Abstract
Colorectal cancer (CRC) and nonalcoholic fatty liver disease (NAFLD) have high prevalence rates and place a considerable burden on the health-care industry. The association between both diseases is controversial. Our aim was to examine the association between NAFLD and CRC. Using data extracted from the Taiwan National Health Insurance Research Database (NHIRD) from 2000 to 2015, we enrolled 60 298 patients with NAFLD. Of these, 52,986 met the inclusion criteria. A comparison group was selected using 4-fold propensity score matching by age, sex, and year of index date. The primary outcome was the cumulative incidence of CRC in patients with NAFLD. Over a mean follow-up period of 8.5 years, 160 new cases of CRC were identified. The incidence rate of CRC was higher in the NAFLD group (12.23 per 100,000 person-years) than in the comparison cohort (6.0 per 100,000 person-years). Cox proportional hazards regression analysis revealed that the adjusted hazard ratio (HR) of CRC was 1.259 in the study group (95% confidence interval [CI]: 1.047–1.486, P = .003). Using Kaplan–Meier analysis, we ascertained that the cumulative incidence of CRC was significantly high in the NAFLD group. Patients older than 50 years, with diabetes mellitus (DM), and with chronic liver disease also exhibited a high risk of CRC. NAFLD was associated with a high risk of CRC. CRC occurs more frequently in patients with NAFLD aged between 50 and 59 years and those older than 60 years with comorbidities, including DM and chronic liver disease. Physicians should consider the subsequent risk of CRC when treating patients with NAFLD.
Keywords: colorectal cancer, National Health Insurance Research Database, nonalcoholic fatty liver disease
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is defined as the presence of hepatic steatosis and is diagnosed through imaging or histology after the exclusion of secondary etiology. NAFLD is the most common reason for abnormal liver function test results in adults in the United States, and the global prevalence was reported to be 25.24%.[1] In Taiwan, the prevalence in the general population ranged between 11.5% and 44.5%.[2] The pathogenesis of NAFLD is complex and is modulated by metabolic, environmental, genetic, and microbial factors.[3] Affected individuals are typically asymptomatic; thus, diagnosis is often incidental. If left untreated, NAFLD may progress to fibrosis and even cirrhosis.[4,5] Despite the possibility of progression to hepatocellular carcinoma, the leading cause of death in patients with NAFLD is cardiovascular disease, followed by extrahepatic malignancy.[6]
Colorectal cancer (CRC) commonly affects elderly individuals aged ≥60 years. Globally, it was the third most diagnosed cancer and the second leading cause of cancer death in 2020.[7] According to the 2019 Taiwan Cancer Registry Annual Report, 17,710 CRC cases were newly diagnosed, with 6436 related deaths.[8] The 5-year relative survival rates for localized, regional, and distant disease were 91%, 72%, and 15%, respectively.[9] Most CRCs arise from polyps over a period estimated to be between 10 and 15 years. The risk factors for CRC include hereditary factors, such as family history or hereditary CRC syndromes; nonmodifiable risk factors such as ethnicity, gender, and inflammatory bowel disease (IBD); and modifiable risk factors, including smoking, obesity, excessive red meat consumption, processed meat consumption, and alcohol intake.[10]
The etiology of CRC in patients with NAFLD remains unclear, and different studies have reported controversial results. Lee et al identified a 1.23-fold increased risk of CRC in Korean women with NAFLD.[11] However, other studies have reported a null association between the 2 diseases.[12,13] Therefore, in this study, we analyzed a nationwide database to investigate the association of NAFLD with CRC risk in the Taiwanese population.
2. Methods
2.1. Database
Taiwan National Health Insurance program is a social health insurance scheme established in 1995, with more than 99% of the 23.72 million residents currently enrolled. This scheme provides inpatient, outpatient, mental health, and prescription drug coverage. The Longitudinal Health Insurance Database (LHID) of the National Health Insurance Research Database (NHIRD) comprises the data of 1 million individuals randomly selected from the Registry of Beneficiaries, including their complete medical records. The disease codes used in the LHID correspond to those of International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). This dataset has been validated by the National Health Research Institutes as being representative of the national population in Taiwan, with no statistically significant differences in the distribution of age, sex, or health-care costs between the dataset and the original NHIRD. This study was approved by the Institutional Review Board of the Tri-Service General Hospital, National Defense Medical Center (TSGHIRB No. E202216011).
2.2. Study design
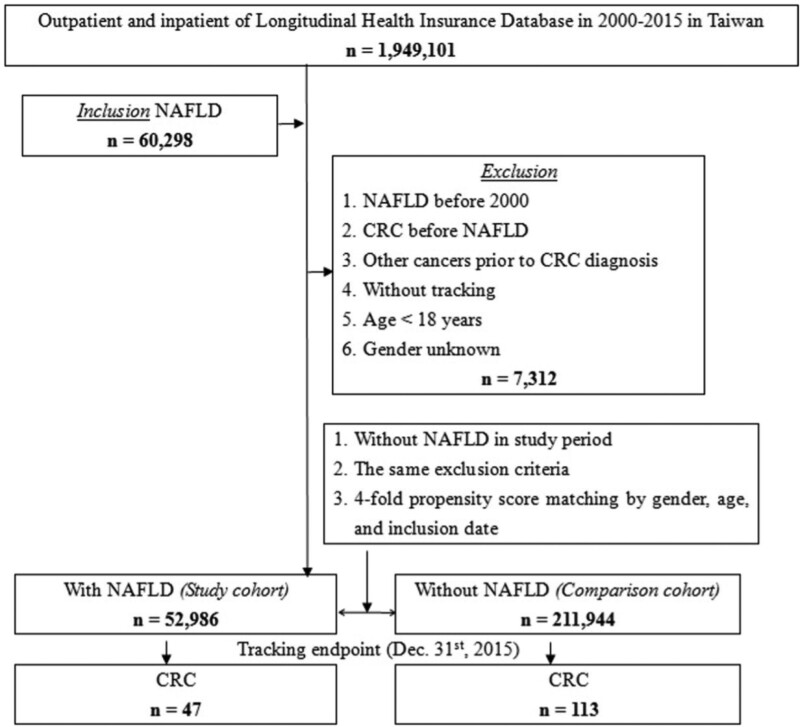
We retrospectively searched the LHID and identified 60,298 patients aged ≥18 years who were diagnosed with NAFLD (ICD-9-CM codes 571.5 and 571.8–571.9) between 2000 and 2015. The index date was defined as the date of NAFLD diagnosis. We defined CRC (ICD-9-CM codes 153–154 and 197.5) as the outcome. The end date was the date of CRC diagnosis or the end of the study period. The exclusion criteria were NAFLD diagnosis before 2000, CRC diagnosis before NAFLD, other malignancies prior to CRC, lack of tracking, age <18 years, and unknown sex. We randomly selected individuals without NAFLD as the comparison cohort, and they were matched with the study group according to age, sex, and index date through 4-fold propensity score matching. The flow diagram is presented in Figure 1.
