytotoxic, suggesting that nuclear expression is essential for the apoptotic effects of Tax. Further, deletion of amino acid residues 81 to 108 (ΔN109) (34), while having little effect on transcriptional activities, resulted in the abrogation of cytotoxicity, indicative that apoptosis observed concomitant with NLS-ΔN81 expression occurred independent of transcriptional activation. Collectively, these results support a role for nuclear expression of amino acid residues 81 to 108 of Tax in mediating programmed cell death.
FIG. 3.
(A) Subcellular localizations of wild-type Tax (WT) and truncation mutants in transfected HeLa cells were determined by immunofluorescence microscopy using an anti-Tax rabbit polyclonal antibody. (B) Cytotoxicity induced by Tax mutants was quantified by transient cell death assays. A CMV-Bax expression construct was included as a positive control for apoptosis. Results shown are representative of four independent transfections; error bars represent standard deviations.
Tax-induced cell death requires caspase activation and is prevented by Bcl-2 expression or serum stimulation.
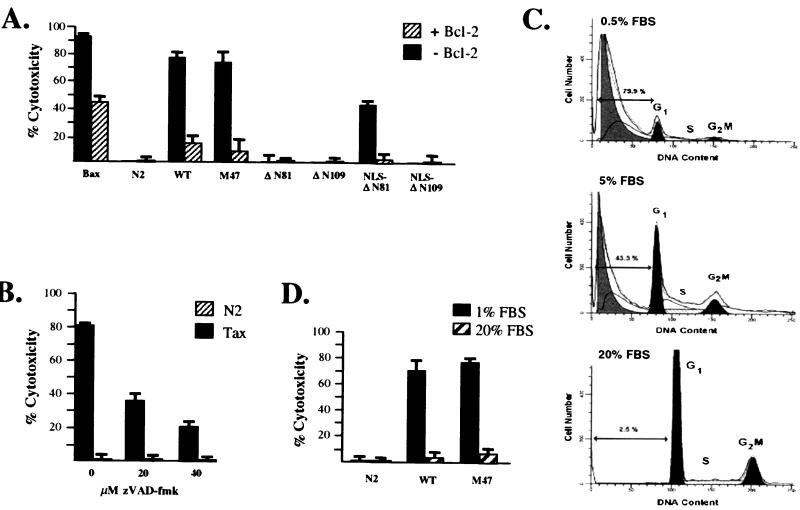
Previous reports have demonstrated that Bcl-2 expression blocks both Tax- and HTLV-1-mediated apoptosis (31, 58). Under the conditions of our transient cell death assay, Bcl-2 coexpression efficiently inhibited cytotoxicity resulting from the expression of the wild-type Tax, M47, and NLS-ΔN81 proteins (Fig. 4A). In agreement with a recent study which showed that Tax-induced apoptosis could be prevented by caspase inhibitors in Jurkat lymphocytes (12), a cell-permeable, peptide analogue inhibitor of the ICE/CED-3-related protease, z-Val-Ala-dl-Asp fluoromethylketone (zVAD-fmk), inhibited Tax-induced apoptosis in HeLa cells in a dosage-dependent manner (Fig. 4B).
FIG. 4.
(A) Tax-induced apoptosis in transient cell death assays was effectively inhibited by coexpression of the antiapoptotic gene product Bcl-2. Results are representative of three independent experiments. Error bars are indicative of standard deviations. WT, wild-type Tax. (B) Tax-mediated cell death was blocked by treatment with increasing concentrations of the chemical caspase inhibitor zVAD-fmk; the empty vector is shown as a control. (C) Apoptosis in the HeLa-M47 clone following 72 h of serum stimulation (0.5, 5, and 20% FBS) was evaluated by propidium iodide staining and FACS analyses. (D) Transient cytotoxicity assays were performed using an empty vector control, CMV-Tax, and CMV-M47 expression constructs under varied serum concentrations (1 and 20% FBS) as described in Materials and Methods; error bars are shown.
Prior observations that increased serum concentrations countered c-Myc-associated apoptosis (19) prompted us to investigate the putative modulatory effects of serum-dependent factors upon Tax-mediated cell death. Puromycin-selected, N2 vector control- and M47-expressing HeLa clones were cultured in media containing varied serum concentrations; after 72 h, both adherent and nonadherent cells were harvested, and their DNA contents were analyzed by FACS. The results shown in Fig. 4C indicate that increased serum stimulation protected Tax-expressing cells from undergoing apoptosis, suggesting that an SRF(s) interferes with the death-effecting signal induced by Tax. No alterations in normal cell cycle progression were observed for the HeLa-N2 cell line (data not shown). Similar results were obtained using both the wild-type Tax and M47 expression constructs in the transient cell death assay, excluding the possibility that signal-responsive cellular pathways may have been adversely affected as a result of the puromycin selection (Fig. 4D).
