Abstract
Plasmacytomas are a rare spectrum of plasma cell neoplasms that are single localized tumours, lacking the clinical features of plasma cell myeloma with no radiographical evidence of additional plasma cell tumours. Two clinical variants of plasmacytomas can be distinguished: solitary plasmacytoma of bone and extramedullary (or extraosseous) plasmacytoma. The latter is rare, representing 1% of all plasma cell neoplasms, occurring most frequently in the upper airways. Ovarian localization is exceptional, with only a few cases being reported in the literature. We herein report a case of an ovarian extramedullary plasmacytoma occurring in a 56-year-old woman who consulted for abdominal pain and abdominal mass, while highlighting the main histological and immunohistochemical features of this rare malignancy, along with a thorough review of literature gathering all cases of ovarian plasmacytomas reported to date.
Keywords: Extramedullary plasmacytoma, ovary, plasma cell neoplasm, case report
Introduction
Extramedullary plasmacytomas are a group of neoplasms resulting from the clonal expansion of mature plasma cells, located in tissues other than bone. They represent 1% of plasma cell neoplasms and occur most often in the upper airways. Ovarian plasmacytomas are an exceptional presentation, with only rare cases reported in the literature. We herein present a case of ovarian plasmacytoma, while highlighting the main histological and immunohistochemical features along with a thorough review of literature, regarding presentation and diagnostic workup.
Patient and observation
