Abstract
Buckwheat is a pseudo-cereal widely grown and consumed throughout the world. Buckwheat is recognized as a good source of nutrients and, in combination with other health-promoting components, is receiving increasing attention as a potential functional food. Despite the high nutritional value of buckwheat, a variety of anti-nutritional features makes it difficult to exploit its full potential. In this framework, sprouting (or germination) may represent a process capable of improving the macromolecular profile, including reducing anti-nutritional factors and/or synthesizing or releasing bioactives. This study addressed changes in the biomolecular profile and composition of buckwheat that was sprouted for 48 and 72 h. Sprouting increased the content of peptides and free-phenolic compounds and the antioxidant activity, caused a marked decline in the concentration of several anti-nutritional components, and affected the metabolomic profile with an overall improvement in the nutritional characteristics. These results further confirm sprouting as a process suitable for improving the compositional traits of cereals and pseudo-cereals, and are further steps towards the exploitation of sprouted buckwheat as a high-quality ingredient in innovative products of industrial interest.
Keywords: buckwheat, germination, starch, protein hydrolysis, lipids, anti-nutritional factors, antioxidants, metabolome
1. Introduction
Buckwheat (Fagopyrum esculentum) is a short-season crop that has recently attracted much interest because of its environmental adaptability [1]. Buckwheat grows well in low-fertility or acidic soils, does not need fertilizers or biocides, and is sustainable for organic and environmentally friendly farming [2]. Buckwheat is also relatively tolerant to UV-B radiation and drought if compared to other crops, due to the high levels of stress-mitigating phenolics [3]. Thus, more intensive exploitation of buckwheat could favor agricultural diversification and minimize environmental degradation, improving food and nutritional security [4]. From a nutritional standpoint, buckwheat has an appreciable protein content (7–21%), and is rich in dietary fibers and bioactive compounds that contribute to its good antioxidant capacity and have multiple positive implications for the consumers’ health [5]. In addition, buckwheat does not contain gluten and is emerging as an alternative to rice and corn in gluten-free formulations [6].
Despite the good nutritional value of buckwheat, the presence of a broad array of anti-nutritional components, such as phytic acid and inhibitors of digestive proteases, has impaired its exploitation [7]. Among the processes that could overcome this limitation, sprouting (or germination) has been reported as an effective and low-cost process with positive effects on the nutritional profile [8]. During sprouting, many biochemical modifications occur and modulate product characteristics, such as bioactivity and flavor [9]. Sprouting reactivates seed metabolism, leading to the catabolism and degradation of macronutrients and anti-nutritional compounds and the biosynthesis of secondary metabolites with potential health benefits, improving the nutritional and health value of sprouted seeds [10]. Actually, most of the studies conducted in buckwheat investigated the Impact of sprouting, taking into consideration only one or few aspects at a time as phenolic profiles and antioxidants [11], starch and protein [12], or fatty acids [13]. In addition, the different experimental conditions adopted in terms of time, temperature, and humidity during sprouting do not allow to have a uniform and comprehensive view of the impact of this technology on changes at the biomolecular level and to draw definitive conclusions.
This study aimed to provide an overview of the changes in the macro- and micromolecular profile and of the properties and content of bioactive compounds in sprouted buckwheat. Sprouting was performed at laboratory-scale level and under controlled conditions for 48 and 72 h to evaluate if the different sprouting time changes the impact of this technology on changes at the biomolecular level. Sprouting-related modifications in the content of anti-nutritional factors, in endogenous α-amylase and protease activity, and the modulation of key digestive enzymes were also investigated. A metabolomic approach (nuclear magnetic resonance, NMR) aimed at a comprehensive view of sprouting-related biochemical events by identifying and quantifying the major metabolites.
2. Materials and Methods
2.1. Materials
Hexane, isopropanol, methanol, chloroform, and isooctane were obtained from VWR Internationa (Radnor, PA, USA). Unless otherwise specified, chemicals and solvents were from Sigma-Aldrich (St. Louis, MO, USA), and were of the highest available analytical grade. Seeds from dehulled common buckwheat (Fagopyrum esculentum) were provided by Molino Filippini s.r.l. (Teglio, Italy), consisting of a mix of different varieties from Eastern Europe harvested at full maturity, even though the plant is still green, in August/September 2020.
2.2. Sprouting Process
Seeds (2.5 kg) were sprouted in a lab-scale climate chamber (IPP110ecoplus, Memmert GmbH + Co. KG, Schwabach, Germany). Seeds were soaked in water (1:3, w/w) for 16 h at 27 °C and sprouted for 48 h (BW-48) and 72 h (BW-72) at 27 °C and 90% relative humidity. Optimal sprouting times were selected on the basis of previous studies that demonstrated that excessive germination determined a substantial loss of flour properties, dough quality, and bread-baking performance [14,15]. After sprouting, seeds were dried at 50 °C for 8 h (Self-Cooking Centerfi, Rational International AG, Heerbrugg, Switzerland). Unsprouted seeds were used as the control (BW-0). All samples were milled into powder (<0.5 mm) in a laboratory mill (IKA Universalmühle M20; IKA Laborteknic, Staufen, Germany). Dry weight (dw) was measured according to AACC 08-01 method and was 0.87 g dw/g powder, 0.90 g dw/g powder, and 0.92 g dw/g powder for BW-0, BW-48, and BW-72, respectively.
2.3. Aqueous Extraction
To avoid interferences due to the vehicle, buckwheat powder was subjected in duplicate to aqueous extraction by suspending 1 g of sample in 10 mL 0.05 M sodium phosphate buffer pH 7 containing 0.1 M NaCl. After 1 h of stirring at 25 °C, the suspension was centrifuged (2500× g, 30 min, 25 °C), and the supernatant was stored at −18 °C.
2.4. Total Starch, Damaged Starch, Fiber, and Glucose Content
Total starch, damaged starch, and fiber content were measured according to AACC 76-13.01, 76-31.01 and AOAC 992-16 methods. Glucose content was evaluated using the D-Glucose Assay Kit (R-Biopharm, Darmstadt, Germany) following the manufacturer’s instructions. Values obtained were normalized for g dw in buckwheat.
2.5. Endogenous α-Amylase Activity
Endogenous α-amylase activity was evaluated using the Cereal α-Amylase Assay Kit (Megazyme, Wicklow, Ireland) following the manufacturer’s instructions.
2.6. SDS-PAGE Analysis of Soluble Proteins
A 0.1 mL volume of aqueous buckwheat extracts was incubated in an equal volume of 0.125 M tris(hydroxymethyl)aminomethane (Tris)-HCl, pH 6.8, 50% glycerol, 1.7% sodium dodecyl sulfate, 0.01% bromophenol blue, in the presence/absence of 0.5% (v/v) 2-mercaptoethanol, and heated at 95 °C for 5 min. Electrophoretic runs were carried out in 12% polyacrylamide gels. Gels were stained with Coomassie Blue R250 and grayscale-imaged on a benchtop scanner. For further analysis, images of each lane were vertically divided into four portions, according to their relative molecular mass (Mr), total (Mr ≤ 97.4 k), high (97.4 < Mr ≤ 45 k), medium (45 < Mr ≤ 21.5 k), and low (Mr < 21.5 k). Image Lab software (Bio-Rad Laboratories, Hercules, CA, USA) was then used for quantitative image analysis.
2.7. Soluble Proteins and Amino Acids/Small Peptides Content
Soluble protein and amino acids/small peptides content in aqueous buckwheat extract were determined spectrophotometrically by the Bradford dye-binding assay [16] and by the o-phthaldialdehyde (OPA) assay for free amino groups [17] using bovine serum albumin and L-isoleucine as the respective standards.
2.8. Endogenous Protease Activity
Endogenous protease activity in aqueous buckwheat extracts was measured using azocasein as a non-specific substrate, according to de Freitas et al. [18], with slight modifications. The reaction mixture contained 0.5 mL of 1% azocasein in 0.5 mM Tris-HCl (pH 5.0) and 0.5 mL of buckwheat extract. The reaction was performed at 37 °C and stopped after 120 min by adding 1 mL of 10% trichloroacetic acid. After centrifugation (10,000× g, 10 min at 25 °C), 0.08 mL of 5 N NaOH was added to 0.4 mL of the supernatant, and the absorbance was measured at 420 nm.
2.9. Lipid Content and Composition
Total lipids were extracted from 0.1 g of buckwheat powder [19]. After methylation [20], the content and profile of fatty acids as methyl esters (FAMEs) were determined by fast GC (GC-2030AF; Shimadzu, Kyoto, Japan), using a capillary column (30 mt, 0.2 μm film thickness) with a programmed temperature gradient (50–250 °C, 10 °C/min) [21]. Chromatographic peaks were identified based on retention time from FAME standard mixture (Sigma-Aldrich, St. Louis, MO, USA) and quantitated using Lab Solution software (Shimadzu, Kyoto, Japan). Peroxidizability and unsaturation indexes were calculated as previously reported [22,23].
2.10. Lipid Peroxidation
Lipid peroxidation was assessed by quantifying conjugated dienes (CD) according to Situnayake et al. [24] with slight modifications. A buckwheat sample (100 mg) was treated with 3.6 mL of hexane/isopropanol (3:2, v/v), followed by thorough mixing and by the addition of 2.4 mL of a 7% solution (w/v) of anhydrous sodium sulfate. After phase separation, the upper layer was collected and evaporated under nitrogen. The lipid residue was dissolved in 5 mL of isooctane, and its absorbance was read at 232 nm against an appropriate blank.
2.11. Phytic Acid Content
Phytic acid was estimated by the Phytic Acid/Total Phosphorus Kit (Megazyme International Ltd., Bray, Ireland) according to the manufacturer’s instructions.
2.12. Pepsin, Trypsin, and Chymotrypsin Activity
Pepsin, trypsin, and chymotrypsin activities were determined according to Urbinati et al. [25], with slight modifications. Bovine blood hemoglobin (0.48 mL, 2% solution at pH 2 for pepsin and pH 7 for trypsin and chymotrypsin) was added to 0.02 mL of aqueous buckwheat extract. The required enzyme was then added (0.1 mL, 0.03 mg/mL) to start the reaction, which was stopped after 10, 20, or 30 min by adding 1 mL of 20% (w/v) trichloroacetic acid. Soluble peptides in the supernatant after centrifugation (14,000× g, 10 min at 25 °C) were detected spectrophotometrically at 280 nm. Readings were corrected by endogenous proteolytic activity, measured without added digestive enzymes.