Tax mutants defective for CBP/p300 interactions exhibit markedly reduced apoptotic phenotypes.
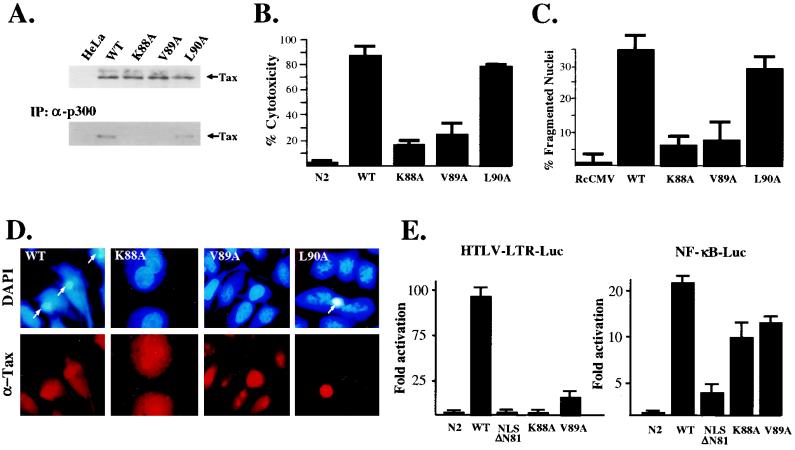
The CBP/p300-binding domain of the HTLV-1 Tax has been previously determined to reside between amino acid residues 76 and 95 (21), a region coinciding with sequences that are shown here to be associated with the viral transactivator's apoptotic function. The Tax mutants K88A and V89A contain single amino acid substitutions and have been characterized as being defective for interactions with CBP, while another mutant located adjacent to this region, L90A, exhibited CBP binding and LTR transactivation comparable to that observed for the wild-type Tax (20, 21). In vivo interactions between these Tax-derived mutants and the p300 transcriptional coactivator were evaluated in transfected HeLa cells. Consistent with their observed binding to CBP, both Tax and L90A were coprecipitated with p300, whereas K88A and V89A were defective for this interaction (Fig. 5A). Comparable levels of p300 in extracts prepared from transfected HeLa cells were verified by immunoblotting (data not shown). In the transient cell death assay, both the wild-type Tax and L90A mutant induced apoptosis, while the cytotoxic effects of the mutants K88A and V89A were markedly reduced (between 10 and 20% [Fig. 5B]). These findings were further confirmed by the overall lack of nuclear fragmentation (pyknosis) in HeLa cells expressing the K88A and V89A mutant proteins, as determined by the absence of atypical DAPI-staining nuclei. By contrast, nuclear fragmentation was associated with wild-type Tax and L90A expression (Fig. 5C and D, arrows). We found that the K88A and V89A Tax mutants significantly transactivated the NF-κB–Luc reporter construct compared to NLS-ΔN81 and the wild-type Tax (Fig. 5E, right) but were impaired in their abilities to activate CREB/ATF-dependent transcription from the viral LTR (Fig. 5E, left). Our data therefore suggest that direct binding of CBP/p300 significantly contributes to the induction of apoptotic cell death by the HTLV-1 Tax.
FIG. 5.
(A) HeLa cells were transfected with RcCMV control vector or the CMV–Tax (WT [wild type]), CMV-K88A, CMV-V89A, or CMV-L90A expression construct. Coimmunoprecipitation (IP) was performed using an anti-p300 antibody, and immunocomplexes containing Tax were detected by immunoblotting using an anti-Tax monoclonal antibody. Comparable levels of Tax expression for each mutant were confirmed by Western blot analysis. (B) Tax-derived mutants K88A and V89A exhibited markedly diminished apoptotic potentials in the transient cell death assay compared to the wild-type Tax or L90A mutant. Results represent average percentages derived from three independent experiments. (C) Nuclear fragmentation induced by HTLV-1 Tax is correlated with coactivator binding. HeLa cells were transfected with Tax-expressing vectors and serum starved (0.5% FBS) for 24 h; Tax expression was detected by immunofluorescence microscopy using an anti-Tax monoclonal antibody. Nuclei were visualized by DAPI staining of DNA. Relative average numbers of fragmented nuclei were quantified from three nonoverlapping fields at an original magnification of ×400 from three independent transfections. (D) Immunofluorescence detection of Tax and Tax-derived mutants K88A, V89A, and L90A at an original magnification of ×1,000 (arrows denote nuclear fragmentation bodies). (E) Transactivation of HTLV-LTR-LUC and NF-κB-Luc reporter constructs by wild-type Tax, K88A, V89A, and NLS-ΔN81 proteins in cotransfected HeLa cells; the empty vector is shown as a control. Error bars represent standard deviations.
