Abstract
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) provides cardio-respiratory support to patients in cardiogenic shock. This comes at the cost of increased left ventricular (LV) afterload that can partly be ascribed to retrograde aortic flow causing LV distension, and leads to complications including cardiac thrombi, arrhythmias and pulmonary edema. LV unloading can be achieved by using an additional circulatory support device to mitigate the adverse effects of mechanical overload that may increase the likelihood of myocardial recovery. Observational data suggest that these strategies may improve outcomes but in whom, when and how LV unloading should be employed is unclear; all techniques require balancing of presumed benefits against the known risk of device-related complications. This review will summarize the current evidence regarding LV unloading with VA-ECMO.
Keywords: VA-ECMO, Unloading, ECPELLA, Afterload, Cardiogenic shock, Venting
Introduction
Cardiogenic shock is a syndrome of inadequate perfusion of vital organs due to primary cardiac dysfunction 1. Despite many advances in acute cardiac care over the last two decades, the mortality following cardiogenic shock remains unacceptably high at around 50%. Early revascularization when cardiogenic shock complicates acute myocardial infarction (AMI) is the only intervention to have shown unequivocal benefit 2. Circulatory support with intra-aortic balloon pump (IABP), percutaneous left ventricular assist devices (pLVAD) or inotropic therapy has not shown a clear survival benefit 3.
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is a mechanical circulatory support (MCS) strategy that provides extracorporeal blood flow of 4–6 liters per minute and sufficient gas exchange to support systemic perfusion in severe cardio-respiratory failure. Support is provided as a bridge to recovery, transplantation, durable MCS, decision or palliation. A significant downside is that VA-ECMO support results in an unphysiological, continuous infusion of blood into the arterial vasculature. In the peripheral configuration (Figure 1) blood flows retrogradely in the aorta, increasing afterload on an already failing left ventricle (LV). If the LV is unable to overcome this increase, rising pressure and volume within the LV result in a vicious circle of LV distension, reduced coronary perfusion pressure, reduced stroke volume and eventually blood stasis within the left heart and aortic root leading to thrombus formation 4. Transmission of increased filling pressures to the pulmonary venous circulation may result in hydrostatic pulmonary edema and even hemorrhage 5 (Figure 2). Adjunctive strategies to decompress the LV include LV unloading (where interventions are focused on reducing LV work) or LV venting (where interventions reduce LV filling pressures but do not necessarily reduce LV work). These strategies are increasingly used to prevent or treat complications of VA-ECMO but lack definitive evidence and can cause adverse outcomes in their own right. The utility of VA-ECMO itself remains unproven in cardiogenic shock, to date a single randomized trial has been reported. The ECMO-CS study (NCT02301819) randomized 122 patients with rapidly deteriorating or severe cardiogenic shock to receive either immediate VA-ECMO or conservative therapy and found no significant difference in the occurrence of a composite primary end point of death, resuscitated cardiac arrest or need for another MCS device at 30 days (63.8% vs 71.2%, p=0.21)6. It is important to note however, that an unloading strategy was only used in 16% of participants and whether protocolized use of unloading may have improved outcomes remains to be proven.
Figure 1: A comparison of Central vs Peripheral cannulation of VA-ECMO.
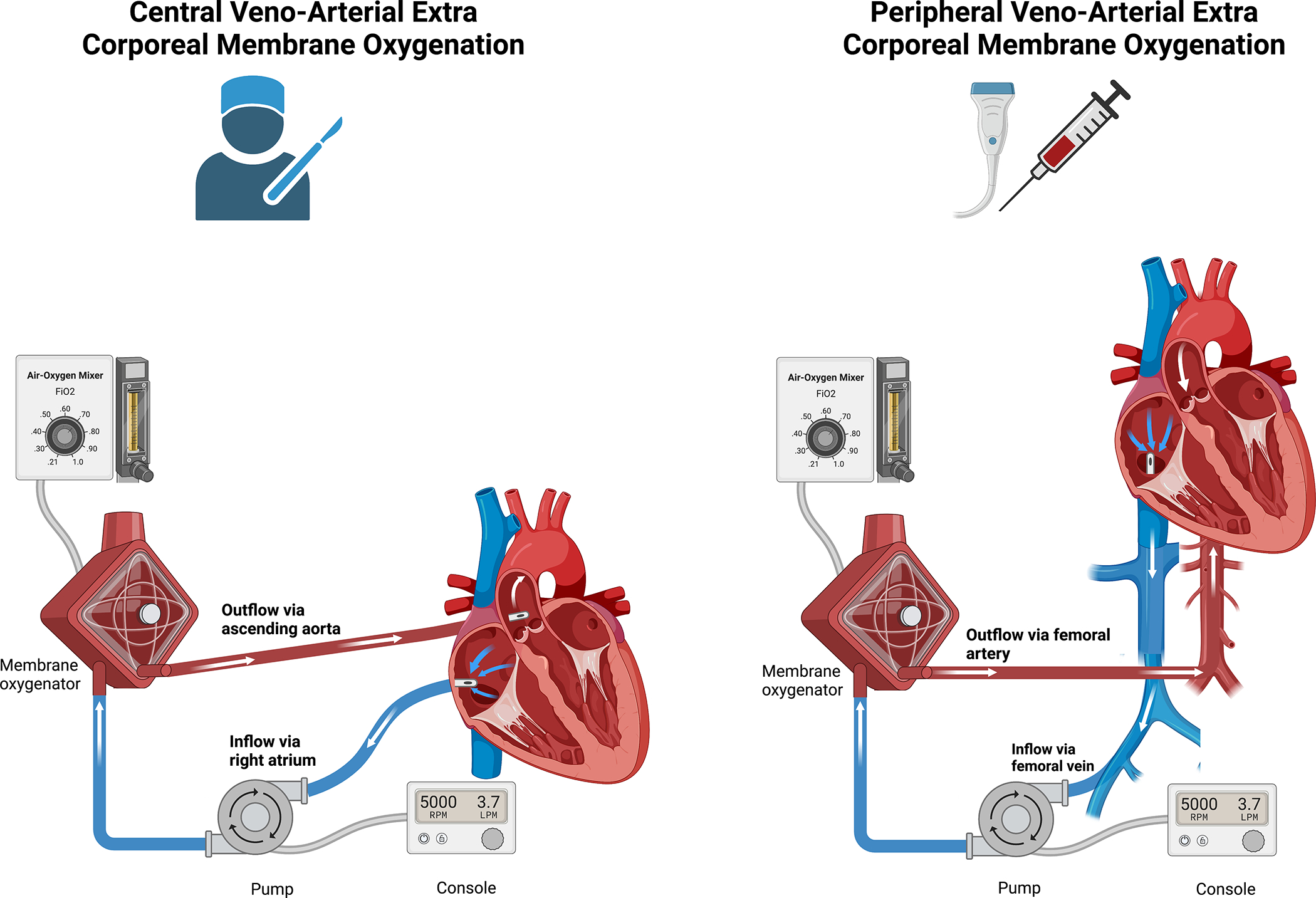
VA-ECMO maybe cannulated centrally via surgery or peripherally percutaneously. In central VA-ECMO the arterial outflow cannula is placed in the ascending aorta resulting in antegrade flow in the aorta in contrast to peripheral VA-ECMO where the outflow cannula is usually sited in the iliac artery resulting in retrograde flow. Different configurations of the venous inflow cannula can be used in both central and peripheral circuits for example femoral vein inflow cannula use in a central VA-ECMO circuit.
Figure 2: Hemodynamic effects of VA-ECMO.
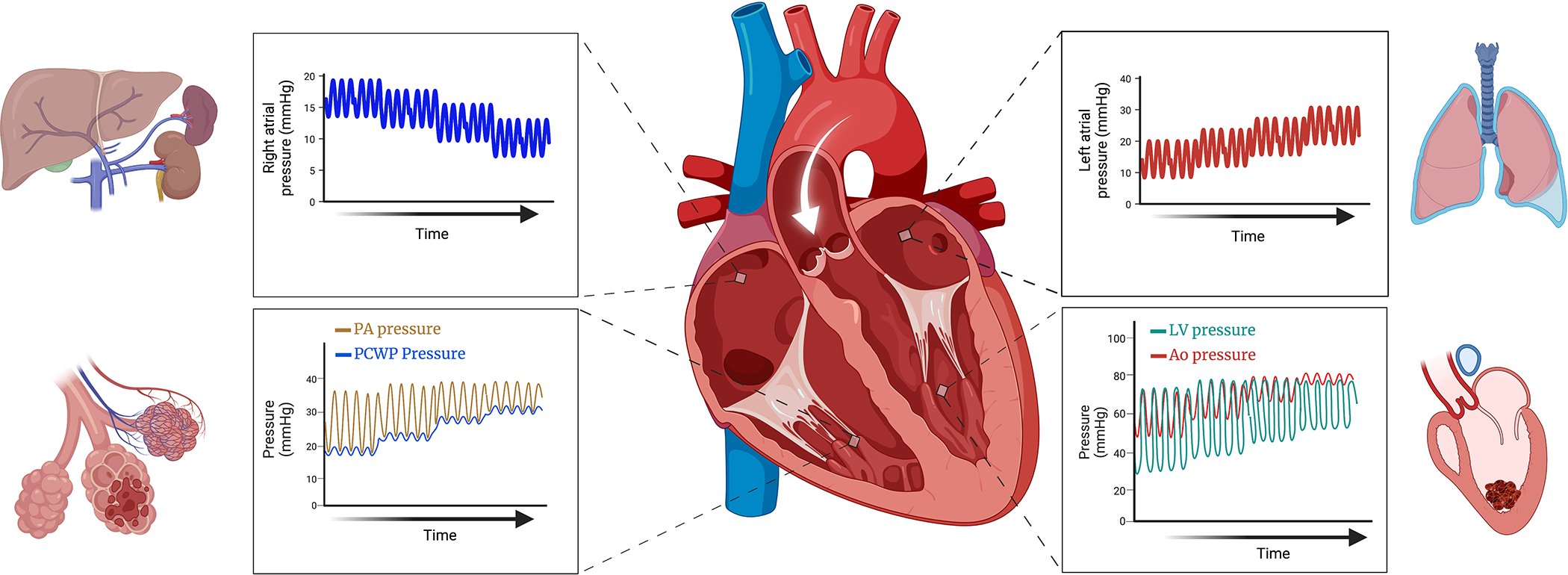
VA-ECMO reduces right atrial pressure, decongesting the liver and kidneys. Mean aortic pressure rises increasing afterload, if the left ventricle (LV) is unable to overcome the increased afterload, stroke volume falls resulting in loss of aortic pulsatility and stagnation of blood potentiating thrombus formation. Rising LV end diastolic pressure transmitted to the left atrium leads to pulmonary congestion. Backwards failure eventually increases pulmonary capillary wedge pressure (PCWP) and pulmonary artery diastolic pressure with loss of pulmonary artery pulsatility and worsening lung injury.
