Abstract
Bacterial RNA polymerases (RNAP) form distinct holoenzymes with different σ factors to initiate diverse gene expression programs. In this study, we report a cryo-EM structure at 2.49 Å of RNA polymerase transcription complex containing a temperature-sensitive bacterial σ factor, σ32 (σ32-RPo). The structure of σ32-RPo reveals key interactions essential for the assembly of E. coli σ32-RNAP holoenzyme and for promoter recognition and unwinding by σ32. Specifically, a weak interaction between σ32 and −35/−10 spacer is mediated by T128 and K130 in σ32. A histidine in σ32, rather than a tryptophan in σ70, acts as a wedge to separate the base pair at the upstream junction of the transcription bubble, highlighting the differential promoter-melting capability of different residue combinations. Structure superimposition revealed relatively different orientations between βFTH and σ4 from other σ-engaged RNAPs and biochemical data suggest that a biased σ4–βFTH configuration may be adopted to modulate binding affinity to promoter so as to orchestrate the recognition and regulation of different promoters. Collectively, these unique structural features advance our understanding of the mechanism of transcription initiation mediated by different σ factors.
Keywords: Cryo-EM, transcription initiation, RNAP, σ32
1. Introduction
Initiation of DNA transcription is one of the main regulatory steps in the control of gene expression. During bacterial transcription initiation, σ factors associate with the RNAP core enzyme, guide the transcription machinery to promoter regions of genes, and engage double-strand promoter DNA [1,2]. The resulting transition from closed RNAP–promoter complex (RPc) to open RNAP–promoter complex (RPo) with melted base pairs (bp) leads to de novo RNA synthesis. Different elements in the promoter DNA, including the −35, extended −10, −10, and discriminator elements, engage intensive interaction with σ factors. Primary σ factor (group 1) is responsible for transcription of housekeeping genes, while alternative σ factors (group 2, group 3, and group 4) control transcription of genes during adaptation to certain intracellular and environmental signals [3,4]. One group of σ factors belonging to the σ54 family is involved in nitrogen metabolism and certain stress responses [5,6].
The group 1 factors, represented by σ70, are the most studied and well-known factors. E. coli σ70 is composed of multiple domains, including σ1.1, σ1.2, σNCR, σ2, σ3.1, σ3.2 and σ4. σ1.1 serves as a gatekeeper, admitting the promoter in and affecting the switching of RNAP properties [7,8,9]. Σ1.2 is responsible for discriminator motif recognition [10]. ΣNCR participates in DNA interaction around the transcription start site [11]. Σ3.1 plays a role in “extended −10 motif” recognition and promoter binding [12,13]. Σ3.2 threads the RNAP and makes extensive interactions in the active center cleft, thereby affecting primer binding and promoter escape [14,15,16]. σ2 contacts the clamp helices of the β’ subunit and mediates sequence-specific interactions with the promoter −10 element, while σ4 contacts the β-flap tip helix (βFTH) and mediates sequence-specific interaction with the promoter −35 element [17]. In contrast, group 2 σ factors (such as E. coli σ38) lack the σNCR domain, group 3 σ factors (such as E. coli σ28 and σ32) lack σ1.1, σNCR, and σ1.2 domains, and group 4 σ factors (such as E. coli σ24 and σ19) contain only two essential domains: σ2 and σ4.
The group 3 σ factor, σ32, plays a pivotal role in the heat shock response (HSR) required by E. coli to sustain protein homeostasis and cope with heat and other stresses [18]. σ32 synthesis is modulated by the secondary structure of the 5′ region of σ32 mRNA, which serves as a “thermometer”, responding to temperature changes [19,20]. When the temperature for growing E. coli cells suddenly increases, σ32 is rapidly synthesized (induction phase) and then directs RNA polymerase to promote transcription of a set of HSR genes to respond to environmental temperature changes [21]. The overproduced σ32 is gradually decreased by a set of conserved chaperones (such as DnaK/DnaJ) and proteases that accumulate during the induction phase [22,23].
In E. coli, the transcription initiation complex structures of σ70 [11,24], σ38 [25], σ28 [26], σ24 [15], and σ54 [27,28,29] have greatly advanced our understanding of how σ factors recognize corresponding promoter elements and initiate transcription. However, the structure of the RNAP holoenzyme or transcription initiation complex that interacts with group 3 σ32 or group 4 σ19 remains unknown. In this study, we assembled the functional E. coli transcribing complex with σ32 and determined the structure at 2.49 Å resolution. The structure shows that σ32 intensively interacts with promoter −10 and −35 element. Furthermore, several unique structural features were observed and the roles of key amino acids involved were validated through mutagenesis studies.
2. Materials and Methods
2.1. E. coli σ32 or σ32 Derivatives
E. coli strain BL21(DE3) (Novagen, Beijing, China) was transformed with plasmid pET28a-rpoH. Single colonies of the resulting transformants were used to inoculate 50 mL LB broth containing 50 μg/mL Kanamycin and cultures were incubated, with shaking, for 16 h at 37 °C. Aliquots (10 mL) were used to inoculate 1 L LB broth containing 50 μg/mL Kanamycin and cultures were incubated, with shaking, at 37 °C until OD600 = 0.6. The cultures were induced by adding IPTG to 1 mM and incubated for 3 h at 37 °C. Then, the cells were harvested by centrifugation (5000 rpm for 10 min at 4 °C), resuspended in 20 mL buffer A (20 mM Tris–HCl, pH 8.0; 0.1 M NaCl; 1.5% glycerol) and lysed using a JN-02C cell disrupter (JNBIO, Guangzhou, China). The lysate was centrifuged (12,000 rpm for 45 min at 4 °C) and the supernatant loaded onto a 2 mL column of Ni-NTA agarose (Qiagen, Wuhan, China) equilibrated with buffer A. The column was washed with 10 mL buffer A containing 0.16 M imidazole and eluted with 10 mL buffer A containing 0.5 M imidazole. The sample was further purified using a 5 mL column of HiTrap Heparin HP (GE Healthcare, Boston, MA, USA) equilibrated in buffer A and eluted using a 100 mL linear gradient of 0.2–1 M NaCl in buffer A. Fractions containing E. coli σ32 or σ32 derivatives were pooled and stored at −80 °C.
2.2. E. coli RNAP Core Enzyme
E. coli RNAP core enzyme was prepared from E. coli strain BL21(DE3) (Invitrogen, Inc., Carlsbad, CA, USA) transformed with plasmid pET28a-rpoA-rpoB-rpoC-rpoZ similar to pIA900 [30]. Single colonies of the resulting transformants were used to inoculate 50 mL LB broth containing 50 μg/mL Kanamycin, and cultures were incubated, with shaking, for 16 h at 37 °C. Aliquots (10 mL) were used to inoculate 1 L LB broth containing 50 μg/mL Kanamycin, cultures were incubated at 37 °C, with shaking, until OD600 = 0.6 and then induced by adding IPTG to 1 mM. The cultures were incubated for 3 h at 37 °C. The cells were subsequently harvested by centrifugation (5000× g for 10 min at 4 °C), resuspended in 20 mL lysis buffer (50 mM Tris-HCl, pH 7.9; 0.2 M NaCl; 2 mM EDTA; 5% glycerol; and 5 mM DTT), and then lysed using a JN-02C cell disrupter. After poly-ethyleneimine precipitation and ammonium sulfate precipitation, the pellet was resuspended in buffer B (10 mM Tris-HCl, pH 7.9; 0.5 M NaCl; and 5% glycerol) and loaded onto a 5 mL column of Ni-NTA agarose equilibrated with buffer B. The column was washed with 25 mL buffer B containing 20 mM imidazole and eluted with 25 mL buffer B containing 0.15 M imidazole. The eluate was diluted in buffer C (10 mM Tris-HCl, pH 7.9; 0.2 M NaCl; and 5% glycerol) and loaded onto a Mono Q 5/10 GL column (GE Healthcare, Boston, MA, USA) equilibrated in buffer C and eluted using a 30 mL linear gradient of 0.3–0.5 M NaCl in buffer C. Fractions containing E. coli RNAP core enzyme were pooled and stored at −80 °C.
