Abstract
Background: The gut microbiota is relatively stable; however, various factors can precipitate an imbalance that is known to be associated with various diseases. We aimed to conduct a systematic literature review of studies reporting the effects of ionizing radiation on the composition, richness, and diversity of the gut microbiota of animals. Methods: A systematic literature search was performed in PubMed, EMBASE, and Cochrane library databases. The standard methodologies expected by Cochrane were utilized. Results: We identified 3531 non-duplicated records and selected twenty-nine studies after considering the defined inclusion criteria. The studies were found to be heterogeneous, with significant differences in the chosen populations, methodologies, and outcomes. Overall, we found evidence of an association between ionizing radiation exposure and dysbiosis, with a reduction of microbiota diversity and richness and alterations in the taxonomic composition. Although differences in taxonomic composition varied across studies, Proteobacteria, Verrucomicrobia, Alistipes, and Akkermancia most consistently reported to be relatively more abundant after ionizing radiation exposure, whereas Bacteroidetes, Firmicutes, and Lactobacillus were relatively reduced. Conclusions: This review highlights the effect of ionizing exposure on gut microbiota diversity, richness, and composition. It paves the way for further studies on human subjects regarding gastrointestinal side effects in patients submitted to treatments with ionizing radiation and the development of potential preventive, therapeutic approaches.
Keywords: microbiome, microbiota, intestinal microbiome, gut microbiota, ionizing radiation, radiotherapy, radiation effects
1. Introduction
The gut microbiota can be defined as the microorganisms (bacteria, viruses, archaea, and protists) that collectively inhabit the intestinal tract’s lumen and mucosal surface. The collection of all genomes of those microorganisms constitutes the intestinal microbiome [1,2].
The gut microbiota’s composition is established early in life, and it’s relatively stable over time. However, an imbalance of the gut microbiota’s composition, also known as dysbiosis, has been linked to a range of factors and diseases, including certain medical conditions such as inflammatory bowel disease, infections, or the overuse of antibiotics [2,3].
Ionizing radiation (IR) refers to energy capable of ionizing atoms or molecules by removing electrons from them. It results from radionuclides decay (unstable atoms) and may take the form of electromagnetic waves or particles [4,5,6,7].
Some of the molecular effects of ionizing radiation include DNA damage by breaking the strands or altering the bases, protein damage by altering the structure and function of proteins, by the production of reactive oxygen species (ROS), which can cause oxidative stress and damage to cells and tissues and by causing the cells to stop dividing and enter in a state of cell cycle arrest [4,5,6,7].
Overall, these molecular effects depend on the type and dose of radiation, as well as the sensitivity of the cells and tissues being exposed, and can lead to temporary cell dysfunction and, ultimately, lead to cell death or senescence [7,8].
The effects of ionizing radiation can be classified into two main categories: deterministic and stochastic effects. Deterministic effects are directly related to the level of radiation dose received. Stochastic effects, on the other hand, are those that are probabilistic and occur randomly without a minimum dose threshold [7]. Both effects are more common in tissues that are highly sensitive to radiation and that have a high rate of cell division, such as the skin, bone marrow and gastrointestinal tract [1,9,10].
Sources of ionizing radiation exposure include medical procedures, naturally occurring radioactive materials such as radon, cosmic radiation, industrial and occupational exposure, nuclear accidents and military activities [7,11,12].
The gut microbiota is a complex and diverse ecosystem of microorganisms, and understanding the effects of ionizing radiation on gut microbiota might provide insights into the causes of the gastrointestinal side effects of the treatments and lead to prophylactic/therapeutic attitudes. Ionizing radiation may induce alterations in the gut microbiota composition, richness, and diversity due to the modulation of microbial gene expression, induction of oxidative stress, and promotion of specific microbial species’ growth and suppression of others [4,5,6,7].
Most published studies evaluating the effects of ionizing radiation on the gut microbiota are in animal models. These studies allow perturbations in the gut microbiota to be studied in a controlled experimental setup and thus help assess the causality of the complex host-microbiota interactions and develop mechanistic hypotheses [13]. Hence, we sought to systematically review the existing evidence of the effects of ionizing radiation on gut microbiota in animal models.
The aim of this study was to undertake a systematic literature review to determine the effects of ionizing radiation on animals’ gut microbiota, namely in its composition, diversity, or richness/abundance.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
A systematic search was carried out using the following electronic databases: PubMed/MedLine (30 November 2022), EMBASE (31 December 2022), and Cochrane library (30 November 2022). Additional articles were identified through the reference list from the included articles and relevant reviews. To ensure that studies were not missed or wrongly excluded and that the search was comprehensive, we also searched gray literature, general search engines, and reference lists of included papers.
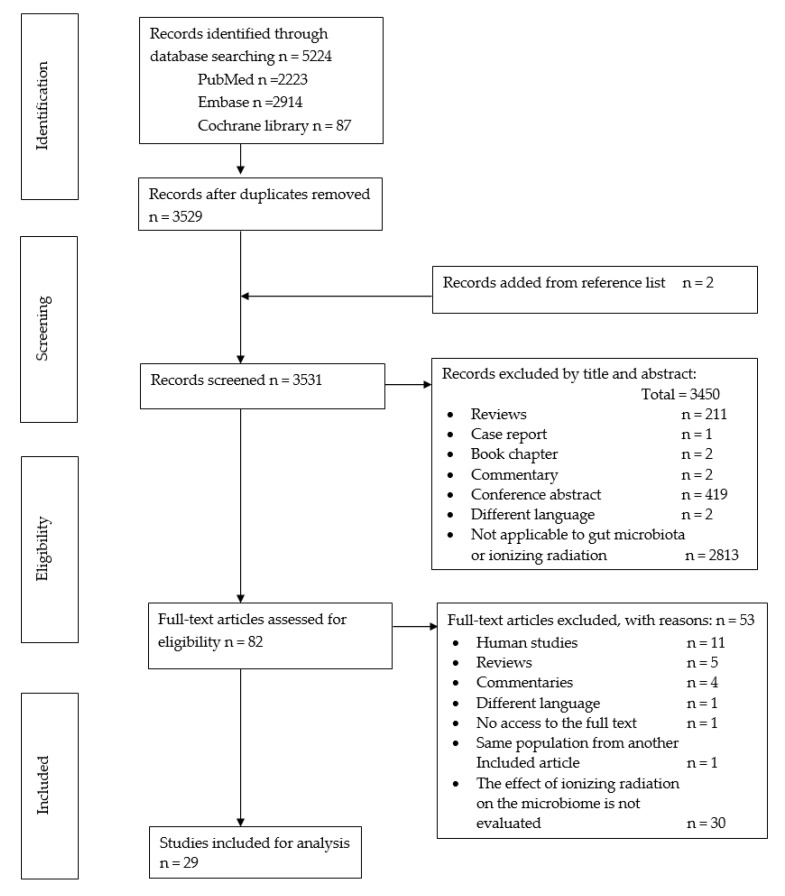
This review was carried out following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines checklist. Additionally, the review protocol was registered on the International PROSPERO review database: PROSPERO 2020: CRD42020210951 (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020210951) (accessed on 5 November 2022) (see Figure 1 for PRISMA diagram and Table 1 for search terms).
Figure 1.
PRISMA flow chart search strategy.
Table 1.
Literature search algorithm—PubMed; EMBASE (via OVID); and Cochrane Library.
| Database | Search Number | Search Terms |
|---|---|---|
| PubMed | Search #1 | “microbiota” OR “gastrointestinal microbiome” OR “microbiome” OR “16s rRNA” |
| Search #2 | “radiation” OR “radiotherapy” | |
| Search #3 | Search #1 AND Search #2 | |
| EMBASE (via OVID) | Search #1 | “microbiota” OR “gastrointestinal microbiome” OR “microbiome” OR “16s rRNA” OR “microflora” |
| Search #2 | “radiation” OR “radiotherapy” | |
| Search #3 | English OR Spanish OR Portuguese | |
| Search #4 | Search #1 AND Search #2 AND Search #3 | |
| Cochrane Library | Search #1 | “microbiota” OR “gastrointestinal microbiome” OR “microbiome” OR “16s rRNA” OR “microflora” |
| Search #2 | “radiation” OR “radiotherapy” | |
| Search #3 | Search #1 AND Search #2 |
We first analyzed the effects of IR on the gut microbiota of humans [14]. During our search, we found that most studies were performed in animal models. In addition to anatomic and physiological differences, human and animal studies present significant methodological differences. Therefore, we consider it relevant to focus this review on animal studies.
The PROSPERO database and Cochrane Library search revealed no similar systematic reviews.
