Background:
Patients with upper limb lymphedema and lower limb lymphedema experience a wide range of physical and psychological symptoms that affect quality of life. The benefits of lymphatic reconstructive surgery for patients with lymphedema are undisputed. However, recording volume reduction alone may be insufficient with regard to postoperative outcome because measurements are often inadequate, depend on many factors, and do not reflect improvement in quality of life.
Methods:
We conducted a prospective single center study patients receiving lymphatic reconstructive surgery. Patients received volume measurements preoperatively and at standardized postoperative intervals. To evaluate patient-reported outcomes, patients completed the following questionnaires: LYMPH-Q Upper Extremity Module, quickDASH, SF 36, Lymphoedema Functioning, Disability and Health Questionnaire for Lower Limb Lymphoedema, and Lower Extremity Functional Scale at the aforementioned intervals.
Results:
We included 55 patients with upper limb lymphedema (24%) and lower limb lymphedema (73%) of lymphedema grades I–III. Patients received lymphovenous anastomosis only (23%), free vascularized lymph node transfer (35%) or a combination of both (42%). Analysis of patient-reported outcome measurements revealed improvements with respect to a broad range of complaints, particularly physical function, symptoms, and psychological well-being. There was no correlation between the extent of volume reduction and improvement in quality of life (Pearson correlation coefficient below ±0.7; P > 0.05).
Conclusions:
Based on a broad range of outcome measurements, we observed an improved quality of life in almost all patients, even in those without measurable volume loss of the extremity operated on, which emphasizes the need for a standardized use of patient-reported outcome measures to evaluate the benefits of lymphatic reconstructive surgery.
Takeaways
Question: The aim of the study was to investigate whether there is a clear correlation between extent of limb volume reduction and improvement in quality of life after lymphatic reconstructive surgery.
Findings: Based on a broad range of outcome measurements, we observed an improved quality of life in almost all patients, even in those without measurable volume reduction of the extremity operated on.
Meaning: The results of our study emphasize the need for a standardized use of patient-reported outcome measures to evaluate the benefits of lymphatic reconstructive surgery.
INTRODUCTION
Reconstructive lymphatic surgery has been one of the most important milestones in lymphedema treatment with a growing body of literature demonstrating a significant and lasting reduction of limb volume.1–7 However, the majority of studies have almost entirely focussed on the objective outcomes after lymphatic reconstruction, such as volume measurements, but only a few included well-validated lymphedema-specific patient-reported outcome measures (PROMs). Given the wide range of physical and psychological complaints that are regularly caused by lymphedema, PROMs are of paramount importance in this patient population.8–11
Currently, many different PROMs are available for patients after lymphatic reconstructive surgery. We previously performed a thorough analysis according to the Consensus-based Standards for the Selection of Health Measurement Instruments of existing lymphedema-specific PROMs for patients with lower limb lymphedema (LLL) and identified the Lymphoedema Functioning, Disability and Health Questionnaire for Lower Limb Lymphoedema (LYMPH-ICF-LL) as superior in terms of its psychometric properties.12,13 Based on a similar approach, Beelen et al claimed that none of the lymphedema-specific PROMs for patients with upper limb lymphedema (ULL) met methodological quality standards and thus, they developed the LYMPH-Q Upper Extremity Module.14–16 After translating the LYMPH-Q into German, we recently demonstrated that patients with arm lymphedema experience significant improvements of function, symptoms and psychological well-being after lymphatic reconstructive surgery, regardless of lymphedema grade, age, BMI, or comorbidities.16 Similar improvements in quality of life (QOL) after lymphatic reconstructive surgery in patients with ULL and LLL were described by other authors.17–20 Although none of these studies analyzed if there is a correlation between volume loss and QOL, greater improvements of QOL were observed in patients after vascularized lymph node transplantation (VLNT) compared with the ones who only received lymphovenous anastomosis (LVAs).
The validity of volume measurements to determine the impact of lymphedema surgery on patients QOL has recently been questioned due to the low standardization of volume loss calculation, high inter-examiner variability, strong dependence on the extent of pre- and postoperative complex decongestive therapy (CDT) and the wearing of compression garments.21–24 The aim of the study was therefore to investigate whether there is a correlation between extent of volume reduction and improvement in QOL. This also raises the question of whether other factors than volume reduction alone potentially influence the postoperative outcome from the patients’ perspective.
MATERIAL AND METHODS
We conducted a prospective study on patients receiving lymphatic reconstructive surgery at the Department of Plastic Surgery and Hand Surgery, University Hospital, Zurich between February 2020 and September 2022. Approval was given by the Cantonal Ethics Committee of Zurich, Switzerland (ethical approval no.: 2020-00110; date: Feb 24, 2020). All patients scheduled for lymphatic reconstructive surgery in our department were consecutively enrolled in the study. Written consent was obtained from all the patients or their parents in the case of minors. Patients that were not able to fill in questionnaires due to insufficient knowledge of the German language, impaired psycho-intellectual abilities, or any psychiatric disorder were only included for baseline and treatment variables.
