Abstract
Childhood-onset systemic lupus erythematosus (SLE) is characterized by increased rates of kidney involvement, termed lupus nephritis. Despite the significant morbidity and mortality associated with this disease, lupus nephritis trials have been plagued by repeated failures to meet clinical endpoints. However, improvements in trial design and the development of targeted approaches have begun to yield promising results, including two new FDA-approved lupus nephritis treatments since 2020. These include belimumab, a monoclonal antibody targeting the B cell survival cytokine BAFF (B cell activating factor), and voclosporin, a cyclosporin analog with improved pharmacokinetic characteristics. In this review, we will summarize the data supporting regulatory approval for these agents in lupus nephritis and highlight ongoing clinical trials targeting the diverse immunologic drivers of renal inflammation in SLE. While pediatric patients remain underrepresented in lupus clinical trials, given the increased severity of childhood-onset SLE and need for long-term protection from kidney damage, we anticipate the need for off-label use of these targeted therapies in the pediatric population. Future studies are needed to define optimal patient selection, drug combinations, and treatment duration in pediatric lupus nephritis.
Keywords: Lupus nephritis, Systemic Lupus Erythematosus, Proliferative lupus nephritis, Immunosuppression, Induction Therapy
Introduction:
Systemic Lupus Erythematosus (SLE) is a chronic autoimmune disease that can affect virtually every organ system in the body. Although most frequently developing in adulthood, childhood-onset SLE accounts for 10-20% of all lupus cases [1]. Pediatric lupus is frequently more severe than adult disease, highlighting the need for effective therapies to control autoimmune inflammation in the pediatric population. Of the many clinical manifestations of SLE, the development of lupus nephritis is associated with a worse prognosis, including the risk of long-term kidney damage and progression to kidney failure. Unfortunately, approximately 35-40% of children with SLE develop lupus nephritis, most often within the first 2 years after diagnosis [2-5]. Compared with adult patients, data describing the long-term prognosis of childhood-onset lupus nephritis is sparse. In the era prior to use of cytotoxic immunosuppression, clinical outcomes in lupus nephritis were poor. However, more recent data report 92% and 86% five- and ten-year kidney survival in pediatric lupus nephritis [6]. Despite these improved renal outcomes, children with SLE and lupus nephritis endure significantly higher morbidity and mortality compared to healthy children. For these reasons, the development of safe and effective treatments for lupus nephritis able to both treat renal inflammation and prevent the accrual of kidney damage is of critical importance.
In this review, we aim to first review the current standard-of-care for initial therapy of pediatric proliferative lupus nephritis. Next, we will summarize the clinical trial data that supported regulatory approval of two new agents for the treatment of adult lupus nephritis, belimumab and voclosporin. Finally, we will highlight new targeted approaches for lupus nephritis, including reported data from an early-stage clinical trial of obinutuzumab in lupus nephritis and new studies registered on ClinicalTrials.gov. Unfortunately, enrollment eligibility for lupus nephritis clinical trials is generally limited to patients greater than 18 years of age. Thus, the generalizability of published data to pediatric patients remains uncertain. However, given the potential for worse renal outcomes for childhood-onset lupus nephritis, developing clinical experience with these novel therapies is an important goal for the pediatric nephrology community.
Lupus nephritis pathogenesis and classification
A detailed overview of the pathogenesis of lupus nephritis is beyond the scope of this manuscript. However, we refer the reader to several reviews on the immunologic mechanisms driving renal inflammation in SLE [7, 8]. To assist with interpretation of clinical trial data, we will briefly review the histopathological classification of lupus nephritis. The initial classification system published by International Society of Nephrology/Renal Pathology Society (ISN/RPS) in 2003 was most recently updated in 2018 and divides lupus nephritis into six classes (Table 1) [9, 10]. Most studies cited in this review, including those that supported the approval of belimumab and voclosporin, used the original 2003 classification in their inclusion criteria. Class III and IV lupus nephritis are predominant in children with lupus nephritis, accounting up to 75% of cases of renal involvement, with 29% classifying as class III and 49% as class IV disease in one study [11]. Because of worse renal outcomes in patients with proliferative disease [12], the majority of clinical trials in lupus nephritis focus on patients with Class III and Class IV disease, with variable inclusion of Class V (membranous) lupus nephritis. For example, the obinutuzumab trials enrolled Class III/IV nephritis with or without concurrent Class V disease, while the belimumab and voclosporin (AURA-LV and AURORA 1) trials also allowed isolated Class V disease. Included in the 2018 ISN/RPS revision of the classification system was the addition of the activity and chronicity indices based on the National Institute of Health index, aimed at better prognosticating progression of renal disease. It is important to note that available recommendations based on evidence are intended to guide treatment for patients with high activity index and low chronicity scores, who should not be managed with the same targeted immunotherapies as patients with low activity index and high chronicity scores.
Table 1:
Histopathologic classification of lupus nephritis (developed by International Society of Nephrology/Renal Pathology Society (ISN/RPS). Adapted from [9].
| Class I | Minimal mesangial lupus nephritis |
|
| Class II | Mesangial proliferative lupus nephritis |
|
| Class III | Focal lupus nephritis |
|
| Class IV | Diffuse lupus nephritis |
|
| Class V | Lupus membranous nephropathy |
|
| Class VI | Advanced sclerosing lupus nephritis |
|
Initial therapy for lupus nephritis: the current standard-of-care.
The treatment of Class III/IV proliferative lupus nephritis has historically been conceptually divided into induction and maintenance phases. While this distinction between treatment phases is somewhat arbitrary, the goal of induction therapy is to achieve remission by controlling inflammation and minimizing parenchymal injury, while the aim of maintenance therapy is to sustain remission and prevent progression of chronic kidney disease. Over the last four decades, significant advances have been made in the treatment of proliferative lupus nephritis, leading to improvements in overall prognosis. A major advance in lupus nephritis therapy was the landmark 1986 trial conducted at the National Institutes of Health (NIH) which showed that cytotoxic chemotherapies, in particular cyclophosphamide, was superior to high-dose prednisone alone in preventing kidney failure in patients with active lupus nephritis [13]. While these findings enhanced clinical outcomes for lupus nephritis patients, the numerous immediate and cumulative adverse effects of cyclophosphamide prompted efforts to discover treatments with improved safety profiles.
In 2000, a trial performed in Hong Kong by Chan et al. demonstrated that mycophenolate mofetil (MMF) resulted in comparable induction of remission compared with IV cyclophosphamide [14]. Despite similar clinical outcomes, MMF treatment was more tolerable, with side-effects such as hair loss and amenorrhea being limited to the cyclophosphamide arm. An important caveat to this study was that few enrolled patients exhibited markers of poor prognosis in lupus nephritis, such as elevated creatinine at time of biopsy, presence of glomerular and tubulointerstitial scarring, and male sex [15]. For this reason, a larger multinational randomized controlled trial, the Aspreva Lupus Management Study (ALMS) group trial, was performed [16]. Using an outcome focused on reduction in urine protein/creatinine ratio and improvement in serum creatinine, the investigators found no significant difference between cyclophosphamide and MMF treatment arms. In addition, no differences were observed in rates of adverse events or infections between treatment groups. An advantage of the ALMS trial was the inclusion of a large and racially diverse population, providing important granular data on differences in response across racial and ethnic groups. The investigators reported that within the subset of black and Hispanic patients, more patients responded to MMF compared to IV cyclophosphamide [16], raising important questions regarding the impact of social determinants of health and structural inequalities on these results. Importantly, while the study did not meet its primary endpoint of showing superior efficacy of MMF compared with IV cyclophosphamide, MMF was found to be consistently effective across all racial/ethnic groups indicating that this therapy is a viable option for initial therapy for proliferative lupus nephritis.