All selected citations were exported from the databases to the reference management software EndNote X20 (Thompson Reuters, New York, NY, USA), and duplicates were excluded.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria were defined using the following components: patient population (P): animals exposed to radiation; exposure of interest (I): ionizing radiation; comparator (C): before and after exposure of the same subject or with controls; outcome (O): changes in the gut microbiome following exposure to radiation and the study design (S) of interest: interventional studies, prospective and retrospective observational cohort studies. The exclusion criteria were other types of studies (e.g., case-report, reviews); human or in vitro studies; and no relevant outcomes reported.
2.3. Study Selection and Data Extraction
All relevant peer-review journal articles in English, Portuguese, and Spanish, indexed until December 2022, were identified. A combination of search terms was used: microbiome, gut microbiota, radiotherapy, ionizing radiation, and 16S rRNA (Table 1, Table 2 and Table 3). The last search was performed on 31 December 2022 by two authors (AF and PB).
According to the defined inclusion and exclusion criteria, relevant studies were independently screened by two reviewers (AF and PB) based on title and abstract. All decisions were recorded on a spreadsheet.
All studies that did not fulfill the defined PICOS characteristics, conference papers, abstracts, and articles from which we could not obtain the full text were excluded.
Full-text papers of all eligible studies were obtained, and the two reviewers independently screened and selected papers a second time.
A tabular summary was developed with the following variables extracted from each eligible study: First author name, date of publication, study design, number of patients and controls, radiation exposure characteristics, type, number, and timepoint of samples, and the most relevant findings (Table 2 and Table 3).
2.4. Risk of Bias in Individual Studies
Two reviewers (AF and PB) assessed the risk of bias in each study independently, with disagreements resolved by consensus. The risk of bias was assessed as described in the Cochrane Handbook [15] by recording the methodology used.
The included studies’ quality was assessed by using the risk of bias tool from the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) for animal studies [16]. Categories for the investigation of quality were as follows: (1) sequence generation; (2) baseline characteristics; (3) allocation concealment; (4) random housing; (5) blinding for the performance bias; (6) random outcome assessment; (7) blinding for the detection bias; (8) incomplete outcome data; (9) selective outcome data; and (10) other sources of bias. Assessment of each category was divided into high, low, or unclear risk of bias.
Table 2.
Summary of study characteristics, demographics, radiation type, sample collection and analysis, and main findings of the eligible studies with mice.
|
Author, Year / Study Design |
Participants / N Irradiated |
Microbiome Assessment Method / Type of Sample / Number of Samples |
Main Findings |
|---|---|---|---|
| Type of Radiation | |||
| Li Y, 2020 [17] / Interventional |
Mice C57BL/6J Male and female 8 weeks N = 5 |
16s rRNA V4 region / Illumina Miseq / Fecal N = 3 Before irradiation, and 6 days and 12 days after irradiation |
Diversity/richness
|
| γ-ray TAI Single dose of 12 Gy or 15 Gy TBI Single dose of 4 or 7 Gy | |||
| Yamanouchi K, 2018 [18] / Interventional |
Mice C57BL/6Njcl Female 8 weeks N = 6 |
DNA Primer PCR / NucleoSpin® DNA Stool / Fecal N = 8 before irradiation, at 1, 2, 6, 12, 24, 48 and 72 h after irradiation |
Composition
|
| X-ray TBI Single dose of 2 Gy and 4 Gy | |||
| Goudarzi M, 2016 [19] / Interventional |
Mice C57BL/6J Male 8 weeks N = 14 7 control |
16s rRNA V4 region / Illumina HiSeq 2500 QIIME version 1.8.0 Phyloseq packages / Fecal N =3 0 and 5 Gy groups (1 day before and 3 and 30 days post-irradiation) N = 2 12 Gy group (1 day before and 3 days post-irradiation) |
Diversity/richness
|
| X-rays TBI Single dose 5 or 12 Gy | |||
| Cui M, 2017 [20] / Interventional |
Mice C57BL/6 Male and Female 6-8 weeks / N = 4 |
16s rRNA V4 region / Illumina Hiseq Uparse / Fecal N = 2 Days 5 and 10 postirradiation |
Diversity
|
| γ-rays TBI Single dose 6.5 Gy | |||
| Sittipo P, 2020 [21] / Interventional |
Mice C57BL/6L Male 8 weeks / N = 10 |
16s rRNA V4-V5 regions / Qubit 2.0 Fluorometer and 2100 Bioanalyzer Ion Torrent PGM platform QIIME v1.9.1 and Microbiome Helper package / Fecal N = 4: -1 day before irradiation -3 (D1, D3 and D10) |
Diversity/richness
|
| γ-rays TBI Single dose 6 Gy | |||
| Gerassy-Vainberg S, 2018 [22] / Interventional |
Mice C57BL/6J Female 6-8 weeks / N = 23 Control n = 22 |
16S rRNA / QIIME V 1.8.0 Illumina Miseq platform Roche 454 Pyrosequencing / Fecal N = 3 1 week before 2 and 6 weeks post radiation |
Composition
|
| γ-rays 4 fractions of 550 cGy Localized internal rectal radiation | |||
| Liu X, 2019 [23] / Interventional |
Mice BALB/c / Male 8 weeks / 4 groups: Control; low-dose 6 mice sampled at each timepoint N = 24 |
16s rRNA V4 / Qiagen Mini Kit Qubit 2.0 fluorometer Illumina Hiseq / Fecal N = 4 Before 7, 21 and 35 Postirradiation |
Diversity/richness
|
| γ-rays TBI group 1–0.5 Gy ×1 dose group 5–0.1 Gy per dose ×5 doses group 10–0.0 Gy per dose ×10 doses | |||
| Johnson LB, 2004 [24] / Interventional |
Mice C57/Bl6 / N = 30 6/per time point controls |
Anaerobic vs. Anaerobic / Viable counts / Tissue samples from the irradiated small intestine N = 5 2, 6, 16, 24 and 48 h |
Composition
|
| X-rays / Single dose 19 Gy | |||
| Lu L, 2019 [25] / Interventional |
Mice C57BL/6 Female 4 to 5 week old / N = 18 6/groups RT only; Control; RT + PC |
16s rRNA V3 and V4 Illumina Hiseq platform / Fecal N = 1 24 h after |
Diversity/richness
|
| X-rays TAI Single dose 12 Gy | |||
| Casero D, 2017 [26] / Interventional |
Mice C57BL/6 Male 6 months / N = 30 10 controls |
16S rRNA V4 region / Illumina HiSeq 2500 QIIME / Fecal N = 2 after 10 and 30 days |
Diversity/richness
|
| TBI 16O (600 MeV/n) at 0.1, 0.25, and 1 Gy | |||
| Kim YS, 2015 [27] / Interventional |
Mice C57BL/6 Male 8–10 week old / N = 3 Control = 2 |
16S rRNA UltraClean® Fecal DNA Isolation Kit / Illumina MiSeq // Fecal (small and large intestine) / N = 1 3 days after irradiation. |
Composition
|
| γ-rays TBI Single dose 8 Gy | |||
| Wang M, 2020 [28] / Interventional |
Mice C57BL/6J Male 8–10 weeks / N = 70 Intestinal group/Survival group Hematopoietic experiments |
16s rRNA V3-V4 / Illumina MiSeq QIIME / Feces removed from the rectum (Small intestines were taken out after three days of irradiation) N = 1 3 days after IR |
Diversity/richness
|
| γ-ray TBI 9.0 Gy—intestinal group 10.0 Gy—survival group 4.0 Gy—hematopoietic group | |||
| Zhao Z, 2020 [29] / Interventional |
Mice C57BL/6 Male 8–10 weeks / N = 4/3 4 pre radiation 3 post radiation |
16S rRNA V4 region / QIIME (v 1.8) PANDAseq (version 2.9) / Fecal Terminal ileum and cecum 3 months after |
Diversity/richness
|
| γ-rays TAI Single dose of 10 Gy | |||
| Wang W, 2020 [30] / Interventional |
Mice / N = 18 Controls = 6 |
16s rRNA / Illumina MiSeq qRT-PCR / Fecal samples directly collected from the lower segment of the colon N = 2 Days 7 and 30 after irradiation |
Diversity/richness
|
| TBI | |||
| Zhao Y, 2019 [31] / Interventional |
Mice C57BL/6J Male 8–12 weeks / N = 5 |
16s rRNA / Illumina Hiseq / Fecal Fresh from rectum 10 month after |
Diversity/richness
|
| γ-rays TBI Single dose 8 Gy | |||
| Li Yiyi, 2020 [32] / Interventional |
Mice C57BL/6J Male 6- to 8-week-old |
16s rDNA / Fecal N = 2 1 week 6 weeks |
Diversity/richness
|
| X-rays Single dose 18 Gy 500 cGy/min for abdominal colorectal localized external radiation | |||
| Raber J, 2020 [33] / Interventional |
Mice C57BL/6 F1 4–6 months / N = 99 |
16S rRNA V4 region / Illumina Miseq / Fecal N = 1 2 months post-radiation |
Diversity/richness
|
| Protons, 4He, 16O, 28Si, 48Ti and 56Fe ions | |||
| Tong JY, 2022 [34] / Interventional |
Mice C57BL/6J Female 3 weeks / N = 24 Controls = 6 |
16S rRNA V4 region / Illumina MiSeq / Fecal |
Diversity/richness
|
| X-rays TBI 5 groups: Test; 0.05, 0.10, 0.15 and 0.20 Gy | |||
| Cheema AK, 2021 [35] / Interventional |
Mice CD2F1 Male 6/7 weeks / N = 16/group |
16S rRNA V3/V4 region / Illumina MiSeq SILVA / Fecal N = 5 7 and 1 days before irradiation and 3, 14 and 30 post-irradiation |
Composition
|
| γ-rays Single dose 9.2 Gy |
Table 3.
