Abstract
Background
Preparticipation screenings have been conceived for the potential to prevent sudden cardiac death in young athletes by early identification of hidden cardiac diseases. Commonly used protocols include family history collection, physical examination, and resting electrocardiogram. Transthoracic echocardiography has been hypothesized to have a primary role in the preparticipation screening.
Aims
The present study aimed to evaluate the additional role of echocardiogram in identifying cardiovascular abnormalities that might be undetected by commonly used preparticipation screening.
Methods
We retrospectively reviewed Ferrari Formula Benessere, a corporate wellness program database, and analyzed data recorded from 2017 to 2022 to compare two medical models: a ‘standard’ preparticipation screening including medical history, physical examination, electrocardiogram and exercise stress testing versus an ‘advanced’ preparticipation screening comprising history, physical examination, electrocardiogram, exercise stress testing and echocardiography.
Results
From an initial sample size of 7500 patients, we included 500 patients (420 male, 33.69 ± 7.9 mean age) enrolled for the first time in the corporate wellness program between 2017 and 2022. Three hundred and thirty-nine (67.8%) patients had no abnormal findings at ‘standard’ preparticipation screening and, even if they would have not required further evaluation, we performed echocardiography anyway (‘advanced’ preparticipation screening): 31 (9.1%) showed some abnormal cardiovascular findings at echocardiography, such as patent foramen ovalis, bicuspid aortic valve, aortic root ectasia or mitral valve prolapse.
Conclusions
Screening echocardiogram showed an additional value (about 10% more) in detecting patients with cardiovascular abnormalities, otherwise undiagnosed with the ‘standard’ preparticipation screening protocol.
Keywords: athletes, echocardiography, preparticipation screening, sudden cardiac death
Introduction
The benefits of sport in improving overall health are undoubted,1 but physical activity has also been shown to be related to an increased risk of cardiovascular events.2,3 The main causes of sudden cardiac death (SCD) in athletes are represented by cardiomyopathies, coronary artery abnormalities, and arrhythmogenic diseases.4,5 It is essential to highlight that sport is not itself the cause of increased mortality, but it can trigger adverse events up to cardiac arrest in patients with underlying cardiac abnormalities, often misunderstood. Preparticipation screening (PPS) of athletes before engaging in sports activities can therefore play a fundamental role in identifying any risk conditions to prevent exercise-related complications.
The PPS examination proposed by the ESC (European Society of Cardiology) includes the collection of medical history, physical examination, and resting electrocardiogram (ECG), reserving further examinations, including echocardiography, for patients with anomalies during the first step evaluation.6 However, about a third of these athletes may have a normal ECG despite having hidden underlying structural abnormalities or initial forms of cardiomyopathy.7,8 Therefore, there is a potential need to find other first-line screening exams, useful in the PPS process.
Echocardiogram could be a noninvasive and accessible tool to increase PPS sensitivity.9 Given the growing adoption of this technique also by noncardiologist physicians, the low cost, and the widespread diffusion of portable ultrasound devices,10,11 its use is increasing. There is currently no unanimous consensus on the inclusion of the echocardiogram in the PPS protocol, even if its role as a first-line exam to detect SCD-related cardiac abnormalities has been recently theorized,12–14 and considering also that the low acoustic impedance of the athletic population makes it possible to obtain high-quality images However, even if a complete echocardiographic study would potentially detect a wide range of structural abnormalities, it could be disadvantageous in terms of time and especially costs.15
The aim of the present paper was to evaluate the potential role of an echocardiogram in identifying cardiovascular abnormalities that might be undetected by commonly used PPS.
Methods
Study design
This is an observational retrospective study, conducted according to STROBE guidelines.16
The Ferrari company delivers its corporate wellness program called ‘Ferrari Formula Benessere’ through Med-Ex, Medicine & Exercise. This project consists of a yearly medical screening, including a complete cardiovascular (CV) evaluation: family and personal medical history, physical examination, resting ECG, exercise stress testing (EST) and echocardiography for all the employees. Its effectiveness has already been proved.17–19 All these data are recorded through an online database.