A final strategy for initial treatment of lupus nephritis that limits adverse effects of cytotoxic agents, is a low-dose cyclophosphamide regimen. The Euro-Lupus Nephritis Trial compared the efficacy and toxicity of low- and high-dose IV cyclophosphamide [17]. In the standard high-dose group, patients received 6 monthly IV cyclophosphamide doses (500mg/m2 titrated up to 1500mg/dose) followed by 2 quarterly doses. In the low-dose group, patients were given 6 fixed-dose 500mg IV cyclophosphamide infusions every 2 weeks before being transitioned to oral azathioprine. Both regimens achieved similar rates of renal remission after initial therapy, with similar rates of relapses but lower rates of adverse infections in the low-dose group. While the Euro-Lupus Nephritis Trial primarily enrolled subjects with European ancestry, its initial findings were supported by the subsequent Abatacept and Cyclophosphamide Combination Efficacy and Safety Study (ACCESS), in which the Euro-Lupus regimen was used as baseline immunosuppression resulting in similar outcomes to historical data in a racially and ethnically diverse cohort [18].
Founded on these data, the current standard of care for initial therapy in proliferative lupus nephritis specify the use of either cyclophosphamide or MMF in conjunction with corticosteroids. Organizations such as European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) and Kidney Disease Improving Global Outcomes (KDIGO) have published recommendations on initial therapy for proliferative lupus nephritis [19, 20]. As noted above, evidence from randomized clinical trials in children with proliferative lupus nephritis is lacking. For this reason, the Childhood Arthritis and Rheumatology Research Alliance (CARRA) used a structured consensus formation process to develop a consensus treatment plan (CTP) for the treatment of childhood-onset lupus nephritis [21]. This approach was adapted from experience in other pediatric diseases, in which clinical trial data is either lacking or challenging to generate because of limited patient population size. Table 2 summarizes the CARRA CTP for initial therapy of newly diagnosed pediatric proliferative lupus nephritis, which recommends the use of either IV Cyclophosphamide every 4 weeks for 6 months or oral MMF twice per day for 6 months, with three separate standardized corticosteroid taper plans. In Europe, the Single Hub and Access point for paediatric Rheumatology in Europe (SHARE) initiative generated a similar set of evidence-based recommendations for the diagnosis and management of childhood lupus nephritis, which outlines a standardized regimen of MMF (1200 mg/m2/day up to 1800 mg/m2/day for poor response) or intravenous cyclophosphamide (high or low dose per Euro-Lupus Trial) in addition to prednisone (1-2 mg/kg/day, maximum 60 mg/day), as summarized in Table 2 [22]. The ultimate goal of both the CARRA CTP and SHARE recommendations was to reduce clinical practice variability in order to allow future comparison of clinical outcomes across standardized treatment approaches.
Table 2:
Consensus treatment plan (CTP) for the initial therapy of childhood-onset proliferative lupus nephritis developed by the Childhood Arthritis and Rheumatology Research Alliance (CARRA; upper panel) and equivalent recommendations generated by the Single Hub and Access point for paediatric Rheumatology in Europe (SHARE; lower panel). Table adapted from [21] and [22].
| Childhood Arthritis and Rheumatology Research Alliance (CARRA) | ||||||
|---|---|---|---|---|---|---|
| Initial regimen (pick one) | ||||||
| Cyclophosphamide IV q 4 weeks x 24 weeks 500mg/m2 body surface area titrated up to 1500mg/dose based on white blood cell (WBC) nadir |
Mycophenolate mofetil 600mg/m2/dose twice per day x 24 weeks (Max 3000mg/d) | |||||
| Steroid regimen (pick one) | ||||||
| Oral steroid regimen |
IV steroid regimen | Mixed regimen | ||||
| Initial dose |
Taper goal |
Initial dose |
Taper goal |
Initial dose |
Taper goal |
|
| Weight >30kg | 60-80mg/d | 20mg/d | Initial pulse then 20mg every 1-4 weeks | 10mg monthly | Mixed oral and IV dosing regiment | |
| Weight <30kg | 2 mg/kg/d | 0.5mg/kg/d | Initial pulse then 10mg every 1-4 weeks | 5mg monthly | Mixed oral and IV dosing regiment | |
| Single Hub and Access point for paediatric Rheumatology in Europe (SHARE) | |
|---|---|
| Initial regimen (pick one) | |
| High-dose IV Cyclophosphamide: 500mg/m2/dose, if tolerated increase to 750mg/m2/dose (Max 1000-2000mg/dose), 6 monthly doses |
Mycophenolate mofetil 1200mg/m2/day x 24 weeks (Max 2000mg/day) When poor response, option to increase to 1800mg/m2/day (Max 3000mg/day) |
| OR | |
| Low-dose IV Cyclophosphamide: 500mg/pulse (in adults) q 2 weeks x 6 pulses |
|
| Steroid regimen | |
| High dose prednisone: 1-2mg/kg/day (Max 60mg/day) | |
Newly approved treatments for lupus nephritis
Despite improved renal outcomes in proliferative lupus nephritis following standardization of cyclophosphamide and MMF treatment regimens, up to 45% of these patients do not achieve remission within the first 6 months of standard therapy [16]. In addition, protocol kidney biopsies have shown continued histologic activity in a significant portion of patients achieving apparent complete clinical remission [23]. These data emphasize the need to develop additional targeted therapies. In recent years, lupus nephritis clinical trials have showed limited success, including large randomized control trials demonstrating no benefit for drugs targeting diverse immune mechanisms such as co-stimulatory blockade (CTLA4-Ig, Abatacept) [18, 24-26], B cell depletion (anti-CD20, Rituximab [27, 28] and Ocrelizumab [29]), and cytokine blockade (anti-IL-6, Sirukumab) [30]. Fortunately, researchers and pharmaceutical companies have continued to pursue alternate approaches to treat this challenging disease. This persistence led to two new FDA-approved therapies for proliferative lupus nephritis since 2020: belimumab and voclosporin. We have included a summary of relevant clinical trials of newly-approved and emerging drugs, along with their primary and secondary endpoints and their definitions in Table 3. All included clinical trials use complete renal remission (CRR) as one of their endpoints, as defined by estimated Glomerular Filtration Rate (eGFR) and reduction in proteinuria. While there is variability in the definition of CRR among the trials, remission as defined by normalization in serum creatinine and resolution of proteinuria after immunosuppressive therapy has been shown in previous studies to be a valid predictor for important long-term outcomes such as renal survival and death in the adult lupus nephritis population [31]. In children with class III and IV lupus nephritis, failure to achieve remission at 6 and 12 months after initiation of therapy has been associated with long-term poor renal prognosis (eGFR<60ml/min/1.73m2 or persistent dialysis) [32].
