Abstract
Background:
Cardiovascular disease (CVD), including elevated blood pressure (BP), is known to promote Alzheimer’s disease (AD) risk. Although brain amyloid load is a recognized hallmark of pre-symptomatic AD, its relationship to increased BP is less known. The objective of this study was to examine the relationship of BP to brain estimates of amyloid-β (Aβ) standard uptake ratio (SUVr). We hypothesized that increased BP is associated with increased SUVr.
Methods:
Using data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI), we stratify BP according to the Seventh Joint National Committee (JNC) on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure Classification (JNC VII). Florbetapir (AV-45) SUVr was derived from the averaged frontal, anterior cingulate, precuneus, and parietal cortex relative to the cerebellum. A Linear Mixed Effects Model enabled the elucidation of amyloid SUVr relationships to BP. The model discounted the effects of demographics, biologics, and diagnosis at baseline within APOE genotype groups. The Least Squares Means procedure was used to estimate the fixed effect means. All analyses were performed using the Statistical Analysis System (SAS).
Results:
In non- ε4 carrier MCI subjects, escalating JNC categories of blood pressure was associated with increasing mean SUVr using JNC-4 as a reference point (low-normal (JNC1) P = 0.018; normal (JNC-1) P = 0.039; JNC-2 P = 0.018 and JNC-3 P = 0.04). A significantly higher brain SUVr was associated with increasing BP despite adjustment for demographics and biological variables in non-ε4 carriers but not in ε4-carriers. This observation supports the view that CVD risk may promote increased brain amyloid load, and potentially, amyloid-mediated cognitive decline.
Conclusion:
Increasing levels of JNC classification of blood pressure is dynamically associated with significant changes in brain amyloid burden in non-ε4 carriers but not in ε4-carrier MCI subjects. Though not statistically significant, amyloid burden tended to decrease with increasing blood pressure in ε4 homozygote, perhaps motivated by increased vascular resistance and the need for higher brain perfusion pressure.
Keywords: Alzheimer’s Disease, Apolipoprotein, Blood Pressure, A-Beta Amyloid, Mild Cognitively Impaired
INTRODUCTION
Epidemiological studies have shown that risk factors for vascular diseases, such as diabetes mellitus and hypertension, are also associated with increased risk for cognitive decline [1–4]. Evidence for an association between late-life high blood pressure and cognition is mixed [5]. However, most have reported that high blood pressure in midlife is associated with more significant late-life cognitive decline, particularly in executive functioning, and attention, and with the development of dementia [6–8]. Similarly, hypertension in healthy adults is associated with poorer cognitive performance [9], increased rate of brain shrinkage [10], degraded white matter connectivity [11], and greater regional brain iron concentration [12]. Thus as an age-related cerebrovascular risk factor, high blood pressure promotes white matter alterations and potentially AD [13]. However, whether and how it influences amyloid deposits in the brain, and therefore, AD development is less understood.
Amyloid-β (Aβ) accumulation in the brain [14] is a pathological feature of AD and underlies cognitive impairment and dementia. Transport of Aβ across the blood-brain barrier is one of the mechanisms regulating the concentration of Aβ in the Central Nervous System [15]. Also, peripheral Aβ interacts with the cerebral vasculature to modulate Aβ deposition in the brain [16]. Elevated levels of Aβ or the intracellular soluble Aβ protein correlate with the loss of neuronal synapses and cognitive impairment [17]. Brain amyloid load is the hallmark of pre-symptomatic AD, such as MCI. Notably, increasing evidence suggests that high blood pressure may directly impact Aβ accumulation. In a recent review, Hughes et al. (2018) provided an overview revealing the complex relationship between increased blood pressure, cognition, and Alzheimer’s Disease [18]. However, Arvanitakis et al. (2018) found little evidence that increased BP increased the odds of amyloid pathology [19]. In another study examining the relationship among hypertension, beta-amyloid and neurodegeneration biomarkers of Alzheimer’s disease (AD), Jeon et al. (2019) [20] concluded that regardless of APOE4 status, AD dementia patients with hypertension had significantly lower Aβ deposition than those without hypertension. Collectively, though several studies support the view that high blood pressure (HBP) may enhance the accumulation of Aβ in the brain [12, 21], the relationship remains inconsistent, and modulating factors need more nuanced understanding [22–25].
Among the multiple genetic variants identified as risk factors for Alzheimer’s Disease (AD), the apolipoprotein E ε4 allele (APOE ε4) is the most consistent genetic polymorphism [26] associated with increased risk for cognitive decline and dementia [27–29], [30–32]. Individuals with two copies of the APOE ε4 allele have a 10 to 12-fold risk for AD compared with ε3 homozygotes [33]. Interestingly, the APOE ε4 polymorphism is also a risk factor for vascular disease [34, 35]. Although the APOE gene regulates the levels of the multifunctional lipid transporter, its relationships to levels of SBP, DBP, and PPR need a more nuanced understanding. Similarly, the differential effects of the APOE gene on the relationship of blood pressure with brain Aβ accumulation need improved understanding. Thus, genetic and vascular risk factors, including Aβ, may work synergistically to influence the neuropathological changes that result in cognitive decline. To test our hypothesis that blood pressure affects Aβ standard uptake ratio (SUVr) in Mild Cognitively Impaired (MCI) subjects, we examined the relationship of blood pressure to SUVr using Alzheimer’s Disease Neuroimaging Initiative (ADNI) data. We hypothesized that increased BP is associated with increased SUVr in patients with mild cognitive impairment (MCI). We also determined whether alleles of the APOE gene differentially influenced blood pressure effects on SUVr.
