Abstract
Background:
Hyaluronic acid dermal fillers are composed of cross-linked viscoelastic particles with high biocompatibility. The performance of the fillers is determined by the viscoelastic properties of particles and the connecting force between particles. However, the relationships among the properties of fillers, the interaction of the gels and the surrounding tissue are not clear enough.
Method:
Four kinds of typical dermal filler were selected in this research to reveal the interaction between the gels and cells. A series of analytical tools was applied to characterize the structure and physicochemical properties of the gel, as well as observing their interaction with the surrounding tissues in vivo and discussing their internal mechanism.
Result:
The large particles internal the gel and the high rheological properties endow the Restylane2 with excellent support. However, these large-size particles have a significant impact on the metabolism of the local tissue surrounding the gel. Juvéderm3 present gel integrity with the high cohesiveness and superior support. The rational matching of large and small particles provides the Juvéderm3 with supporting capacity and excellent biological performance. Ifresh is characterized by small-size particles, moderate cohesiveness, good integrity, lower viscoelasticity and the superior cellular activity located the surrounding tissues. Cryohyaluron has high cohesion and medium particle size and it is prominent in cell behaviors involving localized tissues. Specific macroporous structure in the gel may facilitate the nutrients delivering and removing the waste.
Conclusion:
It’s necessary to make the filler both sufficient support and biocompatibility through the rational matching of particle sizes and rheological properties. Gels with macroporous structured particle showed an advantage in this area by providing a space inside the particle.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13770-023-00533-0.
Keywords: Hyaluronic acid dermal fillers, Gel particle, Rheological properties, Histology, Biocompatibility
Introduction
In the field of cosmetology, hyaluronic acid (HA) dermal fillers are one of the most commonly used products for the non-invasive treatment of imperfections [1–3]. According to the American Academy of Aesthetic Plastic Surgeons, billions of people have received HA injections for facial aesthetic enhancement since the twenty-first century [4]. However, there are some disadvantages of natural HA, such as a short half-life in tissues, easy degradation, and poor stability in the long term, which greatly limit its application in the field of aesthetic medicine [5–7]. Therefore, processing modification became the key to the procedure.
In the early 1960s, Preston et al. [8] began to study the cross-linking procedure between HA. After decades of research, HA fillers are generally cross-linked with a binding agent, 1,4-butanediol diglycidyl ether, which improves the anti-degradation of the product [9, 10]. After cross-linking, the hydrogel was cut into small particle to make it possible to be injected into the skin [11]. Therefore, the particle size and rheological properties of the hydrogel particle largely determine the properties and the in-vivo performance of the gel [12]. Unlike the strong interaction between particles in gel form products, cohesion plays a significant role in granular form with each particle providing scope to support. Under this interaction, gel forms a unity to maintain its supporting performances. However, in fact, the properties are not as high as possible, for example, the excessive properties may lead to unnatural local skin after gel injection, and may also alter local cytological behavior [13, 14].
Therefore, several studies have been conducted to discover the “sweet point” of injectability in hyaluronic acid (HA) dermal filler. Ample studies on the characterization of HA dermal filler have been conducted in recent years as clinicians have been involved in the optimization of existing products. Different manufacturers produce HA gels with various amounts and distinct types of crosslinks. Verpaele et al. [15] and Bogdan Allemann et al. [16] described craft technologies and clinical performances with non-cohesive and cohesive mono-density fillers and proved HA dermal fillers offered a longer-lasting aesthetic effect than the others. While Prager et al. [17] compared the safety of these fillers which belonged to cohesive polydensified fillers with randomized controls in clinical study, the results showed HA fillers present a potentially lower risk of inflammatory reactions. Sébastien Pierre et al. [18] and Hee et al. [19] demonstrated how rheology can be used to help physicians differentiate dermal fillers targeted to certain areas of the face and pointed that viscoelasticity and cohesivity of fillers mainly improved facial volume insufficiency. These two characters also influenced filling tissue integration by the control of gel spreading.
There are many studies investigating the effects of different cross-linking methods and characteristics [20–22], however, the previous research overly focused on macro manifestations and safety dimensions that lack the finding in intrinsic mechanisms of macroscopic manifestations. There is also insufficient knowledge about the status of the gel after injection into the dermal layer and interactions with local tissues, and cells. In the current study, we deliberately selected four typical and distinctive HA fillers: dispersive and large granular production (Restylane2), cohesive and covering-sized particles gel form production (Juvéderm3),cohesive and small particles gel form production (Ifresh) and cohesive and pore structure characteristics gel form production (Cryohyaluron) to analyze the structure properties of gels, physicochemical properties and interactions with local tissues and intrinsic mechanisms in vivo.
