Abstract
The dysregulation of lipid metabolism and alterations in the ratio of monounsaturated fatty acids (MUFAs) to saturated fatty acids (SFAs) have been implicated in cancer progression and stemness. Stearoyl-CoA desaturase 1 (SCD1), an enzyme involved in lipid desaturation, is crucial in regulating this ratio and has been identified as an important regulator of cancer cell survival and progression. SCD1 converts SFAs into MUFAs and is important for maintaining membrane fluidity, cellular signaling, and gene expression. Many malignancies, including cancer stem cells, have been reported to exhibit high expression of SCD1. Therefore, targeting SCD1 may provide a novel therapeutic strategy for cancer treatment. In addition, the involvement of SCD1 in cancer stem cells has been observed in various types of cancer. Some natural products have the potential to inhibit SCD1 expression/activity, thereby suppressing cancer cell survival and self-renewal activity.
Keywords: cancer stem cells, SCD1, phytochemicals, lipid metabolism, cancer progression
1. Introduction
Tumor emergence and progression is an intricate process influenced by various factors. Within the tumor microenvironment, the self-renewal capacity of a tumor relies on a small subset of cells known as tumor-initiating cells and cancer stem cells (CSCs). These cells possess the ability to proliferate, undergo self-renewal, and are frequently associated with the reemergence of tumors following cancer treatment [1]. CSCs adapt their metabolism to facilitate tumor growth. To maintain the function of CSCs, they undergo crucial metabolic shifts such as dysregulation of glycolysis and lipid metabolism. CSCs hold a crucial position in driving the tumor progression, metastasis, infiltration, resistance to drugs, and relapse of cancer cells [2]. Lipid dysfunctions are associated with more aggressive molecular characteristics and increased transcripts related to lipogenesis and cholesterol synthesis pathways, which are linked to unfavorable survival outcomes [3]. Recent studies have been presented suggesting that disruptions in lipid metabolism, caused by the high need for energy and structural components, may have a significant impact on CSCs [4,5].
To maintain energy production, tumor cells need to regulate their nutrient intake and metabolism by reprogramming their metabolic pathways. Lipids play a critical role as a source of energy, structural components of biological membranes, signaling molecules, and regulators of redox homeostasis in cancer cells [6,7]. The dysregulation of lipid metabolism is affected by enzymes and signaling molecules that are involved in the lipid metabolism process. Lipid metabolism can modify the composition of the cell membrane, affect gene expression, and impact downstream signaling pathways that regulate various cellular processes, such as cell proliferation, motility, inflammation, and survival [8]. Lipids are an essential component of cells and organelle membranes, and fatty acids are also necessary for the proliferation of bulk tumor mass and the maintenance of cancer stem cells [9,10]. Alterations in both lipid catabolism and anabolism contribute to acquiring stemness characteristics in cancer cells, including lipid uptake, de novo lipogenesis, lipid desaturation, lipolysis, and fatty acid oxidation [11]. Recent reports implicate lipid desaturation as an essential process for cancer cell survival.
The lipid composition of cellular membranes affects membrane fluidity, cellular signaling, and, consequently, gene expression. Cancer cells have distinctive metabolic phenotypes that are characterized by the altered ratio of saturated to monounsaturated fatty acids (MUFAs) [12,13]. A critical committed step in the biosynthetic pathway of MUFAs is the introduction of the cis double bond between carbons 9 and 10 (Δ-9 position). The endoplasmic reticulum (ER) enzyme stearoyl-coenzyme A desaturase 1 (SCD1) converts saturated fatty acids (SFAs) into MUFAs, hence regulating the ratio of MUFAs to SFAs in cells [14]. This review highlights the role of SCD1, which is essential in the process of lipid desaturation in cancer cells and cancer stem cells [15,16].
2. Structure and Function of SCD1
The crystal structures of SCD1 in both mouse and human species were reported previously [17,18]. Mouse SCD1 shares 84% of its sequence identity with that of humans. SCD1 is an iron-containing transmembrane enzyme that consists of a cone-shaped structure formed by four α-helices. The protein is exclusively located on the ER membrane, with both the N- and C-terminal domains extending into the cytosol. Highly conserved regions surround the iron-containing center, including the eight histidines found in the cytoplasmic loop and C-terminus [17,19,20].
SCD is an integral membrane protein anchored to the ER membrane, where it catalyzes the biosynthesis of MUFAs from dietary or de novo synthesized SFA precursors. Four mouse SCD isoforms (SCD1-4) [21,22,23,24] and two human isoforms (SCD1 and 5) have been identified [25,26]. Two human SCD isoforms share the same enzymatic function but exhibit different tissue distribution patterns. SCD1 is found in almost all tissues, with major levels found in lipogenic tissues such as the liver and adipose tissue [27]; SCD5 is mainly expressed in the brain [28]. SCD1 plays a crucial role in lipid synthesis and the regulation of energy metabolism. SCD1 especially converts stearoyl-CoA (18:0) and palmitoyl-CoA (16:0) into the MUFAs oleoyl-CoA (18:1) and palmitoleoyl-CoA (16:1) through the insertion of a double bond in the Δ-9 position of the substrate [29]. These MUFA products of SCD1 desaturation are used as significant substrates for synthesizing a variety of complex lipids, including phospholipids, triglycerides, cholesteryl esters, wax esters, and other lipid species. SCD1 contributes to the fatty acid composition and fluidity of the membrane, influencing membrane-mediated biological signal transduction for the regulation of cell growth and differentiation [13,30]. It is also critically important in lipid metabolism and energy balance for storing or oxidation of lipids [31]. High SCD1 activity and/or expression has been found in a wide range of diseases, including atherosclerosis [32,33], obesity [34,35], and cancer [36,37]. In particular, reports that the expression and activity of SCD1 can play a key role in the pathogenesis of cancer have been attracting attention. In addition, there is mounting evidence indicating the potential value of SCD1 as a target for novel pharmacological approaches in cancer therapy [12,38,39,40,41,42,43].
3. Role of SCD1 in Cancer
Many studies have demonstrated the relevance of a supporting role for SCD1 in cancer progression of lung, breast, and prostate carcinomas, as well as clear cell renal cell carcinoma (ccRCC) [39,40,43,44,45,46]. The importance of SCD1 in cancer has already been reported in several review articles [12,38,47]. An increased ratio of MUFA/SFA propelled by high SCD1 expression appears to be a marker for the onset of typical traits of malignant behavior such as a high rate of cell proliferation, survival, and invasiveness [47]. It is recognized as a factor contributing to the lipogenic metabolism of cancer cells, followed by the biosynthesis of membrane phospholipids and energy-storage lipids [47]. In lung cancer, the ablation of SCD1 expression reduced the proliferation and invasiveness of cancer cells, consequently impairing tumorigenic capacity [39,48,49]. In addition, treating colon cancer cells with SCD1 inhibitors interrupted the cellular conversion of stearate to oleate in colon cancer cells, resulting in delayed tumor growth [46]. Hypoxia-inducible factor (HIF) is a well-known signaling mechanism that regulates SCD1 activity. In ccRCC, SCD1 was upregulated in response to HIF-2α. Increased SCD1 provides a sufficient substrate for lipid biosynthesis, which is required for rapid cell division. In addition, the upregulation of SCD1 also enhanced the expression of HIF-2α in feedback regulation by cooperating with HIF-1α, ultimately promoting tumorigenesis [50].
Recently, it has been demonstrated that inhibition of SCD1 in tumor cells can have antitumor effects by regulating immune cells in cancer tissues. Administering the SCD1 inhibitor A93957223 in mouse tumor models has been found to enhance antitumor T cells by recruiting dendritic cells (DCs) into tumors. SCD1 inhibition enhanced the production of chemokine C-C motif ligand 4 (CCL4) in cancer cells by reducing Wnt/β-catenin signaling. In addition, inhibiting SCD1 has been found to directly recruit DCs and induce CD8+ T cells induction, leading to increased production of CCL4 due to reduced ER stress [51]. The combination of SCD1 inhibition and anti-PD-1 therapy resulted in a synergistic effect, suggesting that targeting SCD1 may be a promising strategy for enhancing immunotherapy in cancer treatment [51].