MATERIALS AND METHODS
Data for this analysis were downloaded from the ADNI database (http://adni.loni.usc.edu) on 10/12/2012. The ADNI was designed to improve methods for clinical trials by providing an extensive, publicly available database to inform cognitive deterioration leading to AD at an early stage and mark its progress through biomarkers [36]. In part, the goal of ADNI was to test whether neuroimaging, other biological markers, clinical measures, and neuropsychological assessments can be combined to inform cognitive deterioration from cognitively normal (CN) to MCI and AD. Participants in the ADNI study underwent baseline and periodic physical and neurological examinations and standardized neuropsychological assessments and provided biological samples [36]. The physical examinations included height, weight, SBP, and DBP measurements. Seated brachial artery SBP and DBP were obtained using the standard of care approach, and PPR was calculated as SBP minus DBP [37]. Our analysis was a cross-sectional study of longitudinally obtained data from the ADNI cohort.
The ADNI study also provided a rich set of amyloid positron emission tomography (PET) and several clinical and neuropsychological measures acquired from MCIs and other diagnostic categories in participants [38, 39].
Whereas our analysis is limited to 24-month data, the study followed participants over several years with additional years of data acquired in the ADNI-GO, ADNI-2, and now ADNI-3 projects [38]. Participants were classified as meeting the MCI inclusion criteria premise on the following: Mini-Mental State Examination (MMSE) [40] scores between 24 and 30 (inclusive), objective memory loss measured according to education-adjusted scores on the Wechsler Memory Scale Logical Memory II [41], Clinical Dementia Rating of 0.5 [36], absence of significant levels of impairment in other cognitive domains, essentially preserved activities of daily living, and absence of dementia.
Details of the ADNI study, including the acquisition of amyloid PET, have been previously published [41–44]. The analyses included participants who had brain amyloid PET scans at baseline and at 12 and 24 months and 2-year follow-up clinical evaluations. The time of the first amyloid PET scan underscored the baseline visit for each participant.
STATISTICAL ANALYSIS:
The ADNI sample of 1697 participants (at the time of download) consisted of 809 subjects genotyped at the APOE locus (non-ε4 carriers = 465; ε4 heterozygote = 277; and ε4 homozygote = 67). Among these subjects, 466 MCI participants identified for this analysis had data on SUVr. Blood pressure data from the ADNI studies were stratified according to the Seventh Joint National Committee (JNC) on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure Classification (JNC VII). To discern the effects of low blood pressure, the JNC1 was subdivided into two categories: low-normal and normal; and used Univariate analysis to discern unique data characteristics and validate the assumption of normality. The Aβ standard uptake ratio (SUVr) was derived from the averaged frontal, anterior cingulate, precuneus, and parietal cortex relative to the cerebellum. We implemented a Linear Mixed Effects Model (Proc Mixed) with Restricted Maximum Likelihood to elucidate the relationships of amyloid SUVr to BP while accounting for demographics (age, gender, race, education) and biological effects (diastolic blood pressure, pulse pressure, pulse rate, body mass index (BMI), and diagnosis at baseline) variables within APOE genotype groups. To discern fixed effect means, we employed the LS Means procedure. All analyses were performed using the Statistical Analysis System (SAS), Research Triangle North Carolina [45].
RESULTS:
To delineate categories of blood pressure, we used the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7) Classification for systolic and diastolic blood pressure measurements (Table 1). The blood pressure measurements were classified as Low-Normal, Normal, Prehypertension, Hypertension Stage I and II.
Table 1:
Classification of Blood Pressure (mmHg)
| Classification | Systolic BP (mmHg) | Diastolic BP (mmHg) |
|---|---|---|
| Low Normal (JNC 1) | < 100 | < 60 |
| Normal (JNC 1) | 100 – 120 | 60 – 80 |
| Prehypertension (JNC 2) | 120 – 139 | 80 – 89 |
| Hypertension Stage I (JNC 3) | 140 – 159 | 90 – 99 |
| Hypertension Stage II (JNC 4) | ≥ 160 | ≥ 100 |
JNC = Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure Classification
The characteristics of the 860 ADNI participants are presented in Table 2 by blood pressure categories; Low-Normal (56); Normal (152); Pre-HTN (360); Stage I HTN (240), and Stage II HTN (52). The mean age of the participants at baseline ranged from 71 – 75 years. The low-normal group was more educated (mean years of education = 16.55 (SD = 2.68)) compared to the Stage II HTN category (mean years of education = 15.62 (SD = 3.02)). Men had greater representation in all categories than women, and the overall sample is ~87% whites. The sample included non-ε4 carriers (48.39%); ε4 heterozygotes (39.95%); ε4 homozygotes (11.66%). Among those with low-normal blood pressure, the majority were ε4 heterozygotes (45.10%) compared to non-ε4 carriers (43.14%) and ε4 homozygotes (11.76%). The low-normal blood pressure group had a normal mean body mass index (BMI) of 24.52 (3.69). As expected, the sample BMI increased as the blood pressure categories increased, with Stage II HTN having a mean BMI of 28.24(5.77). However, the mean pulse rate was similar across the blood pressure categories, with greater variability (14.26) among Stage II HTN than in the other categories. In addition, cognitive scores (MMSE and ADAS 13) and AV-45 SUVr) were similar across the different blood pressure categories.
