Abstract
Background
There are limited data on the long-term adverse clinical outcomes of adults with metabolic dysfunction-associated fatty liver disease (MAFLD).
Methods
This is a single-centre prospective study of a well-characterized cohort of MAFLD patients who underwent liver biopsy and followed every 6–12 months for adverse clinical outcomes.
Results
The data for 202 patients were analyzed [median age 55.0 (48.0–61.3) years old; male, 47.5%; obese, 88.6%; diabetes mellitus, 71.3%; steatohepatitis, 76.7%; advanced fibrosis, 27.2%]. The median follow-up interval was 7 (4–8) years. The cumulative incidence of liver-related events, cardiovascular events, malignancy and mortality was 0.43, 2.03, 0.60 and 0.60 per 100 person-years of follow-up, respectively. Liver-related events were only seen in patient with advanced fibrosis at 9.1% vs 0% in patient without advanced liver fibrosis (p < 0.001). The cumulative incidence of liver-related events among patients with advanced fibrosis was 1.67 per 100 person-years of follow-up. When further stratified to bridging fibrosis and cirrhosis, the cumulative incidence of liver-related events was 1.47 and 3.85 per 100 person-years of follow-up, respectively. Advanced fibrosis was not significantly associated with cardiovascular events, malignancy or mortality. The cumulative incidence of liver-related events, cardiovascular events, malignancy and mortality were not significantly different between patients with and without steatohepatitis and between obese and non-obese patients. However, liver-related events were only seen among obese patients.
Conclusion
Overall, the cumulative incidence of liver-related event is low in patients with MAFLD, but it is much higher among those with advanced fibrosis. However, there is a relatively high cumulative incidence of cardiovascular event among patients with MAFLD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12072-023-10550-9.
Keywords: MAFLD, Non-alcoholic fatty liver disease, NAFLD, Cardiovascular event, Liver-related event
Introduction
Non-alcoholic fatty liver disease (NAFLD) has been increasing alongside the increasing prevalence of obesity worldwide with a recent meta-analysis estimating its global prevalence to be a staggering 29.8% [1]. In Asia, the prevalence of NAFLD has increased significantly over time from 25.3% between 1999 and 2005, to 28.5% between 2006 and 2011, and 33.9% between 2012 and 2017 [2]. The current understanding of the prognosis of the disease is largely based on retrospective post hoc analyses of existing data sets, mostly from Western populations [3, 4, 5, 6, 7]. Data from prospective cohort studies, especially from Asian populations, is severely lacking. These data are crucial, from being used in estimating the burden of the disease, to informing policy-makers, to the planning of clinical trials, etcetera. Furthermore, in 2020, a new term, metabolic dysfunction-associated fatty liver disease (MAFLD), was proposed for the disease. The diagnosis of MAFLD is made in a person with fatty liver based on imaging, noninvasive score, or histology, if the person is overweight or obese, has type 2 diabetes mellitus (T2DM), or has at least two metabolic risk abnormalities [8, 9]. The rationale for and argument against the new nomenclature is not the focus of this paper, and are covered elsewhere [10, 11]. The new term and its definition have been endorsed by the Asian Pacific Association for the Study of the Liver [12], and the Malaysian Society of Gastroenterology and Hepatology [13, 14], among others. Studies on long-term outcome of the disease using the new term MAFLD are needed. Therefore, the primary objective of this study is to provide long-term clinical outcome data of adult patients with MAFLD. In addition, this study aimed to compare the severity of liver disease and the clinical outcomes of obese and non-obese MAFLD patients, an area that has seen contrasting results in the literature so far [15].
Methods
Adult NAFLD patients (> 18 years old) seen at the Gastroenterology and Hepatology Clinic who underwent a liver biopsy were invited to participate in this study. All subjects had ultrasound evidence of fatty liver with exclusion of significant alcohol intake, consumption of medications that can lead to hepatic steatosis, viral hepatitis B and C infection and other causes of chronic liver disease, where indicated [16]. Liver biopsy was performed when steatohepatitis was suspected (e.g., persistently elevated serum aminotransferase level, high liver stiffness measurement, obese patients with the metabolic syndrome), and if the patient agreed to undergo the procedure.
Much later, following the commencement of the study, the new term MAFLD was conceptualized. In view of this important development, additional analyses were performed to characterize the enrolled subjects based on the new term and its definition. The proportion of subjects with MAFLD and type 2 diabetes mellitus, MAFLD without type 2 diabetes mellitus but who were overweight or obese, lean MAFLD without type 2 diabetes mellitus who had at least 2 metabolic risk abnormalities, and non-MAFLD steatosis was determined [17]. Furthermore, when diabetes mellitus, overweight or obesity, and the presence of at least 2 metabolic risk abnormalities were considered as separate risks, the proportion of subjects who had 1, 2 and 3 risks was determined [18].
Enrolled subjects were followed every 6–12 months. Demographic, clinical, anthropometric and laboratory data were collected using a standard protocol. Height and weight were recorded using standard equipment. Overweight was defined as body mass index (BMI) 23–24.9 kg per m2 while obesity was defined as BMI ≥ 25 kg per m2 [19]. Waist circumference was measured in the standing position at the midpoint between the lowest margin of the least palpable rib and the top of the iliac crest. Central obesity was defined as a waist circumference of > 90 cm for male and > 80 cm for female [20]. Venous blood was sampled after an overnight fast for complete blood count, blood glucose, glycated hemoglobin (HbA1c), lipid profile and liver profile. The diagnosis of diabetes mellitus was either self-reported, or based on the use of medication for diabetes mellitus, fasting blood glucose (FBS) level > 7.0 mmol/L or HbA1c > 6.5% [21]. The diagnosis of hypertension was either self-reported, or based on the use of medication for hypertension, systolic blood pressure (SBP) ≥ 130 mmHg or diastolic blood pressure (DBP) ≥ 85 mmHg [22]. Dyslipidemia was either self-reported, or based on the use of lipid-lowering medication, total cholesterol ≥ 5.2 mmol/L, triglycerides ≥ 1.7 mmol/L, HDL cholesterol < 1.0 mmol/L for male or < 1.3 mmol/L for female or LDL cholesterol ≥ 3.4 mmol/L [23].