The correlation between autophagy and SCD1 expression is also of increasing interest. Huang and colleagues demonstrated that pharmacological inhibition of SCD1 using CAY10566 induces autophagy-dependent apoptosis in human hepatocellular carcinoma (HCC) cells [52]. In addition, treatment involving the use of MF438 to inhibit SCD1 in lung cancer spheroid cells resulted in the activation of the ER stress response accompanied by a significant increase in autophagy, as determined by elevated levels of LC3-II [53]. Blocking SCD1 activity reversed the resistance of lung cancer sphere-forming cells to cisplatin [53]. Autophagy can act as both a cell survival mechanism and a tumor suppressor during tumorigenesis, depending on the context [54]. Ono et al. discovered that inhibiting SCD1 using both the small molecule T-3764518 and SCD1 shRNA in colon cancer HCT-116 cells accelerated the autophagic process and activated AMPK, enabling the cancer cells to escape the cytotoxic effects of SCD1 inhibition [55]. The inhibition of SCD1, leading to the excessive accumulation of saturated fatty acids, can activate the AMP-activated protein kinase (AMPK)-mediated resistance mechanism in HCT-116 cells. Therefore, when cells are exposed to both SCD1 and autophagy inhibitors, the activation of autophagy, known as a survival signal, is inhibited, ultimately resulting in the induction of cancer cell death [55]. In accordance with a previous report, the combination of amodiaquine treatment with SCD1 inhibition using A930572 showed a strong synergistic effect in inhibiting cancer cell proliferation, as demonstrated in lung cancer cells and a mouse xenograft model [56].
The importance of SCD1 function in cancer stem cells as well as cancer has been increasingly highlighted.
4. Role of SCD1 in Cancer Stem Cells
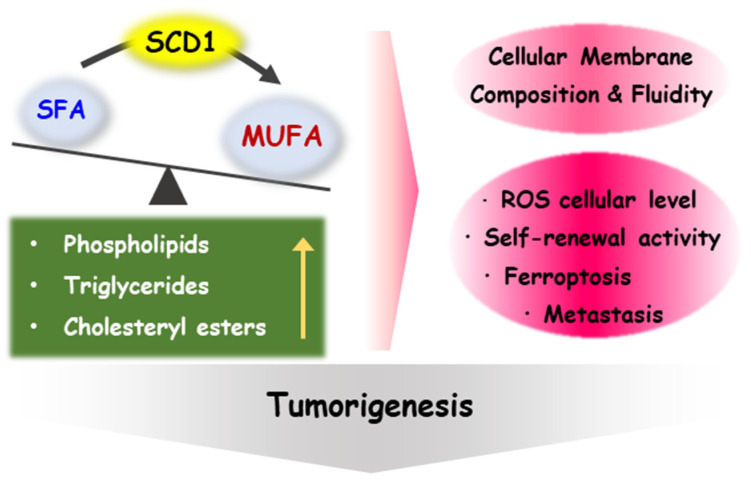
Studies have suggested that CSCs have a higher proportion of MUFAs in their lipid composition compared to non-stem cancer cells, indicating that lipid desaturation could serve as a potential biomarker for CSCs in certain types of tumors, such as ovarian and glioblastoma. [15,57,58]. Membrane fluidity is defined as the degree of the molecular order and motion of membrane constituents, which is dependent on the content of unsaturated lipids [59]. Several studies have demonstrated that reducing membrane fluidity through anti-metastasis drugs can inhibit the metastatic capacity and stemness characteristics of breast cancer cells. This is thought to occur due to changes in membrane properties that affect signaling pathways and gene expression involved in these cellular processes [60]. In addition, Song et al. reported that MUFAs are required for the sphere formation of glioblastoma multiforme cell lines, as assessed by lipidomic profile differences between CSCs and bulk cancer cells [58]. MUFAs such as oleic acid, phosphatidylcholine, and phosphatidylethanolamine are enriched in CSCs compared to their non-stem counterparts [58]. The role of SCD1 in cancer stem cells has expanded to various cancer types, particularly lung, ovarian, breast, and prostate cancer [15,16,53,61]. The possible impact of SCD1 on tumorigenesis is summarized in Figure 1.
Figure 1.
Role of SCD1 in tumorigenesis. MUFAs, as the products of SCD1 desaturation, are used as the building blocks for various types of complex lipids, such as phospholipids, triglyceride, cholesteryl esters, and other types of lipids. Fatty acid composition and the fluidity of the cell membrane are influenced by this factor, which contributes to the regulation of self-renewal activity, ferroptosis, and metastasis by affecting membrane-mediated biological signal transduction. It is also related to the regulation of ROS cellular levels.
4.1. Ovarian Cancer
As mentioned earlier, SCD1 is an enzyme responsible for the desaturation of SFAs to MUFAs, which leads to an increase in the ratio of MUFAs to SFAs. This ratio was also significantly increased in ovarian cancer COV362 and OVCAR5 cells grown as spheres [15]. It is reported that ALDH+/CD133+ cells isolated from COV362 cells possess phenotypic characteristics of cancer stem cells and have a higher level of MUFAs [15]. Inhibition of SCD1 using a small molecule inhibitor or siRNA significantly reduced lipid unsaturation levels in ovarian cancer spheroids and then suppressed sphere-forming ability with decreased levels of ALDH1A1, Nanog, Sox2, and Oct-4 mRNA expression [15]. Nuclear factor kappaB (NF-κB) is reported to be the key signal molecule that regulates SCD1 in ovarian CSCs. When treated with dimethylamino parthenolide, an inhibitor of NF-κB, primary ovarian cancer spheres exhibit reduced levels of both lipid unsaturation and SCD1 mRNA expression [15]. Notably, the mRNA expression of SCD1 was regulated by p65, which binds directly to its promoter region. Conversely, treating SCD1 inhibitors in primary ovarian cancer spheres led to the suppression of NF-κB transcriptional activity.
Spheroids derived from the malignant ascites of ovarian cancer cells exhibit aberrantly elevated expression of SCD1 and fatty acid desaturase 2 (FADS2), positively accelerating lipid metabolic activities [62]. Pharmaceutical inhibitors of SCD1 and FADS2 suppressed sphere formation ability, which was accompanied by a reduction in the expression levels of self-renewal-related markers such as Krüppel-like factor 4 (KLF4) and Bmi-1 [62]. In addition, overexpression of SCD1 or FADS2 led to upregulation of the mesenchymal marker vimentin and epithelial-to-mesenchymal transition (EMT) regulators such as ZEB1, SNAIL, and Slug in ovarian cancer cells [62]. Blocking SCD1/FADS2 contributed to increased cellular reactive oxygen species (ROS) and lipid peroxidation through downregulated glutathione peroxidase 4 (GPX4) and the reduced glutathione/oxidized glutathione (GSH/GSSG) ratio in ascites-derived ovarian cancer cells, thereby promoting ferroptosis [62]. Moreover, co-treatment with SCD1/FADS2-specific inhibitors and cisplatin disrupted the metastatic spindle morphology of ovarian cancer patient-derived organoids [62].
4.2. Lung Cancer
SCD1 is overexpressed in the spheroids of lung cancer NCI-H460 cells and primary tumor cells derived from the malignant pleural effusions of patients with lung adenocarcinoma compared with adherent cultures [63]. Silencing and pharmacological inhibition of SCD1 reduced sphere-forming efficiency and was accompanied by attenuated mRNA expression of stem cell markers such as ALDH1A1, Nanog, and Oct4 [63]. Spheroids treated with the SCD1 inhibitor MF-438 exhibited features of cellular damage, such as cytoplasmic vacuolization, mitochondrial swelling, and apoptotic nuclei [63]. In addition, SCD1 enzymatic inhibition selectively induced apoptosis of ALDH1A1-positive cells. Cells generated from spheroids in the presence of MF-438 show strongly decreased tumorigenic potential with impaired expression of ALDH1A1 [63].