We retrospectively reviewed the Med-Ex database and analyzed data recorded from 2017 to 2022 to compare two medical models: a ‘standard’ PPS including medical history, physical examination, ECG and EST vs. an ‘advanced’ PPS comprising history, physical examination, ECG, EST, and echocardiography. The primary outcome of the study was to assess the efficacy of the ‘advanced’ PPS in detecting CV abnormalities compared to the ‘standard’ PPS.
Participants
A consecutive convenience sample of individuals enrolled for the first time at the Ferrari corporate wellness program ‘Formula Benessere’ was selected between 1 January 2017 and 1 January 2022. Inclusion criteria were represented by the possibility to obtain their medical data from the database: personal and family history, physical examination, resting ECG, resting blood pressure, EST with blood pressure monitoring, and echocardiogram. Exclusion criteria were represented by the presence of any known CV diseases, incomplete medical data through the Med-Ex database, or participants who had been already enrolled in the ‘Formula Benessere’ program. We decided to exclude patients who had been previously screened in the program to verify our hypothesis at the very first visit, without any previous medical visit.
Participation in the program was completely free for employees. Each patient was assessed by medical doctors and allied professionals. Before the visit, each patient signed an informed consent accepting medical procedures and data collection by Med-Ex for scientific purposes. Med-Ex treats these data according to privacy rules. For the present study, data were retrieved through the database by an author with the right to access the data (A.B.). Sensible data were concealed, and each record was numbered and anonymized.
This study was conducted according to the principles of the Helsinki Declaration and its later amendments.
Procedures
Each patient underwent a five-point PPS, under the supervision of a cardiologist or a sports medicine physician:
-
(1)
The family and personal clinical history of participants were recorded and the cardiovascular (CV) risk assessment was performed using the Systemic Coronary Risk Evaluation system, based on age, sex, blood pressure, blood cholesterol, and smoking history.20
-
(2)
Physical examination was conducted according to Bethesda conference recommendations.21
-
(3)
Resting ECG was evaluated considering International ECG criteria.22
-
(4)
A maximal cycle ergometer EST (Daum Ergometer Premium 8i, Daum Electronic Gmbh, Fürth, Germany) was performed. The protocol consisted of 2 min of unloaded cycling, followed by increments of 50 W for men and 30 W for women every 2 min. In the absence of clinical/instrumental alterations requiring exercise cessation, the test was continued until muscle exhaustion. ECG was recorded at rest, during the exercise, and during recovery using Cardioline Cube PC software (Cardioline US, San Diego, CA, USA). Similarly, systolic and diastolic pressures were recorded. All tests were conducted in compliance with the exercise standards for testing.23
-
(5)
All patients underwent 2D echocardiography using a commercially available system (Vingmed Vivid-7, General Electric Vingmed; Milwaukee, WI, USA; or Aplio XV and 400; Toshiba, Japan). The guidelines of European Association of Cardiovascular Imaging served as a guide.24 Three experienced cardiologists performed all studies, which were digitally stored for offline analysis. In the case of disagreement, the entire team reviewed the data.
Statistical analysis
The differences in the frequencies were evaluated with the chi-square test and the Fisher exact test used in cases of small frequencies, for categorical variables. Continuous variables were expressed as the mean ± standard deviation of the differences in the means between the two groups and were assessed by Student's t-test. Analysis of variance (ANOVA) was used to evaluate the differences in the parameters under consideration for variables with three or more categories. Cohen's kappa (κ) statistic was used to measure the agreement between echocardiography and pathology. The chosen level of statistical significance was 0.05. All analyses were performed by using the STATA statistical package14. Stata: Release 14. Statistical Software. College Station, TX: StataCorp LP.
