Abstract
Immune checkpoint inhibitors targeting programmed cell death protein 1 (PD-1) or programmed cell death 1 ligand 1 (PD-L1) have enabled some patients with cancer to experience durable, complete treatment responses; however, reliable anti–PD-(L)1 treatment response biomarkers are lacking. Our research found that PD-L1 K162 was methylated by SETD7 and demethylated by LSD2. Furthermore, PD-L1 K162 methylation controlled the PD-1/PD-L1 interaction and obviously enhanced the suppression of T cell activity controlling cancer immune surveillance. We demonstrated that PD-L1 hypermethylation was the key mechanism for anti–PD-L1 therapy resistance, investigated that PD-L1 K162 methylation was a negative predictive marker for anti–PD-1 treatment in patients with non–small cell lung cancer, and showed that the PD-L1 K162 methylation:PD-L1 ratio was a more accurate biomarker for predicting anti–PD-(L)1 therapy sensitivity. These findings provide insights into the regulation of the PD-1/PD-L1 pathway, identify a modification of this critical immune checkpoint, and highlight a predictive biomarker of the response to PD-1/PD-L1 blockade therapy.
PD-L1 methylation is a previously unidentified predictive biomarker of the response to PD-1/PD-L1 blockade therapy.
INTRODUCTION
Cancer cells exploit programmed cell death 1 ligand 1 (PD-L1) expression to suppress T cell–mediated immunosurveillance (1). Interactions between the extracellular domains of PD-L1 and programmed cell death protein 1 (PD-1) can induce a conformational change in PD-1, which inhibits antitumor immunity by counteracting T cell–activating signals (2). Treatments targeting the PD-1/PD-L1 pathway have been approved for multiple cancers, including non–small cell lung cancer (NSCLC), colorectal cancer, liver cancer, cervical cancer, gastric cancer, and melanoma with durable clinical benefit (3, 4). However, most patients with cancer types do not or only temporarily respond to the treatment that targets the PD-1 and PD-L1 interaction (5, 6). Multiple clinical trials have proposed PD-L1 positivity [defined by immunohistochemistry (IHC) in ≥50% of tumor cells] as a biomarker for the prediction of treatment response to anti–PD-1 (such as nivolumab, pembrolizumab, and durvalumab) or anti–PD-L1 (such as atezolizumab and avelumab) therapies, where objective responses are still less than 50% (7–11). The underlying mechanism of patients with high PD-L1 expression not responding to anti–PD-1 or anti–PD-L1 therapies is not well understood.
Current treatments that target the PD-1/PD-L1 pathway do so by blocking their interactions; thus, checkpoint interaction status may present a key mechanism–based prognostic and predictive biomarker, replacing conventional protein expression readouts for stratifying patients with immune checkpoint inhibitors (ICIs). Recent studies have shown that the PD-1/PD-L1 colocation score is highly predictive of the response to anti–PD-1/PD-L1 immunotherapy (12–14). Hence, it is important to understand the mechanistic pathways that control PD-1/PD-L1 interactions, which can offer a molecular basis for improving the clinical response rate and efficacy of PD-1/PD-L1 blockade in patients with cancer. Over the past several years, investigators have focused on the genetic, transcriptional, and posttranscriptional regulation of the PD-1/PD-L1 pathway. A number of intracellular and extracellular factors, such as interferon-gamma (IFN-γ), transforming growth factor–beta (TGF-β), and Myc proto-oncogene protein (MYC), modulate PD-L1 expression via transcriptional regulation (2, 15–18). Several recent insights explored that PD-L1 and PD-1 are also regulated by protein posttranslational modifications (PTMs), such as ubiquitination, glycosylation, phosphorylation, palmitoylation, and acetylation. These modifications regulate PD-L1 stability and PD-1/PD-L1 interaction or promote PD-L1 translocation from membrane to the nucleus (16, 19–27).
Aberrant histone and nonhistone protein methylation is a well-known hallmark of cancer, which can be methylated from lysine (Lys) and arginine (Arg) residues (28–30). The effects of methylation on protein-protein and protein-DNA interactions, protein stability, and subcellular localization of proteins modulate many cellular processes; hence, several histone methyltransferase inhibitors are currently being evaluated in clinical trials (28, 31–33). Key components of mitogen-activated protein kinase, phosphatidylinositol 3-kinase (PI3K), serine/threonine-protein kinase akt, nuclear factor κB (NF-κB), or estrogen receptor signaling (such as RAS, PI3K, PTEN, E2F1, Rb, NF-κB, or Erα) are also subject to methylation. We revealed that Akt K64 methylation was critical for Akt activation and tumorigenesis, while mothers against decapentaplegic homolog 3 (SMAD3) methylation was crucial for TGF-β pathway activation and cancer metastasis. (33–36).
Hence, in our study, we aim to provide previously unknown insights into the regulation of the PD-1/PD-L1 pathway and develop predictive biomarkers of response to PD-1/PD-L1 blockade therapy. Here, we reported that PD-L1 was methylated at Lys 162 by histone-lysine N-methyltransferase SET domain containing 7 (SETD7), and that demethylation of PD-L1 by lysine-specific histone demethylase 2 (LSD2) triggered the PD-1/PD-L1 interaction. Meanwhile, abnormal interleukin-6 (IL-6) receiving could inhibit SETD7 expression and decrease PD-L1 K162 methylation. Moreover, we confirmed that PD-L1 hypermethylation status contributed to anti–PD-1 treatment resistance and suggested a strategy for predicting the efficacy of PD-1/PD-L1 blockade treatment by detecting PD-L1 K162 methylation levels.
RESULTS
The Lys 162 methylation of PD-L1 restricts PD-L1/PD-1 interactions
To assess the response rate of anti–PD-1/PD-L1 for cancer treatment, we first analyzed 10 NSCLC clinical trials and 9 other kinds of tumors clinical trials (37–58) in which patients received anti–PD-1 or anti–PD-L1 antibody monotherapy and tumor PD-L1 membrane expression level was shown. Results showed that the objective response rate (ORR) was between 17.5 and 48.8%, the overall ORR was 28.7% in NSCLC; besides, results from CheckMate 017 showed that ORR did not increase with the expression level of tumor PD-L1. Furthermore, the ORR is between 10 and 27.9%, except for 52.7% in a skin cutaneous melanoma (SKCM) clinical trial, in other kinds of tumors. Together, we found that most of patients did not benefit from anti–PD-1 or anti–PD-L1 antibody monotherapy although the tumor PD-L1 expression of these patients was positive; the ORR of patients with high tumor PD-L1 expression was still unsatisfactory (less than 30%), such as NCT02087423, NCT03563716, and NCT03084471 (Fig. 1A, fig. S1A, and tables S1 and S2).
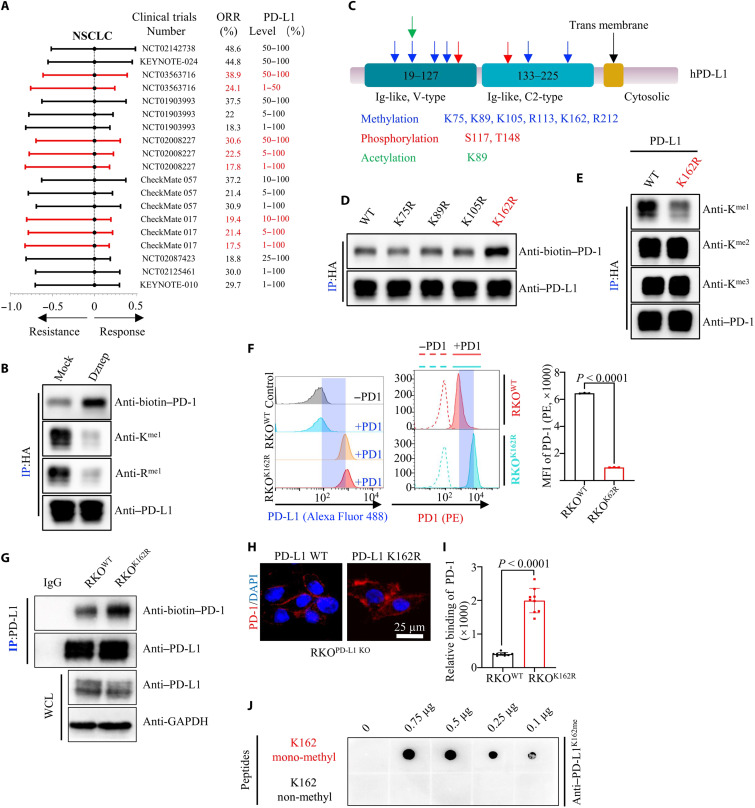
Fig. 1. PD-L1 Lys 162 mono-methylation attenuates PD-1/PD-L1 interaction.
(A) Forest plot of ORR in patients treated with anti–PD-1 or anti–PD-L1 monotherapy versus control in NSCLC. (B) Human embryonic kidney (HEK) 293T cell transfected with hemagglutinin (HA)–PD-L1, and then treated with or without 3-deazaneplanocin A (Dznep), immunoprecipitation (IP) and immunoblot (IB) analysis measuring PD-1/PD-L1 interaction. (C) Presenting potential modification sites from mass spectrometry analysis of PD-L1. (D) HEK293T cell transfected with various HA–PD-L variants and IP and IB analysis measuring PD-1/PD-L1 interaction. (E) HEK293T cells transfected with HA–PD-L wild-type (WT) or HA–PD-L1 K162R variant, followed by IP and IB analysis. (F) Flow cytometry (left) measuring PD-L1 or PD-1 binding on the membrane of RKOPD-L1 WT or RKOPD-L1 K162R cells. Statistical analysis for mean fluorescence intensity (MFI) was shown in the right; n = 3, P < 0.0001. (G) IP and IB analysis measuring PD-1/PD-L1 interaction in RKOPD-L1 WT or RKOPD-L1 K162R cells. WCL, whole cell lysis. (H and I) Representative images (H) for confocal image showing bound PD-1/Fc fusion proteins on the membrane of RKOPD-L1 WT or RKOPD-L1 K162R cells. Statistical analysis for relative binding was shown (I). DAPI, 4′,6-diamidino-2-phenylindole. (J) Ten microliters of double-distilled water containing 0.1 to 0.75 μg of different peptides was added into polyvinylidene difluoride membranes, followed by IB analysis using K162-specific mono-methylation antibody (Anti–PD-L1K162me). All IBs are performed three times, independently, with similar results. Error bars are means ± SD. Statistical significance was assessed using Student’s two-tailed t test.
Given the low response rate of anti–PD-L1/PD-1 treatment, and that PD-1/PD-L1 binding status was high predictive mark for the efficacy of anti–PD-1/PD-L1 immunotherapy, we assumed that certain modifications of PD-L1 might affect the binding of PD-L1 and PD-1. Hence, we used 3-deazaneplanocin A (Dznep, a global protein methylation inhibitor), deacetylase inhibitor cocktail (DIC), phosphatase inhibitor cocktail (PIC), and peptide N-glycosidase F (PNGase F) (as a positive control) to disturb the methylation, acetylation, phosphorylation, and glycosylation level of PD-L1. Dznep application obviously enhanced the binding of PD-L1 and PD-1, while DIC and PIC treatment had no observable impact of the PD-1/PD-L1 interaction (Fig. 1B and fig. S1, B and C). Meanwhile, we also proved that deglycosylated PD-L1 failed to bind to PD-1 (fig. S1D). Next, we seek which lysine or/and arginine methylation could affect the PD-1/PD-L1 interaction. We firstly conducted mass spectrometry (MS) analysis by after a coimmunoprecipitation (co-IP) assay to isolate PD-L1 protein from human embryonic kidney (HEK) 293T cells ectopically expressing hemagglutinin (HA)–PD-L1. Last, six mono-methylation sites (K75, K89, K105, R113, K162, and R212) of PD-L1 were detected (Fig. 1C, fig. S1E, and table S3). Notably, we also found other studies reported PTMs and binding proteins of PD-L1, such as phosphorylation of PD-L1 at S117 (table S3). Subsequently, we constructed methylation-deficient variants of PD-L1 (K75R, K89R, K105R, K162R, R113K, and R212K) and expressed these variants in HEK293T cells. The binding status between PD-1 with these PD-L1 variants was assessed by co-IP assays. Unexpectedly, only PD-L1 K162R variant enhanced the binding of PD-1/PD-L1 (Fig. 1D and fig. S1F). To identify the MS analysis and further validated whether the Lys of PD-L1 can be methylated, we performed a co-IP assay to isolate the PD-L1 protein from RKO and H1975 cells and subsequently used the pan-lysine mono-methylation (pan-K-me1) antibody for detection. Notably, the Lys residue of PD-L1 could be mono-methylated. We also found PD-L1 in the pool of mono-methylated proteins purified by the pan anti–mono-methylated lysine antibody (fig. S1G). Moreover, PD-L1 K162R variant only decreased PD-L1 mono-methylation level, but not di-methylation or tri-methylation level (Fig. 1E). The K162 site was conserved among primates, including humans, chimpanzees, rhesus monkeys, and tree shrews, but not in other species (fig. S1H). To assess the role of PD-L1 K162 methylation in its functional regulation, we constructed PD-L1 knockout (RKOKO) RKO cells using Crispr/Cas9 (fig. S1I) and subsequently stably expressed a methylation-deficient variant of PD-L1 K162R in RKOPD-L1KO cells (RKOK162R) (fig. S1, J and K). Meanwhile, we also proved that PD-L1 K162 methylation did not affect PD-L1 expression level, stability, or membrane translocation compared to RKOWT and RKOK162R cells (fig. S1, J to L). We used an in vitro receptor-ligand binding assay to investigate the interaction between PD-L1 wild type (WT) or PD-L1 K162R and PD-1. In particular, the PD-L1 K162R mutant markedly enhanced the interaction between PD-L1 and PD-1 compared to WT PD-L1 (Fig. 1F). To further confirm this, we generated another IF assay and co-IP assay in RKOWT and RKOK162R cells, which showed that the PD-L1 K162R mutant bound more PD-1 than WT PD-L1, suggesting that PD-L1 K162 methylation limited the PD-1/PD-L1 interaction (Fig. 1, G to I). We then generated a K162-specific mono-methylation antibody (Anti–PD-L1K162me), which specifically recognized PD-L1 K162 mono-methylation, using immunoblot and dot blot assays (Fig. 1J and fig. S1M). Overall, these findings implied that PD-L1 K162 methylation limited PD-L1 and PD-1 interaction.