High SCD1 mRNA and protein levels are associated with tumor progression and poor survival in lung adenocarcinoma [53,64]. High SCD1 expression combined with stemness markers such as CD24, CD133, SOX2, and CD44 is related to lung adenocarcinoma patients with worse prognosis [53]. SCD1 expression has been found to be upregulated in lung cancer spheroids, which are enriched for lung cancer-initiating cells [65]. The increased amount of unsaturated fatty acids caused by SCD1 can act as a substrate for the enzyme porcupine, also known as a membrane-associated O-acyltransferase. Wnt ligand undergoes post-translational acylation, which is mediated by porcupine. Wnt ligand combined with the frizzled class receptor 4 (FZD4) receptor causes inactivation of the destruction complex, ultimately stabilizing β-catenin and YAP/TAZ protein activity [65]. Resistance to cisplatin in lung cancer cells was attenuated through treatment of SCD1 with the pharmacologic inhibitor MF-438, which was confirmed by a sphere-forming assay [53]. In addition, the ALDH1A1high cells isolated from lung cancer spheroids showed higher expression of SCD1 and NANOG, and this effect was abrogated by simultaneous co-treatment with an SCD1 inhibitor [53]. Moreover, SCD1 activity has been linked to the activation of YAP and TAZ through the Wnt/β-catenin axis, thus contributing to the survival and spread of lung cancer stem cells [65]. Silencing of SCD1 caused inhibition in the protein expression of YAP and TAZ, which are required for the spheroid formation of lung cancer cells [65].
Epidermal growth factor receptor (EGFR) directly binds to SCD1 and phosphorylates its Tyr55 residue, which maintains the stability of SCD1 protein and increases MUFA levels to facilitate lung cancer growth [66]. EGFR activation was positively correlated with SCD1 Tyr55 phosphorylation, SCD1 protein expression, and poor patient prognosis in non-small cell lung cancer (NSCLC) tissues [66].
4.3. Liver Cancer
SCD1 mRNA expression was increased in the tumor tissues of approximately 60% of HCC patients compared to their non-tumor counterparts [67]. SCD1 was significantly upregulated in established sorafenib-resistant Bel7402 and Huh7 HCC cell lines and patient-derived tumor xenografts (PDTX) [67]. These cell lines established with shRNA against SCD1 exhibited a decrease in the percentages of CD24 and CD47 markers. Knockdown of SCD1 using shRNA sensitized HCC cells to sorafenib through the induction of ER stress, which is mediated by an increased mRNA expression of CHOP and Bip [67]. Co-treatment with SSI-4 (an inhibitor of SCD1 enzymatic activity) and sorafenib by oral gavage resulted in a synergistic effect on tumor growth inhibition in a PDTX model [67]. Sphere-forming cultures of HCC-enriched subpopulations with stem-cell characteristics are maintained by peroxisome proliferator-activated receptor-gamma (PPARγ) activation, which upregulates SCD1 expression and induces transcriptional activity and nuclear accumulation of β-catenin [68]. Treatment with PPARγ-specific antagonists or SCD1 inhibitors effectively decreased the sphere-forming capacity of HCC Huh7 and Hep3B cells, resulting in the loss of CSC properties through reduced expression of CSC-related markers such as EpCAM, CD133, CD24, KRT19, and ICAM1 [68].
Inhibition of lipid unsaturation using shRNA against SCD1 or chemical inhibitor CAY10566 suppressed the proliferation of HCC JHH7 cells grown under monolayer and sphere cultures, which is mediated by the downregulation of MYCN mRNA expression [69]. In addition, the content of unsaturated fatty acids was increased in MYCNhigh/EpCAM+ CSC-rich HCC cells. It has been known that induction of ER stress causes a loss of intestinal epithelial stemness [70], while its reduction enables the maintenance of functional hematopoietic stem cells [71]. Both MYCNhigh/EpCAM+ CSC-like HCC cells and CSC-rich spheroids showed downregulation of ER stress-induced activating transcription factor 3 (ATF3) gene expression [69]. Treatment with the ER stress chemical inducer stimulated the expression of the ATF3 gene while reducing the expression of MYCN in JHH7 cells [69]. Moreover, the expression of ER stress-inducible transcription factor ATF3 was downregulated in MYCNhigh CSC-like HCC cells, which was rescued through treatment with acyclic retinoid as a modulator of lipid desaturation [69].
4.4. Skin Cancer
In the Human Protein Atlas database, SCD1 is highly expressed in oral and skin squamous carcinoma samples [72]. Runx1 is known to be essential for the growth and survival of human oral and skin squamous cell carcinoma (SCC) cell lines [73]. Runx1 levels affect the membrane fluidity of cultured keratinocytes and human SCC cell lines by regulating SCD1 activity and the concentration of its product, oleate [72]. SCD1 expression induced Wnt activation, which may promote the activation and proliferation of keratinocyte and hair germ cells [72].
4.5. Bladder Cancer
High levels of SCD mRNA and protein have been associated with poor prognosis in patients with bladder cancer. SCD1 expression was upregulated in bladder cancer tissue samples compared with adjacent non-tumor tissues [74]. Inhibition of SCD activity was capable of decreasing the migration and invasion abilities of bladder cancer cell lines [74]. Blockade of SCD1 activity caused cell cycle arrest in the G1/S phase through downregulation of cyclin D1, Rb, Cdk4, and Cdk6. Interestingly, while SCD1 inhibitor A939572 did not induce apoptosis in parental bladder cancer cells, the inhibitor significantly inhibited the growth of sphere-forming cells [74].
4.6. Colon Cancer
The expression of SCD1 protein and mRNA is highly increased in colon adenocarcinoma HCT-15, HCT-116, SW480, and HT-29 cells grown under CSC-enriched culture conditions [75]. The ratios of MUFAs to SFAs and the levels of unsaturated lipids were significantly decreased in HCT116 colon CSCs treated with CAY10566, an SCD1 inhibitor [76]. Treatment with CAY10566 inhibited the spheroid formation of CSCs, indicating that SCD1 activity was associated with the stemness and tumorigenicity of colon CSCs [76]. In addition, the ratios of MUFAs to SFAs were higher in CSCs compared to parent colon HCT116 cancer cells [76]. Another SCD1 inhibitor, MF-438, did not kill the colorectal CSC population cells but regulated the expression of CSC-related signaling genes such as AXIN, LEF1, and Notch1 [77]. Moreover, irinotecan-resistant colon cancer cells led to a decrease in MUFAs with higher levels of SCD1 compared with their parental cells [78]. SCD1 directly regulates the expression of ALDH1A1, which is a CSC biomarker that can stimulate ROS generation and cancer stemness in irinotecan-resistant colon cancer cells [78].
4.7. Glioblastoma
Overexpression of sterol-regulated element-binding protein 1 (SREBP1) has been observed in glioblastoma multiforme (GBM), leading to enhanced lipid metabolism associated with abundant MUFAs [79]. SCD1 is one of the target genes regulated by SREBP1, and it has been shown to be required for tumor growth in several types of cancer [79]. Silencing of SCD1 reduced cell viability in patient-derived glioblastoma stem cells (GSCs), while there was no change in normal human astrocytes [80]. Ectopic expression of SCD1 led to a greater frequency of stem cells in GSCs and promoted cell growth and the formation of secondary spheres. Following intracranial transplantation of GSCs in mice, it was confirmed that more tumors developed in the mice group in which SCD1 was overexpressed [80]. Intranasal delivery of the SCD1 inhibitor CAY10566 into a PDTX mouse model showed the ability to inhibit tumor formation [80]. In addition, activation of SCD1 transcription and the subsequent synthesis of MUFAs plays a cytoprotective role in mitigating ER stress [80]. Using a dataset from The Cancer Genome Atlas, a positive correlation was observed between transcript levels of BiP and SCD1 in the GBM patient group [80]. Inhibition of SCD1 led to the accumulation of SFAs, which in turn exacerbated ER stress. GSCs treated with the SCD1 inhibitor CAY compound displayed an increase in ER stress markers such as Bip, ChOP, sXBP1, and GADD34 [80]. The activity of SCD1 provides GSCs with a survival advantage, making them vulnerable to metabolic targeting via SCD1 inhibition [80].
4.8. Gastric Cancer
SCD1 has been shown to enhance the population of gastric cancer sphere cells through the Hippo/YAP pathway [81]. The use of an inhibitor or siRNA to suppress SCD1 reduced the expression of stemness-associated cell surface markers such as CD44, CD133, and Lgr5 and transcriptional levels of Sox-2, Oct-4, and Nanog. This impairs the sphere-forming ability of patient-derived gastric cancer cells [81]. Inhibition of SCD1 led to disassembly of YAP in the nucleus via decreased YAP phosphorylation, which attenuates the expression of TEA domain family member 1 (TEAD1) and cyclin D1 [81]. In addition, when cells treated with A939572, a pharmacological inhibitor of SCD1, were injected subcutaneously into SCID mice, the ability to form tumors was significantly suppressed [81].