Results
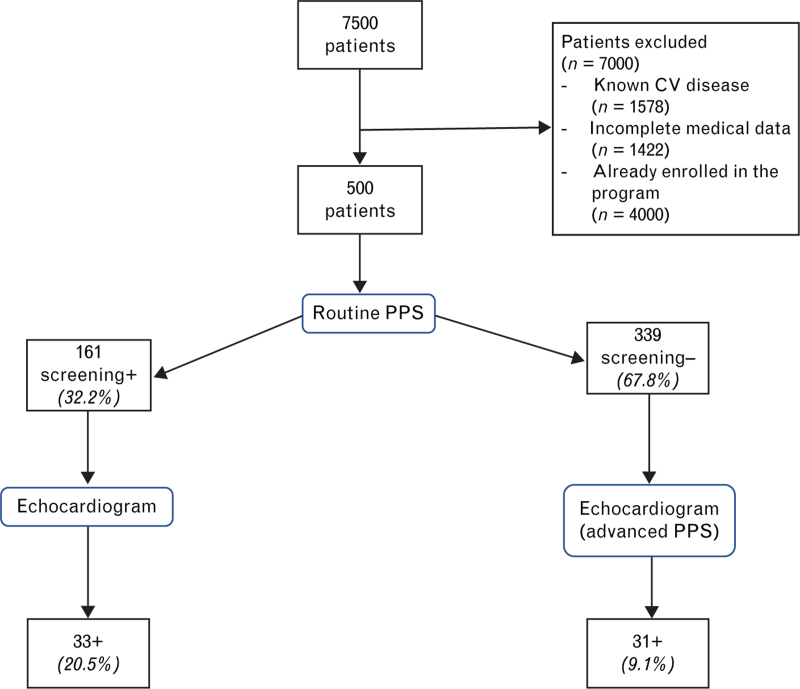
Between January 2017 and January 2022, a total of 7500 patients were screened by the ‘Formula Benessere’ program. After the application of inclusion criteria, 7000 patients were discarded; therefore, data from 500 patients were analyzed. Details of exclusion criteria are shown in Fig. 1.
Fig. 1.
Flow chart of study participants. PPS, preparticipation screening; CV, cardiovascular routine; PPS, personal and family history, physical examination, resting electrocardiogram, resting blood pressure, exercise stress testing with blood pressure monitoring advanced PPS: personal and family history, physical examination, resting electrocardiogram, resting blood pressure, exercise stress testing with blood pressure monitoring and echocardiogram +: abnormal findings; –: normal findings.
Participants were mainly men (84%), with a mean age of 33.69 ± 7.9 years. All the patients underwent the ‘advanced’ PPS protocol including an echocardiogram.
One hundred and sixty-one (32.2%) patients would have shown abnormal findings with the ‘standard’ PPS, thus requiring an echocardiogram evaluation; 339 (67.8%) patients had no abnormal findings at ‘standard’ PPS and, thus, would have not required further evaluation including an echocardiogram.
Thirty-three of 161 (20.5%) of the patients of the ‘standard’ PPS positive group showed some morpho-functional abnormal findings at echocardiography; 31 of the 339 (9.1%) patients of the ‘standard’ PPS negative group showed some abnormal findings at echocardiography (20.5% vs. 9.1% – P < 0.05).
Details of the study design are shown in Fig. 1. Details of abnormal findings in advanced PPS are shown in Table 1. Patent foramen ovale and aortic root ectasia were the two common findings.
Table 1.
Abnormal findings at echocardiography (n = 31) in patients with ‘standard’ PPS negative (n = 339)
| Type | Echocardiographic finding | Number of cases (n, %) | Mean values of measurements |
| Congenital | PFO | 7 (22.6%) | |
| BAV | 1 (3.2%) | ||
| Right chamber | Pulmonary hypertension | 1 (3.2%) | PASP: 45 mmHg |
| Vessels | Aortic root ectasia | 6 (19.3%) | Aortic root diameter: 41.7 mm |
| Valve | MVP | 7 (22.6%) | |
| Left chamber | LA enlargement | 4 (12.9%) | LA dimension: 38.8 ml/m2 |
| Valve | Valvulopathiesa | 3 (9.6%) | VC: 3.4 mm |
| Left chamber | LV concentric remodeling | 1 (3.2%) | RWT: 0.44 |
| Valve | E/A <1 | 1 (3.2%) |
BAV, bicuspid aortic valve; LA, left atrium; LV, left ventricle; MVP, mitral valve prolapse; PASP, pulmonary artery systolic pressure; PFO, patent foramen ovale; RWT, relative wall thickness; VC, vena contracta.
