Abstract
Madin-Darby canine kidney (MDCK) epithelial cells transformed by oncogenic Ras and Raf exhibit cell multilayering and alterations in the actin cytoskeleton. The changes in the actin cytoskeleton comprise a loss of actin stress fibers and enhanced cortical actin. Using MDCK cells expressing a conditionally active form of Raf, we have explored the molecular mechanisms that underlie these observations. Raf activation elicited a robust increase in Rac1 activity consistent with the observed increase in cortical actin. Loss of actin stress fibers is indicative of attenuated Rho function, but no change in Rho-GTP levels was detected following Raf activation. However, the loss of actin stress fibers in Raf-transformed cells was preceded by the induced expression of Rnd3, an endogenous inhibitor of Rho protein function. Expression of Rnd3 alone at levels equivalent to those observed following Raf transformation led to a substantial loss of actin stress fibers. Moreover, cells expressing activated RhoA failed to multilayer in response to Raf. Pharmacological inhibition of MEK activation prevented all of the biological and biochemical changes described above. Consequently, the data are consistent with a role for induced Rnd3 expression downstream of the Raf–MEK–extracellular signal-regulated kinase pathway in epithelial oncogenesis.
The most common fatal malignancies are adenocarcinomas, which arise from polarized epithelial cells of the lung, mammary gland, prostate, colon, pancreas, and urinary tract (77). Activating mutations in Ras are found in 20 to 30% of all human cancers, in particular in adenocarcinomas of the pancreas and the colon (8). Hallmarks of early adenocarcinoma development include cell multilayering and loss of apical polarity (16, 62). When grown on permeable supports as a model system, Madin-Darby canine kidney (MDCK) cells transformed by oncogenic Ras mimic these cellular responses (63). However, little is known about the mechanisms underlying the transformation of polarized epithelial cells that result in loss of apical polarity and cell multilayering.
Multiple effectors of Ras can elicit oncogenic transformation (76); one such is the protein kinase Raf, which binds directly to Ras in a GTP-dependent manner (73), resulting in activation of Raf upon recruitment to the plasma membrane (38, 67). In this work, we have examined the transformation of polarized epithelial cells by the Raf-MEK-extracellular signal-regulated kinase (ERK) pathway. Activated forms of Raf, as well as its downstream effector MEK, can promote oncogenic transformation in cultured cells (13, 41, 58). These transformation events are likely mediated by ERK1 and -2, as no other bona fide effectors of MEK have been identified (39). The Raf-MEK-ERK pathway is thought to be important to carcinomas harboring activating mutations in Ras and is constitutively activated in renal cell carcinomas (47). Direct experimental evidence of a role for the Raf-MEK-ERK pathway in growth of colon tumors in mice has recently been provided through the use of an inhibitor of MEK (65).
Expression of both Ras (6) and, as shown here, Raf can elicit alterations in the actin cytoskeleton associated with oncogenic transformation. Such alterations are suggestive of the involvement of Rho family GTP-binding proteins, which play pivotal roles in cell rearrangements occurring during embryonic development and oncogenic transformation (22, 66, 72). It has recently been suggested that increased expression of Rnd proteins may play a role in oncogenic transformation of cells (46). The Rnd proteins Rnd1, -2, and -3, the latter of which is also known as RhoE (18), comprise an unusual branch of Rho family proteins in that they possess very low intrinsic GTPase activity and are constitutively bound to GTP in the cell (18, 21, 46). Transient expression of Rnd proteins in fibroblasts and MDCK cells results in loss of actin stress fibers and focal adhesions (21, 46). Furthermore, transient expression of Rnd proteins in fibroblasts also leads to cell rounding, hence the name Rnd (46). Interestingly, the effects of Rnd proteins on the actin cytoskeleton and focal adhesions are counteracted by an excess of activated RhoA (21, 46). Recently, Xenopus laevis Rnd1 (XRnd1) was isolated in a screen for genes that perturb secondary axis formation in Xenopus embryos; XRnd1 was found to be transiently expressed, while tissues such as the neural crest and somitogenic mesoderm undergo extensive morphogenetic remodeling (79). Moreover, it was demonstrated that microinjection of XRnd1 mRNA perturbed cell adhesion in early embryogenesis which was fully restored by coinjection of mRNA encoding constitutively active RhoA (V14RhoA) (79). These data support the notion that Rnd and Rho proteins possess antagonistic functions.
Here, we show that activated forms of Raf are sufficient to promote transformation of polarized MDCK cells, resulting in a phenotype similar to that elicited by oncogenic Ras. Activation of Raf results in elevated Rac1 activity without affecting the levels of RhoA-GTP or Cdc42-GTP. We further demonstrate that activation of Raf in polarized MDCK cells is accompanied by the induced expression of Rnd3. Modulation of Rho function appears to be critical for the phenotype of Raf-transformed MDCK cells, as constitutive expression of activated RhoA [RhoA(Q63L)] abrogates cell multilayering. The data presented here thus provide evidence that the regulation of Rnd protein expression may play a role in the transformation of epithelial cells in response to Raf activation.
MATERIALS AND METHODS
Reagents.
The reagents used for these studies were 4-hydroxytamoxifen (4-HT; Calbiochem), blasticidin S hydrochloride (Calbiochem), doxycycline (Sigma), EDTA-free protease inhibitor tablets (Boehringer Mannheim), Geneticin (GibcoBRL), Lipofectamine (GibcoBRL), MEK inhibitor PD098058 (New England Biolabs), MEK inhibitor U0126 (Promega), phleomycin (Sigma), Polybrene (Sigma), and puromycin (Sigma).
Constructs and cell lines.
Medium containing amphotropic virus harboring a retrovirus construct encoding the ecotropic virus receptor and the bleomycin resistance gene was harvested from PT-67 cells. The medium was supplemented with Polybrene (8 μg/ml) and used to infect low-passage MDCK II cells. A pooled population MDCK cells expressing the ecotropic receptor was isolated by phleomycin selection. This MDCK-EcoR cell line was used for subsequent retroviral transductions using replication-defective retrovirus stocks produced by transient transfection of Phoenix-E ecotropic packaging cells as described previously (11). cDNAs encoding K-Ras and Raf-CAAX have previously been described (31, 38). The cDNAs were transferred to pLXSP3/pBabe-Puro3 and expressed in MDCK-EcoR cells. Pooled populations of K-Ras, Raf-CAAX, and vector control cells were selected by resistance to puromycin. MDCK cell clones expressing K-Ras or the vector control, pMV7, have been characterized extensively by others (63).
Constructs based on EGFPΔRaf-1:ER, a conditionally active form of Raf-1 which is fused at the N terminus to enhanced green fluorescent protein (EGFP) (78), have been described in detail elsewhere (78, 81). For the experiments described in this work, MDCK cells expressing the EGFPΔRaf-1[DD]:ER were used. However, similar results were obtained with cells expressing EGFPΔRaf-1[YY]:ER, which possesses lower Raf kinase activity (data not shown). The EGFPΔRaf-1:ER constructs conferring resistance to blasticidin were expressed in MDCK cells by the calcium phosphate coprecipitation method, and drug-resistant clones were isolated by ring cloning as previously described (23). Transfectants were screened by fluorescence microscopy, and clones exhibiting homogeneous green fluorescence were selected for further experimentation. A total of three EGFPΔRaf-1[DD]:ER- and six EGFPΔRaf-1[YY]:ER-expressing clones were used for initial experiments. All clones exhibited similar phenotypes in response to Raf activation. Subsequent experiments were carried out with a representative EGFPΔRaf-1[DD]:ER clone. Key results were verified using a representative EGFPΔRaf-1[YY]:ER clone. The ecotropic virus receptor was expressed in the representative EGFPΔRaf-1[DD]:ER clone by using the methods described above.
pZIP-RhoA(Q63L) was provided by Channing Der (University of North Carolina at Chapel Hill) and has been characterized previously (30). A BamHI fragment encoding human RhoA(Q63L) was transferred to pWZL-Neo and expressed by retroviral transduction in MDCK cells coexpressing EGFPΔRaf-1[DD]:ER and the ecotropic virus receptor. Clones were selected using G418 and screened by Western blotting of whole cell lysates using mouse monoclonal anti-RhoA (26C4; Santa Cruz Biotechnology) and by fluorescence microscopy of cells labeled with fluorescent phalloidin to detect actin stress fibers as detailed below. Three clones expressing RhoA(Q63L) were selected based on the Western blots and by the abundance of actin stress fibers, which is an indicator of the function of activated Rho (28, 56, 68).
To prepare MDCK cell lines expressing Rnd3 under control of the tetracycline-regulatable transactivator (t-TA) (20), a cDNA comprising a Kozak consensus translation initiation sequence and the coding region of Rnd3 was inserted into the pUDH10-3 vector and transfected into the MDCK-T23 clone harboring the Tet-off transactivator (7). For selection, pcDNA6/V5-His/LacZ (Invitrogen) was cotransfected at a 1:100 ratio of the Rnd3 expression plasmid, and 40 clones resistant to blasticidin were isolated. Clones were selected and maintained in the presence of doxycycline (20 ng/ml). Two clones that exhibited inducible regulation of Rnd3 expression were obtained; these were used in parallel for experiments and yielded similar results. To induce Rnd3 expression, doxycycline was removed by washing the cells four times with medium without doxycycline.
Cell culture.
