Abstract
Objectives
How brain MRI lesions associate with outcomes in pediatric anti-NMDA receptor encephalitis (pNMDARE) is unknown. In this study, we correlate T2-hyperintense MRI brain lesions with clinical outcomes in pNMDARE.
Methods
This was a multicenter retrospective cohort study from 11 institutions. Children younger than 18 years with pNMDARE were included. One-year outcomes were assessed by the modified Rankin Score (mRS) with good (mRS ≤2) and poor (mRS ≥3) outcomes.
Results
A total of 175 pNMDARE subjects were included, with 1-year mRS available in 142/175 (81%) and 60/175 (34%) had abnormal brain MRIs. The most common T2-hyperintense lesion locations were frontal, temporal, and parietal. MRI features that predicted poor 1-year outcomes included abnormal MRI, particularly T2 lesions in the frontal and occipital lobes. After adjusting for treatment within 4 weeks of onset, improvement within 4 weeks, and intensive care unit admission, MRI features were no longer associated with poor outcomes, but after multiple imputation for missing data, T2 frontal and occipital lesions associated with poor outcomes.
Discussion
Abnormal frontal and occipital lesions on MRI may associate with 1-year mRS in pNMDARE. MRI of the brain may be a helpful prognostication tool that should be examined in future studies.
Anti-NMDA receptor encephalitis (NMDARE) causes neuropsychiatric symptoms1,2 resulting in morbidity in 20%3 and mortality in 10%.4 NMDARE can be paraneoplastic, occurring in 3% of children with ovarian teratomas.5 Management includes immunotherapy and supportive care.6 Predicting outcomes in NMDARE are challenging, but risk factors for poor outcomes include delayed immunotherapy, younger than 2 or older than 65 years, and extreme delta brush on electroencephalography.2 The anti-NMDA 1-Year Functional Status (NEOS) score, which includes abnormal MRI, can predict 1-year NMDARE outcomes.7 However, in a pediatric NMDARE (pNMDARE) validation study, NEOS was applicable to the entire group, but not in an individual subject.8
MRI abnormalities, usually T2-hyperintense lesions, occur in one-third of children and adults with NMDARE.1,2 Little is known about MRI features and their associated outcomes in NMDARE, especially in children. In 53 NMDARE subjects (17 of which were children), T2-hippocampal lesions associated with worse outcomes in adults, but not in children.9 Here, we assess the association of T2-hyperintense brain MRI lesions and clinical outcomes in a multicenter pNMDARE cohort.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
A multicenter retrospective study with 11 institutions included children younger than 18 years with pNMDARE between January 1, 2008, and September 1, 2022. Diagnosis of pNMDARE was confirmed with positive CSF NMDA receptor (NMDAr) antibodies and at least 1 of 6 neuropsychiatric symptoms.10 Institutional Review Board approval was obtained at each study site, which waived patient consent. Clinical data were collected, including outcomes using the modified Rankin Scale (mRS). NEOS scores were calculated, as a 5-point scale with 1 point for each variable: ICU admission, abnormal MRI, CSF WCC >20, treatment >4 weeks, and lack of improvement <4 weeks7 An MRI lesion was defined as any T2-brain hyperintensity. MRI data were collected from the initial pretreatment brain MRI after neuroradiologist review for clinical purposes; then, lesion location was extracted by a neurologist at each site. Subjects with prior herpes simplex virus encephalitis were excluded. A subset of 36 subjects has been previously published.5,8,11,12
Statistical analysis, including descriptive statistics and comparisons, was performed as appropriate for continuous and discrete data, including for data with normal vs skewed distributions. Significance was set at p < 0.05 with 2-sided hypothesis testing. Multivariable regression modeling with odds ratios with 95% confidence intervals were used to calculate odds of persistent disability based on neuroimaging abnormalities. Initially, complete case analyses were performed. For sensitivity analysis, multiple imputation was performed for missing data. The variables used in the 1-year mRS outcomes to impute values included age of onset, ICU admission, treatment <4 weeks, improvement <4 weeks, and 1-year mRS scores. We also performed mediation and interaction analyses between MRI lesions and ICU admission (SAS 16.0, Cary, NC).
Data Availability
Data are available to qualified researchers based on reasonable request.