Methods
Materials
Four different kinds of HA, namely Cryohyaluron (Eyebright Medical Technology Co. Ltd., [Beijing, China]), Restylane (Restylane2, Medicis Aesthetics Inc., Scottsdale, AZ, USA), Juvéderm Ultra (Juvéderm3, Allergan, Inc., Irvine, CA, USA), and Ifresh (Beijing Aimeike Biotechnology Co., Ltd., [Beijing, China]), were included in the current study.
Scanning electron microscope (SEM)
HA filler samples were placed on the disc and were snap-frozen in a liquid nitrogen bath before they were transferred to a lyophilizer (Sihuan Furui Scientific Instrument Technology Development Co., Ltd. [Beijing, China]) for 8 h. Frozen samples were fractured with a knife, coated by gold with ion sputter, and then visualized by SEM (ZEISS Industrial Metrology, Shanghai, China).
Particle size
Particle size measurement was performed on a Bettersize2600 laser particle size analyzer (Dandong Baite Instrument Co., Ltd., Liaoning, China). HA samples were selected to be mixed with a saline suspension and scanned for the average particle size. The 10% size (D10), average size (D50), and 90% size (D90) of particles were respectively measured.
Extrusion force
All samples were extruded 3 times at a speed of 30 mm/min through the entire length of the syringe with needles on the automated traction bench (MultiTest 1-I [Mecmesin; West Sussex, UK]), and the resulting extrusion forces were recorded.
Swelling degree
Swelling degree was measured by quantitative assay. A dry 500 mesh was selected, and the weight of this state was recorded as m0(g). Following this step, mix 0.5 g of gel well with 6–8 ml of saline. Then, it was brought up to 30 ml. Shook the glass to disperse, removed the mesh after all the particles were swollen, settled for 16 h and placed the filter paper to absorb water. This state was weighted as m1 (g). At last, the mesh was put into the dryer and the sample weight was recorded as m2 (g). The swelling degree (Q) was calculated by the formula below:
Rheological properties
Rheologic measurements were conducted at 25 °C with an automatic Controlled Stress Rheometer (Malvern Instruments LTD, Worcestershire, UK) equipped with a parallel plate geometry (plate diameter, 25 mm; gap, 1.0 mm). The frequency sweep ranged from 10 to 0.1 Hz at 0.1 percent strain. The G′ and the G″ were extracted from two recordings of each sample at a frequency of 0.1 Hz. The experiments were performed within the range of the linear viscoelastic region.
Cohesion
Cohesion was performed as the closest approach to the definition of cohesion claimed by the International Union of Pure and Applied Chemistry [23]. First, HA samples were then prepared with the elimination of air bubbles by centrifugation in 1 ml glass syringes. Then, a Zwick BTC-FR 2.5 materials tester (ZwickRoell GmbH & Co., Ulm, Germany) was adopted to extrude gels at a constant speed of 7.5 mm/min, yielding a volume flow of 0.24 ml/min. Once the constant force was reached, a minimum of 10 drops was collected, and the average drop weight (mg) was calculated.
Animals
4 male New Zealand albino rabbits (250–350 g, aged 6–8 weeks) were housed under standard laboratory conditions, including plastic cages containing pine-sawdust covered floors at 24 °C ± 2 °C, 50 ± 10% relative humidity, 12 h day/night cycles, and allowed free access to food and drink. All the animal experiments were approved by the Institutional Animal Care and Use Committee of Yishengyuan Gene Technology (Tianjin, China) Co., Ltd.
Injection
All 4 male New Zealand albino rabbits were shaved on the back before injection. Four different kinds of HA (50 µl) were intradermally injected (n = 3 points per group) by an experienced anesthesiologist. Another well-trained researcher recorded the skin conditions weekly by using a digital camera (Canon 50D, Tokyo, Japan) Skin images were analyzed by the image analysis software (Image Pro Plus 6.0, Media Cybernetics, Rockville, MD, USA). Animals were randomly sacrificed using an anesthetic overdose at W2, W13, or W26, respectively.
Hematoxylin and eosin stain (H&E stain)
The skin specimens on W2, W13, and W26 (protocol days) were surgically excised after the animals were sacrificed, When the specimens were embedded in paraffin, they were sectioned at 4 μm thickness for histological slide mounting and H&E (Solarbio, Beijing, China) staining. Three microscopic fields were selected for each slide, and photographed at 0.5×, 2×, and 10× magnification. The relationship between gel and surrounding tissues was evaluated [24].
Meanwhile, the slides were scored from 0 to 3, and the binding of gels to surrounding tissues was evaluated. The scoring criteria was as follows: 0. There was no capsule wall, or the capsule wall was thin and only consisted of a few collagen fibers and 1 or 2 layers of fibroblasts; (1) the capsule wall tended to become thinner and denser and consisted of a small amount of collagen fiber cells with occasional fibroblasts; (2) the cystic structure was formed around the experimental sample, which was mainly composed of fibroblasts, collagen fibers, and a small number of collagen fibers; (3) the cystic wall was formed around the experimental sample, and capillaries and fibroblasts were observed. We also analyzed five random fields around filling areas of immune cells at 200× magnification. The immune cells in the epithelial layer and connective tissue were scored of 0–3 as follows: (0) No inflammatory cells were found around the test samples; (1) only a decrease in the number of lymphocytes around the test samples; (2) a few neutrophils and lymphocytes were seen around the experimental sample, and multinucleated foreign-body giant cells were observed occasionally; (3) Inflammatory reactions dominated by neutrophil infiltration were seen around the experimental sample, and histiocytes, phagocytes, capillaries, and small blood vessels were also seen. The final score was calculated from the scores of two blinded observers [25].
