Abstract
Background
Pressure ulcers, also known as bedsores, pressure sores, or pressure injuries, are localised damage to the skin and underlying soft tissue, usually caused by intense or long‐term pressure, shear, or friction. Negative pressure wound therapy (NPWT) has been widely used in the treatment of pressure ulcers, but its effect needs to be further clarified. This is an update of a Cochrane Review first published in 2015.
Objectives
To evaluate the effectiveness of NPWT for treating adult with pressure ulcers in any care setting.
Search methods
On 13 January 2022, we searched the Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations); Ovid Embase, and EBSCO CINAHL Plus. We also searched ClinicalTrials.gov and the WHO ICTRP Search Portal for ongoing and unpublished studies and scanned reference lists of relevant included studies as well as reviews, meta‐analyses, and health technology reports to identify additional studies. There were no restrictions with respect to language, date of publication, or study setting.
Selection criteria
We included published and unpublished randomised controlled trials (RCTs) comparing the effects of NPWT with alternative treatments or different types of NPWT in the treatment of adults with pressure ulcers (stage II or above).
Data collection and analysis
Two review authors independently conducted study selection, data extraction, risk of bias assessment using the Cochrane risk of bias tool, and the certainty of the evidence assessment using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) methodology. Any disagreement was resolved by discussion with a third review author.
Main results
This review included eight RCTs with a total of 327 randomised participants. Six of the eight included studies were deemed to be at a high risk of bias in one or more risk of bias domains, and evidence for all outcomes of interest was deemed to be of very low certainty. Most studies had small sample sizes (range: 12 to 96, median: 37 participants).
Five studies compared NPWT with dressings, but only one study reported usable primary outcome data (complete wound healing and adverse events). This study had only 12 participants and there were very few events; only one participant was healed in the study (risk ratio (RR) 3.00, 95% confidence interval (CI) 0.15 to 61.74, very low‐certainly evidence). There was no evidence of a difference in the number of participants with adverse events in the NPWT group and the dressing group, but the evidence for this outcome was also assessed as very low certainty (RR 1.25, 95% CI 0.64 to 2.44, very low‐certainty evidence). Changes in ulcer size, pressure ulcer severity, cost, and pressure ulcer scale for healing (PUSH) sores were also reported, but we were unable to draw conclusions due to the low certainly of the evidence.
One study compared NPWT with a series of gel treatments, but this study provided no usable data. Another study compared NPWT with 'moist wound healing', which did not report primary outcome data. Changes in ulcer size and cost were reported in this study, but we assessed the evidence as being of very low certainty; One study compared NPWT combined with internet‐plus home care with standard care, but no primary outcome data were reported. Changes in ulcer size, pain, and dressing change times were reported, but we also assessed the evidence as being of very low certainty.
None of the included studies reported time to complete healing, health‐related quality of life, wound infection, or wound recurrence.
Authors' conclusions
The efficacy, safety, and acceptability of NPWT in treating pressure ulcers compared to usual care are uncertain due to the lack of key data on complete wound healing, adverse events, time to complete healing, and cost‐effectiveness.
Compared with usual care, using NPWT may speed up the reduction of pressure ulcer size and severity of pressure ulcer, reduce pain, and dressing change times. Still, trials were small, poorly described, had short follow‐up times, and with a high risk of bias; any conclusions drawn from the current evidence should be interpreted with considerable caution. In the future, high‐quality research with large sample sizes and low risk of bias is still needed to further verify the efficacy, safety, and cost‐effectiveness of NPWT in the treatment of pressure ulcers. Future researchers need to recognise the importance of complete and accurate reporting of clinically important outcomes such as the complete healing rate, healing time, and adverse events.
Keywords: Adult, Humans, Bandages, Negative-Pressure Wound Therapy, Negative-Pressure Wound Therapy/methods, Pressure Ulcer, Pressure Ulcer/therapy, Surgical Wound Infection, Ulcer
Plain language summary
Negative pressure wound therapy for treating pressure ulcers
Key messages
What are pressure ulcers?
Pressure ulcers, also known as bedsores, decubitus ulcers, and pressure injuries, are areas of injury to the skin, the tissue that lies underneath, or both. Pressure ulcers can be painful, may become infected, and affect people's quality of life. People at risk of developing pressure ulcers include those with spinal cord injuries and those who are immobile or who have limited mobility.
How are pressure ulcers managed?
There is a wide variety of treatment options available for pressure ulcers, such as dressings, reconstructive surgery, redistribution of pressure, electrical stimulation, and negative pressure wound therapy (NPWT). NPWT is a technology that is used widely and is promoted for use on wounds, including pressure ulcers. In NPWT, a machine that exerts carefully controlled suction (negative pressure) is attached to a wound dressing that covers the pressure ulcer. This sucks any wound and tissue fluid away from the treated area into a canister. The researchers tried to discover whether NPWT works well as a treatment for pressure ulcers.
What did we want to find out?
The aim of this review is to find out whether the use of NPWT is effective in the treatment of pressure ulcers in any care setting. We wanted to assess the benefits (complete wound healing; healing time) and risks (adverse events) of NPWT with alternative treatments or different types of NPWT in the treatment of pressure ulcers. We also cared about several other outcomes including quality of life, wound infection, change in ulcer size and severity, pain, cost, resource use, and wound recurrence.
What did we do?
We searched the medical literature for published and unpublished robust medical studies (randomised controlled studies) that assessed NPWT for treating pressure ulcers, with no restrictions on language, date of publication, or study setting. We compared and summarised their results, and rated our confidence in the evidence according to research methods, scale, and other factors.
What did we find?
We found eight studies published between 2002 and 2022 involving a total of 327 participants with pressure ulcers at Category/Stage III or above. Five studies compared NPWT with dressings. Only one study with a total of 12 participants reported usable primary outcome data (complete wound healing; adverse events) and found that there was no evidence of a difference in the number of participants with complete wound healing and adverse events in the NPWT group and the dressing group. Three studies reported that NPWT may reduce the size of pressure ulcers compared with dressing, but the results were not reported clearly and the certainty of evidence was very low. One study with a total of 60 participants compared NPWT combined with Internet‐plus home care compared with standard care. This study reports that NPWT combined with Internet‐plus home care may reduce the surface area of ulcers, pain, and dressing change times compared with standard care, but due to the risk of bias in the study, we downgraded the certainty of evidence to a very low level. One study compared NPWT with a series of topical treatments and one study compared it with what was described only as 'moist wound healing', but no useful data were obtained.
What are the limitations of the evidence?
The current evidence on the efficacy of NPWT in the treatment of pressure ulcers is limited, and most studies were small (median 37 participants), poorly reported, of fairly short or unclear duration, and contained little in the way of useful data. We were not able to draw any conclusions about the benefits or harms of NPWT in treating pressure ulcers based on existing evidence. High‐quality research is still needed to help decision‐makers judge the value of NPWT in the treatment of pressure ulcers.
How up‐to‐date is this evidence?
This evidence is current to January 2022.
Summary of findings
Summary of findings 1. NPWT compared with standard dressings therapy.
| Population: adults with pressure ulcers Setting: hospitals and home‐based care Intervention: NPWT Comparison: standard dressings therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard dressings | Risk with NPWT | |||||
|
The proportion of pressure ulcers healed Follow‐up: 24 weeks |
Study population | |||||
| 0 per 1000 | 167 per 1000 (29 to 925) | RR 3.00 (0.15 to 61.74) | 12 (1 RCT) | ⨁⊝⊝⊝ Very lowa | ||
| Time to complete wound healing | Not estimable |
|||||
|
Adverse events Follow‐up: 24 weeks |
667 per 1000 | 857 per 1000 (231 to 992) | RR 1.25 (0.64 to 2.44) |
12 (1 RCT) |
⨁⊝⊝⊝ Very lowb | |
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; NPWT: negative pressure wound therapy; RR: Risk ratio
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect.
aDowngraded once for risk of bias and twice for serious imprecision: the study was not powered to detect a difference in wound healing, and there was such huge imprecision around the estimates that neither a positive nor negative effect of NPWT can be ruled out.
b Downgraded once for risk of bias and twice for serious imprecision. Again the study was underpowered and findings were imprecise largely as it was not designed to assess relative treatment effects.
Summary of findings 2. NPWT compared with moist wound healing.
| Population: adults with pressure ulcers Setting: hospitals Intervention: NPWT Comparison: moist wound healing | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with NPWT (plus standard care) | Risk with NPWT (plus standard care) | |||||
| The proportion of pressure ulcers healed | This outcome was not reported for this comparison. |
|||||
| Time to complete wound healing | This outcome was not reported for this comparison. |
|||||
|
Adverse events |
This outcome was not reported for this comparison. |
|||||
CI: Confidence interval; NPWT: negative pressure wound therapy
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect.
Summary of findings 3. NPWT compared with the Healthpoint system.
| Population: adults with pressure ulcers Setting: hospitals Intervention: NPWT Comparison: Health point system | ||||||
|
Outcomes |
Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with NPWT (plus standard care) | Risk with NPWT (plus standard care) | |||||
| The proportion of pressure ulcers healed | See comments | See comments | 28 (1 RCT) |
Participants with multiple wounds in the trial with data being reported at the wound rather than participant level, but the included study did not clearly report the number of participants randomised to each group. Data were not analysed further due to these units of analysis issues | ||
| Time to complete wound healing | This outcome was not reported for this comparison. |
|||||
|
Adverse events |
This outcome was not reported for this comparison. |
|||||
CI: Confidence interval; NPWT: negative pressure wound therapy; RCT: randomised controlled trial
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect.
Summary of findings 4. NPWT combined with internet‐plus home care compared with standard care.
| Population: adults with pressure ulcers Setting: hospitals and home‐based care Intervention: NPWT combined with internet‐plus home care Comparison: standard care | ||||||
|
Outcomes |
Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with NPWT (plus standard care) | Risk with NPWT (plus standard care) | |||||
| The proportion of pressure ulcers healed | 60 (1 RCT) |
The author only reported the proportion of effective treatment (the wound is completely covered with fresh granulation tissue), which we did not include in the analysis. | ||||
| Time to complete wound healing | This outcome was not reported for this comparison. |
|||||
|
Adverse events |
This outcome was not reported for this comparison. |
|||||
CI: Confidence interval; NPWT: negative pressure wound therapy; RCT: randomised controlled trial
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect.
Background
Description of the condition
Pressure ulcers (also known as bedsores, pressure sores, and pressure injuries), are localised damage to the skin and underlying soft tissue, usually over a bony prominence or caused by external forces such as pressure or pressure combined with shear (EPUAP/NPUAP/PPPIA 2019).
Populations at risk of pressure ulcers include those with spinal cord injuries (Gefen 2014), and those immobilised or with limited mobility such as elderly people and people with acute or chronic conditions that might limit movement or bodily sensation, or both (Allman 1997; Bergstrom 1998; Berlowitz 1990; Berlowitz 1997; Brandeis 1994). Incontinence can also increase the risk of ulceration by producing a detrimental environment for the skin (Brandeis 1994). Impaired nutritional status may also increase risk (Allman 1997; Donini 2005), however, there is currently limited evidence for the effectiveness of nutritional intake interventions for preventing or treating pressure ulcers (Langer 2003; Smith 2013). A systematic review summarised that the most frequently independent predictors of pressure ulcers development were: a lack of mobility/activity; poor perfusion (including diabetes) and worsening skin/pressure ulcer status; other factors including body temperature and immunity, skin moisture, age, nutrition, and general health status are also considered to be potential risk factors for the occurrence of pressure ulcers (Coleman 2013). Other studies found that oedema may also increase the risk for the development of pressure ulcers, which predicted early tissue damage (Chaboyer 2022); overweight was associated with a lower risk of pressure ulcers, while underweight significantly increased the risk of pressure ulcers (Alipoor 2021).
Mobility produces relief from pressure within the body through regular, often sub‐conscious, shifts in positions when sitting or lying. Tissue tolerance is the ability of the skin and its supporting structures to tolerate the effects of pressure by distributing it (cushioning) and by the transfer of pressure loads from the skin surface to the skeleton (EPUAP/NPUAP/PPPIA 2019). These movements, triggered by a reduction in oxygen levels at pressure points and possible discomfort, distribute pressure from contact at the surface, thus reducing the compression of soft tissue against bone (Gebhardt 2002). Populations with limited autonomous movement or conditions that dull body sensation, or both (as described above), are at risk of failing to achieve adequate pressure relief. Prolonged exposure of an area of the body to pressure or compression can interrupt the local blood circulation and trigger a cascade of biochemical changes that may lead to tissue damage and ulceration. Immobility can also lead to increased damage from shear and friction, for example, when people are pulled into positions in chairs and beds.
Pressure ulcers vary in severity. One of the most widely recognised systems for categorising pressure ulcers is that of the National Pressure Ulcer Advisory Panel which is summarised below (NPUAP 2016). Pressure ulcers are staged/categorised from I‐IV, with IV being the most severe.
Stage/category I: non‐blanchable erythema of intact skin
Stage/category II: partial‐thickness skin loss with exposed dermis
Stage/category III: full‐thickness skin loss
Stage/category IV: full‐thickness skin and tissue loss
Unstageable pressure ulcer: obscured full‐thickness skin and tissue loss
Deep tissue pressure ulcer: persistent non‐blanchable deep red, maroon, or purple discolouration.
Pressure ulcers are relatively common, but complex, wounds. Prevalence estimates vary according to the clinical setting being assessed, the data collection methods used, and decisions about whether or not stage I pressure ulcers should be included (since there is no open wound at this stage, but evidence of possible tissue damage). A study in the USA assessed the overall prevalence of pressure ulcers in all facilities. The sample for this study was 918,621 patients, and the overall prevalence between 2006 and 2015 ranged from 9.3% to 13.5% (VanGilder 2017). A cross‐sectional observational study of a community setting in the North of England estimated that the prevalence rate of pressure ulcers (Grade ≥ 1) was 0.77 per 1000 (Stevenson 2013). The prevalence of pressure ulcers in Swedish hospital patients decreased significantly from 17.0 %to 11.4% between 2011 and 2020 and hospital‐acquired pressure ulcers decreased from 8.1% to 6.4% between 2018 and 2020 (Källman 2022). Intensive care unit (ICU) patients are particularly susceptible to developing pressure ulcers. A point‐prevalence study recruited 13,254 patients from 1117 ICUs in 90 countries and estimated that the overall prevalence of pressure ulcer patients was 26.6% with 18.0% of stage II or worse (Labeau 2021). A recent retrospective cohort study showed that the incidence of COVID‐19 patients hospitalised with pressure ulcers was 6.9% (Rrapi 2021).
Pressure ulcers have a large impact on those affected; the ulcers can be painful and may become seriously infected or malodorous. It has been shown that ‐ after adjustment for age, sex, and co‐morbidities ‐ people with pressure ulcers have a lower health‐related quality of life than those without pressure ulcers (Essex 2009). The financial cost of treating ulcers in the UK was recently estimated as being between GBP 1214 for a stage I ulcer, to GBP 14,108 for a stage IV ulcer (Dealey 2012). Pressure ulcers have been shown to increase the length of hospital stay, readmission, and mortality rates (Lyder 2012), and to add considerably to the cost of an episode of hospital care (Chan 2013). In the USA, the annual cost of treating pressure ulcers is USD17.8 billion with approximately 3 million people affected (Hajhosseini 2020); Costs to Australian public hospitals for treating pressure ulcers have been estimated at USD 3.59 billion in 2020 (Nghiem 2022). A systematic review reported that the treatment cost of pressure ulcers per patient per day ranged from EUR 1.71 to EUR 470.49 (Demarré 2015). In addition to the increasing length of hospital stay, discomfort, and pain experienced, but also increases the cost of medical services, risk of death, changes in body image, and quality of life (Walker 2017).
Description of the intervention
Negative pressure wound therapy (NPWT) is a technology that is currently used widely in wound care and is promoted for use on complex wounds (e.g. Guy 2012). NPWT involves the application of a wound dressing through which a negative pressure (or vacuum) is applied, often with the wound and tissue fluid drawn away from the area being collected in a canister. The intervention was developed in the 1990s, and the uptake of NPWT in the healthcare systems of developed countries has been dramatic. A US Department of Health report estimated that between 2001 and 2007, Medicare payments for NPWT pumps and associated equipment increased from USD 24 million to USD 164 million (an increase of almost 600%; Department of Health and Human Services 2009). Initially only one NPWT manufacturer supplied NPWT machines (the VAC system: Kinetic Concepts Inc (KCI), San Antonio, Texas), however, as the NPWT market has grown, a number of different commercial NPWT systems have been developed, with machines becoming smaller and more portable. Indeed, the most recent introduction to the market is a single‐use, or 'disposable', negative‐pressure product. Ad hoc, non‐commercial, negative pressure devices are also used, especially in resource‐poor settings. These devices tend to use simple wound dressings, such as gauze, or transparent occlusive (non‐permeable) dressings, with negative pressure generated in the hospital by vacuum suction pumps.
A number of different healthcare professionals prescribe and apply NPWT, and it is now used both in secondary and primary (community) care, particularly following the introduction of ambulatory systems, and prophylactically, to prevent surgical site infection. Whilst the NPWT systems outlined above differ in a number of respects ‐ such as the type of pressure (constant or cyclical) applied to the wound, the material in contact with the surface of the wound, and also the type of dressing used ‐ the principle of applying negative pressure to the wound in a closed environment is the same for all products.
How the intervention might work
NPWT can collect high volumes of wound exudate, so may reduce the frequency of dressing changes, and subsequent exposure of the wound to the environment. This collection of exudate ostensibly assists in the management of anatomically‐challenging wounds, keeps wounds clean, and reduces wound odour. Manufacturers, however, also suggest that the application of negative pressure (suction) to the wound actually promotes healing by drawing together the wound edges, increasing perfusion (oxygenated blood in the tissues) and removing infectious material and exudate (Kinetic Concepts Inc 2012).