2.13. Tocols Extraction and Determination by HPLC–FLD
Tocols were determined as previously reported [26]. Then, 100 mg of extracted lipids were dissolved in 1 mL hexane and filtered through a 0.2 μm nylon filter. A 2.5 μL aliquot of the hexane solution was injected in a HPLC 1200 series equipped with a fluorimeter detector (λex = 290 nm, λem = 325 nm) (Agilent Technologies, Palo Alto, CA, USA) fitted with a HILIC Poroshell 120 (3 × 100 mm, 2.7 μm) from Agilent Technologies (Palo Alto, CA, USA). An n-hexane/ethyl acetate/acetic acid (97.3:1.8:0.9 v/v/v) mobile phase was used for isocratic elution (0.8 mL/min). A calibration curve was constructed with α-tocopherol (Sigma-Aldrich, St. Louis, MO, USA), as previously reported [26,27,28,29].
2.14. Extraction and Determination of Free and Bound Phenolic Compounds
Free phenolics compounds were extracted twice from 2 g of buckwheat powder using ethanol/water (4:1, v/v) in an ultrasonic bath [30]. The supernatants were collected, evaporated, and reconstituted with 2 mL of methanol/water (1:1, v/v). The extracts were stored at −18 °C until use. Residues of free phenolic extraction were shaken overnight (20 h) with 200 mL of 2 M NaOH at room temperature under nitrogen to obtain the bound phenolic fraction. The hydrolyzed solution was acidified to pH 2 by adding 10 M hydrochloric acid in an ice bath. The final solution was extracted five times with 100 mL of ethyl acetate, and the pooled organic fractions were evaporated to dryness. The bound phenolic compounds were reconstituted in 2 mL of methanol/water (1:1, v/v). Separation of free and bound phenolic compounds from buckwheat powder was carried out using a C-18 column (Poroshell 120, SB-C18, 3.0 × 100 mm, 2.7 µm from Agilent Technologies, Palo Alto, CA, USA) and an Agilent HPLC 1200 series equipped with auto-sampler and a binary pump, according to the methods reported by Gomez-Caravaca et al. [31]. MS/MS analysis (MRM mode) was performed on 6420 Triple Quadrupole (Agilent Technologies, Santa Clara, CA, USA) using an electrospray ionization (ESI) interface in negative and positive ionization mode. Ferulic acid, catechin, and rutin were used as standards for quantitative purposes.
2.15. Total Antioxidant Capacity (TAC) and Ferric Reducing Antioxidant Power (FRAP)
TAC and FRAP were assessed by measuring the ability of the antioxidant molecules in the sample to reduce the radical cation of 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+) [32] and the Fe (III)/tripyridyltriazine complex [33], respectively, using 0.01 mL of the aqueous buckwheat extract. Values obtained were compared to the concentration–response curve of a standard solution of 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox).
2.16. 1H(HR)-NMR Spectra Acquisition and Processing
Aqueous buckwheat extracts were thawed and centrifuged at 2300× g for 5 min at 4 °C to eliminate the coarsest particles and subsequently at 50,000× g for 5 min at 4 °C to eliminate the finest particles. A 0.75 mL aliquot of the supernatant was added to 0.12 mL of 100 mM phosphate buffer containing 10 mM trimethylsilylpropanoic acid (TSP, as internal standard) and brought to pH 7. HR-NMR spectra were recorded at 298 K on a Bruker US+ Avance III spectrometer, as reported elsewhere [26]. Signals were identified by comparing their chemical shift and multiplicity with Chenomx Profiler software data bank (ver. 8.1, Edmonton, AB, Canada). Before statistical analysis, the NMR spectra underwent several pre-processing procedures, such as spectra alignment, removal of some irrelevant signals, normalization, and a final binning [34]. Some parts of spectra lacking metabolic information were removed, including (i) the noise-only regions from 20.00 to 9.40 ppm and from −20.00 to −0.50 ppm; and (ii) the region from 4.69 to 5.05 ppm where water was highly interfering. The new dataset was normalized by applying the Probabilistic Quotient Normalization (PQN) [35], based on the calculation of a most probable dilution factor by looking at the distribution of the quotients of the amplitudes of the samples’ spectra to reference one. Further crucial data reduction was performed by using a binning (or bucketing) algorithm [36]. Spectra were reduced to 348 bins of 150 data points, each bin corresponding to a spectral region of 0.0274 ppm.
2.17. Statistical Analysis
Statistically significant differences were determined by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test and considering p < 0.05 as significant. After pre-processing, NMR data underwent multivariate analyses (PCA) first and then univariate (ANOVA and Tukey’s post hoc test). Statistical analyses were carried out by using the R software environment for statistical computing (version 4.1.0).
3. Results and Discussion
3.1. Carbohydrates
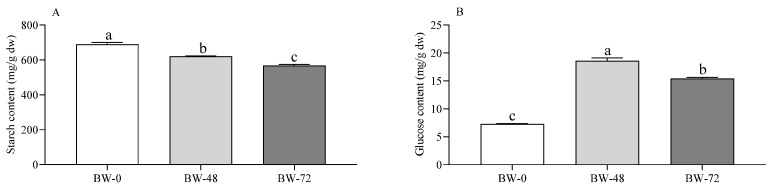
As reported for cereals, pseudo-cereals, and legumes [37,38,39], sprouting significantly promoted the hydrolysis of starch to glucose due to α-amylase activation in the scutellum and aleurone in response to providing energy for seed development [40]. Additionally, in the case of buckwheat, the total starch content decreased with increasing sprouting time (Figure 1A), leading to a release of free glucose (Figure 1B). The increase in damaged starch confirms the starch hydrolysis (Figure 1C), which is an indicator of susceptibility to α-amylase hydrolysis [41], and endogenous α-amylase activity as well (Figure 1D).
Figure 1.
Starch content (A), glucose content (B), damaged starch content (C), and endogenous α-amylase activity (D) in unsprouted buckwheat (BW-0), and sprouted buckwheat (48 h, BW-48; 72 h, BW-72). Data are expressed as mg/g dw (A–C) and U/g dw (D) and are mean ± SD of two different extractions and at least duplicate assays on each extract. Statistical analysis was by one-way ANOVA (always: p < 0.05) with Tukey’s post hoc test (different letters in the same panel indicate significant differences).
Furthermore, damaged starch, which represents the fraction of starch readily accessible to amylase hydrolysis, increased in sprouted buckwheat, likely due to the presence of some holes in the outermost regions of the granules, as observed in other species [42]. Together, these results could suggest an increased starch digestibility in sprouted flours. In support of this hypothesis, Sharma et al. [43] recently showed in millet that sprouting gradually lowers the fractions of resistant and slowly digestible starch while increasing the rapidly digestible starch. More recently, Molska et al. [12] demonstrated that sprouted buckwheat possesses a higher rate of starch hydrolysis during in vitro digestion. Cooking is needed before consumption of starch-based foods and this process, together with milling, modifies the digestibility of the starch [44,45]. Therefore, more studies evaluating the digestibility of sprouted grain foods are needed before conclusions can be drawn. Anyway, it is noteworthy that despite the increased glucose content, in vitro and in vivo studies have reported that sprouted grains do not alter the glycemic index and improve fasting blood glucose, making them a good candidate for blood sugar control [46,47]. Sprouting within 72 h did not modify the content of dietary fibers. This confirms that starch is the main glucidic fraction targeted by endogenous hydrolytic enzymes at the sprouting time considered in buckwheat.
3.2. Proteins
In buckwheat proteins, the percentage of albumins and globulins (45%) is higher than in cereal proteins, with a matching lower content in glutelins (15%) and prolamins (3%) [48]. Buckwheat globulins consist of a major 13S legumin-like and a minor 8S vicilin-like fractions made up of proteins with Mr ranging from 68 kDa to 26 kDa. Buckwheat albumins represent about 25% of the total proteins and consist mainly of single-chain polypeptides with Mr in the 8–16 kDa range [49,50].
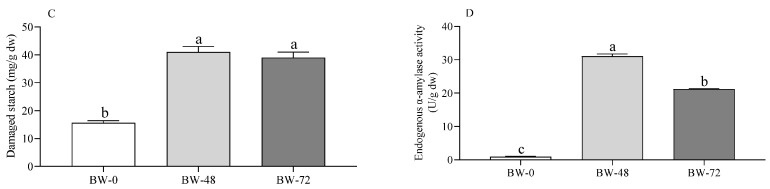
The various panels of Figure 2 report the SDS-PAGE tracings of soluble proteins present in buckwheat aqueous extracts when run in the absence (Figure 2A) and in the presence (Figure 2C) of disulfide reducing agents. Confirming previous reports, the most substantial protein bands in unsprouted buckwheat had at Mr values of 67, 35, 21, and 16 kDa under non-reducing conditions [51]. Under reducing conditions, the bands at Mr 67 and 35 kDa disappeared and were replaced by novel bands at Mr around 55, 25, and 10 kDa.
Figure 2.
SDS-PAGE tracings (A,C) and relative band intensity at Mr < 21.5 kDa (B,D) of proteins in the absence (A,B) and in the presence (C,D) of disulfide reducing agents in aqueous extracts from unsprouted (BW-0), and sprouted buckwheat (48 h, BW-48; 72 h, BW-72). Band intensity is expressed as the percent of unsprouted buckwheat (assigned as 100%), considering at least two individual SDS-PAGE runs for each condition. Statistical analysis was by one-way ANOVA (always: p < 0.05) with Tukey’s post hoc test (different letters indicate significant differences).
Sprouting was accompanied by a time-dependent proteolysis of the large soluble aggregates evident under non-reducing conditions to produce species of Mr around 40 kDa, likely along with smaller peptides that may have escaped detection. The 40 kDa species were not present in the extracts from unsprouted buckwheat and were not observed in the presence of disulfide reductants, suggesting they are formed by disulfide bound peptides originating from nicking of the original aggregates upon sprouting.
As shown in Figure 2B, the largest peptides evident in the unsprouted sample under reducing conditions were quite insensitive to sprouting-dependent proteolysis, and there was a marked difference with the time-dependent decrease in the intensity of smaller bands. The progressive disappearance of the polypeptide at Mr around 35 kDa is particularly striking, as this component appears to be present in a nicked form at 48 h and to be almost completely degraded at 72 h. Finally, it is remarkable that the endogenous proteases activated during sprouting can break down even the smallest proteins in buckwheat, regardless of whether they were present in a free-living form rather than disulfide-linked to larger proteins. Indeed, analysis of band intensity in the regions that comprise polypeptides with Mr < 21.5 kDa (Figure 2B,D) indicates that the products of the proteolytic breakdown of larger species did not accumulate. On the contrary, they were progressively disappearing—at the equivalent rate—in samples analyzed in the presence and absence of disulfide reductants.
