Abstract
Sphingosine-1-phosphate (S1P) is a bioactive lysophospholipid that induces a variety of biological responses in diverse cell types. Many, if not all, of these responses are mediated by members of the EDG (endothelial differentiation gene) family G protein-coupled receptors EDG1, EDG3, and EDG5 (AGR16). Among prominent activities of S1P is the regulation of cell motility; S1P stimulates or inhibits cell motility depending on cell types. In the present study, we provide evidence for EDG subtype-specific, contrasting regulation of cell motility and cellular Rac activity. In CHO cells expressing EDG1 or EDG3 (EDG1 cells or EDG3 cells, respectively) S1P as well as insulin-like growth factor I (IGF I) induced chemotaxis and membrane ruffling in phosphoinositide (PI) 3-kinase- and Rac-dependent manners. Both S1P and IGF I induced a biphasic increase in the amount of the GTP-bound active form of Rac. In CHO cells expressing EDG5 (EDG5 cells), IGF I similarly stimulated cell migration; however, in contrast to what was found for EDG1 and EDG3 cells, S1P did not stimulate migration but totally abolished IGF I-directed chemotaxis and membrane ruffling, in a manner dependent on a concentration gradient of S1P. In EDG5 cells, S1P stimulated PI 3-kinase activity as it did in EDG1 cells but inhibited the basal Rac activity and totally abolished IGF I-induced Rac activation, which involved stimulation of Rac-GTPase-activating protein activity rather than inhibition of Rac-guanine nucleotide exchange activity. S1P induced comparable increases in the amounts of GTP-RhoA in EDG3 and EDG5 cells. Neither S1P nor IGF I increased the amount of GTP-bound Cdc42. However, expression of N17-Cdc42, but not N19-RhoA, suppressed S1P- and IGF I-directed chemotaxis, suggesting a requirement for basal Cdc42 activity for chemotaxis. Taken together, the present results demonstrate that EDG5 is the first example of a hitherto-unrecognized type of receptors that negatively regulate Rac activity, thereby inhibiting cell migration and membrane ruffling.
Cell migration plays a critical role in a wide variety of physiological and pathological phenomena, including morphogenic processes during embryogenesis, inflammatory responses, wound healing, atherosclerosis, and tumor cell dissemination (38, 60). Chemotaxis is a directed movement of cells toward a positive gradient of a soluble chemoattractant. A number of chemokines, other inflammatory mediators, growth factors, and cytokines have been shown to have activities as chemoattractants (38, 60). Chemoattractant receptors, upon ligand binding, activate a complex and a not yet fully defined array of signaling cascades involving protein tyrosine kinases, phospholipases, lipid kinases, and the low-molecular-weight GTP-binding (G) proteins to regulate actin organization and myosin motor function, which constitute essential processes for cell migration (6, 13, 38, 60). Among the low-molecular-weight G proteins, the Rho family GTPases have received much interest as regulators of the actin cytoskeleton (13, 22, 38). Thus, Rho mediates stress fiber formation and focal adhesion, while Rac and Cdc42 direct peripheral actin assembly that results in the formation of lamellipodia and filopodia, respectively, at the leading edge (22). Expression of a dominant-negative Rac mutant has been shown to inhibit chemoattractant-directed migration in several cell types (3, 7, 42). Inhibition of Rac activity in the Drosophila melanogaster embryo results in morphogenic defects (43). Conversely, expression of active Rac and Tiam1, which is a known activator of Rac, has been shown to promote cell migration in several cell types (32, 42, 61). In addition, p65PAK, a known downstream effector of Rac and Cdc42, is implicated in the regulation of cell motility (1, 14, 64). On the other hand, it has been suggested that lipid kinase phosphoinositide (PI) 3-kinase acts upstream of Rac in chemoattractant-activated signaling for lamellipodium formation as well as cell migration (2, 24, 27, 45, 58). In addition, a number of studies also implicate Cdc42 and Rho in cell migration (3, 6, 38, 69). Thus, the signaling pathway comprising PI 3-kinase, Rac, and other Rho family G protein members and their downstream effectors including p65PAK appears to play a critical role in the regulation of cell migration (6, 10, 13, 38, 60).
Sphingosine-1-phosphate (S1P) is a lysophospholipid with a remarkably wide variety of biological activities, including stimulation of mitogenesis, cell differentiation, and smooth muscle contraction; regulation of cell migration; and inhibition of tumor cell invasion (for reviews, see references 5, 15, 26, 29, 49, 65, 66, 68). Recent identification of cell surface heptahelical receptors for S1P and its structurally related lysophospholipid, lysophosphatidic acid (LPA), which are collectively designated EDG (for endothelial differentiation gene) or LP (for lysophospholipid) receptors, strongly suggests that a diversity of S1P-induced responses are mediated through the EDG receptors (5, 15, 26, 49, 65, 66, 68), although some biological activities of S1P were reported to be mediated through its intracellular actions (16, 54, 57, 58, 65, 66, 70, 74). Among the EDG receptors, EDG1, EDG3, EDG5 (AGR16 or H218), and EDG8 are identified as receptors specific for S1P (8, 20, 30, 34, 35, 44, 46, 47, 49, 65, 66, 71), while EDG2, EDG4, and EDG7 are receptors specific for LPA (5, 15, 26, 49). EDG1, EDG3, and EDG5 are widely expressed in various tissues (25, 53, 76), whereas expression of EDG8 is confined to the central nervous system (30). We and others have shown that the S1P receptors exhibit distinct signaling characteristics. EDG1 is coupled exclusively via the heterotrimeric Gi protein to multiple signaling pathways including phospholipase C activation, Ca2+ mobilization, Ras-mitogen activated protein kinase (MAPK)–extracellular signal-regulated protein kinase (ERK) activation, and inhibition of adenylate cyclase (8, 34, 39, 51, 52, 70, 75). EDG3 is coupled via Gi to Ras-MAPK activation and adenylate cyclase inhibition and also to phospholipase C activation largely via Gq/11 (4, 8, 34, 51, 75). EDG5 is coupled to Ras-MAPK activation, to phospholipase C activation, and to adenylate cyclase stimulation via Gi, Gq/11, and Gs, respectively (4, 8, 20, 34, 51, 71, 75). In addition, EDG3 and EDG5, but not EDG1, mediate S1P-induced stress fiber formation (20, 40).
Of particular interest in the diverse S1P actions are the effects of S1P on cell motility. Strikingly, S1P inhibits, rather than stimulates, the migration of vascular smooth muscle cells (12) and neutrophils (31) and also the invasiveness of some tumor cells including melanoma cells (59, 77), osteosarcoma cells (59), and breast carcinoma cells (72, 74). The inhibitory actions of S1P on motility of melanoma cells are suggested to be mediated by cell surface action, because immobilized S1P inhibits migration as effectively as soluble S1P (77). On the other hand, intracellular actions of S1P in inhibiting migration of breast cancer cells were suggested on the basis of the effectiveness of intracellularly introduced photolyzable S1P and overexpressed S1P-synthesizing enzyme sphingosine kinase (74). In contrast to these cases, S1P was recently demonstrated to direct the chemotaxis of vascular endothelial cells (40, 41, 75). While this paper was in preparation, it was shown that Chinese hamster ovary (CHO) cells (34) and HEK293 cells (73) that selectively express either EDG1 or EDG3, but not cells that express EDG5 or nontransfected cells, exhibited chemotaxis toward S1P. To date, it remains unsettled whether S1P-induced inhibition of cell motility involves a receptor-mediated process and, if so, which EDG receptor mediates inhibition of cell migration in response to S1P and what cellular mechanisms underlie the inhibitory actions of S1P on cell motility.
We demonstrate here that EDG5 mediates a complete inhibition of cell migration toward chemoattractant insulin-like growth factor I (IGF I), while EDG1 and EDG3 trigger chemotaxis. EDG5 mediates a complete inhibition of IGF I-induced, PI 3-kinase-dependent Rac activation, which is required for IGF I-directed chemotaxis, whereas EDG1 and EDG3 mediate stimulation of Rac activation. We further show that the site of S1P action in inhibiting Rac in EDG5-expressing cells is distal to PI 3-kinase and involves stimulation of a GTPase-activating protein (GAP) for Rac, rather than inhibition of Rac-guanine nucleotide exchange protein (GEF) or an increase in cellular cyclic AMP.
MATERIALS AND METHODS
Cells.
CHO-K1 (CHO) and Swiss 3T3K cells, obtained from RIKEN Cell Bank (Tsukuba, Japan) and Enrique Rozengurt (Imperial Cancer Research Fund, London, United Kingdom), respectively, were grown in Ham's F-12 medium (CHO) and Dulbecco modified Eagle medium (Swiss 3T3K cells) supplemented with 10% fetal calf serum (Equitech-Bio, Ingram, Tex.), 100 U of penicillin/ml, and 100 μg of streptomycin (Wako Pure Chemicals, Osaka, Japan)/ml. CHO cells overexpressing EDG1, EDG3, or EDG5 were described previously (20, 51, 52). Before each experiment, cells were switched to the respective medium supplemented with 1% fetal calf serum.
Plasmids and transfections.
EDG1, EDG3, and EDG5 cDNAs were described previously (20, 51, 53). The expression vectors for V12-Rac1, myc-tagged V12-Rac1, N17-Rac1, myc-tagged N17-Rac1, myc-tagged wild-type Rac1, and myc-tagged PAK1 (pME18S-V12-Rac1, pME18S-myc-V12-Rac1, pME18S-N17-Rac1, pME18S-myc-N17-Rac1, pME18S-myc-Rac1, and pCMV6M-PAK1, respectively) were described previously (17, 48, 64, 67). N17-Cdc42 and myc-N19-RhoA cDNAs were obtained by PCR as described previously (17, 48) and ligated onto pME18S to generate pME18S-N17-Cdc42 and pME18S-myc-N19-RhoA. β-Galactosidase expression vector pCAGGS-LacZ was obtained from I. Saito (Institute of Medical Sciences, University of Tokyo) and J. Miyazaki (Osaka University Medical School). Replication-deficient adenoviruses encoding N17-Ras and N17-Rac1 were generated and amplified as described previously (17). The cells were infected with adenoviruses at a multiplicity of infection of 100, which conferred successful gene transduction in more than 95% of cells. Transfection for transient gene expression was carried out by using Lipofectamine (Life Technologies, Inc.). Cells used in the migration assay were cotransfected with either the expression plasmids for the dominant-negative small G proteins or the empty vector and pCAGGS-LacZ 48 h before the migration assay (42). For producing recombinant proteins in Escherichia coli, human wild-type Rac1 cDNA, mouse rhotekin (amino acids 7 to 89) cDNA, and rat α-PAK (amino acids 75 to 131) cDNA (the last two were obtained from mouse and rat brain, respectively, by reverse transcription-PCR) were ligated onto pGEX-2T (Amersham Pharmacia Biotech) at the BamH1-EcoR1 sites to generate pGEX-2T-Rac, pGEX-2T-rhotekin, and pGEX-2T-PAK.
Materials.
S1P, dihydro-S1P and sphingosylphosphorylcholine (SPC) were obtained from Biomol (Plymouth Meeting, Pa.). Sphingosine was purchased from Sigma. These sphingolipids were dissolved in dimethyl sulfoxide at 2 × 10−3 M, aliquoted, and stored at −80°C. Final solvent concentrations did not exceed 0.1%. Recombinant human IGF I was purchased from R&D Systems.
Chemotaxis.