All patients received manual circumference measurements by the physiotherapist with subsequent calculation of limb volume according to the method described by Kuhnke,25 preoperatively and at standardized intervals (2 weeks, 6 weeks, 6 months and 12 months) after the surgery. If patients were operated on at two different anatomic locations (eg, both legs), each leg was considered independently to allow for a more precise assessment of the outcome.
Percentage volume difference between limbs for each patient was calculated using the following formula21:
The percentage change in volume differential was calculated as follows:
To evaluate the patient-reported outcome, patients with ULL completed the LYMPH-Q Upper Extremity Module, quick Disabilities of the Arm, Shoulder and Hand (quickDASH) and Short form Health Survey 36 (SF 36) preoperatively and at the aforementioned intervals. Patients with LLL received the LYMPH-ICF-LL, Lower Extremity Functional Scale (LEFS) and SF 36 at the same intervals. Use of the LYMPH-Q Upper Extremity Module, authored by Drs. Klassen, Pusic and Cano, was made under license from Memorial Sloan Kettering Cancer Center, N.Y. Additionally, we collected patient characteristics and surgical details.
Data were then analyzed using Microsoft Excel, version 14.3.6. (Microsoft Corp., Redmond, Wash.) and GraphPad Prism, version 7.04 (GraphPad, La Jolla, Calif.). A t test or one-way ANOVA was performed to compare for continuous parametric data. Pearson correlation was run to analyze if there is a correlation between volume reduction and QOL score. A P value of less than 0.05 was defined as significant.
RESULTS
We included 33 (60%) female and 22 (40%) male patients (Table 1) with a mean age of 43.7 (± 17.3). Most patients (70%) presented with lymphedema grade II, whereas lymphedema grade I (19%) and III (11%) were less common (Fig. 1). The majority of the patients had lymphedema of the lower extremity (73%), followed by lymphedema of the arm (24%) and the genitals (3%).
Table 1.
Patient Characteristics
| N | ||
|---|---|---|
| Patients (N) | 55 | |
| Age, y (mean ± SD) | 43.7 | (±17.3) |
| BMI, kg/m2 (mean ± SD) | 25.5 | (±5.1) |
| Lymphedema stage (N = extremity operated on) (%)* | ||
| I | 12 | (19%) |
| II | 45 | (70%) |
| III | 7 | (11%) |
| Duration until surgery, y (mean ± SD) | 9.9 | (±9.0) |
| Cause of lymphedema (N = patient) (%) | ||
| Primary | 26 | (47%) |
| Secondary | 29 | (53%) |
| Affected anatomic region (N = patient) (%) | ||
| Lower extremity | 40 | (72.7%) |
| Upper extremity | 13 | (23.6%) |
| Genitals | 2 | (3%) |
| Laterality (N = patient) (%) | ||
| Unilateral | 40 | (73%) |
| Bilateral | 15 | (27%) |
| Recurrent erysipelas (N = patient) (%) | 13 | (24%) |
| Prior surgery (N = extremity operatred on) (%)* | ||
| Liposuction | 3 | (5%) |
| LVA | 4 | (6%) |
| VLNT | 1 | (2%) |
| Concomitant disease (N = patient) (%)* | ||
| None | 36 | (65%) |
| Vascular, hypertension, diabetes, coronary artery disease | 10 | (18%) |
| Venous disease (venous insufficiency, thrombosis, varicose veins) | 4 | (7%) |
| Metabolic (adipositas, thyroid, metabolic syndrome) | 8 | (15%) |
| Inflammatory disease | 2 | (4%) |
| Arthritis/osteoporosis | 2 | (4%) |
| Pulmonary, asthma | 2 | (4%) |
More than one value is possible for every patient or extremity operated on.