Table 2:
Characteristics of Participants by Blood Pressure Classification
| Characteristics | Low Normal (N = 56) | Normal (N = 152) | Pre HTN (N = 360) | Stage I HTN (N = 240) | Stage II HTN (N = 52) |
|---|---|---|---|---|---|
| Age at Baseline (Years) | 70.94 (8.46) | 72.21 (8.14) | 72.79 (7.60) | 73.90 (6.99) | 75.00 (7.79) |
| Education (Years) | 16.55 (2.68) | 15.87 (2.89) | 15.96 (2.88) | 15.85 (2.76) | 15.62 (3.02) |
| Gender (% Men) | 35 (62.50%) | 87 (57.24%) | 221 (61.39%) | 136 (56.67%) | 32 (61.54%) |
| Race (% White) | 51 (91.07%) | 147 (96.71%) | 336 (93.33%) | 221 (92.08%) | 47 (90.38%) |
| Race (% Black) | 4 (7.14%) | 3 (1.97%) | 12 (3.33%) | 8 (3.33%) | 3 (5.77%) |
| APOE4 Status (%) | |||||
| non–ε4 Carriers | 22 (43.14%) | 80 (53.33%) | 168 (47.06%) | 130 (54.17%) | 23 (44.23%) |
| ε4 Heterozygote | 23 (45.10%) | 54 (36.00%) | 147 (41.18%) | 89 (37.08%) | 21 (40.38%) |
| ε4 Homozygote | 6 (11.76%) | 16 (10.67%) | 42 (11.76%) | 21 (8.75%) | 8 (15.38%) |
| BMI | 24.52 (3.69) | 25.78 (3.74) | 27.33 (4.80) | 26.94 (4.47) | 28.24 (5.77) |
| Systolic (mmHg) | 92.78 (4.52) | 110.99 (6.19) | 128.88 (6.28) | 145.76 (6.60) | 168.69 (11.29) |
| Diastolic (mmHg) | 54.89 (3.95) | 66.04 (6.07) | 73.26 (7.63) | 77.76 (9.31) | 84.37 (10.04) |
| Pulse Pressure (mmHg) | 37.89 (6.57) | 44.95 (8.08) | 55.63 (9.46) | 68.00 (11.61) | 84.32 (14.26) |
| Pulse Rate (beats/min) | 65.00 (12.01) | 64.52 (9.16) | 64.44 (9.71) | 66.55 (11.60) | 65.67 (11.26) |
| MMSE | 27.63 (1.72) | 27.84 (1.75) | 27.59 (1.83) | 27.51 (1.76) | 27.19 (2.09) |
| ADAS 13 | 17.47 (7.40) | 15.64 (6.66) | 16.41 (6.79) | 16.87 (6.70) | 17.64 (6.47) |
| Amyloid Load (AV45 SUVr) | 1.27 (0.26) | 1.17 (0.23) | 1.18 (0.22) | 1.24 (0.21) | 1.19 (0.25) |
Values are mean ± SD when appropriate; MMSE: Mini Mental State Examination; BMI = Body Mass Index.
ADAS 13 = The Alzheimer’s Disease Assessment Scale; HTN = Hypertension
To test the relationship between SUVr and the different blood pressure categories by APOE ε4 status, we performed a Linear Mixed Effects Model (Table 3). To discount the effect of important confounders, all fixed effects estimates included adjustments for Demographics (Age, Gender, Race, Education), Biologics (Diastolic Blood Pressure, Systolic Blood Pressure, Pulse Rate, Body Mass Index (BMI), and Diagnosis at Baseline. Among non-ε4 carriers (n = 256), with the Stage II HTN as a reference, the Low-Normal group had a statistically significant lower SUVr than the Stage II HTN group SUVr (p-value = 0.018). Similarly, the remaining blood pressure groups had a statistically significantly lower SUVr than the Stage II HTN group: Normal (p-value = 0.036), Pre-HTN (p-value = 0.017), and Stage I HTN (p-value = 0.036) among non-ε4 carriers.