Liver biopsy and histological assessment
At baseline, ultrasonography-guided percutaneous liver biopsies were performed by experienced operators (W.K.C, S.M.) using 18G Temno II semiautomatic biopsy needle (Cardinal Health, Dublin, OH). Liver biopsy slides were stained with hematoxylin and eosin (H&E) and Masson’s trichrome. An experienced histopathologist (N.R.N.M) who was blinded to the clinical data examined the liver biopsy slides and reported the findings based on the Non-alcoholic Steatohepatitis (NASH) Clinical Research Network (CRN) Scoring System [24]. Steatohepatitis was defined as the presence of steatosis, lobular inflammation, and hepatocyte ballooning (≥ grade 1 each), while advanced liver fibrosis was defined as histological fibrosis stage 3 or 4.
Outcomes
Outcomes that occurred during the follow-up period and the date of occurrence were recorded as per reported by patient and corroborated with electronic medical records if the patient was admitted to our hospital. The outcomes included liver-related events (i.e., gastroesophageal varices, variceal bleeding, ascites, hepatocellular carcinoma, spontaneous bacterial peritonitis, hepatorenal syndrome, hepatic encephalopathy), cardiovascular events (i.e., acute myocardial infarction, revascularization, congestive cardiac failure and stroke), extra-hepatic malignancies, and mortality. Patients with history of cardiovascular event prior to enrolment into the study were excluded from the analysis on cardiovascular events. The cause of death was based on information provided by the deceased’s family member. Data was collected until November 2021. Telephone call was made if a subject was not seen in the clinic for > 12 months. A letter was sent to re-establish contact if a subject was uncontactable after telephone call attempts on three different days.
Statistical analysis
A standard statistical software (SPSS 26.0; SPSS, Chicago, IL, USA) was used for data analysis. Continuous variables were expressed as mean ± standard deviation or median (interquartile range) and analyzed using t test or Mann–Whitney test, where appropriate. Categorical variables were expressed as percentages and analyzed using Chi Square test or Fisher’s exact test, where appropriate. The cumulative incidence of outcome events was determined by dividing the number of incident events during follow-up by the number of person-years at risk. Analysis of cumulative incidence of outcome events was further stratified based on the presence of steatohepatitis, advanced liver fibrosis and whether subjects were obese or non-obese. Comparison of outcome events between groups were performed using Kaplan–Meier curves and log-rank test. Cox proportional hazards regression analysis was used to identify independent risk factors associated with outcomes of interest, where appropriate. A p value of < 0.05 was considered statistically significant for all analyses.
Results
Baseline patient characteristics
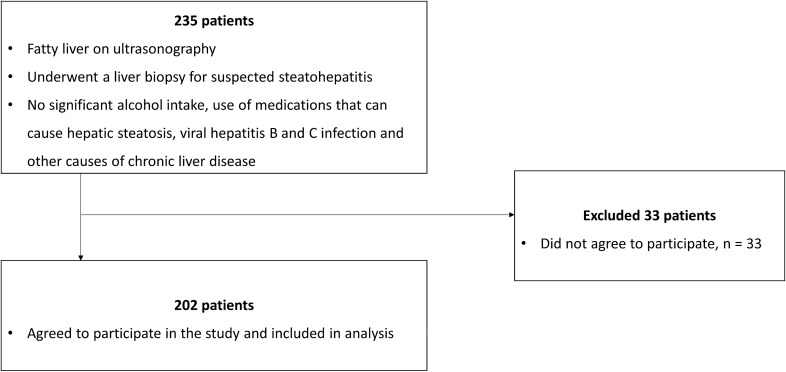
Subject enrolment commenced in May 2012 and ended in January 2018. Majority (86%) of patients who underwent liver biopsy during the enrolment period agreed to participate in the study. The study flowchart is shown in Fig. 1. The data for 202 patients were analyzed. Baseline patient characteristics are presented in Table 1. The median age of the study population was 55 (48–61) years, and 47.5% were male. The study population mainly consisted of ethnic Malays (47%), followed by Chinese (28.2%), and Indians (23.8%). Majority of patients were obese (88.6%) and centrally obese (95.5%) with a median BMI of 29.3 (27.1–32.4) kg per m2. The proportion of patients who were lean and overweight was 3.0% and 8.4%, respectively. Central obesity was present in 66.7% and 83.3% of patients who were lean and overweight, respectively. Majority of patients had diabetes mellitus (71.3%), hypertension (85.6%) and dyslipidemia (94.1%), while prior cardiovascular event was present in 9.4% of the patients. Majority of the patients had steatohepatitis (76.7%), and the distribution of fibrosis stages is as follows: F0, 25.2%; F1 40.6%; F2, 6.9%; F3, 24.8%; and F4, 2.5%. Advanced liver fibrosis was present in 27.3% of the patients. All patients fulfilled the criteria for MAFLD. The proportion of patients with MAFLD and diabetes mellitus, MAFLD without diabetes mellitus but who were overweight or obese, and lean MAFLD without diabetes mellitus who had at least 2 metabolic risk abnormalities were 71.3%, 27.2% and 1.5%, respectively. Furthermore, when we considered diabetes mellitus, overweight or obesity, and the presence of at least 2 metabolic risk abnormalities as separate risks, we found that 11.9%, 26.2% and 61.9% of our study population had 1, 2 and 3 risks, respectively.
Fig. 1.
Study flowchart
Table 1.