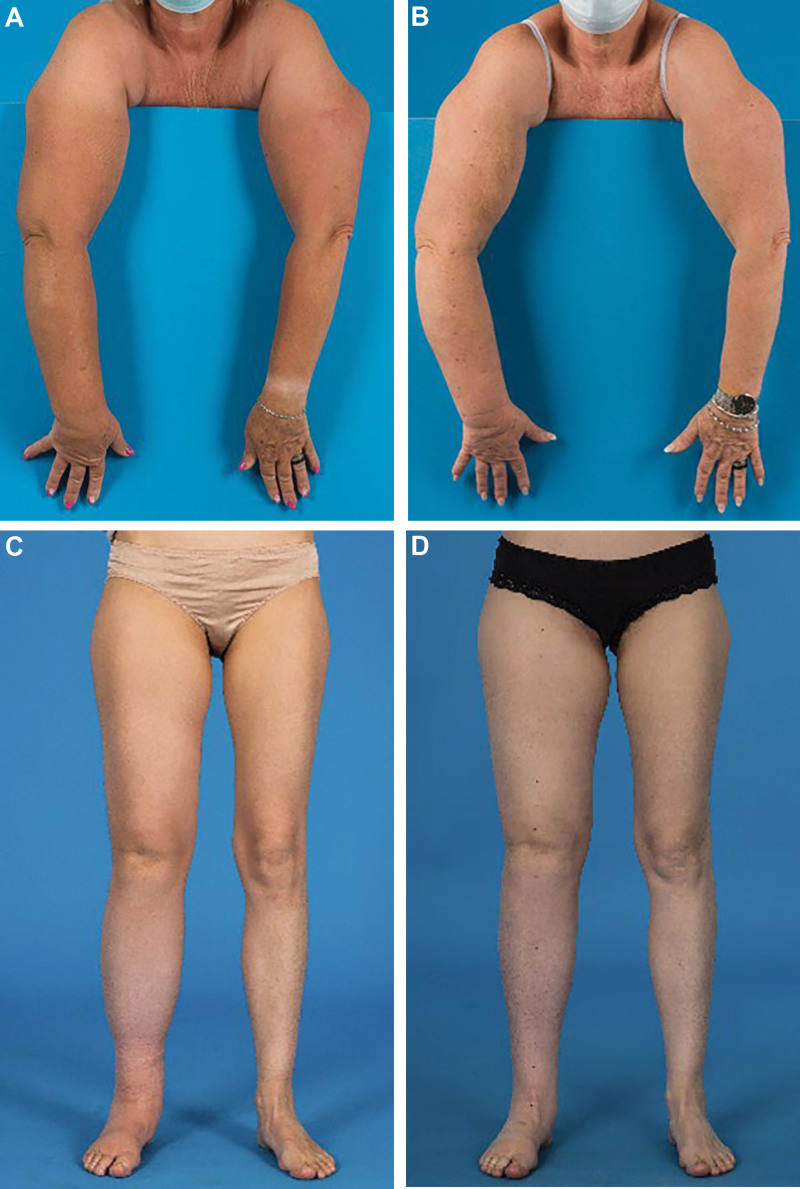
Fig. 1.
A, Preoperative picture of a 50-year-old patient with secondary upper limb lymphedema grade III after axillary lymph node dissection due to breast cancer. B, A volume reduction of 22% was seen 12 months after gastroepiploic lymph node transfer to the right axillary region and liposuction of the upper arm. C, Preoperative picture of a 39-year-old patient with secondary LLL grade II. D, A volume reduction of 11% was seen 6 months after gastroepiploic lymph node transfer to the right groin, two LVAs and liposuction of the thigh.
Overall, 42% of the patients received combined LVA and VLNT, either as laparoscopic right gastroepiploic lymph node transfer or as taking lymph nodes transplant from the thoracic or the abdominal wall after reverse lymphatic mapping (Table 2). The remaining patients underwent LVA (23%) or VLNT (30%), while 11% of the patients, who were diagnosed with bilateral lymphedema, received simultaneous bilateral VLNT. A unilateral double VLNT was performed in a growing number patients (9%) to reconstruct lymph drainage at two different anatomic locations, for example, by transplanting lymph nodes both in the inguinal area and in the knee or ankle of the same limb. From our experience over the past 2 years, this technique is particularly effective to receive timely volume reduction in patients with primary lymphedema. In 33% of the patients, additional liposuction was performed during the same surgery. Complications occurred in a small number of patients, including seroma formation (14%), wound infections or delayed wound healing (8%), cellulitis (3%), and hematoma (8%).
Table 2.
Operation Details
| Surgical technique (N = extremity operated on) | ||
|---|---|---|
| LVA | 15 | (23%) |
| Mean number of LVAs in LVA only (range) | 6 | (5–10) |
| VLNT | 19 | (30%) |
| VLNT + LVA | 27 | (42%) |
| DIEP + VLNT | 3 | (5%) |
| Double VLNT in patients (N = patient) (%) | 11 | (20%) |
| Unilateral | 5 | (9%) |
| Bilateral | 6 | (11%) |
| Additional liposuction (N = extremity operated on) (%) | 21 | (33%) |
| Length of surgery, min (mean ± SD) | 374.3 | (±116.4) |
| LVA | 278 | (±79.2) |
| VLNT | 373 | (±67.1) |
| VLNT + LVA | 381 | (±111.9) |
| DIEP + VLNT | 595.7 | (±57.7) |
| Length of hospital stay, days (mean ± SD) | 4.1 | (±1.7) |
| LVA | 2.5 | (±0.8) |
| VLNT | 4.4 | (±1.2) |
| VLNT + LVA | 4.2 | (±1.4) |
| DIEP + VLNT | 7.6 | (±1.2) |
| Compression class change (N = extremity operated on with known pre- and postoperative compression class)* | ||
| No changes | 14 | (58.3%) |
| Decrease in compression class | 8 | (33.3%) |
| Increase in compression class | 2 | (8.3%) |
| Complications (N = extremity operated on) (%)† | ||
| Wound infection/healing issues | 5 | (8%) |
| Seroma | 9 | (14%) |
| Cellulitis | 2 | (3%) |
| Hematoma | 5 | (8%) |
| Other | 6 | (9%) |
The information was available for 24 of the extremities operated on.