Table 3:
Amyloid Standard Uptake Ratio (SUVr) and JNC Categories of Blood Pressure in Mild Cognitively Impaired (MCI) by Apolipoprotein (APOE) ε4 Status
| Fixed Effects | Standard Error | P-Value | ||
|---|---|---|---|---|
| non–ε4 Carriers (N = 256) | Overall Model (Residual) | 0.0004 | ||
| Intercept | 1.731 | 0.389 | <.0001 | |
| JNC 1: Low Normal | −0.456 | 0.192 | 0.018 | |
| JNC 1: Normal | −0.275 | 0.131 | 0.036 | |
| JNC 2: Prehypertension | −0.243 | 0.101 | 0.017 | |
| JNC 3: Hypertension Stage I | −0.165 | 0.078 | 0.036 | |
| JNC 4: Hypertension Stage II | . | . | . | |
| ε4 Heterozygote (N = 168) | Overall Model (Residual) | 0.002 | ||
| Intercept | 0.447 | 0.492 | 0.366 | |
| JNC 1: Low Normal | −0.009 | 0.255 | 0.971 | |
| JNC 1: Normal | 0.049 | 0.164 | 0.763 | |
| JNC 2: Prehypertension | 0.099 | 0.124 | 0.423 | |
| JNC 3: Hypertension Stage I | 0.096 | 0.095 | 0.317 | |
| JNC 4: Hypertension Stage II | . | . | . | |
| ε4 Homozygote (N = 42) | Overall Model (Residual) | 0.131 | ||
| Intercept | −0.724 | 1.181 | 0.545 | |
| JNC 1: Low Normal | 0.736 | 0.488 | 0.143 | |
| JNC 1: Normal | 0.528 | 0.303 | 0.092 | |
| JNC 2: Prehypertension | 0.287 | 0.228 | 0.219 | |
| JNC 3: Hypertension Stage I | 0.165 | 0.180 | 0.366 | |
| JNC 4: Hypertension Stage II | . | . | . | |
Fixed Effects Estimates Modeling Adjusted for Demographics (Age, Gender, Race, Education), Biologics (Diastolic Blood Pressure, Systolic Blood Pressure, Pulse Rate, Body Mass Index (BMI), and Diagnosis at Baseline
In a similarly adjusted Fixed Effects model, participants’ age was associated with increasing SUVr only among the non-ε4 carriers (p = 0.008) and ε4 heterozygotes (p < 0.0001). Further, diastolic blood pressure was significantly associated with decreased SUVr (p-value = 0.014) among the non-ε4 carriers. However, we observed no consistently discernable relationship of JNC-7 blood pressure categories to SUVr among ε4 heterozygote (n = 168) and ε4 homozygote (n = 42).
Figure 1 shows Least Squares Mean (LS-Mean) estimates from the linear mixed-effects model on the association of SUVr with categories of blood pressure by APOE ε4 status. Among the non-ε4 carriers, the SUVr LS-mean tended to increase with increasing JNC-7 blood pressure categories from Low-Normal to Stage II HTN. This trend was reversed among the ε4 homozygotes with LS means decreasing from Low-Normal to Stage II HTN participants, though not significant in the Fixed Effect model. Among ε4 heterozygotes, we observed no directional relationship between SUVr LS means and BP.
Figure 1:
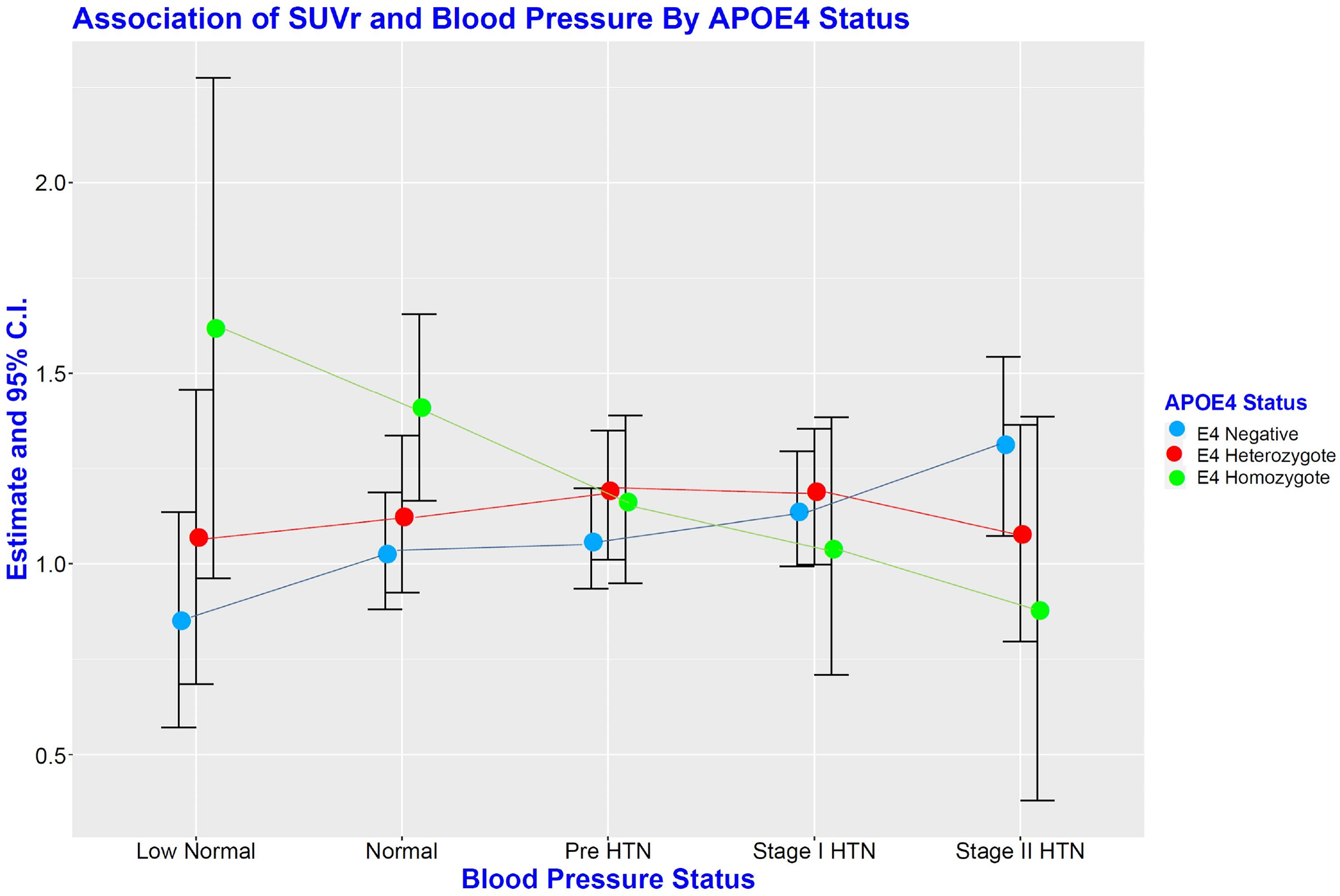
Least Squares Mean (LS-Mean) estimates from a Linear mixed-effects model showing Estimates and 95% CI of the Association of SUVr with categories of blood pressure by APOE ε4 status. HTN = Hypertension; APOE ε4 = Apolipoprotein ε4.
DISCUSSION