Figure 1.
Flow diagram of this study.
Comorbidities included myocardial infarction (MI, ICD-9-CM code 410), peripheral vascular disease (ICD-9-CM code 443), diabetes mellitus (DM, ICD-9-CM code 250), dementia (ICD-9-CM codes 290 and 294.1), rheumatic disease (ICD-9-CM codes 394–395, 397.1, and 397.9), congestive heart failure (CHF, ICD-9-CM codes 402.01, 402.11, 402.91, 404.03, and 428), cerebrovascular disease (CVD, ICD-9-CM codes 430–438), chronic obstructive pulmonary disease (COPD, ICD-9-CM codes 490–496), liver disease (ICD-9-CM code 571), human immunodeficiency virus (ICD-9-CM code 042), chronic kidney disease (CKD, ICD-9-CM codes 580–589), colorectal polyps (ICD-9-CM codes 211.3–211.4 and 569.0), and IBD (ICD-9-CM codes 555–556).
2.3. Statistical analysis
Statistical analyses were performed using SPSS version 21 (Asia Analytics Taiwan Ltd., Taipei, Taiwan). Categorical parameters were assessed using the chi-square (χ2) test and Fisher exact test. Continuous parameters were analyzed using the Student t test. Associations between time-to-event outcomes were examined using the Kaplan–Meier method, and factors related to CRC were assessed using a Cox proportional regression model. The results are presented as adjusted hazard ratios (HRs) with corresponding 95% confidence intervals (CIs). Statistical significance was indicated by P < .05.
3. Results
A total of 52,986 and 211,944 patients were included in the study and comparison cohorts, respectively. The demographic and clinical characteristics of both cohorts at baseline are presented in Table 1. The mean age of patients with and without NAFLD was 42.18 ± 18.56 years and 42.33 ± 19.72 years, respectively. No significant differences in sex and age were observed. Patients with NAFLD had a higher prevalence of comorbidities, namely MI, DM, dementia, rheumatic disease, CHF, CVD, COPD, liver disease, CKD, colorectal polyps, and IBD (all P < .001).
Table 1.
Participants’ demographic and characteristics at baseline.
| NAFLD | Total | With | Without | P | |||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | |
| Total | 264,930 | 52,986 | 20.00 | 211,944 | 80.00 | ||
| Gender | .999 | ||||||
| Male | 144,880 | 54.69 | 28,976 | 54.69 | 115,904 | 54.69 | |
| Female | 120,050 | 45.31 | 24,010 | 45.31 | 96,040 | 45.31 | |
| Age (yr) | 42.30 ± 19.49 | 42.18 ± 18.56 | 42.33 ± 19.72 | .113 | |||
| Age groups (yr) | .999 | ||||||
| 18–29 | 34,360 | 12.97 | 6872 | 12.97 | 27,488 | 12.97 | |
| 30–39 | 68,775 | 25.96 | 13,755 | 25.96 | 55,020 | 25.96 | |
| 40–49 | 61,155 | 23.08 | 12,231 | 23.08 | 48,924 | 23.08 | |
| 50–59 | 53,280 | 20.11 | 10,656 | 20.11 | 42,624 | 20.11 | |
| ≥60 | 47,360 | 17.88 | 9472 | 17.88 | 37,888 | 17.88 | |
| MI | <.001 | ||||||
| Without | 253,944 | 95.85 | 50,601 | 95.50 | 203,343 | 95.94 | |
| With | 10,986 | 4.15 | 2385 | 4.50 | 8601 | 4.06 | |
| PVD | .468 | ||||||
| Without | 248,505 | 93.80 | 49,665 | 93.73 | 198,840 | 93.82 | |
| With | 16,425 | 6.20 | 3321 | 6.27 | 13,104 | 6.18 | |
| DM | <.001 | ||||||
| Without | 212,343 | 80.15 | 40,585 | 76.60 | 171,758 | 81.04 | |
| With | 52,587 | 19.85 | 12,401 | 23.40 | 40,186 | 18.96 | |
| Dementia | <.001 | ||||||
| Without | 259,132 | 97.81 | 51,709 | 97.59 | 207,423 | 97.87 | |
| With | 5798 | 2.19 | 1277 | 2.41 | 4521 | 2.13 | |
| Rheumatic disease | <.001 | ||||||
| Without | 253,310 | 95.61 | 50,388 | 95.10 | 202,922 | 95.74 | |
| With | 11,620 | 4.39 | 2598 | 4.90 | 9022 | 4.26 | |
| CHF | <.001 | ||||||
| Without | 251,252 | 94.84 | 49,961 | 94.29 | 201,291 | 94.97 | |