Baseline patient characteristics
| Overall population, n = 202 | |
|---|---|
| Age, years | 55.0 (48.0–61.3) |
| Male, n (%) | 96 (47.5) |
| Ethnicity, n (%) | |
| Malay | 95 (47) |
| Chinese | 57 (28.2) |
| Indian | 48 (23.8) |
| Others | 2 (1) |
| Diabetes mellitus, n (%) | 144 (71.3) |
| Hypertension, n (%) | 173 (85.6) |
| Dyslipidemia, n (%) | 190 (94.1) |
| Prior cardiovascular event, n (%) | 19 (9.4) |
| Body mass index, kg per m2 | 29.7 (27.1–32.6) |
| Waist circumference, cm | 99.7 ± 10.9 |
| Lean, n (%) | 6 (3.0) |
| Overweight, n (%) | 17 (8.4) |
| Obesity, n (%) | 179 (88.6) |
| Central obesity, n (%) | 193 (96.0) |
| SBP, mmHg | 138 (128–148) |
| DBP, mmHg | 82 (75–89) |
| FBS, mmol/L | 6.3 (5.3–8.3) |
| HbA1c, % | 7.1 (5.9–8.0) |
| TG, mmol/L | 1.6 (1.2–2.1) |
| TC, mmol/L | 4.6 (4.0–5.2) |
| HDL, mmol/L | 1.2 (1.0–1.4) |
| LDL, mmol/L | 2.5 (2.0–3.2) |
| Albumin, g/L | 41.2 ± 3.6 |
| Bilirubin, µmol/L | 10 (8–14) |
| ALP, U/L | 76 (61–93) |
| ALT, U/L | 52 (35–88) |
| AST, U/L | 36 (26–54) |
| GGT, U/L | 70 (38–124) |
| Creatinine, µmol/L | 72 (59–90) |
| Platelet, × 109/L | 267 (224–306) |
| Steatosis, n (%) | |
| 0 | 3 (1.5) |
| 1 | 64 (31.7) |
| 2 | 109 (54) |
| 3 | 26 (12.9) |
| Lobular inflammation, n (%) | |
| 0 | 1 (0.5) |
| 1 | 129 (63.9) |
| 2 | 67 (33.2) |
| 3 | 5 (2.5) |
| Hepatocyte ballooning, n (%) | |
| 0 | 45 (22.3) |
| 1 | 105 (52.0) |
| 2 | 52 (25.7) |
| Fibrosis, n (%) | |
| 0 | 51 (25.2) |
| 1 | 82 (40.6) |
| 2 | 14 (6.9) |
| 3 | 50 (24.8) |
| 4 | 5 (2.5) |
| Advanced fibrosis, n (%) | 55 (27.2) |
SBP systolic blood pressure, DBP diastolic blood pressure, FBS fasting blood sugar, HbA1c hemoglobin A1c, TG triglyceride, TC total cholesterol, HDL high-density lipoprotein, LDL low-density lipoprotein, ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, GGT gamma-glutamyl transferase
Factors associated with steatohepatitis
Patients with steatohepatitis had greater BMI and waist circumference and were more likely to have central obesity, diabetes mellitus and hypertension (Supplementary Table 1). They have higher serum HbA1c, ALT, AST and GGT levels and a lower serum albumin level. Patients with steatohepatitis were more likely to have advanced liver fibrosis. On multivariate analysis, independent factors associated with steatohepatitis were presence of diabetes mellitus [odds ratio (OR) 2.38; 95% confidence interval (CI) 1.01–5.60; p value = 0.048] and advanced fibrosis (OR 14.39; 95% CI 1.81–114.06; p value = 0.012). (Supplementary Table 2).
Factors associated with advanced liver fibrosis
Patients with advanced liver fibrosis were older, had greater waist circumference and were more likely to have diabetes mellitus (Supplementary Table 3). They have higher serum FBS, HbA1c, AST and GGT levels and lower serum creatinine and platelet levels. Patients with advanced liver fibrosis were more likely to have steatohepatitis. On multivariate analysis, independent factors associated with advanced liver fibrosis were age (OR 1.05; 95% CI 1.01–1.10; p value = 0.027), presence of diabetes mellitus (OR 3.23; 95% CI 1.10–9.54; p value = 0.034), platelet count (OR 0.99; 95% CI 0.99–1.00; p value = 0.025) and presence of steatohepatitis (OR 15.63; 95% CI 1.93–126.84; p value = 0.010) (Supplementary Table 4).
Incidence of liver-related events, cardiovascular events, malignancy and mortality
The median follow-up interval was 7 (4–8) years and the total follow-up was 1164 person-years. Five patients (2.5%) developed liver-related events (gastroesophageal varices detected on screening endoscopy, n = 3; variceal hemorrhage, n = 1; and ascites and hepatocellular carcinoma, n = 1). Cardiovascular events developed in 22 patients (12.0%; acute myocardial infarction, n = 9; stroke, n = 8; revascularization, n = 4; and congestive cardiac failure, n = 1). Seven patients (3.5%) developed extra-hepatic malignancy (hematological malignancy, n = 2; colon cancer, n = 1; breast cancer; n = 1; cervical cancer, n = 1; thyroid cancer, n = 1; and ureteric cancer, n = 1). Seven patients (3.5%) died (causes of death included acute myocardial infarction, n = 2; liver cancer, n = 1; pneumonia, n = 1; thyroid cancer, n = 1; coronavirus disease, n = 1; unknown, n = 1). The cumulative incidence of liver-related events, cardiovascular events, malignancy and mortality was 0.43, 2.03, 0.60, and 0.60 per 100 person-years of follow-up, respectively.
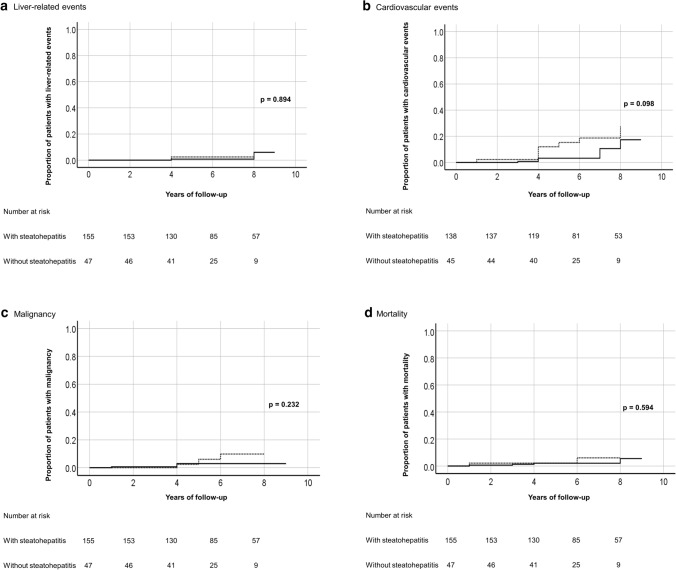
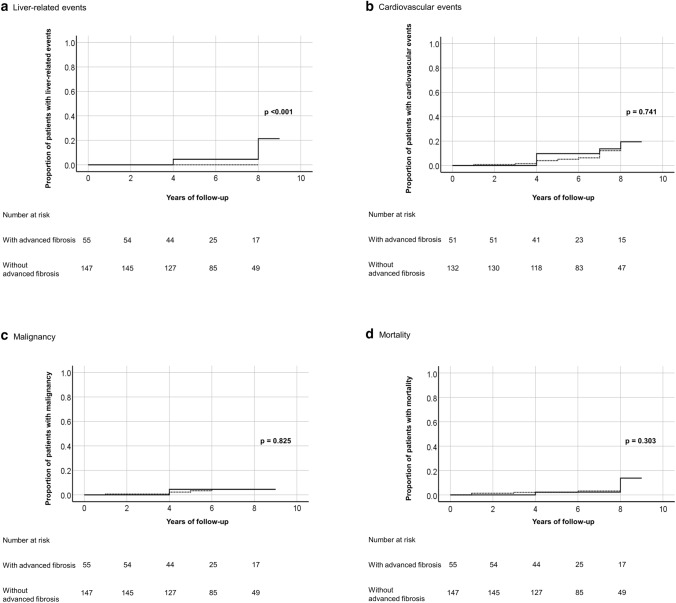
The number of liver-related events, cardiovascular events, malignancy and mortality and the corresponding cumulative incidence per 100 person-years of follow-up are shown in Table 2. There was no significant difference in the cumulative incidence of liver-related events (p = 0.894), cardiovascular events (p = 0.098), malignancy (p = 0.232) and mortality (p = 0.594) between patients with and without steatohepatitis (Fig. 2). However, liver-related events were only seen in patients with advanced liver fibrosis at 9.1% vs 0% in patient without advanced liver fibrosis (p < 0.001). When further stratified to bridging fibrosis and cirrhosis, the cumulative incidence of liver-related events was 1.47 and 3.85 per 100 person-years of follow-up, respectively. There was no significant difference in the cumulative incidence of cardiovascular events (p = 0.741), malignancy (p = 0.825), and mortality (p = 0.303) between patients with and without advanced liver fibrosis (Fig. 3).
