Abstract
Background:
Immunoglobulin E (IgE)-mediated food allergies (FAs) are increasingly common among US children and adults. Not only can living with FA impose considerable physical health impacts, it also imposes economic burden and can negatively impact quality of life. Limited data indicate that allergy to multiple foods (multi-FA) also may be common, but much remains unknown about its distribution and determinants.
Objective:
We aimed to characterize the prevalence, characteristics, determinants, psychosocial burden, and distribution of multi-FA among a large, nationally representative sample of US children and adults.
Methods:
A US population-based survey was administered. Estimates of multi-FA prevalence, conditional frequencies of multi-FA combinations and associated factors were derived. Latent class analyses (LCA) were conducted using 9 dichotomized indicators of specific FA prevalence, which were used to determine factors associated with latent class membership and characterize FA-related psychosocial burden within each class.
Results:
Surveys were completed for 38,408 children and 40 443 adults. Among children and adults meeting established symptom-report criteria for FA, an estimated 40% and 48% had multi-FA, respectively. Among pediatric and adult populations with convincing FAs, the lifetime reported prevalence of physician-diagnosed atopic comorbidities increased significantly as the number of reported current convincing FAs increased, as did the proportion reporting multi-FA-related healthcare utilization and higher perceived psychosocial burden. LCA suggested the existence of four key latent phenotypes of multi-FA: milk/egg-dominant, seafood-dominant, peanut/tree nut-dominant, and broadly multi-food allergic.
Conclusion:
The US population-level burden of multi-FA is high among both children and adults and data indicate the presence of four major phenotypes of multi-FA in both populations.
Keywords: food allergy, multi-FA, psychosocial burden, allergy to multiple foods, burden of disease, epidemiology
Introduction
In recent decades, food allergy (FA) has become a chronic condition of increasing public health concern in the United States (US) owing to its growing prevalence, and the corresponding increase in its physical and psychological impacts.1 Recent survey-based prevalence estimates based upon parent/patient-reported reaction symptomology indicate that immunoglobulin E (IgE)-mediated FA currently affects 7.6% of US children and 10.8% of adults, although these rates attenuate to 4.7% and 5.1%, respectively, if cases are limited to those reporting FA that is physician-diagnosed and confirmed via allergy testing. In contrast, 11.4% of children have parent-reported FA and 19.0% of adults report being allergic to at least one food, suggesting that the perceived burden of disease and corresponding public health burden are each substantial.1 Some clinical reports2,3 suggest that survey assessment of food allergy significantly overestimates food allergy prevalence, even when symptom-report criteria are applied; however, the impact that living with a FA—real or perceived—can impose on daily life is well-documented4 and can be profound given that successful management of IgE-mediated FA requires strict allergen avoidance and constant vigilance to avoid accidental allergen exposure, with potentially life-threatening consequences. Not only can living with FA impose considerable physical health impacts as a result of allergic reactions and their sequelae, it also imposes economic burden,5 causes emotional distress, and can negatively impact quality of life—even among patients who are able to successfully avoid exposure to trigger foods.6,7
Data from selected samples also indicate that FA burden may be greater among patients who experience greater dietary and/or social restriction from having allergies to multiple foods (i.e., multi-FA). For example, greater impairment of FA-related quality of life (QoL) was reported in children with multi-FA4,8–10 and their caregivers4,11,12 compared to those with a single FA. Patients with multi-FA have also been shown to have higher rates of adverse clinical outcomes including increased prevalence of other atopic diseases,13 severity of food-related adverse reactions,14 and risk of anaphylaxis.15,16
While the current US-population level burden of multi-FA is poorly understood, a few notable studies have provided some insights. For instance, a retrospective review of a random sample of 602 pediatric charts from an urban pediatric FA referral population estimated that 78% exhibited evidence of multi-FA.13 Around this same time, a national FA prevalence survey of children from the general US population (i.e., not a selected clinical sample) estimated that 30% of US children were allergic to multiple foods.17 However, in this survey, individuals with multiple tree nut, finfish, or shellfish allergies were only classified as having a single FA to each corresponding group, owing to survey design. Since recent evidence suggests that many tree nut or shellfish allergic children are allergic to multiple tree nuts18 and/or multiple shellfish19—30% was likely an underestimate of the true prevalence of multi-FA. In contrast, a more recent national FA prevalence survey by the same research group estimated that 40% of US children and 45% of US adults with FA are allergic to multiple foods.14,20 Despite these efforts to understand the prevalence and burden of multi-FA in the US, little remains known about multi-FA phenotypes, and their characteristics and phenotypic prevalence among US pediatric and adult populations. Better understanding multi-FA phenotypes can inform future treatment and prevention efforts and help clinicians support and guide their patients regarding disease prognosis. To achieve these goals, this study aimed to characterize the prevalence, characteristics, determinants, psychosocial burden, and distribution of multi-FA among a large, nationally representative sample of US children and adults.
Methods
Survey Sampling, Administration, and Weighting
As previously detailed, a national cross-sectional FA questionnaire was IRB-approved and administered via web and telephone from October 1, 2015 through September 31, 2016 via a dual-sample approach.14,20 English and/or Spanish-speaking adults (≥18 years) residing in a US household were eligible to participate. This study relied upon NORC at the University of Chicago’s nationally-representative, probability-based AmeriSpeak Panel for US population-level inference—where a survey completion rate of 51.2% was observed. AmeriSpeak is a probability-based panel, with households selected from a documented randomly selected list of households in the US are sampled by address, and then contacted by U.S. mail and by NORC telephone and field interviewers. It provides at least 97 percent sample coverage of the U.S. population. To increase precision, these population-weighted AmeriSpeak responses were augmented by calibration-weighted, non-probability-based responses obtained through Survey Sampling International. Detailed information regarding the complex survey sampling, weighting, and analysis methods used for population-level inference was previously published.20–24 In total, survey responses were obtained for 38,408 children and 40,443 adults.
IgE-mediated Food Allergy Case Definitions and Outcomes
The primary outcome measures for the present study were the estimated prevalence of IgE-mediated current allergy to multiple foods in the US pediatric and adult populations. Food allergies that resolved or were “outgrown” prior to survey administration (i.e. allergies to specific foods that the respondent reported currently being able to consume without allergic reaction), were not considered towards the tabulation of multiple food allergies. Individuals with allergies to multiple tree nuts (i.e. cashew and walnut) were considered to have multiple FAs, as were those with allergies to multiple shellfish and finned fish. Reported FAs were considered to be convincingly IgE-mediated (i.e., “convincing”) if the most severe reaction reported to that food included at least one symptom on the stringent symptom list developed by our expert panel, even if such allergies were reported to be physician-diagnosed. Convincing FAs for which a physician’s diagnosis was reported, were considered physician-confirmed. A severe reaction history to a food was indicated by report of multiple specific stringent symptoms occurring within two or more of the following four organ systems (skin/oral mucosa, gastrointestinal, cardiovascular, and respiratory) in response to the question “Think back to the most severe allergic reaction to milk that you/your child ever had. What were your/your child’s symptoms?” Complete descriptions of the methods used for survey development, testing, and categorization of allergy type can be found in our previous publications.19,20
To assess the psychosocial burden of living with FA, the food allergy independent measure (FAIM)-Adult Form was administered to all respondents reporting a current FA and the FAIM-Parent-Form was completed by parents who proxy-reported a current pediatric FA. This validated measure is comprised of 6 questions, which are scored on a 1-to-7-point scale with higher scores indicative of greater psychosocial burden and lower FA-related QoL. FAIM scores were calculated as described by the scale authors.25
Understanding Latent Classes of Multi-FA
Complex survey weighted conditional frequencies of specific multi-FA combinations were calculated and presented descriptively. Latent class analyses (LCA) were conducted using 9 dichotomized indicators of FA prevalence (i.e., peanut, any tree nut, egg, cow’s milk, any shellfish, any fin fish, wheat, soy, sesame) to identify homogeneous, mutually exclusive latent classes of multi-FA risk hypothesized to exist within the study population. Models were also fit that included specific shellfish, fin fish and tree nut allergens, but very high levels of correlation were observed within these three allergen groups, which did not otherwise impact the observed class structure.
LCA was carried out via MPlus 7.4 using the 3-step procedure, which accounts for classification uncertainty (i.e., measurement error) while ensuring that latent class formation is not influenced by observed predictors of class membership. A robust maximum likelihood estimator was used, and complex survey weights were applied. To determine specific factors predictive of class membership, participant characteristics were added to the model to test independent associations via multilevel multinomial logistic regression, which included covariate adjustment for race and ethnicity, age, household income, sex, educational attainment, and nativity.
Each LCA model was run at least 10 times with more than 10,000 iterations to obtain and replicate the best loglikelihood value (i.e., the global maximum). To determine the most appropriate number of FA behavioral risk classes, we used an iterative process where the model was fit with an increasing number of classes, beginning with a single class. As visualized in eFigure 1, Bayesian information criteria (BIC) for the 5-class solution demonstrated very limited improvement relative to the 4-class solution, suggesting that a 4-class solution is most parsimonious. Moreover, Lo-Mendell-Rubin adjusted likelihood ratio tests confirmed the optimality of the four-class solution, which provided significantly improved model fit relative to the 3-class solution in both the pediatric and adult subpopulations (Pediatric aLRT=439, p<.001; Adult aLRT=387, p<.001). Conversely, adding a 5th class did not significantly improve overall model fit relative to the 4-class solution (Pediatric aLRT=83.2, p=.75; Adult aLRT=48, p=.28). Average latent class probabilities for the 4-class model were high in both subpopulations (Pediatric=0.73-0.91; Adult= 0.83-0.97), indicating that specific multi-FA phenotypes were more homogeneous among participants assigned to the same class relative to participants assigned to different classes. Posterior probabilities of latent class membership for each participant were then calculated using the 4-class model. Each participant was assigned to the class for which the participant had the maximum posterior probability.