In the current study, increasing JNC-7 classification of blood pressure levels is dynamically associated with significant changes in brain amyloid burden (measured by SUVr) in non-ε4 carriers but not in ε4-carrier MCI subjects. The dynamic relationship of blood pressure to brain amyloid burden is similarly influenced by ε4 carrier status. This suggests that increasing blood pressure may harm the brain by enabling increased amyloid accumulation in APOE non-ε4 carriers at the transitional stage of neurodegeneration. Paradoxically, and evidenced by the LS-Mean, increasing blood pressure may be advantageous in ε4 homozygote, though the threshold is undetermined. Increased vascular resistance promoted by amyloid deposition and the need for higher brain perfusion pressure may underlie this effect up to a specific blood pressure threshold [21]. This finding is consistent with our previously published observation that changes in brachial artery pulse pressure (PPR), systolic blood pressure (SBP), and diastolic blood pressure (DBP) differentially influenced hippocampal volumes depending on the cognitive phenotype and APOE genotypic categories [46].
Data is scanty on the relationship of blood pressure to brain amyloid load, and evidence on the relationship of blood pressure to the cognitive phenotype is inconsistent. For example, Faraco, Park et al. reported that the effects of Aβ on both mean diffusivity (MD) alterations and global white matter hyperintensity (WMH) were independent of hypertension status [47]. The implication is that mediators besides hypertensive small vessel disease may account for the observed effects [47] in APOE ε4 carriers. Therefore, APOE ε4 may overwhelmingly motivate amyloid deposition in ε4 carriers, while hypertension promotes increased amyloid burden in non-ε4 carriers. Thus, increasing blood pressure may harm the brain by enabling increased amyloid accumulation in non-ε4 carriers at the MCI transitional stage of neurodegeneration but not in ε4 carriers. Another study [48], with a relatively large sample size (n = 1406, aged 60–95 years), investigated the relationship between hypertension and the modulating effect of APOE-ε4. Their findings suggested that hypertension was not associated with either ε2 or ε4 alleles in the model adjusted for age and gender or with the inclusion of other confounders. However, the investigators did not study the interaction between Aβ and APOE. Further, Rodrigue et al. showed that hypertensive APOE ε4 carriers did not have significantly different amyloid burden compared to normotensive non-carriers [21]. Because their result is congruent with our current results in MCI subjects (Figure 1), it is possible that in APOE-ε4 carriers with Aβ mediated elevated vascular resistance, increased blood pressure may promote perfusion and potentially mitigate Aβ effect on brain function.