Physiological basis of LV unloading
Left ventricular afterload is an indirect measure of the mechanical forces imposed on the myocardium during systole. Cardiac mechanical load is often considered synonymous with left ventricular wall stress, a continuous measure throughout the cardiac cycle that is proportional to left ventricular intracavitary pressure and radius and inversely proportional to ventricular wall thickness 7. Continuous LV intracavitary pressure and volume readings can be used to generate LV pressure-volume (PV) loops, that provide estimates of afterload by metrics such as arterial elastance (Ea). Ea is the ratio of ventricular systolic pressure (at end systole) to stroke volume and reflects arterial load (Figure 3a). Arterial load is closely related to cardiac afterload and, if increased, impairs ventricular performance by reducing stroke volume and hence cardiac output, if contractility and end-diastolic volume (EDV) remain constant (Figure 3c). In (patho-)physiological conditions a significant increase in afterload is accompanied by backward failure, venous congestion and higher preload. A higher preload may increase LV EDV, that should in turn increase stroke volume via the Frank-Starling mechanism (Figure 3d). The ability to increase stroke volume by increasing LVEDV is known as the preload reserve, if this is exhausted the LV becomes sensitive to increased afterload particularly in the context of limited contractile reserve 8,9. When preload reserve is exhausted the PV loop shifts upwards and to the right, increasing the pressure volume area (PVA) and myocardial oxygen consumption (MVO2). Increased contractility represents a second compensatory mechanism by which the LV can maintain stroke volume but at the cost of increased PVA and therefore MVO2 (Figure 3d), thereby potentially adversely impacting on myocardial recovery. In cardiogenic shock the LV usually lacks the preload and contractile reserve to overcome the increased afterload associated with VA-ECMO, thus increasing myocardial oxygen demand on a failing ventricle.
Figure 3: Pressure volume (PV) loop basics.
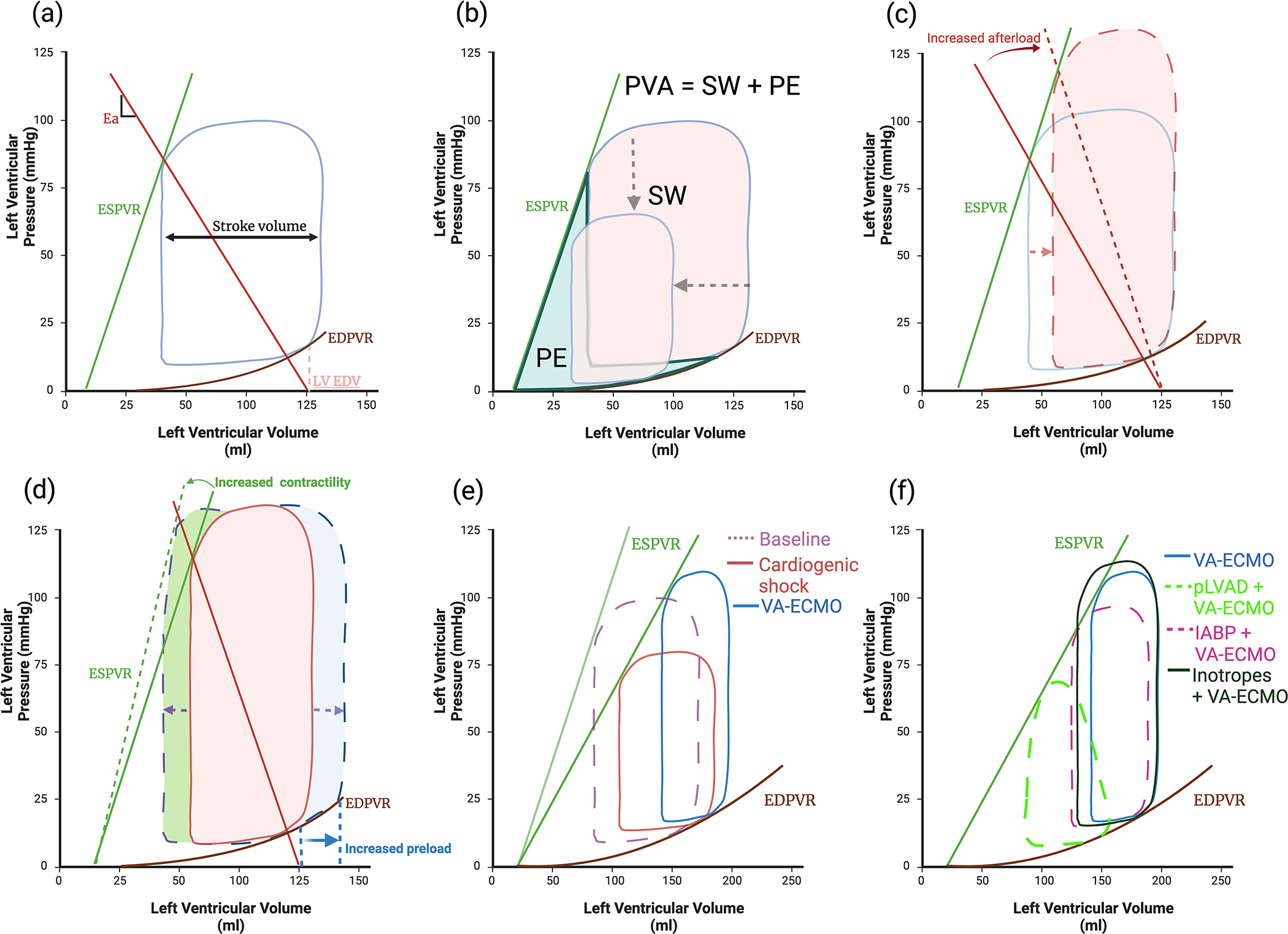
(a) Normal PV loop, boundaries are created by the end systolic pressure volume relationship (ESPVR) and the non-linear end diastolic pressure volume relationship (EDPVR). Effective arterial elastance (Ea) reflects afterload and is the slope of the line between the end diastolic volume (LV EDV) and the ESPVR. (b) Stroke work (SW) is the work required to eject blood, Potential energy (PE) is energy generated during contraction but not converted to SW. Pressure volume area (PVA) correlates linearly with myocardial oxygen consumption (MVO2) and is the sum of the SW and PE. Ventricular unloading is defined by a reduction in the PVA. (c) Increased afterload whilst maintaining the same level of contractility and preload reduces stroke volume (SV) (d) SV can be increased by either increasing preload, however in a dilated ventricle this will cause a significant rise in end diastolic pressure due to the non-linear EDPVR or by increasing contractility but this also increases MVO2. (e) Cardiogenic shock results in loss of contractility and increases end diastolic pressure (EDP) and volume (EDV). (b) VA-ECMO raises systolic pressure, EDP and afterload thereby increasing pressure volume area (PVA), whilst further reducing SV (f) IABP reduces afterload, increasing SV without significantly reducing EDP, EDV or PVA. In contrast pLVAD actively unloads the ventricle reducing afterload, EDV and EDP thereby significantly reducing PVA. Inotropes increase contractility improving SV; however, this increases PVA.
LV unloading refers to strategies that reduce PVA and hence reduce MVO2 (3b) 10. Unloading has two key goals: firstly, to prevent or treat clinically manifest complications and secondly to promote LV recovery, even in those without apparent complications. LV venting in contrast refers to a reduction in left ventricular end diastolic pressure (LVEDP) with the goal of reducing pulmonary congestion, not all patients will therefore require venting in the absence of elevated LVEDP but may still benefit from unloading 11. Emerging evidence suggests unloading may offer cardioprotection beyond mechanical effects, with reduced inflammatory cytokine expression, as found in fulminant myocarditis 12. In a swine model of AMI, LV unloading reduced infarct size 13 with genomic studies identifying a cardioprotective shift in gene expression leading to preserved mitochondrial integrity 14.
Whom to unload?
There is a lack of high-quality data to guide which patients supported with VA-ECMO may benefit from LV venting or unloading. The development of overt complications of increased afterload (such as pulmonary edema or failure of aortic valve opening) is a widely accepted indication for unloading (Table 1). The decision to unload primarily to facilitate LV recovery in the absence of overt complications is more difficult, due to the fine balance between risk and benefit of placing additional MCS devices. Unloading device use can be associated with significant bleeding and vascular complications, hemolysis and coagulation disorders in a population of patients who are already prone to profound coagulopathy and systemic inflammatory response syndrome 15,16. The use of additional MCS for unloading also adds practical complexity, not only in managing device settings but also anticoagulation, monitoring vascular access sites and difficulty in repositioning the patient. These risks need to be balanced against the potential benefits of unloading as approximately 30–70% of patients treated with VA-ECMO develop increased afterload, which is associated with increased mortality 17. LV unloading in these patients has been associated with a higher rate of recovery or bridge to advanced therapies 18, more successful weaning from VA-ECMO 19 and lower in-hospital mortality 16.
Table 1.
Indications for left ventricular unloading
| Hemodynamic • Pulmonary capillary wedge pressure > 18mmHg • Lack of arterial line pulsatility (pulse pressure <15mmHg) |
| Echocardiographic • Increasing left ventricular dimensions • Stasis of blood within the left ventricular cavity • Left ventricular thrombus • Absence of aortic valve opening • Left ventricular outflow tract velocity time integral <10 cm |
| Clinical • Development of pulmonary edema • Refractory ventricular arrhythmia |
The decision to use a mechanical unloading strategy, and if so, the timing of insertion and choice of device will be determined by the treatment goals for an individual patient as well as the experience of the multidisciplinary team, guided by the contemporary evidence base. Hemodynamic, echocardiographic and clinical features may help identify those at risk for developing complications or identify these complications.
Hemodynamic predictors
Reduced arterial pulsatility is a readily available measure of increased afterload in VA-ECMO patients; a pulse pressure <15mmHg correlates strongly with reduced native cardiac output of <1 Liter/min 20 whilst a pulse pressure of <20mmHg has been associated with reduced survival 21. Binary cut off values for reduced pulsatility are designed to aid decision making in clinical practice but there is likely a continuous spectrum of risk as pulsatility decreases 22.