Chemotactic migration of cells was measured in a modified Boyden chamber as described previously (80). Briefly, polycarbonate filters with 8-μm pores (Neuroprobe) were coated with 10 μg of fibronectin (Sigma)/ml in Dulbecco's phosphate-buffered saline (PBS) for 60 min and dried. The coated filter was then placed on a 96-blind-well chamber (Neuroprobe) containing S1P and IGF I, and the CHO cells (8 × 104 cells in 200 μl per well) were loaded into the upper wells. Ligand solutions and the cell suspension were prepared in Ham's F-12 medium containing 0.1% fatty acid-free bovine serum albumin (Sigma). After incubation at 37°C in 5% CO2 for 4 h, the filter was disassembled. The cells on the filter were fixed with methanol and stained with a Diff-Quick staining kit (International Reagents Corp.). The upper side of the filter was then scraped free of cells. The number of cells that migrated to the lower side of the filter was determined by measuring optical densities at 595 nm using a 96-well microplate reader, model 3550 (Bio-Rad). In the migration assay using cells cotransfected with a β-galactosidase expression vector and other vectors, the migratory cells attached to the lower side of the membrane were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) substrate (42). The number of X-Gal stain-positive migratory cells was determined using a microplate reader as described above.
GAP activity assay.
CHO cells expressing EDGs were treated with or without S1P (0.1 μM) and/or IGF I (100 ng/ml) as described in the figure legends. The cells were washed with ice-cold buffer A containing 20 mM Tris-HCl (pH 7.6), 150 mM NaCl, 20 mM MgCl2, 5 mM NaF, 1 mM Na3VO4, and 5 mM EGTA and lysed in a modified buffer A supplemented with 5 μg of leupeptin and aprotinin/ml, 0.6 mM phenylmethylsulfonyl fluoride (PMSF), and 0.5% Triton X-100. Cell lysate was collected with a cell scraper and centrifuged at 18,000 × g at 4°C for 10 min. The supernatants were used as cell extracts. The glutathione S-transferase (GST)-Rac1 fusion protein was produced in strain DH5α of E. coli transformed with pGEX-2T-Rac1 and cleaved with thrombin (62). Recombinant Rac1 (1 μg) was preloaded with [γ-33P]GTP (2,000 to 4,000 Ci/mmol; NEN) in 25 μl of loading buffer (20 mM Tris-HCl [pH 7.6], 25 mM NaCl, 0.1 mM dithiothreitol, 4 μM GTP, 4 mM EDTA) for 10 min at 30°C (63). Cell extracts (40 μl) were then added to 5 μl of the preloaded protein in the presence of 1 mM unlabeled GTP and 17 mM MgCl2 at 20°C. Samples (10 μl) were removed at time zero and at 5- and 10-min intervals and diluted with 3 ml of cold assay buffer (50 mM Tris-HCl [pH 7.6], 100 mM NaCl, 20 mM MgCl2), followed by immediate filtration through a prewetted nitrocellulose filter (NC 45; 0.45-μm pore size; Schleicher & Schuell). After two washes with 3 ml of cold assay buffer, the amount of remaining radioactivity bound to the protein was determined by scintillation counting.
GEF activity assay.
After stimulation, CHO cells expressing either of the EDG subtypes were lysed in 300 μl of lysis buffer (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 0.5 mM NaF, 0.5 mM Na3VO4, 100 nM okadaic acid, 5 μg of leupeptin and aprotinin/ml, 1 mM PMSF). The lysates were centrifuged at 15,000 × g at 4°C for 10 min, and the supernatants were assayed for GEF activity (63). Recombinant Rac1 (2 μg) was preloaded with [3H]GDP (10 Ci/mmol; NEN) in 80 μl of a loading buffer (20 mM Tris-HCl [pH 8.0], 100 mM NaCl, 0.2 mM dithiothreitol, 2 mM EDTA, 100 μM 5-adenylylimido-diphosphate (AMP-PNP), 10 μM GDP) for 20 min at 20°C. Cell lysates (80 μl) were added to 20 μl of the preloaded protein in the presence of 1 mM unlabeled GTP and 10 mM MgCl2 at 20°C. Samples (20 μl) were removed at time zero and at 10- and 20-min intervals, diluted with 1 ml of cold termination buffer (20 mM Tris-HCl [pH 8.0], 100 mM NaCl, 10 mM MgCl2), and filtrated for determining the amount of radioactivity bound to the protein, as described above for the GAP assay.
Determination of the activity of Rho, Rac, and Cdc42.
Cells were washed with ice-cold Ca2+- and Mg2+-free Dulbecco's PBS and lysed in a lysis buffer (50 mM Tris-HCl [pH 7.2], 500 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 5 μg of leupeptin and aprotinin/ml, 0.1 mM PMSF). Cell lysates were clarified by centrifugation at 18,000 × g at 4°C for 10 min, and equal volumes of lysates were incubated with GST-rhotekin (for determination of Rho activity) (54) or GST–p21-activated kinase (PAK) (for determination of Rac and Cdc42 activities) (61) bound to glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech) at 4°C for 45 min. The beads were washed three times with a washing buffer (50 mM Tris-HCl [pH 7.2], 150 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 5 μg of leupeptin and aprotinin/ml, 0.1 mM PMSF). Bound Rho, Rac, and Cdc42 proteins were quantitatively detected by Western blotting using monoclonal antibodies against RhoA (Santa Cruz Biotechnology), Rac (Upstate Biotechnology, Inc.), and Cdc42 (Santa Cruz Biotechnology), respectively, and by densitometry of the corresponding bands using the Quantity One image analyzing system (PDI, Inc.).
PAK kinase assay.
For measurement of the PAK activity, cells were transiently transfected with pCMV6M-myc-PAK1 and lysed in a lysis buffer (40 mM HEPES [pH 7.4], 100 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 25 mM NaF, 1 mM Na3VO4, 5 μg of leupeptin and aprotinin/ml, 0.1 mM PMSF). myc-tagged PAK1 was immunoprecipitated by using a mouse monoclonal anti-myc epitope antibody (clone 9E10). PAK activity associated with the immune complex was assayed in vitro using myelin basic protein (Sigma) as the substrate, as described previously (10).
PI 3-kinase assay.
The assay of PI 3-kinase activity was carried out as described previously (67). PI 3-kinase activity was measured in immunoprecipitates with an antiphosphotyrosine antibody (PY20) (ICN), using PI as the substrate.
Fluorescence microscopy.
Cells cultured in 35-mm-diameter dishes were fixed in 3.7% formaldehyde, permeabilized in 0.25% Triton X-100, and blocked with 3% bovine serum albumin in PBS (67). After incubation with primary antibodies, the cells were stained with fluorescein isothiocyanate-conjugated sheep anti-mouse antibody (Amersham Pharmacia Biotech). Tetramethyl rhodamine B isothiocyanate (TRITC)-labeled phalloidin (Sigma) was used to visualize F-actin. The cells were observed under an inverted fluorescence microscope (IX70; Olympus, Tokyo, Japan).
RESULTS
EDG5 selectively mediates S1P inhibition of cell migration toward IGF I, whereas EDG1 and EDG3 mediate chemotaxis toward S1P.
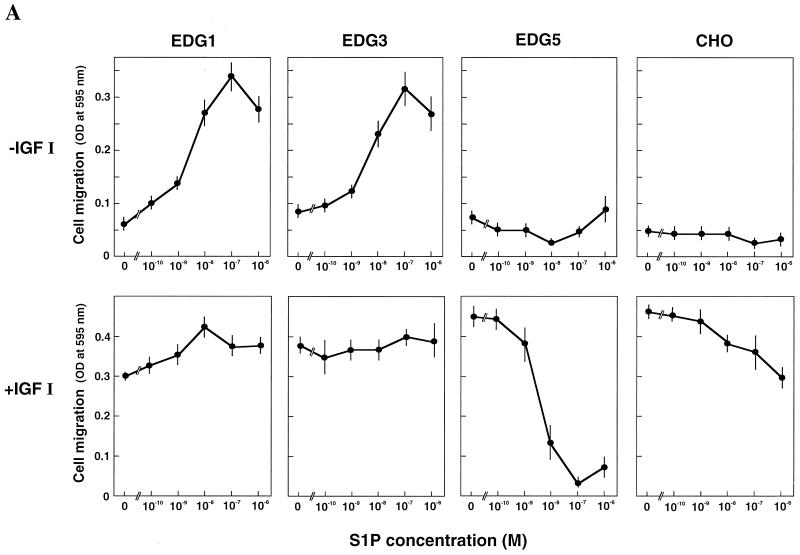
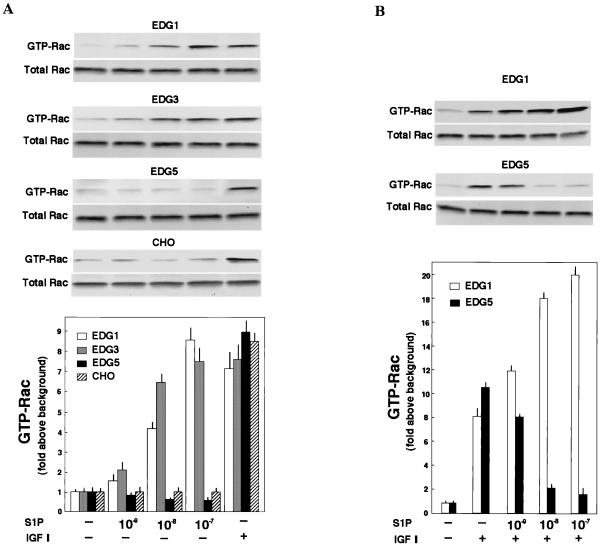
We previously established CHO cell lines which stably express EDG1, EDG3, or EDG5 S1P receptors, i.e., EDG1 cells, EDG3 cells, and EDG5 cells (20, 51, 52). They showed active chemotaxis toward IGF I in standard multiwell Boyden chamber assays comparable to that shown by parental nontransfected CHO cells (Fig. 1A, bottom), indicating that the effector pathway for chemotaxis is not compromised by the expression of the EDG receptors. To clarify the biological activity of each isoform of the EDG receptors for cell migration, we first measured the movement of cells across a porous membrane in response to a concentration gradient of S1P by including S1P only in the lower chamber (Fig. 1A, top). Both EDG1 cells and EDG3 cells showed dose-dependent migration toward S1P. The effects were readily detectable at a concentration as low as 1 nM S1P, with a peak response observed at 0.1 μM and a decline of the responses at supramaximal concentrations of S1P. The maximal migration toward S1P was comparable to the maximal effect induced by IGF I (100 ng/ml). The results are consistent with recently reported studies of vascular endothelial cells expressing endogenous EDG1 and EDG3 receptors (40, 41) and HEK293 cells (73) and CHO cells (35) overexpressing EDG1 and EDG3 receptors. In the presence of 0.1 μM S1P on both sides of the membrane, migration of either EDG1 cells or EDG3 cells across the membrane was reduced to less than 30% of the values in the presence of S1P only in the lower chamber (data not shown). The results indicate that the major portions of the S1P-induced migration in EDG1 and EDG3 cells reflected chemotaxis rather than chemokinesis (random motility).
FIG. 1.
(A) EDG5 mediates inhibition by S1P of cell migration, whereas EDG1 and EDG3 mediate chemotaxis toward S1P. Migration across the porous filter of CHO cells expressing either EDG1, EDG3, or EDG5 and nontransfected CHO cells was determined in the presence of S1P alone at various concentrations (top) and of S1P plus IGF I (100 ng/ml) (bottom) in the lower wells of the Boyden chamber. OD, optical density. (B) EDG5-mediated inhibition of chemotaxis is dependent on a S1P concentration gradient. Migration of EDG5 cells was determined in the presence or absence of S1P (0.1 μM) and IGF I (100 ng/ml) in the upper and lower chambers. All data are means ± standard errors of three determinations and are representative of three independent experiments with similar results.
In sharp contrast to these cell types, EDG5 cells did not show migration in response to a concentration gradient of S1P except at very high concentrations (≧1 μM), but rather demonstrated a reduction in migration below a basal value in the presence of 0.01 μM S1P (Fig. 1A, top). Nontransfected parental CHO cells, which express a low level of endogenous EDG5 receptors but not a detectable level of EDG1 or EDG3 (51), also showed a slight inhibition of cell migration at S1P concentrations of ≧0.1 μM.