Table 2.
Cumulative incidence of liver-related event, cardiovascular event, malignancy and mortality of overall populations, MAFLD patients without and with steatohepatitis, MAFLD patients without and with advanced liver fibrosis, and non-obese and obese MAFLD patients
| Event | Overall population | Without steatohepatitis | With steatohepatitis | Without advanced liver fibrosis | With advanced liver fibrosis | Non-obese | Obese | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Cumulative incidence per 100 person-year | n (%) | Cumulative incidence per 100 person-year | n (%) | Cumulative incidence per 100 person-year | n (%) | Cumulative incidence per 100 person-year | n (%) | Cumulative incidence per 100 person-year | n (%) | Cumulative incidence per 100 person-year | n (%) | Cumulative incidence per 100 person-year | |
| Liver-related event | 5 (2.5) | 0.43 | 1 (2.1) | 0.38 | 4 (2.6) | 0.44 | 0 (0) | 0.00 | 5 (9.1) | 1.67 | 0 (0) | 0.00 | 5 (2.9) | 0.49 |
| Ascites and hepatocellular carcinoma | 1 (0.5) | 0.09 | 0 (0) | 0.00 | 1 (0.6) | 0.11 | 0 (0) | 0.00 | 1 (1.8) | 0.33 | 0 (0) | 0.00 | 1 (0.6) | 0.10 |
| Gastroesophageal varices | 3 (1.5) | 0.26 | 1 (2.1) | 0.38 | 2 (1.3) | 0.22 | 0 (0) | 0.00 | 3 (5.5) | 1.00 | 0 (0) | 0.00 | 3 (1.7) | 0.29 |
| Variceal bleeding | 1 (0.5) | 0.09 | 0 (0) | 0.00 | 1 (0.6) | 0.11 | 0 (0) | 0.00 | 1 (1.8) | 0.33 | 0 (0) | 0.00 | 1 (0.6) | 0.10 |
| Hepatorenal syndrome | 0 (0) | 0.00 | 0 (0) | 0.00 | 0 (0) | 0.00 | 0 (0) | 0.00 | 0 (0) | 0.00 | 0 (0) | 0.00 | 0 (0) | 0.00 |
| Hepatic encephalopathy | 0 (0) | 0.00 | 0 (0) | 0.00 | 0 (0) | 0.00 | 0 (0) | 0.00 | 0 (0) | 0.00 | 0 (0) | 0.00 | 0 (0) | 0.00 |
| SBP | 0 (0) | 0.00 | 0 (0) | 0.00 | 0 (0) | 0.00 | 0 (0) | 0.00 | 0 (0) | 0.00 | 0 (0) | 0.00 | 0 (0) | 0.00 |
| Cardiovascular event | 22 (12.0) | 2.03 | 8 (17.8) | 3.11 | 14 (10.1) | 1.70 | 16 (12.1) | 1.99 | 6 (2.2) | 2.15 | 1 (4.5) | 0.77 | 21 (13.0) | 2.21 |
| Acute MI | 9 (4.9) | 0.83 | 2 (4.4) | 0.78 | 7 (5.1) | 0.85 | 8 (6.1) | 1.00 | 1 (2.0) | 0.36 | 1 (4.5) | 0.77 | 8 (5.0) | 0.84 |
| CCF hospitalization | 1 (0.5) | 0.09 | 0 (0) | 0.00 | 1 (0.7) | 0.12 | 0 (0) | 0.00 | 1 (2.0) | 0.36 | 0 (0) | 0.00 | 1 (0.6) | 0.11 |
| Revascularization | 4 (2.2) | 0.37 | 1 (2.2) | 0.39 | 3 (2.2) | 0.36 | 2 (1.5) | 0.25 | 2 (3.9) | 0.72 | 0 (0) | 0.00 | 4 (2.5) | 0.42 |
| Stroke | 8 (4.4) | 0.74 | 5 (11.1) | 1.95 | 3 (2.2) | 0.36 | 6 (4.5) | 0.75 | 2 (3.9) | 0.72 | 0 (0) | 0.00 | 8 (5.0) | 0.84 |
| Malignancy | 7 (3.5) | 0.60 | 3 (6.4) | 1.14 | 4 (2.6) | 0.44 | 5 (3.4) | 0.58 | 2 (3.6) | 0.67 | 2 (8.6) | 1.49 | 5 (2.8) | 0.49 |
| Colon | 1 (0.5) | 0.09 | 1 (2.1) | 0.38 | 0 (0) | 0.00 | 1 (0.7) | 0.12 | 0 (0) | 0.00 | 0 (0) | 0.00 | 1 (0.6) | 0.10 |
| Breast and ovarian | 1 (0.5) | 0.09 | 0 (0) | 0.00 | 1 (0.6) | 0.11 | 0 (0) | 0.00 | 1 (1.8) | 0.33 | 0 (0) | 0.00 | 1 (0.6) | 0.10 |
| Cervical | 1 (0.5) | 0.09 | 0 (0) | 0.00 | 1 (0.6) | 0.11 | 1 (0.7) | 0.12 | 0 (0) | 0.00 | 0 (0) | 0.00 | 1 (0.6) | 0.10 |
| Thyroid | 1 (0.5) | 0.09 | 1 (2.1) | 0.38 | 0 (0) | 0.00 | 1 (0.7) | 0.12 | 0 (0) | 0.00 | 0 (0) | 0.00 | 1 (0.6) | 0.10 |
| Ureteric | 1 (0.5) | 0.09 | 0 (0) | 0.00 | 1 (0.6) | 0.11 | 1 (0.7) | 0.12 | 0 (0) | 0.00 | 1 (4.3) | 0.75 | 0 (0) | 0.00 |
| Hematologic malignancy | 2 (1.0) | 0.17 | 1 (2.1) | 0.38 | 1 (0.6) | 0.11 | 0 (0) | 0.00 | 1 (1.8) | 0.33 | 1 (4.3) | 0.75 | 1 (0.6) | 0.10 |
| Mortality | 7 (3.5) | 0.60 | 2 (4.3) | 0.76 | 5 (3.2) | 0.55 | 4 (2.7) | 0.46 | 3 (5.5) | 1.00 | 2 (8.6) | 1.49 | 5 (2.8) | 0.49 |
| Liver cancer | 1 (0.5) | 0.09 | 0 (0) | 0.00 | 1 (0.6) | 0.11 | 0 (0) | 0.00 | 1 (1.8) | 0.33 | 0 (0) | 0.00 | 1 (0.6) | 0.10 |
| Acute MI | 2 (1.0) | 0.17 | 1 (2.1) | 0.38 | 1 (0.6) | 0.11 | 2 (1.4) | 0.23 | 0 (0) | 0.00 | 0 (0) | 0.00 | 2 (1.1) | 0.19 |
| Pneumonia | 1 (0.5) | 0.09 | 0 (0) | 0.00 | 1 (0.6) | 0.11 | 1 (0.7) | 0.12 | 0 (0) | 0.00 | 1 (4.3) | 0.75 | 0 (0) | 0.00 |
| Thyroid cancer | 1 (0.5) | 0.09 | 1 (2.1) | 0.38 | 0 (0) | 0.00 | 1 (0.7) | 0.12 | 0 (0) | 0.00 | 0 (0) | 0.00 | 1 (0.6) | 0.10 |