Table 3:
Clinical trials of newly-approved and emerging drug targets and their endpoints
| Drug | Trial | Phase | Treatment | Primary Endpoint | Secondary Endpoint |
|---|---|---|---|---|---|
| Belimumab | BLISS-LN | III | IV belimumab (10mg/kg) vs placebo + standard therapy | Primary efficacy renal response at 104 weeks:
|
CRR at 104 weeks:
|
| Voclosporin | AURA-LV | II | Voclosporin 23.7 mg BID vs. 39.5 mg BID vs. matched placebo + MMF (2 g/d) + rapidly tapered corticosteroids | CRR at 24 weeks:
|
CRR at 48 weeks |
| Voclosporin | AURORA-1 | III | Voclosporin 23.7 mg BID vs. placebo + MMF (2g/d) + rapidly tapered low oral corticosteroids | CRR at 52 weeks:
|
|
| Obinutuzumab | NOBILITY | II | IV obinutuzumab 1000mg vs. placebo on day1 and weeks 2, 24, 26 + MMF + corticosteroids | CRR at 52 weeks:
|
PRR at 52 weeks:
|
Belimumab:
Belimumab (Benlysta) is a monoclonal antibody inhibiting the activity of the B cell survival cytokine BAFF (B cell activating factor), also known as BlyS (B-lymphocyte stimulator) [33, 34]. The rationale for targeting this molecule in SLE is supported by several lines of evidence linking elevated serum BAFF to lupus pathogenesis. These include lupus-like disease in BAFF overexpression murine models [35, 36], increased serum BAFF levels in human lupus patients [37], and a genetic risk polymorphism in the TNFSF13B gene associated with the development of SLE [38]. These observations spurred the development of BAFF inhibition as a strategy to treat renal and non-renal SLE. In 2011, the FDA approved belimumab for the treatment of extra-renal SLE, based on two large phase III randomized clinical trials, BLISS-52 and BLISS-76. Both studies, involving >1500 total combined patients, demonstrated improved clinical outcomes, as assessed by improvement in SRI (SLE Responder Index), reduced flares, reduced steroid use, and improved serologic activity at 52 and 76 weeks, without increase in rates of serious adverse events [39, 40]. Notably, belimumab was also studied in children with SLE in a phase-2, randomized, double-blinded study examining efficacy, safety, and pharmacokinetics (PLUTO Part A). Children aged 5 to 17 years with active SLE were randomized to receive intravenous belimumab 10 mg/kg every 4 weeks or placebo in combination with standard therapy for 52 weeks. A higher proportion of children in the treatment arm (52.8%) met the primary endpoint of SLE Responder Index (SRI4) response rate compared to with placebo (43.6%) without an increase in adverse events [41].
A notable caveat in both the BLISS and PLUTO trials was that patients with severe active lupus nephritis were excluded. However, a pooled post-hoc analysis of subjects in the BLISS trials with evidence of kidney involvement at enrollment demonstrated more frequent and rapid onset of renal remission, greater reductions in proteinuria, and reduced renal flares in the belimumab arms compared with standard therapy [42]. For this reason, a large multinational, multicenter randomized controlled trial of belimumab in biopsy-confirmed active lupus nephritis was conducted (BLISS-LN). 448 patients were randomized to belimumab 10mg/kg IV q 4 weeks vs. placebo in addition to standard initial therapy (MMF or cyclophosphamide plus corticosteroids). Primary efficacy renal response was defined as urine protein to creatinine ratio (uPCR) <0.7, eGFR less that 20% below baseline (or >60mL/min/1.73m2), and no use of rescue therapy. The secondary endpoint of complete renal response was defined as uPCR<0.5 and eGFR <10% of baseline or >90mL/min/1.73m2. At 104 weeks, a significantly greater proportion of belimumab-treated patients demonstrated primary renal efficacy (43% vs 32%; P=0.03) and complete renal response (30% vs 20%; P=0.02) compared to the placebo group. Although the absolute rate of clinical improvement in this and other BLISS trials was small (~10%), belimumab has an excellent long-term safety profile and is known to reduce disease flares and damage accrual in SLE [43, 44]. Belimumab and potentially other BAFF inhibitors thus have an important role as adjunctive therapy for lupus nephritis. Based on these data, the US FDA approved belimumab for adult patients with active lupus nephritis who are receiving standard therapy in December 2020. Although experience with BAFF blockade in childhood-onset lupus nephritis remains limited, randomized trials of non-renal SLE have demonstrated equivalent efficacy and safety of belimumab in pediatric and adult patients [41, 45]. These pediatric data have facilitated regulatory approval for the treatment of extra-renal lupus from age 5. In summary, while important questions remain regarding appropriate patient selection and duration of treatment, BAFF inhibition is a welcome addition to the repertoire of available therapies to treat proliferative lupus nephritis.
Voclosporin
Calcineurin inhibitors (CNI), particularly tacrolimus and cyclosporin, have been long explored as alternate therapies for the treatment of lupus nephritis. Potential benefits for this class of medications include both inhibition of T cell activation and also direct anti-proteinuric effects via stabilization of the podocyte cytoskeleton [46, 47]. Owing to its more favorable side effect profile and higher potency, tacrolimus is generally preferred over cyclosporin in the treatment of lupus nephritis [48, 49]. A 2015 meta-analysis demonstrated that tacrolimus monotherapy was comparable to MMF and more effective than IV cyclophosphamide at inducing complete remission, while combined MMF and tacrolimus exerted greater clinical benefit than IV cyclophosphamide [50]. However, complex pharmacokinetics requiring frequent drug level monitoring and the potential for both acute and chronic nephrotoxicity have presented challenges in the use of tacrolimus in lupus nephritis [51].
Voclosporin is a cyclosporin analog with an improved pharmacokinetic profile that does not require drug level monitoring [52]. Since the initial discovery of this compound in the mid 1990’s, voclosporin has been studied for multiple conditions, including uveitis [53], plaque psoriasis [54], and kidney transplantation [55]. In each case, promising clinical data was not translated into regulatory approval. This changed after Aurinia Pharmaceuticals acquired the rights to pursue voclosporin as a treatment for autoimmune conditions and designed the phase II (AURA-LV) and phase III (AURORA 1) randomized controlled trials of voclosporin for active lupus nephritis. In AURA-LV, 265 adults with active lupus nephritis were randomized to receive two voclosporin doses (23.7 mg or 39.5 mg BID) or placebo in addition to standard MMF and low dose corticosteroid initial therapy. The primary endpoint was complete renal response (CRR) at 24 weeks, defined as uPCR <0.5 plus eGFR >60mL/min/1.73m2 with less than 20% decrease from baseline eGFR. Based on this metric, voclosporin was superior to standard therapy at both 24 weeks (CRR: 32.6% (low-dose) vs 27.3% (high-dose) vs 19.3% (placebo)) and 48 weeks (CRR: 49.4% (low-dose) vs 39.8% (high-dose) vs 23.9% (placebo)). Kaplan-Meier analysis also showed more rapid initial of complete renal response in both voclosporin doses compared with placebo. Unfortunately, these clinical benefits were offset by more serious adverse events, including increased deaths in the low-dose voclosporin group (11.2% (low-dose) vs 2.3% (high-dose) vs 1.1% (placebo)) [56].
Analysis of these data informed the design of a subsequent phase III clinical trial of the lower voclosporin dose (23.7 mg twice per day) in active lupus nephritis (AURORA 1). In this 52-week study, 357 adults with biopsy-confirmed lupus nephritis were randomized to receive voclosporin or placebo in addition to standard MMF and low-dose prednisone initial therapy. The primary endpoint was similar to the phase II AURA-LV study, a composite complete renal response endpoint of uPCR <0.5, eGFR >60mL/min/1.73m2 (or less than 20% decrease from baseline), no need for rescue medications, and steroid dose of 10mg prednisone per day or less. Complete renal response rates were significantly higher in the voclosporin arm (41% voclosporin vs. 23% placebo; odds ratio 2.65 (95% CI 1.6 – 4.3); p<0.0001), whereas adverse events were reassuringly similar in both groups, including no new onset hypertension, hyperkalemia, or hypomagnesemia [57].
