Abstract
Interleukin-3 (IL-3), IL-5, and granulocyte-macrophage colony-stimulating factor regulate the survival, proliferation, and differentiation of hematopoietic lineages. Phosphatidylinositol 3-kinase (PI3K) has been implicated in the regulation of these processes. Here we investigate the molecular mechanism by which PI3K regulates cytokine-mediated proliferation and survival in the murine pre-B-cell line Ba/F3. IL-3 was found to repress the expression of the cyclin-dependent kinase inhibitor p27KIP1 through activation of PI3K, and this occurs at the level of transcription. This transcriptional regulation occurs through modulation of the forkhead transcription factor FKHR-L1, and IL-3 inhibited FKHR-L1 activity in a PI3K-dependent manner. We have generated Ba/F3 cell lines expressing a tamoxifen-inducible active FKHR-L1 mutant [FKHR-L1(A3):ER*]. Tamoxifen-mediated activation of FKHR-L1(A3):ER* resulted in a striking increase in p27KIP1 promoter activity and mRNA and protein levels as well as induction of the apoptotic program. The level of p27KIP1 appears to be critical in the regulation of cell survival since mere ectopic expression of p27KIP1 was sufficient to induce Ba/F3 apoptosis. Moreover, cell survival was increased in cytokine-starved bone marrow-derived stem cells from p27KIP1 null-mutant mice compared to that in cells from wild-type mice. Taken together, these observations indicate that inhibition of p27KIP1 transcription through PI3K-induced FKHR-L1 phosphorylation provides a novel mechanism of regulating cytokine-mediated survival and proliferation.
Cytokines of the interleukin-3 (IL-3)/IL-5/granulocyte-macrophage colony-stimulating factor (GM-CSF) family are important regulators of proliferation, differentiation and effector functions of various hematopoietic cell lineages and their precursors (2, 15). IL-3 and GM-CSF regulate the proliferation and survival of multiple hematopoietic lineages, whereas IL-5 has a more restricted role in the differentiation of eosinophils and basophils, as well as of murine B cells (15, 51). Phosphatidylinositol 3-kinase (PI3K) and its downstream target protein kinase B (PKB) have been linked to regulation of proliferation and survival in a variety of hematopoietic systems (14, 16, 26). PI3K activity is negatively regulated by the PTEN phosphatase, which specifically dephosphorylates the D3 position of phosphatidylinositol, thus inhibiting the action of PI3K (22, 36, 50, 62). Several mechanisms have been proposed to explain the requirement for PI3K activity in cytokine-mediated cell survival. For example, IL-3 regulates PKB-induced phosphorylation of the proapoptotic Bcl-2 family member BAD, inhibiting its proapoptotic activity (14, 16). However, it has recently been shown that this phosphorylation does not correlate well with cell survival (24). Another target of PKB possibly accounting for its antiapoptotic effect is the apoptotic protease caspase-9, which is inactivated upon phosphorylation by PKB (9). However, this phosphorylation site is not evolutionarily conserved (18), leaving its relevance in vivo to be demonstrated. More recently, PKB was demonstrated to be involved in negatively regulating the activity of the forkhead family of transcription factors, which can mediate apoptosis as well as proliferation (6, 30, 52).
To identify a potential mechanism by which PI3K could exert its proliferative and antiapoptotic effects, we focused on cyclin-dependent kinase (CDK) inhibitor p27KIP1. Upregulation of p27KIP1 is linked to cell cycle arrest in G0/G1 through its interaction with CDK-cyclin complexes (53). Regulation of p27KIP1 levels has been described as occurring predominantly posttranslationally, by cyclin E-CDK2-mediated phosphorylation, which subsequently targets p27KIP1 for degradation by the proteasome (23, 46, 53, 55). p27KIP1 in turn also inhibits cyclin E-CDK2 complexes, suggesting that the balance of p27KIP1 and cyclin E-CDK2 is important for G1 progression. Mitogens upregulate cyclin D levels, subsequently sequestering away p27KIP1 from cyclin E-CDK2 complexes and thereby activating these complexes (11). Interestingly, p27KIP1 has also been implicated in the regulation of immunoglobulin M (IgM)-induced B-cell apoptosis, which can be rescued by CD40 ligand engagement (17, 61). The exact mechanism by which cytokines are able to regulate p27KIP1 levels and what the importance of this is for mediating its proliferative and antiapoptotic effects in hematopoietic cells are largely unknown.
Here we show that an important means by which cytokine-mediated proliferation and survival are regulated is through downregulation of p27KIP1. Transcriptional induction of p27KIP1 is regulated by the forkhead-related transcription factor FKHR-L1. Activation of FKHR-L1 is sufficient to elevate p27KIP1 mRNA and protein levels, as well as to induce apoptosis. Importantly, apoptosis of bone marrow-derived hematopoietic stem cells from p27KIP1 null-mutant mice is decreased upon cytokine withdrawal compared to that of cells from wild-type mice, demonstrating the importance of regulating p27KIP1 levels in vivo for cell survival. Our data provide a novel mechanism by which cytokines can both regulate cell cycle progression and inhibit apoptosis by the PI3K-PKB-mediated downregulation of p27KIP1. We propose that the regulation of p27KIP1 transcription by forkhead-related transcription factors may be a general mechanism by which hematopoietic cells can respond appropriately to their environmental conditions, resulting in survival, proliferation, or differentiation.
MATERIALS AND METHODS
Cell culture.
Ba/F3 cells were cultured in RPMI 1640 supplemented with 8% Hyclone serum (Gibco) and recombinant mouse IL-3 produced in COS cells (8). Peripheral blood eosinophils from healthy volunteers obtained from the Blood Bank (Utrecht, The Netherlands) were isolated as described previously (29). Fetal liver-derived myeloid cultures were prepared from day-17 mouse embryos by culture of suspension cells in RPMI 1640 supplemented with IL-3, IL-6, and stem cell factor (SCF) as previously described (45).
Bone marrow cells were flushed out of mouse femurs and resuspended in Iscove's modified Eagle medium containing 20% Myclone Super Plus fetal calf serum, and red blood cells were lysed by diluting them 1:1 with acetic acid-phosphate-buffered saline (PBS). Sca1-positive cells were isolated using Sca1 antibody microbeads (Miltenyi, Gladbach, Germany). Cells were cultured for 5 days in medium supplemented with murine IL-3, IL-6, SCF (R&D, Abingdon, United Kingdom) before analyzing apoptosis upon cytokine withdrawal. Twenty-four hours after cytokine withdrawal, cells were washed with ice-cold PBS, resuspended in binding buffer (10 mM HEPES [pH 7.4], 140 mM NaCl, 2.5 mM CaCl2). Cells were then incubated with fluorescein isothiocyanate-conjugated annexin-V for 10 min at room temperature, washed, and resuspended in binding buffer containing 1 μg of propidium iodide (PI)/ml, and fluorescence was analyzed by fluorescence-activated cell sorter (FACS).
Reagents and antibodies.
LY294002, PD098059, and SB203580 were from Alexis (San Diego, Calif.). Rapamycin was a kind gift from N. Lomax from the Drug Synthesis and Chemistry Branch of the National Cancer Institute (Bethesda, Md.). pRC-p27KIP1 (mouse) was a kind gift from R. Bernards (Netherlands Cancer Institute, Amsterdam, The Netherlands), and spectrin-linked green fluorescent protein (GFP) was a kind gift from A. Beavis and T. Sheck (Princeton, N.J.) and has been described previously (25). myrPKB:ER* was a kind gift from A. Klippel (Chiron Corporation, Emeryville, Calif.). pSG5-mycPTENcaax was obtained by PCR amplification of PTEN from human neutrophil cDNA and shuttling through pGEM-T_caax (33) before subsequent cloning into pSG5. FKHR-L1 constructs were a kind gift from M. E. Greenberg (Boston, Mass.) (6); pCDNA3-FKHR-L1(A3):ER* was generated by cloning FKHR-L1(A3) without the stop codon into a pCDNA3 vector containing the hormone-binding domain of the estrogen receptor (pCDNA3-ER). Constructs for hemagglutinin (HA)-PKB, gagPKB, cyclin D1, cyclin D1 promoter, kinase-dead CDK4, and the low-affinity nerve growth factor receptor (LNGFR) have been described previously (7, 37, 38). The pGL2-p27KIP1 luciferase promoter construct (31) was a kind gift from I. P. Touw (Erasmus University, Rotterdam, The Netherlands). Histone H1 and actinomycin D were purchased from Sigma, and protein A-agarose was purchased from Boehringer GmbH (Mannheim, Germany). p27KIP1 and RACK1 monoclonal antibodies were purchased from Transduction Laboratories (Lexington, Kentucky). PKB, cyclin E, CDK2, ERK1, and ERK2 antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, Calif.). Phospho-Ser473 PKB antibodies were from New England Biolabs (Beverly, Mass.), while FKHR-L1 and phospho-Thr32 FKHR-L1 were from Upstate Biotechnology, Inc. (Lake Placid, N.Y.).