Patient information: a 56-year-old woman with a history of breast neoplasia for which she had a mastectomy, consulted for abdominal pain.
Clinical findings: physical examination revealed abdominal tenderness and an abdominal mass.
Timeline of the current episode: on October 2021, an abdominal-pelvic computed tomography (CT) scan was performed revealing a right ovarian mass, along with a normal biological workup. A few days after, the patient underwent a right adnexectomy.
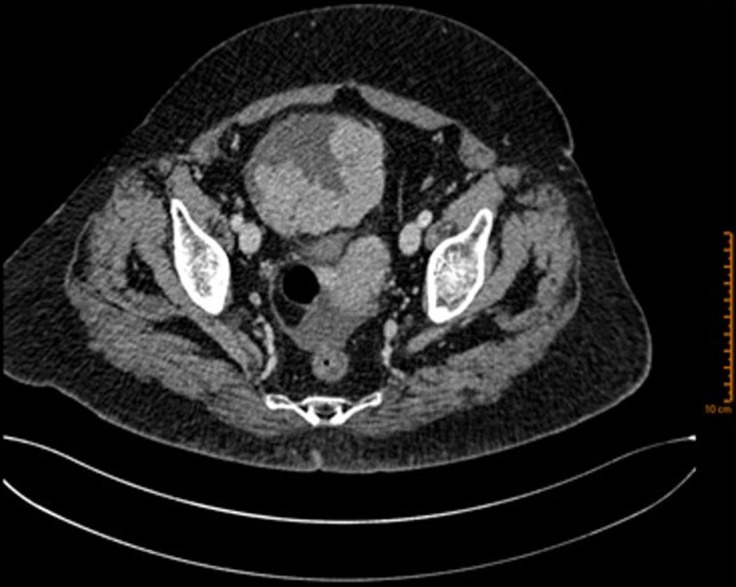
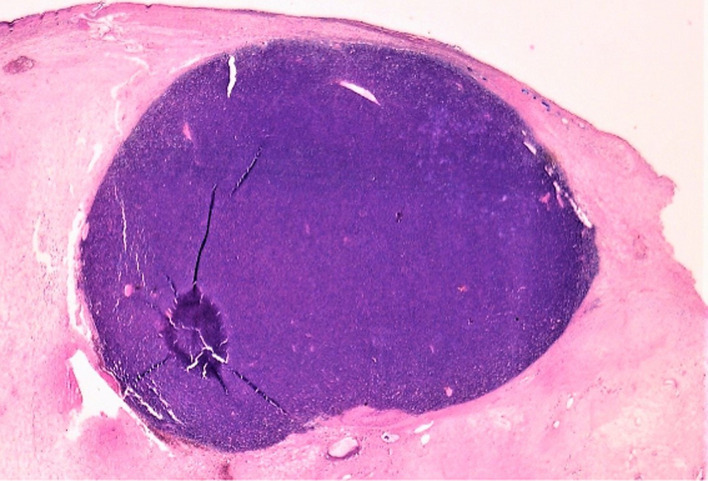
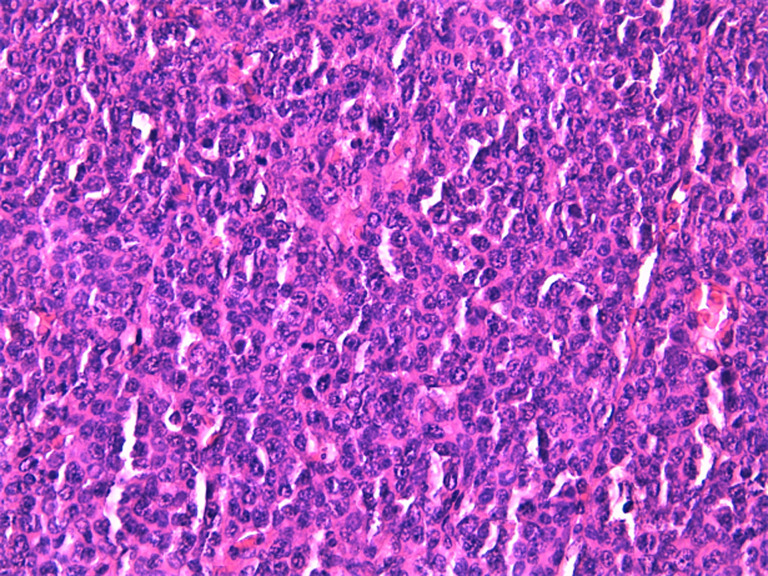
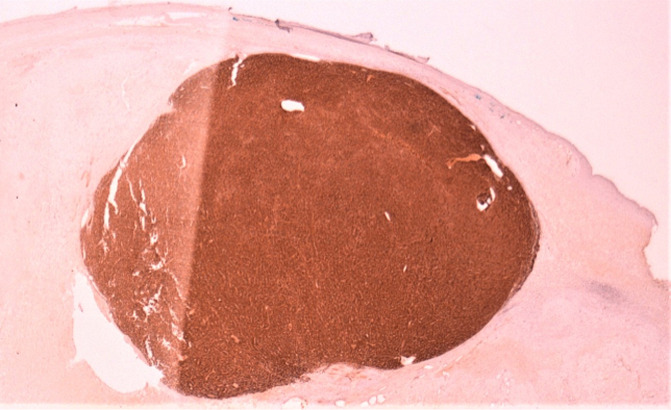
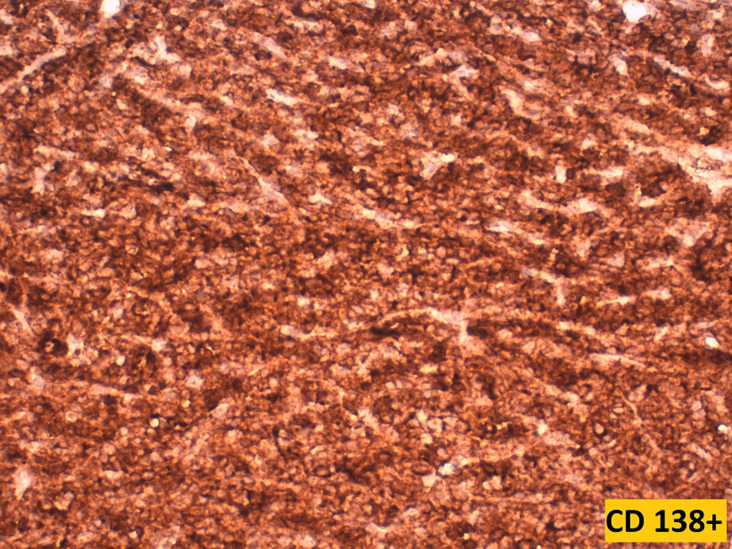
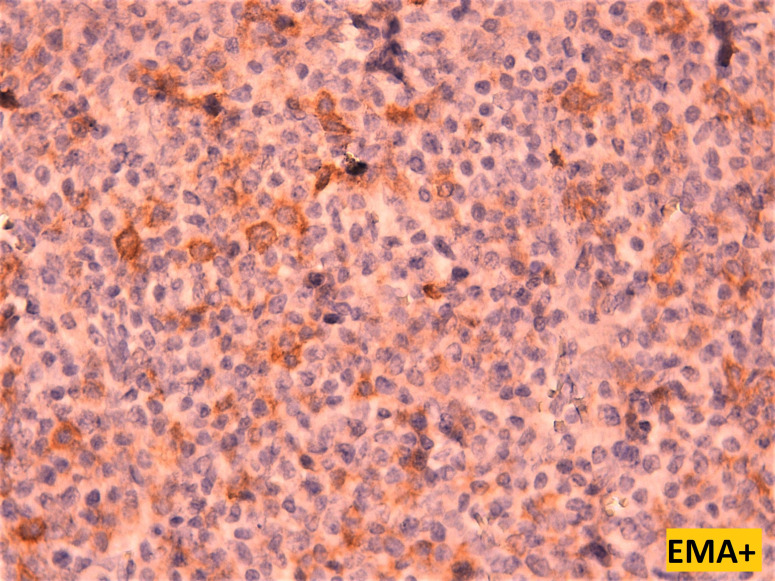
Diagnostic assessment: the abdominal-pelvic CT scan revealed a right ovarian mass, displaying a prevailing cystic component and a solid component enhanced after injection of contrast agent (Figure 1). The biological workup was normal, including the absence of a monoclonal gamma peak on protein electrophoresis, and a bone marrow plasmacytosis estimated at 2%. A right adnexectomy was performed. Macroscopically, the ovarian mass measured 20 x 12 x 7 cm, showing a solid and multilocular cystic appearance at the cut, with yellowish serous content. The wall of the cysts was the site of multiple scattered whitish nodules. Histological examination showed a tumoral proliferation arranged in well-limited nodules (Figure 2), made of sheets of tumoral cells showing a plasmocytic morphology, with abundant eosinophilic cytoplasm and rounded eccentric nucleus with coarsely clumped chromatin (Figure 3). The tumoral cells showed diffuse membranous expression of CD138 (Figure 4, Figure 5), as well as an expression of epithelial membrane antigen (EMA) in a more heterogeneous manner (Figure 6). Ki67 was estimated at 80% (Figure 7).