All MDCK II cell lines were grown in phenol red-free Dulbecco Modified Eagle medium supplemented with 5% fetal calf serum, 2 mM l-glutamine, penicillin, and streptomycin and maintained at 37°C in 5% CO2. The cell lines were split 1:10 once a week with two changes of medium in between. For experiments, MDCK cell lines were plated on either 12-, 24-, or 72-mm-diameter Transwell polycarbonate filters (0.4-μm pore size; Costar) at a density of 2.5 × 105 cells/cm2 and grown for 7 days, with changes of medium every 2 days and the day before experimentation. For biochemical experiments, 100-mm-diameter dishes were used in some instances, but results were confirmed with cells grown on 72 mm-diameter Transwells. Cells expressing EGFPΔRaf-1:ER constructs were treated with 1 μM 4-HT to activate Raf for up to 66 h before experimentation. Treatment with the MEK inhibitors PD098059 (1) and U0126 (14) was started 1 h before the addition of 4-HT. Controls were incubated with the appropriate concentrations of solvent. Transepithelial resistance (TER) measurements were carried out as previously described (23). To quantify the cell number, filters were excised and trypsinized. Cell counts were subsequently performed essentially as previously described (24).
Detection of Rnd3 by immunoprecipitation and Western blotting.
Antisera against recombinant Rnd1 have been described previously (46). Antisera detecting Rnd2 and Rnd3 were raised against recombinant proteins as described for Rnd1. The reactivity of anti-Rnd antisera was determined by Western blotting of recombinant Rnd proteins. For detection of Rnd3, MDCK cells from 72-mm-diameter Transwell filters or 100-mm-diameter dishes were rinsed twice with phosphate-buffered saline (PBS) and scraped into 1 ml of ice-cold modified Gold lysis buffer (1% Triton X-100, 20 mM Tris [pH 8.0], 137 mM NaCl, 10 mM MgCl2, 15% glycerol) containing protease inhibitors. Samples were rotated for 30 to 60 min at 4°C to allow complete cell lysis. Triton X-100-insoluble material was pelleted by centrifugation, and the supernatant was recovered. Protein concentrations were determined by the bicinchoninic acid protein assay (Pierce) and equalized among samples in a given experiment. One-milliliter aliquots of precleared lysate typically containing 2 mg of total protein were incubated overnight at 4°C with a 1:300 dilution of anti-Rnd3#1, which detects recombinant Rnd1, -2, and -3 on Western blots. For the last hour, 20 μl of a 50% slurry of protein A-Sepharose was added. Immunoprecipitates were washed three times with ice-cold lysis buffer. Precipitated proteins were resolved on 16% Tris-glycine gels and transferred to nitrocellulose. Membranes were blocked in BLOTTO (5% nonfat dry milk and 0.1% Triton X-100 in Tris-buffered saline) and probed for 1 h at room temperature with a 1:1,000 dilution of anti-Rnd3#2, which specifically detects Rnd3. Finally, membranes were incubated 30 min at room temperature with a 1:5,000 dilution of protein A-horseradish peroxidase (HRP) (Amersham Pharmacia Biotech), and antigen-antibody complexes were visualized by enhanced chemiluminescence (Renaissance; Dupont NEN) using Hyperfilm (Amersham Pharmacia Biotech).
When antisera specific for Rnd1 or -2 on Western blots were substituted for anti-Rnd3#2, no specific reactivity was detected. Substitution in the immunoprecipitation step with antiserum specific for Rnd1, -2, or -3 confirmed these results. Only when reagents detecting Rnd3 were included in both the immunoprecipitation and the Western blotting was a reaction at the specific mobility obtained. To test the possibility that upregulation of Rnd3 expression by Raf was not an artifact resulting from Rnd3 moving from a Triton X-100-insoluble to a soluble fraction, we repeated key experiments using lysates prepared with 0.5% sodium dodecyl sulfate lysis buffer according to a previously described protocol (23). The results were identical to those obtained with Gold lysis buffer.
Phospho-MEK and phospho-ERK Western blots.
MDCK cells on 12-mm-diameter Transwell filters were rinsed twice in PBS and lysed directly in 200 μl of 2× Laemmli buffer. Lysates were boiled for 3 min and vortexed for 20 min at room temperature to shear DNA. Aliquots of 10 μl, corresponding to approximately 5 μg of total protein, were electrophoresed on 10% Tris-glycine gels (Novex, San Diego, Calif.) and transferred to nitrocellulose. Membranes were blocked in BLOTTO and probed with antibodies as follows: affinity-purified rabbit anti-phospho-MEK1/2 (Ser217/221) (New England Biolabs), 1:1,000; affinity-purified rabbit anti-MEK1/2 (New England Biolabs), 1:1,000; mouse monoclonal anti-phospho-p44/42 mitogen-activated protein kinase (Thr202/Tyr204) (New England Biolabs), 1:2,500; and rabbit anti-ERK1/2 (Santa Cruz Biotechnology), 1:5,000. Secondary reagents comprised goat anti-mouse-HRP and goat anti-rabbit-HRP (Jackson ImmunoResearch Laboratories, West Grove, Pa.), used at a dilution of 1:10,000. Western blots were developed as described above.
Rhotekin RBD and Pak3 CRIB assays.
Bacterial expression vectors encoding glutathione S-transferase (GST) fusions of the Rho binding domain (RBD) of rhotekin and the Cdc42 Rac interacting binding (CRIB) domain of human Pak3 were provided by Xiang-Dong Ren and Martin A. Schwartz (University of California, San Diego) and by Shubha Bagrodia and Richard A. Cerione (Cornell University, Ithaca, N.Y.), respectively. Fusion proteins were bound to glutathione beads according to previously published protocols (3, 54). Each batch of fusion protein was assessed for activity on lysates of NIH 3T3 cells which had been either been transiently transfected with expression vectors encoding activated Rho or Rac or treated with cytotoxic necrotizing factor 1 (CNF1). Fusion proteins were subsequently used to determine Rac, Rho, and Cdc42 activities in lysates of confluent MDCK cell monolayers essentially as described previously (3, 54). In brief, cells were rinsed twice in ice-cold PBS, drained thoroughly, and then lysed in ice-cold lysis buffer. For rhotekin RBD assays, cells were lysed in XDR-cell lysis buffer (1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris [pH 7.2], 500 mM NaCl, protease inhibitors). Pak3 CRIB assays were performed on cells lysed in high-salt modified Gold lysis buffer (1% Triton X-100, 20 mM Tris [pH 8.0], 500 mM NaCl, 10 mM MgCl2, 15% glycerol, 0.5 mM dithiothreitol, protease inhibitors). Lysates were cleared by centrifugation for 2 min and then applied to 25 μg of fusion protein per sample. Lysates were rotated at 4°C for 30 min, rinsed twice in ice-cold lysis buffer, and drained with a Hamilton syringe. The samples were resuspended in 20 μl of 2× Laemmli sample buffer, heated to 50°C for 10 min, and electrophoresed on 16% Tris-glycine gels (Novex). Aliquots comprising 1.25% of whole cell lysate were run simultaneously. Following transfer to nitrocellulose, membranes were blocked with BLOTTO and probed with mouse monoclonal antibodies (MAbs) specific for RhoA (26C4; Santa Cruz Biotechnology), Rac1 (clone 102; Transduction Laboratories), and Cdc42 (clone 44; Transduction Laboratories). In addition, phospho-ERK and ERK blots were performed to control for Raf activation and loading, respectively.
Confocal fluorescence microscopy.
Mouse MAbs against the 114-kDa apical protein and the 58-kDa basolateral protein were provided by Karl Matlin (Beth Israel Hospital) and have previously been characterized (4). Mouse MAb against E-cadherin (rr1) and rat MAb against ZO-1 (R40.76) were obtained and used as previously described (5). Secondary reagents comprised Alexa Fluor 488/594 goat anti-mouse immunoglobulin G (heavy plus light chain) [IgG (H+L)] conjugate and Alexa Fluor 594 goat anti-rat IgG (H+L) conjugate (Molecular Probes). To detect polymerized actin, cells were labeled with Alexa Fluor 488/594 phalloidin (Molecular Probes). Both Alexa Fluor 488-phalloidin and Alexa Fluor 488–anti-mouse IgG (H+L) conjugates yielded such a strong signal that interference from the EGFPΔRaf-1:ER construct could be entirely eliminated by appropriate settings of the confocal microscope. Samples were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The blocking buffer consisted of 10% normal goat serum–0.2% fish skin gelatin–0.1% saponin in PBS and was also used for incubations with antibodies and phalloidin probes. Samples were mounted in FluorSave (Calbiochem) and imaged on a Bio-Rad MRC1024 confocal microscope. Image processing and preparation was carried out as described previously (51). X-Z sections were sampled at the center of the corresponding X-Y sections unless otherwise indicated. All figures included in this work are representative of at least three independent experiments.
BrdU incorporation assay.
MDCK cells grown on 12-mm-diameter Transwell filters as described above were incubated with 50 mM bromodeoxyuridine (BrdU) in growth medium for 3 h, after which cells were washed with PBS and fixed for 20 min in 4% paraformaldehyde in 0.1 M sodium phosphate buffer. Cells were then washed with PBS and incubated in 2 N HCl for 1 h. Following neutralization with 0.1 M Na2B4O7, the cells were processed for immunofluorescence microscopy as described above. Cells were incubated overnight at 4°C with a mouse MAb against BrdU (Calbiochem). As secondary antibody, AlexaFluor 488 goat anti-mouse IgG (H+L) was used (Molecular Probes). After incubation with secondary antibody, the cells were washed and treated for 10 min with 0.1 mg of boiled RNase per ml in blocking buffer, followed by a 30-min incubation with the nuclear stain TO-PRO3 (1:500; Molecular Probes). The cells were then rinsed once with PBS, mounted, and examined by confocal microscopy as described above. The percentage of cells that had incorporated BrdU was quantified by determining the ratio of BrdU-positive nuclei and the total number of nuclei in 25 systematically sampled microscopic fields. As a positive control, cells were seeded at subconfluent density (2 × 104 per 12-mm filter) 24 h before the BrdU assay was carried out.