| With | 13,678 | 5.16 | 3025 | 5.71 | 10,653 | 5.03 | |
| CVD | <.001 | ||||||
| Without | 245,274 | 92.58 | 48,761 | 92.03 | 196,513 | 92.72 | |
| With | 19,656 | 7.42 | 4225 | 7.97 | 15,431 | 7.28 | |
| COPD | <.001 | ||||||
| Without | 241,023 | 90.98 | 47,944 | 90.48 | 193,079 | 91.10 | |
| With | 23,907 | 9.02 | 5042 | 9.52 | 18,865 | 8.90 | |
| Liver disease | <.001 | ||||||
| Without | 239,253 | 90.31 | 47,210 | 89.10 | 192,043 | 90.61 | |
| With | 25,677 | 9.69 | 5776 | 10.90 | 19,901 | 9.39 | |
| HIV | .400 | ||||||
| Without | 264,365 | 99.79 | 52,865 | 99.77 | 211,500 | 99.79 | |
| With | 565 | 0.21 | 121 | 0.23 | 444 | 0.21 | |
| CKD | <.001 | ||||||
| Without | 238,004 | 89.84 | 46,261 | 87.31 | 191,743 | 90.47 | |
| With | 26,926 | 10.16 | 6725 | 12.69 | 20,201 | 9.53 | |
| Colorectal polyp | <.001 | ||||||
| Without | 259,060 | 97.78 | 50,092 | 94.54 | 208,968 | 98.60 | |
| With | 5870 | 2.22 | 2894 | 5.46 | 2976 | 1.40 | |
| IBD | <.001 | ||||||
| Without | 263,256 | 99.37 | 52,410 | 98.91 | 210,846 | 99.48 | |
| With | 1674 | 0.63 | 576 | 1.09 | 1098 | 0.52 | |
CHF = congestive heart failure, CKD = chronic kidney disease, COPD = chronic pulmonary disease, CVD = cerebrovascular disease, DM = diabetes mellitus, HIV = human immunodeficiency virus, IBD = inflammatory bowel disease, MI = myocardial infarction, NAFLD = nonalcoholic fatty liver disease, PVD = peripheral vascular disease.
*P values < .05 were considered statistically significant.
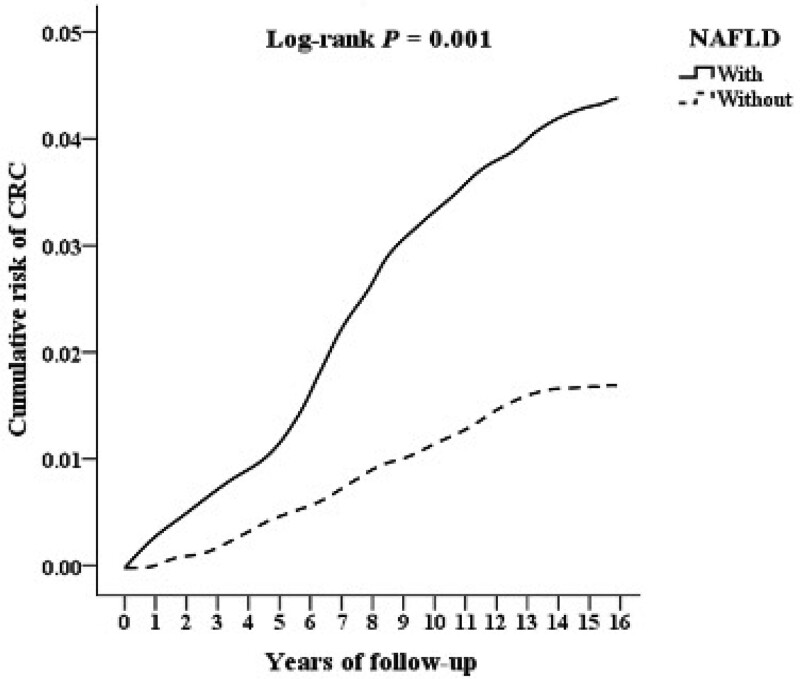
By the end date, 47 and 113 patients had been diagnosed with CRC in the NAFLD group and comparison group, respectively, with a significantly higher incidence (0.09% vs 0.05%, P = .005) in the NAFLD group. Overall, the mean follow-up period was 8.55 ± 6.44 years (Supplemental Table S1, http://links.lww.com/MD/J20, which illustrates the mean follow-up time of this study). The mean time for CRC development was 6.86 ± 4.82 years in the NAFLD group and 7.95 ± 5.61 years in the comparison group (Supplemental Table S2, http://links.lww.com/MD/J21, which shows the mean time for CRC development in this study). As displayed in Figure 2, the cumulative risk of CRC was higher in the NAFLD cohort than in the comparison cohort (log-rank test P = .001). Patients with NAFLD also exhibited a significantly increased incidence of CRC than patients without NAFLD according to the Cox regression analysis (adjusted HR = 1.259, 95% CI = 1.047–1.486, P = .003) (Table 2). We also categorized our patients into 3 groups. These groups include those who were diagnosed by a gastroenterologist, those who had been to the outpatient department more than 3 times or had been hospitalized, and those who had been to the outpatient department more than 3 times or had been hospitalized and were also diagnosed by a gastroenterologist in order to improve the accuracy of the diagnosis of NAFLD (Supplemental Table S3, http://links.lww.com/MD/J22 which supports the positive correlation between these groups and CRC risk). Furthermore, male sex (adjusted HR = 1.543, 95% CI = 1.024–2.060, P = .025), age between 50 and 59 years (adjusted HR = 1.246, 95% CI = 1.060–1.565, P < .001), age ≥60 years (adjusted HR = 1.697, 95% CI = 1.245–1.974, P < .001), DM (adjusted HR = 2.480, 95% CI = 1.224–3.472, P < .001), CVD (adjusted HR = 1.801, 95% CI = 1.003–2.781, P = .036), liver disease (adjusted HR = 2.765, 95% CI = 2.565–3.894, P < .001), and CKD (adjusted HR = 1.561, 95% CI = 1.013–2.090, P < .036) were all associated with the risk of CRC.