2.3. Nucleic Acid Scaffolds and E. coli σ32-RPo Assembly
Nucleic acid scaffolds of the σ32 consensus promoter dnaKp1 for cryo-EM study of E. coli σ32-RPo and for fluorescence polarization study were prepared from synthetic oligos (F: 5′-CCCCCTTGAAGACGTGGTTTACGACCCCATTTAGTAGTCAACCGCAGTGAGTGA-3′; R: 5′-TCACTCACTGCGGTTGACTACTAAATGGGGTCGTAAACCACGTCTTCAAGGGGG-3′) [31] using an annealing procedure (95 °C for 5 min followed by 2 °C step cooling to 25 °C) in annealing buffer (5 mM Tris-HCl, pH 8.0; 200 mM NaCl; and 10 mM MgCl2).
E. coli σ32-RPo, the E. coli RNAP core enzyme, E. coli σ32, and nucleic acid scaffolds were mixed at 1:4:1.2 molar ratios and incubated at 4 °C overnight. The RPo complexes were purified using a Hiload 16/60 Superdex 200 column (GE Healthcare, Boston, MA, USA) and stored in 20 mM Tris–HCl pH 8.0, 0.1 M NaCl, 1% glycerol, and 1 mM DTT with a concentration of 5 mg/mL.
2.4. In Vitro Transcription Assay
In vitro Mango-based transcription assays [32] were carried out by incubating the E. coli RNAP core enzyme, E. coli σ32 or σ32 derivatives, and dnaKp1-mango scaffold. Transcription assays were performed using a 96-well microplate format. Reaction mixtures (40 µL) contained 0.1 μM E. coli RNAP holoenzyme, 0.1 μM E. coli σ32 or σ32 derivatives, 50 nM dnaKp1-mango scaffold, 1 μM TO1-Biotin, 0.1 mM NTP mix (ATP, UTP, GTP and CTP), 40 mM Tris–HCl, pH 8.0, 50 mM NaCl, and 10 mM MgCl2. First, the E. coli RNAP core enzyme, E. coli σ32 or σ32 derivatives, and dnaKp1-mango scaffold were incubated for 10 min at 37 °C. Following incubation with NTP mix and TO1-biotin for another 10 min at 37 °C, fluorescence emission intensities were measured using a multimode plate reader (EnVision, PerkinElmer Inc., Waltham, MA, USA; excitation wavelength = 510 nm; emission wavelength = 535 nm). Relative transcription activities of σ32 derivatives were calculated as described in a previous report [32].
2.5. Fluorescence Polarization Assay
We measured the affinity of two FAM-labeled holoenzyme DNA molecules. The shorter DNA (containing the −35 element) was used to validate the role of DNA-contacted amino acids in σ4 (Figure 1E and Figure 2B), while the longer DNA (containing the sequence from the −35 element to the −10 bubble) was used to validate the effect of σ4–βFTH configuration changes on affinity (Figure 3F). The sequence of the shorter 6-FAM-labeled DNA template strand was 5′-CCTTGAAGACGTGG-3′ and the nontemplate strand was 5′-CCACGTCTTCAAGG-3′; the sequence of the longer 6-FAM-labeled DNA template strand was 5′-CCTTGAAGACGTGGTTTACGACCCCATTTAGTAG-3′ and the nontemplate strand was 5′-GGGGTCGTAAACCACGTCTTCAAGG-3′. The labeled template strand and the nontemplate strand were annealed in a 1:1 ratio in 10 mM Tris-HCl, pH 7.9 and 0.2 M NaCl and stored at −80 °C. The annealed DNA (5 nM) was incubated with E. coli σ32 or σ32 derivatives holoenzyme (10, 20, 40, 80, 160, and 320 nM) in 100 μL FP-A buffer (10 mM Tris-HCl, pH 7.7; 100 mM NaCl; 1 mM DTT; 1% glycerol; and 0.025% Tween-20) in a 96-well plate (Corning, NYC, USA) for 5 min at room temperature. The fluorescence polarization (FP) signals were measured using a plate reader (SPARK, TECAN, Switzerland) at excitation wavelength = 494 nm and emission wavelength = 518 nm. The calculated Kd is an apparent Kd obtained from the best-fit curve by fitting our plots to a nonlinear regression equation [33,34].
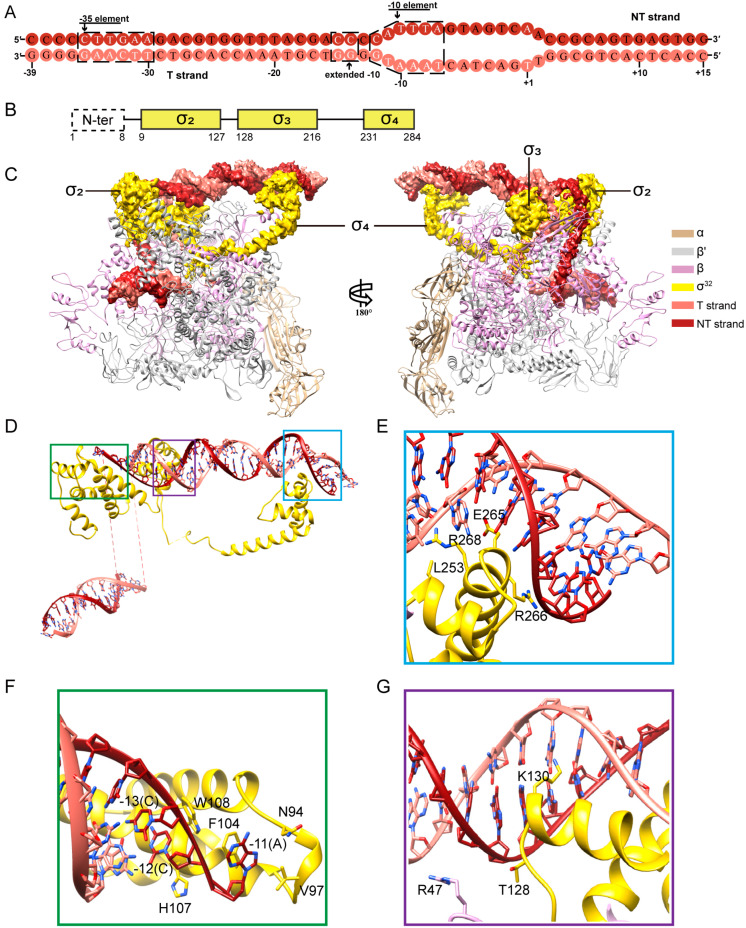
Figure 1.
Cryo-EM structure of the E. coli σ32-RPo complex and interaction between σ32 and promoter DNA. (A) Nucleic acid scaffold. A schematic representation of the synthetic promoter DNA scaffold (54-bp) in the σ32-RPo. The −35, −10, and the extended −10 elements are annotated by a dashed box. Positions are numbered relative to the transcription start site. (B) Domain architecture of σ32, N-ter fragment of σ32 in the empty frame was not modeled in our structure. (C) Overviews of the structure and partial cryo-EM reconstruction maps of the E. coli σ32-RPo. The subunits of β’, β, α, T strand, NT strand, and σ32 are colored light brown, purple, gray, yellow, hot pink, and maroon, respectively. The individual domain of σ32 is labeled. (D) Overall interaction between σ32 and promoter DNA. The dashed boxes indicate the interaction regions. (E) Detailed interaction between σ4 and −35 element of promoter DNA. (F) Detailed interaction between σ2 and −10 element of promoter DNA. (G) Detailed interaction between σ3 and −35/−10 spacer region of promoter DNA.
