Abstract
Agents that elevate intracellular cyclic AMP (cAMP) levels promote neuronal survival in a manner independent of neurotrophic factors. Inhibitors of phosphatidylinositol 3 kinase and dominant-inactive mutants of the protein kinase Akt do not block the survival effects of cAMP, suggesting that another signaling pathway is involved. In this report, we demonstrate that elevation of intracellular cAMP levels in rat cerebellar granule neurons leads to phosphorylation and inhibition of glycogen synthase kinase 3β (GSK-3β). The increased phosphorylation of GSK-3β by protein kinase A (PKA) occurs at serine 9, the same site phosphorylated by Akt. Purified PKA is able to phosphorylate recombinant GSK-3β in vitro. Inhibitors of GSK-3 block apoptosis in these neurons, and transfection of neurons with a GSK-3β mutant that cannot be phosphorylated interferes with the prosurvival effects of cAMP. These data suggest that activated PKA directly phosphorylates GSK-3β and inhibits its apoptotic activity in neurons.
Neurons require continuous exposure to extracellular trophic factors for survival, and those that fail to receive sufficient trophic factor support undergo apoptotic cell death (34). Among the extracellular factors shown to influence neuronal survival are the neurotrophins, which include nerve growth factor, brain-derived neurotrophic factor, neurotrophin 3 and neurotrophin 4, the fibroblast growth factors, ciliary growth factor, insulin, and insulin-like growth factors (2, 31). Agents that elevate intracellular cyclic AMP (cAMP) also promote neuronal survival in a manner independent of neurotrophic factors (24, 38). Substantial progress has been made over the last several years in delineating signal transduction pathways that mediate trophic factor-induced cell survival. Less is known about the survival pathways activated by cAMP in neurons.
Recent reports have established a key role for phosphatidylinositol (PI)-3 kinase in regulating trophic factor-dependent survival of neurons (18, 22, 23). The Akt protein kinase (also termed proteins kinase B [PKB] and Rac) has been implicated as the transducer of PI-3 kinase-dependent survival signals generated by serum and certain growth factors (6, 22, 28). In response to PI-3 kinase activation, Akt binds to phosphorylated membrane lipids via its pleckstrin homology domain and is phosphorylated at threonine 308 and serine 473 (17). Phosphorylation of Akt at these two sites leads to its activation and the propagation of an antiapoptotic signal. Several downstream targets of Akt implicated in cell survival include the Bcl-2 family member BAD (13), caspase 9 (8), and FKHRL1, a member of the Forkhead family of transcription factors (5). Another Akt substrate recently implicated in cell fate decisions is glycogen synthase kinase 3 (GSK-3). Mammalian GSK-3 exists as two isoforms termed α (51 kDa) and β (47 kDa), each encoded by a distinct gene (45–47). The GSK-3 isoforms share 85% homology at the amino acid level and are ubiquitously expressed (45–47). Although GSK-3 was originally identified as a kinase that phosphorylates glycogen synthase, subsequent studies have revealed that GSK-3 has a broader role in the cell (11, 45–47). It phosphorylates a number of substrates not involved in glycogen metabolism, including the initiation factor eIF2B (44), the microtubule-associated protein tau (26), and the transcription factors CREB (21), c-myc (37), c-jun (4), and β-catenin (40). Recently, GSK-3β was shown by complementation to be the mammalian homologue of the shaggy gene from Drosophila melanogaster, which regulates cell fate decisions during axial patterning and neurogenesis (45). GSK-3β homologues in Dictyostelium (25) and Xenopus (27) also appear to regulate cell fate in development. The role of GSK-3β in mammalian cell development is less clear, although recent evidence suggests that it may be a downstream target of the PI-3 kinase-Akt antiapoptotic signaling pathway. Overexpression of a dominant-negative mutant of GSK-3β prevents apoptosis following inhibition of PI-3 kinase, whereas catalytically active GSK-3β induces apoptosis of both rat-1 and PC12 cells (36).
One possible mechanism by which cAMP could promote survival is by activating the PI-3 kinase-Akt pathway. Indeed, agents that elevate intracellular cAMP levels stimulate the activity of Akt when the enzyme is overexpressed in 292 cells (20, 39). The activation of Akt by cAMP is independent of PI-3 kinase activity, does not require the pleckstrin homology domain of Akt, and is dependent on T308 phosphorylation but not S473 phosphorylation. In cerebellar granule neurons, inhibition of PI-3 kinase completely blocked the survival effects of insulin-like growth factor I (IGF-I) but had no effect on cAMP-mediated survival (33). Likewise, in sympathetic ganglion neurons, expression of either a dominant-negative PI-3 kinase or a dominant-negative Akt blocked survival mediated by depolarization but not by cAMP (12). The inability of PI-3 kinase inhibitors or dominant-negative Akt mutants to block the prosurvival effects of cAMP in both neuronal types suggests that cAMP promotes neuronal survival by mechanism independent of PI-3 kinase-Akt activation. In this report, we show that elevation of intracellular cAMP levels in rat cerebellar granule neurons leads to phosphorylation and inhibition of GSK-3β independent of Akt activation. The increased phosphorylation of GSK-3β by PKA occurs at serine 9, the site phosphorylated by Akt. Inhibitors of GSK-3 block apoptosis in these cells, and transfection of neurons with a GSK-3 mutant that cannot be phosphorylated interferes with the prosurvival effects of cAMP.
MATERIALS AND METHODS
Antibodies.
The monoclonal anti-GSK-3β antibody was from Transduction Laboratories (Lexington, Ky.). The polyclonal phosphorus-specific GSK-3β (Ser9) antibody was obtained from Calbiochem (La Jolla, Calif.). The polyclonal phosphorus-specific antibodies for Akt (Ser473 and Thr308), p90 ribosomal S6 protein kinase (RSK) (Ser381), GSK-3α/β (Ser21 and Ser9), GSK-3β (Ser9), and mitogen-activated protein kinase (Thr202 and Tyr204), the polyclonal phosphorus-independent Akt antibody, the immobilized Akt antibody, and extracellular signal-regulated kinase (ERK) antibodies were from New England BioLabs (Beverly, Mass.). The polyclonal anti-β-galactosidase (β-Gal) antibody was purchased from 5′-3′ Inc. (Boulder, Colo.). Cy3-conjugated goat antibody to rabbit immunoglobulin G (IgG) and fluorescein-conjugated goat antibody to mouse IgG were purchased from Chemicon International, Inc. (Temecula, Calif.). The monoclonal antibody (clone 12CA5) against the hemagglutinin (HA) epitope was obtained from Boehringer Mannheim (Indianapolis, Ind.).
Materials.