RESULTS
The Raf-MEK-ERK pathway is sufficient to transform polarized MDCK cells.
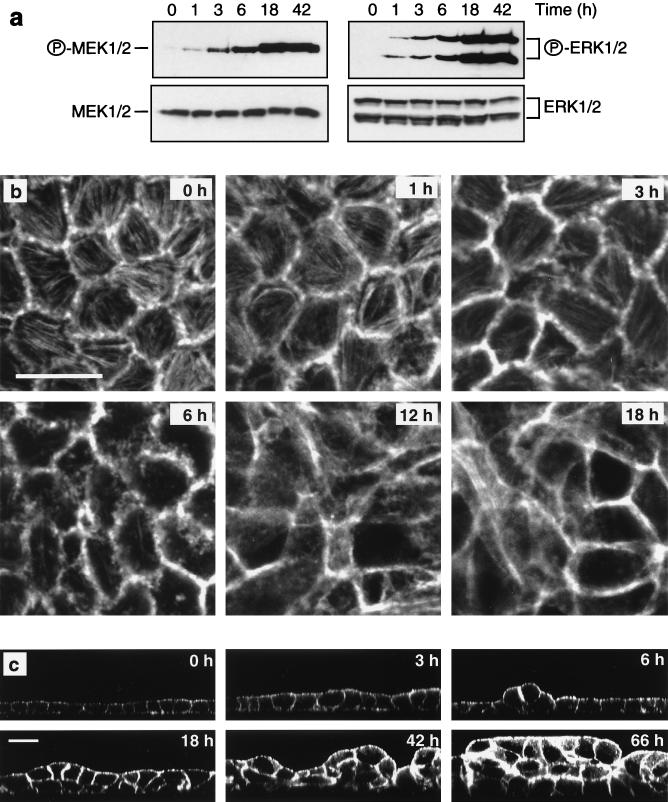
To examine the role of the Raf-MEK-ERK pathway in oncogenesis of polarized epithelial cells, we established clonal MDCK cell lines expressing EGFPΔRaf-1:ER (78). The kinase activity of EGFPΔRaf-1:ER is regulated by estrogen and its analogs such as 4-HT. Activation of EGFPΔRaf-1:ER in polarized MDCK cells resulted in a rapid and sustained activation of MEK and ERK, as detected by Western blotting of whole cell lysates using antibodies specific for activated forms of these proteins (Fig. 1a). The overall levels of MEK and ERK expression were unaffected by Raf activation (Fig. 1a).
FIG. 1.
Time course of Raf-induced transformation of MDCK cells. Polarized MDCK cells expressing EGFPΔRaf-1:ER were treated with 1 μM 4-HT for the indicated periods of time. (a) Activation of MEK and ERK following Raf activation was assayed by Western blotting of whole cell lysates using activation-specific antibodies as described in Materials and Methods. (b and c) Confocal microscopy of MDCK cells labeled with fluorescent phalloidin revealed that the morphological alterations in response to Raf activation comprised progressive changes in cell shape (b and c), loss of actin stress fibers (b), increased cortical actin (b and c), and multilayering (c). (b) X-Y sections; scale bar, 20 μm. (c) X-Z sections; scale bar, 20 μm. All X-Y sections were sampled at the base of the cell monolayers.
Analysis by confocal microscopy of optical sections parallel to the substratum (X-Y sections) of phalloidin-stained MDCK cells following Raf activation revealed alterations in cell morphology beginning within 60 min (Fig. 1b). Initially, we observed discrete aberrations from the normal pentagonal and hexagonal shape of polarized MDCK cells. After 3 h, borders between some cells became poorly demarcated, with phalloidin staining patterns suggestive of membrane ruffling. These changes continued until 12 h after Raf activation, by which time the cell shape had become highly irregular and the cell borders were partially obscured (Fig. 1b). The changes in cell shape that occurred after Raf activation were accompanied by alterations in the actin cytoskeleton. Polarized MDCK cells possess abundant actin stress fibers located along the basal membrane. Within the first 3 h after Raf activation, the number of actin stress fibers appeared to remain constant, but significant thinning of the fibers was evident. The stress fibers then gradually disappeared and were largely absent within 6 to 12 h after activation of Raf (Fig. 1b).
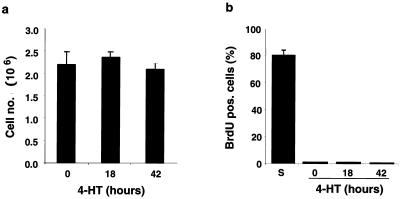
Six hours after Raf activation, cells protruding above the monolayer were observed in optical sections perpendicular to the filter (X-Z sections [Fig. 1c]). Such cells were characterized by intense cortical actin staining. Cell multilayering was evident 12 h after activation of Raf and extensive after 18 h, at which time clusters of cells two to three layers thick covered 20 to 30% of the filter. The multilayering progressed over the next 48 h, with more than 60% of the filter being covered by clusters of cells up to five layers thick (Fig. 1c) as previously described for MDCK cells expressing oncogenic Ras (63). However, the multilayering did not result from an increase in cell number or cell proliferation. In multiple determinations, the number of cells per filter remained constant and BrdU labeling revealed that cells were not transiting through S phase in either the absence or presence of active Raf (Fig. 2). Thus, multilayering of epithelial cells in response to oncogenic transformation can occur without cell division. We also did not detect any change in the number of apoptotic nuclear profiles as detected by TO-PRO staining (data not shown). The multilayering following Raf activation thus appears to comprise cell-cell rearrangements, probably resulting from increased cell motility conferred by activation of the Raf-MEK-ERK pathway (33), and an increase in cell size (Fig. 1c) as previously reported for Raf transformed NIH 3T3 cells (29). The transformation of polarized MDCK cells expressing EGFPΔRaf-1:ER was entirely dependent on Raf activation. No signs of transformation were observed in control MDCK cells or in cells expressing either the estrogen receptor moiety or EGFP alone in the absence or presence of 4-HT (data not shown). Moreover, MDCK cells transduced with Raf-CAAX, a constitutively activated form of Raf, also exhibited cell multilayering as well as the alterations in the actin cytoskeleton described above (data not shown).
FIG. 2.
Confluent MDCK cells are arrested both with and without Raf activation. (a) Cell counts evidenced that Raf activation in polarized MDCK cells does not result in a significant change in cell number. (b) BrdU incorporation to determine the fraction of cells in S phase carried out as described in Materials and Methods showed that polarized MDCK effectively remain arrested following activation of Raf. For comparison, BrdU incorporation in a subconfluent culture is shown (S).
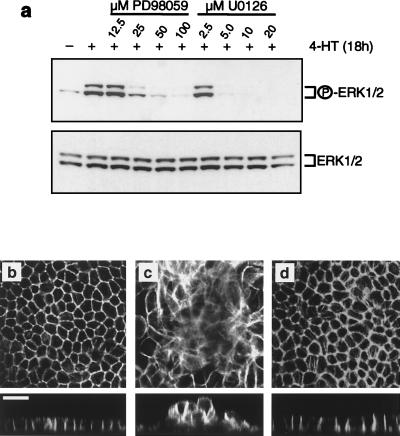
To address the role of downstream effectors of Raf in epithelial oncogenesis, we used the pharmacological inhibitors of MEK, PD098059 and U0126, at concentrations that were sufficient to abrogate Raf-induced ERK activation (Fig. 3a). Preincubation with either PD098059 (50 μM [Fig. 3b to d) or U0126 (10 μM [data not shown]) prior to activation of Raf abrogated the changes in cell shape and the effects of Raf on the actin cytoskeleton as well as cell multilayering. These results indicate that oncogenic transformation of MDCK cells by Raf is dependent on activation of MEK. Since the only known bona fide targets of MEK are ERK1 and -2, the data implicate ERK1 and -2 activation in epithelial oncogenesis by Raf.
FIG. 3.
MEK inhibitors abrogate transformation of MDCK cells by Raf. (a) MDCK cells expressing EGFPΔRaf-1:ER were either untreated or treated with 1 μM 4-HT for 18 h in the absence or presence of the indicated concentrations of the MEK inhibitor PD098059 or U0126. Activation of ERK was assayed by Western blotting of whole cell lysates using activation-specific antibodies as described in Materials and Methods. (b to d) EGFPΔRaf-1:ER-expressing MDCK cells were either untreated (b) or treated with 1 μM 4-HT for 18 h alone (c) or with 1 μM 4-HT for 18 h in the presence of 50 μM PD098059 (d). The cells were then fixed and labeled with fluorescent phalloidin and analyzed by confocal microscopy. All X-Y sections were sampled at the base of the cell monolayers. Scale bar, 20 μm.
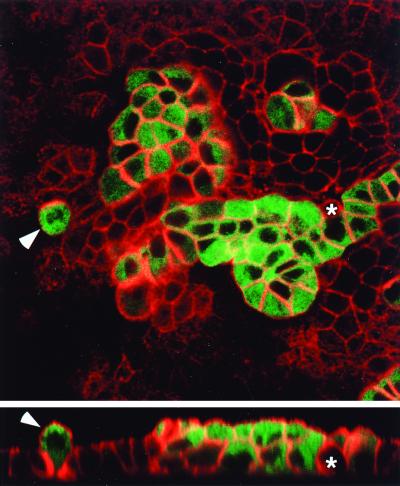
Previous work has shown that Raf-transformed cells secrete autocrine growth factors such as heparin-binding epidermal growth factor (42), which might contribute to the phenotype of MDCK cells described above. To test this possibility, we established cocultures of cells expressing EGFPΔRaf-1:ER and parental MDCK cells plated on permeable supports at ratios ranging from 1:9 to 1:49. With appropriate settings of the confocal microscope, the EGFPΔRaf-1:ER-expressing cells are readily identified based on their green fluorescence. Addition of 4-HT to cocultures of cells expressing EGFPΔRaf-1:ER and normal MDCK cells led to alterations in the actin cytoskeleton and multilayering only in the Raf-expressing population of cells, even when such cells were immediately next to normal cells (Fig. 4). Thus, the phenotype induced by activation of the Raf-MEK-ERK pathway in MDCK cells exhibited a considerable degree of cell autonomy and was not due simply to the release of an autocrine growth factor.