Yet, studies reporting conflicting observations must be noted. In contrast to our observations in APOE ε4 carriers, Oberlin et al. (2015) noted that the combined role of APOE ε4 and elevated systolic blood pressure (SBP) may synergistically compromise memory function long before the appearance of clinically significant impairment [49]. These observations suggest that interventions targeting blood pressure in APOE ε4 carriers during midlife may reduce the risk of cognitive decline in APOE ε4 carriers [49]. Likewise, in a cross-sectional study to discern risk factors for Aβ deposition in cognitively healthy middle-aged and older adults (aged 47–89 yrs.), Rodrigue et al. reported that hypertension interacts with APOE ε4 allele to increase amyloid deposition in cognitively healthy middle-aged and older adults [21]. Accordingly, the mean cortical amyloid level was lowest in the normotensive APOE ε4 positive group, followed closely by the normotensive APOE ε4-negative group and the hypertensive APOE ε4-negative group. The hypertensive APOE ε4-positive group (mean age = 75 yrs.) had significantly greater amyloid deposition than all other groups (P = 0.05), suggesting an association between vascular risk and APOE ε4-positive in the elderly. However, in contrast to our analysis of the ADNI data, the study had a small sample size and did not include participants with MCI.
It is possible that other unknown factors may yet modulate the interaction of blood pressure and the APOE gene on brain amyloid burden. Further, whether treatment of hypertension influence the combined effect of APOE-ε4 status and hypertension on cognitive function is a subject of an ongoing investigation. For example, Kim et al. (2019) examined the interaction between APOE genotypes in both treated and untreated hypertension on cognitive function in a recent analysis of Nurses’ Health Study data [50]. Women with hypertension and at least one APOE-ε4 allele had worse average cognitive function than women without hypertension with the ε3/ε3 genotype; an observation amplified among APOE-ε4 allele carriers with untreated hypertension. Unfortunately, the study did not discern the interactive effect of amyloid load. In our current study, adjusting for age and DBP were associated with increased brain amyloid burden in non-ε4. The association of DBP with SUVr in the context of age was observed only among the non-ε4 carriers, suggesting that having the homozygote allele may exert effects over and beyond the effects of aging and brain amyloid burden.
We conclude that the disadvantageous effects of blood pressure were most noticeable in non-ε4 carriers, attenuated in ε4 heterozygote, and may be compensatory in ε4 homozygote to enhance perfusion pressure and potentially Aβ clearance in the groups at the highest risk of AD. This observation suggests the existence of an interaction between the APOE-ε4 allele and high blood pressure, which, if controlled in subjects with MCI, may delay the onset or risks of AD. Analysis of larger sample sizes is needed to validate our findings and address determinants of amyloid deposition in the aging brain and AD. In addition, similar studies in cognitively healthy persons might provide further insight into the relationship between SUVr and blood pressure. Such studies will further inform additional mediating risk factors such as treatment of hypertension and duration and inflammation.
STRENGTHS AND LIMITATIONS
The approach used in this study enhances the understanding of the relationship between brain amyloid load, recognized as the hallmark of AD, with increased BP in MCI subjects. Potential limitations of this study include a small sample size in the ε4 homozygote group. In addition, blood pressure was not an a priori outcome in the ADNI study; hence assessing blood pressure did not employ a unified procedure but followed current JNC 1–4 clinical standards. Furthermore, the inclusion of a diverse population in such studies may improve the generalization of the results. Nonetheless, our observation is unique and provides important insight into the field’s current understanding. Future work on the longitudinal relationship between hypertension, APOE, and cerebral amyloidosis would undoubtedly increase our current understanding of the impact of SUVr and hypertension on AD.
ACKNOWLEDGMENT:
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). Data collection and sharing for this project were funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research provided funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Details of the ADNI’s co-sponsors have been previously published [36, 39, 40].
FUNDING SOURCES:
The ADNI data was supported by the National Institute on Aging at the NIH (grant U01 AG024904 to M.W. Weiner of the ADNI. This analysis was supported by grants 5R01AG031517-2 and 5RO1AG045058 to T.O. Obisesan and, in part, by the National Center for Advancing Translational Sciences/NIH through the Clinical and Translational Science Award Program (CTSA; grant UL1TR000101). The sponsors played no role in the study’s design, data collection, preparation and interpretation.
ABBREVIATIONS:
- AD
Alzheimer’s Disease
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
- APOE ε4
Apolipoprotein ε4 Allele
- BP
Blood Pressure
- HTN
Hypertension
- MCI
Mild Cognitively Impaired
- MMSE
Mini-Mental State Examination
- SUVr
Aβ Standardized Uptake Value Ratio
Footnotes
CONFLICT OF INTEREST STATEMENT: The authors have no commercial associations that might be a conflict of interest in this article.
STATEMENT OF ETHICS: According to ADNI protocols, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee. The standards were also in accord with the 1964 Helsinki declaration and its ensuing amendments. Before the enrollment of participants, written informed consent forms approved by the participating Institutional Review Boards were used to inform and obtain consent from prospective participants. Details can be found at https://adni.loni.usc.edu/ (No studies with human participants were performed by any of the authors are included in this manuscript).