Figure 2.
Kaplan–Meier curve for the cumulative risk of colorectal cancer stratified by NAFLD. NAFLD = nonalcoholic fatty liver disease.
Table 2.
Risk analysis for colorectal cancer by using Cox regression.
| Variables | Crude HR | 95% CI | 95% CI | P | Adjusted HR | 95% CI | 95% CI | P |
|---|---|---|---|---|---|---|---|---|
| NAFLD | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.297 | 1.052 | 1.533 | .001 | 1.259 | 1.047 | 1.486 | .003 |
| Gender | ||||||||
| Male | 1.663 | 1.197 | 2.479 | <.001 | 1.543 | 1.024 | 2.060 | .025 |
| Female | Reference | Reference | ||||||
| Age groups (yr) | ||||||||
| 18–29 | Reference | Reference | ||||||
| 30–39 | 1.329 | 0.924 | 1.674 | .076 | 1.005 | 0.831 | 1.297 | .172 |
| 40–49 | 1.341 | 0.999 | 1.688 | .051 | 1.014 | 0.865 | 1.303 | .138 |
| 50–59 | 1.652 | 1.234 | 2.335 | <.001 | 1.246 | 1.060 | 1.565 | <.001 |
| ≥60 | 2.030 | 1.456 | 2.996 | <.001 | 1.697 | 1.245 | 1.974 | <.001 |
| MI | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.456 | 0.933 | 2.010 | .094 | 1.065 | 0.656 | 1.689 | .389 |
| PVD | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.588 | 0.972 | 2.265 | .075 | 1.176 | 0.782 | 1.781 | .251 |
| DM | ||||||||
| Without | Reference | Reference | ||||||
| With | 2.989 | 1.452 | 3.897 | <.001 | 2.480 | 1.224 | 3.472 | <.001 |
| Dementia | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.286 | 0.652 | 1.885 | .335 | 1.189 | 0.533 | 1.740 | .489 |
| Rheumatic disease | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.186 | 0.925 | 1.565 | .086 | 1.104 | 0.892 | 1.489 | .154 |
| CHF | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.388 | 1.018 | 1.706 | .032 | 1.265 | 0.985 | 1.572 | .064 |
| CVD | ||||||||
| Without | Reference | Reference | ||||||
| With | 2.445 | 1.174 | 3.376 | <.001 | 1.801 | 1.003 | 2.781 | .046 |
| COPD | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.675 | 0.883 | 2.480 | .203 | 1.464 | 0.672 | 2.289 | .397 |
| Liver disease | ||||||||
| Without | Reference | Reference | ||||||
| With | 2.986 | 1.765 | 4.030 | <.001 | 2.765 | 2.565 | 3.894 | <.001 |
| HIV | ||||||||
| Without | Reference | Reference | ||||||
| With | 0.000 | - | - | .999 | 0.000 | - | - | .999 |
| CKD | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.704 | 1.146 | 2.259 | <.001 | 1.561 | 1.013 | 2.090 | .036 |
| Colorectal polyp | ||||||||
| Without | Reference | Reference | ||||||
| With | 7.010 | 0.756 | 18.986 | .310 | 4.895 | 0.342 | 9.101 | .722 |
| IBD | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.892 | 1.024 | 2.701 | .025 | 1.783 | 0.975 | 2.601 | .066 |
Adjusted HR = Adjusted variables listed in the table, CHF = congestive heart failure, CI = confidence interval, CKD = chronic kidney disease, COPD = chronic pulmonary disease, CVD = cerebrovascular disease, DM = diabetes mellitus, HIV = human immunodeficiency virus, HR = hazard ratio, IBD = inflammatory bowel disease, MI = myocardial infarction, NAFLD = nonalcoholic fatty liver disease, PVD = peripheral vascular disease.
*P values < .05 were considered statistically significant.
Table 3 presents the stratified analysis of patients with and without NAFLD according to demographic factors and comorbidities. The overall incidence of CRC was 12.23 per 100,000 person-years in the NAFLD cohort and 6.00 per 100,000 person-years in the comparison cohort. In the comorbidity analysis, patients with NAFLD had a higher risk of developing CRC than patients without NAFLD (adjusted HR = 1.259, 95% CI = 1.047–1.486, P = .003), regardless of the presence of MI, peripheral vascular disease, dementia, rheumatic disease, CHF, CVD, COPD, CKD, and IBD.
Table 3.
Incidence and adjusted HR for CRC stratified by demographic, characteristics between NAFLD and without NAFLD.