Complex survey-weighted means and proportions were calculated to estimate US population prevalence of multi-FA and other related demographic and clinical characteristics using STATA 17 svy: prefix. The FAIM exhibited excellent internal consistency (Cronbach’s α >.8), and confirmatory factor analysis of the FAIM concluded that a two-factor solution exhibited excellent fit24 to the data (Confirmatory Fit Index [CFI]≥950; Root Mean Square Error of Approximation [RMSEA]<08). Two-sided hypothesis tests with a two-sided P < .05 were used to indicate statistical significance. Covariate-adjusted predicted FAIM scores were evaluated by fitting multilevel linear regression models adjusting for respondent race and ethnicity, age, household income, sex, educational attainment, and nativity.
Results
Surveys were completed for 38,408 children (mean=8.7 years; 95% confidence interval (CI) (8.5-8.8) and by 40 443 adults (mean=46.8 years; 95%CI (46.3-47.2). The weighted distributions of respondents by age, sex, and race and ethnicity have been previously reported14,20 and are consistent with 2016 national estimates from the US Census Bureau’s Current Population Survey.27 Selected demographic and clinical characteristics of individuals meeting established multi-FA case definitions are provided in Table 1.
Table 1.
Distribution of weighted sample demographics among the entire pediatric and adults samples, as well as among individuals meeting specific case definitions of reported, convincing, and physician-confirmed, convincing food allergy
| All US Children | Children with Multiple Reported Food Allergies | Children with Multiple Convincing Food Allergies | Children with Multiple Physician-Confirmed Convincing Food Allergies | All US Adults | Adults with Multiple Reported Food Allergies | Adults with Multiple Convincing Food Allergies | Adults with Multiple Physician-Confirmed Convincing Food Allergies | |
|---|---|---|---|---|---|---|---|---|
| N=38,416 % (95% CI) |
N=2,291 % (95% CI) |
N=1,409 % (95% CI) |
N=861 % (95% CI) |
(N=40,455) % (95% CI) |
N=4,927 % (95% CI) |
N=2,951 % (95% CI) |
N=1,335 % (95% CI) |
|
| Race/ethnicity | ||||||||
| Asian, non-Hispanic | 3.2 (2.8-3.7) | 3.3 (2.6-4.3) | 3.6 (2.6-5.1) | 4.1 (2.7-6.1) | 3.9 (3.6-4.1) | 4.6 (3.9-5.4) | 3.2 (2.6-4.0) | 3.3 (2.4-4.5) |
| Black, non-Hispanic | 13.2 (12.3-14.2) | 18.1 (14.9-22.0) | 20.8 (16.3-26.2) | 22.4 (16.0-30.5) | 11.7 (11.3-12.1) | 12.1 (10.9-13.3) | 13.4 (11.8-15.2) | 12.0 (9.8-14.7) |
| White, non-Hispanic | 52.8 (51.2-54.4) | 48.0 (44.0-52.1) | 45.3 (40.4-50.3) | 44.0 (37.9-50.3) | 64.9 (64.2-65.6) | 60.0 (58.0-61.9) | 60.0 (57.5-62.5) | 62.1 (58.6-65.5) |
| Hispanic | 24.1 (222.5-25.7) | 24.0 (20.6-27.8) | 23.2 (19.5-27.4) | 23.2 (18.6-28.4) | 15.5 (14.9-16.1) | 18.2 (16.5-20.0) | 18 (15.9-20.3) | 18.5 (15.9-21.4) |
| Multiple/other | 6.6 (6.1-7.3) | 6.6 (5.2-8.2) | 7.1 (5.3-9.5) | 6.4 (4.1-9.8) | 4.1 (3.8-4.4) | 5.2 (4.4-6.1) | 5.3 (4.4-6.5) | 4.1 (2.9-5.6) |
| Born in the US | ||||||||
| Yes | 97.8 (97.4-98.1) | 97.7 (96.5-98.5) | 97.6 (95.6-98.7) | 97.3 (94.3-98.8) | 91.6 (91.2-92.0) | 91.4 (90.1-92.5) | 92.9 (91.6-94.1) | 93.0 (90.9-94.7) |
| No | 2.2 (1.9-2.6) | 2.3 (1.5-3.5) | 2.4 (1.3-4.4) | 2.7 (1.2-5.7) | 8.4 (8.1-8.8) | 8.7 (7.5-9.9) | 7.1 (5.9-8.4) | 7.0 (5.3-9.1) |
| Sex | ||||||||
| Female | 48.9 (47.8-50.0) | 47.5 (43.6-51.4) | 47.5 (42.8-52.3) | 45.7 (39.2-52.3) | 51.7 (51.0-52.4) | 65.5 (63.7-67.4) | 66.5 (64.2-68.8) | 65.4 (62.1-68.6) |
| Male | 51.1 (50.0-52.2) | 52.5 (48.6-56.4) | 52.5 (47.7-57.3) | 54.3 (47.7-60.8) | 48.3 (47.6-49.0) | 34.5 (32.6-36.3) | 33.5 (31.2-35.8) | 34.6 (31.4-37.9) |
| Age | ||||||||
| 0-2 years | 15.9 (15.1-16.8) | 12.2 (9.9-15.0) | 12.5 (9.7-16.0) | 10.9 (7.9-14.7) | ||||
| 3-5 years | 16.2 (15.5-17.0) | 16.3 (13.6-19.5) | 18.2 (14.3-22.9) | 16.2 (12.6-20.6) | ||||
| 6-10 years | 27.9 (26.9-28.8) | 28.5 (25.3-31.9) | 30.2 (26.3-34.4) | 31.8 (26.9-37.1) | ||||
| 11-13 years | 16.6 (15.9-17.4) | 18.7 (15.8-22.2) | 17.5 (14.7-20.8) | 19.3 (15.3-23.9) | ||||
| 14-17 years | 23.4 (22.4-24.4) | 24.3 (21.2-27.6) | 21.5 (18.1-25.3) | 21.9 (17.3-27.4) | ||||
| 18-29 years | 21.5 (20.8-22.1) | 24.6 (22.9-26.4) | 23.9 (21.8-26.2) | 24.4 (21.4-27.6) | ||||
| 30-39 years | 17.0 (16.5-17.5) | 20.2 (18.7-21.8) | 20.8 (18.9-22.8) | 23.7 (20.8-26.8) | ||||
| 40-49 years | 16.8 (16.3-17.3) | 16.2 (14.8-17.7) | 16.7 (15.0-18.5) | 18.7 (16.2-21.5) | ||||
| 50-59 years | 18.0 (17.5-18.5) | 18.7 (17.2-20.2) | 19.4 (17.5-21.4) | 20.0 (17.2-23.1) | ||||
| 60+ years | 26.8 (26.2-27.4) | 20.3 (18.8-21.9) | 19.2 (17.3-21.3) | 13.3 (11.1-15.8) | ||||
| Household income, $ | ||||||||
| <25,000 | 16.1 (14.9-17.3) | 13.6 (11.0-16.6) | 15.5 (12.0-19.9) | 13.2 (9.6-17.8) | 16.6 (16.2-17.1) | 15.5 (14.2-16.9) | 16.1 (14.5-17.9) | 12.7 (10.5-15.3) |
| 25,000-49,000 | 22.2 (20.9-23.5) | 21.2 (18.1-24.7) | 21.7 (17.8-26.2) | 17.1 (13.4-21.7) | 22.0 (21.4-22.5) | 21.7 (20.3-23.2) | 22.5 (20.7 (24.5) | 22.0 (19.5-24.8) |
| 50,000-99,999 | 31.1 (29.8-32.5) | 33.7 (30.0-37.6) | 34.6 (29.8-39.7) | 38.3 (31.7-45.3) | 30.9 (30.3-31.5) | 34.1 (32.3-36.0) | 34.7 (32.4-37.1) | 34.5 (31.2-37.9) |
| 100,000-149,000 | 19.2 (18.0-20.5) | 21.1 (17.9-24.6) | 18.2 (15.4-21.3) | 20.5 (16.8-24.8) | 19.6 (19.0-20.2) | 19.3 (17.7-21.1) | 18.4 (16.3-20.8) | 22.2 (19.1-25.6) |
| ≥150,000 | 11.4 (10.3-12.6) | 10.5 (8.1-13.4) | 10.1 (7.5-13.4) | 10.9 (7.2-16.2) | 10.9 (10.4-11.5) | 9.4 (8.2-10.8) | 8.2 (6.9-9.7) | 8.7 (6.9-10.9) |
| Other Conditions | ||||||||
| Asthma | 12.2 (11.4-13.0) | 38.5 (34.4-42.8) | 43.5 (38.5-48.8) | 49.5 (43.0-55.9) | 12.3 (11.8-12.7) | 23.6 (22.0-25.3) | 27.5 (25.3-29.8) | 29.5 (26.4-32.8) |
| Eczema | 5.9 (5.3-6.5) | 16.9 (13.9-20.4) | 17.0 (13.7-20.8) | 18.5 (14.7-23.1) | 6.7 (6.4-7.1) | 12.8 (11.5-14.2) | 13.5 (12.0-15.3) | 13.7 (11.6-16.1) |
| Environmental Allergies | 12.8 (12.0-13.6) | 35.5 (31.8-39.3) | 38.3 (34.1-42.7) | 42.9 (37.8-48.2) | 21.4 (20.9-22.0) | 35.4 (33.6-37.3) | 37.0 (34.6-39.4) | 39.6 (36.0-43.2) |
| Latex Allergy | 1.0 (0.8-1.3) | 7.8 (5.5-11.0) | 8.8 (6.4-12.0) | 9.2 (6.7-12.7) | 6.6 (5.8-7.6) | 8.3 (7.0-9.8) | 9.7 (7.8-11.9) | 7.8 (6.8-8.8) |
| Insect Sting/Venom Allergy | 2.2 (1.9-2.6) | 7.6 (5.7-10.0) | 8.4 (6.5-10.9) | 9.8 (7.3-13.2) | 3.8 (3.6-4.1) | 7.8 (6.8-8.8) | 10.4 (8.9-12.0) | 9.1 (7.4-11.3) |
| EoE | 0.16 (0.12-0.22) | 0.99 (0.54-1.81) | 1.17 (0.65-2.12) | 1.30 (0.62-2.70) | 0.18 (0.14-0.23) | 0.60 (0.41-0.87) | 0.90 (0.59-1.36) | 1.42 (0.88-2.28) |
| FPIES | 0.51 (0.42-0.62) | 5.48 (4.32-6.94) | 7.75 (5.99-9.95) | 8.34 (6.29-10.98) | 0.22 (0.17-0.28) | 1.01 (0.73-1.39) | 1.23 (0.87-1.73) | 2.06 (1.36-3.10) |
| Medication Allergy | 4.2 (3.71-4.65) | 10.0 (7.5-13.0) | 10.8 (8.4-13.8) | 12.1 (9.1-16.0) | 13.4 (13.0-13.9) | 22.7 (21.1-24.4) | 24.1 (22.0-26.2) | 23.2 (20.2-26.4) |
Prevalence of Multi-FA
Overall, among the 11.4% (95%CI: 10.8%–12.0%) of US children reporting one or more current FAs–irrespective of clinical history, 45% reported multiple current FAs. Among the 7.6% (95%CI: 7.1%-8.1%) of US children reporting one or more current FAs that fulfilled previously published criteria for convincingly IgE-mediated FA,33,34 40% reported multiple current convincingly IgE-mediated FAs. Finally, among the 4.7% (95%CI: 4.3%-5.0%) of US children reporting one or more physician-diagnosed current FAs that met established symptom-report criteria for convincingly IgE-mediated FA, 38% reported multiple physician-confirmed FAs.