PD-L1 K162 methylation limits its immunosuppressive function
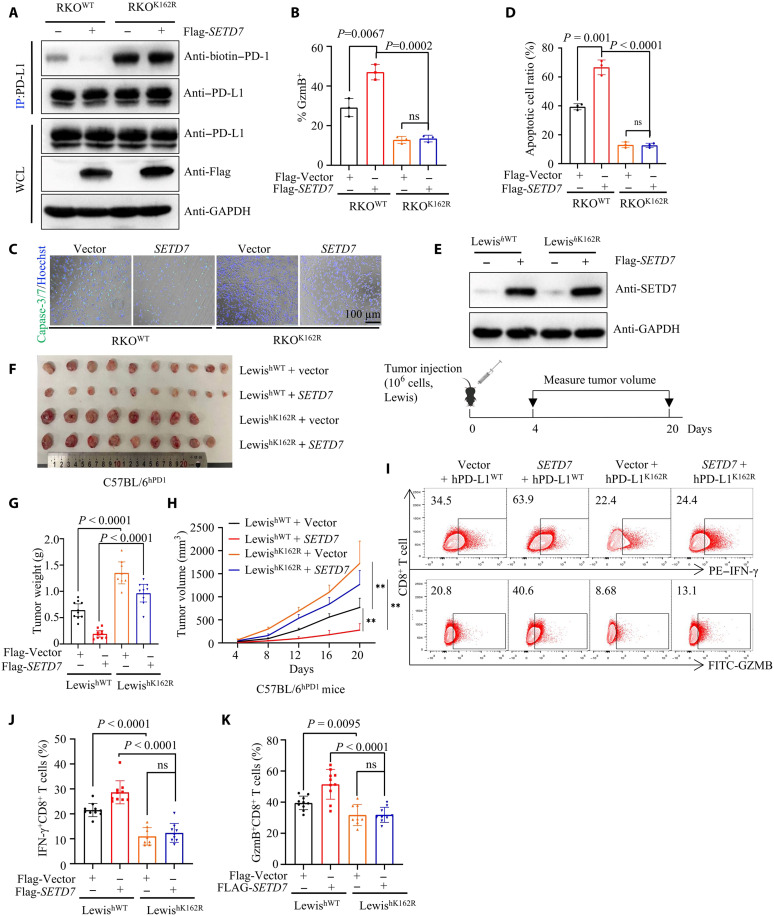
To validate these data, we performed T cell–mediated tumor killing assays by coculturing activated primary human T cells and RKOWT/RKOK162R cells. The results showed that the PD-L1 K162R mutant obviously inhibited T cell cytotoxicity in tumor cells (Fig. 2, A to D). We also evaluated T cell function by measuring granzyme B (GZMB) and IFN-γ expression in T cells. Cells expressing PD-L1 K162R showed lower GZMB and IFN-γ expression in activated primary human T cells (Fig. 2, E and F and fig. S2A).
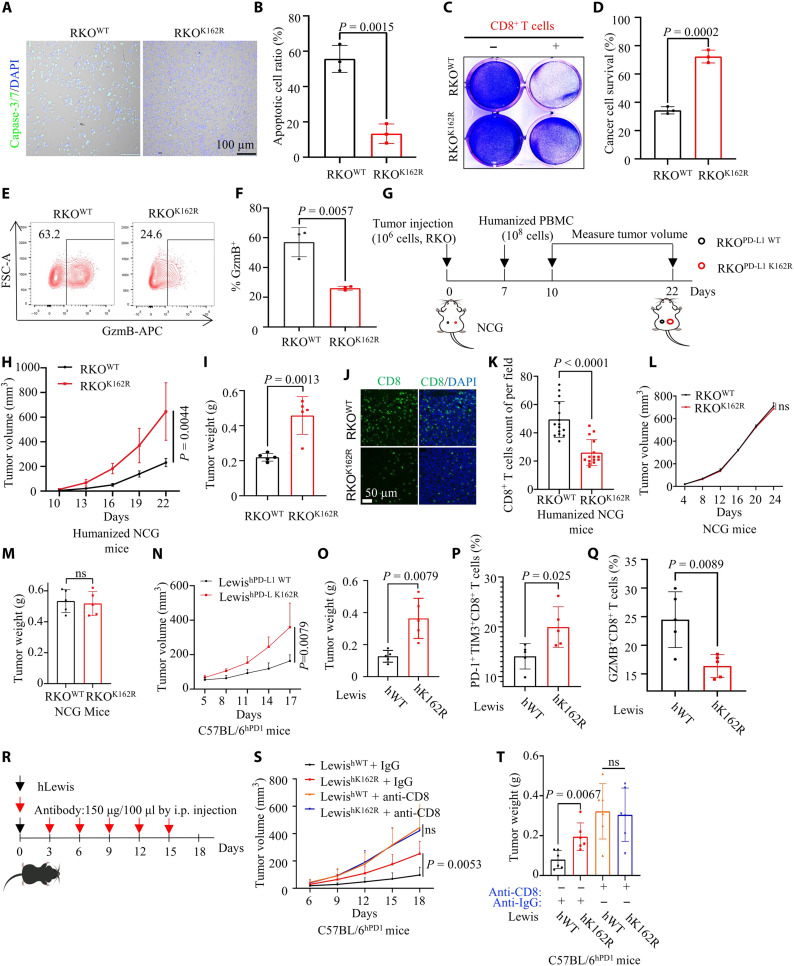
Fig. 2. PD-L1 Lys 162 methylation restricts its immunosuppressive function.
(A and B) T cell–mediated tumor cell killing assay in RKOPD-L1 WT or RKOPD-L1 K162R cells. The representative images (A) and the quantitative ratio (B) of dead cells were shown; n = 3, P = 0.0015. (C and D) Activated T cells cocultured with pretreated RKO cells. The representative images (C) and statistical analysis (D) were shown; n = 3, P = 0.0002. Ig, immunoglobulin. (E and F) Measuring granzyme B (GZMB) of T cells [from experiment (A)] by flow cytometry. The representative images (E) and statistical analysis (F) were shown; n = 3, P = 0.0057. FSC-A, forward scatter area. (G to I) In vitro xenograft tumor assays in immunocompetent humanized peripheral blood mononuclear cell (huPBMC)-NCG mice. The treatment protocol was summarized (G); the tumor growth rate (H) and weight (I) were shown; n = 3, P = 0.0044 (H), P = 0.0013 (I). (J and K) Representative images for number of intratumor CD8+ T cells by immunofluorescence (IF) (J). Statistical analysis was shown (K); n = 3. (L and M) In vitro xenograft tumor assays in immunodeficient NCG mice. The tumor growth rate (L) and weight (M) were shown. (N and O) In vitro xenograft tumor assays using LewishWT or LewishK162R cells in C57BL/6hPD1 mice; the tumor growth rate (N) and weight (O) were shown; n = 5, P = 0.0079 (N), P = 0.0079 (O). (P and Q) Flow cytometry measuring PD-1+ TIM3+ and GZMB expression of tumor-infiltrating CD8+ T cells; statistical analysis was shown; n = 5, P = 0.025 (P), P = 0.0089 (Q). (R to T) In vitro xenograft tumor assays using LewishWT or LewishK162R cells; anti-CD8 antibody to block CD8+ T cell. The treatment protocol was summarized (R); the tumor growth rate (S) and weight (T) were shown; n = 6, P = 0.0053 (O) or ns, P = 0.0067 or ns (P). Error bars are means ± SD. Statistical significance was assessed using Student’s two-tailed t test. ns, not significant.
Next, we examined the tumorigenesis of RKO cells expressing PD-L1 WT or PD-L1 K162R in immunocompetent humanized peripheral blood mononuclear cell (huPBMC)–NCG (NOD/ShiLtJGpt-Prkdcem26Cd52 Il2rgem26Cd22/Gpt) mice and nonobese diabetic/severe combined immunodeficient NCG mice (Fig. 2G). RKOK162R tumors grew faster than RKOWT tumors in huPBMC-NCG mice (Fig. 2, H and I and fig. S2B). Consistently, IF staining of tumors in huPBMC-NCG mice confirmed a lower number of intratumor CD8+ T cells in RKOK162R tumors than in RKOWT tumors (Fig. 2, J and K). Also, tumor growth and weight were not obviously different between RKOK162R and RKOWT tumors in immunodeficient NCG mice (Fig. 2, L and M, and fig. S2C), which implied that PD-L1 K162 methylation did not affect the proliferation of tumor cells themselves, and tumor induced by PD-L1 K162 methylation deficiency relied on the immune system of mice. We generated humanized Lewis lung adenocarcinoma cells expressing human PD-L1 WT (LewishWT) or PD-L1 K162R (LewishK162R). We subcutaneously injected LewishWT and LewishK162R into the backs of humanized PD-1 C57BL/6 mice (C57BL/6hPD1). Result showed that LewishK162R tumors grew faster than LewishWT tumors in C57BL/6hPD1 mice (Fig. 2, N and O, and fig. S2D). Furthermore, we detected cytotoxicity in tumor-infiltrating CD8+ T cells and coexpression levels of PD-1 and T cell immunoglobulin (Ig) domain and mucin domain-3 (TIM-3) on CD8+ T cells. Flow cytometry analysis showed that the LewishK162R tumor had a lower GZMB expression of CD8+ T cells, and a higher ratio of PD-1 + TIM-3+ exhausted CD8+ T cells than that from the LewishWT tumors (Fig. 2, P and Q, and fig. S2, E and F). Also, we then used an anti-CD8 deletion antibody to disable the endogenous CD8+ T cells (Fig. 2R). The results showed that LewishK162R tumors grew obviously faster than LewishWT tumors; however, CD8 depletion negated this effect (Fig. 2, S and T, and fig. S2G). Collectively, these findings revealed that PD-L1 K162 methylation weakened its immunosuppressive function.
PD-L1 Lys 162 methylation is catalyzed by SETD7 and abrogated by IL-6
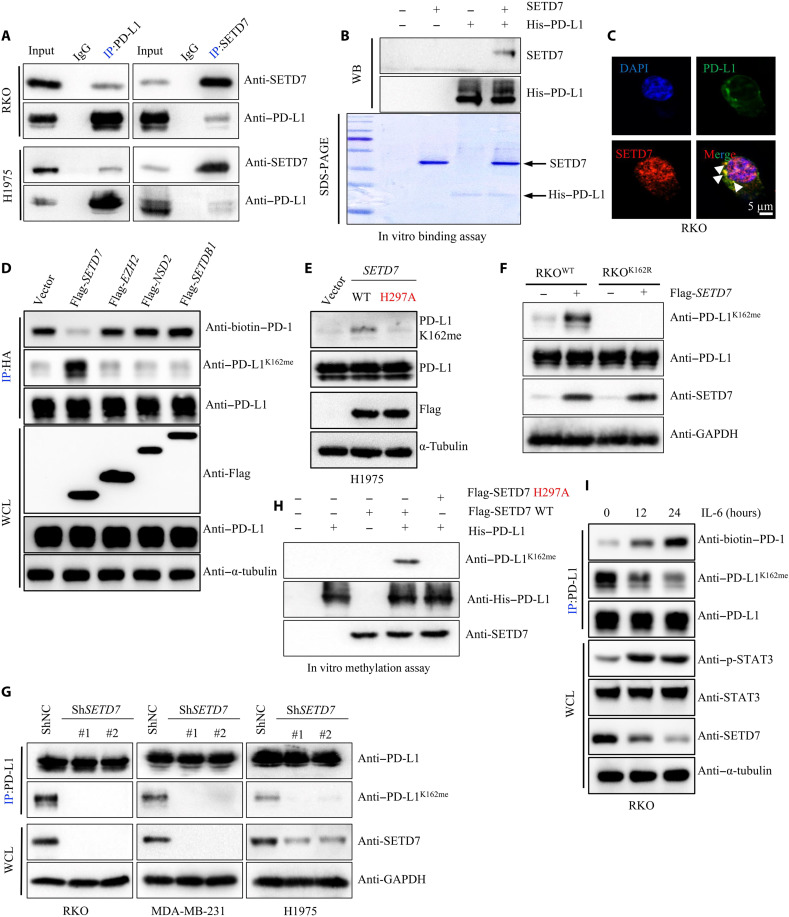
On the basis of this observation, we determined the methyltransferase(s) that mediated PD-L1 methylation. In the PD-L1 binding proteins profile, a physical interaction between SETD7 and PD-L1 was identified (table S4). SETD7 (also named SET7/9) belongs to the SET domain family of Lys methyltransferases (PKMTs) and was identified to catalyze histone H3 Lys 4 mono-methylation (H3K4me). We confirmed the physical interaction between SETD7 and PD-L1 by using another co-IP assay at the endogenous level in RKO and H1975 cells (Fig. 3A). The in vitro His-pulldown assay further demonstrated direct binding between SETD7 and PD-L1 (Fig. 3B), while immunofluorescence (IF) and co-IP assays in an extracted cytoplasm fraction showed that PD-L1 interacted with SETD7 in the cytoplasm (Fig. 3C and fig. S3A). Next, we attempted to determine whether SETD7 could methylate PD-L1. We transfected Flag-SETD7 and Flag-SETD7 H297A (a methyltransferase activity–deficient mutant) into RKO or H1975 cells and subsequently pulled down PD-L1 while using a pan-K-me1 antibody for detection. We found that PD-L1 mono-methylation was up-regulated in cells expressing ectopic SETD7, but not in the STED7 H297A mutant (fig. S3, B and C). Short hairpin RNA (shRNA)–mediated knockdown of SETD7 in RKO and H1975 cells markedly reduced PD-L1 mono-methylation compared to the control and was fully consistent with the SETD7 inhibitor PFI-2 (fig. S3, D and E), suggesting that SETD7 triggered PD-L1 mono-methylation.
Fig. 3. PD-L1 is methylated at Lys 162 by SETD7.