Western blotting.
For the detection of p27KIP1, cells were lysed in Lowry sample buffer and the protein concentration was determined as described previously (39). Equal amounts of each protein sample were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting with p27KIP1 antibody. Blots were subsequently probed with RACK1 antibody (or ERK1 and -2 for eosinophils) to confirm equal protein loading. For the analysis of CDK2 levels, equal amounts of protein of cells lysed in ELB buffer (39) together with inhibitors (see the description of kinase assays below) were analyzed in parallel with cyclin E-associated kinase activity. For detection with phosphospecific antibodies, cells were lysed in ELB buffer together with inhibitors and equal amounts of protein were run on gel, blotted, and probed with phosphospecific antibodies.
Northern blotting.
Ba/F3 cells were cultured with IL-3 and then starved for various times or were starved for IL-3 overnight and subsequently stimulated with IL-3. In some experiments cells were cultured with IL-3 and 4-hydroxy tamoxifen (4-OHT) was added. Total RNA was isolated as described previously (47). Twenty micrograms of total RNA was used for Northern blotting and hybridized with a p27KIP1 probe consisting of full-length p27KIP1 cDNA. Equal RNA loading was verified by stripping and reprobing the blots with a 1.4-kb cDNA fragment of the human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene.
Apoptosis and proliferation assays.
For apoptosis assays cells were counted, washed twice with PBS, resuspended in RPMI 1640 containing 8% Hyclone, and seeded in 24-well dishes (0.4 × 106 cells per well). After 2 h inhibitors were added, and after a further 30 min cytokines were added. After 48 h cells were harvested, washed twice in PBS, and fixed for at least 2 h in 300 μl of PBS–700 μl of ethanol. Cells were spun down gently and permeabilized in 200 μl of 0.1% Triton X-100–0.045 M Na2HPO4–0.0025 M sodium citrate at 37°C for 20 min. Next, 750 μl of apoptosis buffer (0.1% Triton X-100, 10 mM PIPES [piperazine-N,N′-bis{2-ethanesulfonic acid}], 2 mM MgCl2, 40 μg of RNase/ml, 20 μg of propidium iodide/ml) was added, and cells were incubated for 30 min in the dark. The percentage of apoptotic cells was analyzed by FACS as the percentage of cells (10,000 cells counted) with a DNA content of <2N. Thresholds were set to gate out cellular debris. For Ba/F3 cells transfected with GFP-spectrin, 5,000 GFP-positive cells were analyzed. Cell cycle profiles were determined using a FACScalibur (Becton Dickson, Mountain View, Calif.) and analyzed using Cell Quest and MofFit software. For cell proliferation assays Ba/F3 cells were seeded in 24-well dishes (0.1 × 106 cells per well) together with IL-3 with or without inhibitors and the viable cells were counted every 24 h by trypan blue exclusion.
Transient electroporations and generation of stable cell lines.
For transient transfection, Ba/F3 cells were electroporated (0.28 kV; capacitance, 960 μF) and 2 h after electroporation dead cells were removed by separation through a Ficoll gradient (2,500 rpm for 20 min). Cells were harvested 24 h after electroporation and analyzed by FACS as described above. For the generation of polyclonal transfectants constructs were electroporated into Ba/F3 cells together with pSG5 conferring neomycin resistance and maintained in 500 μg of G418 (Boehringer GmbH)/ml in the presence of IL-3. Clonal cell lines were generated by limited dilution, and results shown are representative of at least two separate clones. For the analysis of p27KIP1 levels in cells transiently overexpressing p27KIP1, cells were electroporated together with LNGFR as a marker (37). Dead cells were removed, and LNGFR-expressing cells were separated using monoclonal LNGFR antibody 20.4 (37) and goat anti-mouse microbeads (Miltenyi Biotech). Equal protein concentrations were analyzed by p27KIP1 Western blotting.
Cyclin E-CDK2 kinase assays.
Cyclin E-associated kinase activity was determined as described previously (39), using histone H1 as a substrate. CDK2 levels were determined in parallel by Western blotting.
Luciferase assays.
Ba/F3 cells were electroporated with the pGL2-p27KIP1 luciferase promoter construct (31), a pGL2 thymidine kinase luciferase construct or a pGL2 control luciferase construct, the internal transfection control (pRL-TK; Promega), and expression plasmids. Twenty-four hours after transfection cells were harvested and luciferase activity was measured. Values were corrected for transfection efficiency and growth and represent the means of at least three independent experiments (± standard errors of the means).
RESULTS
Signaling pathways regulating cytokine-mediated proliferation and survival.
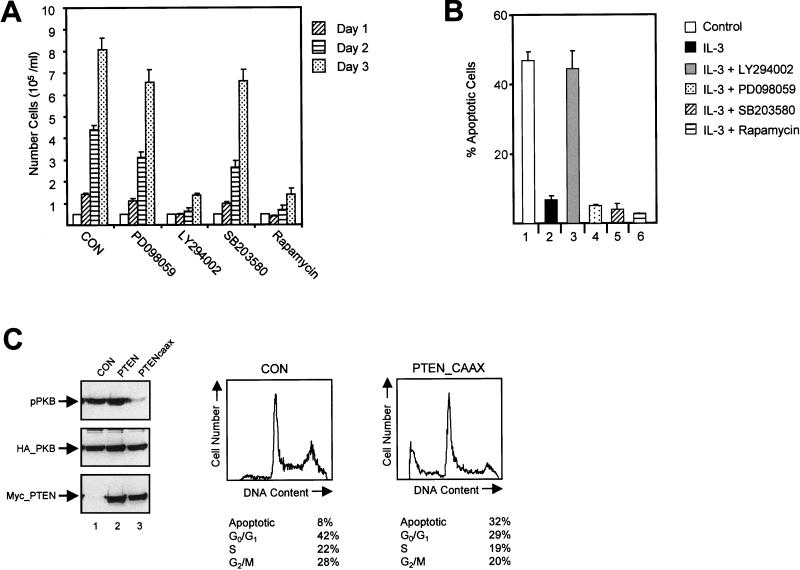
Lymphoid and myeloid lineages require cytokines and growth factors to both induce cell division and act as survival factors. The mouse pre-B-cell line Ba/F3 requires IL-3 to proliferate as well as to overcome a default apoptotic program. To define signaling pathways critically involved in mediating the proliferative response to IL-3, we analyzed the effect of various pharmacological inhibitors on Ba/F3 cells cultured with IL-3. Cells were cultured for 72 h, and the number of trypan blue-excluding cells was determined every 24 h. Proliferation was not affected when the cells were cultured with IL-3 in the presence of mitogen-activated protein kinase (MAPK) kinase inhibitor PD098059 (1) or with SB203580, an inhibitor of p38 MAPK (13), indicating that the proliferative response is not affected by inhibition of MAPKs (Fig. 1A). Activation of ERK and p38 kinases was potently inhibited under these conditions (data not shown). IL-3-dependent proliferation was profoundly inhibited when the cells were cultured in the presence of either PI3K inhibitor LY294002 (56) or rapamycin, an inhibitor of the activation of p70S6K, a target of PI3K signaling.
FIG. 1.