LVEDP (more often estimated by pulmonary capillary wedge (PCWP) rather than measured directly) is a useful metric. Patients with raised PCWP prior to the initiation of VA-ECMO are particularly vulnerable to the impact of increased afterload as their preload reserve is exhausted. A PCWP of >15mmHg measured at the time of VA-ECMO initiation was found to predict response to unloading with IABP 23, whilst others have suggested a threshold of >18mmHg 24. A target LVEDP of 12–18mmHg suggests adequate LV decompression when unloading has been initiated 25. Right and left ventricular interdependence is an important consideration particularly in patients with preserved right ventricular function, who maybe be at increased risk of complications due to a higher volume of transpulmonary blood flow resulting in increased LV filling pressures 26.
Echocardiographic predictors
Transthoracic echocardiography provides detailed non-invasive information on LV geometry and performance to inform the decision on unloading. Serial assessments are recommended, though achieving accurate and reproducible measurements can be challenging in this patient population. Where limited echocardiographic assessment is available, focused assessments of aortic valve opening and the left ventricular outflow tract velocity time integral (LVOT VTI) may provide the most objective markers. Transesophageal echocardiography can be considered in patients with particularly limited transthoracic imaging windows, acknowledging that there is often limited provision for serial reassessment using this technique.
Left ventricular ejection fraction (LVEF) before instituting VA-ECMO is predictive of afterload sensitivity, with lower LVEF associated with raised PCWP and reduced stroke volume on initiation of VA-ECMO 9 and increased risk of death 27. Given the importance of LV geometry in determining afterload and contractility, the finding of increasing LV dimensions despite a fixed ECMO flow rate may prompt consideration of unloading 28. The LVOT VTI can be used to estimate stroke volume and cardiac output; a LVOT VTI <10 cm reflects an insufficient intrinsic cardiac output and therefore predicts unsuccessful weaning from VA-ECMO and sensitivity to increased afterload 29.
Echocardiography can also be used to detect complications including blood stasis, LV thrombus and/or absence of aortic valve opening. Worsening secondary or diastolic mitral regurgitation may reflect rising LV pressures and is an early marker of impending pulmonary edema. A study of 98 patients supported with VA-ECMO found 22% had spontaneous echo contrast on echocardiography, this was associated with a lower LVEF and pulsatility index with a higher incidence of intracardiac thrombus (46% vs 13%) and stroke (36% vs 7.9%) 30; the development of spontaneous echo contrast therefore identifies a high risk group who should be considered for immediate unloading.
Clinical predictors
Whilst hemodynamics and cardiovascular function will be the primary determinants of complications of increased afterload, the etiology of shock will have important influences on these parameters and merits consideration in deciding which patients should receive LV venting or unloading.
In shock complicating AMI, increased LV pressure results in reduced subendocardial perfusion. In addition to the deleterious effects on infarct size and genomic changes, this impairment of subendocardial perfusion can exacerbate ischemia and further impair ventricular performance 31. Data from a meta-analysis of 62 studies including 7581 patients suggest that AMI is the pathology with the greatest mortality benefit with unloading with an absolute risk reduction of 6.65% giving a number needed to treat of 15 to prevent one death 32.
Patients with chronic heart failure often have an elevated PCWP at baseline, making them particularly vulnerable to increased afterload as their preload reserve will be exhausted 33. The use of an IABP in shock caused by acute on chronic heart failure significantly reduces systemic vascular resistance and augments cardiac output by 23% compared with 10% in AMI shock 34. This group would potentially benefit from prophylactic unloading given their vulnerability to increased afterload with demonstrable improvements in cardiac output by reducing afterload and reduction in the incidence of hydrostatic pulmonary edema 35.
How to unload?
A number of mechanical circulatory strategies can be used to achieve LV unloading or venting if conservative measures prove insufficient. Each method is associated with its own contra-indications, risks, cost and potential advantages. The section below outlines the characteristics, hemodynamic effects and clinical evidence for each technique.
Non-invasive and pharmacological approaches
Efforts should be made to reduce preload and afterload by conservative measures before considering invasive LV unloading strategies 36. Extracorporeal blood flow increases mean arterial pressure and hence afterload, therefore the optimal VA-ECMO flow rates should be adequate to provide systemic perfusion whilst minimizing afterload. Lower flow rates (<2.2 L/min/m2) can provide adequate systemic perfusion while reducing LV distension 37, though caution must be exercised as very low rates (particularly <1.5 liter/minute) may increase the risk of thrombotic complications within the ECMO circuit, with potentially severe consequences 38. In patients requiring higher extracorporeal blood flow to maintain perfusion, intravenous vasodilators may reduce mean arterial pressure and restore ventricular ejection 39. A trial of fluid optimization with diuresis and/or hemofiltration may be tried in patients with raised LV filling pressures who have not developed overt complications of increased afterload: a lower total fluid balance has been associated with better outcomes and may avoid the need for mechanical LV unloading yet may not be achievable in all patients 40.
Inotropes may be utilized to increase LV contractility and therefore stroke volume. This approach is often employed as an initial strategy either to counteract mild degrees of increased LV afterload or as a bridge to definitive unloading in those who have developed or are at high risk of developing complications 36. Inotropes however, increase myocardial oxygen demand (Figure 3f), and the use of inotropes has been associated with increased mortality in observational studies 41. A propensity matched cohort of 231 VA-ECMO patients found a significantly worse 30-day survival (25% vs 48%) in those treated with epinephrine in the first 24 hours compared to either no inotropes or inodilators 42.
Venting
Catheters
Percutaneous catheters can be placed into the LV cavity, left atrium, or pulmonary artery 43–46 and connected to the inflow cannula of the VA-ECMO circuit. 7Fr Pigtail catheters inserted into the LV under transesophageal echocardiography guidance have been used to achieve transaortic unloading with a reduction in LV dimensions and volume 43. The use of 5Fr and 6Fr LV catheters has been reported, showing reduction in LV dimensions and increases in mean arterial pressure and pulse pressure. However, the size of percutaneous catheters limits the maximum flow that can be achieved, with a higher risk of hemolysis and therefore this approach is not routinely recommended 44,47.
Unloading
Percutaneous atrial septostomy
Percutaneous atrial septostomy is created with the use of a percutaneous blade or balloon, typically under fluoroscopic or transesophageal echocardiographic guidance. Whilst widely utilized in pediatric populations, limited data are available in adults. Reductions in left atrial pressure, resolution of LV distention and pulmonary edema have been reported 48. A porcine model of cardiogenic shock supported with VA-ECMO demonstrated that atrial septostomy reduced PVA driven by a reduction in stroke volume 49. This could, however, increase the risk of a non-ejecting LV and potential LV or aortic root thrombus due to the lack of forward flow through the aortic valve 50. Furthermore, the residual atrial septal defect may increase the risk of stroke after removal of the venous cannula, particularly after prolonged periods of VA-ECMO support.
Recently, left atrial VA-ECMO (LAVA-ECMO) has been described, where a multistage venous drainage cannula is placed in the left atrium via transseptal puncture. This allows simultaneous bi-atrial drainage thereby unloading the right and left ventricles, with a reduction in PCWP demonstrated 51. This approach may be particularly helpful in patients with LV thrombus or unilateral peripheral vascular disease that precludes a second large-bore femoral arterial access. It may be the preferred choice in patients with severe aortic stenosis or a mechanical aortic valve replacement, where transaortic device placement is contraindicated.
An alternative method to achieve left atrial drainage is with the use of TandemHeart (LivaNova Plc., London, UK), this centrifugal pump-based system uses a 21Fr transseptal cannula sited in the left atrium forming the inflow limb with the outflow limb sited in the femoral artery. An oxygenator can be added to the system or alternatively the left atrial cannula can be connected into the inflow limb of a VA-ECMO circuit via a Y connector achieving bi-atrial drainage with subsequent bi-ventricular unloading52.
Pulmonary Artery Drainage
Placement of an ECMO inflow cannula into the pulmonary artery can indirectly reduce LV pressure and volume by decreasing preload. Single-stage or multi-stage cannulas can be used for this purpose. The ProTek Duo (CardiacAssist Inc., Pittsburgh, PA) dual lumen cannula can also be used as a method to drain both the right atrium and pulmonary artery when both lumens are spliced together as an inflow circuit for VA-ECMO. Protek Duo is available in 29 or 31Fr cannula sizes and is inserted via the jugular vein, enabling flows of up-to 4.5L and is commonly used as a right atrium to pulmonary artery bypass circuit to support the right ventricle.
IABP
The IABP is the most frequently used adjunct MCS device for LV unloading 53. It is placed in a standard position in the descending aorta, with deflation in systole decreasing afterload during LV ejection and promoting forward flow through the aortic valve, whilst inflation in diastole improves coronary blood flow 54. The IABP provides less unloading compared to a pLVAD with an animal model finding a PVA reduction of 12% driven by a reduction in potential energy as opposed to stroke work (3f) 55. The use of an IABP has also been shown to improve cerebral blood flow in patients with cardiogenic shock on VA-ECMO with a pulse pressure of >10mmHg prior to initiation of the IABP whilst reducing it in those with a pulse pressure of <10mmHg 56. These findings suggest IABP may be most effective in those who retain a degree of native LV ejection, an observation consistent with the known physiological effects of counterpulsation in patients with cardiogenic shock where IABP was used as the sole MCS device.
No randomized data exist to support the use of IABP for unloading, whilst observational data are conflicting. In AMI shock the use of IABP unloading is associated with improved short-term mortality (OR 0.82, 95% CI 0.75–0.89, p=<0.001) at the cost of increased major bleeding (OR 1.09, 95% CI 1.0–1.18, p=0.03) compared with VA-ECMO alone 57. A meta-analysis of 2251 patients with post cardiotomy shock found mortality was similar with the combination of VA-ECMO + IABP compared to VA-ECMO alone 58. A larger meta-analysis of 4,653 patients found similar short-term mortality in patients who did or did not receive an IABP, except for in the subgroup of AMI shock where mortality was lower with IABP 59. The heterogenous populations and observational confounding included in these meta-analysis may explain the differing results. The findings may also suggest a differential effect of IABP based on the etiology of shock, with ischemic patients gaining additional benefit from augmentation of coronary perfusion; though this should be confirmed through more robust, randomized studies.
pLVAD
The combination of a percutaneous left ventricular assist device (pLVAD) and VA-ECMO is being increasingly used. pLVADs are transvalvular microxial flow pumps that continuously displace blood from the LV cavity to the aortic root, resulting in a significant decrease in stroke work, PVA (Figure 3f) and therefore MVO2 60. The Impella CP (Abiomed, Danvers, CA) is most commonly used for LV unloading, a configuration referred to as ECMELLA or ECPELLA. The pLVAD is usually inserted percutaneously via the contralateral femoral artery to the ECMO outflow cannula, though surgical implantation via the axillary artery and percutaneous transcaval insertion can be considered 61. Contra-indications to the use of pLVADs include mechanical aortic valve replacement, severe aortic regurgitation, LV thrombus and peripheral vascular disease. The Impella CP device requires large bore arterial access (14 French), as well as anticoagulation delivered directly through the device purge solution, however recent evidence suggests a bicarbonate-based purge solution maybe a safe alternative in patients with bleeding concerns 62,63.
