Abstract
The activity of the transcription factor CREB is regulated by extracellular stimuli that result in its phosphorylation at a critical serine residue, Ser133. Phosphorylation of Ser133 is believed to promote CREB-dependent transcription by allowing CREB to interact with the transcriptional coactivator CREB-binding protein (CBP). Previous studies have established that the domain encompassing Ser133 on CREB, known as the kinase-inducible domain (KID), interacts specifically with a short domain in CBP termed the KIX domain and that this interaction depends on the phosphorylation of Ser133. In this study, we adapted a recently described Escherichia coli-based two-hybrid system for the examination of phosphorylation-dependent protein-protein interactions, and we used this system to study the kinase-induced interaction between the KID and the KIX domain. We identified residues of the KID and the KIX domain that are critical for their interaction as well as two pairs of oppositely charged residues that apparently interact at the KID-KIX interface. We then isolated a mutant form of the KIX domain that interacts more tightly with wild-type and mutant forms of the KID than does the wild-type KIX domain. We show that in the context of full-length CBP, the corresponding amino acid substitution resulted in an enhanced ability of CBP to stimulate CREB-dependent transcription in mammalian cells. Conversely, an amino acid substitution in the KIX domain that weakens its interaction with the KID resulted in a decreased ability of full-length CBP to stimulate CREB-dependent transcription. These findings demonstrate that the magnitude of CREB-dependent transcription in mammalian cells depends on the strength of the KID-KIX interaction and suggest that the level of transcription induced by coactivator-dependent transcriptional activators can be specified by the strength of the activator-coactivator interaction.
Environmental stimuli induce changes in gene expression by activating intracellular signaling pathways that are comprised of kinase cascades which culminate in the phosphorylation and activation of critical transcription factors within the nucleus. The cyclic AMP (cAMP) response element binding protein (CREB) is one of the best characterized transcription factors whose activity is regulated by phosphorylation in response to extracellular signals. Stimuli that activate receptor tyrosine kinases and those that increase either intracellular Ca2+ or intracellular cAMP can all activate CREB by inducing CREB phosphorylation at a specific residue, Ser133 (11, 20, 24, 53). This phosphorylation event is required for CREB to activate transcription in a heterologous system (24) as well as from endogenous promoters that bear CREB-binding sites (CREs) within their upstream regulatory regions (54, 57).
Ser133 lies within the kinase-inducible domain (KID) of CREB, a domain of approximately 60 amino acids (positions 100 to 160) that is critical for the activation of CREB in response to extracellular stimuli (23, 49). The phosphorylation of CREB at Ser133 leads to association of CREB via its KID with a coactivator protein termed the CREB-binding protein (CBP) (9) and CBP is required for extracellular stimulus-induced transcription of CRE-dependent reporter genes (2, 32).
Given the importance of the interaction of phosphorylated CREB with CBP for the induction of CREB-dependent gene expression, considerable effort has been directed towards characterization of the CREB-CBP interaction and the role of CBP in transcriptional activation. The region of CBP that is required for binding to the phosphorylated KID of CREB is a short 94-amino-acid segment of CBP termed the KIX domain (45). The recently solved nuclear magnetic resonance structure of the KIX domain bound to the phosphorylated KID (50) has suggested that the kinase-induced KID-KIX interaction is stabilized by both hydrophobic interactions formed between a helix of KID and a pocket of the KIX domain and electrostatic interactions formed between specific charged residues in the KID and specific charged residues in the KIX domain. However, functional analysis of the proposed interactions has been limited.
It is not clear if CBP recruitment to CREB represents the only stimulus-dependent step that is required for CREB-dependent transcription, or if extracellular stimuli that activate CREB regulate CREB-dependent transcription by targeting additional sites on CBP or on CBP-associated proteins. How CBP activates transcription when recruited to the promoters of target genes by CREB or other transcription factors is also not yet clear. CBP interacts both directly and indirectly with elements of the basal transcription machinery, including TFIIB and the polymerase II (Pol II) RNA polymerase (RNAP) (33, 41), suggesting that one function of CBP may be to bring the Pol II transcription complex to the promoter. Consistent with this possibility is the finding that phosphorylation of CREB at Ser133 leads to the recruitment of Pol II to CREB in vitro and in vivo (29, 42). CBP also possesses an intrinsic histone acetyltransferase (HAT) activity (3, 43) and can associate directly with other HAT- containing proteins (64), suggesting that CBP recruitment to the promoter may contribute to transcriptional activation by remodeling chromatin structure in the vicinity of the target gene. In addition to binding to CREB, CBP associates with a large number of stimulus-dependent as well as stimulus-independent transcription factors (for a review, see reference 22). A critical and as yet unanswered question is how the activities of CBP may be modulated so that various CBP-regulated genes are expressed at the appropriate levels in specific cell types.
Here we describe the use of a bacterial two-hybrid system to examine the phosphorylation-dependent protein-protein interaction of the CREB KID and the CBP KIX domain. Using this assay, we identify residues in both the KID and the KIX domain that are critical for the interaction, and we provide genetic evidence that a pair of oppositely charged residues participate in a critical electrostatic interaction at the protein-protein interface. We then adapt the Escherichia coli system for use in a selection-based assay and, using this selection, we identify a novel KIX domain mutant that interacts more strongly with the KID than does the wild-type KIX domain. We introduce into the KIX domain of full-length CBP substitutions that either strengthen or weaken the KID-KIX interaction, and we show that the strength of binding of the KIX domain to the KID correlates with the ability of CBP to activate CREB-dependent transcription in mammalian cells. Finally, we use these CBP mutants to present evidence that recruitment of CBP to CREB may be sufficient for transcriptional activation.
MATERIALS AND METHODS
Plasmids.
Plasmid pBRα-KID is a derivative of the previously described pBRαLN vector (24) and encodes residues 1 to 248 of the α-subunit of E. coli RNAP followed by three alanine residues fused to residues 100 to 170 of rat α-CREB. Plasmid pACλcI-KIX is a derivative of the previously described pACλcI32 vector (27) and encodes λcI residues 1 to 236 followed by three alanine residues fused to residues 574 to 686 of murine CBP. Point mutants of α-KID and cI-KIX were generated by PCR.
To generate inducible bacterial expression vectors for the mammalian kinases, the region coding for the protein kinase A α (PKA-α) catalytic subunit (36), the region coding for residues 1 to 291 of murine CaMKII (47), or the region coding for residues 1 to 313 of murine CaMKIV (either wild type or E75K) (39) was amplified by the PCR and cloned downstream of a lacUV5 promoter derivative (5P2) to generate, respectively, the vectors 5P2/PKA, 5P2/CaMKII, and 5P2/CaMKIV. A fragment from each of these vectors containing both the lac promoter and the downstream kinase coding region was then cloned into the pACλcI-KIX vector to yield the vectors pACλcI-KIX/PKA, pACλcI-KIX/CaMKII, and pACλcI-KIX/CaMKIV.
Plasmid pBRstar encodes residues 1 to 248 of the α-subunit of E. coli RNAP followed by three additional alanine residues. This vector confers resistance to tetracycline and is a derivative of pALTER-1 (Promega) that contains the modified rpoA gene and control region from pBRαLN. For use in the carbenicillin selection experiments, plasmid pBRstarα-KID was made by cloning the appropriate fragment from pBRα-KID into pBRstar. The PCR was then used to clone 11 mutants of the KID (at positions 137 or 138) into pBRstar from vector pLacVP16-CREB (55).
To make the selection strain, a plasmid was first assembled containing the λ operator, OR2, centered 62 bp upstream of a modified lac promoter driving expression of the bla gene (from pBR322) followed by a portion of the lacZ gene. This plasmid, pFWO62SD+bla, was subsequently used to transfer this reporter construct to an F′ episome (see below).
The hemagglutinin (HA)-CBP mammalian expression vector is derived from pRc/RSV and contains full-length murine CBP tagged at its C terminus with the HA epitope. GAL4-CREBΔLZ and GAL4-Myb have both been previously described (46, 53). The GAL4-luciferase reporter (1) consists of a luciferase gene driven by five GAL4 binding sites upstream of the E1b TATA box. Point mutations in HA-CBP were generated by using the QuickChange PCR system (Stratagene).
Reporter strains.
Quantitative two-hybrid interaction analyses were performed by using the previously described E. coli reporter strain KS1 (14). Growth conditions and β-galactosidase assays were as described previously (14). Selection strain US3F′3.1 was made in two steps. First, strain CSH100 was transformed with plasmid pFWO62SD+bla, and the promoter-bla-lacZ fusion was recombined onto an F′ episome and mated into strain FW102 (60). Second, the resulting F′ was mated into E. coli strain US3recA− to create US3F′3.1.
Cell culture and transfections.
Human embryonic kidney HEK293 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (FCS), glutamine (2 mM), and antibiotics. Transfections were performed with calcium phosphate–HEPES-buffered saline and were carried out within 24 h of plating with 0.5 μg of GAL4-CREB (or GAL4-Myb), 1.0 μg of HA-CBP, and 0.25 μg of GAL4-luciferase per well. Approximately 22 h after transfection, cells were stimulated with 300 μM 8-(4-chlorophenylthio) adenosine 3′:5′-cAMP (CPT-cAMP) (Sigma) and harvested 5 to 7 h later for luciferase assay (Promega).
Protein analysis.
To examine fusion protein expression levels and the extent of α-KID phosphorylation in the E. coli cells, an aliquot of the same bacterial culture used for the β-galactosidase (β-Gal) assay (post isopropyl-β-d-thiogalactopyranoside [IPTG] induction) was boiled in Laemmli sample buffer and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blotting was carried out as previously described (4, 21). The antibodies directed against Ser133-phosphorylated CREB and total CREB have been described previously (21), and the anti-KIX antibody was purchased from Santa Cruz Biotechnology.
RESULTS
Experimental system for studying phosphorylation-dependent protein-protein interactions.
To characterize the nature of the KID-KIX interaction and identify the structural features within the KID and KIX domain that are critical for their interaction, we developed a novel method that facilitates the analysis of phosphorylation-dependent protein-protein interactions in E. coli.
