Abstract
Background:
Air pollution is negatively associated with cardiovascular health. Impediments to efficient regulation include lack of knowledge about which sources of air pollution contributes most to health burden and few studies on effects of the potentially more potent ultrafine particles (UFP).
Objective:
The authors aimed to investigate myocardial infarction (MI) morbidity and specific types and sources of air pollution.
Methods:
We identified all persons living in Denmark in the period 2005–2017, age y and never diagnosed with MI. We quantified 5-y running time-weighted mean concentrations of air pollution at residencies, both total and apportioned to traffic and nontraffic sources. We evaluated particulate matter (PM) with aerodynamic diameter (), (UFP), elemental carbon (EC), and nitrogen dioxide (). We used Cox proportional hazards models, with adjustment for time-varying exposures, and personal and area-level demographic and socioeconomic covariates from high-quality administrative registers.
Results:
In this nationwide cohort of 1,964,702 persons (with person-years of follow-up and 71,285 cases of MI), UFP and were associated with increased risk of MI with hazard ratios (HRs) per interquartile range (IQR) of 1.040 [95% confidence interval (CI): 1.025, 1.055] and 1.053 (95% CI: 1.035, 1.071), respectively. HRs per IQR of UFP and from nontraffic sources were similar to the total (1.034 and 1.051), whereas HRs for UFP and from traffic sources were smaller (1.011 and 1.011). The HR for EC from traffic sources was 1.013 (95% CI: 1.003, 1.023). from nontraffic sources was associated with MI (; 95% CI: 1.034, 1.062) but not from traffic sources. In general, nontraffic sources contributed more to total air pollution levels than national traffic sources.
Conclusions:
and UFP from traffic and nontraffic sources were associated with increased risk of MI, with nontraffic sources being the dominant source of exposure and morbidity. https://doi.org/10.1289/EHP10556
Introduction
A recent review of experimental and clinical studies concluded that air pollution can cause oxidative stress as well as local and systemic inflammation and that it affects vascular tone, fibrinolysis, platelet activity, and plaque formation.1 Correspondingly, many epidemiological studies have found associations between air pollution and a range of cardiovascular end points with stronger evidence for particulate matter (PM) than gaseous components.2 With regard to incident myocardial infarction (MI), some studies found higher risk in association with exposure to nitrogen dioxide (),3–7 whereas other studies did not.8–14 For PM with aerodynamic diameter (), a recent meta-analysis found a hazard ratio (HR) of 1.08 per (95% confidence intervals (CI): 0.99, 1.18), with high heterogeneity between studies and concluded that there was suggestive evidence that long-term exposure to is associated with MI.15 Most subsequent studies have reported an elevated risk14,16–19 with a few exceptions.8,9
Ultrafine particles (UFP), PM , may have health effects disproportionate to their mass contribution to , because they have a large reactive surface area and may penetrate beyond the respiratory tract.20–22 Studies of short-term exposure to UFP suggest an association with stroke, blood pressure, systemic inflammation, and autonomic tone.21–24 Few epidemiological studies have investigated long-term cardiovascular effects of UFP. Two studies found associations between UFP and risk of ischemic heart disease and stroke,25,26 two studies found associations between UFP and risk of MI,9,11 and one study found an association between UFP and hypertension.27 These previous studies all indicate an association between UFP and cardiovascular diseases, but they each have their limitations, e.g., small size, exposure assessed only at single point in time or with at spatial resolution at the kilometer scale, or having only area-level covariates. Several studies have linked air pollution from elemental carbon (EC) or the closely related measures black smoke (BS) and absorbance, with higher CVD mortality.28,29 Two studies showed associations between absorbance and coronary heart disease,30,31 whereas another study found no association between black carbon and ischemic heart disease.32
The chemical composition and toxicity of air pollution depends on the emission source, as does the relative contribution to total air pollution exposure.33 Knowledge about the relative contribution to disease burden of sources such as traffic may therefore facilitate efficient political regulation to improve public health.34 Most long-term studies have, however, not made such subdivisions, and the few results relating to MI are inconsistent.10,26
In a nationwide cohort with extensive data on personal and area-level covariates, we assessed time-varying total and source-specific air pollution at individual residences with a state-of-the-art multiscale air pollution model system and evaluated the association of UFP, , elemental carbon (EC), and with risk of MI.
Methods
The study was conducted in Denmark, where a unique personal identification number has been allocated to all Danish residents since 1968. The identification number allows all residents to be traced in and across all health and administrative registers.35 We identified Danish residents in the Civil Registration System,36 which also provided complete residential histories (including dates of moving in and out) and information about emigration from Denmark.
We identified all who were born after 1920 (information on education is not available for those born before 1921), who lived in Denmark from birth or 1979 onward, and who were present in Denmark on 1 January 2005, and were 50 y of age or older any time after that date. From this population (), we excluded 60,967 with prevalent MI at baseline (see below) and 22,613 with missing information about one or more covariates, leaving a final cohort of 1,964,702 persons.
The cohort was followed up from 1 January 2005 to 31 December 2017. Thus, study entry (baseline) of cohort members was 1 January 2005 or the date of turning 50 y of age, whichever came last.
In accordance with Danish law, entirely register-based studies do not require ethical approval.
Outcome
We identified all cases of MI [International Classification of Diseases, 8th Revision (ICD8) (ICD8: 410 or ICD10: I21)] recorded as primary diagnosis in the Danish National Patient Register37 or with the same codes as primary cause of death in the Danish Register of Cause of Death.38 Individuals registered with an MI in one of those registries, from start of the registration (patient registry: 1977; mortality registry: 1970) and until study entry were excluded as prevalent cases. All other people with a first, incident diagnosis of MI after study entry (age 50 y or 1 January 2005, whichever came last) were counted as cases.
Exposure
We established exact geocodes of all residential addresses in Denmark from the Building and Housing Registry and modeled outdoor air pollution for the period 1995–2017 for these dwellings. We used the fully deterministic Danish DEHM/UBM/AirGIS modeling system,39 which is based on detailed emission inventories and meteorological data from a weather forecast model and then physical (atmospheric transport and dispersion) and chemical processes in the atmosphere are modeled. The system adds contributions to air pollution concentrations at three scales: a) the long-range transported regional background, modeled with the Danish Eulerian Hemispheric Model (DEHM) assessing the contribution from emissions from the entire northern hemisphere40; b) the local background contribution at a resolution calculated from Danish emissions of air pollution41 (more details below) modeled with the Urban Background Model (UBM)42; and c) the contribution from the traffic on the address street modeled with the Operational Street Pollution Model (OSPM), with detailed input parameters including traffic load, composition, and emission factors as well as street and building configuration, and meteorology.39,43 For each address one final estimate was achieved by adding DEHM and UBM, and for addresses with vehicles per day, contribution from local traffic calculated by OSPM was also added. In this coupling of models, it was ensured that emission sources were not counted twice.39,43,44 For all investigated pollutants hourly, address-level concentrations of air pollutants were then aggregated as monthly means. We modeled , EC, and mass concentration as well as particle number concentration (PNC) as an indicator for UFP (denoted in this article as UFP); further details about the modeling system can be found elsewhere.45 In brief, we modeled PNC based on an extension of the DEHM/UBM/AirGIS system with the aerosol dynamics module M7.46 The M7 module represents the aerosol in four size fractions: Nucleation mode (typical size below ), Aitken mode (), Accumulation Mode () and Coarse Mode (); and it handles conversion between size modes. The modes output from the DEHM feed into the UBM and OSPM models without including the particle dynamics. The size range was chosen to facilitate validation against measurement data. We scaled UFP particle number emissions to source-specific and EC mass emissions to derive a consistent emission inventory for PNC.