Regulation of IL-3-mediated proliferation and survival. (A) Ba/F3 cells were cultured in the presence of IL-3 without inhibitors or with PD098059 (50 μM), LY294002 (10 μM), SB203580 (10 μM), or rapamycin (20 ng/ml), and cells were counted every 24 h as indicated. (B) Ba/F3 cells were cultured in the absence of IL-3 (bar 1) or in the presence of IL-3 either alone (bar 2) or with LY294002 (10 μM; bar 3) PD098059 (50 μM; bar 4), SB203580 (10 μM; bar 5), or rapamycin (20 ng/ml; bar 6), and the percentages of apoptotic cells were determined after 48 h. (C, left) COS cells were transfected with 8 μg of either the empty vector or the myc-PTEN or myc-PTENcaax vector together with 2 μg of the HA-PKB vector. HA-PKB was immunoprecipitated with an HA antibody (12CA5) and analyzed for activity by immunoblotting with phospho-Ser473 PKB antibody (top). Expression of HA-PKB and mycPTEN was verified by immunoblotting with either 12CA5 (middle) or myc antibody (9E10; bottom). (Right) Ba/F3 cells were electroporated with 2 μg of the spectrin-GFP vector together with either 18 μg of empty vector (pSG5) or 18 μg of the myc-tagged PTENcaax vector. Dead cells were removed 2 h after electroporation by separation through a Ficoll gradient. Twenty-four hours after electroporation cells were fixed and stained with PI, and the DNA content of 5,000 GFP-positive cells was analyzed by FACS. The data depicted are representative of several independent experiments.
To determine whether the inhibition of proliferation may be due to a decrease in cell survival, we analyzed the effect of pharmacological inhibitors on apoptosis. For this purpose we used FACS analysis of PI-labeled cells and marked cells containing less than 2N DNA content as apoptotic. These results were also confirmed by DNA laddering (data not shown). As expected, addition of PD098059 or SB203580 did not affect cell survival, implying no significant role for MAPKs in the regulation of apoptosis (Fig. 1B). However, IL-3-induced rescue from apoptosis was abrogated when cells were incubated with LY294002. Although rapamycin could efficiently block proliferation, it had no effect on IL-3-mediated rescue from apoptosis, demonstrating that inhibition of cell cycle progression is in itself insufficient to initiate the apoptotic program. Identical results were also found in 32D cells, a murine IL-3-dependent cell line, cultured with IL-3 (data not shown).
To exclude aspecific effects introduced by using pharmacological inhibitors of PI3K, we developed a novel inhibitory tool using the 3-phosphatidylinositol lipid phosphatase PTEN. Although the mechanisms of PTEN regulation are unclear, regulation by membrane localization has been suggested by the recent analysis of its crystal structure (32). We generated a PTEN construct containing a C-terminal CAAX box derived from Ki-Ras (33), resulting in constitutive membrane association (PTENcaax). In contrast to what was found for wild-type PTEN, phosphorylation of PKB was largely abrogated upon expression of this construct, demonstrating that PTENcaax is capable of potently inhibiting PI3K activity (Fig. 1C; left, lane 3). To analyze whether PTEN could affect cytokine-mediated rescue from apoptosis, we electroporated cells with PTEN expression vectors. We observed a minor increase in apoptosis in Ba/F3 cells overexpressing wild-type PTEN (data not shown). Ba/F3 cells ectopically expressing PTENcaax exhibited a much higher percentage of apoptosis than control Ba/F3 cells expressing GFP-spectrin alone (Fig. 1C; right). This observation clearly demonstrates the importance of PI3K-generated phosphatidylinositol lipids for cell survival.
p27KIP1 protein levels correlate with induction of apoptosis.
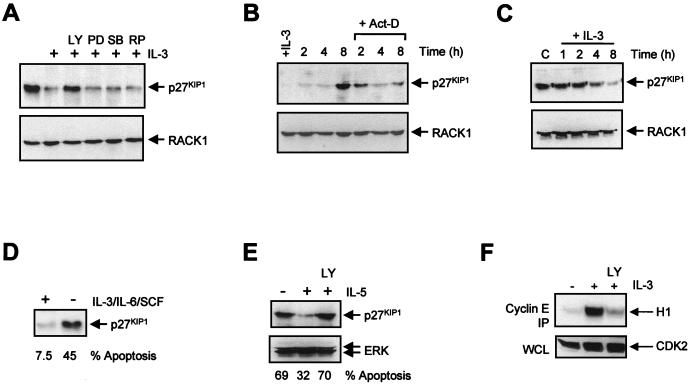
The CDK inhibitor (CKI) p27KIP1 is the only CKI whose expression declines upon mitogenic stimulation, as demonstrated for IL-2 and platelet-derived growth factor (42, 58, 60). Upregulation of p27KIP1 levels has been correlated not only with a decrease in proliferation but also with induction of apoptosis, suggesting that PI3K activity might be associated with a decrease in p27KIP1 levels. To determine whether IL-3 can regulate p27KIP1 levels, Ba/F3 cells were cultured with or without IL-3 and after 24 h the level of p27KIP1 expression was determined by Western blotting. Equal protein loading was confirmed by probing the blot with a RACK1 antibody. Cells cultured without cytokines or with IL-3 in the presence of LY294002 exhibited a significant increase in p27KIP1 expression, whereas inhibition of ERK MAPK, p38 MAPK, or p70S6K had no significant effect (Fig. 2A), correlating with a lack of effect of these inhibitors on apoptosis. Expression of another CKI, p21CIP1, was unaffected (data not shown), suggesting that upregulation of p27KIP1 upon induction of apoptosis may be specific for this CKI.
FIG. 2.
Upregulation of p27KIP1 protein levels correlates with apoptosis. (A) Ba/F3 cells were cultured overnight in the absence or presence of IL-3 without inhibitors or with LY294002 (LY; 10 μM), PD098059 (PD; 50 μM), SB203580 (SB; 10 μM), or rapamycin (RP; 20 ng/ml). Equal amounts of protein were loaded, and the levels of p27KIP1 (top) and RACK1 (bottom) were determined by immunoblotting as described in Materials and Methods. (B) Ba/F3 cells were cultured overnight with IL-3 and cytokine starved for the indicated times in the presence or absence of actinomycin D (5 μg/ml), and p27KIP1 levels were analyzed as for panel A. (C) Ba/F3 cells were cytokine starved overnight and were stimulated with IL-3 for the indicated times, and levels of p27KIP1 were analyzed as for panel A. (D) Mouse fetal liver cultures were treated with or without cytokines for 24 h. The percentages of apoptotic cells were measured, and equal amounts of protein were analyzed for p27KIP1 expression. (E) Human peripheral blood eosinophils were cultured without cytokines, with IL-5, or with IL-5 and LY294002 (10 μM). Equal amounts of protein were analyzed for levels of p27KIP1 (top) or ERK1 and -2 (bottom) by Western blotting. The percentages of apoptotic cells are shown below. (F) Ba/F3 cells were either cytokine starved or cultured with IL-3 or IL-3 together with LY294002 (10 μM) overnight, equal amounts of protein were immunoprecipitated (IP) with cyclin E antibody, and associated kinase activity was analyzed (top). Equal protein loading was verified by analyzing CDK2 expression (bottom). WCL, whole-cell lysate.
Next, we wished to determine the kinetics by which p27KIP1 levels changed upon IL-3 withdrawal and the role of transcription therein. Cells were treated with or without the transcription inhibitor actinomycin D, and IL-3 was withdrawn. Levels of p27KIP1 increased after IL-3 withdrawal, which precedes induction of the apoptotic program in these cells (data not shown). However, this increase was completely blocked in cells treated with actinomycin D (Fig. 2B), indicating that transcriptional regulation is important for elevating p27KIP1 levels following IL-3 withdrawal. Addition of IL-3 to cells that were cytokine starved overnight resulted in a decrease in p27KIP1 levels (Fig. 2C). To determine if cytokine-mediated regulation of p27KIP1 levels is a more general phenomenon, we analyzed primary mouse fetal liver cells cultured in the presence or absence of survival factors (45). Indeed, in cells cultured without cytokines a striking increase in p27KIP1 levels also correlated with an induction of apoptosis (Fig. 2D).
These data raise the possibility that repression of p27KIP1 levels through cytokine-mediated PI3K activation is required for cell survival. To separate a role for p27KIP1 in survival from its role in proliferation, we utilized freshly isolated peripheral blood human eosinophils. Since these terminally differentiated quiescent cells no longer divide, any regulation of p27KIP1 will be independent of cellular proliferation. Again, either removal of the cytokine or inhibition of PI3K resulted in both a decrease in cell survival and an induction of p27KIP1 (Fig. 2E). We could not detect any expression of the CKI p21CIP1 in these cells (data not shown), suggesting a specific function of p27KIP1 distinct from the regulation of cellular proliferation.
Finally, to determine if the increased levels of p27KIP1 were indeed functional, we analyzed whether this increase resulted in a decrease in cyclin E-associated kinase activity. In cells cultured without IL-3, little cyclin E-associated CDK2 activity was observed (Fig. 2F, top). Similarly, addition of LY294002 substantially blocked cyclin E-associated CDK2 activity, correlating with an increase in p27KIP1 levels. Together these data demonstrate that PI3K represses the expression of functional p27KIP1 and that this strongly correlates with cellular survival.