Van Gieson/colloidal iron stain (VG/CI stain)
12% acetic acid rinsed slides were used and pipetted into colloidal iron dyestuff for 1 h at 37 °C. Slides were rinsed with distilled water and immersed in ferrocyanide–hydrochloric acid solution at room temperature for 20 min. Slides were rinsed again and counterstained with the nuclear fast red solution for 5 min. Subsequently, samples were dehydrated well in absolute alcohol, cleared, and mounted for conventional light microscopy.
Degradability measurement
To evaluate the degradability of HA in vivo and in vitro, 100 µl (100µ/ml) of HA enzyme (HAse) was separately injected for each filler sample (n = 3 point per group) and mixed with 10 ml HAse solution, and incubated with 0.5 ml sample at 42 °C at W13. The histological slides of skin and gel residues were collected as previously mentioned. Three microscopic fields were selected from each slide and photographed at 0.5×, and the gel residue changes were compared in vivo.
Picro sirius red staining
We viewed histological sections stained with the 1% Picro sirius technique [26], and then the sections were observed under a polarizing microscope. Under polarized light, collage I or thick fibers acted as yellow-orange birefringence, and collage III or thin fibers act as green birefringence. 10 random fields (80× magnification) per group were visualized using a binocular microscope with Axiocam 208 color camera (ZEISS Industrial Metrology). The densities of Type I and III collagen fibers (per mm2) were recorded using the Image software (Media Cybernetics, L.P., Silver Springs, MD, USA).
Statistical analysis
All the data were expressed as mean ± standard deviations (SDs). Statistical significance was calculated by the analysis of variance (ANOVA) followed by the SNK-q post-test by SPSS version 21 software (SPSS Inc., Chicago, IL, USA). The graphics were drawn by GraphPad Prism 9 program (GraphPad Software Inc., San Diego, CA, USA) (*p < 0.05 was considered to be statistically significant).
Results
SEM analysis of four HA fillers
Differences from the slides of the transversal surface in morphologic structure were visible among Cryohyaluron, Juvéderm3, Restylane2, and Ifresh. As shown in Fig. 1, larger size networks and thicker apertures were found in Cryohyaluron compared with the other three gels. Juvéderm3 exhibited a unique structure that appeared heterogeneous from the other three gels, with some regions of less porosity and other regions of larger average pore size existing at the same time. Restylane2 images showed multiple pore sizes that dispersed with various densities in the sample. No additional evidence of discrete particles was found in the other three gels. In contrast, Restylane2 contained diverse irregular particles that were surrounded by a diffuse network of HA. The appearance of the Ifresh pore was distributed relatively evenly in the images, and the space of the aperture was the smallest in the four gels.
Fig. 1.
The SEM images of four different kinds of HA gel fillers. A series of scanned images from the slides of the transversal surfaces was presented at increasing magnification from 500 multiples to 1000 multiples, respectively, for Cryohyaluron (first column), Juvéderm3 (second column), Restylane2 (third column) and Ifresh (fourth column). Significant disparities in the thickness and the space of the HA aperture were noticeable in the 4 different HA fillers
Comparison of physical properties of four HA fillers
Examination illustrated sizes of all four particles at three levels (D10, D50, D90), demonstrating that Restylane2 (682.04 ± 15.74 μm) contained larger-sized particles, and Ifresh (136.62 ± 13.74 μm) presented smaller-sized particles. The particle size span of Juvederm3 was well-defined, as the particle size was concentrated at both ends (138.12 ± 13.59–915.06 ± 8.14 μm). Besides, the size of Cryohyaluron gels (602.84 ± 6.33 μm) was in the medium of the four gels. Meanwhile, profiles of the extrusion force were shown in Table 1. For the Juvederm3 and the Restylane2 gels, the extrusion forces were smoother and evenly distributed, however, Ifresh was more tough and inhomogeneous. The swelling force displayed that the gel with lower capacity taking up additional fluid was Restylane2 (37.40 ± 3.75), and the other three gels with the greater fluid uptake capacity showed a similar degree (Cryohyaluron: 50.51 ± 2.47 < Ifresh: 56.04 ± 3.87 < Juvederm3: 59.96 ± 3.33).
Table 1.