ECPELLA has been shown to reduce PCWP, improve pulmonary flow by reducing right ventricular afterload, and reduce LV dimensions 64,65, however as with IABP, no randomized data exist to support the use of pLVAD for LV unloading. A registry of 510 propensity matched patients found ECPELLA was associated with 21% lower mortality at 30 days compared with VA-ECMO alone, despite increased rates of severe bleeding (38.4 % vs 17.9%) and hemolysis (33.6% vs 22.4%) 15. These findings highlight the potential tradeoff that needs to be considered when utilizing pLVAD with respect to reducing mortality (with a number needed to treat of 15 in this observational study) balanced against the risk of increased bleeding and limb ischemia (number needed to harm 5 and 11, respectively). The use of distal perfusion cannula incorporated into the ECMO outflow circuit maybe considered in patients displaying signs of limb ischemia distal to the pLVAD access site, but further increases the complexity of the MCS system and risk of complications.
A critical aspect of managing patients treated with ECPELLA is balancing flow rates of both the VA-ECMO circuit and the pLVAD device to achieve adequate systemic perfusion and LV unloading. This balance must be managed in a dynamic manner, with a tendency to higher VA-ECMO flow to achieve adequate systemic perfusion during the initial stabilization followed by a gradual transition to increased pLVAD support to aid cardiac recovery and ultimately weaning preferentially from VA-ECMO. Particular attention must be given to patients with hypoxemic respiratory failure where high pLVAD flow rates may draw deoxygenated blood from the left ventricle into the systemic, coronary and cerebral circulation, exaggerating a phenomenon well described as the “Harlequin” or “North-South syndrome” 66; in this situation it is preferable to set the pLVAD flow at a lower level until an improvement in respiratory function allows titration of pLVAD and weaning of ECMO flow. The same phenomenon can classically be seen with LV recovery and ongoing pulmonary edema. Careful counterbalancing is also required to ensure sufficient LV preload to prevent suction at the pLVAD inflow, that may cause hemolysis. Those with biventricular shock may have lower LV preload due to poor RV function and therefore unloading with pLVAD may be less effective and associated with more frequent complications, though recent experimental data from a porcine model of cardiogenic shock found pLVAD use resulted in septal shift towards the LV, resulting in an increase in RV stroke work and cardiac output without increasing LV stroke work 67.
Initial pLVAD flow rates are generally lower than those that can be achieved by the devices and limited data exists to guide adjusting flow to hemodynamic targets. End-tidal carbon dioxide has been found to directly correlate with increased pulmonary flow and reduced LV dimensions with increasing pLVAD flow and may offer a convenient non-invasive method of assessing the effects of LV unloading 64.
A larger bore version of the Impella device (Impella 5.5) can provide up to six liters per minute of antegrade flow, providing enough forward flow to support systemic perfusion in isolation in most cases. When used as an initial unloading device, the Impella 5.5 may therefore allow earlier weaning of VA-ECMO or lower VA-ECMO flow rates to support the right ventricle and oxygenation until improvement allows decannulation of VA-ECMO. The device is inserted surgically via the axillary artery and can remain in situ for significantly longer than the Impella CP device, with durations of up to 83 days reported, though current licensing permits 30 days in Europe and 14 days in the USA68. This development has potential advantages in allowing patient ambulation and reduced hemolysis and thrombosis 69, acting as a potential bridge to transplant or durable LVAD implantation after ECMO decannulation 70. This approach may be advantageous in patients with decompensated chronic heart failure or in whom the chances of LV recovery are considered low, or if it is anticipated MCS is likely to be required for a prolonged duration. The benefits of these devices must be balanced against the risk, complexity and expense of a large-bore surgically implanted device. It is important therefore in such cases that the multidisciplinary shock team make a careful assessment of potential futility before committing to mechanical unloading.
Surgical approaches
Central VA-ECMO is typically instituted in post-cardiotomy shock or with graft failure following heart transplantation where a sternotomy has already been performed. Although cannulation of the ascending aorta avoids the retrograde flow associated with peripheral VA-ECMO, ventricular distension and reduced forward flow through the left ventricular outflow tract and aortic root may still occur due to impaired LV contractility. This may be potentiated by prolonged ischemic time during cardio-pulmonary bypass with a canine model of hypothermic cardioplegia finding impaired subendocardial perfusion and ventricular performance in those with distended ventricles (LVEDP >20mmHg) 31. These considerations have led some centres to routinely unload all patients treated with peripheral or central VA-ECMO for post-cardiotomy shock71, with a meta-analysis of 2324 patients finding a non-significant (RR 0.93 0.85–1.01, p = 0.09) reduction in mortality but a significantly higher chance of weaning from ECMO32.
Incorporating a surgical venting cannula (16–20 Fr) sited at the LV apex, pulmonary vein or pulmonary artery in to the venous drainage limb of the VA-ECMO circuit via a Y connector can provide effective bi-ventricular unloading53. Minimally invasive surgical techniques have been described utilizing a subxiphoid and anterolateral thoracotomy approach 72. Limited data exists regarding outcomes of these methods in adult patients, one study retrospectively compared 23 patients with a surgical vent to 22 patients with a pLVAD finding similar 30-day mortality 73. Both methods significantly reduced pulmonary artery diastolic pressure and had a similar rate of complications.
Choice of strategy
The mechanism of action of each unloading strategy is likely to be distinct 36 and there are no reliable head-to-head comparisons of clinical effectiveness. Registry data suggest that the IABP may be suitable for less severe degrees of increased afterload, however, in cases where there is a dilated non ejecting ventricle with significantly raised filling pressures active unloading with either a pLVAD or surgical vent is likely to be required 74.
The HERACLES trial (ISRCTN82431978) will be the first randomized comparison of unloading strategies, randomizing 36 patients supported with VA-ECMO to unloading with either IABP or pLVAD and comparing the physiological impact of each device on coronary flow and ventricular pressures/volumes. Whether a tailored approach utilizing hemodynamic and clinical criteria to individualize the unloading strategy may improve outcomes remains to be seen. Whilst randomized data are urgently required to make firm recommendations on choice of unloading method, at the present time decisions should be made in a pragmatic manner, based on local expertise and the strategies available to the particular multidisciplinary shock team (Figure 4).
Figure 4: Left ventricular unloading criteria and methods.
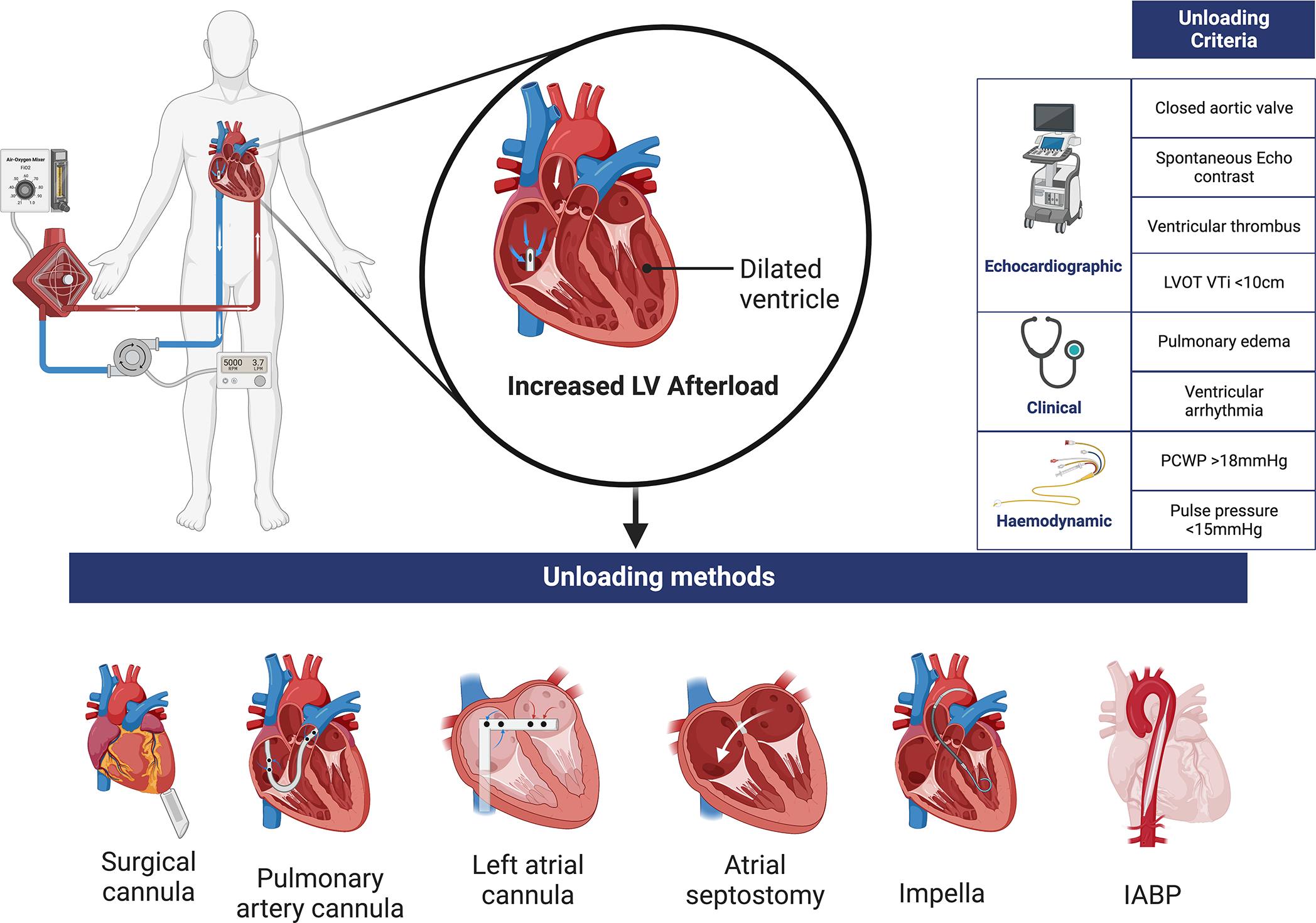
LVOT VTi: Left ventricular outflow tract velocity time integral, PCWP: Pulmonary capillary wedge pressure.