IL-3 downregulates p27KIP1 mRNA levels in a PI3K-dependent manner.
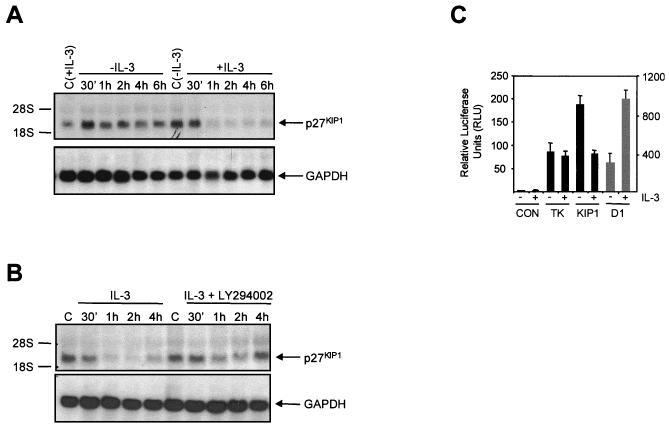
The regulation of p27KIP1 protein expression by phosphorylation, resulting in its degradation by the ubiquitin system, has been extensively studied (41, 54). As upregulation of p27KIP levels upon IL-3 withdrawal was completely abrogated by inhibiting transcription, we investigated whether IL-3 is also capable of regulating p27KIP1 mRNA levels. We observed a very rapid upregulation of p27KIP1 mRNA upon IL-3 withdrawal, whereas addition of IL-3 rapidly downregulated p27KIP1 mRNA (Fig. 3A). To establish a potential role for PI3K in downregulating p27KIP1 mRNA, cytokine-starved Ba/F3 cells were either left untreated or were preincubated with LY294002 before IL-3 stimulation. In agreement with the findings for p27KIP1 protein expression, p27KIP1 mRNA expression was also dependent on PI3K activity, since preincubation with LY294002 was found to significantly abrogate downregulation of p27KIP1 mRNA expression by IL-3 (Fig. 3B).
FIG. 3.
Cytokine-mediated regulation of p27KIP1 transcription requires PI3K. (A) Ba/F3 cells were either IL-3 starved or IL-3 starved overnight and subsequently stimulated with IL-3 for the indicated times. Twenty micrograms of total RNA was used for Northern blotting and hybridized with a p27KIP1 probe (top). Equal RNA loading was verified by GAPDH reprobing (bottom). (B) Ba/F3 cells were IL-3 starved overnight and restimulated with IL-3 for the indicated times with or without preincubation with LY294002 (10 μM) and analyzed as for panel A. (C) Ba/F3 cells were electroporated with 10 μg of either pGL2 (CON), pGL2-TK (TK), pGL2-p27KIP1 (KIP1), or cyclin D1 (D1) luciferase constructs together with 500 ng of tk-renilla plasmid, cultured with or without IL-3 for 24 h, and luciferase activity was analyzed as described in Materials and Methods.
In addition to analyzing p27KIP1 mRNA, we also examined p27KIP1 promoter regulation by IL-3, utilizing a p27KIP1 promoter luciferase construct (31). In agreement with the upregulation of p27KIP1 mRNA in cells cultured without IL-3, p27KIP1 promoter activity was upregulated in cytokine-starved cells compared to that in cells cultured with IL-3 (Fig. 3C). Addition of LY294002 inhibited IL-3-mediated downregulation of p27KIP1 luciferase activity (data not shown). Luciferase activity of control plasmids was unaltered upon IL-3 addition, whereas cyclin D1 promoter activity was upregulated. These data indicate that IL-3 represses p27KIP1 transcription in a PI3K-dependent fashion.
FKHR-L1 is inhibited by PI3K-PKB and elevates p27KIP1 promoter activity.
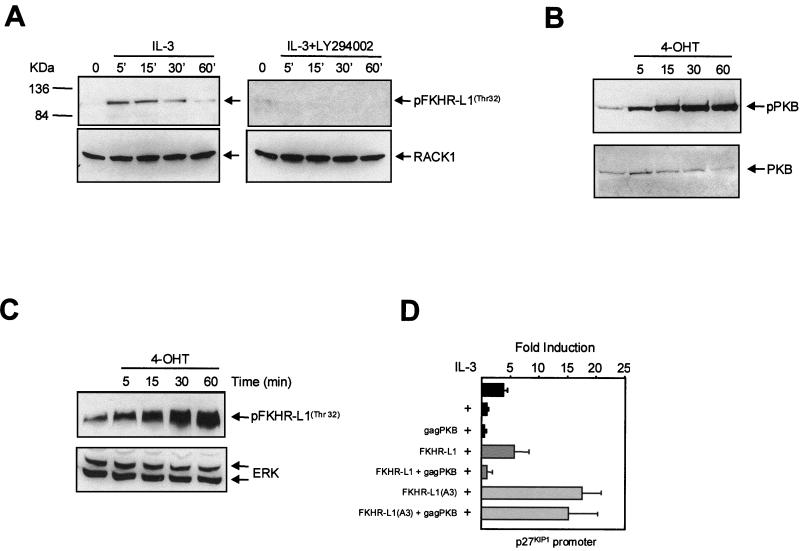
The data obtained so far raise the possibility that PI3K activity results in inactivation of a transcription factor responsible for p27KIP1 transcription. To identify a possible molecular mechanism by which PI3K could regulate p27KIP1 transcription, we focused on the forkhead-related transcription factor FKHR-L1, which has recently been identified as a target of PI3K signaling (6). The activity of FKHR-L1 is inhibited upon phosphorylation by PKB, resulting in nuclear exclusion (6). First we analyzed whether IL-3 could regulate the activity of this transcription factor in PI3K-dependent manner. Indeed, IL-3 stimulation resulted in a rapid transient phosphorylation of endogenous FKHR-L1 (Fig. 4A, left), whereas preincubation of cells with LY294002 completely abrogated this phosphorylation (Fig. 4A, right).
FIG. 4.
Analysis of FKHR-L1 phosphorylation and activity in Ba/F3 cells. (A) Ba/F3 cells were cytokine starved and stimulated with IL-3 for the indicated times (left) or pretreated with LY294002 (10 μM) for 20 min prior to IL-3 stimulation (right). FKHR-L1 phosphorylation was analyzed using a FKHR-L1(Thr32)-specific antibody (top). Equal protein loading was verified by RACK1 reprobing (bottom). (B) Ba/F3 cells stably expressing myrPKB:ER* were cytokine starved overnight and stimulated with 4-OHT (100 nM) for the indicated times. Phosphorylated myrPKB-ER was analyzed using a PKB(Ser473)-specific antibody (top). Equal PKB levels were verified by reprobing the blot with a PKB antibody (bottom). (C) Ba/F3 cells stably expressing myrPKB:ER* were cytokine starved overnight and stimulated with 4-OHT (100 nM) for the indicated times, and FKHR-L1 phosphorylation was analyzed using an FKHR-L1(Thr32)-specific antibody (top). (D) Ba/F3 cells were electroporated with 12 μg of p27KIP1 luciferase construct together with 4 μg of pSG5-gagPKB, FKHR-L1(wt), or FKHR-L1(A3) or combinations thereof as indicated. The DNA concentration was adjusted to 20 μg with pSG5. Cells were cultured with IL-3, and luciferase activity was analyzed 24 h later as described in Materials and Methods.
Since PKB has been shown to critically regulate FKHR-L1, we wished to determine whether in Ba/F3 cells FKHR-L1 is phosphorylated in a PKB-dependent fashion. To address this, we constructed a 4-OHT-inducible active-PKB Ba/F3 cell line (myrPKB:ER*) (28). Concomitant with PKB activation (Fig. 4B), FKHR-L1 phosphorylation was greatly increased upon 4-OHT addition (Fig. 4C). PKB activation was also sufficient to rescue cells from cytokine withdrawal-induced apoptosis (data not shown). This demonstrates that ligand-independent activation of PKB alone is sufficient for FKHR-L1 phosphorylation in Ba/F3 cells.