FIG. 4.
Raf transformation of polarized MDCK cells is cell autonomous. MDCK cells expressing EGFPΔRaf-1:ER were mixed with the parental MDCK cell line at a ratio of 1:9; 42 h prior to experimentation, 1 μM 4-HT was added to the culture to activate Raf in the EGFPΔRaf-1:ER-expressing MDCK cells. With appropriate settings on the confocal microscope, EGFPΔRaf-1:ER-expressing MDCK cells can be identified based on the green fluorescence. In addition, the sample was labeled with fluorescent phalloidin to visualize the actin cytoskeleton (in red) in both parental and EGFPΔRaf-1:ER-expressing MDCK cells. The X-Y section was sampled close to the apical surface of the parental monolayer. Under these conditions, the EGFPΔRaf-1:ER-expressing MDCK cells invariably undergo morphological transformation and multilayer even when completely surrounded by parental cells (arrowhead). In contrast, the parental cells, except for changes in shape (asterisk) apparently inflicted by neighboring EGFPΔRaf-1:ER-expressing MDCK cells, show no signs of transformation and retain growth in monolayers. Scale bar, 20 μm.
Effects of Raf activation on apical and basolateral polarity.
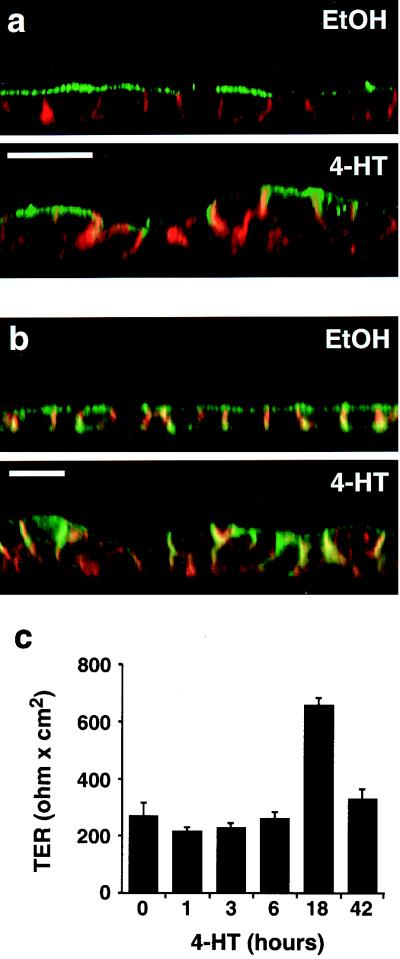
In addition to multilayering and alterations in cell shape, MDCK cells transformed by activated Ras exhibit a redistribution of apical membrane proteins such as the 114-kDa protein (4) to the basolateral surface (63). To compare the effects of Ras and Raf transformation of MDCK cells, we examined the localization of the 114-kDa apical marker protein in MDCK cells following Raf activation. We observed extensive redistribution of the 114-kDa protein from the apical to the basolateral surface of MDCK cells following Raf activation (Fig. 5a). We subsequently determined the effects of Raf on other biological parameters that have been examined in MDCK cells transformed by oncogenic Ras. The cell adhesion molecule E-cadherin was to a large extent retained at areas of cell-cell contact (Fig. 5b). Some intracellular E-cadherin was observed in multilayering MDCK cells, probably as a result of internalization of adherens junction constituents from areas of the plasma membrane no longer engaged in cell-cell contact (Fig. 5b and data not shown). Moreover, the 58-kDa basolateral marker (4) remained confined to the basolateral surface (data not shown). Finally, Raf-transformed MDCK cells maintained functional tight junctions, as evidenced by measurements of TER (Fig. 5c) and by immunolocalization of the tight junction protein ZO-1 (data not shown). Thus, activated Raf largely mimicked the previously described effects of oncogenic Ras in MDCK cells (63). In this respect, MDCK cells seem to differ from rat intestinal epithelial (RIE-1) cells, in which activation of the Raf-MEK-ERK pathway is reported to be insufficient for Ras transformation (48).
FIG. 5.
Effects of Raf activation on cell polarity and on adherens and tight junctions. MDCK cells expressing EGFPΔRaf-1:ER were either untreated or treated with 1 μM 4-HT for 42 h. (a) The cells were fixed and labeled with fluorescent phalloidin to outline cell borders (in red) and immunolabeled to detect the 114-kDa apical marker (in green). Note the extensive redistribution of the 114-kDa apical marker to the lateral cell borders after Raf activation, where it colocalizes with cortical actin (in yellow-orange). Scale bar, 20 μm. (b) Cells were labeled with fluorescent phalloidin to outline cell borders (in green) and immunolabeled to detect the adherens junction constituent E-cadherin (in red). Note that despite the extensive cell-cell rearrangements occurring after Raf activation, E-cadherin still colocalizes with cortical actin at areas of cell-cell contact (in yellow-orange). Scale bar, 20 μm. (c) TER measurements in MDCK cells after activation of Raf for 0 to 42 h. As shown, the cells retain TER after Raf activation. The peak in TER observed after 18 h of Raf activation was observed in all six experiments carried out. Error bars indicate standard deviations (n = 3).
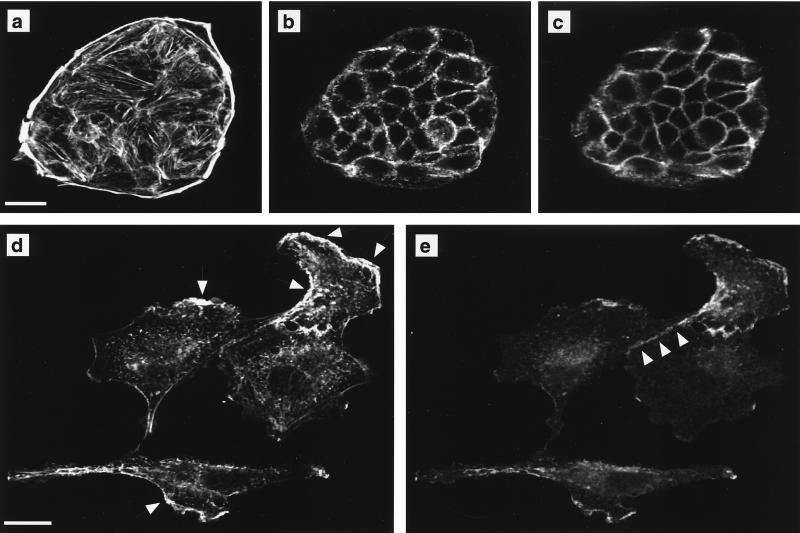
In some respects, our observations regarding E-cadherin localization differ from those of Zondag et al. (82), who showed that E-cadherin was downregulated from the adherens junction upon constitutive expression of Raf-CAAX in MDCK cells. A major difference between the two studies is that our experiments are conducted on confluent polarized MDCK cells rather than subconfluent cultures. We therefore tested whether cell density could influence the effect of Raf activation on E-cadherin localization. MDCK cells grown at subconfluent densities form cell clusters in which E-cadherin is absent from the plasma membrane in the basal region of the cell and colocalizes with cortical actin at areas of cell-cell contact in the apical region of the cell (Fig. 6a to c). Addition of 1 μM 4-HT for 42 h to subconfluent cultures of EGFPΔRaf-1:ER-expressing MDCK cells resulted in cell spreading and scattering, as well as loss of E-cadherin staining from the plasma membrane except in areas colocalizing with phalloidin staining patterns indicative of membrane ruffling (Fig. 6d and e). In addition, E-cadherin was retained at remaining areas of cell-cell contact in sparse cultures of Raf-transformed MDCK cells. These results demonstrate that cell-cell contact resulting from high cell density stabilizes E-cadherin at the plasma membrane in Raf-transformed MDCK cells compared to subconfluent cultures. However, the data do not rule out that long-term strong activation of the Raf-MEK-ERK cascade will eventually lead to downregulation of E-cadherin in confluent cultures of MDCK cells.
FIG. 6.
Raf transformation leads to spreading and scattering of MDCK cells. MDCK cells expressing EGFPΔRaf-1:ER grown at low cell density were either untreated (a to c) or treated with 1 μM 4-HT for 42 h (d and e). The cells were then fixed and labeled with fluorescent phalloidin to detect polymerized actin and immunolabeled to localize E-cadherin. (a and b) Phalloidin staining in optical sections through the basal (a) and apical (b) regions of untreated cells. (c) E-cadherin localization in the apical region of untreated cells. No E-cadherin labeling is detected in at the base of untreated cells (not shown). (d and e) Following activation of Raf, subconfluent MDCK cells become flattened, and both phalloidin (d) and E-cadherin (e) labeling is evident in a single optical section. Arrows in panel d indicate areas of apparent membrane ruffling. Note that E-cadherin colocalizes with polymerized actin at these areas. Arrowheads in panel e illustrate that E-cadherin is retained at areas of cell-cell contact in subconfluent Raf-transformed MDCK cells. Scale bars, 20 μm.