Summary of study characteristics, demographics, radiation type, sample collection and analysis, and main findings of the eligible studies in animals (except mice).
| Animals | Author, Year / Interventional |
Participants / N Irradiated |
Microbiome Assessment Method / Type of Sample / Number of Samples |
Main Findings |
|---|---|---|---|---|
| Type of Radiation | ||||
| Rats | Rentea RM, 2016 [36] / Interventional |
Rats WAG/RijCmer Male 5 weeks / N = 15 5—Nonirradiated; 5—irradiated; 5—intestinal alkaline phosphatase (RT + IAP) |
16s rRNA / Real-time PCR / Fecal N = 2 D0 and 4 days after irraiation |
Composition
|
| X-rays 13 Gy—single dose / Intestinal lower hemibody radiation | ||||
| Lam V, 2012 [37] / Interventional |
Rats WAG/RijCmcr (Wistar) Male 5 weeks / N = 10 (n = 5/group) |
qPCR and 16S rRNA / Second Genome Inc. G3 PhyloChipe 16S rRNA microarray-based assay / Fecal N = 4 D0 and days 4, 11, and 21 post-irradiation |
Composition
|
|
| X-rays TBI Single dose 10.0 Gy Multiple-fraction 18.0 Gy | ||||
| Wild rodent: Bank Vole Myodes glareolus |
Lavrinienko A, 2018 [38] / Observational |
Wild rodent: Bank Vole Myodes glareolus / N = 137 |
16S rRNA V4 / Illumina MiSeq platform at BGI / Fecal |
Diversity/richness
|
| 3 study areas of environmental radiation: (1) high (CH) and (2) low (CL and KL) | ||||
| Lavrinienko, 2020 [39] / Observational |
Wild rodent: Bank Vole Myodes glareolus / 28 individuals provided fecal (CL1 n = 3, CL2 n = 13; CH1 n = 8, CH2 n = 4). (84–43 Recapture) |
16s rRNA V4 / Illumina MiSeq platform at BGI / Fecal N = 1 |
Diversity/richness
Second capture CL:
|
|
| Ambient radiation Chernobyl High Radiation (CH) and Chernobyl Low radiation (CL) | ||||
| Göttingen minipigs and Chinese rhesus macaques |
Carbonero F, 2018 [40] / Interventional |
Göttingen Minipigs and Chinese rhesus Macaques 8 Minipigs 8 Macaques |
16s rRNA / Illumina MiSeq / Fecal N = 2 -2/3 days before -3 days after |
Minipigs Diversity/richness
Diversity/richness
|
| 6 MV linear accelerator (LINAC) 80 ± 2.5 Gy/min 1.8 Gy Minipigs 6.8 Gy Macaques | ||||
| Carbonero F, 2018 [41] / Interventional |
Göttingen minipigs Chinese rhesus macaques / N = 74 male Chinese rhesus macaques 50 Minipigs |
16s rRNA / Illumina MiSeq QIAGEN / Fecal / Minipigs: collected on days 0 and 3 Macaque fecal samples were collected 24 h before irradiation, between 1–3 h postirradiation and on days 3 and 14 postirradiation |
Macaques Diversity/richness
Minipigs Richness
|
|
| Macaques 5.9 Gy (n = 12); 6.3 Gy (n = 14); 6.8 Gy (n = 16); 7.2 Gy (n = 16); and 7.7 Gy (n = 16) Minipigs 1.65 Gy (n = 9); 1.80 Gy (n = 10); 1.95 Gy (n = 11); 2.10 Gy (n = 13); and 2.25 Gy (n = 7) | ||||
|
Chinese rhesus macaques,
Macaca mulatta |
Kalkeri R, 2021 [42] / Interventional |
Chinese rhesus macaques, Macaca Mulatta / N = 19 |
Fecal samples / N = 3 1 day prior and 1 and 4 days after exposure |
Diversity/richness
|
| Gamma-rays 7.4 Gy | ||||
| Flies | Cai Z, 2018 [43] / Interventional |
Flies Males Bactrocera dorsalis 3000 pupae irradiated 15 guts irradiated 15 guts control |
16s rRNA V4 / Illumina MiSeq QIIME v1.8 / Gut / Irradiation 48h before eclosion Day1 Day7 Day14 Post eclosion |
Diversity/richness
|
| 100Gy gamma ray Gammacell 220 60Co With an activity of 9435 × 1015 Bq Central dose of 8Gy/min at the beginning of the test | ||||
| Ben Ami, 2020 [44] / Interventional |
Flies Vienna 8 Wild C capitata pupae / 150 bacterial colonies from non irradiated 150 colonies from 5-day-old irradiated flies and 100 colonies from field flies |
16s rRNA / PCR-DGGE |
Diversity
Non-irradiated vs. irradiated vs. irradiated mass 5 day-read
|
|
| Delta irradiation | ||||
| Woruba DN [45] / Interventional |
Flies Queensland fruit fly, Bactrocera Tryoni 54 = (3 × 18) |
16S rRNA V3 and V4 regions QIIME / Intact gut dissections / N = 2 1 and 14 days after irradiation |
Diversity/richness
|
|
| Delta irradiation |
No formal statistical analysis was undertaken due to the small number of retrieved eligible studies and the heterogeneity of the data and outcomes presented.
3. Results
3.1. Search Results
A total of 5224 citations were identified: 2852 through PubMed, 2914 through EMBASE and 87 through Cochrane library (Figure 1). After removing duplicates and adding two citations from reference lists, 3531 papers were screened for inclusion based on their titles and abstracts. A total of 3450 were excluded, and the full text of the remaining 82 studies was evaluated; a further 53 were then excluded (eleven were studies in humans, thirty did not report the effect of ionizing radiation in microbiota, five were literature revisions; four were commentaries; one was written in a language unreadable by the authors, and the authors were not able to access one article full text). The two reviewers found a final total of 29 studies eligible for review, with a perfect agreement between them (κ = 1).
3.2. Study Characteristics
Twenty-seven interventional studies and two observational studies were included. The analyzed studies were quite heterogeneous regarding population, study methodology, and outcomes. A summary of the characteristics of the studies is presented in Table 2 and Table 3.
3.2.1. Animal Models
Most studies analyzed the gut microbiota from mice (15 used substrains of C57BL/6 [17,18,19,20,21,22,26,27,28,32,33,34], one used BALB/c [23], one used CD2F1, [35] and one other study did not specify the strain [30]), two studies used rats (WAG/RijC) [36,37], two used wild bank voles (Myodes glareolus) [38,39], three used Chinese rhesus macaques [40,41,42], two used Göttingen minipig, [40,41] and three analyzed the gut microbiota of flies [43,44,45].
Nineteen studies evaluated the shift of the gut microbiota of mice, and three studies evaluated rats. Given their small size, low maintenance costs, relatively stable embryonic cells and pliability for genetic manipulations and gene editing, mice are considered the preferable animal model to study human gene functions. However, rats are physiologically, morphologically and genetically closer to humans than mice, which makes rats ideal models for biomedical and clinical studies [46].
Three studies used minipigs and nonhuman primate models. These animals represent large pre-clinical models which have demonstrated physiologic, anatomic, proteomic and genomic similarities to humans [40,47,48]
3.2.2. Radiation Exposure Characteristics
The type of radiation exposure varied throughout the studies. Most researchers evaluated the effect of ionizing radiation from artificial exposure to X-rays [18,19,24,25,32,36,37] or gamma rays [17,20,21,22,23,28,29,31,43,45] in either single or multiple doses, while one used delta radiation [44].
Most researchers used standard total body irradiation (TBI) models [17,19,20,26,30,31,37,42], while some used total abdominal irradiation (TAI) [17,25,29] or localized internal rectal irradiation [22] models to study the effects of irradiation on the gut microbiome. The gamma and X-rays doses ranged from 10 to 18 Gy in TAI studies and 0.1 to 12.0 Gy in TBI. Total abdominal irradiation and total body irradiation ranged from 0.1 Gy to 19.0 Gy in mice studies, from 5.9 Gy to 7.7 Gy in macaques and from 1.8 Gy to 2.25 Gy in minipigs.