(A) Whole cell lysis (WCL) were collected for IP with PD-L1 or SETD7 antibody, followed by IB analysis. (B) Coincubating His–PD-L1 and SETD7 proteins, followed by IB analysis. (C) Fixed RKO cells stained with PD-L1 and SETD7 antibodies, followed by immunofluorescence (IF) assays. (D) HEK293T cells transfected with HA–PD-L1, and then cotransfected with Flag-SETD7, Flag-EZH2, Flag-NSD2, or Flag-SETDB1, followed by IP and IB analysis. (E) Transfecting Flag-SETD7 or Flag-SETD7 H297A into H1975 cells, WCE were collected for IB analysis. (F) Transfecting Flag-SETD7 into RKOPD-L1 WT or RKOPD-L1 K162R cells, WCE were collected for IB analysis. (G) Silencing cellular SETD7 expression, WCE were collected for IP with PD-L1 antibody, followed by IB analysis. (H) Immunoprecipitated SETD7 WT or SETD7 H297A protein from HEK293 cells was incubated with S-adenosyl-l-methionine along with His–PD-L1 protein for in vitro methylation assay. PD-L1 methylation was analyzed by IB analysis using with PD-L1 K162 mono-methylation–specific antibody. (I) RKO cells treated by IL-6 (10 ng/ml) in a time-dependent manner, followed by IP and IB analysis. All IBs are performed three times, independently, with similar results.
Furthermore, we used the K162-specific mono-methylation antibody to validate these findings. We first transfected Flag-SETD7 or other lysine methyltransferase, Histone-lysine N-methyltransferase EZH2 (EZH2), Histone-lysine N-methyltransferase NSD2 (NSD2), and Histone-lysine N-methyltransferase SETDB1 (SETDB1) as a negative control, into HEK293T cells ectopically expressing HA–PD-L1. Results showed that only SETD7 could catalyze PD-L1 mono-methylation at K162 (Fig. 3D). Next, we investigated whether other lysine of PD-L1 could be methylated by SETD7 and transfected by methylation-deficient variants of PD-L1 (K75R, K89R, K105R, and K162R) into HEK293T cells with or without SETD7 cotransfection. Results confirmed that only the K162R mutant completely abrogated SETD7-mediated PD-L1 mono-methylation up-regulation (fig. S3F). Moreover, we detected the PD-L1 K162 mono-methylation level in cells expressing the ectopic vector or SETD7 WT/SETD7 H297A. The data showed that PD-L1 K162 mono-methylation was increased in cells ectopicly expressing SETD7, but not in the STED7 H297A mutant (Fig. 3, E and F, and fig. S3G). Consistently, knockdown of SETD7 decreased PD-L1 K162 mono-methylation levels (Fig. 3G). The in vitro methylation assay confirmed that SETD7 methylated PD-L1 at the K162 site; however, the SETD7 H296A mutant failed to trigger PD-L1 K162 mono-methylation by using the PD-L1 K162–specific mono-methylation antibody (Anti–PD-L1K162me) (Fig. 3H and fig. S3H). Furthermore, we investigated which factors could trigger PD-L1 K162 non-methylation. After browsing the published studies, we found that IL-6 could suppress SETD7 expression (59). Hence, we try to assess whether IL-6 could regulate PD-L1 K162 methylation. Consistently, we proved that gain of IL-6 could inhibit SETD7 expression level, decrease PD-L1 K162 mono-methylation, and enhanced the binding between PD-L1 and PD-1 (Fig. 3I and fig. S3I). Collectively, these observations confirmed that PD-L1 K162 could be mono-methylated by SETD7, while PD-L1 K162 mono-methylation could be abrogated by IL-6 abnormal receiving.
SETD7 regulates PD-L1 and PD-1 interaction and antitumor immunity
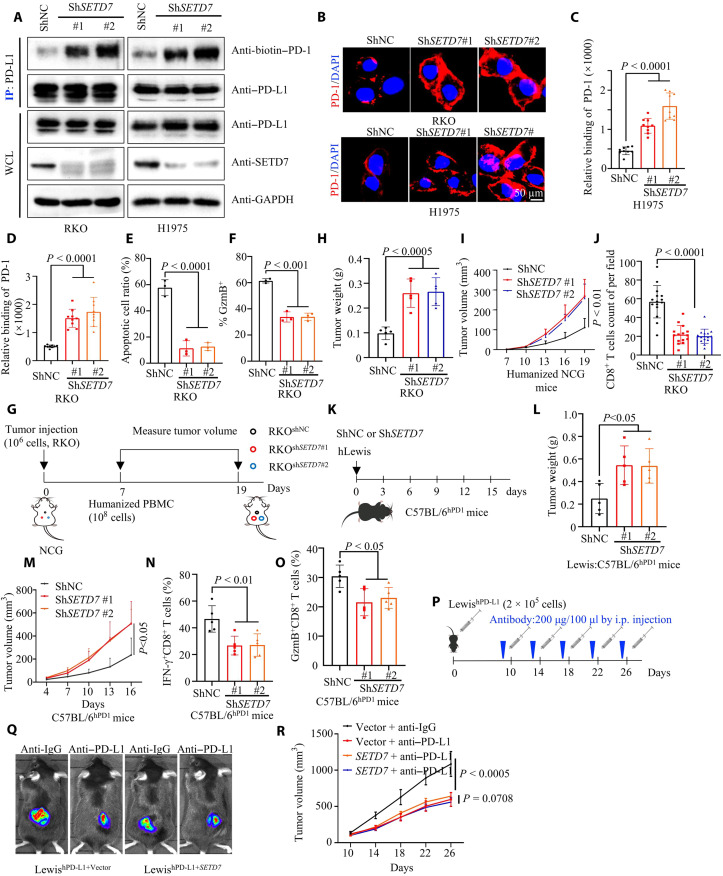
To further validate the biological function of PD-L1 K162 methylation, we first carried out another in vitro receptor-ligand binding assay, which showed that knockdown of SETD7 enhanced PD-L1 and PD-1 interaction, whereas overexpression of SETD7 abrogated the binding of PD-1 to PD-L1 (Fig. 4, A to D, and fig. S4, A to C). Cells expressing shRNA-mediated knockdown of SETD7 were more sensitive to activated T cell–mediated apoptosis (from PBMCs) (Fig. 4E and fig. S4D). Consistently, knockdown of SETD7 inhibited GZMB expression in activated primary human T cells (Fig. 4F and fig. S4E).
Fig. 4. SETD7 regulates PD-L1 and PD-1 interaction and antitumor immunity.
(A) IP and IB analysis measuring PD-1/PD-L1 interaction in short hairpin RNA–negative control (shNC) or short hairpin RNA–SETD7 (shSETD7) cells. (B to D) Representative images (B) showing bound PD-1/Fc fusion proteins on the membrane of shNC or shSETD7 cells. Statistical analysis was shown (C and D); n = 9, P < 0.0001 (C), P < 0.0001 (D). (E) T cell–mediated tumor cell killing assay in shNC or shSETD7 cells. The quantitative ratio of dead cells was showed; n = 3, P < 0.0001. (F) Activated T cells cocultured with shNC or shSETD7 cells; measuring GZMB of T cell by flow cytometry. Statistical analysis was shown; n = 3, P < 0.001. (G to I) In vitro xenograft tumor assays using shNC or shSETD7 cells in immunocompetent huPBMC-NCG mice. The treatment protocol was summarized (G); the tumor weight (H) and growth rate (I) were shown; n = 5, P < 0.0005 (H), P < 0.01 (I). (J) Immunohistochemistry analysis for number of intratumor CD8+ T cells. statistical analysis was shown; n = 15, P < 0.0001. (K to M) In vitro xenograft tumor assays using shNC or shSETD7 LewishWT cells in C57BL/6hPD1 mice. The treatment protocol was summarized (K); the tumor weight (L) and growth rate (M) were shown; n = 5, P < 0.0001 (L) and (M). (N and O) Measuring IFN-γ and GZMB expression of tumor-infiltrating CD8+ T lymphocytes; statistical analysis was shown; n = 5, P < 0.01 (N), P < 0.05 (O). (P to R) In vitro xenograft tumor assays using vector or SETD7 LewishWT cells in C57BL/6hPD1 mice. Anti–PD-L1 antibody was used at indicated time. The treatment protocol was summarized (P). The representative bioluminescence images (Q) and tumor growth rate (R) were shown; n = 5, P < 0.0005 or = 0.0708. All IBs are performed three times, independently, with similar results. Error bars are means ± SD. Statistical significance was assessed using Student’s two-tailed t test.
Furthermore, in immunocompetent huPBMC-NCG mice, we found that knockdown of SETD7 enhanced tumor growth compared to the control (Fig. 4, G to I, and fig. S4F). Further histological staining of tumors in huPBMC-NCG mice confirmed a lower number of intratumoral CD8+T cells in SETD7 knockdown tumors (Fig. 4J and fig. S4G). In addition, we knocked down SETD7 in LewishWT cells which down-regulated K162 methylation of humanized PD-L1 in Lewis cells (fig. S4H). Next, we used an animal study in humanized PD-1 C57BL/6 mice (C57BL/6hPD1) (Fig. 4K), which showed that SETD7 knockdown LewishWT tumors grew faster and weighed more than the control group (Fig. 4, L and M, and fig. S4I). Last, we detected cytotoxicity in tumor-infiltrating CD8+T cells using flow cytometry. CD8+CTLs from SETD7 knockdown LewishWT tumors showed lower GZMB and IFN-γ expression than CD8+CTLs from the control group tumors (Fig. 4, N and O, and fig. S4J). To further assess whether SETD7 regulates anti–PD-L1 therapeutic efficacy, we injected LewishWT-expressing ectopic vector or mouse SETD7 into the back of humanized PD-1 C57BL/6 mice (C57BL/6hPD1) and treated with hPD-L1 antibody (anti–hPD-L1). Results showed no difference between the anti–hPD-L1 group and the anti-IgG group in LewishWT-expressing mouse SETD7, while anti–hPD-L1 treatment could inhibit tumor growth induced by LewishWT-expressing vector. The ORR was 0.55 ± 0.087 in tumor induced by LewishWT-expressing vector, while the ORR was 0.91 ± 0.067 in tumor induced by LewishWT-expressing mouse SETD7 (P < 0.0001), suggesting that SETD7 overexpression triggered anti–hPD-L1 treatment resistance (Fig. 4, P to R). Collectively, our data showed that SETD7 regulated PD-L1 and PD-1 interactions and triggered anti–hPD-L1 resistance.
Methylation of PD-L1 K162 is required for SETD7-mediated antitumor immunity
To further validate the relationship between PD-L1 K162 methylation and SETD7 in the tumor immune response, we stably overexpressed SETD7 in RKOWT and RKOK162R cells, followed by in vitro receptor-ligand binding assays. Data showed that SETD7 weakened the interaction between WT PD-L1 and PD-1 but had no such effect on the PD-L1 K162R mutant (fig. S5A). In addition, the co-IP assay showed that SETD7 failed to attenuated the PD-L1 K162R mutant binding to PD-1 (Fig. 5A). Consistently, T cell-mediated tumor killing assays asserted that SETD7 increased T cell cytotoxicity and induced higher GZMB expression in PBMCs of RKOWT cells, but not in RKOK162R cells (Fig. 5, B to D, and fig. S5B). Next, we generated a stably expressing vector or SETD7 in LewishWT or LewishK162R cells, followed by an animal experiment (Fig. 5E). In vivo xenograft tumors revealed that the tumor growth of LewishK162R cells was obviously increased compared to that of LewishWT cells in C57BL/6hPD1 mice, while the antitumor effect of SETD7 was lower in LewishK162R cells than in LewishWT cells (Fig. 5, F to H). Consistently, LewishK162R tumors exhibited a dramatic decrease in the GZMB and IFN-γ expression of CD8+CTLs (Fig. 5, I to K), and SETD7 obviously enhanced CD8+ CTL immunity activity in LewishWT tumors, while this effect was disabled in LewishK162R tumor (Fig. 5, F to K). Together, these data strongly suggested that PD-L1 K162 methylation was required for SETD7-mediated antitumor effects.
Fig. 5. Methylation of PD-L1 K162 is required for SETD7-mediated antitumor immunity.
(A) Transfecting vector or Flag-SETD7 plasmids into RKOPD-L1 WT or RKOPD-L1 K162R cells and IP and IB analysis measuring PD-1/PD-L1 interaction. (B) Activated T cells cocultured with indicated RKO cells; isolating activated T cells; measuring GZMB of T cell by flow cytometry. Statistical analysis was shown; n = 3, P = 0.0067 or 0.0002. (C and D) T cell–mediated tumor cell killing assay in indicated RKO cells; the representative images (C) and the quantitative ratio of dead cells (D) were shown; n = 3, P = 0.001 or < 0.0001. (E) The successful construction of vector or SETD7 LewishWT cells (top) and the treatment protocol were summarized (bottom). (F to H) In vitro xenograft tumor assays using indicated cells in C57BL/6hPD1 mice. The tumor images (F), tumor weight (G), and tumor growth rate (H) were shown; n = 8 or 9 or 10, P < 0.0001 (G), P < 0.05 (H). (I to K) Measuring IFN-γ and GZMB expression of tumor-infiltrating CD8+ T lymphocytes by flow cytometry; representative results (I) and statistical analysis (J) and (K) were shown; n = 8 or 9 or 10, P < 0.0001 or ns (J), P = 0.0095 or < 0.0001 or not significant (ns) (K). All IBs are performed three times, independently, with similar results. Error bars are means ± SD. Statistical significance was assessed using Student’s two-tailed t test.