Figure 1.
abdominal-pelvic CT scan showing an oval, well-limited right ovarian mass, with a prevailing cystic component and a solid component enhanced after injection of contrast agent
Figure 2.
low power view showing a tumoral proliferation arranged in a well limited nodule within the ovarian cyst´s wall (hematoxylin and eosin stain, magnification x4)
Figure 3.
high power view showing diffuse sheets of tumoral cell displaying a plasmacytoid morphology, with rounded eccentric nucleus, coarse clumped chromatin, and abundant eosinophilic cytoplasm (hematoxylin and eosin stain, magnification x40)
Figure 4.
low power view demonstrating diffuse expression of CD138 by the tumoral cells (magnification x4)
Figure 5.
high power view showing strong and diffuse membranous expression of CD138 by tumoral cells (magnification x40)
Figure 6.
high power view showing patchy membranous and cytoplasmic staining by EMA of the tumoral cells (magnification x40)
Figure 7.
Ki67 estimated at 80%
Diagnosis: the diagnosis of ovarian extramedullary plasmacytoma was sustained, based on morphological and immunohistochemical features, after ruling out a plasma cell myeloma.
Therapeutic interventions: the patient underwent a right adnexectomy.
Follow-up and outcome of interventions: to date, the patient remains asymptomatic and in excellent clinical condition, with no reported complications.
Patient perspective: the patient remarked “I feel well”.
Informed consent: the patient gave informed consent.
