Abstract
Smad proteins are effector molecules that transmit signals from the receptors for the transforming growth factor β (TGF-β) superfamily to the nucleus; of the Smad proteins, Smad2 and Smad4 are essential components for mouse early embryogenesis. We demonstrated that Hgs, a FYVE domain protein, binds to Smad2 in its C-terminal half and cooperates with another FYVE domain protein, the Smad anchor for receptor activation (SARA), to stimulate activin receptor-mediated signaling through efficient recruitment of Smad2 to the receptor. Furthermore, a LacZ knock-in allele of the C-terminal half-deletion mutant of mouse Hgs was created by gene targeting. The introduced mutation causes an embryonic lethality between embryonic days 8.5 and 10.5. Mutant cells showed significantly decreased responses to stimulation with activin and TGF-β. These findings suggest that the two FYVE domain proteins, Hgs and SARA, are prerequisites for receptor-mediated activation of Smad2.
Members of the transforming growth factor β (TGF-β) superfamily, such as activin, nodal, and bone morphogenetic proteins (BMPs), bind to their specific cell surface receptors, which are composed of two distinct subfamilies retaining serine/threonine kinases and are known as the type I and type II receptors (2, 7, 12, 19). Upon ligand binding, the type II receptor kinase phosphorylates and activates the type I receptor kinase, which induces phosphorylation and activation of signal-transducing effector molecules known as Smad1, -2, -3, -5, and -8; subsequently, these receptor-regulated Smad proteins form complexes with Smad4, a collaborating Smad protein. The TGF-β–activin and BMP receptor kinases activate Smad2 or Smad3 and Smad1 or Smad5, respectively, followed by formation of a complex of each Smad with Smad4, which transmits signals from the receptor complex in the plasma membrane to the nucleus (2, 7, 12, 19). Mouse embryos with a homozygous mutation of Smad2 fail to form an organized egg cylinder and lack mesoderm (21, 29, 30). Similar embryonic malformations were seen in mice with mutations of Smad4 (24, 32), nodal (6, 13), the type I activin receptor, ActRIB (9), and the type I BMP receptor, ALK-3 (20). Thus, the Smad activation signalings through TGF-β superfamily receptors exert crucial effects on the generation and patterning of the mesoderm during gastrulation in mice as well as in Xenopus (10).
Antagonistic Smad proteins, such as Smad6 and Smad7, negatively regulate activation of the receptor-regulated Smads by competitively binding to the receptor complexes (2, 7, 12, 19). However, the regulatory mechanism for anchoring the Smads to the receptor complexes has been elusive. A FYVE domain protein named SARA (Smad anchor for receptor activation) was previously cloned and demonstrated to interact directly with Smad2 and Smad3 as well as function to recruit Smad2 to the TGF-β receptor complex (28). So far, the FYVE fingers derived from several proteins, including yeast Vac1p, Vps27p, Fab1p, Pib1p, and the mammalian early endosomal antigen 1 (EEA1), have been shown to bind to the membrane lipid phosphatidylinositol-3-phosphate [PtdIns(3)P] (5, 8, 22, 25), which is important for vesicular transport (27). These findings suggest that proteins containing FYVE fingers possibly contribute to the membrane trafficking of molecules associated with them (5).
Hgs is a FYVE domain protein which was originally cloned as Hrs (hepatic growth factor-regulated tyrosine kinase substrate) (15), and the Hrs gene has recently been given an approved symbol of Hgs by the Human Nomenclature Committee. We previously detected Hgs as an interleukin-2-induced phosphotyrosine protein, overexpression of which induced suppression of interleukin-2-mediated cell growth (1). Hgs was shown to be localized to early endosomes and to be homologous to Vps27p (16), which is essential for protein trafficking through a prevacuolar compartment in yeast (23). Indeed, the FYVE domain of Hgs has been shown to bind PtdIns(3)P (5, 8, 22), suggesting a possible involvement of Hgs in the membrane trafficking of various molecules. Furthermore, Hrs-2, a possible splice variant of Hgs (Hrs) containing an additional 150 amino acid residues at the carboxyl terminus, binds SNAP-25, a component of the protein complexes underlying vesicle docking and fusion, and contributes to calcium-regulated noradrenaline release from permeabilized PC12 cells, implicating a functional role of Hrs-2 in secretion of neurotransmitters through modulation of vesicle-trafficking protein complexes (4). Recently, Hrs (Hgs) null knockout mice were reported to be embryonically lethal with an underlying defect in ventral folding morphogenesis, which may be caused by dysfunction of vesicular transport (17). We here provide evidence that Hgs functions to recruit Smad2 and Smad3 to the activin receptor complex in cooperation with SARA, resulting in modulation of Smad signaling which is indispensable for mouse early embryonic development.
MATERIALS AND METHODS
Targeted disruption of Hgs.
Genomic clones of the mouse Hgs gene were isolated from a λFix II mouse 129/Sv genomic library (Stratagene, La Jolla, Calif.). An 8-kb HindIII genomic fragment encompassing two exons containing the coiled-coil sequence was used to generate a positive/negative targeting vector. The vector was constructed by inserting a neomycin resistance (Neor) cassette (LacZ-PGKNeo) at a XhoI site interrupting the codon for amino acid 455, and the diphtheria toxin A gene was added to the 5′ end to allow negative selection. The targeting vector was linearized and introduced into J1 embryonic stem (ES) cells, which were derived from the 129/SV-ter line, by electroporation. G418-resistant colonies were picked, and targeted ES clones for the Hgs gene were confirmed by using Southern blot hybridization. The targeted ES clones were injected into C57BL/6J blastocysts and transferred to foster mothers to obtain chimeric mice. Chimeric male offspring were mated to C57BL/6J females, and the F1 mice were genotyped by using PCR and Southern blot analysis for the Hgs gene, with specific probes. F1 mice heterozygous for the Hgs allele were intercrossed. Whole embryos of heterozygous matings were genotyped between 7.5 and 8.5 days postcoitum and between 9.5 and 14.5 days with yolk sac, by using PCR. The primers used for genotyping were Hgs sense strand primer (CCTGCAGAATGCCGTGAGCACTTTT), Hgs antisense strand primer (TAGCTGTCTCTGCACCTCCAGGTACT), and LacZ antisense strand primer (TGAGCGAGTAACAACCCGTCGGATT).
