Abstract
The retinoblastoma protein (RB) has been shown to suppress RNA polymerase (Pol) III transcription in vivo (R. J. White, D. Trouche, K. Martin, S. P. Jackson, and T. Kouzarides, Nature 382:88–90, 1996). This regulation involves interaction with TFIIIB, a multisubunit factor that is required for the expression of all Pol III templates (C. G. C. Larminie, C. A. Cairns, R. Mital, K. Martin, T. Kouzarides, S. P. Jackson, and R. J. White, EMBO J. 16:2061–2071, 1997; W.-M. Chu, Z. Wang, R. G. Roeder, and C. W. Schmid, J. Biol. Chem. 272:14755–14761, 1997). However, it has not been established why RB binding to TFIIIB results in transcriptional repression. For several Pol II-transcribed genes, RB has been shown to inhibit expression by recruiting histone deacetylases, which are thought to decrease promoter accessibility. We present evidence that histone deacetylases exert a negative effect on Pol III activity in vivo. However, RB remains able to regulate Pol III transcription in the presence of the histone deacetylase inhibitor trichostatin A. Instead, RB represses by disrupting interactions between TFIIIB and other components of the basal Pol III transcription apparatus. Recruitment of TFIIIB to most class III genes requires its binding to TFIIIC2, but this can be blocked by RB. In addition, RB disrupts the interaction between TFIIIB and Pol III that is essential for transcription. The ability of RB to inhibit these key interactions can explain its action as a potent repressor of class III gene expression.
The retinoblastoma susceptibility gene encodes the important tumor suppressor retinoblastoma protein (RB) (12, 15, 37, 57). Inactivating mutations in this gene are found in many human cancers, including retinoblastomas, many sarcomas, and bladder and small-cell lung carcinomas (12, 15, 37, 57). In a large proportion of other human malignancies the Rb gene is of the wild type, but its function is disrupted. For example, the cyclin-dependent kinases that switch off RB are hyperactive in many tumors (12, 15, 37, 57). Indeed, it has been suggested that the regulatory pathway involving RB may be compromised in all human malignancies (57). It is therefore of considerable importance to obtain a clear understanding of the mechanisms used by RB to influence cellular activity.
RB is a highly abundant protein that has been shown to bind and regulate a variety of transcription factors (12, 15, 37, 48). The best-characterized example is the factor E2F, which controls several genes that are transcribed by RNA polymerase (Pol) II (11, 14). Indeed, RB was thought for some time to control only Pol II-transcribed genes. However, recent advances have demonstrated that RB can also regulate transcription by Pols I and III (7, 58, 62). Pol I synthesizes large rRNA, whereas Pol III synthesizes a variety of small stable RNAs, including 5S rRNA and tRNA; together Pols I and III can be responsible for up to 80% of all nuclear transcription (39).
Experiments using knockout mice revealed a major role for endogenous RB in regulating Pol III. Primary fibroblasts from Rb−/− mice were found to have a fivefold higher Pol III transcriptional activity than equivalent cells from wild-type mice, when assayed in vitro or in vivo (28, 62). Furthermore, overexpression of RB can inhibit Pol III transcription in transfected cells or in a system reconstituted with partially purified factors (8, 28, 62). This repression involves binding of RB to the Pol III-specific factor TFIIIB (8, 28). TFIIIB is a multisubunit complex which contains the TATA-binding protein (TBP), a TBP-associated factor (TAF) called BRF, and at least one other essential TAF (59). Although genes encoding human TBP and BRF have been cloned, the remaining component(s) of mammalian TFIIIB has yet to be characterized (36, 56). TFIIIB serves to recruit Pol III and position it over the initiation site (23). Bacterially expressed recombinant RB interacts with TFIIIB and represses it specifically (8, 28, 29, 46). Furthermore, immunoprecipitation and cofractionation experiments revealed that a population of endogenous RB molecules associates with TFIIIB at physiological ratios (28, 29, 46). This interaction is diminished or abolished in SAOS2 cells, which contain only a truncated mutant form of RB (29). In addition, the activity of TFIIIB was found to be elevated specifically in primary fibroblasts from RB-knockout mice (28). These results established that TFIIIB is a bona fide target for repression by endogenous RB.
TFIIIB is required for all Pol III transcription, which provides a potential explanation as to why every Pol III template tested can be regulated by RB. These include tRNA, 5S rRNA, U6 snRNA, and the Alu repetitive gene family (8, 28, 62). In contrast, only a small minority of Pol II-transcribed genes respond to RB. This is seen clearly in the RB-knockout mice, where the overall level of Pol II transcription does not increase (62). Most Pol II promoters do not contain binding sites for an RB-responsive factor such as E2F and are therefore resistant to RB. Thus, RB is a gene-specific regulator of Pol II transcription but a general regulator of Pol III transcription. A diploid human cell contains ∼2,600 tRNA genes, ∼600 5S rRNA genes, ∼200 U6 snRNA genes, around a million Alu genes, and a range of other less abundant Pol III templates (59). Since RB appears to be able to regulate all Pol III promoters but only a small minority of Pol II promoters, it seems likely that the templates transcribed by Pol III constitute its most abundant set of genetic targets.
Despite substantial evidence that RB can regulate Pol III transcription through interaction with TFIIIB, the mechanism of repression has yet to be established. This contrasts with the other polymerase systems, where the mode of RB action is much better characterized. In the case of Pol I, RB has been reported to bind the factor UBF and prevent this from recognizing the rRNA promoter (7, 53). Repression by RB of E2F-directed Pol II transcription has been shown to involve at least two distinct mechanisms. The simplest of these is based on the ability of RB to bind and mask the activation domain of E2F, thereby blocking its capacity to recruit the basal factor TFIID (42). In addition, RB can repress Pol II transcription by recruiting histone deacetylase (HDAC) complexes to promoters (3, 4, 34, 35). Acetylation of histone tails can help transcription factors gain access to DNA (30, 51, 52). By deacetylating histones, HDACs can reverse this process and thereby silence gene expression (25). The two mechanisms used by RB to control Pol II transcription are selective, since many E2F-dependent promoters can be repressed by RB in the absence of HDAC activity (34). Other repressors, such as MeCP2, can also utilize alternative HDAC-dependent and HDAC-independent mechanisms to regulate transcription (22, 38).
In the present study we have investigated how the binding of RB to TFIIIB inhibits the expression of class III genes. A novel mode of action that involves disruption of key interactions between components of the basal transcription apparatus is revealed; this mechanism will allow RB to interfere with the recruitment of Pol III to its templates.
MATERIALS AND METHODS
Cell culture.
Cells of the BALB/c 3T3 murine fibroblast line A31, the human osteosarcoma cell line SAOS2, and the human cervical carcinoma line C33A and mouse embryonic fibroblasts were grown in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% fetal calf serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml and were harvested when subconfluent.
RT-PCR and Northern blotting.
Total cellular RNA was extracted using TRI reagent (Sigma), according to the manufacturer's instructions. Reverse transcription (RT) was performed for 1 h at 42°C using 3 μg of RNA, 200 ng of random hexamers (Promega), and 400 U of Superscript II reverse transcriptase (Life Technologies) in a total volume of 40 μl of 1× First Strand buffer (Life Technologies) containing 10 mM dithiotreitol (DTT) and a 0.5 mM concentration of each deoxynucleoside triphosphate (dNTP). PCRs were carried out using a PTC-100 programmable thermal controller (MJ Research Inc.). Two microliters of cDNA was amplified with 20 pmol of either dihydrofolate reductase (DHFR) primers (5′-TAGAGAACTCAAAGAACCAC-3′ and 5′-GCCTTTTTCCTCCTGGACC-3′) to obtain a 295-bp product or acidic ribosomal phosphoprotein P0 (ARPP P0) primers (5′-GACCTGGAAGTCCAACTACTTC-3′ and 5′-TGAGGTCCTCCTTGGTGAACAC-3′) to obtain a 268-bp product. Amplification reaction mixtures contained 0.5 U of Taq DNA polymerase (Promega) in a total volume of 1× Taq DNA polymerase buffer (Promega) containing 1.5 mM MgCl2, 1 μCi of [α-32P]dCTP, and a 0.2 mM concentration of each dNTP. PCR was performed under the following cycling parameters: for DHFR, 95°C for 3 min, then 30 cycles of 95°C for 1 min, 46°C for 30 s, and 72°C for 1 min, followed by 72°C for 5 min; for ARPP P0, 95°C for 2 min, then 25 cycles of 95°C for 1 min, 58°C for 30 s, and 72°C for 1 min, followed by 72°C for 3 min. Reaction products were resolved on a 3.5% polyacrylamide gel and detected by autoradiography. Agarose gel electrophoresis and Northern transfer and hybridization were carried out as described previously (5). The B2 gene probe was a 240-bp EcoRI-PstI fragment from pTB14 (61). The ARPP P0 probe was a 1-kb EcoRI-HindIII fragment from the mouse cDNA (18).