The PKA inhibitor H-89 dihydrochloride, the cell-permeative myristoylated PKI inhibitor, the cAMP-elevating agents forskolin and chlorophenylthiol (CPT)-cAMP, the PKC inhibitor bisindolylmaleimide, the PI-3 kinase inhibitor wortmannin, and the MEK inhibitor PD98059 were from Calbiochem. The recombinant GSK-3α and GSK-3β proteins were from New England BioLabs. The PKA catalytic subunit purified from bovine heart was from Promega (Madison, Wis.). Valproic acid and lithium chloride were purchased from Sigma (St. Louis, Mo.). Plasmid purification kits were from Qiagen (Valencia, Calif.), and calcium phosphate transfection kits were from Promega. The Akt kinase assay kit was from New England BioLabs. [γ-32P]ATP (3,000 Ci/mmol) was purchased from Amersham Pharmacia Biotechnology and l-pyruvate kinase was purchased from Sigma. The phosphoglycogen synthase peptide 2 was obtained from Upstate Biotechnology.
Neuronal culture and induction of apoptosis.
Rat cerebellar granule neurons were prepared from 7- to 8-day-old Sprague-Dawley rat pups (15 to 19 g) as described previously (35). Briefly, neurons were dissociated from freshly dissected cerebella by mechanical disruption in the presence of trypsin and DNase and were then plated on poly-l-lysine-coated Nunc culture plates (Fisher, Pittsburgh, Pa.). Cells were seeded at a density of 2.0 × 106/ml in basal modified Eagle medium (BME) containing 10% fetal bovine serum, 25 mM KCl, 2 mM glutamine, and penicillin (10 U/ml)-streptomycin (10 μg/ml). Cytosine arabinoside (10 μM) was added to the culture medium 24 h after plating to limit the growth of nonneuronal cells. With this protocol, 95 to 99% of the cultured cells were granule neurons. After 7 or 8 days in culture, apoptosis was induced by removing serum and reducing the extracellular potassium concentration from 25 to 5 mM. Neurons were rinsed two times in serum-free BME containing 5 mM KCl and then maintained in the same medium in the presence or absence of various drugs. Control cultures were treated identically but were maintained in serum-free medium supplemented with 25 mM KCl. When inhibitors were used for signaling assays, cells were treated with the inhibitor 30 min before the addition of the stimulus. Cells that did not receive drugs received a control vehicle (dimethyl sulfoxide [DMSO] for forskolin, H-89 dihydrochloride, wortmannin, and PD98059 and water for CPT-cAMP, valproic acid, and lithium chloride). The final concentration of DMSO was less than 0.1%.
Immunoblotting assay.
Cerebellar granule neurons were cultured on poly-l-lysine-coated 35-mm plates for 7 or 8 days. After stimulation with drugs (the times are indicated in the figures), neurons were lysed by adding sodium dodecyl sulfate (SDS) sample buffer (62.5 mM Tris-HCl [pH 6.8], 2% [wt/vol] SDS, 10% glycerol, 50 mM dithiothreitol [DTT], 0.1% [wt/vol] bromphenol blue) and were immediately scraped off the plate. Samples were resolved by SDS–10% polyacrylamide gel electrophoresis and transferred to Hybond-P membranes (polyvinylidene difluoride; Amersham, Arlington Heights, Ill.). Membranes were incubated for an hour in a blocking buffer containing 5% (wt/vol) nonfat dry milk in TBST (10 mM Tris-HCl, 140 mM NaCl [pH 7.4], 0.1% Tween 20) and then incubated overnight at 4°C with appropriate primary antibody diluted (1:1,000) in TBST containing 5% bovine serum albumin (BSA). The membranes were washed for 15 min in TBST and then incubated at room temperature for 60 min with horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibody diluted 1:2,000 in TBST containing 5% nonfat dry milk. After being washed extensively for 30 min in TBST, membranes were processed for 1 min using an ECL chemiluminescent substrate kit (Amersham) and exposed to autoradiographic film (Kodak, Rochester, N.Y.). Quantitation was performed using Bio-Rad Quantity One software.
Calcium phosphate transfection of neurons.
Cerebellar granule neurons were transfected using a calcium phosphate coprecipitation method (34). All plasmids were prepared and purified with a Qiagen Plasmid Maxi kit (catalog no. 12163) according to the manufacturer's instructions. Briefly, neurons were cultured for 5 to 6 days in 24-well plates or 35-mm dishes. The DNA-calcium phosphate precipitate was prepared by mixing 1 volume of DNA in 250 mM CaCl2 with an equal volume of 2× HBS (50 mM HEPES, 280 mM NaCl, 1.5 mM Na2HPO4 [pH 7.1]). Plasmid pcDNA3, pCMV5, pAkt (K179M), pcDNA3 GSK-3β (wt), pcDNA3 GSK-3β (S9A), or pcDNA GSK-3β (KI) contained in the precipitate was at a final concentration of 40 μg/ml together with 8 μg of an expression vector encoding β-Gal (pCMV-βgal) per ml to allow detection of the transfected cells. The calcium phosphate-DNA precipitate was incubated at room temperature for 30 min before it was added to the cultures. The conditioned culture medium was removed and saved. The cultures were washed two times with BME, and then 1.5 ml of transfection medium (BME, no addition of glutamine and antibiotics, 37°C, pH 7.4) was added to cultures, immediately followed by the addition of the calcium phosphate-DNA precipitate. Plasmids were added to the transfection medium at a final concentration of 4 to 5 μg/ml. Plates were incubated (37°C, 5% CO2) for 30 min, and then the transfection medium was aspirated. After two washes with fresh transfection medium, the saved conditioned medium was added back to the cultures. Transfection efficiency was assessed by determining the percentage of cells expressing β-Gal by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining or immunostaining (24 to 48 h later). Glycerol or DMSO shock did not increase transfection efficiency but did result in cell damage. Experimental treatments were initiated 24 h after transfection.
Immunostaining.
Neurons were stained 2 days after transfection to identify cells expressing the proteins encoded by the transgenes. Cultures were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min at room temperature and permeabilized with 0.1% Triton X-100. The fixed cells were incubated at room temperature for 15 min in 5% goat serum in TBS-Triton (10 mM Tris-HCl, 140 mM NaCl [pH 7.4], 0.1% Triton X-100) to block nonspecific interactions of the antibodies and then incubated with the appropriate primary antibody diluted in 3% BSA in Tris-buffered saline overnight at 4°C. After being washed three times with TBS-Triton for 15 min, the cells were incubated at room temperature for 60 min with Cy3- or fluorescein-conjugated secondary antibodies in Tris-buffered saline containing 3% BSA. Expression of β-Gal was detected by immunostaining with a polyclonal antibody to β-Gal (1:500 dilution) followed by a Cy3-conjugated goat antibody to rabbit IgG (1:500). Neurons transfected with the HA epitope-tagged GSK-3β were immunostained with a monoclonal antibody to HA (1:500) followed by a fluorescein-conjugated goat antibody to mouse IgG (1:500). Stained cells were visualized by digital deconvolution fluorescence microscopy to confirm that β-Gal was coexpressed with wild-type (wt) GSK-3β, S9A GSK-3β, or kinase-inactive (KI) GSK-3β. To visualize the nuclei of transfected neurons, we included the DNA dye Hoechst 33258 (5.0 μg/ml) in the wash after the secondary antibody incubation.
In vitro phosphorylation of GSK-3 by PKA.