Results
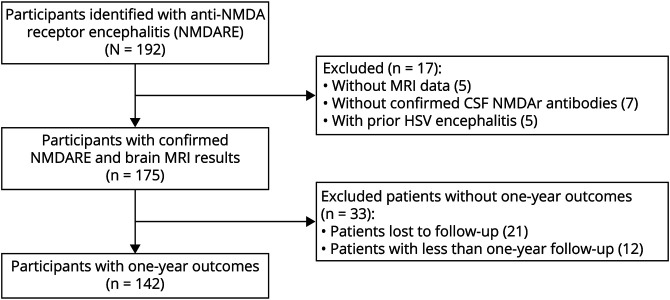
Data were collected from 192 pNMDARE subjects at 11 institutions. Seventeen subjects were excluded: 5 subjects had unavailable MRI data, 7 subjects did not have confirmed CSF NMDAr antibodies, and 5 subjects had prior HSV encephalitis (Figure). A total of 175 subjects were included, with an average age of 11.6 years (SD: 5.0 years) and 70% were female (Table 1).
Figure. Flow Diagram of Pediatric Anti-NMDA Receptor Encephalitis Subjects Included and Excluded From This Study.
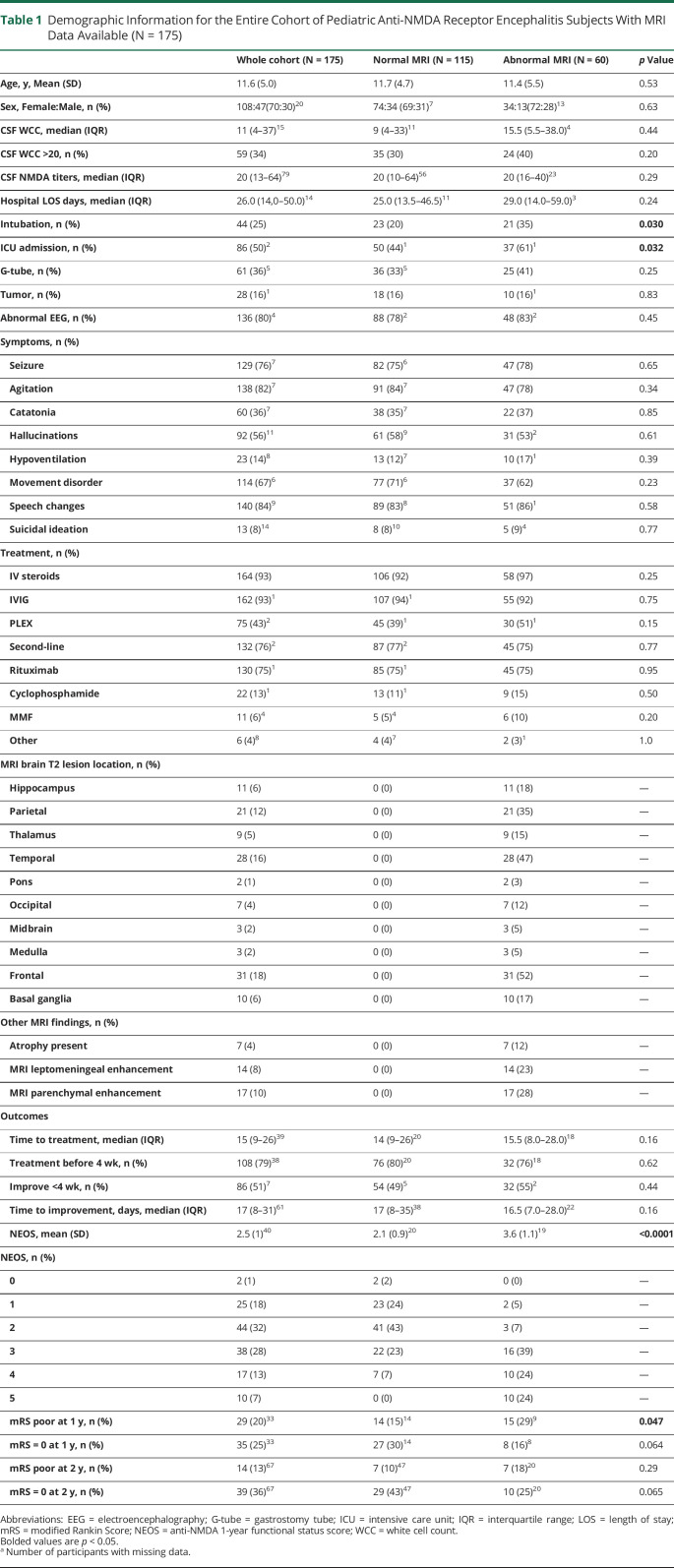
Table 1.