As input data for the modeling system, we used high-resolution emission inventories for Denmark for the period 1990–2020 that are based on registers of high quality and detail,41 e.g., the National Road and Traffic Database.47 The emission inventories are subject to an annual review from international experts to ensure that the calculations are done in accordance with best practices and meet the internationally established quality criteria. The inventories are based on the standardized European Selected Nomenclature for Air Pollution (SNAP) code classification of emission categories.48 The Danish emission estimations are done at the most detailed SNAP level and can be aggregated to the 11 SNAP main categories (Table S1). For large point sources such as power plants or road traffic high-quality estimates are ensured by detailed measurements and detailed information on technology and abatement. This allowed us to model air pollution both with and without the emissions from Danish road traffic (SNAP 07 codes) to the local background (UBM) and the traffic on the address street. Subtracting the two modeling results from each other enabled apportioning of total pollutant levels into national road traffic (denoted as “traffic” in this article) and nontraffic sources, with the latter also including traffic and other sources from surrounding countries. The contribution from road traffic to secondary particles (e.g., nitrate) is therefore not included in the Danish road traffic scenario in this setup. Based on available emission factors, the contribution to from national traffic was further divided into contributions from tailpipe (from motorized road transport vehicles) and nontailpipe (resuspended dust, dust from brakes, clutches, tires, road abrasion, and evaporation from vehicles) by running air pollution models with and without these emissions.
Using modeled monthly mean concentrations, we then calculated running mean exposure 1, 5, and 10 y back in time for each cohort member. These calculations were done as monthly updated, running averages of air pollution at the residential address(es) held by each cohort member. These averages took into account the time each person stayed at each address and provided time-weighted averages (TWA). The running 5-y TWA was our a priori main exposure metric. The final result of the complete modeling exercise was time-varying estimates of , UFP, EC, and in total and from traffic and nontraffic sources. For , traffic estimates were further subdivided into tailpipe and nontailpipe emissions. For all 14 estimates, we estimated TWA over 1, 5, and 10 y, with 5 y being the primary averaging period.
Comparisons between modeled and measured air pollution concentrations show generally good performance of the Danish DEHM/UBM/AirGIS modeling system. For total and EC, the correlation coefficients were in the range of 0.67–0.85 and 0.77–0.79, respectively, across different locations and measurement periods,49 and the correlations were similarly high for .50 For annual means of UFP, correlations between predictions and measurements were 0.86, 0.87, and 0.95 for validations at the regional, urban, and street scale, respectively.51 The source apportioning cannot be validated directly against measurements, but as described previously, the input data are of highest standard, and the model reproduces concentrations in high traffic roads well (Figure S1).41,52,53
Covariates
We selected potential confounders a priori. Statistics Denmark provided annually updated information about country of origin (“Danish origin” defined as being a Danish citizen or having at least one Danish citizen parent; all other classified as “other country of origin”), civil status (married/cohabiting, other), highest attained education (mandatory, short: vocational training, secondary or y of postsecondary education, medium/long: y of postsecondary education), occupational status (high-level white-collar, low-level white-collar, blue-collar, unemployed, retired), personal income (quintiles), and household per capita income (quintiles). Sex- and calendar year–specific quintiles were used for personal and household income to allow for inflation and sex-specific income disparities. In 2017, the 20th, 40th, 60th, and 80th personal income percentiles in Danish kroner (kr) were 142,926, 198,363, 249,053, and 312,717 kr for women and 147,235, 219,142, 281,097, and 370,134 kr for men. The corresponding percentiles of household income per adult in household were 174,497, 229,476, 286,079, and 365,263 kr for women and 171,817, 232,821, 290,162, and 369,949 kr for men.
To account for potential residual confounding and potential socioenvironmental disparities, we included a comprehensive array of parish-level covariates reflecting aspects of deprivation. In the year 2017, there were 2,160 parishes in Denmark with a mean area of and a median population of 1,032 persons. For socioeconomic area-level covariates, we obtained annually updated information at the parish level about the proportion of inhabitants with a criminal record, living in single-parent households, with only basic education, with manual labor, with income in lowest quartile, living in social housing, and with non-Western background. A Western background was defined as being a citizen of or having at least one parent who is a citizen of a Western country (Denmark and the rest of the EU, the UK, Norway, Iceland, Liechtenstein, Switzerland, Andorra, San Marino, the Vatican, Australia, New Zealand, the United States, and Canada). Persons with missing covariates were excluded.
Statistical Methods
We used Spearman rank correlation coefficient to evaluate correlations between air pollutants and covariates. Cox-proportional hazards models with age as time axis were used to calculate HRs and associated 95% CI for 5-y TWA air pollution and MI.
Cohort members were followed from the age of 50 y or 1 January 2005, whichever came last, until death, MI, more than 14 consecutive days of unknown address, emigration, or 31 December 2017, whichever came first. We evaluated associations linearly per interquartile range (IQR) of exposure to facilitate comparison of risk estimates between pollutants (our primary analyses) and per fixed increment to facilitate comparison with results from other studies. We also performed categorical analysis based on percentiles of exposure (, , , , , , and 95–100) as a graphical illustration of the shape of the exposure–response function. We used Akaike information criteria (AIC) to compare the model fit of linear and categorical representation of pollutants.
We evaluated three models with increasing levels of adjustment. Model 1 was the crude model only adjusted for age, sex, and calendar year (categorical, in 2-y categories). Model 2 was additionally adjusted for personal covariates (country of origin, civil status, educational level, occupational status, personal and household income). Model 3 (our main model) was additionally adjusted for all eight parish-level factors: proportion of inhabitants with non-Western background, living in single-parent households, with only basic education, with manual labor, with personal income in the lowest quartile, with household income in the lowest quartile, living in social housing, and with a criminal record.
All variables (except sex and country of origin) were time-dependent, and subjects were allowed to change between levels of exposure and covariates over time. Therefore, exposures and covariates changed with time and, at any moment during follow-up, reflected exactly the conditions pertaining to that specific instance.
In sensitivity analyses, we evaluated shorter (1-y) and longer (10-y) TWA periods, and we evaluated effect stratified by age (50–69, 70–80, y) and sex. Because both air pollution and stroke incidence decreased over the study period, we ran three models as sensitivity analyses: a) including exact year of entry as strata; b) including running calendar year of observation (2-y period) as strata rather than as a covariate; and c) analysis restricted to those entering the study in the year 2005.
All statistical analyses were performed in SAS (version 9.4; SAS Institute Inc.).