Effects of Raf activation on Rho family GTPases.
The loss of actin stress fibers and increased cortical actin and lateral membrane ruffling in Raf-transformed MDCK cells suggested that Raf activation might influence the activation state of the Rho family GTPases Rho, Rac, and Cdc42. Moreover, it was recently reported that in NIH 3T3 fibroblasts, activation of Rac and Cdc42 can lead to downregulation of RhoA-GTP levels (59), raising the possibility that diminished Rho activity in Raf-transformed MDCK cells might contribute to the observed phenotypic effects. We therefore measured the levels of RhoA-GTP using the rhotekin RBD assay and the levels of Rac1-GTP and Cdc42-GTP using the Pak3 CRIB binding assay (see Materials and Methods).
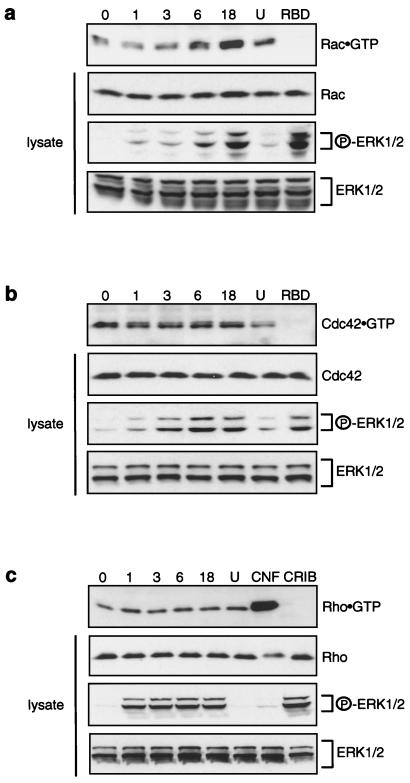
Following Raf activation, we observed a substantial increase in the cellular levels of Rac1-GTP, which was detectable within 3 to 6 h after Raf activation (Fig. 7a) and thus preceded the increased cortical actin (Fig. 7b and c). The elevation in Rac1.GTP levels was significantly reduced by preincubation with 10 μM U0126 (Fig. 7a). Cdc42.GTP levels did not notably change following Raf transformation (Fig. 7b). It should be mentioned that while Rac1-GTP levels were readily detectable with the Pak3 CRIB binding assay, it was difficult to detect Cdc42-GTP in MDCK cells both before and after Raf activation. Strikingly, we did not detect any change in RhoA-GTP levels in Raf-transformed MDCK cells (Fig. 7c). To rule out the possibility that the lack of change in RhoA-GTP levels was due to saturation of the rhotekin RBD assay, we treated MDCK cells with CNF1. CNF1 activates the endogenous pool of RhoA by deamidating asparagine in RhoA, hence mimicking the Q63L mutation (17, 61). This treatment resulted in the detection of highly elevated levels of RhoA-GTP (Fig. 7c). Thus, Raf activation in MDCK cells results in attenuated Rho function without influencing the levels of RhoA-GTP.
FIG. 7.
Effect of Raf activation on Rac1-, Cdc42-, and RhoA-GTP levels. Lanes represent GTP loading on endogenous Rac1 (a), Cdc42 (b), and RhoA (c) in MDCK cells following Raf activation for 0 to 18 h. Rac1-GTP and Cdc42-GTP levels were determined by the Pak3 CRIB assay (a and b, top panels). RhoA-GTP levels were assessed using the rhotekin RBD assay (c, top panel). Each experiment included one sample (lane U) where the cells were treated with 10 μM MEK inhibitor U0126 in addition to 1 μM 4-HT for 18 h. The rhotekin RBD assay (c) also included a sample (lane CNF) which was treated for 18 h with CNF1 only. Moreover, each experiment included one sample, in which Raf had been activated for 18 h, that was probed with the rhotekin RBD GST fusion protein for the CRIB assay (a and b, lanes RBD) and with the Pak3 CRIB GST fusion protein in the rhotekin RBD assay (c, lane CRIB). In addition, Western blots were carried out to detect Rac1, Cdc42, and RhoA in whole cell lysates, as well as phospho-ERK and total ERK (ERK1/2), and indicated on the right.
Raf-induced expression of Rnd3 elicits alterations in the actin cytoskeleton associated with cell transformation.
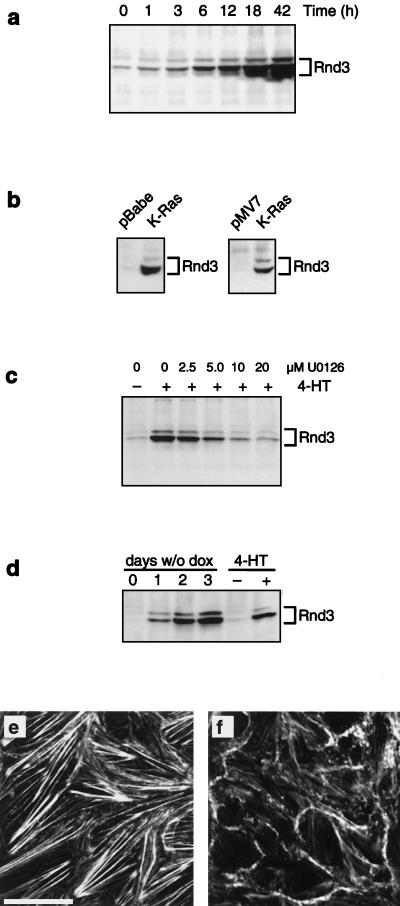
Loss of actin stress fibers following microinjection or transient expression of Rnd proteins in MDCK cells and other cell lines has been described (21, 46). Moreover, Rnd proteins have been suggested to play a role in oncogenic transformation (46). We therefore examined whether Raf transformation influenced the expression of Rnd proteins. Although we detected no alterations in the expression of Rnd1 or -2 (data not shown), we observed that Rnd3 was strongly induced upon Raf activation (Fig. 8a). No induction of Rnd3 expression was observed in parental MDCK cells treated with 4-HT (data not shown). Induced Rnd3 expression was detected after 1 h and strongly increased 3 to 6 h following activation of Raf (Fig. 8a). Rnd3 protein accumulated continuously up to 42 h of Raf activation, the latest time point examined (Fig. 8a). Constitutive expression of K-Ras in both a pooled population of MDCK cells as well as an extensively characterized cell line (63) also induced the expression of Rnd3 (Fig. 8b).
FIG. 8.
Induced expression of Rnd3 following Raf activation. (a) MDCK cells expressing EGFPΔRaf-1:ER were either untreated or treated with 1 μM 4-HT for the indicated periods of time, at which point the expression of Rnd3 was assessed by immunoprecipitation and Western blotting as described in Materials and Methods. (b) MDCK cells expressing K-Ras in either pBabe-Puro3 (pooled population) or pMV7 (clone) (63) were grown to confluency, and expression of Rnd3 was assessed by immunoprecipitation and Western blotting. (c) MDCK cells expressing EGFPΔRaf-1:ER were either untreated or treated with 1 μM 4-HT for 18 h in the absence or presence of the indicated concentrations of the MEK inhibitor U0126. The expression of Rnd3 was assessed by immunoprecipitation and Western blotting. (d) MDCK cells expressing Rnd3 under the control of the Tet-off system were cultured in the presence (0) or absence of doxycycline for 1 to 3 days, at which time the expression of Rnd3 was assessed by immunoprecipitation and Western blotting. As a control, the expression of Rnd3 was measured in extracts of MDCK cells expressing EGFPΔRaf-1:ER that were either untreated or treated with 1 μM 4-HT for 42 h. (e and f) MDCK cells expressing Rnd3 under the control of the Tet-off system were cultured in the presence (e) or absence (f) of doxycycline for 2 days. At this time, the cells were labeled with fluorescent phalloidin to detect polymerized actin and analyzed by confocal microscopy. Both optical sections were sampled at the base of the cell monolayers. Scale bar, 20 μm.
It should be noted that the induction of Rnd3 preceded the effects of Raf on the actin cytoskeleton (Fig. 1b and c). Interestingly, multiple Rnd3 cross-reacting bands were detected in Raf-transformed MDCK cells. Similar heterogeneity was also observed in Western blots of recombinant Rnd3 prepared from Escherichia coli as well as Rnd3 immunoprecipitated from COS7 cells transiently transfected with an Rnd3 expression vector (data not shown). The reasons for such heterogeneity are not clear. As both the transformation of MDCK cells by Raf and the accompanying alterations in the actin cytoskeleton were dependent on MEK activation, we examined whether induction of Rnd3 by Raf similarly required the activation of MEK. Indeed, preincubation with U0126 (Fig. 8c) or PD098059 (data not shown) abrogated Raf-induced Rnd3 expression, indicating a requirement for MEK activation in the induction of Rnd3 by Raf.
To test the effects of Rnd3 directly, we generated MDCK cells expressing Rnd3 under control of the t-TA in the Tet-off system (20). Removal of doxycycline from cells grown on coverslips or in culture dishes at either subconfluent or confluent densities led to efficient induction of Rnd3. The level of Rnd3 expression observed after 42 to 48 h of induction was equivalent to that achieved in response to Raf activation for a similar time period (Fig. 8d). Under these conditions, we observed that Rnd3 expression elicited a significant loss of actin stress fibers and moderately increased cortical actin accompanied by changes in cell morphology reminiscent of those associated with oncogenic transformation of MDCK cells by Raf (Fig. 8e and f). However, the effects of Rnd3 expression in MDCK cells were less dramatic than the alterations that occur following activation of Raf. Clearly other effectors of Raf signaling must cooperate with Rnd3 to yield the phenotype of Ras- and Raf-transformed MDCK cells. For reasons we do not understand, Rnd3 failed to express with the t-TA system when cells were cultured on filters.