Preparation of cell suspensions from mouse embryos.
Yolk sac cells of Hgs+/+ embryos were obtained as described elsewhere (29), and Hgs−/− embryonic cells, which are mostly yolk sac cells, were also prepared. Briefly, timed-mated Hgs+/− mice were killed by cervical dislocation. The uterine horns were removed and embryonic day 8.5 (E8.5) embryos were harvested. Yolk sac cells and embryonic cells from Hgs+/+ and Hgs−/− embryos, respectively, were transferred to 24-well plates and treated with 0.1% collagenase (Sigma) in Dulbecco's minimal essential medium (DMEM) containing 20% fetal calf serum for 60 min at 37°C. Following the digestion, dispersed cells were drawn through a 23-gauge needle into a syringe, deposited into a polystyrene tube, and pelleted at 500 × g for 10 min. Resultant viable cells were counted and plated at ×104 cells/well into 96-well microplates with DMEM containing 10% fetal calf serum.
Whole-mount LacZ staining of Hgs mutant embryos.
Embryos were fixed in phosphate-buffered saline (PBS) containing 2% formaldehyde, 0.2% glutaraldehyde, and 0.75% NP-40 for 1 to 2 h at 4°C. Embryos were then washed with PBS and stained with 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 2 mM MgCl2, and 1 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) per ml in PBS for 24 h at room temperature. The embryos were kept in 4% formaldehyde in PBS for subsequent photography.
Whole-mount in situ hybridization.
Embryos were fixed in 4% paraformaldehyde on ice for several hours and processed for whole-mount in situ hybridization. Antisense RNAs of Brachyury (T) were labeled with digoxigenin UTP (Boehringer Mannheim) and used as probes.
Histological analysis.
Embryos were fixed in 4% paraformaldehyde for several hours on ice, dehydrated with ethanol, and embedded in paraffin wax. Five-micrometer sections were cut and stained with hematoxylin and eosin.
Immunoprecipitation and immunoblotting.
COS7 and 293T cells transfected with the indicated plasmids were treated or untreated with 100 μg of dithiobis(succinimidyl propionate) (DSP), a reducible chemical cross-linker, per ml for 20 min and lysed with cell extraction buffer (1% NP-40, 20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, and 20 μg of aprotinin/ml). The cell lysates were immunoprecipitated with the indicated antibodies, and the immunoprecipitates were then separated in a reduced condition by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride filters (Millipore, Yonezawa, Japan) (26). After incubation in PBS containing 10% fetal calf serum, the filters were probed with the indicated antibodies and visualized using the ECL detection system (Amersham, Little Chalfont, England).
Phosphorylation of Smad proteins.
After transfection with expression plasmids for Flag-tagged Smad2, Smad3, and Smad4 together with the other indicated plasmids, COS7 cells were immunoprecipitated with anti-Flag antibody and the immunoprecipitates that had been separated by gel electrophoresis were then immunoblotted with antiphosphoserine antibody (14).
Luciferase assays.
Cell suspensions of Hgs+/+ and Hgs−/− embryos at E8.5 were prepared as described above. The single cell suspensions (106 cells/ml) were transfected with 10 μg of plasmid DNA of p3TP-Lux, which is inducible by activin (3, 31), or pHXLuc, which is inducible by epidermal growth factor (EGF), by electroporation at 150 V and 950 μF and then cultured in DMEM containing 10% fetal calf serum. After 16 to 20 h, the suspensions were stimulated with 100 ng of activin A (Ajinomoto Co., Kawasaki, Japan) per ml or 100 ng of EGF per ml for 16 h and then assayed for luciferase activity. The viability of the embryonic cells after overnight culture was almost 100%.
R4-2 and HepG2 cells were transfected with the indicated plasmids along with p3TP-Lux (3 μg) or pARE-Lux (3 μg), which are luciferase reporter plasmids that are inducible by activin, in addition to FAST-1 plasmids and β-galactosidase expression plasmids (pENL), by using electroporation (200 V, 950 μF) (11). The cells were stimulated or unstimulated with 2 ng of activin A/ml for 16 h and then assayed for luciferase and β-galactosidase activities.
RESULTS
Hgs interacts with Smads and contributes to their activation.
During the search for molecules which bind to Smad2, we detected Hgs by using yeast two-hybrid assays (data not shown). We first confirmed the association between Hgs and Smads by their coimmunoprecipitation. COS7 cells were cotransfected with Flag-tagged Smads and either HA-tagged wild-type Hgs, an Hgs-dC2 mutant (amino acids 1 to 451) lacking the C-terminal half, or an Hgs-dFYVE mutant lacking the FYVE finger domain, and the lysates were immunoprecipitated with anti-Flag antibody and immunoblotted with anti-HA or anti-Flag antibody. The wild-type Hgs coimmunoprecipitated with Smad1, Smad2, and Smad3 but only marginally with Smad4 and Smad6 (Fig. 1A). Furthermore, the Hgs-dFYVE mutant but not the Hgs-dC2 mutant coimmunoprecipitated with at least Smad2 and Smad3 (Fig. 1B). These data suggest that the C-terminal half of Hgs mediates association with Smad2 and Smad3 and maybe also Smad1. Coimmunoprecipitation of the wild-type Hgs and the Smads was detectable in the presence of either the kinase-active or -inactive form of the activin receptor complex (Fig. 1C). These results together with the yeast two-hybrid assays suggest that the associations between Hgs and the Smads are direct and are independent of ligand stimulation.
FIG. 1.
