Abstract
Introduction:
Cancer immunotherapies have limited efficacy in prostate cancer due to the immunosuppressive prostate microenvironment. Prostate specific membrane antigen (PSMA) expression is prevalent in prostate cancer, preserved during malignant transformation, and increases in response to anti-androgen therapies, making it a commonly targeted tumor associated antigen for prostate cancer. JNJ-63898081 (JNJ-081) is a bispecific antibody targeting PSMA-expressing tumor cells and CD3-expressing T cells, aiming to overcome immunosuppression and promoting antitumor activity.
Patients and Methods:
We conducted a phase 1 dose escalation study of JNJ-081 in patients with metastatic castration-resistance prostate cancer (mCRPC). Eligible patients included those receiving ≥1 prior line treatment with either novel androgen receptor targeted therapy or taxane for mCRPC. Safety, pharmacokinetics, and pharmacodynamics of JNJ-081, and preliminary anti-tumor response to treatment were evaluated. JNJ-081 was administered initially by intravenous (IV) then by subcutaneous (SC) route.
Results:
Thirty-nine patients in 10 dosing cohorts received JNJ-081 ranging from 0.3 μg/kg to 3.0 μg/kg IV and 3.0 μg/kg to 60 μg/kg SC (with step-up priming used at higher SC doses). All 39 patients experienced ≥1 treatment-emergent AE, and no treatment-related deaths were reported. Dose limiting toxicities were observed in 4 patients. Cytokine release syndrome (CRS) was observed at higher doses with JNJ-081 IV or SC; however, CRS and infusion-related reaction (IRR) were reduced with SC dosing and step-up priming at higher doses. Treatment doses >30 μg/kg SC led to transient PSA decreases. No radiographic responses were observed. Antidrug antibody responses were observed in 19 patients receiving JNJ-081 IV or SC.
Conclusions:
JNJ-081 dosing led to transient declines in PSA in patients with mCRPC. CRS and IRR could be partially mitigated by SC dosing, step-up priming, and combination of both strategies. T-cell redirection for prostate cancer is feasible and PSMA is a potential therapeutic target for T-cell redirection in prostate cancer.
Keywords: PSA, immunotherapy, cytokine release syndrome, dose escalation, metastatic castration-resistant prostate cancer
MICROABSTRACT:
PSMA expression is preserved throughout all stages of prostate cancer. In this phase 1 study of JNJ-63898081, a bispecific antibody binding to PSMA-expressing tumor cells and CD3-expressing T cells, patients with metastatic castration-resistant prostate cancer had frequent cases of cytokine release syndrome, which were manageable, and transient declines in PSA. Treatment-related deaths and radiographic responses were not observed. PSMA is a potential therapeutic target for T-cell redirection in prostate cancer.
INTRODUCTION
Initial treatment for men with advanced prostate cancer is androgen-deprivation therapy (ADT). Despite the effectiveness of ADT, disease progression is inevitable with approximately 10–50% of men progressing to castration-resistant disease within 3 years of initial diagnosis.1 Moreover, men with castration-resistant prostate cancer are at greater risk of developing metastatic disease especially to bone (called metastatic castration-resistant prostate cancer, or mCRPC).2, 3 Available treatments for mCRPC have shown to improve outcomes, and includes chemotherapy (docetaxel, cabazitaxel), inhibitors targeting androgen receptor signaling, androgen synthesis, and poly ADP ribose polymerase (PARP), radiotherapy (radium-223) and radioligand therapy (177lutetium PSMA-617).4–7
Immune checkpoint inhibitors provide durable responses in various solid tumors but have limited efficacy in prostate cancer.8–11 This is largely attributed to the immunosuppressive prostate tumor microenvironment characterized by the complex dynamics between cellular (including tumor stroma, regulatory T cells, tumor-associated macrophages) and molecular (including programmed cell death-ligand 1 [PD-L1], programmed cell death protein 1 [PD-1], transforming growth factor beta [TGF-beta], vascular endothelial growth factor [VEGF]) components.12, 13 Therapeutic strategies are needed to help overcome immunosuppression and allow prostate tumors to become more amenable to immunotherapy.
Prostate-specific membrane antigen (PSMA) is a transmembrane protein that is expressed in normal prostatic tissue. PSMA expression is preserved in prostate cancer cells, especially in castration-resistant disease. In addition, its expression is increased in tumors progressing on current anti-androgen therapies.14–18 The tumor microenvironment in PSMA-positive prostate cancer may lack sufficient immune presence, perhaps explaining the limited efficacy of monotherapy with immune checkpoint inhibitors in prostate cancer. Because of the unique expression profile of PSMA in prostate cancer, several therapeutic platforms that target PSMA have been explored and validated, including CD3-redirection approaches to enhance immunogenicity.19, 20
JNJ-63898081 (JNJ-081) is a humanized immunoglobulin G4 proline, alanine, alanine (IgG4 PAA) bispecific antibody, in which one arm binds to PSMA expressed on cell surface, while the other arm binds to the CD3 receptor complex on T cells to promote anti-tumor activity. Here, we report results from a first-in-human, open-label, multicenter, phase 1 dose escalation study (NCT03926013) evaluating the safety and preliminary anti-tumor activity of JNJ-081 in patients with mCRPC.
PATIENTS AND METHODS
Patients
Adults ≥18 years of age with a diagnosis of mCRPC and histologically confirmed adenocarcinoma were included. Adenocarcinoma with small-cell or neuroendocrine features were also allowed. Patients were required to have progressive disease per The Prostate Cancer Clinical Trials Working Group 3 (PCWG3) criteria, measurable or evaluable disease, received ≥1 line of prior novel androgen receptor signaling inhibitor (abiraterone acetate, apalutamide, enzalutamide) or taxane regimen for mCRPC, ECOG PS 0–1, and adequate organ function.