More than one value is possible for every patient or extremity operated on.
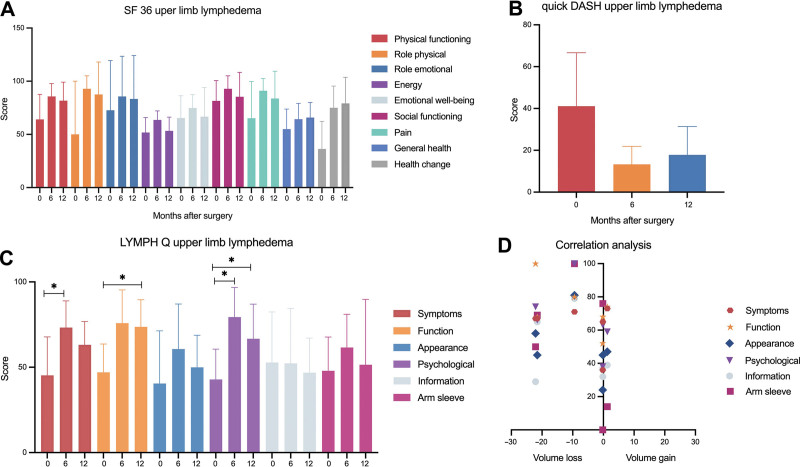
For patients with ULL, all eight patients who had reached their 6–12 months follow-up completed their questionnaires (response rate of 100%) (Fig. 2). The SF 36 (Fig. 2 A) revealed the following improvements: physical functioning, 64.1 ± 23.5 versus 81.7 ± 17.5; role physical, 50.0 ± 50.0 versus 87.5 ± 30.6; role emotional, 72.7 ± 46.7 versus 83.3 ± 40.8; energy, 51.8 ± 14.0 versus 53.3 ± 12.9; emotional well-being, 65.5 ± 20.9 versus 66.7 ± 27.3; social functioning, 81.6 ± 18.9 versus 85.4 ± 22.9; pain, 65.2 ± 34.6 versus 83.8 ± 25.6; general health, 55.0 ± 18.8 versus 65.8 ± 14.3; health change, 36.4 ± 25.9 versus 79.2 ± 24.6. Of note, mean scores after 6 months showed greater improvements compared with those 12 months postoperatively. Based on the LYMPH-Q (Fig. 2C), patients with ULL experienced statistically significant improvements of symptoms 6 months postoperatively (45.3 ± 22.5 versus 73.3 ± 15.6, P = 0.02) and for function (47.0 ± 16.7 versus 73.7 ± 15.9, P = 0.01) and psychological well-being twelve months postoperatively (42.8 ± 17.8 versus 66.7 ± 20.3, P = 0.04). Patients were also more satisfied regarding appearance (40.5 ± 30.9 versus 50.0 ± 18.8) and arm sleeve (47.9 ± 19.8 versus 51.5 ± 38.3), even if these values were not statistically significant. The remaining domain on information showed a decrease of the score after twelve months, pointing towards an overall unsatisfactory perception of the patients’ information regarding the disease (52.7 ± 29.6 versus 46.8 ± 20.3). In line with the scores for physical function, the quick DASH score (Fig. 2B: 41.1 ± 25.3 versus 17.8 ± 13.6) confirmed improved arm function after lymphatic reconstructive surgery.
Fig. 2.
A, The mean score for the different domains of the SF 36, preoperatively as well as 6 and 12 months after surgery, showed an improvement for all domains. B, Analysis of the quickDASH score revealed an improvement in arm function, without being statistically significant. C, LYMPH-Q showed significant improvements regarding symptoms, function and psychological well-being. For the remaining domains, no statistically significant difference could be observed. D, Correlation analysis confirmed that there is no relationship between change in limb volume and improvement in QOL, which is shown for the LYMPH-Q.