Hgs associates with the Smad proteins. (A, B) Coimmunoprecipitation between Hgs and Smad proteins. COS7 cells exogenously expressing HA-tagged wild-type Hgs (Hgs), Hgs-dC2 mutant (dC2), Hgs-dFYVE mutant (dFYVE), or empty vector (pKU) along with Flag-tagged Smad1, Smad2, Smad3, Smad4, or Smad6 were treated with reducible chemical cross-linker DSP, and lysates were immunoprecipitated (IP) with anti-Flag antibody and immunoblotted (IB) with anti-HA and anti-Flag antibodies. (C) Coimmunoprecipitation of Hgs and Smad proteins in the presence of the activin receptor complex. COS7 cells exogenously expressing the wild-type Hgs (Hgs), and Flag-tagged Smad1, Smad2, and Smad3, were combined with wild-type ActRIB(WT) or kinase-inactive ActRIB(KR) and ActRII. After 10 min of stimulation with 2 ng of activin A/ml, lysates were immunoprecipitated with anti-Flag antibody and immunoblotted with anti-Hgs, antiphosphoserine (anti-P-Ser), and anti-Flag antibodies.
Since phosphorylation of the C-terminal serine residues of Smad2 and Smad3 is essential for their activation (12, 18), we next examined the effect of Hgs on serine phosphorylation of Smads in COS7 cells. Expression of the wild-type Hgs or Hgs-dFYVE mutant significantly increased the phosphorylation of Smad2 and Smad3 but not Smad4 upon cotransfection with the wild-type activin receptors, while in cells expressing the Hgs-dC2 mutant and kinase-negative activin receptors we failed to observe increased phosphorylation of Smads (Fig. 2A). Next, luciferase reporter gene assays were performed with activin-responsive p3TP-Lux and pARE-Lux plasmids plus FAST-1-expressing plasmids to assess the effect of Hgs on activin receptor-mediated signaling in Mv1Lu mutant (R4-2) cells. Transfection with the wild-type Hgs and Hgs-dFYVE mutant but not the Hgs-dC2 mutant increased the activities of both p3TP-Lux and pARE-Lux in R4-2 cells expressing the wild-type activin receptors by about fourfold compared to the control vector (Fig. 2B). No such enhancement of luciferase activity was seen in R4-2 cells expressing the kinase-negative activin receptors (data not shown). Similar results were obtained with HepG2 cells. Transfection with the wild-type Hgs and Hgs-dFYVE mutant but not the Hgs-dC2 mutant increased the activities of both p3TP-Lux and pARE-Lux in HepG2 cells upon activin stimulation (Fig. 2C). These results lend support to the contributory role of the C-terminal half of Hgs in signaling mediated by the activin receptor.
FIG. 2.
Hgs contributes to activation of the Smads. (A) Effects of Hgs and its mutants on enhancement of Smad phosphorylation mediated by the activin receptor complex. COS7 cells were exogenously introduced with HA-Hgs, HA-dC2, HA-dFYVE, or pKU and with Flag-tagged Smad2, Smad3, or Smad4, together with the activin receptors, the wild-type ActRIB(WT) or kinase-inactive ActRIB(KR), and ActRII. The cells were immunoprecipitated (IP) with anti-Flag antibody and immunoblotted (IB) with anti-P-Ser and anti-Flag antibodies. (B) Effects of Hgs and its mutants on expression of the activin-responsive luciferase reporter genes in R4-2 cells. R4-2 cells were transfected with plasmids coding for wild-type Hgs (Hgs), Hgs-dC2 (dC2), Hgs-dFYVE (dFYVE), or pCXN2, along with p3TP-Lux or pARE-Lux plus FAST-1 and pENL, together with the wild-type ActRIB(WT) and ActRII. After incubation for 16 h, the cells were assayed for luciferase activity. (C) Effects of Hgs and its mutants on signaling for expression of the activin-responsive luciferase reporter genes in HepG2 cells. HepG2 cells were transfected with Hgs, dC2, dFYVE, or pCXN2, along with p3TP-Lux or pARE-Lux plus FAST-1 and pENL. After stimulation with 2 ng of activin A/ml for 16 h, the cells were assayed for luciferase activity.
To investigate the mechanism of Hgs-mediated activation of Smad2 and Smad3, we examined the effect of Hgs on recruitment of Smad2 and Smad3 to the kinase-negative activin receptor complex in COS7 cells. While transfection with the wild-type Hgs and Hgs-dFYVE mutant increased the association between the Smads and the activin receptor complex, transfection with the Hgs-dC2 mutant did not increase the association or did so only marginally (Fig. 3). Any association between the Smads and the activin receptor complex was not seen in the presence of the kinase-positive receptor complex (Fig. 3). These results suggest that Hgs contributes to the efficient recruitment of Smads to the activin receptor complex and that the C-terminal half of Hgs, which is required for association with Smads, is indispensable. In contrast, the FYVE finger domain of Hgs, which binds to membranes through interaction with PtdIns(3)P (5, 8, 22), is dispensable. These results are consistent with those of the luciferase reporter gene assays.
FIG. 3.
Effects of Hgs and its mutants on recruitment of the Smads to the activin receptor complex. COS7 cells were exogenously introduced with the wild-type Hgs (Hgs), Hgs-dC2 (dC2), Hgs-dFYVE (dFYVE), or empty vector (pCXN2) together with Flag-tagged Smad2 or Smad3 and the HA-tagged kinase-inactive ActRIB(KR) or wild-type ActRIB(WT) and ActRII. The cells were immunoprecipitated (IP) with anti-HA or anti-Hgs (bottom panel) antibody and immunoblotted (IB) with anti-Flag, anti-HA, and anti-Hgs antibodies as indicated.
Cooperation between Hgs and SARA in signaling mediated by the activin receptor complex.