In summary, independent clinical trials showed benefit for a new fixed dose calcineurin inhibitor in active lupus nephritis. In January 2021, the FDA approved voclosporin in combination with background immunosuppression for the treatment of adults with active lupus nephritis. While this new therapeutic option for patients is welcome, important questions remain regarding the use of volcosporin for lupus nephritis. First, although chronic rejection and not direct drug toxicity is likely a major driver of progressive allograft fibrosis in CNI-treated kidney transplant recipients, long-term CNI treatment has been linked with chronic renal fibrosis in non-renal transplant populations [58, 59]. Whether voclosporin treatment carries a similar risk of chronic nephrotoxicity has not been determined, but this concern may be more pertinent to childhood-onset SLE given the need to maintain kidney function for decades. Reassuringly, preliminary data from the AURA2 long-term extension study shows no decline in eGFR following 2 years of volcosporin treatment [60]. Second, it is not yet clear whether the rapid decline in proteinuria in the AURA-LV and AURORA 1 studies is explained by induction of immunologic remission or via podocyte-specific anti-proteinuric effects. If the latter, reduced medication adherence in real world settings may be accompanied by frequent relapse of proteinuric kidney disease. Ultimately, these theoretical concerns are offset by the welcome addition of a new FDA-approved calcineurin inhibitor able to induce remission in patients with proliferative lupus nephritis without requiring frequent drug level monitoring.
New and Emerging Therapies
In addition to the two new FDA-approved therapies, multiple other medications are currently under development for the treatment of lupus nephritis. For the majority of these medications, clinical trials are ongoing and limited data are available to suggest potential efficacy. For this reason, we will describe recently published data on the use of obinutuzumab in lupus nephritis in detail. The remaining active lupus nephritis clinical trials registered at ClinicalTrials.gov are summarized in Table 4 and Figure 1.
Table 4:
Ongoing clinical trials of emerging therapies in lupus nephritis
| Drug | Trial Phase | Target |
ClinicalTrials.gov Identifier: |
|---|---|---|---|
| Immune cell crosstalk | |||
| Iscalimab | Phase 2 | Anti-CD40 | NCT03610516 |
| BI 655064 | Phase 2 | CD40 | NCT03385564 |
| Itolizumab | Phase 1 | CD6 | NCT04128579 |
| Cytokine inhibition | |||
| Anifrolumab | Phase 3 | Type I INF receptor | NCT05138133 |
| Anifrolumab | Phase 2 | Type I INF receptor | NCT02547922 |
| Vunakizumab | Phase 2 | IL-17A | NCT04924296 |
| Secukinumab | Phase 3 | IL-17A | NCT04181762 |
| Guselkumab | Phase 2 | IL-23 | NCT04376827 |
| Plasma cell / antibody targeted | |||
| Daratumumab | Phase 2 | Anti-CD38 | NCT04868838 |
| Nipocalimab | Phase 2 | FcRn | NCT04883619 |
| KZR-616 | Phase 1/2 | Immunoproteasome | NCT03393013 |
| Complement cascade inhibition | |||
| Ravulizumab | Phase 2 | C5 complement | NCT04564339 |
| Vemircopan | Phase 2 | Complement factor D | NCT05097989 |
| Cell signaling inhibitors | |||
| Zanubrutinib | Phase 2 | BTK | NCT04643470s |
| Artesunate | Phase 4 | Antimalarial | NCT03214731 |
| Mizoribine | Phase 3 | Purine antagonist | NCT02256150 |
| B cell targeted | |||
| Ianalumab | Phase 3 | BAFF | NCT05126277 |
| Obinutuzumab | Phase 3 | Anti-CD20 | NCT04221477 |
Active lupus nephritis trials registered on ClinicalTrials.gov. Excluded are studies resulted/terminated before 2018, non-drug therapies, interventions terminated due to insufficient evidence for efficacy, drugs tested for SLE only (not lupus nephritis), and medications already discussed in body of this review.
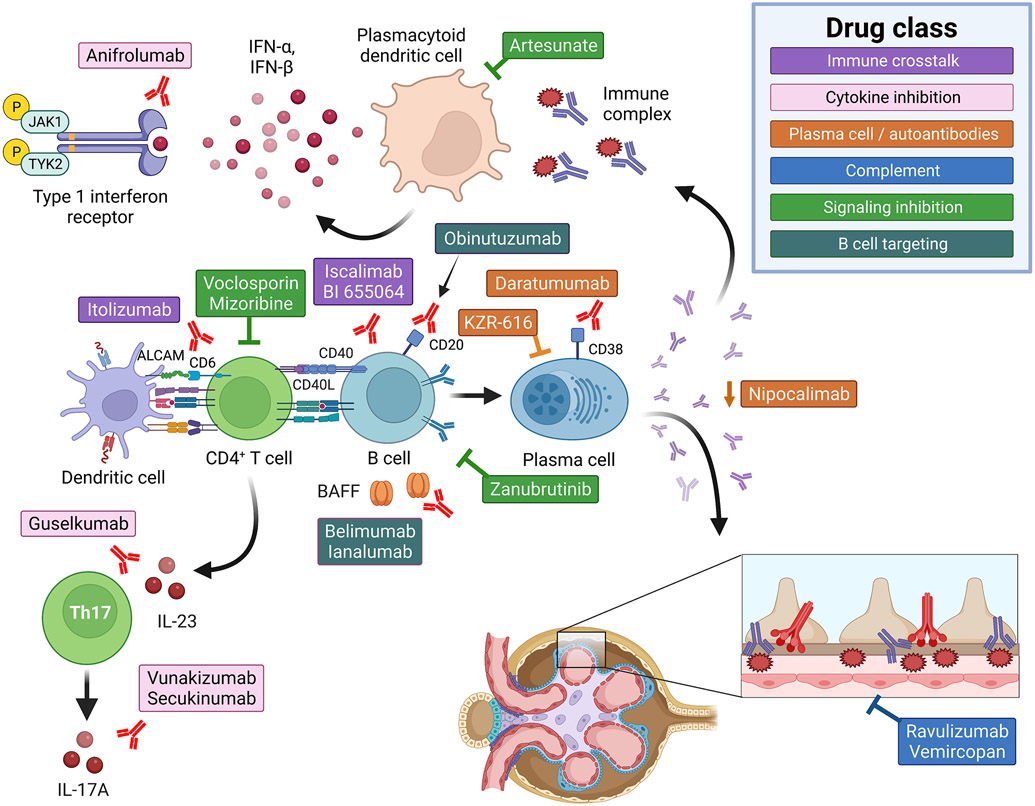
Figure 1: Emerging drug targets for the treatment of lupus nephritis.
Diagram depicting the multiple strategies currently being studies for the treatment of lupus nephritis, divided into five groups. 1) Inhibition of immune cell crosstalk: two separate drugs (Iscalimab and BI 655064) are examining whether blocking CD40:CD40L costimulatory signals prevents autoantibody formation and ameliorates lupus nephritis. Itolizumab targets the T cell activation molecule CD6. 2) Cytokine inhibition: multiple pro-inflammatory cytokines have been implicated in the pathogenesis of SLE and lupus nephritis. Studies are ongoing examining whether blocking type 1 interferon signals (Anifrolumab), BAFF activity (Lanalumab), or Th17 biology (IL-23: Guselkumab; IL-17A: Vunakizumab and Secukinumab) is an effective treatment strategy in lupus nephritis. 3) Plasma cell and/or autoantibody targeted therapies: the intra-glomerular deposition of autoantibody:autoantigen immune-complexes (IC) promotes glomerulonephritis. For this reason, independent strategies are being pursued to either deplete plasma cells (Daratumumab and KZR-616) or reduced serum IgG levels via blockade of the neonatal Fc receptor (FcRn; Nipocalimab). 4) Inhibition of the complement cascade: Given the putative role for complement as a driver of renal inflammation, complement C5 (Ravulizumab) and complement factor D (Vemircopan) inhibitors are being studied in active lupus nephritis. 5) Cell signaling inhibitors: Specific targets being pursued include: Bruton’s tyrosine kinase, a critical mediator of B cell receptor and Fc receptor signaling (Zanubrutinib); endosomal toll-like receptor signaling pathways (the antimalarial Artesunate); and the anti-metabolite Mizoribine. 6) B cell targeted therapies: including BAFF inhibitors (belimumab, ianalumab) and anti-CD20 depleting monoclonal Obinutuzumab.