Space travel is associated with continuous low-dose-rate exposure to radiation that might affect the gut microbiota. Two studies evaluated the effect of space-type radiation, exposing mice to high-energy transfer protons and ions [26,49].
The Chornobyl disaster provides a unique environmental opportunity to explore the impacts of chronic exposure to low-dose radioactive contaminants. Lavrinienko et al. conducted two studies to evaluate the gut microbiota of wild bank voles (Myodes glareolus) exposed to natural environmental radiation in areas of the Chornobyl exclusion zone that differed in the level of radionuclide contamination. Myodes glareolus is a small rodent that is an important mammalian wild model of the biological effects of exposure to ionizing radiation because it combines ecological relevance with laboratory tractability [38,39].
Finally, ionizing irradiation is often used to sterilize insects. However, it may have negative side effects on male insects’ fitness, resulting in reduced competitiveness. Three studies analyzed the shifts of the gut microbiota of flies, exposing them to high doses of gamma-rays (from 65 Gy to 100 Gy) [43,44,45].
3.2.3. Sampling and Microbiota Analysis
Most studies performed in mammals included in this review characterized the gut microbiota through fecal samples collected from the cages [17,18,21,35,42] or removed directly from the terminal ileum, cecum or rectum [28,29,30]. Differently, Johnson et al. analyzed tissue samples from the irradiated small intestine [24]. The studies performed in flies analyzed intact gut dissections [43,44,45].
Fecal samples are considered the most convenient collection method. They are easier to sample frequently, are non-invasive and have long been used for the analysis of the distal gut microbiota. Fecal samples have the disadvantages that they might contain inactive bacteria, bacteria from other gastrointestinal tract compartments, and less controlled sampling variables when compared to biopsy [50].
The number of obtained samples was very heterogeneous between studies varying from one to eight samples at different time points from the same animal. Some studies only collected one sample and compared it to controls, while other studies compared before and after exposure to ionizing radiation.
The sampling collection times within the studies were also very heterogeneous, ranging from after exposure to up to 10 months post-exposure.
Furthermore, the methodology used to study microbiota varied in the different studies.
Most studies chose 16S rRNA-based sequencing [17,20,21,27], whereas a few used qPCR [18,30]. In one study, an older method was used based on bacterial culture colony-forming units [24].
Richness, assessed by the number of OTUs/species, and diversity (alpha diversity and beta diversity) were parameters evaluated in most of the reviewed studies.
Most studies calculated alpha diversity through the Chao1 index, Shannon’s index and Simpson’s index. For beta diversity Bray–Curtis dissimilarity, Un-weighted UniFrac and Weighted UniFrac were used.
3.3. Quality Assessment
During the quality assessment, the reviewers verified that none of the selected studies reported methods of sequence generation or concealed allocation. Regarding the same baseline characteristics, most studies chose animals of the same ages and sex, but few specifically mentioned the weight of the different animals. One of the assessed studies did not specify the used animal’s baseline characteristics.
Regarding random housing, the reviewers considered that it is unlikely that the outcome measurement was influenced by not randomly housing the animals as they all followed the ethical rules for animal studies. None of the studies reported the blinding of the caregivers/investigators. The reviewers considered that although the outcome assessor was not blinded, the outcome, due to its characteristics, is not likely to be influenced by a lack of blinding.
Regarding attrition bias, most studies are not clear regarding how many animals were considered initially, so it was impossible to determine if all the considered animals were analyzed.
Finally, the reviewers considered that there was a low reporting bias.
Detailed information regarding t the quality assessment of the included studies are presented in Table S1 (Supplementary File).
3.4. Findings
The analyzed studies suggest that ionizing radiation causes significant changes in the composition, diversity, and richness of the gut microbiota. The key findings of the studies are organized in Table 4.
Table 4.
Key findings from selected studies.
| Key Findings from the Studies | |
|---|---|
| Diversity | |
| |
| ACE index | |
| α diversity | |
| |
| Shannon index |
|
| Simpson diversity index | |
| Chao1 index |
|
| Beta diversity | |
| Richness | |
| Number of OTUs/Taxa number |
|
| Altered composition/Dysbiosis | |
| |
| Anaerobic counts | |
|
|
| Aerobic counts | |
|
|
| Phylum | |
| Ratio Firmicutes/Bacteroidetes | |
| Actinobacteria | |
| Bacteroidetes |
|
| Epsilonbacteraeota |
|
| Firmicutes |
|
| Proteobacteria |
|
| Verrucomicrobia |
|
| Spirochaetes |
|
| Class | |
| Clostridia | |
| Bacteroida | |
| Betaproteobacteria |
|
| Unidentified_Saccharibacteria |
|
| Epsilonproteobacteria |
|
| Deltaproteobacteria |
|
| Erysipelotrichia |
|
| Order | |
| Clostridiales | |
| Bifidobacteriales |
|
| Coriobacteriales |
|
| Verrucomicrobiales |
|
| Lactobacillales |
|
| Bacteroidales |
|
| Family | |
| Desulfovibrionaceae | |
| Staphylococcaceae |
|
| Lactobacillacea | |
| Prevotellacea |
|
| Clostridiaceae | |
| Lachnospiracea |
|
| Moraxellaceae |
|
| Ruminococcaceae | |
| Porphyromonadaceae |
|
| Rikenellaceae |
|
| Eggerthellaceae |
|
| Enterobacteriaceae | |
| Flavobacteriaceae |
|
|
Muribaculaceae
S24-7 family |
|
| Bacillaceae |
|
| Xanthomonadaceae |
|
| Sphingobacteriaceae |
|
| Aeromonadacea |
|
| Peptostreptococcaceae |
|
| Veillonellaceae |
|
| Genus | |
| Acinetobacter |
|
| Aerococcus |
|
| Actinobacillus |
|
| Actinobacteria major genera |
|
| Akkermansia | |
| Alloprevotella | |
| Alistipes |
|
| Anaerotruncus |
|
| Bacteroides |
|
| Barnesiella | |
| Betaproteobacteria members (Desulfovibrio and Bilophila) |
|
| Bacillus spp. | |
| Bifidobacterium | |
| Butyricimonas |
|
| Blautia | |
| Citrobacter sp. |
|
| Collinsella |
|
| Coprococcus_1 |
|
| Corynebacterium |
|
| Clostridium | |
| Clostridium cluster IV, XIVa and XIVb | |
| Dubosiella |
|
| Elusimicrobium |
|
| Enterobacter sp. |
|
| Enterococcus |
|
| Escherichia-Shigella | |
| Eubacterium_coprostanoligenes_group |
|
| Faecalibacterium |
|
| Helicobacter | |
| Klebsiella sp. | Decreased (Ben Ami, 2020) [44] |
| Lactobacillus |
|
| Mucispirilum |
|
| Olsenella |
|
| Oscillibacter | |
| Parabacteroides | |
| Paraprevotella | |
| Pseudomonas sp. |
|
| Pseudoflavonifractor |
|
| Prevotella | |
| Providencia sp. |
|
| Quinella |
|
| Ralstonia sp. |
|
| Roseburia | |
| Ruminococcus | |
| Slackia |
|
| Streptococcus | |
| Suterella spp. |
|
| Treponema | |
| Veillonella |
|
| Species | |
| Adlercreutzia unclassified |
|
| Akkermansia muciniphila |
|
| Clostridiaceae species |
|
| Eubacterium biforme |
|
| Mollicutes species (Tenericutes phylum) |
|
| Prevotellaceae_UCG-001 | |
| Ruminococcaceae_UCG-014 | |
| Ruminococcus gnavus | |
| S24–7 unclassified species |
|
| Unclassified Lactobacillus |
|
| uncultured_bacterium_g_Acinetobacter, |
|
| uncultured_bacterium_o_, Mollicutes_RF39, |
|
| uncultured_bacterium_g_Citrobacter, |
|
| uncultured_bacterium_g_Lactococcus—decreased |
|
| Streptococcus_gallolyticus |
|
3.4.1. Diversity and Richness Analysis
Overall, studies reported that the diversity of the gut microbiota was altered by ionizing radiation. The α diversity, measured by Shannon, Simpson, ACE and/or Chao1 indexes, decreased in most studies that evaluated diversity (13 in 21 studies) [17,19,21,23,25,26,29,32,40,44], and five studies described increases in α diversity.
β diversity was evaluated in 6 studies, and 5 found significant differences [17,23,26,38,39,42].