Among US children, using the “convincing” case definition tailored to estimate the true population-level prevalence of FA, 4.6% (95%CI:4.2%-5.0%) reported a single convincing FA, while 1.8% (95%CI:1.6%-2.1%) reported 2-3 convincing FAs, and 1.2% (95%CI:1.0%-1.4%) reported >3 convincing FAs. With respect to the US adult population, among the 19.0% (95%CI: 18.5%–19.5%) of US adults reporting one or more current FAs, irrespective of clinical history, 48% reported multiple FAs. Among the 10.8% (95%CI:10.4%-11.1%) of US adults reporting one or more current FAs that met established symptom-report criteria for convincingly IgE-mediated FA, 46% reported multiple current convincingly IgE-mediated FAs. Finally, among the 5.1% (95%CI: 4.9%-5.4%) of US adults reporting ≥1 physician-diagnosed current FAs that met established symptom-report criteria for convincingly IgE-mediated FA, 42% reported multiple physician-confirmed FAs. Among US adults, using the “convincing” case definition believed to reflect the true population-level prevalence of FA, 5.9% (95%CI: 5.6%-6.2%) reported a single convincing FA, while 3.1% (95%CI: 2.9%-3.3%) reported 2-3 convincing FAs, and 1.7% (95%CI: 1.6%-1.9%) reported >3 convincing FAs.
Multi-FA Prevalence by Demographic Factors
The prevalence of multi- vs. mono- convincing FA did not differ significantly across pediatric age strata (X2=0.87; p=.48), with approximately 33% of children with convincing FA under age 3 reporting multiple FAs compared to approximately 40% of those age 3 and older. When the mean number of convincing FAs was examined, a similar pattern emerged, with rates ranging from 2.0 to 2.6 FAs across all pediatric age groups (F=0.9; p=.44).
In contrast, the prevalence of multi-vs. mono-convincing FA differed significantly across adult age strata (F=2.9; p=.01), with approximately 48% of adults with convincing FA between the ages of 18-49 reporting multiple FAs. This declined to 44.5% among 50-59-year-olds, 41.2% among 60-69-year-olds and 37.5% among 70+ year-olds. When the mean number of convincing FAs was examined, a similar pattern was observed, with adults between the ages of 18-49 reporting an average of 2.6 FAs then declining to 2.4 FAs among 50-59 year-olds, 2.2% among 60-69 year-olds and 1.9 FAs among 70+ year-olds (F=6.8; p<.001). Significant differences in multi-vs-mono FA were observed across racial/ethnic strata for both children (F=5.6; p<.001) and adults (F=3.4; p=.001), with Non-Hispanic White children and adults less likely to have multiple food allergies than their Non-Hispanic Black counterparts.
Atopic Comorbidities
Among pediatric and adult populations with convincing FAs, the lifetime reported prevalence of physician-diagnosed atopic comorbidities increased significantly as the number of reported current convincing FAs increased (p<.001 for all; presented in Table 2, which reports data stratified by number of current convincing FAs: 0; 1; 2-3; >3)
Table 2.
Number of Current Food Allergies by Demographic Characteristics
| US Children | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NUMBER OF CURRENT CONVINCING FOOD ALLERGIES (column) | |||||||||||
| No FA (N=35,000) | 1 FA (N=2,025) | 2-3 FAs (N=829) | >3 FAs (N=562) | Total (N=38,416) | P VALUE | ||||||
| RACE/ETHNICITY | % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | |
| White NH (N=26243) | 53.2 | [51.56,54.82] | 50.39 | [45.99,54.78] | 49.76 | [42.74,56.8] | 36.96 | [31.48,42.8] | 52.81 | [51.24,54.38] | <.001 |
| Black NH (N=3456) | 13.05 | [12.08,14.08] | 11.9 | [9.747,14.45] | 19.91 | [13.56,28.27] | 22.54 | [17.26,28.88] | 13.23 | [12.29,14.24] | |
| Asian NH (N=1371) | 3.27 | [2.813,3.798] | 2.179 | [1.6,2.962] | 2.642 | [1.678,4.137] | 5.186 | [3.181,8.347] | 3.231 | [2.802,3.724] | |
| Hispanic (N=4594) | 23.89 | [22.26,25.59] | 28.49 | [23.92,33.55] | 20.25 | [16.13,25.11] | 28.68 | [22.22,36.14] | 24.09 | [22.52,25.73] | |
| Multiple (N=2752) | 6.601 | [6.012,7.243] | 7.044 | [5.472,9.023] | 7.433 | [4.994,10.92] | 6.632 | [4.126,10.49] | 6.637 | [6.069,7.253] | |
| SEX | |||||||||||
| Male (N=19730) | 51.05 | [49.93,52.17] | 51.55 | [47.22,55.86] | 49.66 | [43.1,56.24] | 55.3 | [48.67,61.74] | 51.1 | [50.04,52.16] | 0.71 |
| Female (N=18686) | 48.95 | [47.83,50.07] | 48.45 | [44.14,52.78] | 50.34 | [43.76,56.9] | 44.7 | [38.26,51.33] | 48.9 | [47.84,49.96] | |
| AGE (in years) | |||||||||||
| 0-2 (N=5770) | 16.01 | [15.16,16.9] | 16.79 | [13.11,21.25] | 12.98 | [9.252,17.92] | 12.09 | [8.268,17.35] | 15.94 | [15.13,16.79] | 0.71 |
| 3-5 (N=6164) | 16.12 | [15.33,16.94] | 16.76 | [13.84,20.16] | 18.98 | [13.65,25.77] | 17.93 | [12.75,24.62] | 16.22 | [15.47,17] | |
| 6-10 (N=10524) | 27.73 | [26.73,28.75] | 28.9 | [25.32,32.76] | 27.96 | [23.02,33.5] | 32.56 | [26.63,39.1] | 27.85 | [26.89,28.82] | |
| 11-13 (N=6663) | 16.64 | [15.86,17.46] | 15.49 | [12.5,19.05] | 18.77 | [14.71,23.65] | 16.7 | [13.11,21.04] | 16.63 | [15.89,17.4] | |
| 14-17 (N=9295) | 23.5 | [22.44,24.58] | 22.06 | [19.02,25.43] | 21.31 | [16.69,26.79] | 20.72 | [16.23,26.06] | 23.36 | [22.36,24.39] | |
| ANNUAL HOUSEHOLD INCOME | |||||||||||
| <$25K (N=4775) | 16.13 | [14.96,17.39] | 15.13 | [12.26,18.53] | 14.08 | [9.869,19.69] | 18.62 | [12.81,26.28] | 16.08 | [14.93,17.29] | 0.31 |
| $25K-49K (N=8827) | 22.07 | [20.73,23.47] | 24.7 | [21.36,28.36] | 22.55 | [17.16,29.03] | 19.02 | [14.47,24.59] | 22.16 | [20.88,23.5] | |
| $50K-99K (N=15037) | 31.1 | [29.68,32.55] | 29.59 | [26.31,33.1] | 36.93 | [30.06,44.37] | 30.42 | [25.03,36.42] | 31.12 | [29.76,32.52] | |
| $100K-14 (N=6431) | 19.13 | [17.9,20.42] | 21.74 | [17.41,26.81] | 16.8 | [13.34,20.94] | 21.04 | [16.62,26.27] | 19.23 | [18,20.51] | |
| $150K+ (N=3346) | 11.57 | [10.38,12.88] | 8.841 | [6.6,11.75] | 9.65 | [6.116,14.9] | 10.89 | [7.836,14.94] | 11.4 | [10.27,12.64] | |
| COMORBID CONDITIONS | |||||||||||
| Asthma (N=4402) | 10.51 | [9.702,11.37] | 25.75 | [22.33,29.48] | 41.57 | [34.36,49.17] | 46.08 | [39.54,52.75] | 12.19 | [11.4,13.03] | <.001 |
| Eczema (N=2036) | 5.146 | [4.58,5.779] | 13.2 | [10.15,17] | 14.41 | [10.32,19.77] | 21.09 | [16.12,27.11] | 5.873 | [5.288,6.517] | <.001 |