4.9. Breast Cancer
SCD1 is overexpressed and correlates with poor prognosis in breast cancer patients [82]. CD10 is known to degenerate osteogenic growth peptide (OGP), which is recognized as an anti-tumoral peptide, and is expressed in cancer-associated fibroblasts to support tumor stemness and induce chemoresistance [83]. CD10 sustains the characteristics of cancer stem cells by cleaving the active domain of OGP in mammosphere formation [83]. In addition, OGP treatment of MCF-7 mammosphere cells reduced SCD1 expression and subsequently impaired lipid desaturation [83].
Table 1 provides a summary of the consequences of inhibiting SCD1 in cancer stem cells of various types.
Table 1.
The effects of inhibiting SCD1 on different types of cancer cell lines.
| Cell Type | Regulated Genes or Proteins |
Phenotypic Effects | Ref. |
|---|---|---|---|
| COV362, OVCAR5 | ALDH1A1, Nanog, Sox2, Oct-4↓ | Sphere-forming ability↓ | [15] |
| Ascites-derived ovarian cancer cells | KLF4↓, ROS↑, GPX4↓, GSH/GSSG ratio↓ |
Ferroptosis↑, EMT↓, sphere-forming ability↓ |
[62] |
| NCI-H460 | ALDH1A1, Nanog, Oct-4↓ | Sphere-forming ability↓ | [63] |
| Patient-derived lung cancer tissue | YAP, TAZ activity↓ | Sphere-forming ability↓ | [65] |
| Sorafenib-resistant Bel7402 and Huh7 cells | CD24, CD47↓ CHOP, Bip↑ |
ER stress↑, sphere-forming ability↓ |
[67] |
| Huh7 and Hep3B cells | EpCAM, CD133, CD24, KRT19, ICAM1↓ | Sphere-forming ability↓ | [68] |
| JHH7 cells | ATF3, MYCN↓ | Sphere-forming ability↓ | [69] |
| UMUC3 and RT4 cells | Cyclin D1, Rb, Cdk4, Cdk6↓ | Cell cycle arrest↑, migration and invasion↓, sphere-forming ability↓ |
[74] |
| Patient-derived glioblastoma stem cells | Bip, ChOP, sXBP1, GADD34↑ | Cell viability↓ sphere-forming ability↓ |
[80] |
| Gastric cancer stem-like HSC034 cells | Sox2, Oct4, Nanog, CD44, Lgr5, CD133↓, YAP, TEAD1↓ |
Sphere-forming ability↓, metastasis↓ |
[81] |
5. Regulation of SCD1 Expression by Anti-Carcinogenic Natural Compounds
5.1. Betulinic Acid
Betulinic acid (BetA), a lupine-type pentacyclic triterpenoid saponin from tree bark, is known to exhibit cytotoxicity against various cancer cells but not normal cells [84,85]. BetA inhibited the activity of SCD1, which regulates the saturation level of cardiolipin, a lipid known for mitochondrial structural function [86]. BetA caused an increase in cardiolipin saturation within the mitochondria membrane, resulting in the release of cytochrome c and triggering apoptosis in HeLa cells. Under the same experimental condition, this was also confirmed by SCD1 knockdown using siRNA [86]. In addition, BetA inhibited the proliferation of gallbladder cancer NOZ cells by disturbing mitochondrial membrane potential [87]. Additionally, BetA suppressed SCD1 expression at both the mRNA and protein levels in NOZ cells. Administration of BetA decreased tumor size in xenograft mice injected with NOZ cells, and SCD1 expression was also suppressed [87]. Moreover, in primary colorectal cancer stem cells, betulinic acid induced apoptosis as well as a loss of clonogenicity, which is caused by SCD1 inhibition [88].
5.2. Curcumin
Curcumin is a dietary polyphenol compound derived from rhizomes of turmeric (curcuma longa). It has been shown to have anti-cancer effects in in vitro, in vivo, and pre-clinical studies [89]. Curcumin inhibited primary and secondary mammosphere formation based on breast cancer MCF-7 and SUM149 cells. In addition, curcumin treatment resulted in downregulated expression of SCD1 in ALDH+ populations sorted from primary breast cells [90]. Moreover, the regulatory effects of curcumin on lipogenic enzymes have attracted increasing attention as a potential means of inhibiting or reversing tumor growth [90].
5.3. Trichothecin
Trichothecin is a sesquiterpenoid isolated from the endophytic fungus of the herbal plant Maytenus hookeri Loes. SCD1, which is highly expressed in aggressive colorectal carcinoma, was attenuated by trichothecin treatment and restored saturated fatty acid levels [91]. In addition, the transcriptional activity of the SCD1 promoter region was reduced through trichothecin treatment in colon cancer LOVO and HCT116 cells, contributing to the anti-invasive effect [91]. It can be suggested that the anti-tumor effects of trichothecin may be related to SCD1-mediated fatty acid metabolite alterations.
5.4. Icaritin
Icaritin is a prenylflavonoid derivative from the Chinese herbal medicine Eimedii Herba and has been known to exert anti-cancer effects. The possibility of the direct binding of icaritin to SCD1 is suggested by computer-aided drug design [82]. Icaritin inhibited the growth of MCF-7 and SK-BR-3 cells in the mitochondrial pathway by reducing the expression and activity of SCD1 [82]. An icaritin derivative induced autophagy through AMPK/mTOR and mitogen-activated protein kinase signaling pathways in MCF-7 cells. Icaritin-induced autophagy was alleviated by overexpression of SCD1 in MCF-7 cells.
6. Concluding Remarks and Future Perspectives
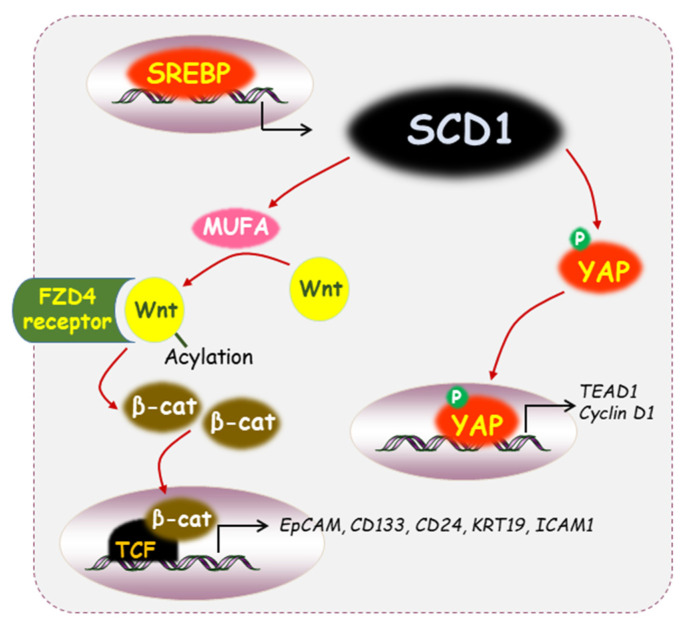
High SCD1 expression has been reported in many malignancies, including cancer stem cells [15,16,53,61]. Some dietary and natural anti-cancer compounds regulate the expression/activity of the SCD1 gene/protein, which results in restoration of the proportion of SFAs. Downregulation of SCD1 expression is associated with inhibited proliferation, migration, metastasis, and growth of cancer (stem) cells. However, the molecular mechanisms underlying restoration of SCD1 function by natural anti-cancer compounds remain largely unknown. Several key signaling mechanisms involving SCD1 in cancer stem cells are presented in Figure 2, which are expected to have the potential to lead to the discovery of phytochemicals or synthetic inhibitors. Our research team achieved experimental results indicating that thymoquinone can act as a growth inhibitor by inhibiting the expression of SCD1 in breast cancer cells, and related research is still in progress.
Figure 2.