Discussion
Plasmacytomas are a group of neoplasms resulting from a clonal expansion of mature plasma cells, typically synthesizing monoclonal immunoglobulin and causing a monoclonal gammapathy. They usually lack the clinical features of plasma cells myeloma, with no clinical or radiological evidence of additional plasma cell tumors. Two clinical variants of plasmacytomas can be distinguished: solitary plasmacytoma of bone and extramedullary (or extraosseous) plasmacytoma [1]. Extramedullary plasmacytoma is rare, accounting for 1% of all plasma cell neoplasm. They occur most commonly in the upper aerodigestive tract, but other localizations are possible such as lymph nodes, bladder, breasts, thyroid, testes, parotid glands, skin, and central nervous system [2]. The ovarian localization remains exceptional. Only a few cases of ovarian plasmacytomas have been reported in literature, all of them being reported as single cases. In our review of the bibliography, we have listed 18 cases, including our present case (Table 1) [2-20].
Table 1.
cases of ovarian plasmacytomas reported by literature in chronological order
| Author | Age | Symptoms | Ovary | Size | Macroscopic appearance | Immunohistochemistry |
|---|---|---|---|---|---|---|
| Voght, 1938 | 30 | Unilateral | Size of a fist | |||
| Bambirra, 1982 | 44 | Abdominal pain | Bilateral | R 14.3 x 5.3 x 4 cm ; L 12.3 x 9 x 36 cm | ||
| Hautzer, 1984 | 56 | Abdominal mass | Left | 24 x 14 x 11 cm | ||
| Talerman, 1987 | 35 | Abdominal mass | Unilateral | 15 x 12 x 9 cm | ||
| Cook, 1988 | 63 | Abdominal pain, distension abdominal mass | Left | 12 x 10 x 7 cm | Smooth outer surface; cut surface solid and lobulated, pale yellow | Alpha heavy chains+, kappa light chains+ |
| Andze, 1993 | 12 | Pelvic mass | Left | 12.3 x 8 x 3.8 cm | ||
| Emery, 1999 | 54 | Abdominal swelling and tenderness | Left | 15 x 12.5 x 7.6 cm | Smooth outer surface; on cut section, the ovarian parenchyma is replaced by a soft, tan, neoplasm with focal areas of hemorrhage | CD43+, kappa light chains+, CD20-, CD45- , lambda immunoglobulin light chains - |
| Ben Salah, 2011 | 36 | Pelvic mass | Bilateral | 20x 18 x 16 cm | ||
| Naourez, 2011 | 36 | Abdominal pain, abdominal, distension and metrorrhagia | Bilateral | Left: 35 cm; right: 30 cm | Solid at cut, with lobulated appearance; tumor capsule was intact | CD138+, lambda light chains+ |
| Zhong YP, 2012 | 54 | Abdominal pain | Right | 12 x 12 x 10 cm | The tumor capsule was intact, with thick walls; gray color | CD38+, CD138+, CD45+, CD99+, Ki-67: 90% |
| Shakuntala, 2012 | 35 | Abdominal mass and intermittent pain | Right | 14 x 13.5 x 6 cm | Solid and cystic areas | CD138+, CK+, Lambda light chains+, EMA focally +; negative for CD79a and kappa light chains |
| Feldman, 2015 | 84 | Weight loss, constipation, dizziness, palpitation, loss of appetite; abdominal mass | Right | 14 cm | Smooth capsule focally calcified; cut surface shows multiple cysts with myxoid, rubbery and hemorrhagic septa and contents | CD138+, kappa light chains+; CK-, CD20-, CD3-, CD5-, BCL1-PAX5- |
| Mondal, 2015 | 47 | Abdominal pain | Left | 10 x 9.5 x 6 cm | Exclusively cystic with yellowish content; thickness of cyst wall is 2.1 cm; inner wall showed multiple tiny nodules and striations; necrotic material was seen; within the thickened portion of cyst wall | CD138+, CD38+, CD45+, CK-, inhibin-, synaptophysin, desmin-, CD99- |
| Tomaselli, 2016 | 46 | Abdominal mass and pain | Right | 15x14x10cm | Very vascular ovarian mass with solid cystic areas | CD138+, lambda light chains+, CD20- |
| Cardenas, 2020 | 48 | Abdominal pain | Bilateral | 10 cm each | ||
| Fares, 2021 | 52 | Ovarian mass detected at pelvic ultrasound for recurring episodes of metrorrhagia | Left | 4 x 3.5 x 3 cm | Well-circumscribed, encapsulated, and white tumor | CD138+, EMA+, lambda light chain restriction; CK-, SMA-, inhibin- H-caldesmone-; Ki67: 10% |
| El amouri, 2022 | 42 | Abdominal distension | Left | 18 x 8 x 5 cm | Grayish-brown solid surface with some cystic changes; smooth outer surface | CD138+, CD45+, CD79a focal+; kappa light chains+; CD20-, CD3-, MPO-, Inhibin-CK-, CD68- Ki67: 40% |
| Present case, 2022 | 56 | Abdominal mass and pain | Right | 20 x 12 x 7 cm | Solid and multilocular cystic appearance at cut; yellow whitish, yellowish serous content | CD138+; EMA+ heterogenous; CK-, Chromogranin- inhibin- CD20-, PAX5-, CD3- |