Comparison of four kinds of HA particle size, extrusion force, and swelling degreet
| Cryohyaluron | Juvederm3 | Restylane2 | Ifresh | |
|---|---|---|---|---|
| D10 (μm) | 229.42 ± 10.52 | 138.12 ± 13.59 | 299.83 ± 15.36 | 65.57 ± 7.12 |
| D50 (μm) | 602.84 ± 6.33 | 514.05 ± 6.27 | 682.04 ± 15.74 | 136.62 ± 13.74 |
| D90 (μm) | 950.46 ± 6.11 | 915.06 ± 8.14 | 1044.20 ± 10.85 | 955.04 ± 10.25 |
| Extrusion force (N) | 17.12 ± 1.04 | 12.71 ± 1.75 | 13.14 ± 1.13 | 20.21 ± 0.96 |
| Swelling degree | 50.51 ± 2.47 | 59.96 ± 3.33 | 37.40 ± 3.75 | 56.04 ± 3.87 |
Comparison of rheological properties of four HA fillers
The rheological properties for all four products evaluated were listed in Table 2. Among the 4 products measured, Cryohyaluron, Juvéderm3, and Ifresh were identified as granular-form gels. In contrast, Restylane2 was classified as a gel-form filler. The granular-form gel sample evaluated with the lowest G′ was Ifresh (131.67 ± 12.05 Pa), whereas the highest sample was Cryohyaluron (178.16 ± 10.11 Pa), and Juvederm3 (145.79 ± 12.39 Pa) was recorded as the mid-range. The gel-form gel HA (Restylane2) was recorded as 396.14 ± 24.45 Pa. Similarly, the overall complex modulus (G″) was lowest for Ifresh (30.97 ± 5.02 Pa), and highest for Cryohyaluron (80.56 ± 8.10 Pa), which was higher than Juvederm3 (39.89 ± 7.12 Pa). Restylane2 (96.94 ± 9.75 Pa) was also found higher than granular-form gels. Cryohyaluron (0.44 ± 0.16) was measured with the highest Tan G′/G″, and the other three gels were essentially similar in viscosity. Cohesion showed that the least cohesive property sample was Restylane2 (0.78 ± 0.12 N). Those with the higher cohesive properties were Juvederm3 (5.00 ± 1.13 N) and Cryohyaluron (4.75 ± 0.78 N).
Table 2.
Measurements of the storage modulus (G′), loss modulus (G″), Tan G′/G″, and cohesion of four kinds of HA fillers at a frequency of 1 Hz at 25 °C
| Sample | Rheological properties | Cohesion (N) | ||
|---|---|---|---|---|
| G′ (Pa) | G″ (Pa) | Tan G′/G″ | ||
| Cryohyaluron | 178.16 ± 10.11 | 80.56 ± 8.10 | 0.44 ± 0.16 | 4.75 ± 0.78 |
| Juvederm3 | 145.79 ± 12.39 | 39.89 ± 7.12 | 0.27 ± 0.07 | 5.00 ± 1.13 |
| Restylane2 | 396.14 ± 24.45 | 96.94 ± 9.75 | 0.24 ± 0.04 | 0.78 ± 0.12 |
| Ifresh | 131.67 ± 12.05 | 30.97 ± 5.02 | 0.21 ± 0.03 | 2.89 ± 0.36 |
Injection
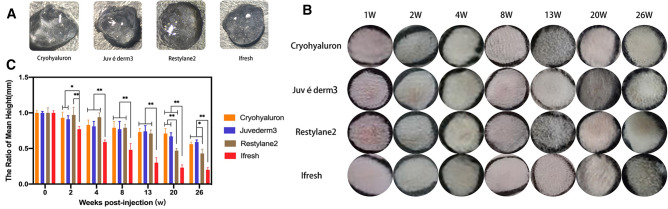
Papule formation was observed following injections of Cryohyaluron, Juvéderm3, Restylane2, and Ifresh. All the gels were injected into the dermis and formed less symmetrical and homogeneous papules. The shapes of the results were affected by certain factors like dermal resistance or positions. After 1 week, all these papules were visible and relatively smooth. As shown in Fig. 2B, C, during the 26 weeks, Ifresh gels were absorbed more than others, and the other types of gels maintained their predetermined shape in vivo. No known adverse reactions like infection, inflammation, or allergic reaction were observed at any time point.
Fig. 2.
A Macro state of four gels at room temperature. B Photo panels of the four treatment groups from the top at 7 time points. No known adverse events were noted at any time point. C Average mean heights over the 26-week were recorded for four HA fillers (*p < 0.05; **p < 0.01)
Histology
Hematoxylin–eosin staining
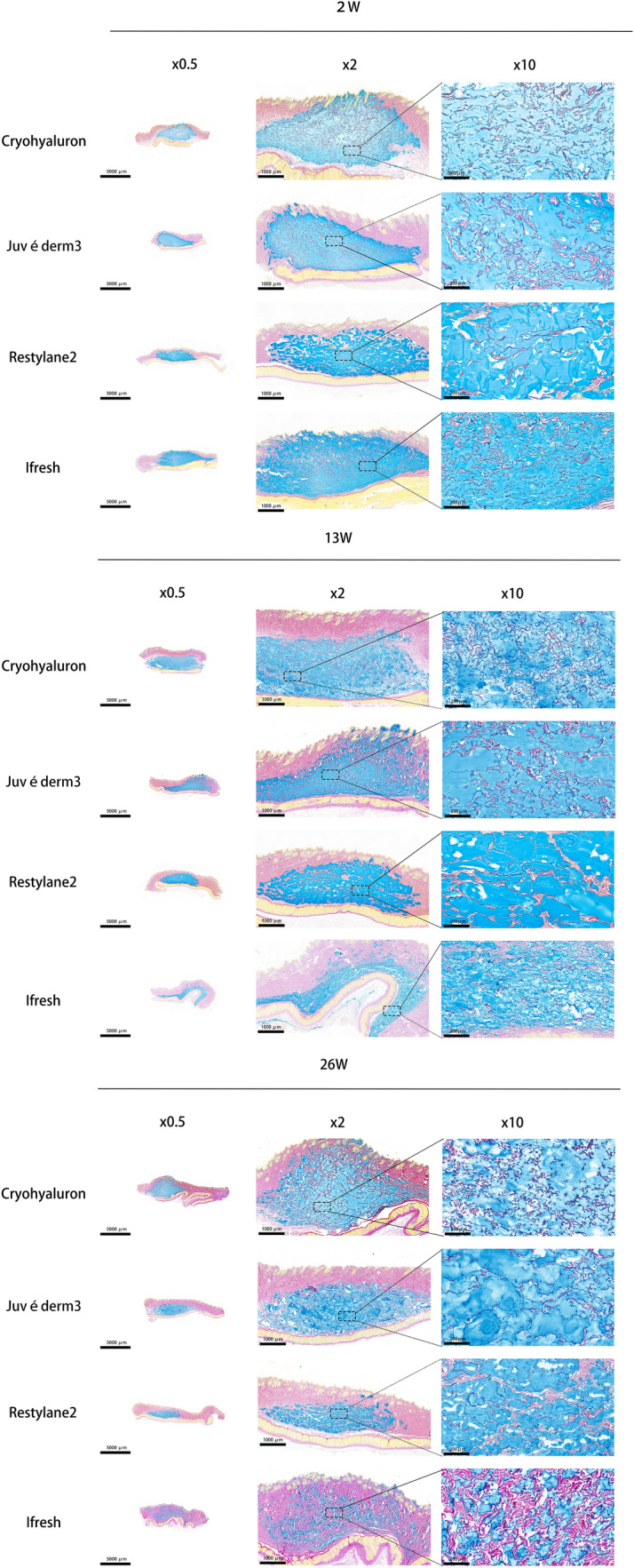
Representative images of dermal skin samples after injection were shown in Figs. 3, 4, 5 and Supplementary Figs. 1-3. To make sure the reactions with foreign bodies in vivo were recorded at different time points, skin tissue samples were selected at 2 weeks (W2), 13 weeks (W13), and 26 weeks (W26). H&E tissue structure was stained in pink and gels in blue-purple, which were observed at 0.5×, 2×, and 10× magnification using an optical microscope. As shown in Fig. 3 and Supplemenatary Fig. 1, the gels diffused around and were evenly distributed in the dermis. No epidermal or dermal alterations or infection infiltrates were found during the 26 weeks. The gels were clearly visible in the subcutaneous tissue, and gels from different manufacturers could be distinguished based on the differences in the shapes and sizes of gels.
Fig. 3.