When to unload
Unloading devices may be inserted before, at the same time or shortly after initiation of VA-ECMO. This prophylactic approach may help protect the vulnerable ventricle and promote myocardial recovery by avoiding exposure to increased afterload, weighed against the potential risks of increased complications and cost 75. Alternatively, unloading maybe reactive when undertaken only in response to adverse hemodynamic, clinical or echocardiographic manifestations of increased afterload, reducing the risk of complications from the unloading device itself but potentially jeopardizing myocardial recovery.
In a series of 106 consecutive ECPELLA patients, survival was similar in those who had concomitant pLVAD implantation at the time of VA-ECMO cannulation and those where implantation was delayed 76. A larger registry of 337 ECPELLA patients found that early pLVAD implantation improved short term mortality whereas delayed unloading, i.e., >2 hours after VA-ECMO implantation, did not 15. Finally, a propensity matched cohort of 74 patients found a significantly lower mortality in those in whom an pLVAD was inserted prior to VA-ECMO compared to VA-ECMO first 77.
A retrospective analysis comparing prophylactic LAVA-ECMO to reactive unloading with either percutaneous or surgical methods found a lower 30-day mortality and transition to durable VAD or cardiac transplantation in the prophylactic unloading group 78. A meta-regression of observational studies found that prophylactic unloading had a significant inverse relationship with mortality 79. However, these data are confounded with differing indications for prophylactic unloading and definitions for reactive unloading. Although current data suggest prophylactic unloading up to 2 hours after the initiation of VA-ECMO appears beneficial, randomized trials are needed to establish treatment efficacy (Table 2).
Table 2.
Current randomized clinical trials of VA-ECMO)
| Trial Name | Inclusion Criteria | Participant number | Intervention | Control | Institution | Primary outcome | Key Secondary outcomes | Estimated study completion |
|---|---|---|---|---|---|---|---|---|
| EARLY-UNLOAD (NCT04775472) |
Cardiogenic shock | 116 | VA-ECMO + atrial septostomy within 12 hours | VA-ECMO alone | Chonnam National University Hospital, Korea | All-cause mortality at 30 days | Rate of atrial septostomy in control group Incidence of cardiac death |
October 2023 |
| REVERSE (NCT03431467) |
Cardiogenic shock | 96 | VA-ECMO + Impella CP | VA-ECMO alone | Multi-center, USA | Recovery from cardiogenic shock at 30 days (survival free from MCS, transplant or inotropic support) | Survival to hospital discharge | January 2025 |
| ECLS-SHOCK (NCT03637205) |
Cardiogenic shock secondary to acute myocardial infarction | 420 | VA-ECMO +/− LV unloading | Standard care (Escalation to other MCS e.g., IABP or pLVAD allowed) | Multi-center, Germany | All-cause mortality at 30 days | Time to death at 6 and 12 month follow up Duration of catecholamine therapy |
November 2023 |
| ANCHOR (NCT04184635) |
Cardiogenic shock secondary to acute myocardial infarction | 400 | VA-ECMO + IABP | Standard care (no MCS device allowed) | Multi-center, France | Treatment failure at 30 days (death in ECMO group or rescue ECMO in the control group) | Mortality at 30 days MACE at 30 days |
November 2024 |
| HERACLES (ISRCTN82431978) |
Cardiogenic shock being treated with VA-ECMO | 36 | VA-ECMO + Impella CP | VA-ECMO + IABP | Multi-center, UK | Change in device coronary flow reserve | Change in LVEDP Time to VA-ECMO decannulation |
February 2025 |
The EARLY-UNLOAD RCT (NCT04775472) is aiming to recruit 116 participants comparing early unloading (within 12 hours of VA-ECMO) versus bailout unloading, with atrial septostomy as the mode of unloading in both groups. The primary outcome is all cause mortality at 30 days, whilst secondary outcome measures including need for bailout atrial septostomy, need for long term heart replacement therapies and resolution of pulmonary edema are likely to be informative. The REVERSE study (NCT03431467) will recruit 96 patients comparing early (within 10 hours) unloading with Impella to VA-ECMO alone with a primary outcome of recovery from cardiogenic shock at 30 days; the chosen primary endpoint is of particular interest given the aim of unloading is not just to treat or avoid complications of increased afterload but also to facilitate myocardial recovery.
The ECLS-SHOCK trial (NCT03637205) will not specifically address the question of unloading, but may also provide informative data. The trial will enroll 430 patients with AMI cardiogenic shock and randomize them to VA-ECMO or standard care. The primary outcome will be all-cause mortality. Escalation to other MCS including IABP and pLVAD is permitted in cases of hemodynamic deterioration in the standard care group 80. In the VA-ECMO group, unloading is only advised in response to lack of arterial pulsatility, lack of aortic valve opening, LVOT VTI <10 cm2 or increasing LV dimensions on echocardiography. The impact of this reactive unloading approach, utilizing subgroup analyses comparing these patients to the standard care group and to the non-unloaded group, will be of considerable interest.
Unloading targets
Once unloading has been initiated continued close monitoring of the patient is essential to ensure any clinical complications such as pulmonary edema which triggered unloading resolve. ECMO flows and unloading device parameters should be continually optimized targeting a MAP adequate to provide systemic perfusion, PCWP <15mmHg and consistent aortic valve opening on echocardiography. The echocardiogram will also provide key information on right ventricular function and recovery of native myocardial contractility that will be reflected with an improvement in arterial pulsatility, as the hemodynamics improve efforts should be made to wean vasoactive medications. If there is evidence of cardiac recovery (pulse pressure >10mmHg, MAP >60mmHg on low doses of vasoactive medication, LVEF >30%) then weaning of VA-ECMO maybe considered 22. Where an additional MCS device such as pLVAD or IABP has been utilized, if possible, VA-ECMO should be weaned first thereby reducing afterload and MVO2 potentially improving the chances of myocardial recovery. This is particularly pertinent in cases where improvement in right ventricular function and oxygenation allow de-escalation to isolated support of the LV. If myocardial recovery is expected and weaning of VA-ECMO is not possible consideration can be given to longer term support either with Impella 5.5 or a durable VAD. In cases where myocardial recovery is considered unlikely VA-ECMO maybe continued as a bridge to transplantation. Finally, some patients will fail to show meaningful improvements despite unloading or develop multi organ failure, early involvement of the palliative care team is encouraged in such cases.
Conclusion
VA-ECMO is a powerful MCS strategy able to support systemic perfusion and provide oxygenation, but the additional hemodynamic load on the failing ventricle may cause complications and impair myocardial recovery. Patients with a limited contractile reserve or who have exhausted preload reserve are particularly vulnerable to developing overt complications of increased afterload and may benefit from prophylactic LV unloading. Whether prophylactic unloading improves myocardial recovery and reduces mortality in patients without adverse consequences of LV afterload is unclear, and potential benefits must be weighed against the risks of an additional MCS device. Finally, the choice of unloading method depends on local expertise with a paucity of data to guide the choice of one method over another. Detailed physiology studies may help personalize this decision. We present a suggested unloading algorithm (Figure 5) whilst awaiting randomized trials to determine when, in whom and how best to unload the LV in VA-ECMO supported patients.
Figure 5: Left ventricular unloading Algorithm.
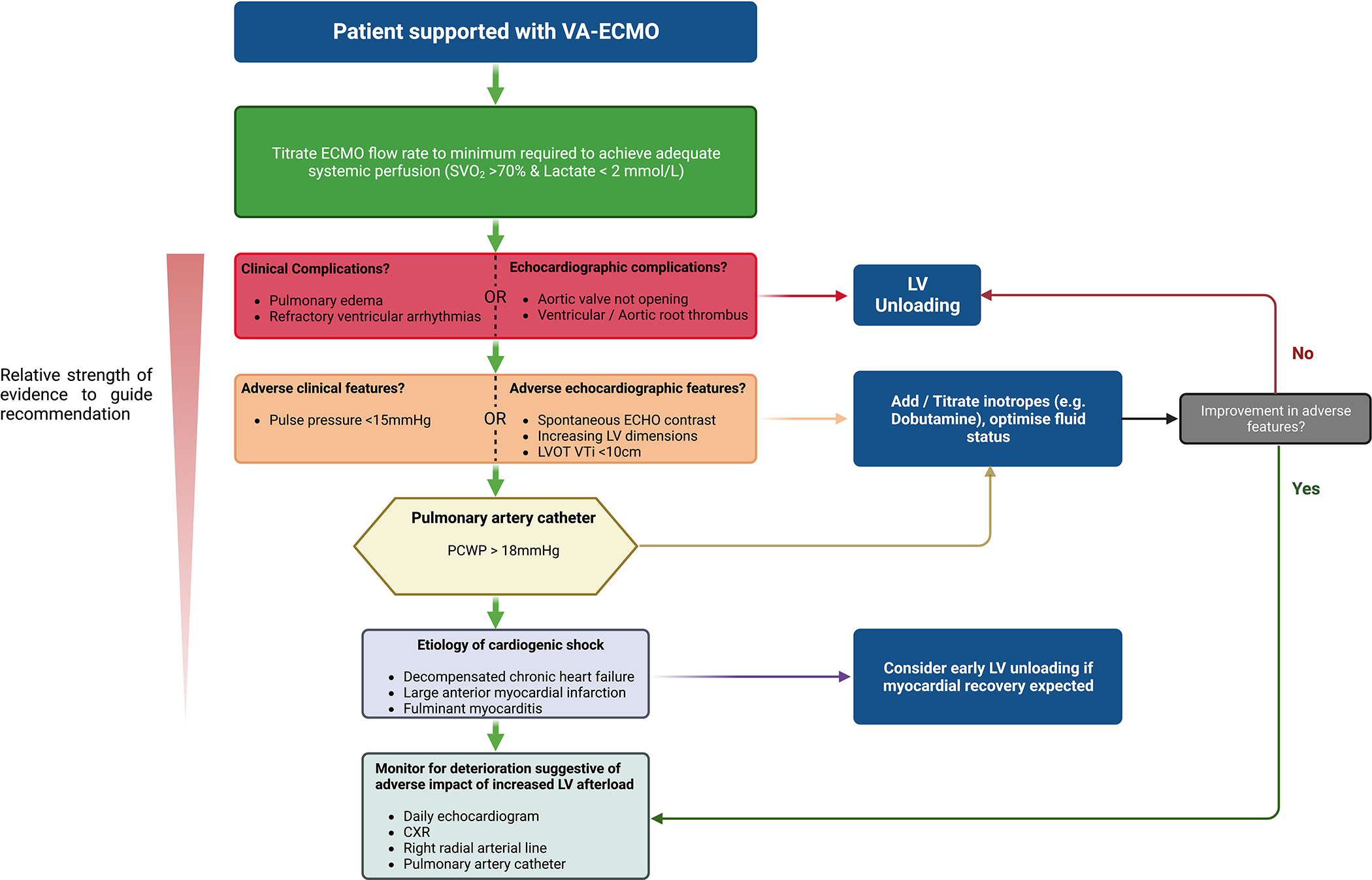
CXR: Chest radiograph, LVOT VTi: Left ventricular outflow tract velocity time integral, PCWP: Pulmonary capillary wedge pressure, SVO2: Mixed venous oxygen saturation
Acknowledgements
Figures were created with Biorender.com
Sources of Funding
The authors receive funding from the British Heart Foundation (FS/CRTF/21/24118), National Institute for Health Research (NIHR130593) and the National Institutes for Health (R01HL139785-01). The authors received support from the King’s Health Partners Cardiovascular and Respiratory Partnership’s Clinical Academic Innovation Fund.