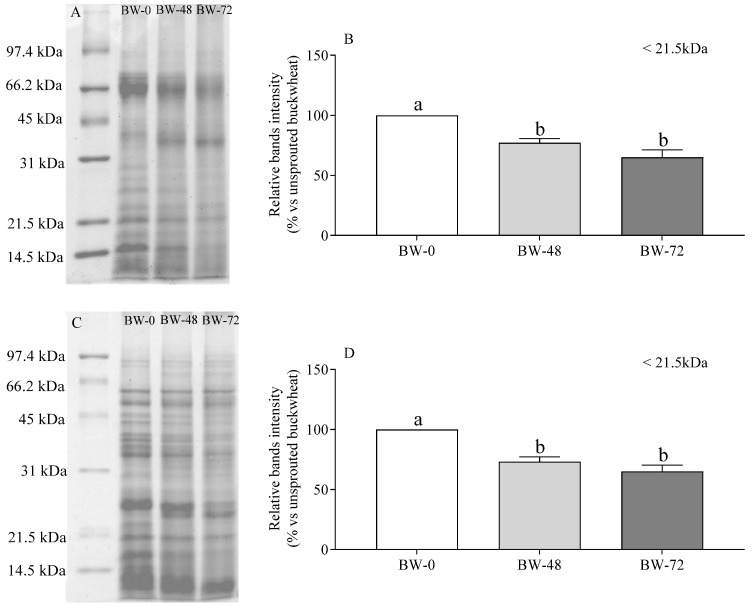
The overall pattern of events hypothesized above was confirmed by dye-binding and OPA assays, which can detect large proteins and small peptides, respectively. Results of the two assays at various sprouting times evidenced how a time-dependent decrease in the content of soluble proteins (Mr > 3 kDa, Figure 3A) [52] was accompanied by increased release of very small peptides and/or individual amino acids [53] (Figure 3B). The measurement of the endogenous protease activity on a convenient non-plant-protein substrate (Figure 3C) confirmed that the activity of endogenous proteases increased steadily in seeds during the sprouting period considered in this study. In buckwheat, protease activation was reported to increase up to day four of germination [54]. As in other grains, protein breakdown is essential to provide the amino acids required for embryo growth and plant development [8]. The nature, number, specificity, and activation mechanism of endogenous enzymes involved in this process remains to be assessed in buckwheat and other grains.
Figure 3.
Soluble protein (A), peptides/amino acids (B), and endogenous protease activity (C) in unsprouted (BW-0) and sprouted buckwheat (48 h, BW-48; 72 h, BW-72). Data are expressed as mg/g dw (A,B), and mg azocasein/h/g dw (C) and are means ± SD of two different extractions and at least duplicate assays. Statistical analysis was by one-way ANOVA (always: p < 0.05) with Tukey’s post hoc test (different letters indicate significant differences).
3.3. Lipids
As previously reported [55], the main fatty acids in buckwheat were linoleic > oleic > palmitic acid, which, together, accounted for approximately 90% of total fatty acids. Sprouting modulated the fatty acid composition of the buckwheat powder (Table 1). The sprouting-related decrease in total fatty acid content targeted mostly saturated fatty acids (SFA) and monounsaturated fatty acids (MUFA), and it was likely related to the re-activation of β-oxidation in the glyoxysomes to fulfill the energy needs of the growing seed [56]. It is of note that germination did not modify the total content of polyunsaturated fatty acids (PUFA), presumably due to their preferential use as a structural component of cellular membranes [57], thus imparting a healthier profile to the fats in sprouted buckwheat although increasing their peroxidability. The unsaturation index (UI) and peroxidability index (PI) increased in sprouted buckwheat (Table 1). A representative chromatogram of BW-0 has been included as Supplementary Material (Figure S1).
Table 1.
Fatty acid methyl esters and conjugated diene content in unsprouted (BW-0) and sprouted buckwheat (48 h, BW-48; 72 h, BW-72).
| FAME | BW-0 | BW-48 | BW-72 |
|---|---|---|---|
| C14:0 | 0.07 ± 0.01a | 0.05 ± 0.00a | 0.03 ± 0.04a |
| C16:0 | 3.96 ± 0.02a | 3.30 ± 0.18b | 2.81 ± 0.34c |
| C16:1 n-7 | 0.07 ± 0.10a | 0.05 ± 0.00a | 0.04 ± 0.06a |
| C17:0 | 0.03 ± 0.04a | 0.04 ± 0.00a | 0.02 ± 0.03a |
| C18:0 | 0.21 ± 0.07a | 0.08 ± 0.03b | 0.04 ± 0.05c |
| C18:1 n-9 | 8.09 ± 0.39a | 6.59 ± 0.36a | 4.72 ± 0.97b |
| C18:2 n-6 | 8.79 ± 0.60a | 9.14 ± 0.59a | 7.63 ± 1.25a |
| C18:3 n-3 | 0.52 ± 0.02b | 0.68 ± 0.04a | 0.65 ± 0.04a |
| C20:0 | 0.32 ± 0.06a | 0.28 ± 0.01a | 0.22 ± 0.09a |
| C20:1 n-9 | 0.68 ± 0.09a | 0.59 ± 0.01a | 0.39 ± 0.07b |
| C22:0 | 0.46 ± 0.19a | 0.31 ± 0.01a | 0.27 ± 0.11a |
| ΣSFA | 5.04 ± 0.27a | 4.06 ± 0.17b | 3.39 ± 0.42b |
| ΣMUFA | 8.84 ± 0.38a | 7.23 ± 0.37a | 5.15 ± 0.98b |
| ΣPUFA | 9.31 ± 0.62a | 9.82 ± 0.63a | 8.28 ± 1.3a |
| Σn-6/Σn-3 | 16.74 ± 0.49a | 13.46 ± 0.12b | 11.65 ± 1.15b |
| Total | 23.20 ± 1.27a | 21.11 ± 1.17ab | 16.81 ± 2.70b |
| UI | 120.65 ± 0.50c | 130.47 ± 0.65b | 132.97 ± 0.16a |
| PI | 43.35 ± 0.44c | 50.57 ± 0.39b | 53.92 ± 0.55a |
| CD | 100.00 ± 2.65c | 174.03 ± 20.29b | 252.00 ± 20.40a |
Fatty acids and conjugated dienes content are expressed as mg FAME/g dw and as percent (%) of unsprouted buckwheat flour (assigned as 100%), respectively. Data are means ± SD of two different extractions and duplicate gas-chromatographic analysis. Statistical analysis was by one-way ANOVA (C16:0, C18:0, C18:1 n-9, C18:3 n-3, C20:1 n-9, ΣSFA, ΣMUFA, Σn-6/Σn-3, total FAME, PI, UI, CD: p < 0.05) with Tukey’s post hoc test (different letters indicate significant differences). CD: conjugated diene; FAME: fatty acid methyl esters; SFA: saturated fatty acids; MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids; PI: peroxidability index; UI: unsaturation index.
Lipid peroxidation was evaluated by monitoring the concentration of CD-containing lipids [58], which increased significantly during sprouting. It is conceivable that the increased lipid peroxidation reflects the transition from seed dormancy to germination, a physiological process regulated by diverse endogenous factors, including reactive oxygen species, which promote the release of seed dormancy by biomolecules oxidation, testa weakening, and endosperm decay [59].
3.4. Bioactive Compounds
3.4.1. Tocols
Although buckwheat exhibits levels of tocopherols similar to wheat, barley, oats, and rye, with γ-tocopherol being the main isoform present, typically in a >10-fold excess with respect to α- and δ-tocopherols [60], buckwheat and corn bran, and wheat germ were dominated by tocopherols, whereas the oat, rice, rye, spelt, and wheat bran oils were rich in tocotrienols [61]. Accordingly, in this study, the main tocols in buckwheat were γ- > δ- ≃ α-tocopherol (Table 2). Sprouting did not affect the α-, γ-, and total tocopherol levels but showed a slight but significant decrease in the δ -isoform. Previous studies also reported sprouting-related changes in the content of tocopherols homologues [62] in various cereals [63,64].
Table 2.
Tocols content in unsprouted (BW-0) and sprouted buckwheat (48 h, BW-48; 72 h, BW-72).
| Tocols 1 | BW-0 | BW-48 | BW-72 |
|---|---|---|---|
| α-tocopherol | 2.11 ± 0.10a | 2.70 ± 0.32a | 2.48 ± 0.12a |
| γ-tocopherol | 56.07 ± 0.16a | 55.31 ± 5.03a | 44.55 ± 1.61a |
| δ-tocopherol | 3.79 ± 0.03a | 3.52 ± 0.05b | 2.95 ± 0.03c |
| Total tocols | 61.97 ± 0.26a | 61.53 ± 5.2a | 49.98 ± 2.10a |
Data are expressed as µg/g dw and are means ± SD of two extractions and duplicate chromatographic runs. Statistical analysis was by one-way ANOVA (δ-tocopherol: p < 0.05) with Tukey’s post hoc test (different letters indicate significant differences). 1 Calculated using the calibration curve of α-tocopherol.
3.4.2. Free and Bound Polyphenols
Phenolic compounds are present in plants either in free or bound form, the latter most commonly being ester-linked to structural cell wall polymers [65]. Although the major portion of phenolics in grains is in the bound form [66], buckwheat contains most of its phenolic compounds in the more bio-accessible free form [67,68].
In this study, 29 free phenolic compounds were identified and quantified in buckwheat. As summarized in Table 3, the buckwheat phenolics were representative of five classes, 6 phenolic acids; 15 flavan-3-ols; 3 flavonols; 3 flavones; and 2 proanthocyanidins. Among them, six were also found in a bound form (four phenolic acids, one flavonol, and one flavone). In unsprouted buckwheat, the most representative free phenolic classes were flavan-3-ols > proanthocyanidins > flavones, all together accounting for about 96% of the total free phenolics content. The main classes found in the bound form were phenolic acids and flavones, which accounted for about 92% of total bound phenolics. The total content in free phenolic compounds was 30 times higher than that of the bound species. The resulting relative abundance was in the order flavan-3-ols > free proanthocyanidins > free flavones > free phenolic acids > bound phenolic acids > free flavonols > bound flavones > bound flavonols > bound flavan-3-ols = bound proanthocyanidins.
Table 3.