LSD2 demethylates PD-L1 K162 methylation
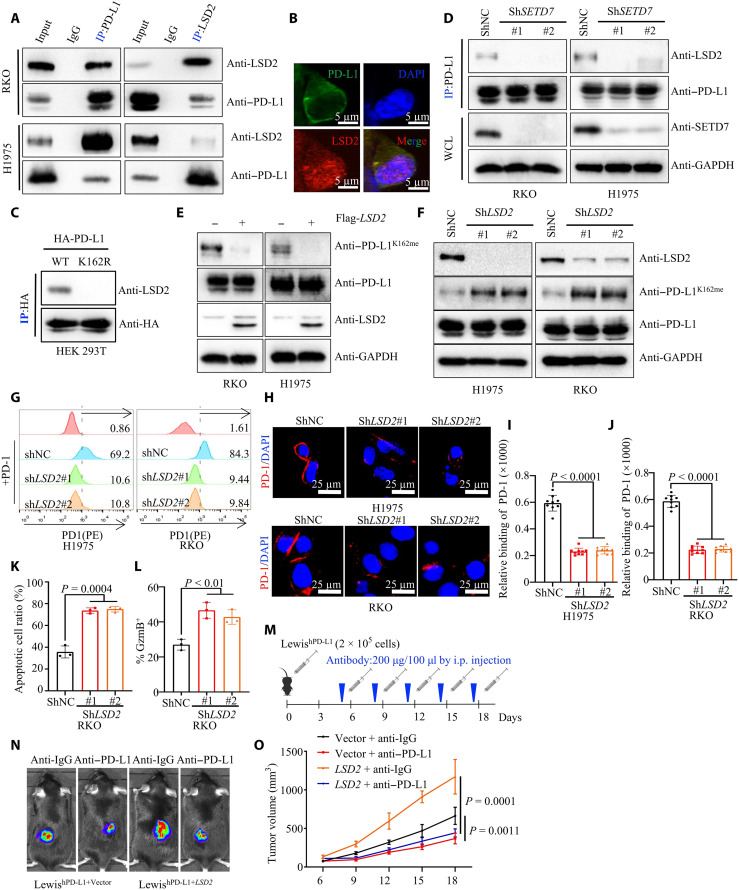
To further clarify the regulatory mechanism of PD-L1 K162 methylation in tumor cells, we focused on the demethylase that catalyzes PD-L1 K162 demethylation. On the basis of previous mass spectrum analysis, we searched PD-L1 binding protein profiles and found that LSD2, a KDM1 family demethylase, was a potential interacting protein of PD-L1 (table S4). The co-IP results were further confirmed that LSD2, but not LSD1 (lysine-specific histone demethylase 1, a homolog of LSD2), interacted with PD-L1 in RKO and H1975 cells (Fig. 6A and fig. S6A). Consistently, the IF assay showed that LSD2 colocalized with PD-L1 in the cytoplasm (Fig. 6B).
Fig. 6. LSD2 demethylates PD-L1 K162 methylation.
(A) WCE were collected for IP with PD-L1 or LSD2 antibody, followed by IB analysis with (top) or without (bottom) PNGase F reaction. (B) Fixed RKO cells stained with PD-L1 and LSD2 antibodies, followed by IF assays. (C) Transfecting HA–PD-L1 WT or HA–PD-L1 K162R plasmids into HEK293T cells, WCE were collected for IP assays with HA antibody, followed by IB analysis. (D) IP and IB analysis measuring LSD-2/PD-L1 interaction in shNC or shSETD7 cells. (E) IP and IB analysis measuring PD-1K162me level in vector or LSD2 cells. (F) WCE were collected from shNC or shLSD2 cells, followed by IB analysis. (G) Flow cytometry measuring PD-L1/PD-1 binding on the membrane of shNC or shLSD2 cells. (H to J) Representative images (H) for confocal image showing bound PD-1/Fc fusion proteins on the membrane of shNC or shLSD2 cells. Statistical analysis was shown (I and J); n = 9, P < 0.0001 (I), P < 0.0001 (J). (K) T cell–mediated tumor cell killing assay in indicated RKO cells; the quantitative ratio of dead cells was shown; P = 0.0004; n = 3, P = 0.0004. (L) Activated T cells cocultured with indicated RKO cells; measuring GZMB of T cell by flow cytometry. Statistical analysis was shown; n = 3, P < 0.01. (M to O) In vitro xenograft tumor assays using vector or LSD2 LewishWT cells in C57BL/6hPD1 mice. Anti–PD-L1 antibody was used at indicated time. The treatment protocol was summarized (M). The representative bioluminescence images (N) and tumor growth rate (O) were shown; n = 5, P = 0.0011 or = 0.0001. All IBs are performed three times, independently, with similar results. Error bars are means ± SD. Statistical significance was assessed using Student’s two-tailed t test.
To validate whether LSD2 demethylated PD-L1 K162 methylation, we used another co-IP assay which showed that LSD2 specifically interacted with WT PD-L1 but not with the PD-L1 K162R mutant (Fig. 6C). Notably, the interaction between PD-L1 and LSD2 was abrogated in SETD7 knockdown cells, whereas ectopic expression of SETD7 enhanced the binding of LSD2 and PD-L1 (Fig. 6D and fig. S6B). More directly, ectopic expression of LSD2 completely removed PD-L1 K162 mono-methylation in RKO and H1975 cells (Fig. 6E), whereas PD-L1 K162 mono-methylation was induced in LSD2 knockdown cells (Fig. 6F and fig. S6C). Furthermore, we transfected Flag-LSD2 or other lysine demethylases, LSD1, lysine-specific demethylase 4B (KDM4B), and lysine-specific demethylase 4D (KDM4D) as a negative control, into HEK293T cells ectopically expressing HA–PD-L1. Results showed that only LSD2 could demethylate PD-L1 K162 methylation and enhanced PD-L1/PD-1 interaction (fig. S6D). These data support the notion that LSD2 demethylates PD-L1 K162 methylation.
Subsequently, in vitro receptor-ligand binding assays and IF staining showed that knockdown of LSD2 inhibited the PD-1 and PD-L1 interaction (Fig. 6, G to J). T cell–mediated tumor killing assays also confirmed that knockdown of LSD2 enhanced T cell cytotoxicity and inversely induced GZMB expression in PBMCs (Fig. 6, K to L, and fig. S6, E and F). To further evaluate whether LSD2 regulates anti–PD-L1 therapeutic efficacy, we used an animal study where we injected LewishWT-expressing ectopic vector or mouse LSD2 into the backs of humanized PD-1 C57BL/6 mice (C57BL/6hPD1) and treated them with hPD-L1 antibody (anti–hPD-L1). There was dramatic tumor growth inhibition under anti–hPD-1 treatment compared to the anti-IgG group in tumor induced by LewishWT-expressing mouse LSD2 (P = 0.0001), while anti–hPD-L1 treatment could also inhibit tumor growth induced by LewishWT-expressing vector (P = 0.0011). However, the ORR was 0.37 ± 0.046 in tumor induced by LewishWT-expressing mouse LSD2, and the ORR was 0.56 ± 0.104 in tumor induced by LewishWT-expressing vector (P = 0.0078), suggesting that LSD2 overexpression enhanced anti–hPD-L1 treatment sensitivity (Fig. 6, M to O). Collectively, these findings demonstrated that LSD2 might function as a negative regulator, antagonizing SETD7-mediated PD-L1 K162 methylation and antitumor immunity.
PD-L1 K162 hypermethylation is a predictive biomarker of PD-1/PD-L1 blockade resistance therapy
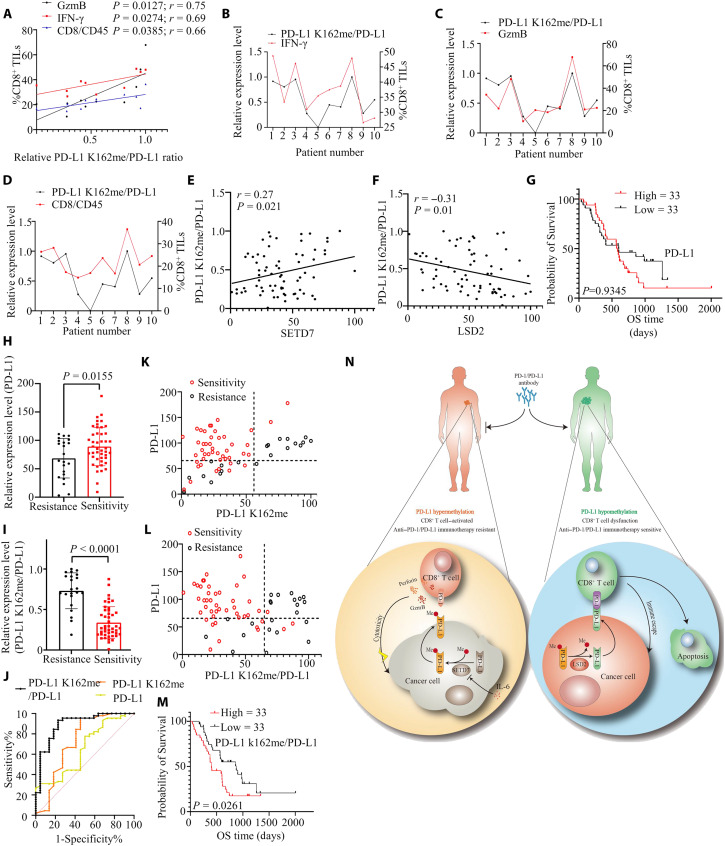
Several clinical trials have reported that PD-L1 expression levels cannot predict anti–PD-(L)1 treatment sensitivity (6). We confirmed that PD-L1 K162 methylation inhibited PD-L1/PD-1 interaction, while PD-L1/PD-1 binding was the key step for switching off T cell activity and tumor immune escape, suggesting that high PD-L1 expression did not predict more PD-L1/PD-1 interaction under PD-L1 K162 hypermethylation. In turn, we hypothesized that anti–PD-L1 treatment might be more sensitive in patients with PD-L1 hyperexpression and low levels of PD-L1 K162 methylation. To test our hypothesis, we first analyzed the TIMER/GSCA database and found that the expression level of SETD7 was positively related to the number of intratumor CD8+ T cells in different types of cancers, and the expression level of LSD2 was negatively correlated with the number of intratumor CD8+ T cells (fig. S7, A and B). Next, immunoblots and flow cytometry analysis were used to investigate the PD-L1 K162 methylation level, total PD-L1 expression level, and cytotoxicity of tumor-infiltrating CD8+ T cells in tumor specimens of patients with NSCLC. We found in several cases that PD-L1 was highly expressed followed by high PD-L1 K162 methylation levels (Case 1, 2, and 3), whereas, in some cases, PD-L1 showed high expression while the PD-L1 K162 methylation level was low (cases 4, 5, and 9) (fig. S8A). Further analysis showed that PD-L1 expression level was not related to the number and cytotoxicity of tumor-infiltrating CD8+ T cells, whereas PD-L1 K162 methylation:PD-L1 ratio (MPR) positively correlated with the number and cytotoxicity of tumor-infiltrating CD8+ T cells (Fig. 7, A to D, and fig. S8, B to E), suggesting that the MPR might be a better factor in response to tumor immune status. To further confirm this result, we analyzed the expression levels of PD-L1 K162 methylation, PD-L1, SETD7, and LSD2 in 70 tumor tissues from patients with NSCLC receiving anti–PD-1 treatment (table S5). Clinical data showed that patients who were sensitive to anti–PD-1 treatment had better overall survival (OS) (fig. S9A), suggesting that anti–PD-1 therapy was effective in sensitive patients. Next, we used IF assays and demonstrated that the MPR was positively correlated with SETD7 expression levels while it negatively correlated with LSD2 expression, and PD-L1 interacted with SETD7/LSD2 in NSCLC tissues (Fig. 7, E and F, and fig. S9, B and C). Using clinical data, we found that the expression level of PD-L1 was not related to OS after anti–PD-1 therapy (Fig. 7G). Consistently, patients who responded to anti–PD-L1 therapy had a higher expression level of LSD2 and PD-L1, and a lower expression level of PD-L1 K162 methylation, SETD7, and the MPR compared with anti–PD-1 therapy-resistant patients (Fig. 7, H and I, and fig. S9, D and E).
Fig. 7. PD-L1 K162 hypermethylation is a predictive biomarker of PD-1/PD-L1 blockade resistance therapy.
(A to D) Ten NSCLC samples and its WCE were collected; measuring PD-L1 and PD-L1 k162 methylation level by IB analysis; investigating cytotoxicity of tumor-infiltrating CD8+ T cells in tumor specimens by flow cytometry analysis. Pearson’s correlation between PD-L1 K162me/PD-L1 ratio and the number/cytotoxicity of tumor-infiltrating CD8+ T cells was shown; n = 10. (E and F) Collecting 70 clinic samples from patients with NSCLC receiving anti–PD-1 treatment, IF assays measuring PD-L1, PD-L1 K162me, SETD7, and LSD2 expression level; Pearson’s correlation between PD-L1 K162me/PD-L1 ratio and SETD7 (E) or LSD2 (F) was shown; n = 70, P = 0.021 (E), P = 0.01 (F). (G) Kaplan-Meier survival analysis was shown. Patients were grouped by PD-L1 expression level; n = 33 (high), n = 33 (low), P = 0.9345. (H) Shown is the PD-L1 relative expression level of patients with anti–PD-1 treatment resistance or sensitivity; n = 45 (sensitivity), n = 22 (resistance), P = 0.0155. (I) Shown is the PD-L1 K162me/PD-L1 relative ratio of patients with anti–PD-1 treatment resistance or sensitivity; n = 45 (sensitivity), n = 22 (resistance), P < 0.0001. (J) Receiver operating characteristic curve analysis for the indicated parameters in patients receiving anti–PD-1 treatment. (K) Shown is the PD-L1 and PD-L1 K162me expression level of patients with NSCLC receiving anti–PD-1 treatment; red (sensitivity), black (resistance); the axes were separated by the Youden index. (L) Shown is the PD-L1 and PD-L1 K162me/PD-L1 expression level of patients with NSCLC receiving anti–PD-1 treatment; red (sensitivity), black (resistance); the axes were separated by the Youden index. (M) Kaplan-Meier survival analysis was shown; patients were grouped by the PD-L1 K162me/PD-L1 ratio; n = 33 (high), n = 33 (low), P = 0.0261. (N) Working model of SETD7-catalyzed LSD2-antagonized PD-L1 K162 methylation cross-talk with host antitumor immunity and anti-PD-(L)1 treatment prognosis. Error bars are means ± SD. Statistical significance was assessed using Student’s two-tailed t test.