Cell-multilayering is abrogated by constitutive expression of activated RhoA.
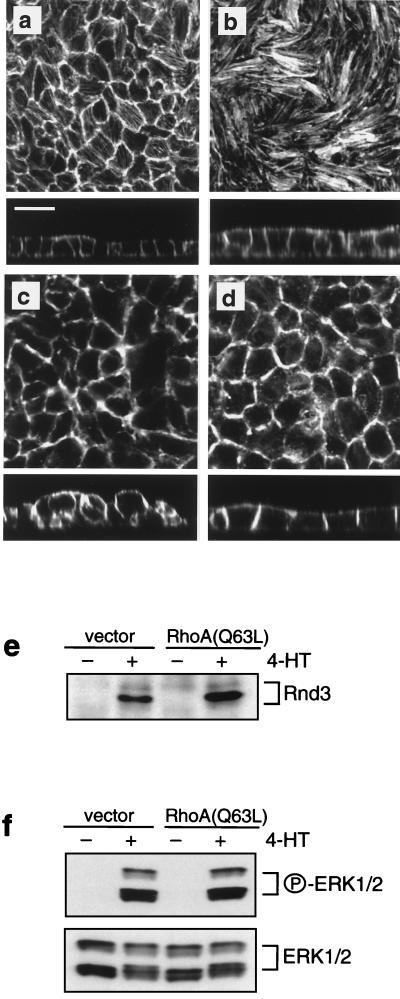
To assess whether alterations in Rho signaling influence the capacity of Raf to transform MDCK cells, we took advantage of the fact that activated RhoA can counterbalance the effects of Rnd3 (21, 46, 79). We thus expressed RhoA(Q63L) in MDCK cells that already express conditionally active Raf-1. MDCK cell clones expressing RhoA(Q63L) displayed a marked increase in the thickness and number of actin stress fibers at the base of cell monolayers (compare X-Y sections of Fig. 9a and b). In this region, the cells furthermore interdigitated extensively, thus partially obscuring cell boundaries. In each of three clones expressing activated RhoA, the activation of Raf failed to elicit the multilayering phenotype (Fig. 9a to d and data not shown). In contrast, clones as well as pooled populations of MDCK cells transfected with the control vector alone exhibited multilayering equivalent to that of parental cells. The inhibition of multilayering in cells expressing RhoA(Q63L) was not due to lack of ERK activation or failure to induce Rnd3 expression. Raf-induced ERK activation and Rnd3 expression was as robust in RhoA(Q63L)-expressing cells as it was in the vector control cells (Fig. 9e and f). Remarkably, despite the expression of activated RhoA, most of the stress fibers were lost in all of the RhoA(Q63L) clones following activation of Raf (Fig. 9d). A possible explanation for this result is that higher levels of RhoA(Q63L) are required to antagonize the effects of Rnd3 induction on actin stress fibers. Alternatively, direct inactivation by Raf of downstream effectors of RhoA such as Rho-kinase and Diaphonous (p140mDia) (2, 44, 69, 74, 75) may cooperate with Rnd3 to elicit a loss of actin stress fibers in MDCK cells transformed by Raf. While the use of RhoA(Q63L) to antagonize Rnd3 function provides indirect evidence for the importance of Rnd3 in oncogenic transformation, the data demonstrate a requirement for alterations in Rho effector function in epithelial oncogenesis by the Raf-MEK-ERK pathway.
FIG. 9.
Constitutive expression of RhoA(Q63L) prevents Raf-induced multilayering. (a to d) MDCK cells expressing EGFPΔRaf-1:ER were transduced with either empty vector, pWZLNeo (a and c), or the same vector encoding RhoA(Q63L) (b and d), and clonal cell lines were derived. The cells were then either untreated (a and b) or treated with 1 μM 4-HT for 42 h (c and d), at which time the cells were labeled with fluorescent phalloidin and analyzed by confocal microscopy. (e and f) The cells described above were either untreated or treated with 1 μM 4-HT for 42 h, at which time the expression of Rnd3 (e) and activation of ERK1 and -2 (f) were assessed as described in Materials and Methods. All X-Y sections were sampled at the base of the cell monolayers. Scale bar, 20 μm.
DISCUSSION
In this work we show that activation of the Raf-MEK-ERK pathway can elicit oncogenic transformation of MDCK cells in a manner that is similar to the effects of activated Ras (63). These data are consistent with the observations that expression of activated Raf in the rat parotid Pa-4 cell line grown on permeable supports results in cell multilayering (40) and that activated MEK1 can promote dedifferentiation of MDCK cells (64). Furthermore, activation of c-Jun and c-Fos, downstream targets of the Raf-MEK-ERK pathway (12), in filter-grown mammary epithelial cells also leads to multilayering (15, 53). Collectively, these studies and the present work emphasize the importance of the Raf-MEK-ERK pathway in epithelial oncogenesis.
The extent to which activated Raf is capable of mimicking the effects of oncogenic Ras may seem surprising given that activated forms of phosphatidylinositol 3′-kinase (PI3′-kinase) have been reported to result in morphological transformation of MDCK cells (31). It is possible that Ras elicits transformation of MDCK cells by the coordinate activation of several pathways but that strong activation of any one of these pathways can mimic the effects of Ras (50). Moreover, our results do not exclude a requirement for endogenous PI3′-kinase activity in Raf-induced epithelial oncogenesis, as has been described for the induction of cyclin D1 by conditionally active forms of MEK1 (70). This possibility will be addressed in future experiments using pharmacological inhibitors of the PI3′-kinase pathway as well as regulated forms of PI3′-kinase (34) and Akt (protein kinase B) (36, 43).
Here, we have begun to dissect the mechanisms by which the Raf-MEK-ERK pathway promotes epithelial oncogenesis. We have demonstrated that activation of Raf in polarized MDCK cells results in a loss of actin stress fibers and increased cortical actin suggestive of membrane ruffling. Consistent with these observations and previous reports (28, 57, 68), we found that Raf activation leads to a robust increase in Rac1 activity. Loss of actin stress fibers is associated with attenuated Rho function either through direct inactivation of RhoA (56) or by inactivation of Rho effector molecules. However, we detected no change in RhoA-GTP levels following Raf activation, thus strengthening the possibility that the effects of Raf on polarized MDCK cells are elicited downstream of Rho.
These results differ substantially from those recently reported by Zondag et al. (82), who found that constitutive expression of activated Ras (V12Ras) and Raf (Raf-CAAX) in MDCK cells results in attenuation of Rac activity and elevated levels of Rho-GTP. The reasons for such disparate observations are unclear. The differences are not due merely to the use of constitutive as opposed to inducible activation of the Raf-MEK-ERK pathway or clonal versus pooled cell populations, as we have reproduced our findings with pooled populations of Raf-CAAX-expressing MDCK cells (data not shown). It is possible that the growth conditions, or the extent to which the cells are polarized when the Raf-MEK-ERK pathway is activated, affect the activity state of Rho family GTPases in MDCK cells. In addition, our data do not exclude that elevated levels of Rac1-GTP in Raf-transformed MDCK cells may contribute to the loss of actin stress fibers. However, constitutive expression of activated Rac in MDCK cells results in increased cortical actin without loss of actin stress fibers (68). Second, Rac-mediated depletion of actin stress fibers would be expected to occur through Pak activation and inactivation of myosin light chain kinase (60). Yet we do not observe loss of actin stress fibers in MDCK cell lines expressing activated Pak1(T423E) under control of the t-TA (M. M. P. Zegers and S. H. Hansen, unpublished observations).
The induced expression of Rnd3 by Raf suggests a mechanism by which Rho effectors may be inactivated in Raf-transformed cells. Based on previous studies of Rnd proteins and the fact that the effector interaction domains of Rnd and Rho proteins are similar (46), it is possible that Rnd proteins may antagonize the effects of Rho proteins by competing for the same (or a subset of) effector molecules. Binding of Rnd3 to Rho effector molecules like Rho kinase or Diaphonous might lead to their sequestration in a state that prevents isometric contraction of actin filaments or directly to the disassembly of focal adhesions which are required for the formation of actin stress fibers (45). This mechanism represents an alternative to the paradigm that regulation of the function of small GTPases occurs solely by affecting the GTP-GDP cycle directly through GTPase-activating proteins, guanine nucleotide exchange factors, or GDP dissociation inhibitors (72). A precedent for such a mechanism exists in the case of the small GTP-binding protein Krev-1/Rap1A, which was isolated in a screen for suppressors of Ras transformation (32). Rap1A possesses an effector domain identical to that of Ras (19), but contrary to Ras, binding of Rap1A to Raf-1 and Ral-GDS does not lead to their activation (26, 71).
The observation that activated RhoA largely abrogated Raf-induced multilayering of MDCK cells suggests that attenuation of Rho function is required for epithelial oncogenesis by the Raf-MEK-ERK pathway. This notion might seem to contradict the hypothesis that RhoA can cooperate with Ras and Raf to transform cells (30, 52). However, given the pleiotropic roles of RhoA in cell physiology, it is possible that some functions of RhoA cooperate with Ras and Raf transformation whereas others may serve to antagonize it. One way in which RhoA might cooperate with Ras and Raf to transform cells could be to alleviate the cell cycle arrest imposed by strong activation of the Raf-MEK-ERK pathway that leads to induction of the cell cycle inhibitor p21Cip1 (49, 78). In addition, it has been reported that expression of activated RhoA in Ras-transformed epithelial cells elicits alterations in cell shape resulting in a fibroblast-like morphology (80). However, others have found that inhibition of the Rho-Rho-kinase pathway may contribute to oncogenic Ras-induced transformation (27). Moreover, as shown here, when tested in an assay of oncogenic transformation of polarized epithelial cells (i.e., multilayering), activated RhoA prevents transformation by the Raf-MEK-ERK pathway. It is also possible that activation of Rho effectors to different extents can elicit opposing phenotypes as has been observed for the Raf-MEK-ERK pathway (78). This scenario might explain some of the apparently contradictory data that have been published in this area.