Fourteen studies described the effect of IR on richness and most demonstrated that ionizing radiation decreases richness, as measured by the number of OTUs/taxa number and richness/Chao1 index [21,23,29,31,32,43]. Two studies reported that the richness and diversity remained unchanged [40,45]. The studies that reported the increase in richness were the studies with flies [43,45].
The study that used the cultured-based method could not assess these parameters, which was expectable [24].
3.4.2. Gut Microbial Composition
Almost all studies reported changes in the microbiota composition after exposure to IR, suggesting that localized irradiation dramatically altered gut microbial composition [17,20,22,25,30,32]. However, the methodology of results reporting was widely variable among them. Some only analyzed alterations at the phylum or genus level, while only three studies analyzed species level [19,26,31]. The qPCR and culture-based studies had limited results of the specific taxa analyzed [18,24,30].
At the phylum level, one of the most consistent findings was the increase of the Proteobacteria following radiation exposure (90% of the studies that reported changes in Proteobacteria relative abundance) [23,25,27,28,29,32,36,37,41]. The most significant increases were found in Lu L et al.’s research (rise of 20%) [25] and in Zhao Z et al.’s (raised from 7.4 to 22.0%) [29]. In Lam V et al.’s research, the abundance increased almost 1000-fold 4 days after 10 Gy of total-body irradiation but then returned to control values [37]. Additionally, the family Desulfovibrionaceae, from the Proteobacteria phylum, showed a significant increase in two studies [17,39].
Contrarily, the relative abundance of Firmicutes decreased in most studies [19,21,22,25,30,36,40,42]. In Li Yiyi et al. study, Firmicutes decreased one week after but increased six weeks after [32]. In another study, the abundance in the large intestine tended to be lower but increased the amount in the small intestine by approximately 18 percentage points [27].
The relative abundance of the phylum Bacteroidetes decreased in four studies, [23,28,32,40] increased in one study [27] and was not significantly affected in another two [36,37].
The ratio Firmicutes to Bacteroidetes decreased in four studies [21,28,38,42] and increased in one study but without significance [34].
The abundance of Verrucomicrobia increased in 75% of the studies [19,27,30,40,41]. Contrarily, in Zhao Z et al.’s research, the abundance decreased from 2.9 to 0.0006% [29].
Finally, the abundance of Actinobacteria decreased in two studies [27,40] and increased in one study [25]. In Li Y et al. research, the abundance decreased one week after exposure and increased six weeks after [32].
At the genus level, the findings were less consistent. Lactobacillus decreased in most studies [18,21,24,28,35] and increased in two studies [27,32]. Four studies showed a decrease in Bacteroides [23,27,30,41] and an increase in two [28,41].
The abundance of Akkermansia increased in three studies [27,30,32]. Alistipes increased in four studies [28,30,41] and decreased in one study [25]. Interestingly, in Kim Y et al.’s research, there was an increase of the genus in the large intestine and a decrease in the small intestine [27].
Bifidobacterium decreased after exposure in three studies [18,26,41]. In the study performed by Yamanouchi et al., a mixed response was found. In the 2 Gy–irradiated group Bifidobacterium presented a decreasing trend from 6 h after irradiation, which continued until 72 h. But the 4 Gy–irradiated group presented an increase of ~10 times after 48 h, reaching 28 times after 72 h [18].
4. Discussion
Animal models are a powerful tool for studying the underlying mechanisms of gut-microbiota-associated diseases and might help to understand the shifts after exposure to ionizing radiation.
This review provides a detailed overview of the pre-clinical studies describing the effect of ionizing radiation on the gut microbiota diversity, richness, and composition of animals. Most studies consist of controlled laboratory assays on small animals, especially on mice.
The mouse and human microbiota are quite similar at the phylum level, with Firmicutes and Bacteroidetes being the most frequent. However, most of the gut composition is unique. At least 85% of the sequences representing genera in mice are not detected in humans [13,51,52], and some important genera that are frequent in humans are not detected in some laboratory mice, such as Faecalibacterium [53]. Nevertheless, animal models provide some relevant insights into the direct effect of radiation, namely for identifying the most radiosensitive bacteria.
These models allow a detailed study of the inflammatory process and of the complex interactions occurring between the host and the intestinal microbiota [51]. Other limitations of animal models include differences in enzyme activity, concentrations of putrefactive products, and immunological activation by the feces content [51,54].
Overall, the analyzed animal experiments confirm that ionizing radiation causes significant changes in gut microbiota composition, diversity and richness.
Interestingly, despite multiple different outcome measures, some concordant results emerged.
Most studies showed a decrease in diversity (especially alpha diversity), the most common finding in dysbiosis, [55] with multiple studies describing lower diversity as being associated with various diseases such as inflammatory bowel disease, [56,57] type 1 diabetes, [58] and obesity [59].
Concerning composition, at the phylum level, the gut microbiota of the analyzed animals was mainly composed of Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria and Verrucomicrobia [60]. Although different results were found throughout the studies, one of the most consistent findings following exposure to ionizing radiation, also observed in human studies [14], was the increased relative abundance of Proteobacteria [23,25,27,28,29,36,37]. The enrichment of Proteobacteria is considered a sign of dysbiosis and has been associated with multiple pathologies, including inflammation [28,29].
The decrease in the relative abundance of both Bacteroidetes [23,28,40] and Firmicutes [19,22,25,30,36,40] was another frequent finding. Like in human studies, four experiments described a decrease in the Firmicutes/Bacteroidetes ratio [21,28,38,42]. The association between these two dominant phyla has been related to several pathological conditions, including obesity [61]. However, the F/B ratio is considered a controversial measure since it only focuses on a high-level taxonomic rank. More recent studies that also analyzed other taxa levels (genus, species, or strain) suggest that the complexity of disease modulation by gut microbiome is much more complex than only an imbalance of these two phyla [62].
Other frequent findings were the increase of the Verrucomicrobia phylum [19,27,30,40,41] of its genus Akkermansia and of the specie Akkermansia muciniphila (A. muciniphila) [26,27,30,32]. Akkermansia is known to have an important value in improving host metabolic functions and immune responses [63], and several studies reported a reduction in the abundance of A. muciniphila in various human diseases, including inflammatory bowel disease, autism, atopy and obesity [64,65].
At the genus level, despite conflicting findings (showing either an increase or a decrease in each genus), the most consistent finding was the decrease in the relative abundance of the genera Bifidobacterium [18,26,40] and Lactobacillus [18,21,24,28,66] well known for their probiotic effects and shown to be beneficial for the host, being used in clinical practice for gastrointestinal diseases [18,67,68]. Lactobacillus has also been linked to an increase in survival rates after IR exposure [69].
The increase in the genus Alistipes was another consistent finding [27,28,30,41]. Alistipes have been seen to have a protective role in multiple diseases, including colitis, autism spectrum disorder and fibrotic liver disorders, but have also been found to contribute to disease [70,71,72].
Regarding other taxa levels, such as order, family, genus, species, or strain, multiple significant findings were found but were dispersed and are summarized in Table 4.
Although gamma rays are typically more energetic than X-rays, so they have a more ionizing effect compared to X-rays, we did not find differences in the effect on gut microbiota.
Concerning the radiation doses, interestingly, Casero et al. found a higher sensitivity of the gut microbiota to lower doses—0.1 and 0.25 Gy as compared to the highest dose—1 Gy, suggesting that at higher doses, DNA repair mechanisms were fully in effect and resulted in a seeming reduction in radiosensitivity [26]. It should be taken into account that some microorganisms are resistant to higher levels of ionizing radiation. Bacterial survival and adaptation to stressors include a complex regulation network, including post-transcriptional regulators, such as small RNAs, which may enhance bacterial resistance to ionizing radiation when adequately combined [73,74].
Ionizing radiation can have significant molecular effects on the gut microbiota, leading to microbial composition, metabolism, and function alterations. The possible molecular effect of ionizing radiation on the gut microbiota is the induction of oxidative stress by generating reactive oxygen and nitrogen species (ROS/RNS) that can damage cellular components and impair the cellular functions of the bacteria. ROS/RNS can also alter the gut microbiota by changing the redox state of the intestinal environment and affecting microbial growth, survival, and metabolism. Another possible major effect of IR on the gut microbiota is the modulation of microbial gene expression that can lead to alterations in microbial metabolism and function [4,5,6,7]. IR can also modify the gut microbiota’s composition by promoting certain microbial species’ growth and suppressing others.
When intestinal inflammation occurs after IR exposure, the oxygen levels are increased. This event leads to an increase in facultative aerobes such as Proteobacteria. It has also been described that oxidative stress actively stimulates the enrichment of Proteobacteria [35]. Unlike obligate anaerobic members of the gut microbiota, the facultative anaerobic can use nitrate, S-oxides and N-oxides as terminal electron acceptors for anaerobic respiration. In the present review, we found that after IR exposure, the relative abundance of the two major groups of anaerobes, Firmicutes and Bacteroidetes, decreased, and the relative abundance of Proteobacteria, an important group of facultative anaerobic bacteria, increased [35,75,76].