| COVID19 | 1 (0.5) | 0.09 | 0 (0) | 0.00 | 1 (0.6) | 0.11 | 0 (0) | 0.00 | 1 (1.8) | 0.33 | 0 (0) | 0.00 | 1 (0.6) | 0.10 |
| Unknown | 1 (0.5) | 0.09 | 0 (0) | 0.00 | 1 (0.6) | 0.11 | 0 (0) | 0.00 | 1 (1.8) | 0.33 | 1 (4.3) | 0.75 | 0 (0) | 0.00 |
SBP spontaneous bacterial peritonitis, CCF congestive cardiac failure, MI myocardial infarction
Fig. 2.
Kaplan–Meier curves showing comparison of outcome events, namely a liver-related events, b cardiovascular events, c malignancy, and d mortality between MAFLD patients with and without steatohepatitis (━━━, with steatohepatitis; - - - -, without steatohepatitis)
Fig. 3.
Kaplan–Meier curves showing comparison of outcome events, namely a liver-related events, b cardiovascular events, c malignancy, and d mortality between MAFLD patients with and without advanced liver fibrosis (━━━, with advanced fibrosis; - - - -, without advanced fibrosis)
Factors associated with cardiovascular events
On univariate analysis, factors associated with cardiovascular events were age, ethnicity, presence of diabetes mellitus, waist circumference, fasting blood glucose, HbA1c, triglyceride, HDL cholesterol, albumin, ALT and creatinine level (Table 3). On multivariate analysis, only serum creatinine level was found to be associated with cardiovascular events with a hazard ratio of 1.02 (95% CI 1.00–1.05, p value = 0.019). Analyses on factors associated with liver-related events, malignancy and mortality were not performed due to the small number of these events.
Table 3.
Univariate and multivariate cox proportional hazards regression analyses of factors associated with cardiovascular events in patients with MAFLD
| HR (95% CI) | p value | Adjusted HR (95% CI) | p value | |
|---|---|---|---|---|
| Age | 1.06 (1.01–1.11) | 0.021 | 1.06 (0.99–1.13) | 0.119 |
| Ethnicity | ||||
| Malay | – | – | – | – |
| Chinese | 1.20 (0.38–3.78) | 0.758 | 0.78 (0.21–2.93) | 0.710 |
| Indian | 3.12 (1.16–8.41) | 0.024 | 1.18 (0.38–3.67) | 0.777 |
| Others | 7.79 (0.94–64.58) | 0.057 | – | 0.989 |
| Diabetes mellitus | 2.98 (1.01–8.85) | 0.049 | – | – |
| Waist circumference, cm | 1.04 (1.01–1.08) | 0.017 | 1.04 (1.00–1.09) | 0.070 |
| Fasting blood glucose, mmol/L | 1.20 (1.07–1.35) | 0.002 | – | – |
| HbA1c, % | 1.34 (1.09–1.66) | 0.007 | 1.05 (0.80–1.36) | 0.739 |
| TG, mmol/L | 1.40 (1.07–1.85) | 0.016 | 1.18 (0.87–1.60) | 0.276 |
| HDL cholesterol, mmol/L | 0.12 (0.02–0.76) | 0.024 | 0.41 (0.04–4.51) | 0.467 |
| Albumin, g/L | 0.87 (0.77–0.98) | 0.024 | 0.95 (0.82–1.11) | 0.538 |
| ALT, U/L | 0.99 (0.97–1.00) | 0.033 | 0.99 (0.98–1.01) | 0.203 |
| Creatinine, µmol/L | 1.04 (1.02–1.06) | < 0.001 | 1.03 (1.00–1.05) | 0.017 |
HbA1c was entered into multivariate analysis as it was considered a better indicator of glycemic profile and control than diabetes mellitus and fasting blood glucose
TG triglyceride, ALT alanine aminotransferase, HR hazard ratio, 95% CI 95% confidence interval
Severity of liver disease at baseline and outcomes in non-obese patients
Among non-obese patients, the percentage of patients with steatohepatitis and advanced liver fibrosis was 60.9% and 21.7%, respectively. The corresponding percentages among obese patients were 78.8% and 27.9%, respectively. Non-obese patients were older, less likely to have central obesity and had lower waist circumference and higher serum albumin level. Among non-obese patients, the cumulative incidence of liver-related events, cardiovascular events, malignancy and mortality was 0.00, 0.77, 1.49, and 1.49 per 100 person-years of follow-up, respectively. The corresponding cumulative incidence among obese patients was 0.49, 2.21, 0.49 and 0.49 per 100 person-years of follow-up, respectively. The cumulative incidence of liver-related events, cardiovascular events, malignancy and mortality were not significantly different between obese and non-obese patients (p = 0.385, 0.240, 0.161 and 0.178, respectively).