The phosphorylation reaction was performed in 50 μl of kinase buffer (50 mM Tris [pH 7.2], 10 mM MgCl2, 1 mM DTT) containing 4 U of PKA catalytic subunit (bovine heart; Promega), 0.3 μg of GSK-3β protein, and 100 μM ATP. Reactions were carried out in the absence and presence of the PKA inhibitor H-89 (5 μM) or the PKI inhibitor (2.5 μM). The phosphorylation reaction was allowed to proceed for 30 min at 30°C and was stopped by adding 3× SDS sample buffer. The phosphorylation of GSK-3β was measured by Western blotting using a GSK-3β (Ser9) antibody. In experiments where the Km and Vmax were determined, 50 μM [γ-32P]ATP (5 μCi/nmol) was added to the kinase reaction mixture. At the end of the phosphorylation reactions, these samples were solubilized in Laemmli's sample buffer and analyzed by SDS-polyacrylamide gel electrophoresis. Gels were dried and subjected to autoradiography. The incorporation of phosphate into GSK-3β and l-pyruvate kinase (a known substrate of GSK-3) was determined by Cerenkov counting of excised SDS-polyacrylamide gel slices.
Assay of GSK-3β.
After stimulation with forskolin or IGF-I, neurons were washed with cold PBS, and neuronal extracts were prepared in cell lysis buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 5 mM β-glycerolphosphate, 1 mM Na3VO4, 1 μg of leupeptin per ml, 1 mM phenylmethylsulfonyl fluoride, 1 μM microcystin) for 15 min at 4°C. After brief sonication, the lysates were clarified by centrifugation at 15,000 × g for 10 min at 4°C, and GSK-3β from 200 μg of cell extract was immunoprecipitated with 1.0 μg of GSK-3β antibody for 2 h at 4°C with rotation. Protein G Plus/Protein A-agarose (20 μl of a 50% suspension) was then added, and the incubation was continued for 1 h at 4°C with rotation. Immune complexes were recovered by centrifugation at 4°C and were washed three times with extraction buffer and twice with kinase buffer. Kinase activity of the immunoprecipitated GSK-3 was assayed in a total volume of 40 μl containing 25 mM sodium glycerophosphate, 20 mM Tris-HCl [pH 7.4], 10 mM MgCl2, 5 mM DTT, 20 μM phosphoglycogen synthase peptide 2, and 50 μM [γ-32P]ATP (1 μCi). After 10 min of incubation at 30°C, reaction mixtures were centrifuged for 1 min, and 20 μl of the supernatant was spotted onto Whatman P81 phosphocellulose paper. Filters were washed in four changes of 175 mM phosphoric acid for a total of 20 min, rinsed in acetone, and dried, and the radioactivity was determined by Cerenkov counting. Background values obtained from reactions lacking cell lysate were subtracted from all values.
In vitro Akt kinase assay.
After stimulation with the reagents indicated below, neuronal extracts were prepared by solubilizing the neurons in cell lysis buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 5 mM β-glycerolphosphate, 1 mM Na3VO4, 1 μg of leupeptin per ml, 1 mM phenylmethylsulfonyl fluoride, 1 μM microcystin) for 15 min at 4°C. After brief sonication, the lysates were clarified by centrifugation at 15,000 × g for 10 min at 4°C, and Akt from 200 μl of cell extract was immunoprecipitated with 20 μl of immobilized Akt antibody cross-linked to agarose hydrazide beads. After the beads were washed three times with cell lysis buffer and three times with kinase buffer (25 mM Tris-HCl, 10 mM MgCl2, 1 mM DTT [pH 7.4]), kinase activity was assayed with GSK-3α fusion protein as a substrate (1 μg) in kinase buffer containing 100 μM ATP and 2.5 μM PKI inhibitor. The phosphorylation reaction was allowed to proceed for 30 min at 30°C and stopped by adding 3× SDS sample buffer. Phosphorylation of GSK-3α was measured by Western blotting using phospho-GSK-3α/β (Ser21 and Ser9) antibody.
Quantitation of apoptosis by nuclear morphological changes.
Cerebellar granule neurons were cultured in 35-mm culture dishes and 24-well plates as described above. After removal of the medium, the neurons were rinsed once with cold PBS, pH 7.2, fixed for 10 min with 4% paraformaldehyde in PBS at 4°C, washed with distilled water, and dried at room temperature. Cells were stained with Hoechst 33258 (5 μg/ml) for 5 min, washed, and dried. Apoptosis was quantified by scoring the percentage of cells in the adherent cell population with condensed or fragmented nuclei. To obtain unbiased counting, cells were scored without knowledge of their prior treatment.
RESULTS
Forskolin and CPT-cAMP stimulate GSK-3β phosphorylation.
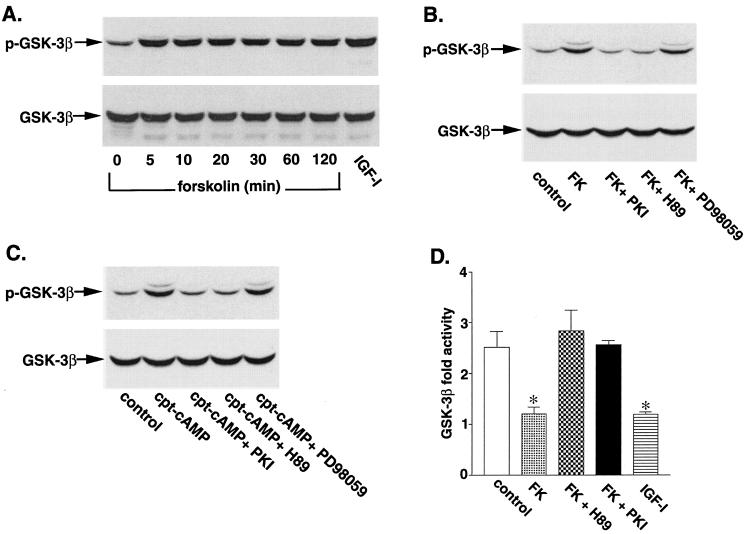
Cultures of newborn rat cerebellar granule neurons, the interneurons of the cerebellum, provide a good model for investigating signaling pathways that regulate neuronal apoptosis because of the high degree of cellular homogeneity. These neurons survive and differentiate in vitro in the presence of serum and depolarizing concentrations of KCl (25 mM). If the medium is changed to serum-free medium containing 5 mM KCl, the neurons undergo apoptotic cell death. Previous studies have shown that apoptosis of cerebellar granule neurons is inhibited by a variety of molecules which raise cAMP levels (7, 15, 33, 42). To investigate the potential role of GSK-3β in mediating the protective effects of cAMP, we examined whether agents that elevate cAMP levels regulate the phosphorylation state of GSK-3β. After 7 days in culture, rat cerebellar granule neurons were incubated in serum-free medium containing 5 mM KCl for 4 h and then incubated with forskolin (10 μM), an activator of adenylate cyclase, to elevate intracellular cAMP levels. The neurons were then solubilized, and the phosphorylation state of GSK-3β was measured by Western blotting using phosphospecific GSK-3β (Ser9) antibodies. Forskolin rapidly increased the phosphorylation of GSK-3β (47 kDa) on serine 9 (Fig. 1A). The extent of GSK-3β phosphorylation by forskolin was similar to that obtained with a maximal concentration of IGF-I (50 ng/ml). The phosphorylation of GSK-3β by forskolin was completely blocked when neurons were preincubated for 30 min with the cell-permeative myristoylated PKI inhibitor (25 μM) and with H-89 (10 μM), a selective inhibitor of PKA (Fig. 1B and C). Preincubation of neurons with PD98059, a MEK inhibitor that blocks the ERK pathway, had no significant effect on the phosphorylation of GSK-3β by forskolin. The ability of forskolin to stimulate phosphorylation of GSK-3β was mimicked by incubating the neurons with a cell permeative cAMP analog (CPT-cAMP) (Fig. 1C). As seen with forskolin, the phosphorylation of GSK-3β by CPT-cAMP was blocked by the PKI inhibitor and H-89 but not by PD98059 (Fig. 1C).