Obinutuzumab
Dysregulated B cell activation is key driver of lupus pathogenesis [34, 61]. For this reason, B cell depletion has long been considered a promising therapeutic strategy in SLE. However, despite promising data from small initial studies, rituximab, a monoclonal depleting antibody targeting the B cell antigen CD20, failed to meet primary efficacy endpoints in two large randomized controlled studies in extra-renal SLE and lupus nephritis, respectively (EXPLORER and LUNAR) [27, 28]. A separate anti-CD20 depleting antibody, ocrelizumab, demonstrated numerically but not statistically significantly improvements in lupus outcomes compared to standard therapy, but higher rates of infectious complications limited further development of this agent [29].
These clinical failures raised important questions regarding the role for B cells in lupus disease. Perhaps patient heterogeneity and challenges in designing appropriate endpoints in lupus clinical trials masked the efficacy of B cell depletion therapies [62]. Alternatively, since disease-defining anti-nuclear antibodies can predate lupus clinical symptoms by years [63], the primary role for B cells may be to initiate an inflammatory cascade in SLE, rather than sustain tissue damage in established disease. Finally, rituximab-resistant CD20neg plasma cells may be an underappreciated driver of lupus pathogenesis [64]. While these factors may have contributed to negative trial results, both animal and human data suggest that the failure to completely eliminate CD20+ B cells is a major contributor to the clinical inefficacy of rituximab in SLE. For example, post-hoc analysis of the LUNAR lupus nephritis trial showed that the depth of B cell depletion correlated with improved renal responses [65]. This is relevant since tissue-resident CD20+ B cells are known to resist depletion following rituximab treatment in both human lupus patients and in murine lupus models [66].
For these reasons, new “type II” anti-CD20 monoclonal antibodies have been engineered for more potent B cell cytotoxicity. One such agent, obinutuzumab, exhibits greater direct cell killing and antibody-dependent cellular cytotoxicity (ADCC) compared with rituximab, resulting in enhanced B cell ablation in follicular lymphoma, rheumatoid arthritis, and SLE [67-69]. These improved pharmacologic characteristics prompted the design of a phase II trial of Obinutuzumab in active lupus nephritis (NOBILITY). Notably, addition of obinutuzumab to background MMF resulted in increased complete renal response rates at both 52 weeks (35% (obinutuzumab) vs 23% (placebo), p=0.115) and 104 weeks (41% vs 23%, p=0.026) [70]. Treatment was well-tolerated with only modestly increased rates of infusion reactions and no serious adverse events. An ongoing phase III study of obinutuzumab (REGENCY trial) aims to examine the efficacy, safety, and pharmacokinetics of obinutuzumab compared to placebo in combination with standard therapy in class III and IV lupus nephritis. Patients will be randomized to two different Obinutuzumab regimens (1000 mg IV at baseline and weeks 2, 24, 26, 50 and 52 or 1000 mg IV at baseline and weeks 2, 24, 26 and 52) or placebo in addition to MMF and prednisone and followed for a primary endpoint of complete renal response at week 76 (NCT04221477). Placed in the context of the failed LUNAR trial of rituxiumab in lupus nephritis, these data suggest that B cell targeting may yet have a role in the treatment of lupus nephritis, provided anti-CD20 agents are selected for their ability to induce deep and durable B cell depletion.
Active lupus nephritis trials
Table 4 summarizes the active lupus nephritis clinical trials registered at ClinicalTrials.gov. As an overall framework, we have divided these approaches into five main groups: 1) disruption of immune cell crosstalk; 2) specific cytokine blockade; 3) plasma cell and/or autoantibody targeted therapies; 4) complement cascade inhibitors; 5) cell signaling inhibitors; and 6) B cell targeted agents. Figure 1 summarizes each of these therapeutic approaches which may prove beneficial for the treatment of lupus nephritis.
Concluding remarks
After decades of minimal advancement, the past few years have heralded major achievements in the ability to treat proliferative lupus nephritis, one of the most severe clinical manifestations of SLE. This is particularly important for pediatric patients who exhibit a higher risk for developing lupus nephritis and have a longer potential to accrue kidney damage. Although the studies described above report promising efficacy and safety in adults, questions remain regarding the use of these new therapeutic agents in children-onset lupus nephritis including the potential for pediatric specific drug toxicity. For example, the use of B cell targeted therapies prior to completion of primary immunization schedule may increase the risk of poor vaccine response. Moreover, while Janus kinase (JAK) signaling inhibition has shown promise in adult-onset extra-renal SLE, blocking growth hormone (GH)-mediated JAK2 phosphorylation might theoretically decrease linear growth in childhood [71]. Alternatively, these new biologic agents may carry pediatric-specific benefits by sparing children from long-term treatment with corticosteroids or cytotoxic agents, which are known to negatively affect growth, development, and fertility. Relying on off-label use and extrapolating dosing guidelines from adult studies subjects children to treatment without clearly understanding the age-specific risk-benefit ratio. For these reasons, efforts must be made to promote inclusion of children and adolescents in drug-development trials for glomerular diseases. The Pediatric Working Group (of the NephCure Kidney International Gateway Initiative) is a group that is spearheading the initiative to facilitate the inclusion of pediatric patients in clinical trials for glomerular diseases through engagement with regulatory authorities in the United States and Europe [72].
Despite this lack of pediatric-specific data, regulatory approval of belimumab, volcosporin, and potentially obinutuzumab in the future, will provide multiple new treatment options for the pediatric nephrology community. In addition, academic clinicians and pharmaceutical companies are pursuing multiple independent strategies which may yield additional benefits to patients. Given the heterogeneity of human SLE and the lack of pediatric clinical trial data, a major challenge will be to identify which patients are likely to gain the most benefit from each of these novel agents. Future observational studies are needed to provide additional guidance on clinical decision making on optimal timing and duration of therapy as well as appropriate patient selection for these new agents. Finally, improved biomarkers of disease activity and remission are also needed to inform the duration of therapy and balance effective control of inflammation with long-term drug toxicity.
Summary points.
The current standard of care for initial therapy in pediatric proliferative lupus nephritis specify the use of cyclophosphamide or MMF in conjunction with corticosteroids.
Belimumab, a monoclonal antibody against BAFF (B cell activating factor), and voclosporin, a cyclosporin analog with improved pharmacokinetic characteristics, are two newly FDA-approved drugs for the treatment of adult lupus nephritis.
Obinutuzumab is a new drug under development for treatment of lupus nephritis and has been shown in phase II trials to be effective and well-tolerated.
There are numerous ongoing clinical trials examining the diverse immunologic drivers of renal inflammation and potential drug targets lupus nephritis.
Acknowledgements:
This work was supported by the National Institutes of Health under award numbers: R01AR073938 (SWJ), R01AR075813 (SWJ), 5T32DK007662 (MK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support provided by the Arthritis National Research Foundation (ANRF) Eng Tan Scholar Award (SWJ); and by a Lupus Research Alliance, Novel Research Grants (SWJ). Figures were created with BioRender.com. The authors declare that no conflict of interest exists.
Multiple choice questions
- What is the current standard of care for initial therapy for pediatric proliferative lupus nephritis?
- MMF and corticosteroids
- Cyclophosphamide and corticosteroids
- Tacrolimus and corticosteroids
- Corticosteroids only
- A and B
- Which of the following statements is FALSE regarding the Aspreva Lupus Management Study (ALMS) trial?