Transient-transfection assays.
pU6/Hae/RA.2 (31) and the RB expression constructs pSG5L-HA-RB(wt) and pSG-RB;567L (45) have been described previously. pCAT (Promega) contains the chloramphenicol acetyltransferase (CAT) gene driven by the simian virus 40 promoter and enhancer. Cells were transiently transfected using the calcium phosphate precipitation method. DNA precipitates were left on the plates overnight and then the cells were washed with phosphate-buffered saline and cultured for 24 h before harvesting. Total RNA was extracted using TRI reagent (Sigma), according to the manufacturer's instructions. It was then analyzed by primer extension using labeled primers specific for VA1 (5′-CACGCGGGCGGTAACCGCATG-3′), pU6/Hae/RA.2 (5′-GGGTCTGAGTCACCTGGACAACCTC-3′), and CAT (5′-CGATGCCATTGGGATATATCA-3′). Primer extension reactions were conducted as previously described (62).
Recombinant proteins, cell extracts, and transcription assays.
Glutathione S-transferase (GST) fusion proteins were expressed in bacteria and purified on glutathione-agarose as described previously (28). Histidine-tagged RB was expressed in bacteria and purified by chromatography on nickel-nitrilotriacetic acid (Qiagen), according to the manufacturer's specifications. Both GST-RB and histidine-tagged RB contained RB residues 379 to 928. HeLa cell nuclear extracts were purchased from the Computer Cell Culture Center (Mons, Belgium). Transcription reactions were carried out as described previously (61), except that pBR322 was not included and the incubations were performed for 60 min at 30°C. The pVA1 template plasmid contains the adenovirus VA1 gene (9). The pLeu template contains a human tRNALeu gene (61).
Antibodies and Western blotting.
Antiserum 4286 was raised by immunizing rabbits with synthetic peptide RPGFSPTSHRLLPTP (human TFIIIC110 residues 897 to 911) coupled to keyhole limpet hemocyanin. Antisera 128 and 330 were raised against residues 533 to 547 and 664 to 677 of human BRF, respectively, and have been described and characterized previously (1, 5, 29). SL30 is a monoclonal antiserum against TBP that was generously provided by Nouria Hernandez (33). Antiserum against BN51 was generously provided by Michael Ittmann (20, 21). Antibodies F-7 against the hemagglutinin (HA) tag, M-19 against TAFI48, and BF683 against cyclin A were purchased from Santa Cruz. Western immunoblot analyses were performed as previously described (60).
Immunoprecipitation.
Whole-cell extract (150 μg) was incubated for 3 h at 4°C on an orbital shaker with 20 μl of protein A-Sepharose beads carrying equivalent amounts of prebound immunoglobulin G. Samples were then pelleted, supernatants were removed, and the beads were washed five times with 150 μl of LDB buffer (20 mM HEPES-KOH [pH 7.9], 17% glycerol, 100 mM KCl, 12 mM MgCl2, 0.1 mM EDTA, 2 mM DTT). The bound material was analyzed by Western blotting. In the experiments that involved radiolabeled BRF, reticulocyte lysate (Promega) was used to synthesize BRF in the presence of [35S]Met and [35S]Cys, according to the manufacturer's specifications; this lysate (15 μl) was then mixed with nuclear extract (150 μg) prior to immunoprecipitation. In these cases, the precipitated material was analyzed by autoradiography rather than Western blotting.
When immunoprecipitations were carried out after transfection, C33A cells were washed twice with ice-cold phosphate-buffered saline and then scraped into a microextraction buffer (20 mM HEPES [pH 7.8], 450 mM NaCl, 50 mM NaF, 25% glycerol, 0.2 mM EDTA, 0.5 mM DTT, 0.5 mM phenylmethylsulfonyl fluoride, 0.5 μg of leupeptin per ml, 0.7 μg of pepstatin per ml, 1.0 μg of trypsin inhibitor per ml, 0.5 μg of aprotinin per ml, 40 μg of ubenimex [Bestatin] per ml) containing 1 mM sodium vanadate, 50 mM β-glycerophosphate, and 0.5% Triton X-100. After incubation on ice for 15 min, the lysates were cleared by centrifugation at 4°C for 10 min in a microcentrifuge and then used for immunoprecipitation.
Polymerase assays.
Assays of RNA polymerization activity were conducted as described previously (41). Each reaction mixture contained 5 μg of poly(dA-dT) template and α-amanitin at a final concentration of 2 μg/ml.
RESULTS
TSA induces B2 gene expression in vivo.
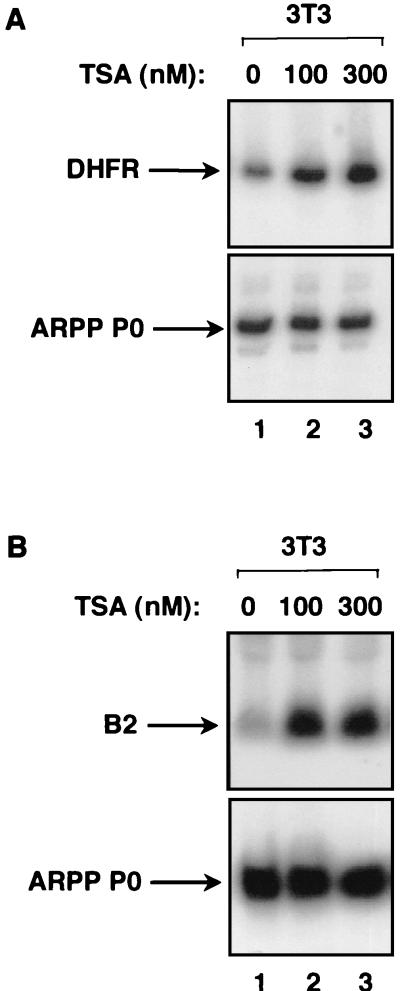
The involvement of HDACs in regulating transcription can be investigated using trichostatin A (TSA), a drug which inhibits HDACs potently and specifically (47, 64). For example, TSA was used to show that HDACs regulate the gene encoding DHFR in human osteosarcoma cells (34). We have used a similar approach with regard to Pol III transcription in untransformed murine fibroblasts. Control experiments confirmed that TSA is effective in this system. Thus, DHFR expression is stimulated when fibroblasts are treated with 100 or 300 nM TSA (Fig. 1A, top panel). This response is specific, since the gene encoding ARPP P0 is unaffected by TSA (Fig. 1A, bottom panel). Having confirmed the efficacy of TSA in murine fibroblasts, we examined how a chromosomal Pol III template responds to the drug. The B2 family was chosen for this analysis, since previous studies have shown that Pol III transcription of these middle repetitive genes is heavily repressed by histones in chromatin from murine fibroblasts (6, 43). Consistent with these findings, treatment with TSA produced a strong induction of B2 transcripts (Fig. 1B). After normalization to the ARPP P0 mRNA, B2 expression was found to be stimulated seven- to eightfold by TSA. This result provides the first evidence that HDAC function contributes to the control of class III gene expression in vivo, although it does not show whether the effect is direct. We therefore investigated whether RB exploits HDAC activity to repress Pol III transcription.
FIG. 1.
TSA stimulates the expression of a Pol III template in vivo. (A) RT-PCR analysis of cDNAs prepared from total RNA extracted from BALB/c 3T3 fibroblasts cultured without TSA (lane 1) or in the presence of 100 nM TSA (lane 2) or 300 nM TSA (lane 3). The cDNAs were PCR amplified using primers specific for DHFR and ARPP P0. (B) Northern blot analysis of the same total RNA (10 μg) samples as were used for panel A, which had been extracted from BALB/c 3T3 fibroblasts cultured without TSA (lane 1) or in the presence of 100 nM TSA (lane 2) or 300 nM TSA (lane 3). The upper panel shows the blot probed with a B2 gene and the lower panel shows the same blot stripped and reprobed with the ARPP P0 gene.
HDAC activity is not required for RB to repress Pol III transcription.