| Allergic Rhinitis (N=5381) | 11.34 | [10.57,12.15] | 25.45 | [21.95,29.29] | 38.59 | [32.88,44.64] | 37.83 | [31.82,44.25] | 12.79 | [12.02,13.61] | <.001 |
| Latex Allergy (N=482) | 0.5747 | [.4408,.749] | 5.349 | [3.204,8.798] | 8.888 | [5.494,14.07] | 8.303 | [5.886,11.59] | 1.036 | [.8417,1.275] | <.001 |
| Sting/Venom Allergy (N=878) | 1.905 | [1.599,2.267] | 4.651 | [3.563,6.05] | 7.593 | [5.311,10.74] | 9.702 | [6.495,14.25] | 2.226 | [1.928,2.57] | <.001 |
| EoE (N=74) | 0.1158 | [.0762,.1759] | 0.3433 | [.1752,.6715] | 1.103 | [.4497,2.682] | 1.315 | [.6281,2.733] | 0.1584 | [.115,.2181] | <.001 |
| FPIES (261) | 0.1908 | [.1315,.2768] | 2.175 | [1.523,3.097] | 7.015 | [4.764,10.21] | 8.845 | [6.373,12.15] | 0.5083 | [.4181,.6178] | <.001 |
| Medication Allergy (N=1617) | 3.671 | [3.224,4.177] | 9.539 | [6.94,12.98] | 9.851 | [6.768,14.12] | 12.19 | [8.822,16.62] | 4.153 | [3.706,4.652] | <.001 |
| US Adults | |||||||||||
| NUMBER OF CURRENT CONVINCING FOOD ALLERGIES (column) | |||||||||||
| No FA | 1 FA | 2-3 FAs | >3 FAs | Total | |||||||
| (N=34,210) | (N=3,361) | (N=1,817) | (N=1,067) | (N=40,455) | P VALUE | ||||||
| RACE/ETHNICITY | % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | |
| White NH (N=28747) | 65.4 | [64.65,66.14] | 61.68 | [59.13,64.16] | 62.94 | [59.75,66.02] | 56.04 | [51.87,60.13] | 64.94 | [64.25,65.63] | <.001 |
| Black NH (N=4231) | 11.61 | [11.15,12.09] | 11.22 | [9.768,12.85] | 12.86 | [10.93,15.07] | 13.94 | [11.24,17.17] | 11.67 | [11.24,12.11] | |
| Asian NH (N=1748) | 3.806 | [3.544,4.086] | 4.88 | [3.954,6.009] | 2.986 | [2.254,3.947] | 3.432 | [2.478,4.737] | 3.837 | [3.594,4.097] | |
| Hispanic (N=4042) | 15.34 | [14.69,16.01] | 15.55 | [13.6,17.71] | 15.83 | [13.32,18.71] | 21.43 | [17.87,25.47] | 15.47 | [14.87,16.09] | |
| Multiple/Other (N=1687) | 3.849 | [3.585,4.133] | 6.684 | [5.27,8.443] | 5.389 | [4.27,6.781] | 5.155 | [3.557,7.415] | 4.086 | [3.828,4.361] | |
| SEX | |||||||||||
| Male (N=17843) | 50.05 | [49.31,50.79] | 33.83 | [31.51,36.22] | 30.55 | [27.7,33.56] | 37.14 | [33.32,41.12] | 48.27 | [47.58,48.96] | <.001 |
| Female (N=22612) | 49.95 | [49.21,50.69] | 66.17 | [63.78,68.49] | 69.45 | [66.44,72.3] | 62.86 | [58.88,66.68] | 51.73 | [51.04,52.42] | |
| AGE (in years) | |||||||||||
| 18-29 (N=8336) | 21.27 | [20.59,21.98] | 21.14 | [19.2,23.21] | 23.61 | [20.88,26.58] | 25.54 | [22.08,29.35] | 21.41 | [20.78,22.06] | <.001 |
| 30-39 (N=7803) | 16.59 | [16.08,17.11] | 19.43 | [17.51,21.51] | 20.31 | [17.9,22.96] | 21.88 | [18.73,25.39] | 16.96 | [16.48,17.45] | |
| 40-49 (N=6289) | 16.89 | [16.35,17.45] | 14.88 | [13.23,16.69] | 15.24 | [13.21,17.51] | 19.09 | [16.12,22.47] | 16.76 | [16.26,17.28] | |
| 50-59 (N=7799) | 17.83 | [17.32,18.36] | 20.41 | [18.42,22.55] | 20.29 | [17.86,22.96] | 17.24 | [14.41,20.5] | 18.05 | [17.56,18.54] | |
| 60+ (N=10218) | 27.41 | [26.78,28.06] | 24.15 | [22.15,26.27] | 20.54 | [18.05,23.29] | 16.24 | [13.46,19.47] | 26.82 | [26.23,27.41] | |
| ANNUAL HOUSEHOLD INCOME | |||||||||||
| <$25K (N=8168) | 16.64 | [16.15,17.13] | 16.66 | [15,18.46] | 15.46 | [13.52,17.62] | 17.4 | [14.4,20.87] | 16.61 | [16.16,17.07] | 0.03 |
| $25K-49K (N=10826) | 21.89 | [21.33,22.47] | 22.03 | [20.23,23.95] | 22.66 | [20.27,25.23] | 21.9 | [19,25.1] | 21.93 | [21.4,22.46] | |
| $50K-99K (N=13500) | 30.68 | [30.04,31.34] | 32.16 | [29.94,34.47] | 35.66 | [32.68,38.74] | 32.78 | [29.14,36.64] | 30.96 | [30.36,31.57] | |
| $100K-149K (N=5204) | 19.66 | [19.01,20.32] | 19.68 | [17.56,21.98] | 18.17 | [15.38,21.34] | 19 | [15.77,22.72] | 19.6 | [19,20.22] | |
| $150K+ (N=2757) | 11.12 | [10.56,11.72] | 9.469 | [7.92,11.28] | 8.061 | [6.488,9.975] | 8.926 | [6.722,11.76] | 10.9 | [10.37,11.44] | |
| COMORBID CONDITIONS | |||||||||||
| Asthma (N=5108) | 10.88 | [10.41,11.36] | 20.75 | [18.85,22.79] | 27.91 | [25.02,30.98] | 26.87 | [23.6,30.42] | 12.26 | [11.81,12.72] | <.001 |
| Eczema (N=2682) | 6.101 | [5.751,6.472] | 10.84 | [9.311,12.59] | 12.5 | [10.63,14.66] | 15.15 | [12.45,18.31] | 6.733 | [6.396,7.087] | <.001 |
| Allergic Rhinitis (N=8783) | 19.92 | [19.35,20.51] | 31.75 | [29.51,34.09] | 38.07 | [35,41.25] | 36.24 | [32.45,40.22] | 21.46 | [20.91,22.02] | <.001 |
| Latex Allergy (N=1052) | 1.827 | [1.655,2.016] | 4.433 | [3.59,5.461] | 8.508 | [6.874,10.49] | 8.123 | [6.184,10.6] | 2.294 | [2.118,2.485] | <.001 |
| Sting/Venom Allergy (N=1565) | 3.312 | [3.061,3.583] | 6.308 | [5.257,7.553] | 10.39 | [8.52,12.61] | 10.47 | [8.261,13.19] | 3.829 | [3.582,4.092] | <.001 |
| EoE (N=89) | 0.1245 | [.0858,.1805] | 0.3873 | [.1911,.7831] | 0.6668 | [.3548,1.25] | 1.331 | [.7522,2.346] | 0.1774 | [.1353,.2326] | <.001 |
| FPIES (N=113) | 0.1299 | [.0913,.1849] | 0.5098 | [.2549,1.017] | 0.838 | [.4857,1.442] | 2.043 | [1.318,3.155] | 0.2071 | [.1623,.2641] | <.001 |
| Medication Allergy (N=5652) | 12.26 | [11.8,12.73] | 22.21 | [20.24,24.31] | 24.71 | [22.06,27.57] | 23.7 | [20.48,27.26] | 13.42 | [12.98,13.87] | <.001 |
Healthcare Utilization
The reported number of FA-related emergency department (ED) visits in the past 12 months and over the respondent’s lifetime was associated with the number of current convincing FAs (p<.001 for both), with a greater number of FAs associated with greater odds of reporting ED visits (Table 3). Similarly, the likelihood of reporting a severe FA reaction as well as a history of epinephrine auto-injector (EAI) use for treatment of an acute FA reaction also increased commensurate with the number of current convincing FAs (p<.001). Patient-report of a current EAI prescription was also associated with a greater number of current convincing FAs. Notably, multiple logistic regression analyses adjusting for whether or not the patient has a current EAI prescription as well as their race and ethnicity, sex, age, household income, nativity (US vs. other), educational attainment, and the presence of at least one physician-confirmed FA found that patients with 2-3 FAs had double the odds of EAI use [OR=2.1 (95%CI: 1.6-2.6)]; while patients with >3 FAs had triple the odds of reporting prior EAI use [OR=3.0 (95%CI: 2.3-3.9)].
Table 3.