Rac1, Cdc42, and RhoA all promote the formation and maintenance of E-cadherin-based adherens junctions in epithelial cells (9, 10, 25, 28, 35). One mechanism by which Rac1 and Cdc42 regulate adherens junctions is through the effector IQGAP1 (37). In contrast, the effector molecules of RhoA, which regulate adherens junction assembly and maintenance, are not known. It would be of interest to identify these molecules, as they are likely effectors of Rnd proteins in cell adhesion. Rnd1 is localized to adherens junctions in confluent, quiescent fibroblasts (46). Similarly, in MDCK cells microinjected with a Rnd3 expression vector, Rnd3 has been found to localize to areas of cell-cell contact (21). Another important finding to support a role for Rnd proteins in cell-cell adhesion is the finding that microinjection of mRNA encoding XRnd1 into the animal pole of Xenopus embryos results in cell dissociation (79). Given their role in cell adhesion, it is not surprising that constitutively active derivatives of Rac1, Cdc42, and RhoA also inhibit scattering of MDCK cells in response to hepatocyte growth factor/scatter factor (HGF/SF) or phorbol ester (3, 55). However, activated RhoA may additionally inhibit cell scattering by promoting formation of a dense network of actin stress fibers (55). Rnd3 also increases the rate of cell migration in response to HGF/SF (21), again providing evidence of antagonism between Rho and Rnd proteins.
It has not been possible by site-directed mutagenesis to derive dominant-interfering forms of Rnd proteins. It appears that the equivalent of the classical dominant negative mutation corresponding to the N17 mutant in Ras fails to bind nucleotide and hence does not yield a dominant-negative Rnd protein (46, 79; P. Chardin, unpublished observation). Similarly, our attempts to use antisense technology to inhibit Rnd3 expression in Raf-transformed MDCK cells have been unsuccessful. Moreover, since effectors of Rnd family members have not been identified, it is not possible to use conventional strategies to address whether induction of Rnd3 is required for oncogenesis of MDCK cells downstream of Raf. However, in the context of the previously published work on Rnd proteins and their antagonism of Rho function, the present study supports a role for Rnd3 in epithelial oncogenesis downstream of Raf. Rnd3 may possess dual functions in this response: first, by promoting reorganization of the actin cytoskeleton and changes in cell shape necessary for cell motility, and second, by attenuating cell adhesion to permit translation of the increased cell motility into cell-cell rearrangements occurring during multilayering. Evidently, further progress in understanding how Rnd proteins function and how they contribute to oncogenic transformation of polarized epithelial cells will require the identification of the mechanisms by which Rnd proteins antagonize the function of the classical Rho proteins in the cell.
ACKNOWLEDGMENTS
We are grateful to Patrice Bouquet (INSERM, Nice, France) for purified CNF1, Channing Der (University of North Carolina at Chapel Hill) for RhoA expression vectors, and Karl Matlin for MAbs against the 58- and 114-kDa marker proteins as well as MDCK cells expressing K-Ras. We also acknowledge Shubha Bagrodia and Richard A. Cerione (Cornell University, Ithaca, N.Y.), and Xiang-Dong Ren and Martin A. Schwartz (University of California, San Diego) for generously providing reagents and advice for the Pak3 CRIB and rhotekin RBD assays, respectively. In addition, we thank Yen Hoang Nguyen and Tyler Rootlieb for excellent technical assistance and David Dankort (UCSF Cancer Research Institute) for critical reading of the manuscript.
S.H.H. is the recipient of a senior research fellowship from the Weimann Foundation, Denmark, a grant from the Novo Nordic Foundation, and an award from the Danish Medical Research Council. This investigation was supported by a fellowship from the California Division of the American Cancer Society (2-7-99 to M.M.P.Z.) and by a DAMD grant (17-97-1-7326 to K.E.M.). M.M. acknowledges the generous support of the UCSF Cancer Research Institute for funding to support these studies.
Footnotes
This paper is dedicated to Lizzi Nauman.
REFERENCES
- 1.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27894. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 2.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 3.Bagrodia S, Taylor S J, Jordon K A, Van Aelst L, Cerione R A. A novel regulator of p21-activated kinases. J Biol Chem. 1998;273:23633–23636. doi: 10.1074/jbc.273.37.23633. [DOI] [PubMed] [Google Scholar]
- 4.Balcarova-Ständer J, Pfeiffer S E, Fuller S D, Simons K. Development of cell surface polarity in the epithelial Madin-Darby canine kidney (MDCK) cell line. EMBO J. 1984;3:2687–2694. doi: 10.1002/j.1460-2075.1984.tb02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balkovetz D F, Pollack A L, Mostov K E. Hepatocyte growth factor alters the polarity of Madin-Darby canine kidney cell monolayers. J Biol Chem. 1997;272:3471–3477. doi: 10.1074/jbc.272.6.3471. [DOI] [PubMed] [Google Scholar]
- 6.Bar-Sagi D, Feramisco J R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233:1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- 7.Barth A I, Pollack A L, Altschuler Y, Mostov K E, Nelson W J. NH2-terminal deletion of beta-catenin results in stable colocalization of mutant beta-catenin with adenomatous polyposis coli protein and altered MDCK cell adhesion. J Cell Biol. 1997;136:693–706. doi: 10.1083/jcb.136.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bos J L. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. . (Erratum, 50:1352, 1990.) [PubMed] [Google Scholar]
- 9.Braga V M, Del Maschio A, Machesky L, Dejana E. Regulation of cadherin function by Rho and Rac: modulation by junction maturation and cellular context. Mol Biol Cell. 1999;10:9–22. doi: 10.1091/mbc.10.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braga V M, Machesky L M, Hall A, Hotchin N A. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen D, Heath V, O'Garra A, Johnston J, McMahon M. Sustained activation of the Raf-MEK-ERK pathway elicits cytokine unresponsiveness in T cells. J Immunol. 1999;163:5796–5805. [PubMed] [Google Scholar]
- 12.Cook S J, Aziz N, McMahon M. The repertoire of Fos and Jun proteins expressed during the G1 phase of the cell cycle is determined by the duration of mitogen-activated protein kinase activation. Mol Cell Biol. 1999;19:330–341. doi: 10.1128/mcb.19.1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 14.Favata M F, Horiuchi K Y, Manos E J, Daulerio A J, Stradley D A, Feeser W S, Van Dyk D E, Pitts W J, Earl R A, Hobbs F, Copeland R A, Magolda R L, Scherle P A, Trzaskos J M. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–16832. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 15.Fialka I, Schwarz H, Reichmann E, Oft M, Busslinger M, Beug H. The estrogen-dependent c-JunER protein causes a reversible loss of mammary epithelial cell polarity involving a destabilization of adherens junctions. J Cell Biol. 1996;132:1115–1132. doi: 10.1083/jcb.132.6.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fish E M, Molitoris B A. Alterations in epithelial polarity and the pathogenesis of disease states. N Engl J Med. 1994;330:1580–1588. doi: 10.1056/NEJM199406023302207. [DOI] [PubMed] [Google Scholar]
- 17.Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Fiorentini C, Boquet P. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- 18.Foster R, Hu K Q, Lu Y, Nolan K M, Thissen J, Settleman J. Identification of a novel human Rho protein with unusual properties: GTPase deficiency and in vivo farnesylation. Mol Cell Biol. 1996;16:2689–2699. doi: 10.1128/mcb.16.6.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frech M, John J, Pizon V, Chardin P, Tavitian A, Clark R, McCormick F, Wittinghofer A. Inhibition of GTPase activating protein stimulation of Ras-p21 GTPase by the Krev-1 gene product. Science. 1990;249:169–171. doi: 10.1126/science.2164710. [DOI] [PubMed] [Google Scholar]
- 20.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guasch R M, Scambler P, Jones G E, Ridley A J. RhoE regulates actin cytoskeleton organization and cell migration. Mol Cell Biol. 1998;18:4761–4771. doi: 10.1128/mcb.18.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 23.Hansen S H, Casanova J E. Gs alpha stimulates transcytosis and apical secretion in MDCK cells through cAMP and protein kinase A. J Cell Biol. 1994;126:677–687. doi: 10.1083/jcb.126.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen S H, Sandvig K, van Deurs B. Internalization efficiency of the transferrin receptor. Exp Cell Res. 1992;199:19–28. doi: 10.1016/0014-4827(92)90457-j. [DOI] [PubMed] [Google Scholar]
- 25.Hordijk P L, ten Klooster J P, van der Kammen R A, Michiels F, Oomen L C, Collard J G. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- 26.Hu C D, Kariya K, Kotani G, Shirouzu M, Yokoyama S, Kataoka T. Coassociation of Rap1A and Ha-Ras with Raf-1 N-terminal region interferes with ras-dependent activation of Raf-1. J Biol Chem. 1997;272:11702–11705. doi: 10.1074/jbc.272.18.11702. [DOI] [PubMed] [Google Scholar]
- 27.Izawa I, Amano M, Chihara K, Yamamoto T, Kaibuchi K. Possible involvement of the inactivation of the Rho-Rho-kinase pathway in oncogenic Ras-induced transformation. Oncogene. 1998;17:2863–2871. doi: 10.1038/sj.onc.1202213. [DOI] [PubMed] [Google Scholar]
- 28.Jou T S, Nelson W J. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J Cell Biol. 1998;142:85–100. doi: 10.1083/jcb.142.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerkhoff E, Rapp U R. High-intensity Raf signals convert mitotic cell cycling into cellular growth. Cancer Res. 1998;58:1636–1640. [PubMed] [Google Scholar]