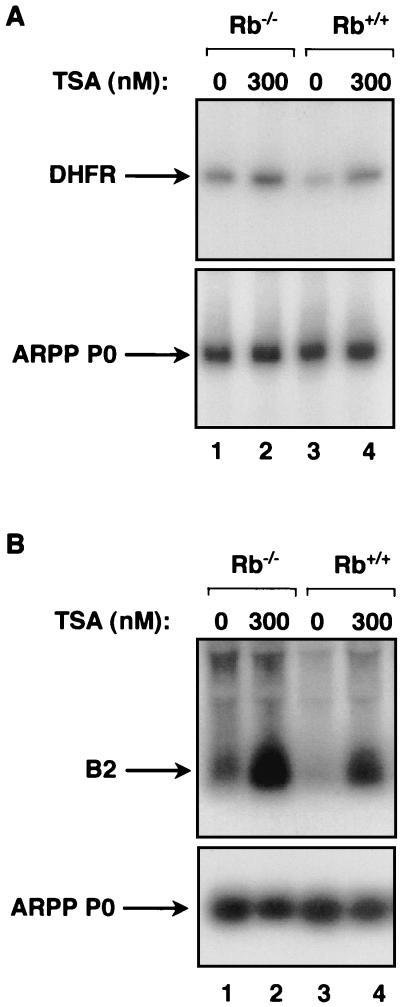
To examine the action of endogenous RB in vivo, we made use of matched embryonic fibroblasts derived from either wild-type or RB-knockout mice. Control experiments confirmed that DHFR gene expression is sensitive to TSA in these cells (Fig. 2A, upper panel). Furthermore, DHFR transcript levels are elevated in Rb−/− fibroblasts compared with matched Rb+/+ cells, consistent with the fact that the DHFR promoter is bound by E2F and repressed by RB (11). These effects are specific, since the ARPP P0 gene does not respond to the presence of RB or TSA (Fig. 2A, bottom panel). As for the DHFR mRNA, B2 transcripts made by Pol III are overexpressed in R−/− fibroblasts relative to the wild-type controls (Fig. 2B). This indicates that B2 genes are subject to repression by endogenous RB in vivo, consistent with previous demonstrations that Pol III activity increases in RB-knockout cells (28, 62). As seen in the 3T3 cells, B2 transcripts are induced when the embryonic fibroblasts are cultured in the presence of TSA to block HDAC function (Fig. 2B). The increases in B2 RNA levels in response to 300 nM TSA are 3.5-fold in the knockout cells and 4.1-fold in the wild-type cells, after normalization against the ARPP P0 mRNA. Thus, B2 expression remains responsive to TSA even in the absence of RB. Furthermore, the Rb−/− cells continue to express higher levels of B2 RNA than the corresponding wild-type cells when HDAC function is blocked with TSA (Fig. 2B, compare lanes 2 and 4). This demonstrates that endogenous RB is able to repress class III gene expression in the absence of HDAC activity. The data suggest that RB can use an HDAC-independent mechanism to regulate Pol III transcription in vivo.
FIG. 2.
Repression of a Pol III template by RB in vivo is maintained in the presence of TSA. (A) RT-PCR analysis of cDNAs prepared from total RNA extracted from Rb−/− fibroblasts or matched wild-type fibroblasts that were cultured in the presence or absence of 300 nM TSA. The cDNAs were PCR amplified using primers specific for DHFR and ARPP P0. (B) Northern blot analysis of the same total RNA (10 μg) samples as in panel A, which had been extracted from Rb−/− fibroblasts or matched wild-type fibroblasts that were cultured in the presence or absence of 300 nM TSA. The upper panel shows the blot probed with a B2 gene, and the lower panel shows the same blot stripped and reprobed with the ARPP P0 gene.
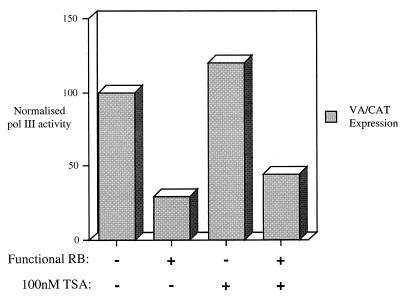
To test this possibility further, we carried out transient-transfection assays with cells grown in the presence or absence of TSA. Primer extension analysis was used to monitor VA1 gene transcription in SAOS2 cells that had been cotransfected with RB expression vectors. To normalize for transfection efficiency, we included as internal control a CAT reporter gene driven by the simian virus 40 early promoter. In contrast to the 567L RB control, an inactive null mutant from a retinoblastoma patient (63), wild-type RB clearly produced repression of VA1 (Fig. 3). Treatment of cells with 100 nM TSA caused a slight increase in VA1 expression, whether or not functional RB was present. The response to TSA was much weaker in this experiment than had been seen with the endogenous B2 genes (Fig. 1 and 2); this may be due to the use of different promoters but might also reflect the fact that the chromatin structure of a transfected template is likely to be different from that of a chromosomal gene. Nevertheless, it is clear that the TSA did not prevent wild-type RB from inhibiting the Pol III reporter. Repression by RB was also maintained in the presence of 200 nM TSA (T. R. P. Brown and R. J. White, unpublished observations). These data provide further evidence that HDAC activity is not required for RB to regulate Pol III transcription in vivo.
FIG. 3.
RB can repress Pol III transcription of a transfected VA1 gene in the presence of TSA. SAOS2 cells were transfected with pVA1 (0.5 μg), pCAT (4 μg), and 8 μg of pSG5L-HA-RB(wt), encoding wild-type RB (bars 2 and 4), or 8 μg of pSG-RB;567L, encoding an inactive RB null mutant (bars 1 and 3). Cells depicted by bars 3 and 4 were maintained in 100 nM TSA for 24 h prior to harvesting. VA1 and CAT RNA levels were assayed by primer extension and then quantitated using a phosphorimager. Values shown represent the signal for VA1 normalized to the levels of CAT expression to correct for transfection efficiency; they are expressed relative to the value obtained in the absence of TSA or functional RB (bar 1), which is designated 100%, and are means from two experiments.
HDACs are thought to function primarily by altering chromatin structure through the deacetylation of histones (25). However, transcription factors can also be regulated by direct acetylation and might therefore respond to HDACs in the absence of nucleosomes (13, 19, 44). To investigate this less likely possibility, we tested in vitro, using naked DNA templates, whether TSA might influence Pol III transcription and its control by RB. Recombinant RB (residues 379 to 928) was produced in bacteria as a GST fusion protein and then purified using glutathione-agarose beads. When added to cell extracts, the GST-RB produced potent repression of VA1 gene transcription by Pol III, as demonstrated previously (8, 28, 29, 62) (Fig. 4A). This repression was maintained when TSA was included at concentrations of 165 and 330 nM. Similar results were obtained using a tRNA gene as a template (Fig. 4B). Indeed, RB was able to repress in the presence of ∼500 nM TSA, even though a concentration of 100 nM is sufficient to block HDAC function. We conclude that RB can inhibit Pol III transcription efficiently by a mechanism that does not require HDAC activity.
FIG. 4.
Recombinant RB represses Pol III transcription in vitro despite the presence of TSA. (A) pVA1 (250 ng) was transcribed using 10 μg of HeLa cell extract that had been preincubated for 15 min at 30°C with 250 ng of GST or GST-RB. Lanes 3 and 4 also contained 165 nM TSA and lanes 5 and 6 contained 330 nM TSA. (B) pLeu (250 ng) was transcribed using 10 μg of HeLa cell extract that had been preincubated for 15 min at 30°C without addition (lanes 1, 2, and 11), with 250 ng of GST (lanes 3 through 6), or with 250 ng of GST-RB (lanes 7 through 10). Lanes 4 and 8 contained 165 nM TSA, lanes 5 and 9 contained 331 nM TSA, and lanes 2, 6, and 10 contained 496 nM TSA.
RB disrupts the interaction between TFIIIB and TFIIIC2.
RB has been shown to inhibit Pol III transcription by associating specifically with TFIIIB (8, 28). We therefore investigated whether RB can influence the interactions made by TFIIIB with other components of the Pol III machinery. One of the key contacts is with TFIIIC2, the DNA-binding factor which is responsible for promoter recognition at most class III genes; once TFIIIC2 has bound to DNA, it serves to recruit TFIIIB through protein-protein interactions (39, 59).