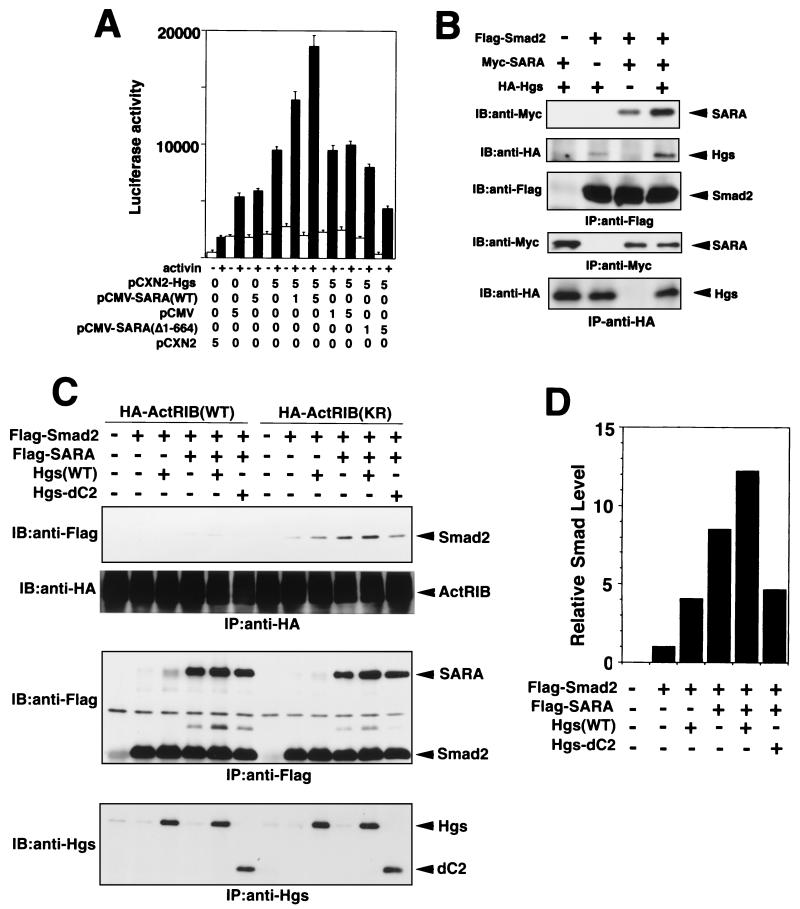
SARA, a FYVE domain protein, was previously reported to interact directly with Smad2 and Smad3 and contribute to recruitment of Smad2 to the TGF-β receptor complex (28). Although the functional characteristics of SARA are similar to those of Hgs, there is little amino acid sequence homology between the two proteins except for their FYVE domains, which have a 42% identity (1, 15, 28). Hence, we investigated the functional relationship between Hgs and SARA in activin receptor-mediated signaling. For this, we analyzed activation of pARE-Lux in HepG2 cells by transiently expressing activin receptors plus FAST-1 and combinations of the wild-type Hgs, wild-type SARA, and mutant SARA (Δ1-644), which inhibits TGF-β signaling (28). Cotransfection of the wild-type Hgs and SARA engendered a marked increase in pARE-Lux activity that was dependent on the dose of transfected SARA, while cotransfection with the mutant SARA (Δ1-644) reduced the luciferase activity to a level lower than that of the control vector (Fig. 4A). These results suggest that Hgs and SARA are cooperatively involved in the activin-induced signaling.
FIG. 4.
Cooperation between Hgs and SARA in the activin receptor-mediated signaling and recruitment of Smad2 to ActRIB. (A) Cooperative effect between Hgs and SARA on signaling for activation of pARE-Lux expression. HepG2 cells were transfected with the plasmids coding for the wild-type Hgs (Hgs) and/or Myc-tagged wild-type SARA (SARA), SARA mutant (Δ1-644), or empty vector (pCMV or pCXN2), together with ActRIB, ActRII, pARE-Lux, FAST-1 plasmid, and pENL. After stimulation with 2 ng of activin A/ml for 16 h, cells were assayed for luciferase activity. (B) Cooperative effect between Hgs and SARA on their association with Smad2, 293T cells were exogenously introduced with combinations of the HA-tagged wild-type Hgs (Hgs), Myc-tagged wild-type SARA (SARA), and Flag-tagged Smad2. The cells were immunoprecipitated (IP) with anti-Flag antibody (top three panels), anti-Myc (middle panel), and anti-HA (bottom panel) antibodies and immunoblotted (IB) with anti-Myc, anti-HA, and anti-Flag antibodies as indicated. (C) Cooperative effect between SARA and Hgs or Hgs-dC2 on the recruitment of Smad2 to the activin receptor complex. 293T cells were exogenously introduced with combinations of the wild-type Hgs (Hgs), Hgs-dC2, Flag-tagged wild-type SARA (SARA), and Flag-tagged Smad2, together with HA-tagged kinase-inactive ActRIB(KR) or wild-type ActRIB(WT) and ActRII. The cells were immunoprecipitated (IP) with anti-HA (top two panels), anti-Flag (middle panel), and anti-Hgs (bottom panel) antibodies and immunoblotted (IB) with anti-Flag, anti-HA, and anti-Hgs antibodies as indicated. (D) Relative Smad levels in Smad2 bands that coimmunoprecipitated with HA-ActRIB(KR) (shown in panel C) were quantified by a densitometer.
Next, we examined complex formation between Smad2, Hgs, and SARA in 293T cells. The binding of Smad2 to Hgs and SARA was significantly enhanced when both SARA and Hgs were simultaneously transfected with Smad2, as compared with transfection of either SARA or Hgs (Fig. 4B). Furthermore, recruitment of Smad2 to the activin receptor complex was similarly enhanced by cotransfection of Hgs with SARA, in contrast to transfection of either Hgs or SARA alone (Fig. 4C and D). These results are again consistent with those of the luciferase assays, suggesting that Hgs and SARA promote activin signaling by cooperatively recruiting Smad2 to the receptor complex. Furthermore, cotransfection with SARA and the Hgs-dC2 mutant impeded the SARA-induced association between Smad2 and the activin receptor complex (Fig. 4C and D), suggesting that the Hgs-dC2 mutant has a suppressive effect on the SARA-mediated association of Smad2 with the receptor complex.
Hgs mutants are embryonically lethal early.