The age of presentation is quite variable, ranging from 30 to 84 years old, with a mean age of 46 years. Only one case affecting a 12-year-old child was described [3]. The left ovary appears to be slightly more affected, although cases with bilateral localization have also been reported. When comparing all cases reported in the literature including our case, 7 cases involved the left ovary, 5 cases involved the right ovary, and 4 other cases were bilateral. Only two cases were reported as unilateral, with no data regarding the laterality of the tumor [4,5]. Clinically, patients usually present with symptoms related to ovarian mass, such as pelvic pain and abdominal mass. The tumor is usually diagnosed at a large size. The largest reported size was a 35 cm long axis, while the minimal reported size was a 4 cm long axis, which is somewhat reminiscent of the first case reported by Voegt in 1938 who described the tumor as the size of a fist [5]. The large size at the time of the presentation is more likely due to the insidious progression of this tumor. In our case, the patient complained of abdominal pain with an abdominal mass on physical examination. The abdominal CT scan revealed a right ovarian mass measuring 20 x 12 x 7 cm, which is consistent with the literature data.
Ovarian plasmacytoma generally presents as a mass with a smooth outer surface, usually with a thick capsule, which may focally be calcified. At incision, the ovarian parenchyma can be partially or completely replaced by a lobulated solid mass of soft consistency, ranging in colors from tan to yellow, to white or gray, brown. It can also be exclusively cystic with thick walls, or have a dual component, with both solid and cystic areas. Hemorrhagic, necrotic, and myxoid areas may also occur (Table 1). In our case, the mass displayed a solid and multiloculated cystic appearance on a cut, of yellowish-white color, with a serious yellowish content.
The histological appearance of the tumoral proliferation was broadly consistent in all cases reported in literature, with tumor cells displaying a plasmacytoid morphology with an eccentric nucleus and abundant cytoplasm. The main issue remains to think of a plasmacytoma at first glance. Since ovarian localization of this tumor is relatively rare, pathologists may be reluctant to make this diagnosis based on morphology alone and would be more tempted to rule out the most common diagnoses first, such as a granulosa tumor. Therefore, the definitive diagnosis of ovarian plasmacytoma has always been made after immunochemistry, along with a correlation of clinical, radiological, and biological findings.
Plasma cell differentiation can be evaluated by the expression of CD138, CD38, CD79a and MUM1. A further characteristic is the strong expression of restricted cytoplasmic light chains (kappa or lambda) in almost all cases, which can be useful in distinguishing a monotypic plasmacytoma from polytypic reactive plasma cell infiltrates. Usually, mature neoplastic plasma cells will not express pan-B-cell markers, such as CD20, PAX5. However, CD20 can be expressed in 20% of plasma cell myeloma, and Cyclin D1 is positive in cases that harbors t (11;14) and in some case with hyperdiploidy [6]. Unlike normal plasma cells, CD45 is usually negative or expressed at low levels, while CD56 can be seen in 70% of plasma cell myeloma [7]. The immunophenotype of extramedullary plasmacytomas appears to be similar to that of plasma cell myeloma. However, extraosseous plasmacytoma typically lacks the expression of CD56 and cyclin D1. In our literature review, we noticed that several different panels were used, probably depending on the initial suspicion of the pathologist when confronted with a tumor proliferation made of cells of such a monomorphic appearance. CD138 was widely used to confirm plasmacytoid differentiation of the tumor cells, along with CD38 in two cases, and CD79a in one case. EMA was reported to be focally expressed in 3 cases, including ours. CD45 when performed, was positive in 3 cases, and negative in two cases. EMA was reported to be focally expressed in 3 cases, including our own case. Kappa light chains and lambda light chains were respectively positive in 4 cases each (Table 1).