Discussion
In this prospective study on a well-characterized cohort of MAFLD patients with baseline liver biopsy who had a median follow-up of 7 years, we found the occurrence of liver-related events, cardiovascular events, malignancy and mortality to be 0.43, 2.03, 0.60, and 0.60 per 100 person-years of follow-up, respectively. The cumulative incidence of liver-related events is relatively low at 0.43 per 100 person-years of follow-up. This is despite our study including only patients who had undergone a liver biopsy, which indicates that they were suspected to have more severe liver disease clinically at baseline. This translated to the relatively high percentage of patients with histological steatohepatitis and advanced fibrosis at 76.7% and 27.3%, respectively, which is consistent with that seen in tertiary care biopsy populations [25]. The finding of our study is similar to a multi-centre study by Sanyal and colleagues in the United States [26], which found the cumulative incidence of liver-related events to be 0.46 per 100 person-years of follow-up. Their study included 1773 patients (definite or borderline steatohepatitis, 75%; advanced fibrosis, 30%) with median follow-up of 4 years. This relatively low cumulative incidence of liver-related events is an important consideration in the planning of clinical trials because the Food and Drug Administration requires a study drug (following conditional approval based on histological response) to demonstrate reduction in liver-related events for it to be granted full approval. For this, clinical trials will need to include an adequately large number of patients with more severe liver disease to see a sufficient number of liver-related events within the time frame of the study. In fact, we only observed liver-related events among patients with advanced fibrosis in our study, and the incidence of liver-related events increased from bridging fibrosis to cirrhosis. This exponential increase in cumulative incidence of liver-related events is similar to that observed by Sanyal and colleagues [26], with the cumulative incidence being 0.99 and 2.69 per 100 person-years of follow-up among patients with bridging fibrosis and cirrhosis, respectively. This is also consistent with the results of a meta-analysis, which found that fibrosis stage is the single most important predictor of liver-related mortality with the risk increasing exponentially with increasing fibrosis stage [4].
Although the cumulative incidence of liver-related events is low, MAFLD contributes significantly to the burden of chronic liver disease due to its very high prevalence in the general population. In Malaysia, over half of its adult population is overweight or obese [27], and the prevalence of NAFLD has been estimated to be 22.7% based on a study on health check individuals [28], and as high as 37.4% in a similar and more recent study [29]. NAFLD is now the most common etiology of cirrhosis and/or hepatocellular carcinoma among patients seen in our clinic [30], and a recent study found an over two-fold increase in the proportion of hepatocellular carcinoma due to cryptogenic cause (most of which is recognized to be due to NAFLD), from 16.4% in an earlier study to 34.2%. Furthermore, this was not including the 7.4% of patients who had a diagnosis of nonalcoholic steatohepatitis (NASH) prior to the diagnosis of hepatocellular carcinoma [31, 32]. NASH is already recognized as one of the leading etiologies among adults awaiting liver transplantation and the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the United States [33, 34]. The burden of NAFLD has been projected to continue to increase globally [35, 36]. However, besides liver-related complications, MAFLD patients are also at risk of cardiovascular events due to its close association with the metabolic syndrome [37]. In fact, cardiovascular disease has long been recognized as the leading cause of mortality in patients with NAFLD [37, 38]. In our study, we found cardiovascular disease to be the leading cause of mortality and the cumulative incidence of cardiovascular events to be 2.03 per 100 person-years of follow-up with no significant difference between patients with and without steatohepatitis, advanced fibrosis or obesity. The cumulative incidence of cardiovascular events in our study is much higher than the cumulative incidence of cardiovascular events of 0.83 per 100 person-years of follow-up reported by Sanyal and colleagues, which may be due to ethnic and genetic differences as well as differences in the burden and optimal treatment of concomitant metabolic risk factors. Asians are known to develop metabolic complications at lower BMI than their Caucasian counterparts [39]. Besides that, a retrospective study and a subsequent prospective study in several Asian centres found high rates of suboptimal treatment of metabolic risk factors among patients with NAFLD [40, 41].
All in all, there is an urgent need to coordinate and streamline the care of patients with MAFLD. Patients with more severe liver disease (i.e., advanced fibrosis or cirrhosis) need to be identified and referred to specialist for further management. On the other hand, patients with milder disease should remain in primary care, where they are best managed [14]. An example of this is by using serum alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) level among patients with type 2 diabetes mellitus, who are at higher risk of more severe liver disease [14, 21]. As seen in this study, diabetes mellitus is an independent factor associated with both steatohepatitis and advanced fibrosis. As serum ALT and AST level may be normal in patients with NASH [42], simultaneous assessment of liver fibrosis (e.g., with a two-step approach i.e., Fibrosis-4 index, followed by liver stiffness measurement for patients with elevated Fibrosis-4 index) [14, 21, 43, 44] will complement the evaluation and serves as a safety net to identify patients with more severe liver disease but normal serum ALT and/or AST level.