The increase in A. muciniphila can be explained by two factors. First, this bacterium can tolerate a small amount of oxygen; additionally, it belongs to the mucin-degrading bacterial family and can generate energy by decomposing mucin secreted by the gut mucosa. A. muciniphila uses mucin as its sole carbon and nitrogen source and produces enzymes that destroy mucin. Due to these facts, when more mucin is present, A. muciniphila has a competitive advantage over other bacteria and increases its relative levels on the local microbiota [64,77].
There were limitations in this review. The primary limitation is that the number of irradiated animals varied greatly across the studies and that most trials had small sample sizes (most studies that exposed mammals to radiation included less than ten animals), [17,18,27] which may condition the study results and their interpretation. The studies with the higher number of animals were those which included flies [43,44,45] and those that analyzed wild rodents exposed to environmental radiation [38,39].
In addition to the inclusion of different types of animals, there were also sex and age differences. Interestingly, one study that analyzed both female and male mice found significant outcome differences between the sexes [20]. Most studies only analyzed males or females; only one other study included both female and male mice and did not refer to differences in the results [17]. More studies including animals of both sexes and addressing possible differences in gut microbiota response to irradiation would be important.
Furthermore, the type, dosage, and duration of radiation exposure varied. Most studies analyzed the effect of acute artificial exposure to low-dose gamma or X-rays. Two studies analyzed acute exposure to high-energy space-type radiation [26,33], and two others analyzed the effect of chronic exposure in contaminated areas near Chornobyl [38,39]. It is known that radiation effects depend on the dose, dose rate, dose fractionation, irradiated volume and type of radiation. The interpretation of results from the different studies should take into consideration the type and characteristics of radiation exposure.
Another factor that should be considered is the method used for microbiota characterization. One study used a cultured-based method, and few used qPCR and primers. The latter has the disadvantage of limiting the information to the selected genera [18,24,30]. Most of the remaining studies chose 16S rRNA sequencing to study gut microbiota’s taxonomic distribution and diversity. In fact, 16S rRNA is a cost-effective semi-quantitative method [2]. Even though it is the most commonly utilized method, 16S rRNA presents some disadvantages. For instance, the identification accuracy depends on the size of the reference database, and the resolution power is only at the species level. However, most of the included studies only analyzed genus levels [78].
Methodologies such as metagenomics, metatranscriptomics, metaproteomics and metabolomics can be used to study functional gut microbiota. Shotgun metagenomics, a quantitative method that provides a large amount of functional information, allows identification at the strain level (low-level taxonomic rank describing genetic variants or species subtypes). However, it is costly and not used frequently in these studies [2].
Most studies used fecal samples. However, despite being the most common sampling method used, it may only partially represent the structure of the whole gut microbiota.
Finally, the time points of feces collection after exposure also varied, and several studies did not evaluate long-term effects [18,25,36]. Most of the studies that had long-term evaluations reported changes immediately after exposure to ionizing radiation but found they were not permanent [18,19,21,23,26,30,37].
5. Conclusions
Animal models allow the investigation of the effect of ionizing radiation without some of the confounding factors and limitations that exist in human studies. The studies included herein demonstrated that dysbiosis occurs after exposure to ionizing radiation. All studies demonstrated shifts in composition, richness, or diversity, highlighting the importance of considering the effects of ionizing radiation exposure on the gut microbiota.
Overall, several limitations were identified as the population, methodology and the reporting of outcomes were highly variable throughout the included studies, which renders comparisons of the multiple findings rather difficult, with multiple conflicting outcome measures.
Despite the mentioned limitations, consistent and convincing evidence was found: diversity and richness are reduced after ionizing radiation exposure. Some consistent findings were also found regarding composition. At the phylum level, Firmicutes and Bacteroidetes’ relative abundance decreased, while Proteobacteria’ and Verrucomicrobia’ increased. At the genus level, Alistipes and Akkermancia increased in most studies, while Lactobacillus decreased. These findings should be further explored and considered, especially when considering the side effects of medical treatments and further embracing prophylactic/therapeutic attitudes.
Notably, significant coincident findings between human and animal studies were found, namely the decrease of alfa diversity and richness; the decrease of the ratio Firmicutes/Bacteroidetes; the decrease of Firmicutes; the increase of Proteobacteria. At the genus level, in most studies, the decrease in Lactobacillus [14].
Importantly, we did not find significant contradictory results. In animal studies, we have relevant results in Verrucomicrobia, Alistipes and Akkermansia, but in human studies, these groups of bacteria were not evaluated.
More extensive, better-designed studies and longer time horizons are needed to better understand and characterize the process and the influence of IR on the gut microbiome.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cimb45050249/s1, Table S1—Risk of Bias of the analyzed interventional studies.
Author Contributions
Conceptualization, A.F., R.S. and P.B.; methodology, A.F. and P.B.; writing—original draft preparation, A.F.; writing—review and editing, A.F., A.O., R.S. and P.B.; supervision, R.S. and P.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kumagai T., Rahman F., Smith A.M. The Microbiome and Radiation Induced-Bowel Injury: Evidence for Potential Mechanistic Role in Disease Pathogenesis. Nutrients. 2018;10:1405. doi: 10.3390/nu10101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch S.V., Pedersen O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 3.Marchesi J.R., Adams D.H., Fava F., Hermes G.D., Hirschfield G.M., Hold G., Quraishi M.N., Kinross J., Smidt H., Tuohy K.M., et al. The gut microbiota and host health: A new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nam Y.D., Kim H.J., Seo J.G., Kang S.W., Bae J.W. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing. PLoS ONE. 2013;8:e82659. doi: 10.1371/journal.pone.0082659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford P.A., Gordon J.I. Microbial regulation of intestinal radiosensitivity. Proc. Natl. Acad. Sci. USA. 2005;102:13254–13259. doi: 10.1073/pnas.0504830102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang A., Ling Z., Yang Z., Kiela P.R., Wang T., Wang C., Cao L., Geng F., Shen M., Ran X., et al. Gut microbial dysbiosis may predict diarrhea and fatigue in patients undergoing pelvic cancer radiotherapy: A pilot study. PLoS ONE. 2015;10:e0126312. doi: 10.1371/journal.pone.0126312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desouky O., Ding N., Zhou G. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015;8:247–254. doi: 10.1016/j.jrras.2015.03.003. [DOI] [Google Scholar]
- 8.Molla M., Panes J. Radiation-induced intestinal inflammation. World J. Gastroenterol. 2007;13:3043–3046. doi: 10.3748/wjg.v13.i22.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leibowitz B.J., Wei L., Zhang L., Ping X., Epperly M., Greenberger J., Cheng T., Yu J. Ionizing irradiation induces acute haematopoietic syndrome and gastrointestinal syndrome independently in mice. Nat. Commun. 2014;5:3494. doi: 10.1038/ncomms4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth C., Tudor G., Tudor J., Katz B.P., MacVittie T.J. Acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys. 2012;103:383–399. doi: 10.1097/HP.0b013e318266ee13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andreyev J. Gastrointestinal complications of pelvic radiotherapy: Are they of any importance? Gut. 2005;54:1051–1054. doi: 10.1136/gut.2004.062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauer-Jensen M., Wang J., Boerma M., Fu Q., Denham J.W. Radiation damage to the gastrointestinal tract: Mechanisms, diagnosis, and management. Curr. Opin. Support. Palliat. Care. 2007;1:23–29. doi: 10.1097/SPC.0b013e3281108014. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen T.L., Vieira-Silva S., Liston A., Raes J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015;8:1–16. doi: 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes A., Oliveira A., Soares R., Barata P. The Effects of Ionizing Radiation on Gut Microbiota, a Systematic Review. Nutrients. 2021;13:3025. doi: 10.3390/nu13093025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins J., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane. 2021. [(accessed on 30 October 2022)]. Available online: www.training.cochrane.org/handbook.