| NAFL`D | With | Without (Reference) | With vs Without (Reference) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stratified | Events | PYs | Rate (per 105 PYs) | Events | PYs | Rate (per 105 PYs) | Adjusted HR | 95%CI | 95%CI | P |
| Total | 47 | 384,148.50 | 12.23 | 113 | 1882,062.72 | 6.00 | 1.259 | 1.047 | 1.486 | .003 |
| Gender | ||||||||||
| Male | 27 | 210,506.11 | 12.83 | 63 | 1029,701.11 | 6.12 | 1.295 | 1.077 | 1.529 | <.001 |
| Female | 20 | 173,642.39 | 11.52 | 50 | 852,361.61 | 5.87 | 1.213 | 1.009 | 1.432 | .040 |
| Age groups (yr) | ||||||||||
| 18–29 | 4 | 48,756.22 | 8.20 | 11 | 223,050.24 | 4.93 | 1.028 | 0.855 | 1.213 | .151 |
| 30–39 | 9 | 94,148.43 | 9.56 | 27 | 472,298.11 | 5.72 | 1.033 | 0.859 | 1.219 | .148 |
| 40–49 | 9 | 87,072.39 | 10.34 | 25 | 425,360.80 | 5.88 | 1.087 | 0.904 | 1.282 | .095 |
| 50–59 | 10 | 77,118.24 | 12.97 | 23 | 382,053.19 | 6.02 | 1.331 | 1.107 | 1.571 | <.001 |
| ≥60 | 15 | 77,053.22 | 19.47 | 27 | 379,300.38 | 7.12 | 1.690 | 1.405 | 1.994 | <.001 |
| MI | ||||||||||
| Without | 44 | 366,268.53 | 12.01 | 107 | 1804,610.08 | 5.93 | 1.252 | 1.041 | 1.477 | .008 |
| With | 3 | 17,879.97 | 16.78 | 6 | 77,452.64 | 7.75 | 1.338 | 1.113 | 1.579 | <.001 |
| PVD | ||||||||||
| Without | 43 | 359,788.25 | 11.95 | 106 | 1774,806.53 | 5.97 | 1.236 | 1.028 | 1.459 | .027 |
| With | 4 | 24,360.25 | 16.42 | 7 | 107,256.19 | 6.53 | 1.554 | 1.293 | 1.835 | <.001 |
| DM | ||||||||||
| Without | 27 | 293,565.84 | 9.20 | 84 | 1524,380.58 | 5.51 | 1.031 | 0.858 | 1.217 | .144 |
| With | 20 | 90,582.66 | 22.08 | 29 | 357,682.14 | 8.11 | 1.683 | 1.399 | 1.986 | <.001 |
| Dementia | ||||||||||
| Without | 45 | 374,810.26 | 12.01 | 110 | 1840,593.04 | 5.98 | 1.241 | 1.032 | 1.465 | .016 |
| With | 2 | 9338.24 | 21.42 | 3 | 41,469.68 | 7.23 | 1.829 | 1.521 | 2.159 | <.001 |
| CHF | ||||||||||
| Without | 44 | 362,280.19 | 12.15 | 107 | 1787,153.47 | 5.99 | 1.253 | 1.042 | 1.479 | .008 |
| With | 3 | 21,868.31 | 13.72 | 6 | 94,909.25 | 6.32 | 1.341 | 1.115 | 1.582 | <.001 |
| CVD | ||||||||||
| Without | 42 | 352,966.41 | 11.90 | 104 | 1743,491.95 | 5.97 | 1.232 | 1.025 | 1.455 | .023 |
| With | 5 | 31,182.09 | 16.03 | 9 | 138,570.77 | 6.49 | 1.525 | 1.268 | 1.800 | <.001 |
| COPD | ||||||||||
| Without | 41 | 346,968.30 | 11.82 | 101 | 1713,580.49 | 5.89 | 1.239 | 1.030 | 1.462 | .020 |
| With | 6 | 37,180.20 | 16.14 | 12 | 168,482.23 | 7.12 | 1.400 | 1.164 | 1.652 | <.001 |
| Liver disease | ||||||||||
| Without | 36 | 342,162.22 | 10.52 | 102 | 1705,010.59 | 5.98 | 1.087 | 0.904 | 1.283 | .095 |
| With | 11 | 41,986.28 | 26.20 | 11 | 177,052.13 | 6.21 | 2.605 | 2.167 | 3.075 | <.001 |
| HIV | ||||||||||
| Without | 47 | 383,249.33 | 12.26 | 113 | 1878,102.47 | 6.02 | 1.259 | 1.047 | 1.486 | <.001 |
| With | 0 | 899.17 | 0.00 | 0 | 3960.25 | 0.00 | - | - | - | - |
| CKD | ||||||||||
| Without | 40 | 334,635.72 | 11.95 | 102 | 1701,895.50 | 5.99 | 1.232 | 1.025 | 1.454 | .023 |
| With | 7 | 49,512.78 | 14.14 | 11 | 180,167.22 | 6.11 | 1.431 | 1.190 | 1.689 | <.001 |
| Colorectal polyp | ||||||||||
| Without | 41 | 362,549.84 | 11.31 | 110 | 1853,871.72 | 5.93 | 1.178 | 0.979 | 1.390 | .077 |
| With | 6 | 21,598.66 | 27.78 | 3 | 28,191.00 | 10.64 | 1.613 | 1.341 | 1.904 | <.001 |
| IBD | ||||||||||
| Without | 44 | 379,826.65 | 11.58 | 111 | 1872,082.58 | 5.93 | 1.207 | 1.004 | 1.425 | .045 |
| With | 3 | 4321.85 | 69.41 | 2 | 9980.14 | 20.04 | 2.140 | 1.780 | 2.526 | <.001 |
Adjusted HR = Adjusted Hazard ratio: Adjusted for variables in Table 3.
CHF = congestive heart failure, CI = confidence interval, CKD = chronic kidney disease, COPD = chronic pulmonary disease, CVD = cerebrovascular disease, DM = diabetes mellitus, HIV = human immunodeficiency virus, IBD = inflammatory bowel disease, MI = myocardial infarction, NAFLD = nonalcoholic fatty liver disease, PVD = peripheral vascular disease, PYs = person-yr.
*P values < .05 were considered statistically significant.
CRC was further classified into 3 subtypes according to the anatomic region, namely right-sided colon cancer, left-sided colon cancer, and rectum cancer. The incidence and adjusted HRs of CRC in both groups are presented in Table 4. Patients with NAFLD were more likely to develop malignancy in all anatomic regions (right-sided colon: adjusted HR = 1.234, 95% CI = 1.027–1.456; left-sided colon: adjusted HR = 1.405, 95% CI = 1.165–1.658; rectum: adjusted HR = 1.378, 95% CI = 1.144–1.629).
Table 4.