Diverse signaling mechanisms through SCD1 in cancer stem cells. SCD1 is one of the target genes regulated by SREBP1. Decreased YAP phosphorylation resulting from SCD1 inhibition caused the disassembly of YAP in the nucleus, leading to a reduction in the expression of TEAD1 and cyclin D1. Post-translational acylation of the Wnt ligand, which MUFA mediates, enables its binding with the FZD4 receptor, resulting in the stabilization of β-catenin and leading to the expression of CSC regulatory genes.
It becomes particularly interesting to investigate the role of SCD1 in cancer (stem) cells, which is associated with obesity, lipid accumulation, and inflammation [92,93]. In addition, tumorigenesis is linked to insulin-resistant glucose metabolism [94]. Under the condition of insulin resistance, expression of glucose transporter type 4 is blocked, resulting in elevated blood glucose levels and increased β-oxidation of fatty acids [95]. As SCD1 is linked with insulin resistance in morbidly obese patients [96], SCD1 may serve as a connection in the association between insulin resistance and cancer.
High SCD1 expression is a major cause of the increased ratio of MUFAs/SFAs, which contributes to the fatty acid composition and fluidity of the membrane. This influences membrane-mediated biological signal transduction to regulate cell growth and differentiation [13,30]. Cellular membrane composition of the MUFA/SFA ratio can be modulated by SCD1 and is crucial for lipid metabolism and energy balance for the storage or oxidation of lipids. Reducing membrane fluidity using anti-metastasis drugs in cancer cells inhibits metastatic capacity and stemness characteristics. Downregulation of SCD1 expression in tumor cells is a reliable therapeutic strategy to treat cancer. Therefore, natural anti-cancer compounds may contribute to maintaining cellular membrane status through the regulation of SCD1 expression or activity, which could be an alternative therapeutic target for chemoresistant cancer cells or cancer stem cells.
Abbreviations
AMPK, AMP-activated protein kinase; ATF3, activating transcription factor 3; CCL4, chemokine C-C motif ligand 4; ccRCC, clear cell renal cell carcinoma; CSCs, cancer stem cells; DCs, dendritic cells; ER, endoplasmic reticulum; EGFR, epidermal growth factor receptor; FADS2, fatty acid desaturase 2; FZD4, frizzled class receptor 4; GBM, glioblastoma multiforme; GSH/GSSG, reduced glutathione/oxidized glutathione; GPX4, glutathione peroxidase 4; HCC, hepatocellular carcinoma; HIF, hypoxia-inducible factor; KLF4, Krüppel-like factor 4; MUFAs, monounsaturated fatty acids; NF-κB, nuclear factor kappaB; OGP, osteogenic growth peptide; PDTX, patient-derived tumor xenografts; PPARγ, proliferator-activated receptor-gamma; ROS, reactive oxygen species; SCC, squamous cell carcinoma; SCD1, stearoyl-coenzyme A desaturase 1; SFAs, saturated fatty acids; SREBP1, sterol-regulated element-binding protein 1; TEAD1, TEA domain family member 1.
Author Contributions
Conceptualization, J.-Y.M. and D.-H.K.; writing—original draft preparation, J.-Y.M. and D.-H.K.; visualization, J.-Y.M. and D.-H.K.; supervision, D.-H.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by a Kyonggi University Research Grant (2020).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Reya T., Morrison S.J., Clarke M.F., Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Jung Y., Kim W.Y. Cancer stem cell targeting: Are we there yet? Arch. Pharm. Res. 2015;38:414–422. doi: 10.1007/s12272-015-0570-2. [DOI] [PubMed] [Google Scholar]
- 3.Vargas T., Moreno-Rubio J., Herranz J., Cejas P., Molina S., Gonzalez-Vallinas M., Mendiola M., Burgos E., Aguayo C., Custodio A.B., et al. ColoLipidGene: Signature of lipid metabolism-related genes to predict prognosis in stage-II colon cancer patients. Oncotarget. 2015;6:7348–7363. doi: 10.18632/oncotarget.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H., Feng Z., He M.L. Lipid metabolism alteration contributes to and maintains the properties of cancer stem cells. Theranostics. 2020;10:7053–7069. doi: 10.7150/thno.41388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mancini R., Noto A., Pisanu M.E., De Vitis C., Maugeri-Sacca M., Ciliberto G. Metabolic features of cancer stem cells: The emerging role of lipid metabolism. Oncogene. 2018;37:2367–2378. doi: 10.1038/s41388-018-0141-3. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S., Roy A., Dwarakanath B.S. Metabolic cooperation and competition in the tumor microenvironment: Implications for therapy. Front. Oncol. 2017;7:68. doi: 10.3389/fonc.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ventura R., Mordec K., Waszczuk J., Wang Z., Lai J., Fridlib M., Buckley D., Kemble G., Heuer T.S. Inhibition of de novo palmitate synthesis by fatty acid synthase induces apoptosis in tumor cells by remodeling cell membranes, inhibiting signaling pathways, and reprogramming gene expression. EBioMedicine. 2015;2:808–824. doi: 10.1016/j.ebiom.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harayama T., Riezman H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell. Biol. 2018;19:281–296. doi: 10.1038/nrm.2017.138. [DOI] [PubMed] [Google Scholar]
- 9.Folmes C.D., Park S., Terzic A. Lipid metabolism greases the stem cell engine. Cell. Metab. 2013;17:153–155. doi: 10.1016/j.cmet.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Wang T., Fahrmann J.F., Lee H., Li Y.J., Tripathi S.C., Yue C., Zhang C., Lifshitz V., Song J., Yuan Y., et al. JAK/STAT3-regulated fatty acid beta-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 2018;27:136–150.e5. doi: 10.1016/j.cmet.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yi M., Li J., Chen S., Cai J., Ban Y., Peng Q., Zhou Y., Zeng Z., Peng S., Li X., et al. Emerging role of lipid metabolism alterations in cancer stem cells. J. Exp. Clin. Cancer Res. 2018;37:118. doi: 10.1186/s13046-018-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igal R.A. Stearoyl-CoA desaturase-1: A novel key player in the mechanisms of cell proliferation, programmed cell death and transformation to cancer. Carcinogenesis. 2010;31:1509–1515. doi: 10.1093/carcin/bgq131. [DOI] [PubMed] [Google Scholar]
- 13.Ntambi J.M. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J. Lipid Res. 1999;40:1549–1558. doi: 10.1016/S0022-2275(20)33401-5. [DOI] [PubMed] [Google Scholar]
- 14.Ntambi J.M. Dietary regulation of stearoyl-CoA desaturase 1 gene expression in mouse liver. J. Biol. Chem. 1992;267:10925–10930. doi: 10.1016/S0021-9258(19)50107-7. [DOI] [PubMed] [Google Scholar]
- 15.Li J., Condello S., Thomes-Pepin J., Ma X., Xia Y., Hurley T.D., Matei D., Cheng J.X. Lipid Desaturation Is a Metabolic Marker and Therapeutic Target of Ovarian Cancer Stem Cells. Cell Stem Cell. 2017;20:303–314.e5. doi: 10.1016/j.stem.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peck B., Schug Z.T., Zhang Q., Dankworth B., Jones D.T., Smethurst E., Patel R., Mason S., Jiang M., Saunders R., et al. Inhibition of fatty acid desaturation is detrimental to cancer cell survival in metabolically compromised environments. Cancer Metab. 2016;4:6. doi: 10.1186/s40170-016-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai Y., McCoy J.G., Levin E.J., Sobrado P., Rajashankar K.R., Fox B.G., Zhou M. X-ray structure of a mammalian stearoyl-CoA desaturase. Nature. 2015;524:252–256. doi: 10.1038/nature14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H., Klein M.G., Zou H., Lane W., Snell G., Levin I., Li K., Sang B.C. Crystal structure of human stearoyl-coenzyme A desaturase in complex with substrate. Nat. Struct. Mol. Biol. 2015;22:581–585. doi: 10.1038/nsmb.3049. [DOI] [PubMed] [Google Scholar]