Healthcare utilization among US children and adults with different number of current convincing food allergies
| Number of Current Convincing Food Allergies among US Children | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 FA (N=2,025) | 2-3 FAs (N=829) | >3 FAs (N=562) | Total | P VALUE | |||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | ||
| ED last 12 mo (N=615) | 13.95 | [10.62,18.12] | 24.62 | [17.85,32.94] | 30.47 | [24.31,37.42] | 19.02 | [16.09,22.34] | <.001 |
| ED visit in lifetime (N=1470) | 34.05 | [30.03,38.33] | 50.89 | [43.87,57.88] | 60.06 | [53.53,66.25] | 42.04 | [38.76,45.4] | <.001 |
| EAI used to treat (N=1015) | 17.52 | [15.02,20.33] | 30.95 | [25.25,37.28] | 51.71 | [45.13,58.23] | 26.29 | [23.81,28.93] | <.001 |
| Severe Reaction History (N=1523) | 31.26 | [27.74,35.01] | 51.26 | [44.2,58.28] | 71.33 | [65.6,76.45] | 42.25 | [39.12,45.44] | <.001 |
| Epinephrine Prescription (N=1529) | 33.91 | [30.02,38.03] | 41.72 | [35.37,48.36] | 64.44 | [57.49,70.83] | 40.72 | [37.6,43.92] | <.001 |
| Number of Current Convincing Food Allergies among US Adults | |||||||||
| 1 FA (N=3,361) | 2-3 FAs (N=1,817) | >3 FAs (N=1,067) | Total | P VALUE | |||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | ||
| ED last 12 mo (N=555) | 5.066 | [3.992,6.41] | 9.583 | [8.002,11.44] | 18.87 | [15.85,22.32] | 8.57 | [7.638,9.604] | <.001 |
| ED visit in lifetime (N=2507) | 28.88 | [26.74,31.11] | 45.34 | [42.2,48.52] | 58.9 | [54.83,62.86] | 38.3 | [36.63,40] | <.001 |
| EAI used to treat (N=1473) | 11.76 | [10.38,13.29] | 26.17 | [23.67,28.83] | 42.7 | [38.77,46.72] | 20.87 | [19.59,22.21] | <.001 |
| Severe Reaction History (N=3199) | 37.29 | [34.92,39.72] | 61.39 | [58.25,64.43] | 80.12 | [76.89,83] | 51.04 | [49.27,52.8] | <.001 |
| Epinephrine Prescription (N=1697) | 16.15 | [14.61,17.83] | 27.68 | [25.07,30.45] | 43.66 | [39.73,47.67] | 23.87 | [22.53,25.27] | <.001 |
Complex survey-weighted conditional probabilities (displayed as percentages) of having specific FAs among US children and adults with at least one convincing FA were calculated and are presented in Figure 1. These data indicate substantial variation in the extent to which the top 9 allergens co-occur—ranging from an estimated 61% of US adults with a convincing fin-fish allergy also reporting a convincing shellfish allergy to only 2% of US adults with a shellfish allergy also reporting a convincing sesame allergy. Among children with peanut allergy (PA), 33.1% had tree nut (TN) co-allergy and 61% of those with TN allergy had PA. In contrast, among children with sesame allergy, 55.2% and 43.9% had PA and TN co-allergy, respectively. Table 4 compares the number of current convincing food allergies among individuals with specific food allergies.
Figure 1.

Bivariate conditional frequencies of specific Convincingly IgE-mediated Multifood Allergies Among US Children. Conditional formatting is applied to each matrix such that higher rates of comorbidity are indicated with red, moderate rates of comorbidity are indicated with yellow shading and low rates of comorbidity are indicated with green. The data are presented as conditional frequencies (from 0-100%) of individuals with particular column food allergies who also have specific row food allergies. For example 8% of children with a tree nut allergy also have a sesame allergy.
Figure 1a. Bivariate conditional frequencies of specific Convincingly IgE-mediated Multifood Allergies Among US Children
Conditional probabilities of individuals with food allergies labeled within each column, who are also allergic to the specific food listed in the corresponding row. For example, 61% of children with a tree nut allergy are also allergic to peanut, while 33% of children who are allergic to peanut are also allergic to tree nut.
Figure 1b. Bivariate conditional frequencies of specific Convincingly IgE-mediated Multifood Allergies Among US Adults
Conditional probabilities of individuals with food allergies labeled within each column, who are also allergic to the specific food listed in the corresponding row. For example, 50% of adults with a tree nut allergy are also allergic to peanut, while 33% of adults who are allergic to peanut are also allergic to tree nut.
Table 4.
Number of Current Food Allergies among US Children and Adults with Specific Convincing Food Allergies
| Number of Current Convincing Food Allergies among US Children | Number of Current Convincing Food Allergies among US Adults | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 FA (N=2,025) | 2-3 FAs (N=829) | >3 FAs (N=562) | 1 FA (N=3,361) | 2-3 FAs (N=1,817) | >3 FAs (N=1,067) | ||||||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | ||
| Peanut (N=1101) | 45.21 | [39.66,50.88] | 21.51 | [17.64,25.97] | 33.28 | [28.66,38.24] | Peanut (N=1184) | 32.31 | [28.79,36.03] | 28.98 | [25.69,32.5] | 38.71 | [35,42.56] |
| Almond (N=341) | 3.288 | [1.937,5.528] | 15.9 | [11.55,21.5] | 80.81 | [75.05,85.5] | Almond (N=437) | 4.27 | [2.49,7.23] | 18.4 | [14.2,23.5] | 77.3 | [71.95,81.94] |
| Cashew (N=332) | 4.212 | [2.115,8.213] | 19.22 | [13.53,26.57] | 76.57 | [69.06,82.71] | Cashew (N=341) | 3.771 | [2.001,6.994] | 16.41 | [11.66,22.59] | 79.82 | [73.47,84.97] |
| Hazelnut (N=262) | 5.04 | [1.259,18.09] | 10.91 | [5.335,21.02] | 84.05 | [72.19,91.45] | Hazelnut (N=346) | 3.76 | [1.773,7.795] | 13.88 | [9.968,19] | 82.36 | [76.64,86.92] |
| Pecan (N=279) | 0 | 6.339 | [3.386,11.56] | 93.66 | [88.44,96.61] | Pecan (N=326) | 0 | 9.108 | [5.843,13.93] | 90.89 | [86.07,94.16] | ||
| Pistachio (N=235) | 1.967 | [.8396,4.54] | 15.26 | [9.032,24.63] | 82.77 | [73.58,89.23] | Pistachio (N=267) | 3.019 | [1.458,6.148] | 7.814 | [4.831,12.4] | 89.17 | [84.13,92.74] |
| Walnut (N=299) | 5.868 | [3.177,10.59] | 10.72 | [7.015,16.05] | 83.41 | [77.15,88.21] | Walnut (N=406) | 4.925 | [3.015,7.948] | 15.16 | [11.3,20.05] | 79.91 | [74.63,84.32] |
| Any Tree Nut (N=587) | 10.51 | [7.024,15.43] | 27.65 | [22.37,33.63] | 61.84 | [55.53,67.78] | Any Tree Nut (N=744) | 9.693 | [7.407,12.59] | 28.52 | [24.52,32.87] | 61.79 | [57.16,66.22] |
| Sesame (N=102) | 13.58 | [5.265,30.76] | 18.86 | [11.73,28.92] | 67.56 | [53.34,79.14] | Sesame (N=149) | 19.71 | [11.08,32.59] | 22.42 | [15.46,31.34] | 57.87 | [46.91,68.12] |
| Milk (N=837) | 56.94 | [49.03,64.52] | 29.95 | [22.77,38.27] | 13.11 | [9.954,17.07] | Milk (N=1125) | 39.89 | [35.81,44.11] | 38.19 | [34.18,42.38] | 21.92 | [18.7,25.51] |
| Egg (N=418) | 24.21 | [18.32,31.28] | 47.65 | [37.13,58.39] | 28.13 | [21.04,36.51] | Egg (N=490) | 34.56 | [27.96,41.81] | 35.02 | [28.42,42.25] | 30.42 | [25.24,36.16] |
| Any Fin Fish (N=237) | 16.13 | [9.419,26.22] | 11.24 | [7.364,16.8] | 72.63 | [62.49,80.87] | Fin Fish (N=551) | 9.999 | [7.268,13.61] | 20.72 | [16.61,25.55] | 69.28 | [64.03,74.07] |