Disclosures
Dr Donker reports speaker fees from Getinge-Maquet and Fresenius-Xenios-NovaLung and research cooperation with Getinge-Maquet and FreseniusXenios-NovaLung. Dr Pappalardo reports personal fees from Abiomed unrelated to the submitted work. Dr Kapur has received institutional research support and speaker/consulting honoraria from Abbott, Abiomed, Boston Scientific, Getinge, LivaNova, Medtronic, MDStart, Precardia, and Zoll. Prof Perera reports receiving speaker fees/honoraria/research grant support from Abiomed, Getinge, Abbott Vascular and Philips.
Abbreviations
- AMI
acute myocardial infarction
- IABP
intra-aortic balloon pump
- LAVA-ECMO
left atrial veno-arterial extra corporeal membrane oxygenation
- LV
left ventricle
- LVEDP
left ventricular end diastolic pressure
- MCS
mechanical circulatory support
- MVO2
myocardial oxygen consumption
- PCWP
pulmonary capillary wedge pressure
- pLVAD
percutaneous left ventricular assist device
- VA-ECMO
Veno-arterial Extra Corporeal Membrane Oxygenation
References
- 1.van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 2.Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901 [DOI] [PubMed] [Google Scholar]
- 3.Thiele H, de Waha-Thiele S, Freund A, Zeymer U, Desch S, Fitzgerald S. Management of cardiogenic shock. EuroIntervention. 2021;17:451–465. doi: 10.4244/EIJ-D-20-01296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams B, Bernstein W. Review of Venoarterial Extracorporeal Membrane Oxygenation and Development of Intracardiac Thrombosis in Adult Cardiothoracic Patients. J Extra Corpor Technol. 2016;48:162–167. [PMC free article] [PubMed] [Google Scholar]
- 5.Boulate D, Luyt CE, Pozzi M, Niculescu M, Combes A, Leprince P, Kirsch M. Acute lung injury after mechanical circulatory support implantation in patients on extracorporeal life support: an unrecognized problem. Eur J Cardiothorac Surg. 2013;44:544–549; discussion 549–550. doi: 10.1093/ejcts/ezt125 [DOI] [PubMed] [Google Scholar]
- 6.Ostadal P, Rokyta R, Karasek J, Kruger A, Vondrakova D, Janotka M, Naar J, Smalcova J, Hubatova M, Hromadka M, et al. Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock: Results of the ECMO-CS Randomized Clinical Trial. Circulation. 2022. doi: 10.1161/circulationaha.122.062949 [DOI] [PubMed] [Google Scholar]
- 7.Vest AR, Heupler F. Afterload. In: Anwaruddin S, Martin JM, Stephens JC, Askari AT, eds. Cardiovascular Hemodynamics: An Introductory Guide. Totowa, NJ: Humana Press; 2013:29–51. [Google Scholar]
- 8.Ross J Afterload mismatch and preload reserve: A conceptual framework for the analysis of ventricular function. Progress in Cardiovascular Diseases. 1976;18:255–264. doi: 10.1016/0033-0620(76)90021-9 [DOI] [PubMed] [Google Scholar]
- 9.Dickstein ML. The Starling Relationship and Veno-Arterial ECMO: Ventricular Distension Explained. ASAIO J. 2018;64:497–501. doi: 10.1097/MAT.0000000000000660 [DOI] [PubMed] [Google Scholar]
- 10.Uriel N, Sayer G, Annamalai S, Kapur NK, Burkhoff D. Mechanical Unloading in Heart Failure. J Am Coll Cardiol. 2018;72:569–580. doi: 10.1016/j.jacc.2018.05.038 [DOI] [PubMed] [Google Scholar]
- 11.Jain P, Salama M, Everett K, Reyelt L, Kapur NK. To Vent or Not to Vent: A Loaded Question During Venoarterial Extracorporeal Membrane Oxygenation Support for Cardiogenic Shock. Circ Cardiovasc Interv. 2021;14:e010537. doi: 10.1161/CIRCINTERVENTIONS.121.010537 [DOI] [PubMed] [Google Scholar]
- 12.Spillmann F, Van Linthout S, Schmidt G, Klein O, Hamdani N, Mairinger T, Krackhardt F, Maroski B, Schlabs T, Soltani S, et al. Mode-of-action of the PROPELLA concept in fulminant myocarditis. Eur Heart J. 2019;40:2164–2169. doi: 10.1093/eurheartj/ehz124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briceno N, Annamalai SK, Reyelt L, Crowley P, Qiao X, Swain L, Pedicini R, Foroutanjazi S, Jorde L, Yesodharan G, et al. Left Ventricular Unloading Increases the Coronary Collateral Flow Index Before Reperfusion and Reduces Infarct Size in a Swine Model of Acute Myocardial Infarction. J Am Heart Assoc. 2019;8:e013586. doi: 10.1161/JAHA.119.013586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esposito ML, Zhang Y, Qiao X, Reyelt L, Paruchuri V, Schnitzler GR, Morine KJ, Annamalai SK, Bogins C, Natov PS, et al. Left Ventricular Unloading Before Reperfusion Promotes Functional Recovery After Acute Myocardial Infarction. J Am Coll Cardiol. 2018;72:501–514. doi: 10.1016/j.jacc.2018.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrage B, Becher PM, Bernhardt A, Bezerra H, Blankenberg S, Brunner S, Colson P, Cudemus Deseda G, Dabboura S, Eckner D, et al. Left Ventricular Unloading Is Associated With Lower Mortality in Patients With Cardiogenic Shock Treated With Venoarterial Extracorporeal Membrane Oxygenation: Results From an International, Multicenter Cohort Study. Circulation. 2020;142:2095–2106. doi: 10.1161/CIRCULATIONAHA.120.048792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grandin EW, Nunez JI, Willar B, Kennedy K, Rycus P, Tonna JE, Kapur NK, Shaefi S, Garan AR. Mechanical Left Ventricular Unloading in Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation. J Am Coll Cardiol. 2022;79:1239–1250. doi: 10.1016/j.jacc.2022.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Truby LK, Takeda K, Mauro C, Yuzefpolskaya M, Garan AR, Kirtane AJ, Topkara VK, Abrams D, Brodie D, Colombo PC, et al. Incidence and Implications of Left Ventricular Distention During Venoarterial Extracorporeal Membrane Oxygenation Support. ASAIO J. 2017;63:257–265. doi: 10.1097/MAT.0000000000000553 [DOI] [PubMed] [Google Scholar]
- 18.Pappalardo F, Schulte C, Pieri M, Schrage B, Contri R, Soeffker G, Greco T, Lembo R, Mullerleile K, Colombo A, et al. Concomitant implantation of Impella((R)) on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail. 2017;19:404–412. doi: 10.1002/ejhf.668 [DOI] [PubMed] [Google Scholar]
- 19.Aso S, Matsui H, Fushimi K, Yasunaga H. The Effect of Intraaortic Balloon Pumping Under Venoarterial Extracorporeal Membrane Oxygenation on Mortality of Cardiogenic Patients: An Analysis Using a Nationwide Inpatient Database. Crit Care Med. 2016;44:1974–1979. doi: 10.1097/CCM.0000000000001828 [DOI] [PubMed] [Google Scholar]
- 20.Mourad M, Eliet J, Zeroual N, Saour M, Sentenac P, Manna F, Molinari N, Gandet T, Colson PH, Gaudard P. Pulse pressure and end-tidal carbon dioxide for monitoring low native cardiac output during veno-arterial ECLS: a prospective observational study. Crit Care. 2020;24:569. doi: 10.1186/s13054-020-03280-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT, Hodgson C, Scheinkestel C, Cooper DJ, Thiagarajan RR, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J. 2015;36:2246–2256. doi: 10.1093/eurheartj/ehv194 [DOI] [PubMed] [Google Scholar]
- 22.Lorusso R, Shekar K, MacLaren G, Schmidt M, Pellegrino V, Meyns B, Haft J, Vercaemst L, Pappalardo F, Bermudez C, et al. ELSO Interim Guidelines for Venoarterial Extracorporeal Membrane Oxygenation in Adult Cardiac Patients. ASAIO J. 2021;67:827–844. doi: 10.1097/MAT.0000000000001510 [DOI] [PubMed] [Google Scholar]
- 23.Petroni T, Harrois A, Amour J, Lebreton G, Brechot N, Tanaka S, Luyt CE, Trouillet JL, Chastre J, Leprince P, et al. Intra-aortic balloon pump effects on macrocirculation and microcirculation in cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation*. Crit Care Med. 2014;42:2075–2082. doi: 10.1097/CCM.0000000000000410 [DOI] [PubMed] [Google Scholar]
- 24.Alkhouli M, Narins CR, Lehoux J, Knight PA, Waits B, Ling FS. Percutaneous Decompression of the Left Ventricle in Cardiogenic Shock Patients on Venoarterial Extracorporeal Membrane Oxygenation. J Card Surg. 2016;31:177–182. doi: 10.1111/jocs.12696 [DOI] [PubMed] [Google Scholar]
- 25.Moller JE, Hassager C, Bonello L, Delmas C, Pappalardo F. Pump flow setting and assessment of unloading in clinical practice. Eur Heart J Suppl. 2021;23:A23–A26. doi: 10.1093/eurheartj/suab004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donker DW, Sallisalmi M, Broome M. Right-Left Ventricular Interaction in Left-Sided Heart Failure With and Without Venoarterial Extracorporeal Membrane Oxygenation Support-A Simulation Study. ASAIO J. 2021;67:297–305. doi: 10.1097/MAT.0000000000001242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalra R, Bartos JA, Kosmopoulos M, Carlson C, John R, Shaffer A, Martin C, Raveendran G, Yannopoulos D. Echocardiographic evaluation of cardiac recovery after refractory out-of-hospital cardiac arrest. Resuscitation. 2020;154:38–46. doi: 10.1016/j.resuscitation.2020.06.037 [DOI] [PubMed] [Google Scholar]