We used the MPR as a predictive biomarker for sensitivity to PD-1/PD-L1 blockade therapy. The receiver operating characteristic curve showed that, among all the parameters tested, the level of the MPR could distinguish anti–PD-1 treatment sensitivity in patients with NSCLC from anti–PD-L1 treatment resistant patients with NSCLC (Fig. 7J). Results revealed the cutoff value of the MPR, determined by the Youden index [J = sensitivity-(1-specificity)], less than 0.65 predicted 93.33% sensitivity and 77.27% specificity of anti–PD-1 therapy, which is an improvement from using PD-L1 or PD-L1 K162 methylation as the predictive markers (table S6). We subsequently reanalyzed the above clinical data and found that the MPR could serve as a predictive biomarker of sensitivity to PD-1/PD-L1 blockade therapy; a low MPR (less than 0.65) indicated a better OS when patients received anti–PD-1 therapy (Fig. 7, K to M). If patients had high PD-L1 K162 methylation expression level, the cytotoxic function of tumor-infiltrating CD8+ T cells would be not inhibited by PD-L1/PD-1 binding, because PD-L1 K162 methylation attenuated the interaction of PD-L1 and PD-1. In this situation, other therapeutic strategies (such as Anti-CTLA4, Anti-Tim3, chemotherapy, radiotherapy, and antiangiogenic therapy) might be the first choice. Together, our data hinted that PD-L1 hypomethylated status was required for anti–PD-L1 or anti–PD-1 treatment sensitivity, and MPR might function as a better mark to guide anti–PD-L1 or anti–PD-1 treatment.
DISCUSSION
Immune suppression and evasion of cancer cells by various mechanisms are considered one of the hallmarks of cancer (5). ICIs have heralded an era in cancer treatment, and antibodies that block the PD-1-PD–L1 axis have clinical activity in nearly 20 different cancer types as of December 2021, enabling the possibility of long-term survival in patients with late-stage disease and providing therapeutic indications in earlier-stage settings (60–62). However, although some patients with cancer experience durable and complete treatment responses from antagonistic antibodies to PD-1 or PD-L1, most fail to respond, suggesting the existence of PD-L1–related immunopathogenesis and treatment resistance mechanisms (6–8). In this study, we demonstrated that PD-L1 methylation is a critical modification for its function and that PD-L1 hypermethylation tumors are resistant to anti–PD-L1 and anti–PD-1 therapy. We confirmed that SETD7 triggered PD-L1 methylation at K162, which was demethylated by LSD2. Meanwhile, we also proved that IL-6 could inhibit SETD7 expression level and suppress PD-L1 K162 methylation.
Currently, despite the direct evaluation of PD-L1 expression as biologically plausible and the best available biomarker for predicting tumor response and survival prognosis, many PD-L1–positive tumors do not respond to the PD-L1–PD-1 blockade (27). The PTMs that contribute to the regulation of PD-L1 expression and function included phosphorylation, palmitoylation, glycosylation, acetylation, and ubiquitination (2, 63). Considering our current findings, we highlighted that PD-L1 K162 methylation was important for its immunosuppressive function, and in vitro and in vivo data confirmed that methylation of PD-L1 at K162 played a negative role in PD-1 and PD-L1 interaction, supporting an alternative immune suppression and evasion mechanism in PD-L1 hypermethylation cancers. Hence, PD-L1 hypermethylated tumors are resistant to anti–PD-L1 or anti–PD-1 therapies. Further in vivo and clinical sample analyses also showed that PD-L1 K162 hypermethylation predicted the efficiency of anti–PD-L1 or anti–PD-1 therapy resistance. The SETD7 expression level was negatively related to the response to anti–PD-L1 therapy and positively related to the number of intratumor CD8+ T cells in different types of cancers, while the expression level of LSD2 was positively related to the response to anti–PD-L1 therapy and negatively related to the number of intratumor CD8+ T cells.
Currently, reliable anti–PD-L1 or anti–PD-1 treatment response biomarkers are lacking, which leads to obvious side effects, financial costs, and health care burden, with unsatisfactory clinical benefits in most treated patients (5, 11). Several studies and trials have investigated the use of multiple biomarkers to predict patient response or harm; however, none are comprehensive in predicting potential benefit (64). In this study, we demonstrate a previously unknown biologic marker to predict anti–PD-L1 or anti–PD-1 treatment response. Results showed that the MPR was a superior biomarker to predict a sensitive patient who may receive the anti–PD-L1 or anti–PD-1 therapy. Considering 0.65 of the cutoff values of MPR, we predicted 93.33% sensitivity and 77.27% specificity of anti–PD-1 therapy for patients with NSCLC.
In summary, we identified PD-L1 K162 methylation induced by SETD7 as a critical modification for PD-1 and PD-L1 interaction; thus, PD-L1 K162 hypermethylation tumors were resistant to anti–PD-L1 and anti–PD-1 therapy (Fig. 7N). Our study identified PD-L1 as a nonhistone substrate of SETD7, whereas PD-L1 K162 hypomethylation contributed to cancer immune surveillance. Furthermore, our study revealed that abnormal IL-6 stimulation obviously inhibited SETD7 expression and PD-L1 K162 methylation, and SETD7-mediated PD-L1 K162 methylation not only served as a critical step for PD-1/PD-L1 interaction but also represented a negative predictive marker for anti–PD-L1 and anti–PD-1 treatment in patients with NSCLC.
MATERIALS AND METHODS
Cell lines and cell culture
Human H1975, MDA-MB-231, RKO, HEK293T, and mouse Lewis cell lines were purchased from American Type Culture Collection. RKO, HEK293T, and Lewis cells were maintained in Dulbecco’s modified Eagle’s medium basal medium (KeyGEN Biotech). MDA-MB-231 cells were cultured in L-15 medium (HyClone; GE Healthcare). H1975 cells were cultured in RPMI 1640 medium (KeyGEN Biotech). All media were mixed with 10% (v/v) fetal bovine serum (Gibco; Thermo Fisher Scientific Inc.). MDA-MB-231 cells were cultured at 37°C in a 100% air incubator, and the other cells were cultured at 37°C in a 5% (v/v) CO2 incubator.
Antibodies and chemicals
The primary antibodies for PD-L1 (29122 and 13684; Cell Signaling Technology and CSB-MA878942A0m; CUSABIO), pan phospho-serine/threonine rabbit mAb (AP0893; ABclonal), pan phospho-tyrosine rabbit mAb (AP1162; ABclonal), ABclonal), pan mono-methyl lysine (A18293; ABclonal), SETD7 (A9225; ABclonal), LSD2(A19820; ABclonal), glyceraldehyde-3-phosphate dehydrogenase (sc-47724; Santa Cruz Biotechnology), H3K4me1 (A2355; ABclonal), flag tag (AE063; ABclonal and 14793S; Cell Signaling Technology), pan acethyl lysine (ab190479, abcam), HA tag (AE036; ABclonal and 3724; Cell Signaling Technology), α-tubulin (AC007; ABclonal), PCNA (A12427; ABclonal), biotin (5597; Cell Signaling Technology, 20684; ABclonal), caveolin-1 (A19006; ABclonal), and CD8 (ab237709; Abcam) were purchased from commercial company. The PD-L1 K162 mono-methylation rabbit antibody was prepared by ABclonal, China (https://abclonal.com.cn). Secondary antibodies for horseradish peroxidase (HRP) goat anti-rabbit IgG (H+L) (GM01H; sibEnzyme), HRP goat anti-mouse IgG (H+L) (GRB01H; sibEnzyme), HRP-conjugated AffiniPure goat anti-rabbit IgG light chain (AS062; ABclonal), and HRP-conjugated AffiniPure Mouse Anti-Rabbit IgG light chain (AS061; ABclonal) were commercially available. The secondary antibodies used for IF assays were as follows: DyLight 549, goat anti-rabbit IgG (A23320; Abbkine); DyLight 488, goat anti-mouse IgG (A23210; Abbkine); DyLight 488, goat anti-rabbit IgG (A23220; Abbkine); and DyLight 549, and goat anti-mouse IgG (A23310; Abbkine). Antibodies for flow cytometry were listed: phycoerythrin (PE)-conjugated anti-human PD-1 antibody (367403; BioLegend), Alexa Fluor 647– conjugated anti-human GZMB antibody (IC2906R; R&D Systems), PE-conjugated anti-human PD-L1 antibody (374512; BioLegend), antigen-presenting cell allophycocyanin (APC)–conjugated anti-mouse PD-L1 antibody (124311; BioLegend), PE-conjugated anti-mouse CD45 antibody (147711; BioLegend), APC-conjugated anti-human CD45 antibody (305011; BioLegend), fluorescein isothiocyanate (FITC)–conjugated anti-human/mouse GZMB antibody (515403; BioLegend), PE-conjugated anti-mouse IFN-γ antibody (505808; BioLegend), PerCP/Cyanine5.5–conjugated anti-human CD45 antibody (304028; BioLegend), PE-conjugated anti-human CD8 antibody (344706; BioLegend), FITC-conjugated anti-human CD3 antibody (300305; BioLegend), Zombie NIR Fixable Viability Kit (423106; BioLegend), APC-conjugated anti-mouse CD3 antibody (100236; BioLegend), PerCP/Cyanine5.5-conjugated anti-mouse CD45 antibody(147706; BioLegend), PE/Cy7-conjugated anti-mouse CD8 antibody (552877; BD Pharmingen), PE-conjugated anti-mouse CD45 antibody(147712; BioLegend), and PerCP/Cyanine5.5–conjugated anti-mouse IFN-γ antibody (505821; BioLegend). PFI-2 (HY-18627) was bought from MedChemExpress company. Deacetylase inhibitor cocktail (P1112, Beyotime), phosphatase inhibitor cocktail (P1082, Beyotime), monensin (420701; BioLegend), brefeldin A (420601; BioLegend), ionomycin (abx282481; Abbexa), and phorbol 12-myristate 13-acetate (abx282551; Abbexa) were obtained from the indicated companies.
Plasmid construction
Plasmids expressing PCDNA3.1-flag-hSETD7, PCDNA3.1-flag-mSETD7, PCDNA3.1-flag-hLSD2, and PCDNA3.1-ha-PD-L1 were purchased from AUGCT (www.augct.com). Plasmids for pLKO-AS3W-ha-PD-L1, pLKO-AS3W- flag-mSETD7, pLKO-AS3W-flag-hSETD7, and pLKO-AS3W-flag-hLSD2 were constructed by inserting the indicated DNA into the pLKO-AS3W vector. Plasmids expressing PD-L1 and SETD7 mutants were constructed using the Mut Express II Fast Mutagenesis Kit V2 (C214-01; Vazyme). The target sequences of shRNAs were as followed: shhSETD7#1 (5′-GCCTTGTAGGAGAAGTAAA-3′); shhSETD7#2 (5′-GGGTTTAUGTTGCTGAATC-3′); shhSETD7#3 (5′-CCGCACTTTATGGGAAATT-3′); shhLSD2#1 (5′-AAGACATTCAAGGAACCGTCT-3′); shhLSD2#2 (5′- GAGAAGAAGTACAGGAAAT-3′); shhLSD2#3 (5′- TTAACAACCCAGTAGCATTAA-3′); shmSETD7#1 (5′-CCTAATACTGTTATGTCGTTT-3′); shmSETD7#2 (5′- CCGTGTTCAGAGATACCAAAT-3′); shmSETD7#3 (5′- CGGAGTGTGTTGGATCCATTA-3′); shmLSD2#1 (5′-CCTGGCTTTGAGAAACCTCAT-3′); shmLSD2#2 (5′- GCCAAGAAACTTCAGTTGATA-3′); shmLSD2#3 (5′- GCAAGCAAGATTGCAGCCTTT-3′); shmPD-L1#1 (5-GGTCAACGCCACAGCGAAT-3); shmPD-L1#2 (5-GGCGTTTACTGCTGCATAATC-3); shmPD-L1#2 (5-AGACGTAAGCAGTGTTGAA-3). Plasmids containing pLKO.1-shRNAs were constructed by inserting the indicated shRNA sequences into the pLKO.1 vector.
Construction of indicated cell lines
Luciferase virus was purchased from Genechem Shang Hai. pLKO.1-shRNAs, pMD2.G, and psPAX three-package system were used to construct a silencing-expression lentivirus. We generated high-expression viruses using the pLKO-AS3W-DNAs, pMD2.G, and psPAX three-package system. The CRISPR-Cas9 system was used to construct hPD-L1 knockout cell lines. Single-guide RNAs (sgRNAs) for hPD-L1 were as follows: 5′-GTCCAGATGACTTCGGCCTT-3′;5′-GGATGACCAATTCAGCTGTA-3′;5′-GACACATTCAGAATATTACC-3′; sgRNAs for mPD-L1 were as follows: 5′-GCCAGTGGCAGGTGAGTCTC-3′;5′-GCCTGCTGTCACTTGCTACG-3′ and 5′-GAGGTTGGACAAGGCTTCCG-3′. All viruses were transfected into the indicated cells. After 48 hours, puromycin was used to select the infected cells. Immunoblot assays were performed to validate the successful construction.
MS analysis
MS assays were conducted at the Protein Chemistry and Proteomics Facility of Tsinghua University Technology Center for Protein Research. Proteins samples were separated by SDS–polyacrylamide gel electrophoresis. The bands of interest were excised from the gel, reduced with dithiothreitol (5 mM), alkylated with iodoacetamide (11 mM), and digested with sequencing-grade modified trypsin in ammonium bicarbonate (50 mM) at 37°C overnight. The peptides were extracted using 0.1% trifluoroacetic acid in 50% acetonitrile aqueous solution. The peptides were redissolved in 25 μl of 0.1% trifluoroacetic acid and analyzed using an Orbitrap Fusion mass spectrometer. For liquid chromatography–tandem MS (LC-MS/MS) analysis, the peptides were separated using a Thermo Dionex Ultimate 3000 HPLC system directly interfaced with an Orbitrap Q Exactive mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). An LTQ-Orbitrap mass spectrometer was operated in data-dependent acquisition mode using the Xcalibur 4.0.27.10 software, followed by 3-s data-dependent MS/MS scans in an ion routing multipole at 30 normalized collision energy (HCD). The MS/MS spectra from each LC-MS/MS run were searched using an in-house Proteome Discoverer (Version PD1.4, Thermo Fisher Scientific, USA). Static peptide modifications include methylation, acetylation, and phosphorylation.
