Abstract
Postoperative chyle leak is a rare but serious complication of head and neck surgery. Chyle leak can lead to a systemic metabolic imbalance, a prolonged wound healing and longer hospital stay. Early identification and treatment are crucial for good surgical outcome. The diagnosis can be made intraoperatively or in the early postoperative period. Various treatment options described in the literature can be divided into conservative and surgical modalities. Currently, there is no evidence that any approach is superior to the other due to relatively small number of studies describing chyle leak management. There are no official guidelines for the treatment of postoperative chyle leak. The aim of this article is to present the therapeutic possibilities and to offer an algorithm for chyle leak management.
Key words: Chyle, Neck dissection, Thoracic duct, Algorithm
Introduction
A lymphatic leakage is a loss of lymph fluid from a damaged lymphatic vessel. In head and neck surgery, there is a difference between right and left postoperative lymphatic leakage. The distinction is based on differences in anatomy and physiology and reflected in terminology. In the strictest sense lymphatic leakage is a consequence of right duct injury. On the other hand, the injury of thoracic duct on the left side of neck and leakage of its content is defined as chyle leak. The rate of iatrogenic lymphatic injuries is 2-8% after neck dissection and 0.5-1.4% after thyroidectomy (1). Thoracic duct injuries are more common and present about 75-92% of neck lymphatic injuries (1, 2). Chyle leak is a more serious complication compared to lymphatic leakage. Chyle leak can lead to a systemic metabolic imbalance, a prolonged wound healing and longer hospital stay (3).
The diagnosis can be made intraoperatively (visualization of aqueous or milky fluid in operative field) or in the early postoperative period (increased accumulation of lymph in the drainage).
Chyle leak is a rare but dangerous complication, and various treatment options described in the literature can be divided into conservative and surgical modalities (4). Currently, there is no evidence that any approach is superior to the other due to relatively small number of studies describing chyle leak management (5).
ANATOMY OF THE LYMPH SYSTEM
The lymphatic system consists of lymphatic capillaries, lymphatic vessels, lymph nodes, lymphatic ducts, tonsils, spleen, thymus, and other tissues in the body. It runs parallel to the blood vessels and permits lymph to circulate around the body.
The thoracic duct is the main and largest lymph duct that drains the lymph from the legs, pelvic organs, abdominal wall, abdominal organs, lymph of the left half of the chest, left lung, left arm, left thoracic wall, breast and left half of the head and neck.
It starts from the upper end of the cisterna chyli and ends in the left venous angle (the junction of the left subclavian vein and the left internal jugular vein). Topographically, its course is divided into the abdominal, thoracic, and cervical part. The abdominal part is in retroperitoneal space. The thoracic part is located in the posterior mediastinum. Proximally, thoracic duct rotates to the left and then comes to the left of the oesophagus where it is in close proximity to the left laryngeal nerve, then crosses the aortic arch, ascends behind the left subclavian vein, passes through the superior thoracic aperture and reaches the lower neck (5, 6). In the cervical region, the thoracic duct at level C7 rotates laterally and forward and forms the thoracic duct arc, located in the triangle of the vertebral artery. This deep neck triangle is bounded by the subclavian artery, anterior scalene muscle and longus colli muscle. In this area the duct crosses from medial to lateral, behind the left common carotid artery, left vagal nerve, and left internal jugular vein, and finally flows into the venous angle (2, 5, 6). At this point it can have multiple terminations in venous system (1).
The right lymphatic duct is shorter in its length and carries lymph only from the right side of the head and neck, right arm, right side of the chest and breast, right lung, right half of the mediastinum, and flows into the right venous angle (6).
PHYSIOLOGY AND FUNCTION OF THE LYMPHATIC SYSTEM
The main functions of the lymphatic system are homeostasis of body fluids, fatty acid absorption, and immune system modulation.