Nucleus-directed expression of the CBP/p300-binding peptide of Tax causes apoptosis.
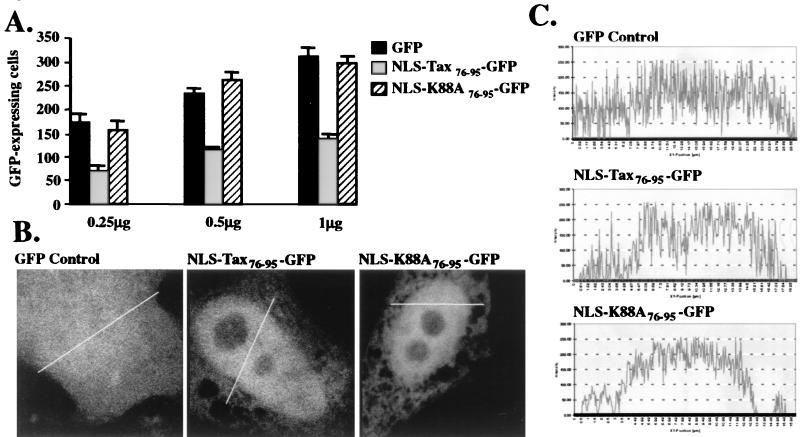
To directly assess the death-inducing potential of the coactivator-binding domain of Tax, amino acid residues 76 to 95 were expressed as an amino-terminal GFP fusion, localized to the nucleus by incorporation of the SV40 large T-antigen-derived NLS (NLS-Tax76–95-GFP). The CBP/p300-binding defective mutation K88A was also expressed in the same context (NLS-K88A76–95-GFP). As shown in Fig. 6A, the NLS-Tax76–95-GFP expression construct was associated with significant cytotoxicity in transient cell death assays versus the GFP vector control. Importantly, the NLS-K88A76-95-GFP construct resulted in no detectable apoptosis. We also observed that the NLS-Tax peptide-GFP fusion-expressing construct appeared somewhat reduced in its cytotoxic potential relative to the full-length wild-type Tax protein (data not shown). This decrease could have resulted from protein folding effects as a result of GFP sequences; alternatively, the absence of residues required for Tax dimerization may alter cytotoxic effects (53). The NLS-Tax76–95-GFP and NLS-K88A76–95-GFP fusions were predominantly expressed in the nuclei of transfected HeLa cells, as opposed to the generally diffuse expression pattern detected for the GFP control using quantitative, confocal fluorescence microscopy (Fig. 6B). Line quantification of relative fluorescence intensities confirmed that GFP-Tax peptide fusions were expressed at comparable levels in transfected cells (Fig. 6C). These data indicate that nuclear localization of the coactivator-binding peptide of Tax is responsible for the transactivator's death-inducing properties.
FIG. 6.
(A) Expression of the NLS-Tax76–95-GFP fusion was associated with cytotoxicity in transient cell death assays; the NLS-K88A76–95-GFP and GFP control plasmids did not cause apoptosis. Results depict relative numbers of GFP-expressing cells under serum deprivation conditions and are representative of triplicate experiments. Error bars are indicative of standard deviations. (B) GFP fusions were detected in transfected HeLa cells by quantitative confocal microscopy as described in Materials and Methods. The lines shown in the micrographs (B) indicate the regions of cells quantified according to relative fluorescence intensities (C).
Ectopic expression of the p300 coactivator partially prevents Tax-induced cell death.