We next examined whether each isoform of the S1P receptors modulated chemotaxis toward IGF I. When S1P was included together with IGF I in the lower chamber, a marked inhibition of chemotaxis toward IGF I was observed in EDG5 cells but not in EDG1 cells or EDG3 cells (Fig. 1A, bottom). The S1P inhibition observed in EDG5 cells was dose dependent, with a nearly complete inhibition at 0.1 μM. Nontransfected CHO cells again exhibited a slight inhibition of IGF I-induced chemotaxis in response to S1P. In EDG5 cells, S1P was effective in inhibiting IGF I-induced chemotaxis only when S1P was present in the lower well (Fig. 1B). Thus, EDG5-mediated inhibition of chemotaxis toward IGF I was dependent on an S1P concentration gradient. Besides S1P, dihydro-S1P, which is also a potent agonist for EDG5 (71), inhibited IGF I-induced chemotaxis with a potency similar to that of S1P (Table 1). SPC, a weaker agonist for EDG5 (4, 20, 52, 75), inhibited IGF I-induced chemotaxis with less potency than S1P and dihydro-S1P. Sphingosine, which does not serve as an agonist for EDG5 (8, 20, 71), was essentially ineffective at the doses employed. We tested the possibility that S1P would affect the adhesion of EDG5 cells to the fibronectin-coated substratum, thereby affecting cell migration across the membrane. However, this was not the case: S1P did not affect the adhesion of EDG5 cells to the fibronectin-coated substratum (data not shown).
TABLE 1.
Effects of various sphingolipids on chemotaxis of EDG5 cells toward IGF I
| Concn (M) | Migration (% control) in the presence ofa:
|
|||
|---|---|---|---|---|
| S1P | Dihydro-S1P | SPC | Sphb | |
| 10−9 | 52 ± 5** | 70 ± 4* | 96 ± 4 | 99 ± 1 |
| 10−8 | 18 ± 6** | 40 ± 5** | 83 ± 4 | 94 ± 2 |
| 10−7 | 6 ± 1** | 5 ± 2** | 29 ± 3** | 86 ± 4 |
Migration of EDG5 cells was determined in the presence of IGF I (100 ng/ml) and various concentrations of indicated sphingolipids in the lower chambers. The values are expressed as a percentage of the control value (optical density value at 595 nm, 0.42) in the presence of IGF I alone and are means ± standard errors of three determinations. The results are representative of two experiments with similar results. ∗ and ∗∗, statistically significant compared to the value in the presence of IGF I alone at P values of <0.05 and <0.01, respectively, by one-way analysis of variance ANOVA combined with Bonferroni's test.
Sph, sphingosine.
EDG5 mediates formation of stress fibers and inhibition of IGF I-induced membrane ruffling, whereas EDG1 mediates membrane ruffling.
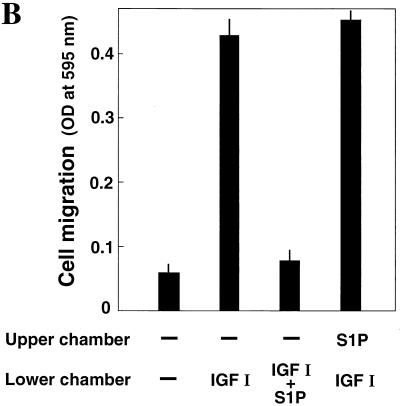
We found that EDG5 and EDG1 regulate the reorganization of the actin cytoskeleton in totally different ways. Shown in Fig. 2A are EDG1 cells and EDG5 cells stained with TRITC-labeled phalloidin to visualize F-actin. In a basal unstimulated state EDG5 cells (b) showed slightly enhanced stress fiber formation compared to EDG1 cells (a). In both cell types IGF I induced membrane ruffling as well as a reduction in stress fiber formation (c and d). In EDG1 cells S1P was as effective as IGF I in inducing membrane ruffling and reducing stress fibers (e). In sharp contrast to EDG1 cells, EDG5 cells responded to S1P with augmented formation of stress fibers but not of membrane ruffles (f). In addition, when S1P was added together with IGF I to EDG5 cells, it completely abolished IGF I-induced membrane ruffling (h). The simultaneous addition of S1P and IGF I to EDG1 cells did not strengthen membrane ruffling compared to the addition of either alone (g). We further examined whether S1P stimulation of EDG5 had any effect on membrane ruffling induced by the expression of a GTPase-deficient, constitutively active myc-tagged V12-Rac (Fig. 2B). We observed that EDG5 stimulation did not inhibit V12-Rac-induced membrane ruffling (compare a and c).
FIG. 2.
(A) EDG5 mediates formation of stress fibers and inhibition of IGF I-induced membrane ruffling in response to S1P stimulation, whereas EDG1 mediates membrane ruffling. EDG1 and EDG5 cells were nonstimulated (a and b) or stimulated with either IGF I (100 ng/ml) (c and d), S1P (0.1 μM) (e and f), or IGF I plus S1P (g and h) for 30 min and then fixed and stained for F-actin with TRITC-phalloidin. (B) EDG5 does not mediate inhibition of V12-Rac-induced membrane ruffling in response to S1P stimulation. EDG5 cells were transiently transfected with an expression vector for myc-tagged V12-Rac 48 h prior to stimulation with S1P (0.1 μM) for 30 min. The cells were then fixed and stained for visualizing F-actin and myc-tagged V12-Rac protein with TRITC-phalloidin (a and c) and 9E10 antimyc antibody (b and d), respectively. Arrows, cells expressing myc-tagged V12-Rac. The results shown are representative of three experiments with similar results.
S1P- and IGF I-induced chemotaxis requires the activities of Rac, Cdc42, PI 3-kinase, and a protein tyrosine kinase.
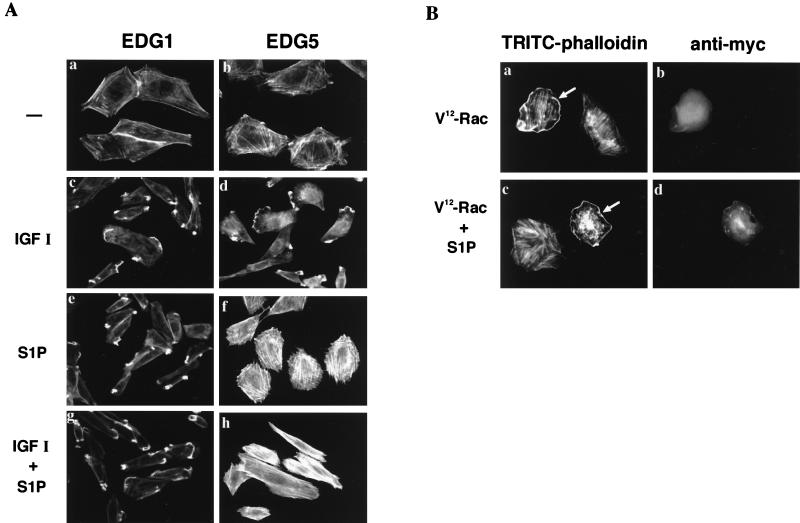
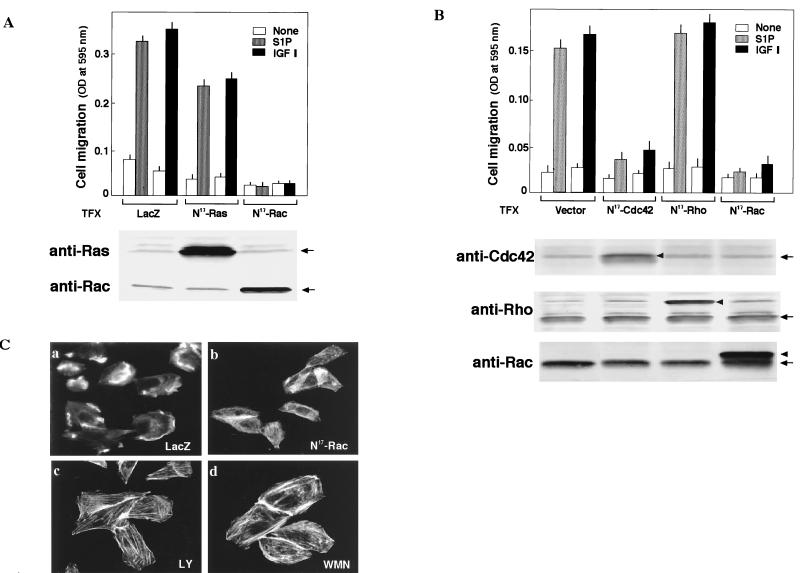
Previous investigations (3, 7, 18, 22, 36, 38) demonstrated roles for Ras and the Rho family GTPases, especially Rac, in chemotaxis. We therefore determined the effects of adenovirus-mediated expression of dominant-negative forms of H-Ras and Rac1, N17-Ras and N17-Rac, respectively, on migration toward S1P (Fig. 3A). The expression of N17-Rac, but not of LacZ, totally abolished the migration of EDG1 cells toward S1P and IGF I (Fig. 3A, top). However, the expression of N17-Ras, which totally abolished S1P-induced ERK activation as previously reported by us (51), only partially (approximately 25%) reduced chemotaxis toward S1P and IGF I. The expression of N17-Ras and N17-Rac in transfected cells was confirmed by Western blotting (Fig. 3A, bottom). We also determined the effects of the expression of dominant-negative forms of Cdc42 and RhoA, N17-Cdc42 and N19-RhoA, respectively, in a transient transfection assay using plasmid expression vectors. The coexpression of N17-Cdc42 and β-galactosidase nearly abolished the chemotaxis of transfected cells toward S1P and IGF I, as did N17-Rac expression (Fig. 3B). In contrast, the expression of N19-RhoA, which abolished S1P-induced stress fiber formation in EDG3 and EDG5 cells (data not shown), did not inhibit chemotaxis toward S1P and IGF I. The expression of dominant-negative small G proteins in transfected cells was confirmed by Western blotting (Fig. 3B, bottom).
FIG. 3.
Chemotaxis induced by S1P via EDG1 and by IGFI is dependent on Rac and Cdc42 but not Rho. (A) EDG1 cells were transduced with adenoviral vectors encoding either LacZ, N17-Ras, or N17-Rac and 48 h later were assayed for chemotaxis toward S1P (0.1 μM) and IGF I (100 ng/ml). Parallel dishes were processed for immunodetection of N17-Ras (anti-Ras) and N17-Rac (anti-Rac). OD, optical density. (B) EDG1 cells were cotransfected with a vector encoding N17-Cdc42, myc-N19-Rho, or myc-N17-Rac or the empty vector and the β-galactosidase (LacZ) expression vector 48 h before the migration assay. LacZ-expressing migrating cells were counted as described in Materials and Methods. The expression of the dominant-negative GTPases (arrowheads) and endogenous wild-type GTPases (arrows) was detected by Western blotting. The data in panels A and B are means ± standard errors of three determinations. (C) EDG1 cells were transduced with adenoviruses encoding LacZ or N17-Rac or pretreated with LY294002 (LY; 30 μM) or wortmannin (WMN, 0.1 μM) for 20 min and then stimulated with S1P (0.1 μM) for 30 min. The cells were fixed and stained for visualizing F-actin with TRITC-phalloidin. The results are representative of three experiments with similar results.