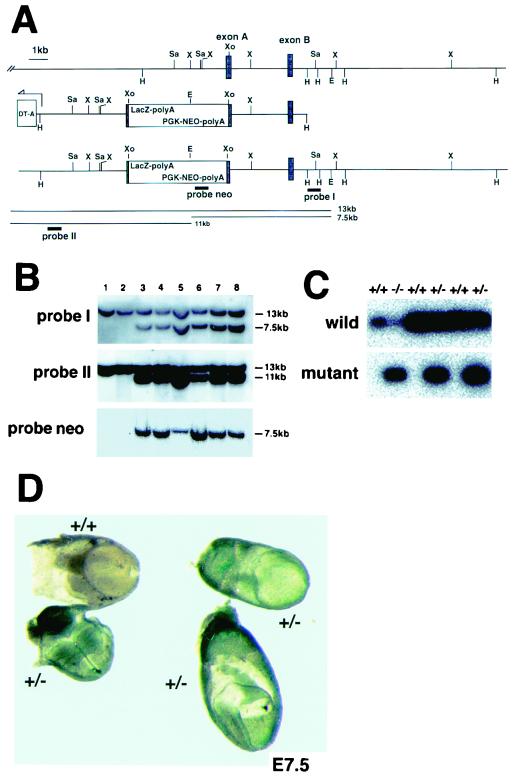
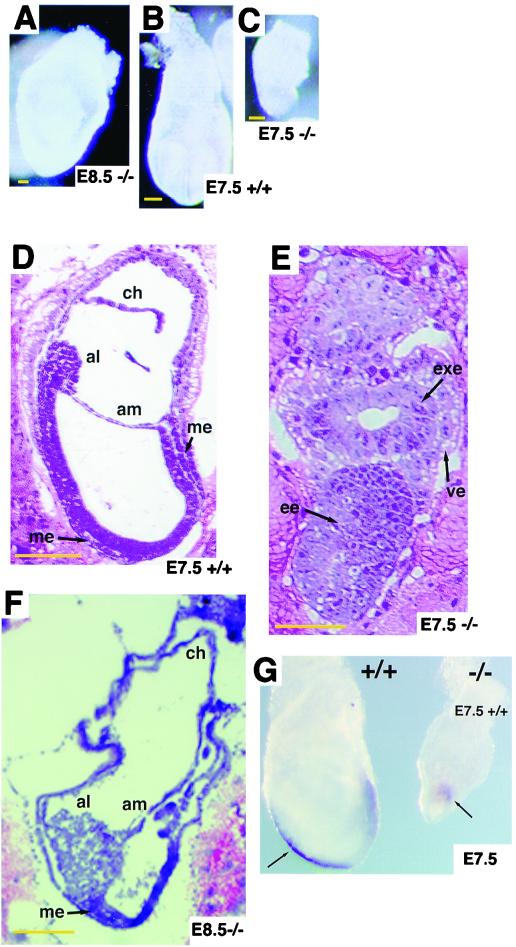
To determine the significance of the C-terminal half of Hgs in vivo, we generated through gene targeting a knock-in mouse line which harbors an Hgs mutant lacking the C-terminal half. The construct of the targeting vector was designed to express a mutant Hgs lacking the C-terminal half (amino acids 455 to 777) in association with a LacZ marker (Fig. 5A). This Hgs mutant (amino acids 1 to 454) is almost identical to the Hgs-dC2 mutant (amino acids 1 to 451). Targeted ES cell clones were used to obtain chimeric mice that transmitted the mutant allele through the germ line (Fig. 5B). Genotypes of progeny of the heterozygote (Hgs+/−) intercrosses were analyzed by PCR (Fig. 5C) and showed that 41% were wild type (Hgs+/+), 59% were Hgs+/−, and none were homozygous (Hgs−/−) (Table 1). Hgs−/− embryos were recovered at Mendelian ratios at E7.5 through E9.5, but none of the 20 embryos at E10.5 were homozygous (Table 1), indicating that Hgs−/− embryos are lethal by E10.5. LacZ staining showed that Hgs expression is ubiquitous in embryos at E7.5 (Fig. 5D), as reported by Komada and Soriano (17). The mutant embryos showed growth retardation at E7.5, and the embryo proper seemed not to develop at E8.5 (Fig. 6A through C). Histological analyses at E7.5 showed that Hgs−/− embryos, unlike the wild-type embryos, retained the appearance of the egg cylinder (Fig. 6D and E) and extraembryonic structures developed in most of the mutants at E8.5 (Fig. 6F), although a Brachyury (T) marker analysis at E7.5 showed development of the primitive streak (Fig. 6G).
FIG. 5.
Targeted disruption of the Hgs gene. (A) Restriction map of the Hgs genomic fragment containing two exons (top line); structure of the Hgs targeting vector construct containing a neomycin resistance (Neor) cassette (LacZ-PGKNeo) and diphtheria toxin A (DT-A) at the 5′ end (middle line); and structure of the targeted Hgs allele (bottom line). Exon B contains the coiled-coil sequence (amino acids 470 to 497). The 3′ probe (probe I) detects a 13-kb EcoRI band corresponding to the wild-type allele and a 7.5-kb band corresponding to the mutated allele. The 5′ probe (probe II) detects a 13-kb band of the wild-type allele and an 11-kb band of the mutated allele. (B) Southern blot analyses of genomic DNA from the Hgs wild-type (+/+) ES clone (lane 1) and embryo (lane 2) and the heterozygous mutant (+/−) ES clones (lanes 3 and 4) and embryos (lanes 5 through 8) at E10.5. (C) PCR analyses of genomic DNA from embryos. The Hgs wild-type (+/+), heterozygous (+/−), and homozygous (−/−) embryos at E7.5 were genotyped for the wild-type and mutant alleles. (D) Whole-mount LacZ staining of Hgs heterozygous (+/−) mutant and wild-type (+/+) embryos at E7.5.
TABLE 1.