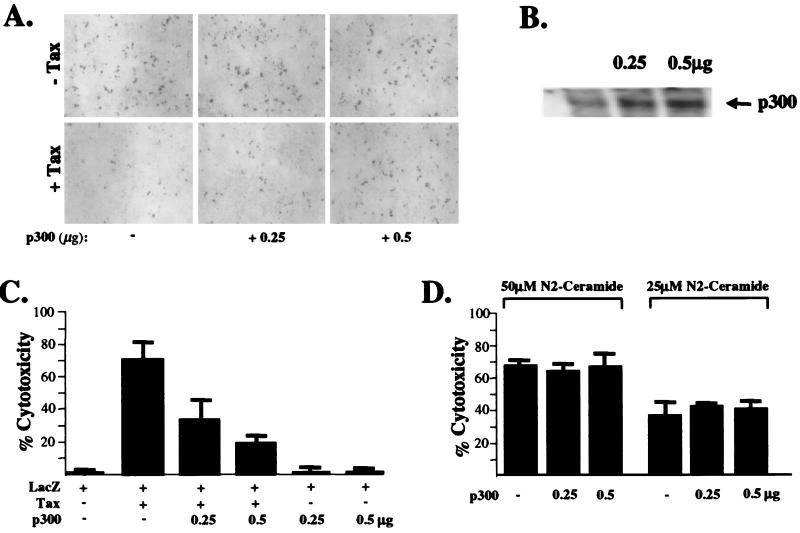
Recent studies have demonstrated a potential role for the p300 coactivator in apoptosis mediated by c-Fos, the tumor suppressor p53, and χ irradiation (43, 56, 61). Consistent with a model whereby sequestration of the intranuclear coactivator pool by Tax causes apoptosis, coexpression of CBP/p300 might be expected to inhibit the ability of Tax to promote cell death. This hypothesis was tested by expressing a constant level of the wild-type Tax protein in HeLa cells in the presence of increasing concentrations of a CMV-driven p300 expression construct. Nuclear fragmentation bodies induced by Tax expression, following 24 h of serum deprivation, were visualized and quantified by DAPI staining and by UV and fluorescence microscopy. The results indicated that coexpression of p300 and Tax significantly reduced the induction of apoptotic bodies or pyknosis in a dosage-dependent manner compared to the vector control in absence of p300 (data not shown). In the transient cell death assay, increased p300 expression, in the presence of Tax, resulted in a dosage-dependent increase in the number of β-galactosidase-expressing cells (Fig. 7A and C). Exogenous p300 did not significantly increase the number of β-galactosidase-positive cells in the vector control experiment (CMV-lacZ), thus ruling out the possibility that the observed increase in the number of blue cells may have resulted from enhanced CMV promoter activation due to increased p300 levels (Fig. 7A and C). Increases in p300 levels in transfected HeLa cells were detected by Western blotting (Fig. 7B). Ectopic p300 expression did not affect apoptosis induced by treatment with the sphingolipid C2-ceramide (Fig. 7D), suggesting that both p300-responsive and unresponsive pathways influence cell fate.
FIG. 7.
(A) Ectopic expression of p300 blocks Tax-induced apoptosis in a dose-dependent manner. HeLa cells were transfected either with an RcCMV control or CMV-Tax expression construct in the presence of increasing concentrations of a CMV-p300 expression vector. β-Galactosidase-expressing cells were detected by staining with an X-Gal solution. (B) Western blot analysis of increased p300-FLAG expression in transfected HeLa cells using a monoclonal antibody against human recombinant p300. (C) Average percentages of Tax-associated cytotoxicity in the presence of increasing concentrations of CMV-p300 were derived from transient cell death assays performed in triplicate. (D) Increased p300 expression did not affect apoptosis induced by C2-ceramide (Sigma) in transient cell death assays; results are representative of duplicate experiments. Error bars indicate standard deviations.
DISCUSSION
The HTLV-1 Tax has previously been shown by others to induce programmed cell death in lymphoid and nonlymphoid cells through an ill-defined mechanism (11, 12, 18, 28, 31, 36, 58). In this respect, Tax resembles other cellular and viral oncogene products (e.g., c-Myc, c-Jun, adenovirus E1A 12S protein, polyomavirus T antigen, and human papillomavirus E7 protein) and certain regulators of cellular proliferation (e.g., E2F, pRB, p53, and cyclin D) which also possess apoptosis-inducing properties (1, 5, 8, 38, 39, 41, 57). Thus, by understanding the molecular mechanisms involved in oncogene-associated cell death, we may gain further insight regarding the identities of key genetic defects, preceding the establishment of certain malignancies, which are required to support tumor survival.
Apoptosis induced by the HTLV-1 Tax was observed in HeLa clonal populations that expressed the Tax-derived mutants M47 and NLS-ΔN81. FACS analyses revealed the presence of distinct sub-G1 populations that were correlated with oligonucleosomal DNA fragmentation, not observed in HeLa-N2 control cells. The relative cytotoxic potentials for various Tax-derived mutants were determined using a transient cell death assay. Interestingly, results from these experiments suggested that apoptotic cell death occurred only when amino acid residues 81 to 108 of Tax were expressed in the nucleus. As this domain encompasses the transcriptional coactivator-binding domain of Tax (20, 21), two mutants defective for CBP/p300 binding were assayed for their abilities to induce apoptosis and nuclear fragmentation. The fact that mutations in Tax resulting in impairment of p300 interactions severely hindered Tax-induced cell death is suggestive that constitutive formation of Tax-CBP/p300 complexes might perturb critical cell survival signals. Indeed, mutually exclusive occupancy of these coactivators by nuclear hormone receptors is believed to play an important role in the potent apoptosis-inducing properties of retinoid and glucocorticoid, anticancer therapeutic compounds (9, 10, 22, 26). Nuclear expression of the coactivator-binding peptide spanning residues 76 to 95 of Tax as a GFP fusion caused significant cell death and nuclear fragmentation in transient assays. Its counterpart, containing the K88A mutation, did not induce apoptosis. In support of a role for CBP/p300 in mediating apoptotic responses, ectopic expression of the p300 coactivator prevented Tax-induced programmed cell death in a dosage-dependent manner. These results suggest that the HTLV-1 transactivator might promote caspase activation and apoptosis, either through direct coactivator interactions or by inducing numerous nuclear factors to collectively overwhelm the cellular transcriptional machinery. Alternatively, an unknown protein(s) might interact with residues found within the coactivator binding domain of Tax to induce cell death; overexpression of p300 could then competitively displace such factors.