DATA AVAILABILITY STATEMENT:
Data used to prepare this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (https://adni.loni.usc.edu/). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
REFERENCES:
- 1.DeCarli C: Vascular factors in dementia: an overview. Journal of the neurological sciences 2004, 226(1–2):19–23. [DOI] [PubMed] [Google Scholar]
- 2.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ: The association between midlife blood pressure levels and late-life cognitive function: the Honolulu-Asia Aging Study. Jama 1995, 274(23):1846–1851. [PubMed] [Google Scholar]
- 3.Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ: Midlife blood pressure and dementia: the Honolulu–Asia aging study☆. Neurobiology of aging 2000, 21(1):49–55. [DOI] [PubMed] [Google Scholar]
- 4.Shah NS, Vidal J-S, Masaki K, Petrovitch H, Ross GW, Tilley C, DeMattos RB, Tracy RP, White LR, Launer LJ: Midlife blood pressure, plasma β-amyloid, and the risk for Alzheimer disease: the Honolulu Asia Aging Study. Hypertension (Dallas, Tex : 1979) 2012, 59(4):780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Low L-F, Yap MH, Brodaty H: Will testing for apolipoprotein E assist in tailoring dementia risk reduction? A review. Neuroscience & Biobehavioral Reviews 2010, 34(3):408–437. [DOI] [PubMed] [Google Scholar]
- 6.Novak V, Hajjar I: The relationship between blood pressure and cognitive function. Nature Reviews Cardiology 2010, 7(12):686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gąsecki D, Kwarciany M, Nyka W, Narkiewicz K: Hypertension, brain damage, and cognitive decline. Current hypertension reports 2013, 15(6):547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jennings JR, Zanstra Y: Is the brain the essential in hypertension? Neuroimage 2009, 47(3):914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raz N, Rodrigue KM, Acker JD: Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behavioral neuroscience 2003, 117(6):1169. [DOI] [PubMed] [Google Scholar]
- 10.Raz N, Rodrigue KM, Kennedy KM, Acker JD: Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology 2007, 21(2):149. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy KM, Raz N: Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain research 2009, 1297:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigue KM, Haacke EM, Raz N: Differential effects of age and history of hypertension on regional brain volumes and iron. Neuroimage 2011, 54(2):750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC: Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nature communications 2016, 7(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M: ABAD directly links Aß to mitochondrial toxicity in Alzheimer’s disease. Science 2004, 304(5669):448–452. [DOI] [PubMed] [Google Scholar]
- 15.Zlokovic BV: Clearing amyloid through the blood–brain barrier. Journal of neurochemistry 2004, 89(4):807–811. [DOI] [PubMed] [Google Scholar]
- 16.Zlokovic BV: New therapeutic targets in the neurovascular pathway in Alzheimer’s disease. Neurotherapeutics 2008, 5(3):409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Mims PN, Roman RJ, Fan F: Is beta-amyloid accumulation a cause or consequence of Alzheimer’s disease? Journal of Alzheimer’s parkinsonism & dementia 2016, 1(2). [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes TM, Lockhart SN, Smagula SF: Blood pressure’s role in Alzheimer disease pathology. The American Journal of Geriatric Psychiatry 2018, 26(1):23–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arvanitakis Z, Capuano AW, Lamar M, Shah RC, Barnes LL, Bennett DA, Schneider JA: Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology 2018, 91(6):e517–e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeon SY, Byun MS, Yi D, Lee JH, Choe YM, Ko K, Sohn BK, Choi HJ, Lee J-Y, Lee DY: Influence of hypertension on brain amyloid deposition and Alzheimer’s disease signature neurodegeneration. Neurobiology of aging 2019, 75:62–70. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Diaz-Arrastia R, Park DC: Risk factors for β-amyloid deposition in healthy aging: vascular and genetic effects. JAMA neurology 2013, 70(5):600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Oliveira FF, Chen ES, Smith MC, Bertolucci PHF: Associations of blood pressure with functional and cognitive changes in patients with Alzheimer’s disease. Dementia and geriatric cognitive disorders 2016, 41(5–6):314–323. [DOI] [PubMed] [Google Scholar]
- 23.Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A: Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA neurology 2014, 71(10):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obisesan TO, Ferrell RE, Goldberg AP, Phares DA, Ellis TJ, Hagberg JM: APOE genotype affects black-white responses of high-density lipoprotein cholesterol subspecies to aerobic exercise training. Metabolism 2008, 57(12):1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razay G, Williams J, King E, Smith AD, Wilcock G: Blood pressure, dementia and Alzheimer’s disease: the OPTIMA longitudinal study. Dementia and geriatric cognitive disorders 2009, 28(1):70–74. [DOI] [PubMed] [Google Scholar]
- 26.Coon KD, Myers AJ, Craig DW, Webster JA, Pearson JV, Lince DH, Zismann VL, Beach TG, Leung D, Bryden L: A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. 2007. [DOI] [PubMed]