FIG. 1.
Phosphorylation and inhibition of GSK-3β by forskolin and CPT-cAMP. (A) Cerebellar granule neurons were cultured for 7 days, washed twice with BME, and then placed in serum-free medium containing 5 mM KCl. Two hours later, the cells were either left untreated or treated with 10 μM forskolin for the indicated times. Some neurons were incubated with IGF-I (50 ng/ml) for 30 min as a positive control. Cell lysates were analyzed by immunoblotting with a phosphospecific GSK-3β (Ser9) antibody. (B) Neurons were pretreated for 30 min in the absence or presence of 25 μM cell-permeative PKI inhibitor, 10 μM H-89, or 30 μM PD98059 prior to incubation with 10 μM forskolin (FK) for 30 min. Cell lysates were immunoblotted with anti-GSK-3β (Ser9) antibody. (C) Neurons were pretreated for 30 min in the absence or presence of 25 μM cell-permeative PKI inhibitor, 10 μM H-89, or 30 μM PD98059 prior to incubation with 30 μM CPT-cAMP (cpt-cAMP) for 30 min. Results shown are representative of at least three experiments. (D) After serum and 25 mM KCl starvation in 5 mM KCl medium, neurons were incubated for 30 min with 10 μM forskolin in the presence or absence of 10 μM H-89, 25 μM PKI inhibitor, or IGF-I (50 ng/ml). After incubation, the neurons were lysed, GSK-3β was immunoprecipitated, and its activity was determined as described in Materials and Methods. Control neurons were washed similarly and then placed in serum-containing conditioned medium. The results are expressed as fold activity of control neurons and are mean values ± SEMs from three experiments. *, statistical significance according to Student's t test (P < 0.05 versus the value for 5 mM KCl).
Phosphorylation of GSK-3β at serine 9 is known to inhibit enzyme activity. To confirm this, in vitro kinase assays were carried out following immunoprecipitation of GSK-3β using phosphoglycogen synthase peptide 2 as the substrate (Fig. 1D). Treatment of neurons with forskolin led to about a 50% decrease in GSK-3β activity, similar to the decrease in activity observed with IGF-I. The cell-permeative PKI inhibitor and H-89 blocked the forskolin-induced decrease in GSK-3β activity.
Phosphorylation of GSK-3β by forskolin is not mediated by the ERK pathway.
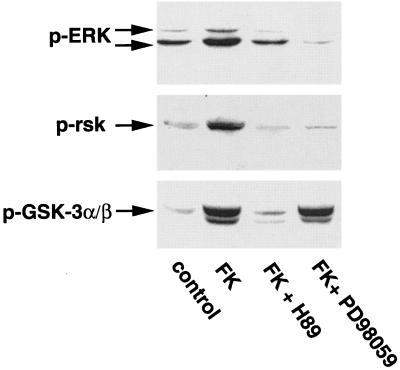
The inability of the MEK inhibitor PD98059 to block phosphorylation of GSK-3β by forskolin suggested that the ERK pathway was not involved in the phosphorylation event. However, cAMP has been previously shown to activate the ERK pathway (19), and recent data have indicated that RSK can directly phosphorylate GSK-3β (41). To examine the potential relationship between activation of the ERK pathway and phosphorylation of GSK-3β by forskolin, we first determined whether forskolin activates the ERK pathway in rat cerebellar granule neurons. Incubation of neurons with forskolin (10 μM) for 30 min led to increased phosphorylation of ERK on threonine 202 and tyrosine 204 (Fig. 2, top panel) and RSK on serine 381 (Fig. 2, middle panel). The phosphorylation of ERK and RSK by forskolin was completely inhibited by the MEK inhibitor PD98059 (Fig. 2, top and middle panels). However, although PD98059 completely blocked the activation of ERK and RSK by forskolin, it had no effect on the phosphorylation of GSK-3β by forskolin (Fig. 2, bottom panel). As expected, H-89 inhibited the phosphorylation of ERK and GSK-3β by forskolin. Thus, although the activation of PKA stimulates the ERK pathway in neurons, this pathway does not lead to GSK-3β phosphorylation in cerebellar granule neurons.
FIG. 2.
Phosphorylation of GSK-3 by cAMP-PKA does not require activation of the ERK pathway. Neurons were treated as described for Fig. 1A and then pretreated for 30 min in the absence or presence of 10 μM H-89 or 30 μM PD98059 prior to incubation with 10 μM forskolin (FK) for 30 min. Cell lysates were immunoblotted with phosphospecific antibodies against ERK (Thr202 and Tyr204) (top), p90 RSK (Ser381) (middle), and GSK-3α/β (Ser21 and Ser9) (bottom). Results shown are representative of at least three experiments.
Phosphorylation of GSK-3β by forskolin is not mediated by the Akt pathway.
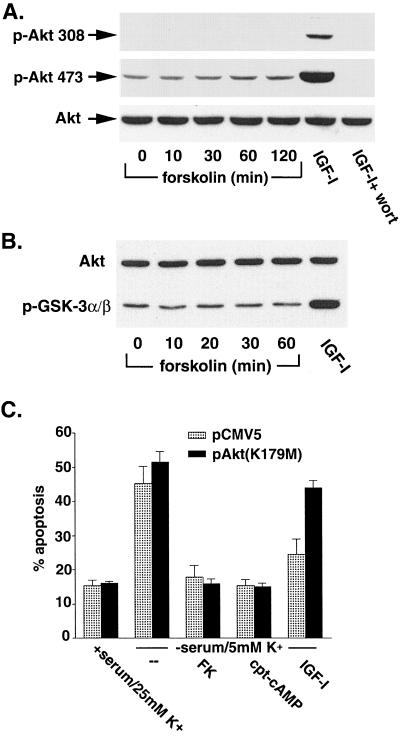
To investigate the potential role of the Akt pathway in the regulation of GSK-3β by forskolin, we examined whether forskolin could activate Akt in the cerebellar granule neurons. Neurons were incubated with forskolin, and the phosphorylation state of Akt was determined using phosphospecific (Ser473) antibodies (Fig. 3A). Forskolin had no effect on Akt phosphorylation at serine 473. In contrast, IGF-I (50 ng/ml) stimulated Akt phosphorylation at this site, and the increased phosphorylation of Akt by IGF-I was blocked by wortmannin. Similar results were obtained when phosphospecific (Ser308) antibodies were used (Fig. 3A), indicating that forskolin was unable to phosphorylate Akt at the two critical residues that activate the kinase. The activity of Akt was directly measured after immunoprecipitation with phosphorylation-independent Akt antibodies using a GSK-3α fusion protein as the substrate. Phosphorylation of GSK-3α was then detected by Western blotting using phosphospecific GSK-3α/β (Ser21 and Ser9) antibodies (Fig. 3B). Consistent with the data obtained with phosphospecific Akt antibodies, forskolin had no effect on Akt activity. However, in the control samples, IGF-I markedly increased the activity of Akt.