Demographic Information for the Entire Cohort of Pediatric Anti-NMDA Receptor Encephalitis Subjects With MRI Data Available (N = 175)
Thirty-four percent (60/175) had abnormal brain MRIs with the most common abnormalities including T2-hyperintense frontal (31/60 = 52%), temporal (28/60 = 47%), and parietal (21/60 = 35%) lesions (Table 1). Abnormal brain MRI was associated with ICU admission, intubation, higher NEOS score, and poor 1-year mRS (mRS ≥3) scores.
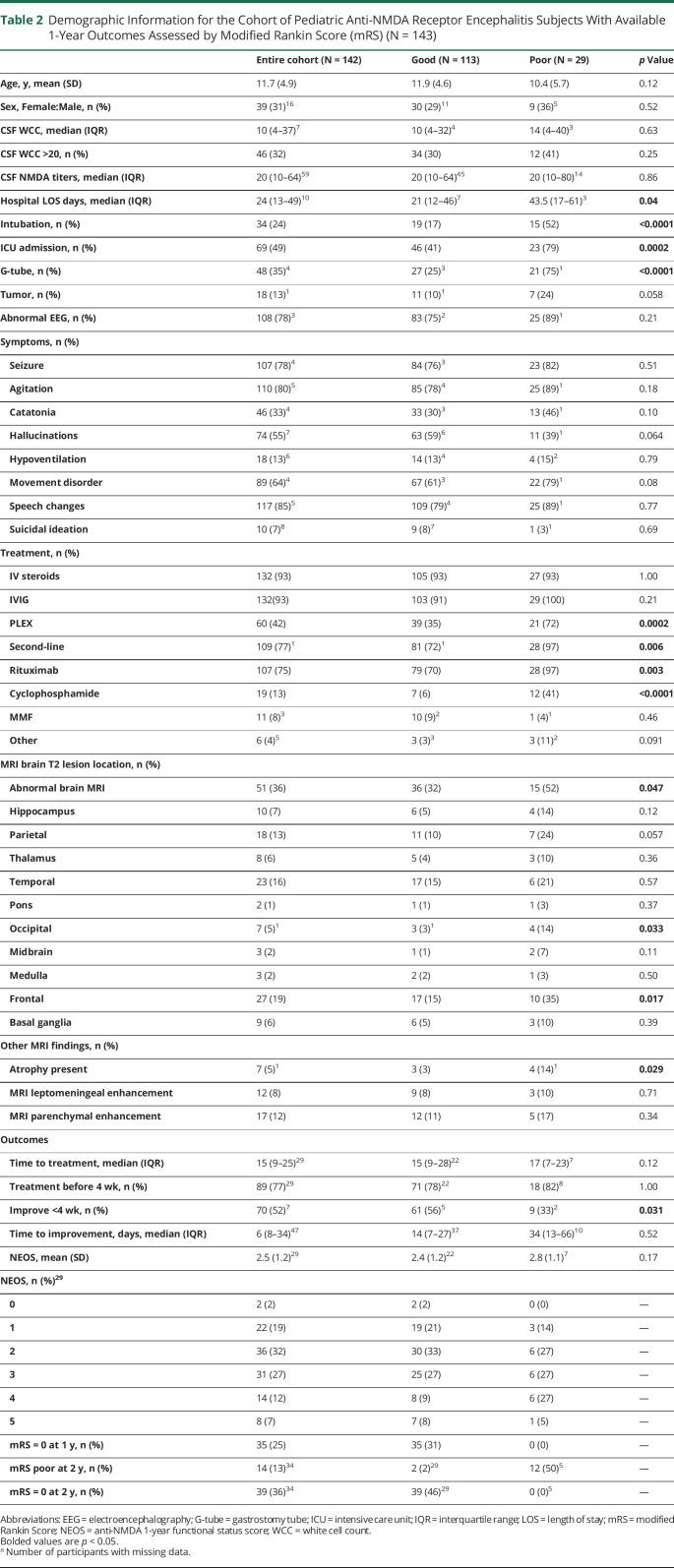
For 1-year outcomes, 142 participants had available data, with poor (mRS ≥3) outcomes in 29 and 113 had good (mRS ≤2) outcomes (Table 2). Abnormal brain MRI correlated with poor 1-year outcomes (OR 2.9; 95% CI 1.2–7.0), as did frontal (OR 4.2; 95% CI 1.5–11.6) and occipital lobe T2-hyperintense lesions (OR 6.8; 95% CI 1.1–43.3). Other variables associated with poor 1-year outcomes included prolonged hospital length of stay, intubation, ICU admission, gastrostomy placement, plasma exchange and/or second-line treatments (including rituximab and cyclophosphamide), and no improvement <4 weeks from symptom onset (Table 2). Data from 12 patients were not included because 1 year had not passed from symptom onset. We also assessed those lost to 1-year follow-up by assessing faster recovery or milder disease by comparing mRS at 3 and 6 months or improvement <4 weeks. No differences were observed in these characteristics between those included vs excluded at the 1-year follow-up.