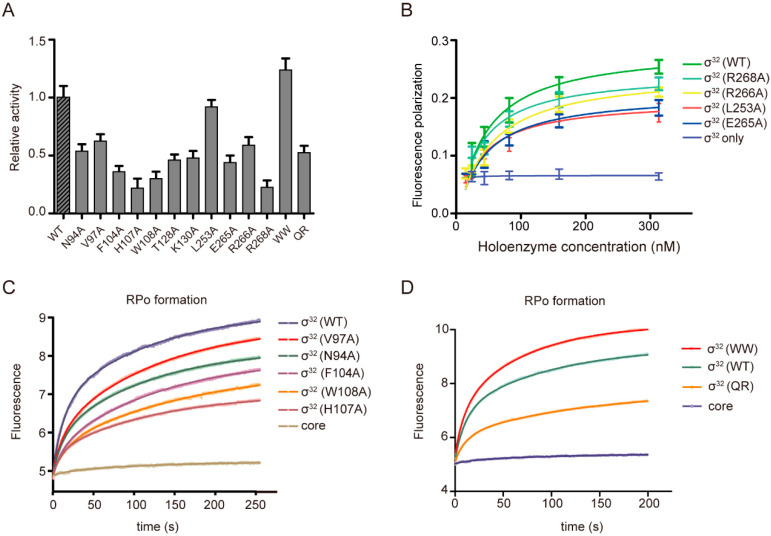
Figure 2.
In vitro transcription activity, DNA binding affinity, and promoter unwinding activity of σ32 and σ32 derivatives. (A) In vitro transcription activity of σ32 and σ32 derivatives. The experiments were conducted in triplicate (mean± SEM; 3 determinations). Error bars represent mean± SEM of n = 3 experiments. (B) Binding affinities of wild-type (WT) σ32 or σ32 derivatives holoenzyme and promoter −35 element measured using fluorescence polarization (FP) assay. (C) Promoter unwinding activity of wild-type (WT) σ32 or σ32 derivatives holoenzyme (σ32 derivatives in the “A-11” pocket) measured using stopped-flow assay. (D) Promoter unwinding activity of σWW, σHW (WT), and σQR measured using stopped-flow assay.
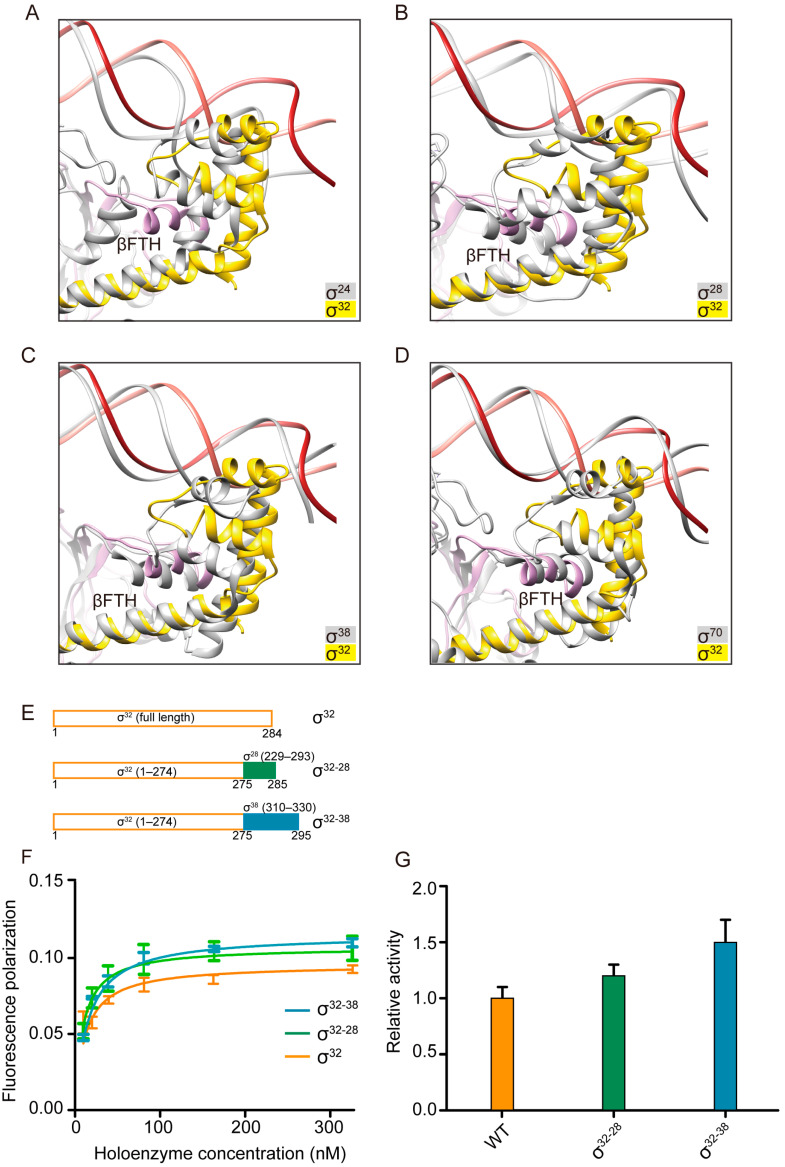
Figure 3.
Superimposition and characteristics of βFTH–σ4 interactions. (A–D) Superimposition of βFTH–σ4 interactions between σ32 and σ24 (PDB: 6JBQ), σ28 (PDB: 6PMI), σ38 (PDB: 6OMF), and σ70 (PDB: 6CAO), respectively. (E) Schematic diagram of the WT, chimera σ32–28, and chimera σ32–38. (F) Binding affinities of wild-type σ32 or σ32 derivative holoenzyme and promoter measured using the FP assay. (G) In vitro transcription activities of WT, chimera σ32–28, and chimera σ32–38 (mean ± SEM; 3 determinations). Error bars represent mean± SEM of n = 3 experiments.
Fluorescence polarization was calculated using the formula:
| P = (IVV − IVH)/(IVV + IVH) |
where IVV and IVH are fluorescence intensities with the excitation polarizer at the vertical position and the emission polarizer at the vertical position and the horizontal position, respectively. The equilibrium dissociation constant, Kd, was calculated using the equation:
| P = Pf + {(Pb − Pf) × [T]/(Kd + [T])} |
where P is the fluorescence polarization at a given concentration of holoenzyme, Pf is the fluorescence polarization of free 6-FAM-labeled promoter DNA, Pb is the fluorescence polarization of bound 6-FAM-labeled promoter DNA, and [T] is the concentration of holoenzyme harboring WT or mutant σ32.
2.6. Stopped-Flow Assay
The promoter for the stop flow assay was prepared as previously reported [15]. The Cy3-amido-dT-modified nontemplate-strand primer (dnaKp1-Cy3-nontemplate: 5′-GCATCTCCCCCTTGAAGACGTGGTTTACGACCCCATTTAGTAGTC/iCy3dT/ACCGCAGT-3′; 10 µM, Sangon Biotech) and the template-strand primer (dnaKp1-template: 5′-GGCACGTACGAATATACCACATACCAATCCTTCCTTCGTACGTGCACTCACTCACTGCGGTTGACTACTAAAT-3′; 10 µM, Sangon Biotech) were processed as previously reported [15]. To monitor the efficiency of RPo formation of E. coli RNAP holoenzymes comprising wild-type or derivatives of E. coli σ32, 60 µL σ32-RNAP holoenzyme (200 nM) and 60 µL Cy3- PdnaKp1 (4 nM) in 10 mM Tris–HCl (pH 7.7), 20 mM NaCl, 10 mM MgCl2, and 1 mM DTT were rapidly mixed and the change in Cy3 fluorescence was monitored in real time using a stopped-flow instrument (SX20, Applied Photophysics Ltd., UK) equipped with an excitation filter (515/9.3 nm) and a long-pass emission filter (570 nm). The data were plotted in SigmaPlot (Systat software, San Jose, CA, USA) and fitted to eqn: F = F0 + a1 × (1 − exp × (−kobs,1 × t)) + a2× (1 − exp × (−kobs,2 × t)). The amplitudes (a1 and a2) and observed rates (kobs,1 and kobs,2) were estimated as previously reported [15,35].
2.7. Cryo-EM Grid Preparation
Grids (Quantifoil 1.2/1.3 Au 300 mesh) were glow-discharged for 25 s at 15 mA prior to application of 4 μL complex at a concentration of 1.2 mg/mL, then plunge-frozen in liquid ethane using a Vitrobot (FEI, Valley, SD, USA.) with 100% chamber humidity at 4 °C.