Transcription factor binding site analysis of the p27KIP1 promoter sequence revealed consensus forkhead transcription factor binding sites, suggesting that FKHR-L1 may regulate p27KIP1 expression. To investigate whether p27KIP1 promoter activity could also be enhanced by FKHR-L1, we expressed either wild-type FKHR-L1 or an “active” FKHR-L1 mutant in which all three PKB phosphorylation sites were mutated to alanine [FKHR-L1(A3)] (6). Ectopic expression of FKHR-L1 increased p27KIP1 promoter activity, which was further enhanced when FKHR-L1(A3) was expressed (Fig. 4D). To determine whether PKB could regulate FKHR-L1-induced promoter activity, we cotransfected a constitutively active PKB mutant (gagPKB) with FKHR-L1 expression vectors (7). Cotransfection of gagPKB completely inhibited p27KIP1 promoter activity induced by wild-type FKHR-L1, whereas the increase in promoter activity induced by FKHR-L1(A3) was unaffected (Fig. 4D).
Transcriptional activity of FKHR-L1 directly induces p27KIP1 expression.
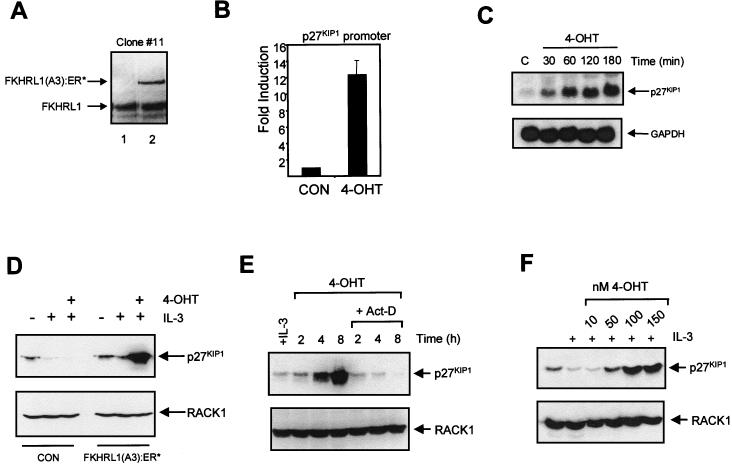
Previous studies investigating the function of forkhead-related transcription factors have all utilized transient overexpression of these proteins (6, 40). To allow us to specifically analyze the consequence of FKHR-L1 activation in more detail, we generated several clonal Ba/F3 cell lines expressing a 4-OHT-inducible FKHR-L1(A3) construct, FKHR-L1(A3):ER*. Expression levels of FKHR-L1(A3):ER* in all cell lines were approximately one-third to one-fifth of that of endogenous FKHR-L1 (Fig. 5A). Similar to what was found in the cotransfection experiments (Fig. 4D), p27KIP1 promoter activity was upregulated upon 4-OHT addition (Fig. 5B). Furthermore, addition of 4-OHT resulted in a striking upregulation of p27KIP1 mRNA within 30 to 60 min (Fig. 5C), providing compelling evidence for direct FKHR-L1 transcriptional regulation of p27KIP1 expression in vivo. In accordance with induction of p27KIP1 mRNA, p27KIP1 protein levels were also highly elevated in cells treated with 4-OHT (Fig. 5D). To confirm that upregulation of p27KIP1 levels was indeed a result of FKHR-L1-mediated transcription, actinomycin D was added prior to 4-OHT addition. As shown in Fig. 5E, this completely abrogated upregulation of p27KIP1 protein, as well as mRNA (data not shown). Finally, we analyzed levels of p27KIP1 with various concentrations of 4-OHT; the levels were elevated in a dose-dependent fashion (Fig. 5F).
FIG. 5.
FKHR-L1 directly regulates p27KIP1 transcription. (A) Expression of FKHR-L1 in Ba/F3 cells or Ba/F3 cells stably expressing FKHR-L1(A3):ER* was verified by immunoblotting with FKHR-L1 antibody. (B) Ba/F3 cells stably expressing FKHR-L1(A3):ER* were electroporated with 12 μg of p27KIP1 luciferase construct together with 8 μg of pSG5. Cells were cultured with IL-3 (CON) or with IL-3 and 4-OHT (100 nM), and luciferase activity was analyzed 24 h later as described in Materials and Methods. (C) Ba/F3 cells stably expressing FKHR-L1(A3):ER* were treated with 4-OHT (100 nM) for the indicated times; 20 μg of total RNA was used for Northern blotting and hybridized with a p27KIP1 probe (top). Equal RNA loading was verified by GAPDH reprobing (bottom). (D) Ba/F3 cells and Ba/F3 cells stably expressing FKHR-L1(A3):ER* were cytokine starved overnight and were cultured with IL-3 or with IL-3 and 4-OHT (100 nM). Equal amounts of protein were loaded, and the levels of p27KIP1 (top) and RACK1 (bottom) were determined by immunoblotting as described in Materials and Methods. (E) Ba/F3 cells stably expressing FKHR-L1(A3):ER* were treated with 4-OHT (100 nM) in the absence or presence of actinomycin D (5 μg/ml) for the indicated times and analyzed as for panel D. (F) Ba/F3 cells stably expressing FKHR-L1(A3):ER* were cultured in the absence or presence of IL-3 or IL-3 together with various concentrations 4-OHT overnight and were analyzed as for panel D.
Regulation of p27KIP1 expression is important for maintenance of cell survival.
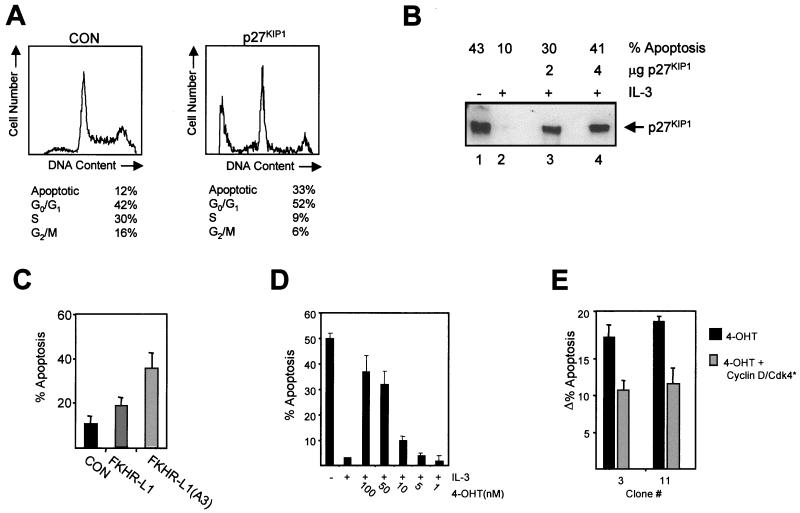
The data described above suggest that repression of p27KIP1 levels through PKB-mediated FKHR-L1 phosphorylation may be necessary for cytokine-mediated survival and proliferation. To address whether mere ectopic expression of p27KIP1 is sufficient to induce apoptosis, we introduced an expression plasmid for p27KIP1 in Ba/F3 cells, together with spectrin-GFP as a marker for transfected cells. Twenty-four hours after electroporation, cells were fixed and stained with PI and the DNA content of the spectrin-GFP-expressing cells was analyzed. Cells transfected with both spectrin-GFP and p27KIP1 exhibited a significantly higher percentage of apoptotic cells and cells in G0/G1 than control cells (Fig. 6A). To exclude the possibility that supraphysiological levels of p27KIP1 expression alone cause cells to undergo apoptosis, p27KIP1 levels in transfected cells were analyzed. This was performed by coexpressing LNGFR (47), sorting LNGFR-expressing cells by magnetic cell sorting, and analyzing p27KIP1 expression levels in corrected protein samples. Levels of p27KIP1 inducing apoptosis in transfected cells did not exceed the levels in IL-3-starved cells (Fig. 6B). Thus uncontrolled expression of physiological levels of p27KIP1 is sufficient to induce apoptosis in cytokine-dependent cells.
FIG. 6.