- 27.Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JB: Fibrillar amyloid-β burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proceedings of the National Academy of Sciences 2009, 106(16):6820–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Hara R, Yesavage JA, Kraemer HC, Mauricio M, Friedman LF, Murphy GM Jr: The APOE∍ 4 allele Is Associated with Decline on Delayed Recall Performance in Community-Dwelling Older Adults. Journal of the American Geriatrics Society 1998, 46(12):1493–1498. [DOI] [PubMed] [Google Scholar]
- 29.Caselli RJ, Reiman EM, Locke DE, Hutton ML, Hentz JG, Hoffman-Snyder C, Woodruff BK, Alexander GE, Osborne D: Cognitive domain decline in healthy apolipoprotein E ε4 homozygotes before the diagnosis of mild cognitive impairment. Archives of neurology 2007, 64(9):1306–1311. [DOI] [PubMed] [Google Scholar]
- 30.Mucke L, Selkoe DJ: Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harbor perspectives in medicine 2012, 2(7):a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahley RW: Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 1988, 240(4852):622. [DOI] [PubMed] [Google Scholar]
- 32.Davignon J, Gregg RE, Sing CF: Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology 1988, 8(1):1–21. [DOI] [PubMed] [Google Scholar]
- 33.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small G, Roses A, Haines J, Pericak-Vance MA: Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261(5123):921–923. [DOI] [PubMed] [Google Scholar]
- 34.Haan MN, Mayeda ER: Apolipoprotein E genotype and cardiovascular diseases in the elderly. Current cardiovascular risk reports 2010, 4(5):361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song Y, Stampfer MJ, Liu S: Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease. Annals of internal medicine 2004, 141(2):137–147. [DOI] [PubMed] [Google Scholar]
- 36.Wyman BT, Harvey DJ, Crawford K, Bernstein MA, Carmichael O, Cole PE, Crane PK, DeCarli C, Fox NC, Gunter JL: Standardization of analysis sets for reporting results from ADNI MRI data. Alzheimer’s & Dementia 2013, 9(3):332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bender AR, Raz N: Age-related differences in memory and executive functions in healthy APOE ε4 carriers: The contribution of individual differences in prefrontal volumes and systolic blood pressure. Neuropsychologia 2012, 50(5):704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moffat S, Szekely C, Zonderman A, Kabani N, Resnick S: Longitudinal change in hippocampal volume as a function of apolipoprotein E genotype. Neurology 2000, 55(1):134–136. [DOI] [PubMed] [Google Scholar]
- 39.Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack CR, Jagust W, Trojanowski JQ, Toga AW, Beckett L: Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimer’s & Dementia 2005, 1(1):55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiner MW, Aisen PS, Jack CR, Jagust WJ, Trojanowski JQ, Shaw L, Saykin AJ, Morris JC, Cairns N, Beckett LA: The Alzheimer’s disease neuroimaging initiative: progress report and future plans. Alzheimer’s & Dementia 2010, 6(3):202–211. e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scale—Fourth WDWM: Edition (WMS-IV). New York: Psychological Corporation; 2009. [Google Scholar]
- 42.Nation DA, Edmonds EC, Bangen KJ, Delano-Wood L, Scanlon BK, Han SD, Edland SD, Salmon DP, Galasko DR, Bondi MW: Pulse pressure in relation to tau-mediated neurodegeneration, cerebral amyloidosis, and progression to dementia in very old adults. JAMA neurology 2015, 72(5):546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jack CR, Bernstein MA, Borowski BJ, Gunter JL, Fox NC, Thompson PM, Schuff N, Krueger G, Killiany RJ, DeCarli CS: Update on the magnetic resonance imaging core of the Alzheimer’s disease neuroimaging initiative. Alzheimer’s & Dementia 2010, 6(3):212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Folstein MF, Folstein SE, McHugh PR: “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research 1975, 12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 45.Institute S: SAS/IML 9.3 user’s guide: Sas Institute; 2011. [Google Scholar]
- 46.Ngwa JS, Fungwe TV, Ntekim O, Allard JS, Johnson SM, Castor C, Graham L, Nadarajah S, Gillum RF, Obisesan TO et al. : Associations of Pulse and Blood Pressure with Hippocampal Volume by APOE and Cognitive Phenotype: The Alzheimer’s Disease Neuroimaging Initiative (ADNI). Dement Geriatr Cogn Disord 2018, 45(1–2):66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faraco G, Park L, Zhou P, Luo W, Paul SM, Anrather J, Iadecola C: Hypertension enhances A β-induced neurovascular dysfunction, promotes β-secretase activity, and leads to amyloidogenic processing of APP. Journal of Cerebral Blood Flow & Metabolism 2016, 36(1):241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fuzikawa AK, Peixoto S, Taufer M, Moriguchi EH, Lima-Costa M: Association of ApoE polymorphisms with prevalent hypertension in 1406 older adults: the Bambuí Health Aging Study (BHAS). Brazilian Journal of Medical and Biological Research 2008, 41(2):89–94. [DOI] [PubMed] [Google Scholar]
- 49.Oberlin LE, Manuck SB, Gianaros PJ, Ferrell RE, Muldoon MF, Jennings JR, Flory JD, Erickson KI: Blood pressure interacts with APOE ε4 to predict memory performance in a midlife sample. Neuropsychology 2015, 29(5):693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim IY, Grodstein F, Kraft P, Curhan GC, Hughes KC, Huang H, Kang JH, Hunter DJ: Interaction between apolipoprotein E genotype and hypertension on cognitive function in older women in the Nurses’ Health Study. PloS one 2019, 14(11):e0224975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used to prepare this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (https://adni.loni.usc.edu/). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf