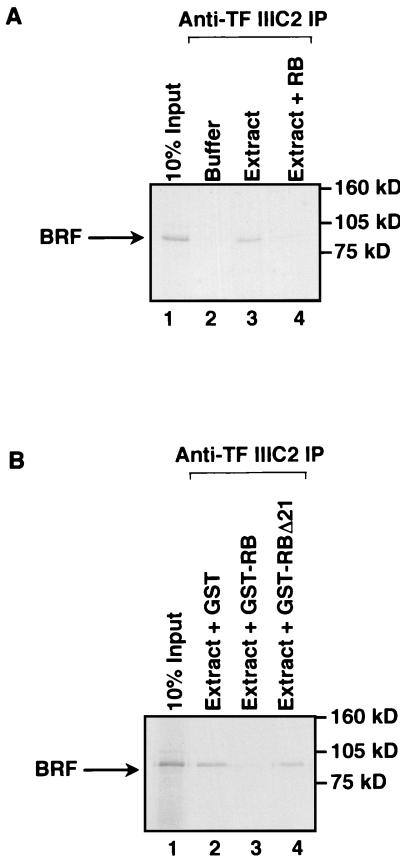
Coimmunoprecipitation assays were carried out to investigate whether the presence of RB can interfere with the association between TFIIIB and TFIIIC2. The BRF subunit of TFIIIB was 35S labeled and then mixed with a HeLa cell extract. Immunoprecipitation reactions carried out with antiserum against TFIIIC2 were found to coprecipitate the radiolabeled BRF; this is due to the binding of TFIIIB to TFIIIC2, rather than fortuitous cross-reaction, since little or no BRF was immunoprecipitated in the absence of cell extract (Fig. 5A, lanes 2 and 3). Addition of recombinant histidine-tagged RB (residues 379 to 928) resulted in a substantial decrease in the amount of BRF that coprecipitated with TFIIIC2 (Fig. 5A, lanes 3 and 4). To exclude the possibility that this was a nonspecific response to added protein, we repeated these experiments in the presence of GST-RB or an equal amount of GST alone. Relative to GST, GST-RB produced a significant decrease in the proportion of BRF that was coprecipitated (Fig. 5B). As a further test of specificity, we examined the effect of an equal amount of GST-RB carrying the Δ21 null mutation, which arose in a small-cell lung carcinoma (16). The RBΔ21 mutant, in which 35 residues have been deleted, was found to have lost the ability to disrupt the interaction between TFIIIB and TFIIIC2 (Fig. 5B, lane 4).
FIG. 5.
RB disrupts the interaction between TFIIIB and TFIIIC2. (A) Reticulocyte lysate (15 μl) containing in vitro-translated BRF was immunoprecipitated (IP) in the presence of buffer or 150 μg of HeLa nuclear extract using anti-TFIIIC2 antibody 4286. Recombinant histidine-tagged RB (100 ng) was included in lane 4. Proteins retained after extensive washing were resolved on a sodium dodecyl sulfate (SDS)–7.8% polyacrylamide gel and then visualized by autoradiography. Lane 1 shows 10% of the input reticulocyte lysate containing in vitro-translated BRF. (B) Reticulocyte lysate (15 μl) containing in vitro-translated BRF was immunoprecipitated in the presence of 150 μg of HeLa nuclear extract (lanes 2 to 4) using anti-TFIIIC2 antibody 4286. Lanes 2, 3, and 4 contained 200 ng of GST, GST-RB, and GST-RBΔ21, respectively. Proteins retained after extensive washing were resolved on an SDS–7.8% polyacrylamide gel and then visualized by autoradiography. Lane 1 shows 10% of the input reticulocyte lysate containing in vitro-translated BRF.
As an alternative approach to adding radiolabeled BRF, we employed an anti-TBP antibody to monitor the effect of RB on the binding of endogenous TFIIIB to TFIIIC2. These factors have been reported to associate in the absence of DNA as part of a holoenzyme complex (55). TBP is an integral component of the TFIIIB complex and, accordingly, could be coprecipitated from nuclear extract with antiserum against TFIIIC2 but not with the corresponding preimmune serum (Fig. 6A). This interaction was abolished by the inclusion of GST-RB. The effect was specific, since association was maintained in the presence of an equal amount of GST-RB carrying the Δ21 null mutation. These combined data provide evidence that RB is able to dissociate TFIIIB from its functional association with TFIIIC2.
FIG. 6.
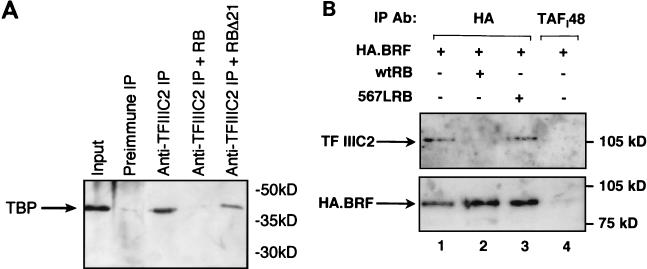
RB disrupts the interaction between TFIIIB and endogenous TFIIIC2 both in vitro and in vivo. (A) HeLa cell extract (150 μg) was immunoprecipitated (IP) using antiserum 4286 against TFIIIC2 (lanes 3 through 5) or the corresponding preimmune serum (lane 2). Lanes 3, 4, and 5 contained, respectively, 200 ng of GST, GST-RB, and GST-RBΔ21. Precipitated material was resolved on a sodium dodecyl sulfate (SDS)–7.8% polyacrylamide gel and then analyzed by Western blotting with anti-TBP antibody SL30. Lane 1 shows 10% of the input nuclear extract. (B) C33A cells were transfected with pcDNA3HA.BRF (2 μg) along with 2 μg of pSG5L vector (lanes 1 and 4), pSG5L-HA-RB(wt) (lane 2), or pSG-RB;567L (lane 3). Transfected cells were immunoprecipitated using anti-HA antibody (Ab) F-7 (lanes 1 to 3) or control antibody M-19 against TAFI48 (lane 4). Immunoprecipitated material was resolved on an SDS–7.8% polyacrylamide gel and then analyzed by Western blotting with antibody 4286 against the TFIIIC110 subunit of TFIIIC2 and antibody F-7 against the HA tag on transfected BRF.
To test whether RB acts in a similar manner in vivo, we carried out coimmunoprecipitations from transfected cells. RB-null cells were transfected with a vector encoding BRF that had been tagged with HA. When an anti-HA antibody was used for immunoprecipitation from lysates of the transfected cells, TFIIIC2 was coprecipitated with the tagged BRF, as revealed by Western blotting (Fig. 6B, lane 1). This reflects a specific interaction, since TFIIIC2 was not detected when an irrelevant antibody against TAFI48 was used for a negative control immunoprecipitation (Fig. 6B, lane 4). Furthermore, the anti-HA antibody failed to coprecipitate TFIIIC2 when cells were transfected with an empty vector encoding the HA tag without BRF attached (P. H. Scott and R. J. White, unpublished observations). We then tested whether expressing RB affects the interaction between transfected HA-BRF and endogenous TFIIIC2. When a vector encoding wild-type RB was included in the transfection, very little TFIIIC2 was coimmunoprecipitated with the anti-HA antibody, despite the fact that HA-BRF was expressed efficiently (Fig. 6B, lane 2). After normalization to the amount of HA-BRF in each immunoprecipitate, wild-type RB was found to reduce the interaction with endogenous TFIIIC2 ∼17-fold. In contrast, mutant RB carrying the 567L substitution did not compromise the binding of TFIIIC2 to HA-BRF (Fig. 6B, lane 3). These data demonstrate that RB can disrupt the interaction between TFIIIB and TFIIIC2 in vivo. They also suggest that this ability is dependent on wild-type RB function, as it can be abolished by a single-residue inactivating substitution.
RB does not prevent BRF from binding to TBP.
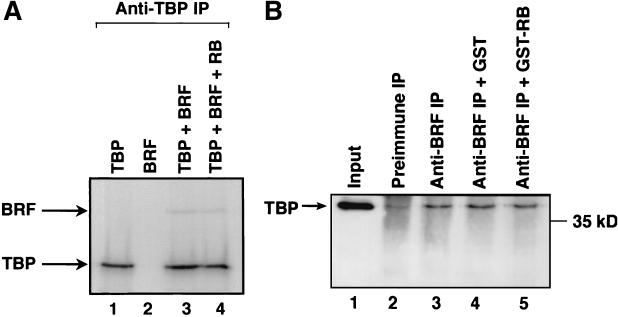
As a control for the specificity of these effects, we tested the ability of RB to influence the interaction between TBP and BRF that is fundamental to the integrity of the TFIIIB complex. As expected, an anti-TBP antibody immunoprecipitated 35S-labeled TBP but not 35S-labeled BRF (Fig. 7A, lanes 1 and 2). However, the antibody did coimmunoprecipitate BRF when it was mixed with TBP (Fig. 7A, lane 3), by virtue of the interaction between these two proteins. Addition of recombinant RB did not diminish the binding of TBP to BRF (Fig. 7A, lane 4). Similarly, endogenous TBP was coimmunoprecipitated from cellular extracts using antiserum against BRF (Fig. 7B, lanes 2 and 3). Again, the interaction showed little or no response to the inclusion of recombinant RB (Fig. 7B, lanes 4 and 5). These observations rule out the possibility that RB has a general effect on protein-protein interactions and suggest instead that the dissociation of TFIIIB from TFIIIC2 is a specific response.
FIG. 7.
Binding of TBP to BRF is maintained in the presence of RB. (A) Anti-TBP antibody SL30 was used to immunoprecipitate (IP) reticulocyte lysate (15 μl) containing in vitro-translated TBP and/or in vitro-translated BRF. Histidine-tagged recombinant RB (100 ng) was added to lane 4. Proteins retained after extensive washing were resolved on a sodium dodecyl sulfate (SDS)–7.8% polyacrylamide gel and then visualized by autoradiography. (B) HeLa cell extract (150 μg) was immunoprecipitated using antiserum 128 against BRF (lanes 3 to 5) or the corresponding preimmune serum (lane 2). Lanes 4 and 5 received 200 ng of GST and GST-RB, respectively. Precipitated material was resolved on an SDS–7.8% polyacrylamide gel and then analyzed by Western blotting with antibody SL30 against TBP. Lane 1 shows 10% of the input nuclear extract.
RB can repress a TFIIIC2-independent U6 snRNA gene promoter in vivo.
TFIIIC2 is essential for the recruitment of TFIIIB to the majority of class III genes (39, 59). However, the promoters of some vertebrate U6 snRNA genes have upstream TATA boxes that are recognized directly by TBP, allowing them to recruit TFIIIB without any requirement for TFIIIC2 (2, 27, 32, 54). If RB represses Pol III transcription solely by disrupting the interaction between TFIIIB and TFIIIC2, then it would not be expected to regulate a TFIIIC2-independent U6 snRNA gene. However, it has been shown previously that U6 transcription in a nuclear extract is inhibited potently by recombinant RB (28). Since the evidence that RB regulates U6 gene expression is to date derived solely from in vitro work, we decided to test if the same was true in vivo. The reporter construct used was pU6/Hae/RA.2, which contains all of the U6 upstream promoter, including the TATA box; however, the entire coding sequence was replaced by a fragment from the β-globin gene, thereby eliminating the possibility of any cryptic internal recognition site for TFIIIC2 (31). This construct was transfected into SAOS2 cells, along with a control for transfection efficiency and expression vectors encoding either wild-type RB or the 567L null mutant. Relative to the nonfunctional control, wild-type RB produced an approximately fourfold repression of U6 promoter activity, after normalization to the internal control (Fig. 8, bars 1 and 2). This is comparable to the repression observed with VA1 under these conditions (Fig. 3). We therefore conclude that the U6 snRNA promoter external to the gene is a bona fide target for regulation by RB both in vitro and in vivo. Since this promoter does not utilize TFIIIC2, the repression mechanism in this case must be distinct from the effect on the TFIIIB/TFIIIC2 interaction which we have observed. We therefore tested whether TSA might influence the ability of RB to regulate U6. However, as with the other class III genes, RB remained able to repress U6 expression in the presence of TSA. We therefore sought an alternative mechanism that might explain the effect of RB on TFIIIC2-independent class III genes.
FIG. 8.
RB represses a U6 snRNA gene promoter in vivo, even in the presence of TSA. SAOS2 cells were transfected with pU6/Hae/RA.2 (0.5 μg), pCAT (4 μg), and 8 μg of pSG5L-HA-RB(wt), encoding wild-type RB (lanes 2 and 4), or 8 μg of pSG-RB;567L, encoding an inactive RB null mutant (lanes 1 and 3). In lanes 3 and 4, the cells were maintained in 200 nM TSA for 24 h prior to harvesting. Levels of RNA derived from pU6/Hae/RA.2 and pCAT were assayed by primer extension and then quantitated using a phosphorimager. Values shown represent the signal for U6 normalized to the levels of CAT expression to correct for transfection efficiency; they are expressed relative to the value obtained in the absence of TSA or functional RB (lane 1), which is designated 100%, and are means from two experiments.
RB disrupts the interaction between TFIIIB and Pol III.
Once TFIIIB has been attracted to a promoter, it serves to recruit Pol III and position it over the initiation site (23). This activity is believed to be essential for the expression of any class III gene (59). We therefore examined whether the presence of RB has any influence on the ability of TFIIIB to bind to Pol III. An antiserum against the Pol III-specific subunit BN51 (20) coimmunoprecipitated 35S-labeled BRF after being mixed with a HeLa cell extract (Fig. 9A). This reflects the well-documented interaction between TFIIIB and Pol III, rather than a fortuitous cross-reaction, since only a low background level of BRF was coprecipitated when the HeLa extract was replaced by buffer (Fig. 9A, lane 2). We therefore tested whether the binding of TFIIIB to Pol III could be disrupted by the presence of RB. This proved to be the case, since inclusion of GST-RB reduced the amount of BRF that was coimmunoprecipitated to background levels seen in the absence of extract (Fig. 9A, lane 5). The effect is specific, since an equal amount of GST did not diminish the interaction (Fig. 9A, lane 4). To examine whether this activity requires functional RB, we made use of the Δ21 null mutant (Fig. 9B). Whereas wild-type RB again reduced the interaction between BRF and Pol III to background levels (Fig. 9B, lane 4), an equal amount of the Δ21 mutant had little effect in this assay (lane 5).
FIG. 9.
RB disrupts the interaction between TFIIIB and Pol III. (A) Reticulocyte lysate (15 μl) containing in vitro-translated BRF was immunoprecipitated (IP) in the presence of buffer (lane 2) or 150 μg of HeLa nuclear extract (lanes 3 to 5) using antiserum against the Pol III-specific subunit BN51. Lanes 4 and 5 contained 200 ng of GST and GST-RB, respectively. Proteins retained after extensive washing were resolved on a sodium dodecyl sulfate (SDS)–7.8% polyacrylamide gel and then visualized by autoradiography. Lane 1 shows 10% of the input reticulocyte lysate containing in vitro-translated BRF. (B) Reticulocyte lysate (15 μl) containing in vitro-translated BRF was immunoprecipitated in the presence of buffer (lane 2) or 150 μg of HeLa nuclear extract (lanes 3 to 5) using antiserum against the Pol III-specific subunit BN51. Lanes 3, 4, and 5 contained 200 ng of GST, GST-RB, and GST-RBΔ21, respectively. Proteins retained after extensive washing were resolved on an SDS–7.8% polyacrylamide gel and then visualized by autoradiography. Lane 1 shows 10% of the input reticulocyte lysate containing in vitro-translated BRF.
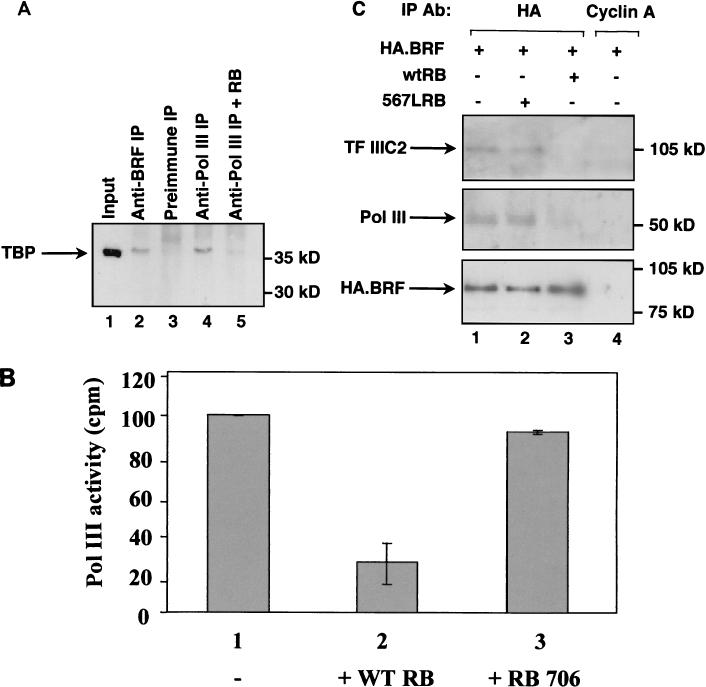
We used a monoclonal antibody against TBP to monitor the interactions made by endogenous TFIIIB. This revealed that TBP could be coimmunoprecipitated from HeLa cell extract using antisera against either the BRF subunit of TFIIIB or the BN51 subunit of Pol III, whereas only background amounts were detected with preimmune serum (Fig. 10A, lanes 2 through 4). This is consistent with the report that endogenous TFIIIB and Pol III associate in the absence of DNA (55). The interaction was substantially diminished by the inclusion of recombinant histidine-tagged RB; indeed, addition of RB reduced the amount of TBP that coprecipitated with Pol III to a level that was close to the low background level observed with preimmune serum (Fig. 10A, lane 5). These data show that RB can disrupt the association of endogenous TFIIIB and Pol III.