Study design
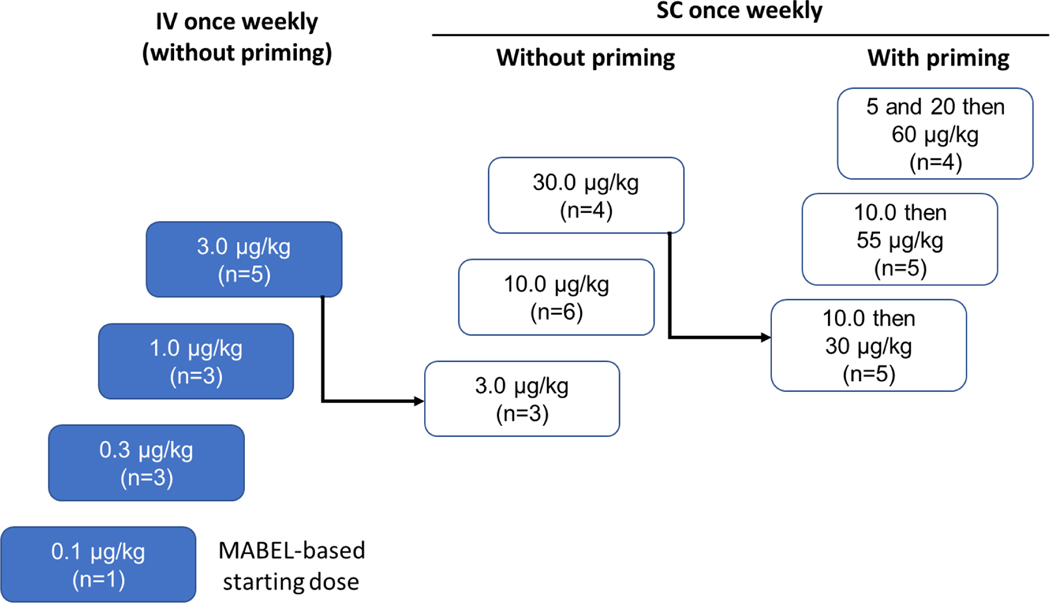
Study 63898081EDI1001 was a first-in-human, open-label, multicenter, phase 1 study of JNJ-081 comprising a dose-escalation phase (Part 1) and dose expansion phase (Part 2). This study was conducted in accordance with the International Council for Harmonization Good Clinical Practice Standards and the Declaration of Helsinki. The protocol was approved by institutional and ethics committees. All patients provided written informed consent. Part 1 was initiated with an accelerated titration phase, followed by a standard titration phase with or without priming dose(s) (Figure 1). More than one priming dose was possible.
Figure 1. Dose escalation phase.
IV, intravenous; MABEL; minimum anticipated biological effect level; SC, subcutaneous.
Dose escalation was initiated at 0.1 μg/kg JNJ-081 administered intravenously (IV) once a week. Subsequent dose levels were determined using a continuous reassessment method based on a Bayesian regression model. The dose escalation transitioned from accelerated to standard dose escalation when Grade ≥2 non-hematologic toxicity or Grade ≥3 hematologic toxicity occurred. After a dose level was deemed safe, drug dosage for patients enrolled in prior cohorts could be increased to that dose level. Subcutaneous (SC) injection as well as priming dose strategies were implemented in standard titration phase to mitigate Grade ≥2 cytokine release syndrome (CRS). To mitigate the potential for infusion related reactions (IRR) or CRS, all patients received their first dose of JNJ-081 with premedication consisting of corticosteroid, antipyretic, and antihistamine. Corticosteroid could be weaned for subsequent doses.
Study assessments
Efficacy assessments using chest, abdomen, and pelvis imaging by CT/MRI and bone scan (99mTc) were performed every 8 weeks for the first 24 weeks, followed by every 12 weeks while on treatment. Pharmacokinetic, immunogenicity, or biomarker analyses were performed using serum and biopsy tissue. Pharmacokinetic and immunogenicity assays were performed using an electrochemiluminescence-based immunoassay format on the MSD® platform. Immunogenicity or anti-drug antibody testing used multi-tiered testing approach (screening, specificity, titration) with biotinylated JNJ-081 and SulfoTag-labelled JNJ-081 as capture and detection antibodies, respectively. Serum prostate-specific antigen (PSA) levels and systemic cytokine concentrations were also evaluated. Adverse events (AEs), including dose limiting toxicities (DLTs) were recorded. AEs including CRS (AE of special interest) were graded using National Cancer Institute Common Terminology Criteria for Adverse Events, v5.0.
Statistical analysis
Response to treatment was evaluated by investigator. Overall response rate (ORR) was defined as the proportion of participants who had a partial response (PR) or better according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1/PCWG3 criteria and was to be supplied with two-sided 90% confidence interval. Duration of response (DOR) was defined as the interval between the date of initial documentation of a response (PR or better) to the date of first documented evidence of progressive disease, or death due to any cause. Time to response (TTR) was defined as the time from the date of first dose of study drug to the date of first documented response.
All safety and efficacy analyses were performed on data consisting of patients receiving at least 1 dose of study drug and summarized using descriptive statistics. Additional statistical methods were provided in the study protocol.
RESULTS
Patients
Thirty-nine patients with mCRPC were enrolled in the study, of which 38 patients (97.4%) were heavily pretreated with ≥3 lines of prior therapies (Table 1). All enrolled patients had adenocarcinoma; however, one included adenocarcinoma with small cell features. The dose escalation part of the study had 10 cohorts with treatment dose levels ranging from 0.1 μg/kg to 3 μg/kg IV and from 3 μg/kg to 60 μg/kg SC (Table 1). To mitigate CRS observed in the 30 μg/kg SC cohort, a single priming dose of 10 μg/kg was implemented on day 1, followed by treatment doses of either 30 μg/kg or 55 μg/kg SC once weekly in 2 subsequent dosing cohorts. Multi-dose priming was implemented with 5 μg/kg and 20 μg/kg on days 1 and 3, respectively, followed by doses of 60 μg/kg on day 8 and subsequently once a week. All 39 enrolled patients discontinued treatment predominantly due to progressive disease (89.7%; n=35). Other reasons for discontinuation were withdrawal of consent by patient (5.1%; n=2), AEs, and lost to follow-up (2.6%; n=1).
Table 1.