Valvulopathies: mitral valve (at least moderate regurgitation, at least mild stenosis), tricuspid valve (at least moderate regurgitation, at least mild stenosis), aortic valve (at least mild regurgitation, at least mild stenosis), pulmonary valve (at least moderate regurgitation, at least mild stenosis).
Discussion
The main results of the present study highlight that the implementation of the ‘standard’ PPS with an echocardiogram showed an increase of about 10% in the detection of cardiovascular abnormalities not previously identified. We found that this minority of patients who have a negative ‘standard’ PPS and therefore would have not been further investigated showed some form of abnormal findings by using echocardiography.
Earlier detection of CV abnormalities in asymptomatic patients, in particular in the young, allows an appropriate and prompt approach, preventing long-term complications and possible adverse events, other than influencing their sport eligibility.11
For instance, the detection of a patent foramen ovale (22.6% of abnormal findings), clinically silent in the vast majority of cases but easily documented by using ultrasound imaging, allows specific contraindications for SCUBA diving (echocardiography with bubble contrast).25 The prevalence of this cardiac abnormality found in our cohort is in line with that reported by the European position paper on the management of patients with patent foramen ovale (25%).25
Also, the finding of a bicuspid aortic valve makes it possible to investigate the presence of other dangerous conditions often associated, such as aortic coarctation, progressive dilation of the bulb and ascending aorta, significant aortic valve regurgitation and an increased risk of aortic dissection.26 Although this congenital defect may remain without clinical consequences for a lifetime, it can deteriorate in aortic valve stenosis and regurgitation and aortic dilatation, which could represent a life-risk for the patient, other than reasons of noneligibility for sports activity.27
About 22% of negative ‘standard’ PPS patients had significant valvulopathies, deserving regular monitoring for the risk of evolution over time, according to the recent position statement of the European Association of Preventive Cardiology.28 Indeed, the presence of mitral valve prolapse, common in our cohort, seems to be related to an increased risk of SCD, even if a recent report would question that.29
Other conditions found may be related to a hypertensive state (such as diastolic dysfunction, left atrium enlargement, or left ventricle concentric remodeling): the early detection of organ damage in a hypertensive patient is important to stratify the CV risk and to provide correct indications about the appropriate physical exercise to practice and the therapeutic strategy to adopt.30
Moreover, a PPS echocardiography might be of even more critical importance in detecting cardiomyopathies at risk of SCD (hypertrophic, dilated, arrhythmogenic or left-ventricle noncompaction), coronary artery abnormalities, and other congenital defects (patent ductus arteriosus, aortic coarctation)11 that were not detected in our sample size.
Some studies suggested the inclusion of echocardiography in the PPS to detect the most widespread cardiac abnormalities related to SCD.13,14 Fuller15 pointed out that a complete echocardiographic study would potentially detect a wide range of structural abnormalities but could be disadvantageous: diagnostic necessity, time constraints, and cost-effectiveness are unfavorable reasons for its use.31 Therefore, a focused echocardiogram could reduce the time and cost needed to run the exam,32,33 and this is a topic well explored in scientific literature. Lots of authors have analyzed the primary role of echocardiography during the medical examination sports screening in terms of early diagnosis against various abnormalities,34 trying to find different solutions such as the 5-min screening of Wyman et al.,12 a single echocardiography view of Weidenbener et al.,35 or the specific research of a CV condition of Pelliccia et al.36 Niederseer et al.37 proposed to include double screening echocardiography in the athlete instead of a yearly seriate use: in adolescence to rule out structural heart disease, and over the age of 30 to evaluate abnormal cardiac remodeling to exercise, cardiomyopathies and wall motion anomalies. This could represent an interesting solution and a valid line of research for future studies.