The excess amounts of interstitial fluid and proteins from the tissues that cannot be returned through the blood vessels are returned by the lymphatic system. The lymphatic system is a low-pressure system without a central pump, so lymph flows more slowly than blood. The lymph flow is enhanced by pumping, which can be result of extrinsic or adjacent tissue movement, or intrinsic contractions of specialized muscle cells in lymphatic vessel walls (7).
The lymphatic system is important in the intestinal regulation and function. It helps with lipid transport, fights against infections, and removes excess fluid. The lacteals are lymph capillaries in the small intestine villi responsible for absorption of the fats and fat-soluble vitamins. Lacteals produce chyle, a lipid-rich, milky white fluid containing chylomicrons (lipoproteins responsible for transport of fats in aqueous medium). The intestinal chyle drains exclusively to the cisterna chyli. Chyle consists of blood plasma-like fluid, protein (2-4.5%), white blood cells, electrolytes, fat-soluble vitamins, trace elements, glucose, and large amounts of chylomicrons (8). Its electrolyte composition is similar to plasma. The main lipid component is triglycerides. Chyle flow rates are dependent on the diet, intestinal function, peristalsis, physical activity, respiratory movements, coughing, pulsation of adjacent arteries and changes in intra-abdominal and intrathoracic pressure (1, 9).
There is a huge content difference between chyle from the thoracic duct and lymph from the right lymphatic duct. Lymph production rate is estimated at 2mL/kg/min approximately resulting in 2-4 litres daily.
PATHOPHYSIOLOGY OF CHYLE LEAK
When the lymphatic system is disrupted, proteins accumulate in the intercellular space and cause oedema.
Chyle leak and the consequent loss of its components can lead to the potentially life-threatening malnutrition, electrolyte and fluid imbalances and other systemic complications. The loss of large volume of protein, fat and electrolyte-rich fluid results in primary hypoproteinemia, hyponatremia, hypokalemia and hypocalcemia. Hypoproteinemia may complicate primary hypovolemia from fluid loss due to fluid shifts in third space. In addition, chyle leak can result in loss of leukocytes leading to immunosuppression that increases risk of delayed wound healing, wound infection or breakdown, fistula formation and sepsis (10).
Chyle fistulas are space-filling that exert pressure on surrounding tissues. Local pressure effect of chyle beneath skin flaps can interrupt tissue perfusion causing poor wound healing and promoting flap necrosis (10). The consequences can range from negligible to life-threatening conditions like chylothorax or chylomediastinum (11).
All this can lead to an extended hospital stay, higher treatment costs and a less favourable surgical outcome. In extreme cases, it can be lethal (12). According to a study by Dongbin et al. chyle leak complications occur in 40% of cases, mainly in the form of electrolyte and protein imbalance, primarily hyponatremia and hypoalbuminemia (13).
DIAGNOSIS OF CHYLE LEAK
Iatrogenic chyle leak can be observed intraoperatively or in the early postoperative period.
INTRAOPERATIVE RECOGNITION OF CHYLE LEAK
If the leak is identified at the time of surgery, all actions must be taken to stop it immediately. A special attention should be paid to careful examination of venous angle region at the end of a head and neck procedure, particularly if it involves dissection of level IV neck nodes. If secretion of lymph is observed during surgery, a source must be found (10). Use of surgical loupes or operative microscope can visualization which may be difficult due to variable course and collapsibility of the thoracic duct (10). Placement of the patient in the Trendelenburg position and increase of intrathoracic pressure can help the surgeon to find the source of the leak (10). As described by Cernea et al. an increase of intraabdominal pressure can also help, it is achieved by compressing the abdomen with the upper body of the assistant (9).
Intraoperative damage to the lymphatic duct may go unnoticed due to empty lymphatic system as result of routine preoperative fasting.
POSTOPERATIVE IDENTIFICATION OF CHYLE LEAK
In the postoperative course chyle leak is suspected when unexpectedly high accumulation of the white fluid in the drainage is found or when there is a sudden increase of drainage output after feeding. Lymphedema or erythema can be seen in the neck, and the liquid collection can sometimes be palpated in supraclavicular region (14). Generally, this is enough to make a diagnosis, but in case of any doubt biochemical assay may be helpful for confirmation.