Patient demographics and baseline characteristics
| Characteristics | JNJ-081 Weekly IV (n=12) | JNJ-081 Weekly SC (n=27) | Total (n=39) |
|---|---|---|---|
| Age, median (range), yrs | 67.5 (43–82) | 67 (50–80) | 67 (43–82) |
| ECOG PS, n (%) | |||
| 0 | 7 (58.3) | 9 (33.3) | 16 (41.0) |
| 1 | 5 (41.7) | 18 (66.7) | 23 (59.0) |
| Gleason score at initial diagnosis, n (%) | |||
| 7 | 6 (50.0) | 9 (33.3) | 15 (38.5) |
| ≥8 | 6 (50.0) | 14 (51.9) | 20 (51.3) |
| Unknown | 0 | 4 (14.8) | 4 (10.3) |
| Metastases stage at initial diagnosis, n (%) | |||
| M0 | 4 (33.3) | 10 (37.0) | 14 (35.9) |
| M1 | 6 (50.0) | 12 (44.4) | 18 (46.2) |
| Unknown | 2 (16.7) | 5 (18.5) | 7 (17.9) |
| Prior cancer related therapy, n (%) | |||
| Prostatectomy | 5 (41.7) | 9 (33.3) | 14 (35.9) |
| Radiotherapy | 7 (58.3) | 21 (77.8) | 28 (71.8) |
| Hormonal therapy | 12 (100) | 27 (100) | 39 (100) |
| Orchietomy | 1 (8.3) | 0 | 1 (2.6) |
| GnRHa | 11 (91.7) | 23 (85.2) | 34 (87.2) |
| First-generation antiandrogen | 7 (58.3) | 18 (66.7) | 25 (64.1) |
| Novel ARSI | 12 (100) | 26 (96.3) | 38 (97.4) |
| Chemotherapy – taxane-based | 8 (66.7) | 22 (81.5) | 30 (76.9) |
| Othera | 9 (75.0) | 23 (85.2) | 32 (82.1) |
Therapies other than hormonal therapy and chemotherapy, including atezolizumab, dexamethasone, dutasteride, granulocyte macrophage colony stimulating factor, niraparib, olaparib, pembrolizumab, prednisone, and zoledronic acid.
ARSI, androgen receptor signaling inhibitor; ECOG PS, Eastern Cooperative Oncology Group Performance Status; GnRHA, gonadotropin-releasing hormone agonist; IV, intravenous; SC, subcutaneous.
Safety
All 39 patients experienced at least one treatment-emergent AE (TEAE). DLTs were observed in 4 patients and included Grade 3 transaminitis (N=2, following 30 μg/kg SC or 55 μg/kg SC doses), Grade 2 disseminated intravascular coagulation (N=1, following fourth full treatment dose [60 μg/kg SC dose]), and Grade 3 hemolysis (N=1, following 60 μg/kg SC dose). All DLT occurred following a Grade 2 CRS events, except for hemolysis AE.
Serious TEAEs occurred in 18 (46.2%) patients (Table 2), with the most common being CRS (20.5%), pyrexia (7.7%), and back pain (5.1%). TEAEs leading to treatment discontinuation occurred in one patient each in priming with 10 μg/kg then treatment dose at 55 μg/kg SC (lethargy and pyrexia), and priming of 5 and 20 μg/kg then treatment dose of 60 μg/kg SC (disseminated intravascular coagulation). No treatment-related death was reported.
Table 2.
Treatment-emergent adverse events
| n (%) | Weekly IV | Weekly SC (without priming) | Weekly SC (with priming) | Total (n=39) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 μg/kg (n=1) | 0.3 μg/kg (n=3) | 1 μg/kg (n=3) | 3 μg/kg (n=5) | 3 μg/kg (n=3) | 10 μg/kg (n=6) | 30 μg/kg (n=4) | 10 then 30 μg/kg (n=5) | 10 then 55 μg/kg (n=5) | 5 and 20, then 60 μg/kg (n=4) | ||
| Any TEAEs | 1 (100) | 3 (100) | 3 (100) | 5 (100) | 3 (100) | 6 (100) | 4 (100) | 5 (100) | 5 (100) | 4 (100 | 39 (100) |
| Related | 1 (100) | 3 (100) | 3 (100) | 5 (100) | 3 (100) | 6 (100) | 4 (100) | 5 (100) | 5 (100) | 4 (100) | 39 (100) |
| Grade 3–4 | 0 | 1 (33.3) | 1 (33.3) | 4 (80.0) | 0 | 2 (33.3) | 2 (50.0) | 4 (80.0) | 2 (40.0) | 1 (25.0) | 17 (43.6) |
| Most common TEAEs (>40% in total group) | |||||||||||
| Pyrexia | 0 | 1 (33.3) | 1 (33.3) | 3 (60.0) | 2 (66.7) | 4 (66.7) | 4 (100) | 3 (60.0) | 5 (100) | 4 (100) | 27 (69.2) |
| CRS | 0 | 0 | 1 (33.3) | 3 (60.0) | 2 (66.7) | 4 (66.7) | 4 (100) | 3 (60.0) | 5 (100) | 4 (100) | 26 (66.7) |
| Injection site erythema | 0 | 0 | 0 | 0 | 1 (33.3) | 5 (83.3) | 3 (75.0) | 4 (80.0) | 5 (100) | 4 (100) | 22 (56.4) |
| Chills | 0 | 3 (100) | 1 (33.3) | 3 (60.0) | 0 | 4 (66.7) | 1 (25.0) | 1 (20.0) | 2 (40.0) | 3 (75.0) | 18 (46.2) |
| Fatigue | 1 (100) | 1 (33.3) | 2 (66.7) | 4 (80.0) | 0 | 3 ( 50.0) | 1 (25.0) | 2 (40.0) | 2 (40.0) | 0 | 16 (41.0) |
| Serious TEAEs | 0 | 1 (33.3) | 1 (33.3) | 3 (60.0) | 0 | 2 (33.3) | 3 (75.0) | 3 (60.0) | 3 (60.0) | 2 (50.0) | 18 (46.2) |
| Related | 0 | 1 (33.3) | 0 | 3 (60.0) | 0 | 0 | 2 (50.0) | 1 (20.0) | 3 (60.0) | 2 (50.0) | 12 (30.8) |
| AEs leading to discontinuation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (20.0) | 0 | 1 (25.0) | 2 (5.1) |
| Related | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25.0) | 1 (2.6) |
CRS, cytokine release syndrome; IV, intravenous; SC, subcutaneous; TEAE, treatment-emergent adverse event.
Database captured both the syndrome term CRS and individual signs and symptoms of CRS. Signs and symptoms of CRS are presented in Table 3 in addition to the report of CRS.
CRS was observed at higher doses with IV or SC administration (Table 3). No Grade ≥3 CRS events were reported. No notable CRS-related neurotoxicity was observed. Grade 2 CRS treated with tocilizumab generally resolved after a single dose. SC administration as well as step-up priming helped mitigate CRS during escalation to higher doses (Table 3). Grade 1 or 2 injection site reaction (ISR) were also reported for SC administration.
Table 3.