FIG. 3.
Phosphorylation of GSK-3 by cAMP-PKA does not require activation of Akt. (A) Cerebellar granule neurons were cultured for 7 days, washed twice, and placed in serum-free medium containing 5 mM KCl. Two hours later, the cells were either left untreated or treated with 10 μM forskolin for the indicated times. Some neurons were incubated with IGF-I (50 ng/ml) in the absence or presence of wortmannin (wort) (100 nM) for 30 min as positive controls. Cell lysates were immunoblotted with antibodies to phospho-S473 Akt and phospho-T308 Akt. The membrane was then stripped and reprobed with antibodies to phosphorylation-independent Akt. (B) Neurons were treated as described for panel A. Akt was immunoprecipitated from cell lysates with an immobilized Akt antibody, and kinase activity was determined by an in vitro kinase assay using 1 μg of recombinant GSK-3α fusion protein as the substrate. Phosphorylation of GSK-3α was detected by immunoblotting with phosphospecific GSK-3α/β (Ser21 and Ser9) antibody (bottom). The membranes were stripped and reprobed with the antibody to phosphorylation-independent Akt (top). (C) Neurons were transfected with the indicated expression vectors (along with pCMV-β-Gal), and 24 h later they were placed in medium containing 25 mM KCl (K+) plus serum or in deprivation medium (5 mM KCl, no serum) in the presence or absence of 10 μM forskolin (FK), 500 μM CPT-cAMP, or 50 ng/ml of IGF-I per ml. After 20 h, the neurons were fixed and costained with an antibody to β-Gal and Hoechst 33258. Apoptosis was quantified by scoring the percentage of transfected neurons in the adherent cell population with condensed or fragmented nuclei. Data are from three experiments and are the means ± SEMs.
Additional experiments were done to examine whether a dominant-inactive Akt construct, pAkt (K179M), could block the protective effects of cAMP on cell survival (Fig. 3C). As previously reported, dominant-inactive Akt had no effect on the ability of forskolin or CPT-cAMP to protect rat cerebellar granule neurons from apoptosis induced by lowering extracellular potassium. On the other hand, dominant-inactive Akt significantly blocked the ability of IGF-I to protect these neurons from the same apoptotic stimulus.
Purified PKA can phosphorylate GSK-3β in vitro.
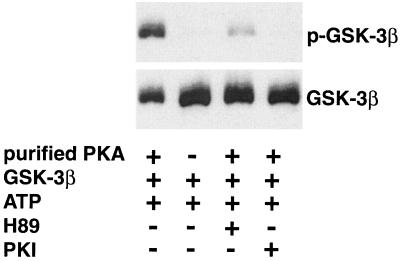
Since forskolin did not appear to phosphorylate GSK-3β through the protein kinases known to directly phosphorylate GSK-3, we questioned whether PKA itself could directly phosphorylate GSK-3β. To address this question, an in vitro kinase assay was performed using purified PKA and recombinant GSK-3β. Phosphorylation of GSK-3β was detected by Western blotting with phosphospecific antibodies (Fig. 4). In the presence of ATP, the purified PKA catalytic subunit phosphorylated GSK-3β. The site phosphorylated by PKA in GSK-3β (Ser9) resides in the Akt (or PKB) consensus site. These data suggest that the Akt consensus site in GSK-3β (RTTSF) is similar enough to the PKA consensus site (RRXSF) for PKA phosphorylation. There was no detectable phosphorylation in the absence of purified PKA. The H-89 PKA inhibitor and the PKI inhibitor significantly blocked the in vitro phosphorylation of recombinant GSK-3β. Assays were carried out to determine the Km and Vmax for human recombinant GSK-3β and a known substrate, l-pyruvate kinase. The Km and Vmax values for GSK-3β and l-pyruvate kinase were 7.24 μM and 7.23 μM/min/mg of protein and 19.18 μM and 30.48 μM/min/mg of protein, respectively. These values were determined by nonlinear regression analysis of data plotted by Michaelis-Menton kinetics. The relatively low Km for the in vitro phosphorylation of GSK-3β raises the possibility that PKA directly phosphorylates GSK-3β in vivo. To date, we have been unable to demonstrate a direct interaction between PKA and GSK-3β in neurons by coimmunoprecipitation (data not shown). This implies that the interaction between PKA and GSK-3β is not strong enough to detect by immunoprecipitation or that they do not interact in vivo because there is another kinase downstream of PKA that directly phosphorylates GSK-3β.
FIG. 4.
In vitro phosphorylation of GSK-3 by purified PKA. The phosphorylation reaction mixture consisted of 4 U of purified PKA catalytic subunit, 1 μg of GSK-3β fusion protein, and 100 μM ATP. Reactions were carried out in the absence and presence of the PKA inhibitor H-89 (5 μM) and the PKI inhibitor (2.5 μM). The phosphorylation reaction was allowed to proceed for 30 min at 30°C and was stopped by adding 3× SDS sample buffer. The phosphorylation of GSK-3β (top) was measured by Western blotting with phospho-GSK-3β (Ser9) antibody. The membrane was stripped and reprobed with a monoclonal phosphorylation-independent antibody to GSK-3β (bottom).
Inhibitors of GSK-3β protect neurons from apoptosis.
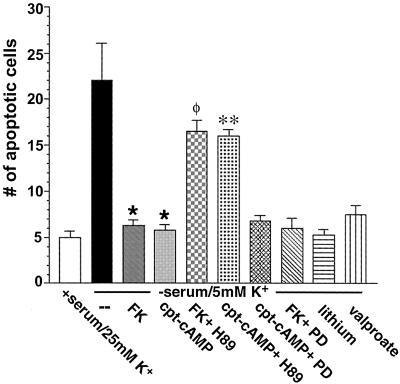
To determine the role of GSK-3β in the neuroprotective effects of cAMP we first examined the effects of various inhibitors of GSK-3β on apoptosis in rat cerebellar granule neurons. In agreement with previous studies, forskolin and CPT-cAMP markedly inhibited apoptosis induced by withdrawal of serum and lowering of the KCl concentration (Fig. 5). If cells are preincubated with PKA inhibitor H-89 prior to the addition of forskolin or CPT-cAMP, the protective effects are blocked. Consistent with results demonstrating that the ERK pathway does not mediate the phosphorylation of GSK-3β by forskolin or CPT-cAMP, the MEK inhibitor PD98059 did not influence the ability of forskolin and CPT-cAMP to protect neurons from apoptosis. Lithium and valproate, two direct inhibitors of GSK-3β (41, 42), blocked apoptosis to the same extent as forskolin and CPT-cAMP. These data suggest that GSK-3β mediates the apoptotic effects induced by serum withdrawal and that inhibition of GSK-3β by phosphorylation protects neurons from apoptosis.