A triglyceride concentration of >100 mg/dL or a triglyceride level greater in drainage fluid than that of serum would support the diagnosis of chyle leak in uncertain cases (15). Presence of chylomicrons in lipid analysis of the neck drainage may indicate chyle leak, but may also be present due to breakdown of adipose tissue in the wound.
Cholesterol levels also can put suspicion on chyle leak, but triglyceride levels are more specific for chyle (16). Lee et al. have found in their study that the portable SD LipidoCare test system (SD Biosensor, Suwon, South Korea) can be used for quick and accurate screening for chyle leak. The SD LipidoCare test system is a portable instrument that can measure various lipid profiles (total cholesterol, triglycerides, high-density lipoprotein, and low-density lipoprotein), all within 3 minutes by dropping aspirated fluid from neck drainage with a mini pipette into the system (12).
REVIEW OF THE LITERATURE
There are numerous proposed conservative and surgical methods to stop chyle leak (Table 1.).
Table 1. Conservative and surgical methods to stop chyle leak.
| CONSERVATIVE MANAGEMENT | SURGERY |
|---|---|
| first line low-fat diet (nutrients rich in medium-chain triglycerides) total parenteral nutrition via central venous line daily correction of electrolytes, vitamins and oligo-elements |
first line – neck exploration ligation: surgical suture, haemoclips local/regional myofascial flap local sclerosing agents: tetracycline or OK-432 local application: fibrin glue, vicryl nets |
| second line somatostatin/octreotide pancreatic lipase inhibitor (orlistat) |
second line transthoracic approach (thoracoscopic or open thoracotomy): thoracic duct ligation transabdominal approach (percutaneous thoracic duct embolization - TDE) |
CONSERVATIVE APPROACH
Almost all authors recommend conservative approach as first line of treatment regardless of leakage amount (1-4).
Change of nutrition to low-fat diet rich in medium-chain triglycerides is one of measures (17, 18). Medium-chain triglycerides are mostly water-soluble and enter the circulation through the hepatic system, thus avoiding the flow through the gastrointestinal lymphatic system. Through the central venous pathway, it is necessary to continuously replace electrolytes, vitamins, and oligoelements.
Total parenteral nutrition which bypasses the gastrointestinal lymphatic system and provides all necessary nutrients to the patient is another option (19, 20). Some authors describe it as first choice treatment, while the others recommend it when low-fat diet does not give results or in patients with higher lymph outflow.
Different diet changes can resolve more than 70% of chyle leak in period of 2 months and there is no statistical difference in success among them (19).
Further conservative methods include octreotide, a long-acting analogue of the hormone somatostatin, and/or pancreatic lipase inhibitor orlistat/tetrahydrolipstatin.
Somatostatin reduces the production of lymphatic contents by inhibiting the production of pancreatic and gastrointestinal enzymes (21). It also reduces lymph production and decreases its flow by contracting the smooth muscles of the digestive and lymphatic systems. Somatostatin has a very short half-life and requires continuous intravenous administration. Administration of long-acting analogue octreotide (100 µg/day for 2-3 days subcutaneously) can stop low and medium volume chyle leak within 5-7 days (22, 23). It is recommended to continue the use for 1-2 days after chyle leak resolution (1). Some authors describe good results even in high volume chyle leak after prolonged treatment up to 30 days (1), and some recommend it as first line treatment (21). Octreotide should be used carefully, especially in patients with previous cardiovascular and hepatic disorders. The most commonly described mild side effects are nausea and diarrhea, and possible severe complications are hypoglycemia and cholecystitis with gastrointestinal bleeding (24).
Orlistat/tetrahydrolipstatin inactivates pancreatic enzymes and prevents the resorption of fat cells in the intestine, and their entry into the enteral circulation (24).