Cellular cytoplasm and nucleus isolation
Cellular cytoplasmic and nuclear fractions were separated using the Nuclear and Cytoplasmic Protein Extraction Kit (16B01A06; BOSTER).
Immunoblot assays and IP
The indicated cells were lysed with NP-40 lysis buffer for 30 min at 4°C, and the protein concentration was measured using a bicinchoninic acid assay kit (Thermo Fisher Scientific). A 5× protein sample buffer [250 mM tris-HCl (pH 6.8),10% SDS, 30% glycerol, 5% β-mercaptoethanol, and bromophenol blue] was mixed with protein, and then samples were boiled at 100°C for 5 to 10 min. Proteins were separated by electrophoresis in an 8 to 12% premade SDS-polyacrylamide minigel, and then transferred to a polyvinylidene difluoride membrane. Membranes were incubated with the indicated primary antibodies overnight at 4°C, and then incubated with HRP-conjugated secondary antibodies for 2 hours at room temperature. The membranes were analyzed using chemiluminescence. For IP, magnetic beads were washed three times with NP-40 lysis buffer, and then primary antibodies were incubated with magnetic beads (HY-K0205; MCE) overnight at 4°C, subsequently rotated with cell lysis and immunoblot assays.
In vitro methylation assays and his-pulldown assays
Proteins for SETD7 (11209-H07E, ab51285, Abcam) and His-tagged PD-L1 (10084-H08H, ab126688, Abcam) were obtained from the indicated companies. FLAG-tagged SETD7 was isolated from HEK293T cells. Biotin-tagged PD-L1 peptides were produced by ABclonal. For in vitro methylation assays, 1 μg of SETD7, 5 μl of 5 × PKMT buffer [10 mM tris-HCl (pH 8), 2% glycerol, 0.8 mM KCl, and 1 mM MgCl2], 13 μM S-adenosyl-l-methionine, His–PD-L1–purified protein, or biotin-tagged PD-L1 peptides as substrates and H2O were added to a final volume of 25 μl. The reaction tubes were then incubated at 37°C for 10 hours. The reaction was stopped by adding 5× protein sample buffer [250 mM tris-HCl (pH 6.8),10% SDS, 30% glycerol, 5% β-mercaptoethanol, and bromophenol blue], followed by boiling the samples at 95°C for 10 min. For His-pulldown assays, magnetic beads His-tag (C650033, Sangon Biotech) were washed three times with NP-40 buffer, and then His-tagged PD-L1 protein and purified SETD7 protein were incubated and rotated with magnetic beads His-tag overnight at 4°C. The washed magnetic beads were His-tagged five times with wash buffer, followed by immunoblot assays.
Immunohistochemistry
Clinical samples were obtained from the Wuhan Tongji Hospital. This study was approved by the Ethics Committee of the Huazhong University of Science and Technology and the Institutional Ethics Committee of Shanghai Pulmonary Hospital. Written informed consent was obtained from all participants. For IHC, tissue specimens were cut into 4-μm section and fixed with 4% paraformaldehyde for 15 min at room temperature. The samples were stained with the indicated primary antibodies overnight at 4°C, followed by incubation with secondary antibodies at room temperature for 2 hours. Two independent experts were blinded to assess each result.
Immunofluorescence
For IF, 4-μm tissues specimens and the indicated cells were fixed with 4% paraformaldehyde for 15 min at room temperature. Samples were stained with the indicated primary antibodies overnight at 4°C, followed by incubation with Alexa Fluor 549/488–conjugated secondary antibodies at room temperature for 2 hour. The cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Images were obtained using a fluorescence microscope or confocal laser scanning microscope. For tissue samples, the mean fluorescence intensity (MFI) was calculated using the ImageJ software (version 1.8.0). The MFI for LSD2, SETD7, and PD-L1 K162me was standardized to the score of 1 to 100. The maximum MFI (PD-L1 K162me)/MFI (PD-L1) was standardized to 1, and the other MFIs of PD-L1 for tissues were standardized.
Immune receptor and ligand interaction assay
For PD-L1 and PD-1 protein interaction, cells were incubated with biotinylated PD-1 (SinoBiological, China) for 1 hour, and then fixed with 4% paraformaldehyde at room temperature for 15 min. The cells were then incubated with an anti-biotin antibody for 2 hours at room temperature, followed by incubation with Alexa Fluor 549–conjugated secondary antibodies. The cell nuclei were stained with DAPI. Cell images were obtained using a fluorescence microscope or confocal laser scanning microscope. Flow cytometry assays were also conducted to detect the binding between PD-L1 and PD-1.
Deglycosylation of PD-L1
Glycosylated PD-L1 protein was deglycosylated using PNGase F (P0704; BioLabs), according to the manufacturer’s instructions.
T cell–mediated tumor cell killing assay
Assays were conducted as described previously (65–67). Briefly, dendritic cells (DCs) were isolated from PBMCs and cocultured with the indicated cancer cells. PBMCs were activated using pretreated DCs. To analyze T cell–mediated tumor cell killing, pretreated PBMCs were reactivated with IL-2 (100 IU/ml), anti-CD3 antibody (0.1 μg/ml), and anti-CD28 (0.1 μg/ml) antibodies. We cocultured the indicated cells with activated primary human T cells at a 10:1 ratio. On the one hand, cells were stained with caspase-3/7 Activity Apoptosis Assay Kit *Green Fluorescence (E607103; Sangon Biotech) according to the instruction books after 24 to 48 hours. Cell nuclei were stained with Hoechst33342. Cell images were obtained using a fluorescence microscope. On the other hand, T cells and cell debris were washed with phosphate-buffered saline (PBS) three times after 3 days, and the left cells were fixed with 4% paraformaldehyde for 15 min at room temperature. The cells were then stained with crystal violet, and the optical density value was quantified using a spectrophotometer at 570 nm.
Analysis of T cell activation
After coculturing the indicated cancer cells and activated primary human T cells, T cells were collected, stained, and analyzed by flow cytometry.
Enzyme-linked immunosorbent assay
The tumor necrosis factor–α (TNF-α) concentration in the medium was measured using a Human TNF-alpha ELISA Kit (RK00030; ABclonal) according to the manufacturer’s instructions.
Real-time PCR
Total RNA was extracted from the indicated cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. cDNA was synthesized using the ABScript III RT Master Mix for quantitative polymerase chain reaction (qPCR) with a gDNA Remover kit (RK20429; ABclonal). Quantitative real-time PCR was performed using ABScript III RT Master Mix for qPCR (RK20429; ABclonal) and a QuantStudio 3 Real-Time PCR Instrument (Thermo Fisher Scientific). The primers used were 5′-TCGGTAACTGACTTGAATGTCCA-3′ (forward) and 5′-TCGCTTCCCTGTTTTAGCTGC-3′ (reverse).
Isolation of tumor-infiltrating lymphocytes and flow cytometry analysis
Tumor tissues were gently split into small pieces, and then digested using 6 ml of PBS containing collagenase IV (50 μl 25 mg/ml; 17104019, Invitrogen) and deoxyribonuclease I (25 μl 10 mg/ml; 10104159001, Roche) for 1 hour at 37°C. Tumor cells and tumor-infiltrating lymphocytes were separated, harvested, and isolated using a Percoll gradient (17-0891-01, GE Healthcare) according to the manufacturer’s instructions. Fluorochrome-labeled antibodies were used for the flow cytometric analysis. For cellular cytokine analysis, cells were cocultured with phorbol myristate acetate, ionomycin, and brefeldin A for 4 to 8 hours. The Zombie UV Fixable Viability Kit (423106; BioLegend) was used to exclude dead cells. Fixed buffer and intracellular staining perm wash buffer (421002, BioLegend) were used for intracellular cytokine staining.
Animal study
All mouse studies were approved by the Animal Care and Use Committee of Tongji Hospital. The sample sizes were justified by statistical considerations and statistical power analyses. Mice were randomly divided into groups. The assignment was blinding during the experiment and outcome assessment. NCG mice were purchased from GemPharmatech company, NCG mice was knocked out Prkdc (protein kinase, DNA activated, catalytic polypeptide) and Il2rg from NOD/ShiltJGpt mice by gene editing technology, which was a severe immunodeficient mouse strain. For xenograft tumor assays in huPBMC-NCG mouse, 6- to 8-week-old NCG mice were injected with cancer cells (1 × 106). A total of 10 × 106 PBMCs were injected into the tail vein 7 days after cancer cell injection. The tumor volume was measured at the indicated time, and tumor volume was calculated using the formula: V = 0.5 × major axis × minor axis × minor axis. Six- to 8-week-old humanized PD-1 C57BL/6 mice [B6/JGpt-Pdcd1em1Cin(hPDCD1)/Gpt; GemPharmatech] were injected with humanized PD-L1 Lewis cells. Antibodies for IgG (BE0088; BioXcell), CTLA-4 (BE0131; BioXcell), CD8 (BE0223; BioXcell), and PD-L1 (durvalumab; IMFINZI) were intraperitoneally injected at the indicated times (shown in the figures). All mice were euthanized after the experiments. Tumor images were obtained, and the tumor tissues were further analyzed.
Statistical analysis
Statistical analysis was carried out using SPSS 22.0 and GraphPad Prism 8.0. The data are presented as means ± SD. The differences were analyzed using Student’s t test or one-way analysis of variance (ANOVA). Differences were considered statistically significant at P < 0.05.
Acknowledgments
We thank the Medical Subcenter of HUST Analytical & Testing Center for data acquisition. We were grateful to W. Zhang and H.-K. Lin for suggestions on our work.
Funding: This work was supported by NSFC (nos. 81974432 to G.W., 81922053 to G.W., and 81874186 to J.H.), startup funding from Tongji Hospital for G.W., and National Key Research and Development Program of China (no. 2022YFA1105303 G.W.)
Author contributions: G.W. conceived the project. C.H., J.H., and G.W. contributed to the design of the project and extensive discussions. C.H., Y.C., and A.L. performed the animal experiments. C.H., Q.W., and D.S. performed ex vivo T cell experiments and biochemistry experiments. C.H., F.H., and J.L. analyzed the mass spectrometry samples. C.H. performed chromatin immunoprecipitation and quantitative PCR with reverse transcription experiments. S.R., T.J., Q.C., J.W., and C.Z. provided human NSCLC samples and clinical analysis. X.Z. provided human PBMCs. P.L. purified the proteins. Y.L., S.R., L.S., and X.L. provided technical assistance. C.H., J.H., and G.W. wrote the manuscript and the other authors revised it. C.H. and G.W. made the figures. J.H. helped to proofread the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S9
Tables S1 to S3, S5 and S6
Other Supplementary Material for this manuscript includes the following:
Table S4
REFERENCES AND NOTES
- 1.A. Kalbasi, A. Ribas, Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 20, 25–39 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.C. Sun, R. Mezzadra, T. N. Schumacher, Regulation and function of the PD-L1 checkpoint. Immunity 48, 434–452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.A. M. Scott, J. D. Wolchok, L. J. Old, Antibody therapy of cancer. Nat. Rev. Cancer 12, 278–287 (2012). [DOI] [PubMed] [Google Scholar]
- 4.X. He, C. Xu, Immune checkpoint signaling and cancer immunotherapy. Cell Res. 30, 660–669 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.P. S. Hegde, D. S. Chen, Top 10 challenges in cancer immunotherapy. Immunity 52, 17–35 (2020). [DOI] [PubMed] [Google Scholar]