Results
The final cohort of 1,964,702 persons accrued a total of 18,309,318 person-years of follow-up and 71,285 MI events.
Most (71%) cohort members entered the cohort in 2005 (Figure S2). Table 1 describes the cohort members at entry, where median 5-y average concentrations of air pollutants were: (10th–90th percentile: 9.1–12.2), (10th–90th percentile: 7,963–15,695), (10th–90th percentile: 0.5–1.0), and (10th–90th percentile: 10.5–23.7). High concentrations of UFP or were associated with higher age, being retired, being female, not living with a spouse, not being a blue-collar worker, and being of non-Danish origin. Further, higher concentrations at the residence were associated with living in a parish with more social housing and non-Western immigrants (Table S2).
Table 1.
Cohort () characteristics at baseline (last of: 1 January 2005 and age 50 y), including time-weighted average air pollution concentrations over previous 5 y Denmark, 2005–2017.
| Baseline characteristics | Total |
|---|---|
| Individual level variables | |
| Women [ (%)] | 1,037,088 (53) |
| Age (y) [median (10%–90%)] | 58 (50–76) |
| Country of origin [ (%)] | |
| Denmark | 1,927,035 (98) |
| Other | 37,667 (2) |
| Civil status [ (%)] | |
| Married/cohabiting | 1,438,200 (73) |
| Other | 526,502 (27) |
| Educationa [ (%)] | |
| Mandatory | 711,579 (36) |
| Short | 888,013 (45) |
| Medium or long | 365,110 (19) |
| Occupational statusb [ (%)] | |
| White-collar, high level | 200,884 (10) |
| White-collar, low level | 296,628 (15) |
| Blue-collar | 590,213 (30) |
| Unemployed | 75,957 (4) |
| Retired | 801,020 (41) |
| Personal income: quintilesc [ (%)] | |
| 1st (low) | 489,663 (25) |
| 2nd | 414,704 (21) |
| 3rd | 327,020 (17) |
| 4th | 337,208 (17) |
| 5th (high) | 396,107 (20) |
| Household income: quintilesd [ (%)] | |
| 1st (low) | 406,916 (21) |
| 2nd | 355,572 (18) |
| 3rd | 328,899 (17) |
| 4th | 391,770 (20) |
| 5th (high) | 481,544 (25) |
| Air pollution levels (5-y mean) [median (10%–90%)] | |
| total () | 11.2 (9.1–12.2) |
| nontraffic () | 10.9 (8.9–11.6) |
| traffic () | 0.2 (0.1–0.8) |
| UFP total () | 11,106 (7,963–15,695) |
| UFP nontraffic () | 9,757 (7,452–12,032) |
| UFP traffic () | 1,202 (357–3,649) |
| EC total () | 0.7 (0.5–1.0) |
| EC nontraffic () | 0.5 (0.4–0.6) |
| EC traffic () | 0.1 (0.0–0.4) |
| total () | 15.3 (10.5–23.7) |
| nontraffic () | 11.2 (8.5–13.4) |
| traffic () | 4 (1.4–10.9) |
| Area level variables [median (10%–90%)] | |
| % Non-Western background | 3 (1–12) |
| % Only basic education | 11 (6–15) |
| % Manual labor | 13 (8–17) |
| % Unemployed | 2 (1–3) |
| % Low income | 4 (2–7) |
| % Social housinge | 14 (1–43) |
| % Sole-providers | 6 (4–8) |
| % with criminal record | 0.4 (0.2–0.9) |
Note: EC, elemental carbon; UFP, ultrafine particles: .
Short: vocational training, secondary education or y of postsecondary education. Medium or long y of postsecondary education.
White-collar, high level: Managers/directors with employees and people with employment that requires high-level skills. White-collar, low level: Managers with 0–4 employees and people with employment that requires intermediate level skills. Blue-collar: employment that requires low-level skills.
We applied age and calendar-year specific quintiles to account for inflation and income disparities by sex. In 2017, the 20th, 40th, 60th, and 80th percentile in Danish kroner were 142,926, 198,363, 249,053, and 312,717, respectively, for women and 147,235, 219,142, 281,097, and 370,134 for men.
We applied age and calendar year specific quintiles to account for inflation and income disparities by sex for singles. In 2017, the 20th, 40th, 60th, and 80th percentile per adult in household, in Danish kroner, were 174,497, 229,476, 286,079, and 365,263, respectively, for women and 171,817, 232,821, 290,162, and 369,949, respectively, for men.
Publicly funded nonprofit housing estates, where one-third of the apartments can be used by municipalities for persons in need.
For the vast majority of addresses, national road traffic sources contributed less to air pollution than nontraffic sources, which was most pronounced for UFP and (Table 1; Figure S3).
Spearman rank correlations between pollutants ranged from 0.26 to 0.99. Contributions to from traffic tailpipes and traffic nontailpipe sources were highly correlated with each other and with their sum ( from traffic) () but only moderately correlated with from all sources () (Table S3). The correlation between area-level covariates and air pollutants and individual socioeconomic covariates were generally very low to moderate (Table S4). Pollutants averaged over 1, 5, and 10 y were highly correlated () (Table S5).
Results from our main model (full adjustment) showed HRs per IQR of 1.053 (95% CI: 1.035, 1.071) per , 1.040 (95% CI: 1.025, 1.055) per UFP, 1.027 (95% CI: 1.013, 1.040) per , and 1.009 (95% CI: 1.000, 1.019) per EC (Table 2). Results from the crude model showed HRs below 1.
Table 2.
Associations between MI () and 5-y averages of air pollutants, total and by source. Denmark, 2005–2017, . Person-years: 18,309,319.
| IQR | Model 1a per IQR | Model 2b. per IQR | Model 3c per IQR | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Total | 0.983 (0.969, 0.998) | 1.019 (1.004, 1.034) | 1.053 (1.035, 1.071) | |
| Nontraffic | 0.989 (0.973, 1.006) | 1.032 (1.015, 1.049) | 1.051 (1.032, 1.069) | |
| Traffic | 0.991 (0.985, 0.998) | 0.996 (0.990, 1.003) | 1.011 (1.003, 1.018) | |
| Tailpipe | 0.989 (0.982, 0.996) | 0.996 (0.989, 1.003) | 1.012 (1.004, 1.020) | |
| Nontailpipe | 0.995 (0.990, 1.000) | 0.997 (0.992, 1.002) | 1.008 (1.002, 1.014) | |
| UFP | ||||
| Total | 0.961 (0.950, 0.972) | 1.001 (0.990, 1.013) | 1.040 (1.025, 1.055) | |
| Nontraffic | 0.974 (0.964, 0.985) | 1.014 (1.003, 1.024) | 1.034 (1.022, 1.046) | |
| Traffic | 0.963 (0.953, 0.972) | 0.984 (0.974, 0.994) | 1.011 (0.999, 1.024) | |
| EC | ||||
| Total | 0.966 (0.957, 0.975) | 0.989 (0.981, 0.998) | 1.009 (1.000, 1.019) | |
| Nontraffic | 0.969 (0.960, 0.977) | 0.994 (0.987, 1.001) | 1.001 (0.996, 1.007) | |
| Traffic | 0.981 (0.973, 0.989) | 0.992 (0.984, 1.000) | 1.013 (1.003, 1.023) | |
| Total | 0.969 (0.960, 0.979) | 0.994 (0.984, 1.004) | 1.027 (1.013, 1.040) | |
| Nontraffic | 0.984 (0.973, 0.996) | 1.025 (1.014, 1.037) | 1.048 (1.034, 1.062) | |
| Traffic | 0.972 (0.963, 0.981) | 0.986 (0.977, 0.995) | 1.009 (0.998, 1.020) | |
Note: CI, confidence interval; EC, elemental carbon; HR, hazard ratio; IQR, interquartile range; MI, myocardial infarction; UFP, ultrafine particles: .
Adjusted for age, sex, and calendar year (in 2-y categories).
Model 1 with further adjustment for marital status, education, occupational status, ethnicity, and personal and household income.
Model 2 with additional adjustment for area-level percentage of population: living in social housing, being sole providers, of non-Western origin, having low income, being unemployed, having blue-collar work, having only basic education, and having a criminal record.
The HRs per IQR were stronger for the nontraffic contributions than for the traffic contributions to , UFP, and . The pattern was opposite for EC, where only the association with the contribution from traffic deviated from the null. The tailpipe and nontailpipe contributions to from traffic showed similar associations with MI. Table S6 shows HRs per fixed increase in air pollution.
Results based on categories for , UFP, and , indicated increase in risk over the entire exposure range (Figure 1A–D), which was similar for the nontraffic contribution to UFP and . For from nontraffic sources, there was some indication of a leveling off at higher exposure levels (Figure S4A–D; Table S7). For traffic contributions, categorical analysis indicated a lower threshold for the associations with the traffic contributions. (Figure S5 and S6; Tables S7 and S8). Generally, the linear representation of exposure offered a better or only marginally worse fit of data than the categorical model (: 0.00–1.54). Exceptions included traffic, tailpipe and nontailpipe, and nontraffic, where the categorical model was superior (Table S9).
Figure 1.
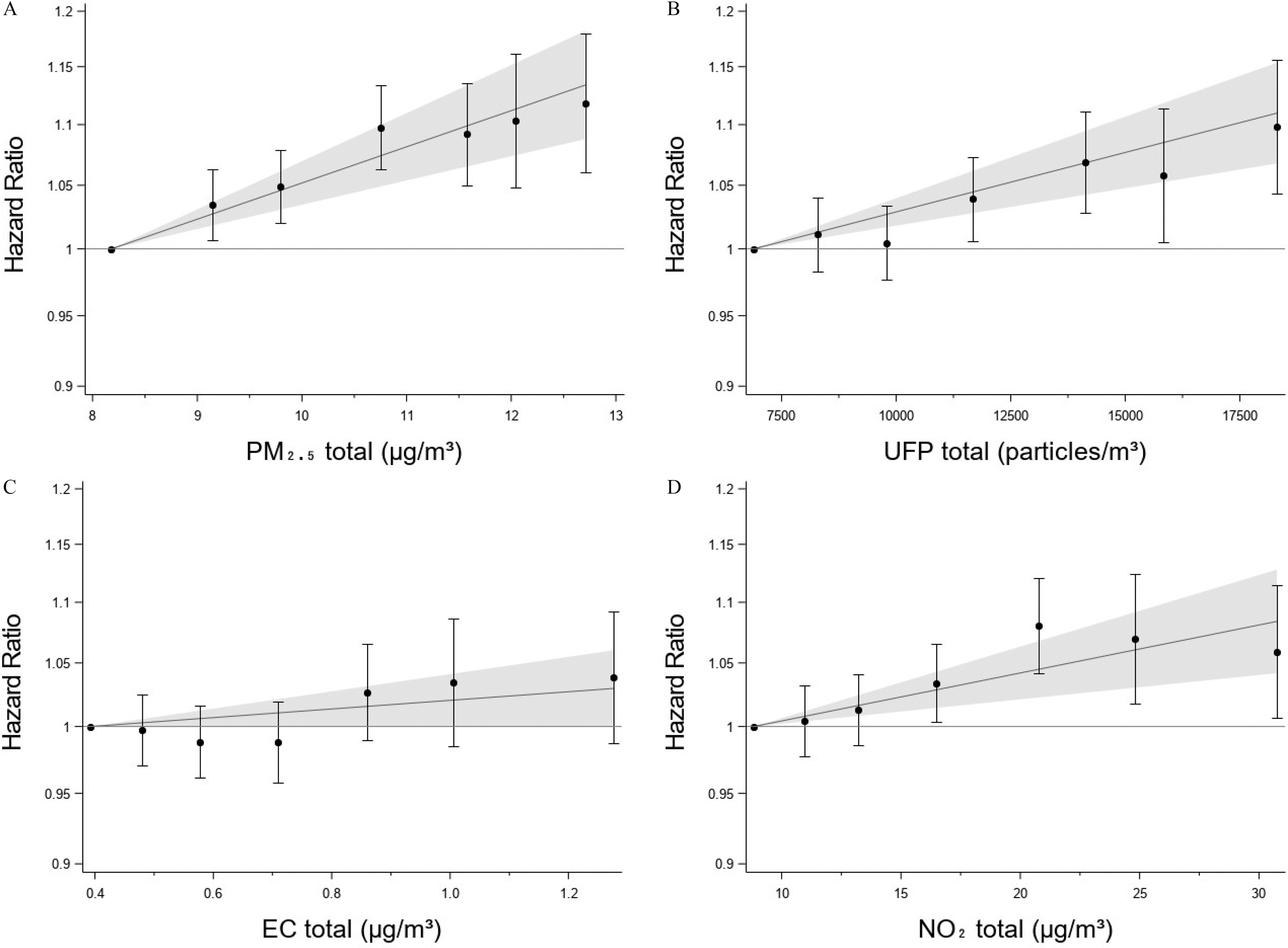
Associations between MI and 5-y time-weighted averages of (A) , (B) UFP, (C) EC, and (D) specified both in categories and as linear variables in the fully adjusted model 3. Categories defined from percentile of exposure: (reference), 10%–25%, 25%–50%, 50%–75%, 75%–90%, 90%–95%, and ; HRs and 95% CI plotted at the median of each category. The linear estimates from Table 2 with 95% CIs are plotted with the median of the categorical reference category as the null (). (Table S3 holds the same information in tabulated form). Denmark, 2005–2017, . Note: CI, confidence interval; EC, elemental carbon; MI, myocardial infarction; UFP, ultrafine particles.
Sensitivity analyses showed that the association between air pollution and MI was stronger in older age groups, with only little evidence of an association before age 70 y (Table 3). Except for , associations tended to be stronger in males, albeit with widely overlapping CIs. HRs changed little when averaging air pollution over 1 or 10 y (Table S10). Including year of entry or running calendar year as strata produced results identical to those of the main model (Table S11).
Table 3.