Treatment-emergent adverse events of CRS and IRR/ISR
| n (%) | Weekly IV | Weekly SC (without priming) | Weekly SC (with priming) | Total (n=39) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 μg/kg (n=1) | 0.3 μg/kg (n=3) | 1 μg/kg (n=3) | 3 μg/kg (n=5) | 3 μg/kg (n=3) | 10 μg/kg (n=6) | 30 μg/kg (n=4) | 10 then 30 μg/kg (n=5) | 10 then 55 μg/kg (n=5) | 5 and 20, then 60 μg/kg (n=4) | ||
| CRS | 0 | 0 | 1 (33.3) | 3 (60.0) | 2 (66.7) | 4 (66.7) | 4 (100) | 3 (60.0) | 5 (100) | 4 (100) | 26 (66.7) |
| Grade 2 | 0 | 0 | 0 | 3 (60.0) | 0 | 1 (16.7) | 3 (75.0) | 1 (20.0) | 4 (80.0) | 1 (25.0 | 13 (33.3) |
| Most common CRS symptoms * | |||||||||||
| Pyrexia | 0 | 0 | 1 (33.3) | 3 (60.0) | 2 (66.7) | 4 (66.7) | 4 (100) | 3 (60.0) | 5 (100) | 4 (100) | 26 (66.7) |
| Hypotension | 0 | 0 | 0 | 2 (40.0) | 0 | 1 (16.7) | 2 (50.0) | 1 (20.0) | 4 (80.0) | 1 (25.0) | 11 (28.2) |
| Chills | 0 | 0 | 0 | 0 | 0 | 3 (50.0) | 1 (25.0) | 1 (20.0) | 2 (40.0) | 3 (75.0) | 10 (25.6) |
| Tachycardia | 0 | 0 | 0 | 2 (40.0) | 0 | 1 (16.7) | 1 (25.0) | 0 | 1 (20.0) | 1 (25.0) | 6 (15.4) |
| Hypoxia | 0 | 0 | 0 | 2 (40.0) | 0 | 0 | 0 | 1 (20.0) | 1 (20.0) | 1 (25.0) | 5 (12.8) |
| IRR/ISR | 0 | 2 (66.7) | 0 | 4 (80.0) | 3 (100) | 6 (100) | 4 (100) | 5 (100) | 5 (100) | 4 (100) | 33 (84.6) |
| Most common IRR/ISR symptoms * | |||||||||||
| Injection site erythema | 0 | 0 | 0 | 0 | 1 (33.3) | 5 (83.3) | 3 (75.0) | (80.0) | 5 (100) | 4 (100.0) | 22 (56.4) |
| Injection site pruritus | 0 | 0 | 0 | 0 | 0 | 4 (66.7) | 2 (50.0) | 2 (40.0) | 1 (20.0) | 1 (25.0) | 10 (25.6) |
| Injection site pain | 0 | 0 | 0 | 0 | 1 (33.3) | 3 (50.0) | 0 | 0 | 2 (40.0) | 1 (25.0) | 7 (17.9) |
| Chills | 0 | 1 (33.3) | 0 | 3 (60.0) | 0 | C | 0 | 0 | 0 | 0 | 4 (10.3) |
More than 10% of patients in total group, as reported by the investigator. CRS, cytokine release syndrome; ISR, infusion/injection site reaction; IRR, infusion/injection related reaction; IV, intravenous; SC, subcutaneous; TEAE, treatment-emergent adverse event.
The most frequent laboratory abnormalities reported as TEAEs were AST increased (15.4%), creatinine increased (15.4%), lipase increased (15.4%; no pancreatitis observed), ALT increased (12.8%), and neutrophils increased (12.8%). Among these laboratory TEAEs, grade ≥3 was reported for ALT and AST increased (7.7% each), and lipase increased (5.1%).
Efficacy
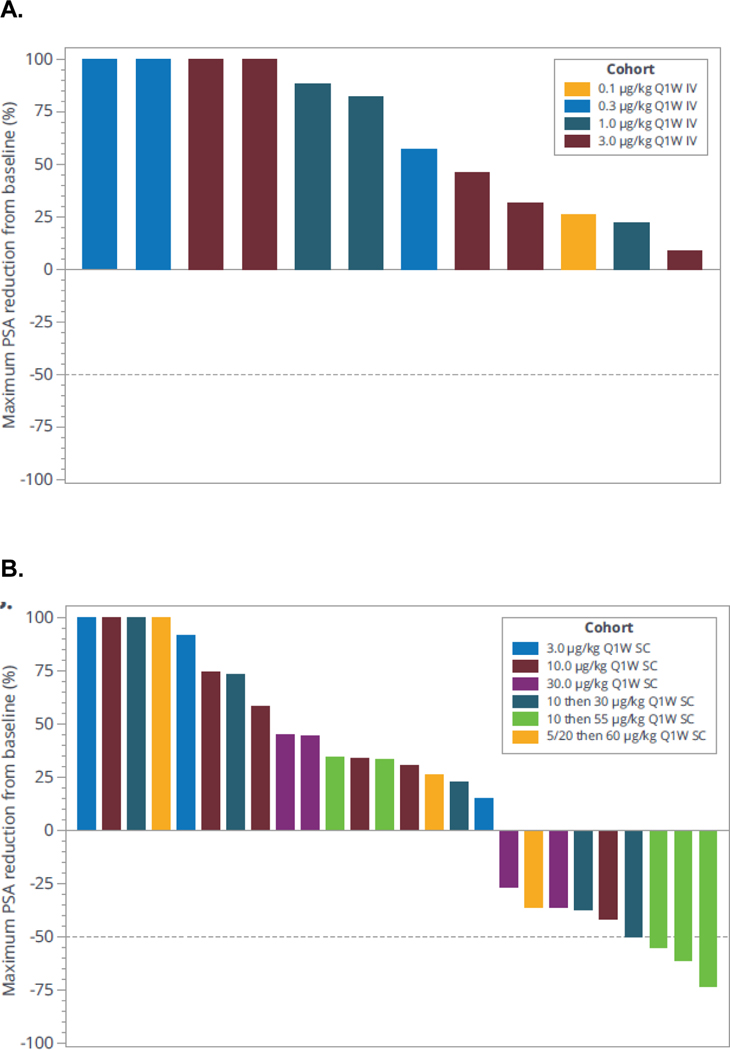
Of the 39 mCRPC participants exposed, 23 had RECIST evaluable disease. Overall, there were no radiographic responses (complete or partial response) per RECIST 1.1. The best overall response observed was stable disease in 2 of 23 participants (13.0%) who had baseline target lesions. Transient PSA decreases were observed at treatment doses greater than 30 μg/kg SC. Two patients treated with 55 μg/kg had confirmed PSA decline of >50% (Figure 2A and 2B).