2.8. Cryo-EM Data Acquisition and Processing
Grids were transferred to a 300 kV Titan Krios electron microscope equipped with a Gatan K3 summit direct electron detector and a GIF energy filter with a slit width of 20 eV. All the images were auto-collected via EPU [36] with a magnification of 105 k and exposure time of 2.5 s using a 0.851 Å pixel size and a defocus ranging from −1.0 to−1.5 μm. The total dose was about 54 e/Å2. A total of 8555 movies were imported and processed using Relion. All 40 frames were aligned using MotionCor2 [37] before contrast transfer function (CTF) estimations. The contrast transfer function was estimated for each summed image using CTFFIND4 [38]. From the summed images, ~4,000,000 particles were auto-picked and subjected to 2D classification in RELION [39]. 2D averages of the best classes were used as templates for auto-picking in RELION. Auto-picked particles were manually inspected and then subjected to 2D classification in RELION. Poorly populated classes were removed, resulting in a dataset of 641,734 particles. These particles were re-extracted without binning and processed further using 3D refinement and CTF refinement. The final density map was post-processed in RELION with a resolution of 2.49 Å. Focus refinement with mask and signal subtract improved local resolution of the DNA-σ32 interaction area to 3.40 Å.
2.9. Cryo-EM Model Building and Refinement
The coordinates of core RNAP and promoter DNA from the structure of E. coli core RNAP (PDB: 7MKP) [40] were fitted into the cryo-EM density map using chimera [41] and manually modified in COOT [42]. The coordinates of σ32 were manually built in COOT [42]. The coordinates were real-space refined with secondary structure restraints in Phenix [43]. Due to the weak map signal, the region between the −10 bubble and downstream double-stranded DNA was not modeled. The portions of chains C (residues 233–332 and 974–1025) and F (residues 960–1126), which are in low-resolution maps and not the main focus of our work, were also not modeled.
3. Results
3.1. The Cryo-EM Structure of E. coli σ32-RPo
To determine the structure of E. coli σ32-RPo, we reconstituted the complex with the E. coli RNAP core enzyme, σ32, and a nucleic acid scaffold (Figure 1). The nucleic acid scaffold (−39 to +15; +1 as transcription start site) is composed of a 28-bp upstream double-stranded DNA (dsDNA) with consensus sequences of the −35 element, a 13-bp transcription bubble (complementary sequences on both strands), and a 13-bp downstream dsDNA (Figure 1A).
We obtained a cryo-EM map at 3.0 Å for the E. coli σ32-RPo complex with local resolution at the active-center cleft of RNAP around 2.49 Å (Figure 1C and Figure S1 and Table 1). The map shows clear densities for residues of σ2 (residues 47–127) and σ4 (residues 231–279) (Figure 1B,C). The map also shows clear densities for the upstream part of the transcription bubble and the downstream dsDNA (Figure 1C,D). Similar to studies on other σ factors [11,44], the overall structure of RNAP shows minimal structural change upon σ32 engagement. Structural superimposition between RNAP core (PDB 7MKP) and σ32-RNAP results in an RMSD of 1.633 Å across 3268 residues [45]. Similar to other σ factors, the RNAP clamp in the structure of σ32-RPo remains in a closed conformation (Figure 1C). The omega subunit is absent from the solved structure, probably due to low binding affinity.
Table 1.
Statistics of cryo-EM data and structure refinement.
| Data Collection and Processing | RPo |
|---|---|
| Magnification | 105,000 |
| Voltage (kV) | 300 |
| Electron exposure (e−/Å2) | 54 |
| Defocus range (μm) | −1.0~−1.5 |
| Pixel size (Å) | 0.851 |
| Symmetry Imposed | C1 |
| Number of micrographs | 8557 |
| Initial particle projections (no.) | 3,854,813 |
| Final particle projections (no.) | 641,734 |
| Map resolution (Å) | 2.49 |
| FSC threshold | 0.143 |
| Map resolution range | 2.3–7.0 |
| Refinement | |
| Initial model used | PDB 7MKP |
| Model resolution (Å) | 2.64 |
| FSC threshold | 0.143 |
| Map sharpening B factor (Å2) | −10 |
| Model composition | |
| Nonhydrogen atoms | 28,255 |
| Protein residues | 3386 |
| Nucleotides | 83 |
| B factors (Å2) | |
| Protein | 8.12/205.39/64.84 |
| Nucleic acids | 94.01/275.47/208.74 |
| Validation | |
| MolProbity score | 1.54 |
| Clashscore | 6.05 |
| Poor rotamers (%) | 0.03 |
| Ramachandran plot | 0.00 |
| Favored (%) | 96.67 |
| Allowed (%) | 3.33 |
| Model to Map | |
| Map CC | 0.8358 |
In the structure of σ32-RPo, the σ2 and σ4 domains bind to the surface of RNAP (Figure 1C). The σ2 domain attaches to the clamp helices of the β′ subunit of RNAP via a polar surface. The interface residues include D77, E81, I84, and M87 of σ32, and R275, R278, R281, L282, L285, I291, E295, and M298 of clamp helix of the β′ subunit (Figure S3A). The σ4 domain of σ32 uses a distinct hydrophobic surface to bind the βFTH of RNAP. The interface residues include A221, L225, W244, L245, L278 and E283 of the σ4 domain and E898, K900, L901, I905 and F906 of the βFTH (Figure S3B). Furthermore, σ32 strongly interacts with different DNA elements in the promoter DNA (Figure 1D–G). The N-terminus of σ32 was not modeled in the structure, possibly due to the flexibility caused by the presence of intrinsically disordered regions.
3.2. The Interactions between σ4 Domain and the −35 Element
σ32-regulated promoters have a distinct consensus sequence at their −35 elements (5′-CTTGAA-3′; from −35 to −30). In the structure, σ4 adopts a helix-turn-helix fold (Figure 1D), inserts into the major groove of dsDNA around the −35 element, and potentially makes base-specific polar interactions with nucleotides (Figure 1E). The interactions are important, as substituting E265, R266, or R268 with alanine resulted in substantial loss of transcription activity (Figure 2A). The effect of L253A is modest compared with that of other residues. Furthermore, the fluorescence polarization assay shows that the substitution of these residues with alanine profoundly decreases binding affinity between σ32 and −35 element. The calculated KD values of L253A, E265A, R266A, and R268A mutants are 38.75 nM, 45.88 nM, 54.87 nM, and 46.72 nM, respectively, while the KD value of the wild-type σ factor is 28.41 nM, justifying the functional importance of these interactions (Figure 2B). It is worth noting that while σ32-RNAP holoenzyme readily interacts with promoter −35 element DNA, the sole σ32 fails to bind DNA (Figure 2B), which is consistent with previous reports that sole σ subunits adopt a closed conformation and are incapable of binding DNA [46,47].
3.3. The Interactions between σ32 and −35/−10 Spacer
The interactions between σ factors and −35/−10 spacer were established to stabilize the conformation of the upstream DNA duplex, promoting the engagement of the upstream duplex with σ4 and σ2 for subsequent promoter unwinding. The σ32-RPo complex contacts the phosphate backbones of the spacer region between the −35 and the −10 elements at three positions (Figure 1D). Beside the reported interaction between R47 of the RNAP-β′ zipper domain and phosphate backbones of nucleotides between G−17 and C−18 on the nontemplate DNA strand, σ32 was also found to interact with −35/−10 spacer. Specifically, T128 contacts the same phosphate backbones of nucleotides between G−17 and C−18. K130 of σ32 makes a H-bond with N7 atoms of G−15 on the template DNA strand, which together with G−14 were identified to be the “extend −10” element [48] (Figure 1G). Substituting T128 and K130 with alanine causes a substantial loss of transcription activity (Figure 2A), highlighting the importance of these interactions [48].
3.4. The Promoter-DNA-Unwinding Function of σ32
σ70, σ38, and σ28 of E. coli unwind promoter DNA at the −11/−12 junction [26,44,49], while σ24 unwinds promoter DNA at a position 1 bp upstream of the transcription bubble [15]. Our structure shows that the C:G bases at position −12 are paired, while the A:T bases at position −11 are unwound (Figure 1F). This observation strongly suggests that σ2 unwinds promoter DNA at the −11/−12 junction, which is consistent with that of σ70 [49,50]. Specifically, H107 blocks the path upstream dsDNA and serves as a wedge to disrupt base stacking between positions A−11 and C−12 (Figure 1F). The unwound nucleotide A−11 on the nontemplate DNA strand is stabilized by a pocket created by N94, V97, F104, and W108 on the “specificity loop” of σ2 domain [51] (Figure 1F). A similar pocket is reported where different bases are recognized [11,15]. The functional importance of these residues in transcription was validated by our experiment, which showed that substitution with alanine resulted in significant loss of transcription activity (Figure 2A).
To explore the contributions of these residues in promoter unwinding, we modified a stopped-flow assay to monitor RPo formation by E. coli σ32-RNAP, in which the fluorescence of a Cy3 fluorophore at +1 position on the nontemplate DNA strand increases upon RPo formation. As shown in Figure 2C and Table S1, the kinetics of RPo equilibration of σ32 (H107A)-RNAP holoenzyme is significantly slower than that of wild-type σ32-RNAP. Mutations in the protein pockets of A−11 (N94A, V97A, W108A and F104A) also exhibited slowed RPo equilibration. It is worth noting that the curves could well fit to a double exponential where the amplitudes and observed rates for the fast (a1, kobs,1) and slow (a2, kobs,2) phases were fixed [35] (Table S1), suggesting the existence of a significant intermediate (RPi) on the path to RPo formation. The amplitudes (a1, a2) describe that phase’s contribution to the total fluorescent enhancement signal. As show in Table S1, the amplitude of the curve is dominated by the amplitude of the slow rate, which implies that the slow phase contributes more to final RPo formation. As fluorescence enhancement serves as a reporter of the open complex, we conclude that substituting these residues with alanine significantly destabilizes the open E. coli RNAP complex. Specifically, our results show that substituting W108, H107, and F104 with alanine greatly destabilizes the RPo, while substituting V97 and N94 with alanine only slightly destabilizes the RPo (Table S1).
Sequence alignment and structural analysis of other E. coli σ factors indicate that diverse residues lie in the same position in different σ factors and play the same role as H107–W108 of σ32 (Figures S2 and S4I–L). To compare the efficiency of these residues at promoter unwinding, we substituted the HW residues with WW (σ70 and σ38) and QR (σ28), and tested the corresponding transcription activity using a Mango-dependent in vitro transcription assay. As shown in Figure 2A, the rankings in transcription activity differ as follows: σWW > σHW (WT) > σQR. Similarly, the RPo formation activity was ranked as σWW > σHW (WT) > σQR (Figure 2D, Table S2), indicating that distinct wedge residues were employed by different σ factors to orchestrate the recognition and unwinding of different promoters. We speculate that the HW motif in σ32 evolved from the WW motif in σ70 with the purpose of facilitating selective expression of different genes to adapt to changes in internal and external environments. One possibility is that the WH motif, beside its role in helping promoter DNA melting, and together with adjacent residues, may provide a unique structural feature that allows σ32 to interact with specific promoters and regulate the expression of heat shock genes.
Notably, the curves and parameters (kobs,1~0.1s−1, kobs,2~0.01s−1) in Figure 2C,D are similar and resemble those in E. coli σ24-RNAP [15] rather than those in M. tuberculosis CarD-RNAP [52], which means that the observed fast rates (kobs,1~1s−1) report on protein–DNA interaction is not related to opening (i.e., binding) and the observed slow rate (kobs,2~0.1s−1) is related to RPo equilibration. These results indicated the importance of RPo formation for transcription, and also highlighted the multiplicity of RPo equilibration in different bacteria.
3.5. βFTH Adopts a Distinct Conformation to Orchestrate σ32-Promoter Recognition
To date, structures of four σ factors that belong to the σ70 family have been resolved, including σ70 [11,50], σ38 [25,44], σ28 [26], and σ24 [15]. We compared these published structures to dissect more details about the mechanism of promoter recognition. Structure superimposition revealed that the relative orientation between βFTH and σ4 in σ32-engaged RNAP differ between these σ-engaged RNAPs (Figure 3A–D). To further explore the role of βFTH in σ-mediated DNA engagement, we designed two chimeric σ factors to validate DNA binding affinity to the corresponding holoenzymes. Considering that (1) σ38 recognizes promoters overlap with σ70 but σ70 is larger than the other σ factors; (2) σ28 and σ38 belong to the same group 3 category; and (3) the tail of σ24 did not observably interact with βFTH, we chose σ28 and σ38 as our research targets. The chimera of σ32 and σ28, designated as σ32–28, was designed by replacing the βFTH-interacting σ4 segment of σ32 with the βFTH-interacting σ4 segment of σ28. The chimera of σ32 and σ38, designated as σ32–38, was designed by replacing the βFTH-interacting σ4 region of σ32 with the βFTH-interacting σ4 region of σ38 (Figure 3E). The calculated KD values of WT, chimera σ32–28, and chimera σ32–38 are 14.95 nM, 9.22 nM, 8.34 nM, respectively. Owing to the larger binding area, the binding affinity in this experiment is higher than that in Figure 2B, and σ32–28 and σ32–38 both enhanced the affinity of promoter DNA binding to the holoenzyme as shown by the result of the fluorescence polarization assay (Figure 3F). Similarly, both chimeras enhanced transcription activities (Figure 3G). These results indicate the importance of σ4–βFTH interaction in σ32-promoter recognition, and also highlighted the important role of βFTH in orchestrating the recognition of other σ promoters. Collectively, our structural and biochemical results suggest that biased σ4–βFTH configurations in different σs may be adopted to modulate binding affinity to promoters so as to orchestrate the recognition and regulation of different promoters.
4. Discussion
Bacterial cells sense and respond to different environmental conditions by regulating different expression regulons via activation of distinct σ-RNAP holoenzymes [53]. Certain RNAPs harboring group 1 and group 2 σ factors can transcribe the same promoter sets [54]. E. coli σ70 shares a high degree of protein sequence similarity with σ38 and prefers binding to similar DNA sequences at the −10 and −35 promoter elements [55]. In contrast, the protein sequences and target DNA sequence of other σ factors are less similar.
Our structure revealed that the recognition between σ32-RPo and −35 element closely resembles that of σ24-RPo [15] rather than that of σ28-RPo [26], despite the fact that both σ32 and σ28 belong to group 3 (Figure 1E and Figure S4). By comparison, σ70-RPo and σ38-RPo strongly interact with −35 element and the spacer region, with both having six to eight residues participating in the interactions (Figure S4C,D,G,H), while σ24-RPo interacts modestly with the −35 element and the spacer region, with four residues participating in the interactions (Figure S4A,E). It is worth noting that the interaction between σ28 and −35 element is the weakest (Figure S4B), consistent with the finding that β’ZBD is relocated to help strength the interaction with the −35 element [26]. Furthermore, our study revealed a relatively weak interaction between σ32 and −35/−10 spacer region mediated by T128 and K130 of σ32 (Figure 1G), which is in congruence with a previous study [48]. Collectively, these structural features are consistent with the fact that group 2 σs are more similar to housekeeping group 1 σs, and group 1 and group 2 σs account for the transcription of a majority of genes in bacteria [2].
Our structure may also help solve or explain some previous questions. Based on our structure, we speculate that phosphorylation of Y260 may inhibit σ32 activity [56] by attenuating the interaction between adjacent residues and the −35 element or by triggering an unpopular σ4–βFTH configurations. Our structure also connects a series of disordered regions in σ32 previously reported in research on HSR: residues 47–55, termed feedback control region [57,58,59], are located in σ2.1 and are partially protected by residues 57–66 of the clamp helix of β‘, which is the binding site for DnaKJ chaperones [60,61]. Sandwiched between σ1.2 and σ3.0, residues 122–144 (Region C) [62] and residues 263–284 (The C-terminal region) [63] are mainly responsible for binding with RNAP. In addition, as σ32 I54 (which was shown to play a crucial role in membrane-targeted instability of σ32 during HSR) [57] was fixed and protected by the helix of σ2.2 and the clamp helix of the β’ subunit, we speculate that cytoplasmic σ32 faces two mutually exclusive fates: either bind to RNAP to exert their active function or be inactivated through membrane localization.