Rel/NF-κB family members have been shown to play essential roles in modulating apoptotic pathways (2, 3). Whether NF-κB promotes apoptosis, or acts in a protective manner, appears dependent on the nature of stimulation, the identity of Rel-related subunits activated, and the duration of the stimulating signal (2, 3). In contrast to tumor necrosis factor alpha, IL-1, and lipopolysaccharides that trigger transient NF-κB activation, Tax expression is known to result in prolonged induction of NF-κB-dependent transcription through the targeted activation of IκB-kinase complex components and constitutive phosphorylation or degradation of IκBα and IκBβ (50). The aberrant, persistent activation of NF-κB transcription by Tax could exert a proapoptotic function (28). Indeed, long-term expression of Tax, in either Jurkat or rat fibroblast (5R)-derived cell lines, has led to the selection of clones that can no longer respond to NF-κB-inducing signals (47, 59). Consistent with these findings, our results underscore the importance of Tax-mediated NF-κB responses for the induction of apoptosis under conditions of cellular stress. Tax mutants defective in NF-κB activation exhibited reduced apoptosis-inducing activities and inhibition of Tax-mediated NF-κB transactivation partially inhibited cell death. Rel/NF-κB subunits utilize CBP/p300 in a phosphorylation-dependent manner and stimulate the expression of numerous other factors that also require the recruitment of coactivators for their transcription functions. It is tempting to speculate that persistent, Tax-mediated NF-κB transactivation could indirectly increase the demands for CBP/p300 through cascade effects; and in cells in which these demands are not adequately satisfied, uncoupled signaling pathways lead to cellular death.
A striking feature of HTLV-1-infected lymphocytes in vivo is the absence of detectable viral gene expression. Thus, in response to T-cell activation, increases in Tax protein levels within subpopulations of HTLV-1-infected cells may be accompanied by a burst of viral expression, followed by immediate apoptotic cell death and/or clearance by immune responses. Selective pressures for the maintenance of reduced Tax expression, therefore, potentially may contribute to viral latency, allowing infected cells to evade host immune surveillance mechanisms. The late onset of many cancers, including acute or lymphoma-stage ATLL, is thought to result from a genetic, selective process whereby a clonal neoplastic cell population that avoids or bypasses apoptotic cell death is the cause of neoplasia. The data presented here indicate that direct and/or indirect titration of the transcriptional coactivator p300 by the HTLV-1 Tax results in apoptosis, raising the intriguing possibility that limiting concentrations of nuclear CBP/p300 may pose an early barrier to oncogenic transformation.
ACKNOWLEDGMENTS
This work was supported by G. Franchini (Basic Research Laboratory, Division of Basic Sciences, National Cancer Institute, National Institutes of Health) and in part by C. Z. Giam (Department of Microbiology and Immunology, Uniformed Services University of the Health Sciences; grants RO1 CA48709 and RO1 CA/GM 75688).
We acknowledge James McNally and Tatiana Karpova, Laboratory of Receptor Biology and Gene Expression (LRBGE), Fluorescence Imaging Facility, National Cancer Institute, NIH, for use of the Leica TCS confocal fluorescence microscope and quantitation software. Jeremy Hansen is thanked for technical assistance.