FIG. 10.
RB disrupts the interaction between TFIIIB and endogenous Pol III both in vitro and in vivo. (A) HeLa cell extract (150 μg) was immunoprecipitated (IP) using antiserum 128 against BRF (lane 2), anti-BN51 antiserum against Pol III (lanes 3 and 4), or the corresponding preimmune serum (lane 3). Recombinant histidine-tagged RB (100 ng) was included in lane 5. Precipitated material was resolved on a sodium dodecyl sulfate (SDS)–7.8% polyacrylamide gel and then analyzed by Western blotting with anti-TBP antibody SL30. Lane 1 shows 10% of the input nuclear extract. (B) HeLa cell extract (150 μg) was immunoprecipitated using antiserum 128 against BRF. Lanes 2 and 3 contained, respectively, 200 ng of wild-type (WT) GST-RB and GST-RB carrying an inactivating substitution at residue 706. After extensive washing, coprecipitated Pol III was detected using the random polymerization assay. Values shown are means of four experiments; error bars indicate the standard deviation. (C) C33A cells were transfected with pcDNA3HA.BRF (2 μg) along with 2 μg of pSG5L vector (lanes 1 and 4), pSG-RB;567L (lane 2), and pSG5L-HA-RB(wt) (lane 3). Transfected cells were immunoprecipitated (IP) using anti-HA antibody (Ab) F-7 or control antibody BF683 against cyclin A. Immunoprecipitated material was resolved on an SDS–7.8% polyacrylamide gel and then analyzed by Western blotting with antiserum 4286 against TFIIIC2 (top panel), anti-BN51 antiserum against Pol III (middle panel), and antibody F-7 against the HA tag on transfected BRF (bottom panel).
Polymerization assays using poly(dA-dT) as a template provide a sensitive method for detecting RNA polymerases in the absence of transcription factors. This approach was used as an alternative method for monitoring the interaction between endogenous TFIIIB and Pol III. An antiserum against BRF coimmunoprecipitated from cell extracts, Pol III activity that was readily detectable using the polymerization assay (Fig. 10B). When recombinant RB was added to the extract, the amount of Pol III that coprecipitated was diminished fourfold. This effect was specific, since it was not seen with an equal amount of RB carrying an inactivating point mutation at residue 706 (24). These results therefore provide independent confirmation that the essential interaction between TFIIIB and Pol III can be compromised by the presence of functional RB.
Transient transfections with HA-tagged BRF were carried out to test the effect of RB on the ability of TFIIIB to bind to Pol III in vivo. Western blotting revealed that both TFIIIC2 and Pol III can be coprecipitated from the transfected cells using an anti-HA antibody (Fig. 10C, lanes 1 through 3); these interactions are specific, since neither was detected in control immunoprecipitations carried out using an irrelevant antibody against cyclin A (Fig. 10C, lane 4). Cotransfection with a vector encoding wild-type RB (Fig. 10C, lane 3) caused a substantial decrease in the amount of TFIIIC2 that was bound to HA-tagged BRF, as before. Furthermore, the amount of Pol III bound was also reduced significantly. Quantitation revealed that wild-type RB produced an approximately eightfold decrease in the binding of transfected BRF to endogenous Pol III, after normalization to the amount of HA-BRF immunoprecipitated. In contrast, null mutant RB carrying the 567L substitution had little or no effect on either interaction (Fig. 10C, lane 2). We conclude that RB is able in vivo to disrupt the binding of TFIIIB to both TFIIIC2 and Pol III.
DISCUSSION
Expression of a class III gene requires multiple contacts between components of the basal transcription apparatus. At least two of these interactions can be disrupted by RB, which can account for its potent capacity to repress the Pol III system. With only a few exceptions, recruitment of TFIIIB to a promoter is dependent upon its binding to TFIIIC2 (59). Interference with the contact between TFIIIB and TFIIIC2 will therefore provide a powerful mechanism for inhibiting the expression of most class III genes. However, a minority of Pol III templates, such as some U6 snRNA genes, have upstream TATA sequences that are recognized directly by TBP, allowing TFIIIB to be recruited in a TFIIIC2-independent manner (59). Nevertheless, a TATA-containing U6 promoter is subject to repression by RB both in vitro and in vivo. This may be explained by the capacity of RB to disrupt interactions between Pol III and TFIIIB. Since recruitment of Pol III by TFIIIB is an essential step in transcription initiation, this second mechanism can potentially account for the fact that RB represses every class III gene tested.
The HDAC inhibitor TSA blocks the ability of RB to regulate Pol II transcription of the DHFR gene (34). This is considered to reflect a process in which RB brings HDAC molecules to a promoter, which then reduce chromatin accessibility by removing acetyl groups from the tails of histones (3, 4, 34, 35). Studies in vitro have shown previously that histone acetylation can facilitate Pol III transcription from a chromatin template (30, 51, 52). Indeed, subunits of TFIIIC2 have been found to acetylate histones (17, 26). The physiological relevance of these data is supported strongly by our observation that TSA stimulates the expression of B2 genes in living cells. As far as we are aware, this provides the first evidence that acetylation can influence Pol III activity in vivo. Nevertheless, TSA does not block the ability of RB to regulate class III genes in vitro or in vivo. Since RB disrupts the interaction between TFIIIB and TFIIIC2, it is unlikely to stay associated with a Pol III promoter and may therefore not affect the chromatin state of class III genes in situ; this contrasts with the Pol II situation, where an E2F-RB-HDAC complex remains stably bound to DNA (3, 4, 34, 35). Our data do not exclude the possibility that HDACs might play a subsidiary role in Pol III repression by RB. Indeed, we consistently observed that the difference in B2 expression between Rb+/+ and Rb−/− fibroblasts is diminished slightly (11 to 17%) in the presence of TSA. However, it is clear that RB can inhibit Pol III transcription efficiently in the presence of TSA, which indicates that HDAC activity is not required for regulation of this system.
Although a few promoters with E2F recognition sites are repressed in an HDAC-dependent fashion, in the majority of cases RB can inhibit Pol II transcription irrespective of the presence of TSA (34). E2F is able to recruit TFIID to a promoter in a step that requires TFIIA, but this can be blocked by the addition of RB (42). Since TFIID and TFIIA are both general factors, this effect can account for the inhibitory influence of RB on many Pol II promoters. An analogy may be drawn between this mechanism and the ability of RB to disrupt the TFIIIB-TFIIIC2 interaction. In both cases, the targeted step involves recruitment of a TBP-containing complex (TFIID or TFIIIB) by a DNA-binding factor (E2F or TFIIIC2).
Association with RB not only prevents TFIIIB from binding to TFIIIC2, but it also interferes with its ability to recruit Pol III. We are not aware of any precedent for the second mechanism, since RB has not been reported to influence the polymerase recruitment step in the class I or class II systems. Whereas a few Pol III promoters do not require TFIIIC2, the interaction between TFIIIB and Pol III is believed to be essential for initiation of transcription at any class III gene (39, 59). The ability of RB to disrupt this step may account for its inhibitory effect in vitro (28) and in vivo (Fig. 8) on a U6 snRNA gene that does not utilize TFIIIC2. However, since U6 transcription involves a specialized complex called PTF or SNAPc, it is possible that RB also influences additional steps in initiation at a U6 promoter. Indeed, we cannot exclude the possibility that RB can affect aspects of TFIIIB function besides its interactions with TFIIIC2 and Pol III. For example, TFIIIB is likely to contact TFIIIC1, an uncharacterized factor that is required for Pol III transcription in mammals (10, 65); this interaction may be inhibited by RB, if it occupies an extensive area of the TFIIIB surface. Human TFIIIB is believed to contain at least one uncharacterized component, besides BRF and TBP, which probably corresponds to the B" subunit found in yeast TFIIIB (33, 36, 49, 50). Since molecular reagents are not available for this polypeptide, we are unable to test if its association with TFIIIB is also disrupted by RB. However, we have shown that binding of BRF to TBP is maintained in the presence of RB. This is consistent with the fact that these subunits can be coimmunoprecipitated using anti-RB antibodies (28). The ability of RB to interfere with TFIIIB binding to TFIIIC2 and Pol III therefore constitutes a specific effect.