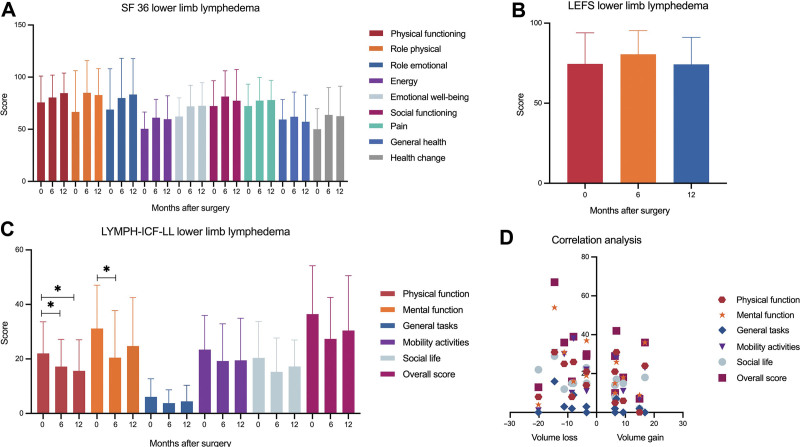
For patients with LLL, 22 of 28 patients, giving a response rate of 79%, completed their questionnaires after 6 to 12 months (Fig. 3). SF 36 (Fig. 3 A) showed improvements in all domains (physical functioning, 75.7 ± 25.3 versus 84.7 ± 19.3; role physical, 66.7 ± 39.6 versus 82.8 ± 25.4; role emotional, 68.9 ± 39.1 versus 83.4 ± 34.4; energy, 50.5 ± 16.1 versus 59.7 ± 22.4; emotional well-being, 62.3 ± 17.9 versus 72.5 ± 22.2; social functioning, 72.3 ± 24.2 versus 77.4 ± 29.9; pain, 72.3 ± 21.1 versus 78.1 ± 18.9; health change, 50.0 ± 19.7 versus 62.5 ± 28.9), except in the one with questions regarding general health (59.3 ± 19.2 versus 57.2 ± 25.7). Given a maximum score of 80, the average LEFS score (Fig. 3B) remained almost unchanged in patients with LLL 12 months postoperatively (74.6 ± 19.4 versus 74.3 ± 16.9). In contrast, the LYMPH-ICF-LL (Fig. 3C) showed significant improvements regarding physical function 12 months postoperatively (22.0 ± 11.6 versus 15.6 ± 11.2; P = 0.02) and mental function six months postoperatively (31.2 ± 15.9 versus 20.5 ± 17.3; P = 0.01). Lower scores, which for this type of questionnaire pointing toward less impairment caused by lymphedema, were also observed for general tasks (6.1 ± 6.6 versus 4.4 ± 5.9), mobility activities (23.4 ± 12.5 versus 19.5 ± 15.4), social life (20.4. ± 13.4 versus 17.3 ± 9.7), and also in the overall score (36.5 ± 17.8 versus 30.4 ± 20.1).
Fig. 3.
A, The average score for the different domains of the SF 36, preoperatively as well as 6 and 12 months after surgery, showed an improvement for all domains except general health. B, Analysis of the LEFS score hints toward no relevant difference in limb function after lymphatic reconstructive surgery. C, LYMPH-ICF-LL showed significant improvements regarding physical and mental function. Mean scores for general tasks, mobility activities, social life and the overall score also revealed an improvement in QOL without being statistical significant. D, Correlation analysis confirmed that there is no relationship between change in limb volume and improvement in QOL, which is shown for the LYMPH-ICF-LL.
All patients received standardized CDT for at least 1 year preoperatively and an intensive treatment phase in the month before the operation. Based on this, only mild to moderate edema was present at the time of preoperative circumference measurements in most patients. Preoperative percentage volume difference to the unaffected extremity was 20.1% (range 4.0–35.8) for ULL and 18.8% (range 2.3–49.3) for LLL.
In 34 patients (62%), limb volume measurements could be obtained at the 6–12 months follow-up. At this point in time, 83% of patients with ULL and 50% of patients with LLL showed a volume reduction. In these patients, average percentage change in volume differential was 67.7% for ULL and 39.8% for LLL. Thus, we observed an average percentage change in volume differential of 52.2% in comparison with the unaffected limb.
Altogether, we observed an absolute volume reduction ranging from a minimum of 0.01% to a maximum of 22.1% and a mean volume reduction of 8.4% (±7.4%) with respect to volume reduction of the diseased extremity when compared with preoperative volume.
Although compression class was reduced in eight patients, compression garments remained unchanged in 58% of the patients. Improvement of QOL scores was seen in almost all patients as described above, irrespective of measurable volume reduction. This was also confirmed by additional correlation analysis including data of 20 patients who completed volume measurements and all questionnaires pre- and postoperatively. In fact, for all domains of the LYMPH-Q, SF 36, LYMPH-ICF-LL and LEFS, correlation analysis revealed that there is no correlation between volume loss and improvement in QOL or lower limb function (Figs. 2D, 3D). (See table, Supplemental Digital Content 1, which shows correlation analysis upper limb lymphedema. http://links.lww.com/PRSGO/C593.) (See table, Supplemental Digital Content 2, which shows correlation analysis lower limb lymphedema. http://links.lww.com/PRSGO/C594.) However, regarding arm function after lymphatic reconstructive surgery, we observed a negative correlation between quickDASH and volume reduction (r = – 0.81, P = 0.048), which confirms an improved arm function in less arm volume.