Potential negative consequences of NPWT include wound maceration (softening due to exposure to liquid), and retention of dressing materials that may cause wound infection, as well as other injuries (FDA 2011). NPWT devices are usually worn continually by patients during treatment. They can interfere with mobility, and, anecdotally, are often noisy, which prevents some patients from sleeping.
Why it is important to do this review
There would be benefits to stakeholders (e.g. patients, clinicians, and policy‐makers) in establishing whether NPWT improves the healing of pressure ulcers. However, recommendations on pressure ulcer treatment guidelines have been inconsistent. Previous review work has found little evidence about the effects of NPWT on severe pressure ulcers (Soares 2013). A Japanese Society of Pressure Ulcers (JSPU) guideline (2016) considers “NPWT as an early adjunct therapy for reducing the size and depth of Stage III and IV pressure ulcers“ (JSPU 2016). The National Institute for Health and Care Excellence (NICE) guideline (2014) suggests “do not routinely offer adults NPWT to treat a pressure ulcer, unless it is necessary to reduce the number of dressing changes” (NICE 2014). The American College of Physicians (ACP) guideline (2015) does not recommend NPWT as an effective therapy for pressure ulcer treatment (ACP 2015). The Wound healing society (WHS) guideline (2015 update) considers “using NPWT for stage III or IV pressure ulcers that fail to progress in healing with conventional therapy” (WHS 2015). The Wound, Ostomy and Continence Nurses Society‐Wound (WOCN) guideline (2016) considers the “use of NPWT which may increase complete wound closure compared to standard wound dressings and is associated with lower risk of secondary infections” (WOCN 2016). The International Clinical Practice Guideline (2019 edition) considered NPWT as an early adjunct therapy for reducing the size and depth of Category/Stage III and IV pressure injuries (EPUAP/NPUAP/PPPIA 2019). The production of a robust and updated systematic review can contribute to this aim by identifying, appraising, and synthesising the evidence base to inform decision‐makers and possibly guide future research.
Objectives
To assess the effects of negative pressure wound therapy (NPWT) for treating adults with pressure ulcers in any care setting.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs), including cluster RCTs, irrespective of the language of report. Cross‐over trials were only included if they reported outcome data at end of the first treatment period, before cross‐over. Studies using quasi‐randomisation were excluded.
Types of participants
We included studies recruiting adults with a pressure ulcer (category II or above), managed in any care setting. We excluded trials of participants with category I ulcers. We accepted the study authors' definitions of what they classed as category II or above unless it was clear that wounds with unbroken skin were included. Studies that recruited participants with category II or higher pressure ulcers alongside people with other types of wounds were included if the results for people with relevant pressure ulcers were presented separately (or were available from the study authors).
Types of interventions
Intervention: any brand of NPWT (both commercial and non‐commercial treatments) was included. We included any RCT in which the NPWT during the treatment period was the only systematic difference between treatment groups.
Comparison: standard care (such as dressings and gauze) or other treatments (including different types/brands of NPWT). We anticipated likely comparisons would include the use of NPWT during the care pathway compared with no use of NPWT, or a comparison of different types/brands of NPWT used during the care pathway.
Types of outcome measures
We list the primary and secondary outcomes below. If a study was apparently eligible (i.e. correct study design, population, and intervention/comparator), but did not report a listed outcome, we contacted the study authors where possible to establish whether an outcome of interest here was measured, but not reported.
We reported outcome measures at the latest time point available for a study (assumed to be the length of follow‐up if not specified) and the time point specified in the methods as being of primary interest (if this was different from the latest time point available). For all outcomes we categorised outcomes from (consistent with previous versions):
under a week to eight weeks as short‐term;
over eight weeks to 26 weeks as medium‐term; and
over 26 weeks as long‐term.
Primary outcomes
The proportion of ulcers healed (frequency of complete healing)
Time to complete wound healing: we recorded whether this had been correctly analysed using censored data and with adjustment for prognostic covariates such as baseline size.
Adverse events (generic): reported data were extracted on adverse events classed as 'serious adverse events' and 'non‐serious adverse events' where a clear methodology for the collection of adverse event data was provided. This methodology needed to make it clear whether events were reported at the participant level or, where multiple events/persons were reported, that an appropriate adjustment had been made for data clustering. Individual types of adverse events such as pain or infection that require specific assessment were not extracted under this outcome ‐ rather this is the assessment of any event classed as adverse by the patient and or health professional during the trial.
Where both the outcomes above were reported, we present all data in a summary outcome table for reference. Where equal amounts of information were available, we anticipated focusing on time to healing as the key outcome measure. We accepted the authors' definitions of what constituted a healed wound.
Secondary outcomes
Change (and rate of change) in ulcer size, with adjustment for baseline size: we contacted study authors to request adjusted means when these were not presented. Where change or rate of change in wound size was reported without adjustment for baseline size, we documented the use of the outcome in the study but did not summarise the data in the narrative or use them in any meta‐analysis.
Change in the severity of ulcers: pressure ulcer healing status assessment tool; that includes measures such as the pressure ulcer scale for healing (PUSH) tool, Sussman wound healing tool, and pressure sore status tool.
Participant health‐related quality of life/health status: measured using a standardised generic questionnaire such as EQ‐5D, SF‐36, SF‐12 or SF‐6 or wound‐specific questionnaires such as the Cardiff wound impact schedule. We did not include ad hoc measures of quality of life that were not likely to be validated and would not be common to multiple trials.
Wound infection: as defined by the author.
Mean pain scores: (including pain at dressing change) we included this information only where the data were reported as either a presence or absence of pain or as a continuous outcome using a validated scale such as a visual analogue scale (VAS).
Resource use: including measurements of resource use such as number of dressing changes, nurse visits, length of hospital stay, re‐admission, and re‐operation/intervention.
Costs: any costs applied to resource use.
Wound recurrence: as defined by the study author.
Search methods for identification of studies
Electronic searches
For this first update of the review, we searched the following electronic databases to identify reports of relevant clinical trials.
The Cochrane Wounds Specialised Register (searched 13 January 2022);
The Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 12) in the Cochrane Library (searched 13 January 2022);
Ovid MEDLINE including In‐Process & Other Non‐Indexed Citations (1946 to 13 January 2022);
Ovid EmbaseE(1974 to 13 January 2022);
EBSCO CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature; 1937 to 13 January 2022).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase, and EBSCO CINAHL Plus can be found in Appendix 1. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (Lefebvre 2021). We combined the EMBASE search with the Ovid EMBASE filter developed by the UK Cochrane Centre (Lefebvre 2021). We combined the CINAHL Plus search with the trial filter developed by Glanville 2019. There were no restrictions with respect to language, date of publication, or study setting.
We also searched the following clinical trials registries:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) (searched 18 January 2022);
World Health Organisation (WHO) International Clinical Trials Registry Platform (https://www.who.int/clinical‐trials‐registry‐platform ) (searched 18 January 2022).
Search strategies for clinical trial registries can be found in Appendix 1.
Searching other resources
Searching reference lists of included trials and relevant reviews
We aimed to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, as well as relevant systematic reviews, meta‐analyses, and health technology assessment reports.
Searching by contacting individuals or organisations
When necessary, we contacted authors of key papers and abstracts to request further information about their trials.
Adverse effects
We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Two review authors independently screened for possibly relevant studies by titles and abstracts of the citations retrieved by the searches. After this initial evaluation, we obtained all full texts of potentially relevant studies and the same two review authors independently checked whether the full‐text papers met the inclusion criteria. Any disagreement was resolved by a discussion with third review author. We recorded all reasons for the exclusion of studies for which we had obtained full copies of the text. We have completed a PRISMA flowchart to summarise this process (Figure 1; Liberati 2009).
1.
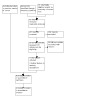
Study flow diagram.
Where required, we obtained all relevant publications when studies were reported more than once. Whilst the study was included only once in the review, all reports were examined to ensure the maximal extraction of relevant data.
Data extraction and management
We extracted and summarised details of the eligible studies. Two review authors extracted data independently and resolved disagreements by discussion, drawing on a third review author where required. Where data were missing from reports, we attempted to contact the study authors to obtain this information. Had a study with more than two intervention arms been included, we would have extracted only those data from intervention and control groups that met the eligibility criteria.
We extracted the following data where possible, by treatment group, for the pre‐specified interventions and outcomes in this review. Outcome data were collected for relevant time points as described in Types of outcome measures.
Country of origin
Type of wound and surgery
Unit of randomisation (per patient) ‐ single wound or multiple wounds on the same patient
Unit of analysis
Trial design e.g. parallel cluster
Care setting
Number of participants randomised to each trial arm
Trial registration number or protocol
Eligibility criteria and key baseline participant data
Details of the treatment regimen received by each group
Duration of treatment
Details of any co‐interventions
Primary and secondary outcome(s) (with definitions)
Outcome data for primary and secondary outcomes (by group)
Duration of follow‐up
Number of withdrawals (by group)
Publication status of study; and
Source of funding for the trial
Assessment of risk of bias in included studies
The risk of bias was assessed according to the criteria described in the Cochrane Collaboration tool for assessing the risk of bias (Higgins 2011), which considered the following six domains for included studies evaluated: bias arising from the random sequence generation (selection bias); bias due to allocation concealment (selection bias); bias due to blinding of outcome assessment (detection bias); bias due to incomplete outcome data (attrition bias); bias in the selection of the reported result (reporting bias); other bias. Each of the items was evaluated by two review authors as having low risk of bias, some concerns, and high risk of bias. Any disagreement was resolved by a discussion with a third review author. In this review we recorded issues with unit of analysis, for example, where a cluster trial has been undertaken but analysed at the individual level in the study report (Appendix 2) In this review we recorded issues with a unit of analysis.
For trials using cluster randomisation, we assessed the risk of bias considering recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and comparability with individually randomised trials (Higgins 2022; Appendix 3).
Measures of treatment effect
For dichotomous outcomes we calculated the risk ratio (RR) with 95% confidence intervals (CI). For continuously distributed outcome data we used the mean difference (MD) with 95% CIs, for trials that used the same assessment scale. If trials used different assessment scales, we used the standardised mean difference (SMD) with 95% CIs. We only considered mean or median time to healing without survival analysis as a valid outcome if reports specified that all wounds healed (i.e. if the trial authors regarded time to healing as a continuous measure, as there is no censoring). Time‐to‐event data (e.g. time‐to‐complete wound healing), were reported as hazard ratios (HR) where possible in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022). If studies reporting time‐to‐event data (e.g. time to healing) did not report a hazard ratio, then, where feasible, we planned to estimate this using other reported outcomes, such as the numbers of events, through the application of available statistical methods (Parmar 1998).
Unit of analysis issues
Where studies randomised at the participant level and measured outcomes at the wound level, for example for wound healing, and the number of wounds appeared to be equal to the number of participants, we treated the participant as the unit of analysis.
We had anticipated a possible unit of analysis issue if individual participants with multiple wounds were randomised, the allocated treatment used on the multiple wounds per participant (or perhaps only on some participants) and then data were presented and analysed by wound not person. This is a type of clustered data and presents a unit of analysis error which inflates precision. In cases where included studies contained some or all clustered data we planned to report this alongside whether data had been (incorrectly) treated as independent. We recorded this as part of the risk of bias assessment. We did not plan to undertake further calculation to adjust for clustering.
We also planned to record when randomisation and allocation had been undertaken at the wound level – that is a split‐site or split‐body design, and assess whether the correct paired analysis had been undertaken in the study, issues would have been recorded in the risk of bias section.
Dealing with missing data
It is common to have data missing from trial reports. Excluding participants post‐randomisation from the analysis, or ignoring those participants who are lost to follow‐up compromises the randomisation, and potentially introduces bias into the trial. Where there were data missing that we thought should be included in the analyses, we contacted the relevant study authors to enquire whether these data were available.
Where data for 'proportion of wounds healed' remained missing, we assumed that if randomised participants were not included in an analysis, their wound did not heal (i.e. they would be considered in the denominator but not the numerator).
In a time‐to‐healing analysis using survival analysis methods, dropouts should be accounted for as censored data, so we took no action regarding missing data.
For continuous variables, for example length of hospital stay, and for all secondary outcomes, we presented the data available from the study reports/study authors and did not plan to impute missing data. We calculated missing measures of variance where possible. If the calculation was not possible, we contacted the study authors. Where these measures of variation were not available the study was excluded from any relevant meta‐analyses that were conducted.
Assessment of heterogeneity
Assessment of heterogeneity can be a complex, multi‐faceted process. Where assessment of heterogeneity was required we firstly considered clinical and methodological heterogeneity: that is the degree to which the included studies varied in terms of participant, intervention, outcome and characteristics such as length of follow‐up. This assessment of clinical and methodological heterogeneity was supplemented by information regarding statistical heterogeneity ‐ assessed using the Chi² test (a significance level of P < 0.10 was considered to indicate statistically significant heterogeneity) in conjunction with the I² measure (Higgins 2003). I² examines the percentage of total variation across RCTs that is due to heterogeneity rather than chance (Higgins 2003). It is generally considered that I² values of 25% or less may mean a low level of heterogeneity (Higgins 2003), and values of 75% or more indicate very high heterogeneity (Deeks 2022). Where there was evidence of high heterogeneity we planned to explore this further where possible: see Data synthesis.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of the results. Publication bias is one of a number of possible causes of 'small‐study effects', that is, a tendency for estimates of the intervention effect to be more beneficial in smaller RCTs. Funnel plots allow a visual assessment of whether small‐study effects may be present in a meta‐analysis. A funnel plot is a simple scatter plot of the intervention effect estimates from individual RCTs against some measure of each trial's size or precision (Page 2022). We planned to present funnel plots for meta‐analyses comprising 10 RCTs or more using RevMan 5 (RevMan 2020).
Data synthesis
Details of included studies were combined in a narrative review according to the type of comparator, possibly by location of/type of wound and then by outcomes by time period. Where appropriate and required clinical and methodological heterogeneity was considered, and we anticipated pooling data when studies appeared appropriately similar in terms of wound type, intervention type, duration of follow‐up, and outcome type, thus synthesis was considered viable.
Our standard approach for meta‐analytical analyses was to employ a random‐effects model. Our preference for the more conservative random‐effects model is because statistical assessments can miss potentially important between‐study heterogeneity in small samples, (Kontopantelis 2012).
A fixed‐effect analysis was only planned when, in the judgement of the review authors, there was minimal clinical heterogeneity and this was supported by an X2 value estimated to be statistically non‐significant and an I2 of 0% (Kontopantelis 2013). In all other circumstances, a random‐effects model wound be adopted. If relevant, where clinical heterogeneity was thought to be acceptable or of interest, we planned to meta‐analyse even when statistical heterogeneity is high – attempting to interpret the causes behind this heterogeneity – use of meta‐regression or subgroup analyses for that purpose would also be considered (Thompson 1999; Thompson 2002).
Data were presented using forest plots where possible. For dichotomous outcomes present the summary estimate as a risk ratio (RR) with 95% CI. Where continuous outcomes were measured in the same way across studies, we planned to present a pooled mean difference (MD) with 95% CI; we planned to pool MD estimates where studies measured the same outcome using different methods. For time‐to‐event data, we planned to plot (and, if appropriate, pool) estimates of hazard ratios (HRs) and 95% CIs as presented in the study reports using the generic inverse variance method in RevMan 5.3. Where time to healing was analysed as a continuous measure, but it was not clear if all wounds healed, use of the outcome in the study would be documented, but data would not be summarised or used in any meta‐analysis.
Pooled estimates of treatment effect would be obtained using Cochrane RevMan software 5 (RevMan 2020).
Subgroup analysis and investigation of heterogeneity
Had there been sufficient included trials and data, we had planned to assess potential heterogeneity across the following areas where there was evidence of between‐trial heterogeneity. We envisaged conducting subgroup analyses for:
category of the ulcer;
features of the negative pressure system and/or vacuum cycle protocol used;
duration of NPWT treatment;
methodological features of studies (allocation adequately concealed versus not reported or inadequate) and type of randomisation (truly randomised with the adequate method of generating the randomisation sequence versus not reported).
Sensitivity analysis
When possible, we planned to exclude RCTs with high risk for one or more domains from meta‐analysis to explore the impact on the research results. However, we did not conducted any sensitivity analyses.
Summary of findings and assessment of the certainty of the evidence
We planned to present the main results of the review in the summary of findings tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2022). Summary of findings tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach. The GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The certainty of a body of evidence involves consideration of the risk of bias, directness of evidence, heterogeneity, precision (or imprecision) of effect estimates, and risk of publication bias (Schunemann 2022).
We presented the following outcomes in the summary of findings tables.
Time to complete wound healing where analysed using appropriate survival analysis methods.
The proportion of wounds completely healing during the trial period.
Adverse events.
For other outcomes, we conducted a GRADE assessment and presented the results in narrative format in the results section.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies
Results of the search
This 2022 update obtained 264 citations from the electronic search, left 191 from electronic databases, and 67 from trial registries, we also assessed 21 potentially eligible records by scanning reference lists and relevant reviews, 11 of records were duplicates. In addition to the four studies previously included, we identified 35 references as potentially eligible and obtained 32 studies as full ext.
The initial version of this review included four studies (Ashby 2012; de Laat 2011; Ford 2002; Niezgoda 2004), and we added four studies in this first update (Dwivedi 2016; Dwivedi 2017; Şahin 2022; Tang 2019); we excluded 28 studies and three are awaiting assessment as we still have been unable to obtain the full texts for two (Pruksapong 2011; Yu 2012), and one study did not have obvious outcome data, but the graphs presented require more exploration for the data on change in wound volume at two weeks (Wanner 2003).
Included studies
Types of studies
Eight randomised controlled trials (RCTs) of negative pressure wound therapy (NPWT) for the treatment of pressure ulcers, containing 327 participants, are included in this review (Ashby 2012; de Laat 2011; Dwivedi 2016; Dwivedi 2017; Ford 2002; Niezgoda 2004; Şahin 2022; Tang 2019), all of those studies were two‐armed RCTs using a parallel‐group.