On the basis of our data, we are able to propose a model to explain why the expression of mammalian class III genes is potently repressed by RB. This model is summarized in Fig. 11. It suggests that RB binds to TFIIIB and thereby disrupts protein-protein interactions that are essential for Pol III transcription. Binding of RB to TFIIIB would seem to be relatively tight, since the association can withstand extensive washing at elevated salt concentrations (28). An avid interaction would obviously help it to compete with Pol III and TFIIIC2 for contact with TFIIIB. Competition must also be facilitated by the very high concentrations of endogenous RB; while there may be tens of thousands of TFIIIB molecules in a mammalian cell (40), RB may be more abundant than this by 2 orders of magnitude (57). Genetic analysis has shown that RB exercises a potent influence over Pol III transcription in vivo; this can now be understood in terms of its considerable abundance, its affinity for TFIIIB, and its ability to disrupt interactions that are essential for the function of the initiation complex.
FIG. 11.
Model to explain the repression of Pol III transcription by RB. Specific initiation at class III genes is dependent on the interaction between TFIIIB and Pol III; in most cases TFIIIB must be recruited to promoters through binding to TFIIIC2. RB associates with TFIIIB and prevents it from interacting with Pol III and TFIIIC2. In this way, RB is able to disrupt preinitiation complex formation and inhibit transcription.
ACKNOWLEDGMENTS
We thank Bill Kaelin for RB expression vectors, Michael Ittmann for antisera against BN51, and Nouria Hernandez for pU6/Hae/RA.2 and monoclonal antibody SL30 against TBP.
This work was funded by project grant 17/C10311 to R.J.W. from the Biotechnology and Biological Sciences Research Council. P.H.S. is a Wellcome Trust Research Fellow, and R.J.W. is a Jenner Research Fellow of the Lister Institute of Preventive Medicine.
REFERENCES
- 1.Alzuherri H M, White R J. Regulation of a TATA-binding protein-associated factor during cellular differentiation. J Biol Chem. 1998;273:17166–17171. doi: 10.1074/jbc.273.27.17166. [DOI] [PubMed] [Google Scholar]
- 2.Bernues J, Simmen K A, Lewis J D, Gunderson S I, Polycarpou-Schwarz M, Moncollin V, Egly J-M, Mattaj I. Common and unique transcription factor requirements of human U1 and U6 snRNA genes. EMBO J. 1993;12:3573–3585. doi: 10.1002/j.1460-2075.1993.tb06031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brehm A, Kouzarides T. Retinoblastoma protein meets chromatin. Trends Biochem Sci. 1999;24:142–145. doi: 10.1016/s0968-0004(99)01368-7. [DOI] [PubMed] [Google Scholar]
- 4.Brehm A, Miska E A, McCance D J, Reid J L, Bannister A J, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- 5.Cairns C A, White R J. p53 is a general repressor of RNA polymerase III transcription. EMBO J. 1998;17:3112–3123. doi: 10.1093/emboj/17.11.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey M F, Singh K. Enhanced B2 transcription in simian virus 40-transformed cells is mediated through the formation of RNA polymerase III transcription complexes on previously inactive genes. Proc Natl Acad Sci USA. 1988;85:7059–7063. doi: 10.1073/pnas.85.19.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavanaugh A H, Hempel W M, Taylor L J, Rogalsky V, Todorov G, Rothblum L I. Activity of RNA polymerase I transcription factor UBF blocked by Rb gene product. Nature. 1995;374:177–180. doi: 10.1038/374177a0. [DOI] [PubMed] [Google Scholar]
- 8.Chu W-M, Wang Z, Roeder R G, Schmid C W. RNA polymerase III transcription repressed by Rb through its interactions with TFIIIB and TFIIIC2. J Biol Chem. 1997;272:14755–14761. doi: 10.1074/jbc.272.23.14755. [DOI] [PubMed] [Google Scholar]
- 9.Dean N, Berk A J. Ordering promoter binding of class III transcription factors TFIIIC1 and TFIIIC2. Mol Cell Biol. 1988;8:3017–3025. doi: 10.1128/mcb.8.8.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean N, Berk A J. Separation of TFIIIC into two functional components by sequence specific DNA affinity chromatography. Nucleic Acids Res. 1987;15:9895–9907. doi: 10.1093/nar/15.23.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 12.Grana X, Garriga J, Mayol X. Role of the retinoblastoma protein family, pRB, p107 and p130 in the negative control of cell growth. Oncogene. 1998;17:3365–3383. doi: 10.1038/sj.onc.1202575. [DOI] [PubMed] [Google Scholar]
- 13.Gu W, Roeder R G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 14.Helin K. Regulation of cell proliferation by the E2F transcription factors. Curr Opin Genet Dev. 1998;8:28–35. doi: 10.1016/s0959-437x(98)80058-0. [DOI] [PubMed] [Google Scholar]
- 15.Herwig S, Strauss M. The retinoblastoma protein: a master regulator of cell cycle, differentiation and apoptosis. Eur J Biochem. 1997;246:581–601. doi: 10.1111/j.1432-1033.1997.t01-2-00581.x. [DOI] [PubMed] [Google Scholar]
- 16.Horowitz J M, Park S-H, Bogenmann E, Cheng J-C, Yandell D W, Kaye F J, Minna J D, Dryja T P, Weinberg R A. Frequent inactivation of the retinoblastoma anti-oncogene is restricted to a subset of human tumor cells. Proc Natl Acad Sci USA. 1990;87:2775–2779. doi: 10.1073/pnas.87.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh Y-J, Kundu T K, Wang Z, Kovelman R, Roeder R G. The TFIIIC90 subunit of TFIIIC interacts with multiple components of the RNA polymerase III machinery and contains a histone-specific acetyltransferase activity. Mol Cell Biol. 1999;19:7697–7704. doi: 10.1128/mcb.19.11.7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurford R K, Cobrinik D, Lee M-H, Dyson N. pRB and p107/p130 are required for the regulated expression of different sets of E2F responsive genes. Genes Dev. 1997;11:1447–1463. [Google Scholar]
- 19.Imhof A, Yang X-J, Ogryzko V V, Nakatani Y, Wolffe A P, Ge H. Acetylation of general transcription factors by histone acetyltransferases. Curr Biol. 1997;7:689–692. doi: 10.1016/s0960-9822(06)00296-x. [DOI] [PubMed] [Google Scholar]
- 20.Ittmann M, Ali J, Greco A, Basilico C. The gene complementing a temperature-sensitive cell cycle mutant of BHK cells is the human homologue of the yeast RPC53 gene, which encodes a subunit of RNA polymerase C (III) Cell Growth Differ. 1993;4:503–511. [PubMed] [Google Scholar]
- 21.Jackson A J, Ittmann M, Pugh B F. The BN51 protein is a polymerase (Pol)-specific subunit of RNA Pol III which reveals a link between Pol III transcription and pre-rRNA processing. Mol Cell Biol. 1995;15:94–101. doi: 10.1128/mcb.15.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones P L, Veenstra G J C, Wade P A, Vermaak D, Kass S U, Landsberger N, Strouboulis J, Wolffe A P. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 23.Kassavetis G A, Braun B R, Nguyen L H, Geiduschek E P. S. cerevisiae TFIIIB is the transcription initiation factor proper of RNA polymerase III, while TFIIIA and TFIIIC are assembly factors. Cell. 1990;60:235–245. doi: 10.1016/0092-8674(90)90739-2. [DOI] [PubMed] [Google Scholar]
- 24.Kaye F J, Kratzke R A, Gerster J L, Horowitz J M. A single amino acid substitution results in a retinoblastoma protein defective in phosphorylation and oncoprotein binding. Proc Natl Acad Sci USA. 1990;87:6922–6926. doi: 10.1073/pnas.87.17.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knoepfler P S, Eisenman R N. Sin meets NuRD and other tails of repression. Cell. 1999;99:447–450. doi: 10.1016/s0092-8674(00)81531-7. [DOI] [PubMed] [Google Scholar]
- 26.Kundu T K, Wang Z, Roeder R G. Human TFIIIC relieves chromatin-mediated repression of RNA polymerase III transcription and contains an intrinsic histone acetyltransferase activity. Mol Cell Biol. 1999;19:1605–1615. doi: 10.1128/mcb.19.2.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagna G, Kovelman R, Sukegawa J, Roeder R G. Cloning and characterization of an evolutionarily divergent DNA-binding subunit of mammalian TFIIIC. Mol Cell Biol. 1994;14:3053–3064. doi: 10.1128/mcb.14.5.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larminie C G C, Cairns C A, Mital R, Martin K, Kouzarides T, Jackson S P, White R J. Mechanistic analysis of RNA polymerase III regulation by the retinoblastoma protein. EMBO J. 1997;16:2061–2071. doi: 10.1093/emboj/16.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larminie C G C, Sutcliffe J E, Tosh K, Winter A G, Felton-Edkins Z A, White R J. Activation of RNA polymerase III transcription in cells transformed by simian virus 40. Mol Cell Biol. 1999;19:4927–4934. doi: 10.1128/mcb.19.7.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee D Y, Hayes J J, Pruss D, Wolffe A P. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993;72:73–84. doi: 10.1016/0092-8674(93)90051-q. [DOI] [PubMed] [Google Scholar]