The yeast two-hybrid assay (19) has been used extremely effectively to study protein-protein interactions in a variety of ways (37). However, the yeast system has not been particularly useful for examining the effect of phosphorylation on mammalian protein-protein interactions, because many mammalian proteins are constitutively phosphorylated in yeast due to the presence of yeast protein kinases that are homologous to mammalian protein kinases (17, 25, 44). To circumvent this limitation of the yeast two-hybrid system, we adapted a recently developed E. coli two-hybrid system to study phosphorylation-dependent protein-protein interactions. This seemed a reasonable approach because previous studies have shown that components of mammalian signal transduction cascades are unphosphorylated when expressed in E. coli (30), presumably due to the absence in these bacteria of serine/threonine and tyrosine kinases that mimic the effects of mammalian protein kinases (66).
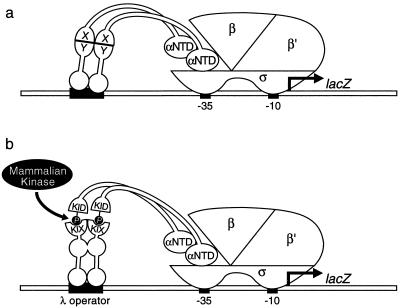
The E. coli two-hybrid system is based on the observation that any sufficiently strong protein-protein interaction can activate transcription in E. coli, provided one of the interacting components is tethered to the DNA via a DNA-binding domain and the other is tethered to a subunit of RNAP (13, 14). Furthermore, previous work has shown that the strength of the protein-protein interaction determines the magnitude of the activation (14), an observation that is consistent with the notion that these arbitrarily selected protein-protein interactions function by stabilizing the binding of RNAP to the promoter. In this two-hybrid system, one of the protein domains to be tested is fused to the bacteriophage λ cI protein (or to another sequence-specific DNA-binding protein), while the other protein domain under investigation is fused to the α or ω subunit of the bacterial RNAP. Compatible plasmids directing the synthesis of the λ cI and the α (or ω) fusion proteins are introduced into a suitable E. coli strain containing a test promoter driving the expression of a linked reporter gene (e.g., the lacZ gene) (Fig. 1a). The test promoter bears a λ cI binding site (OR2) in the upstream regulatory region, so that if the two fused protein domains can interact, the λ cI fusion protein will stabilize the binding of RNAP (containing the α fusion protein) to the test promoter, thereby stimulating expression of the reporter gene. The level of reporter gene expression, which reflects the strength of the protein-protein interaction, can be assayed either quantitatively, using a liquid β-Gal assay, or qualitatively by examining colony color on indicator medium containing the chromogenic substrate X-Gal (5-bromo-4-chloro-3-indolyl-β-d-thiogalactopyranoside).
FIG. 1.
Schematic of E. coli two-hybrid system. (a) Interaction between protein domains X and Y activates transcription in E. coli. Two proteins to be studied, X and Y, are fused to the α-subunit of RNA polymerase and the λ cI repressor, respectively. These two fusion proteins are introduced into bacteria harboring a lacZ reporter gene under the control of a promoter with a λ cI binding site (OR2) upstream of its −10 and −35 regions. If the two proteins to be tested (X and Y) interact, the binding of RNA polymerase to the promoter is stabilized, resulting in increased transcription of the lacZ gene. The strength of the protein-protein interaction correlates with the level of lacZ expression, which can be measured with either quantitative or qualitative β-galactosidase assays. (b) Interaction between the KID of CREB and the KIX domain of CBP activates transcription in E. coli. Depicted are the α-KID and λ cI-KIX fusion proteins. When the α-KID fusion protein is phosphorylated at Ser133 of the KID moiety by a constitutively active mammalian kinase, the KID and the KIX moieties can interact, resulting in increased transcription from the test promoter and increased expression of the lacZ reporter gene.
Generation of expression vectors for analyzing the KID-KIX interaction in E. coli.
To determine if the E. coli two-hybrid system could be employed to study phosphorylation dependent protein-protein interactions in general and the KID-KIX interaction in particular, the KID and KIX domain were fused, respectively, to the α-subunit of RNAP and the bacteriophage λ cI protein (Fig. 1b). The first fusion protein, termed α-KID, contains the KID of CREB (amino acids 100 to 170) fused via an alanine linker at its N terminus to amino acids 1 to 248 of the α-subunit of the E. coli RNAP. The second fusion protein, termed cI-KIX, contains the KIX domain of CBP (amino acids 574 to 686) fused via an alanine linker at its N terminus to amino acids 1 to 236 of the λ cI protein. The chimeric genes encoding α-KID and cI-KIX were cloned into bacterial expression vectors, and the vector encoding the cI-KIX fusion protein also contained a gene encoding the catalytic subunit of hamster PKA under the control of an IPTG-inducible promoter.
Inducible phosphorylation of CREB at Ser133 by PKA in E. coli.
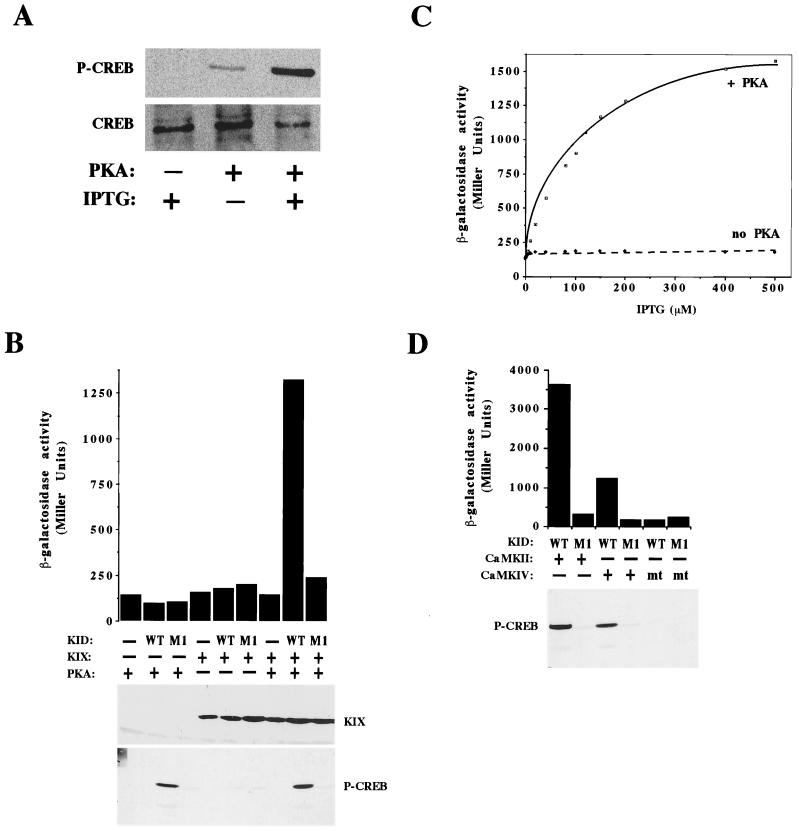
We first asked whether a mammalian kinase could phosphorylate the CREB KID at Ser133 in E. coli cells. As shown in Fig. 2A, the use of an antibody specific for Ser133-phosphorylated CREB (21) indicated that in the absence of PKA, the α-KID fusion protein is not phosphorylated at Ser133. However, induction of PKA gene expression by exposure of the bacteria to IPTG resulted in the phosphorylation of α-KID at Ser133. The increase in α-KID detection by the anti-phospho-Ser133 antibody was not due simply to the induction of α-KID gene expression (lower panel), since Western blotting with an antibody that recognized CREB regardless of its state of phosphorylation revealed that exposure of the E. coli cells to IPTG did not significantly affect the level of α-KID in these cells. Thus, we conclude that PKA can phosphorylate the α-KID fusion protein at Ser133 in E. coli cells.
FIG. 2.
Mammalian kinases induce the phosphorylation of Ser133 of CREB in E. coli and induce a Ser133-dependent KID-KIX interaction in E. coli. (A) The E. coli reporter strain KS1 was cotransformed with the α-KID expression vector and another vector encoding either PKA (lanes 2 and 3) or no kinase (lane 1). In this experiment, the PKA vector, termed 5P2/PKA, does not carry the gene encoding the cI-KIX fusion (see Materials and Methods). Cells were induced with 50 μM IPTG as indicated, grown to log phase, and lysed. Samples were analyzed by Western blotting with either an antibody that recognizes Ser133-phosphorylated CREB (P-CREB [top panel]) or an antibody that recognizes CREB regardless of its phosphorylation state (bottom panel). (B) The E. coli reporter strain KS1 was cotransformed with expression vectors encoding α-KID, cI-KIX, and PKA as indicated. WT, wild-type KID; M1, α-KID with the S133A substitution. Bacteria were induced with 100 μM IPTG, grown to log phase, and harvested for either β-Gal assay (top) or Western blotting (bottom). Western blotting was performed with either an antibody that recognizes the KIX domain (upper panel) or the anti-CREB-P-Ser133 antibody (lower panel). (C) KS1 bacteria were cotransformed with vectors encoding α-KID (wild-type), cI-KIX, and either PKA (solid line) or no kinase (dashed line). Bacteria were induced with various concentrations of IPTG as indicated, grown to log phase, and harvested for β-Gal assay. (D) The E. coli reporter strain KS1 was cotransformed with expression vectors encoding cI-KIX, α-KID (either wild type or M1), and constitutively active forms of either CaMKII or CaMKIV as indicated. For CaMKIV, a kinase-inactive mutant form (mt) containing the K75E substitution was also tested. Bacteria were induced with 100 μM IPTG, grown to log phase, and harvested for either β-galactosidase assay (top) or Western blotting with the anti-CREB-P-Ser133 antibody (bottom).