Of all included studies, two studies were undertaken in the USA ( Ford 2002; Niezgoda 2004), one in the UK (Ashby 2012), one in Turkey (Şahin 2022), two in India (Dwivedi 2016; Dwivedi 2017), one in China (Tang 2019), and one in the Netherlands (de Laat 2011). From the information available it seems that four studies used an NPWT machine from the same Vacuum‐Assisted Closure device (V.A.C.® Therapy System Patient Support – KCI) (Ashby 2012; de Laat 2011; Ford 2002; Şahin 2022); two studies used the same low‐cost device (no detailed for the manufacturer) (Dwivedi 2016; Dwivedi 2017); it was not clear what type of NPWT machine was used in other two studies (Niezgoda 2004; Tang 2019).
Types of participants
Ulcers were category III and IV in seven studies, Ashby 2012; Dwivedi 2016; Dwivedi 2017; Ford 2002; Niezgoda 2004; Şahin 2022; and category IV only in de Laat 2011.
Types of interventions
NPWT was compared with:
wet‐to‐dry dressing in Şahin 2022, follow‐up time eight weeks: quote: "The device was operated at 125mmHg pressure for 5 min with and 2 min without active vacuum. Wound dressings were changed every 48 h."
standard care in Tang 2019, including wound assessment, instrument debridement combined with autolytic debridement, wet dressing, and health education, follow‐up time three months: quote: "NPWT dressings were changed five to seven days, negative pressure setting range ‐80 to ‐120 mm Hg. After the patient brought the machine home, the ostomy therapist observed and guided the use of NPWT through WeChat video, voice, and other internet platforms".
wet‐to‐moist gauze dressings in Dwivedi 2017, follow‐up time nine weeks: quote:"The NPWT dressing was changed weekly or more if the dressing became saturated or loss of suction occurred, patients and caregivers were taught how to charge the NPWT device and advised to charge it after every 5 to 6 hours."
wet‐to‐moist gauze dressings in Dwivedi 2016, follow‐up time nine weeks: quote:"NPWT was changed every week or earlier if required. The dressing was changed by resident staff with the help of research staff, Patients and caregivers were taught how to charge the Romovac and advised to charge it after every 5–6 hours."
a choice of three standard dressing types in Ashby 2012, follow‐up time 24 weeks: quote:"Devices were used in accordance with the manufacturer's guidance. The duration of treatment was determined by the nurse treating the patient and also the patient, in accordance with current practice."
a wet‐to‐moist dressing with a sodium hypochlorite 0.25% solution in de Laat 2011, follow‐up time six weeks: quote:"The fluid connection system was changed at least once a week. Negative pressure mode of 125 mm Hg."
and to moist wound healing with no further definition in Niezgoda 2004, follow‐up time 6 weeks: no further details.
the Healthpoint system (which uses three gel treatments) in Ford 2002, follow‐up time unclear: quote:"NPWT dressings were changed Mondays, Wednesdays, and Fridays (manufacturer recommends dressing changes every 48 hours)."
Ashby 2012 described as a pilot study. Niezgoda 2004 was presented as an interim analysis; no further data were available from the study authors, who confirmed that the study had not been published in full. Tang 2019 was designed to compare the effectiveness of NPWT combined with internet‐plus home care to standard care.
Outcomes
Eight studies provided data on six of our outcomes of interest. For the primary outcome, only one study reported adverse events, and the proportion of pressure ulcers healed (Ashby 2012). For the secondary outcomes, five measured changes in the size of pressure ulcers (de Laat 2011; Dwivedi 2016; Dwivedi 2017; Şahin 2022; Tang 2019), three reported pressure ulcer severity (Dwivedi 2016; Dwivedi 2017; Şahin 2022), and three studies reported the cost of treatment (Dwivedi 2016; Niezgoda 2004; Tang 2019). Only Tang 2019 study reported pain and resource use (time of dressing change). No study reported time to complete healing, health‐related quality of life, wound infection, or wound recurrence.
Care settings
Participants were from two types of settings, including four studies conducted in hospitals (de Laat 2011; Dwivedi 2016; Dwivedi 2017; Ford 2002); three studies were conducted in‐home and in hospitals (Ashby 2012; Şahin 2022; Tang 2019), and one study did not provide enough data (Niezgoda 2004).
Funding sources
One study did not receive any funding (Şahin 2022), three studies received full or partial funding from the public (Ashby 2012; de Laat 2011; Ford 2002), and four studies did not provide information about any source of funding (Dwivedi 2016; Dwivedi 2017; Niezgoda 2004; Tang 2019).
Excluded studies
Twenty‐eight studies were excluded from the review for the following reasons:
not a randomised controlled trial (10 studies; Andrianasolo 2018; Dwivedi 2020; Hampton 2015; Kumar 2021; Leonardi 2017; McCallon 2015; Mullner 1997; Papp 2018; Srivastava 2016; Tauro 2007);
NPWT was not the only systematic difference between study groups (nine studies; Baek 2020; Chen 2018; Ciliberti 2016; Gao 2015; Liu 2021; Mari 2019; Mohammed 2020; Wagstaff 2014; Zhang 2012);
no outcomes relevant to this review reported or obtained from study authors to date (one study; Wild 2008);
study population had mixed wounds and data on the treatment of pressure ulcers were not available separately (six studies; Braakenburg 2006; Hu 2009; Joseph 2000; Mody 2008; Schwarz 2012; Ali 2015);
study population was not relevant (one study; Moues 2007);
we were unable to obtain any further information regarding the study (no abstract or publication; one study; Greer 1999).
Risk of bias in included studies
We assessed the methodological quality of all eight included studies according to the Cochrane Collaboration tool for assessing the risk of bias. The results are presented in the risk of bias summary (Figure 2) and risk of bias graph (Figure 3).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Random sequence generation
All included studies specified that participants were randomly allocated to treatments. Four studies specified the method of generating the sequence leading to random allocation, whether by computer or random number table (Ashby 2012; Dwivedi 2016; Dwivedi 2017; Tang 2019), but we assessed Tang 2019 as unclear risk for this domain due to the indistinct description of random methods. The remaining four studies were classed as being at unclear risk of selection bias as they reported limited information about the methods employed (de Laat 2011; Ford 2002; Niezgoda 2004; Şahin 2022).
Concealed allocation
We classed one study as being at low risk for this domain (Ashby 2012). This study reported that "the research nurse telephoned a secure and remote randomisation service". The remaining seven studies did not provide sufficient details to make a judgement, and we classified these as unclear for this domain..
Blinding
We deemed two studies to be at low risk of detection bias for wound healing as they undertook blinded outcome assessment (Ashby 2012; Ford 2002). It was noted in de Laat 2011 that blinded outcome assessment was not possible for healing outcomes, so we classed this study as being at a high risk of detection bias. Five studies did not report any information about blinding being used in the study, and we classed them as being at unclear risk of detection bias (Dwivedi 2016; Dwivedi 2017; Niezgoda 2004; Şahin 2022; Tang 2019).
Incomplete outcome data
We classed four studies as being at low risk of attrition bias (Ashby 2012; de Laat 2011; Şahin 2022; Tang 2019), and four studies as at high risk of attrition bias (Ford 2002; Niezgoda 2004; Dwivedi 2016; Dwivedi 2017). Ford 2002 enrolled 28 participants with 41 wounds; 22 participants with 35 wounds completed the study. Niezgoda 2004 seemed to have presented an interim analysis both in terms of only some participants having been randomised and not all participants completed follow‐up. Dwivedi 2016 had a dropout rate of 30 % in the NPWT group and 23.3 % in the control group. Dwivedi 2017 had a dropout rate of 31.3 % in the NPWT group and 21.4 % in the control group.
Selective reporting
We classed all the studies as being at low risk of reporting bias except for Niezgoda 2004, which we classed as being at unclear risk due to the limited information available about it.
Other potential sources of bias
The results of Tang 2019 must be viewed with caution, as the patients in this study took the NPWT device home, and the investigators used the internet for guidance and observation, detailed information was very limited, so we considered this study to be high‐risk in this domain. We classed the Ford 2002 study as high risk of bias due to unit of analysis issues. We found that the data used in the two studies (Dwivedi 2016; Dwivedi 2017) were similar, but there was little difference in the number of people included in the intervention group and the control group. We contacted the author to request more detailed information, but no reply was received. We can not confirm whether this study is a duplicate of published data or a different design RCT, so we classed these studies as high risk of bias. We classed three studies (Ashby 2012; de Laat 2011, Niezgoda 2004) as having unclear risk of bias for this domain.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
See Table 1 for negative pressure wound therapy (NPWT) compared with standard dressings therapy; Table 2 for NPWT compared with moist wound healing; Table 3 for NPWT compared with the Healthpoint system; Table 4 for NPWT combined with internet‐plus home care compared with standard care.
See Table 5 for a summary of included studies and outcome data.
1. Study outcomes.
| Study | Comparison | Length of follow‐up | Time points of data presented |
Pressure Ulcer healed |
Adverse events | Change in ulcer size | Pressure ulcers severity | Wound infection and pain | Resource use | Cost |
| Dwivedi 2016 |
Group A: Negative pressure device (NPD) (N = 21) Group B: Wet‐to‐moist gauze dressings (n = 23) "nine patients withdrew from The NPWT group and seven from the control group." |
9 weeks |
1、2、3、4、5、6、7、8、9 weeks |
Not reported | Not reported | Data could not be used as they were reported as length, width, and depth The length and width were significantly decreased in Group A than in Group B from weeks 5 to 9 The depth was significantly decreased in Group A than in Group B at week 9 |
PUSH scores (reported as depth, exudate, and tissue type separately). | Not reported | Not reported | The total NPWT and SC cost of one representative PU was determined by multiplying the daily cost by the number of days required to achieve wound granulation. Group A: USD 105 Group B: USD 200 The total cost of a 9‐week treatment of one PU in Group B was significantly higher than Group A |
| Dwivedi 2017 |
Group A: NPWT (N=22) Group B: wet‐to‐moist gauze dressings (n = 22) "Ten participants were withdrawn from the NPWT group, and six participants withdrew from the comparison group and refused additional treatment. " |
9 weeks |
3、6、9 weeks |
Not reported | Not reported | Data could not be used as they were reported as length, width, and depth The length was significantly decreased in Group A than in Group B at week 6 and week 9 The width and depth were significantly decreased in Group A than in Group B at week 9 |
PUSH scores (reported as depth, exudate, and tissue type separately). | Not reported | Not reported | Not reported |
| Şahin 2022 |
Group A: NPWT (N = 15) Group B: wet‐to‐dry dressing (n = 15) |
8 weeks |
8 weeks |
Not reported | Not reported |
Change in wound size: Group A: mean ‐18.47 (SD 14.95); Group B: mean ‐3.8 (SD 14.95) |
Change in PUSH score: Group A: mean ‐4.597 (standard deviation 2.63); Group B: mean ‐1.067 (standard deviation 2.63) |
Not reported | Not reported | Not reported |
| Tang 2019 |
Group A: NPWT (N = 30) Group B: standard care (n = 30); Including wound assessment, wound debridement, wet dressing, and health education |
3 month | 1、2、3 month | Not reported |
Not reported |
Number with 50% (or greater) reduction in wound size Group A: 16/30 Group B: 10/30 Rate of change in wound size Group A: mean ‐67.51 (SD 3.52); Group B: mean ‐52.75 (SD 4.52) |
Not reported |
Pain Group A: mean 3.04 (standard deviationSD 1.02); Group B: mean 4.19 (SD 0.37) The measurement time and method are not reported clearly |
Time of dressing change Group A: mean 11.06 (standard deviationSD 1.12); Group B: mean 37.36 (SD 5.24) |
Total dressing change cost Group A: RMB 35 000. 03 ± 15. 31 Group B: RMB 34993. 65 ± 16.39 |
| Ashby 2012 |
Group A: NPWT (n = 6) Group B: standard dressings (N = 6) "One of the following, chosen by the treating nurse: a spun hydrocolloid (fibrous hydrocolloid) dressing, a foam dressing or an alginate dressing (all non‐silver)" |
24 weeks | 24 weeks |
Group A: 1/6 Group B: 0/6 |
The number of participants with an AE: Group A: 5/6 Group B: 4/6 Serious AE (number of events): Group A: 4 Group B: 4 Non‐serious AE (number of events): Group A: 12 Group B: 8 |
Not reported | Not reported | Not reported | The number of trial treatment visits was reported but not extracted as the duration of treatments was different | Not reported |
|
de Laat 2011 |
Group A: NPWT (N = 6; 9 ulcers) Group B: conventional dressing therapy (n = 6; 7 ulcers) |
6 weeks | 6 weeks | Not reported |
Not reported for pressure ulcer group separately |
Number with 50% (or greater) reduction in wound size: Group A: 5/6 Group B: 5/6 Median treatment time in weeks until 50% wound volume reduction (IQR): Group A: 2 (1‐2) Group B: 3 (3‐4) |
Not reported | Not reported | Not reported |
Not reported |
| Ford 2002 |
Group A: NPWT Group B: Healthpoint system Total of 28 participants ‐ the number allocated to each group was not presented |
3‐10 months | Not clear what time point outcomes were presented for |
Group A: 2 ulcers healed Group B: 2 ulcers healed |
Not reported clearly: 1 lateral malleolar ulcer complicated by sepsis, requiring amputation |
Data reported on the Mean % reduction in volume could not be used as they were not clear if some participants had data considered in both trial groups | Not reported | Not reported | Not reported |
Not reported |
| Niezgoda 2004 |
Group A: NPWT (n = 54) Group B: moist wound healing (no further details) (n = 43) |
42 days | 42 days | Not reported |
Not reported |
Unadjusted Reported that wounds in Group A had a mean reduction in the area of 12.7cm² (SD 93.7). Wounds in Group B had a mean increase in the area of 23.5cm² (SD 261.2cm²). |
Not reported | Not reported | Not reported |
Mean cost of care per day (including materials, labour, debridements, and length of stay): Group A: USD 130 Group B: USD 132 No standard deviations reported |
Abbreviations
AE: adverse event(s); IQR: inter‐quartile range; NPWT: negative pressure wound therapy; SD: standard deviation
Comparison 1: NPWT compared with standard dressings; short‐term follow‐up (two studies, 42 participants)
One study with six weeks of follow‐up compared NPWT with standard dressings in people with spinal cord injury and pressure ulcers (de Laat 2011). The study was deemed to be at high risk of detection bias. Another study with a total of 30 participants (Şahin 2022) compared NPWT with standard dressings (wet to dry dressing) with eight weeks follow‐up. We judged the study as being at unclear risk of bias for selection bias and detection bias.
Primary outcomes
TheseBoth studies (de Laat 2011; Şahin 2022) did not report complete wound healing or adverse events.
Secondary outcome: change in ulcer size
The de Laat 2011 study reported that there was no evidence of a difference between groups in the number of pressure ulcers considered to have a 50% (or more) reduction in wound volume at the end of the six‐week follow‐up with 83% (5/6) participants recorded as reaching this endpoint in each group (risk ratio (RR) 1.00, 95% confidence interval (CI) 0.60 to 1.66; Analysis 1.1). This study reported a median time to reach a 50% (or greater) reduction in wound volume of two weeks (interquartile range (IQR) 1 to 2) in the NPWT‐treated group compared with three weeks (IQR 3 to 4) in the dressing‐treated group. We have not analysed the data further here. GRADE assessment: very low‐certainty evidence due to risk of bias, indirectness of the outcome (complete healing is preferable), and imprecision.
1.1. Analysis.

Comparison 1: NPWT compared with standard dressings: short‐term follow‐up, Outcome 1: Proportion of ulcers with 50% or greater reduction in wound area
The Şahin 2022 study reported that the change of wound area reduction in the NPWT group was significantly higher than in the control group at the end of the eight‐week follow‐up (mean difference (MD) ‐14.67, 95% CI ‐25.37 to ‐3.97; Analysis 1.2), and the wound area was measured by a mobile 3DWM device. This study only reported the mean and P value. We tried to contact the author to obtain more detailed data, but no reply was received. We calculated the missing standard deviation (SD) of variance where possible according to the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022). GRADE assessment: very low‐‐certainty evidence due to risk of bias, indirectness of the outcome, and imprecision.
1.2. Analysis.

Comparison 1: NPWT compared with standard dressings: short‐term follow‐up, Outcome 2: Change in wound size
Secondary outcomes: pressure ulcers severity
The Şahin 2022 study reported that the decrease of PUSH sore in the NPWT group was significantly higher than in the control group (MD ‐3.53, 95% CI ‐5.41 to ‐1.65; Analysis 1.3), the reduction in the PUSH score represents a decrease in the severity of pressure ulcers. We calculated the missing SD of variance where possible according to the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022). GRADE assessment: Very low‐‐certainty evidence due to risk of bias, indirectness of the outcome, and imprecision.
1.3. Analysis.

Comparison 1: NPWT compared with standard dressings: short‐term follow‐up, Outcome 3: PUSH score
Other secondary outcomes
de Laat 2011 and Şahin 2022 did not report on health‐related quality of life; pressure ulcer severity; wound infection; pain; resource use; cost; or wound recurrence.
Comparison 2: NPWT compared with standard dressings therapy; medium‐term follow‐up (three studies, 100 participants)
One study with a total of 12 participants compared NPWT with standard dressings with 24 weeks of follow‐up (Ashby 2012). The study was a pilot study that explicitly stated that it was not designed or powered to detect treatment effects. This study may have a potential bias in data collection. The study compared NPWT with standard dressings (alginate, foam, or hydrofibre ‐ the choice of these being left to health professionals). Two studies with a total of 88 participants compared NPWT with standard dressings within nine weeks of follow‐up (Dwivedi 2016; Dwivedi 2017). We judged both studies as being at high risk of bias for attrition bias and other biases.
Primary outcome: proportion of wounds healed
One study with a total of 12 participants (12 pressure ulcers) examined the proportion of pressure ulcers healed (Ashby 2012). There was no evidence of a difference in the number of wounds healed in the NPWT group (1/6) and the dressing group (0/6) (RR 3.00, 95% CI 0.15 to 61.74; Analysis 2.1). The study was not powered to detect a difference in wound healing, and there was such huge imprecision around the estimates that neither a positive nor negative effect of NPWT can be ruled out. GRADE assessment: very low‐certainty evidence due to serious imprecision and risk of bias.
2.1. Analysis.

Comparison 2: NPWT compared with standard dressings: medium‐term follow‐up, Outcome 1: Proportion of wounds healed
Dwivedi 2016 and Dwivedi 2017 did not report the proportion of wounds healed.
Primary outcome: adverse events
One study with a total of 12 participants reported adverse events (Ashby 2012). There was no evidence of a difference in the number of participants with adverse events in the NPWT group (83%, 5/6) and the dressing group (67%, 4/6) (RR: 1.25, 95% CI 0.64 to 2.44; Analysis 2.2). Again, the study was underpowered and findings were imprecise largely as it was not designed to assess relative treatment effects. GRADE assessment: very low‐certainty evidence due to serious imprecision and risk of bias. The Dwivedi 2017 study reported that 10 patients withdrew from the NPWT group due to adverse events, or owing to the refusal of additional treatment, but it did not provide detailed information.
2.2. Analysis.

Comparison 2: NPWT compared with standard dressings: medium‐term follow‐up, Outcome 2: Adverse events
The other study (Dwivedi 2016) did not provide any information on adverse events.
Secondary outcomes: change in ulcer size
Two studies (Dwivedi 2016; Dwivedi 2017) reported that length, width, and depth were significantly (P < 0.01) decreased in the NPWT group as compared with the standard care group at week nine. We tried to contact the author to obtain data on the change in the surface area, but no reply was received. We have not analysed the data further here. GRADE assessment: very low‐certainty evidence due to risk of bias, indirectness of the outcome, and imprecision.
Ashby 2012 did not report the change in ulcer size.
Secondary outcomes: pressure ulcer severity
Dwivedi 2017 and Dwivedi 2016 used the PUSH tool but did not provide sufficient data to be included in the analyses, we did not analyse further here.
Ashby 2012 did not report the pressure ulcer severity.
Secondary outcomes: cost
The total cost in the NPWT‐treated group was USD 105 compared with USD 200 in the standard dressing group (Dwivedi 2016). This study used innovative low‐cost devices, and the calculated cost may not represent the overall cost of commonly used NPWT devices. The data in the study were reported poorly and are not analysed further here. GRADE assessment: very low‐certainty evidence due to risk of bias, Indirectness, and imprecision. The other studies in this comparison (Ashby 2012; Dwivedi 2017) did not report economic outcomes.
Other secondary outcomes: health‐related quality of life, resource use, wound infection, pain, resource use, or wound recurrence
No studies were found that reported on: health‐related quality of life; resource use; wound infection; pain; or wound recurrence. The Ashby 2012 study did report on the number of trial treatment visits that were made, but we did not extract these data as the duration of treatments differed (Table 5).
Comparison 3: NPWT compared with moist wound healing; short‐term follow‐up (one study, 97 participants)
One study compared NPWT with 'moist wound healing', but provided few details about the comparator intervention (Niezgoda 2004). The only information available came from a conference abstract; no further published information was available. The study was presented as an interim analysis at a point when recruitment and follow‐up of recruited participants were not complete. We considered it to be at a high risk of attrition bias.
Primary outcomes
The Niezgoda 2004 study did not report on complete wound healing or adverse events.
Secondary outcome: change in ulcer size
Niezgoda 2004 reported only unadjusted data for changes in ulcer size (Table 5) which we did not consider further as per our methods.
Secondary outcome: cost
The mean cost of care (including materials, labour, debridements, and length of stay) in the NPWT‐treated group was USD 130 compared with USD 132 in the moist wound healing group. No information about the variation around these estimates was presented and the data are not analysed further here.
Other secondary outcomes
Niezgoda 2004 did not report on: health‐related quality of life; wound infection; pressure ulcer severity; resource use, pain; or wound recurrence.
Comparison 4: NPWT compared with the Healthpoint system (one study, 28 participants)
One study compared NPWT with the Healthpoint system (Ford 2002). The Healthpoint System consists of three gel products: Accuzyme®, Iodosorb®, and Panafil®. The study reports that of the choice of three treatments available ‐ participants whose wounds showed substantial exudate received Iodosorb® or Iodoflex®; those patients whose ulcers were clean and granulating received Panafil®. Accuzyme® was not used. We considered the study to be at high risk of attrition bias; it also had unit of analysis issues. It should be noted that the study was reported to be an interim analysis and that the length of follow‐up was unclear.
Primary outcome data: proportion of ulcers healed
The study reported that two ulcers healed in each treatment arm. However, whilst the number of participants for the study was reported (n = 28), the number in each treatment arm was not, and there were 41 wounds in the study. It was not clear whether one or two participants healed in each group. Due to these units of analysis issues, we have not analysed the data further here.
Primary outcome data: adverse events
Secondary outcomes
Ford 2002 did not report on changes in ulcer size; health‐related quality of life; pressure ulcer severity; wound infection; pain; resource use; cost; or wound recurrence.
Comparison 5: NPWT combined with internet‐plus home care compared with standard care (one study, 60 participants)
One study with a total of 60 participants (Tang 2019) compared NPWT (in‐home) with standard care with a 3‐month follow‐up.
Primary outcomes:
The Tang 2019 study did not report on complete wound healing or adverse events. The author only reported the proportion of significant effective treatment (the wound is of pressure ulcers healed completely covered with fresh granulation tissue), which we did not include in the analysis. We also note that covered with granulation tissue is not 'healed' so we would also question this definition of healing.
Secondary outcomes: change in ulcer size
The Tang 2019 study reported that the rate of wound area reduction in the NPWT group was significantly higher than in the control group at 1 month (MD ‐5.96%, 95% CI ‐6.74% to ‐5.18% to; Analysis 3.1) and 3 months after the intervention (MD ‐14.76%, 95% CI ‐16.81% to ‐12.71%; Analysis 4.1). We downgraded the evidence to very low certainty: once for risk of bias, once for indirectness, and once for imprecision.
3.1. Analysis.

Comparison 3: NPWT combined with internet‐plus home care compared with standard care: short‐term follow‐up, Outcome 1: Rate of change in ulcer size
4.1. Analysis.

Comparison 4: NPWT combined with internet‐plus home care compared with standard care: medium‐term follow‐up, Outcome 1: Rate of change in ulcer size
The study also reported a higher effective rate (effective definition is that the ulcer size is reduced by more than 50%, with fresh granulation tissue growing, and the coverage rate of the wound is more than 70%) in the treatment group (16/30) than those in the control group (10/30), we have not analysed the data further here.
Secondary outcomes: pain
Tang 2019 reported that the severity of pain reported in the intervention group was lower than that in the control group (MD ‐1.15 points, 95% CI ‐1.54 to ‐0.76; Analysis 4.2), but this study did not report detailed information on pain measurement methods and time. We downgraded the evidence to very low certainty: once for risk of bias, once for indirectness, and once for imprecision.
4.2. Analysis.

Comparison 4: NPWT combined with internet‐plus home care compared with standard care: medium‐term follow‐up, Outcome 2: Pain
Secondary outcomes: resource use
Tang 2019 reported that the frequency of dressing changes in the intervention group was lower than that in the control group. The point estimate for the MD was ‐26.3 (95% CI ‐28.22 to ‐24.38; Analysis 4.3). We downgraded the evidence to very low certainty: once for risk of bias, once for indirectness, and once for imprecision.
4.3. Analysis.

Comparison 4: NPWT combined with internet‐plus home care compared with standard care: medium‐term follow‐up, Outcome 3: Frequency of dressing change
Secondary outcomes: cost
Tang 2019also reported the cost of dressing changes in the NPWT group (RMB 35000.03) and in the standard care group (RMB 34993.65). No details information about the variation around these estimates and the data are not analysed further here.
Secondary outcomes
Tang 2019did not report on health‐related quality of life; pressure ulcer severity; wound infection; or wound recurrence.
Discussion
Summary of main results
This updated review included eight studies with 327 participants which predominantly assess negative pressure wound therapy NPWT to treat pressure ulcers. Only Ashby 2012, which was a pilot/feasibility study, reported primary outcome data on both the proportion of wounds healed and adverse events that we report fully in the review. Whilst there was no evidence of a difference between NPWT and standard dressings for these outcomes, the study was hugely underpowered having only 12 participants and so its estimates were very imprecise and inconclusive. The fact that only one participant healed during the 24‐week follow‐up period highlights the need for adequate follow‐up in studies that evaluate treatments for severe pressure ulcers.
We classed the seven remaining studies as being at unclear or high risk of bias for at least one risk of bias domain (de Laat 2011; Dwivedi 2016; Dwivedi 2017; Ford 2002; Niezgoda 2004; Şahin 2022; Tang 2019); they also had a small sample size and poorly reported outcomes. One of these studies, which also had 12 participants (de Laat 2011), reported no evidence of a difference in the number of wounds achieving a 50% (or greater) reduction in wound volume over a six‐week follow‐up period. This was a surrogate outcome and the comparison was again underpowered and imprecise. Three studies reported that NPWT can reduce the surface area of pressure ulcers compared with dressing, but the results of these studies have not been completely reported, and the certainty of evidence is considered to be very low, so we cannot draw reliable conclusions (Dwivedi 2016; Dwivedi 2017; Şahin 2022). Three studies reported a composite measure of pressure ulcer severity, but data was poorly reported, and it was not possible to draw a conclusion (Dwivedi 2016; Dwivedi 2017; Şahin 2022).
One study reported that NPWT combined with internet‐plus home care can reduce the surface area of ulcers, pain, and dressing change times compared with standard care, but we downgraded the certainty of evidence to a very low level due to the risk of bias, indirectness, and imprecision (Tang 2019).
Overall completeness and applicability of evidence
With the help of the Cochrane Wounds, we comprehensively searched and screened the current evidence comparing the effects of NPWT for treating pressure ulcers. While we added four randomised controlled trials (RCTs) since the previous version of this review, the data were still insufficient to draw a clear conclusion. Most studies did not (or not fully) report our pre‐established key outcomes: complete healing of pressure ulcers, time to healing data, and adverse events. Most of the included studies have unclear, inconsistent, and non‐standard outcome reporting. For example, although some studies reported changes in wound size, they only provided changes in length and width, and such results were prone to measurement bias compared to complete wound area measurements. We contacted the authors several times to obtain further data but did not obtain the data we expected.
All the included studies had small sample sizes with short or medium follow‐up times, which resulted in a lack of statistical power to detect treatment effects. The NPWT devices used in the included studies were also different. Some studies used self‐made NPWT devices, which was also an important factor limiting the promotion of research results. In addition, we were unable to perform subgroup analysis due to missing data.
Quality of the evidence
We rated the certainty of the evidence according to the GRADE approach. We found the certainty of the evidence to be very low for all outcomes. Low confidence in the evidence means that the results of included RCTs need to be cautiously interpreted.
RCTs need to be adequately powered so that they are able to detect treatment effects of a specified size, should they exist. Additionally, trials should have adequate follow‐up periods so that there is enough time in which important outcome events, such as complete wound healing, can occur. However, only one included study with 12 participants reported the primary outcome (complete wound healing and adverse events). The short follow‐up time may be the main reason for the failure to observe wound healing in other studies. In addition, most included studies were small and presented limited outcome data with estimates that were imprecise and had wide confidence intervals. In the future, RCTs should calculate the sample size through appropriate methods and observe important outcomes during a sufficiently long follow‐up period.
Rigorous RCTs in wound care are feasible ‐ they must follow good practice conduct and reporting guidelines, for example, CONSORT (Schulz 2010). Key areas of good practice are the robust generation of a randomised sequence and the use of blinded outcome assessment, but only one study claimed they used reported proper random methods, allocation concealed, and blinded outcome assessment. Additionally, studies should report clearly how they planned to collect adverse event data and how this process was standardised for both treatment arms. In terms of analysis, where possible, data from all participants should be included ‐ that is an intention‐to‐treat (ITT) analysis should be conducted ‐ and measures of variation such as the standard deviation (SD) or standard error (SE) should be presented around measures where appropriate. Half of the included studies had attrition bias due to the loss of participants; steps should be taken while a trial is being conducted to prevent the occurrence of missing data as far as possible.
Potential biases in the review process
There were several potential biases in this review process.
We attempted to contact the authors included in the study to obtain more information, it is unfortunate that not all the authors responded. Missing data and information may lead to bias.
The review considered as much evidence as it was possible to obtain, including studies that were not published in English‐language journals. It is possible that there may be unpublished data that we have not been able to access and there is a potential for publication bias, however, this is likely to be a limited issue in this review.
Our search in January 2022 did not identify any ongoing studies, so we consider our findings up‐to‐date at the time of publication.
Agreements and disagreements with other studies or reviews
There are two systematic reviews that have examined the effect of NPWT on the treatment of pressure ulcers. The previous Cochrane Review concluded that no rigorous RCT evidence is available regarding the effects of NPWT compared with alternatives for the treatment of pressure ulcers (Dumville 2015). Our updated review draws the same conclusions. A new meta‐analysis published in 2021 concluded that NPWT can significantly improve pressure ulcer complete healing rate, shorten healing time and reduce cost for stage III or IV pressure ulcers (Song 2021). We do not agree with the newly published meta‐analysis and consider it to have limitations. The meta‐analysis included 16 RCTs, which is inconsistent with other reviews. Some of the included studies did not distinguish pressure ulcers from other wounds (lower limb ulcers and diabetic foot ulcers), and the authors did not distinguish the type of ulcer well when analysing and interpreting the results. For example, the meta‐analysis includes a study that was withdrawn for ethical reasons and does not distinguish between pressure ulcers and other ulcers (Ali 2017). The results of this included study are not consistent with most other reviews.
NICE guidelines (NICE 2014) suggest "do not routinely offer adults NPWT to treat a pressure ulcer, unless it is necessary to reduce the number of dressing changes” (for example, in a wound with a large amount of exudate)", and maintain this suggestion in 2018 edition. (rating B, NICE 2018). Our results are consistent with the recommendations from the NICE, although we included two additional RCTs.
The American College of Physicians (ACP) guideline does not recommend NPWT as an effective therapy for pressure ulcer treatment (low level, ACP 2015). This review included three studies; we excluded two of these because they were not prospective RCTs. Although we believe that the current evidence is insufficient to prove that NPWT is ineffective, it is consistent with our conclusions in the ACP guideline also considers that the quality of the evidence is low.
The Wound Healing Society (WHS) guideline (2015 update) considers “using NPWT for stage III or IV pressure ulcers that fail to progress in healing with conventional therapy” (Level 1, WHS 2015). We excluded a non‐RCT that they included, and included four additional RCTs in this review. We do not think that this guideline defines the current quality of evidence as level 1 (they described it as high). At the same time, when using NPWT, we should not only consider whether pressure ulcers fail to progress in healing, but also comprehensively consider the amount of exudate, wound size, and cost‐effectiveness.
The Wound, Ostomy and Continence Nurses Society‐Wound (WOCN) guideline (2016) considers the “use of NPWT which may increase complete wound closure compared to standard wound dressings and is associated with lower risk of secondary infections” (rating B, WOCN 2016). Japanese Society of Pressure Ulcers (JSPU) guideline (2016) considered "NPWT as an early adjunct therapy for reducing the size and depth of Stage III and IV pressure ulcers" (rating C1, JSPU 2016). Similarly, the International Clinical Practice Guideline (2019 edition) considered NPWT as an early adjunct therapy for reducing the size and depth of Category/Stage III and IV pressure injuries (rating B, EPUAP/NPUAP/PPPIA 2019); this guideline includes some non‐RCTs. The recommendations of these guidelines can be combined with the opinions of clinical experts, but the current evidence is not sufficient to support the routine recommendation of NPWT for the treatment of pressure ulcers.
Authors' conclusions
Implications for practice.
Most randomised controlled trials (RCT) included only a small number of patients and did not clearly report clinically important outcomes (such as the proportion of wounds healed, time to ulcer healing, and adverse events). The current evidence is of insufficient quality to be able to draw conclusions about the effectiveness and safety of NPWT for pressure ulcers. More high‐quality RCTs are still needed to determine the effect of negative pressure wound therapy (NPWT) on key outcomes. Practitioners should fully consider the uncertainty of this evidence and choose appropriate treatment methods in combination with patients' skin status and cost‐effectiveness.
Implications for research.
We have the following suggestions for future research.
Most of the included studies did not report the primary outcome or provide enough information to perform a meta‐analysis, which is an important limitation of this study. Future studies need to report key outcomes for evaluating the effectiveness and safety of NPWT, such as the proportion of ulcer healing, healing time, and adverse events, and should also use recognised methods to evaluate cost, resource use, pain, change in ulcer size, the severity of pressure ulcers, and quality of life, which are important for patients and clinical decision‐making.
Problems such as the lack of standardised outcome measurement, poor reporting of outcomes, and inconsistency of outcomes measured between studies can be improved by developing and implementing a core outcome set (Schmitt 2019). Future researchers should develop a core outcome set for the treatment of pressure ulcers.
Future research should adhere to CONSORT reporting guidelines to improve the transparency and accuracy of reporting and interpreting clinical trial results (Schulz 2010). Most of the included studies did not adhere to these guidelines, which made it difficult for uses of their evidence to judge the studies' risk of bias.
Most of the included studies were small with short follow‐up times. Large and robust RCTs are still required to judge the efficacy of NPWT in treating pressure ulcers.
Cost‐effectiveness needs to be fully considered before NPWT is widely used, there is still a lack of research focus on the cost‐effectiveness of NPWT.
Future research should further clarify the parameter settings of NPWT, such as therapeutic frequency, duration, and location. Meanwhile, NPWT is not applicable to pressure ulcer patients at all stages, so it is necessary to clarify which states of pressure ulcer are more suitable for NPWT.
Future research should pay attention to concealed allocation, and make sure to blind assessors to avoid bias.
Future researchers should register or publish their study protocol in advance to improve transparency, adhere to their protocol and justify deviations from their study protocol.
What's new
| Date | Event | Description |
|---|---|---|
| 26 May 2023 | New citation required but conclusions have not changed | Updated. Conclusions not changed. |
| 26 May 2023 | New search has been performed | For this update, the co‐authors have been changed to Jiyuan Shi, Ya Gao, Jinhui Tian, Jiang Li, Jianguo Xu, Fan Mei, and Zheng Li. We have updated our search strategy and included four new randomised controlled trials (RCTs) with a total of 178 participants, in addition to the four studies identified in the original review which had a total of 149 participants. For this update, we have not changed the previous version’s conclusions due to the very low certainty of evidence (GRADE) for all outcomes. Thus, more high‐quality RCTs are still necessary to determine the effect of negative pressure wound therapy (NPWT) on essential benefits and adverse outcomes. We will continue to monitor newly published trials in this field and update this review as needed. |
History
Protocol first published: Issue 10, 2014 Review first published: Issue 5, 2015
| Date | Event | Description |
|---|---|---|
| 2 June 2015 | Amended | Acknowledgements completed and updated |
Acknowledgements
Cochrane Wounds supported the authors in the development of this updated review. The authors are grateful to Sophie Bishop (who ran the searches), Tom Patterson (who supported the authors and the editorial team), and Gill Rizzello (who provided editorial guidance to the authors and supported the editorial team).
The authors are also grateful to the following editors and peer reviewers for their time and conducted the review and editorial process for this article:
Sign‐off Editor (final editorial decision): Neil O'Connell; Managing Editor (selected peer reviewers provided editorial guidance to authors, edited the article): Helen Wakeford, Cochrane Central Editorial Service; Editorial Assistant (collated peer‐reviewer comments, conducted editorial policy checks, and supported editorial team): Lisa Wydrzynski; Cochrane Central Editorial Service Copy Editor (copy editing and production): Heather Maxwell; Peer‐reviewers (provided comments and recommended an editorial decision): Georgina Gethin, Alliance for Research and Innovation in Wounds, Professor of Nursing in the School of Nursing and Midwifery, National University of Ireland, Galway; Nancy Munoz, Assistant Chief, Nutrition and Food Service VA Sothern Nevada Healthcare System, US; Tania Philips, Professor of Dermatology, Boston University School of Medicine; Director, Dermatology Wound Clinic, Boston Medical Center, US (clinical/content review), Jennifer Hilgart, Cochrane (methods review), Steve McDonald, Cochrane Australia (search review).
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure and Cochrane Programme Grant funding to the Cochrane Wounds Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Search strategies
Cochrane Wounds Specialised Register 1 MESH DESCRIPTOR Negative‐Pressure Wound Therapy EXPLODE ALL AND INREGISTER 2 MESH DESCRIPTOR Suction EXPLODE ALL AND INREGISTER 3 MESH DESCRIPTOR vacuum EXPLODE ALL AND INREGISTER 4 ("negative pressure" or negative‐pressure or TNP or NPWT or NWPT) AND INREGISTER 5 (sub‐atmospheric or subatmospheric) AND INREGISTER 6 ((seal* next surface*) or (seal* next aspirat*)) AND INREGISTER 0 7 (wound near2 suction*) AND INREGISTER 8 (wound near5 drainage) AND INREGISTER 9 (foam next suction*) or (suction* next dressing*) AND INREGISTER 1 10 vacuum‐assisted AND INREGISTER 11 ((vacuum next therapy) or (vacuum next dressing*) or (vacuum next seal*) or (vacuum next closure) or (vacuum next assist*) or (vacuum next compression) or (vacuum next pack*) or (vacuum next drainage) or (suction* adj drainage) or VAC) AND INREGISTER 12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 13 MESH DESCRIPTOR Pressure Ulcer EXPLODE ALL AND INREGISTER 14 (pressure next (ulcer* or sore* or injur*)) AND INREGISTER 15 (decubitus next (ulcer* or sore*)) AND INREGISTER 16 ((bed next sore*) or bedsore*) AND INREGISTER 17 #14 OR #13 OR #15 OR #16 18 #12 AND #17
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL) #1 MeSH descriptor: [Negative‐Pressure Wound Therapy] explode all trees #2 MeSH descriptor: [Suction] explode all trees #3 MeSH descriptor: [Vacuum] explode all trees #4 ("negative pressure" or negative‐pressure or TNP or NPWT or NWPT):ti,ab,kw #5 (sub‐atmospheric or subatmospheric):ti,ab,kw #6 ((seal* next surface*) or (seal* next aspirat*)):ti,ab,kw #7 (wound near/2 suction*):ti,ab,kw #8 (wound near/5 drainage):ti,ab,kw #9 (foam next suction*) or (suction* next dressing*):ti,ab,kw #10 vacuum‐assisted:ti,ab,kw #11 ((vacuum next therapy) or (vacuum next dressing*) or (vacuum next seal*) or (vacuum next assist*) or (vacuum next closure) or (vacuum next compression) or (vacuum next pack*) or (vacuum next drainage) or (suction* adj drainage) or VAC):ti,ab,kw #12 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11) #13 MeSH descriptor: [Pressure Ulcer] explode all trees #14 (pressure next (ulcer* or sore* or injur*)):ti,ab,kw #15 (decubitus next (ulcer* or sore*)):ti,ab,kw #16 ((bed next sore*) or bedsore*):ti,ab,kw #17 #13 or #14 or #15 or #16 #18 #12 and #17 in Trials
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL) via CRS 1 MESH DESCRIPTOR Negative‐Pressure Wound Therapy EXPLODE ALL AND CENTRAL:TARGET 2 MESH DESCRIPTOR Suction EXPLODE ALL AND CENTRAL:TARGET 3 MESH DESCRIPTOR vacuum EXPLODE ALL AND CENTRAL:TARGET 4 ("negative pressure" or negative‐pressure or TNP or NPWT or NWPT) AND CENTRAL:TARGET 5 (sub‐atmospheric or subatmospheric) AND CENTRAL:TARGET 6 ((seal* next surface*) or (seal* next aspirat*)) AND CENTRAL:TARGET 7 (wound near2 suction*) AND CENTRAL:TARGET 8 (wound near5 drainage) AND CENTRAL:TARGET 9 (foam next suction*) or (suction* next dressing*) AND CENTRAL:TARGET 10 vacuum‐assisted AND CENTRAL:TARGET 11 ((vacuum next therapy) or (vacuum next dressing*) or (vacuum next seal*) or (vacuum next closure) or (vacuum next assist*) or (vacuum next compression) or (vacuum next pack*) or (vacuum next drainage) or (suction* adj drainage) or VAC) AND CENTRAL:TARGET 12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 AND CENTRAL:TARGET 13 MESH DESCRIPTOR Pressure Ulcer EXPLODE ALL AND CENTRAL:TARGET 14 (pressure next (ulcer* or sore* or injur*)) AND CENTRAL:TARGET 15 (decubitus next (ulcer* or sore*)) AND CENTRAL:TARGET 16 ((bed next sore*) or bedsore*) AND CENTRAL:TARGET 17 #14 OR #13 OR #15 OR #16 AND CENTRAL:TARGET 18 #12 AND #17 AND CENTRAL:TARGET 19 (NCT0* or ACTRN* or ChiCTR* or DRKS* or EUCTR* or eudract* or IRCT* or ISRCTN* or JapicCTI* or JPRN* or NTR0* or NTR1* or NTR2* or NTR3* or NTR4* or NTR5* or NTR6* or NTR7* or NTR8* or NTR9* or SRCTN* or UMIN0*):AU AND CENTRAL:TARGET 20 http*:SO AND CENTRAL:TARGET 21 #19 OR #20 AND CENTRAL:TARGET 22 #18 AND #21
Ovid MEDLINE
1 exp Negative‐Pressure Wound Therapy/
2 exp Suction/ 3 exp Vacuum/ 4 (negative pressure or negative‐pressure or TNP or NPWT or NWPT).tw. 5 (sub‐atmospheric or subatmospheric).tw. 6 ((seal* adj surface*) or (seal* adj aspirat*)).tw. 7 (wound adj2 suction*).tw. 8 (wound adj5 drainage).tw. 9 ((foam adj suction*) or (suction* adj dressing*)).tw. 10 vacuum‐assisted.tw. 11 ((vacuum adj therapy) or (vacuum adj dressing*) or (vacuum adj seal*) or (vacuum adj closure) or (vacuum adj assist*) or (vacuum adj compression) or (vacuum adj pack*) or (vacuum adj drainage) or (suction* adj drainage) or VAC).tw. 12 or/1‐11 13 exp Pressure Ulcer/ 14 (pressure adj (ulcer* or sore* or injur*)).tw. 15 (decubitus adj (ulcer* or sore*)).tw. 16 (bedsore* or bed sore*).tw. 17 or/13‐16 18 12 and 17 19 randomized controlled trial.pt. 20 controlled clinical trial.pt. 21 randomized.ab. 22 placebo.ab. 23 drug therapy.fs. 24 randomly.ab. 25 trial.ab. 26 groups.ab. 27 or/19‐26 28 exp animals/ not humans.sh. 29 27 not 28 30 18 and 29
Ovid Embase 1 exp suction drainage/ 2 exp vacuum assisted closure/ 3 (negative pressure or negative‐pressure or TNP or NPWT or NWPT).tw. 4 (sub‐atmospheric or subatmospheric).tw. 5 ((seal* adj surface*) or (seal* adj aspirat*)).tw. 6 (wound adj2 suction*).tw. 7 (wound adj5 drainage).tw. 8 ((foam adj suction*) or (suction* adj dressing*)).tw. 9 vacuum‐assisted.tw. 10 ((vacuum adj therapy) or (vacuum adj dressing*) or (vacuum adj seal*) or (vacuum adj assist*) or (vacuum adj closure) or (vacuum adj compression) or (vacuum adj pack*) or (vacuum adj drainage) or (suction* adj drainage) or VAC).tw. 11 or/1‐10 12 exp decubitus/ 13 (pressure adj (ulcer* or sore* or injur*)).tw. 14 (decubitus adj (ulcer* or sore*)).tw. 15 (bedsore* or bed sore*).tw. 16 or/12‐15 17 11 and 16 18 Randomized controlled trial/ 19 Controlled clinical study/ 20 Random$.ti,ab. 21 randomization/ 22 intermethod comparison/ 23 placebo.ti,ab. 24 (compare or compared or comparison).ti. 25 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 26 (open adj label).ti,ab. 27 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 28 double blind procedure/ 29 parallel group$1.ti,ab. 30 (crossover or cross over).ti,ab. 31 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 orintervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 32 (assigned or allocated).ti,ab. 33 (controlled adj7 (study or design or trial)).ti,ab. 34 (volunteer or volunteers).ti,ab. 35 human experiment/ 36 trial.ti. 37 or/18‐36 38 (random$ adj sampl$ adj7 (cross section$ or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.) 39 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.) 40 (((case adj control$) and random$) not randomi?ed controlled).ti,ab. 41 (Systematic review not (trial or study)).ti. 42 (nonrandom$ not random$).ti,ab. 43 Random field$.ti,ab. 44 (random cluster adj3 sampl$).ti,ab. 45 (review.ab. and review.pt.) not trial.ti. 46 we searched.ab. and (review.ti. or review.pt.) 47 update review.ab. 48 (databases adj4 searched).ab. 49 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/ 50 Animal experiment/ not (human experiment/ or human/) 51 or/38‐50 52 37 not 51 53 17 and 52
EBSCO CINAHL Plus
S42 S18 AND S41 S41 S40 NOT S39 S40 S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 S39 S37 NOT S38 S38 MH (human) S37 S34 OR S35 OR S36 S36 TI (animal model*) S35 MH (animal studies) S34 MH animals+ S33 AB (cluster W3 RCT) S32 MH (crossover design) OR MH (comparative studies) S31 AB (control W5 group) S30 PT (randomized controlled trial) S29 MH (placebos) S28 MH (sample size) AND AB (assigned OR allocated OR control) S27 TI (trial) S26 AB (random*) S25 TI (randomised OR randomized) S24 MH cluster sample S23 MH pretest‐posttest design S22 MH random assignment S21 MH single‐blind studies S20 MH double‐blind studies S19 MH randomized controlled trials S18 S12 AND S17 S17 S13 OR S14 OR S15 OR S16 S16 TI ( bed sore* or bedsore* ) or AB ( bed sore* or bedsore* ) S15 TI ( decubitus ulcer* or decubitus sore* or decubitus injur*) or AB ( decubitus ulcer* or decubitus sore* or decubitus injur* ) S14 TI ( pressure ulcer* or pressure sore* or pressure injur*) or AB ( pressure ulcer* or pressure sore* or pressure injur* ) S13 (MH "Pressure Ulcer+") S12 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 S11 TI ( foam suction* or suction* dressing* or suction* drainage ) OR AB ( foam suction* or suction* dressing* or suction* drainage ) S10 TI vacuum‐assisted OR AB vacuum‐assisted S9 TI ( vacuum therapy or vacuum dressing* or vacuum seal* or vacuum closure or vacuum compression or vacuum pack or vacuum drainage or vacuum assist* or VAC ) OR AB ( vacuum therapy or vacuum dressing* or vacuum seal* or vacuum closure or vacuum compression or vacuum pack or vacuum drainage or vacuum assist* or VAC ) S8 TI (wound N5 drainage) OR AB (wound N5 drainage) S7 TI (wound N2 suction*) OR AB (wound N2 suction*) S6 TI ( (seal* N1 surface* or seal* N1 aspirat*) ) OR AB ( (seal* N1 surface* or seal* N1 aspirat*) ) S5 TI ( sub‐atmospheric or subatmospheric ) OR AB ( sub‐atmospheric or subatmospheric ) S4 TI ( negative pressure or negative‐pressure or TNP or NPWT or NWPT ) OR AB ( negative pressure or negative‐pressure or TNP or NPWT or NWPT ) S3 (MH "Negative Pressure Wound Therapy") S2 (MH "Vacuum") S1 (MH "Suction+")
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
negative pressure OR vacuum assisted OR NPWT OR TNP | Pressure Ulcer OR decubitus
World Health Organization International Clinical Trials Registry Platform pressure ulcer OR decubitus [Title] AND negative pressure OR vacuum assisted OR NPWT OR TNP [Intervention] pressure ulcer OR decubitus [Condition] AND negative pressure OR vacuum assisted OR NPWT OR TNP [Intervention] pressure injury [Title] AND negative pressure OR vacuum assisted OR NPWT OR TNP [Intervention] pressure injury [Condition] AND negative pressure OR vacuum assisted OR NPWT OR TNP [Intervention]
Appendix 2. 'Risk of bias' assessment (individually randomised controlled trials)
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process provided to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Either of the following.
Insufficient information available to permit a judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
'As‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following.
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following.
The study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the study's pre‐specified primary outcomes have been reported.
One or more primary outcomes are reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information available to permit a judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Appendix 3. 'Risk of bias' assessment (cluster‐randomised controlled trials)
In cluster‐randomised trials, particular biases to consider include the following.
Recruitment bias. This can occur when individuals are recruited to the trial after the clusters have been randomised, as the knowledge of whether each cluster is an 'intervention' or 'control' cluster could affect the types of participants recruited.
Baseline imbalance. Cluster‐randomised trials often randomise all clusters at once, so lack of concealment of an allocation sequence should not usually be an issue. However, because small numbers of clusters are randomised, there is a possibility of chance baseline imbalance between the randomised groups, in terms of either the clusters or the individuals. Although not a form of bias as such, the risk of baseline differences can be reduced by using stratified or pair‐matched randomisation of clusters. Reporting of the baseline comparability of clusters, or statistical adjustment for baseline characteristics, can help reduce concern about the effects of baseline imbalance.
Loss of clusters. Occasionally complete clusters are lost from a trial and have to be omitted from the analysis. Just as for missing outcome data in individually randomised trials, this may lead to bias. In addition, missing outcomes for individuals within clusters may also lead to a risk of bias in cluster‐randomised trials.
Incorrect analysis. Many cluster‐randomised trials are analysed by incorrect statistical methods, not taking the clustering into account. Such analyses create a 'unit of analysis error' and produce over‐precise results (the standard error of the estimated intervention effect is too small) and P values that are too small. They do not lead to biased estimates of effect. However, if they remain uncorrected, they will receive too much weight in a meta‐analysis.
Comparability with individually randomised trials. In a meta‐analysis including both cluster and individually randomised trials, or including cluster‐randomised trials with different types of clusters, possible differences between the intervention effects being estimated need to be considered. For example, in a vaccine trial of infectious diseases, a vaccine applied to all individuals in a community would be expected to be more effective than if the vaccine was applied to only half of the people. Another example is provided by a Cochrane Review of hip protectors. The cluster trials showed a large positive effect whereas individually randomised trials did not show any clear benefit. One possibility is that there was a 'herd effect' in the cluster‐randomised trials (which were often performed in nursing homes, where compliance with using the protectors may have been enhanced). In general, such 'contamination' would lead to underestimates of the effect. Thus, if an intervention effect is still demonstrated despite contamination in those trials that were not cluster‐randomised, a confident conclusion about the presence of an effect can be drawn. However, the size of the effect is likely to be underestimated. Contamination and 'herd effects may be different for different types of clusters.
Data and analyses
Comparison 1. NPWT compared with standard dressings: short‐term follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Proportion of ulcers with 50% or greater reduction in wound area | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.60, 1.66] |
| 1.2 Change in wound size | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐14.67 [‐25.37, ‐3.97] |
| 1.3 PUSH score | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐3.53 [‐5.41, ‐1.65] |
Comparison 2. NPWT compared with standard dressings: medium‐term follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Proportion of wounds healed | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.15, 61.74] |
| 2.2 Adverse events | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.64, 2.44] |
Comparison 3. NPWT combined with internet‐plus home care compared with standard care: short‐term follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Rate of change in ulcer size | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐5.96 [‐6.74, ‐5.18] |
Comparison 4. NPWT combined with internet‐plus home care compared with standard care: medium‐term follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Rate of change in ulcer size | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐14.76 [‐16.81, ‐12.71] |
| 4.2 Pain | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐1.54, ‐0.76] |
| 4.3 Frequency of dressing change | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐26.30 [‐28.22, ‐24.38] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ashby 2012.
| Study characteristics | ||
| Methods | 2‐arm RCT Conducted in home and hospital settings in 1 geographical location in the UK Duration of follow‐up was a maximum of 24 weeks |
|
| Participants | 44 participants: all included in the analysis Inclusion criteria listed: pressure ulcer graded III or IV according to the European Pressure Ulcer Advisory Panel Grading System; must receive primary care via Leeds Primary Care Trust; ulcer should contain at least 80% viable tissue or have a very thin layer of slough (nonviable tissue) requiring no further debridement prior to use of NPWT Exclusion criteria listed: the presence of unclear undermining in the pressure ulcer cavity precluding the use of NPWT; pressure ulcer with necrotic tissue, eschar, or necrotic bone present; the patient has limited life expectancy; pressure ulcer located where, in the opinion of the treating clinician, a vacuum seal could not be obtained; pressure ulcer too close to exposed blood vessels or organs, or both, anastomotic sites or nerves, or both; patient unable to give valid informed consent because of incapacity; the patient was unable to consent as trial materials were not available in a suitable language; the patient did not wish to consent to participation within a trial; a clinical judgment was made that the patient was not receiving adequate nutrition to allow treatment with NPWT; other reasons, in the clinical judgment of the treating clinician or nurse, which excluded the patient from the trial |
|
| Interventions | Group A: the wound was closed using a Wound Care Kit that includes foam dressing (V.A.C. Granu Foam Silver), film drape, TRAC pad with tubing and a drainage canister. Steril foam material was placed inside the wound and was attached to the canister through tubing. The canister was attached to the Vacuum‐Assisted Closure device (V.A.C.® Therapy System Patient Support – KCI), which is a portable device that applies intermittent or continuous negative pressure. The device was operated at 125 mmHg pressure for 5 min with and 2 min without active vacuum. Wound dressings were changed every 48 h. The wound area was measured after all three rounds of treatment. Offloading of the sore was performed by position change, airflow mattress usage and it was taken into consideration not to raise the head of the bed, more than 30◦ Group B: The wound was initially evaluated for any necrotic findings and debrided if needed, and then washed with an antiseptic solution. A culture specimen was obtained from the wound. 3DWM was used to measure the pressure sores by taking pictures. The length and width of wounds were measured with disposable paper rulers. Wound depth was determined in centimetres with a sterile cotton‐tip applicator by measuring against a ruler. Wounds were finally covered with gauze dressing soaked with saline. Wounds were treated three times a day, and measurements were repeated every 48 h. Co‐interventions: none described |
|
| Outcomes | Primary outcomes: complete wound healing (% ulcers healed) Secondary outcomes: adverse events |
|
| Notes | Pilot study Only one ulcer per participant was followed Duration of follow‐up differed between groups: mean duration was 3.8 months for Group A and 5.0 months for Group B Funding: Supported by the Medical Research Council |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was conducted using pre‐generated random permuted blocks (block sizes of four and six). A data manager at the York Trials Unit, who was completely independent of the research team, created the randomisation programme" Comment: adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "the research nurse telephoned a secure and remote randomisation service, located at the York Trials Unit (University of York, UK)." Comment: central allocation was used to conceal allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Wound healing Quote: "We piloted a blinded outcome assessment process using digital photographs of the wound taken using the mobile camera phone" Comment: blinding of key study personnel used and unlikely that the blinding could have been broken Adverse events Comment: not blinded Resource use Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: flow chart shows that all participant data were included in analysis |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes identified in the methods section were reported in the results (and were outcomes that would be expected to be included in such a study). Protocol not seen. Only 1 participant's pressure ulcer healed so not possible to calculate mean 'time to healing' |
| Other bias | Unclear risk | No unit of analyses issues Quote: "Whilst the research nurse was primarily responsible for data collection, this responsibility was also delegated to nurses treating patients in acute and community settings." Comment: there may have been inconsistency in reporting between community nurses |
de Laat 2011.
| Study characteristics | ||
| Methods | 2‐arm RCT Multi‐centred, conducted in hospital settings in the Netherlands Duration of follow‐up was a maximum of 6 weeks |
|
| Participants | 24 participants (with a total of 28 wounds). 12 of these participants had pressure ulcers. Data were extracted and presented for the pressure ulcer subgroup only. Inclusion criteria listed: patients ≥ 18 years who were admitted to the study hospitals with difficult‐to‐heal surgical wounds, or paraplegic and tetraplegic patients with pressure ulcers grade IV according to the European Pressure Ulcer Advisory Panel grading system Exclusion criteria listed: patients with bleeding disorders; thrombolytic treatment; fistulas to organs or body cavities; malignant disease; untreated osteomyelitis; life expectancy of < 1 year; radiation or chemical exposure; pregnant or lactating women; people unable to comply with 1 of the interventions or who had been treated with 1 of the study treatments in the past 30 days |
|
| Interventions | Group A: NPWT (VAC system, vacuum‐assisted closure; KCI, San Antonio, TX, USA; n = 6, 7 pressure ulcers). "Devices were used in accordance with the manufacturer's guidance. The foam dressings and the TRAC Pad were changed 3 times a week (Monday morning, and Wednesday and Friday in the afternoon). The fluid connection system was changed at least once a week. Negative pressure mode of 125 mm Hg." Group B: conventional dressing therapy (n = 6, 9 pressure ulcers) with a sodium hypochlorite 0.25% solution. "This wet‐to‐moist dressing was changed 2 to 3 times a day, depending on the wound debris. The sodium hypochlorite solution was prepared by one hospital pharmacist." Co‐intervention: wound debridement took place when considered clinically necessary before the start of the therapy and during treatment. Participants received (medical) care as needed |
|
| Outcomes | Primary outcomes: adverse events Secondary outcomes: change in wound size |
|
| Notes | Some participants had more than one ulcer, so there was potential for unit of analysis issues although this is not clear from the report Funding source: the surgical department of Nijmegen University Medical Centre |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A prospective stratified randomised controlled trial was carried out …" Comment: method of generating of random schedule is not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients in the difficult‐to‐heal surgical wounds group or the spinal cord injury patients with pressure ulcer group, were both allocated randomly to either the topical negative pressure group or the sodium hypochlorite group by using sealed envelopes." Comment: whilst sealed envelopes were used to conceal allocation it is not clear whether these were numbered to ensure sequential opening, or opened by an independent person |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
The median treatment time to 50% reduction of wound volume Quote: "Because of the striking foam imprints in the wound of patients with topical negative pressure therapy blinding was not possible." Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: flow chart shows that all participant and all wounds data were included in analysis; the presentation of the data and methods outlined show that an ITT analysis was done considering all randomised participants |
| Selective reporting (reporting bias) | Low risk | Comment: based on paper only, protocol not obtained |
| Other bias | Unclear risk | Comment: it was not clear whether there were unit of analyses issues |
Dwivedi 2016.
| Study characteristics | ||
| Methods | 2‐arm RCT Conducted in hospital settings in 1 geographical location in India The duration of follow‐up was a maximum of 9 weeks |
|
| Participants | 60 participants enrolled: stated that 44 participants with 44 wounds completed the study Inclusion criteria listed: traumatic paraplegia; age 16–60 years; Either gender; Stage III‐IV PU as defined by the European Pressure Ulcer Advisory Panel (EPUAP); subjects able to give informed consent. Exclusion criteria listed: necrotic tissue unlikely to tolerate debridement; chronic osteomyelitis not treatable by antibiotics alone; exposed blood vessels and nerves; comorbidities such as diabetes mellitus, rheumatoid disease, vasculitis, neuropathy, chemotherapy, radiation therapy; poor nutritional status as determined by a Braden scale nutritional assessment score of 2 or 1; Serum albumin<2.5g/L, haemoglobin <9.0g/L |
|
| Interventions | Group A: negative pressure device (NPD) was a low‐cost device, comprises of a low‐power continuous‐suction apparatus consisting of a bellow unit of 800ml capacity, a connecting tube with clamp, a ‘Y’ connector, a curved needle with matching catheter, and a spare perforated catheter (ROMOVAC SETGS‐5002 SIZE‐10; Romsons Scientific and Surgical Industries Pvt. Ltd. Agra, U.P., India), a sterilized piece of foam and a transparent polyurethane adhesive dressing (Opsite; G. Surgiwear Ltd., Shahjahanpur, U.P., India). NPD was changed every week or earlier if required. The dressing was changed by resident staff with the help of research staff. Group B: the pressure ulcers were cleaned with normal saline and packed with sterilized gauze to cover the wound. The dressing was changed once or twice daily depending on the absorbency of the dressing. |
|
| Outcomes | Primary outcomes: none Secondary outcomes: Surface area (reported as length and width separately). PUSH scores (reported as depth, exudate, and tissue type separately). |
|
| Notes | Funding source: no details of funding sources | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated random table was obtained and used to allocate participants to one of the two treatment groups: either NPWT or standard care (SC)" Comment: adequate |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Treatment was allocated on an individually named patient basis and participants commenced their allocated treatment immediately following randomization. Participants were assigned an identification number, which was used to identify them throughout the trial. Allocation of participants was done by one of the co‐authors." Comment: no detailed information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Weekly assessment of PUs for every outcome measure and clinical photography was carried out by the same co‐author throughout the trial." Comment: no detailed information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "There were nine patients withdrawn from the NPWT group and seven from the standard group. Data from 44 patients were analyzed." Comment: missing data |
| Selective reporting (reporting bias) | Low risk | Comment: reported data on all outcomes stated in the methods |
| Other bias | High risk | We found that the data used in the two studies were similar (CTRI/2014/09/0050), but there was a difference in the number of people included in the intervention group and the control group. We contacted the author to request more detailed information, but no reply was received. |
Dwivedi 2017.
| Study characteristics | ||
| Methods | 2‐arm RCT Single‐centred, conducted in hospital settings at Boston Medical in India Duration of follow‐up was a maximum of 9 weeks |
|
| Participants | 60 participants enrolled: stated that 44 participants with 44 wounds completed the study Ten participants were withdrawn from the NPD group due to deterioration of pressure ulcer with possible wound infection, or because the individual’s natal cleft area disrupted the vacuum, or owing to the refusal of additional treatment. In addition, 6 participants withdrew from the comparison group and refused additional treatment. Therefore, data from 44 patients were analysed Inclusion criteria listed: individuals with spinal cord injury and paraplegia; patients aged 16 to 60 years and stage III to IV pressure ulcer as defined by the National Pressure Ulcer Advisory Panel (NPUAP) 2016 taxonomy for pressure ulcer staging Exclusion criteria listed: pressure ulcers with necrotic tissue that could not be removed on baseline debridement, chronic osteomyelitis, exposed blood vessels and nerves in the wound, and specific comorbid conditions likely to impair wound healing such as diabetes mellitus, rheumatoid disease, and vasculitis; patients undergoing chemotherapy or radiation therapy and persons with severe nutritional deficits (Braden Scale score for Pressure Sore Risk Nutrition subscale score of ≤2, a serum.albumin level of <2.5 g/L, or a haemoglobin level of <9.0 g/L) |
|
| Interventions | Group A: negative pressure device (NPD). The unit also includes a connecting tube with clamp, a “Y” connector, a curved needle with a matching catheter, and a spare perforated catheter (Romo Vac Set GS‐5002 SIZE‐10; Romsons Scientific and Surgical Industries Pvt Ltd, Agra, Uttar Pradesh, India). The wound dressing was a sterilized piece of foam and transparent polyurethane adhesive dressing (Opsite; G. Surgiwear Ltd, Shahjahanpur, Uttar Pradesh, India) to ensure the airtight seal needed to exert negative pressure. The NPD dressing was changed weekly or more often if the dressing became saturated or loss of suction occurred. The NPD dressing was changed by the same investigator throughout the study. Group B: The wound was gently cleansed to remove debris from the wound bed, and saline‐soaked gauze was placed lightly inside the bed, which was covered with a sterile dry dressing. Dressings were changed once or twice daily depending upon the volume of exudate and saturation of the dressing. |
|
| Outcomes | Primary outcomes: none Secondary outcomes: surface area (reported as length and width separately). PUSH scores (reported as depth, exudate, and tissue type separately). |
|
| Notes | Funding source: no details of funding sources. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Study group location was completed using a computer‐generated random table." Comment: adequate |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All clinical assessments on a given patient were made by the same investigator throughout the study, and all data collectors received standardized training concerning data collection procedures." Comment: no detailed information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Ten participants were withdrawn from the NPD group due to deterioration of PI with possible wound infection, or because the individual’s natal cleft area disrupted the vacuum, or owing to refusal of additional treatment. In addition, 6 participants withdrew from the comparison group and refused additional treatment. " |
| Selective reporting (reporting bias) | Low risk | Comment: reported data on all outcomes stated in the methods |
| Other bias | High risk | We found that the data used in the two studies were similar (CTRI/2014/09/0050), but there was a difference in the number of people included in the intervention group and the control group. We contacted the author to request more detailed information, but no reply was received. |
Ford 2002.
| Study characteristics | ||
| Methods | 2‐arm RCT Single‐centred, conducted in hospital settings at Boston Medical in the USA Duration of follow‐up unclear – stated as ranging from 3 to 10 months not clear if it different between trial groups |
|
| Participants | 28 participants with 41 wounds enrolled: stated that 22 participants with 35 wounds completed the study Inclusion criteria listed: presence of stage III or IV ulcer for ≥ 4 weeks; albumin ≥ 2.0; age 21–80 years; ulcer volume after debridement = 10 mL–150 mL Exclusion criteria listed: fistulas to organs or body cavities; malignancy in the wound; pregnant or lactating women; Hashimoto thyroiditis; Graves' disease; iodine allergy; systemic sepsis; electrical burns; radiation exposure; chemical exposure; cancer; connective tissue disease; chronic renal or pulmonary disease; uncontrolled diabetes; corticosteroids or immunosuppressive agents; cardiac pacemaker; ferromagnetic clamps; recent placement of orthopaedic hardware |
|
| Interventions | Group A: NPWT (number of participants in the trial group not reported; n = 20 ulcers). The report suggests using the KCI VAC product. The duration of treatment was 6 weeks. "NPWT dressings were changed Mondays, Wednesdays, and Fridays (manufacturer recommends dressing changes every 48 hours)." Group B: Healthpoint System (which consists of three gel products: Accuzyme®, Iodosorb®, and Panafil®). The study reports that of the choice of three treatments available ‐ participants whose wounds showed substantial exudate received Iodosorb® or Iodoflex®; those patients whose ulcers were clean and granulating received Panafil®. Because all wounds were debrided surgically as appropriate, Accuzyme® was not used. The number of participants in the trial groups was not reported; n = 15 ulcers. The duration of treatment was 6 weeks. "HP [Healthpoint] dressings were changed once or twice daily, depending on the degree of wound drainage." |
|
| Outcomes | Primary outcomes: Complete wound healing (% ulcers healed) Adverse events Secondary outcomes: not reported |
|
| Notes | Some participants had more than one ulcers: potential unit of analysis issue Funding source: the Plastic Surgery Education Foundation and Kinetic Concepts, San Antonio, TX |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients underwent ulcer debridement as necessary, followed by random assignment to 6 weeks of treatment with either VAC or HP. Randomization was based on a table of random letters, V or H, generated before the trial began." Comment: method of generation of random schedule not clear |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Wound healing Quote: "Blinded clinic staff, including nurses, medical students, and interns, measured wounds and obtained plaster impressions." Comment: blinding of key study personnel used Adverse events Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "28 participants with 41 wounds were enrolled; 22 participants with 35 wounds completed the study." Comment: report suggest 6 participants with 1 wound each were lost to follow‐up. It is not clear which trial group these participants were from. The paper also reports that the average patient age was 41.7 years in Group A and 54.4 years in Group B. It is not clear if this was before or after the loss of 6 participants, but there seems to be imbalance |
| Selective reporting (reporting bias) | Low risk | Comment: based on paper only, protocol not obtained |
| Other bias | High risk | Comment: possible unit of analysis issue is due to participants with multiple wounds in the trial with data being reported at the wound rather than participant level. No information on the number of participants randomised to each group. Duration of follow‐up and any differences in trial groups of follow‐up times were not clear |
Niezgoda 2004.
| Study characteristics | ||
| Methods | 2‐arm RCT Conducted in USA Follow‐up (at time results presented) 42 days (6 weeks) |
|
| Participants | 97 participants. Inclusion criteria listed: stage III and IV pressure ulcers located on the trunk or trochanter regions Exclusion criteria listed: none listed |
|
| Interventions | Group A: NPWT (VAC) n = 54 Group B: moist wound healing (no further information) n = 43 |
|
| Outcomes | Primary outcomes: none Secondary outcomes: Change in ulcer size (unadjusted) Cost |
|
| Notes | Conference abstract; interim analyses; abstract notes that full follow‐up was planned as 82 days Authors contacted via e‐mail and confirmed that the full study was not published and that outcome data were not available to us Funding not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Interim analysis in terms of both participant numbers and length of follow‐up for those participants recruited |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information; interim analysis ‐ not clear why further work not available |
Tang 2019.