Phenols content in unsprouted (BW-0) and sprouted buckwheat (48 h, BW-48; 72 h, BW-72).
| Compounds | [M − H]− | MS Fragments | Q. T. | Free Phenolic Compounds | Anova | Bound Phenolic Compounds | Anova | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BW-0 | BW-48 | BW-72 | BW-0 | BW-48 | BW-72 | ||||||
| Phenolic acids | |||||||||||
| Protocatechuic-4-O-glucoside acid | 315 | 153 | 315→153 | 30.25 ± 1.01c | 59.31 ± 0.73b | 82.90 ± 4.24a | p < 0.05 | 11.92 ± 0.09a | 3.39 ± 0.61b | 2.93 ± 0.10b | p < 0.05 |
| Caffeic acid hexose | 341 | 251 | 341→251 | 1.88 ± 0.22a | 2.15 ± 0.03a | 2.09 ± 0.25a | n.s. | n.d. | n.d. | n.d. | |
| Caffeic acid hexose | 341 | 251 | 341→251 | n.d.c | 5.82 ± 0.85b | 13.30 ± 0.56a | p < 0.05 | n.d. | n.d. | n.d. | |
| p-Coumaric acid | 163 | 119 | 163→119 | 3.25 ± 0.60b | 8.76 ± 0.52a | 9.08 ± 0.92a | p < 0.05 | n.d.b | 0.25 ± 0.04b | 3.28 ± 0.59a | p < 0.05 |
| Swertiamacroside | 487 | 451, 179 | 487→179 | 0.93 ± 0.15b | 3.95 ± 0.67a | 4.44 ± 0.62a | p < 0.05 | 22.99 ± 1.64a | 10.37 ± 0.67b | 15.48 ± 1.22b | p < 0.05 |
| Ferulic acid | 193 | 178 | 193→178 | n.d.c | 2.49 ± 0.15b | 3.84 ± 0.49a | p < 0.05 | n.d.b | 0.32 ± 0.03b | 3.67 ± 0.37a | p < 0.05 |
| Total phenolic acids | 36.31 ± 1.53c | 82.48 ± 1.43b | 115.65 ± 2.82a | p < 0.05 | 34.90 ± 1.55a | 14.34 ± 1.27c | 25.36 ± 1.54b | p < 0.05 | |||
| Flavan-3-ols | |||||||||||
| Catechin-glucoside | 451 | 289 | 451→289 | 118.76 ± 2.96c | 202.06 ± 28.76b | 356.54 ± 0.70a | p < 0.05 | n.d. | n.d. | n.d. | |
| Catechin | 289 | 203 | 289→203 | 0.87 ± 0.05c | 36.42 ± 2.88b | 60.88 ± 5.11a | p < 0.05 | n.d. | n.d. | n.d. | |
| (Epi)afzelchin-(epi)catechin isomer A | 561 | 543, 435, 425, 407, 289, 271 |
561→289 | 38.70 ± 1.04a | 2.12 ± 0.06c | 6.78 ± 0.23b | p < 0.05 | n.d. | n.d. | n.d. | |
| Catechin-glucoside | 451 | 289 | 451→289 | 95.00 ± 0.88b | 117.91 ± 2.69a | 35.68 ± 3.91c | p < 0.05 | n.d. | n.d. | n.d. | |
| Epicatechin | 289 | 244 | 289→244 | 59.42 ± 1.13a | 20.65 ± 0.21b | 16.25 ± 1.36c | p < 0.05 | n.d. | n.d. | n.d. | |
| Catechin-glucoside | 451 | 289 | 451→289 | 17.66 ± 2.96b | 38.20 ± 6.74a | 29.02 ± 2.10ab | p < 0.05 | n.d. | n.d. | n.d. | |
| (Epi)afzelchin-(epi)catechin isomer B | 561 | 543, 435, 425, 407, 289, 271 |
561→289 | 124.29 ± 0.46a | 55.97 ± 1.6b | 21.66 ± 1.41c | p < 0.05 | n.d. | n.d. | n.d. | |
| Epiafzelchin-epiafzelchin-epicatechin | 833 | 561, 543, 289, 271 | 833→561 | 143.23 ± 9.48a | 140.22 ± 0.02a | 125.91 ± 2.01a | n.s | n.d. | n.d. | n.d. | |
| (Epi)afzelchin-(epi)catechin isomer C | 561 | 543, 435, 425, 407, 289, 271 |
561→289 | 45.04 ± 8.17a | 10.89 ± 2.09b | 13.65 ± 0.63b | p < 0.05 | n.d. | n.d. | n.d. | |
| Epicatechin-gallate | 441 | 289, 169 | 441→169 | 9.62 ± 1.12b | 20.15 ± 1.38a | 10.18 ± 0.04b | p < 0.05 | n.d. | n.d. | n.d. | |
| Epiafzelchin-epicatechin-O-methyl gallate | 727 | 561, 455, 289, 271 | 727→289 | 125.94 ± 3.21a | 128.91 ± 1.63a | 119.97 ± 5.65a | n.s. | n.d. | n.d. | n.d. | |
| (-)-Epicatechin-3-(3″-O-methyl) gallate | 455 | 289, 183 | 4555→183 | 149.20 ± 1.76a | 48.49 ± 7.36b | 43.41 ± 0.79b | p < 0.05 | n.d. | n.d. | n.d. | |
| (Epi)afzelchin-(epi)catechin isomer D | 561 | 543, 435, 425, 407, 289, 271 |
561→289 | 32.32 ± 2.90a | 8.32 ± 0.23b | 0.67 ± 0.03c | p < 0.05 | n.d. | n.d. | n.d. | |
| Epiafzelchin-epicatechin-O-dimethyl gallate | 741 | 469, 319, 271 | 741→469 | 78.74 ± 4.91a | 32.42 ± 0.97b | 34.20 ± 1.14b | p < 0.05 | n.d. | n.d. | n.d. | |
| Epicatechin-O-3,4-dimethyl gallate | 469 | 319, 271, 125 | 469→271 | 126.39 ± 4.50a | 25.58 ± 0.79b | 19.49 ± 1.08b | p < 0.05 | n.d. | n.d. | n.d. | |
| Total flavan-3-ol | 1165.17 ± 14.29a | 888.32 ± 37.45b | 894.29 ± 13.16b | p < 0.05 | n.d. | n.d. | n.d. | ||||
| Flavonols | |||||||||||
| Quercitrin | 447 | 301, 179, 151 | 447→301 | 4.64 ± 0.50b | 3.20 ± 0.13b | 8.61 ± 1.41a | p < 0.05 | n.d. | n.d. | n.d. | |
| Rutin | 609 | 301 | 609→301 | 9.91 ± 0.84c | 15.59 ± 0.14b | 23.01 ± 1.15a | p < 0.05 | n.d. | n.d. | n.d. | |
| Quercetin | 301 | 178, 151 | 30→151 | 3.39 ± 0.15b | 21.37 ± 1.02a | 28.12 ± 2.93a | p < 0.05 | 4.29 ± 0.01a | n.d.b | n.d.b | p < 0.05 |
| Total flavonols | 17.93 ± 1.19c | 40.17 ± 1.29b | 59.74 ± 2.66a | p < 0.05 | 4.29 ± 0.01a | n.d.b | n.d.b | p < 0.05 | |||
| Flavones | |||||||||||
| Orientin | 447 | 357, 327 | 447→357 | 2.42 ± 0.18c | 16.27 ± 0.54b | 24.20 ± 1.52a | p < 0.05 | n.d. | n.d. | n.d. | |
| Isorientin | 447 | 357, 327 | 447→357 | 9.56 ± 1.73c | 403.42 ± 22.51b | 1419.45 ± 144.04a | p < 0.05 | n.d. | n.d. | n.d. | |
| Vitexin | 431 | 311 | 431→311 | 75.78 ± 4.48c | 1966.23 ± 246.04b | 5263.67 ± 352.42a | p < 0.05 | 15.60 ± 1.58c | 303.08 ± 8.66b | 1050.46 ± 63.59a | p < 0.05 |
| Total flavones | 87.76 ± 6.03a | 2385.92 ± 269.09b | 6707.32 ± 206.86a | p < 0.05 | 15.60 ± 1.58c | 303.08 ± 8.66b | 1050.46 ± 63.59a | p < 0.05 | |||
| Proanthocyanidins | |||||||||||
| Procyanidin B2-3-O-gallate | 729 | 577, 289 | 729→577 | 65.68 ± 4.47b | 67.15 ± 0.24b | 139.11 ± 4.20a | p < 0.05 | n.d. | n.d. | n.d. | |
| Procyanidin B2 | 577 | 425, 407, 289 | 577→425 | 48.03 ± 0.39c | 176.24 ± 5.77b | 491.72 ± 20.12a | p < 0.05 | n.d. | n.d. | n.d. | |
| Total proanthocyanidins | 113.71 ± 4.86c | 243.39 ± 6.01b | 630.83 ± 24.31a | p < 0.05 | n.d. | n.d. | n.d. | ||||
| Total phenols compounds | 1420.89 ± 3.06c | 3640.28 ± 234.93b | 8407.83 ± 163.85a | p < 0.05 | 54.79 ± 3.12c | 317.42 ± 9.93b | 1075.82 ± 62.05a | p < 0.05 | |||
Data are expressed µg/g dw and are means ± SD of two extraction and duplicate chromatographic analysis. Statistical analysis was by one-way ANOVA with Tukey’s post hoc test (different letters indicate significant differences). Q. T.: Quantification transition.
In 48 h and 72 h sprouted buckwheat, the primary free phenolic classes were flavones > flavan-3-ols > proanthocyanidins, accounting for approximately 98% of total free phenolic compounds. The main bound phenolic classes were flavones, which accounted for about 97% of total bound phenolic compounds. Total free phenolic compounds were approximately 11 and 8 times higher than the bound counterpart in 48 and 72 h sprouted buckwheat, respectively. The main phenolic classes in 48 h sprouted buckwheat were free flavones > free flavan-3-ols > bound flavones > free proanthocyanidins > free phenolic acids > free flavonols > bound phenolic acids > bound flavan-3-ols = bound flavonols = bound proanthocyanidins. The main phenolic classes in 72 h sprouted buckwheat were free flavones > bound flavones > free flavan-3-ols > free proanthocyanidins > free phenolic acids > free flavonols > bound phenolic acids > bound flavan-3-ols = bound flavonols = bound proanthocyanidins. Sprouting determined an increase in total free phenolic acids, total free flavonols, total free flavones, total free proanthocyanidins, total free phenols compounds, total bound flavones, and total bound phenols compounds content. On the contrary, sprouting caused a diminished content of total free flavan-3-ol, total bound phenolic acids, and total bound flavonols.
De novo synthesis and transformation could be responsible for the dramatically increased levels of polyphenols during germination. The primary building block for the synthesis of phenolic compounds is glucose, and several crucial molecular signaling pathways, including the oxidative pentose phosphate pathway, glycolysis, acetate/malonate pathway, shikimate pathway, phenylpropanoid pathway, and hydrolyzable tannin pathway, are involved in the synthesis and transformation of polyphenols during the earliest phases of plant growth [69]. Although we have considered the seeds as a whole, a previous study showed that polyphenols compounds are mainly accumulated in cotyledons and hypocotyls during buckwheat germination [70]. In addition, de novo synthesis could result from the activation of phenylalanine ammonia lyase (PAL), the key enzyme in phenolic biosynthesis involved in forming phenylpropanoids, hydroxycinnamates, flavonoids, proanthocyanidins, hydroxystilbenes, coumarins, lignans, and lignins [71]. During buckwheat germination, a positive linear correlation between PAL activity and flavonoids and phenolic accumulation was evidenced, suggesting that the variation in PAL activity was probably involved with phenolic (or flavonoid) accumulation [72].