Genotype of offspring and embryos from Hgs heterozygote crossesa
| Age | No. of embryos or offspring analyzed | No. with genotype
|
||
|---|---|---|---|---|
| +/+ | +/− | −/− | ||
| E6.5 | 23 | 5 | 12 | 6 |
| E7.5 | 20 | 4 | 11 | 5 |
| E8.5 | 42 | 10 | 22 | 10 |
| E9.5 | 16 | 5 | 8 | 3 |
| E10.5 | 20 | 7 | 13 | 0 |
| E14.5 | 14 | 4 | 10 | 0 |
| Newborn | 111 | 46 (41%) | 65 (59%) | 0 |
Hgs genotypes of embryos and newborns derived from heterozygous intercrosses were identified by PCR and Southern blot analysis as described in Materials and Methods.
FIG. 6.
Morphological and histological appearance of Hgs+/+ and Hgs−/− embryos. (A through C) External appearance of Hgs+/+ and Hgs−/− embryos at E7.5 and E8.5. (D through F) Hematoxylin-and-eosin-stained sections of Hgs+/+ and Hgs−/− embryos at E7.5 and E8.5. Arrows indicate embryonic ectoderm (ee), extraembryonic ectoderm (exe), chorion (ch), allantois (al), amnion (am), and embryonic mesoderm (me). Bars, 50 μm (A through C) and 100 μm (D through F). (G) Whole-mount in situ hybridization for Brachyury (T) genes was carried out with Hgs+/+ and Hgs−/− at E7.5.
Hgs mutant cells show decreased responses to stimulation with activin and TGF-β.
To investigate whether signaling mediated by activin or TGF-β is impaired in Hgs−/− embryos, we conducted luciferase reporter gene assays with activin- and TGF-β-responsive p3TP-Lux. Hgs+/+ and Hgs−/− embryos at E8.5 were transfected with p3TP-Lux or the c-myc promoter-driven luciferase reporter (pHXLuc) plasmids. After stimulation with activin A, TGF-β, or EGF, luciferase activities were assessed. The luciferase activity driven by p3TP-Lux was more than 10-fold higher in Hgs+/+ embryos compared with Hgs−/− embryos upon stimulation with activin and TGF-β (Fig. 7A). In contrast, the activities of p3TP-Lux as well as pHXLuc were significantly increased upon stimulation with EGF in both embryos (Fig. 7A). These results suggest that Hgs plays a critical role in signaling mediated by activin and TGF-β in mouse embryos.
FIG. 7.
Decreased responses to stimulation with activin and TGF-β in Hgs−/− embryos at E8.5. (A) Luciferase assays of E8.5 Hgs+/+ and Hgs−/− embryonic cells transfected with p3TP-Lux or pHXLuc and preincubated for 16 to 20 h. The cells were then stimulated with 100 ng of activin A/ml, 10 ng of TGF-β/ml, or 100 ng of EGF/ml for 16 h and assayed for luciferase activity. Results are representative of three comparable experiments. (B) Rescue of unresponsiveness of Hgs−/− embryonic cells to activin stimulation by cotransfection of Hgs and SARA. E8.5 Hgs+/+ and Hgs−/− embryonic cells were transfected with expression plasmids of SARA and/or Hgs together with activin-responsive pARE-Lux and FAST-1. Cells were then stimulated with 100 ng of activin A/ml for 8 h and assayed for luciferase activity. To calibrate the total DNA transfected, the backbone vectors pCMV and pCXN2 were used for SARA and Hgs, respectively.
Next, we examined whether or not transfection with Hgs and/or SARA is able to overcome the unresponsiveness of Hgs mutant embryos to activin. The wild-type and Hgs mutant embryos were transfected with both Hgs and SARA, or with either Hgs or SARA along with an activin-responsive luciferase reporter plasmid, pARE-Lux, and then stimulated with activin. The wild-type embryos showed significant increases in luciferase activities irrespective of transfection of Hgs and SARA (Fig. 7B). On the other hand, the simultaneous transfection of Hgs and SARA induced the maximum level of luciferase activity in the Hgs mutant embryos, whereas luciferase activity was barely increased without transfection of Hgs and SARA (Fig. 7B). The transfection of either SARA alone or Hgs alone induced significant increases in the luciferase activity of the Hgs mutant embryos, and the SARA-induced increase was much higher than the Hgs-induced increase (Fig. 7B). These results indicate that the unresponsiveness of Hgs mutant embryos to activin can be rescued partially by overexpression of either Hgs or SARA and rescued completely by simultaneous overexpression of Hgs and SARA, suggesting that Hgs and SARA have additive effects on activin-mediated signaling.
DISCUSSION
The present study showed an empirical association of Hgs with Smad2 and Smad3, effector molecules associated with the activin receptor complex. The C-terminal half of Hgs was revealed to be essential for the activin-mediated signaling for activation of p3TP-Lux and pARE-Lux. The involvement of Hgs in activin-mediated signaling can be accounted for by the observation that the binding of Smads with the C-terminal half of Hgs elicits efficient recruitment of Smads to the type I activin receptor, ActRIB, resulting in an increase of phosphorylation and activation of the Smads. Such functions of Hgs are reminiscent of SARA, which is able to bind to Smad2 and recruit it to the TGF-β receptor complex (28). We revealed that the coexpression of Hgs and SARA augments the activin-mediated signaling for activation of pARE-Lux, in which Hgs contributes to the formation of a tighter complex between SARA and Smad2 as compared with the expression of SARA alone (Fig. 4B). The coexpression of SARA with wild-type Hgs also increases the association between Smad2 and ActRIB, while the coexpression of SARA with the Hgs-dC2 mutant appreciably suppresses Smad2 association with the receptor, compared with the expression of SARA alone, indicating that the Hgs-dC2 mutant, which contains the FYVE domain but lacks the binding site to Smad2, has an inhibitory effect on SARA-mediated Smad2 association with ActRIB (Fig. 4C). Similarly, the SARA mutant (Δ1-664) suppressed the Hgs-induced enhancement of activin signaling (Fig. 4A). These results suggest that the two FYVE domain proteins, Hgs and SARA, cooperate in the initiation of activin signaling by recruiting Smad2 to the activin receptor complex, thus facilitating receptor-dependent phosphorylation and activation of Smad2 by the receptor.