FIG. 5.
Effects of various agents and inhibitors on apoptosis of rat cerebellar granule neurons. Cerebellar granule neurons were cultured for 7 days, washed twice with BME, and placed in serum-free medium containing 5 mM KCl in the absence or presence of forskolin (FK) (10 μM), CPT-cAMP (500 μM), forskolin plus H-89 (10 μM), CPT-cAMP plus H-89 (10 μM), CPT-cAMP plus PD98059 (PD) (30 μM), forskolin plus PD98059 (30 μM), lithium (15 mM), or valproate (15 mM). After 24 h neurons were stained with Hoechst 33258 (5 μg/ml) for 5 min, and apoptosis was quantified by scoring the percentage of neurons in the adherent cell population with condensed or fragmented nuclei. To obtain unbiased counting, cells were scored without knowledge of their prior treatment. Data are presented as means ± SEMs (n = 4). ∗, P < 0.001 versus apoptotic (serum-free, 5 mM KCl) medium; φ, P < 0.001 versus apoptotic medium plus forskolin; ∗∗, P < 0.001 versus apoptotic medium plus CPT-cAMP (Student's t test).
Transfection of cerebellar granule neurons with wt-GSK-3β, a KI GSK-3β mutant, and a Ser9→Ala9 GSK-3β mutant.
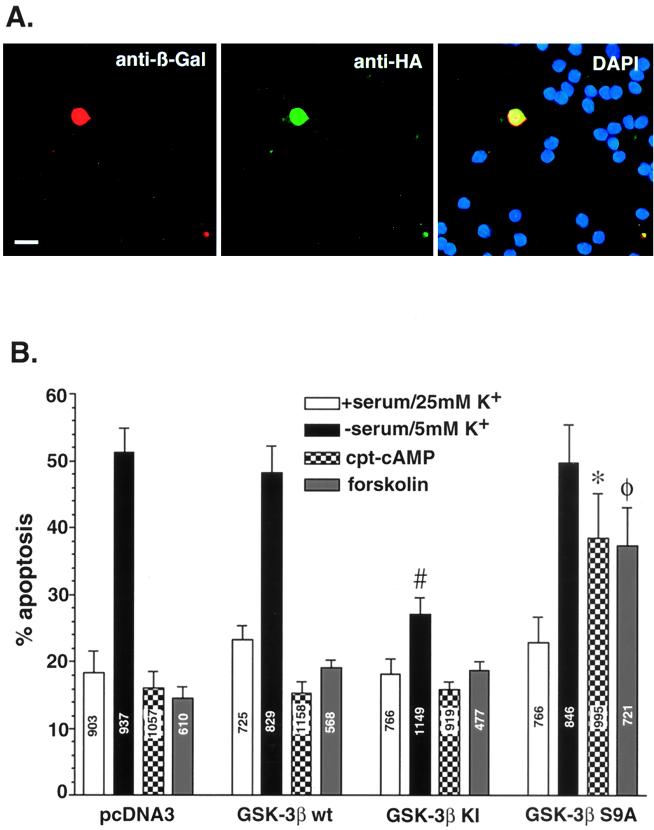
To confirm the results from the inhibitor studies described above, neurons were transfected with GSK-3β plasmids to determine whether a kinase-dead mutant of GSK-3β would block apoptosis induced in this model system. Transfections were also carried out with wt GSK-3β and a mutant of GSK-3β that cannot be phosphorylated at serine 9. In all of the transfection experiments, neurons were cotransfected with β-Gal as an indicator of transfection. The cultures were coimmunostained with rabbit antibodies against β-Gal (secondary antibody conjugated to Cy3) and mouse antibodies against HA, the epitope tag on the GSK constructs (secondary antibody conjugated to fluorescein isothiocyanate). DAPI (5′, 6′-diamidino-2-phenylindole) stain was included in the final wash of the cultures to determine nuclear morphology (Fig. 6A). Costained neurons were scored as apoptotic if they had one or more lobes of condensed chromatin. Seven hundred to 1,200 neurons were counted in each experimental group. The results from these experiments are shown in Fig. 6B. In cells transfected with the control vector, 18% ± 3% (mean ± standard error of the mean [SEM]) of the total neurons were apoptotic in the presence of serum. Serum withdrawal and lowering of the KCl concentration led to a threefold increase in the percentage of apoptotic neurons (51% ± 3%). Incubation of the neurons with CPT-cAMP or forskolin at the time of serum withdrawal prevented the increase in apoptosis. Similar data were obtained when neurons were transfected with wt GSK-3β. In contrast, transfection of neurons with KI GSK-3β blunted the increase in apoptosis induced by serum and KCl withdrawal (27% ± 4% versus 51% ± 3%) but had no effect on basal apoptosis or the ability of CPT-cAMP and forskolin to protect against apoptosis. On the other hand, transfection of neurons with a GSK-3β mutant that cannot be phosphorylated at serine 9 interfered with the ability of cAMP and forskolin to protect neurons from apoptosis but had little effect on basal apoptosis.
FIG. 6.
Transfection of cerebellar granule neurons with wt-GSK-3β, a KI GSK-3β mutant, and a Ser9→Ala9 GSK-3β mutant. Neurons were cotransfected with the control vector, wt GSK-3β, (KI) GSK-3β, (S9A) GSK-3β along with pCMV-β-Gal. One day after transfection, the neurons were placed in complete medium (serum, 25 mM KCl) or switched to serum-free medium containing 5 mM KCl, with or without cAMP (500 μM) or forskolin (10 μM). After 24 h the transfected neurons were fixed and immunostained with an antibody to β-Gal (Cy3-coupled secondary antibody) and 12CA5 antibody to HA (fluorescein isothiocyanate-coupled secondary antibody). To reveal nuclear morphology, neurons were also stained with DAPI. (A) Demonstration of the triple-staining method in neurons grown in serum and 25 mM KCl. Bar, 10 μm. (B) Effects of GSK-3β constructs on neuronal survival. The β-Gal-positive neurons were scored as healthy or apoptotic as described for Fig. 3C. Data are presented as means ± SEMs (n = 4). #, P < 0.001 versus pcFNA3 in pcDNA3 in apoptotic (serum-free, 5 mM KCl) medium; ∗, P < 0.001 versus pcDNA3 in apoptotic medium plus CPT-cAMP; φ, P < 0.001 versus pcDNA3 in apoptotic medium plus forskolin (Student's t test). Numbers on the bars indicate the total number of neurons counted.