Additional conservative measure is limiting physical activity in order to decrease pressure in the lymphatic system (25). Cough control with antitussives and stool regulation will help prevent high intrathoracic and abdominal pressure.
Extracorporeal compression of the neck in the projection of lymphatic leakage with the removal of the negative drainage system remains a clinically questionable approach (2).
SURGICAL APPROACH
Chyle leak can be recognized at time of initial surgery or as postoperative complication.
Intraoperative identification and ligation of the open duct is the most effective way to stop chyle leak. Delay of enteral feeding for patients with intraoperatively recognized chyle leak is recommended (3).
There is no consensus on revision surgery regarding indication defined by chyle volume loss or timing. Some authors recommend revision surgery when chyle output is higher than 500 mL/day for 4 days, while the others describe possibility of waiting up to 30 days with chyle output of more than 1000 mL/day (1-4). Chyle leak causes significant nutritional and immune compromise, so early surgical intervention is recommended when conservative therapy is likely to fail or take too long to resolve leakage (19).
Recommended suture materials for ligation of thoracic duct are absorbable polyfilament suture, non-absorbable monofilament sutures (1, 3, 4, 8) and titanium clips. After thoracic duct ligation, safety of ligation should be checked by raising the intraabdominal or intrathoracic pressure.
Covering of the repaired lesion with an additional muscle layer can be helpful. The sternocleidomastoid muscle flap is the most commonly used, the use of scalene or infrahyoid muscles is less common. Use of the pectoralis major flap is also an option. Numerous surgical procedures have been described in the literature aiming to secure the site of lymphatic leakage additionally.
The use of local sclerosing agents such as tetracycline or OK-432 (Picibanil, Chungai Pharmaceutical Co., Tokyo, Japan) can stop chyle leak by causing an additional local inflammatory response (26-28). Topical application or installation via a drainage system may be a possible route of administration (29, 30). However, the possible complication is neurotoxicity for nerves in proximity, phrenic and brachial plexus (31). If this therapeutic approach fails to stop chyle leak, partial sclerosis can further complicate new surgical exploration (32).
Positive experiences after the local application of fibrin glue or polygalaktin 910 (Vicryl [Ethicon US]) nets have been described in the literature (33).
If the first line of surgical intervention, neck revision surgery, does not give the desired clinical results, it is rational to ligate the duct by the transthoracic or transabdominal approach. Access is through the right chest, thoracoscopic or open thoracotomy, between the aorta and the azygos vein (34). The procedure is very effective, some authors propose it as first line surgical intervention in patients with more than 1000 mL/day leak (8). The patient’s general condition, compromised by prolonged chyle leak, can further be decreased with this procedure. Through percutaneous thoracic duct embolization (TDE), it is possible to access the chyle tank and close it with the help of sclerosing agents (Histoacryl with or without coils). Unfortunately, this procedure sometimes requires multiple revisions (35).