Despite our best effort, this study has several limitations. First, we only included patients suspected to have more severe liver disease clinically who had undergone a liver biopsy. Therefore, the findings from our study are only applicable to a similar population and not to NAFLD or MAFLD patients in general. Secondly, although all included patients in our study fulfilled the MAFLD criteria, the findings cannot be generalized to patients with MAFLD and other concomitant liver disease because our study population only included patients who fulfilled both NAFLD and MAFLD criteria (i.e., overlapping NAFLD and MAFLD patients) [11]. We acknowledge that there is a need to look at the clinical outcomes of MAFLD patients with other concomitant liver disease (i.e., MAFLD only patients). We believe that such studies should look at each of the other concomitant causes of chronic liver disease separately, and that comparison should be made between patients with and without MAFLD for each of the other causes of chronic liver disease. Besides that, comparison should be made between patients with MAFLD without other concomitant liver disease and patients with MAFLD and each of the other causes of chronic liver disease. A systematic review and meta-analysis that focused on the non-overlapping groups found the prevalence of fibrosis (based on liver stiffness measurement or the fibrosis-4 score) to be highest in the MAFLD only group, intermediate in the overlapping NAFLD and MAFLD group, and lowest in the NAFLD only group [45]. In another study that was based on the National Health and Nutrition Survey III in the United States, the overall, cardiovascular and liver-related mortality was found to be not significantly different between NAFLD and MAFLD patients [46]. However, this was likely due to the large proportion of overlapping NAFLD and MAFLD patients at 73.2%. The overlapping NAFLD and MAFLD group has been estimated to make up 79.9% (95% confidence interval 75.3–83.9%) of patients with fatty liver disease [45]. In a subsequent study using the same dataset from the National Health and Nutrition Survey III, MAFLD was associated with increased mortality risk in participants with and without excessive alcohol consumption, but participants with both MAFLD and excessive alcohol consumption expressed the highest mortality risk [47]. It is interesting to note that all NAFLD patients included in this study fulfilled the MAFLD criteria naturally. This is not unexpected as the NAFLD patients included in this study were those who underwent a liver biopsy due to suspicion of having more severe liver disease, and NAFLD patients with more severe liver disease are likely to fulfil the criteria for MAFLD as well. Indeed, studies have shown that the serum ALT and AST levels and the prevalence of fibrosis were not significantly different between NAFLD patients who did not fulfil the criteria for MAFLD (i.e., NAFLD only patients) and subjects with no fatty liver disease [45], and that NAFLD only patients have extremely low risk of liver-related mortality [46].
Thirdly, we depended on patients’ self-reporting for outcome events during follow-up, which may be affected by recall bias. However, we corroborated with documentations in the electronic medical records for patients who were admitted in our hospital. Otherwise, discharge documents were reviewed, where available. Similarly, we depended on family members’ reporting on the date and cause of death for patients who have died. The cause of death was uncertain for only one of the patients. Fourth, the relatively small number of outcome events has limited our analysis on associated factors. Fifth, we aimed to compare between obese and non-obese patients, but the small number of non-obese patients as well as the relatively small number of outcome events has limited our analysis. However, we did not observe any liver-related events among non-obese patients, despite a substantial proportion of them having steatohepatitis and advanced fibrosis at baseline, which is due to the nature of their selection and inclusion into the study. Further studies with a larger number of patients are needed. In this study, we collected longitudinal anthropometric and laboratory data, including various non-invasive tests, but the results will be presented in separate papers due to the large amount of analysis, interpretation and discussion associated with them. There is an urgent need to demonstrate that non-invasive tests could act as a surrogate for clinical endpoints due to the various limitations of liver biopsy in clinical trials. Besides that, temporal change in anthropometry and laboratory indices are difficult to capture and data on their impact on clinical outcomes, especially liver-related outcomes, is scarce in the literature.
In conclusion, we reported the cumulative incidence of liver-related events, cardiovascular events, malignancy and mortality in a cohort of well-characterized MAFLD patients. The cumulative incidence of liver-related events is relatively low despite a high proportion of patients with steatohepatitis and advanced fibrosis at baseline. However, patients with advanced fibrosis are at increased risk of liver-related events, supporting the need for a simple assessment and referral pathway for patients with MAFLD. Furthermore, cardiovascular disease is the leading cause of mortality with a relatively high cumulative incidence of cardiovascular events, highlighting the need to improve the control of metabolic risk factors among patients with MAFLD. Further in-depth studies on this cohort may provide evidence for the use of non-invasive tests as surrogate for clinical endpoints and enable us to assess the impact of temporal changes in anthropometry and laboratory indices on liver-related outcomes in patients with MAFLD.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
WKC conceptualized the study. WLC, SEC, FC, NRNM, SM and WKC contributed to the data. WLC drafted the manuscript. All authors reviewed the draft manuscript, contributed to important intellectual content, and approved the final manuscript.
Funding
This study was funded by the University of Malaya Research Grant (Project No.: RG536-13HTM) and the University of Malaya Special Research Fund (Project No.: BKS067-2017).
Data availability
Request for data sharing should be made to the corresponding author. Individual patient-level data containing confidential or identifiable patient information are subject to patient privacy and cannot be shared.
Declarations
Conflict of interest
WKC has served as a consultant or advisory board member for Roche, Abbvie, Boehringer Ingelheim and Novo Nordisk; and a speaker for Viatris and Hisky Medical. The other authors have no conflict of interest to declare.
Ethical approval
This is a single-centre prospective study of a well-characterized cohort of NAFLD patients. The study followed the ethical guidelines of the 1975 Helsinki Declaration, and ethical approval was obtained from our institutional review board prior to the commencement of the study (MECID. No.: 20168124134).