From first engaging with promoter DNA to finally producing RNA, RNAP holoenzymes harboring promoters undergo several sequential conformational states, including promoter recognition (RPc), promoter melting (RPi), and bubble formation (RPo) [64]. Promoter melting is nucleated by the wedge residue, as shown in our study and in previous reports [15,65]. The hallmark of RPo maturation is the formation of an approximately 12-nt “bubble” around the promoter −10 element, which allows initial RNA synthesis and NTPs incorporation. W-dyad was found to wedge the transcription bubble in σ70. While the presence of W-dyad is common in group 1 σ factors [66,67], residues harboring bulky hydrophobic sidechains are favored at corresponding sites in other σ factors [67]. Given the robust DNA-melting capacity of group 1 σ factors, it was believed that the W-dyad would be optimal for supporting the upstream transcription bubble. Our structure shows that H107 in σ32 replaces W433 in the W-dyad in σ70 to wedge the upstream transcription bubble, while W108 in σ32 resembles W434 in the W-dyad of σ70. Our data also show that different σ factors employ distinct wedge residues in initiating transcription with the following activity ranking: WW > HW > QR. This result is consistent with the result of the stopped-flow assay. Our result is in congruence with previous hypothesis that the W-dyad is the optimal choice for wedging the upstream ds/ss junction of the transcription bubble [65], giving its powerful DNA-melting capacity. Other residues such as H, N, and Q (Figure 1F and Figure S4I–J) with inferior promoter melting abilities may allow alternative σ factors to form RPo at a slower rate (Figure 2C,D), fine-tuning their specificity [15]. Variations in the residues at this very position may lead to differences in DNA-melting capabilities of different σ factors, leading to differential transcription activity and gene regulation [68].
During transcription initiation, conserved σ4 contacts the βFTH and mediates recognition of the promoter −35 element [69]. Previous reports established that variations in spacer lengths greatly affects transcription activity and leads to the concurrent rotation of the σ4–βFTH module, which plays a significant role in promoter recognition [70,71,72]. However, whether the σ4–βFTH modules of different σs can allosterically affect promoter recognition has not been explored. Here, two chimeric σ factors (σ32–28 and σ32–38) with partial σ4 domain replaced were designed to test the impact on DNA binding affinity and transcription activity, and it was surprising to find variation in DNA binding affinity and enhanced transcription activity with the chimeric proteins (Figure 3F,G). These results indicated the importance of σ4–βFTH interaction in σ32-promoter recognition and also highlighted the important role of βFTH in orchestrating the recognition of other σ promoters. Although our study shows that the σ4–βFTH interaction affects transcription activity by influencing the binding affinity of σ4 to the promoter, it seems that other steps such as promoter escape and transcription pausing may also play a role [16,49]. Nevertheless, our study indicates that the σ4–βFTH interaction is tuned to balance the distinct transcription efficiencies of different σ factors.
In summary, our structure and supporting biochemical data identified a different promoter recognition and promoter melting mode for transcription initiation, including (1) a relatively weak interaction between σ32 and −35/−10 spacer that may account for less efficient promoter recognition [2]; (2) a histidine with inferior promoter melting ability that may allow σ32 to form RPo at a slower rate, fine-tuning its specificity; (3) a biased σ4–βFTH configuration to modulate the affinity of binding to the promoter so as to orchestrate the recognition and regulation of different promoters. Our work will deepen our understanding of transcription initiation mechanisms of different σ32 factors as well as the biological function of σ32.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom13050738/s1, Figure S1: Purification of E. coli σ32-RPo complex and Cryo-EM of the E. coli σ32-RPo complex; Figure S2: Sequence alignment of σ factors; Figure S3: Structural and interacted details of pointed motifs; Figure S4: The detailed interactions between σs (σ24, σ28, σ38 and σ70) and corresponding promoters. Table S1: Kinetic parameters from Figure 2C of RPo formation by E. coli RNAP holoenzyme comprising wild-type or derivatives of E. coli σ32; Table S2: Kinetic parameters from Figure 2D of RPo formation by E. coli RNAP holoenzyme comprising wild-type or derivatives of E. coli σ32.
Author Contributions
S.W. and L.M. conceived and supervised the project. Q.L. and F.W. prepared samples. J.W. performed cryo-EM data acquisition, data processing and model building. Q.L., T.C. and W.Y. conducted the fluorescence polarization assay and the stopped-flow assay. S.W., L.M. and Q.L. conducted structure data analysis and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Structure coordinates and cryo-EM density map of σ32-RPo have been deposited in the Protein Data Bank and Electron Microscopy Data Bank under accession numbers 8HKC and EMD-34849, respectively.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Funding Statement
We thank the Cryo-EM facility of Hubei University for single particle cryo-EM data collection and computation support. This work was supported by a grant from the National Key R&D Program of China (2020YFA0908400) to S.W.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ruff E.F., Record J.M.T., Artsimovitch I. Initial Events in Bacterial Transcription Initiation. Biomolecules. 2015;5:1035–1062. doi: 10.3390/biom5021035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feklistov A., Sharon B.D., Darst S.A., Gross C.A. Bacterial sigma factors: A historical, structural, and genomic perspective. Annu. Rev. Microbiol. 2014;68:357–376. doi: 10.1146/annurev-micro-092412-155737. [DOI] [PubMed] [Google Scholar]
- 3.Chen J., Boyaci H., Campbell E.A. Diverse and unified mechanisms of transcription initiation in bacteria. Nat. Rev. Genet. 2021;19:95–109. doi: 10.1038/s41579-020-00450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruber T.M., Gross C.A. Multiple Sigma Subunits and the Partitioning of Bacterial Transcription Space. Annu. Rev. Microbiol. 2003;57:441–466. doi: 10.1146/annurev.micro.57.030502.090913. [DOI] [PubMed] [Google Scholar]
- 5.Shingler V. Signal sensory systems that impact σ⁵⁴ -dependent transcription. FEMS Microbiol. Rev. 2011;35:425–440. doi: 10.1111/j.1574-6976.2010.00255.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhang N., Jovanovic G., McDonald C., Ces O., Zhang X., Buck M. Transcription Regulation and Membrane Stress Management in Enterobacterial Pathogens. Biophys. Infect. 2016;915:207–230. doi: 10.1007/978-3-319-32189-9_13. [DOI] [PubMed] [Google Scholar]
- 7.Pletnev P., Pupov D., Pshanichnaya L., Esyunina D., Petushkov I., Nesterchuk M., Osterman I., Rubtsova M., Mardanov A., Ravin N., et al. Rewiring of growth-dependent transcription regulation by a point mutation in region 1.1 of the housekeeping sigma factor. Nucleic Acids Res. 2020;48:10802–10819. doi: 10.1093/nar/gkaa798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bae B., Davis E., Brown D., Campbell E.A., Wigneshweraraj S., Darst S.A. Phage T7 Gp2 inhibition of Escherichia coli RNA polymerase involves misappropriation of sigma70 domain 1.1. Proc. Natl. Acad. Sci. USA. 2013;110:19772–19777. doi: 10.1073/pnas.1314576110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vuthoori S., Bowers C.W., McCracken A., Dombroski A.J., Hinton D.M. Domain 1.1 of the sigma(70) subunit of Escherichia coli RNA polymerase modulates the formation of stable polymerase/promoter complexes. J. Mol. Biol. 2001;309:561–572. doi: 10.1006/jmbi.2001.4690. [DOI] [PubMed] [Google Scholar]