Since extramedullary plasmacytomas share with multiple myeloma the same morphological appearance of the tumor cells, and their immunohistochemical profile is relatively similar, the distinction requires a variety of additional clinical, biological, and radiological arguments. Diagnostic criteria for extramedullary plasmacytoma include histologic confirmation of plasma cell proliferation, bone marrow plasmacytosis of less than 5% on bone marrow biopsy specimens, the absence of clinical features of multiple myeloma, and normal bone imaging [6]. The distinction with multiple myeloma is important since more than 60% of patients who are treated for solitary plasmacytoma are cured with only local therapies, while the 5-year survival for patients with plasma cell myeloma is around 35% [8]. Progression to multiple myeloma is the most feared complication, occurring in about 15% of cases, particularly in the setting of minimal bone marrow involvement [6]. Regional recurrences develop in as many as 25% of cases, with the possibility of distant extraosseous metastasis. Thus, active surveillance afterward is indicated. The National Comprehensive Cancer Network (NCCN) guidelines recommend regular complete blood pictures, serum chemistries (LDH, albumin, calcium, beta 2 microglobulin), serum immunoglobulins, serum and urine-free light chain assays, and imaging [9].
Conclusion
Extramedullary plasmacytomas in the ovary remain rare but possible neoplasms, justifying a meticulous morphological examination in the quest for plasmacytoid differentiation, particularly when confronted with a monomorphic tumoral proliferation with eccentric nuclei. The incorporation of plasma cell markers along with the immunoglobulin light chain in the immunohistochemistry panel for such ovarian proliferations can be of great help in challenging cases. It is of utmost importance to eliminate bone marrow plasmacytosis and bone involvement that may be part of multiple myeloma, given the therapeutic and prognostic disparity implied.
Footnotes
Cite this article: Anass Haloui et al. Ovarian plasmacytoma: a case report. Pan African Medical Journal. 2023;44(108). 10.11604/pamj.2023.44.108.37603
Competing interests
The authors declare no competing interests.
Authors' contributions
Manuscript drafting: Anass Haloui; data collection: Anass Haloui, Nassira Karich, Nada Akouh, Noura Seghrouchni, Younesse Najioui, Asmae Aissaoui, El Mehdi Tiabi, Samia Malki, Samah Tahri, Houda Bachir, Abbou Widad, Rim Ouajdi, Imane Kamaoui, Mhand Mohammed, Badr Serji, and Tijani Elharroudi; manuscript revision: Amal Bennani. All the authors read and approved the final version of this manuscript.