- 28.Donker DW, Meuwese CL, Braithwaite SA, Broome M, van der Heijden JJ, Hermens JA, Platenkamp M, de Jong M, Janssen JGD, Balik M, et al. Echocardiography in extracorporeal life support: A key player in procedural guidance, tailoring and monitoring. Perfusion. 2018;33:31–41. doi: 10.1177/0267659118766438 [DOI] [PubMed] [Google Scholar]
- 29.Aissaoui N, Luyt CE, Leprince P, Trouillet JL, Leger P, Pavie A, Diebold B, Chastre J, Combes A. Predictors of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med. 2011;37:1738–1745. doi: 10.1007/s00134-011-2358-2 [DOI] [PubMed] [Google Scholar]
- 30.Unai S, Nguyen ML, Tanaka D, Gorbachuk N, Marhefka GD, Hirose H, Cavarocchi NC. Clinical Significance of Spontaneous Echo Contrast on Extracorporeal Membrane Oxygenation. Ann Thorac Surg. 2017;103:773–778. doi: 10.1016/j.athoracsur.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 31.Lucas SK, Schaff HV, Flaherty JT, Gott VL, Gardner TJ. The Harmful Effects of Ventricular Distention during Postischemic Reperfusion. The Annals of Thoracic Surgery. 1981;32:486–494. doi: 10.1016/s0003-4975(10)61782-1 [DOI] [PubMed] [Google Scholar]
- 32.Kowalewski M, Malvindi PG, Zielinski K, Martucci G, Slomka A, Suwalski P, Lorusso R, Meani P, Arcadipane A, Pilato M, et al. Left Ventricle Unloading with Veno-Arterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock. Systematic Review and Meta-Analysis. J Clin Med. 2020;9. doi: 10.3390/jcm9041039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung M, Shiloh AL, Carlese A. Monitoring of the adult patient on venoarterial extracorporeal membrane oxygenation. ScientificWorldJournal. 2014;2014:393258. doi: 10.1155/2014/393258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malick W, Fried JA, Masoumi A, Nair A, Zuver A, Huang A, Haythe J, Farr M, Rabbani L, Karmpaliotis D, et al. Comparison of the Hemodynamic Response to Intra-Aortic Balloon Counterpulsation in Patients With Cardiogenic Shock Resulting from Acute Myocardial Infarction Versus Acute Decompensated Heart Failure. Am J Cardiol. 2019;124:1947–1953. doi: 10.1016/j.amjcard.2019.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brechot N, Demondion P, Santi F, Lebreton G, Pham T, Dalakidis A, Gambotti L, Luyt CE, Schmidt M, Hekimian G, et al. Intra-aortic balloon pump protects against hydrostatic pulmonary oedema during peripheral venoarterial-extracorporeal membrane oxygenation. Eur Heart J Acute Cardiovasc Care. 2018;7:62–69. doi: 10.1177/2048872617711169 [DOI] [PubMed] [Google Scholar]
- 36.Donker DW, Brodie D, Henriques JPS, Broome M. Left Ventricular Unloading During Veno-Arterial ECMO: A Simulation Study. ASAIO J. 2019;65:11–20. doi: 10.1097/MAT.0000000000000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh SK, Ning Y, Kurlansky P, Kaku Y, Naka Y, Takayama H, Sayer G, Uriel N, Masoumi A, Fried JA, et al. Impact of Venoarterial Extracorporeal Membrane Oxygenation Flow on Outcomes in Cardiogenic Shock. ASAIO J. 2022;68:239–246. doi: 10.1097/MAT.0000000000001462 [DOI] [PubMed] [Google Scholar]
- 38.Ki KK, Passmore MR, Chan CHH, Malfertheiner MV, Fanning JP, Bouquet M, Millar JE, Fraser JF, Suen JY. Low flow rate alters haemostatic parameters in an ex-vivo extracorporeal membrane oxygenation circuit. Intensive Care Med Exp. 2019;7:51. doi: 10.1186/s40635-019-0264-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kondo T, Sawamura A, Okumura T, Kano N, Morimoto R, Watanabe N, Hiraiwa H, Kuwayama T, Sugiura Y, Haga T, et al. Promising method for management of venoarterial extracorporeal membrane oxygenation: A case of severe heart failure successfully stabilized by “high-flow/vasodilation method”. J Cardiol Cases. 2018;18:81–84. doi: 10.1016/j.jccase.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt M, Bailey M, Kelly J, Hodgson C, Cooper DJ, Scheinkestel C, Pellegrino V, Bellomo R, Pilcher D. Impact of fluid balance on outcome of adult patients treated with extracorporeal membrane oxygenation. Intensive Care Med. 2014;40:1256–1266. doi: 10.1007/s00134-014-3360-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basir MB, Lemor A, Gorgis S, Taylor AM, Tehrani B, Truesdell AG, Bharadwaj A, Kolski B, Patel K, Gelormini J, et al. Vasopressors independently associated with mortality in acute myocardial infarction and cardiogenic shock. Catheter Cardiovasc Interv. 2022;99:650–657. doi: 10.1002/ccd.29895 [DOI] [PubMed] [Google Scholar]
- 42.Zotzmann V, Rilinger J, Lang CN, Kaier K, Benk C, Duerschmied D, Biever PM, Bode C, Wengenmayer T, Staudacher DL. Epinephrine, inodilator, or no inotrope in venoarterial extracorporeal membrane oxygenation implantation: a single-center experience. Crit Care. 2019;23:320. doi: 10.1186/s13054-019-2605-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbone A, Malvindi PG, Ferrara P, Tarelli G. Left ventricle unloading by percutaneous pigtail during extracorporeal membrane oxygenation. Interact Cardiovasc Thorac Surg. 2011;13:293–295. doi: 10.1510/icvts.2011.269795 [DOI] [PubMed] [Google Scholar]
- 44.Hong TH, Byun JH, Lee HM, Kim YH, Kang GH, Oh JH, Hwang SW, Kim HY, Park JH, Jung JJ. Initial Experience of Transaortic Catheter Venting in Patients with Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock. ASAIO J. 2016;62:117–122. doi: 10.1097/MAT.0000000000000327 [DOI] [PubMed] [Google Scholar]
- 45.Avalli L, Maggioni E, Sangalli F, Favini G, Formica F, Fumagalli R. Percutaneous left-heart decompression during extracorporeal membrane oxygenation: an alternative to surgical and transeptal venting in adult patients. ASAIO J. 2011;57:38–40. doi: 10.1097/MAT.0b013e3181fe5d0b [DOI] [PubMed] [Google Scholar]
- 46.von Segesser LK, Kwang K, Tozzi P, Horisberger J, Dembitsky W. A simple way to decompress the left ventricle during venoarterial bypass. Thorac Cardiovasc Surg. 2008;56:337–341. doi: 10.1055/s-2008-1038664 [DOI] [PubMed] [Google Scholar]
- 47.Jung JJ, Kang DH, Moon SH, Yang JH, Kim SH, Kim JW, Byun JH. Left Ventricular Decompression by Transaortic Catheter Venting in Extracorporeal Membrane Oxygenation. ASAIO J. 2021;67:752–756. doi: 10.1097/MAT.0000000000001450 [DOI] [PubMed] [Google Scholar]
- 48.Pasrija C, Tran D, Kon ZN. Atrial Septostomy: An Alternative for Left Ventricular Unloading During Extracorporeal Life Support. Ann Thorac Surg. 2018;105:1858. doi: 10.1016/j.athoracsur.2017.10.020 [DOI] [PubMed] [Google Scholar]
- 49.Mlcek M, Meani P, Cotza M, Kowalewski M, Raffa GM, Kuriscak E, Popkova M, Pilato M, Arcadipane A, Ranucci M, et al. Atrial Septostomy for Left Ventricular Unloading During Extracorporeal Membrane Oxygenation for Cardiogenic Shock: Animal Model. JACC Cardiovasc Interv. 2021;14:2698–2707. doi: 10.1016/j.jcin.2021.09.011 [DOI] [PubMed] [Google Scholar]
- 50.Hireche-Chikaoui H, Grubler MR, Bloch A, Windecker S, Bloechlinger S, Hunziker L. Nonejecting Hearts on Femoral Veno-Arterial Extracorporeal Membrane Oxygenation: Aortic Root Blood Stasis and Thrombus Formation-A Case Series and Review of the Literature. Crit Care Med. 2018;46:e459–e464. doi: 10.1097/CCM.0000000000002966 [DOI] [PubMed] [Google Scholar]
- 51.Singh-Kucukarslan G, Raad M, Al-Darzi W, Cowger J, Brice L, Basir MB, O’Neill WW, Alaswaad K, Eng MH. Hemodynamic Effects of Left-Atrial Venous Arterial Extra-Corporeal Membrane Oxygenation (LAVA-ECMO). ASAIO J. 2022;68:e148–e151. doi: 10.1097/MAT.0000000000001628 [DOI] [PubMed] [Google Scholar]
- 52.Jumean M, Pham DT, Kapur NK. Percutaneous bi-atrial extracorporeal membrane oxygenation for acute circulatory support in advanced heart failure. Catheter Cardiovasc Interv. 2015;85:1097–1099. doi: 10.1002/ccd.25791 [DOI] [PubMed] [Google Scholar]
- 53.Donker DW, Brodie D, Henriques JPS, Broome M. Left ventricular unloading during veno-arterial ECMO: a review of percutaneous and surgical unloading interventions. Perfusion. 2019;34:98–105. doi: 10.1177/0267659118794112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madershahian N, Liakopoulos OJ, Wippermann J, Salehi-Gilani S, Wittwer T, Choi YH, Naraghi H, Wahlers T. The impact of intraaortic balloon counterpulsation on bypass graft flow in patients with peripheral ECMO. J Card Surg. 2009;24:265–268. doi: 10.1111/j.1540-8191.2009.00807.x [DOI] [PubMed] [Google Scholar]