- 30.Khosravi-Far R, Solski P A, Clark G J, Kinch M S, Der C J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khwaja A, Rodriguez-Viciana P, Wennström S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989;56:77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- 33.Klemke R L, Cai S, Giannini A L, Gallagher P J, de Lanerolle P, Cheresh D A. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klippel A, Escobedo M A, Wachowicz M S, Apell G, Brown T W, Giedlin M A, Kavanaugh W M, Williams L T. Activation of phosphatidylinositol 3-kinase is sufficient for cell cycle entry and promotes cellular changes characteristic of oncogenic transformation. Mol Cell Biol. 1998;18:5699–5711. doi: 10.1128/mcb.18.10.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kodama A, Takaishi K, Nakano K, Nishioka H, Takai Y. Involvement of Cdc42 small G protein in cell-cell adhesion, migration and morphology of MDCK cells. Oncogene. 1999;18:3996–4006. doi: 10.1038/sj.onc.1202773. [DOI] [PubMed] [Google Scholar]
- 36.Kohn A D, Barthel A, Kovacina K S, Boge A, Wallach B, Summers S A, Birnbaum M J, Scott P H, Lawrence J C, Jr, Roth R A. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J Biol Chem. 1998;273:11937–11943. doi: 10.1074/jbc.273.19.11937. [DOI] [PubMed] [Google Scholar]
- 37.Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, Shoji I, Matsuura Y, Yonehara S, Kaibuchi K. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell-cell adhesion. Science. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- 38.Leevers S J, Paterson H F, Marshall C J. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994;369:411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- 39.Lewis T S, Shapiro P S, Ahn N G. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 40.Li D, Mrsny R J. Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J Cell Biol. 2000;148:791–800. doi: 10.1083/jcb.148.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mansour S J, Matten W T, Hermann A S, Candia J M, Rong S, Fukasawa K, Vande Woude G F, Ahn N G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 42.McCarthy S A, Samuels M L, Pritchard C A, Abraham J A, McMahon M. Rapid induction of heparin-binding epidermal growth factor/diphtheria toxin receptor expression by Raf and Ras oncogenes. Genes Dev. 1995;9:1953–1964. doi: 10.1101/gad.9.16.1953. [DOI] [PubMed] [Google Scholar]
- 43.Mirza A M, Kohn A D, Roth R A, McMahon M. Oncogenic transformation of cells by a conditionally active form of the protein kinase Akt/PKB. Cell Growth Differ. 2000;11:279–292. [PubMed] [Google Scholar]
- 44.Nakano K, Takaishi K, Kodama A, Mammoto A, Shiozaki H, Monden M, Takai Y. Distinct actions and cooperative roles of ROCK and mDia in Rho small G protein-induced reorganization of the actin cytoskeleton in Madin-Darby canine kidney cells. Mol Biol Cell. 1999;10:2481–2491. doi: 10.1091/mbc.10.8.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nobes C D, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 46.Nobes C D, Lauritzen I, Mattei M G, Paris S, Hall A, Chardin P. A new member of the Rho family, Rnd1, promotes disassembly of actin filament structures and loss of cell adhesion. J Cell Biol. 1998;141:187–197. doi: 10.1083/jcb.141.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oka H, Chatani Y, Hoshino R, Ogawa O, Kakehi Y, Terachi T, Okada Y, Kawaichi M, Kohno M, Yoshida O. Constitutive activation of mitogen-activated protein (MAP) kinases in human renal cell carcinoma. Cancer Res. 1995;55:4182–4187. [PubMed] [Google Scholar]
- 48.Oldham S M, Clark G J, Gangarosa L M, Coffey R J, Jr, Der C J. Activation of the Raf-1/MAP kinase cascade is not sufficient for Ras transformation of RIE-1 epithelial cells. Proc Natl Acad Sci USA. 1996;93:6924–6928. doi: 10.1073/pnas.93.14.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olson M F, Paterson H F, Marshall C J. Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature. 1998;394:295–299. doi: 10.1038/28425. [DOI] [PubMed] [Google Scholar]
- 50.Pawson T, Saxton T M. Signaling networks—do all roads lead to the same genes? Cell. 1999;97:675–678. doi: 10.1016/s0092-8674(00)80779-5. [DOI] [PubMed] [Google Scholar]
- 51.Pollack A L, Runyan R B, Mostov K E. Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev Biol. 1998;204:64–79. doi: 10.1006/dbio.1998.9091. [DOI] [PubMed] [Google Scholar]
- 52.Qiu R G, Chen J, McCormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci USA. 1995;92:11781–11785. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reichmann E, Schwarz H, Deiner E M, Leitner I, Eilers M, Berger J, Busslinger M, Beug H. Activation of an inducible c-FosER fusion protein causes loss of epithelial polarity and triggers epithelial-fibroblastoid cell conversion. Cell. 1992;71:1103–1116. doi: 10.1016/s0092-8674(05)80060-1. [DOI] [PubMed] [Google Scholar]
- 54.Ren X D, Kiosses W B, Schwartz M A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ridley A J, Comoglio P M, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ridley A J, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 57.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 58.Samuels M L, Weber M J, Bishop J M, McMahon M. Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human Raf-1 protein kinase. Mol Cell Biol. 1993;13:6241–6252. doi: 10.1128/mcb.13.10.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sander E E, ten Klooster J P, van Delft S, van der Kammen R A, Collard J G. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanders L C, Matsumura F, Bokoch G M, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- 61.Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- 62.Schoenenberger C-A, Matlin K S. Cell polarity and epithelial oncogenesis. Trends Cell Biol. 1991;1:87–92. doi: 10.1016/0962-8924(91)90035-8. [DOI] [PubMed] [Google Scholar]
- 63.Schoenenberger C A, Zuk A, Kendall D, Matlin K S. Multilayering and loss of apical polarity in MDCK cells transformed with viral K-ras. J Cell Biol. 1991;112:873–889. doi: 10.1083/jcb.112.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schramek H, Feifel E, Healy E, Pollack V. Constitutively active mutant of the mitogen-activated protein kinase kinase MEK1 induces epithelial dedifferentiation and growth inhibition in Madin-Darby canine kidney-C7 cells. J Biol Chem. 1997;272:11426–11433. doi: 10.1074/jbc.272.17.11426. [DOI] [PubMed] [Google Scholar]
- 65.Sebolt-Leopold J S, Dudley D T, Herrera R, Van Becelaere K, Wiland A, Gowan R C, Tecle H, Barrett S D, Bridges A, Przybranowski S, Leopold W R, Saltiel A R. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat Med. 1999;5:810–816. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- 66.Settleman J. Getting in shape with Rho. Nat Cell Biol. 2000;2:E7–E9. doi: 10.1038/71390. [DOI] [PubMed] [Google Scholar]
- 67.Stokoe D, Macdonald S G, Cadwallader K, Symons M, Hancock J F. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994;264:1463–1467. doi: 10.1126/science.7811320. . (Erratum, 266:1792–1793.) [DOI] [PubMed] [Google Scholar]
- 68.Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tominaga T, Sahai E, Chardin P, McCormick F, Courtneidge S A, Alberts A S. Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol Cell. 2000;5:13–25. doi: 10.1016/s1097-2765(00)80399-8. [DOI] [PubMed] [Google Scholar]
- 70.Treinies I, Paterson H F, Hooper S, Wilson R, Marshall C J. Activated MEK stimulates expression of AP-1 components independently of phosphatidylinositol 3-kinase (PI3-kinase) but requires a PI3-kinase signal to stimulate DNA synthesis. Mol Cell Biol. 1999;19:321–329. doi: 10.1128/mcb.19.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Urano T, Emkey R, Feig L A. Ral-GTPases mediate a distinct downstream signaling pathway from Ras that facilitates cellular transformation. EMBO J. 1996;15:810–816. [PMC free article] [PubMed] [Google Scholar]
- 72.Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 73.Vojtek A B, Hollenberg S M, Cooper J A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 74.Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol. 1999;1:136–143. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- 75.Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, Saito Y, Nakao K, Jockusch B M, Narumiya S. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997;16:3044–3056. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.White M A, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler M H. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 77.Wingo P A, Ries L A, Giovino G A, Miller D S, Rosenberg H M, Shopland D R, Thun M J, Edwards B K. Annual report to the nation on the status of cancer, 1973–1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999;91:675–690. doi: 10.1093/jnci/91.8.675. [DOI] [PubMed] [Google Scholar]
- 78.Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wünnenberg-Stapleton K, Blitz I L, Hashimoto C, Cho K W. Involvement of the small GTPases XRhoA and XRnd1 in cell adhesion and head formation in early Xenopus development. Development. 1999;126:5339–5351. doi: 10.1242/dev.126.23.5339. [DOI] [PubMed] [Google Scholar]
- 80.Zhong C, Kinch M S, Burridge K. Rho-stimulated contractility contributes to the fibroblastic phenotype of Ras-transformed epithelial cells. Mol Biol Cell. 1997;8:2329–2344. doi: 10.1091/mbc.8.11.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu J, Woods D, McMahon M, Bishop J M. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zondag G C M, Evers E E, ten Klooster J P, Janssen L, van der Kammen R, Collard J G. Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition. J Cell Biol. 2000;149:775–781. doi: 10.1083/jcb.149.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]