Table 2.
Demographic Information for the Cohort of Pediatric Anti-NMDA Receptor Encephalitis Subjects With Available 1-Year Outcomes Assessed by Modified Rankin Score (mRS) (N = 143)
Using multivariable logistic regression, adjusting for ICU admission, improvement <4 weeks, and treatment <4 weeks, abnormal MRI, T2 frontal, and T2 occipital lesions no longer associated with poor outcomes; ICU admission was the only predictor for poor outcomes (eTable 1, links.lww.com/NXI/A860). Interaction and mediation analyses of ICU admission did not affect the relationship between MRI lesions and outcomes. Sensitivity analyses were performed using multiple imputation to fill in missing data for 1-year mRS outcomes in 33 patients, with missing mRS scores (23), missing treatment <4 weeks (8), missing ICU admission (1), and missing ICU admission/treatment <4 weeks (1). After multiple imputation, T2 frontal (OR 2.81, 95% CI 1.10–6.66) and occipital lobe lesions (OR 8.58, 95% CI 1.15–64.3) were associated with poor 1-year outcomes, even when adjusting for ICU admission, treatment <4 weeks, and improvement <4 weeks (eTable 2).
Discussion
In this pNMDARE cohort, abnormal brain MRI was associated with poor 1-year outcomes, particularly T2-hyperintense frontal and occipital lesions. Abnormal brain MRIs were also associated with intubation and ICU admission. This is one of the largest studies to date that examines T2-hyperintense lesion locations and their association with outcomes in pNMDARE.
Despite multiple neurologic symptoms, only 34% of pNMDARE had brain MRI abnormalities. As executive dysfunction and impulsivity are common residual symptoms in NMDARE,1 T2-hyperintense frontal lobe lesions may help to identify those at higher risk for long-term neuropsychological dysfunction. Residual memory problems are also common, but T2-hippocampal/temporal lesions did not associate with outcomes in this study. Surprisingly, T2-hyperintense occipital lobe lesions associated with poor outcomes but may be due to other brain involvement. Although ICU admission altered the associations of MRI lesions with 1-year outcomes and ICU admission did not have a mediation or interaction effect, multiple imputation did demonstrate an association between T2 frontal and occipital lesions with outcomes. This suggests that missing data are affecting the results, which were mitigated by multiple imputation. Moreover, T2 lesions may overlap with demyelinating diseases13 and/or reflect cytotoxic injury, suggestive of more severe disease and affect outcomes.
Limitations include that we performed a descriptive and retrospective study of MRI lesion location without including lesion volume or networks. Multiple observers inputted MRI data, which could introduce bias. Another limitation includes that we cannot confirm that all T2-hyperintense lesions present on acute imaging are related to NMDARE as prior MRIs are unavailable. The timing of MRI from symptom onset or its relationship to the number of abnormalities was not included, which may confound this study. In those without 1-year mRS scores, many of these subjects had not reached 1-year follow-up time and our subjects lost to follow-up appeared random. Compounding this, data were collected from tertiary and quaternary pediatric medical centers, and thus, severity bias and convenience sampling are present in this data set. This could affect the rates of neuroimaging abnormalities and 1-year disability. Finally, mRS was used as a standardized and efficient outcome measure that is consistent across institutions; however, the mRS may not adequately capture residual cognitive/neuropsychiatric symptoms in NMDARE.1,14,15
T2-hyperintense frontal and occipital lobe lesions may associate with poor outcomes in pNMDARE. Future studies should also explore the association of MRI lesions, their locations, and networks with residual neuropsychological outcomes.