- 10.Zenkin N., Kulbachinskiy A., Yuzenkova Y., Mustaev A., Bass I., Severinov K., Brodolin K. Region 1.2 of the RNA polymerase sigma subunit controls recognition of the -10 promoter element. EMBO J. 2007;26:955–964. doi: 10.1038/sj.emboj.7601555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narayanan A., Vago F.S., Li K., Qayyum M.Z., Yernool D., Jiang W., Murakami K.S. Cryo-EM structure of Escherichia coli sigma(70) RNA polymerase and promoter DNA complex revealed a role of sigma non-conserved region during the open complex formation. J. Biol. Chem. 2018;293:7367–7375. doi: 10.1074/jbc.RA118.002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell J.E., Zheng D., Busby S.J., Minchin S.D. Identification and analysis of ‘extended -10’ promoters in Escherichia coli. Nucleic Acids Res. 2003;31:4689–4695. doi: 10.1093/nar/gkg694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A., Malloch R.A., Fujita N., Smillie D.A., Ishihama A., Hayward R.S. The Minus 35-Recognition Region of Escherichia coli Sigma 70 is Inessential for Initiation of Transcription at an “Extended Minus 10” Promoter. J. Mol. Biol. 1993;232:406–418. doi: 10.1006/jmbi.1993.1400. [DOI] [PubMed] [Google Scholar]
- 14.Oguienko A., Petushkov I., Pupov D., Esyunina D., Kulbachinskiy A. Universal functions of the sigma finger in alternative sigma factors during transcription initiation by bacterial RNA polymerase. RNA Biol. 2021;18:2028–2037. doi: 10.1080/15476286.2021.1889254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang C., Li L., Shen L., Shi J., Wang S., Feng Y., Zhang Y. Structures and mechanism of transcription initiation by bacterial ECF factors. Nucleic Acids Res. 2019;47:7094–7104. doi: 10.1093/nar/gkz470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petushkov I., Esyunina D., Mekler V., Severinov K., Pupov D., Kulbachinskiy A. Interplay between σ region 3.2 and secondary channel factors during promoter escape by bacterial RNA polymerase. Biochem. J. 2017;474:4053–4064. doi: 10.1042/BCJ20170436. [DOI] [PubMed] [Google Scholar]
- 17.Murakami K.S., Masuda S., Campbell E.A., Muzzin O., Darst S.A. Structural Basis of Transcription Initiation: An RNA Polymerase Holoenzyme-DNA Complex. Science. 2002;296:1285–1290. doi: 10.1126/science.1069595. [DOI] [PubMed] [Google Scholar]
- 18.Yura T. Regulation of the heat shock response in Escherichia coli: History and perspectives. Genes Genet. Syst. 2019;94:103–108. doi: 10.1266/ggs.19-00005. [DOI] [PubMed] [Google Scholar]
- 19.Nagai H., Yuzawa H., Kanemori M., Yura T. A distinct segment of the sigma 32 polypeptide is involved in DnaK-mediated negative control of the heat shock response in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1994;91:10280–10284. doi: 10.1073/pnas.91.22.10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagai H., Yuzawa H., Yura T. Interplay of two cis-acting mRNA regions in translational control of sigma 32 synthesis during the heat shock response of Escherichia coli. Proc. Natl. Acad. Sci. USA. 1991;88:10515–10519. doi: 10.1073/pnas.88.23.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamath-Loeb A.S., Gross C.A. Translational regulation of sigma 32 synthesis: Requirement for an internal control element. J. Bacteriol. 1991;173:3904–3906. doi: 10.1128/jb.173.12.3904-3906.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guisbert E., Herman C., Lu C.Z., Gross C.A. A chaperone network controls the heat shock response in E. coli. Genes Dev. 2004;18:2812–2821. doi: 10.1101/gad.1219204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herman C., Thévenet D., D’Ari R., Bouloc P. Degradation of sigma 32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc. Natl. Acad. Sci. USA. 1995;92:3516–3520. doi: 10.1073/pnas.92.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakami K.S. X-ray crystal structure of Escherichia coli RNA polymerase sigma70 holoenzyme. J. Biol. Chem. 2013;288:9126–9134. doi: 10.1074/jbc.M112.430900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu B., Zuo Y., Steitz T.A. Structures of E. coli sigmaS-transcription initiation complexes provide new insights into polymerase mechanism. Proc. Natl. Acad. Sci. USA. 2016;113:4051–4056. doi: 10.1073/pnas.1520555113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi W., Zhou W., Zhang B., Huang S., Jiang Y., Schammel A., Hu Y., Liu B. Structural basis of bacterial σ -mediated transcription reveals roles of the RNA polymerase zinc-binding domain. EMBO J. 2020;39:e104389. doi: 10.15252/embj.2020104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danson A.E., Jovanovic M., Buck M., Zhang X. Mechanisms of σ(54)-Dependent Transcription Initiation and Regulation. J. Mol. Biol. 2019;431:3960–3974. doi: 10.1016/j.jmb.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glyde R., Ye F., Darbari V.C., Zhang N., Buck M., Zhang X. Structures of RNA Polymerase Closed and Intermediate Complexes Reveal Mechanisms of DNA Opening and Transcription Initiation. Mol. Cell. 2017;67:106–116.e4. doi: 10.1016/j.molcel.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y., Darbari V.C., Zhang N., Lu D., Glyde R., Wang Y.P., Winkelman J.T., Gourse R.L., Murakami K.S., Buck M., et al. TRANSCRIPTION. Structures of the RNA polymerase-σ54 reveal new and conserved regulatory strategies. Science. 2015;349:882–885. doi: 10.1126/science.aab1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svetlov V., Artsimovitch I. Purification of bacterial RNA polymerase: Tools and protocols. Methods Mol. Biol. 2015;1276:13–29. doi: 10.1007/978-1-4939-2392-2_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helmann J.D., Chamberlin M.J. Structure and function of bacterial sigma factors. Annu. Rev. Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- 32.Wang F., Shi J., He D., Tong B., Zhang C., Wen A., Zhang Y., Feng Y., Lin W. Structural basis for transcription inhibition by E. coli SspA. Nucleic Acids Res. 2020;48:9931–9942. doi: 10.1093/nar/gkaa672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You L., Shi J., Shen L., Li L., Fang C., Yu C., Cheng W., Feng Y., Zhang Y. Structural basis for transcription antitermination at bacterial intrinsic terminator. Nat. Commun. 2019;10:3048. doi: 10.1038/s41467-019-10955-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maziarz M., Garcia-Marcos M. Methods in Cell Biology. Volume 142. Academic Press; New York, NY, USA: 2017. Fluorescence polarization assays to measure interactions between Gα subunits of heterotrimeric G proteins and regulatory motifs; pp. 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.KKo J., Heyduk T. Kinetics of promoter escape by bacterial RNA polymerase: Effects of promoter contacts and transcription bubble collapse. Biochem. J. 2014;463:135–144. doi: 10.1042/BJ20140179. [DOI] [PubMed] [Google Scholar]
- 36.Mastronarde D.N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 2005;152:36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Zheng S.Q., Palovcak E., Armache J.-P., Verba K.A., Cheng Y., Agard D.A. MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohou A., Grigorieff N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 2015;192:216–221. doi: 10.1016/j.jsb.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheres S.H. RELION: Implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 2012;180:519–530. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang J.Y., Olinares P.D., Chen J., Campbell E.A., Mustaev A., Chait B.T., Gottesman M.E., Darst S.A. Structural basis of transcription arrest by coliphage HK022 Nun in an Escherichia coli RNA polymerase elongation complex. eLife. 2017;6:e25478. doi: 10.7554/eLife.25478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera? A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 42.Emsley P., Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 43.Adams P.D., Afonine P.V., Bunkóczi G., Chen V.B., Davis I.W., Echols N., Headd J.J., Hung L.W., Kapral G.J., Grosse-Kunstleve R.W., et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu J., Cui K., Shen L., Shi J., Li L., You L., Fang C., Zhao G., Feng Y., Yang B., et al. Crl activates transcription by stabilizing active conformation of the master stress transcription initiation factor. eLife. 