IL-3-mediated survival requires inactivation of FKHR-L1 and downregulation of p27KIP1 levels. (A) Ba/F3 cells were electroporated with 2 μg of spectrin-GFP vector together with either 18 μg of empty vector (pSG5; left) or 18 μg of p27KIP1 vector (right). Dead cells were removed by separation through a Ficoll gradient. Twenty-four hours after electroporation cells were fixed and stained with PI and the DNA contents of 5,000 GFP-positive cells were analyzed by FACS. The data are representative of several independent experiments. (B) Ba/F3 cells were electroporated with 2 μg of LNGFR DNA or 2 μg LNGFR DNA together with 2 or 4 μg of p27KIP1 DNA, and the total amount of DNA was adjusted to 20 μg with pSG5. Dead cells were removed by separating cells through a Ficoll gradient and LNGFR-expressing cells were analyzed 24 h after transfection as described in Materials and Methods. (C) Ba/F3 cells were electroporated with spectrin-GFP (2 μg) together with 18 μg of pSG5 (CON), FKHR-L1, or FKHR-L1(A3) DNAs and analyzed as for panel A. The data represent three independent experiments (± standard errors of the means). (D) Ba/F3 cells stably expressing FKHR-L1(A3):ER* were cytokine starved and cultured with IL-3 or IL-3 together with various concentrations of 4-OHT, and the percentages of apoptotic cells were determined after 48 h by FACS analysis. (E) FKHR-L1(A3):ER-expressing cell lines were electroporated with either 18 μg of pSG5 and 2 μg of spectrin-GFP DNAs (black bars) or 5 μg of kinase-dead CDK4, 5 μg of cyclin D1, 2 μg of spectrin-GFP, and 8 μg of pSG5 DNAs (grey bars). Dead cells were removed by separation through a Ficoll gradient. Cells were treated with 4-OHT and analyzed 24 h later as for panel A. The data are representative of several independent experiments.
FKHR-L1 function has also been linked with the induction of apoptosis in fibroblasts, cerebellar neurons, and T cells (6). We analyzed the induction of apoptosis upon transient overexpression of either FKHR-L1 or the active mutant FKHR-L1(A3) in Ba/F3 cells. Apoptosis was significantly increased in cells electroporated with FKHR-L1 and was further enhanced when the active mutant FKHR-L1(A3) was overexpressed (Fig. 6C). Next, we analyzed the effect of the addition of increasing 4-OHT concentrations to the FKHR-L1(A3):ER* stable cell lines. 4-OHT addition resulted in the induction of apoptosis in a dose-dependent fashion (Fig. 6D).
Finally, we reasoned that if the elevation of p27KIP1 plays a critical role in FKHR-L1-mediated induction of apoptosis, coexpression of cyclin-CDK complexes should be capable of titrating away the induced p27KIP1 and thereby rescuing cells from apoptosis (40, 53). Indeed, expression of cyclin D together with a kinase-dead form of CDK4 in 4-OHT-treated cells was sufficient to significantly rescue FKHR-L1(A3)-induced apoptosis in two independent clones (Fig. 6E). These data confirm that increases in p27KIP1 levels play a significant role in FKHR-L1-induced apoptosis.
p27KIP1 deficiency increases hematopoietic cell survival after cytokine withdrawal.
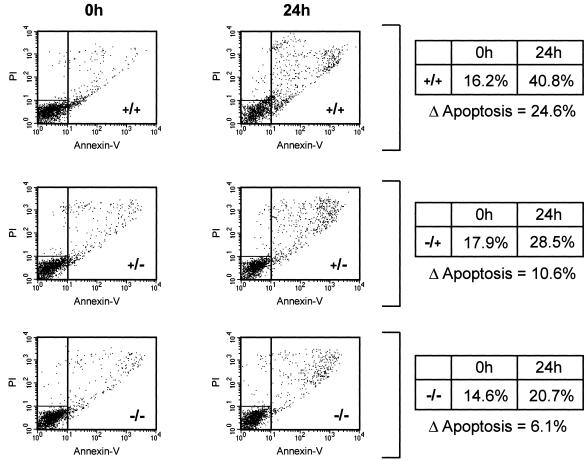
Finally, to examine the importance of p27KIP1 in the regulation of apoptosis in vivo, we utilized hematopoietic stem cells obtained from either wild-type mice or mice lacking one or both p27KIP1 alleles (27). Bone marrow-derived Sca1+ stem cells were cytokine starved and analyzed 24 h later, using annexin-V staining to label apoptotic cells. Strikingly, stem cells obtained from mice lacking one p27KIP1 allele exhibited a moderate protection against cytokine withdrawal-induced apoptosis compared to those from wild-type mice (Fig. 7). This was significantly enhanced in stem cells from mice lacking both alleles. These data demonstrate the importance of regulating p27KIP1 levels in the modulation of hematopoietic cell apoptosis in vivo.
FIG. 7.
Increased survival of p27KIP1 (−/−) hematopoietic cells after cytokine withdrawal. Hematopoietic stem cells were isolated from either wild-type mice (+/+) or mice lacking one (−/+) or both (−/−) alleles of the p27KIP1 gene and cultured as described in Materials and Methods. Cells were cytokine starved for 24 h, and the percentages of apoptotic cells were analyzed by annexin-V staining. The percentage increase in apoptosis after cytokine withdrawal is shown (Δapoptosis). Data are representative of three independent experiments.
DISCUSSION
The control of proliferation and apoptosis by cytokines is critical in the regulation of a variety of hematopoietic lineages (15, 21). Our data demonstrate PI3K signaling to be indispensable in mediating cellular proliferation and survival. The importance of PI3K activity in mediating survival was supported by overexpression the 3-phosphatidylinositol lipid phosphatase PTEN (36), which is a uniquely specific tool for decreasing 3-phosphoinositide levels in cells. Upon overexpression of membrane-localized PTEN, we observed an induction of apoptosis in IL-3-cultured Ba/F3 cells (Fig. 1C). The fact that membrane-targeted PTEN, unlike wild-type PTEN, is potently active (Fig. 1C) suggests that membrane localization is a critical aspect of PTEN regulation in vivo. Mutations in the chromosomal region of PTEN resulting in a loss of function of PTEN have been described in a variety of neoplasias, including lymphoid malignancies (19). These mutations result in the accumulation of PtdIns(3,4,5)P3 in the absence of cellular stimulation. While inhibition of PTEN activity may have deleterious effects on cell proliferation, resulting in a neoplastic phenotype, our data demonstrate that uncontrolled PTEN activity can result in the induction of an apoptotic program.
In search of a potential mechanism by which PI3K could regulate cytokine-mediated cell survival and proliferation, we focused on the CKI p27KIP1. p27KIP1 is an inhibitor of cell cycle progression, exerting its effect through interaction with cyclin-CDK complexes and arresting cells in G0/G1 (53). Furthermore, p27KIP1 has been implicated in the regulation of apoptosis in immature B cells (17, 61). Cross-linking of surface Ig (IgM) on the WEHI-231 B-cell lymphoma, for example, results in growth arrest and eventually induction of an apoptotic program which can be rescued by CD40 ligand engagement. These IgM-induced changes are correlated with an increase in p27KIP1 protein which is inhibited by CD40, although the molecular mechanisms of these observations are unclear (17, 61). A potential role for PI3K in downregulating p27KIP1 levels was suggested by the observation that overexpression of PTEN in glioblastoma cells resulted in enhanced p27KIP1 levels (34). We have explored the IL-3-mediated regulation of p27KIP1 levels and a possible role for PI3K therein. Survival factor withdrawal resulted in an increase of p27KIP1 protein levels in a PI3K-dependent manner (Fig. 2A). In cultures of primary fetal liver cells cytokine withdrawal also resulted in an increase of apoptosis paralleled by upregulation of p27KIP1, suggesting that this may be a common feature of primary lymphocyte lineages (Fig. 2D). Levels of p27KIP1 in primary human eosinophils undergoing apoptosis were also analyzed (Fig. 2E). In eosinophils, both cytokine starvation and inhibition of PI3K resulted in significantly higher levels of p27KIP1, correlating with induction of apoptosis (Fig. 1D). Importantly, induction of p27KIP1 in these nondividing cells suggests an additional cell cycle-independent role for this CKI.