- 19.Man W.C., Miyazaki M., Chu K., Ntambi J.M. Membrane topology of mouse stearoyl-CoA desaturase 1. J. Biol. Chem. 2006;281:1251–1260. doi: 10.1074/jbc.M508733200. [DOI] [PubMed] [Google Scholar]
- 20.Shen J., Wu G., Tsai A.L., Zhou M. Structure and mechanism of a unique diiron center in mammalian stearoyl-CoA desaturase. J. Mol. Biol. 2020;432:5152–5161. doi: 10.1016/j.jmb.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaestner K.H., Ntambi J.M., Kelly T.J., Jr., Lane M.D. Differentiation-induced gene expression in 3T3-L1 preadipocytes. A second differentially expressed gene encoding stearoyl-CoA desaturase. J. Biol. Chem. 1989;264:14755–14761. doi: 10.1016/S0021-9258(18)63763-9. [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki M., Jacobson M.J., Man W.C., Cohen P., Asilmaz E., Friedman J.M., Ntambi J.M. Identification and characterization of murine SCD4, a novel heart-specific stearoyl-CoA desaturase isoform regulated by leptin and dietary factors. J. Biol. Chem. 2003;278:33904–33911. doi: 10.1074/jbc.M304724200. [DOI] [PubMed] [Google Scholar]
- 23.Ntambi J.M., Buhrow S.A., Kaestner K.H., Christy R.J., Sibley E., Kelly T.J., Jr., Lane M.D. Differentiation-induced gene expression in 3T3-L1 preadipocytes. Characterization of a differentially expressed gene encoding stearoyl-CoA desaturase. J. Biol. Chem. 1988;263:17291–17300. doi: 10.1016/S0021-9258(19)77834-X. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Y., Prouty S.M., Harmon A., Sundberg J.P., Stenn K.S., Parimoo S. Scd3-a novel gene of the stearoyl-CoA desaturase family with restricted expression in skin. Genomics. 2001;71:182–191. doi: 10.1006/geno.2000.6429. [DOI] [PubMed] [Google Scholar]
- 25.Beiraghi S., Zhou M., Talmadge C.B., Went-Sumegi N., Davis J.R., Huang D., Saal H., Seemayer T.A., Sumegi J. Identification and characterization of a novel gene disrupted by a pericentric inversion inv(4)(p13.1q21.1) in a family with cleft lip. Gene. 2003;309:11–21. doi: 10.1016/S0378-1119(03)00461-X. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L., Ge L., Parimoo S., Stenn K., Prouty S.M. Human stearoyl-CoA desaturase: Alternative transcripts generated from a single gene by usage of tandem polyadenylation sites. Biochem. J. 1999;340:255–264. doi: 10.1042/bj3400255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ntambi J.M., Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res. 2004;43:91–104. doi: 10.1016/S0163-7827(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 28.Sinner D.I., Kim G.J., Henderson G.C., Igal R.A. StearoylCoA desaturase-5: A novel regulator of neuronal cell proliferation and differentiation. PLoS ONE. 2012;7:e39787. doi: 10.1371/journal.pone.0039787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paton C.M., Ntambi J.M. Biochemical and physiological function of stearoyl-CoA desaturase. Am. J. Physiol. Endocrinol. Metab. 2009;297:E28–E37. doi: 10.1152/ajpendo.90897.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spector A.A., Yorek M.A. Membrane lipid composition and cellular function. J. Lipid Res. 1985;26:1015–1035. doi: 10.1016/S0022-2275(20)34276-0. [DOI] [PubMed] [Google Scholar]
- 31.Flowers M.T., Ntambi J.M. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr. Opin. Lipidol. 2008;19:248–256. doi: 10.1097/MOL.0b013e3282f9b54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park E.I., Paisley E.A., Mangian H.J., Swartz D.A., Wu M.X., O’Morchoe P.J., Behr S.R., Visek W.J., Kaput J. Lipid level and type alter stearoyl CoA desaturase mRNA abundance differently in mice with distinct susceptibilities to diet-influenced diseases. J. Nutr. 1997;127:566–573. doi: 10.1093/jn/127.4.566. [DOI] [PubMed] [Google Scholar]
- 33.Sivaramakrishnan M.R., Pynadath T.I. Increased liver oleic acid synthesis in cholesterol-fed rabbits. Atherosclerosis. 1982;41:21–25. doi: 10.1016/0021-9150(82)90065-X. [DOI] [PubMed] [Google Scholar]
- 34.Enser M. The role of insulin in the regulation of stearic acid desaturase activity in liver and adipose tissue from obese-hyperglycaemic (ob/ob) and lean mice. Biochem. J. 1979;180:551–558. doi: 10.1042/bj1800551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith S.B., Mersmann H.J., Smith E.O., Britain K.G. Stearoyl-coenzyme A desaturase gene expression during growth in adipose tissue from obese and crossbred pigs. J. Anim. Sci. 1999;77:1710–1716. doi: 10.2527/1999.7771710x. [DOI] [PubMed] [Google Scholar]
- 36.Lu J., Pei H., Kaeck M., Thompson H.J. Gene expression changes associated with chemically induced rat mammary carcinogenesis. Mol. Carcinog. 1997;20:204–215. doi: 10.1002/(SICI)1098-2744(199710)20:2<204::AID-MC7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 37.Thai S.F., Allen J.W., DeAngelo A.B., George M.H., Fuscoe J.C. Detection of early gene expression changes by differential display in the livers of mice exposed to dichloroacetic acid. Carcinogenesis. 2001;22:1317–1322. doi: 10.1093/carcin/22.8.1317. [DOI] [PubMed] [Google Scholar]
- 38.Igal R.A. Roles of Stearoyl-CoA desaturase-1 in the regulation of cancer cell growth, survival and tumorigenesis. Cancers. 2011;3:2462–2477. doi: 10.3390/cancers3022462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scaglia N., Igal R.A. Inhibition of Stearoyl-CoA Desaturase 1 expression in human lung adenocarcinoma cells impairs tumorigenesis. Int. J. Oncol. 2008;33:839–850. [PubMed] [Google Scholar]
- 40.von Roemeling C.A., Marlow L.A., Wei J.J., Cooper S.J., Caulfield T.R., Wu K., Tan W.W., Tun H.W., Copland J.A. Stearoyl-CoA desaturase 1 is a novel molecular therapeutic target for clear cell renal cell carcinoma. Clin. Cancer Res. 2013;19:2368–2380. doi: 10.1158/1078-0432.CCR-12-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nashed M., Chisholm J.W., Igal R.A. Stearoyl-CoA desaturase activity modulates the activation of epidermal growth factor receptor in human lung cancer cells. Exp. Biol. Med. 2012;237:1007–1017. doi: 10.1258/ebm.2012.012126. [DOI] [PubMed] [Google Scholar]
- 42.Holder A.M., Gonzalez-Angulo A.M., Chen H., Akcakanat A., Do K.A., Fraser Symmans W., Pusztai L., Hortobagyi G.N., Mills G.B., Meric-Bernstam F. High stearoyl-CoA desaturase 1 expression is associated with shorter survival in breast cancer patients. Breast Cancer Res. Treat. 2013;137:319–327. doi: 10.1007/s10549-012-2354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fritz V., Benfodda Z., Rodier G., Henriquet C., Iborra F., Avances C., Allory Y., de la Taille A., Culine S., Blancou H., et al. Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes with oncogenic signaling and blocks prostate cancer progression in mice. Mol. Cancer Ther. 2010;9:1740–1754. doi: 10.1158/1535-7163.MCT-09-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du X., Wang Q.R., Chan E., Merchant M., Liu J., French D., Ashkenazi A., Qing J. FGFR3 stimulates stearoyl CoA desaturase 1 activity to promote bladder tumor growth. Cancer Res. 2012;72:5843–5855. doi: 10.1158/0008-5472.CAN-12-1329. [DOI] [PubMed] [Google Scholar]
- 45.Roongta U.V., Pabalan J.G., Wang X., Ryseck R.P., Fargnoli J., Henley B.J., Yang W.P., Zhu J., Madireddi M.T., Lawrence R.M., et al. Cancer cell dependence on unsaturated fatty acids implicates stearoyl-CoA desaturase as a target for cancer therapy. Mol. Cancer Res. 2011;9:1551–1561. doi: 10.1158/1541-7786.MCR-11-0126. [DOI] [PubMed] [Google Scholar]