We next examined if the phosphorylation of α-KID induced an interaction of α-KID with the CBP KIX domain in E. coli by monitoring the level of lacZ reporter gene transcription as reflected in the intracellular levels of β-Gal (14). As shown in Fig. 2B, E. coli cells containing the α-KID and cI-KIX fusion proteins exhibited a marked increase in lacZ gene expression in the presence of PKA over that detected in the absence of PKA. The induction of lacZ gene expression required the presence of cI-KIX and α-KID as well as PKA. Furthermore, the induction of lacZ gene expression was dependent on the presence of a serine at position 133 of the KID, because substitution of this residue with an alanine (the M1 mutant) abolished this induction even in the presence of PKA. In addition, in the presence of PKA, increasing concentrations of IPTG led to a dose-dependent increase in β-Gal activity (Fig. 2C). In contrast, in the absence of PKA, even the highest concentration of IPTG failed to elicit any detectable increase in β-Gal activity. At the highest concentration of IPTG, the level of β-Gal activity was nearly eight-fold higher in the presence of PKA than in its absence. Taken together, these experiments indicate that in the presence of PKA, α-KID becomes phosphorylated at Ser133, leading to its interaction with cI-KIX and a concomitant increase in lacZ gene expression.
Other mammalian kinases that target Ser133 of CREB phosphorylate the KID in E. coli and induce a KID-KIX interaction.
To determine if the E. coli system might also be useful for studying phosphorylation events that are mediated by kinases other than PKA, several calcium/calmodulin-dependent kinases (CaMKs) that are known to phosphorylate CREB at Ser133 were tested for their abilities to promote the KID-KIX interaction in E. coli. Although two of these kinases, CaMKII and CaMKIV, have been shown to phosphorylate CREB at Ser133 in vitro as well as in mammalian cells (11, 16, 35, 53, 56), the ability of these kinases to induce a CREB-CBP interaction has not been well characterized. Versions of CaMKII or CaMKIV that are rendered constitutively active due to deletion of a C-terminal inhibitory domain (10, 47) were introduced into E. coli along with α-KID. As shown in Fig. 2D, the presence of either enzymatically active CaMKII or CaMKIV resulted in the phosphorylation of the α-KID protein at Ser133 in E. coli cells and led to an interaction of the KID with the KIX domain. Introduction of a mutant CaMKIV that is specifically defective in its ability to phosphorylate its substrates did not induce phosphorylation of CREB at Ser133 and did not lead to a KID-KIX interaction. We conclude that kinases that target Ser133 of CREB in mammalian cells can also target Ser133 of CREB in E. coli cells. Furthermore, we show that phosphorylation of the KID at Ser133 by these kinases induces an interaction of the KID with the KIX domain.
Taken together, these findings establish the E. coli two-hybrid system as a useful approach for examining phosphorylation-dependent protein interactions and the phosphorylation-dependent KID-KIX interaction in particular. We find that the interaction of the KID and KIX domain in the E. coli system requires that the same conditions be fulfilled as previously demonstrated for the KID-KIX interaction in mammalian cells. The induction of reporter gene expression in E. coli cells depends on the presence of both the KID and KIX domain, a kinase that will phosphorylate Ser133, and an intact phosphoacceptor site at Ser133.
Electrostatic interactions stabilize the KID-KIX complex.
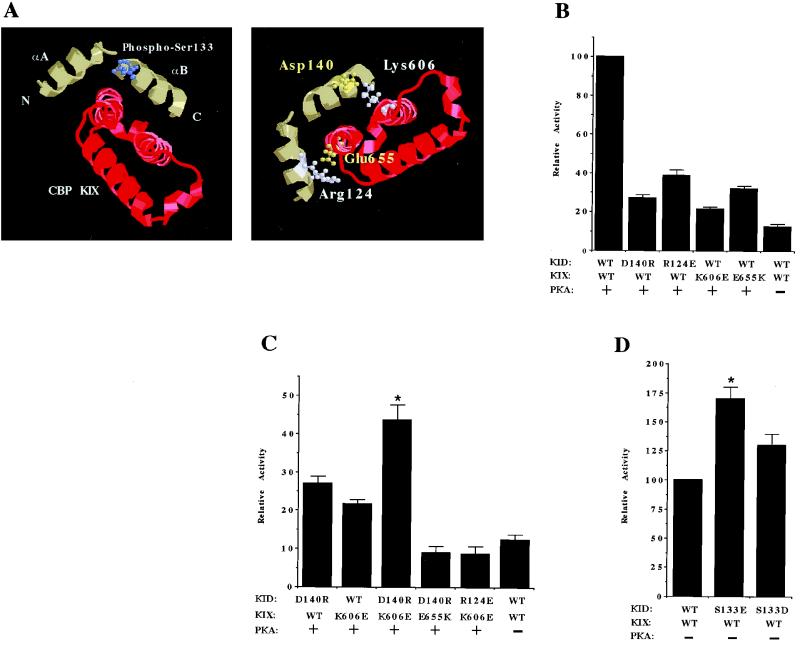
Having established the feasibility of the E. coli two-hybrid system for studying the phosphorylation-dependent KID-KIX interaction, we next applied this technique to probe the structural features of the KID and the KIX domain that are critical for their association. The structural data suggest that upon phosphorylation and association with the KIX domain, the KID forms a highly ordered structure comprised of two α-helices which form a kink close to the phosphorylated Ser133 position (50). The more C-terminal helix, termed αB, stretches approximately from residues 133 to 144 and docks with a hydrophobic pocket created by two of the α-helices in the CBP KIX domain. The structural evidence also indicates that electrostatic interactions may be important for stabilizing the association between the αB helix of KID and the KIX domain. In particular, it has been proposed that within the KID there are two regions of electrostatic interactions that contribute to the stability of the KID-KIX complex (50). One region includes Asp140 and Asp144 of the KID, which have been proposed to interact with Lys606 and His602 of the CBP KIX domain, respectively (50) (Fig. 3A). The second region includes Arg124 of the KID, which has been proposed to interact with Glu655 of the KIX domain (50). We hypothesized that if these electrostatic interactions are important for the KID-KIX interaction, then reversing the charge of either residue in a pair should disrupt the interaction. As shown in Fig. 3B, replacing Asp140 with an Arg (D140R) or replacing Arg124 with a Glu (R124E) significantly reduced the PKA-induced KID-KIX interaction in E. coli. Similarly, in CBP, replacing Lys606 with a Glu (K606E) or replacing Glu655 with a Lys (E655K) greatly reduced the PKA-induced KID-KIX interaction. These results demonstrate that amino acid residues 124 and 140 in the KID and residues 606 and 655 in the KIX domain are each important for the KID-KIX interaction.
FIG. 3.
Electrostatic interactions between residues in the KID and the KIX domain contribute to the stability of the KID-KIX complex. (A [left panel]) Ribbon diagram based on the nuclear magnetic resonance-derived solution structure of the Ser133-phosphorylated KID (green α-helices) bound to the KIX domain (red α-helices) (50). The N and C termini of the KID are indicated, as are the αA and αB helices. The phosphorylated Ser133 residue is shown in blue. (Right panel) Residues of the KID and KIX domain proposed to participate in electrostatic interactions. One proposed region of interaction involves potential salt-bridge contacts between the side chain of Arg 124, located within the αA helix of the KID, and the side chain of Glu655 of the KIX domain. A separate, distinct region of interaction involves putative electrostatic contacts between the side chain of Asp140, located within the αB helix of the KID, and the side chain of Lys606 of the KIX domain. Acidic (negatively charged) residues are depicted in yellow, and basic (positively charged) residues are depicted in white. Not shown are the side chains of two other residues, Asp144 (in the KID αB helix) and His602 (in KIX), that have also been proposed to contribute stabilizing electrostatic interactions to the KID-KIX complex (in the same region as the Asp140-Lys606 contacts). (B) Expression vectors encoding either wild-type (WT) or mutant versions of α-KID and cI-KIX (bearing the indicated substitutions) were cotransformed into the E. coli reporter strain KS1. The cI-KIX vector either did or did not also encode PKA. Cells were induced with IPTG, grown to log phase, and harvested for β-Gal assay. Numbers are plotted as percentages of the wild-type KID-KIX interaction in the presence of PKA and represent the means of at least four independent experiments (error bars represent standard errors). (C) Charge-swap substitutions in the KID and KIX domain can suppress charge-swap substitutions in the partner protein. Mutant versions of α-KID and cI-KIX (bearing the indicated substitutions) were introduced into the E. coli reporter strain KS1 as described in panel A. Numbers are plotted as percentages of the wild-type KID-KIX interaction in the presence of PKA and represent the means of at least two independent experiments (error bars represent standard errors). The asterisk indicates P < 0.0005 (analysis of variance for D140R/K606E versus D140R/WT and D140R/K606E versus WT/K606E). (D) Acidic amino acids at residue 133 in the KID enhance interaction with the KIX domain in the absence of PKA. An expression vector encoding wild-type cI-KIX (but no PKA) was cotransformed into KS1 bacteria with a vector encoding either wild-type or mutant α-KID as indicated. Data are plotted as percentages of the wild-type KID-KIX interaction in the absence of PKA and represent the means from at least two independent experiments (error bars represent standard errors). The asterisk indicates P < 0.005 (analysis of variance for 133E/WT versus WT/WT).
However, these charge-swap experiments do not directly address the question of whether these charged amino acid residues in the KID are critical because they form electrostatic interactions with complementary charged residues in the KIX domain. To address this question, we attempted to suppress the effect of the charge-swap substitution at residue 124 or residue 140 in the KID by swapping the charge of the proposed partner amino acid residue in the KIX domain (either residue 655 or residue 606, respectively). If two charged residues are participating in an electrostatic interaction, then substitution of one of the residues with an oppositely charged residue should inhibit binding, but this inhibition could in principle be rescued, or suppressed, by the complementary charge-swap substitution of the partner residue (61). In contrast, if the two residues are not part of an electrostatic pair, then it is unlikely that the effect of altering the first residue will be suppressed by a compensatory alteration of the second residue.