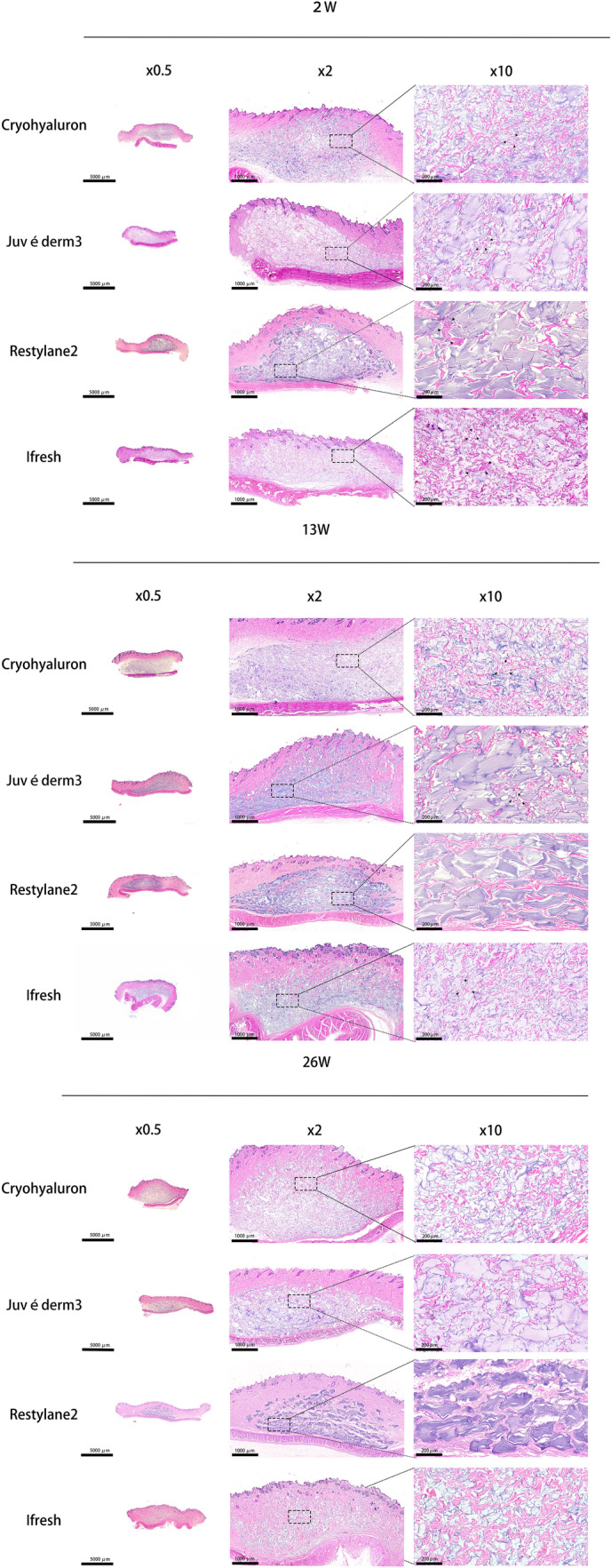
Dermal HA shown by H&E staining at W2, W13, and W26 at three different magnifications (Scale bars = 5000 μm (×0.5), 1000 μm (×2), 200 μm (×10)). Notes The tissue structure was stained in pink and gels in blue-purple by H&E. Black arrow: thick and short fibers; White arrow: thin and long fibers. Black triangle: immune cells
Fig. 4.
Dermal HA indicated by VG/CI staining at W2, W13, and W26 at three different magnifications. (Scale bars = 5000 μm (×0.5), 1000 μm (× 2), 200 μm (× 10)). Notes VG/CI tissue structure was stained in pink and gels in blue
Fig. 5.
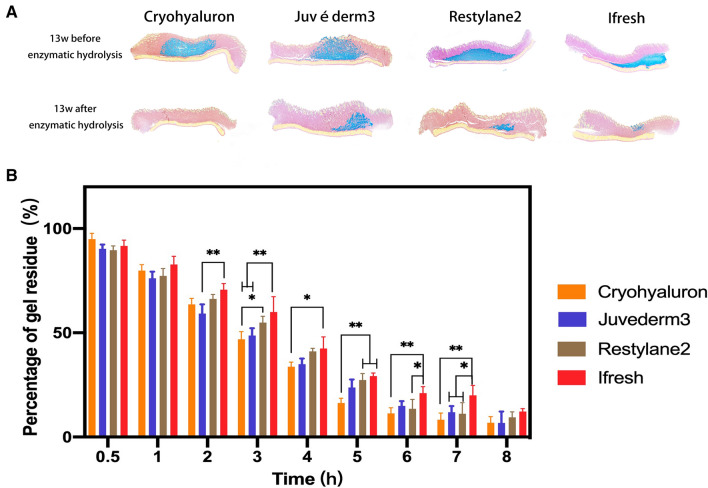
Enzymatic hydrolysis of four HA fillers in vivo (A) and in vitro (B) (*p < 0.05; **p < 0.01)
Restylane2 particles presented irregular polygons with uniform sizes and did not show the obvious interparticle disassembly during the process of injection in vivo. Moreover, a relatively large space between each particle of Restylane2 could be observed and filled with fibrous tissue that appeared in the shape of thinner and longer fibers from the result of Fig. 3. The treatment with Restylane2 resulted in a mild infiltration with comparable numbers of macrophages and lymphocytes between the fibers and gels (black triangle) on W2. In the gels of Juvéderm3, the particles presented a wide range of sizes, and particles of varying sizes had a close relationship with each other, covering both large and small particles. The effect and interaction on the surrounding tissue around the large particles of Juvéderm3 were similar to Restylane2. However, the fibers around small particles were thicker and shorter (black arrow) compared with those around large particles of Juvéderm3 (white arrow). More fibroblasts could be observed to cluster around the small particles of Juvéderm3 that indicated significant dermis regeneration compared to the large particles. Ifresh tissues showed the smallest particle size among four gels and had a significant degradation within 26 weeks. Also, the particles in Ifresh tissues had the same situation as the small particles we just mentioned. Several stubby fibers grew around the particles and some immune cells could be observed (black triangle). Cryohyaluron particles were of medium sizes and the gel aperture was clear in the images. Different from other gel forms, Cryohyaluron gel has obvious separation structure inside. The result indicated that Cryohyaluron maintained its macroporous ultrastructure until 26 weeks after injection and showed signs of cellularization in Fig. 3. Fibers with uniform thickness were evenly distributed in the space between gels and inside gels.
Van Gieson/colloidal iron (VG/CI) staining
Van Gieson/colloidal iron (VG/CI) staining was performed to visualize exogenous HA fillers (Fig. 4 and Supplemenatary Fig. 2). VG/CI tissue structure was stained in pink and gels in blue, also the density of HA can be expressed by color which were observed at 0.5×, 2×, and 10× magnification using an optical microscope. Four gels were shown on different stains, which were more noticeable compared with other gels at W2. Restylane2 presented larger gels and could be clearly distinguished from surrounding tissues. Juvéderm3 showed a mixture of large and small gels consisting of macroscopic physical properties. Ifresh exhibited relatively smaller in four gels, and Cryohyaluron showed the medium-size gel and showed good compatibility with surrounding tissues. In comparison to W2, W13, and W26 at three different magnifications, the distribution patterns of gels had not presented clear differences. The density of HA decreased, and few fibers grew into the space between gels. Therefore, the gels of Ifresh were absorbed more than the others.
Enzymatic degradation
In the experiment in vivo (Fig. 5A and Supplemenatary Fig. 3), it was demonstrated that all four gels were mostly degraded after 2 weeks, and hyaluronidases had a significant effect on the enzymatic hydrolysis performance of the gels. In contrast, in the experiment in vitro (Fig. 5B), the degradation rates of gels increased over time and Ifresh was the most percentage of gel residue in all four gels in vitro. After 9 h, all gels were absorbed by over 90%.
Picro sirius red staining
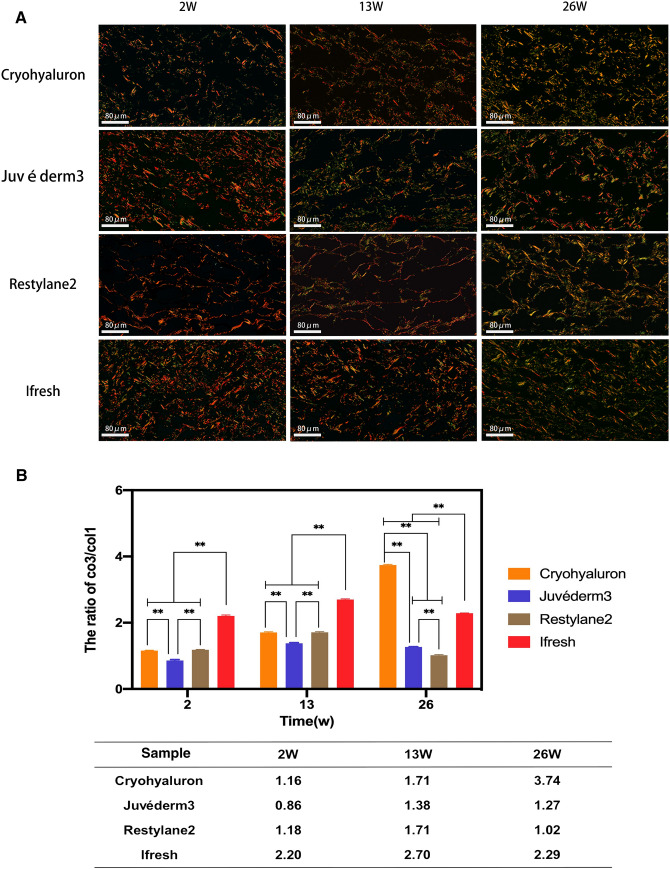
Picro sirius red staining is a qualitative analysis of Type I (col1) and Type III (col3) collagen. Under the polarized light, tissues presented with the appearance and organization of red or orange (col1 mature thicker fibers) and green (col3 new thinner fibers) collagen fibers. As shown in Fig. 6A, at W2, irregularly arranged orange or red col1 fibers were predominant in the four groups. Also, a few green fibrillar structures (col3) were present around reticular dispositions. At W13, col3 fibers showed a considerable number of col3 fibers in samples, and in addition to Cryohyaluron, col3 was concentrated around the aperture in the other three gels. The green fiber began to present reticular dispositions in Cryohyaluron samples. The fibers around Juvéderm3 exhibited aggregation consistent with particle size, and more green fibers grew around the smaller particles. At W26, samples taken from Restylane2 and Juvéderm3 tended to show regularly alignment Type I collagen fibers. The boundaries of Ifresh started to disappear, and it was difficult to divide fibers and gel particles at this time point. The same as 13 weeks, the fiber amount increased in Cryohyaluron, and there were still a number of fibers in reticular dispositions.