- 46.Mason P., Liang B., Li L., Fremgen T., Murphy E., Quinn A., Madden S.L., Biemann H.P., Wang B., Cohen A., et al. SCD1 inhibition causes cancer cell death by depleting mono-unsaturated fatty acids. PLoS ONE. 2012;7:e33823. doi: 10.1371/journal.pone.0033823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Igal R.A. Stearoyl CoA desaturase-1: New insights into a central regulator of cancer metabolism. Biochim. Biophys. Acta. 2016;1861:1865–1880. doi: 10.1016/j.bbalip.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Hess D., Chisholm J.W., Igal R.A. Inhibition of stearoylCoA desaturase activity blocks cell cycle progression and induces programmed cell death in lung cancer cells. PLoS ONE. 2010;5:e11394. doi: 10.1371/journal.pone.0011394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scaglia N., Igal R.A. Stearoyl-CoA desaturase is involved in the control of proliferation, anchorage-independent growth, and survival in human transformed cells. J. Biol. Chem. 2005;280:25339–25349. doi: 10.1074/jbc.M501159200. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y., Wang H., Zhang J., Lv J., Huang Y. Positive feedback loop and synergistic effects between hypoxia-inducible factor-2alpha and stearoyl-CoA desaturase-1 promote tumorigenesis in clear cell renal cell carcinoma. Cancer Sci. 2013;104:416–422. doi: 10.1111/cas.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katoh Y., Yaguchi T., Kubo A., Iwata T., Morii K., Kato D., Ohta S., Satomi R., Yamamoto Y., Oyamada Y., et al. Inhibition of stearoyl-CoA desaturase 1 (SCD1) enhances the antitumor T cell response through regulating beta-catenin signaling in cancer cells and ER stress in T cells and synergizes with anti-PD-1 antibody. J. Immunother. Cancer. 2022;10:e004616. doi: 10.1136/jitc-2022-004616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang G.M., Jiang Q.H., Cai C., Qu M., Shen W. SCD1 negatively regulates autophagy-induced cell death in human hepatocellular carcinoma through inactivation of the AMPK signaling pathway. Cancer Lett. 2015;358:180–190. doi: 10.1016/j.canlet.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 53.Pisanu M.E., Noto A., De Vitis C., Morrone S., Scognamiglio G., Botti G., Venuta F., Diso D., Jakopin Z., Padula F., et al. Blockade of Stearoyl-CoA-desaturase 1 activity reverts resistance to cisplatin in lung cancer stem cells. Cancer Lett. 2017;406:93–104. doi: 10.1016/j.canlet.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 54.Levy J.M.M., Towers C.G., Thorburn A. Targeting autophagy in cancer. Nat. Rev. Cancer. 2017;17:528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ono A., Sano O., Kazetani K.I., Muraki T., Imamura K., Sumi H., Matsui J., Iwata H. Feedback activation of AMPK-mediated autophagy acceleration is a key resistance mechanism against SCD1 inhibitor-induced cell growth inhibition. PLoS ONE. 2017;12:e0181243. doi: 10.1371/journal.pone.0181243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu X., Xiang J., Li Y., Xia Y., Xu S., Gao X., Qiao S. Inhibition of Stearoyl-CoA Desaturase 1 potentiates anti-tumor activity of amodiaquine in non-small cell lung cancer. Biol. Pharm. Bull. 2022;45:438–445. doi: 10.1248/bpb.b21-00843. [DOI] [PubMed] [Google Scholar]
- 57.Mukherjee A., Kenny H.A., Lengyel E. Unsaturated fatty acids maintain cancer cell stemness. Cell Stem Cell. 2017;20:291–292. doi: 10.1016/j.stem.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song M., Lee H., Nam M.H., Jeong E., Kim S., Hong Y., Kim N., Yim H.Y., Yoo Y.J., Kim J.S., et al. Loss-of-function screens of druggable targetome against cancer stem-like cells. FASEB J. 2017;31:625–635. doi: 10.1096/fj.201600953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hagve T.A. Effects of unsaturated fatty acids on cell membrane functions. Scand. J. Clin. Lab. Investig. 1988;48:381–388. doi: 10.1080/00365518809085746. [DOI] [PubMed] [Google Scholar]
- 60.Zhao W., Prijic S., Urban B.C., Tisza M.J., Zuo Y., Li L., Tan Z., Chen X., Mani S.A., Chang J.T. Candidate Antimetastasis Drugs Suppress the Metastatic Capacity of Breast Cancer Cells by Reducing Membrane Fluidity. Cancer Res. 2016;76:2037–2049. doi: 10.1158/0008-5472.CAN-15-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El Helou R., Pinna G., Cabaud O., Wicinski J., Bhajun R., Guyon L., Rioualen C., Finetti P., Gros A., Mari B., et al. miR-600 acts as a bimodal switch that regulates breast cancer stem cell fate through WNT signaling. Cell Rep. 2017;18:2256–2268. doi: 10.1016/j.celrep.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 62.Xuan Y., Wang H., Yung M.M., Chen F., Chan W.S., Chan Y.S., Tsui S.K., Ngan H.Y., Chan K.K., Chan D.W. SCD1/FADS2 fatty acid desaturases equipoise lipid metabolic activity and redox-driven ferroptosis in ascites-derived ovarian cancer cells. Theranostics. 2022;12:3534–3552. doi: 10.7150/thno.70194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noto A., Raffa S., De Vitis C., Roscilli G., Malpicci D., Coluccia P., Di Napoli A., Ricci A., Giovagnoli M.R., Aurisicchio L., et al. Stearoyl-CoA desaturase-1 is a key factor for lung cancer-initiating cells. Cell Death Dis. 2013;4:e947. doi: 10.1038/cddis.2013.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang J., Fan X.X., He J., Pan H., Li R.Z., Huang L., Jiang Z., Yao X.J., Liu L., Leung E.L., et al. SCD1 is associated with tumor promotion, late stage and poor survival in lung adenocarcinoma. Oncotarget. 2016;7:39970–39979. doi: 10.18632/oncotarget.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noto A., De Vitis C., Pisanu M.E., Roscilli G., Ricci G., Catizone A., Sorrentino G., Chianese G., Taglialatela-Scafati O., Trisciuoglio D., et al. Stearoyl-CoA-desaturase 1 regulates lung cancer stemness via stabilization and nuclear localization of YAP/TAZ. Oncogene. 2017;36:4573–4584. doi: 10.1038/onc.2017.75. [DOI] [PubMed] [Google Scholar]
- 66.Zhang J., Song F., Zhao X., Jiang H., Wu X., Wang B., Zhou M., Tian M., Shi B., Wang H., et al. EGFR modulates monounsaturated fatty acid synthesis through phosphorylation of SCD1 in lung cancer. Mol. Cancer. 2017;16:127. doi: 10.1186/s12943-017-0704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma M.K.F., Lau E.Y.T., Leung D.H.W., Lo J., Ho N.P.Y., Cheng L.K.W., Ma S., Lin C.H., Copland J.A., Ding J., et al. Stearoyl-CoA desaturase regulates sorafenib resistance via modulation of ER stress-induced differentiation. J. Hepatol. 2017;67:979–990. doi: 10.1016/j.jhep.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 68.Ma X.L., Sun Y.F., Wang B.L., Shen M.N., Zhou Y., Chen J.W., Hu B., Gong Z.J., Zhang X., Cao Y., et al. Sphere-forming culture enriches liver cancer stem cells and reveals Stearoyl-CoA desaturase 1 as a potential therapeutic target. BMC Cancer. 2019;19:760. doi: 10.1186/s12885-019-5963-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qin X.Y., Su T., Yu W., Kojima S. Lipid desaturation-associated endoplasmic reticulum stress regulates MYCN gene expression in hepatocellular carcinoma cells. Cell Death Dis. 2020;11:66. doi: 10.1038/s41419-020-2257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heijmans J., van Lidth de Jeude J.F., Koo B.K., Rosekrans S.L., Wielenga M.C., van de Wetering M., Ferrante M., Lee A.S., Onderwater J.J., Paton J.C., et al. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Rep. 2013;3:1128–1139. doi: 10.1016/j.celrep.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 71.Miharada K., Sigurdsson V., Karlsson S. Dppa5 improves hematopoietic stem cell activity by reducing endoplasmic reticulum stress. Cell Rep. 2014;7:1381–1392. doi: 10.1016/j.celrep.2014.04.056. [DOI] [PubMed] [Google Scholar]