Associationsa between 5-y averages of air pollutants and MI in entire population and stratified by sex and age. Denmark, 2005–2017, .
| Person-years | Cases | per IQR: | UFP per IQR: | EC per IQR: | per IQR: | |
|---|---|---|---|---|---|---|
| HRa (95% CI) | HRa (95% CI) | HRa (95% CI) | HRa (95% CI) | |||
| Entire study population | 18,309,319 | 71,285 | 1.053 (1.035, 1.071) | 1.040 (1.025, 1.055) | 1.009 (1.000, 1.019) | 1.027 (1.013, 1.040) |
| Sex | ||||||
| Male | 8,369,008 | 43,8s40 | 1.053 (1.033, 1.073) | 1.044 (1.027, 1.061) | 1.011 (1.001, 1.022) | 1.030 (1.014, 1.045) |
| Female | 9,940,311 | 27,445 | 1.052 (1.030, 1.075) | 1.033 (1.013, 1.052) | 1.005 (0.992, 1.019) | 1.022 (1.005, 1.040) |
| Age (y) | ||||||
| Age 50–70 | 11,970,225 | 28,842 | 0.993 (0.972, 1.014) | 1.007 (0.988, 1.026) | 0.992 (0.977, 1.006) | 1.016 (0.998, 1.033) |
| Age 70–80 | 4,308,188 | 23,317 | 1.072 (1.048, 1.097) | 1.049 (1.028, 1.070) | 1.010 (0.997, 1.025) | 1.026 (1.007, 1.045) |
| Age | 2,030,906 | 19,216 | 1.123 (1.095, 1.150) | 1.078 (1.055, 1.102) | 1.028 (1.015, 1.042) | 1.043 (1.023, 1.064) |
Note: CI, confidence interval; EC, elemental carbon; HR, hazard ratio; IQR, interquartile range; MI, myodardial infarction; UFP, ultrafine particles: .
Adjusted for age, sex, calendar year (in 2-y categories), marital status, education, occupational status, ethnicity, personal and household income, and percentage of parish population: living in social housing, being sole providers, of non-Western origin, having low income, being unemployed, having blue-collar work, having only basic education, and having a criminal record.
Discussion
In this nationwide register-based cohort, with person-years of follow-up, 71,285 cases, and exposure levels well below EU-regulatory limits (annual mean: , ), air pollution was associated with a higher risk of MI. The associations were stronger among older people.
and UFP showed the highest HRs per IQR. Contributions to and UFP from nontraffic sources showed higher HRs than contributions from national road traffic when expressed per IQR. In general, nontraffic sources contributed more to total air pollution levels than national road traffic sources.
For ease of comparison, risk estimates from other studies have in the following discussion been rescaled to the IQR of the present study by means of this formula: .
We found to be associated with MI with a HR of 1.053 (95% CI: 1.035, 1.071) per IQR (), which is statistically compatible with the majority of previous studies.,8–10,18,19,26,54–58 There are, however, also studies suggesting both weaker3,14,17 and stronger associations.16,19 The studies with weaker associations generally modeled exposure with lower temporal or spatial resolution than the present study, and two of them found stronger risks after relatively crude adjustment for potential covariates.14,17 Exposure misclassification and residual confounding may thus have driven results toward the null. Furthermore, the study populations in these studies were much younger than those in the present study, which may have attenuated risk, as our and some other studies suggested a stronger association among older age groups.17,59 Stronger associations than those of our study were found in a Dutch cross-sectional study, oversampling elderly participants, and showing to be associated with self-reported heart attack (, 95% CI: 1.12, 1.24)16 and in a Korean cohort dominated by younger people, where was associated with higher risk of MI hospitalization (, 95% CI: 1.38, 2.09).6 The authors of the Korean study hypothesized that the high risk may be attributable to higher levels of or different composition of air pollution in comparison with air pollution levels or composition in Western cities.
The small size of UFP allows systemic penetration beyond the respiratory tract and provides a large reactive surface area. UFPs may therefore be a more potent risk factor for cardiovascular disease than , of which they constitute a weight-wise small fraction.60 The present study showed an association between UFP and higher risk for MI. The few previous studies on long-term exposure to UFP and risk of MI have suggested a positive association with MI,9,11 which is corroborated by results on self-reported stroke/ischemic heart disease25 and ischemic heart disease mortality.26 These results support our UFP and MI findings, although they tended to show weaker associations than those of our study, which may reflect differences in age composition, available covariates, or exposure composition and assessment.
The physical, chemical, and toxicological characteristics of PM depend on their sources, which may have implications for development of cost-efficient prevention strategies. Few other studies have investigated source-specific air pollution in relation to MI. A case–control study from Massachusetts, in the United States, found that the strongest risk factor per IQR for acute MI hospitalization was from area-level sources,10 whereas there was only a weak association with local traffic-dominated sources, which corroborates our observation for . In contrast, in the Californian Teachers Study, and UFP from gasoline and diesel combustion were associated with similar HRs per IQR for ischemic heart disease as total and UFP.26 These studies had smaller populations and more crude exposure assessment than the present study. In the present study, nontraffic sources of and UFP seemed most important when expressed per IQR. Altogether, and UFP from both traffic and nontraffic sources seem associated with risk of MI, with the highest HRs per IQR for nontraffic sources. This finding, combined with nontraffic sources contributing most to exposure, suggests that nontraffic sources may contribute more to the public health burden from MI than national traffic sources. The main nontraffic sources of emissions of and UFP air pollution in Denmark are nonindustrial combustion plants (such as wood stoves) and ships (Figure S7; Table S12). Further, the categorical analyses and to some extent the AIC analyses indicated that some of the linear HRs should be treated with caution due to a possible nonlinear shape of the function.
The present study found an association between and higher risk of MI, which has also been found in several previous studies.3,5,6,11,14,26 We found associations for from other sources than traffic but virtually no association with hailing from traffic sources, indicating that correlated air pollutants rather than per se might have influenced these results for .
We found indicative associations between total EC and traffic EC and risk of MI, but no association with EC from nontraffic sources. One previous study found no association between BC and ischemic heart disease,32 another study found BC to be associated with coronary heart disease,31 and a third study found a higher incidence of coronary events in association with absorbance although not statistically significant. Altogether, the literature is inconclusive concerning a possible association between EC and MI.