The functional significance of FYVE domain family proteins has been assessed; the FYVE domains of several proteins, including Hgs, EEA1, FGD1, Vac1P, Vps27, Fab1p, and Pib1p, bind to a membrane PtdIns(3)P (5, 8, 22) which is important for vesicular transport (27). Indeed, the FYVE domain of EEA1 is required for its localization to early endosomes in HepG2 cells (25). Accordingly, one may consider that Hgs contributes to anchoring Smad2 to the plasma membrane through the function of its FYVE domain, resulting in efficient recruitment of Smad2 to ActRIB. However, this consideration is unconvincing because the Hgs-dFYVE mutant enhanced the activin-mediated signaling and retained the ability for recruitment of the Smads to ActRIB. It is possible that not only the FYVE domain but also another as-yet-unidentified domain may be involved in the Smad recruitment to ActRIB. This possibility is sustained by the fact that another Hgs mutant, with the FYVE domain deleted, still retains the ability for early endosomal localization in HeLa cells (16). These observations suggest that the Hgs FYVE domain is dispensable for the recruitment of Smad2 to the receptor complex.
We prepared Hgs homozygous mutant mice carrying the C-terminal half-deletion mutant of the Hgs gene, which is almost identical to the Hgs-dC2 mutant. The Hgs mutant embryos are lethal between E8.5 and E10.5. Embryonic cells of the Hgs mutant mice showed unresponsiveness to activin A and TGF-β but appreciably responded to EGF to the same extent that the Hgs wild-type embryos responded. These results suggest that the mutant Hgs causes impairment of the signaling mediated by activin and TGF-β in embryos, leading to defective embryonic development. Hgs (Hrs) null mutant embryos also have been reported to be lethal between E10.5 and E11.5 and to show a defect of ventral folding morphogenesis despite development of three germ layers (17). They are significantly different from our Hgs mutant embryos. This discrepancy may be attributed to the different ES cells used to create the mutants (J1 versus Ak7 cells). We are preparing a null mutation of the Hgs gene to answer this question. Alternatively, this mutation in our mice might lead to more severely affected phenotypes than we observed in the Hgs null mice because, as described above, our Hgs mutant allele may have an inhibitory effect on SARA function, which plays a critical role in the Smad2 activation (28). Actually, some Hgs+/− embryos died (the number of Hgs+/− offspring was about 30% lower than expected); this is dependent perhaps on the genetic background, although phenotypic variance was not observed among Hgs−/− embryos.
Overexpression of SARA in Hgs mutant embryonic cells rescued their unresponsiveness to activin in vitro more efficiently than that of the wild-type Hgs (Fig. 7C), suggesting that Hgs is not primarily required for Smad2 activation in activin signaling, whereas SARA may be indispensable for it. However, since the Hgs mutant of the embryonic cells, like Hgs-dC2, should have an inhibitory activity for SARA-mediated association of Smad2 with ActRIB, the activin signaling could be suppressed in the Hgs mutant embryonic cells. Although Hgs may be dispensable for Smad2 association with ActRIB in the presence of SARA, Hgs plays a critical role in the efficient association of Smad2 to ActRI through cooperation with SARA (Fig. 4C). Hence, we speculate that Hgs and SARA, both binding to Smad2, synergistically cooperate in activin signaling by independent but quite similar mechanisms.
The mutant phenotypes of Hgs embryos are apparently similar to ActRIB−/− (9) and Smad2−/− embryos (21, 29, 30) at E7.5 in that they retain the egg cylinder appearance. However, in Hgs mutants primitive streaks seem to develop, and at E8.5 the faint development of embryonic mesoderm and extraembryonic structures was observed. These observations suggest that the loss of Hgs may suppress but not abolish activin signaling, which is still sufficient to induce formation of mesoderm, particularly since SARA is present in the Hgs mutant mice. Further embryological studies may be required to compare Hgs−/− embryos and other mutants in terms of TGF-β family signaling.
ACKNOWLEDGMENTS
We thank H. Takano for his help in the preparation of embryonic tissue sections, T. Noda for providing the J1 ES cell line and mice carrying the Neor gene, J. Massague for providing expression plasmids for ActRII/HA and p3TP-Lux, T. Imamura and M. Kawabata for discussion, and S. Moffatt and L. C. Ndhlovu for critically reading the manuscript.
This work was supported in part by a grant from the Core Research for Evolutional Science and Technology (CREST) program of the Japan Science and Technology Corporation, grants-in-aid for scientific research on priority areas from the Ministry of Education, Science, Sport, and Culture of Japan, and a grant from special coordination funds of the Science and Technology Agency of Japan.