DISCUSSION
Agents that elevate intracellular cAMP levels, such as forskolin (a direct activator of adenylate cyclase), cholera toxin (an activator of Gs proteins), IBMX (a phosphodiesterase inhibitor), and pituitary adenylate cyclase-activating polypeptide (PACAP-38), protect neurons from a variety of apoptotic signals (7, 10, 12, 15, 19, 24, 29, 32, 33, 38, 42, 50). The antiapoptotic effects of cAMP are seen in many types of neuronal systems, including cerebellar granule neurons (7, 15, 24, 33, 38, 42), dopamine neurons (37), septal cholinergic neurons (29), and sympathetic and sensory neurons (1, 12, 43). The mechanisms underlying the antiapoptotic effects of cAMP are not well understood. In previous studies, two signaling pathways that could mediate the neuroprotective effect of cAMP have been explored: the ERK pathway and the PI-3 kinase-Akt pathway. The ERK pathway has been implicated in the survival of sympathetic neurons exposed to cytosine arabinoside (1) and in PC12 cells induced to undergo apoptosis by nerve growth factor withdrawal (49). However, other studies with sympathetic neurons (10, 43) indicate that cAMP promotes neuronal survival through an ERK-independent pathway. In our studies, cAMP stimulated phosphorylation of both ERK and RSK in the cerebellar granule neurons, but activation of this pathway did not lead to protection against apoptosis, since the MEK inhibitors blocked phosphorylation but not the ability of cAMP to protect the neurons from apoptosis. These results concur with the findings of Creedon et al. (10) and indicate that, in rat cerebellar granule neurons, the ERK pathway does not mediate the survival effects of cAMP. As previously discussed, most studies examining the role of PI-3 kinase-Akt in mediating the neuroprotective effects of cAMP have demonstrated that the Akt pathway is not involved. We reexamined the potential involvement of the Akt pathway, since GSK-3β is known to be downstream of Akt. The results from Western blotting with T308 and S473 phosphospecific Akt antibodies and direct Akt kinase assays showed that cAMP had no effect on Akt phosphorylation or activity in cerebellar granule neurons. Consistent with these findings, wortmannin and transfection of cerebellar granule neurons with a dominant-negative Akt mutant failed to diminish the protective effect of cAMP in our cells. Thus, Akt does not mediate the survival effects of cAMP in rat cerebellar granule neurons.
Our data suggest a novel signaling pathway by which cAMP promotes neuronal survival, which is a PKA pathway that leads to inhibition of GSK-3β. Forskolin and CPT-cAMP phosphorylate and inactivate GSK-3β in cerebellar granule neurons. As expected, the phosphorylation of GSK-3β by forskolin and CPT-cAMP is blocked by specific inhibitors of PKA. PKA phosphorylates GSK-3β in vitro at a site known to inhibit its activity, and indeed, forskolin inhibited the kinase activity of GSK-3β. If neurons are transfected with a GSK-3β mutant that cannot be phosphorylated, cAMP is unable to fully protect neurons from apoptosis. Furthermore, direct inhibitors of GSK-3β, valproate and lithium, and a dominant-negative mutant of GSK-3β protect cerebellar granule neurons from apoptosis. These data strongly support the hypothesis that cAMP activates PKA and leads to the phosphorylation and inactivation of GSK-3β, a proapoptotic kinase in cerebellar granule neurons.
The ability of PKA to phosphorylate GSK-3β in vitro supports the notion that PKA directly phosphorylates GSK-3β in vivo. These data suggest that PKA and Akt share a phosphorylation target in cells. Cell localization of PKA, Akt, and GSK-3β may be an important factor in determining which kinase has access to GSK-3β in response to a given stimulus. In addition to PKA and Akt, the protein kinases RSK and integrin-linked kinase also phosphorylate and inactivate GSK-3 (40, 50). Inactivation of GSK-3 by RSK is a proposed mechanism by which the N-methyl d-aspartate (NMDA)-activated ERK pathway opposes apoptosis. Inactivation of GSK-3 by integrin-linked kinase is thought to mediate the antiapoptotic effects of cell attachment (14). Thus, GSK-3β appears to represent a convergence site of multiple signaling pathways involved in cell fate decisions.
ACKNOWLEDGMENTS
We thank Paula Hoffman and Sanjiv Bhave for their assistance in establishing cultures of rat cerebellar granule neurons and Michael Browning for providing purified PKA. We also thank Jenifer Monks for her assistance with digital deconvolution fluorescent microscopy.
This research was supported by grants from the USAMRC (DAMD17-99-1-9481), NIH (NS38619-01A1), and VA (Merit Award and REAP Award).
REFERENCES
- 1.Anderson C N G, Tolkovsky A M. A role for MAPK/ERK in sympathetic neuron survival: protection against a p53-dependent, JNK-independent induction of apoptosis by cytosine arabinoside. J Neurosci. 1999;19:664–673. doi: 10.1523/JNEUROSCI.19-02-00664.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barde Y A. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 3.Beavo J A, Bechtel P J, Krebs E G. Preparation of homogeneous cyclic AMP-dependent protein kinase(s) and its subunits from rabbit skeletal muscle. Methods Enzymol. 1974;38:299–308. doi: 10.1016/0076-6879(74)38046-9. [DOI] [PubMed] [Google Scholar]
- 4.Boyle W J, Smeal T, Defize L H, Angel P, Woodgett J R, Karin M, Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991;64:573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- 5.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 6.Burgering B M T, Coffer P J. Protein kinase B (cAkt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 7.Campard P K, Crochemore C, Rene F, Monnier D, Koch B, Loeffler J P. PACAP type I receptor activation promotes cerebellar neuron survival through the cAMP/PKA signaling pathway. DNA Cell Biol. 1997;16:323–333. doi: 10.1089/dna.1997.16.323. [DOI] [PubMed] [Google Scholar]
- 8.Cardone M H, Roy M, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 9.Chen, G., L.-D. Huang, Y.-M. Jiang, and H. K. Manji. 1999. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. 72:1327–1330. [DOI] [PubMed]
- 10.Creedon D J, Johnson E M, Lawrence J C. Mitogen-activated protein kinase-independent pathways mediate the effects of nerve growth factor and cAMP on neuronal survival. J Biol Chem. 1996;271:20713–20718. doi: 10.1074/jbc.271.34.20713. [DOI] [PubMed] [Google Scholar]
- 11.Cross D A E, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 12.Crowder R J, Freeman R S. The survival of sympathetic neurons promoted by potassium depolarization, but not cAMP, requires phosphatidylinositol 3-kinase and Akt. J Neurochem. 1999;73:466–475. [PubMed] [Google Scholar]