- 6.S. L. Topalian, F. S. Hodi, J. R. Brahmer, S. N. Gettinger, D. C. Smith, D. F. McDermott, J. D. Powderly, R. D. Carvajal, J. A. Sosman, M. B. Atkins, P. D. Leming, D. R. Spigel, S. J. Antonia, L. Horn, C. G. Drake, D. M. Pardoll, L. Chen, W. H. Sharfman, R. A. Anders, J. M. Taube, T. L. McMiller, H. Xu, A. J. Korman, M. Jure-Kunkel, S. Agrawal, D. McDonald, G. D. Kollia, A. Gupta, J. M. Wigginton, M. Sznol, Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.J. R. Brahmer, S. S. Tykodi, L. Q. M. Chow, W. J. Hwu, S. L. Topalian, P. Hwu, C. G. Drake, L. H. Camacho, J. Kauh, K. Odunsi, H. C. Pitot, O. Hamid, S. Bhatia, R. Martins, K. Eaton, S. Chen, T. M. Salay, S. Alaparthy, J. F. Grosso, A. J. Korman, S. M. Parker, S. Agrawal, S. M. Goldberg, D. M. Pardoll, A. Gupta, J. M. Wigginton, Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.M. A. Gubens, M. Davies, NCCN guidelines updates: New immunotherapy strategies for improving outcomes in non-small cell lung cancer. J. Natl. Compr. Canc. Netw. 17, 574–578 (2019). [DOI] [PubMed] [Google Scholar]
- 9.J. D. Wolchok, H. Kluger, M. K. Callahan, M. A. Postow, N. A. Rizvi, A. M. Lesokhin, N. H. Segal, C. E. Ariyan, R. A. Gordon, K. Reed, M. M. Burke, A. Caldwell, S. A. Kronenberg, B. U. Agunwamba, X. Zhang, I. Lowy, H. D. Inzunza, W. Feely, C. E. Horak, Q. Hong, A. J. Korman, J. M. Wigginton, A. Gupta, M. Sznol, Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.J. C. Hassel, Ipilimumab plus nivolumab for advanced melanoma. Lancet Oncol. 17, 1471–1472 (2016). [DOI] [PubMed] [Google Scholar]
- 11.D. J. Olson, Z. Eroglu, B. Brockstein, A. S. Poklepovic, M. Bajaj, S. Babu, S. Hallmeyer, M. Velasco, J. Lutzky, E. Higgs, R. Bao, T. C. Carll, B. Labadie, T. Krausz, Y. Zha, T. Karrison, V. K. Sondak, T. F. Gajewski, N. I. Khushalani, J. J. Luke, Pembrolizumab plus ipilimumab following anti-PD-1/L1 failure in melanoma. J. Clin. Oncol. 39, 2647–2655 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.N. Gavrielatou, Y. Liu, I. Vathiotis, J. Zugazagoitia, T. N. Aung, S. Shafi, A. Fernandez, K. Schalper, A. Psyrri, D. L. Rimm, Association of PD-1/PD-L1 co-location with immunotherapy outcomes in non-small cell lung cancer. Clin. Cancer Res. 28, 360–367 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.I. Girault, J. Adam, S. Shen, S. Roy, C. Brard, S. Faouzi, E. Routier, J. Lupu, S. Warren, K. Sorg, S. F. Ong, P. Morel, J.-Y. Scoazec, S. Vagner, C. Robert, A PD-1/PD-L1 proximity assay as a theranostic marker for PD-1 blockade in patients with metastatic melanoma. Clin. Cancer Res. 28, 518–525 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Q. Li, Z. W. Zhou, J. Lu, H. Luo, S. N. Wang, Y. Peng, M. S. Deng, G. B. Song, J. M. Wang, X. Wei, D. Wang, K. D. Westover, C. X. Xu, PD-L1(P146R) is prognostic and a negative predictor of response to immunotherapy in gastric cancer. Mol. Ther. 30, 621–631 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.M. Yi, M. Niu, L. Xu, S. Luo, K. Wu, Regulation of PD-L1 expression in the tumor microenvironment. J. Hematol. Oncol. 14, 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.X. Dai, Y. Gao, W. Wei, Post-translational regulations of PD-L1 and PD-1: Mechanisms and opportunities for combined immunotherapy. Semin. Cancer Biol. 85, 246–252 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.J. M. Hsu, C. W. Li, Y. J. Lai, M. C. Hung, Posttranslational modifications of PD-L1 and their applications in cancer therapy. Cancer Res. 78, 6349–6353 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.G. Z. Wang, L. Zhang, X.-C. Zhao, S.-H. Gao, L.-W. Qu, H. Yu, W.-F. Fang, Y.-C. Zhou, F. Liang, C. Zhang, Y.-C. Huang, Z. Liu, Y.-X. Fu, G.-B. Zhou, The Aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy. Nat. Commun. 10, 1125 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D. Zhu, R. Xu, X. Huang, Z. Tang, Y. Tian, J. Zhang, X. Zheng, Deubiquitinating enzyme OTUB1 promotes cancer cell immunosuppression via preventing ER-associated degradation of immune checkpoint protein PD-L1. Cell Death Differ. 28, 1773–1789 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.J. Zhang, X. Bu, H. Wang, Y. Zhu, Y. Geng, N. T. Nihira, Y. Tan, Y. Ci, F. Wu, X. Dai, J. Guo, Y. H. Huang, C. Fan, S. Ren, Y. Sun, G. J. Freeman, P. Sicinski, W. Wei, Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature 553, 91–95 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.H. Yao, J. Lan, C. Li, H. Shi, J. P. Brosseau, H. Wang, H. Lu, C. Fang, Y. Zhang, L. Liang, X. Zhou, C. Wang, Y. Xue, Y. Cui, J. Xu, Inhibiting PD-L1 palmitoylation enhances T-cell immune responses against tumours. Nat. Biomed. Eng. 3, 306–317 (2019). [DOI] [PubMed] [Google Scholar]
- 22.R. Mezzadra, C. Sun, L. T. Jae, R. Gomez-Eerland, E. de Vries, W. Wu, M. E. W. Logtenberg, M. Slagter, E. A. Rozeman, I. Hofland, A. Broeks, H. M. Horlings, L. F. A. Wessels, C. U. Blank, Y. Xiao, A. J. R. Heck, J. Borst, T. R. Brummelkamp, T. N. M. Schumacher, Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature 549, 106–110 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Y. Gao, N. T. Nihira, X. Bu, C. Chu, J. Zhang, A. Kolodziejczyk, Y. Fan, N. T. Chan, L. Ma, J. Liu, D. Wang, X. Dai, H. Liu, M. Ono, A. Nakanishi, H. Inuzuka, B. J. North, Y. H. Huang, S. Sharma, Y. Geng, W. Xu, X. S. Liu, L. Li, Y. Miki, P. Sicinski, G. J. Freeman, W. Wei, Acetylation-dependent regulation of PD-L1 nuclear translocation dictates the efficacy of anti-PD-1 immunotherapy. Nat. Cell Biol. 22, 1064–1075 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.M. L. Burr, C. E. Sparbier, Y. C. Chan, J. C. Williamson, K. Woods, P. A. Beavis, E. Y. N. Lam, M. A. Henderson, C. C. Bell, S. Stolzenburg, O. Gilan, S. Bloor, T. Noori, D. W. Morgens, M. C. Bassik, P. J. Neeson, A. Behren, P. K. Darcy, S. J. Dawson, I. Voskoboinik, J. A. Trapani, J. Cebon, P. J. Lehner, M. A. Dawson, CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature 549, 101–105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.J. M. Hsu, W. Xia, Y. H. Hsu, L. C. Chan, W. H. Yu, J. H. Cha, C. T. Chen, H. W. Liao, C. W. Kuo, K. H. Khoo, J. L. Hsu, C. W. Li, S. O. Lim, S. S. Chang, Y. C. Chen, G. X. Ren, M. C. Hung, STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat. Commun. 9, 1908 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.L. C. Chan, C. W. Li, W. Xia, J. M. Hsu, H. H. Lee, J. H. Cha, H. L. Wang, W. H. Yang, E. Y. Yen, W. C. Chang, Z. Zha, S. O. Lim, Y. J. Lai, C. Liu, J. Liu, Q. Dong, Y. Yang, L. Sun, Y. Wei, L. Nie, J. L. Hsu, H. Li, Q. Ye, M. M. Hassan, H. M. Amin, A. O. Kaseb, X. Lin, S. C. Wang, M. C. Hung, IL-6/JAK1 pathway drives PD-L1 Y112 phosphorylation to promote cancer immune evasion. J. Clin. Invest. 129, 3324–3338 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.C. W. Li, S. O. Lim, E. M. Chung, Y. S. Kim, A. H. Park, J. Yao, J. H. Cha, W. Xia, L. C. Chan, T. Kim, S. S. Chang, H. H. Lee, C. K. Chou, Y. L. Liu, H. C. Yeh, E. P. Perillo, A. K. Dunn, C. W. Kuo, K. H. Khoo, J. L. Hsu, Y. Wu, J. M. Hsu, H. Yamaguchi, T. H. Huang, A. A. Sahin, G. N. Hortobagyi, S. S. Yoo, M. C. Hung, Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1. Cancer Cell 33, 187–201.e10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.S. Lanouette, V. Mongeon, D. Figeys, J. F. Couture, The functional diversity of protein lysine methylation. Mol. Syst. Biol. 10, 724 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.M. Albert, K. Helin, Histone methyltransferases in cancer. Semin. Cell Dev. Biol. 21, 209–220 (2010). [DOI] [PubMed] [Google Scholar]
- 30.E. L. Greer, Y. Shi, Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 13, 343–357 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.K. K. Biggar, S. S.-C. Li, Non-histone protein methylation as a regulator of cellular signalling and function. Nat. Rev. Mol. Cell Biol. 16, 5–17 (2015). [DOI] [PubMed] [Google Scholar]
- 32.M. Yamagishi, K. Uchimaru, Targeting EZH2 in cancer therapy. Curr. Opin. Oncol. 29, 375–381 (2017). [DOI] [PubMed] [Google Scholar]
- 33.M. Rodriguez-Paredes, F. Lyko, The importance of non-histone protein methylation in cancer therapy. Nat. Rev. Mol. Cell Biol. 20, 569–570 (2019). [DOI] [PubMed] [Google Scholar]
- 34.C. Huang, F. Hu, D. Song, X. Sun, A. Liu, Q. Wu, X. She, Y. Chen, L. Chen, F. Hu, F. Xu, X. Luo, Y. Feng, X. Yang, J. Hu, G. Wang, EZH2-triggered methylation of SMAD3 promotes its activation and tumor metastasis. J. Clin. Invest. 132, e152394 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.G. Wang, J. Long, Y. Gao, W. Zhang, F. Han, C. Xu, L. Sun, S. C. Yang, J. Lan, Z. Hou, Z. Cai, G. Jin, C. C. Hsu, Y. H. Wang, J. Hu, T. Y. Chen, H. Li, M. G. Lee, H. K. Lin, SETDB1-mediated methylation of Akt promotes its K63-linked ubiquitination and activation leading to tumorigenesis. Nat. Cell Biol. 21, 214–225 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.S. M. Carlson, O. Gozani, Nonhistone lysine methylation in the regulation of cancer pathways. Cold Spring Harb. Perspect. Med. 6, a026435 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.M. Reck, D. Rodríguez-Abreu, A. G. Robinson, R. Hui, T. Csőszi, A. Fülöp, M. Gottfried, N. Peled, A. Tafreshi, S. Cuffe, M. O'Brien, S. Rao, K. Hotta, T. A. Leal, J. W. Riess, E. Jensen, B. Zhao, M. C. Pietanza, J. R. Brahmer, Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J. Clin. Oncol. 39, 2339–2349 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.M. Reck, D. Rodríguez-Abreu, A. G. Robinson, R. Hui, T. Csőszi, A. Fülöp, M. Gottfried, N. Peled, A. Tafreshi, S. Cuffe, M. O’Brien, S. Rao, K. Hotta, M. A. Leiby, G. M. Lubiniecki, Y. Shentu, R. Rangwala, J. R. Brahmer, Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375, 1823–1833 (2016). [DOI] [PubMed] [Google Scholar]
- 39.B. C. Cho, D. R. Abreu, M. Hussein, M. Cobo, A. J. Patel, N. Secen, K. H. Lee, B. Massuti, S. Hiret, J. C. H. Yang, F. Barlesi, D. H. Lee, L. P. Ares, R. W. Hsieh, N. S. Patil, P. Twomey, X. Yang, R. Meng, M. L. Johnson, Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): Primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 23, 781–792 (2022). [DOI] [PubMed] [Google Scholar]
- 40.L. Fehrenbacher, A. Spira, M. Ballinger, M. Kowanetz, J. Vansteenkiste, J. Mazieres, K. Park, D. Smith, A. Artal-Cortes, C. Lewanski, F. Braiteh, D. Waterkamp, P. He, W. Zou, D. S. Chen, J. Yi, A. Sandler, A. Rittmeyer, Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 387, 1837–1846 (2016). [DOI] [PubMed] [Google Scholar]
- 41.A. Rittmeyer, F. Barlesi, D. Waterkamp, K. Park, F. Ciardiello, J. von Pawel, S. M. Gadgeel, T. Hida, D. M. Kowalski, M. C. Dols, D. L. Cortinovis, J. Leach, J. Polikoff, C. Barrios, F. Kabbinavar, O. A. Frontera, F. de Marinis, H. Turna, J. S. Lee, M. Ballinger, M. Kowanetz, P. He, D. S. Chen, A. Sandler, D. R. Gandara; OAK Study Group , Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 389, 255–265 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.H. Borghaei, L. Paz-Ares, L. Horn, D. R. Spigel, M. Steins, N. E. Ready, L. Q. Chow, E. E. Vokes, E. Felip, E. Holgado, F. Barlesi, M. Kohlhäufl, O. Arrieta, M. A. Burgio, J. Fayette, H. Lena, E. Poddubskaya, D. E. Gerber, S. N. Gettinger, C. M. Rudin, N. Rizvi, L. Crinò, G. R. Blumenschein Jr., S. J. Antonia, C. Dorange, C. T. Harbison, F. Graf Finckenstein, J. R. Brahmer, Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373, 1627–1639 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.J. Brahmer, K. L. Reckamp, P. Baas, L. Crinò, W. E. E. Eberhardt, E. Poddubskaya, S. Antonia, A. Pluzanski, E. E. Vokes, E. Holgado, D. Waterhouse, N. Ready, J. Gainor, O. Arén Frontera, L. Havel, M. Steins, M. C. Garassino, J. G. Aerts, M. Domine, L. Paz-Ares, M. Reck, C. Baudelet, C. T. Harbison, B. Lestini, D. R. Spigel, Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373, 123–135 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.M. C. Garassino, B. C. Cho, J. H. Kim, J. Mazières, J. Vansteenkiste, H. Lena, J. Corral Jaime, J. E. Gray, J. Powderly, C. Chouaid, P. Bidoli, P. Wheatley-Price, K. Park, R. A. Soo, Y. Huang, C. Wadsworth, P. A. Dennis, N. A. Rizvi, L. Paz-Ares Rodriguez, S. Novello, S. Hiret, P. Schmid, E. Laack, R. Califano, M. Maemondo, S. W. Kim, J. Chaft, D. Vicente Baz, T. Berghmans, D. W. Kim, V. Surmont, M. Reck, J. Y. Han, E. Holgado Martin, C. Belda Iniesta, Y. Oe, A. Chella, A. Chopra, G. Robinet, H. Soto Parra, M. Thomas, P. Cheema, N. Katakami, W. C. Su, Y. C. Kim, J. Wolf, J. S. Lee, H. Saka, M. Milella, I. Ramos Garcia, A. Sibille, T. Yokoi, E. J. Kang, S. Atagi, E. Spaeth-Schwalbe, M. Nishio, F. Imamura, N. Gabrail, R. Veillon, S. Derijcke, T. Maeda, D. Zylla, K. Kubiak, A. Santoro, M. N. Uy, S. Lucien Geater, A. Italiano, D. Kowalski, F. Barlesi, Y. M. Chen, D. Spigel, B. Chewaskulyong, R. Garcia Gomez, R. Alvarez, C. H. Yang, T. C. Hsia, F. Denis, H. Sakai, M. Vincent, K. Goto, J. Bosch-Barrera, G. Weiss, J. L. Canon, C. Scholz, M. Aglietta, H. Kemmotsu, K. Azuma, P. Bradbury, R. Feld, A. Chachoua, J. Jassem, R. Juergens, R. Palmero Sanchez, A. Malcolm, N. Vrindavanam, K. Kubota, C. Waller, D. Waterhouse, B. Coudert, Z. Mark, M. Satouchi, G. C. Chang, C. Herzmann, A. Chaudhry, S. Giridharan, P. Hesketh, N. Ikeda, R. Boccia, N. Iannotti, M. Haigentz, J. Reynolds, J. Querol, K. Nakagawa, S. Sugawara, E. H. Tan, T. Hirashima, S. Gettinger, T. Kato, K. Takeda, O. Juan Vidal, A. Mohn-Staudner, A. Panwalkar, D. Daniel, K. Kobayashi, G. E. I. Ladrera, C. Schulte, M. Sebastian, M. Cernovska, H. Coupkova, L. Havel, N. Pauk, J. Singh, S. Murakami, T. Csoszi, G. Losonczy, A. Price, I. Anderson, M. Iqbal, V. Torri, E. Juhasz, S. Khanani, L. Koubkova, B. Levy, R. Page, C. Bocskei, L. Crinò, D. Einspahr, C. Hagenstad, N. Juat, L. Overton, M. Garrison, Z. Szalai, Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): An open-label, single-arm, phase 2 study. Lancet Oncol. 