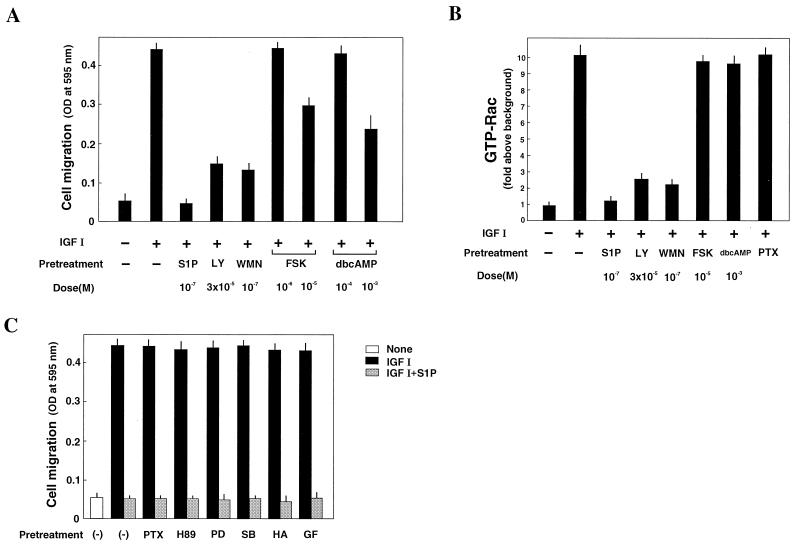
We also explored other intracellular signaling molecules that are required for chemotaxis induced by S1P and IGF I. IGF I-directed chemotaxis in four CHO cell types was approximately 70% reduced after pretreatment with the PI 3-kinase inhibitors LY294002 (30 μM) and wortmannin (0.1 μM) and was nearly completely abolished by tyrosine kinase inhibitor genistein (100 μM). Pertussis toxin (PTX) pretreatment was ineffective in inhibiting migration toward IGF I. Similar to chemotaxis toward IGF I, chemotaxis toward S1P of EDG1 and EDG3 cells was potently inhibited by the PI 3-kinase inhibitors and genistein. Unlike chemotaxis toward IGF I however, chemotaxis toward S1P in EDG1 and EDG3 cells was totally abolished by PTX pretreatment, which was consistent with previous reports (50, 60, 80) showing that, among the heptahelical G protein-coupled type of receptors, Gi-coupled receptors preferentially mediate chemotaxis. We also observed that chemotaxis toward either S1P or IGF I was not sensitive to PD98059 (30 μM), SB203580 (10 μM), HA1077 (10 μM), or GF109203X (5 μM); these are the inhibitors for MAPK kinase, p38MAPK, Rho kinase, and protein kinase C (PKC), respectively. These composite results indicate that the chemotactic signals elicited by Gi-coupled heptahelical receptors EDG1 and EDG3 and IGF I receptor tyrosine kinase converge before the common pathway comprising Rac and Cdc42, tyrosine kinases, and PI 3-kinase.
We also observed that PI 3-kinase inhibitors LY294002 and wortmannin and adenovirus-mediated expression of N17-Rac, but not LacZ, abolished S1P-induced membrane ruffling in EDG1 cells (Fig. 3C), similar to IGF I stimulation in EDG1 and EDG5 cells (data not shown). In addition, treatment of EDG1 cells with the PI 3-kinase inhibitors augmented stress fibers. The results indicate that PI 3-kinase and Rac are required for S1P- and IGF I-induced membrane ruffling as well.
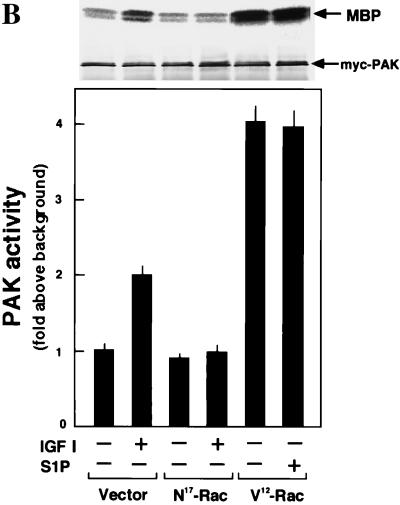
EDG5 mediates inhibition of IGF I stimulation of Rac and p65PAK, whereas EDG1 and EDG3 mediate activation of Rac and p65PAK.
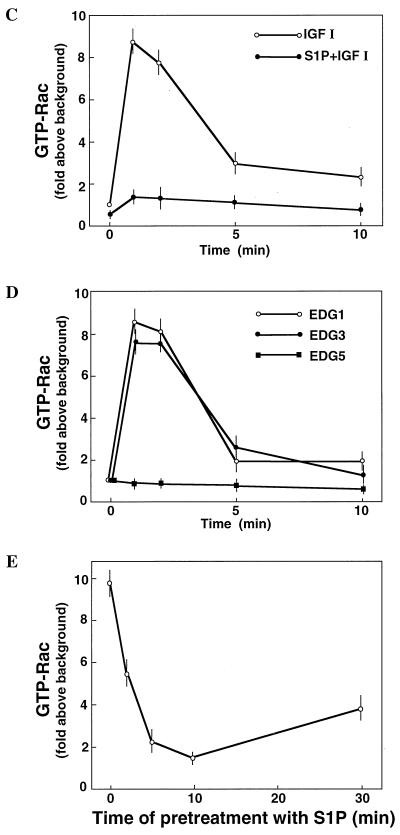
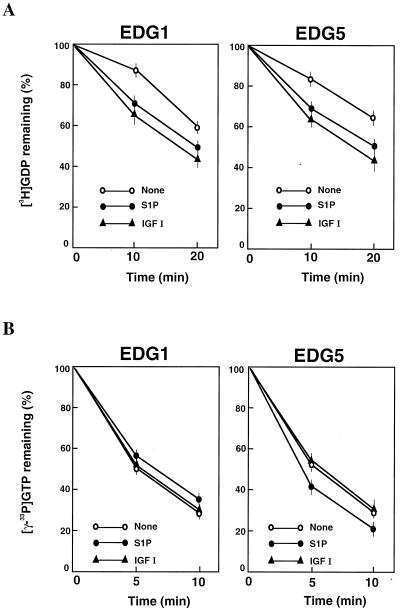
Since Rac seemed to be located downstream of PI 3-kinase (24, 27, 45, 58), we first determined whether or not EDG5-mediated signaling affected cellular Rac activity. The Rac activation assay using recombinant GTP-Rac binding fusion protein GST-PAK1(75–131) (61) showed that IGF I induced similar increases in the amounts of GTP-loaded Rac in all of the four CHO cell types (Fig. 4A). IGF I-induced Rac activation was biphasic with a peak activation at 1 min followed by a lower level of sustained stimulation for at least 10 min (Fig. 4C). In EDG1 cells and EDG3 cells, but not in parental CHO cells, S1P also induced biphasic increases in the amount of GTP-loaded Rac (Fig. 4D) with a dose-response relationship similar to that observed for S1P-induced migration (compare Fig. 1A and 4D). The maximal effects and time courses of S1P-induced and IGF I-induced Rac activation in EDG1 cells and EDG3 cells were comparable. In EDG5 cells, by sharp contrast, S1P slightly reduced the amount of GTP-loaded Rac below a basal level (Fig. 4A and D). When EDG5 cells were stimulated with IGF I together with S1P, Rac activation was strongly suppressed (Fig. 4B, C, and E). Thus, in EDG5 cells S1P dose-dependently inhibited IGF I-stimulated Rac activation, with a nearly complete inhibition at 0.1 μM S1P; in EDG1 cells, by contrast, the addition of S1P together with IGF I caused up to a 2.5-fold-greater increase in the amount of GTP-bound Rac than the addition of IGF I alone (Fig. 4B). The inhibitory effect of S1P on IGF I-induced Rac activation was dependent on the time length of S1P pretreatment (Fig. 4E); the inhibition by S1P was maximal with a 10-min pretreatment. Dihydro-S1P induced responses in the three EDG cell types similar in magnitude to those induced by S1P (Table 2).
FIG. 4.
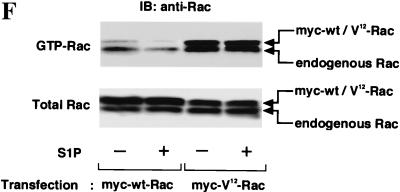
EDG1 and EDG3 mediate activation of Rac, whereas EDG5 mediates inhibition of IGF I-stimulation of Rac. (A) EDG cells and CHO cells were stimulated with various concentrations of S1P or 100 ng of IGF I/ml for 1 min, and the amount of GTP-loaded Rac (GTP-Rac) was determined as described in Materials and Methods. A portion (1/20) of the cell lysate was subjected to Western analysis for evaluating the amount of total Rac in each sample. (B) EDG1 and EDG5 cells were stimulated with IGF I (100 ng/ml) plus various concentrations of S1P and analyzed as described for panel A. (C) Time-dependent increase in the amount of GTP-Rac in IGF I-stimulated EDG5 cells in the presence and absence of S1P. The cells were pretreated with S1P (0.1 μM) for 10 min or left unpretreated and then stimulated with IGF I (100 ng/ml) for the indicated time periods. (D) Time-dependent increase in the amount of GTP-Rac in S1P (0.1 μM)-stimulated EDG1, EDG3, and EDG5 cells. (E) Time-dependent decrease in the amount of GTP-Rac in S1P-pretreated, IGF I-stimulated EDG5 cells. EDG5 cells were treated with S1P (0.1 μM) for the indicated time periods. IGF I (100 ng/ml) was present for the last 1 min of the incubation. The data in panels A to E are means ± standard errors of three determinations. (F) EDG5 cells were transiently transfected with expression vectors for myc-tagged wild-type (wt) Rac or V12-Rac and 48 h later were stimulated with S1P (0.1 μM) for 5 min or were not stimulated and assayed for the amounts of GTP-bound forms of Rac and V12-Rac. The results are representative of two or three experiments with similar results. IB, immunoblot.
TABLE 2.
Effects of S1P and dihydro-S1P on activation of Rac and Rho in EDG cells
| Cells and protein | Mean level (fold above background) ± SE when stimulated witha:
|
||
|---|---|---|---|
| None | S1P | Dihydro-S1P | |
| EDG5 | |||
| GTP-Rac | 1.0 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| GTP-Rac (+ IGF I) | 8.7 ± 0.1 | 1.7 ± 0.2* | 2.6 ± 0.2* |
| GTP-Rho | 1.0 ± 0.1 | 8.8 ± 0.2* | 8.6 ± 0.2* |
| EDG1 | |||
| GTP-Rac | 1.0 ± 0.1 | 8.2 ± 0.2* | 10.3 ± 0.3* |
| EDG3 | |||
| GTP-Rac | 1.0 ± 0.1 | 7.2 ± 0.3* | 6.8 ± 0.1* |
| GTP-Rho | 1.0 ± 0.1 | 8.2 ± 0.2* | 8.3 ± 0.2* |
EDG cells were stimulated with S1P (10−7 M) or dihydro-S1P (10−7 M) for 1 min, except IGF I-stimulated EDG5 cells, which were pretreated with S1P or dihydro-S1P for 10 min and then stimulated with IGF I for 1 min. The amounts of GTP-bound Rac and Rho were determined as described in Materials and Methods. ∗, statistically significant compared to the value in the absence of a stimulus (P < 0.01).
In EDG5 cells transiently transfected with an expression vector for myc-tagged wild-type Rac, S1P reduced the amounts of the GTP-bound forms of both endogenous and exogenously expressed wild-type Rac (Fig. 4F). In EDG5 cells transiently transfected with myc-tagged V12-Rac, by contrast, S1P failed to reduce the amount of the GTP-loaded form of V12-Rac (Fig. 4F), as for V12-Rac-induced membrane ruffling (Fig. 2B). Unexpectedly, we found that the expression of V12-Rac in EDG5 cells led to a considerable increase in the level of the GTP-bound form of endogenous Rac, compared to the level in cells expressing exogenous wild-type Rac. Moreover, S1P failed to detectably reduce the level of the GTP-bound form of endogenous Rac in cells expressing V12-Rac.