- 31.Lobo S M, Hernandez N. A 7 bp mutation converts a human RNA polymerase II snRNA promoter into an RNA polymerase III promoter. Genes Dev. 1989;58:55–67. doi: 10.1016/0092-8674(89)90402-9. [DOI] [PubMed] [Google Scholar]
- 32.Lobo S M, Lister J, Sullivan M L, Hernandez N. The cloned RNA polymerase II transcription factor IID selects RNA polymerase III to transcribe the human U6 gene in vitro. Genes Dev. 1991;5:1477–1489. doi: 10.1101/gad.5.8.1477. [DOI] [PubMed] [Google Scholar]
- 33.Lobo S M, Tanaka M, Sullivan M L, Hernandez N. A TBP complex essential for transcription from TATA-less but not TATA-containing RNA polymerase III promoters is part of the TFIIIB fraction. Cell. 1992;71:1029–1040. doi: 10.1016/0092-8674(92)90397-u. [DOI] [PubMed] [Google Scholar]
- 34.Luo R X, Postigo A A, Dean D C. Rb interacts with histone deacetylase to repress transcription. Cell. 1998;92:463–473. doi: 10.1016/s0092-8674(00)80940-x. [DOI] [PubMed] [Google Scholar]
- 35.Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Lorain S, Le Villain J P, Troalen F, Trouche D, Harel-Bellan A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature. 1998;391:601–604. doi: 10.1038/35410. [DOI] [PubMed] [Google Scholar]
- 36.Mital R, Kobayashi R, Hernandez N. RNA polymerase III transcription from the human U6 and adenovirus type 2 VAI promoters has different requirements for human BRF, a subunit of human TFIIIB. Mol Cell Biol. 1996;16:7031–7042. doi: 10.1128/mcb.16.12.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulligan G, Jacks T. The retinoblastoma gene family: cousins with overlapping interests. Trends Genet. 1998;14:223–229. doi: 10.1016/s0168-9525(98)01470-x. [DOI] [PubMed] [Google Scholar]
- 38.Nan X, Ng H-H, Johnson C A, Laherty C D, Turner B M, Eisenman R N, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 39.Paule M R, White R J. Transcription by RNA polymerases I and III. Nucleic Acids Res. 2000;28:1283–1298. doi: 10.1093/nar/28.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pombo A, Jackson D A, Hollinshead M, Wang Z, Roeder R G, Cook P R. Regional specialization in human nuclei: visualization of discrete sites of transcription by RNA polymerase III. EMBO J. 1999;18:2241–2253. doi: 10.1093/emboj/18.8.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roeder R G. Multiple forms of deoxyribonucleic acid-dependent ribonucleic acid polymerase in Xenopus laevis. J Biol Chem. 1974;249:241–248. [PubMed] [Google Scholar]
- 42.Ross J F, Liu X, Dynlacht B D. Mechanism of transcriptional repression of E2F by the retinoblastoma tumor suppressor protein. Mol Cell. 1999;3:195–205. doi: 10.1016/s1097-2765(00)80310-x. [DOI] [PubMed] [Google Scholar]
- 43.Russanova V R, Driscoll C T, Howard B H. Adenovirus type 2 preferentially stimulates polymerase III transcription of Alu elements by relieving repression: a potential role for chromatin. Mol Cell Biol. 1995;15:4282–4290. doi: 10.1128/mcb.15.8.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakaguchi K, Herrera J E, Saito S, Miki T, Bustin M, Vassilev A, Anderson C W, Appella E. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 1998;12:2831–2841. doi: 10.1101/gad.12.18.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sellers W R, Novitch B G, Miyake S, Heith A, Otterson G A, Kaye F J, Lassar A B, Kaelin W G., Jr Stable binding to E2F is not required for the retinoblastoma protein to activate transcription, promote differentiation, and suppress tumor cell growth. Genes Dev. 1998;12:95–106. doi: 10.1101/gad.12.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutcliffe J E, Cairns C A, McLees A, Allison S J, Tosh K, White R J. RNA polymerase III transcription factor IIIB is a target for repression by pocket proteins p107 and p130. Mol Cell Biol. 1999;19:4255–4261. doi: 10.1128/mcb.19.6.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taunton J, Hassig C A, Schreiber S L. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science. 1996;272:408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- 48.Taya Y. RB kinases and RB-binding proteins: new points of view. Trends Biochem Sci. 1997;22:14–17. doi: 10.1016/s0968-0004(96)10070-0. [DOI] [PubMed] [Google Scholar]
- 49.Teichmann M, Dieci G, Huet J, Ruth J, Sentenac A, Seifart K H. Functional interchangeability of TFIIIB components from yeast and human cells in vitro. EMBO J. 1997;16:4708–4716. doi: 10.1093/emboj/16.15.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teichmann M, Seifart K H. Physical separation of two different forms of human TFIIIB active in the transcription of the U6 or the VAI gene in vitro. EMBO J. 1995;14:5974–5983. doi: 10.1002/j.1460-2075.1995.tb00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tse C, Sera T, Wolffe A P, Hansen J C. Disruption of higher-order folding by core histone acetylation dramatically enhances transcription of nucleosomal arrays by RNA polymerase III. Mol Cell Biol. 1998;18:4629–4638. doi: 10.1128/mcb.18.8.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ura K, Kurumizaka H, Dimitrov S, Almouzni G, Wolffe A P. Histone acetylation: influence on transcription, nucleosome mobility and positioning, and linker histone-dependent transcriptional repression. EMBO J. 1997;16:2096–2107. doi: 10.1093/emboj/16.8.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Voit R, Schäfer K, Grummt I. Mechanism of repression of RNA polymerase I transcription by the retinoblastoma protein. Mol Cell Biol. 1997;17:4230–4237. doi: 10.1128/mcb.17.8.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waldschmidt R, Wanandi I, Seifart K H. Identification of transcription factors required for the expression of mammalian U6 genes in vitro. EMBO J. 1991;10:2595–2603. doi: 10.1002/j.1460-2075.1991.tb07801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Z, Luo T, Roeder R G. Identification of an autonomously initiating RNA polymerase III holoenzyme containing a novel factor that is selectively inactivated during protein synthesis inhibition. Genes Dev. 1997;11:2371–2382. doi: 10.1101/gad.11.18.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z, Roeder R G. Structure and function of a human transcription factor TFIIIB subunit that is evolutionarily conserved and contains both TFIIB- and high-mobility-group protein 2-related domains. Proc Natl Acad Sci USA. 1995;92:7026–7030. doi: 10.1073/pnas.92.15.7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 58.White R J. Regulation of RNA polymerases I and III by the retinoblastoma protein: a mechanism for growth control? Trends Biochem Sci. 1997;22:77–80. doi: 10.1016/s0968-0004(96)10067-0. [DOI] [PubMed] [Google Scholar]
- 59.White R J. RNA polymerase III transcription. Berlin, Germany: Springer-Verlag; 1998. [Google Scholar]
- 60.White R J, Gottlieb T M, Downes C S, Jackson S P. Mitotic regulation of a TATA-binding-protein-containing complex. Mol Cell Biol. 1995;15:1983–1992. doi: 10.1128/mcb.15.4.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White R J, Stott D, Rigby P W J. Regulation of RNA polymerase III transcription in response to F9 embryonal carcinoma stem cell differentiation. Cell. 1989;59:1081–1092. doi: 10.1016/0092-8674(89)90764-2. [DOI] [PubMed] [Google Scholar]
- 62.White R J, Trouche D, Martin K, Jackson S P, Kouzarides T. Repression of RNA polymerase III transcription by the retinoblastoma protein. Nature. 1996;382:88–90. doi: 10.1038/382088a0. [DOI] [PubMed] [Google Scholar]
- 63.Yandell D W, Campbell T A, Dayton S H, Petersen R, Walton D, Little J B, McConkie-Rosell A, Buckley E G, Dryja T P. Oncogenic point mutations in the human retinoblastoma gene: their application to genetic counseling. N Engl J Med. 1989;321:1689–1695. doi: 10.1056/NEJM198912213212501. [DOI] [PubMed] [Google Scholar]
- 64.Yoshida M, Kijimia M, Akita M, Beppu T. Potent and specific inhibition of histone deacetylase both in vitro and in vivo by trichostatin A. J Biol Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- 65.Yoshinaga S K, Boulanger P A, Berk A J. Resolution of human transcription factor TFIIIC into two functional components. Proc Natl Acad Sci USA. 1987;84:3585–3589. doi: 10.1073/pnas.84.11.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]