- 13.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 14.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/Akt by integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Mello S R, Galli C, Ciotti T, Calissano P. Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proc Natl Acad Sci USA. 1993;23:10989–10993. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dominguez I, Itoh K, Sokol S Y. Role of glycogen synthase kinase 3 β as a negative regulator of dorsoventral axis formation in Xenopus embryos. Proc Natl Acad Sci USA. 1995;92:8498–8502. doi: 10.1073/pnas.92.18.8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 18.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 19.Dugan L L, Kim J S, Zhang Y, Bart R D, Sun Y, Holtzman D M, Gutmann D H. Differential effects of cAMP in neurons and astrocytes. Role of B-raf. J Biol Chem. 1999;36:25842–25848. doi: 10.1074/jbc.274.36.25842. [DOI] [PubMed] [Google Scholar]
- 20.Filippa M, Sable C L, Filloux C, Hemmings B, Obberghen E V. Mechanism of protein kinase B activation by cyclic AMP-dependent protein kinase. Mol Cell Biol. 1999;19:4989–5000. doi: 10.1128/mcb.19.7.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foil C J, Williams J S, Chou C-H, Wang Q M, Roach P J, Andrisani O M. A secondary phosphorylation of CREB341 at Ser129 is required for the cAMP-mediated control of gene expression. J Biol Chem. 1994;269:32187–32193. [PubMed] [Google Scholar]
- 22.Franke T F, Yang S, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 23.Fruman D A, Meyers R E, Cantley C C. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 24.Hanson M G, Jr, Shen S, Wiemelt A P, McMorris F A, Barres B A. Cyclic AMP elevation is sufficient to promote the survival of spinal motor neurons in vitro. J Neurosci. 1998;18:7361–7371. doi: 10.1523/JNEUROSCI.18-18-07361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harwood A J, Plyte S E, Woodgett J, Strutt H, Kay R R. Glycogen synthase kinase 3 regulates cell fate in dictyostelium. Cell. 1995;80:139–148. doi: 10.1016/0092-8674(95)90458-1. [DOI] [PubMed] [Google Scholar]
- 26.Hong M, Lee V M-Y. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem. 1997;272:19547–19553. doi: 10.1074/jbc.272.31.19547. [DOI] [PubMed] [Google Scholar]
- 27.Itoh K, Tang T L, Neel B G, Sokol S Y. Specific modulation of ectodermal cell fates in Xenopus embryos by glycogen synthase kinase. Development. 1995;121:3979–3988. doi: 10.1242/dev.121.12.3979. [DOI] [PubMed] [Google Scholar]
- 28.Jones P F, Jakubowicz T, Pitossi F J, Maurer F, Hemmings B A. Molecular cloning and identification of a serine threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci USA. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kew J N, Smith D W, Sofroniew M V. Nerve growth factor withdrawal induces the apoptotic death of developing septal cholinergic neurons in vitro: protection by cyclic AMP analogue and high potassium. Neuroscience. 1996;70:329–339. doi: 10.1016/0306-4522(95)00365-7. [DOI] [PubMed] [Google Scholar]
- 30.Klein P S, Melton D A. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levi-Monalcini R. The nerve growth factor: thirty-five years later. EMBO J. 1987;6:1145–1154. doi: 10.1002/j.1460-2075.1987.tb02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mena M A, Casarejos M J, Bonin A, Ramos J A, Garcia Yebenes J. Effects of dibutyryl cyclic AMP and retinoic acid on the differentiation of dopamine neurons: prevention of cell death by dibutyryl cyclic AMP. J Neurochem. 1995;65:2612–2620. doi: 10.1046/j.1471-4159.1995.65062612.x. [DOI] [PubMed] [Google Scholar]
- 33.Miller T M, Tansey M G, Johnson E M, Jr, Creedon D J. Inhibition of phosphatidylinositol 3-kinase activity blocks depolarization and insulin-like growth factor I-mediated survival of cerebellar granule cells. J Biol Chem. 1997;272:9847–9853. doi: 10.1074/jbc.272.15.9847. [DOI] [PubMed] [Google Scholar]
- 34.Narayanan V. Apoptosis in development and disease of the nervous system. 1. Naturally occurring cell death in the developing nervous system. Pediatr Neurol. 1997;16:9–13. doi: 10.1016/s0887-8994(96)00257-3. [DOI] [PubMed] [Google Scholar]
- 35.Novelli A, Reilly J A, Lysio P G, Henneberry R C. Glutamate becomes neurotoxic via the N-methyl-d-aspartate receptor when intracellular energy levels are reduced. Brain Res. 1988;451:205–212. doi: 10.1016/0006-8993(88)90765-2. [DOI] [PubMed] [Google Scholar]
- 36.Pap M, Cooper G M. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 37.Plyte S E, Hughes K, Nikolakaki E, Pulverer B J, Woodgett J R. Glycogen synthase kinase-3: functions in oncogenesis and development. Biochim Biophys Acta. 1992;1114:147–162. doi: 10.1016/0304-419x(92)90012-n. [DOI] [PubMed] [Google Scholar]
- 38.Rydel R E, Greene L A. cAMP analogs promote survival and neurite outgrowth in cultures of rat sympathetic and sensory neurons independently of nerve growth factor. Proc Natl Acad Sci USA. 1988;85:1257–1261. doi: 10.1073/pnas.85.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sable C L, Filippa N, Hemmings B, Van Obberghen E. cAMP stimulates protein kinase B in a wortmannin-insensitive manner. FEBS Lett. 1997;409:253–257. doi: 10.1016/s0014-5793(97)00518-8. [DOI] [PubMed] [Google Scholar]
- 40.Seeling J M, Miller J R, Gil R, Moon R T, White R, Virshup D M. Regulation of beta-catenin signaling by the B56 subunit of protein phosphatase 2A. Science. 1999;283:2089–2091. doi: 10.1126/science.283.5410.2089. [DOI] [PubMed] [Google Scholar]
- 41.Torres M A, Eldar-Finkelman H, Krebs E G, Moon R T. Regulation of ribosomal S6 protein kinase-p90 (rsk), glycogen synthase kinase 3, and beta-catenin in early Xenopus development. Mol Cell Biol. 1999;19:1427–1437. doi: 10.1128/mcb.19.2.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villalba M, Bockaert J, Journot L. Pituitary adenylate cyclase-activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway. J Neurosci. 1997;17:83–90. doi: 10.1523/JNEUROSCI.17-01-00083.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Virdee K, Tolkovsky A M. Activation of p44 and p42 MAP kinases is not essential for the survival of rat sympathetic neurons. Eur J Neurosci. 1995;7:2159–2169. doi: 10.1111/j.1460-9568.1995.tb00637.x. [DOI] [PubMed] [Google Scholar]
- 44.Welsh G I, Proud C G. Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem J. 1993;294:625–629. doi: 10.1042/bj2940625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welsh G I, Wilson C, Proud C G. GSK3: a SHAGGY frog story. Trends Cell Biol. 1996;6:274–279. doi: 10.1016/0962-8924(96)10023-4. [DOI] [PubMed] [Google Scholar]
- 46.Woodgett J R. A common denominator linking glycogen metabolism, nuclear oncogenes and development. Trends Biochem Sci. 1991;16:177–181. doi: 10.1016/0968-0004(91)90071-3. [DOI] [PubMed] [Google Scholar]
- 47.Woodgett J R, Plyte S E, Pulverer B J, Mitchell J A, Hughes K. Roles of glycogen synthase kinase-3 in signal transduction. Biochem Soc Trans. 1993;21:905–907. doi: 10.1042/bst0210905. [DOI] [PubMed] [Google Scholar]
- 48.Xia Z, Dudek H, Miranti C K, Greenberg M E. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J Neurosci. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 50.Yan G M, Lin S Z, Irwin R P, Paul S M. Activation of G proteins bidirectionally affects apoptosis of cultured cerebellar granule neurons. J Neurochem. 1995;65:2425–2431. doi: 10.1046/j.1471-4159.1995.65062425.x. [DOI] [PubMed] [Google Scholar]