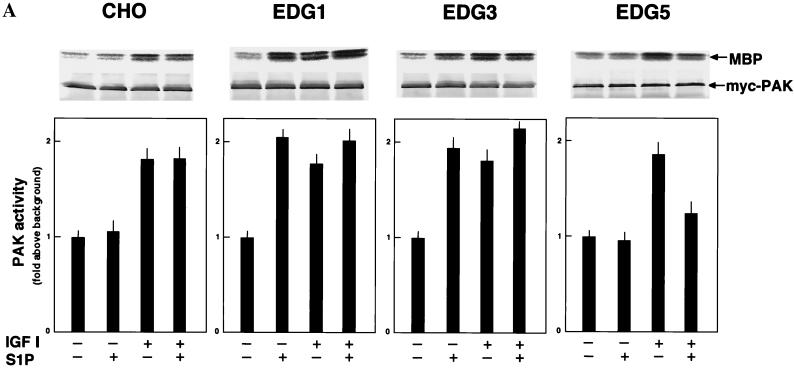
p65PAK is implicated as an downstream effector of Rac in the signaling leading to cell migration (14). Moreover, it was recently demonstrated that p65PAK can be activated by sphingosine (11, 33). In all four CHO cell types, IGF I indeed stimulated PAK activity in a Rac-dependent manner (Fig. 5). In EDG1 cells and EDG3 cells S1P also stimulated PAK activity (Fig. 5A), whereas in EDG5 cells S1P did not stimulate PAK activity by itself and inhibited IGF I-induced PAK activation by 60% (Fig. 5A). S1P failed to inhibit PAK activation induced by the expression of V12-Rac in EDG5 cells (Fig. 5B), as for membrane ruffling induced by V12-Rac (Fig. 2B).
FIG. 5.
EDG5 mediates inhibition of IGF I-induced p65PAK activation. (A) CHO cells and EDG cells were pretreated with S1P (0.1 μM) for 5 min or left unpretreated and then stimulated with S1P (0.1 μM) or IGF I (100 ng/ml) for a further 5 min and assayed for p65PAK activity as described in Materials and Methods. Autoradiographs and anti-myc Western blots are shown at the top. MBP, myelin basic protein. (B) EDG5 cells were transiently transfected with the empty vector or expression vectors for N17-Rac and V12-Rac and 48 h later were treated with S1P (0.1 μM) or IGF I (100 ng/ml) for 5 min or were left untreated. All data are means ± standard errors of three determinations. The results are representative of two experiments with similar results.
The activities of EDGs on activation of Rho and Cdc42.
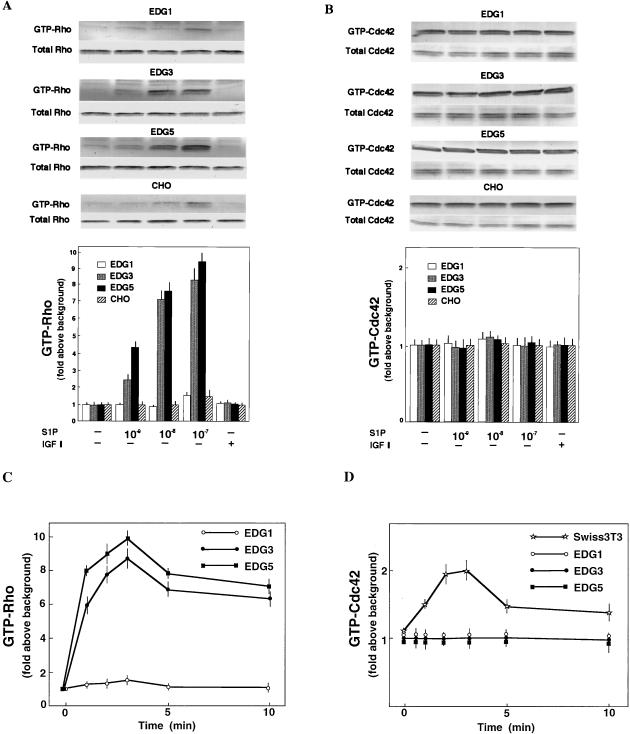
We further determined the effects of S1P on the activity of Rho and Cdc42, which are also implicated in cell migration (3, 6, 13, 22, 38). Consistent with our previous observation (20) that S1P induces stress fiber formation via EDG5 (Fig. 2A), S1P increased the amount of GTP-loaded Rho in EDG5 cells in a dose-dependent manner (Fig. 6A). S1P also increased the amount of GTP-Rho in EDG3 cells. S1P-induced Rho activation in both EDG3 cells and EDG5 cells was rapid and reached a peak at 3 min, followed by a lower level of sustained activation (Fig. 6C). In EDG1 cells and parental CHO cells S1P failed to increase the amount of GTP-Rho except at the highest concentration employed. IGF I did not change the level of GTP-Rho in any of the four cell types. Dihydro-S1P induced magnitudes of Rho activation similar to those induced by S1P in both EDG3 cells and EDG5 cells (Table 2). The amount of GTP-bound Cdc42 was at a readily detectable level in an unstimulated state in all four cell types (Fig. 6B). Neither S1P nor IGF I altered the level of GTP-loaded Cdc42 in these cell types (Fig. 6B and D). In Swiss 3T3 cells, however, bradykinin, a G protein-coupled receptor agonist which has been demonstrated to induce filopodium formation in this cell type (36), evoked a time-dependent Cdc42 activation (Fig. 6D).
FIG. 6.
EDG5 and EDG3 mediate activation of Rho, but EDG1, EDG3, and EDG5 do not mediate activation of Cdc42. (A and B) EDG cells and CHO cells were stimulated with S1P at various concentrations or IGF I (100 ng/ml) for 3 min. (C and D) EDG cells and Swiss 3T3 cells were stimulated with S1P (0.1 μM) or bradykinin (0.1 μM), respectively, for the indicated times. The amounts of GTP-bound Rho and Cdc42 were determined as described in Materials and Methods. A portion (1/20) of the cell lysate was subjected to Western analysis for evaluating the amounts of total Rho and Cdc42 in each sample. The data are means ± standard errors of three determinations. The results are representative of two or three experiments with similar results.
EDG5-mediated inhibition of chemotaxis does not involve cyclic AMP, Gi, ERK, p38, Rho kinase, or PKC.
We previously showed that EDG5 is weakly coupled to stimulation of adenylate cyclase (20). A rise in the intracellular cyclic AMP level has been shown to lead to inhibition of cell migration in neutrophils and other cell types (19, 23, 37). However, cyclic AMP does not likely contribute to EDG5-mediated inhibition of chemotaxis in CHO cells. This notion is based on the following observations. First, the S1P dose-response curve for the cyclic AMP increase in EDG5 cells is shifted to the right approximately 100-fold compared to that for inhibition of chemotaxis (compare Fig. 1B and Fig. 9 of reference 20). Second, the adenylate cyclase activator forskolin did not inhibit IGF I-induced chemotaxis or Rac activation in EDG5 cells at the dose that causes an increase in cellular cyclic AMP similar to that caused by S1P at 0.1 μM, which is a maximally inhibitory dose for chemotaxis (Fig. 7A). Third, we observed that although higher concentrations of forskolin, which induced a considerable rise in cellular cyclic AMP above a level achieved with S1P, and dibutyryl cyclic AMP inhibited chemotaxis toward IGF I, they did not inhibit IGF I-induced Rac activation (Fig. 7A and B). Finally, the protein kinase A inhibitor H-89 failed to abolish inhibition by S1P of IGF I-induced Rac activation and chemotaxis (Fig. 7C).
FIG. 7.
Cyclic AMP, protein kinase A, ERK, p38MAPK, Rho kinase, PKC, and Gi protein are not involved in S1P inhibition of IGF I-induced chemotaxis and Rac activation. (A) Forskolin, dibutyryl cyclic AMP (dbcAMP), and PI 3-kinase inhibitors reduces chemotaxis toward IGF I. (B) PI 3-kinase inhibitors, but not forskolin, dbcAMP, or PTX, reduce IGF I-induced Rac activation. (C) Various protein kinase inhibitors and PTX do not block S1P inhibition of chemotaxis toward IGF I. EDG5 cells were pretreated with PTX (100 ng/ml), forskolin (FSK) (1 or 10 μM), dbcAMP (0.1 or 1 mM), LY294002 (LY) (30 μM), wortmannin (WMN) (0.1 μM), PD98059 (PD) (30 μM), SB203580 (SB) (10 μM), HA1077 (HA) (10 μM), or GF109203X (GF) (5 μM) for 20 min except for PTX, where the treatment time was 24 h, or with S1P (0.1 μM) for 10 min and then stimulated with IGF I (100 ng/ml). In the migration assay, all drugs except PTX were present throughout the 4-h incubation period. The data are means ± standard errors of three determinations. The results are representative of two experiments with similar results. OD, optical density.
In addition to the cyclic AMP signaling pathway, we explored the possibility of the involvement of other signaling pathways in EDG5-mediated inhibition of chemotaxis. However, none of the inhibitors, which include PTX, the Rho kinase inhibitor HA1077, the MAPK kinase inhibitor PD98059, the p38MAPK inhibitor SB203580, and the PKC inhibitor GF109203X, affected S1P-induced inhibition of chemotaxis (Fig. 7C).
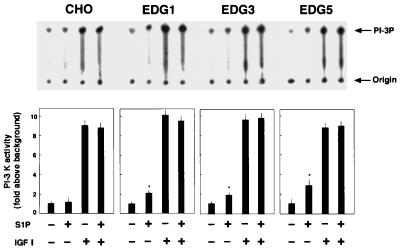
EDG5 signaling interferes with IGF I-induced Rac activation at a site downstream of PI 3-kinase.
Like IGF I-induced chemotaxis and membrane ruffling (Fig. 7A), IGF I-induced Rac activation in EDG5 cells was sensitive to LY294002 and wortmannin (Fig. 7B), indicating the dependence of Rac activation on PI 3-kinase in IGF I-stimulated cells. This observation and results showing that IGF I-induced chemotaxis and membrane ruffling are dependent on cellular Rac activity indicate that PI 3-kinase is acting upstream of Rac in the IGF I signaling leading to chemotaxis and membrane ruffling. To delineate a site(s) at which EDG5 negatively regulates the IGF I signaling pathway, we studied and compared the effects of S1P on PI 3-kinase activity in all EDG cell types. As shown in Fig. 8, treatment with S1P at 0.1 μM of any EDG cell type, but not of CHO cells, stimulated the PI 3-kinase activity approximately twofold over a basal value. IGF I induced even stronger (10-fold) stimulation of PI 3-kinase activity in the four CHO cell types. Importantly, S1P did not inhibit this effect of IGF I on the PI 3-kinase activity in EDG5 cells, as in the other cell types. These observations clearly indicate that EDG5 exerts inhibition on the IGF I-induced signaling at a site beyond PI 3-kinase.
FIG. 8.
EDG5 does not mediate inhibition of IGF I-induced PI 3-kinase activation. CHO cells and EDG cells were stimulated with S1P (0.1 μM) and/or IGF I (100 ng/ml) for 5 min and assayed for PI 3-kinase activity as described in Materials and Methods. Autoradiographs of thin-layer chromatography plates and quantitated results are shown. The data are means ± standard errors of three determinations. The results are representative of two experiments with similar results. ∗, statistically significant (P < 0.05) compared to the value for nonstimulated cells analyzed as described for Table 1.
S1P stimulates Rac-GAP activity, but does not inhibit Rac-GEF activity, through EDG5.