- 16.Hooijmans C.R., Rovers M.M., de Vries R.B.M., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., Dong J., Xiao H., Zhang S., Wang B., Cui M., Fan S. Gut commensal derived-valeric acid protects against radiation injuries. Gut Microbes. 2020;11:789–806. doi: 10.1080/19490976.2019.1709387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamanouchi K., Tsujiguchi T., Sakamoto Y., Ito K. Short-term follow-up of intestinal flora in radiation-exposed mice. J. Radiat. Res. 2019;60:328–332. doi: 10.1093/jrr/rrz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goudarzi M., Mak T.D., Jacobs J.P., Moon B.H., Strawn S.J., Braun J., Brenner D.J., Fornace A.J., Jr., Li H.H. An Integrated Multi-Omic Approach to Assess Radiation Injury on the Host-Microbiome Axis. Radiat. Res. 2016;186:219–234. doi: 10.1667/RR14306.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui M., Xiao H., Li Y., Zhou L., Zhao S., Luo D., Zheng Q., Dong J., Zhao Y., Zhang X., et al. Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol. Med. 2017;9:448–461. doi: 10.15252/emmm.201606932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sittipo P., Pham H.Q., Park C.E., Kang G.U., Zhi Y., Ji H.J., Jang A., Seo H.S., Shin J.H., Lee Y.K. Irradiation-Induced Intestinal Damage Is Recovered by the Indigenous Gut Bacteria Lactobacillus acidophilus. Front. Cell Infect. Microbiol. 2020;10:415. doi: 10.3389/fcimb.2020.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerassy-Vainberg S., Blatt A., Danin-Poleg Y., Gershovich K., Sabo E., Nevelsky A., Daniel S., Dahan A., Ziv O., Dheer R., et al. Radiation induces proinflammatory dysbiosis: Transmission of inflammatory susceptibility by host cytokine induction. Gut. 2018;67:97–107. doi: 10.1136/gutjnl-2017-313789. [DOI] [PubMed] [Google Scholar]
- 23.Liu X., Zhou Y., Wang S., Guan H., Hu S., Huang R., Zhou P. Impact of Low-dose Ionising Radiation on the Composition of the Gut Microbiota of Mice. Toxicol. Sci. 2019;171:258–268. doi: 10.1093/toxsci/kfz144. [DOI] [PubMed] [Google Scholar]
- 24.Johnson L.B., Riaz A.A., Adawi D., Wittgren L., Back S., Thornberg C., Osman N., Gadaleanu V., Thorlacius H., Jeppsson B. Radiation enteropathy and leucocyte-endothelial cell reactions in a refined small bowel model. BMC Surg. 2004;4:10. doi: 10.1186/1471-2482-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu L., Li W., Sun C., Kang S., Li J., Luo X., Su Q., Liu B., Qin S. Phycocyanin Ameliorates Radiation-Induced Acute Intestinal Toxicity by Regulating the Effect of the Gut Microbiota on the TLR4/Myd88/NF-κB Pathway. JPEN J. Parenter Enteral. Nutr. 2020;44:1308–1317. doi: 10.1002/jpen.1744. [DOI] [PubMed] [Google Scholar]
- 26.Casero D., Gill K., Sridharan V., Koturbash I., Nelson G., Hauer-Jensen M., Boerma M., Braun J., Cheema A.K. Space-type radiation induces multimodal responses in the mouse gut microbiome and metabolome. Microbiome. 2017;5:105. doi: 10.1186/s40168-017-0325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y.S., Kim J., Park S.J. High-throughput 16S rRNA gene sequencing reveals alterations of mouse intestinal microbiota after radiotherapy. Anaerobe. 2015;33:1–7. doi: 10.1016/j.anaerobe.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Wang M., Dong Y., Wu J., Li H., Zhang Y., Fan S., Li D. Baicalein ameliorates ionizing radiation-induced injuries by rebalancing gut microbiota and inhibiting apoptosis. Life Sci. 2020;261:118463. doi: 10.1016/j.lfs.2020.118463. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Z., Cheng W., Qu W., Shao G., Liu S. Antibiotic Alleviates Radiation-Induced Intestinal Injury by Remodeling Microbiota, Reducing Inflammation, and Inhibiting Fibrosis. ACS Omega. 2020;5:2967–2977. doi: 10.1021/acsomega.9b03906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W., Hu L., Chang S., Ma L., Li X., Yang Z., Du C., Qu X., Zhang C., Wang S. Total body irradiation-induced colon damage is prevented by nitrate-mediated suppression of oxidative stress and homeostasis of the gut microbiome. Nitric Oxide. 2020;102:1–11. doi: 10.1016/j.niox.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y., Zhang J., Han X., Fan S. Total body irradiation induced mouse small intestine senescence as a late effect. J. Radiat. Res. 2019;60:442–450. doi: 10.1093/jrr/rrz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y., Yan H., Zhang Y., Li Q., Yu L., Li Q., Liu C., Xie Y., Chen K., Ye F., et al. Alterations of the Gut Microbiome Composition and Lipid Metabolic Profile in Radiation Enteritis. Front. Cell Infect. Microbiol. 2020;10:541178. doi: 10.3389/fcimb.2020.541178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raber J., Fuentes Anaya A., Torres E.R.S., Lee J., Boutros S., Grygoryev D., Hammer A., Kasschau K.D., Sharpton T.J., Turker M.S., et al. Effects of Six Sequential Charged Particle Beams on Behavioral and Cognitive Performance in B6D2F1 Female and Male Mice. Front. Physiol. 2020;11:959. doi: 10.3389/fphys.2020.00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong J.Y., Jiang W., Yu X.Q., Wang R., Lu G.H., Gao D.W., Lv Z.W., Li D. Effect of low-dose radiation on thyroid function and the gut microbiota. World J. Gastroenterol. 2022;28:5557–5572. doi: 10.3748/wjg.v28.i38.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheema A.K., Li Y., Singh J., Johnson R., Girgis M., Wise S.Y., Fatanmi O.O., Kaytor M.D., Singh V.K. Microbiome study in irradiated mice treated with BIO 300, a promising radiation countermeasure. Anim. Microbiome. 2021;3:71. doi: 10.1186/s42523-021-00132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rentea R.M., Lam V., Biesterveld B., Fredrich K.M., Callison J., Fish B.L., Baker J.E., Komorowski R., Gourlay D.M., Otterson M.F. Radiation-induced changes in intestinal and tissue-nonspecific alkaline phosphatase: Implications for recovery after radiation therapy. Am. J. Surg. 2016;212:602–608. doi: 10.1016/j.amjsurg.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Lam V., Moulder J.E., Salzman N.H., Dubinsky E.A., Andersen G.L., Baker J.E. Intestinal microbiota as novel biomarkers of prior radiation exposure. Radiat. Res. 2012;177:573–583. doi: 10.1667/RR2691.1. [DOI] [PubMed] [Google Scholar]
- 38.Lavrinienko A., Mappes T., Tukalenko E., Mousseau T.A., Møller A.P., Knight R., Morton J.T., Thompson L.R., Watts P.C. Environmental radiation alters the gut microbiome of the bank vole Myodes glareolus. ISME J. 2018;12:2801–2806. doi: 10.1038/s41396-018-0214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavrinienko A., Tukalenko E., Kesäniemi J., Kivisaari K., Masiuk S., Boratyński Z., Mousseau T.A., Milinevsky G., Mappes T., Watts P.C. Applying the Anna Karenina principle for wild animal gut microbiota: Temporal stability of the bank vole gut microbiota in a disturbed environment. J. Anim. Ecol. 2020;89:2617–2630. doi: 10.1111/1365-2656.13342. [DOI] [PubMed] [Google Scholar]
- 40.Carbonero F., Mayta-Apaza A.C., Yu J.Z., Lindeblad M., Lyubimov A., Neri F., Szilagyi E., Bartholomew A. A comparative analysis of gut microbiota disturbances in the Gottingen minipig and rhesus macaque models of acute radiation syndrome following bioequivalent radiation exposures. Radiat. Environ. Biophys. 2018;57:419–426. doi: 10.1007/s00411-018-0759-0. [DOI] [PubMed] [Google Scholar]
- 41.Carbonero F., Mayta A., Bolea M., Yu J.Z., Lindeblad M., Lyubimov A., Neri F., Szilagyi E., Smith B., Halliday L., et al. Specific Members of the Gut Microbiota are Reliable Biomarkers of Irradiation Intensity and Lethality in Large Animal Models of Human Health. Radiat. Res. 2019;191:107–121. doi: 10.1667/RR14975.1. [DOI] [PubMed] [Google Scholar]
- 42.Kalkeri R., Walters K., Van Der Pol W., McFarland B.C., Fisher N., Koide F., Morrow C.D., Singh V.K. Changes in the gut microbiome community of nonhuman primates following radiation injury. BMC Microbiol. 2021;21:93. doi: 10.1186/s12866-021-02146-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai Z., Yao Z., Li Y., Xi Z., Bourtzis K., Zhao Z., Bai S., Zhang H. Intestinal probiotics restore the ecological fitness decline of Bactrocera dorsalis by irradiation. Evol. Appl. 2018;11:1946–1963. doi: 10.1111/eva.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ben Ami E., Yuval B., Jurkevitch E. Manipulation of the microbiota of mass-reared Mediterranean fruit flies Ceratitis capitata (Diptera: Tephritidae) improves sterile male sexual performance. ISME J. 2010;4:28–37. doi: 10.1038/ismej.2009.82. [DOI] [PubMed] [Google Scholar]