3.5. Antioxidant Capacity
The sprouting-dependent increase in the content of phenolics also brought forward an increase in antioxidant capacity. As shown in Table 4, FRAP in aqueous buckwheat extracts increased progressively during sprouting, whereas TAC was statistically significant only after 72 h. Albeit not statistically significant, a close linear correlation was present between TAC and free phenolic content (Pearson r = 0.996, r2 = 0.992, p = 0.057). The feebler correlation between free phenolic content and antioxidant activity is probably because the antioxidant activity results from a different type of extractable bioactive component with antioxidant activity, such as citric, ferulic, and ascorbic acids, frequently present in cereal seed [73]. In addition to the polyphenols content, the structure–activity relationship and the in vitro assay adopted should be also deeply considered in evaluating the polyphenols’ antioxidant activity [74]. Hydroxyl groups on ring-B and the presence of a 3-hydroxyl group on ring-C in flavonoids increased TAC and FRAP but phenolic acids lacking a 3-hydroxyl group had significantly lower FRAP [75].
Table 4.
Total antioxidant capacity (TAC) and ferric reducing antioxidant power (FRAP) of unsprouted (BW-0) and sprouted buckwheat (48 h, BW-48; 72 h, BW-72).
| BW-0 | BW-48 | BW-72 | |
|---|---|---|---|
| TAC | 26.21 ± 4.23b | 31.09 ± 2.81b | 46.99 ± 3.21a |
| FRAP | 9.91 ± 0.45c | 13.63 ± 0.20b | 17.98 ± 0.40a |
TAC and FRAP are expressed as µmol trolox eq/g dw. Data are means ± SD of two different extractions and triplicate spectrophotometric analysis. Statistical analysis was by one-way ANOVA (always: p < 0.05) with Tukey’s post hoc test (different letters indicate significant differences).
Augmented levels in antioxidants, mainly in the most bioaccessibility-free form of polyphenols, may have a major nutritional backlash related to their antioxidant ability. Merendino et al. [76] evidenced in spontaneously hypertensive (SHR) and Wistar-Kyoto rats fed with pasta made with 30% of sprouted buckwheat powder an amended plasma antioxidant capacity and reduced oxidative markers and genotoxic effects respect rats fed with commercial pasta. Moreover, oxidatively stressed SHR rats fed with sprouted buckwheat powder-enriched pasta also determined a significant decrease in DNA damage and a more efficient DNA repair than the control diet [77].
3.6. Metabolome
The NMR spectroscopy is an essential tool that provides information for the molecular characterization of natural products due to its intrinsic ability of quantifying all detectable components in complex mixtures, directly without a preliminary separation. Particularly, 1H NMR-based metabolomics have proven effective and efficient because 1H atoms are ubiquitous and in high isotopic abundance, thus allowing high-throughput acquisition of spectra to identify and quantify most metabolites [78]. Using an untargeted approach, it was tested whether sprouting determined a time-dependent increase in free amino acid, sugars, and organic acid levels, indicating the re-activation of seed metabolism upon germination.
The effect of sprouting on the metabolome has been evaluated by an NMR-based metabolomics approach in combination with Foodomics and Chemometrics techniques [79]. Unsupervised principal component analysis was carried out on a binned dataset. The obtained model was used to select metabolites mainly involved in the source of diversification between samples collected in the whole experimental set, by applying the ANOVA on the total bins. This approach allowed for assessing the time course of changes in the metabolic profile at various sprouting times. A total of 15 metabolites, including 7 free amino acids, 3 sugar, 3 organic acids, 1 nucleotide, and 1 nicotinic acid derivative were identified throughout the 1H NMR spectra. Table 5 lists the essential bins that changed their integral area as the concentration levels of the corresponding metabolites changed during sprouting. In summary, sprouting is associated with an increased concentration of tryptophan, glutamine, alanine, valine, isoleucine, glucose, fructose, acetate, and lactate, whereas a decrease in trigonelline concentration was observed upon sprouting.
Table 5.
Metabolomic profile of unsprouted (BW-0) and sprouted buckwheat (48 h, BW-48; 72 h, BW-72).
| Metabolites | ppm (δ) | BW-0 | BW-48 | BW-72 |
|---|---|---|---|---|
| Tryptophan | 7.735 (d), 7.754 (d), 7.265 (t), 7.201 (t) | 90 ± 1.25b | 106 ± 12.7b | 130 ± 12.7a |
| Phenylalanine | 7.431 (m), 7.379 (m), 7.344 (d) | 719 ± 53a | 772 ± 50a | 737 ± 26a |
| Tyrosine | 7.194 (d) | 269 ± 51.4a | 280 ± 10a | 272 ± 20a |
| Glutamine | 2.475 (m), 2.155 (m) | 1305 ± 32b | 1870 ± 211b | 3876 ± 159a |
| Alanine | 1.480 (d) | 570 ± 14b | 770 ± 12a | 719 ± 50a |
| Valine | 1.042 (d), 0.978 (d) | 213 ± 21b | 384 ± 38a | 344 ± 68a |
| Isoleucine | 1.010 (d), 0.950 (t) | 183 ± 11b | 284 ± 36a | 237 ± 63a |
| Sucrose | 5.412 (d) | 872 ± 202a | 1451 ± 796a | 1536 ± 771a |
| Glucose | 5.237 (d) | 300 ± 20.2b | 2476 ± 202a | 2320 ± 202a |
| Fructose | 4.107 (d) | 753 ± 397b | 1044 ± 329ab | 1581 ± 605a |
| Acetate | 1.949 (s) | 291 ± 32b | 310 ± 32ab | 330 ± 27a |
| Lactate | 1.331 (d) | 329 ± 5b | 519 ± 55a | 497 ± 79a |
| GABA | 3.023 (t), 2.313 (t), 1.905 (m) | 987 ± 17a | 985 ± 29a | 955 ± 30.6a |
| NADP+ | 9.341 (s), 9.103 (d), 8.841 (d), 8.452 (s) | 24.5 ± 2.06a | 20.8 ± 2.65a | 20.4 ± 3.26a |
| Trigonelline | 9.090 (s), 8.840 (m) | 68 ± 4a | 52 ± 11ab | 47 ± 13b |
Data are expressed as signal area and are means ± SD of five spectroscopic analyses. Statistical analysis was by one-way ANOVA (tryptophan, glutamine, alanine, valine, isoleucine, glucose, fructose, acetate, lactate, trigonelline: p < 0.05) with Tukey’s post hoc test (different letters indicate significant differences). For convenience, only one signal is reported for sucrose, glucose, and fructose. The letters in brackets indicate multiplicity (s: singlet; d: doublet; t: triplet; m: multiplet). GABA: γ-aminobutyric acid; NADP+: nicotinamide adenine dinucleotide phosphate.
Although NMR has just been used to identify the major metabolites in sprouted legumes [80,81], to the best of our knowledge, this is the only study that used it to evaluate the impact of sprouting on the metabolome in germinated buckwheat. The increased glucose levels in the sprouted samples confirm the relevance of starch hydrolysis during germination. In addition, the increased levels of fructose, acetate, and lactate appear likely associated with a burst in glucose metabolism and enzymes activation [54,82]. Along the same line of reasoning, substantial endogenous proteolysis during sprouting increases (almost 3-fold, at 72 h) the levels of several free amino acids and glutamine, the latter is an amino acid abundant in storage proteins and relevant to nitrogen metabolism. These results indicate that metabolites dynamics during seeds germination are pretty complicated as substances are continuously synthesized, recycled, and degraded due to metabolism reactivation. This demonstrates that analyzing the chemical diversity and the wide range of metabolite concentrations in plants necessitates authoritative analytical approaches. In this complicated but intriguing scenario, 1H-NMR spectroscopy could be considered a powerful analytical tool, offering the opportunity for reliable metabolite detection and investigating the whole set of metabolites, which are essentially nongenetically encoded substrates, intermediates, and products of biochemical pathways [81,83,84].
3.7. Anti-Nutritional Factors
Sprouting decreased steadily the content of phytic acid but the analytical difference was statistically significant only after 72 h of germination (Table 6).
Table 6.
Phytic acid content of unsprouted (BW-0) and sprouted buckwheat (48 h, BW-48; 72 h, BW-72).
| BW-0 | BW-48 | BW-72 | |
|---|---|---|---|
| Phytic acid | 13.34 ± 0.27a | 12.09 ± 0.38ab | 11.38 ± 0.34b |
Data are expressed as mg/g dw. Data are means ± SD of two different extractions and triplicate spectrophotometric analysis. Statistical analysis was by one-way ANOVA (p < 0.05) with Tukey’s post hoc test (different letters indicate significant differences).
The process of sprouting can decrease phytate concentrations present through the activation and de novo synthesis of phytase, which release myo-inositol, phosphate, and other minerals for plant growth [85], as well as the leaching of water-soluble phytate during soaking [86].
Since cereal grains are a natural source of protease inhibitors [87], the effect of sprouting on the activity of the major digestive enzymes was evaluated to the same enzymes without buckwheat extracts. As shown in Table 7, unsprouted buckwheat aqueous extracts almost blocked trypsin activity, and the removal of trypsin inhibitors upon sprouting was non-linear, with more than 50% inhibitory capacity still present at 72 h of sprouting. Chymotrypsin activity was not affected by the addition of any of the buckwheat extracts, whereas BW-72 extract significantly enhanced pepsin activity.
Table 7.
Pepsin, trypsin, and chymotrypsin activity in the absence or presence of unsprouted (BW-0) and sprouted buckwheat (48 h, BW-48; 72 h, BW-72).
| w/o BW Extract | BW-0 | BW-48 | BW-72 | |
|---|---|---|---|---|
| Pepsin activity | 89.44 ± 4.81b | 92.41 ± 5.16b | 107.41 ± 20.50ab | 152.22 ± 27.76a |
| Trypsin activity | 180.37 ± 49.83a | 6.39 ± 2.03c | 65.56 ± 14.35bc | 82.96 ± 8.26b |
| Chymotrypsin activity | 540.28 ± 71.10a | 360.54 ± 62.22a | 372.78 ± 63.79a | 359.81 ± 46.65a |
Pepsin, trypsin, and chymotrypsin activities are expressed as U/mg enzymes. Data are means ± SD of two different extractions and at least triplicate activity measurements. Statistical analysis was by one-way ANOVA (pepsin and trypsin: p < 0.05) with Tukey’s post hoc test (different letters indicate significant differences).