Figure 2. Maximum change in PSA.
(A) IV dose and (B) SC dose. IV, intravenous; PSA, prostate-specific antigen; Q1W, once a week; SC, subcutaneous.
Pharmacokinetics, pharmacodynamics, and immunogenicity
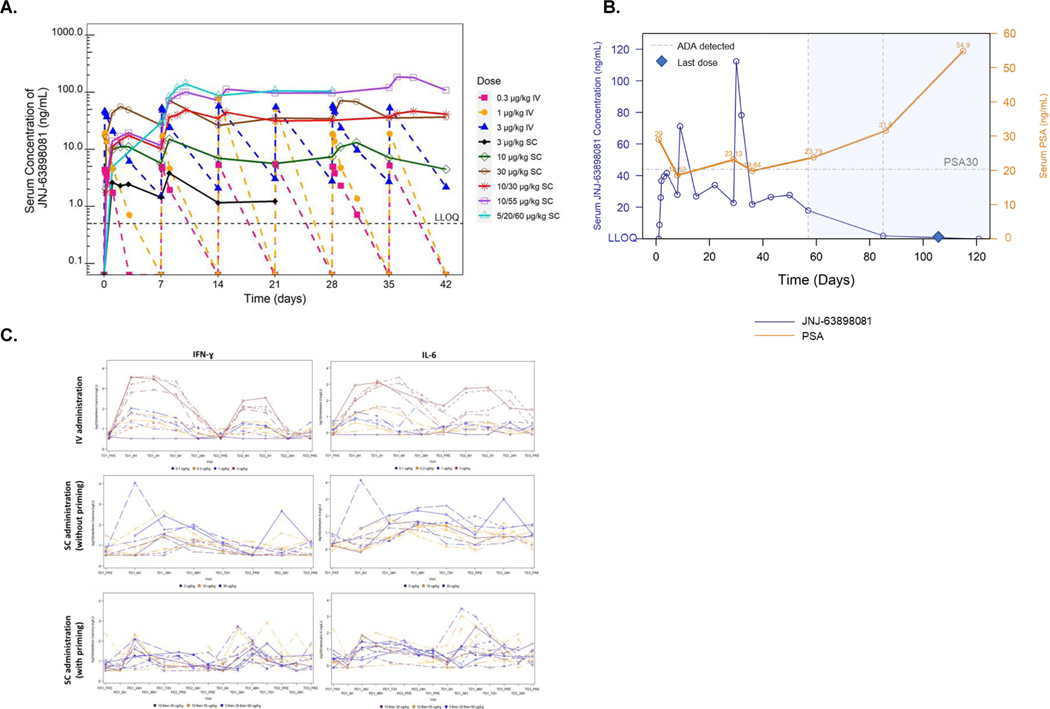
Once weekly IV administration of JNJ-081 (0.1–3 μg/kg) led to increased Cmax and AUC values (Figure 3A). The median tmax values were reached at the end of infusion (EOF) or EOF + 2 hrs with IV administration, and from 24 to 168 hrs following SC administration. Mean t1/2 was 43.7 hrs following 3 μg/kg IV dose and appear longer following SC administration. Cmax and AUC values increased dose proportionally after once weekly SC administration of 3–30 μg/kg and 30–60 μg/kg following priming dose.
Figure 3. Pharmacokinetics and pharmacodynamics of JNJ-081.
(A) Mean serum concentration-time profiles of JNJ-081 after IV or SC administration. Data not shown when n<3. (B) Serum concentration-time profiles of JNJ-081 (blue, left y-axis) and PSA (orange, right y-axis) of a participant who was ADA positive on Days 57 and 85. (C) Changes in peripheral cytokines with IV and SC doses. H, hours; IL-6, Interleukin 6; IFN-γ, Interferon gamma; IV, intravenous; PD, priming dose; PRE, predose; PSA, prostate-specific antigen; Q1W, once a week; SC, subcutaneous; TD, treatment dose.
Anti-drug antibodies (ADA) were detected in 2 of 12 (16.7%) patients with IV administration and 17 of 27 (63.0%) patients with SC administration (Table 4). Positive ADA status was observed in patients receiving 3 μg/kg IV (maximum titer 10), and in patients across all SC doses (maximum titer of 10485760). ADA generally corresponded with decrease in serum concentrations of JNJ-081 in with SC doses, particularly for patients with high titers. Intrapatient dose escalation in 3 patients did not overcome the reduced exposure after ADA seropositivity. A patient receiving 30 μg/kg had a PSA decrease of >30%; however, the decrease was transient, and the return to baseline and subsequent increase coincided with the onset of ADA seropositivity and a corresponding decrease in JNJ-081 serum levels (Figure 3B).
Table 4.
ADA incidence by cohort
| Cohort | Dose | ADA-positive/Total Patients |
|---|---|---|
| 1 | IV 0.1 μg/kg | 0/1 |
| 2 | IV 0.3 μg/kg | 0/3 |
| 3 | IV 1 μg/kg | 0/3 |
| 4 | IV 3 μg/kg | 2/5 |
| 5 | SC 3 μg/kg | 2/3 |
| 6 | SC 10 μg/kg | 4/6 |
| 7 | SC 30 μg/kg | 3/4 |
| 8 | SC 10 then 30 μg/kg | 3/5 |
| 9 | SC 10 then 55 μg/kg | 2/5 |
| 10 | SC 5 and 20, then 60 μg/kg | 3/4 |
ADA, anti-drug-antibodies; IV, intravenous; SC, subcutaneous.
Dose dependent cytokine increases were observed with IV administration. Cytokine increases after SC dosing were more variable and with lower magnitudes compared to IV administration at similar exposure levels. Serum cytokine levels of IFN-γ and IL-6 were two of the more notable and robust cytokines that demonstrated an increase in production after dosing with JNJ-081 (Figure 3C).
DISCUSSION
In this study, we observed that JNJ-081 exhibited a manageable safety profile, and patients with mCRPC dosed with JNJ-081 had transient PSA responses. However, CRS events were observed at higher doses with IV or SC administration, but could be partially mitigated by SC dosing, step-up priming, and the combination of both strategies. In addition, ADA formation led to decreased JNJ-081 serum levels, which impacted drug exposure and efficacy.