References
- 1.Ben Salah H, Hdiji S, Makni S, Ghorbel AM, Boudawara T, Elloumi M, et al. [Extramedullary plasmocytomas] Cancer Radiother. 2012 Jul-Aug;16(4):282–7. doi: 10.1016/j.canrad.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Feldman A, Isrow D, Schultz D, Inamdar K, Rasool N, Elshaikh MA. Solitary ovarian plasmacytoma. A case report and review of literature. Gynecol Oncol Rep. 2015 May 19;13:20–2. doi: 10.1016/j.gore.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andze G, Pagbe JJ, Tchokoteu PF, Mbakop A, Yomi J, Juimo AG, et al. Le plasmocytome solitaire extraosseux ovarien: à propos d'un cas exceptionnel chez une enfant de 12 ans. J Chir (Paris) 1993 Mar;130(3):137–40. [PubMed] [Google Scholar]
- 4.Talerman A, Path FR. Nonspecific tumors of the ovary, including mesenchymal tumors and malignant lymphoma. Blaustein´s Pathology of the Female Genital Tract. In: RJ Kurman., editor. 3rd edn. New York: Springer-Verlag; 1987. pp. 722–41. [Google Scholar]
- 5.Voegt H. Extramedullary plasmacytoma. Virchows Arch Pathol Anat. 1938;302:497–508. [Google Scholar]
- 6.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. Lyon: IARC; 2017. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (Revised 4th edition) p. 4. [Google Scholar]
- 7.Dabbs DJ. Diagnostic immunohistochemistry: Theranostic and genomic application. Elsevier. 2019;5 [Google Scholar]
- 8.Mondal SK, Chatterjee S, Mandal S, Bhattacharjee D. Primary extramedullary plasmacytoma of ovary: Report of a rare neoplasm. J Cancer Res Ther. 2015 Oct-Dec;11(4):923–4. doi: 10.4103/0973-1482.165876. [DOI] [PubMed] [Google Scholar]
- 9.Anderson KC, Alsina M, Bensinger W, Biermann JS, Chanan-Khan A, Cohen AD, et al. NCCN clinical practice guidelines in oncology: multiple myeloma. J Natl Compr Canc Netw. 2009 Oct;7(9):908–42. doi: 10.6004/jnccn.2009.0061. [DOI] [PubMed] [Google Scholar]
- 10.Bambirra EA, Miranda D, Magalhaes GMC. Plasma cell myeloma simulating Krukenberg's tumor. South Med J. 1982 Apr;75(4):511–2. doi: 10.1097/00007611-198204000-00046. [DOI] [PubMed] [Google Scholar]
- 11.Hautzer NW. Primary plasmacytoma of ovary. Gynecol Oncol. 1984;18(1):115–8. doi: 10.1016/0090-8258(84)90014-3. [DOI] [PubMed] [Google Scholar]
- 12.Cook HT, Boylston AW. Plasmacytoma of the ovary. Gynecol Oncol. 1988;29(3):378–81. doi: 10.1016/0090-8258(88)90239-9. [DOI] [PubMed] [Google Scholar]
- 13.Emery JD, Kennedy AW, Tubbs RR, Castellani WJ, Hussein MA. Plasmacytoma of the ovary: a case report and literature review. Gynecol Oncol. 1999;73(1):151–4. doi: 10.1006/gyno.1998.5246. [DOI] [PubMed] [Google Scholar]
- 14.Gouiaa N, Ellouze S, Bellaaj H, Louati D, Abid H, Mnif H, et al. Bilateral ovarian plasmocytoma: A case report. Imagerie de la Femme. 2011;21:111–114. [Google Scholar]
- 15.Zhong YP, Zhang JJ, Huang XN. Multiple myeloma with rupture of ovarian plasmacytoma. Chin Med J (Engl) 2012 Aug;125(16):2948–50. [PubMed] [Google Scholar]
- 16.Shakuntala P, Praveen S, Shankaranand B, Rajshekar K, Umadevi K, Bafna U. A rare case of plasmacytoma of the ovary: a case report and literature review. Ecancermedicalscience. 2013;7:288. doi: 10.3332/ecancer.2013.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomaselli T, Rollo D, Tarsitano F, Trezzi R, Crescini C. Lymphoplasmacytic lymphoma or plasmacytoma of the ovary? A case report and a literature review. Ital J Gynaecol Obstet. 2016;28(2):17–20. [Google Scholar]
- 18.Cárdenas-Perilla R, Urrego-Meléndez OM. Bilateral ovarian plasmacytoma detected on 18F-FDG PET/CT. Rev Esp Med Nucl Imagen Mol (Engl Ed) 2020;39(2):96–7. doi: 10.1016/j.remn.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Fares S, Taoufik A, Maataoui A, Qamouss O, Sokori K. Solitary Ovarian Plasmocytoma: A Rare localization of Extramedullary Plasmocytoma. A case Report. Arch Med Case Rep Case Study. 2021;4(5) [Google Scholar]
- 20.Elamouri JS, Shaffouh AY, Torjman FA. Plasmacytoma of the ovary. Ibnosina J Med Biomed Sci. 2021;13:227–30. [Google Scholar]