Although various studies evidenced an ameliorative effect of sprouting on trypsin inhibition and phytates content due to enzymatic degradation [88,89,90], to the best of our knowledge, this is the first study evaluating the effect of sprouting on pepsin and chymotrypsin activity in buckwheat. The impact on digestive proteases reported here confirms recent reports on improved in vitro protein digestibility in sprouted brown finger millet [91] and sprouted wheat [92]. These results appear to be of particular importance in light of the use and exploitation of germinated cereals to obtain foods with a higher digestibility.
4. Conclusions
Sprouting caused significant changes in the composition of buckwheat seeds. While some changes were already maximal after 48 h, 72 h appeared to be the best sprouting duration after this method had been adopted. The increased release of free peptides/amino acids and phenolic compounds with antioxidant activity, and the substantial decrease in anti-nutritional factors, suggest sprouting as a suitable process to improve buckwheat nutritional properties, thus obtaining a high-quality ingredient. With this in mind, the final food products should carefully monitor some changes observed after sprouting. In fact, increased perishability could result from increased oxidation of lipids.
Further studies, including evaluating the digestibility of sprouted material, the presence of bioactive species in proteolytic fragments, and the effect on the gut microbiota are needed to boost the industrial exploitation of buckwheat and traditional buckwheat foods.
Acknowledgments
The Authors thank Mattia Gardella for his skillful assistance and Francesco Bonomi for reading and commenting on the manuscript.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods12102047/s1.
Author Contributions
Conceptualization, A.B. (Alberto Barbiroli), A.M., S.I. and M.D.N.; Data curation, S.M.B., E.C., F.P., G.P., S.M. and M.D.N.; funding acquisition, A.M. and S.I.; investigation, S.M.B., E.C., F.P., G.P., S.M. and M.D.N.; methodology, S.M.B., E.C., F.P., G.P., S.M. and M.D.N.; supervision, A.B. (Alberto Barbiroli), A.M., S.I. and M.D.N.; validation, S.M.B., E.C., F.P., G.P., S.M., F.C., A.B. (Alessandra Bordoni), A.B. (Alberto Barbiroli), A.M., S.I. and M.D.N.; writing—original draft, S.M.B. and M.D.N.; writing—review and editing, S.M.B., E.C., F.P., G.P., S.M., F.C., A.B. (Alessandra Bordoni), A.B. (Alberto Barbiroli), A.M., S.I. and M.D.N. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research is part of the following projects (1) “MIND FoodS HUB (Milano Innovation District Food System Hub): Innovative concept for the eco-intensification of agricultural production and for the promotion of dietary patterns for human health and longevity through the creation in MIND of a digital Food System Hub”, co-funded by POR FESR 2014–2020_BANDO Call HUB Ricerca e Innovazione, Regione Lombardia; (2) One Health Action Hub: University Task Force for the resilience of territorial ecosystems, funded by Università degli Studi di Milano (PSR 2021-GSA-Linea 6).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kreft M. Buckwheat phenolic metabolites in health and disease. Nutr. Res. Rev. 2016;29:30–39. doi: 10.1017/S0954422415000190. [DOI] [PubMed] [Google Scholar]
- 2.Aubert L., Konrádová D., Barris S., Quinet M. Different drought resistance mechanisms between two buckwheat species Fagopyrum esculentum and Fagopyrum tataricum. Physiol. Plant. 2021;172:577–586. doi: 10.1111/ppl.13248. [DOI] [PubMed] [Google Scholar]
- 3.Germ M., Gaberščik A. Chapter twenty one—The Effect of Environmental Factors on Buckwheat. In: Zhou M., Kreft I., Woo S.-H., Chrungoo N., Wieslander G., editors. Molecular Breeding and Nutritional Aspects of Buckwheat. Academic Press; Cambridge, MA, USA: 2016. pp. 273–281. [Google Scholar]
- 4.Singh M., Malhotra N., Sharma K. Buckwheat (Fagopyrum sp.) genetic resources: What can they contribute towards nutritional security of changing world? Genet. Resour. Crop Evol. 2020;67:1639–1658. doi: 10.1007/s10722-020-00961-0. [DOI] [Google Scholar]
- 5.Sturza A., Păucean A., Chiș M.S., Mureșan V., Vodnar D.C., Man S.M., Urcan A.C., Rusu I.E., Fostoc G., Muste S. Influence of Buckwheat and Buckwheat Sprouts Flours on the Nutritional and Textural Parameters of Wheat Buns. Appl. Sci. 2020;10:7969. doi: 10.3390/app10227969. [DOI] [Google Scholar]
- 6.Giménez-Bastida J.A., Piskuła M., Zieliński H. Recent advances in development of gluten-free buckwheat products. Trends Food Sci. Technol. 2015;44:58–65. doi: 10.1016/j.tifs.2015.02.013. [DOI] [Google Scholar]
- 7.Mattila P.H., Pihlava J.-M., Hellström J., Nurmi M., Eurola M., Mäkinen S., Jalava T., Pihlanto A. Contents of phytochemicals and antinutritional factors in commercial protein-rich plant products. Food Qual. Saf. 2018;2:213–219. doi: 10.1093/fqsafe/fyy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saithalavi K.M., Bhasin A., Yaqoob M. Impact of sprouting on physicochemical and nutritional properties of sorghum: A review. J. Food Meas. Charact. 2021;15:4190–4204. doi: 10.1007/s11694-021-00969-9. [DOI] [Google Scholar]
- 9.Ikram A., Saeed F., Afzaal M., Imran A., Niaz B., Tufail T., Hussain M., Anjum F.M. Nutritional and end-use perspectives of sprouted grains: A comprehensive review. Food Sci. Nutr. 2021;9:4617–4628. doi: 10.1002/fsn3.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemmens E., Moroni A.V., Pagand J., Heirbaut P., Ritala A., Karlen Y., Lê K.-A., Van den Broeck H.C., Brouns F.J.P.H., De Brier N., et al. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2019;18:305–328. doi: 10.1111/1541-4337.12414. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez-Jubete L., Wijngaard H., Arendt E.K., Gallagher E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010;119:770–778. doi: 10.1016/j.foodchem.2009.07.032. [DOI] [Google Scholar]
- 12.Molska M., Reguła J., Zielińska-Dawidziak M., Tomczak A., Świeca M. Starch and protein analysis in buckwheat (Fagopyrum esculentum Moench) sprouts enriched with probiotic yeast. LWT. 2022;168:113903. doi: 10.1016/j.lwt.2022.113903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemzer B., Al-Taher F. Analysis of Fatty Acid Composition in Sprouted Grains. Foods. 2023;12:1853. doi: 10.3390/foods12091853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Setia R., Dai Z., Nickerson M.T., Sopiwnyk E., Malcolmson L., Ai Y. Properties and bread-baking performance of wheat flour composited with germinated pulse flours. Cereal Chem. 2020;97:459–471. doi: 10.1002/cche.10261. [DOI] [Google Scholar]
- 15.Sharanagat V.S., Nema P.K. Bread preparation by partial replacement of wheat by germinated sorghum. Food Sci. Technol. Int. 2023;29:13–24. doi: 10.1177/10820132211058002. [DOI] [PubMed] [Google Scholar]
- 16.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.Di Nunzio M., Loffi C., Chiarello E., Dellafiora L., Picone G., Antonelli G., Di Gregorio C., Capozzi F., Tedeschi T., Galaverna G., et al. Impact of a Shorter Brine Soaking Time on Nutrient Bioaccessibility and Peptide Formation in 30-Months-Ripened Parmigiano Reggiano Cheese. Molecules. 2022;27:664. doi: 10.3390/molecules27030664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freitas C., Souza D., Araújo E., Cavalheiro M., Oliveira L., Ramos M. Anti-oxidative and proteolytic activities and protein profile of laticifer cells of Cryptostegia grandiflora, Plumeria rubra and Euphorbia tirucalli. Braz. J. Plant Physiol. 2009;22:11–22. doi: 10.1590/S1677-04202010000100002. [DOI] [Google Scholar]
- 19.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- 20.Stoffel W., Insull W., Jr., Ahrens E.H., Jr. Gas-liquid chromatography of highly unsaturated fatty acid methyl esters. Proc. Soc. Exp. Biol. Med. 1958;99:238–241. doi: 10.3181/00379727-99-24307. [DOI] [PubMed] [Google Scholar]
- 21.Bub A., Malpuech-Brugère C., Orfila C., Amat J., Arianna A., Blot A., Di Nunzio M., Holmes M., Kertész Z., Marshall L., et al. A Dietary Intervention of Bioactive Enriched Foods Aimed at Adults at Risk of Metabolic Syndrome: Protocol and Results from PATHWAY-27 Pilot Study. Nutrients. 2019;11:1814. doi: 10.3390/nu11081814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Nunzio M., van Deursen D., Verhoeven A.J., Bordoni A. n-3 and n-6 Polyunsaturated fatty acids suppress sterol regulatory element binding protein activity and increase flow of non-esterified cholesterol in HepG2 cells. Br. J. Nutr. 2010;103:161–167. doi: 10.1017/S000711450999167X. [DOI] [PubMed] [Google Scholar]
- 23.Di Nunzio M., Valli V., Bordoni A. Pro- and anti-oxidant effects of polyunsaturated fatty acid supplementation in HepG2 cells. Prostaglandins Leukot. Essent. Fat. Acids. 2011;85:121–127. doi: 10.1016/j.plefa.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Situnayake R.D., Crump B.J., Zezulka A.V., Davis M., McConkey B., Thurnham D.I. Measurement of conjugated diene lipids by derivative spectroscopy in heptane extracts of plasma. Pt 3Ann. Clin. Biochem. 1990;27:258–266. doi: 10.1177/000456329002700313. [DOI] [PubMed] [Google Scholar]
- 25.Urbinati E., Di Nunzio M., Picone G., Chiarello E., Bordoni A., Capozzi F. The Effect of Balsamic Vinegar Dressing on Protein and Carbohydrate Digestibility is Dependent on the Food Matrix. Foods. 2021;10:411. doi: 10.3390/foods10020411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Nunzio M., Picone G., Pasini F., Chiarello E., Caboni M.F., Capozzi F., Gianotti A., Bordoni A. Olive oil by-product as functional ingredient in bakery products. Influence of processing and evaluation of biological effects. Food Res. Int. 2020;131:108940. doi: 10.1016/j.foodres.2019.108940. [DOI] [PubMed] [Google Scholar]