Therapies that harness the immune system to combat cancer have become more prevalent and viable in recent years.19, 21, 22 One such approach is through bispecific molecules that facilitate a synapse between T cells and tumor antigens, leading to T cell activation and causing T cell-mediated lysis of target cells without the need for TCR-MHC interactions. This approach was first successfully used with blinatumomab for Philadelphia chromosome-negative relapsed or refractory acute lymphoblastic leukemia and has shown great promises with several bispecific agents for other hematologic malignancies19, 20, 23–27 and tebentafusp for unresectable or metastatic uveal melanoma.28
The tumor microenvironment in mCRPC may lack a sufficient immune presence, which perhaps explains the lack of efficacy of checkpoint inhibitor monotherapy in prostate cancer.29, 30 T cell redirection could be an important approach to enhance the immune presence in the microenvironment of such tumors. Several other CD3-redirecting approaches targeting PSMA with mechanisms of action similar to that intended with JNJ-081 have entered clinical research for treating prostate cancer. These include BAY2010112 (also known as pasotuxizumab, a non-Fc-bearing CD3-PSMA bispecific T cell engager [BiTE] molecule),31 AMG 160 (a PSMA-targeted, half-life extended BiTE molecule),32 ES414 (an Fc-competent bivalent bispecific CD3-PSMA molecule),19, 32 and HPN424 (a tri-specific T cell-activating construct targeting CD3, PSMA, and human serum albumin).33 Preliminary clinical data from a phase 1 study of pasotuxizumab indicated that this strategy was tolerated at doses up to 80 μg/day with continuous intravenous infusion and induced radiographic responders in patients with castration-resistant prostate cancer.31 Efficacy with AMG 160 was also reported with a confirmed PSA 50 response of 27.6% and a confirmed radiographic partial response of 13.3% in patients with mCRPC who had measurable lesions at baseline.34
Although PSMA expression in normal tissues other than the prostate is relatively low, its RNA or protein expression is observed in normal epithelial tissues including kidney, liver, small intestine, salivary, and mammary gland as well as in endothelial cells.14, 26 This expression pattern of PSMA enables bispecific agents, such as JNJ-081 and other PSMA-target CD3 redirecting agents, to engage T cells and PSMA-expressing cells, which could cause T-cell activation beyond tumor sites and CRS events. Preliminary data reported for all investigational PSMA-targeted CD3 redirecting agents indicate frequent observations of CRS, mainly manifested as pyrexia and occasionally accompanied with hypotension. Therefore, administration of these redirecting agents require corticosteroid premedication at least with initial dosing. Reports of immune effector cell-associated neurotoxicity syndrome (ICANS) were also rare for these group of agents unlike the CD3 redirecting agents for hematologic targets.31–33
The most commonly reported serious TEAE for JNJ-081 was CRS, which emerged in the third dosing cohort after initiating the study. Based on preclinical reports that the in vivo efficacy of JNJ-081 was observed at certain dose levels, we investigated various strategies to mitigate CRS. In this study, JNJ-081 was administered by IV and SC to determine the effects of routes of administration on CRS. Grade 2 CRS repeatedly occurred at a dose level of JNJ-081 that was 10-fold higher when administered by SC than IV, and furthermore, was approximately 2-fold higher with priming. As a result, SC administration, priming, and the combination of these strategies coupled with corticosteroid premedication could greatly mitigate CRS to achieve higher treatment doses. Further research is needed to better refine the necessary number of priming doses, and the increments between priming doses and the frequency of priming doses.
A gradual increase in serum concentration of JNJ-081 was observed following SC administration with Tmax ranging from 24 to 168 hrs. SC administration appeared to prolong the apparent t1/2 of JNJ-081 compared with IV administration as shown by a shallower decline of the terminal slope in the PK curve, resulting in more sustained exposure with smaller peak to trough concentration ratio. This phenomenon is often observed with extravascular administration of drugs when the rate of absorption is slower than the rate of elimination.35 The gradual PK increase from SC absorption and relatively stable JNJ-081 levels compared with IV administration likely contributed to a decrease in cytokine release.36 As shown in Figure 3C, the change from IV to SC administration introduced more muted and variable IFN-γ and IL-6 production with JNJ-081.
Based on the emerging ADA that negatively impacted PK exposure, this clinical study was terminated early. Full patient enrollment was not achieved for Part 1, and no patients were enrolled in Part 2. The limited enrollment data is a constraint on generalizing safety and anti-tumor efficacy profiles. However, the sample size is customary with early-phase oncology clinical trials evaluating first-in-human investigational agents and provides insights into the safety, tolerability, and efficacy of PSMA-targeted CD3 redirecting agents. In this study, all patients eventually discontinued treatment, primarily due to disease progression. Moreover, early termination of the study prevented many planned pharmacodynamic and biomarker assessments. Despite the small number of patients in cohorts from this study, the evidence suggests that the introduction of SC step-up dosing could be a viable option to manage cytokine, and mitigate CRS in future solid tumor CD3 redirector clinical trials.
At doses greater than 30 μg/kg SC, JNJ-081 demonstrated a transient, substantial decrease in PSA levels. Higher dosing of JNJ-081 yielded larger PSA reduction, with greater than 50% decreases observed with doses over 55 μg/kg. These results were generally consistent with reported data regarding other PSMA-targeted CD3 redirecting bispecifics for mCRPC that are currently in development.13, 31
Compared with BiTEs, ES414 and HPN424, JNJ-081 more resembled an endogenous human IgG antibody. However, immunogenicity (as measured by the presence of ADA) still occurred and was more prevalent with SC administration at higher doses. Further, systemic exposure of JNJ-081 was greatly reduced after ADA formation which could not be restored with intra-patient dose escalation. Loss of systemic exposure could also be responsible for transient PSA decreases. These results are consistent with previous findings.31, 34, 37 As with other bispecific antibodies, increased immunogenicity associated with SC administration of JNJ-081 may be the result of slower distribution, the difference in volume compared with IV administration, and greater variability in drug exposure levels between individuals. Moreover, skin cells possess highly specialized apparatus for foreign antigen presentation.38
The transient decreases in PSA in 2 patients receiving JNJ-081 and the reported clinical activity of other PSMA-directed bispecific antibodies13 suggest that PSMA remains a viable target for T cell engagement to treat mCRPC. Further investigation may target methods to reduce CRS and, presumably, increase the likelihood of achieving maximum tolerated dose and optimal anti-tumor efficacy. These may include antibody component manipulation to further increase half-life, optimal dosing delivery for SC administration, and prophylactic (rather than symptomatic) treatment for CRS.