- MMF exhibited consistent efficacy across all racial/ethnic groups.
- More Black and Hispanic patients responded to MMF compared to IV cyclophosphamide
- Rates of adverse events were higher in the IV cyclophosphamide group
- MMF was found to be equally effective as IV cyclophosphamide, but not superior
- Which of the following molecules does Belimumab target?
- B cell activating factor
- CD20
- IL-6
- Type I INF receptor
- Complement factor D
- Which of the following drugs is FDA-approved for the treatment of adult active lupus nephritis in combination with background immunosuppression after being shown to induce higher rates of complete renal remission compared to standard therapy alone?
- Obinutuzumab
- Voclosporin
- Ocrelizumab
- Rituximab
- Tacrolimus
- Which of the following is true regarding Obinutuzumab?
- It failed to induce complete renal response rates higher than MMF in patients with lupus nephritis in the phase II trial
- It is an anti-CD20 monoclonal antibody
- Long term treatment carries risk of chronic renal fibrosis
- Rituximab has been shown to exhibit greater antibody-dependent cellular cytotoxicity than obinutuzumab
- It is only effective in the treatment of extra-renal SLE
Answers: 1. E; 2. C; 3. A; 4. B; 5. B
References:
- 1.Malattia C, Martini A (2013) Paediatric-onset systemic lupus erythematosus. Best Pract Res Clin Rheumatol 27:351–362. [DOI] [PubMed] [Google Scholar]
- 2.Pinheiro SVB, Dias RF, Fabiano RCG, Araujo SA, Silva A (2019) Pediatric lupus nephritis. J Bras Nefrol 41:252–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenderfer SE, Chang JC, Goodwin Davies A, Luna IY, Scobell R, Sears C, Magella B, Mitsnefes M, Stotter BR, Dharnidharka VR, Nowicki KD, Dixon BP, Kelton M, Flynn JT, Gluck C, Kallash M, Smoyer WE, Knight A, Sule S, Razzaghi H, Bailey LC, Furth SL, Forrest CB, Denburg MR, Atkinson MA (2022) Using a Multi-Institutional Pediatric Learning Health System to Identify Systemic Lupus Erythematosus and Lupus Nephritis: Development and Validation of Computable Phenotypes. Clin J Am Soc Nephrol 17:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiraki LT, Feldman CH, Liu J, Alarcon GS, Fischer MA, Winkelmayer WC, Costenbader KH (2012) Prevalence, incidence, and demographics of systemic lupus erythematosus and lupus nephritis from 2000 to 2004 among children in the US Medicaid beneficiary population. Arthritis Rheum 64:2669–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiorot FJ, Islabao AG, Pereira RM, Terreri MT, Saad-Magalhaes C, Novak GV, Molinari BC, Sakamoto AP, Aikawa NE, Campos LM, Peracchi OA, Appenzeller S, Ferriani VP, Silva MF, Fonseca AR, Sztajnbok FR, Paim LB, Fraga MM, Okuda EM, Bica BE, Sena EG, Moraes AJ, Rolim AM, Spelling PF, Scheibel IM, Cavalcanti AS, Matos EN, Robazzi TC, Guimaraes LJ, Santos FP, Ramos VC, Carneiro-Sampaio M, Bonfa E, Silva CA, Brazilian Childhood-onset Systemic Lupus Erythematosus G (2019) Disease presentation of 1312 childhood-onset systemic lupus erythematosus: influence of ethnicity. Clin Rheumatol 38:2857–2863. [DOI] [PubMed] [Google Scholar]
- 6.Demir S, Gulhan B, Ozen S, Celegen K, Batu ED, Tas N, Orhan D, Bilginer Y, Duzova A, Ozaltin F, Topaloglu R (2021) Long Term Renal Survival of Pediatric Patients with Lupus Nephritis. Nephrol Dial Transplant. [DOI] [PubMed] [Google Scholar]
- 7.Lech M, Anders HJ (2013) The pathogenesis of lupus nephritis. J Am Soc Nephrol 24:1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yung S, Yap DY, Chan TM (2020) A review of advances in the understanding of lupus nephritis pathogenesis as a basis for emerging therapies. F1000Res 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M (2004) The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15:241–250. [DOI] [PubMed] [Google Scholar]
- 10.Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, D'Agati VD, Ferrario F, Haas M, Jennette JC, Joh K, Nast CC, Noel LH, Rijnink EC, Roberts ISD, Seshan SV, Sethi S, Fogo AB (2018) Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 93:789–796. [DOI] [PubMed] [Google Scholar]
- 11.Hiraki LT, Benseler SM, Tyrrell PN, Hebert D, Harvey E, Silverman ED (2008) Clinical and laboratory characteristics and long-term outcome of pediatric systemic lupus erythematosus: a longitudinal study. J Pediatr 152:550–556. [DOI] [PubMed] [Google Scholar]
- 12.Tunnicliffe DJ, Palmer SC, Henderson L, Masson P, Craig JC, Tong A, Singh-Grewal D, Flanc RS, Roberts MA, Webster AC, Strippoli GF (2018) Immunosuppressive treatment for proliferative lupus nephritis. Cochrane Database Syst Rev 6:CD002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin HA 3rd, Klippel JH, Balow JE, le Riche NG, Steinberg AD, Plotz PH, Decker JL (1986) Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med 314:614–619. [DOI] [PubMed] [Google Scholar]
- 14.Chan TM, Li FK, Tang CS, Wong RW, Fang GX, Ji YL, Lau CS, Wong AK, Tong MK, Chan KW, Lai KN (2000) Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong-Guangzhou Nephrology Study Group. N Engl J Med 343:1156–1162. [DOI] [PubMed] [Google Scholar]
- 15.Falk RJ (2000) Treatment of lupus nephritis--a work in progress. N Engl J Med 343:1182–1183. [DOI] [PubMed] [Google Scholar]
- 16.Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, Li LS, Mysler E, Sanchez-Guerrero J, Solomons N, Wofsy D, Aspreva Lupus Management Study G (2009) Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 20:1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houssiau FA, Vasconcelos C, D'Cruz D, Sebastiani GD, Garrido Ed Ede R, Danieli MG, Abramovicz D, Blockmans D, Mathieu A, Direskeneli H, Galeazzi M, Gul A, Levy Y, Petera P, Popovic R, Petrovic R, Sinico RA, Cattaneo R, Font J, Depresseux G, Cosyns JP, Cervera R (2002) Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum 46:2121–2131. [DOI] [PubMed] [Google Scholar]
- 18.Group AT (2014) Treatment of lupus nephritis with abatacept: the Abatacept and Cyclophosphamide Combination Efficacy and Safety Study. Arthritis Rheumatol 66:3096–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I, Boletis J, Frangou E, Houssiau FA, Hollis J, Karras A, Marchiori F, Marks SD, Moroni G, Mosca M, Parodis I, Praga M, Schneider M, Smolen JS, Tesar V, Trachana M, van Vollenhoven RF, Voskuyl AE, Teng YKO, van Leew B, Bertsias G, Jayne D, Boumpas DT (2020) 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis 79:713–723. [DOI] [PubMed] [Google Scholar]
- 20.Rovin BH, Caster DJ, Cattran DC, Gibson KL, Hogan JJ, Moeller MJ, Roccatello D, Cheung M, Wheeler DC, Winkelmayer WC, Floege J, Conference P (2019) Management and treatment of glomerular diseases (part 2): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 95:281–295. [DOI] [PubMed] [Google Scholar]