Incidence and aHR for CRC stratified by different sites.
| NAFLD | With | Without (Reference) | With vs without (Reference) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CRC subgroups | Events | PYs | Rate (per 105 PYs) | Events | PYs | Rate (per 105 PYs) | Adjusted HR | 95%CI | 95%CI | P |
| Overall CRC | 47 | 384,148.50 | 12.23 | 113 | 1882,062.72 | 6.00 | 1.259 | 1.047 | 1.486 | .003 |
| Colon cancer, right side | 11 | 384,148.50 | 7.29 | 27 | 1882,062.72 | 1.43 | 1.234 | 1.027 | 1.456 | .024 |
| Colon cancer, left side | 19 | 384,148.50 | 4.69 | 41 | 1882,062.72 | 2.18 | 1.405 | 1.165 | 1.658 | <.001 |
| Rectal cancer | 5 | 384,148.50 | 0.26 | 11 | 1882,062.72 | 0.58 | 1.378 | 1.144 | 1.629 | <.001 |
Adjusted HR = Adjusted Hazard ratio: Adjusted for variables in Table 3.
CI = confidence interval, NAFLD = nonalcoholic fatty liver disease, PYs = person-yr.
4. Discussion
In this retrospective population-based cohort study, we enrolled 60,298 patients with NAFLD from a large database to clarify whether NAFLD is associated with a risk of CRC in the Taiwanese population that was monitored for a mean follow-up period of 8 years. We observed that patients with NAFLD had a higher risk of CRC than controls, with an adjusted HR of 1.295. The incidence of CRC in patients with NAFLD was 12.23 per 100,000 person-years, which was 2 times higher than that in the comparison group, after adjustment for age, sex, and comorbidities.
The mechanisms underlying the development of CRC in patients with NAFLD are not fully elucidated yet. Currently, 4 hypotheses have been postulated, the most well-known being insulin resistance caused by hyperinsulinemia and the insulin-like growth factor-1 axis, which leads to antiapoptotic and proliferative effects. Another hypothesis is imbalanced adipokine secretion due to adipose tissue dysfunction. For example, adiponectin has anticarcinogenic effects, and leptin has proneoplastic effects. Because the balance of adipokines is disrupted in NAFLD, a neoplasm may develop.[14] A third hypothesis is that chronic inflammation induces the release of several proinflammatory cytokines such as interleukin-6 and tumor necrosis factor-α, which stimulate cell proliferation and promote tumor progression.[15] The final hypothesis is dysbiosis of the gut microbiota, which has been recognized to play a key role in CRC development.[16] The majority of CRCs begin as aberrant crypt foci, which evolve into polyps and eventually progress to CRC over an estimated period of 10 to 15 years.[10] Previous studies have reported a connection between several types of colon polyps and NAFLD.[17,18] However, the association between NAFLD and CRC is controversial, although most studies have reported a positive correlation. Ahn et al recruited 26,540 asymptomatic patients who underwent first-time colonoscopy and abdominal ultrasonography on the same day. They observed that patients with NAFLD had a higher prevalence of advanced colorectal neoplasia (defined as invasive cancer, adenoma >10 mm in diameter, and high-grade dysplasia) or had villous histological characteristics compared with those without NAFLD (2.8% vs 1.9%, P < .001).[19] In a large cohort study conducted in China, Lin et al investigated the relationship between NAFLD and colorectal malignant neoplasm. They observed a higher prevalence of colorectal malignant neoplasm in patients with NAFLD (29.3% vs 18.0%, P < .05), especially sigmoid colon cancer and highly differentiated cancer.[20] However, other studies have reported contrasting findings. Basyigit et al conducted a prospective observational study involving 127 participants and reported that the prevalence of CRC was significantly lower in patients with NAFLD (odds ratio = 7.380, 95% CI = 3.069–7.961, P = .010) and was significantly associated with the presence of insulin resistance (odds ratio = 5.023, 95% CI = 1.789–9.789, P = .001). They attributed this finding to the catabolism of tumor cells, which led to decreased hepatic fat deposition and further NAFLD.[12]
Several established risk factors, including central obesity, metabolic syndrome, excessive alcohol consumption, and type 2 DM, are associated with CRC.[21–24] In our study, patients with DM in the NAFLD group had a significantly higher risk of CRC (adjusted HR = 1.683, 95% CI = 1.399–1.986, P < .001) than those in the comparison group. Previous research has indicated that insulin is a crucial growth factor of colonic epithelial cells and is a mitogen of tumor cell growth; hence, hyperinsulinemia may promote colon carcinogenesis.[25] Evidence suggests that exogenous insulin may increase the risk of CRC, whereas metformin decreases the incidence of CRC.[26,27] In 2011, Yuhara et al conducted a meta-analysis of 14 studies and concluded that DM increases CRC risk. Their study further demonstrated that the association of DM with cancer risk was stronger for colon cancer (RR: 1.38, 95% CI: 1.26–1.51) than for rectal cancer (RR 1.20, 95% CI: 1.09–1.31).[24]
In Taiwan, the prevalence of chronic hepatitis B virus (HBV) infection was between 15% and 20% prior to the introduction of the national HBV vaccination program in 1984. This high prevalence was reduced to <1% in people born after 1922.[28] Notwithstanding this successful vaccination program, HBV hepatitis (28.5%) remains the second most common cause of abnormal liver function test results in Taiwan after NAFLD (33.6%).[29] In our study, patients with concomitant liver disease had a higher risk of CRC (adjusted HR = 2.605, 95% CI = 2.167–3.075, P < .001). This finding is supported by the results of Su et al.[30] Their population-based case–control study enrolled 139,496 Taiwanese patients, and they concluded that chronic HBV infection was associated with CRC development. The association between chronic HBV infection and CRC was also validated in other studies involving Asian populations.[31–33]
In our subgroup analysis, we observed that in the NAFLD group, patients aged between 50 and 59 years and those older than 60 years had an increased risk of CRC. Several previous studies have suggested that patients with NAFLD may develop colorectal neoplasia at an earlier age. Wong et al observed that both female and male patients with NAFLD aged between 40 and 50 years had a higher prevalence of colorectal adenoma, adenoma with villous architecture, high grade dysplasia, and cancer. In another retrospective study published in 2020, Lesmana et al confirmed that NAFLD was associated with an increased risk of any histological type of colon polyps, including adenomatous polyp, dysplasia, and adenocarcinoma, implying the need to perform screening colonoscopy in patients with NAFLD. Additionally, they stated that screening colonoscopy should be started earlier because 25% of patients younger than 50 years presented with polyps.[34] Other major modifiable risk factors for CRC include obesity, excessive alcohol consumption, sedentary lifestyle, diabetes, and high consumption of red meat and processed meat.[35] Neither the latest international CRC screening guidelines (e.g., the United States Preventive Services Taskforce guidelines) nor the updated Asia Pacific Consensus Recommendations for Colorectal Cancer Screening regards NAFLD as a major risk factor that would necessitate altering screening recommendations.[36,37] Our study revealed that patients with NAFLD who were younger than 50 years were not at risk of CRC. Because colonoscopy is an invasive procedure accompanied by the risks of bloating, hemorrhage, splenic rupture, and perforation, weighing the cost-effectiveness of earlier screening program is critical.