| Salmon (N=105) | 9.211 | [2.42,29.33] | 3.439 | [1.471,7.83] | 87.35 | [70.05,95.32] | Salmon (N=245) | 3.226 | [1.454,7.004] | 9.924 | [5.913,16.19] | 86.85 | [80.32,91.45] |
| Tuna (N=103) | 7.047 | [2.71,17.1] | 6.448 | [3.137,12.79] | 86.51 | [76.04,92.83] | Tuna (N=237) | 6.166 | [3.063,12.02] | 6.686 | [3.886,11.27] | 87.15 | [80.77,91.63] |
| Halibut (N=73) | 0 | 0.6705 | [.0906,4.783] | 99.33 | [95.22,99.91] | Halibut (N=229) | 0.8749 | [.1232,5.938] | 11.8 | [6.627,20.13] | 87.33 | [78.95,92.68] | |
| Anchovy (N=107) | 4.211 | [1.305,12.75] | 6.092 | [2.883,12.42] | 89.7 | [80.3,94.9] | Anchovy (N=277) | 0.6493 | [.2273,1.841] | 11.97 | [8.228,17.09] | 87.38 | [82.25,91.19] |
| Cod (N=93) | 7.43 | [2.567,19.65] | 3.655 | [1.342,9.568] | 88.91 | [77.14,95.02] | Cod (N=232) | 1.2 | [.4081,3.473] | 9.538 | [5.37,16.38] | 89.26 | [82.47,93.63] |
| Catfish (N=109) | 0.4087 | [.0559,2.921] | 4.679 | [2.22,9.593] | 94.91 | [89.89,97.51] | Catfish (N=250) | 2.61 | [1.138,5.873] | 6.524 | [3.539,11.72] | 90.87 | [85.39,94.42] |
| Anchovy (N=107) | 4.211 | [1.305,12.75] | 6.092 | [2.883,12.42] | 89.7 | [80.3,94.9] | Anchovy (N=277) | 0.6493 | [.2273,1.841] | 11.97 | [8.228,17.09] | 87.38 | [82.25,91.19] |
| Shellfish (N=599) | 27.12 | [21.91,33.05] | 32.43 | [26.38,39.12] | 37.1 | [30.68,44] | Shellfish (N=1732) | 32.971 | [28.97,35.64] | 36.38 | [33.23,39.66] | 30.64 | [27.76,33.68] |
| Shrimp (N=457) | 25.95 | [20.32,32.52] | 32.69 | [26.79,39.18] | 41.36 | [34.04,49.08] | Shrimp (N=1183) | 23.98 | [20.36,28.02] | 38.94 | [35.16,42.86] | 37.08 | [33.41,40.91] |
| Crab (N=290) | 9.116 | [4.64,17.13] | 29.5 | [22.4,37.76] | 54.5 | [44.66,64.01] | Crab (N=833) | 16.474 | [13.651,19.06] | 36.66 | [32.22,41.34] | 46.87 | [42.23,51.57] |
| Lobster (N=266) | 3.929 | [1.949,7.764] | 34.58 | [26.61,43.53] | 61.49 | [52.24,69.97] | Lobster (N=800) | 5.91 | [3.937,8.781] | 43.37 | [38.62,48.26] | 50.72 | [45.9,55.52] |
| Mollusk (N=276) | 8.969 | [5.813,13.59] | 37.3 | [27.19,48.65] | 53.73 | [42.81,64.31] | Mollusk (N=931) | 19.1 | [15.29,23.61] | 36.9 | [32.58,41.44] | 44 | [39.52,48.57] |
| Soy (N=204) | 25.27 | [16.01,37.51] | 36.12 | [21.6,53.72] | 38.6 | [26.42,52.4] | Soy (N=376) | 18.8 | [14.07,24.67] | 41.67 | [35.33,48.29] | 39.53 | [32.99,46.47] |
| Wheat (N=205) | 33.45 | [22.21,46.96] | 34.07 | [21.02,50.08] | 32.48 | [22.44,44.43] | Wheat (N=441) | 31.59 | [25.77,38.07] | 36.23 | [30.11,42.83] | 32.18 | [26.44,38.51] |
A greater number of reported convincing FAs was also associated with greater perceived FA severity and corresponding FA-related psychosocial burden (eFigure 2), as assessed by the FAIM. Significant positive adjusted associations between the number of current convincing FAs were observed among the adult population overall, as well as for each of the FAIM’s constituent indicators before and after adjusting for covariates. However, among the pediatric population, there was no association observed between the number of convincing FAs and perceived risk of accidental allergen exposure (FAIM item 1), as well as a somewhat attenuated association with the reported likelihood that a food-allergic reaction would be appropriately treated in the event of accidental allergen exposure (FAIM item 4).
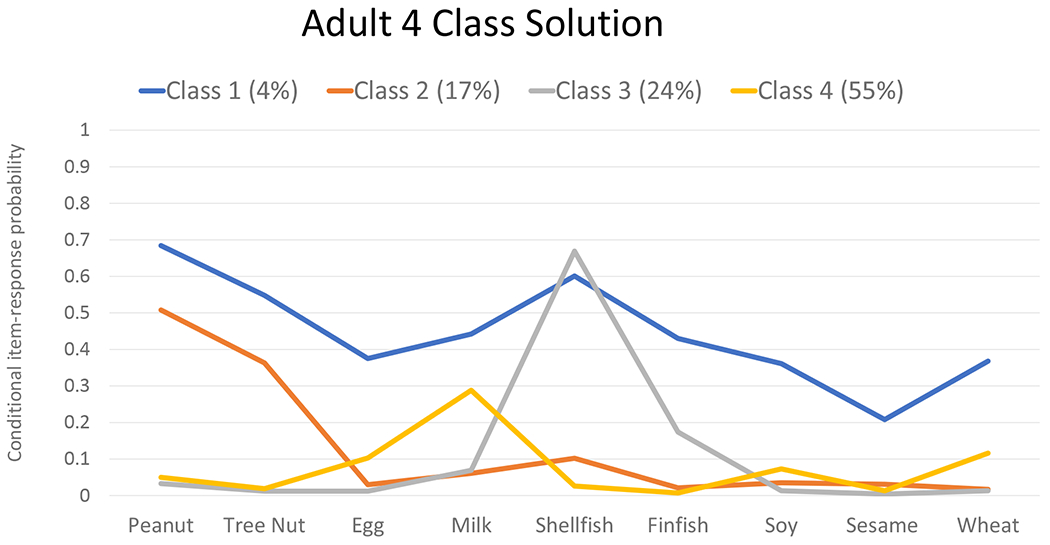
Latent Class Analysis of Multi-FA
For both the pediatric and adult subpopulations, a 4-class model provided the best fit to the data and showed similar patterns of specific co-allergen clustering within class members (Figure 2). Class 1 (i.e., the broadly multi-allergic class) represents a subgroup with elevated probabilities of each top 9 FA and comprised an estimated 5%/4% of US children/adults with FA. Class 2 (i.e., the peanut/treenut-dominant class) represents a subgroup with elevated probabilities of PA and TN allergies and comprised an estimated 28%/17% of US children/adults with FA. Class 3 (i.e., the seafood-dominant class) was characterized by elevated probabilities of shellfish and fin fish allergies and comprised an estimated 16%/24% of US children/adults with FA. Finally, class 4 (i.e., the milk/egg dominant class) was characterized by elevated probabilities of milk allergy, and to a lesser degree egg allergy. Class 4 comprised an estimated 51%/55% of US children/adults with FA.
Figure 2. Estimated Latent Classes of Multi-food allergy among children and adults with at least one convincingly IgE-mediated food allergy.

Line graphs of convincingly IgE-mfood allergen-specific probabilities within 4 latent classes of multi food allergy, stratified by pediatric (0-17 years) and adult (>17 years) respondents
Multinomial logistic regression analyses, conducted to assess multivariate predictors of latent class membership (eTable 1), estimated that children with physician-diagnosed asthma were more likely to belong to the broadly multi-FA- (Class 1) and peanut/treenut-dominant (Class 2) classes vs. the milk/egg dominant class (Class 4). Children with eczema were also more likely to belong to the peanut/treenut-dominant class (Class 2) compared to the milk/egg-dominant class (Class 4). Children with at least one biological parent who had eczema and/or FA were more likely to fall into the broadly multi-FA class (Class 1). In contrast, among adult FA patients, asthma and eczema status were not associated with class membership, but allergic rhinitis was associated with greater probability of membership within the nut-dominant class relative to the milk/egg-dominant class. Non-Hispanic Black, Non-Hispanic Asian, and Hispanic children were each significantly more likely to be in the seafood-dominant class (Class 3) compared to the milk/egg-dominant class, while non-Hispanic Black and Hispanic adults were more likely to be in the broadly multi-FA class compared to the milk/egg-dominant class. Finally, children and adults who were born in the US were generally less likely to be in the broadly multi-FA class than their non-native US born counterparts.
When mean scores were estimated for each of the six FAIM indicators across the four latent classes, among US children and adults a clear pattern of greatest reported FA-related psychosocial burden was observed for members of the broadly multi-FA class, with individuals in the peanut/tree-nut dominant class reporting the second greatest burden (Figure 3). With respect to class 3 (seafood-dominant)) and class 4 (milk/egg dominant), children comprising class 3 reported the lowest scores on item 1 (which assesses perceived risk of accidental exposure) and comparable scores to those in class 4 on items 5 and 6 (which assess FA-related dietary and social limitations). In contrast, adults in class 3 reported the lowest scores on items 1, 5 and 6.
Figure 3. Differences in Pediatric and Adult FAIM Scores by latent multi-food allergy class.