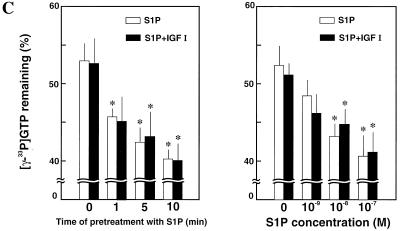
The activities of the small GTPases including the Ras and Rho family proteins are regulated by the actions of GEFs and GAPs. We tried to identify the molecular mechanism by which EDG5 negatively regulates Rac activation. Treatment of EDG1 cells and EDG5 cells with S1P led to stimulation of Rac-GEF activity in cell lysates (Fig. 9A). IGF I also stimulated Rac-GEF activity in both cell types. On the other hand, treatment of EDG1 cells with S1P tended to inhibit Rac-GAP activity in cell lysates (Fig. 9B, left). In contrast, treatment of EDG5 cells with S1P stimulated Rac-GAP activity. IGF I was without a significant effect on Rac-GAP activity in either cell type (Fig. 9B, right). The stimulatory effect of S1P on GAP activity in EDG5 cells was time dependent: stimulation of GAP activity by S1P was maximal after 10 min of treatment in the presence and absence of IGF I (Fig. 9C, left). The stimulatory effect of S1P on GAP activity was also dose dependent, with a maximal stimulation observed at 0.1 μM (Fig. 9C, right).
FIG. 9.
EDG5 mediates stimulation of Rac-GAP activity but not inhibition of Rac-GEF activity. (A and B) EDG1 and EDG5 cells were stimulated with S1P (0.1 μM) or IGF I (100 ng/ml) for 1 min (GEF assay) or 10 min (GAP assay) or were not stimulated. (C, left) EDG5 cells were treated with S1P (0.1 μM) for the indicated times. IGF I (100 ng/ml) was present for the last 1 min of the incubation when indicated. (Right) EDG5 cells were stimulated with various concentrations of S1P with or without IGF I (100 ng/ml) for 10 min. Cell lysate was prepared. Rac-GEF activity in cell lysate was assayed using [3H]GDP-loaded recombinant Rac protein as described in Materials and Methods. Similarly, Rac-GAP activity in cell lysate was determined using [γ-33P]GTP-loaded Rac. The values at 5 min in the Rac-GAP assay are shown. All values are means ± standard errors of three determinations. The results are representative of two or three experiments with similar results. ∗ (C), statistically significant (P < 0.05) compared to the value for nonstimulated cells analyzed as described for Table 1.
DISCUSSION
To date, there are only several reports (9, 19, 23, 37, 46) on inhibitors for chemotaxis, i.e., chemorepellants. These include a μ-opioid agonist, prostaglandin E, and pituitary adenylate cyclase-activating peptide, which reportedly act mainly on leukocytes via cell surface receptors. Cellular mechanisms by which the chemorepellants suppress cell migration have remained largely unknown. S1P is unique and distinct from these chemorepellants in that it bidirectionally regulates cell migration, i.e., S1P acts as a chemoattractant for certain cell types (34, 40, 41, 73), while it strongly inhibits cell migration in others (12, 31, 59, 72, 77); the latter include several tumor-derived cell types. S1P is a normal constituent of the blood and is present at a substantially high concentration (>0.1 μM), most of which is bound to albumin (79). This suggests that blood-borne mediator S1P is capable of regulating cell motility in various tissues. S1P is released from activated platelets (76). S1P also likely acts as a paracrine regulator for cell migration under certain circumstances. There exist at least four different isoforms of the G protein-coupled cell surface receptor for S1P with signaling characteristics distinct from each other (44, 66, 68). Thus, it is quite reasonable to postulate that many of pleiotropic actions of S1P, including bidirectional effects on cell migration, are mediated by the EDG family receptors (5, 26, 29, 44, 49, 66, 68).
In the present study we identified EDG5 as a receptor to mediate inhibition of chemotaxis, whereas the other two, EDG1 and EDG3, are receptors mediating chemotaxis. Importantly, we demonstrated that EDG5 is the first receptor that negatively regulates the small G protein Rac, a critical regulator of the actin cytoskeleton and cell motility, whereas EDG1 and EDG3 mediate the activation of Rac, as do IGF I and other chemoattractants. In addition, our results indicate that the EDG5-mediated S1P inhibition of Rac activity involves stimulation of Rac-GAP activity.
We demonstrated in the present study that CHO cells overexpressing EDG5, but not EDG1 or EDG3, exhibited inhibition of chemotaxis toward IGF I in response to the S1P concentration gradient (Fig. 1). S1P did not at all affect the adhesion of EDG5 cells to the fibronectin-coated substratum, indicating that EDG5 actually mediated the inhibition of migration. The relative potencies of various sphingolipids in inhibiting cell migration (Table 1) are in good agreement with their previously reported relative agonist potencies for EDG5 (20, 71). Consistent with its inhibitory activity on cell migration, EDG5 also mediated inhibition of IGF I-induced membrane ruffling, whereas EDG1 and EDG3 mediated induction of membrane ruffling (Fig. 2). Nontransfected parental CHO cells, which show a low level of expression of endogenous EDG5 (51), also exhibited S1P inhibition of chemotaxis toward IGF I, although to a lesser extent (Fig. 1A). It was previously demonstrated that human vascular smooth muscle cells (12) and certain tumor cells including B16 melanoma cells (59, 72, 74, 77) respond to S1P with inhibition of directed cell migration. We identified EDG5 as a dominant EDG subtype expressed in vascular smooth muscle cells and B16 melanoma cells (H. Okamoto, H. Yamaguchi, Y. Ryu, and Y. Takuwa, unpublished observations). This suggests the interesting possibility that S1P-induced inhibition of migration of these cell types may be mediated by endogenously expressed EDG5 receptors. On the other hand, intracellular action mechanisms for S1P-induced inhibition of cell motility are suggested (65, 74). It was demonstrated in breast cancer cells that flash photolysis of caged S1P incorporated into cells induced inhibition of cell migration, suggesting an intracellular role for S1P in inhibiting cell migration (74). The same group also demonstrated that overexpression of the S1P-synthesizing enzyme sphingosine kinase in breast cancer cells induced inhibition of cell migration without a significant release of S1P into the extracellular space (74). In addition, it was shown that sphingosine, a precursor of S1P, could alter cellular PAK activity (11) and PDK1 activity (33). It was also noted that higher concentrations of S1P are required for inhibition of cell motility in breast cancer cells (72, 74) than in vascular smooth muscle cells (12) and B16 melanoma cells (59, 77). Moreover, dihydro-S1P, an agonist for the EDG S1P receptors (70, 71), is ineffective in inhibiting the motility of the breast cancer cells (74), which contrasts with the present data for CHO-EDG5 cells (Table 1). Thus, these data suggest that there may be more than a single cellular mechanism for S1P inhibition of migration and that mechanisms for S1P inhibition of cell migration could be different in different cell types.
Accumulated evidence strongly suggests that Rac plays an important role in chemotactic signaling. Thus, the expression of N17-Rac in a variety of cell types inhibits directed cell migration toward diverse chemoattractants, including platelet-derived growth factor (PDGF), LPA, epidermal growth factor (EGF), insulin, and colony-stimulating factor-1 (3, 7, 42). Conversely, the expression of L61-Rac, an active form of Rac, in COS-7 cells was shown to enhance migration toward EGF (32, 42), although others (28, 61) reported that expression of an active Rac instead reduced cell motility. It was also shown that expression of Tiam1, a GEF for Rac, promoted random motility of the epithelial MDCK cells in a matrix-dependent manner (61) and also haptotaxis of COS-7 cells (42). Moreover, it was recently shown that the chemoattractants N-formyl-Met-Leu-Phe and leukotriene B4 activated Rac in neutrophils via Gi protein (2). We observed in the present study that migration and membrane ruffling induced by either S1P or IGF I were equally abolished by adenoviral vector-mediated expression of N17-Rac (Fig. 2 and 3A). Very recently, it was demonstrated that endogenous EDG1 in endothelial cells mediates cortical actin filament assembly in a Rac-dependent manner (40). These results together confirm critical roles for Rac in the signaling for chemotaxis and membrane ruffling that is elicited by both G protein-coupled heptahelical receptors and receptor tyrosine kinases. Consistent with the Rac dependence of the S1P effects in EDG1 and EDG3 cells, we observed that S1P activated Rac in these cell types, while IGF I activated Rac in any of the three EDG cell types (Fig. 4A). One of the most striking findings in the present study was that the S1P effects on Rac activity in EDG5 cells were exactly the opposite of those in EDG1 and EDG3 cells. In EDG5 cells, S1P by itself did not induce Rac activation, chemotaxis, or membrane ruffling, but rather inhibited these responses. More impressively, S1P abolished all of the responses induced by IGF I (Fig. 1, 2, and 4). Significantly, the S1P dose-response relationships for inhibition of Rac activation (Fig. 4B) and chemotaxis (Fig. 1A) in EDG5 cells are similar. In addition, consistent with the suppressing action of S1P on Rac activity in EDG5 cells, S1P inhibited IGF I-induced activation of PAK (Fig. 5), a known downstream effector of Rac (47). All these results provide further support for the notion that Rac plays an essential role in regulating cell migration and highlight EDG5 as a unique receptor that negatively controls Rac.
Previous reports (3, 5, 13, 21) implicate the other two members of the Rho family G proteins, Rho and Cdc42, in cell migration. We observed in the present study that expression of N17-Cdc42, but not of N19-RhoA, abolished chemotaxis toward IGF I (Fig. 3B), indicating the essential role for Cdc42. The unstimulated level of GTP-bound Cdc42 was readily detectable in the CHO cell types, although neither S1P nor IGF further activated Cdc42 in any of the four CHO cell types (Fig. 6B). The fact that we observed bradykinin-induced stimulation of Cdc42 activity above an unstimulated level in Swiss 3T3 cells (Fig. 6D) confirms the validity of our assay procedure for GTP-bound Cdc42. Thus, the present observations suggest that a basal level of activity of Cdc42 is likely important for chemotaxis in CHO cells, although it also remains possible that expression of N17-Cdc42 could inhibit Rac-GEF and result in inhibition of Rac. On the other hand, the present results for N19-RhoA expression (Fig. 3A) and measurements of cellular RhoA-GTP content (Fig. 6A) indicate that RhoA is not likely to be involved in either S1P induction or inhibition of chemotaxis. S1P similarly induces an increase in RhoA-GTP in both EDG3 cells and EDG5 cells (Fig. 6A), while it stimulates and inhibits migration in EDG3 cells and EDG5 cells, respectively. Our results for S1P-induced Rho activation in EDG cells are consistent with previous observations that EDG3 and EDG5 mediate stress fiber formation in endothelial cells (40) and CHO cells (20) and neurite retraction and cell rounding in PC-12 cells (71) and that EDG1 does not mediate stress fiber formation in endothelial cells (40).
In EDG1 cells, the effects of S1P on Rac activation, membrane ruffling, and chemotaxis were all dependent on both Gi and PI 3-kinase, whose properties are compatible with the known properties of chemoattractants acting via the G protein-coupled heptahelical type of receptors (2, 23, 45, 50, 80). IGF I-induced Rac activation, membrane ruffling, and chemotaxis were also dependent on PI 3-kinase but not Gi (Fig. 2 and 7). Previous studies (2, 24) demonstrated that chemoattractant receptors induced stimulation of Rac via activation of PI 3-kinase. We found that levels of Rac activation induced by IGF I in all four CHO cell types and by S1P in EDG1 and EDG3 cells were equally inhibited by the PI 3-kinase inhibitors (Fig. 7), indicating that Rac activation occurs downstream of PI 3-kinase activation. Unexpectedly, we observed that S1P activated PI 3-kinase in EDG5 cells despite its inhibitory effect on Rac activation (Fig. 8). This observation indicates that S1P inhibits Rac activity at a site beyond PI 3-kinase.