CONCLUSIONS
JNJ-081 demonstrated transient decreases in PSA in patients with mCRPC, with a manageable safety profile. Grade 2 CRS was observed at higher doses and was partially mitigated by SC and step-up dosing. ADA formation was frequent with SC administration. Additionally, ADA positivity generally corresponded with decrease in serum JNJ-081 concentrations in patients receiving SC doses, particularly for those with high titers. PSMA remains a potential therapeutic target for T cell redirection in treating prostate cancer.
KEY MESSAGES.
JNJ-63898081 (JNJ-081) is a bispecific antibody designed to form an immune synapse between PSMA-expressing tumor cells and CD3-expressing T cells.
In this phase 1 study, subcutaneous JNJ-081 was associated with prevalent anti-drug-antibody formation.
Although dose escalation did not reach its full potential, this study contributed to our understanding of both the safety profile and tumor targeting with an anti-PSMA and CD3 bispecific in patients with metastatic resistant prostate cancer.
CLINICAL PRACTICE POINTS.
Patients with metastatic castration-resistant prostate cancer (mCRPC) have poor prognosis and although available treatments have improved outcomes in patients, the disease remains uniformly fatal. Although therapies with immune checkpoint inhibitors have shown efficacy in other solid tumors, these immunotherapies lack efficacy in patients with mCRPC. A challenge in developing effective immunotherapies for prostate cancer is likely due to the various immunosuppressive mechanisms present in the prostate tumor microenvironment. A potential therapeutic approach to overcome immunosuppression is inducing or enhancing tumor-specific immune responses through T-cell redirection. Prostate-specific membrane antigen (PSMA) is a membrane bound antigen prevalent in prostate cancer and its expression is preserved during prostate cancer progression, making it a suitable T-cell redirecting target. JNJ-63898081 (JNJ-081) is a bispecific antibody designed to bind PSMA-expressing tumor cells and CD3-expressing T cells to promote anti-tumor activity via immune synapse formation. In this first-in-human, phase 1 dose escalation study, administration of JNJ-081 was associated with transient declines in serum-prostate specific antigen levels in patients with mCRPC. In addition, dose limiting toxicities, mainly cytokine release syndrome which was manageable, were limited in duration and no treatment-related deaths were reported. This study indicates that T-cell redirection is feasible for the treatment of prostate cancer and PSMA remains a potential therapeutic target for T-cell redirection.
Acknowledgements
The authors thank all patients for their participation in this study and acknowledge the collaboration and commitment of the investigators and their staff. Writing assistance was provided by Paul D. Cao, PhD, of Janssen Global Services, LLC. Additional editorial support was provided by Namit Ghildyal, PhD, of Janssen Global Services, LLC.
Funding source
The study was funded by Janssen Research & Development, LLC.
Abbreviations:
- ARSI
androgen receptor synthesis inhibitor
- CRS
cytokine release syndrome
- ECOG PS
European Eastern Cooperative Oncology Group Performance Status
- IRR
infusion-related reaction
- MABEL
minimum anticipated biological effect level
- mCRPC
metastatic castration-resistant prostate cancer
- PSMA
prostate-specific membrane antigen
- PSA
prostate-specific antigen
- PCWG3
Prostate Cancer Clinical Trials Working Group 3
- RECIST
Response Evaluation Criteria in Solid Tumors
Footnotes
Credit author statement
EA Lim: writing–review and editing; MT Schweizer: writing–review and editing; KN Chi: writing–review and editing; R Aggarwal: writing–review and editing; N Agarwal: writing–review and editing; J Gulley: writing–review and editing; J Greger: conceptualization, resources, data curation, supervision, investigation, methodology, project administration, writing-review and editing; E Attiyeh: data curation, formal analysis, methodology, writing-original draft, writing-review and editing; S Wu: formal analysis, supervision, writing-review and editing; P Jaiprasart: data curation, formal analysis, methodology, writing-original draft, writing-review and editing; J Loffredo: data curation, formal analysis, methodology, writing-original draft, writing-review and editing; N Bandyopadhyay: conceptualization, data curation, formal analysis, methodology, writing-original draft, writing-review and editing; H Xie: Conceptualization, data curation, formal analysis, methodology, writing–review and editing; AR Hansen: writing–review and editing.
Competing interest disclosures
EA Lim reports other support from Pfizer outside the submitted work; MT Schweizer reports personal fees from Janssen and Resverlogix, as well as grants from Zenith Epigenetics, Bristol Myers Squibb, Merck, Immunomedics, Janssen, AstraZeneca, Pfizer, Madison Vaccines, Tmunity, and Hoffmann-La Roche outside the submitted work; R Aggarwal reports honoraria from Clovis Oncology, advisory roles for AstraZeneca, Dendreon, and Janssen, research funding from AbbVie, Amgen, Cancer Targeted Technology, Janssen, Merck, Novartis, Xynomic Pharmaceuticals, and Zenith Epigenetics, and compensation for travel, accommodations, and expenses from Xynomic Pharmaceuticals; N Agarwal reports consultancy to Astellas, Astra Zeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Gilead, Janssen, Merck, MEI Pharma, Nektar, Novartis, Pfizer, Pharmacyclics, and Seattle Genetics, and additionally reports institutional research funding from Astra Zeneca, Bavarian Nordic, Bayer, Bristol Myers Squibb, Calithera, Celldex, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Genentech, Glaxo Smith Kline, Immunomedics, Janssen, Medivation, Merck, Nektar, New Link Genetics, Novartis, Pfizer, Prometheus, Rexahn, Roche, Sanofi, Seattle Genetics, Takeda, and Tracon; KN Chi and J Gulley have nothing to disclosed; AR Hansen reports research funding to the institution from GSK, Merck, Pfizer, MedImmune/Genentech, Roche, Janssen, BMS, AstraZeneca, Astellas, Boehringer Ingelheim, and Bayer and consulting role/advisory boards for GSK, Merck, and Eisai; E Attiyeh, J Greger, S Wu, P Jaiprasart, J Loffredo, N Bandyopadhyay, H Xie are employees of Janssen Research and Development