Discussion
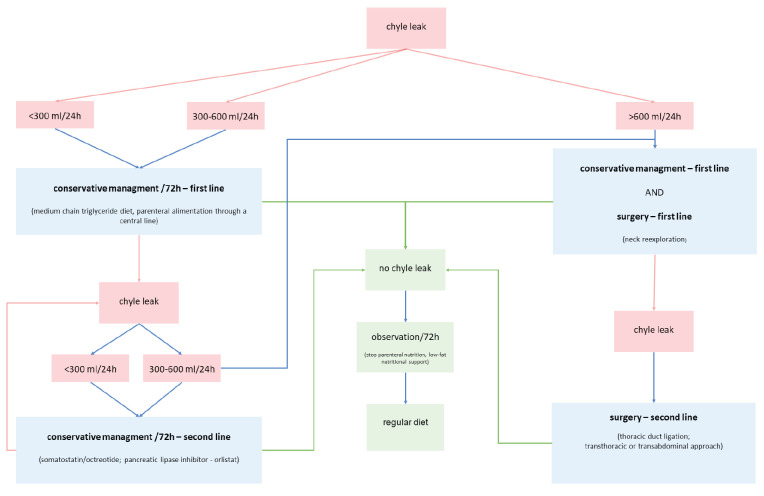
The knowledge of surgical anatomy is the best prevention of lymphatic duct injury (8). Possible anatomical variations of position and branching makes the vulnerable lymphatic duct a place of frequent unintended surgical lesion that results in uncontrolled lymphatic leakage (3). Thoracic duct lesions are more often in an advanced malignant metastatic disease that affects level IV of the left side of neck and spreads toward the upper mediastinum (4), but the fear of chyle leak should not limit proper oncological resection (1). Chyle leak is most often observed on the first postoperative day, a few hours after feeding the patient. It is manifested primarily by the white turbidity of the drainage content. On the right side of the neck, due to the different origin and chemical composition of the lymph, in case of increased drainage of serous or bloodstained content, lymphatic leakage is usually observed after a few days. Accumulation of lymph in surgically treated neck is manifested through a systemic metabolic disorder and loco-regional chemical irritation of musculoskeletal structures (2). Fluid loss and electrolyte imbalance deteriorate general patient condition. The patient’s immune system is disrupted, complicating wound healing process (7). When the chyle leak reaches volume of 1l/24h, then it becomes potentially life threatening condition. So far, there is no algorithm or guidelines that would unambiguously clinically determine the therapeutic approach in unintentional thoracic duct lesion. There are several proposed algorithms suggesting order of possible interventions but without specific time frame recommendations and some with same approach regardless of chyle leak volume (1, 7, 21). Figure 1. shows our recommendations for resolving chyle leak. Based on literature data we tried to create an algorithm that includes all effective methods in specific order according to chyle leak volume. In patients with low volume chyle loss effects on patients general condition is milder and probability of conservative treatment success is higher. In cases with high volume chyle loss patients general condition and local status deteriorates much faster and consequently there is no time to expect results of the above mentioned conservative measures, usually rendering a more proactive approach. It is necessary for the algorithm to include not only possible curative methods but also their time frame and distinction of patients based on chyle volume loss. A conservative or surgical approach (Table 1.) will depend on the general condition of the patient, local status and the volume of chyle leak. According to chyle volume loss per day, patients can be divided in three groups, low chyle leak (up to 300 ml/24 h), medium (from 300-600/24 h), and large (over 600 ml/24 h).
Figure 1.
Our recommendations for resolving chyle leak
First line treatment of patients with postoperative chyle leak is change in diet for patients with low leak. Complete cessation of feeding and total parenteral nutrition is the first choice for patients with medium leak (19). The first effects of diet intervention can be expected within 12 hours after the initiation, and the full therapeutic effect through the second and third day of treatment. With the cessation of chyle leak, after 72 hours, the total parenteral nutrition can be replaced with a low-fat diet for the next 72 hours. Adequate clinical finding on the neck allows the patient to return to normal nutrition. After 72 hours of treatment, if the chyle leak is not decreased or stopped, second line of conservative or surgical approach should be considered.
As a second-line conservative treatment octreotide and/or pancreatic lipase inhibitor orlistat/tetrahydrolipstatin can be used. The effect of somatostatin/octreotide treatment can be expected within 24 hours. Continuation of chyle leak after 72 hours of the second-line treatment cannot be treated with conservative methods only.
Extracorporeal compression of the neck in the projection of lymphatic leakage with the removal of the negative drainage system is not recommended, especially in the case of microvascular anastomosis present on that side of the neck. The entire lymphatic system is characterized by low pressure flow. Although negative drainage pressure enforces the outflow of lymph, removal of drainage system increases the possibility of lymph accumulation in the neck. Extracorporeal compression, on the other hand, can contribute to lymph flowing into the thorax instead of the neck, with the consequent formation of chylothorax.