REFERENCES
- 1.Allemand I, Grimber G, Kornprobst M, Bennoun M, Molina T, Briand P, Joulin V. Compensatory apoptosis in response to SV40 large T antigen expression in the liver. Oncogene. 1995;11:2583–2590. [PubMed] [Google Scholar]
- 2.Baichwal V R, Baeuerle P A. Activate NF-kappa B or die? Curr Biol. 1997;7:R94–R96. doi: 10.1016/s0960-9822(06)00046-7. [DOI] [PubMed] [Google Scholar]
- 3.Barkett M, Gilmore T D. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- 4.Bex F, Yin M J, Burny A, Gaynor R B. Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB-binding protein and p300. Mol Cell Biol. 1998;18:2392–2405. doi: 10.1128/mcb.18.4.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blagosklonny M V. A node between proliferation, apoptosis, and growth arrest. Bioessays. 1999;21:704–709. doi: 10.1002/(SICI)1521-1878(199908)21:8<704::AID-BIES10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Borrow J, Stanton V P, Andresen M, Becher R, Behm F G, Chaganti R S K, Civin C I, Disteche C, Dube I, Frischauf A M, Horsman D, Mitelman F, Volinia S, Watmore A E, Housman D E. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14:33–41. doi: 10.1038/ng0996-33. [DOI] [PubMed] [Google Scholar]
- 7.Bossy-Wetzel E, Bakiri L, Yaniv M. Induction of apoptosis by the transcription factor c-Jun. EMBO J. 1997;16:1695–1709. doi: 10.1093/emboj/16.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns T F, El-Deiry W S. The p53 pathway and apoptosis. J Cell Physiol. 1999;181:231–239. doi: 10.1002/(SICI)1097-4652(199911)181:2<231::AID-JCP5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 9.Caelles C, Gonzales-Sancho J M, Munoz A. Nuclear hormone receptor antagonism with AP-1 by inhibition of the JNK pathway. Genes Dev. 1997;11:3351–3364. doi: 10.1101/gad.11.24.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravarti D, LaMorte V J, Nelson M C, Nakajima T, Schulman I G, Juguilon H, Montminy M, Evans R M. Role of CBP/p300 in nuclear receptor signaling. Nature. 1996;383:99–103. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- 11.Chlichlia K, Moldenhauer G, Daniel P T, Busslinger M, Gazzolo L, Schirrmacher V, Khazaie K. Immediate effects of reversible HTLV-I Tax function: T-cell activation and apoptosis. Oncogene. 1995;10:269–277. [PubMed] [Google Scholar]
- 12.Chlichia K, Busslinger M, Peter M E, Walczak H, Krammer P H, Schirrmacher V, Khazaie K. ICE-proteases mediate HTLV-I Tax-induced apoptotic T-cell death. Oncogene. 1997;14:2265–2272. doi: 10.1038/sj.onc.1201070. [DOI] [PubMed] [Google Scholar]
- 13.Colgin M A, Nyborg J K. The human T-cell leukemia virus type 1 oncoprotein Tax inhibits the transcriptional activity of c-Myb through competition for the CREB binding protein. J Virol. 1998;72:9396–9399. doi: 10.1128/jvi.72.11.9396-9399.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckner R, Ewen M E, Newsome D, Gerdes M, DeCaprio J A, Lawrence J B, Livingston D M. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 15.Gessain A, Barin F, Vernant J C, Gout O, Maurs L, Calander A, de The G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 16.Giebler H A, Loring J E, Van Orden K, Colgin M A, Garrus J E, Escudero K, Brauweiler A, Nyborg J K. Anchoring of CREB-binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of Tax transactivation. Mol Cell Biol. 1997;17:5156–5164. doi: 10.1128/mcb.17.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giles R H, Peters D J, Breuning M H. Conjunction dysfunction: CBP/p300 in human disease. Trends Genet. 1998;14:178–183. doi: 10.1016/s0168-9525(98)01438-3. [DOI] [PubMed] [Google Scholar]
- 18.Hall A P, Irvine J, Blyth K, Cameron E R, Onions D E, Campbell M E. Tumors derived from HTLV-I Tax transgenic mice are characterized by enhanced levels of apoptosis and oncogene expression. J Pathol. 1998;186:209–214. doi: 10.1002/(SICI)1096-9896(1998100)186:2<209::AID-PATH162>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 19.Harrington E A, Bennett M R, Fanidi A, Evan G I. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 1994;13:3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrod R, Kuo Y L, Tang Y, Yao Y, Vassilev A, Nakatani Y, Giam C Z. p300 and p300/cAMP-responsive element-binding protein associated factor interact with human T-cell lymphotropic virus type-1 Tax in a multi-histone acetyltransferase/activator-enhancer complex. J Biol Chem. 2000;275:11852–11857. doi: 10.1074/jbc.275.16.11852. [DOI] [PubMed] [Google Scholar]