Embryological derivatives, molecular features, and prognosis differ between right-sided and left-sided colon cancer. The sidedness of CRC also influences the choice of biological therapy. Furthermore, right-sided colon cancer has a poor prognosis than left-sided colon cancer.[10] In a meta-analysis of 11 articles with 12,081 patients, Lin et al concluded that NAFLD in Asian patients exhibited a high risk of right-sided colon tumors.[38] However, in our study, patients with NAFLD had a higher incidence of left-sided colon cancer than of right-sided cancer. The exact mechanisms by which NAFLD is associated with the laterality of CRC are currently uncertain.
The strength of the present study was that we examined the association between NAFLD and CRS in a large sample using a population-based design. We, however, acknowledge several limitations. First, the NHIRD does not provide laboratory, pathology, or imaging information such as that from abdominal ultrasonography, computed tomography, and magnetic resonance imaging. Because fatty liver index, which comprises body mass index, waist circumference, triglyceride, and gamma γ-glutamyl transferase, is assumed to accurately identify NAFLD in Chinese patients,[39] the accuracy of diagnosis cannot be reconfirmed in our study because measurements may not have been rigorously standardized. Second, some confounding factors were present, such as excessive consumption of processed meat or red meat and moderate or heavy alcohol drinking. Such lifestyle factors are risk factors for CRC, but we could not retrieve any related data from the NHIRD. Finally, the results are limited by the uniform ethnic background of the patients.
5. Conclusion
Patients with NAFLD may have a 1.259-fold increased risk of CRC compared with those without NAFLD. This finding might alert physicians to identify NAFLD patients who may require timely screening colonoscopy. Further research is necessary to explore the mechanisms underlying the association between NAFLD and CRC.
Author contributions
Conceptualization: Ying-Hsiang Wang, Yi-Chiao Cheng.
Data curation: Chi-Hsiang Chung.
Formal analysis: Chi-Hsiang Chung.
Methodology: Ying-Hsiang Wang, Wu-Chien Chien.
Resources: Je-Ming Hu.
Supervision: Je-Ming Hu, Wu-Chien Chien, Yi-Chiao Cheng.
Writing – original draft: Po-Hsien Wu.
Writing – review & editing: Po-Hsien Wu.
Supplementary Material
Abbreviations:
- CHF
- congestive heart failure
- CI
- confidence interval
- CKD
- chronic kidney disease
- COPD
- chronic obstructive pulmonary disease
- CRC
- colorectal cancer
- CVD
- cerebrovascular disease
- DM
- diabetes mellitus
- HBV
- hepatitis B virus
- HR
- hazard ratio
- IBD
- inflammatory bowel disease
- ICD-9-CM
- international classification of diseases, 9th revision, clinical modification
- LHID
- longitudinal health insurance database
- MI
- myocardial infarction
- NAFLD
- nonalcoholic fatty liver disease
- NHIRD
- National Health Insurance Research Database
Supplemental Digital Content is available for this article.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
This study was supported by the Tri-Service General Hospital Research Foundation (TSGH-B-112020).
The authors have no conflicts of interest to disclose.
How to cite this article: Wu P-H, Chung C-H, Wang Y-H, Hu J-M, Chien W-C, Cheng Y-C. Association between nonalcoholic fatty liver disease and colorectal cancer: A population-based study. Medicine 2023;102:21(e33867).
Contributor Information
Po-Hsien Wu, Email: jackwu0523@gmail.com.
Chi-Hsiang Chung, Email: g694810042@gmail.com.
Ying-Hsiang Wang, Email: imma031@yahoo.com.tw.
Je-Ming Hu, Email: Jeminghu@gamil.com.
Wu-Chien Chien, Email: chienwu@ndmctsgh.edu.tw.
References
- [1].Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- [2].Chen YY, Yeh MM. Non-alcoholic fatty liver disease: a review with clinical and pathological correlation. J Formos Med Assoc. 2021;120(1 Pt 1):68–77. [DOI] [PubMed] [Google Scholar]
- [3].Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–73. [DOI] [PubMed] [Google Scholar]
- [4].Puri P, Sanyal AJ. Nonalcoholic fatty liver disease: definitions, risk factors, and workup. Clin Liver Dis (Hoboken). 2012;1:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Calzadilla Bertot L, Adams LA. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci. 2016;17:774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–24. [DOI] [PubMed] [Google Scholar]
- [7].Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- [8].Health Promotion Administration Ministry of Health and Welfare, Taiwan. Cancer Registry Annual Report, 2019, HPA, 2022.