Intraoperative identification and ligation of the open duct is the most effective way to stop chyle leak. Visualization of the leakage site and surgical ligation can be very challenging. The thin and vulnerable duct wall, numerous possible anatomical variations, oncological status of the neck, radiation and chemotherapy performed, and possible previous surgeries and reoperations increase the risk of and complicate the repair of an evident chyle leak. Regional inflammation caused by the lymphatic leakage further complicates the identification of anatomical structures.
The preferred ligation material is a pliable polyfilament atraumatic resorptive suture with serous needle. The use of non-resorptive monofilament sutures is not excluded, but it may lead to additional rupture of the duct wall. The use of titanium clips is also an effective way to close the duct. However, if the chyle leak is located at the junction of the neck and the mediastinum, the clips may be potentially dangerous. Moving of the visceral pleura due to an irritating cough can move the metal clip and cause a new lesion of the lymphatic duct system. After thoracic duct ligation, safety of ligation should be checked by raising the intraabdominal or intrathoracic pressure.
The described algorithm has not been tested in a clinical trial but corresponds to the authors long-term surgical experience. Some methods are proposed in the literature review but are not recommended in the algorithm due to low efficacy and undesirable side effects. Methods recommended in algorithm (except transabdominal approach) have all been used in our patients. In our experience the efficacy of the method is observed within two days and is dependent on chyle leak volume. Advantage of our recommendation is that it is easy to remember as rule of number 3 (300, 600 mL/day, each conservative therapy for 3 days). Due to low number of cases, prospective multi-institutional research should be initiated to objectively test our proposed time and volume boundaries.
Conclusion
Identification of lymphatic duct and immediate surgical care of the open lymphatic structures is the best way to avoid postoperative lymphatic leakage. Since there are no official guidelines for the treatment, it is necessary to propose an algorithm.
References
- 1.Delaney SW, Shi H, Shokrani A, Sinha UK. Management of chyle leak after head and neck surgery: Review of Current Treatment Strategies. Int J Otolaryngol. 2017;2017:8362874. 10.1155/2017/8362874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Gier HH, Balm AJ, Bruning PF, Gregor RT, Hilgers FJ. Systematic approach to the treatment of chylous leakage after neck dissection. Head Neck. 1996. July-August;18(4):347–51. [DOI] [PubMed] [Google Scholar]
- 3.Lucente FE, Diktaban T, Lawson W, Biller HF. Chyle fistula management. Otolaryngol Head Neck Surg. 1981;89(4):575–8. 10.1177/019459988108900413 [DOI] [PubMed] [Google Scholar]
- 4.Crumley RL, Smith JD. Postoperative chylous fistula prevention and management. Laryngoscope. 1976. June;86(6):804–13. 10.1288/00005537-197606000-00008 [DOI] [PubMed] [Google Scholar]
- 5.Jalšovec D. Sustavna i topografska anatomija čovjeka. Zagreb, Hrvatska: Školska knjiga; 2004. 852 p. [Google Scholar]
- 6.Srikumar S, Newton JR, Westin TA. Bilateral chylothorax following left-sided radical neck dissection. J Laryngol Otol. 2006. August;120(8):705–7. 10.1017/S0022215106001344 [DOI] [PubMed] [Google Scholar]