2019;8:e50928. doi: 10.7554/eLife.50928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qayyum M.Z., Molodtsov V., Renda A., Murakami K.S. Structural basis of RNA polymerase recycling by the Swi2/Snf2 family of ATPase RapA in Escherichia coli. J. Biol. Chem. 2021;297:101404. doi: 10.1016/j.jbc.2021.101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vishwakarma R.K., Cao A.M., Morichaud Z., Perumal A.S., Margeat E., Brodolin K. Single-molecule analysis reveals the mechanism of transcription activation in M. tuberculosis. Sci. Adv. 2018;4:eaao5498. doi: 10.1126/sciadv.aao5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz E.C., Shekhtman A., Dutta K., Pratt M.R., Cowburn D., Darst S., Muir T.W. A full-length group 1 bacterial sigma factor adopts a compact structure incompatible with DNA binding. Chem. Biol. 2008;15:1091–1103. doi: 10.1016/j.chembiol.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koo B.M., Rhodius V.A., Campbell E.A., Gross C.A. Dissection of recognition determinants of Escherichia coli sigma32 suggests a composite -10 region with an ‘extended -10’ motif and a core -10 element. Mol. Microbiol. 2009;72:815–829. doi: 10.1111/j.1365-2958.2009.06690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brodolin K., Morichaud Z. Region 4 of the RNA polymerase sigma subunit counteracts pausing during initial transcription. J. Biol. Chem. 2021;296:100253. doi: 10.1074/jbc.RA120.016299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saecker R.M., Chen J., Chiu C.E., Malone B., Sotiris J., Ebrahim M., Yen L.Y., Eng E.T., Darst S.A. Structural origins of Escherichia coli RNA polymerase open promoter complex stability. Proc. Natl. Acad. Sci. USA. 2021;118:e2112877118. doi: 10.1073/pnas.2112877118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campagne S., Marsh M.E., Capitani G., Vorholt J.A., Allain F.H. Structural basis for -10 promoter element melting by environmentally induced sigma factors. Nat. Struct. Mol. Biol. 2014;21:269–276. doi: 10.1038/nsmb.2777. [DOI] [PubMed] [Google Scholar]
- 52.Rammohan J., Ruiz Manzano A., Garner A.L., Stallings C.L., Galburt E.A. CarD stabilizes mycobacterial open complexes via a two-tiered kinetic mechanism. Nucleic Acids Res. 2015;43:3272–3285. doi: 10.1093/nar/gkv078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Österberg S., del Peso-Santos T., Shingler V. Regulation of alternative sigma factor use. Annu. Rev. Microbiol. 2011;65:37–55. doi: 10.1146/annurev.micro.112408.134219. [DOI] [PubMed] [Google Scholar]
- 54.Rodrigue S., Provvedi R., Jacques P.E., Gaudreau L., Manganelli R. The sigma factors of Mycobacterium tuberculosis. FEMS Microbiol. Rev. 2006;30:926–941. doi: 10.1111/j.1574-6976.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- 55.Gaal T., Ross W., Estrem S.T., Nguyen L.H., Burgess R.R., Gourse R.L. Promoter recognition and discrimination by EsigmaS RNA polymerase. Mol. Microbiol. 2001;42:939–954. doi: 10.1046/j.1365-2958.2001.02703.x. [DOI] [PubMed] [Google Scholar]
- 56.Klein G., Dartigalongue C., Raina S. Phosphorylation-mediated regulation of heat shock response in Escherichia coli. Mol. Microbiol. 2003;48:269–285. doi: 10.1046/j.1365-2958.2003.03449.x. [DOI] [PubMed] [Google Scholar]
- 57.Lim B., Miyazaki R., Neher S., Siegele D.A., Ito K., Walter P., Akiyama Y., Yura T., Gross C.A. Heat shock transcription factor σ32 co-opts the signal recognition particle to regulate protein homeostasis in E. coli. PLoS Biol. 2013;11:e1001735. doi: 10.1371/journal.pbio.1001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yura T., Guisbert E., Poritz M., Lu C.Z., Campbell E., Gross C.A. Analysis of sigma32 mutants defective in chaperone-mediated feedback control reveals unexpected complexity of the heat shock response. Proc. Natl. Acad. Sci. USA. 2007;104:17638–17643. doi: 10.1073/pnas.0708819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horikoshi M., Yura T., Tsuchimoto S., Fukumori Y., Kanemori M. Conserved region 2.1 of Escherichia coli heat shock transcription factor sigma32 is required for modulating both metabolic stability and transcriptional activity. J. Bacteriol. 2004;186:7474–7480. doi: 10.1128/JB.186.22.7474-7480.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki H., Ikeda A., Tsuchimoto S., Adachi K., Noguchi A., Fukumori Y., Kanemori M. Synergistic binding of DnaJ and DnaK chaperones to heat shock transcription factor σ32 ensures its characteristic high metabolic instability: Implications for heat shock protein 70 (Hsp70)-Hsp40 mode of function. J. Biol. Chem. 2012;287:19275–19283. doi: 10.1074/jbc.M111.331470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodriguez F., Arsène-Ploetze F., Rist W., Rüdiger S., Schneider-Mergener J., Mayer M., Bukau B. Molecular basis for regulation of the heat shock transcription factor sigma32 by the DnaK and DnaJ chaperones. Molecular Cell. 2008;32:347–358. doi: 10.1016/j.molcel.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 62.Arsene F., Tomoyasu T., Mogk A., Schirra C., Schulze-Specking A., Bukau B. Role of region C in regulation of the heat shock gene-specific sigma factor of Escherichia coli, sigma32. J. Bacteriol. 1999;181:3552–3561. doi: 10.1128/JB.181.11.3552-3561.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tomoyasu T., Arsene F., Ogura T., Bukau B. The C terminus of sigma(32) is not essential for degradation by FtsH. J. Bacteriol. 2001;183:5911–5917. doi: 10.1128/JB.183.20.5911-5917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen J., Chiu C., Gopalkrishnan S., Chen A.Y., Olinares PD B., Saecker R.M., Winkelman J.T., Maloney M.F., Chait B.T., Ross W., et al. Stepwise Promoter Melting by Bacterial RNA Polymerase. Mol. Cell. 2020;78:275–288.e276. doi: 10.1016/j.molcel.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bae B., Feklistov A., Lass-Napiorkowska A., Landick R., Darst S.A. Structure of a bacterial RNA polymerase holoenzyme open promoter complex. eLife. 2015;4:e08504. doi: 10.7554/eLife.08504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gruber T.M., Bryant D.A. Molecular systematic studies of eubacteria, using sigma70-type sigma factors of group 1 and group 2. J. Bacteriol. 1997;179:1734–1747. doi: 10.1128/jb.179.5.1734-1747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lonetto M., Gribskov M., Gross C.A. The sigma 70 family: Sequence conservation and evolutionary relationships. J. Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koo B.M., Rhodius V.A., Nonaka G., deHaseth P.L., Gross C.A. Reduced capacity of alternative sigmas to melt promoters ensures stringent promoter recognition. Genes Dev. 2009;23:2426–2436. doi: 10.1101/gad.1843709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geszvain K., Gruber T.M., Mooney R.A., Gross C.A., Landick R. A hydrophobic patch on the flap-tip helix of E. coli RNA polymerase mediates sigma(70) region 4 function. J. Mol. Biol. 2004;343:569–587. doi: 10.1016/j.jmb.2004.08.063. [DOI] [PubMed] [Google Scholar]
- 70.Fang C., Philips S.J., Wu X., Chen K., Shi J., Shen L., Xu J., Feng Y., O’Halloran T.V., Zhang Y. CueR activates transcription through a DNA distortion mechanism. Nat. Chem. Biol. 2021;17:57–64. doi: 10.1038/s41589-020-00653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fang C., Li L., Zhao Y., Wu X., Philips S., You L., Zhong M., Shi X., O’Halloran T., Li Q., et al. The bacterial multidrug resistance regulator BmrR distorts promoter DNA to activate transcription. Nat. Commun. 2020;11:6284. doi: 10.1038/s41467-020-20134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zuo Y., Steitz T.A. Crystal structures of the E. coli transcription initiation complexes with a complete bubble. Mol. Cell. 2015;58:534–540. doi: 10.1016/j.molcel.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Structure coordinates and cryo-EM density map of σ32-RPo have been deposited in the Protein Data Bank and Electron Microscopy Data Bank under accession numbers 8HKC and EMD-34849, respectively.