Strengths and Limitations
The prospective nationwide cohort design was a major strength of our study. Furthermore, we benefited from extensive and continuously updated high-quality public registers providing detailed information on both outcome, residential history, and a wide array of covariates. Similar to two recent state or nationwide studies with millions of participants,3,14 our study could only discern a positive relationship between air pollution and MI after adjusting for individual- and area-level factors. This phenomenon could be partly due to higher air pollution levels at the residences of individuals of higher socioeconomic position in Denmark,61 combined with higher risk of MI among individuals of low socioeconomic position.62 The association of socioeconomic position and risk of MI is likely through lifestyle. We could not adjust our analyses for individual lifestyle factors, such as smoking, diet, and physical activity, which could potentially have confounded our results. In a previous study, we analyzed the consequences of lack of information about such lifestyle factors among 246,766 randomly selected residents in Denmark for whom questionnaire data on lifestyle were also available. We found that when including all individual- and area-level covariates of the present study, information on body mass index, smoking, physical activity, and diet bestowed no additional impact on HRs for air pollution and MI.63 Even so, we cannot entirely rule out that residual confounding from lifestyle may have affected our results in either direction. Air pollution is a complex mixture of many chemical species that might correlate depending on the source pattern. Thus we cannot exclude the possibility that associations with MI, which was identified with one pollutant, might be (partly) due to exposure to correlated pollutants. Two other qualities of our study was the state-of-the-art, address-level exposure modeling covering an entire country and the ability of the modeling to assess source-specific contributions to air pollution. The validity of separation of the traffic and nontraffic contributions to air pollution depends to a high degree on the quality of the emission inventory for road traffic. The uncertainty of the Danish emission inventory depends on the emission sector and the pollutant considered. For some sectors, e.g., large point sources such as power plants and road transport, the data quality is high because a large share of the emission is covered by direct measurements or very detailed information on technology and abatement. For other sectors, such as small-scale combustion, the uncertainty is higher due to a large number of small installations with limited knowledge about technology and abatement measures. Regarding pollutants, the uncertainty for is lower than for PM and EC because of a higher data quality of main emitters of than of PM. Greater uncertainty may also have affected UFP estimates because there are no inventory standards and no mandatory national registration. However, correlations with measurement data were similar to those for other pollutants. Direct validation against measurements was not possible for source-specific concentrations. Circumstantial evidence for their quality is, however, provided by our model’s ability to predict concentrations also in conditions where traffic is the primary source of air pollution and by the quality of input data inventories.
Our modeling system only allowed separate estimation of primarily emitted national road-traffic pollution, and we can thus not determine to what degree long-distance transported secondary species related to traffic contribute to MI risk. It was a particular strength that our modeling included also UFP, which has not previously been modeled in detail on such a large scale. A limitation of our study is exposure misclassification. First, we did not have information about occupational or other nonresidential exposures. Second, some uncertainty is inevitable when modeling exposure. We would expect the resulting error in the exposure assessment to be a mixture of Berkson and Classical error, which could affect risk estimate in either direction.
We recommend caution when generalizing the results of the present nationwide study to other populations. Potential caveats include differences in the age composition, levels and composition of air pollution, population susceptibility (e.g., due to genetics and comorbidity), and possible differences in correlation between air pollution and sociodemographic characteristics at the individual and area level.
Conclusion
This study adds to the evidence that PM air pollution, including UFP, is associated with higher risk of MI. The association was apparent for PM from both traffic and nontraffic sources, with highest HRs per IQR for nontraffic sources, which also contributed most of the air pollution.
Supplementary Material
Acknowledgments
This work was supported by the Health Effects Institute (HEI) (Assistance Award No. R-82811201). HEI is an organization jointly funded by the U.S. Environmental Protection Agency (U.S. EPA) and certain motor vehicle and engine manufacturers. The contents of this article do not necessarily reflect the views of HEI or its sponsors, nor do they necessarily reflect the views and policies of the U.S. EPA or motor vehicle and engine manufacturers.
References
- 1.Shkirkova K, Lamorie-Foote K, Connor M, Patel A, Barisano G, Baertsch H, et al. 2020. Effects of ambient particulate matter on vascular tissue: a review. J Toxicol Environ Health B Crit Rev 23(7):319–350, PMID: , 10.1080/10937404.2020.1822971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, et al. 2015. Expert position paper on air pollution and cardiovascular disease. Eur Heart J 36(2):83–93b, PMID: , 10.1093/eurheartj/ehu458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Héritier H, Vienneau D, Foraster M, Eze IC, Schaffner E, de Hoogh K, et al. 2019. A systematic analysis of mutual effects of transportation noise and air pollution exposure on myocardial infarction mortality: a nationwide cohort study in Switzerland. Eur Heart J 40(7):598–603, PMID: , 10.1093/eurheartj/ehy650. [DOI] [PubMed] [Google Scholar]
- 4.Gandini M, Scarinzi C, Bande S, Berti G, Carnà P, Ciancarella L, et al. 2018. Long term effect of air pollution on incident hospital admissions: results from the italian longitudinal study within LIFE MED HISS project. Environ Int 121(Pt 2):1087–1097, PMID: , 10.1016/j.envint.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Roswall N, Raaschou-Nielsen O, Ketzel M, Gammelmark A, Overvad K, Olsen A, et al. 2017. Long-term residential road traffic noise and NO2 exposure in relation to risk of incident myocardial infarction – a Danish cohort study. Environ Res 156:80–86, PMID: , 10.1016/j.envres.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Kim H, Kim J, Kim S, Kang SH, Kim HJ, Kim H, et al. 2017. Cardiovascular effects of long-term exposure to air pollution: a population-based study with 900 845 person-years of follow-up. J Am Heart Assoc 6(11):e007170, PMID: , 10.1161/JAHA.117.007170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenlund M, Bellander T, Nordquist T, Alfredsson L. 2009. Traffic-generated air pollution and myocardial infarction. Epidemiology 20(2):265–271, PMID: , 10.1097/EDE.0b013e318190ea68. [DOI] [PubMed] [Google Scholar]