- 45.Woruba D.N., Morrow J.L., Reynolds O.L., Chapman T.A., Collins D.P., Riegler M. Diet and irradiation effects on the bacterial community composition and structure in the gut of domesticated teneral and mature Queensland fruit fly, Bactrocera tryoni (Diptera: Tephritidae) BMC Microbiol. 2019;19:281. doi: 10.1186/s12866-019-1649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bryda E.C. The Mighty Mouse: The impact of rodents on advances in biomedical research. Mo. Med. 2013;110:207–211. [PMC free article] [PubMed] [Google Scholar]
- 47.Bassols A., Costa C., Eckersall P.D., Osada J., Sabrià J., Tibau J. The pig as an animal model for human pathologies: A proteomics perspective. Proteom. Clin. Appl. 2014;8:715–731. doi: 10.1002/prca.201300099. [DOI] [PubMed] [Google Scholar]
- 48.Singh V.K., Olabisi A.O. Nonhuman primates as models for the discovery and development of radiation countermeasures. Expert Opin. Drug Discov. 2017;12:695–709. doi: 10.1080/17460441.2017.1323863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raber J., Yamazaki J., Torres E.R.S., Kirchoff N., Stagaman K., Sharpton T., Turker M.S., Kronenberg A. Combined Effects of Three High-Energy Charged Particle Beams Important for Space Flight on Brain, Behavioral and Cognitive Endpoints in B6D2F1 Female and Male Mice. Front. Physiol. 2019;10:179. doi: 10.3389/fphys.2019.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Claesson M.J., Clooney A.G., O’Toole P.W. A clinician’s guide to microbiome analysis. Nat. Rev. Gastroenterol. Hepatol. 2017;14:585–595. doi: 10.1038/nrgastro.2017.97. [DOI] [PubMed] [Google Scholar]
- 51.Touchefeu Y., Montassier E., Nieman K., Gastinne T., Potel G., Bruley des Varannes S., Le Vacon F., de La Cochetière M.F. Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—Current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 2014;40:409–421. doi: 10.1111/apt.12878. [DOI] [PubMed] [Google Scholar]
- 52.Ley R.E., Bäckhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hugenholtz F., de Vos W.M. Mouse models for human intestinal microbiota research: A critical evaluation. Cell Mol. Life Sci. 2018;75:149–160. doi: 10.1007/s00018-017-2693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imaoka A., Setoyama H., Takagi A., Matsumoto S., Umesaki Y. Improvement of human faecal flora-associated mouse model for evaluation of the functional foods. J. Appl. Microbiol. 2004;96:656–663. doi: 10.1111/j.1365-2672.2004.02189.x. [DOI] [PubMed] [Google Scholar]
- 55.Mosca A., Leclerc M., Hugot J.P. Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front. Microbiol. 2016;7:455. doi: 10.3389/fmicb.2016.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alam M.T., Amos G.C.A., Murphy A.R.J., Murch S., Wellington E.M.H., Arasaradnam R.P. Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut Pathog. 2020;12:1. doi: 10.1186/s13099-019-0341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manichanh C., Rigottier-Gois L., Bonnaud E., Gloux K., Pelletier E., Frangeul L., Nalin R., Jarrin C., Chardon P., Marteau P., et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han H., Li Y., Fang J., Liu G., Yin J., Li T., Yin Y. Gut Microbiota and Type 1 Diabetes. Int. J. Mol. Sci. 2018;19:995. doi: 10.3390/ijms19040995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee C.J., Sears C.L., Maruthur N. Gut microbiome and its role in obesity and insulin resistance. Ann. N. Y. Acad. Sci. 2020;1461:37–52. doi: 10.1111/nyas.14107. [DOI] [PubMed] [Google Scholar]
- 60.Chu M., Zhang X. Bacterial Atlas of Mouse Gut Microbiota. Cell. Microbiol. 2022;2022:5968814. doi: 10.1155/2022/5968814. [DOI] [Google Scholar]
- 61.Magne F., Gotteland M., Gauthier L., Zazueta A., Pesoa S., Navarrete P., Balamurugan R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients. 2020;12:1474. doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tseng C.H., Wu C.Y. The gut microbiome in obesity. J. Formos. Med. Assoc. 2019;118((Suppl. S1)):S3–S9. doi: 10.1016/j.jfma.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 63.Zhang T., Li Q., Cheng L., Buch H., Zhang F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019;12:1109–1125. doi: 10.1111/1751-7915.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Derrien M., Belzer C., de Vos W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017;106:171–181. doi: 10.1016/j.micpath.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Wang L., Christophersen C.T., Sorich M.J., Gerber J.P., Angley M.T., Conlon M.A. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl. Environ. Microbiol. 2011;77:6718–6721. doi: 10.1128/AEM.05212-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Z., Wang Q., Wang X., Zhu L., Chen J., Zhang B., Chen Y., Yuan Z. Gut microbial dysbiosis is associated with development and progression of radiation enteritis during pelvic radiotherapy. J. Cell Mol. Med. 2019;23:3747–3756. doi: 10.1111/jcmm.14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheikh Sajjadieh M.R., Kuznetsova L.V., Bojenko V.B. Dysbiosis in ukrainian children with irritable bowel syndrome affected by natural radiation. Iran J. Pediatr. 2012;22:364–368. [PMC free article] [PubMed] [Google Scholar]
- 68.García-Peris P., Velasco C., Lozano M.A., Moreno Y., Paron L., de la Cuerda C., Bretón I., Camblor M., García-Hernández J., Guarner F., et al. Effect of a mixture of inulin and fructo-oligosaccharide on Lactobacillus and Bifidobacterium intestinal microbiota of patients receiving radiotherapy: A randomised, double-blind, placebo-controlled trial. Nutr. Hosp. 2012;27:1908–1915. doi: 10.3305/nh.2012.27.6.5992. [DOI] [PubMed] [Google Scholar]
- 69.Rosli D., Shahar S., Manaf Z.A., Lau H.J., Yusof N.Y.M., Haron M.R., Majid H.A. Randomized controlled trial on the effect of partially hydrolyzed guar gum supplementation on diarrhea frequency and gut microbiome count among pelvic radiation patients. JPEN J Parenter Enteral Nutr. 2021;45(2):277–286. J. Parenter. Enter. Nutr. 2021;46:475. doi: 10.1002/jpen.1987. [DOI] [PubMed] [Google Scholar]
- 70.Parker B.J., Wearsch P.A., Veloo A.C.M., Rodriguez-Palacios A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020;11:906. doi: 10.3389/fimmu.2020.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dziarski R., Park S.Y., Kashyap D.R., Dowd S.E., Gupta D. Pglyrp-Regulated Gut Microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii Enhance and Alistipes finegoldii Attenuates Colitis in Mice. PLoS ONE. 2016;11:e0146162. doi: 10.1371/journal.pone.0146162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feng Q., Liang S., Jia H., Stadlmayr A., Tang L., Lan Z., Zhang D., Xia H., Xu X., Jie Z., et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat. Commun. 2015;6:6528. doi: 10.1038/ncomms7528. [DOI] [PubMed] [Google Scholar]
- 73.Villa J.K., Han R., Tsai C.-H., Chen A., Sweet P., Franco G., Vaezian R., Tkavc R., Daly M.J., Contreras L.M. A small RNA regulates pprM, a modulator of pleiotropic proteins promoting DNA repair, in Deinococcus radiodurans under ionizing radiation. Sci. Rep. 2021;11:12949. doi: 10.1038/s41598-021-91335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Šiková M., Janoušková M., Ramaniuk O., Páleníková P., Pospíšil J., Bartl P., Suder A., Pajer P., Kubičková P., Pavliš O., et al. Ms1 RNA increases the amount of RNA polymerase in Mycobacterium smegmatis. Mol. Microbiol. 2019;111:354–372. doi: 10.1111/mmi.14159. [DOI] [PubMed] [Google Scholar]
- 75.Winter S.E., Winter M.G., Xavier M.N., Thiennimitr P., Poon V., Keestra A.M., Laughlin R.C., Gomez G., Wu J., Lawhon S.D., et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2014;339:708–711. doi: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morgan X.C., Tickle T., Sokol H., Gevers D., Devaney K.L., Ward D.V., A Reyes J., A Shah S., Leleiko N., Snapper S.B., et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim S., Shin Y.-C., Kim T.-Y., Kim Y., Lee Y.-S., Lee S.-H., Kim M.-N., O E., Kim K.S., Kweon M.-N. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes. 2021;13:1–20. doi: 10.1080/19490976.2021.1892441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sankar S.A., Lagier J.C., Pontarotti P., Raoult D., Fournier P.E. The human gut microbiome, a taxonomic conundrum. Syst. Appl. Microbiol. 2015;38:276–286. doi: 10.1016/j.syapm.2015.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.