DISCUSSION
The results of this study confirm that lymphatic reconstructive surgery leads to an improvement in QOL. Interestingly, we even observed an improved QOL in patients without volume loss of the extremity operated on, which emphasizes that factors other than volume loss only contribute to the positive effect of reconstructive lymphatic surgery. These findings are consistent with the observation of other authors who reported improvement of subjective symptoms in patients without or before measurable volume differences occur.19,26,27
In patients with ULL, the SF 36 and quickDASH revealed improvements with respect to a broad range of physical and psychological complaints. Even though our results were not statistically significant, they are well in line with other studies using the SF 36 and quickDASH in patients with arm lymphedema after lymphatic reconstructive surgery or liposuction alone.28,29 Considering that the SF 36 is a generic questionnaire, and its results may be influenced by comorbidities, only a few authors have used this questionnaire for patients with lymphedema. Given the substantial number of patients with comorbidities, we believe this is one of the main reasons why the results of the SF 36 were not significant. The same accounts for the quickDASH, which has been designed for patients with any kind of arm impairment. Interestingly, we observed a significant correlation between quickDASH score and extent of volume loss, which emphasizes the fact that patients with less arm volume have fewer difficulties with daily upper extremity activities. Indeed, similar observations were made by other authors, who reported about an association between quickDASH score and lymphedema severity.30 This is most likely not only related to limb volume but also to skin and tissue fibrosis that limit range of motion and upper extremity strength.
Regarding upper extremity function, the corresponding domain of the LYMPH-Q revealed a significant improvement twelve months postoperatively. Significant results were also obtained for symptoms and psychological well-being. Although the preoperative mean scores were comparable with that of patients with severe arm swelling in a cohort of 3222 women in the United States and Denmark, the postoperative scores were partially similar to that of patients with mild or moderate arm swelling.15 For all domains, except information, we observed a slight decrease in the mean score 12 months postoperatively compared with six months. We believe that this can be explained by an initial loss of limb volume that leads to a relief of symptoms. However, after a couple of months, the volume typically stagnates until the transplanted lymph nodes begin to function, which might be unsettling for many patients. Thus, it will be necessary to reassess the LYMPH-Q in that patient cohort 2 years postoperatively and to include further patients to draw final conclusions. This is the first study that has assessed the LYMPH-Q in patients after lymphatic reconstructive surgery, and further comparison with other studies was not possible.
Based on the LYMPH-ICF-LL, we observed a significant increase in the mean scores for physical and mental function in patients with LLL after lymphatic reconstructive surgery. To the best of our knowledge, there exists only one further study that analyzed QOL in patients after lymphatic reconstructive surgery based on the LYMPH-ICF-LL. Although they focused on patients with ULL and included only 25 patients with LLL, they also observed significant improvements for physical and mental function only, while the remaining domains showed no statistically significant difference.31 Even though the LYMPH-ICF-LL score showed an improved physical limb function, the LEFS, a self-report questionnaire that can be used for a wide range of lower extremity conditions, remained unchanged. Lee et al have demonstrated that the LEFS score showed no difference in patients with different grades of lymphedema. Considering that our patients with an average score of 74 were only six points below the maximum score of 80, it seems likely that the LEFS does not adequately reflect the limitations of LLL.
Regarding limb volume, 83% of patients with ULL and 50% of patients with LLL showed a volume reduction postoperatively. This is in line with Chang, who reported reduction of limb volume in 60% of patients with LLL.27,32 We observed a volume reduction up to 22% and a mean volume reduction of 8.4% (±7.4%) 6 or 12 months postoperatively, which is similar to other studies.33 Of note, it is well known that further volume improvement after VLNT occurs after 1 year due to shunting and lymphangiogensis.21 Hence, a longer duration of follow-up will be necessary to draw final conclusions. Although most authors only report the so-called excess volume reduction, we additionally calculated the average percentage change in volume differential comparing the volume reduction to the healthy extremity for unilateral lymphedema yielding 52.2%, which is comparable with other studies using this method.18,19,21 Because one-third of our patients had bilateral lymphedema, we can only report the volume reduction of the affected limbs in these patients, which depends on the absolute numbers—ie, large volume reductions can only be achieved in patients with correspondingly large limb volumes. Furthermore, it has to be considered that the majority of our patient cohort had LLL, in which an improvement of lymphatic drainage is, per se, more difficult due to the higher venous pressure. Moreover, almost one half of our patients were diagnosed with primary lymphedema, which tends to show less volume reduction after lymphatic surgery.21,26,34 Other issues leading to an extreme heterogeneity of the current literature are the different grading systems, the etiology and stage of lymphedema, various surgical techniques, and the different pre- and postoperative treatment protocols regarding CDT.33 In Switzerland, patients have to adhere to a formal and strict conservative therapy protocol, for at least 1 year before insurance will cover lymphatic surgery. This includes the wear of fitted compression garments and an optimal support by physiotherapists, including regular CDT and an intensive treatment phase in the month before the surgery. This usually results in a significant preoperative volume reduction, which must be considered when assessing postoperative outcomes. In contrast, greater volume reductions are expected in untreated lymphedema and in patients who receive additional liposuction due to an advanced fibroadipose tissue accumulation. The latter is increasingly recommended in combination with lymphatic surgery, especially in advanced lymphedema.35,36 Patients who had recurrent erysipelas preoperatively did not develop further infections after lymphatic reconstructive surgery, which most likely also contributed to improved quality of life.