- 7.Ilczyszyn A, Ridha H, Durrani AJ. Management of chyle leak post neck dissection: a case report and literature review. J Plast Reconstr Aesthet Surg. 2011. September;64(9):e223–30. 10.1016/j.bjps.2010.12.018 [DOI] [PubMed] [Google Scholar]
- 8.Ahn D, Sohn JH, Jeong JY. Chyle Fistula After Neck Dissection: An 8-Year, Single-Center, Prospective Study of Incidence, Clinical Features, and Treatment. Ann Surg Oncol. 2015. December;22 Suppl 3:S1000–6. 10.1245/s10434-015-4822-7 [DOI] [PubMed] [Google Scholar]
- 9.Cernea CR, Hojaij FC, De Carlucci D, Jr, Tavares MR, Araújo-Filho VJ, Silva-Filho GB, et al. Abdominal compression: a new intraoperative maneuver to detect chyle fistulas during left neck dissections that include level IV. Head Neck. 2012. November;34(11):1570–3. 10.1002/hed.21956 [DOI] [PubMed] [Google Scholar]
- 10.Rodgers GK, Johnson JT, Petruzzelli GJ, Warty VS, Wagner RL. Lipid and volume analysis of neck drainage in patients undergoing neck dissection. Am J Otolaryngol. 1992;13(5):306–9. 10.1016/0196-0709(92)90053-V [DOI] [PubMed] [Google Scholar]
- 11.Erisen L, Coskun H, Basut O. Objective and early diagnosis of chylous fistula in the postoperative period. Otolaryngol Head Neck Surg. 2002. February;126(2):172–5. 10.1067/mhn.2002.121859 [DOI] [PubMed] [Google Scholar]
- 12.Lee DH, Kim HK, Lee JK, Lim SC. Early diagnosis of chyle fistula with SD LipidoCare after neck dissection. J Laryngol Otol. 2021. April;135(4):355–8. 10.1017/S0022215121000888 [DOI] [PubMed] [Google Scholar]
- 13.Andreou Z, Trinidade A, Shakeel M, Argiris K, Panesar J, Kothari P. Severe hypo-osmotic hyponatraemia due to chylous leak following radical neck dissection. J Coll Physicians Surg Pak. 2013. March;23(3):221–3. [PubMed] [Google Scholar]
- 14.Lv S, Wang Q, Zhao W, Han L, Wang Q, Batchu N, et al. A review of the postoperative lymphatic leakage. Oncotarget. 2017. April 20;8(40):69062–75. 10.18632/oncotarget.17297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhardwaj R, Vaziri H, Gautam A, Ballesteros E, Karimeddini D, Wu GY. Chylous Ascites: A Review of Pathogenesis, Diagnosis and Treatment. J Clin Transl Hepatol. 2018. March 28;6(1):105–13. 10.14218/JCTH.2017.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGrath EE, Blades Z, Anderson PB. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med. 2010. January;104(1):1–8. 10.1016/j.rmed.2009.08.010 [DOI] [PubMed] [Google Scholar]
- 17.Campisi CC, Boccardo F, Piazza C, Campisi C. Evolution of chylous fistula management after neck dissection. Curr Opin Otolaryngol Head Neck Surg. 2013. April;21(2):150–6. 10.1097/MOO.0b013e32835e9d97 [DOI] [PubMed] [Google Scholar]
- 18.Martin IC, Marinho LH, Brown AE, McRobbie D. Medium chain triglycerides in the management of chylous fistulae following neck dissection. Br J Oral Maxillofac Surg. 1993. August;31(4):236–8. 10.1016/0266-4356(93)90146-N [DOI] [PubMed] [Google Scholar]
- 19.Steven BR, Carey S. Nutritional management in patients with chyle leakage: a systematic review. Eur J Clin Nutr. 2015. July;69(7):776–80. 10.1038/ejcn.2015.48 [DOI] [PubMed] [Google Scholar]
- 20.Ardicli B, User IR, Ciftci AO, Karnak I, Tanyel FC, Ekinci S. An unusual complication of tumor surgery: chylous leakage. Surg Today. 2022. February;52(2):330–6. 10.1007/s00595-021-02334-1 [DOI] [PubMed] [Google Scholar]