While regulation of p27KIP1 levels has been previously considered to occur predominantly posttranslationally (23, 49), we found a rapid and dramatic effect of IL-3 on p27KIP1 mRNA (Fig. 3A). In addition, IL-3 was also capable of downregulating p27KIP1 promoter activity in a PI3K-dependent manner (Fig. 3C), prompting us to investigate the role of PI3K-regulated transcription factors in this process. Transcription factors of the AFX/FKHR forkhead family are phosphorylated by the PI3K target PKB, resulting in inhibition of their activity (3, 6, 20, 30). One member, FKHR-L1, has been linked to induction of apoptosis, possibly by the upregulation of Fas ligand on cells (6). FKHR-L1 is endogenously expressed in Ba/F3 cells and phosphorylated in a PI3K- and PKB-dependent manner (Fig. 4A and C). Furthermore, overexpression of an active FKHR-L1 mutant resulted in induction of apoptosis (Fig. 6C). Since Fas ligand was unable to induce apoptosis in Ba/F3 cells (data not shown), a role for FKHR-L1 in induction of apoptosis must be mediated by an alternative mechanism. The presence of several forkhead transcription factor binding sites in the p27KIP1 promoter suggested a possible link between FKHR-L1 and transcription of p27KIP1. Indeed, overexpression of FKHR-L1 elevated p27KIP1 promoter activity, which could be inhibited by cotransfection of active PKB (Fig. 4D). To specifically analyze the effect of FKHR-L1 on p27KIP1 transcription, we utilized Ba/F3 cells stably expressing a 4-OHT-inducible active FKHR-L1 construct. Upon FKHR-L1 activation, p27KIP1 mRNA was greatly elevated within 30 to 60 min, concomitant with a spectacular elevation of p27KIP1 protein levels (Fig. 5C to E). These data clearly demonstrate that activation of FKHR-L1 alone is sufficient to induce rapid upregulation of p27KIP1 mRNA in vivo. To determine if p27KIP1 is indeed an important target of FKHR-L1-induced apoptosis, we overexpressed cyclin D-CDK4 complexes to titrate away functional p27KIP1. Indeed, overexpression of cyclin D-CDK4 complexes was sufficient to significantly reduce FKHR-L1-induced apoptosis, thus suggesting that p27KIP1 is an important FKHR-L1 target for the induction of apoptosis. The fact that apoptosis was not completely rescued by overexpression of cyclin D-CDK4 suggests that there are possibly additional targets accounting for FKHR-L1-induced apoptosis. During the preparation of this paper it was reported that FKHR-L1-related transcription factor AFX was able to induce growth suppression through regulation of p27KIP1 expression (40). However, these overexpression studies were performed with cells not normally expressing AFX. We have now been able to demonstrate that regulation of p27KIP1 transcription can be controlled through cytokines and further that this seems to play a role in the regulation of survival.
Here we also provide proof for the importance of p27KIP1 in the induction of apoptosis by utilizing mice lacking one or both alleles of the p27KIP1 gene (27). There was a significant decrease in apoptosis upon cytokine withdrawal in mice lacking one p27KIP1 gene allele (change in apoptosis [Δapoptosis] = 10.6%) compared to that in wild-type mice (Δapoptosis = 24.6%); this decrease was even more striking in mice lacking both alleles (Δapoptosis = 6.1%). While the role of p27KIP1 in regulating growth arrest is fairly well defined, relatively little is known regarding the mechanisms by which this protein may regulate apoptosis. A potential mechanism is suggested by a recent report by Boussiotis et al., who demonstrated that p27KIP1 is capable of directly influencing transcription independently of its ability to block cell cycle progression (5). Increased p27KIP1 levels were found to inhibit IL-2 transcription in T cells through the binding, nuclear export, and subsequent degradation of the Jun transcription factor coactivator JAB1 (12). Potentially inhibition of antiapoptotic gene expression through p27KIP1-mediated degradation of JAB1 could play a role in the induction of apoptosis. In various malignancies it has been shown that reduced levels of p27KIP1 correlate with poor prognosis (10, 35, 48). The levels of p27KIP1 do not, however, correlate with the proliferative status of the tumor cells, suggesting that the benefits of p27KIP1 reflect an additional function such as increased apoptosis. Indeed, decreased p27KIP1 expression in gastric carcinomas correlates with decreased apoptosis and increased aggressiveness of the tumor (44).
The regulation of both proliferation and survival by p27KIP1 has parallels with that by the tumor suppressor protein p53. p53 has a major G1 checkpoint function and can mediate a transient growth arrest in certain situations that favor cell survival, while inducing apoptosis in others (59). Interestingly, one study has demonstrated that overexpression of Bcl-2 can significantly counteract the apoptotic effects of p27KIP1, preventing caspase activation (57). This suggests that p27KIP1 may either inhibit specific antiapoptotic Bcl-2 family members or activate proapoptotic family members such as Bim that have recently been shown to play a critical role in apoptosis induced by cytokine withdrawal (4).
Our findings demonstrate a novel mechanism by which cytokines mediate rescue from apoptosis. This involves the downregulation p27KIP1 levels through the PI3K- and PKB-regulated inactivation of transcription factors of the AFX/FKHR forkhead family. Exposure of hematopoietic cells to cytokines acts to stimulate both survival and proliferation. The regulation of p27KIP1 expression by PI3K allows the modulation of both these processes by altering the levels of a single protein. Our data not only provide insight into the mechanisms of cytokine-mediated signal transduction regulating cell proliferation and survival but also identify critical components regulating p27KIP1 transcription. The mechanism of PI3K-mediated forkhead transcription factor regulation is conserved between the nematode worm Caenorhabditis elegans (43) and mammalian cells. Our data implicate the regulation of p27KIP1 by this evolutionarily conserved signaling pathway as a general mechanism for controlling cell fate decisions regulating survival and proliferation or differentiation.
ACKNOWLEDGMENTS
We thank Tom O'Toole for technical help with the fetal liver cultures, Kris Reedquist for critically reading the manuscript, and Geert Kops for helpful discussions. Thanks also to Ivo Touw for helpful discussions and providing the p27KIP1 luciferase construct, Anke Klippel for providing the myrPKB:ER* construct and M. E. Greenberg for the FKHR-L1 and FKHR-L1(A3) constructs.
Eric W.-F. Lam is supported by the Leukemia Research Fund of Great Britain.
REFERENCES
- 1.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 2.Arai K I, Lee F, Miyajima A, Miyatake S, Arai N, Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- 3.Biggs W H, Meisenhelder J, Hunter T, Cavenee W K, Arden K C. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouillet P, Metcalf D, Huang D C, Tarlinton D M, Kay T W, Kontgen F, Adams J M, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 5.Boussiotis V A, Freeman G J, Taylor P A, Berezovskaya A, Grass I, Blazar B R, Nadler L M. p27kip1 functions as an anergy factor inhibiting interleukin 2 transcription and clonal expansion of alloreactive human and mouse helper T lymphocytes. Nat Med. 2000;6:290–297. doi: 10.1038/73144. [DOI] [PubMed] [Google Scholar]
- 6.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 7.Burgering B M, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 8.Caldenhoven E, van Dijk T, Raaijmakers J A, Lammers J W, Koenderman L, de Groot R P. Activation of the STAT3/acute phase response factor transcription factor by interleukin-5. J Biol Chem. 1995;270:25778–25784. doi: 10.1074/jbc.270.43.25778. [DOI] [PubMed] [Google Scholar]
- 9.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 10.Catzavelos C, Bhattacharya N, Ung Y C, Wilson J A, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I, Kapusta L, Franssen E, Pritchard K I, Slingerland J M. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat Med. 1997;3:227–230. doi: 10.1038/nm0297-227. [DOI] [PubMed] [Google Scholar]
- 11.Cheng M, Sexl V, Sherr C J, Roussel M F. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1) Proc Natl Acad Sci USA. 1998;95:1091–1096. doi: 10.1073/pnas.95.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claret F X, Hibi M, Dhut S, Toda T, Karin M. A new group of conserved coactivators that increase the specificity of AP-1 transcription factors. Nature. 1996;383:453–457. doi: 10.1038/383453a0. [DOI] [PubMed] [Google Scholar]