We are aware that the validity of this study must be considered with caution due to the relatively small number of patients who completed the 6 to 12 months follow-up, including volume measurements and all questionnaires. In addition to enrolling more patients, our future research will focus on objectifying the softness of the limb operated on and evaluating the results 2 years postoperatively.
CONCLUSIONS
Although our study is limited by the relatively small number of patients, it is unique in terms of the broad range of physical and psychological complaints covered by the different domains of six well-validated questionnaires. The thorough analysis of the different PROMs demonstrates that generic questionnaires like the SF-36 and self-reporting scores, namely the LEFS, do not adequately reflect the impairments of lymphedema patients. Hence, surgeons should rely on disease-specific PROMs such as the LYMPH-ICF-LL and the LYMPH-Q. Most importantly, we can conclude that there is no correlation between volume loss and QOL, which underlines the paramount importance of lymphatic reconstructive surgery for patients with lymphedema and emphasizes the need to include PROMs to assess the postoperative outcome.
DISCLOSURE
Nicole Lindenblatt acts as a scientific advisor and symposium speaker for Medical Microinstruments (MMI). All the other authors have no financial interests to declare in relation to the content of this article.
Supplementary Material
Footnotes
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
Drs. Grünherz and Barbon contributed equally to this work.
REFERENCES
- 1.Batista BN, Germain M, Faria JC, et al. Lymph node flap transfer for patients with secondary lower limb lymphedema. Microsurgery. 2017;37:29–33. [DOI] [PubMed] [Google Scholar]
- 2.Travis EC, Shugg S, McEwan WM. Lymph node grafting in the treatment of upper limb lymphoedema: a clinical trial. ANZ J Surg. 2015;85:631–635. [DOI] [PubMed] [Google Scholar]
- 3.Dionyssiou D, Demiri E, Tsimponis A, et al. A randomized control study of treating secondary stage II breast cancer-related lymphoedema with free lymph node transfer. Breast Cancer Res Treat. 2016;156:73–79. [DOI] [PubMed] [Google Scholar]
- 4.Gharb BB, Rampazzo A, Spanio di Spilimbergo S, et al. Vascularized lymph node transfer based on the hilar perforators improves the outcome in upper limb lymphedema. Ann Plast Surg. 2011;67:589–593. [DOI] [PubMed] [Google Scholar]
- 5.Demirtas Y, Ozturk N, Yapici O, et al. Supermicrosurgical lymphaticovenular anastomosis and lymphaticovenous implantation for treatment of unilateral lower extremity lymphedema. Microsurgery. 2009;29:609–618. [DOI] [PubMed] [Google Scholar]
- 6.Becker C, Assouad J, Riquet M, et al. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg. 2006;243:313–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien BM, Sykes P, Threlfall GN, et al. Microlymphaticovenous anastomoses for obstructive lymphedema. Plast Reconstr Surg. 1977;60:197–211. [DOI] [PubMed] [Google Scholar]
- 8.Chachaj A, Malyszczak K, Pyszel K, et al. Physical and psychological impairments of women with upper limb lymphedema following breast cancer treatment. Psychooncology. 2010;19:299–305. [DOI] [PubMed] [Google Scholar]
- 9.De Vrieze T, Gebruers N, Nevelsteen I, et al. Physical activity level and age contribute to functioning problems in patients with breast cancer-related lymphedema: a multicentre cross-sectional study. Support Care Cancer. 2020;28:5717–5731. [DOI] [PubMed] [Google Scholar]
- 10.Morgan PA, Franks PJ, Moffatt CJ. Health-related quality of life with lymphoedema: a review of the literature. Int Wound J. 2005;2:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridner SH. The psycho-social impact of lymphedema. Lymphat Res Biol. 2009;7:109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devoogdt N, De Groef A, Hendrickx A, et al. Lymphoedema functioning, disability and health questionnaire for lower limb lymphoedema (Lymph-ICF-LL): reliability and validity. Phys Ther. 2014;94:705–721. [DOI] [PubMed] [Google Scholar]
- 13.Grunherz L, Hulla H, Uyulmaz S, et al. Patient-reported outcomes following lymph reconstructive surgery in lower limb lymphedema: a systematic review of literature. J Vasc Surg Venous Lymphat Disord. 2021;9:811–819.e2. [DOI] [PubMed] [Google Scholar]