The cellular activity of Rac is regulated by Rac-GEFs as represented by Tiam1 and vav, which have been shown to act downstream of PI 3-kinase (36, 61), and Rac-GAPs (69). Our results for the determination of Rac-GEF activity and Rac-GAP activity showed that, like IGF I, S1P stimulated Rac-GEF activity similarly in EDG5 cells and EDG1 cells but, notably, that S1P stimulated Rac-GAP activity only in EDG5 cells (Fig. 9). These observations suggest that EDG5 negatively regulates Rac activity through a mechanism involving stimulation of Rac-GAP rather than inhibition of Rac-GEF. In support of this notion, in EDG5 cells expressing the GTPase-deficient V12-Rac, S1P failed to inhibit membrane ruffling and PAK activation and to reduce the amount of GTP-bound V12-Rac (Fig. 2B, 4F, and 5). Interestingly, expression of V12-Rac in EDG5 cells caused an increase in the GTP-bound form of endogenous Rac and also prevented its inhibition by S1P (Fig. 4F). A plausible explanation would be that V12-Rac expressed in EDG5 cells could sequester cellular Rac-GAPs and consequently abolish EDG5-mediated, Rac-GAP-dependent inhibition by S1P of Rac activity. However, the extent of EDG5-mediated stimulation of Rac-GAP activity in the present results was rather smaller, although our assay condition might not be optimal. Thus, we speculate that it is possible that other mechanisms, including ones involving guanine nucleotide exchange inhibitor protein and Rac-GEF, could be cooperating.
Another point of interest in the present study is our observation that the expression of N17-Ras only partially inhibited, by 30% at most, migration toward IGF I and S1P (Fig. 3A). The expression of N17-Ras totally blocks the action of endogenous Ras in this cell type under the experimental condition we employed, because we previously observed that the adenovirus-mediated expression of N17-Ras abolished S1P-induced ERK activation (51). This observation is in contrast to a previous study (36) showing that expression of N17-Ras nearly abolished migration of NIH 3T3 cells toward the PDGF B chain and LPA. It was previously shown that microinjection of active Ras in Swiss 3T3 cells and other cell types mimics the effect of active Rac on the actin cytoskeleton (56), which led to the idea that Ras is located upstream of Rac. In support for this notion, a recent study (62) demonstrated that the Ras-GEF, Sos-1, mediates PDGF-induced Rac activation downstream of Ras by forming a complex with two other proteins. However, the present observations in CHO cells indicate that Rac is not always located downstream of Ras, in a linear fashion, suggesting that more than a single mechanism for chemoattractant-induced Rac activation are operative.
In conclusion, EDG5 negatively regulates Rac activity and cell migration. To our knowledge, this is the first report showing that there exists a receptor type associated with inhibition of Rac. Intriguingly, the receptors for previously reported chemorepellants are also of the heptahelical type (9, 19, 23, 37, 46). Cellular mechanisms by which these agonists exert inhibition of cell migration are presently poorly understood. It is an interesting question whether these chemorepellant receptors share the capacity to inhibit Rac activity with EDG5. Another interesting subject is what functional roles the chemorepellant receptor class with Rac-inhibiting activity play in morphogenesis involving cell motility during fetal life and pathological processes including inflammation and tumor invasion and metastasis during adult life.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministry of Education, Science and Culture of Japan, the Japan Society for the Promotion of Science Research for the Future Program, and the Hoh-Ansha Foundation.
We are grateful to J. Chernoff (the Fox Chase Cancer Center) and I. Saito (University of Tokyo) and J. Miyazaki (Osaka University) for providing p65PAK cDNA and pCAGGS-LacZ, respectively. We thank Nobuko Yamaguchi and Yasuhiro Hiratsuka for preparing the manuscript and technical assistance, respectively.
REFERENCES
- 1.Adam L, Vadlamudi R, Kondapaka S B, Chernoff J, Mendelsohn J, Kumar R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J Biol Chem. 1998;273:28238–28246. doi: 10.1074/jbc.273.43.28238. [DOI] [PubMed] [Google Scholar]
- 2.Akasaki T, Koga H, Sunimoto H. Phosphoinositide 3-kinase-dependent and -independent activation of the small GTPase Rac2 in human neutrophils. J Biol Chem. 1999;274:18055–18059. doi: 10.1074/jbc.274.25.18055. [DOI] [PubMed] [Google Scholar]
- 3.Allen W E, Zicha D, Ridley A J, Jones G E. A role for Cdc42 in macrophage chemotaxis. J Cell Biol. 1998;141:1147–1157. doi: 10.1083/jcb.141.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An S, Zheng Y, Bleu T. Sphingosine 1-phosphate-induced cell proliferation, survival, and related signaling events mediated by G protein-coupled receptors Edg3 and Edg5. J Biol Chem. 2000;275:288–296. doi: 10.1074/jbc.275.1.288. [DOI] [PubMed] [Google Scholar]
- 5.An S. Molecular identification and characterization of G protein-coupled receptors for lysophosphatidic acid and sphingosine 1-phosphate. Ann N Y Acad Sci. 2000;905:25–33. doi: 10.1111/j.1749-6632.2000.tb06535.x. [DOI] [PubMed] [Google Scholar]
- 6.Anand-Apte B, Zetter B. Signaling mechanisms in growth factor-stimulated cell motility. Stem Cells. 1997;15:259–267. doi: 10.1002/stem.150259. [DOI] [PubMed] [Google Scholar]
- 7.Anand-Apte B, Zetter B R, Viswanathan A, Qiu R G, Chen J, Ruggieri R, Symons M. Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by the Rac and extracellular signal-regulated kinase pathways. J Biol Chem. 1997;272:30688–30692. doi: 10.1074/jbc.272.49.30688. [DOI] [PubMed] [Google Scholar]
- 8.Ancellin N, Hla T. Differential pharmacological properties and signal transduction of the sphingosine 1-phosphate receptors EDG-1, EDG-3, and EDG-5. J Biol Chem. 1999;274:18997–19002. doi: 10.1074/jbc.274.27.18997. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong R A. Investigation of the inhibitory effects of PGE2 and selective EP agonists on chemotaxis of human neutrophils. Br J Pharmacol. 1995;116:2903–2908. doi: 10.1111/j.1476-5381.1995.tb15943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagrodia S, Taylor S J, Creasy C L, Chernoff J, Cerione R A. Identification of a mouse p21Cdc42/Rac activated kinase. J Biol Chem. 1995;270:22731–22737. doi: 10.1074/jbc.270.39.22731. [DOI] [PubMed] [Google Scholar]
- 11.Bokoch G M, Reilly A M, Daniels R H, King C C, Olivera A, Spioegel S, Knaus U G. A GTPase-independent mechanism of p21-activated kinase activation. Regulation by sphingosine and other biologically active lipids. J Biol Chem. 1998;273:8137–8144. doi: 10.1074/jbc.273.14.8137. [DOI] [PubMed] [Google Scholar]
- 12.Bornfeldt K E, Graves L M, Rainse E W, Igarashi Y, Wayman G, Yamamura S, Yatomi Y, Sidhu J S, Krebs E G, Hakomori S, Ross R. Sphingosine-1-phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cells: spatial and temporal modulation of PDGF chemotactic signal transduction. J Cell Biol. 1995;130:193–206. doi: 10.1083/jcb.130.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bretscher M S. Moving membrane up to the front of migrating cells. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81246-5. [DOI] [PubMed] [Google Scholar]
- 14.Chernoff J. Close encounters of the LIM-kinase. Nat Cell Biol. 1999;1:E115–E117. doi: 10.1038/12942. [DOI] [PubMed] [Google Scholar]
- 15.Chun J, Contos J J, Munroe D. A growing family of receptor genes for lysophosphatidic acid (LPA) and other lysophospholipids (LPs) Cell Biochem Biophys. 1999;30:213–242. doi: 10.1007/BF02738068. [DOI] [PubMed] [Google Scholar]
- 16.Cuvillier O, Pirianov G, Kleuser B, Vanek P G, Coso O A, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 17.Deguchi J, Namba T, Hamada H, Nakaoka T, Abe J, Sato O, Miyata T, Makuuchi M, Kurokawa K, Takuwa Y. Targeting endogenous platelet-derived growth factor B-chain by adenovirus-mediated gene transfer potently inhibits in vivo smooth muscle proliferation after arterial injury. Gene Ther. 1999;6:956–965. doi: 10.1038/sj.gt.3300918. [DOI] [PubMed] [Google Scholar]
- 18.Fox P L, Sa G, Dobrowolski S F, Stacey D W. The regulation of endothelial cell motility by p21 ras. Oncogene. 1994;9:3519–3526. [PubMed] [Google Scholar]
- 19.Garrido E, Delgado M, Martinez C, Gomariz R P, la Fuente M D. Pituitary adenylate cyclase-activating polypeptide (PACAP38) modulates lymphocyte and macrophage functions: stimulation of adherence and opposite effect on mobility. Neuropeptides. 1996;30:583–595. doi: 10.1016/s0143-4179(96)90042-6. [DOI] [PubMed] [Google Scholar]
- 20.Gonda K, Okamoto H, Takuwa N, Yatomi Y, Okazaki H, Sakurai T, Kimura S, Sillard R, Harii K, Takuwa Y. The novel sphingosine 1-phosphate receptor AGR16 is coupled via pertussis toxin-sensitive and -insensitive G-proteins to multiple signalling pathways. Biochem J. 1999;337:67–75. [PMC free article] [PubMed] [Google Scholar]
- 21.Gräler M H, Brenhardt G, Lipp M. EDG6, a novel G-protein-coupled receptor related to receptors for bioactive lysophospholipids, is specifically expressed in lymphoid tissue. Genomics. 1998;53:164–169. doi: 10.1006/geno.1998.5491. [DOI] [PubMed] [Google Scholar]
- 22.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 23.Harvath L, Robbins J D, Russel A A, Seamon K B. cAMP and human neutrophil chemotaxis. Elevation of cAMP differentially affects chemotactic responsiveness. J Immunol. 1991;146:224–232. [PubMed] [Google Scholar]
- 24.Hawkins P T, Eguinoa A, Stokoe D, Cooke F T, Walters R, Wennström S, Claesson-Welsh L, Evans T, Symons M, Stephens L. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 25.Hla T, Maciag T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J Biol Chem. 1990;265:9308–9313. [PubMed] [Google Scholar]
- 26.Hla T, Lee M J, Ancellin N, Liu C H, Thangada S, Thompson B D, Kluk M. Sphingosine-1-phosphate: extracellular mediator or intracellular second messenger? Biochem Pharmacol. 1999;58:201–217. doi: 10.1016/s0006-2952(99)00086-6. [DOI] [PubMed] [Google Scholar]
- 27.Hooshmand-Rad R, Claesson-Welsh L, Wennström S, Yokota K, Siegbahn A, Heldin C H. Involvement of phosphatidylinositide 3′-kinase and Rac in platelet-derived growth factor-induced actin reorganization and chemotaxis. Exp Cell Res. 1997;234:434–441. doi: 10.1006/excr.1997.3636. [DOI] [PubMed] [Google Scholar]
- 28.Hordijk P L, ten Klooster J P, van der Kammen R A, Michiels F, Oomen L C, Collard J G. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- 29.Igarashi Y. Sphingosine-1-phosphate as an intracellular signaling molecule. Ann N Y Acad Sci. 1998;845:19–31. doi: 10.1111/j.1749-6632.1998.tb09659.x. [DOI] [PubMed] [Google Scholar]