Comparing the advantages of carrying out echocardiography at each visit, the cost that this would require and the severity of the CV abnormalities that we found, we hypothesize that it could be reasonable to perform an ultrasound evaluation in the context of the first PPS, limiting subsequent echocardiographic follow-up to patients with detected clinical-instrumental alterations. Once a CV pathology is diagnosed, a multimodality approach, including stress echocardiography, cardiac magnetic resonance and computer tomography, is necessary to better evaluate the athlete and to consider his sports eligibility.38
Our study has some significant limitations. Firstly, it was not possible to carry out a cost-effectiveness analysis because the exams are part of a corporate wellness program free of charge for the employees. In addition, the study group is not represented by athletes, but by a sample of employees of the Ferrari company, with various levels of physical activity. Furthermore, the composition of the sample showed a clear male prevalence (80%). Finally, the echocardiographic examination, even if performed by a highly skilled physician, is very operator-dependent and requires good manual skill, a training period, and echocardiography experience. One of the main problems of this work was to define precisely what was abnormal and what was normal and therefore when to define a ‘positive’ and when ‘negative’ results; to better define this positivity, we referred to the latest European guidelines.
Conclusion
Screening echocardiogram could help to detect patients with CV abnormalities (about 10% more) that might be undetected with ‘standard’ PPS protocol. A focused cardiac ultrasound examination may optimize the cost-effectiveness ratio: early detection of asymptomatic structural heart conditions could have important prognostic implications, especially in the sports eligibility process, thus reducing the risk of SCD.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lee D-C, Pate RR, Lavie CJ, Sui X, Church TS, Blair SN. Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol 2014; 64:472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JH, Malhotra R, Chiampas G, d’Hemecourt P, Troyanos C, Cianca J, et al. Cardiac arrest during long-distance running races. N Engl J Med 2012; 366:130–140. [DOI] [PubMed] [Google Scholar]
- 3.Marijon E, Tafflet M, Celermajer DS, Dumas F, Perier M-C, Mustafic H, et al. Sports-related sudden death in the general population. Circulation 2011; 124:672–681. [DOI] [PubMed] [Google Scholar]
- 4.Empana J-P, Lerner I, Valentin E, Folke F, Böttiger B, Gislason G, et al. Incidence of sudden cardiac death in the European Union. J Am Coll Cardiol 2022; 79:1818–1827. [DOI] [PubMed] [Google Scholar]
- 5.Peterson DF, Kucera K, Thomas LC, Maleszewski J, Siebert D, Lopez-Anderson M, et al. Aetiology and incidence of sudden cardiac arrest and death in young competitive athletes in the USA: a 4-year prospective study. Br J Sports Med 2021; 55:1196–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 2021; 42:17–96. [DOI] [PubMed] [Google Scholar]
- 7.Perez M, Fonda H, Le V-V, Mitiku T, Ray J, Freeman JV, et al. Adding an electrocardiogram to the preparticipation examination in competitive athletes: a systematic review. Curr Probl Cardiol 2009; 34:586–662. [DOI] [PubMed] [Google Scholar]