- 27.Pasini F., Gómez-Caravaca A.M., Blasco T., Cvejić J., Caboni M.F., Verardo V. Assessment of Lipid Quality in Commercial Omega-3 Supplements Sold in the French Market. Biomolecules. 2022;12:1361. doi: 10.3390/biom12101361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marzocchi S., Caboni M.F., Pasini F. Co-milling process of olives and oleaginous matrices with high nutritional value: A preliminary characterisation of the obtained oils. Int. J. Food Sci. Nutr. 2022;73:1057–1066. doi: 10.1080/09637486.2022.2128309. [DOI] [PubMed] [Google Scholar]
- 29.Bombai G., Pasini F., Verardo V., Sevindik O., Di Foggia M., Tessarin P., Bregoli A.M., Caboni M.F., Rombolà A.D. Monitoring of compositional changes during berry ripening in grape seed extracts of cv. Sangiovese (Vitis vinifera L.) J. Sci. Food Agric. 2017;97:3058–3064. doi: 10.1002/jsfa.8151. [DOI] [PubMed] [Google Scholar]
- 30.Martín-García B., Pasini F., Verardo V., Gómez-Caravaca A.M., Marconi E., Caboni M.F. Distribution of Free and Bound Phenolic Compounds in Buckwheat Milling Fractions. Foods. 2019;8:670. doi: 10.3390/foods8120670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gómez-Caravaca A.M., Verardo V., Berardinelli A., Marconi E., Caboni M.F. A chemometric approach to determine the phenolic compounds in different barley samples by two different stationary phases: A comparison between C18 and pentafluorophenyl core shell columns. J. Chromatogr. A. 2014;1355:134–142. doi: 10.1016/j.chroma.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Di Nunzio M., Bordoni A., Aureli F., Cubadda F., Gianotti A. Sourdough Fermentation Favorably Influences Selenium Biotransformation and the Biological Effects of Flatbread. Nutrients. 2018;10:1898. doi: 10.3390/nu10121898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones A., Acquaviva A., Suktham T., Dennis G.R., Shalliker R.A., Soliven A. Total Antioxidant Capacity with Peak Specificity via Reaction Flow Chromatography and the Ferric Reducing Antioxidant Power Assay. Food Anal. Methods. 2020;13:608–616. doi: 10.1007/s12161-019-01675-5. [DOI] [Google Scholar]
- 34.Hatzakis E. Nuclear Magnetic Resonance (NMR) Spectroscopy in Food Science: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019;18:189–220. doi: 10.1111/1541-4337.12408. [DOI] [PubMed] [Google Scholar]
- 35.Dieterle F., Ross A., Schlotterbeck G., Senn H. Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in 1H NMR Metabonomics. Anal. Chem. 2006;78:4281–4290. doi: 10.1021/ac051632c. [DOI] [PubMed] [Google Scholar]
- 36.Craig A., Cloarec O., Holmes E., Nicholson J.K., Lindon J.C. Scaling and Normalization Effects in NMR Spectroscopic Metabonomic Data Sets. Anal. Chem. 2006;78:2262–2267. doi: 10.1021/ac0519312. [DOI] [PubMed] [Google Scholar]
- 37.Ma X., Liu Y., Liu J., Zhang J., Liu R. Changes in starch structures and in vitro digestion characteristics during maize (Zea mays L.) germination. Food Sci. Nutr. 2020;8:1700–1708. doi: 10.1002/fsn3.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chinma C.E., Abu J.O., Adedeji O.E., Aburime L.C., Joseph D.G., Agunloye G.F., Adebo J.A., Oyeyinka S.A., Njobeh P.B., Adebo O.A. Nutritional composition, bioactivity, starch characteristics, thermal and microstructural properties of germinated pigeon pea flour. Food Biosci. 2022;49:101900. doi: 10.1016/j.fbio.2022.101900. [DOI] [Google Scholar]
- 39.Suárez-Estrella D., Bresciani A., Iametti S., Marengo M., Pagani M.A., Marti A. Effect of Sprouting on Proteins and Starch in Quinoa (Chenopodium quinoa Willd.) Plant Foods Hum. Nutr. 2020;75:635–641. doi: 10.1007/s11130-020-00864-6. [DOI] [PubMed] [Google Scholar]
- 40.Ussenov Y.A., Akildinova A., Kuanbaevich B.A., Serikovna K.A., Gabdullin M., Dosbolayev M., Daniyarov T., Ramazanov T. The Effect of Non-Thermal Atmospheric Pressure Plasma Treatment of Wheat Seeds on Germination Parameters and α-Amylase Enzyme Activity. IEEE Trans. Plasma Sci. 2022;50:330–340. doi: 10.1109/TPS.2022.3145831. [DOI] [Google Scholar]
- 41.Marti A., Caramanico R., Bottega G., Pagani M.A. Cooking behavior of rice pasta: Effect of thermal treatments and extrusion conditions. LWT—Food Sci. Technol. 2013;54:229–235. doi: 10.1016/j.lwt.2013.05.008. [DOI] [Google Scholar]
- 42.Marchini M., Marti A., Folli C., Prandi B., Ganino T., Conte P., Fadda C., Mattarozzi M., Carini E. Sprouting of Sorghum (Sorghum bicolor [L.] Moench): Effect of Drying Treatment on Protein and Starch Features. Foods. 2021;10:407. doi: 10.3390/foods10020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma B., Gujral H.S. Modifying the dough mixing behavior, protein & starch digestibility and antinutritional profile of minor millets by sprouting. Int. J. Biol. Macromol. 2020;153:962–970. doi: 10.1016/j.ijbiomac.2019.10.225. [DOI] [PubMed] [Google Scholar]
- 44.Xie X., Qi L., Xu C., Shen Y., Wang H., Zhang H. Understanding how the cooking methods affected structures and digestibility of native and heat-moisture treated rice starches. J. Cereal Sci. 2020;95:103085. doi: 10.1016/j.jcs.2020.103085. [DOI] [Google Scholar]
- 45.Li F., Guan X., Li C. Effects of degree of milling on the starch digestibility of cooked rice during (in vitro) small intestine digestion. Int. J. Biol. Macromol. 2021;188:774–782. doi: 10.1016/j.ijbiomac.2021.08.079. [DOI] [PubMed] [Google Scholar]
- 46.Jiménez-Pulido I.J., Rico D., Martinez-Villaluenga C., Pérez-Jiménez J., Luis D.D., Martín-Diana A.B. Sprouting and Hydrolysis as Biotechnological Tools for Development of Nutraceutical Ingredients from Oat Grain and Hull. Foods. 2022;11:2769. doi: 10.3390/foods11182769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu T.F., Kise M., Wang M.F., Ito Y., Yang M.D., Aoto H., Yoshihara R., Yokoyama J., Kunii D., Yamamoto S. Effects of pre-germinated brown rice on blood glucose and lipid levels in free-living patients with impaired fasting glucose or type 2 diabetes. J. Nutr. Sci. Vitaminol. 2008;54:163–168. doi: 10.3177/jnsv.54.163. [DOI] [PubMed] [Google Scholar]
- 48.Zhu F. Buckwheat proteins and peptides: Biological functions and food applications. Trends Food Sci. Technol. 2021;110:155–167. doi: 10.1016/j.tifs.2021.01.081. [DOI] [Google Scholar]
- 49.Alonso-Miravalles L., O’Mahony J.A. Composition, Protein Profile and Rheological Properties of Pseudocereal-Based Protein-Rich Ingredients. Foods. 2018;7:73. doi: 10.3390/foods7050073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazza G., Oomah B.D. Buckwheat. Volume 2. Elsevier; Amsterdam, The Netherlands: 2003. pp. 692–699. [Google Scholar]
- 51.Guo X., Xiong Y.L. Characteristics and functional properties of buckwheat protein–sugar Schiff base complexes. LWT—Food Sci. Technol. 2013;51:397–404. doi: 10.1016/j.lwt.2012.12.003. [DOI] [Google Scholar]
- 52.Di Nunzio M., Loffi C., Montalbano S., Chiarello E., Dellafiora L., Picone G., Antonelli G., Tedeschi T., Buschini A., Capozzi F., et al. Cleaning the Label of Cured Meat; Effect of the Replacement of Nitrates/Nitrites on Nutrients Bioaccessibility, Peptides Formation, and Cellular Toxicity of In Vitro Digested Salami. Int. J. Mol. Sci. 2022;23:12555. doi: 10.3390/ijms232012555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egger L., Schlegel P., Baumann C., Stoffers H., Guggisberg D., Brügger C., Dürr D., Stoll P., Vergères G., Portmann R. Physiological comparability of the harmonized INFOGEST in vitro digestion method to in vivo pig digestion. Food Res. Int. 2017;102:567–574. doi: 10.1016/j.foodres.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 54.Guzmán-Ortiz F., Castro-Rosas J., Gomez-Aldapa C., Mora-Escobedo R., Rojas-León A., Rodriguez M., Cortés R., Alma Delia R.-G. Enzyme activity during germination of different cereals: A review. Food Rev. Int. 2018;35:177–200. doi: 10.1080/87559129.2018.1514623. [DOI] [Google Scholar]
- 55.Sinkovič L., Kokalj D., Vidrih R., Meglič V. Milling fractions fatty acid composition of common (Fagopyrum esculentum Moench) and tartary (Fagopyrum tataricum (L.) Gaertn) buckwheat. J. Stored Prod. Res. 2020;85:101551. doi: 10.1016/j.jspr.2019.101551. [DOI] [Google Scholar]
- 56.Faraoni P., Sereni E., Gnerucci A., Cialdai F., Monici M., Ranaldi F. Glyoxylate cycle activity in Pinus pinea seeds during germination in altered gravity conditions. Plant Physiol. Biochem. 2019;139:389–394. doi: 10.1016/j.plaphy.2019.03.042. [DOI] [PubMed] [Google Scholar]
- 57.Ferreri C., Masi A., Sansone A., Giacometti G., Larocca A.V., Menounou G., Scanferlato R., Tortorella S., Rota D., Conti M., et al. Fatty Acids in Membranes as Homeostatic, Metabolic and Nutritional Biomarkers: Recent Advancements in Analytics and Diagnostics. Diagnostics. 2017;7:1. doi: 10.3390/diagnostics7010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Nunzio M., Valli V., Bordoni A. PUFA and oxidative stress. Differential modulation of the cell response by DHA. Int. J. Food Sci. Nutr. 2016;67:834–843. doi: 10.1080/09637486.2016.1201790. [DOI] [PubMed] [Google Scholar]