| Study characteristics | ||
| Methods | 2‐arm RCT Conducted in‐home and hospital settings in China The duration of follow‐up was a maximum of 3 months |
|
| Participants | 60 participants: all included in the analysis Inclusion criteria listed: Pressure ulcer graded III or IV according to the European Pressure Ulcer Advisory Panel Grading System; the blood glucose level and nutritional index level were well controlled, and the body mass index (BMI) was at the same level; the patient's age was > 60 years old Exclusion criteria listed: Patients with malignant tumours; the wound has large blood vessel exposure and nerve exposure; the wound has active bleeding, necrotic tissue, and contraindications to debridement, no debridement or secondary osteomyelitis without treatment, or there is an undetected sinus; there are systemic complications affecting wound repair, such as autoimmune diseases, multiple organ failure and so on |
|
| Interventions | Group A: the wound was initially evaluated for the whole body and wound; implement conservative instrument debridement combined with autolytic debridement; selected the appropriate new dressing according to the evaluation results of the wound, adopted the wet dressing change method, and determined the frequency of dressing change and dressing change according to the wound exudation until the rotten meat in the wound bed disappeared Group B: wound evaluation, cleaning, and debridement were the same as those in the control group; the dressing was cut according to the size of the wound, and the sealing film was trimmed to completely cover the dressing and exceed the area of 3~5cm beyond the edge of the wound. The operator pinched the sealing film with his index finger and thumb, cut a small hole with a diameter of 1~2cm on the film, and then pasted a suction cup; connected the NPWT treatment host, and set the pressure through the touch screen of the NPWT treatment instrument. The pressure setting range of pressure ulcers was ‐80 to ‐120 mm Hg; guided the patient's family members on the operation, observation, and possible troubleshooting of the machine setting, and give health education; after the patient took the machine home, the stoma therapist observed and guided the effect of negative pressure treatment through WeChat video, voice, and other Internet platforms. If there was a fault, it would be eliminated in time. For problems that could not be solved remotely, the stoma therapist would provide on‐site service; the dressing shall be changed according to the drainage fluid and wound conditions, and the dressing shall be changed once in the outpatient department in 5~7 days until the patient's wound bed was free of rotten meat and the wound red granulation tissue was completely covered |
|
| Outcomes | Primary outcome: none Second outcome: Times of dressing change Rate of change in wound size Pain |
|
| Notes | Funding source: no details of funding sources | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Pairing based on patients' age, basic physical conditions, and the stage of pressure ulcers, each pair was divided into the control group and intervention group with 30 patients in each group according to the random number table". Comment: there are no further details of the randomisation process, we can not make a judgment according to the author's description. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The ostomy therapist observed the effect and guided the use of NPWT treatment through WeChat video, voice, and other Internet platforms". Comment: no detailed information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No participants withdraw from the study" Comment: all participant data were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes identified in the methods section were reported in the results (and were outcomes that would be expected to be included in such a study). Protocol not seen. |
| Other bias | High risk | Quote: "After the patient took the machine home, the ostomy therapist observed and guided the use of NPWT through WeChat video, voice, and other Internet programs" Comment: researchers may not be able to control the potential bias risk. |
Şahin 2022.
| Study characteristics | ||
| Methods | 2‐arm RCT Conducted in‐home and hospital settings in 1 geographical location in the UK The duration of follow‐up was a maximum of 24 weeks |
|
| Participants | 30 participants: all included in the analysis Inclusion criteria listed: (1) being ≥18 years old; (2) not having time/access constraints; (3) voluntariness Exclusion criteria listed:(1) Stage I and Stage II pressure ulcers; (2) cancer; (3) increased risk of bleeding; (4) osteomyelitis; (5) corticosteroid treatment; (6) contraindications of NPWT (fistula, necrotic tissue in the wound, burn wound, cardiorespiratory disease) |
|
| Interventions | Group A: the wounds were initially evaluated for any necrotic findings and debrided if needed, and then washed with an antiseptic solution. Wound depth was determined in centimetres with a sterile cotton‐tip applicator by measuring against a ruler. The wound was closed using a Wound Care Kit that includes foam dressing (V.A.C. Granu Foam Silver), film drape, TRAC pad with tubing, and a drainage canister. Steril foam material was placed inside the wound and was attached to the canister through the tubing. The canister was attached to the Vacuum‐Assisted Closure device (V.A.C.® Therapy System Patient Support – KCI), which is a portable device that applies intermittent or continuous negative pressure. The device was operated at 125 mmHg pressure for 5 min with and 2 min without an active vacuum. Wound dressings were changed every 48 h. Group B: the wound was initially evaluated for any necrotic findings and debrided if needed, and then washed with an antiseptic solution. 3DWM was used to measure the pressure sores by taking pictures. Wounds were finally covered with gauze dressing soaked with saline. Wounds were treated three times a day, and measurements were repeated every 48 h. |
|
| Outcomes | Primary outcomes: none Secondary outcomes: PUSH Tool Change in wound size |
|
| Notes | Funding source: no financial support | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the subjects were divided into experimental and control groups according to their respective protocol numbers. The protocol numbers ending with odd digits were assigned to the control group, and those ending with even digits were assigned to the experimental group." Commend: information cannot determine how the protocol number is generated. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | quote: "Its consort trial number is NCT04311229." Commend: the study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in a prespecified way |
| Other bias | Low risk | Comment: no other clear sources of bias was found |
Abbreviations
h: hour; ITT: intention to treat; min: minute; NPD: negative pressure device; NPWT: negative pressure wound therapy; PUSH: pressure ulcer scale for healing; RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ali 2015 | Study population had range of wounds |
| Andrianasolo 2018 | Not an RCT |
| Baek 2020 | NPWT was not the only systematic difference between groups: the trial applied NPWT in both comparison groups |
| Braakenburg 2006 | Study population had range of wounds ‐ authors contacted to try and obtain pressure ulcer data |
| Chen 2018 | NPWT was not the only systematic difference between groups: the trial applied NPWT in both comparison groups |
| Ciliberti 2016 | NPWT was not the only systematic difference between groups: the trial applied NPWT in both comparison groups |
| Dwivedi 2020 | Non‐randomised study |
| Gao 2015 | NPWT was not the only systematic difference between groups: the trial applied NPWT in both comparison groups |
| Greer 1999 | Unable to locate an abstract or full‐text publication |
| Hampton 2015 | Not an RCT |
| Hu 2009 | Study population had range of wounds ‐ based on translation |
| Joseph 2000 | Study population had range of wounds ‐ authors contacted to try and obtain pressure ulcer data |
| Kumar 2021 | Not an RCT |
| Leonardi 2017 | Not an RCT |
| Liu 2021 | NPWT was not the only systematic difference between groups: the trial applied NPWT in both comparison groups |
| Mari 2019 | NPWT was not the only systematic difference between groups: the trial applied NPWT in both comparison groups |
| McCallon 2015 | Not an RCT |
| Mody 2008 | Study population had range of wound wounds ‐ authors contacted to try and obtain pressure ulcer data. 11/48 wounds were pressure ulcers, but it seems that only 2 were in the NPWT group |
| Mohammed 2020 | NPWT was not the only systematic difference between groups: the trial applied NPWT in both comparison groups |
| Moues 2007 | Not corrected study population. Confirmed by study author |
| Mullner 1997 | Not an RCT |
| Papp 2018 | not RCT |
| Schwarz 2012 | Study population had range of wound ‐ authors contacted to try and obtain pressure ulcer data |
| Srivastava 2016 | Not an RCT |
| Tauro 2007 | Not an RCT |
| Wagstaff 2014 | NPWT was not the only systematic difference between groups: the trial applied NPWT in both comparison groups |
| Wild 2008 | No relevant outcome data reported ‐ authors contacted. RCT contained 10 participants in total |
| Zhang 2012 | NPWT was not the only systematic difference between groups: the trial applied NPWT in both comparison groups |
Abbreviations
NPWT: negative pressure wound therapy; RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Pruksapong 2011.
| Methods | States it is an RCT |
| Participants | People with chronic wounds |
| Interventions | Portable vacuum dressing |
| Outcomes | |
| Notes | Unable to obtain paper to date. Abstract notes that 30 participants with chronic wounds were recruited and describes the intervention as a vacuum dressing. It is possible that these wounds are pressure ulcers and that the treatment is NPWT. This need to be confirmed using the full text which we have been unable to obtain to date. |
Wanner 2003.
| Methods | Describes that participants were randomly put into groups ‐ no further detail |
| Participants | People with pressure ulcers |
| Interventions | NPWT |
| Outcomes | Time to 50% reduction in wound area |
| Notes | The outcome data is unclear we have contacted the authors to ask for more information |
Yu 2012.
| Methods | Described as RCT in title |
| Participants | People with pressure ulcers |
| Interventions | NPWT |
| Outcomes | |
| Notes | Unable to obtain this conference abstract or any associated publication to date |
NPWT: negative pressure wound therapy; RCT: randomised controlled trial
Differences between protocol and review
We expanded the list of extracted data from the previews version to include:
trial registration number or protocol.
We have added the outcome of pressure ulcer severity, which was not previously planned.
We present each of the primary outcomes using the GRADE approach of the review in a summary of findings table. For other outcomes, we conducted a GRADE assessment and presented the results in narrative format in the results section.
We added: "When possible, we planned to exclude RCTs with high risk for one or more domains from meta‐analysis to explore the impact on the research results. But we have not conducted any sensitivity analysis." This was not planned in the previous version.
Contributions of authors
Jiyuan Shi: conceived, designed, and coordinated the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; produced the first draft of the review; contributed to writing or editing the review; advised on the review; approved the final review prior to submission, and is a guarantor of the review, and is a guarantor of the review. Ya Gao: extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; performed statistical analysis; checked the quality of the statistical analysis; contributed to writing or editing the review; advised on the review;
Jianguo Xu: extracted data; checked quality assessment; contributed to writing or editing the review; advised on the review; and approved the final review prior to submission.
Zheng Li: conceived and designed the review; undertook and checked quality assessment; contributed to writing or editing the review; advised on the review, approved the final review prior to submission; and is a guarantor of the review. Jinhui Tian: checked quality assessment; contributed to writing or editing the review; advised on the review; and approved the final review prior to submission.
Fan Mei: checked quality assessment; contributed to writing or editing the review; advised on the review; and approved the final review prior to submission.
Jiang Li: checked quality assessment; contributed to writing or editing the review; advised on the review; and approved the final review prior to submission.
Sources of support
Internal sources
-
None, Other
None
External sources
-
None, Other
None
Declarations of interest
Jiyuan Shi: nothing to declare.
Ya Gao: nothing to declare.
JinHui Tian: nothing to declare.
Jiang Li: nothing to declare.
JianGuo Xu: nothing to declare.
Fan Mei: nothing to declare.
Zheng Li: nothing to declare.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ashby 2012 {published data only}
- Ashby R, Dumville J, Soares M, McGinnis E, Stubbs N, Adderley U et al. A pilot study of negative pressure wound therapy for the treatment of grade III/IV pressure ulcers. Trials 2012;13:119. [DOI: 10.1186/1745-6215-13-119] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Laat 2011 {published data only}
- Laat EH, den Boogaard MH, Spauwen PH, Kuppevelt DH, Goor H, Schoonhoven L. Faster wound healing with topical negative pressure therapy in difficult-to-heal wounds: a prospective randomized controlled trial. Annals of Plastic Surgery 2011;67:626-31. [DOI] [PubMed] [Google Scholar]
Dwivedi 2016 {published data only}
- Dwivedi MK, Srivastava RN, Bhagat AK, Agarwal R, Baghel K, Jain A, Raj S. Pressure ulcer management in paraplegic patients with a novel negative pressure device: a randomised controlled trial. Journal of Wound Care 2016;25(4):199-200, 202-4, 206-7. [DOI] [PubMed] [Google Scholar]
Dwivedi 2017 {published data only}
- Dwivedi MK, Bhagat AK, Srivastava RN, Jain A, Baghel K, Raj S. Expression of MMP-8 in Pressure Injuries in Spinal Cord Injury Patients Managed by Negative Pressure Wound Therapy or Conventional Wound Care: A Randomized Controlled Trial. Journal of Wound, Ostomy, & Continence Nursing 2017;44(4):343-349. [DOI] [PubMed] [Google Scholar]
Ford 2002 {published data only}
- Ford CN, Reinhard ER, Yeh D, Syrek D, De Las Morenas A, Bergman SB et al. Interim analysis of a prospective, randomized trial of vacuum-assisted closure versus the Healthpoint system in the management of pressure ulcers. Annals of Plastic Surgery 2002;49:55-61. [DOI] [PubMed] [Google Scholar]
Niezgoda 2004 {published data only}
- Niezgoda JA. A comparison of vacuum assisted closure therapy to moist wound care in the treatment of pressure ulcers: preliminary results of a multicenter trial. In: 2nd World Union of Wound Healing Societies Meeting; 2004 July 8-13; Paris. 2004.
Şahin 2022 {published data only}
- Şahin E, Rizalar S, Özker E 2022. Effectiveness of negative-pressure wound therapy compared to wet-dry dressing in pressure injuries. J Tissue Viability 2022;31(1):164-172. [DOI] [PubMed] [Google Scholar]
Tang 2019 {published data only}
- Tang Yanhua, Wang Jiaqi, Yang Juan. Application of negative pressure wound therapy combined with internet-plus home care for elderly patients with severe pressure injuries. Nursing of Integrated Traditional Chinese and Western Medicine 2019;5:8. [Google Scholar]
References to studies excluded from this review
Ali 2015 {published data only}
- Ali Z, Anjum A, Khurshid L. Evaluation of low-cost custom made VAC therapy compared with conventional wound dressings in the treatment of non-healing lower limb ulcers in lower socio-economic group patients of Kashmir valley. JJournal of Orthopaedic Surgery and Research 2015;10:183. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Andrianasolo 2018 {published data only}
- Andrianasolo J, Ferry T, Boucher F, Chateau J, Shipkov H, Daoud F, et al. Pressure ulcer-related pelvic osteomyelitis: evaluation of a two-stage surgical strategy (debridement, negative pressure therapy and flap coverage) with prolonged antimicrobial therapy. BMC Infectious Diseases 2018;18(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baek 2020 {published data only}
- Baek W, Lee N, Han EJ, Roh TS, Lee WJ. A prospective randomized study: the usefulness and efficacy of negative pressure wound therapy with lipidocolloid polyester mesh compared to traditional negative pressure wound therapy for treatment of pressure ulcers. Pharmaceutics 2020;12(9):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braakenburg 2006 {published data only}
- Braakenburg A, Obdeijn MC, Feitz R, Rooij IA, Griethuysen AJ, Klinkenbijl JH. The clinical efficacy and cost effectiveness of the vacuum-assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plastic and Reconstructive Surgery 2006;118:390-400. [DOI] [PubMed] [Google Scholar]
Chen 2018 {published data only}
- Chen CS, Yu CC. Prospective randomized control study comparing gauze-based with foam-based negative pressure wound therapy for the stage 4 pressure injuries. Formosan Journal of Surgery 2018;51(6):223-7. [Google Scholar]
Ciliberti 2016 {published data only}
- Ciliberti M, De Lara F, Serra G, Tafuro F, Iazzetta FM, Filosa A, et al. The effect of a bacteria- and fungi- binding mesh dressing on the bacterial load of pressure ulcers treated with negative pressure wound therapy: a pilot study. Wounds-A Compendium of Clinical Research & Practice 2016;28(11):408-20. [PubMed] [Google Scholar]
Dwivedi 2020 {published data only}
- Dwivedi MK, Bhagat A, Srivastava RN, Raj L . Management of Grade IV pressure ulcers with a novel negative pressure device in traumatic paraplegia subjects. Cureus 2020;12(7):e9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gao 2015 {published data only}
- Gao YB, Tong SL, Pan F, Yu JH. [Myogenic elephant skin cream combined with Vacuum sealing drainage (VSD) for the treatment of bedsores]. Zhongguo Gushang 2015;28(2):150-4. [PubMed] [Google Scholar]
Greer 1999 {published data only}
- Greer SE, Longaker MT, Margiotta M. Preliminary results from a multicenter, randomized, controlled study of the use of subatmospheric pressure dressing for pressure ulcer healing. In: Wound Repair and Regeneration. Vol. 7. 1999:Abstract A255.
Hampton 2015 {published data only}
- Hampton J. Providing cost-effective treatment of hard-to-heal wounds in the community through use of NPWT. British Journal of Community Nursing 2015;Community Wound Care (Supplement):S14, S16-20. [DOI] [PubMed] [Google Scholar]
Hu 2009 {published data only}
- Hu KX, Zhang HW, Zhou F, Yao G, Shi JP, Wang LF. Observation on the therapeutic effects of negative-pressure wound therapy on the treatment of complicated and refractory wounds. Zhonghua Shao Shang Za Zhi [Chinese Journal of Burns] 2009;4:249-52. [PubMed] [Google Scholar]
Joseph 2000 {published data only}
- Joseph E, Hamori CA, Bergman S, Roaf E, Swann NF, Anastasi GW. A prospective randomized trial of vacuum-assisted closure versus standard therapy of chronic nonhealing wounds. Wounds 2000;12:60-7. [Google Scholar]
Kumar 2021 {published data only}
- Kumar V, Rasouliyan L, Long S, Rao MB. PIT1 negative pressure wound therapy for pressure ulcer: effect on outcomes and utilization using real-world data. Value in Health 2021;24(Supplement 1):S122. [Google Scholar]
Leonardi 2017 {published data only}
- Leonardi F. Experience with a new negative pressure incision dressing in pressure sore reconstruction. Journal of Wound Care 2017;26(SUPPL 6):395. [Google Scholar]
Liu 2021 {published data only}
- Liu Q, Zhang N, Li Z, He H. Efficacy of autologous platelet-rich plasma gel in thetreatment of refractory pressure injuries and its effecton wound healing time and patient quality of life . Clinics 2021;5:76:e2355 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Mari 2019 {published data only}
- Mari W, Younes S, Naqvi J, Issa AA, Oroszi TL, Cool DR, et al. Use of a natural porcine extracellular matrix with negative pressure wound therapy hastens the healing rate in Stage 4 pressure ulcers. Wounds-A Compendium of Clinical Research & Practice 2019;31(5):117-22. [PubMed] [Google Scholar]
McCallon 2015 {published data only}
- McCallon S K, Frilot C. A retrospective study of the effects of clostridial collagenase ointment and negative pressure wound therapy for the treatment of chronic pressure ulcers. Wounds-A Compendium of Clinical Research & Practice 2015;27(3):44-53. [PubMed] [Google Scholar]
Mody 2008 {published data only}
- Mody GN, Nirmal IA, Duraisamy S, Perakath B. A blinded, prospective, randomized controlled trial of topical negative pressure wound closure in India. Ostomy Wound Management 2008;12:36-46. [PubMed] [Google Scholar]
Mohammed 2020 {published data only}
- Mohammed A H, Hamed S A, Abdelghany A I. Comparison between two different protocols of negative pressure therapy for healing of chronic ulcers. Journal of Tissue Viability 2020;29(1):37-41. [DOI] [PubMed] [Google Scholar]
Moues 2007 {published data only}
- Moues CM, Van Den Bemd GJ, Meerding WJ, Hovius SE. Cost analysis comparing vacuum-assisted closure wound therapy to conventional moist gauze therapy. In: 2nd World Union of Wound Healing Societies Meeting 8-13 July; Paris. Vol. 87, Abstract no. A008. 2004.