- 21.Harrod R, Tang Y, Nicot C, Lu H S, Vassilev A, Nakatani Y, Giam C Z. An exposed KID-like domain in human T-cell lymphotropic virus type-1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol Cell Biol. 1998;18:5052–5061. doi: 10.1128/mcb.18.9.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helmberg A, Auphan N, Caelles C, Karin M. Glucocorticoid-induced apoptosis of human leukemic cells is caused by the repressive function of the glucocorticoid receptor. EMBO J. 1995;14:452–460. doi: 10.1002/j.1460-2075.1995.tb07021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horvai A E, Xu L, Korzus E, Brard G, Kalafus D, Mullen T M, Rose D W, Rosenfeld M G, Glass C K. Nuclear integration of JAK/STAT and Ras/AP-1 signaling by CBP and p300. Proc Natl Acad Sci USA. 1997;94:1074–1079. doi: 10.1073/pnas.94.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janknecht R, Hunter T. Nuclear fusion of signaling pathways. Science. 1999;284:443–444. doi: 10.1126/science.284.5413.443. [DOI] [PubMed] [Google Scholar]
- 25.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 26.Kawasaki H, Eckner R, Yao T P, Taira K, Chiu R, Livingston D M, Yokoyama K K. Distinct roles of the co-activators p300 and CBP in retinoic-acid-induced F9-cell differentiation. Nature. 1998;393:284–289. doi: 10.1038/30538. [DOI] [PubMed] [Google Scholar]
- 27.Kwok R P, Laurance M E, Lundblad J R, Goldman P S, Shih H, Connor L M, Marriott S J, Goodman R H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature. 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 28.Los M, Khazaie K, Schulze-Osthoff K, Baeuerle P A, Schirrmacher V, Chlichlia K. Human T cell leukemia virus-I (HTLV-I) Tax-mediated apoptosis in activated T cells requires an enhanced intracellular prooxidant state. J Immunol. 1998;161:3050–3055. [PubMed] [Google Scholar]
- 29.Maestro R, Dei Tos A P, Hamamori Y, Krasnokutsky S, Sartorelli V, Kedes L, Doglioni C, Beach D H, Hannon G J. Twist is a potential oncogene that inhibits apoptosis. Genes Dev. 1999;13:2207–2217. doi: 10.1101/gad.13.17.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miura M, Zhu H, Rotello R, Hartwieg E A, Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C.elegans cell death gene ced-3. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- 31.Nicot C, Astier-Gin T, Guillemain B. Activation of Bcl2 expression in human endothelial cells chronically expressing the human T-cell lymphotropic virus type-I. Virology. 1997;236:47–53. doi: 10.1006/viro.1997.8720. [DOI] [PubMed] [Google Scholar]
- 32.Nicot C, Mahieux R, Opavsky R, Cereseto A, Wolff L, Brady J N, Franchini G. HTLV-I Tax transrepresses the human c-Myb promoter independently of its interaction with CBP or p300. Oncogene. 2000;19:2155–2164. doi: 10.1038/sj.onc.1203536. [DOI] [PubMed] [Google Scholar]
- 33.Nicot C, Mahieux R, Takemoto S, Franchini G. Bcl-X(L) is up-regulated by HTLV-I and HTLV-II in vitro and in ex vivo ATLL samples. Blood. 2000;96:275–281. [PubMed] [Google Scholar]
- 34.Nicot C, Tie F, Giam G Z. Cytoplasmic forms of human T-cell leukemia virus type 1 Tax induce NF-κB activation. J Virol. 1998;72:6777–6784. doi: 10.1128/jvi.72.8.6777-6784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicot C, Astier-Gin T, Edouard E, Legrand E, Moynet D, Vital A, Londos-Gagliardi D, Moreau J P, Guillemain B. Establishment of HTLV-I-infected cell lines from French, Guianese and West Indian patients and isolation of a proviral clone producing viral particles. Virus Res. 1993;30:317–333. doi: 10.1016/0168-1702(93)90099-9. [DOI] [PubMed] [Google Scholar]
- 36.Ohya O, Tomaru U, Yamashita I, Kasai T, Morita K, Ikeda H, Wakisaka A, Yoshiki T. HTLV-I induced myeloneuropathy in WKAH rats: apoptosis and local activation of the HTLV-I pX and TNF-alpha genes implicated in the pathogenesis. Leukemia. 1997;11:255–257. [PubMed] [Google Scholar]
- 37.Osame M, Usuku K, Izumo S, Ljichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 38.Pan H, Griep A E. Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice: implications for tumor suppressor gene function in development. Genes Dev. 1994;8:1285–1299. doi: 10.1101/gad.8.11.1285. [DOI] [PubMed] [Google Scholar]
- 39.Pandey S, Wang E. Cells en route to apoptosis are characterized by the upregulation of c-fos, c-myc, c-jun, cdc2, and RB phosphorylation, resembling events of early cell-cycle traverse. J Cell Biochem. 1995;58:135–150. doi: 10.1002/jcb.240580203. [DOI] [PubMed] [Google Scholar]