- 59.Farooq M.A., Zhang X., Zafar M.M., Ma W., Zhao J. Roles of Reactive Oxygen Species and Mitochondria in Seed Germination. Front. Plant Sci. 2021;12:781734. doi: 10.3389/fpls.2021.781734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chitarrini G., Nobili C., Pinzari F., Antonini A., De Rossi P., Del Fiore A., Procacci S., Tolaini V., Scala V., Scarpari M., et al. Buckwheat achenes antioxidant profile modulates Aspergillus flavus growth and aflatoxin production. Int. J. Food Microbiol. 2014;189:1–10. doi: 10.1016/j.ijfoodmicro.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 61.Górnaś P., Rudzińska M., Raczyk M., Soliven A. Lipophilic bioactive compounds in the oils recovered from cereal by-products. J. Sci. Food Agric. 2016;96:3256–3265. doi: 10.1002/jsfa.7511. [DOI] [PubMed] [Google Scholar]
- 62.Górnaś P., Baškirovs G., Siger A. Free and Esterified Tocopherols, Tocotrienols and Other Extractable and Non-Extractable Tocochromanol-Related Molecules: Compendium of Knowledge, Future Perspectives and Recommendations for Chromatographic Techniques, Tools, and Approaches Used for Tocochromanol Determination. Molecules. 2022;27:6560. doi: 10.3390/molecules27196560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Žilić S., Basić Z., Šukalović V., Maksimović V., Simic M., Filipović M. Can the sprouting process applied to wheat improve the contents of vitamins and phenolic compounds and antioxidant capacity of the flour? Int. J. Food Sci. Technol. 2014;49:1040–1047. doi: 10.1111/ijfs.12397. [DOI] [Google Scholar]
- 64.Zhou X., Hao T., Zhou Y., Tang W., Xiao Y., Meng X., Fang X. Relationships between antioxidant compounds and antioxidant activities of tartary buckwheat during germination. J. Food Sci. Technol. 2015;52:2458–2463. doi: 10.1007/s13197-014-1290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siemińska-Kuczer A., Szymańska-Chargot M., Zdunek A. Recent advances in interactions between polyphenols and plant cell wall polysaccharides as studied using an adsorption technique. Food Chem. 2022;373:131487. doi: 10.1016/j.foodchem.2021.131487. [DOI] [PubMed] [Google Scholar]
- 66.Zeng Z., Liu C., Luo S., Chen J., Gong E. The Profile and Bioaccessibility of Phenolic Compounds in Cereals Influenced by Improved Extrusion Cooking Treatment. PLoS ONE. 2016;11:e0161086. doi: 10.1371/journal.pone.0161086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu H., Liu S., Yao L., Wang L., Li C. Free and Bound Phenolics of Buckwheat Varieties: HPLC Characterization, Antioxidant Activity, and Inhibitory Potency towards α-Glucosidase with Molecular Docking Analysis. Antioxidants. 2019;8:606. doi: 10.3390/antiox8120606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Angelino D., Cossu M., Marti A., Zanoletti M., Chiavaroli L., Brighenti F., Del Rio D., Martini D. Bioaccessibility and bioavailability of phenolic compounds in bread: A review. Food Funct. 2017;8:2368–2393. doi: 10.1039/C7FO00574A. [DOI] [PubMed] [Google Scholar]
- 69.Gan R.-Y., Lui W.-Y., Wu K., Chan C.-L., Dai S.-H., Sui Z.-Q., Corke H. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 2017;59:1–14. doi: 10.1016/j.tifs.2016.11.010. [DOI] [Google Scholar]
- 70.Ling A., Li X., Hu X., Ma Z., Wu K., Zhang H., Hao M., Wei S. Dynamic changes in polyphenol compounds, antioxidant activity, and PAL gene expression in different tissues of buckwheat during germination. J. Sci. Food Agric. 2018;98:5723–5730. doi: 10.1002/jsfa.9119. [DOI] [PubMed] [Google Scholar]
- 71.Liu A.-L., Wang Y.-H., Wang T.-Y., Zhu Y., Wu P., Li L.-J. Comparative metabolomic profiling of secondary metabolites in different tissues of Euryale ferox and functional characterization of phenylalanine ammonia-lyase. Ind. Crops Prod. 2023;195:116450. doi: 10.1016/j.indcrop.2023.116450. [DOI] [Google Scholar]
- 72.Ren S.-C., Sun J.-T. Changes in phenolic content, phenylalanine ammonia-lyase (PAL) activity, and antioxidant capacity of two buckwheat sprouts in relation to germination. J. Funct. Foods. 2014;7:298–304. doi: 10.1016/j.jff.2014.01.031. [DOI] [Google Scholar]
- 73.Álvarez R., Araya H., Navarro-Lisboa R., Lopez de Dicastillo C. Evaluation of Polyphenol Content and Antioxidant Capacity of Fruits and Vegetables Using a Modified Enzymatic Extraction. Food Technol. Biotechnol. 2016;54:462–467. doi: 10.17113/ftb.54.04.16.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnsen P.R., Pinna C., Mattio L., Strube M.B., Di Nunzio M., Iametti S., Dallavalle S., Pinto A., Frøkiær H. Investigation of the Effects of Monomeric and Dimeric Stilbenoids on Bacteria-Induced Cytokines and LPS-Induced ROS Formation in Bone Marrow-Derived Dendritic Cells. Int. J. Mol. Sci. 2023;24:2731. doi: 10.3390/ijms24032731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Csepregi K., Neugart S., Schreiner M., Hideg É. Comparative Evaluation of Total Antioxidant Capacities of Plant Polyphenols. Molecules. 2016;21:208. doi: 10.3390/molecules21020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Merendino N., Molinari R., Costantini L., Mazzucato A., Pucci A., Bonafaccia F., Esti M., Ceccantoni B., Papeschi C., Bonafaccia G. A new “functional” pasta containing tartary buckwheat sprouts as an ingredient improves the oxidative status and normalizes some blood pressure parameters in spontaneously hypertensive rats. Food Funct. 2014;5:1017–1026. doi: 10.1039/C3FO60683J. [DOI] [PubMed] [Google Scholar]
- 77.Meschini R., Filippi S., Molinari R., Costantini L., Bonafaccia G., Merendino N. Pasta containing tartary buckwheat sprouts prevents DNA damage in spontaneously hypertensive rats. Int. J. Food Sci. Nutr. 2015;66:574–578. doi: 10.3109/09637486.2015.1052378. [DOI] [PubMed] [Google Scholar]
- 78.Kil Y.S., Han A.R., Hong M.J., Kim J.B., Park P.H., Choi H., Nam J.W. 1H NMR-Based Chemometrics to Gain Insights Into the Bran of Radiation-Induced Colored Wheat Mutant. Front. Nutr. 2021;8:806744. doi: 10.3389/fnut.2021.806744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ciampa A., Danesi F., Picone G. NMR-Based Metabolomics for a More Holistic and Sustainable Research in Food Quality Assessment: A Narrative Review. Appl. Sci. 2023;13:372. doi: 10.3390/app13010372. [DOI] [Google Scholar]
- 80.Chen L., Wu J.e., Li Z., Liu Q., Zhao X., Yang H. Metabolomic analysis of energy regulated germination and sprouting of organic mung bean (Vigna radiata) using NMR spectroscopy. Food Chem. 2019;286:87–97. doi: 10.1016/j.foodchem.2019.01.183. [DOI] [PubMed] [Google Scholar]
- 81.Farag M.A., Sharaf El-Din M.G., Selim M.A., Owis A.I., Abouzid S.F., Porzel A., Wessjohann L.A., Otify A. Nuclear Magnetic Resonance Metabolomics Approach for the Analysis of Major Legume Sprouts Coupled to Chemometrics. Molecules. 2021;26:761. doi: 10.3390/molecules26030761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nam K.H. Glucose Isomerase: Functions, Structures, and Applications. Appl. Sci. 2022;12:428. doi: 10.3390/app12010428. [DOI] [Google Scholar]
- 83.Chiarello E., Di Nunzio M., Picone G., Antonelli G., Capozzi F., Bordoni A. Insight on Glucose and Fructose Absorption and Relevance in the Enterocyte Milieu. Nutrients. 2022;14:517. doi: 10.3390/nu14030517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Selegato D.M., Pilon A.C., Carnevale Neto F. Plant Metabolomics Using NMR Spectroscopy. In: Gowda G.A.N., Raftery D., editors. NMR-Based Metabolomics: Methods and Protocols. Springer; New York, NY, USA: 2019. pp. 345–362. [DOI] [PubMed] [Google Scholar]
- 85.Bouajila A., Ammar H., Chahine M., Khouja M., Hamdi Z., Khechini J., Salem A.-F.Z.M., Ghorbel A., López S. Changes in phytase activity, phosphorus and phytate contents during grain germination of barley (Hordeum vulgare L.) cultivars. Agrofor. Syst. 2020;94:1151–1159. doi: 10.1007/s10457-019-00443-y. [DOI] [Google Scholar]
- 86.Elliott H., Woods P., Green B.D., Nugent A.P. Can sprouting reduce phytate and improve the nutritional composition and nutrient bioaccessibility in cereals and legumes? Nutr. Bull. 2022;47:138–156. doi: 10.1111/nbu.12549. [DOI] [PubMed] [Google Scholar]
- 87.Kårlund A., Paukkonen I., Gómez-Gallego C., Kolehmainen M. Intestinal Exposure to Food-Derived Protease Inhibitors: Digestion Physiology- and Gut Health-Related Effects. Healthcare. 2021;9:1002. doi: 10.3390/healthcare9081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Modgil R., Sood P. Effect of Roasting and Germination on Carbohydrates and Anti-nutritional Constituents of Indigenous and Exotic Cultivars of Pseudo-cereal (Chenopodium) J. Life Sci. 2017;9:64–70. doi: 10.1080/09751270.2017.1336020. [DOI] [Google Scholar]
- 89.Nkhata S.G., Ayua E., Kamau E.H., Shingiro J.-B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018;6:2446–2458. doi: 10.1002/fsn3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chinma C.E., Adedeji O.E., Etim I.I., Aniaka G.I., Mathew E.O., Ekeh U.B., Anumba N.L. Physicochemical, nutritional, and sensory properties of chips produced from germinated African yam bean (Sphenostylis stenocarpa) LWT. 2021;136:110330. doi: 10.1016/j.lwt.2020.110330. [DOI] [Google Scholar]
- 91.Azeez S., Chinma C., Bassey S., Eze R., Makinde A., Sakariyah A., Okubanjo S., Danbaba N., Adebo O. Impact of germination alone or in combination with solid-state fermentation on the physicochemical, antioxidant, in vitro digestibility, functional and thermal properties of brown finger millet flours. LWT. 2021;154:112734. doi: 10.1016/j.lwt.2021.112734. [DOI] [Google Scholar]
- 92.Singh A., Bobade H., Sharma S., Singh B., Gupta A. Enhancement of Digestibility of Nutrients (In vitro), Antioxidant Potential and Functional Attributes of Wheat Flour through Grain Germination. Plant Foods Hum. Nutr. 2021;76:118–124. doi: 10.1007/s11130-021-00881-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.