- Moues CM, Vos MC, den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum-assisted closure wound therapy. 13th conference of the European Wound Management Association 2003, 22-24 May; Pisa, Italy:Presentation 42.
- Moues CM, Vos MC, den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair and Regeneration 2004;12:11-7. [DOI] [PubMed] [Google Scholar]
- Mouës CM, den Bemd GJ, Heule F, Hovius SE. Comparing conventional gauze therapy to vacuum-assisted closure wound therapy: a prospective randomised trial. Journal of Plastic, Reconstructive and Aesthetic Surgery 2007;60:672-81. [DOI] [PubMed] [Google Scholar]
Mullner 1997 {published data only}
- Mullner T, Mrkonjic L, Kwasny O, Vecsei V. The use of negative pressure to promote the healing of tissue defects: a clinical trial using the vacuum sealing technique. British Journal of Plastic Surgery 1997;50:194-9. [DOI] [PubMed] [Google Scholar]
Papp 2018 {published data only}
- Papp AA. Incisional negative pressure therapy reduces complications and costs in pressure ulcer reconstruction. IInternational Wound Journal 2018 Apr;16(2):394-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwarz 2012 {published data only}
- Rahmanian-Schwarz A, Willkomm LM, Gonser P, Hirt B, Schaller HE. A novel option in negative pressure wound therapy (NPWT) for chronic and acute wound care. Burns 2012;38:573-7. [DOI] [PubMed] [Google Scholar]
Srivastava 2016 {published data only}
- Srivastava RN, Dwivedi MK, Bhagat AK, Raj S, Agarwal R, Chandra A. A non-randomised, controlled clinical trial of an innovative device for negative pressure wound therapy of pressure ulcers in traumatic paraplegia patients. International Wound Journal 2016;13(3):343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tauro 2007 {published data only}
- Tauro LF, Ravikrishnan J, Satish Rao BS, Divakar Shenoy H, Shetty SR, Menezes LT. A comparative study of the efficacy of topical negative pressure moist dressings and conventional moist dressings in chronic wounds. Indian Journal of Plastic Surgery 2007;40:133-40. [Google Scholar]
Wagstaff 2014 {published data only}
- Wagstaff MJ, Driver S, Coghlan P, Greenwood JE. A randomized, controlled trial of negative pressure wound therapy of pressure ulcers via a novel polyurethane foam. Wound Repair and Regeneration 2014;22:205-11. [DOI] [PubMed] [Google Scholar]
Wild 2008 {published data only}
- Wild T, Stremitzer S, Budzanowski A, Hoelzenbein T, Ludwig C, Ohrenberger G. Definition of efficiency in vacuum therapy - a randomised controlled trial comparing with V.A.C. therapy. International Wound Journal 2008;5:641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2012 {published data only}
- Zhang C, Yang JC, Feng YQ, Wang HT, Li X, Chen NJ. Clinical observations of negative pressure wound therapy and basic fibroblast growth factor in the treatment of intractable pressure ulcer. Zhonghua Yi Xue Za Zhi 2012;92:2862-4. [PubMed] [Google Scholar]
References to studies awaiting assessment
Pruksapong 2011 {published data only}
- Pruksapong C. Efficacy of portable vacuum dressing in chronic wound care: a prospective randomized control trial. Journal of the Medical Association of Thailand 2011;94:1212-7. [PubMed] [Google Scholar]
Wanner 2003 {published data only}
- Wanner MB, Schwarzl F, Strub B, Zaech GA, Pierer G. Vacuum-assisted wound closure for cheaper and more comfortable healing of pressure sores: a prospective study. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery 2003;37:28-33. [DOI] [PubMed] [Google Scholar]
Yu 2012 {published data only}
- Yu C. Negative pressure wound therapy using gauze versus polyurethene foam filler in the healing undermining of pressure ulcers, a prospective randomised controlled trial. In: 4th Congress of the World Union of Wound Healing Societies; September 2-6; Yokohama, Japan. 2012.
Additional references
ACP 2015
- Qaseem A, Humphrey LL, Forciea MA, Starkey M, Denberg TD, Clinical Guidelines Committee of the American College of Physicians. Treatment of pressure ulcers: a clinical practice guideline from the American College of Physicians. Annals of Internal Medicine 2015 Mar; 3;162(5):370-9. [DOI] [PubMed] [Google Scholar]
Ali 2017
- Ali Z, Anjum A, Khurshid L, Ahad H, Maajid S, Dhar SA. Retraction Note: Evaluation of low-cost custom made VAC therapy compared with conventional wound dressings in the treatment of non-healing lower limb ulcers in lower socio-economic group patients of Kashmir valley. Journal of Orthopaedic Surgery and Research 2017 Apr 20;12(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alipoor 2021
- Alipoor E, Mehrdadi P, Yaseri M, Hosseinzadeh-Attar M. Association of overweight and obesity with the prevalence and incidence of pressure ulcers: a systematic review and meta-analysis. Clinical Nutrition 2021 Sep;40(9):5089-98. [DOI] [PubMed] [Google Scholar]
Allman 1997
- Allman RM. Pressure ulcer prevalence, incidence, risk factors, and impact. Clinical Geriatric Medicine 1997;13(3):421-36. [PubMed] [Google Scholar]
Bergstrom 1998
- Bergstrom N, Braden B, Champagne M, Kemp M, Ruby E. Predicting pressure ulcer risk: a multisite study of the predictive validity of the Braden Scale. Nursing Research 1998;47(5):261-9. [DOI] [PubMed] [Google Scholar]
Berlowitz 1990
- Berlowitz DR, Wilikin SVB. Risk factors for pressure sores: a comparison of cross-sectional and cohort derived data. Journal of the American Geriatrics Society 1990;37(11):1043-50. [DOI] [PubMed] [Google Scholar]
Berlowitz 1997
- Berlowitz DR, Brandeis GH, Anderson J, Brand HK. Predictors of pressure ulcer healing among long-term care residents. Journal of the American Geriatrics Society 1997;45(1):30-4. [DOI] [PubMed] [Google Scholar]
Brandeis 1994
- Brandeis GH, Ooi WL, Hossain M, Morris JN, Lipsitz LA. A longitudinal study of risk factors associated with the formation of pressure ulcers in nursing homes. Journal of the American Geriatrics Society 1994;42:388-93. [DOI] [PubMed] [Google Scholar]
Chaboyer 2022
- Chaboyer W, Coyer F, Harbeck E, Thalib L, Latimer S, Wan CS, et al. Oedema as a predictor of the incidence of new pressure injuries in adults in any care setting: A systematic review and meta-analysis. International Journal of Nursing Studies 2022 Apr;128:104189. [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan B, Ieraci L, Mitsakakis N, Pham B, Krahn M. Net costs of hospital-acquired and pre-admission PUs among older people hospitalised in Ontario. Journal of Wound Care 2013;22:341-2. [DOI] [PubMed] [Google Scholar]
Coleman 2013
- Coleman S, Gorecki C, Nelson EA, Closs SJ, Defloor T, Halfens R. Patient risk factors for pressure ulcer development: systematic review. International Journal of Nursing Studies 2013;50(7):974-1003. [DOI] [PubMed] [Google Scholar]
Dealey 2012
- Dealey C, Posnett J, Walker A. The cost of pressure ulcers in the United Kingdom. Journal of Wound Care 2012;21:261-2, 264, 266. [DOI] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Demarré 2015
- Demarré L, Van Lancker A, Van Hecke A, Verhaeghe S, Grypdonck M, Lemey J. The cost of prevention and treatment of pressure ulcers: a systematic review. International Journal of Nursing Studies 2015 Nov;52(11):1754-74. [DOI] [PubMed] [Google Scholar]
Department of Health and Human Services 2009
- Department of Health and Human Services, Office of Inspector General. Comparison of prices for negative pressure wound therapy pumps. https://oig.hhs.gov/oei/reports/oei-02-07-00660.pdf 2009;Accessed Dec 2012; OEI-02-07-00660.
Donini 2005
- Donini LM, De Felice MR, Tagliaccica A, De Bernardini L, Cannella C. Nutritional status and evolution of pressure sores in geriatric patients. Journal of Nutrition and Health in Aging 2005;9:446-54. [PubMed] [Google Scholar]
EPUAP/NPUAP/PPPIA 2019
- European Pressure Ulcer Advisory Panel National pressure injury advisory Panel, and Pan Pacific pressure injury alliance, Prevention and Treatment of Pressure ulcers/injuries: clinical Practice guideline(2019). The International Guideline. Emily Haesler (Ed.). EPUAP/NPUAP/PPPIA; 2019.
Essex 2009
- Essex HN, Clark M, Sims J, Warriner A, Cullum N. Health-related quality of life in hospital inpatients with pressure ulceration: assessment using generic health-related quality of life measures. Wound Repair and Regeneration 2009;17:797-805. [DOI] [PubMed] [Google Scholar]
FDA 2011
- US Food and Drug Administration. FDA Safety Communication: Update on serious complications associated with negative pressure wound therapy systems. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm244211.htm 2011;Accessed Nov 2012.
Gebhardt 2002
- Gebhardt K. Pressure ulcer prevention. Part 1. Causes of pressure ulcers. Nursing Times 2002;98:41-4. [PubMed] [Google Scholar]
Gefen 2014
- Gefen A. Tissue changes in patients following spinal cord injury and implications for wheelchair cushions and tissue loading: a literature review. Ostomy Wound Management 2014;60:34-45. [PubMed] [Google Scholar]
Glanville 2019
- Glanville J, Dooley G, Wisniewski S, Foxlee R, Noel-Storr A. Development of a search filter to identify reports of controlled clinical trials within CINAHL Plus. Health Information and Libraries Journal 2019;36(1):73-90. [DOI] [PubMed] [Google Scholar]
Guy 2012
- Guy H, Grothier l. Using negative pressure therapy in wound healing. Nursing Times 2012;108:18, 20. [PubMed] [Google Scholar]
Hajhosseini 2020
- Hajhosseini B, Longaker MT, Gurtner GC. Pressure Injury. Annals of Surgery 2020 Apr;271(4):671-679. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne, JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2022
- Higgins JP, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
JSPU 2016
- Takafumi K, Katsunori F, Yayoi N. JSPU Guidelines for the Prevention and Management of Pressure Ulcers (4th Ed.). Clinical Guideline 2016;16:4.
Kinetic Concepts Inc 2012
- Kinetic Concepts Inc. Science behind wound therapy. http://www.kci1.com/KCI1/sciencebehindwoundtherapy (accessed 11 May 2012).
Kontopantelis 2012
- Kontopantelis E, Reeves D. Performance of statistical methods for meta-analysis when true study effects are non-normally distributed: a simulation study. Statistical Methods in Medical Research 2012;21(4):409-26. [DOI] [PubMed] [Google Scholar]
Kontopantelis 2013
- Kontopantelis E, Springate DA, Reeves D. A re-analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta-analysis. PLOS One 2013;8(7):e69930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Källman 2022
- Källman U, Hommel A, Borgstedt Risberg M, Gunningberg L, Sving E, Bååth C. Pressure ulcer prevalence and prevention interventions - a ten-year nationwide survey in Sweden. International Wound Journal 2022 Nov;19(7):1736-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Labeau 2021
- Labeau SO, Afonso E, Benbenishty J, European Society of Intensive Care Medicine (ESICM) Trials Group Collaborators. Prevalence, associated factors and outcomes of pressure injuries in adult intensive care unit patients: the DecubICUs study. Intensive Care Medicine 2021 Feb;47(2):160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langer 2003
- Langer G, Knerr A, Kuss O, Behrens J, Schlömer GJ. Nutritional interventions for preventing and treating pressure ulcers. Cochrane Database of Systematic Reviews 2003, Issue 4. Art. No: CD003216. [DOI: 10.1002/14651858.CD003216] [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021)Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lyder 2012
- Lyder CH, Wang Y, Metersky M, Curry M, Kliman R, Verzier NR, et al. Hospital-acquired pressure ulcers: results from the national Medicare Patient Safety Monitoring System study. Journal of the American Geriatrics Society 2012;60:1603-8. [DOI] [PubMed] [Google Scholar]
Nghiem 2022
- Nghiem S, Campbell J, Walker RM, Byrnes J, Chaboyer W. Pressure injuries in Australian public hospitals: A cost of illness study. International Journal of Nursing Studies 2022 Jun;130:104191. [DOI] [PubMed] [Google Scholar]
NICE 2014
- National Institute for Health and Clinical Excellence. Pressure ulcers: prevention and management of pressure ulcers. Clinical Guideline 179 April 2014:Accessed 21 Jan 2015.
NICE 2018
- National Institute for Health and Clinical Excellence. 2018 surveillance of pressure ulcers: prevention and management. Clinical Guideline 179. 30 November 2018. [PubMed]
NPUAP 2016
- National Pressure Ulcer Advisory Panel (NPUAP). NPUAP Pressure Injury Stages; 2016.. Available at cdn.ymaws.com/npuap.site-ym.com/resource/resmgr/npuap_pressure_injury_stages.pdf.
Page 2022
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Parmar 1998
- Parmar MK, Torri V, Stewert L. Extracting summary statistics to perform meta-analysis of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815-34. [DOI] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- Review Manager (RevMan) 5.4. Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rrapi 2021
- Rrapi R, Chand S, Lo JA, Gabel CK, Song S, Holcomb Z. The significance of pressure injuries and purpura in COVID-19 patients hospitalized at a large urban academic medical center: a retrospective cohort study. Journal of the American Academy of Dermatology 2021 Aug;85(2):462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmitt 2019
- Schmitt J, Lange T, Kottner J, Prinsen C, Weberschock T, Hahnel E, et al. Cochrane Reviews and DermatologicalTtrials Outcome Concordance: why core outcome sets could make trial results more usable. Journal of Investigative Dermatology 2019 May;139(5):1045-53. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLOS Medicine 2010;7(3):e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schunemann 2022
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Smith 2013
- Smith ME, Totten A, Hickam DH, Fu R, Wasson N, Rahman B et, al. Pressure ulcer treatment strategies: a systematic comparative effectiveness review. Annals of Internal Medicine 2013;159:39-50. [DOI] [PubMed] [Google Scholar]
Soares 2013
- Soares MO, Dumville JC, Ashby RL, Iglesias CP, Bojke L, Adderley U, et al. Methods to assess cost-effectiveness and value of further research when data are sparse: negative-pressure wound therapy for severe pressure ulcers. Medical Decision Making 2013;33:415-36. [DOI] [PubMed] [Google Scholar]
Song 2021
- Song YP, Wang L, Yuan BF. Negative-pressure wound therapy for III/IV pressure injuries: a meta-analysis. Wound Repair and Regeneration 2021 Jan;29(1):20-33. [DOI] [PubMed] [Google Scholar]
Stevenson 2013
- Stevenson R, Collinson M, Henderson V, Wilson L, Dealey C, McGinnis E, et al. The prevalence of pressure ulcers in community settings: an observational study. International Journal of Nursing Studies 2013;50:1550-7. [DOI] [PubMed] [Google Scholar]
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Statistics in Medicine 1999;18:2693-708. [DOI] [PubMed] [Google Scholar]
VanGilder 2017
- VanGilder C, Lachenbruch C, Algrim-Boyle C. The International Pressure Ulcer Prevalence™ Survey: 2006-2015: a 10-Year Pressure Injury Prevalence and Demographic Trend Analysis by Care Setting. Journal of Wound, Ostomy, and Continence Nursing 2017;44:20-8. [DOI] [PubMed] [Google Scholar]
Walker 2017
- Walker RM, Gillespie BM, Thalib L, Higgins NS, Whitty JA. Foam dressings for treating pressure ulcers. Cochrane Database of Systematic Reviews 2017, Issue 12. Art. No: CD011332. [DOI: 10.1002/14651858.CD011332.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHS 2015
- Gould L, Stuntz M, Giovannelli M. Wound Healing Society 2015 update on guidelines for pressure ulcers. Wound Repair and Regeneration 2016;Jan-Feb;24(1):145-62. [DOI] [PubMed] [Google Scholar]
WOCN 2016
- Wound, Ostomy and Continence Nurses (WOCN) Society-Wound Guidelines Task Force. WOCN 2016 Guideline for Prevention and Management of Pressure Injuries (Ulcers): An Executive Summary. Journal of Wound, Ostomy, and Continence Nursing 2016;May/Jun;44(3):241-6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Dumville 2014
- Dumville JC, Webster J, Evans D, Land L. Negative pressure wound therapy for treating pressure ulcers. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No: CD011334. [DOI: 10.1002/14651858.CD011334] [DOI] [PubMed] [Google Scholar]
Dumville 2015
- Dumville JC, Webster J, Evans D, Land L. Negative pressure wound therapy for treating pressure ulcers. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD011334. [DOI: 10.1002/14651858.CD011334.pub2] [DOI] [PubMed] [Google Scholar]