We therefore tested the abilities of the KID D140R and R124E mutants to interact, respectively, with the KIX domain K606E and E655K mutants. As shown in Fig. 3C, the KID D140R and KIX domain K606E mutants interacted more efficiently with one another than either mutant interacted with a wild-type partner. Similar results were obtained with the KID R124E and KIX domain E655K mutant pair, although in this case, the compensatory effect of the two substitutions was more modest (data not shown). As controls, we examined the ability of the KID D140R mutant to interact with the KIX domain E655K mutant and the ability of the KID R124E mutant to interact with the KIX domain K606E mutant. We found that each of these mutant pairs interacted much less efficiently with one another than either mutant interacted with the wild-type partner (Fig. 3B and C). Taken together, these findings provide strong genetic evidence that electrostatic interactions occur between Asp140 in the KID and Lys606 in the KIX domain and between Arg124 in the KID and Glu655 in the KIX domain and that these interactions play an important role in stabilizing the KID-KIX complex.
Negative charge at position 133 of the KID contributes to the stability of the KID-KIX complex.
We next investigated how the phosphorylation of Ser133 functions to stabilize the KID-KIX interaction, and, in particular, if the negative charge of the phosphate group plays an important role. Although previous studies have shown that substitution of Ser133 with an acidic amino acid residue is not sufficient to lead to CREB-dependent transcription (23), the effect of this substitution on the KID-KIX interaction is not known. To address this issue, we replaced Ser133 with either a glutamic or aspartic acid residue and tested whether these KID mutants interacted with the KIX domain in E. coli. As shown in Fig. 2B, in the absence of PKA, wild-type KID does not interact detectably with the KIX domain; however, when Ser133 is replaced with a glutamic acid residue, an interaction of the KID with the KIX domain could be detected (Fig. 3D). Likewise, substitution of Ser133 with an aspartic acid residue led to a detectable interaction between the KID and the KIX domain in the absence of PKA, although this effect was smaller than when Ser133 was replaced with a glutamic acid residue. Although the KID in which Ser133 was replaced with a glutamic acid residue was capable of interacting with the KIX domain, the strength of interaction was significantly less than that of the wild-type KID and KIX domain in the presence of PKA (<25%). This may explain why the substitution of Ser133 with a glutamic acid or aspartic acid residue failed to generate a form of CREB that functions as a constitutively active transcription factor. Although a glutamic acid residue cannot fully mimic the effect of the phosphorylated serine at position 133, our results suggest that the negative charge at this position does contribute significantly to the ability of the KID to interact with the KIX domain.
Role of hydrophobic residues within the KID αB helix in stabilizing the KID-KIX complex.
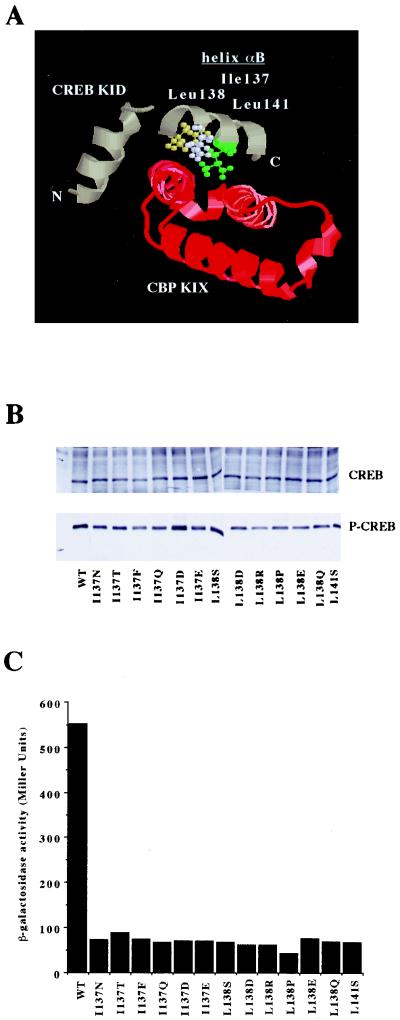
We also used the E. coli two-hybrid system to address the role of hydrophobic interactions in stabilizing the KID-KIX complex. As shown in Fig. 4A, the surface of the KID αB helix that contacts the KIX domain hydrophobic pocket is formed primarily by three residues: Ile137, Leu138, and Leu141 (50). Previous investigations had identified mutations in the corresponding codons that resulted in disruption of the KID-KIX interaction in yeast (55). However, it remained possible that the disruption of the interaction was a secondary effect caused by the inability of the KID mutants to be phosphorylated at Ser133 in the yeast cells. To examine whether the alteration of Ile137, Leu138, or Leu141 disrupts the KID-KIX interaction in the E. coli system, we introduced 13 of these previously identified hydrophobic amino acid substitutions into the KID moiety of the α-KID fusion protein. As shown in Fig. 4B, all 13 α-KID mutants were phosphorylated at Ser133 when introduced together with PKA into E. coli cells, demonstrating that the substitutions did not interfere with substrate recognition by the kinase. However, when introduced together with both the λ cI-KIX fusion protein and PKA, none of the 13 α-KID mutants was able to induce reporter gene expression (Fig. 4C). Taken together, these findings confirm and extend the previous observations and strongly suggest that hydrophobic residues within the αB helix are required for the Ser133-phosphorylated KID to interact with the KIX domain.
FIG. 4.
Helix αB mutations in the KID do not affect PKA-induced Ser133 phosphorylation, but do block the KID-KIX interaction in E. coli. (A) Ribbon diagram of the Ser133-phosphorylated KID (green α-helices [N and C termini are labeled]) bound to the KIX domain (red α-helices) (50). Shown are the side chains of three hydrophobic residues within the KID αB helix—Ile137 (white), Leu138 (yellow), and Leu141 (green)—that have been proposed to interact with the hydrophobic pocket formed by the α-helices of the KIX domain. (B) α-KID expression vectors encoding either wild-type KID (WT) or 1 of 13 separate mutant derivatives bearing substitutions within the αB helix (at either residue 137, 138, or 141) were cotransformed along with a vector encoding PKA into the E. coli reporter strain KS1. Cells were induced with 40 μM IPTG, grown to log phase, and harvested for Western blotting with either an antibody that recognizes total CREB (upper panel) or the anti-CREB–P-Ser133 antibody (lower panel). (C) Each of the 13 α-KID mutants or wild-type α-KID was introduced along with cI-KIX and PKA into the reporter strain KS1. Cells were induced with 50 μM IPTG, grown to log phase, and harvested for the β-Gal assay.
Selection-based generation of KIX domain mutants.
To facilitate the analysis of the mechanism by which CREB and CBP activate transcription in mammalian cells, we sought to identify additional KIX domain mutants that could interact with mutant forms of the KID. Specifically, we wished to identify suppressor KIX domain mutants that might bind to the KID mutants bearing substitutions at the positions of the critical hydrophobic residues in the αB helix (I137, L138, and L141). To permit the efficient isolation of such mutants, we modified the E. coli two-hybrid system so that the test promoter directed transcription of both a selectable gene (the bla gene, which encodes the β-lactamase protein) and the lacZ gene (Fig. 5A). In this case, the activation of reporter gene transcription results in increased expression of the bla gene, rendering the cells resistant to ampicillin. Any ampicillin-resistant clones can then also be assayed qualitatively (with indicator medium) and quantitatively (by liquid β-Gal assay) for lacZ expression.
FIG. 5.
Identification of a KIX domain mutant that binds a KID helix αB mutant. (A) Schematic of the reporter system used for the carbenicillin selection. The promoter region containing the λcI binding site (OR2) as well as the α and cI fusion proteins (here indicated as α-X and cI-Y) are identical to those used in the original reporter system (Fig. 1). In this case, however, the bla gene is inserted directly downstream of the promoter region. If the protein domains X and Y interact, the bacteria harboring these two fusion proteins will express the bla gene at a higher level and will be resistant to higher levels of carbenicillin. Because the lacZ gene is expressed cocistronically with the bla gene, these bacteria will also contain increased levels of β-Gal activity. (B) PKA-induced KID-KIX interaction confers carbenicillin resistance on host E. coli. The bacterial reporter strain US3F′3.1 was cotransformed with an expression vector encoding the wild-type α-KID fusion protein and a second vector encoding the wild-type cI-KIX fusion protein either together with PKA (solid squares) or without PKA (open squares). The graph shows the number of bacterial colonies remaining on an LB agar plate at different concentrations of carbenicillin (in the presence of 100 μM IPTG). (C) A library of cI-KIX expression vectors containing the randomly mutagenized KIX domain was transformed into a pooled mix of bacterial reporter strain US3F′3.1 cells containing 1 of 11 different α-KID mutants bearing substitutions in helix αB (see Materials and Methods). The cI-KIX expression vector also encoded PKA. These bacteria were then plated on carbenicillin-containing plates, and 24 colonies that grew were picked as potential positives. Plasmid DNA was then isolated from 14 of these 24 colonies and used to retransform strain US3F′3.1, and the KID-KIX interaction was assessed by β-Gal assay. Also included as controls in the β-Gal assay were US3F′3.1 bacteria transformed with both wild-type α-KID and wild-type cI-KIX in either the presence (+) or absence (−) of PKA.
We first tested whether the interaction between the wild-type forms of the KIX domain and the KID was sufficiently strong to permit bacteria harboring the cI-KIX and α-KID fusion proteins to tolerate higher concentrations of carbenicillin in the presence of PKA than in its absence. As shown in Fig. 5B, at the two highest concentrations of carbenicillin tested (800 and 1,000 μg/ml), the number of colonies obtained with cells containing the kinase (PKA) was approximately 6 orders of magnitude greater than the number of colonies obtained with cells lacking the kinase.
To identify mutants of the KIX domain that can interact with the KID mutants, the gene fragment encoding the KIX domain of the cI-KIX fusion protein was mutagenized by random PCR mutagenesis. We transformed E. coli cells containing the bla reporter gene construct with a pool of 11 of the KID helix αB mutants, so that each bacterium was transformed with a single α-KID mutant. These bacteria were then transformed with a library of randomly mutagenized cI-KIX hybrid genes representing approximately 3 × 105 independent clones. As in the previous experiments, the vector bearing the mutagenized cI-KIX gene also directed the synthesis of the catalytic subunit of PKA. The bacteria were then plated on Luria-Bertani (LB) agar containing 1,000 μg of carbenicillin per ml and induced with IPTG. From an initial screen of approximately 5 × 106 transformants, we identified 24 colonies which grew within 24 h of plating on carbenicillin. Of the original 24 candidates, 10 failed subsequently to grow in liquid media, suggesting that they were false positives. The DNA was isolated from each of the remaining 14 positives and retransformed into bacteria harboring the identical bla and lacZ reporter genes, and the interaction between the mutant KID and KIX domain was measured with the liquid β-Gal assay. As shown in Fig. 5C, one of the secondary transformants (that containing DNA from positive clone 1) displayed a level of β-Gal activity that was significantly higher than that of the other secondary transformants. We retained this clone for detailed characterization.