- 55.Sauren LD, Reesink KD, Selder JL, Beghi C, van der Veen FH, Maessen JG. The acute effect of intra-aortic balloon counterpulsation during extracorporeal life support: an experimental study. Artif Organs. 2007;31:31–38. doi: 10.1111/j.1525-1594.2007.00337.x [DOI] [PubMed] [Google Scholar]
- 56.Yang F, Jia ZS, Xing JL, Wang Z, Liu Y, Hao X, Jiang CJ, Wang H, Jia M, Hou XT. Effects of intra-aortic balloon pump on cerebral blood flow during peripheral venoarterial extracorporeal membrane oxygenation support. J Transl Med. 2014;12:106. doi: 10.1186/1479-5876-12-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kida H, Sotomi Y, Hikoso S, Nakatani D, Mizuno H, Suna S, Okada K, Kitamura T, Komukai S, Dohi T, et al. Prognostic significance of intra-aortic balloon pumping support in patients with acute myocardial infarction and veno-arterial extracorporeal membrane oxygenation therapy. J Cardiol. 2021. doi: 10.1016/j.jjcc.2021.10.011 [DOI] [PubMed] [Google Scholar]
- 58.Huang D, Xu A, Guan Q, Qin J, Zhang C. Venoarterial extracorporeal membrane oxygenation with intra-aortic balloon pump for postcardiotomy cardiogenic shock: A systematic review and meta-analysis. Perfusion. 2021:2676591211042568. doi: 10.1177/02676591211042568 [DOI] [PubMed] [Google Scholar]
- 59.Vallabhajosyula S, O’Horo JC, Antharam P, Ananthaneni S, Vallabhajosyula S, Stulak JM, Eleid MF, Dunlay SM, Gersh BJ, Rihal CS, et al. Concomitant Intra-Aortic Balloon Pump Use in Cardiogenic Shock Requiring Veno-Arterial Extracorporeal Membrane Oxygenation. Circ Cardiovasc Interv. 2018;11:e006930. doi: 10.1161/CIRCINTERVENTIONS.118.006930 [DOI] [PubMed] [Google Scholar]
- 60.Meani P, Lorusso R, Pappalardo F. ECPella: Concept, Physiology and Clinical Applications. J Cardiothorac Vasc Anesth. 2022;36:557–566. doi: 10.1053/j.jvca.2021.01.056 [DOI] [PubMed] [Google Scholar]
- 61.Afana M, Altawil M, Basir M, Alqarqaz M, Alaswad K, Eng M, O’Neill WW, Lederman RJ, Greenbaum AB. Transcaval access for the emergency delivery of 5.0 liters per minute mechanical circulatory support in cardiogenic shock. Catheter Cardiovasc Interv. 2021;97:555–564. doi: 10.1002/ccd.29235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beavers CJ, Dunn SP, DiDomenico RJ, Moretz J, Jennings DL. BICARBONATE-BASED PURGE SOLUTION DURING IMPELLA SUPPORT: A GROWING ALTERNATIVE. Journal of the American College of Cardiology. 2022;79:633–633. doi: doi: 10.1016/S0735-1097(22)01624-2 [DOI] [Google Scholar]
- 63.Balthazar T, Vandenbriele C, Verbrugge FH, Uil CD, Engström A, Janssens S, Rex S, Meyns B, Mieghem NV, Price S, et al. Managing Patients With Short-Term Mechanical Circulatory Support. Journal of the American College of Cardiology. 2021;77:1243–1256. doi: doi: 10.1016/j.jacc.2020.12.054 [DOI] [PubMed] [Google Scholar]
- 64.Eliet J, Gaudard P, Zeroual N, Rouviere P, Albat B, Mourad M, Colson PH. Effect of Impella During Veno-Arterial Extracorporeal Membrane Oxygenation on Pulmonary Artery Flow as Assessed by End-Tidal Carbon Dioxide. ASAIO J. 2018;64:502–507. doi: 10.1097/MAT.0000000000000662 [DOI] [PubMed] [Google Scholar]
- 65.Lim HS. The Effect of Impella CP on Cardiopulmonary Physiology During Venoarterial Extracorporeal Membrane Oxygenation Support. Artif Organs. 2017;41:1109–1112. doi: 10.1111/aor.12923 [DOI] [PubMed] [Google Scholar]
- 66.Alexis-Ruiz A, Ghadimi K, Raiten J, Mackay E, Laudanski K, Cannon J, Ramakrishna H, Evans A, Augoustides JG, Vallabhajosyula P, et al. Hypoxia and Complications of Oxygenation in Extracorporeal Membrane Oxygenation. J Cardiothorac Vasc Anesth. 2019;33:1375–1381. doi: 10.1053/j.jvca.2018.05.028 [DOI] [PubMed] [Google Scholar]
- 67.Josiassen J, Helgestad OKL, Udesen NLJ, Banke A, Frederiksen PH, Hyldebrandt JA, Schmidt H, Jensen LO, Hassager C, Ravn HB, et al. Unloading using Impella CP during profound cardiogenic shock caused by left ventricular failure in a large animal model: impact on the right ventricle. Intensive Care Med Exp. 2020;8:41. doi: 10.1186/s40635-020-00326-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zaky M, Nordan T, Kapur NK, Vest AR, DeNofrio D, Chen FY, Couper GS, Kawabori M. Impella 5.5 Support beyond 50 Days as Bridge to Heart Transplant in End-Stage Heart Failure Patients. ASAIO J. 2022. doi: 10.1097/MAT.0000000000001796 [DOI] [PubMed] [Google Scholar]
- 69.Ramzy D, Anderson M, Batsides G, Ono M, Silvestry S, D’Alessandro DA, Funamoto M, Zias EA, Lemaire A, Soltese E. Early Outcomes of the First 200 US Patients Treated with Impella 5.5: A Novel Temporary Left Ventricular Assist Device. Innovations (Phila). 2021;16:365–372. doi: 10.1177/15569845211013329 [DOI] [PubMed] [Google Scholar]
- 70.Levine A, Kai M, Ohira S, Panza JA, Pan S, Lanier G, Aggarwal-Gupta C, Gass A. Ecpella 5.5: An Evolution in the Management of Mechanical Circulatory Support. Cardiol Rev. 2022. doi: 10.1097/CRD.0000000000000466 [DOI] [PubMed] [Google Scholar]
- 71.Whitman GJ. Extracorporeal membrane oxygenation for the treatment of postcardiotomy shock. J Thorac Cardiovasc Surg. 2017;153:95–101. doi: 10.1016/j.jtcvs.2016.08.024 [DOI] [PubMed] [Google Scholar]
- 72.Guirgis M, Kumar K, Menkis AH, Freed DH. Minimally invasive left-heart decompression during venoarterial extracorporeal membrane oxygenation: an alternative to a percutaneous approach. Interact Cardiovasc Thorac Surg. 2010;10:672–674. doi: 10.1510/icvts.2009.228346 [DOI] [PubMed] [Google Scholar]
- 73.Tepper S, Masood MF, Baltazar Garcia M, Pisani M, Ewald GA, Lasala JM, Bach RG, Singh J, Balsara KR, Itoh A. Left Ventricular Unloading by Impella Device Versus Surgical Vent During Extracorporeal Life Support. Ann Thorac Surg. 2017;104:861–867. doi: 10.1016/j.athoracsur.2016.12.049 [DOI] [PubMed] [Google Scholar]
- 74.Stephens AF, Wanigasekara D, Pellegrino VA, Burrell AJC, Marasco SF, Kaye DM, Steinseifer U, Gregory SD. Comparison of Circulatory Unloading Techniques for Venoarterial Extracorporeal Membrane Oxygenation. ASAIO J. 2021;67:623–631. doi: 10.1097/MAT.0000000000001268 [DOI] [PubMed] [Google Scholar]
- 75.Russo JJ, Aleksova N, Pitcher I, Couture E, Parlow S, Faraz M, Visintini S, Simard T, Di Santo P, Mathew R, et al. Left Ventricular Unloading During Extracorporeal Membrane Oxygenation in Patients With Cardiogenic Shock. J Am Coll Cardiol. 2019;73:654–662. doi: 10.1016/j.jacc.2018.10.085 [DOI] [PubMed] [Google Scholar]
- 76.Schrage B, Burkhoff D, Rubsamen N, Becher PM, Schwarzl M, Bernhardt A, Grahn H, Lubos E, Soffker G, Clemmensen P, et al. Unloading of the Left Ventricle During Venoarterial Extracorporeal Membrane Oxygenation Therapy in Cardiogenic Shock. JACC Heart Fail. 2018;6:1035–1043. doi: 10.1016/j.jchf.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 77.Radakovic D, Opacic D, Prashovikj E, Marcus-André D, Schramm R, Morshuis M, Kizner L, Rudolph V, Gummert J, Flottmann C. Timing of Left Ventricular Unloading with Impella Device in Patients with VA-ECMO: A Propensity Score–Matched Analysis. Thorac Cardiovasc Surg. 2020:S1–S172. doi: 10.1055/s-0040-1705453v [DOI] [Google Scholar]
- 78.Na SJ, Yang JH, Yang JH, Sung K, Choi JO, Hahn JY, Jeon ES, Cho YH. Left heart decompression at venoarterial extracorporeal membrane oxygenation initiation in cardiogenic shock: prophylactic versus therapeutic strategy. J Thorac Dis. 2019;11:3746–3756. doi: 10.21037/jtd.2019.09.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Wal PS, van Steenwijk MPJ, Montenij LJ, Donker DW, Meuwese CL. Prophylactic versus therapeutic left ventricular unloading during extracorporeal membrane oxygenation, better safe than sorry? J Thorac Dis. 2020;12:6412–6415. doi: 10.21037/jtd-20-2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thiele H, Freund A, Gimenez MR, de Waha-Thiele S, Akin I, Poss J, Feistritzer HJ, Fuernau G, Graf T, Nef H, et al. Extracorporeal life support in patients with acute myocardial infarction complicated by cardiogenic shock - Design and rationale of the ECLS-SHOCK trial. Am Heart J. 2021;234:1–11. doi: 10.1016/j.ahj.2021.01.002 [DOI] [PubMed] [Google Scholar]