- 21.Mina R, von Scheven E, Ardoin SP, Eberhard BA, Punaro M, Ilowite N, Hsu J, Klein-Gitelman M, Moorthy LN, Muscal E, Radhakrishna SM, Wagner-Weiner L, Adams M, Blier P, Buckley L, Chalom E, Chedeville G, Eichenfield A, Fish N, Henrickson M, Hersh AO, Hollister R, Jones O, Jung L, Levy D, Lopez-Benitez J, McCurdy D, Miettunen PM, Quintero-del Rio AI, Rothman D, Rullo O, Ruth N, Schanberg LE, Silverman E, Singer NG, Soep J, Syed R, Vogler LB, Yalcindag A, Yildirim-Toruner C, Wallace CA, Brunner HI, Carra SLES (2012) Consensus treatment plans for induction therapy of newly diagnosed proliferative lupus nephritis in juvenile systemic lupus erythematosus. Arthritis Care Res (Hoboken) 64:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groot N, de Graeff N, Marks SD, Brogan P, Avcin T, Bader-Meunier B, Dolezalova P, Feldman BM, Kone-Paut I, Lahdenne P, McCann L, Ozen S, Pilkington CA, Ravelli A, Royen-Kerkhof AV, Uziel Y, Vastert BJ, Wulffraat NM, Beresford MW, Kamphuis S (2017) European evidence-based recommendations for the diagnosis and treatment of childhood-onset lupus nephritis: the SHARE initiative. Ann Rheum Dis 76:1965–1973. [DOI] [PubMed] [Google Scholar]
- 23.Yo JH, Barbour TD, Nicholls K (2019) Management of refractory lupus nephritis: challenges and solutions. Open Access Rheumatol 11:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merrill JT, Burgos-Vargas R, Westhovens R, Chalmers A, D'Cruz D, Wallace DJ, Bae SC, Sigal L, Becker JC, Kelly S, Raghupathi K, Li T, Peng Y, Kinaszczuk M, Nash P (2010) The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis and rheumatism 62:3077–3087. [DOI] [PubMed] [Google Scholar]
- 25.Furie R, Nicholls K, Cheng TT, Houssiau F, Burgos-Vargas R, Chen SL, Hillson JL, Meadows-Shropshire S, Kinaszczuk M, Merrill JT (2014) Efficacy and safety of abatacept in lupus nephritis: a twelve-month, randomized, double-blind study. Arthritis Rheumatol 66:379–389. [DOI] [PubMed] [Google Scholar]
- 26.Efficacy and Safety Study of Abatacept to Treat Lupus Nephritis; ClinicalTrials.gov Identifier: NCT01714817.
- 27.Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, Maciuca R, Zhang D, Garg JP, Brunetta P, Appel G, Group LI (2012) Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 64:1215–1226. [DOI] [PubMed] [Google Scholar]
- 28.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, Utset TO, Gordon C, Isenberg DA, Hsieh HJ, Zhang D, Brunetta PG (2010) Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis and rheumatism 62:222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mysler EF, Spindler AJ, Guzman R, Bijl M, Jayne D, Furie RA, Houssiau FA, Drappa J, Close D, Maciuca R, Rao K, Shahdad S, Brunetta P (2013) Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheum 65:2368–2379. [DOI] [PubMed] [Google Scholar]
- 30.Rovin BH, van Vollenhoven RF, Aranow C, Wagner C, Gordon R, Zhuang Y, Belkowski S, Hsu B (2016) A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Treatment With Sirukumab (CNTO 136) in Patients With Active Lupus Nephritis. Arthritis Rheumatol 68:2174–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korbet SM, Lewis EJ, Schwartz MM, Reichlin M, Evans J, Rohde RD (2000) Factors predictive of outcome in severe lupus nephritis. Lupus Nephritis Collaborative Study Group. Am J Kidney Dis 35:904–914. [DOI] [PubMed] [Google Scholar]
- 32.Demir S, Gulhan B, Ozen S, Celegen K, Batu ED, Tas N, Orhan D, Bilginer Y, Duzova A, Ozaltin F, Topaloglu R (2022) Long-term renal survival of paediatric patients with lupus nephritis. Nephrol Dial Transplant 37:1069–1077. [DOI] [PubMed] [Google Scholar]
- 33.Jackson SW, Davidson A (2019) BAFF inhibition in SLE-Is tolerance restored? Immunol Rev 292:102–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canny SP, Jackson SW (2021) B Cells in Systemic Lupus Erythematosus: From Disease Mechanisms to Targeted Therapies. Rheum Dis Clin North Am 47:395–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL (1999) Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med 190:1697–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arkatkar T, Jacobs HM, Du SW, Li QZ, Hudkins KL, Alpers CE, Rawlings DJ, Jackson SW (2018) TACI deletion protects against progressive murine lupus nephritis induced by BAFF overexpression. Kidney Int 94:728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stohl W, Metyas S, Tan SM, Cheema GS, Oamar B, Xu D, Roschke V, Wu Y, Baker KP, Hilbert DM (2003) B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum 48:3475–3486. [DOI] [PubMed] [Google Scholar]
- 38.Steri M, Orru V, Idda ML, Pitzalis M, Pala M, Zara I, Sidore C, Faa V, Floris M, Deiana M, Asunis I, Porcu E, Mulas A, Piras MG, Lobina M, Lai S, Marongiu M, Serra V, Marongiu M, Sole G, Busonero F, Maschio A, Cusano R, Cuccuru G, Deidda F, Poddie F, Farina G, Dei M, Virdis F, Olla S, Satta MA, Pani M, Delitala A, Cocco E, Frau J, Coghe G, Lorefice L, Fenu G, Ferrigno P, Ban M, Barizzone N, Leone M, Guerini FR, Piga M, Firinu D, Kockum I, Lima Bomfim I, Olsson T, Alfredsson L, Suarez A, Carreira PE, Castillo-Palma MJ, Marcus JH, Congia M, Angius A, Melis M, Gonzalez A, Alarcon Riquelme ME, da Silva BM, Marchini M, Danieli MG, Del Giacco S, Mathieu A, Pani A, Montgomery SB, Rosati G, Hillert J, Sawcer S, D'Alfonso S, Todd JA, Novembre J, Abecasis GR, Whalen MB, Marrosu MG, Meloni A, Sanna S, Gorospe M, Schlessinger D, Fiorillo E, Zoledziewska M, Cucca F (2017) Overexpression of the Cytokine BAFF and Autoimmunity Risk. The New England journal of medicine 376:1615–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, Leon MG, Tanasescu C, Nasonov E, Lan JL, Pineda L, Zhong ZJ, Freimuth W, Petri MA (2011) Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 377:721–731. [DOI] [PubMed] [Google Scholar]
- 40.Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, Sanchez-Guerrero J, Schwarting A, Merrill JT, Chatham WW, Stohl W, Ginzler EM, Hough DR, Zhong ZJ, Freimuth W, van Vollenhoven RF, Group B-S (2011) A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 63:3918–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunner HI, Abud-Mendoza C, Viola DO, Calvo Penades I, Levy D, Anton J, Calderon JE, Chasnyk VG, Ferrandiz MA, Keltsev V, Paz Gastanaga ME, Shishov M, Boteanu AL, Henrickson M, Bass D, Clark K, Hammer A, Ji BN, Nino A, Roth DA, Struemper H, Wang ML, Martini A, Lovell D, Ruperto N, Paediatric Rheumatology International Trials O, the Pediatric Rheumatology Collaborative Study G (2020) Safety and efficacy of intravenous belimumab in children with systemic lupus erythematosus: results from a randomised, placebo-controlled trial. Ann Rheum Dis 79:1340–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dooley MA, Houssiau F, Aranow C, D'Cruz DP, Askanase A, Roth DA, Zhong ZJ, Cooper S, Freimuth WW, Ginzler EM, Bliss, Study G (2013) Effect of belimumab treatment on renal outcomes: results from the phase 3 belimumab clinical trials in patients with SLE. Lupus 22:63–72. [DOI] [PubMed] [Google Scholar]