- 72.Jain P., Nattakom M., Holowka D., Wang D.H., Thomas Brenna J., Ku A.T., Nguyen H., Ibrahim S.F., Tumbar T. Runx1 role in epithelial and cancer cell proliferation implicates lipid metabolism and Scd1 and Soat1 activity. Stem Cells. 2018;36:1603–1616. doi: 10.1002/stem.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scheitz C.J., Lee T.S., McDermitt D.J., Tumbar T. Defining a tissue stem cell-driven Runx1/Stat3 signalling axis in epithelial cancer. EMBO J. 2012;31:4124–4139. doi: 10.1038/emboj.2012.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Piao C., Cui X., Zhan B., Li J., Li Z., Li Z., Liu X., Bi J., Zhang Z., Kong C. Inhibition of stearoyl CoA desaturase-1 activity suppresses tumour progression and improves prognosis in human bladder cancer. J. Cell. Mol. Med. 2019;23:2064–2076. doi: 10.1111/jcmm.14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu Y., Kim H., Choi S., Yu J., Lee J.Y., Lee H., Yoon S., Kim W.Y. Targeting a lipid desaturation enzyme, SCD1, selectively eliminates colon cancer stem cells through the suppression of Wnt and NOTCH signaling. Cells. 2021;10:106. doi: 10.3390/cells10010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun M., Yang Z. Metabolomic studies of live single cancer stem cells using mass spectrometry. Anal. Chem. 2019;91:2384–2391. doi: 10.1021/acs.analchem.8b05166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi S., Yoo Y.J., Kim H., Lee H., Chung H., Nam M.H., Moon J.Y., Lee H.S., Yoon S., Kim W.Y. Clinical and biochemical relevance of monounsaturated fatty acid metabolism targeting strategy for cancer stem cell elimination in colon cancer. Biochem. Biophys. Res. Commun. 2019;519:100–105. doi: 10.1016/j.bbrc.2019.08.137. [DOI] [PubMed] [Google Scholar]
- 78.Sun M., Chen X., Yang Z. Single cell mass spectrometry studies reveal metabolomic features and potential mechanisms of drug-resistant cancer cell lines. Anal. Chim. Acta. 2022;1206:339761. doi: 10.1016/j.aca.2022.339761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Srivastava N.K., Pradhan S., Gowda G.A., Kumar R. In vitro, high-resolution 1H and 31P NMR based analysis of the lipid components in the tissue, serum, and CSF of the patients with primary brain tumors: One possible diagnostic view. NMR Biomed. 2010;23:113–122. doi: 10.1002/nbm.1427. [DOI] [PubMed] [Google Scholar]
- 80.Pinkham K., Park D.J., Hashemiaghdam A., Kirov A.B., Adam I., Rosiak K., da Hora C.C., Teng J., Cheah P.S., Carvalho L., et al. Stearoyl CoA desaturase is essential for regulation of endoplasmic reticulum homeostasis and tumor growth in glioblastoma cancer stem cells. Stem Cell. Rep. 2019;12:712–727. doi: 10.1016/j.stemcr.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao Y., Li J., Xi H., Cui J., Zhang K., Zhang J., Zhang Y., Xu W., Liang W., Zhuang Z., et al. Stearoyl-CoA-desaturase-1 regulates gastric cancer stem-like properties and promotes tumour metastasis via Hippo/YAP pathway. Br. J. Cancer. 2020;122:1837–1847. doi: 10.1038/s41416-020-0827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang C., Jin Y.Y., Mei J., Hu D., Jiao X., Che H.L., Tang C.L., Zhang Y., Wu G.S. Identification of icaritin derivative IC2 as an SCD-1 inhibitor with anti-breast cancer properties through induction of cell apoptosis. Cancer Cell Int. 2022;22:202. doi: 10.1186/s12935-022-02621-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu S., Lu Y., Su A., Chen J., Li J., Zhou B., Liu X., Xia Q., Li Y., Li J., et al. A CD10-OGP membrane peptolytic signaling axis in fibroblasts regulates lipid metabolism of cancer stem cells via SCD1. Adv. Sci. 2021;8:e2101848. doi: 10.1002/advs.202101848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mullauer F.B., Kessler J.H., Medema J.P. Betulinic acid, a natural compound with potent anticancer effects. Anticancer Drugs. 2010;21:215–227. doi: 10.1097/CAD.0b013e3283357c62. [DOI] [PubMed] [Google Scholar]
- 85.Zuco V., Supino R., Righetti S.C., Cleris L., Marchesi E., Gambacorti-Passerini C., Formelli F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002;175:17–25. doi: 10.1016/S0304-3835(01)00718-2. [DOI] [PubMed] [Google Scholar]
- 86.Potze L., Di Franco S., Grandela C., Pras-Raves M.L., Picavet D.I., van Veen H.A., van Lenthe H., Mullauer F.B., van der Wel N.N., Luyf A., et al. Betulinic acid induces a novel cell death pathway that depends on cardiolipin modification. Oncogene. 2016;35:427–437. doi: 10.1038/onc.2015.102. [DOI] [PubMed] [Google Scholar]
- 87.Wang H., Dong F., Wang Y., Wang X., Hong D., Liu Y., Zhou J. Betulinic acid induces apoptosis of gallbladder cancer cells via repressing SCD1. Acta Biochim. Biophys. Sin. 2020;52:200–206. doi: 10.1093/abbs/gmz148. [DOI] [PubMed] [Google Scholar]
- 88.Potze L., di Franco S., Kessler J.H., Stassi G., Medema J.P. Betulinic Acid Kills Colon Cancer Stem Cells. Curr. Stem Cell Res. Ther. 2016;11:427–433. doi: 10.2174/1574888X11666151203223512. [DOI] [PubMed] [Google Scholar]
- 89.Mansouri K., Rasoulpoor S., Daneshkhah A., Abolfathi S., Salari N., Mohammadi M., Rasoulpoor S., Shabani S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer. 2020;20:791. doi: 10.1186/s12885-020-07256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Colacino J.A., McDermott S.P., Sartor M.A., Wicha M.S., Rozek L.S. Transcriptomic profiling of curcumin-treated human breast stem cells identifies a role for stearoyl-coa desaturase in breast cancer prevention. Breast Cancer Res. Treat. 2016;158:29–41. doi: 10.1007/s10549-016-3854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liao C., Li M., Li X., Li N., Zhao X., Wang X., Song Y., Quan J., Cheng C., Liu J., et al. Trichothecin inhibits invasion and metastasis of colon carcinoma associating with SCD-1-mediated metabolite alteration. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids. 2020;1865:158540. doi: 10.1016/j.bbalip.2019.158540. [DOI] [PubMed] [Google Scholar]
- 92.Kundu J.K., Surh Y.J. Emerging avenues linking inflammation and cancer. Free. Radic. Biol. Med. 2012;52:2013–2037. doi: 10.1016/j.freeradbiomed.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 93.Larson E.A., Dalamaga M., Magkos F. The role of exercise in obesity-related cancers: Current evidence and biological mechanisms. Semin. Cancer Biol. 2023;91:16–26. doi: 10.1016/j.semcancer.2023.02.008. [DOI] [PubMed] [Google Scholar]
- 94.Chiefari E., Mirabelli M., La Vignera S., Tanyolac S., Foti D.P., Aversa A., Brunetti A. Insulin resistance and cancer: In search for a causal link. Int. J. Mol. Sci. 2021;22:11137. doi: 10.3390/ijms222011137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lopaschuk G.D. Fatty acid oxidation and its relation with insulin resistance and associated disorders. Ann. Nutr. Metab. 2016;68((Suppl. 3)):15–20. doi: 10.1159/000448357. [DOI] [PubMed] [Google Scholar]
- 96.Garcia-Serrano S., Moreno-Santos I., Garrido-Sanchez L., Gutierrez-Repiso C., Garcia-Almeida J.M., Garcia-Arnes J., Rivas-Marin J., Gallego-Perales J.L., Garcia-Escobar E., Rojo-Martinez G., et al. Stearoyl-CoA desaturase-1 is associated with insulin resistance in morbidly obese subjects. Mol. Med. 2011;17:273–280. doi: 10.2119/molmed.2010.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.