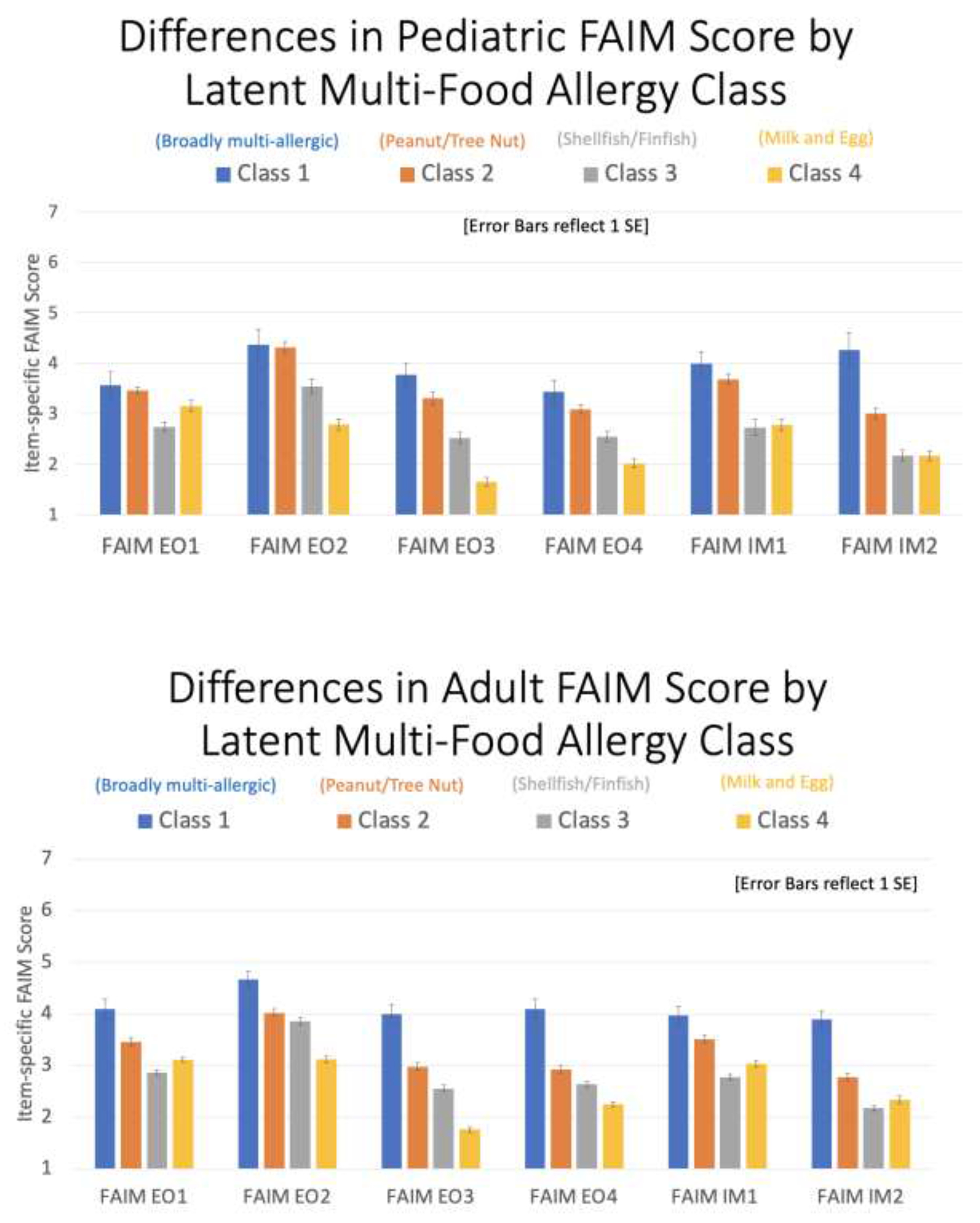
Bar graphs demonstrating mean item-specific FAIM scores estimated for each of the 4 estimated latent multi-food allergy classes stratified by pediatric (0-17 years) and adult (>17 years) respondents
Discussion
These US population-based survey data indicate that nearly half of food-allergic adults are currently allergic to multiple foods. Based on the specific case definition used, between 42%-48% of US adults with FA report allergies to at least 2 foods with a substantial proportion allergic to more than 3 foods. Our analysis identified a positive correlation between a greater number of FAs and a higher multi-faceted burden of FA including ED utilization and history of severe reactions, in line with our experience in clinical practice. The present study also demonstrated that patients with a greater number of FAs are more likely to report atopic comorbidities such as asthma, atopic dermatitis and environmental allergies as well as medication and latex allergy and other gastrointestinal comorbidities including eosinophilic esophagitis and food protein induced enterocolitis syndrome (FPIES). This study also expands upon previous clinical data18 indicating that there are specific patterns of multi-FA that frequently co-occur and suggests that these multi-FA phenotypes are relatively stable across US pediatric and adult populations.
Improving understanding of the frequency and distribution of multi-FA in the US population can help identify patients who may be at elevated risk of more severe FA outcomes, both in terms of greater risk of anaphylaxis and ED utilization, as well as greater psychosocial burden. Understanding the phenotypes of multi-FA and common comorbid FAs in pediatric and adult populations can help inform diagnostic testing in clinical practice and future treatment strategies. These data, which were gleaned from a nationally representative sample of nearly 80,000 US children and adults, and include data from over 14,000 individuals with at least one current reported FA, also clearly identified a monotonic relationship between an increased number of current FAs and increased physical burden of disease, as assessed by patient-reported food-allergic reaction symptomology, physician-diagnosed comorbidities and reported clinical outcomes.
While there are numerous food allergen immunotherapies under clinical investigation,28 the only FDA-approved treatment at present is a PA oral immunotherapy, offered as monotherapy. However, according to the present data, only 6.6% of the US population with ≥1 convincingly IgE-mediated FA and 12.8% of the US population with ≥1 physician-diagnosed convincingly IgE-mediated FA have a single PA. This leaves the vast majority of FA patients with multi-FA lacking FDA-approved treatments. Most food allergen immunotherapeutics currently undergoing phase 2 and 3 trials currently target only a single FA; however, there have been recent efforts to treat multiple FAs simultaneously. For example, a small study by Andorf et al. showed the efficacy of omalizumab, anti-IgE, in combination with multi-FA oral immunotherapy (OIT).18 This pilot study examined a pediatric population (N=48) and showed that the group receiving omalizumab in conjunction with multi-FA OIT displayed higher rates of passing double blind placebo controlled food challenges, compared to placebo groups. Other recent data29–31 indicate that simultaneous OIT to multi-FA is being conducted successfully in private allergy practices, while another study found that study participants allergic to foods with a similar protein structure can gain protection against cross-reactive allergens (e.g., pecan/walnut; cashew/pistachio) after OIT to a single FA.32
The present analysis of data collected from pediatric and adult FA patients clearly estimated 4 major multi-FA phenotypic clusters including milk/egg dominant, seafood dominant, peanut/tree nut dominant, and a broadly multi-food-allergic group. Antigenic similarity may not fully explain the observed clustering; therefore, other clinical features and comorbidities may play important roles in further understanding these observed patterns. The present study demonstrated that atopic dermatitis may have the potential to predict whether a child will develop peanut/tree nut allergies, consistent with previously published research;33 however, it did not reliably predict class membership in the milk/egg dominant group. Interestingly, among adults with multi-FA, allergic rhinitis rather than eczema was associated with higher probability of being in the peanut/tree nut dominant group. It is also notable that individuals reporting Black race were not only more likely to have multiple food allergies in unadjusted analyses than their White counterparts, but Black race was a strong predictor of membership in the broadly multi-food-allergic cluster. This is consistent with previous work highlighting a disparate burden of food allergy within Black households.1
Regardless of the predictors of the phenotypes, it is interesting to note that the same phenotypes were identified in both pediatric and adult subpopulations. Identification of four major latent classes of multi-FA in this large, nationally representative cohort has the potential to inform ongoing efforts to better characterize FA phenotypes and endotypes.34 With the goal to more efficiently and effectively target identification and care for individuals with multi-FAs, the present data may help researchers and pharmaceutical companies develop optimal treatment strategies for patients with multi-FAs. Similarly, the fact that these phenotypes are associated with different levels of psychosocial burden at the population-level can inform patient stratification for targeted interventions that aim to improve FA management and psychosocial outcomes among the most burdened patient populations.
These data also indicate multiple other avenues through which clinical management of food allergy may be improved. For instance, even among individuals meeting study criteria for multiple convincing FAs, remarkably few respondents reported having a current auto-injector prescription—particularly among adult patients. Epinephrine is the only first-line treatment for anaphylaxis and ensuring ready access to EAIs is a cornerstone of food allergy management.35 The present findings of greater psychosocial burden among individuals reporting a greater number of food allergies also highlights the importance of working to ensure patients receive accurate food allergy diagnosis—utilizing oral food challenges (particularly among adults) and working to differentially diagnose IgE-mediated allergy so that patients are not unnecessarily avoiding foods to which they are not allergic.
Our study demonstrates a positive association between multi-FA and the risk of having physician-diagnosed atopic comorbidities. To date, a growing body of evidence supports the existence of an “atopic march”;36 however, whether the course of the atopic march progresses similarly in patients with single vs multiple FAs or in different phenotypes of multi-FA reported in this study has not been investigated. It is important to note that the onset of patient reported physician-diagnosed atopic comorbidities was not assessed in this study, thus longitudinal studies are needed to investigate any correlation or temporal relationship of these atopic comorbidities with the development of specific phenotypes of multi-FA.
Remarkably, we found that a greater number of current FAs was associated with an increased risk of reporting ever receiving a physician-diagnosis of allergy to substances other than food such as latex, Hymenoptera, and/or prescription medications. To determine whether this correlation truly exists or is incidental is outside the scope of the present study given the fact that these physician-diagnosed allergies were only reported by the participants and not confirmed by retrospective chart review or further clinical evaluation due to the survey design of this research study. In any event, given that non-food allergies (e.g. latex, drug, sting/venom allergy) were assessed using the following question stem: “Has your child ever been diagnosed by a doctor with…” the reported estimates likely include substantial numbers of patients whose non-food allergies had resolved by the time of survey assessment, were misdiagnosed, or misinterpreted by the patient in the first place. This is a notable limitation and may indicate over-perception of allergy in these reportedly multi-allergic individuals.
Other noteworthy limitations of this study include the inability to clinically confirm reported allergy cases, which is likely to have led to over-estimation of prevalence of IgE-mediated allergies including those to food, drugs, latex, insect stings and venom.
Conclusion
These data suggest that there are four major phenotypes of multi-FA in both pediatric and adult populations and the population-level burden of multi-FA is high among both children and adults. Given the prevalence in the US, understanding the phenotypes of multi-FA can help inform future research and targeted therapies as well as strategies to minimize the physical and psychosocial burden of this disease.
Supplementary Material
Funding Source:
This study was supported by the grant R21AI135702 from the National Institute of Allergy and Infectious Disease (NIAID) (PI Gupta) and supported in part by the Intramural Research Program of the NIH, NIAID.
Conflict of Interest:
Dr. Warren reports research support from the National Institutes of Health, Food Allergy Research & Education (FARE), and the Sunshine Charitable Foundation. He is currently employed by Northwestern University and is an Assistant Professor of Preventive Medicine at Northwestern University Feinberg School of Medicine.
Dr. Aktas was employed by Northwestern University at the time of this work, and she is currently employed by the National Institute of Allergy and Infectious Disease/NIH.