- 8.Wheeler MT, Heidenreich PA, Froelicher VF, Hlatky MA, Ashley EA. Cost-effectiveness of preparticipation screening for prevention of sudden cardiac death in young athletes. Ann Intern Med 2010; 152:276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gleason CN, Kerkhof DL, Cilia EA, Lanyi MA, Finnoff J, Sugimoto D, et al. Early screening for cardiovascular abnormalities with preparticipation echocardiography: feasibility study. Clin J Sport Med. 2017;27:423–9. [DOI] [PubMed] [Google Scholar]
- 10.D’Ascenzi F, Anselmi F, Mondillo S, Finocchiaro G, Caselli S, Garza MS-D la, et al. The use of cardiac imaging in the evaluation of athletes in the clinical practice: a survey by the Sports Cardiology and Exercise Section of the European Association of Preventive Cardiology and University of Siena, in collaboration with the European Association. Eur J Prev Cardiol England 2021; 28:1071–1077. [DOI] [PubMed] [Google Scholar]
- 11.Palermi S, Serio A, Vecchiato M, Sirico F, Gambardella F, Ricci F, et al. Potential role of an athlete-focused echocardiogram in sports eligibility. World J Cardiol 2021;13:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyman RA, Chiu RY, Rahko PS. The 5-min screening echocardiogram for athletes. J Am Soc Echocardiogr 2008; 21:786–788. [DOI] [PubMed] [Google Scholar]
- 13.Weiner RB, Wang F, Hutter AMJ, Wood MJ, Berkstresser B, McClanahan C, et al. The feasibility, diagnostic yield, and learning curve of portable echocardiography for out-of-hospital cardiovascular disease screening. J Am Soc Echocardiogr 2012; 25:568–575. [DOI] [PubMed] [Google Scholar]
- 14.Rizzo M, Spataro A, Cecchetelli C, Quaranta F, Livrieri S, Sperandii F, et al. Structural cardiac disease diagnosed by echocardiography in asymptomatic young male soccer players: implications for preparticipation screening. Br J Sports Med England 2012; 46:371–373. [DOI] [PubMed] [Google Scholar]
- 15.Fuller CM. Cost effectiveness analysis of screening of high school athletes for risk of sudden cardiac death. Med Sci Sports Exerc 2000; 32:887–890. [DOI] [PubMed] [Google Scholar]
- 16.Cuschieri S. The STROBE guidelines. Saudi J Anaesth 2019; 13:S31–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palermi S, Vecchiato M, Pennella S, Marasca A, Spinelli A, de Luca M, et al. The impact of the COVID-19 pandemic on childhood obesity and lifestyle—a report from Italy. Pediatr Rep 2022; 14:410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biffi A, Gallo G, Fernando F, Sirico F, Signorello MG, de Martino L, et al. Relationship between cardiorespiratory fitness, baseline blood pressure and hypertensive response to exercise in the Ferrari Corporate Population. High Blood Press Cardiovasc Prev 2022; 29:81–88. [DOI] [PubMed] [Google Scholar]
- 19.Palermi S, Annarumma G, Spinelli A, Massa B, Serio A, Vecchiato M, et al. Acceptability and practicality of a quick musculoskeletal examination into sports medicine pre-participation evaluation. Pediatr Rep 2022; 14:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham IM, di Angelantonio E, Huculeci R. New Way to ‘SCORE’ Risk: updates on the ESC Scoring System and Incorporation into ESC Cardiovascular Prevention Guidelines. Curr Cardiol Rep 2022; 24:1679–1684. [DOI] [PubMed] [Google Scholar]
- 21.Pelliccia A, Zipes DP, Maron BJ. Bethesda Conference #36 and the European Society of Cardiology Consensus Recommendations revisited a comparison of U.S. and European criteria for eligibility and disqualification of competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol 2008; 52:1990–1996. [DOI] [PubMed] [Google Scholar]
- 22.Drezner JA, Sharma S, Baggish A, Papadakis M, Wilson MG, Prutkin JM, et al. International criteria for electrocardiographic interpretation in athletes: consensus statement. Br J Sports Med 2017; 51:704–731. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 2013; 128:873–934. [DOI] [PubMed] [Google Scholar]