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akaza H, Procopio G, Pripatnanont C, et al. Metastatic Castration-Resistant Prostate Cancer Previously Treated With Docetaxel-Based Chemotherapy: Treatment Patterns From the PROXIMA Prospective Registry. J Glob Oncol. 2018;4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Facchini G, Caffo O, Ortega C, et al. Very Early PSA Response to Abiraterone in mCRPC Patients: A Novel Prognostic Factor Predicting Overall Survival. Front Pharmacol. 2016;7:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin TT, Chen YH, Wu YP, et al. Risk factors for progression to castration-resistant prostate cancer in metastatic prostate cancer patients. J Cancer. 2019;10:5608–5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N Engl J Med. 2019;381:2506–2518. [DOI] [PubMed] [Google Scholar]
- 5.de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382:2091–2102. [DOI] [PubMed] [Google Scholar]
- 6.Sayegh N, Swami U, Agarwal N. Recent Advances in the Management of Metastatic Prostate Cancer. JCO Oncol Pract. 2022;18:45–55. [DOI] [PubMed] [Google Scholar]
- 7.Crawford ED, Heidenreich A, Lawrentschuk N, et al. Androgen-targeted therapy in men with prostate cancer: evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019;22:24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen AR, Massard C, Ott PA, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol. 2018;29:1807–1813. [DOI] [PubMed] [Google Scholar]
- 9.Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J Clin Oncol. 2020;38:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boudadi K, Suzman DL, Anagnostou V, et al. Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Oncotarget. 2018;9:28561–28571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stultz J, Fong L. How to turn up the heat on the cold immune microenvironment of metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2021;24:697–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heitmann JS, Pfluegler M, Jung G, Salih HR. Bispecific Antibodies in Prostate Cancer Therapy: Current Status and Perspectives. Cancers (Basel). 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinoshita Y, Kuratsukuri K, Landas S, et al. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J Surg. 2006;30:628–636. [DOI] [PubMed] [Google Scholar]
- 15.Queisser A, Hagedorn SA, Braun M, Vogel W, Duensing S, Perner S. Comparison of different prostatic markers in lymph node and distant metastases of prostate cancer. Mod Pathol. 2015;28:138–145. [DOI] [PubMed] [Google Scholar]
- 16.Ross JS, Sheehan CE, Fisher HA, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9:6357–6362. [PubMed] [Google Scholar]
- 17.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 18.Wright GL Jr., Grob BM, Haley C, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–334. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Hoyos G, Sewell T, Bader R, et al. MOR209/ES414, a Novel Bispecific Antibody Targeting PSMA for the Treatment of Metastatic Castration-Resistant Prostate Cancer. Mol Cancer Ther. 2016;15:2155–2165. [DOI] [PubMed] [Google Scholar]
- 20.Klinger M, Benjamin J, Kischel R, Stienen S, Zugmaier G. Harnessing T cells to fight cancer with BiTE(R) antibody constructs--past developments and future directions. Immunol Rev. 2016;270:193–208. [DOI] [PubMed] [Google Scholar]
- 21.Haber L, Olson K, Kelly MP, et al. Generation of T-cell-redirecting bispecific antibodies with differentiated profiles of cytokine release and biodistribution by CD3 affinity tuning. Sci Rep. 2021;11:14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klinger M, Brandl C, Zugmaier G, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119:6226–6233. [DOI] [PubMed] [Google Scholar]
- 23.Burt R, Warcel D, Fielding AK. Blinatumomab, a bispecific B-cell and T-cell engaging antibody, in the treatment of B-cell malignancies. Hum Vaccin Immunother. 2019;15:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budde LE, Sehn LH, Matasar M, et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol. 2022;23:1055–1065. [DOI] [PubMed] [Google Scholar]
- 25.Hutchings M, Morschhauser F, Iacoboni G, et al. Glofitamab, a Novel, Bivalent CD20-Targeting T-Cell-Engaging Bispecific Antibody, Induces Durable Complete Remissions in Relapsed or Refractory B-Cell Lymphoma: A Phase I Trial. J Clin Oncol. 2021;39:1959–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreau P, Garfall AL, van de Donk N, et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2022;387:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verkleij CPM, Broekmans MEC, van Duin M, et al. Preclinical activity and determinants of response of the GPRC5DxCD3 bispecific antibody talquetamab in multiple myeloma. Blood Adv. 2021;5:2196–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan P, Hassel JC, Rutkowski P, et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N Engl J Med. 2021;385:1196–1206. [DOI] [PubMed] [Google Scholar]
- 29.Beer TM, Kwon ED, Drake CG, et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol. 2017;35:40–47. [DOI] [PubMed] [Google Scholar]
- 30.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hummel HD, Kufer P, Grullich C, et al. Pasotuxizumab, a BiTE((R)) immune therapy for castration-resistant prostate cancer: Phase I, dose-escalation study findings. Immunotherapy. 2021;13:125–141. [DOI] [PubMed] [Google Scholar]
- 32.Deegen P, Thomas O, Nolan-Stevaux O, et al. The PSMA-targeting Half-life Extended BiTE Therapy AMG 160 has Potent Antitumor Activity in Preclinical Models of Metastatic Castration-resistant Prostate Cancer. Clin Cancer Res. 2021;27:2928–2937. [DOI] [PubMed] [Google Scholar]
- 33.De Bono JS, Fong L Beer TM, et al. Results of an ongoing phase 1/2a dose escalation study of HPN424, a tri-specific half-life extended PSMA-targeting T-cell engager, in patients with metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology 39, no. 15_suppl (May 20, 2021) 5013. . [Google Scholar]
- 34.Tran B, Horvath L, Rettig M, et al. Phase I study of AMG 160, a half-life extended bispecific T-cell engager (HLE BiTE immune therapy) targeting prostate-specific membrane antigen, in patients with metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology. 38, no. 15_suppl. [Google Scholar]
- 35.Yanez JA, Remsberg CM, Sayre CL, Forrest ML, Davies NM. Flip-flop pharmacokinetics--delivering a reversal of disposition: challenges and opportunities during drug development. Ther Deliv. 2011;2:643–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Kamperschroer C, Wong G, Xuan D. A Modeling Framework to Characterize Cytokine Release upon T-Cell-Engaging Bispecific Antibody Treatment: Methodology and Opportunities. Clin Transl Sci. 2019;12:600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SC, Ma JSY, Kim MS, et al. A PSMA-targeted bispecific antibody for prostate cancer driven by a small-molecule targeting ligand. Sci Adv. 2021;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarvi NL, Balu-Iyer SV. Immunogenicity Challenges Associated with Subcutaneous Delivery of Therapeutic Proteins. BioDrugs. 2021;35:125–146. [DOI] [PMC free article] [PubMed] [Google Scholar]