Dr. Gupta receives research support from the National Institutes of Health (NIH) (R21 ID # AI135705, R01 ID # AI130348, U01 ID # AI138907), Food Allergy Research & Education (FARE), Melchiorre Family Foundation, Sunshine Charitable Foundation, The Walder Foundation, UnitedHealth Group, Thermo Fisher Scientific, and Genentech. She serves as a medical consultant/advisor for Genentech, Novartis, Aimmune LLC, Allergenis LLC, and Food Allergy Research & Education (FARE). Dr. Gupta has ownership interest in Yobee Care, Inc. She is currently employed by Ann & Robert H. Lurie Children’s Hospital of Chicago and is a Professor of Pediatrics & Medicine at Northwestern University Feinberg School of Medicine.
The other authors have no potential conflicts to disclose.
Abbreviations/Acronyms:
- aLRT
adjusted likelihood ratio test
- BIC
Bayesian information criteria
- CI
confidence interval
- CFI
Confirmatory Fit Index
- ED
emergency department
- EAI
epinephrine auto injector
- FA
food allergy
- FAIM
food allergy independent measure
- FDA
US Food and Drug Administration
- FPIES
food protein-inducted enterocolitis syndrome
- IgE
immunoglobulin E
- LCA
latent class analysis
- OIT
oral immunotherapy
- PA
peanut allergy
- QoL
quality of life
- RMSEA
Root Mean Square Error of Approximation
- TN
tree nut
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Warren CM, Jiang J, Gupta RS. Epidemiology and burden of food allergy. Curr Allergy Asthma Rep. 2020. Feb;20(2): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grabenhenrich L, Trendelenburg V, Bellach J, Yürek S, Reich A, Fiandor A, et al. Frequency of food allergy in school-aged children in eight European countries—The EuroPrevall-iFAAM birth cohort. Allergy. 2020. Sep;75(9):2294–308. [DOI] [PubMed] [Google Scholar]
- 3.Lyons SA, Clausen M, Knulst AC, Ballmer-Weber BK, Fernandez-Rivas M, Barreales L, et al. Prevalence of food sensitization and food allergy in children across Europe. The Journal of Allergy and Clinical Immunology: In Practice. 2020. Sep 1;8(8):2736–46. [DOI] [PubMed] [Google Scholar]
- 4.Warren CM, Otto AK, Walkner MM, Gupta RS. Quality of life among food allergic patients and their caregivers. Curr Allergy Asthma Rep. 2016. May; 16(5): 1–8. [DOI] [PubMed] [Google Scholar]
- 5.Bilaver LA, Chadha AS, Doshi P, O’Dwyer L, Gupta RS. Economic burden of food allergy: a systematic review. Ann Allergy Asthma Immunol. 2019. Apr 1;122(4):373–80. [DOI] [PubMed] [Google Scholar]
- 6.Feng C, Kim JH. Beyond avoidance: the psychosocial impact of food allergies. Clin Rev Allergy Immunol. 2019. Aug;57(1):74–82. [DOI] [PubMed] [Google Scholar]
- 7.Warren C, Dyer A, Lombard L, Dunn-Galvin A, Gupta R. The Psychosocial Burden of Food Allergy Among Adults: A US Population-Based Study. J Allergy Clin Immunol Pract. 2021. Jun 1;9(6):2452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DunnGalvin A, De BlokFlokstra BM, Burks AW, Dubois AE, Hourihane JO. Food allergy QoF questionnaire for children aged 0–12 years: content, construct, and cross-cultural validity. Clin Exp Allergy. 2008. Jun;38(6):977–86. [DOI] [PubMed] [Google Scholar]
- 9.Dunn Galvin A, Hourihane JB. Health-related quality of life in food allergy. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz. 2016. Jul; 59(7): 841–8. [DOI] [PubMed] [Google Scholar]
- 10.Wassenberg J, Cochard MM, DunnGalvin A, Ballabeni P, Flokstra-de Blok BM, Newman CJ, et al. Parent perceived quality of life is age-dependent in children with food allergy. Pediatr Allergy Immunol. 2012. Aug;23(5):412–9. [DOI] [PubMed] [Google Scholar]
- 11.Cohen BL, Noone S, Muñoz-Furlong A, Sicherer SH. Development of a questionnaire to measure quality of life in families with a child with food allergy. J Allergy Clin Immunol. 2004. Nov 1;114(5): 1159–63. [DOI] [PubMed] [Google Scholar]
- 12.Sicherer SH, Noone SA, Munoz-Furlong A. The impact of childhood food allergy on quality of life. Ann Allergy Asthma Immunol. 2001. Dec 1;87(6):461–4. [DOI] [PubMed] [Google Scholar]
- 13.Park JH, Ahn SS, Sicherer SH. Prevalence of allergy to multiple versus single foods in a pediatric food allergy referral practice. J Allergy Clin Immunol. 2010. Feb 1;125(2):AB216. [Google Scholar]
- 14.Gupta RS, Warren CM, Smith BM, Blumenstock JA, Jiang J, Davis MM, et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. 2018. Dec 1;142(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yavuz ST, Sahiner UM, Buyuktiryaki B, Soyer OU, Tuncer A, Sekerel BE, et al. Phenotypes of IgE-mediated food allergy in Turkish children. Allergy Asthma Pract. 2011. Nov 1 (Vol. 32, No. 6, p. 47). OceanSide Publications. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman P, Nicklas RA, Randolph C, Oppenheimer J, Bernstein D, Bernstein J, et al. Anaphylaxis—a practice parameter update 2015. Ann Allergy Asthma Immunol. 2015. Nov 1;115(5):341–84. [DOI] [PubMed] [Google Scholar]
- 17.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011. Jul;128(1):e. [DOI] [PubMed] [Google Scholar]
- 18.Andorf S, Borres MP, Block W, Tupa D, Bollyky JB, Sampath V, et al. Association of clinical reactivity with sensitization to allergen components in multifood-allergic children. J Allergy Clin Immunol Pract. 2017. Sep 1;5(5): 1325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang HT, Warren CM, Gupta RS, Davis CM. Prevalence and characteristics of shellfish allergy in the pediatric population of the United States. J Allergy Clin Immunol Pract. 2020. Apr 1;8(4):1359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta RS, Warren CM, Smith BM, Jiang J, Blumenstock JA, Davis MM, et al. Prevalence and severity of food allergies among US adults. JAMA Netw Open. 2019. Jan 4;2(1):e185630- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren CM, Chadha AS, Sicherer SH, Jiang J, Gupta RS. Prevalence and severity of sesame allergy in the United States. JAMA Netw Open. 2019. Aug 2;2(8):e199144-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warren C, Lei D, Sicherer S, Schleimer R, Gupta R. Prevalence and characteristics of peanut allergy in US adults. J Allergy Clin Immunol. 2021. Jun 1;147(6):2263–70. [DOI] [PubMed] [Google Scholar]
- 23.Samady W, Warren C, Wang J, Das R, Gupta RS. Egg allergy in US children. J Allergy Clin Immunol Pract. 2020. Oct 1;8(9):3066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warren CM, Aktas ON, Gupta RS, Davis CM. Prevalence and characteristics of adult shellfish allergy in the United States. J Allergy Clinical Immunol. 2019. Nov 1;144(5):1435–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flokstra-de Blok BM, van der Meulen GN, DunnGalvin A, Vlieg-Boerstra BJ, Oude Elberink JN, Duiverman EJ, et al. Development and validation of the Food Allergy Quality of Life Questionnaire - Adult Form. Allergy. 2009. Aug;64(8): 1209–17. [DOI] [PubMed] [Google Scholar]
- 26.Kline RB. Structural equation modeling. New York: Guilford. 1998. [Google Scholar]
- 27.US Census Bureau’s Current Population Survey. Available from: https://www.census.gov/programs-survevs/cps.html
- 28.Macdougall JD, Burks AW, Kim EH. Current insights into immunotherapy approaches for food allergy. ImmunoTargets Ther. 2021; 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wasserman R, Windom H. Exploiting Nut Cross Reactivity to Facilitate Real World Treatment of Tree Nut Allergy. Ann Allergy Asthma Immunol. 2021; In press [DOI] [PubMed] [Google Scholar]
- 30.Eapen AA, Lavery WJ, Siddiqui JS, Lierl MB. Oral immunotherapy for multiple foods in a pediatric allergy clinic setting. Ann Allergy Asthma Immunol. 2019. Dec;123(6):573–581.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fergeson J. Multi-Food OIT. Food Allergy Support Team 2021 Annual Meeting. 2021. Available from: https://fastoit.org/wp-content/uploads/2021/07/multi-food-oit_fergeson.pdf
- 32.Graham F, Eigenmann PA. Atopic dermatitis and its relation to food allergy. Curr Opin Allergy Clin Immunol. 2020. Jun 1;20(3):305–10. [DOI] [PubMed] [Google Scholar]
- 33.Brough HA, Fiu AH, Sicherer S, Makinson K, Douiri A, Brown SJ, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol. 2015. Jan 1;135(1):164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker MG, Sampson HA. Phenotypes and endotypes of food allergy: A path to better understanding the pathogenesis and prognosis of food allergy. Ann Allergy Asthma Immunol. 2018. Mar 1;120(3):245–53. [DOI] [PubMed] [Google Scholar]
- 35.Shaker MS, Wallace DV, Golden DB, Oppenheimer J, Bernstein JA, Campbell RE, et al. Anaphylaxis—a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. Journal of Allergy and Clinical Immunology. 2020. Apr 1;145(4): 1082–123. [DOI] [PubMed] [Google Scholar]
- 36.Zheng T, Yu J, Oh MH, Zhu Z. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol’Res. 2011. Apr 1;3(2):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