- 24.Galderisi M, Cosyns B, Edvardsen T, Cardim N, Delgado V, di Salvo G, et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Ima. Eur Heart J Cardiovasc Imaging 2017; 18:1301–1310. [DOI] [PubMed] [Google Scholar]
- 25.Germonpré P, Dendale P, Unger P, Balestra C. Patent foramen ovale and decompression sickness in sports divers. J Appl Physiol 1998; 84:1622–1626. [DOI] [PubMed] [Google Scholar]
- 26.Boraita A, Morales-Acuna F, Marina-Breysse M, Heras M-E, Canda A, Fuentes M-E, et al. Bicuspid aortic valve behaviour in elite athletes. Eur Heart J Cardiovasc Imaging 2019; 20:772–780. [DOI] [PubMed] [Google Scholar]
- 27.D’Ascenzi F, Valentini F, Anselmi F, Cavigli L, Bandera F, Benfari G, et al. Bicuspid aortic valve and sports: from the echocardiographic evaluation to the eligibility for sports competition. Scand J Med Sci Sports 2021; 31:510–520. [DOI] [PubMed] [Google Scholar]
- 28.van Buuren F, Gati S, Sharma S, Papadakis M, Adami PE, Niebauer J, et al. Athletes with valvular heart disease and competitive sports: a position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2021; 28:1569–1578. [DOI] [PubMed] [Google Scholar]
- 29.Nalliah CJ, Mahajan R, Elliott AD, Haqqani H, Lau DH, Vohra JK, et al. Mitral valve prolapse and sudden cardiac death: a systematic review and meta-analysis. Heart 2019; 105:144–151. [DOI] [PubMed] [Google Scholar]
- 30.Pescatello LS, Buchner DM, Jakicic JM, Powell KE, Kraus WE, Bloodgood B, et al. Physical activity to prevent and treat hypertension: a systematic review. Med Sci Sports Exerc 2019; 51:1314–1323. [DOI] [PubMed] [Google Scholar]
- 31.Mont L, Pelliccia A, Sharma S, Biffi A, Borjesson M, Brugada Terradellas J, et al. Preparticipation cardiovascular evaluation for athletic participants to prevent sudden death: Position paper from the EHRA and the EACPR, branches of the ESC. Endorsed by APHRS, HRS, and SOLAECE. Eur J Prev Cardiol 2017; 24:41–69. [DOI] [PubMed] [Google Scholar]
- 32.Fishman ML, Shea J, Choi BG, Solomon AJ. Feasibility of focused cardiac ultrasound in preparticipation screening. Sports Exerc Med 2015; 1:011. [Google Scholar]
- 33.Yim ES, Basilico F, Corrado G. Early screening for cardiovascular abnormalities with preparticipation echocardiography: utility of focused physician-operated echocardiography in preparticipation screening of athletes. J Ultrasound Med 2014; 33:307–313. [DOI] [PubMed] [Google Scholar]
- 34.Modaff DS, Hegde SM, Wyman RA, Rahko PS. Usefulness of focused screening echocardiography for collegiate athletes. Am J Cardiol 2019; 123:169–174. [DOI] [PubMed] [Google Scholar]
- 35.Weidenbener EJ, Krauss MD, Waller BF, Taliercio CP. Incorporation of screening echocardiography in the preparticipation exam. Clin J Sport Med 1995; 5:86–89. [DOI] [PubMed] [Google Scholar]
- 36.Pelliccia A, Spataro A, Maron BJ. Prospective echocardiographic screening for coronary artery anomalies in 1,360 elite competitive athletes. Am J Cardiol 1993; 72:978–979. [DOI] [PubMed] [Google Scholar]
- 37.Niederseer D, Rossi VA, Kissel C, Scherr J, Caselli S, Tanner FC, et al. Role of echocardiography in screening and evaluation of athletes. Heart 2020; doi: 10.1136/heartjnl-2020-317996. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38.D’Andrea A, Sperlongano S, Russo V, D’Ascenzi F, Benfari G, Renon F, et al. The role of multimodality imaging in athlete's heart diagnosis: current status and future directions. J Clin Med 2021; 10:5126. [DOI] [PMC free article] [PubMed] [Google Scholar]