- 13.Cuenda A, Rouse J, Doza Y N, Meier R, Cohen P, Gallagher T F, Young P R, Lee J C. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 14.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 15.de Groot R P, Coffer P, Koenderman L. Regulation of proliferation, differentiation and survival by the IL-3/IL-5/GM-CSF receptor family. Cell Signal. 1998;8:12–18. doi: 10.1016/s0898-6568(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 16.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 17.Ezhevsky S A, Toyoshima H, Hunter T, Scott D W. Role of cyclin A and p27 in anti-IgM induced G1 growth arrest of murine B-cell lymphomas. Mol Biol Cell. 1996;7:553–564. doi: 10.1091/mbc.7.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita E, Jinbo A, Matuzaki H, Konishi H, Kikkawa U, Momoi T. Akt phosphorylation site found in human caspase-9 is absent in mouse caspase-9. Biochem Biophys Res Commun. 1999;264:550–555. doi: 10.1006/bbrc.1999.1387. [DOI] [PubMed] [Google Scholar]
- 19.Gronbaek K, Zeuthen J, Guldberg P, Ralfkiaer E, Hou-Jensen K. Alterations of the MMAC1/PTEN gene in lymphoid malignancies. Blood. 1998;91:4388–4390. [PubMed] [Google Scholar]
- 20.Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem. 1999;274:17184–17192. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- 21.Guthridge M A, Stomski F C, Thomas D, Woodcock J M, Bagley C J, Berndt M C, Lopez A F. Mechanism of activation of the GM-CSF, IL-3, and IL-5 family of receptors. Stem Cells. 1998;16:301–313. doi: 10.1002/stem.160301. [DOI] [PubMed] [Google Scholar]
- 22.Haas-Kogan D, Shalev N, Wong M, Mills G, Yount G, Stokoe D. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr Biol. 1998;8:1195–1198. doi: 10.1016/s0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 23.Hengst L, Reed S I. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 24.Hinton H J, Welham M J. Cytokine-induced protein kinase B activation and Bad phosphorylation do not correlate with cell survival of hematopoietic cells. J Immunol. 1999;162:7002–7009. [PubMed] [Google Scholar]
- 25.Kalejta R F, Shenk T, Beavis A J. Use of a membrane-localized green fluorescent protein allows simultaneous identification of transfected cells and cell cycle analysis by flow cytometry. Cytometry. 1997;29:286–291. doi: 10.1002/(sici)1097-0320(19971201)29:4<286::aid-cyto4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 27.Kiyokawa H, Kineman R D, Manova-Todorova K O, Soares V C, Hoffman E S, Ono M, Khanam D, Hayday A C, Frohman L A, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1) Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 28.Klippel A, Escobedo M A, Wachowicz M S, Apell G, Brown T W, Giedlin M A, Kavanaugh W M, Williams L T. Activation of phosphatidylinositol 3-kinase is sufficient for cell cycle entry and promotes cellular changes characteristic of oncogenic transformation. Mol Cell Biol. 1998;18:5699–5711. doi: 10.1128/mcb.18.10.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koenderman L, Kok P T, Hamelink M L, Verhoeven A J, Bruijnzeel P L. An improved method for the isolation of eosinophilic granulocytes from peripheral blood of normal individuals. J Leukoc Biol. 1988;44:79–86. doi: 10.1002/jlb.44.2.79. [DOI] [PubMed] [Google Scholar]
- 30.Kops G J, de Ruiter N D, de Vries-Smits A M, Powell D R, Bos J L, Burgering B M. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 31.Kwon T K, Nagel J E, Buchholz M A, Nordin A A. Characterization of the murine cyclin-dependent kinase inhibitor gene p27Kip1. Gene. 1996;180:113–120. doi: 10.1016/s0378-1119(96)00416-7. [DOI] [PubMed] [Google Scholar]
- 32.Lee J O, Yang H, Georgescu M M, Di Cristofano A, Maehama T, Shi Y, Dixon J E, Pandolfi P, Pavletich N P. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 33.Leevers S J, Paterson H F, Marshall C J. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994;369:411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- 34.Li D M, Sun H. PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. Proc Natl Acad Sci USA. 1998;95:15406–15411. doi: 10.1073/pnas.95.26.15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loda M, Cukor B, Tam S W, Lavin P, Fiorentino M, Draetta G F, Jessup J M, Pagano M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 36.Maehama T, Dixon J E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 37.Mavilio F, Ferrari G, Rossini S, Nobili N, Bonini C, Casorati G, Traversari C, Bordignon C. Peripheral blood lymphocytes as target cells of retroviral vector-mediated gene transfer. Blood. 1994;83:1988–1997. [PubMed] [Google Scholar]
- 38.Medema R H, Herrera R E, Lam F, Weinberg R A. Growth suppression by p16ink4 requires functional retinoblastoma protein. Proc Natl Acad Sci USA. 1995;92:6289–6293. doi: 10.1073/pnas.92.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medema R H, Klompmaker R, Smits V A, Rijksen G. p21waf1 can block cells at two points in the cell cycle, but does not interfere with processive DNA-replication or stress-activated kinases. Oncogene. 1998;16:431–441. doi: 10.1038/sj.onc.1201558. [DOI] [PubMed] [Google Scholar]
- 40.Medema R H, Kops G J, Bos J L, Burgering B M. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 41.Montagnoli A, Fiore F, Eytan E, Carrano A C, Draetta G F, Hershko A, Pagano M. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 1999;13:1181–1189. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nourse J, Firpo E, Flanagan W M, Coats S, Polyak K, Lee M H, Massague J, Crabtree G R, Roberts J M. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 43.Ogg S, Paradis S, Gottlieb S, Patterson G I, Lee L, Tissenbaum H A, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 44.Ohtani M, Isozaki H, Fujii K, Nomura E, Niki M, Mabuchi H, Nishiguchi K, Toyoda M, Ishibashi T, Tanigawa N. Impact of the expression of cyclin-dependent kinase inhibitor p27Kip1 and apoptosis in tumor cells on the overall survival of patients with non-early stage gastric carcinoma. Cancer. 1999;85:1711–1718. [PubMed] [Google Scholar]
- 45.Packham G, White E L, Eischen C M, Yang H, Parganas E, Ihle J N, Grillot D A, Zambetti G P, Nuñez G, Cleveland J L. Selective regulation of Bcl-XL by a Jak kinase-dependent pathway is bypassed in murine hematopoietic malignancies. Genes Dev. 1998;12:2475–2487. doi: 10.1101/gad.12.16.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pagano M, Tam S W, Theodoras A M, Beer-Romero P, Del Sal G, Chau V, Yew P R, Draetta G F, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 47.Pals C M, Verploegen S A, Raaijmakers J A, Lammers J W, Koenderman L, Coffer P J. Identification of cytokine-regulated genes in human leukocytes in vivo. J Allergy Clin Immunol. 2000;105:760–768. doi: 10.1067/mai.2000.104382. [DOI] [PubMed] [Google Scholar]
- 48.Porter P L, Malone K E, Heagerty P J, Alexander G M, Gatti L A, Firpo E J, Daling J R, Roberts J M. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med. 1997;3:222–225. doi: 10.1038/nm0297-222. [DOI] [PubMed] [Google Scholar]
- 49.Sheaff R J, Groudine M, Gordon M, Roberts J M, Clurman B E. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 50.Stambolic V, Suzuki A, de la Pompa J L, Brothers G M, Mirtsos C, Sasaki T, Ruland J, Penninger J M, Siderovski D P, Mak T W. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 51.Takatsu K. Interleukin 5 and B cell differentiation. Cytokine Growth Factor Rev. 1998;9:25–35. doi: 10.1016/s1359-6101(97)00034-8. [DOI] [PubMed] [Google Scholar]
- 52.Tang E D, Nuñez G, Barr F G, Guan K L. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 53.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 54.Tsvetkov L M, Yeh K H, Lee S J, Sun H, Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9:661–664. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- 55.Vlach J, Hennecke S, Amati B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 1997;16:5334–5344. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vlahos C J, Matter W F, Hui K Y, Brown R F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 57.Wang X. p27Kip1 overexpression causes apoptotic death of mammalian cells. Oncogene. 1997;15:2991–2997. doi: 10.1038/sj.onc.1201450. [DOI] [PubMed] [Google Scholar]
- 58.Weber J D, Hu W, Jefcoat S C J, Raben D M, Baldassare J J. Ras-stimulated extracellular signal-related kinase 1 and RhoA activities coordinate platelet-derived growth factor-induced G1 progression through the independent regulation of cyclin D1 and p27. J Biol Chem. 1997;272:32966–32971. doi: 10.1074/jbc.272.52.32966. [DOI] [PubMed] [Google Scholar]
- 59.White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 60.Winston J, Dong F, Pledger W J. Differential modulation of G1 cyclins and the Cdk inhibitor p27kip1 by platelet-derived growth factor and plasma factors in density-arrested fibroblasts. J Biol Chem. 1996;271:11253–11260. doi: 10.1074/jbc.271.19.11253. [DOI] [PubMed] [Google Scholar]
- 61.Wu M, Bellas R E, Shen J, Yang W, Sonenshein G E. Increased p27Kip1 cyclin-dependent kinase inhibitor gene expression following anti-IgM treatment promotes apoptosis of WEHI 231 B cells. J Immunol. 1999;163:6530–6535. [PubMed] [Google Scholar]
- 62.Wu X, Senechal K, Neshat M S, Whang Y E, Sawyers C L. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1998;95:15587–15591. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]