Identification of a hyperactive KIX domain mutant.
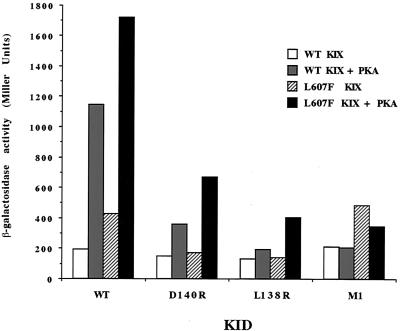
Having isolated the α-KID and cI-KIX plasmids from clone 1, we confirmed that the mutant phenotype mapped to restriction fragments encoding the KID and KIX domain of the fusion proteins. DNA sequencing revealed that the gene fragment encoding the mutant KIX domain moiety contained a single base pair substitution resulting in a Leu-to-Phe substitution at position 607 (L607F) and that the interacting partner was α-KID mutant L138R. To test the specificity of the effect of the KIX domain L607F substitution, the KIX domain L607F mutant was analyzed for its ability to interact with each of the 11 KID hydrophobic mutants with changes at Ile137 or Leu138. The L138R mutant was the only KID mutant that interacted strongly with the KIX domain L607F mutant (data not shown). However, in addition to its ability to interact effectively with the KID L138R mutant, the KIX domain L607F mutant also interacted well with the wild-type KID. Notably, as shown in Fig. 6, in the presence of PKA, the KIX domain L607F mutant was more effective than the wild-type KIX domain in interacting with the wild-type KID or several mutant KIDs (e.g., the charge-swap mutant D140R and the Ser133-to-Ala [M1] mutant). In the case of the wild-type KID and the M1 mutant, the increase also was observed in the absence of PKA, although the interaction of KIX L607F with wild-type KID was still strongly stimulated by the kinase. We conclude that the KIX domain L607F mutant binds more strongly than the wild-type KIX domain to both the phosphorylated and nonphosphorylated wild-type KID.
FIG. 6.
The L607F KIX domain mutant is a hyperactive KID binder. Wild-type (WT) or L607F cI-KIX was introduced into E. coli strain KS1 in either the presence or absence of PKA together with wild-type or mutant forms of α-KID, as indicated. Bacteria were induced with IPTG, grown to log phase, and harvested for the β-Gal assay.
CBP L607F hyperactivates CREB-dependent transcription in mammalian cells.
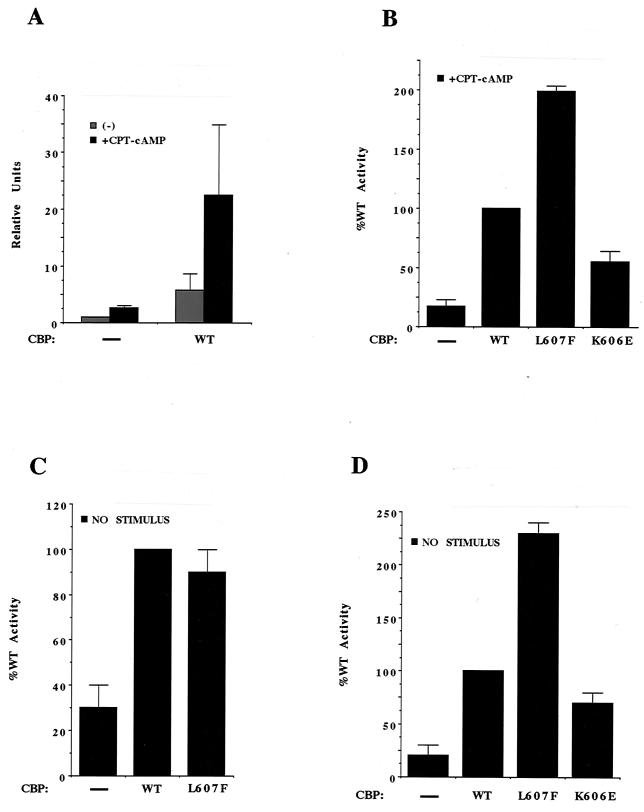
To compare the activity of a CBP containing the L607F substitution with that of wild-type CBP, a gene encoding an HA epitope-tagged variant of the mutant CBP was cloned into a mammalian expression vector. This vector or an otherwise identical vector encoding epitope-tagged wild-type CBP was transfected into HEK293 cells along with a vector encoding GAL4-CREBΔLZ, which contains the GAL4 DNA binding and dimerization domain fused to amino acids 1 to 313 of CREB (lacking the CREB leucine zipper) and a vector bearing a luciferase reporter gene that contains five GAL4 binding sites within its regulatory region. As shown in Fig. 7A, in the absence of cotransfected CBP, the activation of PKA with the cAMP analog CPT-cAMP induced reporter gene expression to a limited extent. However, the cotransfection of wild-type CBP with GAL4-CREBΔLZ led to a substantial increase in both basal and cAMP-induced reporter gene expression, indicating that the amount of CBP available to promote GAL4-CREB-dependent transcription is limiting in these cells. We next examined the ability of the CBP L607F mutant to stimulate reporter gene expression. As shown in Fig. 7B, when compared to wild-type CBP, the CBP L607F mutant was clearly more effective at stimulating GAL4-CREBΔLZ-dependent transcription. The enhanced ability of the CBP L607F mutant to stimulate the activity of GAL4-CREBΔLZ likely reflects the increased ability of the KIX domain bearing this substitution to interact with the CREB KID and suggests that the strength of the KID-KIX interaction may play an important role in determining the level of CREB-dependent transcription.
FIG. 7.
CBP mutants affect CREB-dependent transcription in mammalian cells (A) HEK293 cells were transfected with vectors encoding GAL4-CREBΔLZ and GAL4-luciferase together with either an empty vector or a vector encoding wild-type (WT) HA-CBP. Cells were either unstimulated or stimulated with 300 μM CPT-cAMP for 5 to 7 h and then harvested for luciferase assays. Data are plotted as arbitrary units normalized to the value obtained when empty vector was transfected and cells were not stimulated. The data shown represent the means of three independent experiments performed in triplicate (error bars represent standard errors). (B) 293 cells were transfected with vectors encoding GAL4-CREBΔLZ and GAL4-luciferase together with an empty vector (−) or a vector encoding either wild-type CBP, L607F CBP, or K606E CBP as indicated. Cells were stimulated with CPT-cAMP for 5 to 7 h and then lysed for luciferase assays. Data are plotted as the percentages of the value obtained with wild-type CBP and represent the means of three independent experiments performed in triplicate (error bars represent standard errors [±]). (C) 293 cells were transfected with vectors encoding GAL4-Myb(186–325) and GAL4-luciferase together with an empty vector (−) or a vector encoding either wild-type CBP or L607F CBP as indicated. Cells were left unstimulated and harvested for luciferase assays. Data are plotted as the percentages of the value obtained with wild-type CBP and represent the means of four independent experiments performed in triplicate (error bars represent standard errors [±]). (D) 293 cells were transfected with vectors encoding GAL4-CREBΔLZ and GAL4-luciferase together with an empty vector (−) or a vector encoding either wild-type CBP, L607F CBP, or K606E CBP as indicated. Cells were left unstimulated and harvested for luciferase assays. Data are plotted as the percentages of the value obtained with wild-type CBP and represent the means of four independent experiments performed in triplicate (error bars represent standard errors [±]).
To test further the idea that the strength of the KID-KIX interaction determines the magnitude of CREB- or CBP-dependent transcription, we introduced another KIX domain mutation, K606E, in the context of full-length CBP to generate a form of CBP (CBP K606E) that might be expected to interact with CREB more weakly than does wild-type CBP. As described earlier, the K606E substitution greatly diminishes the ability of the KIX domain to bind to the wild-type KID phosphorylated at Ser133 (Fig. 3B). In contrast to our observation with L607F CBP, introduction of the CBP K606E mutant into HEK293 cells together with GAL4-CREBΔLZ led to a significant reduction in cAMP-induced GAL4-CREB-dependent transcription when compared to the effect of wild-type CBP (Fig. 7B). The differences in the abilities of wild-type CBP, CBP K606E, and CBP L607F to stimulate the activity of GAL4-CREBΔLZ are not due to differences in the amounts of these proteins in the HEK293 cells as determined by Western blot analysis (data not shown). Rather, the level of CBP-mediated transcription correlates with the strength with which each KIX domain variant interacts with the phosphorylated KID in E. coli cells. Preliminary results suggest that the CBP mutants (L607F and K606E) have a similar effect on CRE-dependent transcription (data not shown). It will be critical to determine whether expression of endogenous CREB target genes, such as c-fos (52) and BDNF (54, 57), is differentially affected by the different CBP alleles. Nevertheless, our findings to date suggest that when the amount of CBP is limiting in cells, the level of CREB-dependent gene transcription is determined by the strength of the KID-KIX interaction.
As a control for the specificity of this effect, we examined whether the L607F CBP mutant could increase the activity of another CBP-dependent transcription factor. c-Myb has been shown to interact with CBP via the KIX domain (46). Thus, we asked whether the CBP L607F mutant could lead to increased GAL4-Myb-dependent transcription. As shown in Fig. 7C, endogenous CBP is limiting relative to GAL4-Myb, since the expression of the luciferase reporter gene greatly increases when exogenous, wild-type CBP is introduced together with the GAL4-Myb fusion protein. However, introduction of the CBP L607F mutant with GAL4-Myb does not lead to a further increase in the activation of the GAL4 reporter gene. This indicates that the increase in CREB-dependent transcription mediated by the CBP L607F mutant is not due to a nonspecific increase in CBP function (e.g., an increase in protein stability) and suggests that the effect of the L607F substitution may be specific to CREB and CREB-related proteins.