- 8.Cramer J, Jørgensen JT, Hoffmann B, Loft S, Bräuner EV, Prescott E, et al. 2020. Long-term exposure to air pollution and incidence of myocardial infarction: a Danish Nurse Cohort study. Environ Health Perspect 128(5):57003, PMID: , 10.1289/EHP5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Downward GS, van Nunen EJHM, Kerckhoffs J, Vineis P, Brunekreef B, Boer JMA, et al. 2018. Long-term exposure to ultrafine particles and incidence of cardiovascular and cerebrovascular disease in a prospective study of a Dutch cohort. Environ Health Perspect 126(12):127007, PMID: , 10.1289/EHP3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madrigano J, Kloog I, Goldberg R, Coull BA, Mittleman MA, Schwartz J. 2013. Long-term exposure to PM2.5 and incidence of acute myocardial infarction. Environ Health Perspect 121(2):192–196, PMID: , 10.1289/ehp.1205284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai L, Weichenthal S, Kwong JC, Burnett RT, Hatzopoulou M, Jerrett M, et al. 2019. Associations of long-term exposure to ultrafine particles and nitrogen dioxide with increased incidence of congestive heart failure and acute myocardial infarction. Am J Epidemiol 188(1):151–159, PMID: , 10.1093/aje/kwy194. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson RW, Carey IM, Kent AJ, van Staa TP, Anderson HR, Cook DG. 2013. Long-term exposure to outdoor air pollution and incidence of cardiovascular diseases. Epidemiology 24(1):44–53, PMID: , 10.1097/EDE.0b013e318276ccb8. [DOI] [PubMed] [Google Scholar]
- 13.Lipsett MJ, Ostro BD, Reynolds P, Goldberg D, Hertz A, Jerrett M, et al. 2011. Long-term exposure to air pollution and cardiorespiratory disease in the California Teachers Study cohort. Am J Respir Crit Care Med 184(7):828–835, PMID: , 10.1164/rccm.201012-2082OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bai L, Shin S, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, et al. 2019. Exposure to ambient air pollution and the incidence of congestive heart failure and acute myocardial infarction: a population-based study of 5.1 million Canadian adults living in Ontario. Environ Int 132:105004, PMID: , 10.1016/j.envint.2019.105004. [DOI] [PubMed] [Google Scholar]
- 15.Alexeeff SE, Liao NS, Liu X, Van Den Eeden SK, Sidney S. 2021. Long-term PM2.5 exposure and risks of ischemic heart disease and stroke events: review and meta-analysis. J Am Heart Assoc 10(1):e016890, PMID: , 10.1161/JAHA.120.016890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klompmaker JO, Janssen NAH, Bloemsma LD, Gehring U, Wijga AH, van den Brink C, et al. 2019. Associations of combined exposures to surrounding green, air pollution, and road traffic noise with cardiometabolic diseases. Environ Health Perspect 127(8):87003, PMID: , 10.1289/EHP3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hystad P, Larkin A, Rangarajan S, AlHabib KF, Avezum Á, Calik KBT, et al. 2020. Associations of outdoor fine particulate air pollution and cardiovascular disease in 157 436 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet Planet Health 4(6):e235–e245, PMID: , 10.1016/S2542-5196(20)30103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danesh Yazdi M, Wang Y, Di Q, Zanobetti A, Schwartz J. 2019. Long-term exposure to PM2.5 and ozone and hospital admissions of medicare participants in the southeast USA. Environ Int 130:104879, PMID: , 10.1016/j.envint.2019.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim OJ, Lee SH, Kang SH, Kim SY. 2020. Incident cardiovascular disease and particulate matter air pollution in South Korea using a population-based and nationwide cohort of 0.2 million adults. Environ Health 19(1):113, PMID: , 10.1186/s12940-020-00671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.HEI (Health Effects Institute). 2013. Understanding the Health Effects of Ambient Ultrafine Particles. Boston, MA: Health Effects Institute. [Google Scholar]
- 21.Ohlwein S, Kappeler R, Kutlar Joss M, Künzli N, Hoffmann B. 2019. Health effects of ultrafine particles: a systematic literature review update of epidemiological evidence. Int J Public Health 64(4):547–559, PMID: , 10.1007/s00038-019-01202-7. [DOI] [PubMed] [Google Scholar]
- 22.Schraufnagel DE. 2020. The health effects of ultrafine particles. Exp Mol Med 52(3):311–317, PMID: , 10.1038/s12276-020-0403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu JY, Hsiao TC, Lee KY, Chuang HC, Cheng TJ, Chuang KJ. 2018. Association of ultrafine particles with cardiopulmonary health among adult subjects in the urban areas of northern Taiwan. Sci Total Environ 627:211–215, PMID: , 10.1016/j.scitotenv.2018.01.218. [DOI] [PubMed] [Google Scholar]
- 24.Andersen ZJ, Olsen TS, Andersen KK, Loft S, Ketzel M, Raaschou-Nielsen O. 2010. Association between short-term exposure to ultrafine particles and hospital admissions for stroke in Copenhagen, Denmark. Eur Heart J 31(16):2034–2040, PMID: , 10.1093/eurheartj/ehq188. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Lane K, Corlin L, Patton A, Durant J, Thanikachalam M, et al. 2017. Association of Long-term near-highway exposure to ultrafine particles with cardiovascular diseases, diabetes and hypertension. Int J Environ Res Public Health 14(5):461–477, PMID: , 10.3390/ijerph14050461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostro B, Hu J, Goldberg D, Reynolds P, Hertz A, Bernstein L, et al. 2015. Associations of mortality with long-term exposures to fine and ultrafine particles, species and sources: results from the California Teachers Study Cohort. Environ Health Perspect 123(6):549–556, PMID: , 10.1289/ehp.1408565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai L, Chen H, Hatzopoulou M, Jerrett M, Kwong JC, Burnett RT, et al. 2018. Exposure to ambient ultrafine particles and nitrogen dioxide and incident hypertension and diabetes. Epidemiology 29(3):323–332, PMID: , 10.1097/EDE.0000000000000798. [DOI] [PubMed] [Google Scholar]
- 28.Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, et al. 2013. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environ Health 12(1):43, PMID: , 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strak M, Weinmayr G, Rodopoulou S, Chen J, de Hoogh K, Andersen ZJ, et al. 2021. Long term exposure to low level air pollution and mortality in eight European cohorts within the ELAPSE project: pooled analysis. BMJ 374:n1904, PMID: , 10.1136/bmj.n1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cesaroni G, Forastiere F, Stafoggia M, Andersen ZJ, Badaloni C, Beelen R, et al. 2014. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE project. BMJ 348:f7412, PMID: , 10.1136/bmj.f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf K, Hoffmann B, Andersen ZJ, Atkinson RW, Bauwelinck M, Bellander T, et al. 2021. Long-term exposure to low-level ambient air pollution and incidence of stroke and coronary heart disease: a pooled analysis of six european cohorts within the ELAPSE project. Lancet Planet Health 5(9):e620–e632, PMID: , 10.1016/S2542-5196(21)00195-9. [DOI] [PubMed] [Google Scholar]