- 43.Stohl W, Schwarting A, Okada M, Scheinberg M, Doria A, Hammer AE, Kleoudis C, Groark J, Bass D, Fox NL, Roth D, Gordon D (2017) Efficacy and Safety of Subcutaneous Belimumab in Systemic Lupus Erythematosus: A Fifty-Two-Week Randomized, Double-Blind, Placebo-Controlled Study. Arthritis Rheumatol 69:1016–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urowitz MB, Ohsfeldt RL, Wielage RC, Kelton KA, Asukai Y, Ramachandran S (2019) Organ damage in patients treated with belimumab versus standard of care: a propensity score-matched comparative analysis. Annals of the rheumatic diseases 78:372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunner HI, Abud-Mendoza C, Mori M, Pilkington CA, Syed R, Takei S, Viola DO, Furie RA, Navarra S, Zhang F, Bass DL, Eriksson G, Hammer AE, Ji BN, Okily M, Roth DA, Quasny H, Ruperto N (2021) Efficacy and safety of belimumab in paediatric and adult patients with systemic lupus erythematosus: an across-study comparison. RMD Open 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P (2008) The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14:931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao R, Liu Q, Zheng Z, Fan J, Peng W, Kong Q, He H, Yang S, Chen W, Tang X, Yu X (2015) Tacrolimus Protects Podocytes from Injury in Lupus Nephritis Partly by Stabilizing the Cytoskeleton and Inhibiting Podocyte Apoptosis. PLoS One 10:e0132724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mok CC (2017) Calcineurin inhibitors in systemic lupus erythematosus. Best Pract Res Clin Rheumatol 31:429–438. [DOI] [PubMed] [Google Scholar]
- 49.Takeuchi H, Okuyama K, Konno O, Jojima Y, Akashi I, Nakamura Y, Iwamoto H, Hama K, Iwahori T, Uchiyama M, Ashizawa T, Matsuno N, Nagao T, Hirano T, Oka K (2005) Optimal dose and target trough level in cyclosporine and tacrolimus conversion in renal transplantation as evaluated by lymphocyte drug sensitivity and pharmacokinetic parameters. Transplant Proc 37:1745–1747. [DOI] [PubMed] [Google Scholar]
- 50.Hannah J, Casian A, D'Cruz D (2016) Tacrolimus use in lupus nephritis: A systematic review and meta-analysis. Autoimmun Rev 15:93–101. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez Nieto M, Jayne DR (2016) Con: The use of calcineurin inhibitors in the treatment of lupus nephritis. Nephrol Dial Transplant 31:1567–1571. [DOI] [PubMed] [Google Scholar]
- 52.Sin FE, Isenberg D (2018) An evaluation of voclosporin for the treatment of lupus nephritis. Expert Opin Pharmacother 19:1613–1621. [DOI] [PubMed] [Google Scholar]
- 53.Schultz C (2013) Voclosporin as a treatment for noninfectious uveitis. Ophthalmol Eye Dis 5:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kunynetz R, Carey W, Thomas R, Toth D, Trafford T, Vender R (2011) Quality of life in plaque psoriasis patients treated with voclosporin: a Canadian phase III, randomized, multicenter, double-blind, placebo-controlled study. Eur J Dermatol 21:89–94. [DOI] [PubMed] [Google Scholar]
- 55.Busque S, Cantarovich M, Mulgaonkar S, Gaston R, Gaber AO, Mayo PR, Ling S, Huizinga RB, Meier-Kriesche HU, Investigators P (2011) The PROMISE study: a phase 2b multicenter study of voclosporin (ISA247) versus tacrolimus in de novo kidney transplantation. Am J Transplant 11:2675–2684. [DOI] [PubMed] [Google Scholar]
- 56.Rovin BH, Solomons N, Pendergraft WF 3rd, Dooley MA, Tumlin J, Romero-Diaz J, Lysenko L, Navarra SV, Huizinga RB, Group A-LS (2019) A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int 95:219–231. [DOI] [PubMed] [Google Scholar]
- 57.Rovin BH, Teng YKO, Ginzler EM, Arriens C, Caster DJ, Romero-Diaz J, Gibson K, Kaplan J, Lisk L, Navarra S, Parikh SV, Randhawa S, Solomons N, Huizinga RB (2021) Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 397:2070–2080. [DOI] [PubMed] [Google Scholar]
- 58.Chapman JR (2011) Chronic calcineurin inhibitor nephrotoxicity-lest we forget. Am J Transplant 11:693–697. [DOI] [PubMed] [Google Scholar]
- 59.Karolin A, Genitsch V, Sidler D (2021) Calcineurin Inhibitor Toxicity in Solid Organ Transplantation. Pharmacology 106:347–355. [DOI] [PubMed] [Google Scholar]
- 60.Saxena A, Mina-Osorio P, Mela C, Birardi V, Randhawa S (2021) 514 Voclosporin for lupus nephritis: interim analysis of the AURORA 2 extension study. Lupus Science and Medicine 8:A20–A21. [Google Scholar]
- 61.Lipsky PE (2001) Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol 2:764–766. [DOI] [PubMed] [Google Scholar]
- 62.Merrill JT, Manzi S, Aranow C, Askenase A, Bruce I, Chakravarty E, Chong B, Costenbader K, Dall'Era M, Ginzler E, Hanrahan L, Kalunian K, Merola J, Raymond S, Rovin B, Saxena A, Werth VP (2018) Lupus community panel proposals for optimising clinical trials: 2018. Lupus Sci Med 5:e000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB (2003) Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 349:1526–1533. [DOI] [PubMed] [Google Scholar]
- 64.Hale M, Rawlings DJ, Jackson SW (2018) The long and the short of it: insights into the cellular source of autoantibodies as revealed by B cell depletion therapy. Curr Opin Immunol 55:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gomez Mendez LM, Cascino MD, Garg J, Katsumoto TR, Brakeman P, Dall'Era M, Looney RJ, Rovin B, Dragone L, Brunetta P (2018) Peripheral Blood B Cell Depletion after Rituximab and Complete Response in Lupus Nephritis. Clin J Am Soc Nephrol 13:1502–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ (2007) Depletion of B cells in murine lupus: efficacy and resistance. J Immunol 179:3351–3361. [DOI] [PubMed] [Google Scholar]
- 67.Freeman CL, Sehn LH (2018) A tale of two antibodies: obinutuzumab versus rituximab. Br J Haematol 182:29–45. [DOI] [PubMed] [Google Scholar]
- 68.Reddy V, Klein C, Isenberg DA, Glennie MJ, Cambridge G, Cragg MS, Leandro MJ (2017) Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology (Oxford) 56:1227–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, Phillips E, Sangha R, Schlag R, Seymour JF, Townsend W, Trneny M, Wenger M, Fingerle-Rowson G, Rufibach K, Moore T, Herold M, Hiddemann W (2017) Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N Engl J Med 377:1331–1344. [DOI] [PubMed] [Google Scholar]
- 70.Furie RA, Aroca G, Cascino MD, Garg JP, Rovin BH, Alvarez A, Fragoso-Loyo H, Zuta-Santillan E, Schindler T, Brunetta P, Looney CM, Hassan I, Malvar A (2022) B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 81:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C (1993) Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237–244. [DOI] [PubMed] [Google Scholar]
- 72.Gipson DS, Tarnoff J, Lee L, Vivarelli M, Levtchenko E, Oh J, Smoyer WE, Desmond H, Attalla S, Trachtman H (2021) A pediatric gateway initiative for glomerular disease: introducing PIONEER. Kidney Int 99:515–518. [DOI] [PubMed] [Google Scholar]