- 21.Tulassay Z. Somatostatin and the gastrointestinal tract. Scand J Gastroenterol Suppl. 1998;228:115–21. 10.1080/003655298750026642 [DOI] [PubMed] [Google Scholar]
- 22.Swanson MS, Hudson RL, Bhandari N, Sinha UK, Maceri DR, Kokot N. Use of Octreotide for the Management of Chyle Fistula Following Neck Dissection. JAMA Otolaryngol Head Neck Surg. 2015. August;141(8):723–7. 10.1001/jamaoto.2015.1176 [DOI] [PubMed] [Google Scholar]
- 23.Jain A, Singh SN, Singhal P, Sharma MP, Grover M. A prospective study on the role of octreotide in management of chyle fistula neck. Laryngoscope. 2015. July;125(7):1624–7. 10.1002/lary.25171 [DOI] [PubMed] [Google Scholar]
- 24.Nyquist GG, Hagr A, Sobol SE, Hier MP, Black MJ. Octreotide in the medical management of chyle fistula. Otolaryngol Head Neck Surg. 2003. June;128(6):910–1. 10.1016/S0194-59980300464-9 [DOI] [PubMed] [Google Scholar]
- 25.Belloso A, Saravanan K, de Carpentier J. The community management of chylous fistula using a pancreatic lipase inhibitor (orlistat). Laryngoscope. 2006. October;116(10):1934–5. 10.1097/01.mlg.0000236846.45777.39 [DOI] [PubMed] [Google Scholar]
- 26.Roh JL, Park CI. OK-432 sclerotherapy of cervical chylous lymphocele after neck dissection. Laryngoscope. 2008. June;118(6):999–1002. 10.1097/MLG.0b013e31816b657b [DOI] [PubMed] [Google Scholar]
- 27.Knipping S, Goetze G, Neumann K, Blonching M. Sclerotherapy of cervical cysts with Picibanil (OK-432). Eur Arch Otorhinolaryngol. 2007. April;264(4):423–7. 10.1007/s00405-006-0201-0 [DOI] [PubMed] [Google Scholar]
- 28.Havas TE, Gullane PJ, Kassel RN. The incidence and management of chylous fistulae. Aust N Z J Surg. 1987;57(11):851–4. 10.1111/j.1445-2197.1987.tb01279.x [DOI] [PubMed] [Google Scholar]
- 29.Kassel RN, Havas TE, Gullane PJ. The use of topical tetracycline in the management of persistent chylous fistulae. J Otolaryngol. 1987;16(3):174–8. [PubMed] [Google Scholar]
- 30.Kimura M, Ohto H, Shibata A, Yamada H, Nishiwaki S, Umemura M. Cervical chyloma after neck dissection: a case report. Nagoya J Med Sci. 2017. February;79(1):91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirse DJ, Suen JY, Stern SJ. Phrenic nerve paralysis after doxycycline sclerotherapy for chylous fistula. Otolaryngol Head Neck Surg. 1997. June;116(6 Pt 1):680–3. 10.1016/S0194-5998(97)70249-3 [DOI] [PubMed] [Google Scholar]
- 32.Kirse DJ, Stern SJ, Suen JY, Rudnicki S, Roberson PK, Schaefer RF. Neurotic effects of doxycycline sclerotherapy. Otolaryngol Head Neck Surg. 1998. March;118(3 Pt 1):356–62. [DOI] [PubMed] [Google Scholar]
- 33.Gregor RT. Management of chyle fistulization in association with neck dissection. Otolaryngol Head Neck Surg. 2000. March;122(3):434–9. 10.1067/mhn.2000.95229 [DOI] [PubMed] [Google Scholar]
- 34.Van Natta TL, Nguyen AT, Benharash P, French SW. Thoracoscopic thoracic duct ligation for persistent cervical chyle leak: utility of immediate pathologic confirmation. JSLS. 2009. July-September;13(3):430–2. [PMC free article] [PubMed] [Google Scholar]
- 35.Cope C. Diagnosis and treatment of postoperative chyle leakage via percutaneous transabdominal catheterization of the cisterna chyli: a preliminary study. J Vasc Interv Radiol. 1998. September-October;9(5):727–34. 10.1016/S1051-0443(98)70382-3 [DOI] [PubMed] [Google Scholar]