Informed consent
All participating subjects provided informed consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Le MH, et al. 2019 Global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;20(12):2809–2817.e28. doi: 10.1016/j.cgh.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Li J, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4(5):389–398. doi: 10.1016/S2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- 3.Unalp-Arida A, Ruhl CE. Noninvasive fatty liver markers predict liver disease mortality in the U.S. population. Hepatology. 2016;63(4):1170–1183. doi: 10.1002/hep.28390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dulai PS, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65(5):1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angulo P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–397.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor RS, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. 2020;158(6):1611–1625.e12. doi: 10.1053/j.gastro.2020.01.043. [DOI] [PubMed] [Google Scholar]
- 7.Ekstedt M, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 8.Eslam M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 9.Eslam M, Sanyal AJ, George J. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 10.Wai-Sun Wong V, Kanwal F. On the proposed definition of metabolic-associated fatty liver disease. Clin Gastroenterol Hepatol. 2021;19(5):865–870. doi: 10.1016/j.cgh.2021.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Chan WK, Wong VW. Meaning of non-overlapping patients between the MAFLD and NAFLD definitions. Liver Int. 2022;42(2):271–273. doi: 10.1111/liv.15142. [DOI] [PubMed] [Google Scholar]
- 12.Eslam M, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14(6):889–919. doi: 10.1007/s12072-020-10094-2. [DOI] [PubMed] [Google Scholar]
- 13.Tan SS, et al. Endorsing the redefinition of fatty liver disease. Lancet Gastroenterol Hepatol. 2021;6(3):163. doi: 10.1016/S2468-1253(21)00002-9. [DOI] [PubMed] [Google Scholar]
- 14.Chan WK, et al. Malaysian Society of Gastroenterology and Hepatology consensus statement on metabolic dysfunction-associated fatty liver disease. J Gastroenterol Hepatol. 2022;37(5):795–811. doi: 10.1111/jgh.15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan WK. Comparison between obese and non-obese NAFLD. Clin Mol Hepatol. 2023;29(Suppl):S58–S67. doi: 10.3350/cmh.2022.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong VW, et al. Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 1: Definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33(1):70–85. doi: 10.1111/jgh.13857. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, et al. Are the different MAFLD subtypes based on the inclusion criteria correlated with all-cause mortality? J Hepatol. 2021;75(4):987–989. doi: 10.1016/j.jhep.2021.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Huang J, et al. MAFLD criteria guide the subtyping of patients with fatty liver disease. Risk Manag Healthc Policy. 2021;14:491–501. doi: 10.2147/RMHP.S285880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anuurad E, et al. The new BMI criteria for asians by the regional office for the western pacific region of WHO are suitable for screening of overweight to prevent metabolic syndrome in elder Japanese workers. J Occup Health. 2003;45(6):335–343. doi: 10.1539/joh.45.335. [DOI] [PubMed] [Google Scholar]
- 20.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome–a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 21.MEMS. Clinical practice guidelines on management of type 2 diabetes mellitus. 6th ed. https://mems.my/cpg-management-of-type-2-diabetes-mellitus-6th-edition/ (2020). Accessed 16 Sept 2022
- 22.Unger T, et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 23.Tan EX, et al. Non-obese non-alcoholic fatty liver disease (NAFLD) in Asia: an international registry study. Metabolism. 2022;126:154911. doi: 10.1016/j.metabol.2021.154911. [DOI] [PubMed] [Google Scholar]
- 24.Kleiner DE, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 25.Newsome PN, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5(4):362–373. doi: 10.1016/S2468-1253(19)30383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanyal AJ, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021;385(17):1559–1569. doi: 10.1056/NEJMoa2029349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Health and Morbidity Survey (NHMS) Non-communicable diseases, healthcare demand, and health literacy—key findings. Putrajaya: Institute for Public Health, Ministry of Health Malaysia; 2019. p. 2020. [Google Scholar]
- 28.Goh SC, Ho EL, Goh KL. Prevalence and risk factors of non-alcoholic fatty liver disease in a multiracial suburban Asian population in Malaysia. Hepatol Int. 2013;7(2):548–554. doi: 10.1007/s12072-012-9359-2. [DOI] [PubMed] [Google Scholar]
- 29.Khammas ASA, et al. Prevalence and risk factors of sonographically detected non alcoholic fatty liver disease in a screening centre in Klang Valley, Malaysia: an observational cross-sectional study. Porto Biomed J. 2019;4(2):e31. doi: 10.1016/j.pbj.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim SZ, et al. Epidemiological trends of gastrointestinal and liver diseases in Malaysia: a single-center observational study. J Gastroenterol Hepatol. 2022;37(9):1732–1740. doi: 10.1111/jgh.15905. [DOI] [PubMed] [Google Scholar]
- 31.Goh KL, et al. Liver cancer in Malaysia: epidemiology and clinical presentation in a multiracial Asian population. J Dig Dis. 2015;16(3):152–158. doi: 10.1111/1751-2980.12223. [DOI] [PubMed] [Google Scholar]
- 32.Karuthan SR, et al. Barcelona Clinic Liver Cancer and Hong Kong Liver Cancer staging systems for prediction of survival among Hepatocellular Carcinoma patients. Med J Malaysia. 2021;76(2):199–204. [PubMed] [Google Scholar]
- 33.Wong RJ, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 34.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 35.Estes C, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 36.Estes C, et al. Modelling NAFLD disease burden in four Asian regions-2019-2030. Aliment Pharmacol Ther. 2020;51(8):801–811. doi: 10.1111/apt.15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69(9):1691–1705. doi: 10.1136/gutjnl-2020-320622. [DOI] [PubMed] [Google Scholar]
- 38.Adams LA, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Zhu L, et al. Lean yet unhealthy: Asian American adults had higher risks for metabolic syndrome than Non-Hispanic White adults with the same body mass index: evidence from NHANES 2011–2016. Healthcare (Basel) 2021;9(11):1518. doi: 10.3390/healthcare9111518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khoo S, et al. Suboptimal treatment of dyslipidemia in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2020;35(2):320–325. doi: 10.1111/jgh.14794. [DOI] [PubMed] [Google Scholar]
- 41.Leow YW, et al. Hepatic steatosis and metabolic risk factors among patients with chronic hepatitis B: The multicentre, prospective CAP-Asia study. J Viral Hepat. 2023 doi: 10.1111/jvh.13796. [DOI] [PubMed] [Google Scholar]
- 42.Loo SY, Chan WK. Emerging new standard for non-invasive assessment of liver disease mortality in non-alcoholic fatty liver disease. Hepatobiliary Surg Nutr. 2017;6(2):135–137. doi: 10.21037/hbsn.2017.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan WK, Nik Mustapha NR, Mahadeva S. A novel 2-step approach combining the NAFLD fibrosis score and liver stiffness measurement for predicting advanced fibrosis. Hepatol Int. 2015;9(4):594–602. doi: 10.1007/s12072-014-9596-7. [DOI] [PubMed] [Google Scholar]
- 44.Chan WK, et al. Optimizing use of nonalcoholic fatty liver disease fibrosis score, fibrosis-4 score, and liver stiffness measurement to identify patients with advanced fibrosis. Clin Gastroenterol Hepatol. 2019;17(12):2570–2580.e37. doi: 10.1016/j.cgh.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Ayada I, et al. Systematically comparing epidemiological and clinical features of MAFLD and NAFLD by meta-analysis: focusing on the non-overlap groups. Liver Int. 2022;42(2):277–287. doi: 10.1111/liv.15139. [DOI] [PubMed] [Google Scholar]
- 46.Younossi ZM, et al. Are there outcome differences between NAFLD and metabolic-associated fatty liver disease? Hepatology. 2022;76(5):1423–1437. doi: 10.1002/hep.32499. [DOI] [PubMed] [Google Scholar]
- 47.van Kleef LA, et al. Metabolic dysfunction-associated fatty liver disease improves detection of high liver stiffness: The Rotterdam Study. Hepatology. 2022;75(2):419–429. doi: 10.1002/hep.32131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Request for data sharing should be made to the corresponding author. Individual patient-level data containing confidential or identifiable patient information are subject to patient privacy and cannot be shared.