19, 521–536 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.S. J. Antonia, A. Villegas, D. Daniel, D. Vicente, S. Murakami, R. Hui, T. Kurata, A. Chiappori, K. H. Lee, M. de Wit, B. C. Cho, M. Bourhaba, X. Quantin, T. Tokito, T. Mekhail, D. Planchard, Y. C. Kim, C. S. Karapetis, S. Hiret, G. Ostoros, K. Kubota, J. E. Gray, L. Paz-Ares, J. de Castro Carpeño, C. Faivre-Finn, M. Reck, J. Vansteenkiste, D. R. Spigel, C. Wadsworth, G. Melillo, M. Taboada, P. A. Dennis, M. Özgüroğlu, Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 379, 2342–2350 (2018). [DOI] [PubMed] [Google Scholar]
- 46.R. S. Herbst, P. Baas, D. W. Kim, E. Felip, J. L. Pérez-Gracia, J. Y. Han, J. Molina, J. H. Kim, C. D. Arvis, M. J. Ahn, M. Majem, M. J. Fidler, G. de Castro Jr., M. Garrido, G. M. Lubiniecki, Y. Shentu, E. Im, M. Dolled-Filhart, E. B. Garon, Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 387, 1540–1550 (2016). [DOI] [PubMed] [Google Scholar]
- 47.K. Shitara, M. Özgüroğlu, Y. J. Bang, M. di Bartolomeo, M. Mandalà, M. H. Ryu, L. Fornaro, T. Olesiński, C. Caglevic, H. C. Chung, K. Muro, E. Goekkurt, W. Mansoor, R. S. McDermott, E. Shacham-Shmueli, X. Chen, C. Mayo, S. P. Kang, A. Ohtsu, C. S. Fuchs, G. Lerzo, J. M. O’Connor, G. A. Mendez, J. Lynam, N. Tebbutt, M. Wong, A. Strickland, C. Karapetis, D. Goldstein, P. Vasey, J. L. van Laethem, E. van Cutsem, S. Berry, M. Vincent, B. Muller, F. Rey, A. Zambrano, J. Guerra, M. Krogh, L. Baeksgaard, M. Yilmaz, A. Elme, A. Magi, P. Auvinen, T. Alanko, M. Moehler, V. Kunzmann, T. Seufferlein, P. Thuss-Patience, E. Goekkurt, T. Hoehler, G. Haag, S. E. al-Batran, H. Castro, K. Lopez, M. Aguilar Vasquez, M. Sandoval, K. O. Lam, S. Cuffe, C. Kelly, R. Geva, E. Shacham-Shmueli, A. Hubert, A. Beny, B. Brenner, A. Giuseppe, A. Falcone, E. Maiello, R. Passalacqua, V. Montesarchio, H. Hara, K. Chin, T. Nishina, Y. Komatsu, N. Machida, S. Hironaka, T. Satoh, T. Tamura, N. Sugimoto, H. Cho, Y. Omuro, K. Kato, M. Goto, I. Hyodo, K. Yoshida, H. Baba, T. Esaki, J. Furuse, W. Z. Wan Mohammed, C. Hernandez, J. Casas Garcia, A. Dominguez Andrade, K. Clarke, G. Hjortland, N. Glenjen, T. Kubiatowski, J. Jacek, M. Wojtukiewicz, S. Lazarev, Y. Lancukhay, S. Afanasayev, V. Moiseyenko, V. Kostorov, S. Protsenko, V. Shirinkin, D. Sakaeva, N. Fadeeva, W. P. Yong, C. H. M. Ng, B. Robertson, B. Rapaport, G. Cohen, L. Dreosti, P. Ruff, C. Jacobs, G. Landers, W. Szpak, S. Y. Roh, J. Lee, Y. H. Kim, Y. J. Bang, H. C. Chung, M. H. Ryu, M. Alsina Maqueda, F. Longo Munoz, A. Cervantes Aguilar, E. Aranda Aguilar, P. Garcia Alfonso, F. Rivera, J. Feliu Batle, R. Pazo Cid, K. H. Yeh, J. S. Chen, Y. Chao, C. J. Yen, M. Özgüroğlu, O. Kara, S. Yalcin, D. Hochhauser, I. Chau, A. Benson, V. Shankaran, W. Shaib, P. Philip, V. Sharma, R. Siegel, W. Sun, Z. Wainberg, B. George, A. Bullock, S. Myrick, J. Faruol, R. Siegel, T. Larson, C. Becerra, S. Ratnam, D. A. Richards, S. L. Riche, Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet 392, 123–133 (2018). [DOI] [PubMed] [Google Scholar]
- 48.C. Robert, G. V. Long, B. Brady, C. Dutriaux, M. Maio, L. Mortier, J. C. Hassel, P. Rutkowski, C. McNeil, E. Kalinka-Warzocha, K. J. Savage, M. M. Hernberg, C. Lebbé, J. Charles, C. Mihalcioiu, V. Chiarion-Sileni, C. Mauch, F. Cognetti, A. Arance, H. Schmidt, D. Schadendorf, H. Gogas, L. Lundgren-Eriksson, C. Horak, B. Sharkey, I. M. Waxman, V. Atkinson, P. A. Ascierto, Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330 (2015). [DOI] [PubMed] [Google Scholar]
- 49.C. Robert, A. Ribas, J. Schachter, A. Arance, J. J. Grob, L. Mortier, A. Daud, M. S. Carlino, C. M. McNeil, M. Lotem, J. M. G. Larkin, P. Lorigan, B. Neyns, C. U. Blank, T. M. Petrella, O. Hamid, S. C. Su, C. Krepler, N. Ibrahim, G. V. Long, Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 20, 1239–1251 (2019). [DOI] [PubMed] [Google Scholar]
- 50.D. A. Fennell, S. Ewings, C. Ottensmeier, R. Califano, G. G. Hanna, K. Hill, S. Danson, N. Steele, M. Nye, L. Johnson, J. Lord, C. Middleton, P. Szlosarek, S. Chan, A. Gaba, L. Darlison, P. Wells-Jordan, C. Richards, C. Poile, J. F. Lester, G. Griffiths, G. Price, P. Shaw, J. Cave, J. Naik, A. Ford, T. Geldhart, G. Dancey, D. Papadatos, A. Polychronis, P. Jankowska, A. Scott, J. Gardiner, M. Cominos, L. Campbell, C. MacGregor, L. Mullholand, M. Chitnis, G. Dougherty, Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): A multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 22, 1530–1540 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.R. L. Ferris, G. Blumenschein Jr., J. Fayette, J. Guigay, A. D. Colevas, L. Licitra, K. Harrington, S. Kasper, E. E. Vokes, C. Even, F. Worden, N. F. Saba, L. C. Iglesias Docampo, R. Haddad, T. Rordorf, N. Kiyota, M. Tahara, M. Monga, M. Lynch, W. J. Geese, J. Kopit, J. W. Shaw, M. L. Gillison, Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 375, 1856–1867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.R. L. Ferris, R. Haddad, C. Even, M. Tahara, M. Dvorkin, T. E. Ciuleanu, P. M. Clement, R. Mesia, S. Kutukova, L. Zholudeva, A. Daste, J. Caballero-Daroqui, B. Keam, I. Vynnychenko, C. Lafond, J. Shetty, H. Mann, J. Fan, S. Wildsmith, N. Morsli, J. Fayette, L. Licitra, Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann. Oncol. 31, 942–950 (2020). [DOI] [PubMed] [Google Scholar]
- 53.D. F. McDermott, M. A. Huseni, M. B. Atkins, R. J. Motzer, B. I. Rini, B. Escudier, L. Fong, R. W. Joseph, S. K. Pal, J. A. Reeves, M. Sznol, J. Hainsworth, W. K. Rathmell, W. M. Stadler, T. Hutson, M. E. Gore, A. Ravaud, S. Bracarda, C. Suárez, R. Danielli, V. Gruenwald, T. K. Choueiri, D. Nickles, S. Jhunjhunwala, E. Piault-Louis, A. Thobhani, J. Qiu, D. S. Chen, P. S. Hegde, C. Schiff, G. D. Fine, T. Powles, Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 24, 749–757 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.S. K. Pal, D. F. McDermott, M. B. Atkins, B. Escudier, B. I. Rini, R. J. Motzer, L. Fong, R. W. Joseph, S. Oudard, A. Ravaud, S. Bracarda, C. Suárez, E. T. Lam, T. K. Choueiri, B. Ding, C. Quach, K. Hashimoto, C. Schiff, E. Piault-Louis, T. Powles, Patient-reported outcomes in a phase 2 study comparing atezolizumab alone or with bevacizumab vs sunitinib in previously untreated metastatic renal cell carcinoma. BJU Int. 126, 73–82 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.U. Vaishampayan, P. Schöffski, A. Ravaud, C. Borel, J. Peguero, J. Chaves, J. C. Morris, N. Kotecki, M. Smakal, D. Zhou, S. Guenther, M. Bajars, J. L. Gulley, Avelumab monotherapy as first-line or second-line treatment in patients with metastatic renal cell carcinoma: Phase Ib results from the JAVELIN Solid Tumor trial. J. Immunother. Cancer 7, 275 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.A. B. Apolo, J. A. Ellerton, J. R. Infante, M. Agrawal, M. S. Gordon, R. Aljumaily, T. Gourdin, L. Dirix, K. W. Lee, M. H. Taylor, P. Schöffski, D. Wang, A. Ravaud, J. Manitz, G. Pennock, M. Ruisi, J. L. Gulley, M. R. Patel, Avelumab as second-line therapy for metastatic, platinum-treated urothelial carcinoma in the phase Ib JAVELIN Solid Tumor study: 2-year updated efficacy and safety analysis. J. Immunother. Cancer 8, e001246 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.J. Bellmunt, R. de Wit, D. J. Vaughn, Y. Fradet, J. L. Lee, L. Fong, N. J. Vogelzang, M. A. Climent, D. P. Petrylak, T. K. Choueiri, A. Necchi, W. Gerritsen, H. Gurney, D. I. Quinn, S. Culine, C. N. Sternberg, Y. Mai, C. H. Poehlein, R. F. Perini, D. F. Bajorin; KEYNOTE-045 Investigators , Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 376, 1015–1026 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.G. P. Sonpavde, C. N. Sternberg, Y. Loriot, A. Marabelle, J. L. Lee, A. Fléchon, G. Roubaud, D. Pouessel, V. Zagonel, F. Calabro, G. L. Banna, S. J. Shin, F. E. Vera-Badillo, T. Powles, E. Hellmis, P. A. P. Miranda, A. R. Lima, U. Emeribe, S. M. Oh, S. J. Hotte, Primary results of STRONG: An open-label, multicenter, phase 3b study of fixed-dose durvalumab monotherapy in previously treated patients with urinary tract carcinoma. Eur. J. Cancer 163, 55–65 (2022). [DOI] [PubMed] [Google Scholar]
- 59.J. Wang, J. Chen, B. Zhang, X. Jia, IL-6 regulates the bone metabolism and inflammatory microenvironment in aging mice by inhibiting Setd7. Acta Histochem. 123, 151718 (2021). [DOI] [PubMed] [Google Scholar]
- 60.H. H. Lee, Y. N. Wang, W. Xia, C. H. Chen, K. M. Rau, L. Ye, Y. Wei, C. K. Chou, S. C. Wang, M. Yan, C. Y. Tu, T. C. Hsia, S. F. Chiang, K. S. C. Chao, I. I. Wistuba, J. L. Hsu, G. N. Hortobagyi, M. C. Hung, Removal of N-linked glycosylation enhances PD-L1 detection and predicts anti-PD-1/PD-L1 therapeutic efficacy. Cancer Cell 36, 168–178.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.L. B. Kennedy, A. K. S. Salama, A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 70, 86–104 (2020). [DOI] [PubMed] [Google Scholar]
- 62.D. M. Pardoll, The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.C. W. Li, S. O. Lim, W. Xia, H. H. Lee, L. C. Chan, C. W. Kuo, K. H. Khoo, S. S. Chang, J. H. Cha, T. Kim, J. L. Hsu, Y. Wu, J. M. Hsu, H. Yamaguchi, Q. Ding, Y. Wang, J. Yao, C. C. Lee, H. J. Wu, A. A. Sahin, J. P. Allison, D. Yu, G. N. Hortobagyi, M. C. Hung, Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 7, 12632 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.S. T. Kim, R. Cristescu, A. J. Bass, K. M. Kim, J. I. Odegaard, K. Kim, X. Q. Liu, X. Sher, H. Jung, M. Lee, S. Lee, S. H. Park, J. O. Park, Y. S. Park, H. Y. Lim, H. Lee, M. Choi, A. A. Talasaz, P. S. Kang, J. Cheng, A. Loboda, J. Lee, W. K. Kang, Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 24, 1449–1458 (2018). [DOI] [PubMed] [Google Scholar]
- 65.Z. Liu, X. Wu, Medical Immunological Techniques. (People’s Medical Publishing House, 2014).
- 66.S. K. Wculek, F. J. Cueto, A. M. Mujal, I. Melero, M. F. Krummel, D. Sancho, Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20, 7–24 (2020). [DOI] [PubMed] [Google Scholar]
- 67.C. E. Bryant, S. Sutherland, B. Kong, M. S. Papadimitrious, P. D. Fromm, D. N. J. Hart, Dendritic cells as cancer therapeutics. Semin. Cell Dev. Biol. 86, 77–88 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S9
Tables S1 to S3, S5 and S6
Table S4