Recruitment of CBP to CREB is sufficient for transcriptional activation.
While it is well established that active PKA stimulates CREB-dependent transcription by promoting the phosphorylation of CREB at Ser133 and the subsequent recruitment of CBP to the promoters of CREB-regulated genes, it has been unclear whether the recruitment of CBP is sufficient for CREB-dependent transcription or if PKA also stimulates the activity of the CREB-CBP complex by phosphorylating CBP. To address this issue, we used the CBP KIX domain mutants to determine whether in the absence of PKA stimulation the recruitment of CBP to CREB was sufficient to stimulate CREB-dependent transcription. Wild-type or mutant CBPs were introduced into HEK293 cells together with GAL4-CREBΔLZ and the luciferase reporter gene. As shown in Fig. 7D, in the absence of PKA stimulation, the L607F CBP induced significantly more CREB-dependent transcription than wild-type CBP. In contrast, the K606E CBP behaved similarly to wild-type CBP. These results demonstrate that docking of CBP to CREB is sufficient to activate CREB-dependent transcription even in the absence of a PKA-inducing stimulus. Under the conditions of this experiment, anti-phospho-CREB (Ser133) antibody staining revealed that CREB was not detectably phosphorylated on Ser133 (data not shown). While additional stimulus-induced phosphorylation events may enhance the ability of the CREB-CBP complex to activate transcription, these events appear not to be obligatory. We explicitly tested whether or not CREB-dependent transcription depends on a consensus PKA site in CBP that has been implicated in the ability of CBP to mediate the stimulatory effects of other transcription factors in response to cAMP (63). We found that replacement of Ser1772 of CBP (the phosphoacceptor within this consensus PKA site) with an alanine had no effect on the ability of the CBP L607F mutant to stimulate the transcriptional activity of GAL4-CREBΔLZ in response to a PKA-inducing stimulus (data not shown).
DISCUSSION
In this study, we have utilized a recently described E. coli-based two-hybrid assay to investigate the structural basis for the interaction between Ser133-phosphorylated CREB and CBP. We found that interactions between complementary charged residues in the KID and KIX domain are critical for stabilizing their interaction. Moreover, our analysis has resulted in the identification of CBP mutants that have allowed us to address several important unanswered questions regarding the mechanism of CBP action. We have found that the strength of the interaction between the KID and KIX domain plays a critical role in determining the magnitude of the CREB-dependent transcriptional response. Furthermore, we find that recruitment of CBP to CREB can suffice to mediate CREB-dependent transcription.
The strength of the KID-KIX interaction affects the magnitude of CREB-dependent transcription.
Using the E. coli two-hybrid assay, we identified single point substitutions in the KIX domain of CBP that lead to either an increase or a decrease in the affinity of the KIX domain for the wild-type KID of CREB. When placed in the context of full-length CBP, the same substitutions result in either an increase or a decrease in the ability of CBP to activate CREB-dependent transcription in mammalian cells. Specifically, the L607F substitution in the KIX domain strengthens the KID-KIX interaction, and in the context of full-length CBP, the L607F substitution enhances the ability of CBP to promote CREB-dependent transcription. In contrast, the K606E mutation reduces the ability of the KIX domain to bind to wild-type KID and, in the context of full-length CBP, diminishes the ability of CBP to promote CREB-dependent transcription. It will be important in the future to assess the effect on CREB-dependent transcription of additional CBP KIX domain mutations that result in various levels of either enhanced or reduced binding of the KIX domain to the Ser133-phosphorylated KID. However, the simplest interpretation of our findings to date is that alterations in the strength of the KID-KIX interaction have corresponding effects on the CREB-CBP interaction and that the magnitude of CREB-dependent transcriptional activation reflects the strength of the interaction of CREB and CBP.
Our results suggest a mechanism that may explain how CBP functions endogenously to specify the magnitude of activation of different CBP-dependent target genes in response to different stimuli. The strength of the interaction between CBP and a transcription factor bound to the promoter of the target gene would represent a potential level at which target gene expression could be regulated. In the case of CREB, CBP recruitment is regulated by the stimulus-induced phosphorylation of CREB at Ser133. Our findings demonstrate, however, that the CREB-dependent transcriptional response is not saturated even under stimulatory conditions that result in efficient phosphorylation of Ser133 of CREB. That is, our results suggest that even when CREB is phosphorylated at Ser133, CREB-dependent reporter gene expression can be further augmented by increasing the affinity of CBP for phosphorylated CREB. These observations are consistent with the possibility that CREB-dependent gene expression, which depends on the phosphorylation of CREB at Ser133, may be further modulated by additional modifications or factors that either strengthen or weaken the binding of CBP to CREB.
The existence of multiple mechanisms for varying the strength of the interaction of CBP with specific transcription factors would be expected to facilitate the generation of specific responses to stimulatory signals under various conditions and in different cell types. Indeed, it has been shown that the recruitment of CBP to specific promoters is specified not only by the strength of its interaction with a single DNA-bound transcription factor, but also by the strength of its interactions with multiple DNA-bound transcription factors (34). In the well-characterized example of the IFN-β promoter and enhancer, the evidence indicates that CBP is synergistically recruited to the promoter by multiple contacts with several different DNA-bound transcriptional activators and that the ability of these multiple contacts to recruit CBP to the promoter correlates with the rate of IFN-β transcription in vitro (38, 65).
Previous studies have shown that CBP may be a component of the Pol II holoenzyme complex in mammalian cells (8, 42). This suggests that increasing the strength of the KID-KIX interaction may enhance transcription of CREB-dependent target genes by stabilizing the Pol II complex on the promoter. Support for this idea comes from previous studies with yeast demonstrating that the magnitude of transcription induced by a particular transcriptional activator can be modulated by varying the strength of interaction between the activator and particular components of the general transcriptional machinery (18, 48, 62).
Recruitment of CBP to CREB increases CREB-dependent transcription in the absence of stimulus.
We found that the CBP L607F mutant induced greater CREB-dependent transcription in mammalian cells than did wild-type CBP even in the absence of PKA stimulation. Under these conditions, CREB is not detectably phosphorylated at Ser133 (data not shown). The results of these experiments show that recruitment of CBP to CREB is sufficient to activate CREB-dependent transcription and argue that, in this system, stimulation of the PKA signaling pathway with CPT-cAMP serves primarily to phosphorylate CREB at Ser133 and recruit CBP. Two recent studies have similarly shown that recruitment of CBP to CREB is sufficient to induce CREB-dependent transcription even in the absence of an extracellular stimulus (6, 15).
In apparent contradiction to our findings and those of Cardinaux et al. (6) and Du et al. (15), previous studies have demonstrated that the phosphorylation of CREB at Ser133 does not always correlate with the activation of CREB, because certain stimuli lead to the phosphorylation of Ser133 of CREB but fail to induce CREB-dependent transcription (4, 5, 7, 51, 58). These findings have suggested the existence of a second signaling event, or gating step, that regulates CREB-dependent transcription at a point that is distinct from Ser133 phosphorylation. It is possible that under certain conditions, mechanisms are activated which specifically inhibit CREB-dependent transcriptional activation even when Ser133 is phosphorylated.
CBP mediates the transcriptional effects of another stimulus-dependent transcription factor, Pit-1, and one study has suggested that Pit-1-dependent transcription depends on an intact PKA site encompassing Ser1772 of CBP (63). This suggests that CBP may be directly targeted by PKA to activate transcription and has raised the possibility that the gating effect observed for CREB-dependent transcription may depend on whether or not Ser1772 of CBP is phosphorylated. However, we found that the ability of CBP to mediate CREB-dependent transcriptional activation is unaffected by the disruption of the PKA site at Ser1772. Our results argue that phosphorylation of this residue of CBP is not required for PKA-induced CREB-dependent transcription and are consistent with previous findings demonstrating that the alteration of Ser1772 does not affect the ability of PKA to activate a GAL4-CBP fusion protein (33).
Structural determinants of the KID-KIX interaction.
Using the E. coli two-hybrid system, we have examined the role of electrostatic interactions in stabilizing the KID-KIX complex and have obtained evidence that specific oppositely charged residues interact at the protein-protein interface. We found first that changing particular charged residues in both the KID and the KIX domain to residues carrying the opposite charge (R124E or D140R in the KID and K606E or E655K in the KIX domain) greatly reduced the ability of the mutant domain to bind to its wild-type partner. A previous study in which random mutagenesis was used to identify substitutions in the KIX domain that disrupt its binding to the KID in vitro also uncovered the substitution K606E (45). The existing structural data have suggested that this Lys residue may stabilize the KID-KIX complex, forming a salt bridge with Asp140 in the KID (50), but to date, there have been no functional data to support this proposal. Our results show that it is possible to partially suppress the effect of the K606E substitution in the KIX domain with a complementary charge-swap substitution (D140R) in the KID, and thus provide strong support for the idea that Lys606 of CBP and Asp140 of CREB participate in an electrostatic interaction that stabilizes the KID-KIX complex. Similarly, our results with complementary charge-swap substitutions R124E (in the KID) and E655K (in the KIX domain) provide support for the proposal that Arg124 of CREB and Glu655 of CBP form a salt bridge that stabilizes the KID-KIX complex.
Although, when tested as isolated domains in the E. coli system, the KIX domain K606E and KID D140R mutants interacted significantly more efficiently than either mutant interacted with the wild-type partner domain, the K606E substitution in the context of full-length CBP (CBP K606E) did not enhance transcriptional activation by a GAL4-CREB mutant in which D140 was converted to an arginine residue (data not shown). However, preliminary data suggest that the GAL4-CREB D140R mutant may not be efficiently phosphorylated at Ser133 in response to PKA stimulation in HEK293 cells.