- 14.Beelen LM, van Dishoeck AM, Tsangaris E, et al. Patient-reported outcome measures in lymphedema: a systematic review and COSMIN analysis. Ann Surg Oncol. 2021;28:1656–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klassen AF, Tsangaris E, Kaur MN, et al. Development and psychometric validation of a patient-reported outcome measure for arm lymphedema: the LYMPH-Q upper extremity module. Ann Surg Oncol. 2021;28:5166–5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grunherz L, Angst F, Barbon C, et al. Cultural adaption and multicenter validation of the german version of the LYMPH-Q upper extremity module. J Vasc Surg Venous Lymphat Disord. 2022;10:922–928.e2. [DOI] [PubMed] [Google Scholar]
- 17.Cheng MH, Loh CYY, Lin CY. Outcomes of vascularized lymph node transfer and lymphovenous anastomosis for treatment of primary lymphedema. Plast Reconstr Surg Glob Open. 2018;6:e2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciudad P, Maruccia M, Socas J, et al. The laparoscopic right gastroepiploic lymph node flap transfer for upper and lower limb lymphedema: technique and outcomes. Microsurgery. 2017;37:197–205. [DOI] [PubMed] [Google Scholar]
- 19.Patel KM, Lin CY, Cheng MH. A prospective evaluation of lymphedema-specific quality-of-life outcomes following vascularized lymph node transfer. Ann Surg Oncol. 2015;22:2424–2430. [DOI] [PubMed] [Google Scholar]
- 20.De Brucker B, Zeltzer A, Seidenstuecker K, et al. Breast cancer-related lymphedema: quality of life after lymph node transfer. Plast Reconstr Surg. 2016;137:1673–1680. [DOI] [PubMed] [Google Scholar]
- 21.Garza RM, Beederman M, Chang DW. Physical and functional outcomes of simultaneous vascularized lymph node transplant and lymphovenous bypass in the treatment of lymphedema. Plast Reconstr Surg. 2022;150:169–180. [DOI] [PubMed] [Google Scholar]
- 22.Raju A, Chang DW. Vascularized lymph node transfer for treatment of lymphedema: a comprehensive literature review. Ann Surg. 2015;261:1013–1023. [DOI] [PubMed] [Google Scholar]
- 23.Baltzer HL, Winocour S, Harless C, et al. Lymphaticovenous bypass: adaptations and lessons learned. Plast Reconstr Surg Glob Open. 2017;5:e1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward J, King I, Monroy-Iglesias M, et al. A meta-analysis of the efficacy of vascularised lymph node transfer in reducing limb volume and cellulitis episodes in patients with cancer treatment-related lymphoedema. Eur J Cancer. 2021;151:233–244. [DOI] [PubMed] [Google Scholar]
- 25.Kuhnke E. Volumenbestimmung aus Umfangsmessungen. Folia Angiol. 1976;24:224–232. [Google Scholar]
- 26.Chang DW, Suami H, Skoracki R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg. 2013;132:1305–1314. [DOI] [PubMed] [Google Scholar]
- 27.Chang DW. Lymphaticovenular bypass for lymphedema management in breast cancer patients: a prospective study. Plast Reconstr Surg. 2010;126:752–758. [DOI] [PubMed] [Google Scholar]
- 28.Hoffner M, Bagheri S, Hansson E, et al. SF-36 shows increased quality of life following complete reduction of postmastectomy lymphedema with liposuction. Lymphat Res Biol. 2017;15:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damstra RJ, Voesten HG, van Schelven WD, et al. Lymphatic venous anastomosis (LVA) for treatment of secondary arm lymphedema. A prospective study of 11 LVA procedures in 10 patients with breast cancer related lymphedema and a critical review of the literature. Breast Cancer Res Treat. 2009;113:199–206. [DOI] [PubMed] [Google Scholar]
- 30.Baran E, Yildiz TI, Gursen C, et al. The association of breast cancer-related lymphedema after unilateral mastectomy with shoulder girdle kinematics and upper extremity function. J Biomech. 2021;121:110432. [DOI] [PubMed] [Google Scholar]
- 31.Qiu SS, Pruimboom T, Cornelissen AJM, et al. Outcomes following lymphaticovenous anastomosis (LVA) for 100 cases of lymphedema: results over 24-months follow-up. Breast Cancer Res Treat. 2020;184:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang DW. Combined approach to surgical treatment of lymphedema. Lymphat Res Biol. 2021;19:23–24. [DOI] [PubMed] [Google Scholar]
- 33.Carl HM, Walia G, Bello R, et al. Systematic review of the surgical treatment of extremity lymphedema. J Reconstr Microsurg. 2017;33:412–425. [DOI] [PubMed] [Google Scholar]
- 34.Garza RM, Chang DW. Lymphovenous bypass for the treatment of lymphedema. J Surg Oncol. 2018;118:743–749. [DOI] [PubMed] [Google Scholar]
- 35.Brazio PS, Nguyen DH. Combined liposuction and physiologic treatment achieves durable limb volume normalization in class II–III lymphedema: a treatment algorithm to optimize outcomes. Ann Plast Surg. 2021;86(5S Suppl 3):S384–S389. [DOI] [PubMed] [Google Scholar]
- 36.Burton JS, Sletten AC, Marsh E, et al. Adipose tissue in lymphedema: a central feature of pathology and target for pharmacologic therapy. Lymphat Res Biol. 2023;21:2–7. [DOI] [PubMed] [Google Scholar]