- 32.Ljungman PLS, Andersson N, Stockfelt L, Andersson EM, Nilsson Sommar J, Eneroth K, et al. 2019. Long-term exposure to particulate air pollution, black carbon, and their source components in relation to ischemic heart disease and stroke. Environ Health Perspect 127(10):107012, PMID: , 10.1289/EHP4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Achilleos S, Kioumourtzoglou MA, Wu CD, Schwartz JD, Koutrakis P, Papatheodorou SI. 2017. Acute effects of fine particulate matter constituents on mortality: a systematic review and meta-regression analysis. Environ Int 109:89–100, PMID: , 10.1016/j.envint.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Jin L, Kan H. 2019. Air pollution: a global problem needs local fixes. Nature 570(7762):437–439, PMID: , 10.1038/d41586-019-01960-7. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt M, Pedersen L, Sørensen HT. 2014. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 29(8):541–549, PMID: , 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen CB. 2011. The Danish Civil Registration System. Scand J Public Health 39(7 suppl):22–25, PMID: , 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 37.Lynge E, Sandegaard JL, Rebolj M. 2011. The Danish National Patient Register. Scand J Public Health 39(7 suppl):30–33, PMID: , 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 38.Helweg-Larsen K. 2011. The Danish Register of Causes of Death. Scand J Public Health 39(7 suppl):26–29, PMID: , 10.1177/1403494811399958. [DOI] [PubMed] [Google Scholar]
- 39.Khan J, Kakosimos K, Raaschou-Nielsen O, Brandt J, Jensen SS, Ellermann T, et al. 2019. Development and performance evaluation of new AirGIS – a GIS based air pollution and human exposure modelling system. Atmos Environ 198:102–121, 10.1016/j.atmosenv.2018.10.036. [DOI] [Google Scholar]
- 40.Brandt J, Silver JD, Frohn LM, Geels C, Gross A, Hansen AB, et al. 2012. An integrated model study for Europe and North America using the Danish Eulerian Hemispheric Model with focus on intercontinental transport of air pollution. Atmos Environ 53:156–176, 10.1016/j.atmosenv.2012.01.011. [DOI] [Google Scholar]
- 41.Plejdrup MS, Nielsen O-K, Gyldenkærne S, Bruun HG. 2021. Spatial High-Resolution Distribution of Wmissions to Air – SPREAD 3.0. Aarhus University, DCE – Danish Centre for Environment and Energy: Aarhus University, Department of Environmental Science. [Google Scholar]
- 42.Brandt J, Christensen JH, Frohn LM, Berkowicz R. 2003. Air pollution forecasting from regional to urban street scale – implementation and validation for two cities in Denmark. Phys Chem Earth 28(8):335–344, 10.1016/S1474-7065(03)00054-8. [DOI] [Google Scholar]
- 43.Jensen SS, Ketzel M, Becker T, Christensen J, Brandt J, Plejdrup M, et al. 2017. High resolution multi-scale air quality modelling for all streets in Denmark. Transp Res Part D Transp Environ 52:322–339, 10.1016/j.trd.2017.02.019. [DOI] [Google Scholar]
- 44.Brandt JC, Frohn JH, Palmgren LM, Berkowicz F, Zlatev R. 2001. Operational air pollution forecasts from European to local scale. Atmos Environ 35(suppl 1):S91–S98, 10.1016/S1352-2310(00)00415-5. [DOI] [Google Scholar]
- 45.Frohn LM, Ketzel M, Christensen JH, Brandt J, Ulas I, Massling A, et al. 2021. Modelling ultrafine particle number concentrations at address resolution in Denmark from 1979–2018 – part 1: regional and urban scale modelling and evaluation. Atmos Environ 264:17. 10.1016/j.atmosenv.2021.118631. [DOI] [Google Scholar]
- 46.Vignati E, Wilson J, Stier P. 2004. M7: an efficient size-resolved aerosol microphysics module for large-scale aerosol transport models. J Geophys Res 109(D22):D22202, 10.1029/2003JD004485. [DOI] [Google Scholar]
- 47.Jensen SS, Plejdrup MS, Hilling K. 2019. GIS-Based National Road and Traffic Database 1960–2020. Aarhus, Denmark: Aarhus University, DCE – Danish Centre for Environment and Energy. [Google Scholar]
- 48.EEA (European Environment Agency). 2007. EMEP/CORINAIR Emission Inventory Guidebook–2007. https://www.eea.europa.eu/publications/EMEPCORINAIR5 [accessed 25 January 2022].
- 49.Hvidtfeldt UA, Ketzel M, Sørensen M, Hertel O, Khan J, Brandt J, et al. 2018. Evaluation of the danish AirGIS air pollution modeling system against measured concentrations of PM2.5, PM10, and black carbon. Environ Epidemiol 2(2):e014, 10.1097/EE9.0000000000000014. [DOI] [Google Scholar]
- 50.Ketzel M, Berkowicz R, Hvidberg M, Jensen SS, Raaschou-Nielsen O. 2011. Evaluation of AirGIS: a GIS-based air pollution and human exposure modelling system. Int J Environ Pollut 47(1–4):226–238, 10.1504/IJEP.2011.047337. [DOI] [Google Scholar]
- 51.Ketzel M, Frohn LM, Christensen JH, Brandt J, Massling A, Andersen C, et al. 2021. Modelling ultrafine particle number concentrations at address resolution in Denmark from 1979 to 2018 - part 2: local and street scale modelling and evaluation. Atmos Environ 264:16. 10.1016/j.atmosenv.2021.118633. [DOI] [Google Scholar]
- 52.Geels C, Winther M, Andersson C, Jalkanen J-P, Brandt J, Frohn LM, et al. 2021. Projections of shipping emissions and the related impact on air pollution and human health in the Nordic region. Atmos Chem Phys 21(16):12495–12519, 10.5194/acp-21-12495-2021. [DOI] [Google Scholar]
- 53.European Monitoring and Evaluation Programme, Centre on Emmission Inventories and Projections. 2022. The Emissions Database. https://www.ceip.at/webdab-emission-database [accessed 5 May 2022].
- 54.Beelen R, Stafoggia M, Raaschou-Nielsen O, Andersen ZJ, Xun WW, Katsouyanni K, et al. 2014. Long-term exposure to air pollution and cardiovascular mortality: an analysis of 22 European cohorts. Epidemiology 25(3):368–378, PMID: , 10.1097/EDE.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 55.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, et al. 2007. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med 356(5):447–458, PMID: , 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 56.Puett RC, Hart JE, Suh H, Mittleman M, Laden F. 2011. Particulate matter exposures, mortality, and cardiovascular disease in the health professionals follow-up study. Environ Health Perspect 119(8):1130–1135, PMID: , 10.1289/ehp.1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolf K, Stafoggia M, Cesaroni G, Andersen ZJ, Beelen R, Galassi C, et al. 2015. Long-term exposure to particulate matter constituents and the incidence of coronary events in 11 European cohorts. Epidemiology 26(4):565–574, PMID: , 10.1097/EDE.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 58.Elliott EG, Laden F, James P, Rimm EB, Rexrode KM, Hart JE. 2020. Interaction between Long-term exposure to fine particulate matter and physical activity, and risk of cardiovascular disease and overall mortality in U.S. women. Environ Health Perspect 128(12):127012, PMID: , 10.1289/EHP7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Danesh Yazdi M, Wang Y, Di Q, Wei Y, Requia WJ, Shi L, et al. 2021. Long-term association of air pollution and hospital admissions among Medicare participants using a doubly robust additive model. Circulation 143(16):1584–1596, PMID: , 10.1161/CIRCULATIONAHA.120.050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bevan GH, Al-Kindi SG, Brook RD, Münzel T, Rajagopalan S. 2021. Ambient air pollution and atherosclerosis: insights into dose, time, and mechanisms. Arterioscler Thromb Vasc Biol 41(2):628–637, PMID: , 10.1161/ATVBAHA.120.315219. [DOI] [PubMed] [Google Scholar]
- 61.Raaschou-Nielsen O, Taj T, Poulsen AH, Hvidtfeldt UA, Ketzel M, Christensen JH, et al. 2022. Air pollution at the residence of Danish adults, by socio-demographic characteristics, morbidity, and address level characteristics. Environ Res 208:112714, PMID: , 10.1016/j.envres.2022.112714. [DOI] [PubMed] [Google Scholar]
- 62.Manrique-Garcia E, Sidorchuk A, Hallqvist J, Moradi T. 2011. Socioeconomic position and incidence of acute myocardial infarction: a meta-analysis. J Epidemiol Community Health 65(4):301–309, PMID: , 10.1136/jech.2009.104075. [DOI] [PubMed] [Google Scholar]
- 63.Sørensen M, Hvidtfeldt UA, Poulsen AH, Thygesen LC, Frohn LM, Ketzel M, et al. 2022. The effect of adjustment to register-based and questionnaire-based covariates on the association between air pollution and cardiometabolic disease. Environ Res 203:111886, PMID: , 10.1016/j.envres.2021.111886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