- [9].Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. [DOI] [PubMed] [Google Scholar]
- [10].Dekker E, Tanis PJ, Vleugels JLA, et al. Colorectal cancer. Lancet. 2019;394:1467–80. [DOI] [PubMed] [Google Scholar]
- [11].Lee YI, Lim YS, Park HS. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: a retrospective cohort study. J Gastroenterol Hepatol. 2012;27:91–5. [DOI] [PubMed] [Google Scholar]
- [12].Basyigit S, Uzman M, Kefeli A, et al. Absence of non-alcoholic fatty liver disease in the presence of insulin resistance is a strong predictor for colorectal carcinoma. Int J Clin Exp Med. 2015;8:18601–10. [PMC free article] [PubMed] [Google Scholar]
- [13].Touzin NT, Bush KN, Williams CD, et al. Prevalence of colonic adenomas in patients with nonalcoholic fatty liver disease. Therap Adv Gastroenterol. 2011;4:169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Parizadeh SM, Parizadeh SA, Alizade-Noghani M, et al. Association between non-alcoholic fatty liver disease and colorectal cancer. Expert Rev Gastroenterol Hepatol. 2019;13:633–41. [DOI] [PubMed] [Google Scholar]
- [15].Sanna C, Rosso C, Marietti M, et al. Non-alcoholic fatty liver disease and extra-hepatic cancers. Int J Mol Sci. 2016;17:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fan X, Jin Y, Chen G, et al. Gut microbiota dysbiosis drives the development of colorectal cancer. Digestion. 2021;102:508–15. [DOI] [PubMed] [Google Scholar]
- [17].Blackett JW, Verna EC, Lebwohl B. Increased prevalence of colorectal adenomas in patients with nonalcoholic fatty liver disease: a cross-sectional study. Dig Dis. 2020;38:222–30. [DOI] [PubMed] [Google Scholar]
- [18].Lesmana CRA, Pakasi LS, Sudoyo AW, et al. The clinical significance of colon polyp pathology in Nonalcoholic Fatty Liver Disease (NAFLD) and its impact on screening colonoscopy in daily practice. Can J Gastroenterol Hepatol. 2020;2020:6676294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ahn JS, Sinn DH, Min YW, et al. Non-alcoholic fatty liver diseases and risk of colorectal neoplasia. Aliment Pharmacol Ther. 2017;45:345–53. [DOI] [PubMed] [Google Scholar]
- [20].Lin XF, Shi KQ, You J, et al. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: a large study. Mol Biol Rep. 2014;41:2989–97. [DOI] [PubMed] [Google Scholar]
- [21].Esposito K, Chiodini P, Colao A, et al. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35:2402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Karahalios A, English DR, Simpson JA. Weight change and risk of colorectal cancer: a systematic review and meta-analysis. Am J Epidemiol. 2015;181:832–45. [DOI] [PubMed] [Google Scholar]
- [23].LoConte NK, Brewster AM, Kaur JS, et al. Alcohol and cancer: a statement of the American Society of Clinical Oncology. J Clin Oncol. 2018;36:83–93. [DOI] [PubMed] [Google Scholar]
- [24].Yuhara H, Steinmaus C, Cohen SE, et al. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am J Gastroenterol. 2011;106:1911–21; quiz 1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6:164–79. [DOI] [PubMed] [Google Scholar]
- [26].Chen X, Liang H, Song Q, et al. Insulin promotes progression of colon cancer by upregulation of ACAT1. Lipids Health Dis. 2018;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].González N, Prieto I, Del Puerto-Nevado L, et al. 2017 update on the relationship between diabetes and colorectal cancer: epidemiology, potential molecular mechanisms and therapeutic implications. Oncotarget. 2017;8:18456–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hu YC, Yeh CC, Chen RY, et al. Seroprevalence of hepatitis B virus in Taiwan 30 years after the commencement of the national vaccination program. PeerJ. 2018;6:e4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen C-H, Huang M-H, Yang J-C, et al. Prevalence and etiology of elevated serum alanine aminotransferase level in an adult population in Taiwan. J Gastroenterol Hepatol. 2007;22:1482–9. [DOI] [PubMed] [Google Scholar]
- [30].Su FH, Le TN, Muo CH, et al. Chronic hepatitis B virus infection associated with increased colorectal cancer risk in Taiwanese population. Viruses. 2020;12:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jung YS, Kim NH, Park JH, et al. Correlation between hepatitis B virus infection and colorectal neoplasia. J Clin Med. 2019;8:2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Song C, Lv J, Liu Y, et al. Associations between hepatitis B virus infection and risk of all cancer types. JAMA Netw Open. 2019;2:e195718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu T, Li W, Zhang Y, et al. Associations between hepatitis B virus infection and risk of colorectal cancer: a population-based prospective study. BMC Cancer. 2021;21:1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lesmana CRA, Pakasi LS, Sudoyo AW, et al. The clinical significance of colon polyp pathology in Nonalcoholic Fatty Liver Disease (NAFLD) and its impact on screening colonoscopy in daily practice. Can J Gastroenterol Hepatol. 2020;2020:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gunter MJ, Alhomoud S, Arnold M, et al. Meeting report from the joint IARC-NCI international cancer seminar series: a focus on colorectal cancer. Ann Oncol. 2019;30:510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sung JJ, Ng SC, Chan FK, et al. An updated Asia Pacific Consensus Recommendations on colorectal cancer screening. Gut. 2015;64:121–32. [DOI] [PubMed] [Google Scholar]
- [37].Force UPST. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2021;325:1965–77. [DOI] [PubMed] [Google Scholar]
- [38].Lin X, You F, Liu H, et al. Site-specific risk of colorectal neoplasms in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. PLoS One. 2021;16:e0245921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Huang X, Xu M, Chen Y, et al. Validation of the fatty liver index for nonalcoholic fatty liver disease in middle-aged and elderly Chinese. Medicine (Baltimore). 2015;94:e1682. [DOI] [PMC free article] [PubMed] [Google Scholar]