- 30.Im D S, Heise C E, Ancellin N, O'Dowd B F, Shei G, Heavens R P, Rigby M R, Hla T, Mandala S, McAllister G, George S R, Lynch K R. Characterization of a novel sphingosine 1-phosphate receptor, Edg-8. J Biol Chem. 2000;275:14284–14286. doi: 10.1074/jbc.275.19.14281. [DOI] [PubMed] [Google Scholar]
- 31.Kawa S, Kimura S, Hakomori S, Igarashi Y. Inhibition of chemotactic motility and trans-endothelial migration of human neutrophils by sphingosine 1-phosphate. FEBS Lett. 1997;420:196–200. doi: 10.1016/s0014-5793(97)01516-0. [DOI] [PubMed] [Google Scholar]
- 32.Keely P J, Westwick J K, Whitehead I P, Der C J, Parise L V. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 33.King C C, Zenke F T, Dawson P E, Dutil E M, Newton A C, Hemmings B A, Bokoch G M. Sphingosine is a novel activator of 3-phosphoinositide-dependent kinase 1. J Biol Chem. 2000;275:18108–18113. doi: 10.1074/jbc.M909663199. [DOI] [PubMed] [Google Scholar]
- 34.Kon J, Sato K, Watanabe T, Tomura H, Kuwabara A, Kimura T, Tammama K, Ishizuka T, Murata N, Kanda T, Kobayashi I, Ohta H, Ui M, Okajima F J. Comparison of intrinsic activities of the putative sphingosine 1-phosphate receptor subtypes to regulate several signaling pathways in their cDNA-transfected Chinese hamster ovary cells. J Biol Chem. 1999;274:23940–23947. doi: 10.1074/jbc.274.34.23940. [DOI] [PubMed] [Google Scholar]
- 35.Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kundra V, Anand-Apte B, Feig L A, Zetter B R. The chemotactic response to PDGF-BB: evidence of a role for Ras. J Cell Biol. 1995;130:725–731. doi: 10.1083/jcb.130.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.la Fuente M D, Carrasco M, Rio M D, Hernanz A. Modulation of murine lymphocyte functions by sulfated cholecystokinin octapeptide. Neuropeptides. 1998;32:225–233. doi: 10.1016/s0143-4179(98)90041-5. [DOI] [PubMed] [Google Scholar]
- 38.Lauffenburger D A, Horwitz A F. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 39.Lee M J, Van B J, Thangada S, Liu C H, Hand A R, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 40.Lee M J, Thangada S, Claffey K P, Ancellin N, Liu C H, Kluk M, Volpi M, Sha'afi R I, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 41.Lee O H, Kim Y M, Lee Y M, Moon E J, Lee D J, Kim J H, Kim K W, Kown Y G. Sphingosine 1-phosphate induces angiogenesis: its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1999;264:743–750. doi: 10.1006/bbrc.1999.1586. [DOI] [PubMed] [Google Scholar]
- 42.Leng J, Klemke R L, Reddy A C, Cheresh D A. Potentiation of cell migration by adhesion-dependent cooperative signals from the GTPase Rac and Raf kinase. J Biol Chem. 1999;274:37855–37861. doi: 10.1074/jbc.274.53.37855. [DOI] [PubMed] [Google Scholar]
- 43.Luo L, Liao Y J, Jan L Y, Jan Y N. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;11:1738–1747. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- 44.Lynch K R, Im D S. Life on the edg. Trend Pharmacol Sci. 1999;20:473–475. doi: 10.1016/s0165-6147(99)01401-7. [DOI] [PubMed] [Google Scholar]
- 45.Ma A D, Metjima A, Bagrodia S, Taylor S, Abrams C S. Cytoskeletal reorganization by G protein-coupled receptors is dependent on phosphoinositide 3-kinase γ, a Rac guanosine exchange factor, and Rac. Mol Cell Biol. 1998;18:4744–4751. doi: 10.1128/mcb.18.8.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makman M H, Bilfinger T V, Stefano G B. Human granulocytes contain an opiate alkaloid-selective receptor mediating inhibition of cytokine-induced activation and chemotaxis. J Immunol. 1995;154:1323–1330. [PubMed] [Google Scholar]
- 47.Manser E, Leung T, Salihuddin H, Zhao Z S, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 48.Mitsui H, Takuwa N, Kurokawa K, Exton J H, Takuwa Y. Dependence of activated Gα12-induced G1 to S phase cell cycle progression on both Ras/mitogen-activated protein kinase and Ras/Rac1/Jun N-terminal kinase cascades in NIH3T3 fibroblasts. J Biol Chem. 1997;272:4904–4910. doi: 10.1074/jbc.272.8.4904. [DOI] [PubMed] [Google Scholar]
- 49.Moolenaar W H. Bioactive lysophospholipids and their G protein-coupled receptors. Exp Cell Res. 1999;253:230–238. doi: 10.1006/excr.1999.4702. [DOI] [PubMed] [Google Scholar]
- 50.Neptune E R, Bourne H R. Receptors induce chemotaxis by releasing the βγ subunit of Gi, not by activating Gq or Gs. Proc Natl Acad Sci USA. 1997;94:14489–14494. doi: 10.1073/pnas.94.26.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okamoto H, Takuwa N, Gonda K, Okazaki H, Chang K, Yatomi Y, Shigematsu H, Takuwa Y. EDG1 is a functional sphingosine-1-phosphate receptor that is linked via a Gi/o to multiple signaling pathways, including phospholipase C activation, Ca2+ mobilization, Ras-mitogen-activated protein kinase activation, and adenylate cyclase inhibition. J Biol Chem. 1998;273:27104–27110. doi: 10.1074/jbc.273.42.27104. [DOI] [PubMed] [Google Scholar]
- 52.Okamoto H, Takuwa N, Yatomi Y, Gonda K, Shigematsu H, Takuwa Y. EDG3 is a functional receptor specific for sphingosine 1-phosphate and sphingosylphosphorylcholine with signaling characteristics distinct from EDG1 and AGR16. Biochem Biophys Res Commun. 1999;260:203–208. doi: 10.1006/bbrc.1999.0886. [DOI] [PubMed] [Google Scholar]
- 53.Okazaki H, Ishizaka N, Sakurai T, Kurokawa K, Gonda K, Kumada M, Takuwa Y. Molecular cloning of a novel putative G protein-coupled receptor expressed in the cardiovascular system. Biochem Biophys Res Commun. 1993;190:1104–1109. doi: 10.1006/bbrc.1993.1163. [DOI] [PubMed] [Google Scholar]
- 54.Oliver A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- 55.Ren X D, Kiosses W B, Schwartz M A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall H. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 57.Rius R A, Edsall L C, Spiegel S. Activation of sphingosine kinase in pheochromocytoma PC12 neuronal cells in response to trophic factors. FEBS Lett. 1997;417:173–176. doi: 10.1016/s0014-5793(97)01277-5. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 59.Sadahira Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine 1-phosphate, a specific endogenous signaling molecule controlling cell motility and tumor cell invasiveness. Proc Natl Acad Sci USA. 1992;89:9686–9690. doi: 10.1073/pnas.89.20.9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanchez-Madrid F, del Pozo M A. Leukocyte polarization in cell migration and immune interactions. EMBO J. 1999;18:501–511. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sander E E, van Delft S, ten Klooster J P, Reid T, van der Kammen R A, Michels F, Collard J G. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J Cell Biol. 1998;143:1385–1398. doi: 10.1083/jcb.143.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scita G, Nordstrom J, Carbone R, Tenca P, Giardia G, Gutkind S, Biarnegard M, Betsholtz C, DiFiore P P. EPS8 and E3B1 transduce signals from Ras to Rac. Nature. 1999;401:290–293. doi: 10.1038/45822. [DOI] [PubMed] [Google Scholar]
- 63.Self A J, Hall A. Measurement of intrinsic nucleotide exchange and GTP hydrolysis rates. Methods Enzymol. 1995;256:67–76. doi: 10.1016/0076-6879(95)56010-6. [DOI] [PubMed] [Google Scholar]
- 64.Sells M A, Boyd J T, Chernoff J. p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J Cell Biol. 1999;145:837–849. doi: 10.1083/jcb.145.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spiegel S. Sphingosine 1-phosphate: a prototype of a new class of second messengers. J Leukoc Biol. 1999;65:341–344. doi: 10.1002/jlb.65.3.341. [DOI] [PubMed] [Google Scholar]
- 66.Spiegel S, Milstien S. Functions of a new family of sphingosine-1-phosphate receptors. Biochim Biophys Acta. 2000;1484:107–116. doi: 10.1016/s1388-1981(00)00010-x. [DOI] [PubMed] [Google Scholar]
- 67.Takuwa N, Fukui Y, Takuwa Y. Cyclin D1 expression mediated by phosphatidylinositol 3-kinase through mTOR-p70(S6K)-independent signaling in growth factor-stimulated NIH 3T3 fibroblasts. Mol Cell Biol. 1999;19:1346–1358. doi: 10.1128/mcb.19.2.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takuwa, Y., H. Okamoto, N. Takuwa, K. Gonda, N. Sugimoto, and S. Sakurada. Subtype-specific, differential activities of the EDG family receptors for sphingosine-1-phosphate, a novel lysophospholipid mediator. Mol. Cell. Endocrinol., in press. [DOI] [PubMed]
- 69.van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 70.van Brocklyn J R, Lee M J, Menzeleev R, Olivera A, Edsall L, Cuvillier O, Thomas D M, Coompman P J, Thangada S, Liu C H, Hla T, Spiegel S. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J Cell Biol. 1998;142:229–240. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Brocklyn J R, Tu Z, Edsall L C, Schmidt R R, Spiegel S. Sphingosine 1-phosphate-induced cell rounding and neurite retraction are mediated by the G protein-coupled receptor H218. J Biol Chem. 1999;274:4626–4632. doi: 10.1074/jbc.274.8.4626. [DOI] [PubMed] [Google Scholar]
- 72.Wang F, Nahara K, Olivera A, Thompson E W, Spiegel S. Involvement of focal adhesion kinase in inhibition of motility of human breast cancer cells by sphingosine 1-phosphate. Exp Cell Res. 1999;247:17–28. doi: 10.1006/excr.1998.4327. [DOI] [PubMed] [Google Scholar]
- 73.Wang F, van Brocklyn J R, Hobson J P, Movafagh S, Zukowska-Grojec Z, Milsime S, Spiegel S. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem. 1999;274:35343–35350. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- 74.Wang F, van Brocklyn J R, Edsall L, Nava V E, Spiegel S. Sphingosine-1-phosphate inhibits motility of human breast cancer cells independently of cell surface receptors. Cancer Res. 1999;59:6185–6191. [PubMed] [Google Scholar]
- 75.Windh R T, Lee M J, Hla T, An S, Barr A J, Maning D R. Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the G(i), G(q), and G(12) families of heterotrimeric G proteins. J Biol Chem. 1999;274:27351–27358. doi: 10.1074/jbc.274.39.27351. [DOI] [PubMed] [Google Scholar]
- 76.Yamaguchi F, Tokuda M, Hatase O, Brenner S. Molecular cloning of the novel human G protein-coupled receptor (GPCR) gene mapped on chromosome 9. Biochem Biophys Res Commun. 1996;227:608–614. doi: 10.1006/bbrc.1996.1553. [DOI] [PubMed] [Google Scholar]
- 77.Yamamura S, Yatomi Y, Ruan F, Sweeney E A, Hakomori S, Igarashi Y. Sphingosine 1-phosphate regulates melanoma cell motility through a receptor-coupled extracellular action and in a pertussis toxin-insensitive manner. Biochemistry. 1997;36:10751–10759. doi: 10.1021/bi970926s. [DOI] [PubMed] [Google Scholar]
- 78.Yatomi Y, Ruan F Q, Hakomori S I, Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- 79.Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, Satoh K, Ozaki Y, Kume S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem (Tokyo) 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- 80.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 81.Zondag G C, Postma F R, Etten I V, Verlaan I, Moollenaar W H. Sphingosine 1-phosphate signaling through the G-protein-coupled receptor Edg-1. Biochem J. 1998;330:605–609. doi: 10.1042/bj3300605. [DOI] [PMC free article] [PubMed] [Google Scholar]