REFERENCES
- 1.Asao H, Sasaki Y, Arita T, Tanaka N, Endo K, Kasai H, Takeshita T, Endo Y, Fujita T, Sugamura K. Hrs is associated with STAM, a signal-transducing adaptor molecule. Its suppressive effect on cytokine-induced cell growth. J Biol Chem. 1997;272:32785–32791. doi: 10.1074/jbc.272.52.32785. [DOI] [PubMed] [Google Scholar]
- 2.Attisano L, Wrana J L. Mads and Smads in TGF-β signaling. Curr Opin Cell Biol. 1998;10:188–194. doi: 10.1016/s0955-0674(98)80141-5. [DOI] [PubMed] [Google Scholar]
- 3.Attisano L, Wrana J L, Montalvo E, Massague J. Activation of signalling by the activin receptor complex. Mol Cell Biol. 1996;16:1066–1073. doi: 10.1128/mcb.16.3.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bean A J, Seifert R, Chen Y A, Sacks R, Scheller R H. Hrs-2 is an ATPase implicated in calcium-regulated secretion. Nature. 1997;385:826–829. doi: 10.1038/385826a0. [DOI] [PubMed] [Google Scholar]
- 5.Burd C G, Emr S D. Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to ring FYVE finger domains. Mol Cell. 1998;2:157–162. doi: 10.1016/s1097-2765(00)80125-2. [DOI] [PubMed] [Google Scholar]
- 6.Conlon F L, Lyons K M, Takaesu N, Barth K S, Kispert A, Herrmann B, Robertson E J. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- 7.Derynck R, Feng X H. TGF-β signaling. Biochim Biophys Acta. 1997;1333:F105–F150. doi: 10.1016/s0304-419x(97)00017-6. [DOI] [PubMed] [Google Scholar]
- 8.Gaullier J M, Simonsen A, DíArrigo A, Bremnes B, Stenmark H, Aasland R. FYVE fingers bind PtdIns(3)P. Nature. 1998;394:432–433. doi: 10.1038/28767. [DOI] [PubMed] [Google Scholar]
- 9.Gu Z, Nomura M, Simpson B B, Lei H, Feijen A, van den Eijnden-van Raaij J, Donahoe P K, Li E. The type I activin receptor ActRIB is required for egg cylinder organization and gastrulation in the mouse. Genes Dev. 1998;12:844–857. doi: 10.1101/gad.12.6.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harland R, Gerhart J. Formation and function of Spemann's organizer. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi H, Abdollah S, Qiu Y, Cai J, Xu Y Y, Grinnell B W, Richardson M A, Topper J N, Jr, Gimbrone M A, Wrana J L, Falb D. The MAD-related protein Smad7 associates with the TGF-β receptor and functions as an antagonist of TGF-β signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 12.Heldin C-H, Miyazono K, ten Dijke P. TGF-β signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 13.Iannaccone P M, Zhou X, Khokha M, Boucher D, Kuehn M R. Insertional mutation of a gene involved in growth regulation of the early mouse embryo. Dev Dyn. 1992;194:198–208. doi: 10.1002/aja.1001940305. [DOI] [PubMed] [Google Scholar]
- 14.Kawabata M, Inoue H, Hanyu A, Imamura T, Miyazono K. Smad proteins exist as monomers in vivo and undergo homo- and hetero-oligomerization upon activation by serine/threonine kinase receptors. EMBO J. 1998;17:4056–4065. doi: 10.1093/emboj/17.14.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komada M, Kitamura N. Growth factor-induced tyrosine phosphorylation of Hrs, a novel 115-kilodalton protein with a structurally conserved putative zinc finger domain. Mol Cell Biol. 1995;15:6213–6221. doi: 10.1128/mcb.15.11.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komada M, Masaki R, Yamamoto A, Kitamura N. Hrs, a tyrosine kinase substrate with a conserved double zinc finger domain, is localized to the cytoplasmic surface of early endosomes. J Biol Chem. 1997;272:20538–20544. doi: 10.1074/jbc.272.33.20538. [DOI] [PubMed] [Google Scholar]
- 17.Komada M, Soriano P. Hrs, a FYVE finger protein localized to early endosomes, is implicated in vesicular traffic and required for ventral folding morphogenesis. Genes Dev. 1999;13:1475–1485. doi: 10.1101/gad.13.11.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macias-Silvia M, Abdollah S, Hoodless P A, Pirone R, Attisano L, Wrana J L. MADR2 is a substrate of the TGF-β receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell. 1996;87:1215–1224. doi: 10.1016/s0092-8674(00)81817-6. [DOI] [PubMed] [Google Scholar]
- 19.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 20.Mishina Y, Suzuki A, Ueno N, Behringer R R. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- 21.Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393:786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- 22.Patki V, Lawe D C, Corvera S, Virbasius J V, Chawla A. A functional PtdIns(3)P-binding motif. Nature. 1998;394:433–434. doi: 10.1038/28771. [DOI] [PubMed] [Google Scholar]
- 23.Piper R C, Cooper A A, Yang H, Stevens T H. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J Cell Biol. 1995;131:603–617. doi: 10.1083/jcb.131.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sirard C, Luis de la Pompa J L, Elia A, Itie A, Mirtsos C, Cheung A, Hahn S, Wakeham A, Schwartz L, Kern S E, Rossant J, Mak T W. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev. 1998;12:107–119. doi: 10.1101/gad.12.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stenmark H, Aasland R, Toh B H, D'Arrigo A. Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger. J Biol Chem. 1996;271:24048–24054. doi: 10.1074/jbc.271.39.24048. [DOI] [PubMed] [Google Scholar]
- 26.Takeshita T, Arita T, Higuchi M, Asao H, Endo K, Kuroda H, Tanaka N, Murata K, Ishii N, Sugamura K. STAM, signal transducing adaptor molecule, is associated with Janus kinases and involved in signaling for cell growth and c-myc induction. Immunity. 1997;6:449–457. doi: 10.1016/s1074-7613(00)80288-5. [DOI] [PubMed] [Google Scholar]
- 27.Toker A, Cantley L C. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 28.Tsukazaki T, Chiang T A, Davison A F, Attisano L, Wrana J L. SARA, a FYVE domain protein that recruits Smad2 to the TGF-β receptor. Cell. 1998;95:779–791. doi: 10.1016/s0092-8674(00)81701-8. [DOI] [PubMed] [Google Scholar]
- 29.Waldrip W R, Bikoff E K, Hoodless P A, Wrana J L, Robertson E J. Smad2 signaling in extraembryonic tissues determines anterior-posterior polarity of the early mouse embryo. Cell. 1998;92:797–808. doi: 10.1016/s0092-8674(00)81407-5. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein M, Yang X, Li C, Xu X, Gotay J, Deng C-X. Failure of egg cylinder elongation and mesoderm induction in mouse embryos lacking the tumor suppressor smad2. Proc Natl Acad Sci USA. 1998;95:9378–9383. doi: 10.1073/pnas.95.16.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willis S A, Zimmerman C M, Li L I, Mathews L S. Formation and activation by phosphorylation of activin receptor complexes. Mol Endocrinol. 1996;10:367–379. doi: 10.1210/mend.10.4.8721982. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Li C, Xu X, Deng C. The tumor suppressor SMAD4/DPC4 is essential for epiblast proliferation and mesoderm induction in mice. Proc Natl Acad Sci USA. 1998;95:3667–3672. doi: 10.1073/pnas.95.7.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]