- 40.Petrij F, Giles R H, Dauwerse H G, Saris J J, Hennekam R C, Masuno M, Tommerup N, van Ommen G J, Goodman R H, Peters D J. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature. 1995;376:348–351. doi: 10.1038/376348a0. [DOI] [PubMed] [Google Scholar]
- 41.Pierce A M, Scheinder-Broussard R, Gimenez-Conti I B, Russel J L, Conti C J, Johnson D G. E2F1 has both oncogenic and tumor-suppressive properties in a transgenic model. Mol Cell Biol. 1999;19:6408–6414. doi: 10.1128/mcb.19.9.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Preston G A, Srinivasan D, Barrett J C. Apoptotic response to growth factor deprivation involves cooperative interactions between c-Fos and p300. Cell Death Differ. 2000;7:215–226. doi: 10.1038/sj.cdd.4400637. [DOI] [PubMed] [Google Scholar]
- 44.Ravi R, Mookerjee B, van Hensbergen Y, Bedi G C, Giordano A, El-Deiry W S, Fuchs E J, Bedi A. p53-mediated repression of nuclear factor-kappaB RelA via the transcriptional integrator p300. Cancer Res. 1998;58:4531–4536. [PubMed] [Google Scholar]
- 45.Reed J C. Dysregulation of apoptosis in cancer. Cancer J Sci Am. 1998;4:S8–S14. [PubMed] [Google Scholar]
- 46.Reed J C. Mechanisms of apoptosis avoidance in cancer. Curr Opin Oncol. 1999;11:68–75. doi: 10.1097/00001622-199901000-00014. [DOI] [PubMed] [Google Scholar]
- 47.Ruben S M, Rosen C A. Suppression of signals required for activation of transcription factor NF-kappa B in cells constitutively expressing the HTLV-I Tax protein. New Biol. 1990;2:894–902. [PubMed] [Google Scholar]
- 48.Sheppard K A, Phelps K M, Williams A J, Thanos D, Glass C K, Rosenfeld M G, Gerritsen M E, Collins T. Transcriptional activation by NF-kappaB requires multiple coactivators. J Biol Chem. 1998;273:29291–29294. doi: 10.1074/jbc.273.45.29291. [DOI] [PubMed] [Google Scholar]
- 49.Smith M R, Greene W C. Identification of HTLV-I Tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 50.Sun S C, Ballard D W. Persistent activation of NF-kappaB by the Tax transforming protein of HTLV-1: hijacking cellular IkappaB kinases. Oncogene. 1999;18:6948–6958. doi: 10.1038/sj.onc.1203220. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki T, Uchida-Toita M, Yoshida M. Tax protein of HTLV-1 inhibits CBP/p300-mediated transcription by interfering with recruitment of CBP/p300 onto DNA element of E-box or p53 binding site. Oncogene. 1999;18:4137–4143. doi: 10.1038/sj.onc.1202766. [DOI] [PubMed] [Google Scholar]
- 52.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 53.Tie F, Adya N, Greene W C, Giam C Z. Interaction of the human T-lymphotropic virus type 1 Tax dimer with CREB and the viral 21-base-pair repeat. J Virol. 1996;70:8368–8374. doi: 10.1128/jvi.70.12.8368-8374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Orden K, Yan J-P, Ulloa A, Nyborg J K. Binding of the human T-cell leukemia virus Tax protein to the coactivator CBP interferes with CBP-mediated transcriptional control. Oncogene. 1999;18:3766–3772. doi: 10.1038/sj.onc.1202703. [DOI] [PubMed] [Google Scholar]
- 55.Van Orden K, Giebler H A, Lemasson I, Gonzales M, Nyborg J K. Binding of p53 to the KIX domain of CREB binding protein. J Biol Chem. 1999;274:26321–26328. doi: 10.1074/jbc.274.37.26321. [DOI] [PubMed] [Google Scholar]
- 56.Webster G A, Perkins N D. Transcriptional cross talk between NF-κB and p53. Mol Cell Biol. 1999;19:3485–3495. doi: 10.1128/mcb.19.5.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White E, Sabbatini P, Debbas M, Wold W S, Kuscher D I, Gooding L R. The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor alpha. Mol Cell Biol. 1992;12:2570–2580. doi: 10.1128/mcb.12.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamada T, Yamaoka S, Goto T, Nakai M, Tsujimoto M, Hatanaka M. The human T-cell leukemia virus type I Tax protein induces apoptosis which is blocked by the Bcl-2 protein. J Virol. 1994;68:3374–3379. doi: 10.1128/jvi.68.5.3374-3379.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamaoka S, Courtois G, Bessia C, Whiteside S T, Weil R, Agou F, Kirk H E, Kay R J, Israel A. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 60.Yao T P, Oh S P, Fuchs M, Zhou N D, Ch'ng L E, Newsome D, Bronson R T, Li E, Livingston D M, Eckner R. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell. 1998;93:361–372. doi: 10.1016/s0092-8674(00)81165-4. [DOI] [PubMed] [Google Scholar]
- 61.Yuan Z M, Huang Y, Ishiko T, Nakada S, Utsugisawa T, Shioya H, Utsugisawa Y, Shi Y, Weichselbaum R, Kufe D. Function for p300 and not CBP in the apoptotic response to DNA damage. Oncogene. 1999;18:5714–5717. doi: 10.1038/sj.onc.1202930. [DOI] [PubMed] [Google Scholar]