Appendix. Authors

Study Funding
This work was supported by the National Center for Advancing Translational Sciences (NCATS) of the NIH under Award Nos. UL1TR002378 and KL2TR002381 and the 2021-2022, 2022-2025 Pediatric Epilepsy Research Foundation Grants, Emory School of Medicine Doris Duke Charitable Foundation COVID-19 Fund to Retain Clinical Scientists, and the Georgia CTSA NIH (Award No. UL1-TR002378).
Disclosure
G.Y. Gombolay, J.N. Brenton, J.H. Yang, C.M. Stredny, R. Kammeyer, C. Otten, N. Vu, J.D. Santoro, K. Robles-Lopez, A. Christiana, C. Steriade, M, Morris, M. Gorman, M. Moodley, D. Hardy, A. Kornbluh, I. Kahn, and L. Sepeta have no relevant disclosures. A. Yeshokumar is an employee of Bristol Myers Squibb but does not affect this study. Go to Neurology.org/NN for full disclosures.
References
- 1.Dalmau J, Armangue T, Planaguma J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057. [DOI] [PubMed] [Google Scholar]
- 2.Nosadini M, Eyre M, Molteni E, et al. Use and safety of immunotherapeutic management of N-Methyl-d-aspartate receptor antibody encephalitis: a meta-analysis. JAMA Neurol. 2021;78(11):1333-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zekeridou A, Karantoni E, Viaccoz A, et al. Treatment and outcome of children and adolescents with N-methyl-D-aspartate receptor encephalitis. J Neurol. 2015;262(8):1859-1866. [DOI] [PubMed] [Google Scholar]
- 4.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li JH, Milla SS, Gombolay GY. Rate of anti-NMDA receptor encephalitis in ovarian teratomas. Neuropediatrics. 2021;53(2):133-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nosadini M, Thomas T, Eyre M, et al. International consensus recommendations for the treatment of pediatric NMDAR antibody encephalitis. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balu R, McCracken L, Lancaster E, Graus F, Dalmau J, Titulaer MJ. A score that predicts 1-year functional status in patients with anti-NMDA receptor encephalitis. Neurology. 2019;92(3):e244–e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loerinc LB, Blackwell L, Howarth R, Gombolay G. Evaluation of the anti-N-methyl-D-aspartate receptor encephalitis one-year functional status score in predicting functional outcomes in pediatric patients with anti-N-methyl-D-aspartate receptor encephalitis. Pediatr Neurol. 2021;124:21-23. [DOI] [PubMed] [Google Scholar]
- 9.Zhang T, Duan Y, Ye J, et al. Brain MRI characteristics of patients with anti-N-methyl-D-aspartate receptor encephalitis and their associations with 2-year clinical outcome. AJNR Am J Neuroradiol. 2018;39(5):824-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashyap N, Morris M, Loerinc LB, et al. The neutrophil-to-lymphocyte ratio is associated with intubation in pediatric anti-NMDA receptor encephalitis: a retrospective study. J Neuroimmunol. 2022;370:577931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin J, Elkins K, Bhalla S, et al. Electroencephalography characteristics to predict one-year outcomes in pediatric anti-NMDA receptor encephalitis. Epilepsy Res. 2021;178:106787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Titulaer MJ, Hoftberger R, Iizuka T, et al. Overlapping demyelinating syndromes and anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2014;75(3):411-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Bruijn M, Aarsen FK, van Oosterhout MP, et al. Long-term neuropsychological outcome following pediatric anti-NMDAR encephalitis. Neurology. 2018;90(22):e1997–e2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heine J, Kopp UA, Klag J, Ploner CJ, Pruss H, Finke C. Long-term cognitive outcome in anti-N-Methyl-D-Aspartate receptor encephalitis. Ann Neurol. 2021;90(6):949-961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available to qualified researchers based on reasonable request.