We also examined the role of hydrophobic residues in stabilizing the KID-KIX interaction. Our results indicated that substitutions of residue Ile137, Leu138, or Leu141 within the αB helix of the KID completely abrogated the ability of the Ser133-phosphorylated KID to interact with the wild-type KIX domain. Available structural data suggest that the KID αB helix interacts strongly with a hydrophobic pocket formed by three helices of the KIX domain (50). In addition, replacement of both Ile137 and Leu138 within the KID αB helix with alanine residues disrupts the ability of the KID to bind to the KIX domain in vitro (45). A reverse yeast two-hybrid screen has also identified Ile137, Leu138, and Leu141 within the KID αB helix as being critical for the KID-KIX interaction (55). However, in this study, it was not determined whether these KID mutants were phosphorylated effectively at Ser133, and it remained possible that alteration of residues within the αB helix of the KID prevented the phosphorylation of Ser133. Our results demonstrate that the KID αB helix mutants that do not bind to the KIX domain can still be phosphorylated at Ser133 by PKA in E. coli, supporting the idea that hydrophobic interactions between the Ser133-phosphorylated KID αB helix and the KIX domain are critical for the KID-KIX interaction.
The E. coli two-hybrid system.
In this study, we have used an E. coli-based two-hybrid system for analyzing the kinase-dependent interaction of the KID and the KIX domain. We found that the E. coli system effectively recapitulates the critical features of the KID-KIX interaction and that mutations which disrupt the KID-KIX interaction in other systems disrupt the interaction in E. coli as well. One of the major advantages of the E. coli-based system is that it can be used to characterize phosphorylation-dependent protein-protein interactions without interference from endogenous signaling molecules. Although recent studies show that homologs of eukaryotic signaling kinases exist in some prokaryotes (40, 59, 66), to date no such kinases have been found in E. coli. Therefore, it is unlikely that a mammalian substrate such as CREB would be phosphorylated at Ser133 in E. coli without the addition of PKA (or a CaMK). Indeed, our results demonstrate CREB Ser133 phosphorylation is undetectable in E. coli in the absence of a mammalian kinase. In contrast to the situation in E. coli, the KID and the KIX domain interact constitutively in yeast, presumably due to the presence of a basal yeast Ser133 kinase activity (55). Thus, the E. coli-based system permits the study of the effects of particular eukaryotic kinases on a protein-protein interaction.
Several other studies describing E. coli-based two-hybrid systems have been reported recently (12, 26, 28a, 31, 46a). In addition, a one-hybrid version of the E. coli system employed here has been adapted for use with a different selection strategy to identify Zn finger peptides that can interact with predetermined target sequences (28).
In our study, in addition to demonstrating the utility of the E. coli two-hybrid system for studying phosphorylation-dependent interactions, we have developed a rapid and simple carbenicillin-based selection for detecting protein-protein interactions in E. coli. This selection should facilitate the use of the E. coli two-hybrid system for identifying novel kinases and binding partners that interact with specific phosphoproteins. Given the rapid doubling time of E. coli and the ease with which this organism can be manipulated genetically, it is likely that the E. coli systems will prove to be particularly useful for studying a variety of different protein-protein interactions.
ACKNOWLEDGMENTS
This work was supported by NIH Mental Retardation Research Center grant P30-HD18655 (to M.E.G. and J.M.K.), NIH grant CA43855 (to M.E.G.), NIH grant GM55637 (to A.H.), an established investigatorship from the American Heart Association (to A.H.), and an HMS/Affiliated Hospital Collaborative Seed grant (to A.H. and M.E.G.). A.J.S. was supported by an NIH Medical Scientist Training Program fellowship, and S.L.D. was supported by a Charles A. King Trust postdoctoral fellowship.
We thank P. Brindle for providing the GAL4-Myb expression vector and for useful discussions, F. Whipple for providing the 5P2 vector and for helpful advice, R. Goodman and P. Goldman for providing the KID helix αB mutants, and R. Goodman and R. Kwok for providing the HA-CBP expression vector. We also thank A. Bonni, P. Brindle, R. Goodman, and J. Zeig for critical reading of the manuscript. We thank members of the Greenberg and Hochschild laboratories for their help and advice during the course of these studies, and, in particular, we are especially grateful to A. Brunet for her support and generous help in conducting some of the experiments.
REFERENCES
- 1.Abdollah S, Macias-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana J L. TbetaRI phosphorylation of Smad2 or Ser465 and Ser467 is required for Smad2-Smad4 complex formation and signaling. J Biol Chem. 1997;272:27678–27685. doi: 10.1074/jbc.272.44.27678. [DOI] [PubMed] [Google Scholar]
- 2.Arias J, Alberts A S, Brindle P, Claret F X, Smeal T, Karin M, Feramisco J, Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994;370:226–229. doi: 10.1038/370226a0. [DOI] [PubMed] [Google Scholar]
- 3.Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 4.Bonni A, Ginty D D, Dudek H, Greenberg M E. Serine 133-phosphorylated CREB induces transcription via a cooperative mechanism that may confer specificity to neurotrophin signals. Mol Cell Neurosci. 1995;6:168–183. doi: 10.1006/mcne.1995.1015. [DOI] [PubMed] [Google Scholar]
- 5.Brindle P, Nakajima T, Montminy M. Multiple protein kinase A-regulated events are required for transcriptional induction by cAMP. Proc Natl Acad Sci USA. 1995;92:10521–10525. doi: 10.1073/pnas.92.23.10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardinaux J-R, Notis J C, Zhang Q, Vo N, Craig J C, Fass D M, Brennan R G, Goodman R H. Recruitment of CREB binding protein is sufficient for CREB-mediated gene activation. Mol Cell Biol. 2000;20:1546–1552. doi: 10.1128/mcb.20.5.1546-1552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawla S, Hardingham G E, Quinn D R, Bading H. CBP: a signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science. 1998;281:1505–1509. doi: 10.1126/science.281.5382.1505. [DOI] [PubMed] [Google Scholar]
- 8.Cho H, Orphanides G, Sun X, Yang X-J, Ogryzko V, Lees E, Nakatani Y, Reinberg D. A human RNA polymerase II complex containing factors that modify chromatin structure. Mol Cell Biol. 1998;18:5355–5363. doi: 10.1128/mcb.18.9.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chrivia J C, Kwok R P, Lamb N, Hagiwara M, Montminy M R, Goodman R H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 10.Cruzalegui F H, Means A R. Biochemical characterization of the multifunctional Ca2+/calmodulin-dependent protein kinase type IV expressed in insect cells. J Biol Chem. 1993;268:26171–26178. [PubMed] [Google Scholar]
- 11.Dash P K, Karl K A, Colicos M A, Prywes R, Kandel E R. cAMP response element-binding protein is activated by Ca2+/calmodulin- as well as cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1991;88:5061–5065. doi: 10.1073/pnas.88.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dmitrova M, Younes-Cauet G, Oertel-Buchheit P, Porte D, Schnarr M, Granger-Schnarr M. A new LexA-based genetic system for monitoring and analyzing protein heterodimerization in Escherichia coli. Mol Gen Genet. 1998;257:205–212. doi: 10.1007/s004380050640. [DOI] [PubMed] [Google Scholar]
- 13.Dove S L, Hochschild A. Conversion of the omega subunit of Escherichia coli RNA polymerase into a transcriptional activator or an activation target. Genes Dev. 1998;12:745–754. doi: 10.1101/gad.12.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dove S L, Joung J K, Hochschild A. Activation of prokaryotic transcription through arbitrary protein-protein contacts. Nature. 1997;386:627–630. doi: 10.1038/386627a0. [DOI] [PubMed] [Google Scholar]
- 15.Du K, Asahara H, Jhala U S, Wagner B L, Montminy M. Characterization of a CREB gain-of-function mutant with constitutive transcriptional activity in vivo. Mol Cell Biol. 2000;20:4320–4327. doi: 10.1128/mcb.20.12.4320-4327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enslen H, Sun P, Brickey D, Soderling S H, Klamo E, Soderling T R. Characterization of Ca2+/calmodulin-dependent protein kinase IV. Role in transcriptional regulation. J Biol Chem. 1994;269:15520–15527. [PubMed] [Google Scholar]
- 17.Errede B, Levin D E. A conserved kinase cascade for MAP kinase activation in yeast. Curr Opin Cell Biol. 1993;5:254–260. doi: 10.1016/0955-0674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 18.Farrell S, Simkovich N, Wu Y, Barberis A, Ptashne M. Gene activation by recruitment of the RNA polymerase II holoenzyme. Genes Dev. 1996;10:2359–2367. doi: 10.1101/gad.10.18.2359. [DOI] [PubMed] [Google Scholar]
- 19.Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 20.Ginty D D, Bonni A, Greenberg M E. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994;77:713–725. doi: 10.1016/0092-8674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 21.Ginty D D, Kornhauser J M, Thompson M A, Bading H, Mayo K E, Takahashi J S, Greenberg M E. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- 22.Goldman P S, Tran V K, Goodman R H. The multifunctional role of the co-activator CBP in transcriptional regulation. Recent Prog Horm Res. 1997;52:103–119. [PubMed] [Google Scholar]
- 23.Gonzalez G A, Menzel P, Leonard J, Fischer W H, Montminy M R. Characterization of motifs which are critical for activity of the cyclic AMP-responsive transcription factor CREB. Mol Cell Biol. 1991;11:1306–1312. doi: 10.1128/mcb.11.3.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez G A, Montminy M R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 25.Hanks S K, Quinn A M, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 26.Hays L B, Chen Y S, Hu J C. Two-hybrid system for characterization of protein-protein interactions in E. Coli BioTechniques. 2000;29:288–290. doi: 10.2144/00292st04. , 292–294, 296. [DOI] [PubMed] [Google Scholar]
- 27.Hu J C, Kornacker M G, Hochschild A. Escherichia coli one- and two-hybrid systems for the analysis and identification of protein-protein interactions. Methods. 2000;20:80–94. doi: 10.1006/meth.1999.0908. [DOI] [PubMed] [Google Scholar]
- 28.Joung J K, Ramm E I, Pabo C O. A bacterial two-hybrid selection system for studying protein-DNA and protein-protein interactions. Proc Natl Acad Sci USA. 2000;97:7382–7387. doi: 10.1073/pnas.110149297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Karimova G, Pidoux J, Ullmann A, Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc Natl Acad Sci USA. 1998;95:5752–5756. doi: 10.1073/pnas.95.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kee B L, Arias J, Montminy M R. Adaptor-mediated recruitment of RNA polymerase II to a signal-dependent activator. J Biol Chem. 1996;271:2373–2375. doi: 10.1074/jbc.271.5.2373. [DOI] [PubMed] [Google Scholar]
- 30.Khokhlatchev A, Xu S, English J, Wu P, Schaefer E, Cobb M H. Reconstitution of mitogen-activated protein kinase phosphorylation cascades in bacteria. Efficient synthesis of active protein kinases. J Biol Chem. 1997;272:11057–11062. doi: 10.1074/jbc.272.17.11057. [DOI] [PubMed] [Google Scholar]
- 31.Kornacker M G, Remsburg B, Menzel R. Gene activation by the AraC protein can be inhibited by DNA looping between AraC and a LexA repressor that interacts with AraC: possible applications as a two-hybrid system. Mol Microbiol. 1998;30:615–624. doi: 10.1046/j.1365-2958.1998.01096.x. [DOI] [PubMed] [Google Scholar]
- 32.Korzus E, Torchia J, Rose D W, Xu L, Kurokawa R, McInerney E M, Mullen T M, Glass C K, Rosenfeld M G. Transcription factor-specific requirements for coactivators and their acetyltransferase functions. Science. 1998;279:703–707. doi: 10.1126/science.279.5351.703. [DOI] [PubMed] [Google Scholar]
- 33.Kwok R P, Lundblad J R, Chrivia J C, Richards J P, Bachinger H P, Brennan R G, Roberts S G, Green M R, Goodman R H. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 34.Maniatis T, Falvo J V, Kim T H, Kim T K, Lin C H, Parekh B S, Wathelet M G. Structure and function of the interferon-beta enhanceosome. Cold Spring Harbor Symp Quant Biol. 1998;63:609–620. doi: 10.1101/sqb.1998.63.609. [DOI] [PubMed] [Google Scholar]
- 35.Matthews R P, Guthrie C R, Wailes L M, Zhao X, Means A R, McKnight G S. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol Cell Biol. 1994;14:6107–6116. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maurer R A. Both isoforms of the cAMP-dependent protein kinase catalytic subunit can activate transcription of the prolactin gene. J Biol Chem. 1989;264:6870–6873. [PubMed] [Google Scholar]
- 37.Mendelsohn A R, Brent R. Applications of interaction traps/two-hybrid systems to biotechnology research. Curr Opin Biotechnol. 1994;5:482–486. doi: 10.1016/0958-1669(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 38.Merika M, Williams A J, Chen G, Collins T, Thanos D. Recruitment of CBP/p300 by the IFN beta enhanceosome is required for synergistic activation of transcription. Mol Cell. 1998;1:277–287. doi: 10.1016/s1097-2765(00)80028-3. [DOI] [PubMed] [Google Scholar]
- 39.Miranti C K, Ginty D D, Huang G, Chatila T, Greenberg M E. Calcium activates serum response factor-dependent transcription by a Ras- and Elk-1-independent mechanism that involves a Ca2+/calmodulin-dependent kinase. Mol Cell Biol. 1995;15:3672–3684. doi: 10.1128/mcb.15.7.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munoz-Dorado J, Inouye S, Inouye M. A gene encoding a protein serine/threonine kinase is required for normal development of M. xanthus, a gram-negative bacterium. Cell. 1991;67:995–1006. doi: 10.1016/0092-8674(91)90372-6. [DOI] [PubMed] [Google Scholar]
- 41.Nakajima T, Uchida C, Anderson S F, Lee C G, Hurwitz J, Parvin J D, Montminy M. RNA helicase A mediates association of CBP with RNA polymerase II. Cell. 1997;90:1107–1112. doi: 10.1016/s0092-8674(00)80376-1. [DOI] [PubMed] [Google Scholar]
- 42.Nakajima T, Uchida C, Anderson S F, Parvin J D, Montminy M. Analysis of a cAMP-responsive activator reveals a two-component mechanism for transcriptional induction via signal-dependent factors. Genes Dev. 1997;11:738–747. doi: 10.1101/gad.11.6.738. [DOI] [PubMed] [Google Scholar]
- 43.Ogryzko V V, Schiltz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 44.Ohya Y, Kawasaki H, Suzuki K, Londesborough J, Anraku Y. Two yeast genes encoding calmodulin-dependent protein kinases. Isolation, sequencing and bacterial expressions of CMK1 and CMK2. J Biol Chem. 1991;266:12784–12794. [PubMed] [Google Scholar]
- 45.Parker D, Ferreri K, Nakajima T, LaMorte V J, Evans R, Koerber S C, Hoeger C, Montminy M R. Phosphorylation of CREB at Ser-133 induces complex formation with CREB-binding protein via a direct mechanism. Mol Cell Biol. 1996;16:694–703. doi: 10.1128/mcb.16.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker D, Rivera M, Zor T, Henrion-Caude A, Radhakrishnan I, Kumar A, Shapiro L H, Wright P E, Montminy M, Brindle P K. Role of secondary structure in discrimination between constitutive and inducible activators. Mol Cell Biol. 1999;19:5601–5607. doi: 10.1128/mcb.19.8.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46a.Pelletier J N, Arndt K M, Pluckthun A, Michnick S W. An in vivo library-versus-library selection of optimized protein-protein interactions. Nat Biotechnol. 1999;17:683–690. doi: 10.1038/10897. [DOI] [PubMed] [Google Scholar]
- 47.Planas-Silva M D, Means A R. Expression of a constitutive form of calcium/calmodulin dependent protein kinase II leads to arrest of the cell cycle in G2. EMBO J. 1992;11:507–517. doi: 10.1002/j.1460-2075.1992.tb05081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ptashne M, Gann A. Transcriptional activation by recruitment. Nature. 1997;386:569–577. doi: 10.1038/386569a0. [DOI] [PubMed] [Google Scholar]
- 49.Quinn P G. Distinct activation domains within cAMP response element-binding protein (CREB) mediate basal and cAMP-stimulated transcription. J Biol Chem. 1993;268:16999–17009. [PubMed] [Google Scholar]
- 50.Radhakrishnan I, Perez-Alvarado G C, Parker D, Dyson H J, Montminy M R, Wright P E. Solution structure of the KIX domain of CBP bound to the transactivation domain of CREB: a model for activator:coactivator interactions. Cell. 1997;91:741–752. doi: 10.1016/s0092-8674(00)80463-8. [DOI] [PubMed] [Google Scholar]
- 51.Schwaninger M, Blume R, Kruger M, Lux G, Oetjen E, Knepel W. Involvement of the Ca(2+)-dependent phosphatase calcineurin in gene transcription that is stimulated by cAMP through cAMP response elements. J Biol Chem. 1995;270:8860–8866. doi: 10.1074/jbc.270.15.8860. [DOI] [PubMed] [Google Scholar]
- 52.Sheng M, McFadden G, Greenberg M E. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990;4:571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- 53.Sheng M, Thompson M A, Greenberg M E. CREB: a Ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 54.Shieh P B, Hu S C, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 55.Shih H M, Goldman P S, DeMaggio A J, Hollenberg S M, Goodman R H, Hoekstra M F. A positive genetic selection for disrupting protein-protein interactions: identification of CREB mutations that prevent association with the coactivator CBP. Proc Natl Acad Sci USA. 1996;93:13896–13901. doi: 10.1073/pnas.93.24.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun P, Enslen H, Myung P S, Maurer R A. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994;8:2527–2539. doi: 10.1101/gad.8.21.2527. [DOI] [PubMed] [Google Scholar]
- 57.Tao X, Finkbeiner S, Arnold D B, Shaywitz A J, Greenberg M E. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 58.Thompson M A, Ginty D D, Bonni A, Greenberg M E. L-type voltage-sensitive Ca2+ channel activation regulates c-fos transcription at multiple levels. J Biol Chem. 1995;270:4224–4235. doi: 10.1074/jbc.270.9.4224. [DOI] [PubMed] [Google Scholar]
- 59.Udo H, Munoz-Dorado J, Inouye M, Inouye S. Myxococcus xanthus, a gram-negative bacterium, contains a transmembrane protein serine/threonine kinase that blocks the secretion of beta-lactamase by phosphorylation. Genes Dev. 1995;9:972–983. doi: 10.1101/gad.9.8.972. [DOI] [PubMed] [Google Scholar]
- 60.Whipple F W. Genetic analysis of prokaryotic and eukaryotic DNA-binding proteins in Escherichia coli. Nucleic Acids Res. 1998;26:3700–3706. doi: 10.1093/nar/26.16.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whipple F W, Hou E F, Hochschild A. Amino acid-amino acid contacts at the cooperativity interface of the bacteriophage lambda and P22 repressors. Genes Dev. 1998;12:2791–2802. doi: 10.1101/gad.12.17.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Y, Reece R J, Ptashne M. Quantitation of putative activator-target affinities predicts transcriptional activating potentials. EMBO J. 1996;15:3951–3963. [PMC free article] [PubMed] [Google Scholar]
- 63.Xu L, Lavinsky R M, Dasen J S, Flynn S E, McInerney E M, Mullen T M, Heinzel T, Szeto D, Korzus E, Kurokawa R, Aggarwal A K, Rose D W, Glass C K, Rosenfeld M G. Signal-specific co-activator domain requirements for Pit-1 activation. Nature. 1998;395:301–306. doi: 10.1038/26270. [DOI] [PubMed] [Google Scholar]
- 64.Yang X J, Ogryzko V V, Nishikawa J, Howard B H, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 65.Yie J, Senger K, Thanos D. Mechanism by which the IFN-beta enhanceosome activates transcription. Proc Natl Acad Sci USA. 1999;96:13108–13113. doi: 10.1073/pnas.96.23.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang C C. Bacterial signalling involving eukaryotic-type protein kinases. Mol Microbiol. 1996;20:9–15. doi: 10.1111/j.1365-2958.1996.tb02483.x. [DOI] [PubMed] [Google Scholar]