Fig. 6.
A Picro sirius red-stained sections under polarized light at 2, 13 and 26 weeks. B Graphical presentation of the ratio of col3/col1 fibers in the four HA fillers samples (*p < 0.05; **p < 0.01). Notes The tissues presented with the appearance and organization of red or orange (col1 mature thicker fibers) and green (col3 new thinner fibers) collagen fibers
The quantitative data on collagen fibers were analyzed by Image J, and as shown in Fig. 6B, the density of col1 fibers was higher in all groups at W2 compared to other time points. In contrast with samples taken at W2 and W26, the ratio of col 3/col1 fibers was the highest at W13 besides Cryohyaluron. These fibers were more densely and regularly arranged in the Cryohyaluron samples (1.71) than in the other groups. At W26, there was a greater density of col 3 fibers in the Cryohyaluron (3.74) than the other groups, and Restylane2 (1.02) presented the lowest ratio in all four samples.
Histological analysis
H&E staining was performed to evaluate the process of fusion degree and immune cell expression in different groups (Fig. 3, Table 3). In terms of binding of gels to surrounding tissues, not all groups presented the same score at W2, which illustrated that those fillers were significantly different in fusion degree during the beginning process of injection. At W13, despite slight variations, the histological scores ranged from 0.5 to 2.5, while compared with W2, the average score increased by 0.5. At W26, fusion degree was observed in four groups with a score ranging from 0.5 to 3. The fusion degree of the tissues around the fillings was significantly different (p < 0.05). Therefore, Cryohyaluron (0.5) had the best integration with the organization, and the fiber wrapping of Restylane2 (3) was the most noticeable. In terms of immune response, at W2, all groups presented a score of 2 except Restylane2 (2.5) which represented that the initial immune response of Restylane2 was stronger than others. At W13, the immune response ranged from 1.5 to 2.0, some small blood vessels could be seen in some fields of Ifresh slides, and until W26, this phenomenon still existed. In the other three groups, there was a mixed amount of inflammatory cell infiltration, which showed a continuous downward trend during the whole 26 weeks.
Table 3.
Histological profiles of HA fillers in four groups over time
| Category | 2W | 13W | 26W |
|---|---|---|---|
| Histologic | |||
| Cryohyaluron | 0 (0–0) | 0.5 (0–1) | 0.5 (0–1)** |
| Juvederm3 | 1 (1–1) | 1.5 (1–2) | 2.5 (2–3)*,** |
| Restylane2 | 2 (2–2) | 2.5 (2–3) | 3 (3–3)*,** |
| Ifresh | 0 (0–0) | 1 (1–1) | 1 (1–1)** |
| Immune cells | |||
| Cryohyaluron | 2 (2–2) | 1.5 (1–2) | 1 (1–1)** |
| Juvederm3 | 2 (2–2) | 1.5 (1–2) | 1 (1–1)** |
| Restylane2 | 2.5 (2–3) | 1.5 (1–2) | 1 (1–1)** |
| Ifresh | 2 (2–2) | 2 (1–3) | 2 (1–3)** |
Data were expressed as median (minimum–maximum)
*p < 0.05, compared to D1
**p < 0.05, compared to the same day@@@
Discussion
Stabilized Hyaluronic acid (HA) manufactured using various cross-linking technologies as an injectable gel has been widely used for cosmetic application. Cross-linked HA is essentially a gel particle composition composed of a large number of viscoelastic particulate. Its overall rheological properties of it are determined by the viscoelastic properties of particles and the interaction forces between particles. Different product formulations and design results in HA gels with various characteristic properties such as particle sizes, viscoelasticity, and interaction forces between particles. Furthermore, these properties will affect the performance of HA gel products from disparate dimensions including support capacity, maintenance time, and local biocompatibility. These evaluations of HA gels in previous studies often focus on the biological safety and the macroscopic expression of the fillers. However, the research on the internal mechanism of their macro performance is very limited, and the researchers lack sufficient understanding of the patterns of distribution in the dermis by injecting HA gel, the influence on the surrounding tissue, and the interaction between the gel and the cells. Four kinds of typical dermal filler were selected to analyze in this research to characterize the structure and physicochemical properties of the gel, as well as observe their interaction with the surrounding tissues in vivo and discussed their internal mechanism.
Gel-form products have high cohesion, and strong physical interaction between particles, and product supportability is determined by the physical force between particles and the strength of particles. However, the strength of granular-form products with low or no cohesion is only determined by the strength of particles. Under the microscope, the gel-form HA is soft, smooth, homogeneous, and free of particles, but granular-form HA is granular and uneven. Also, gel-form HA owns better histocompatibility, high cohesion, stable and lasting effect which is distinguished by good viscosity, high elasticity, and ductility in granular-form gels.
Restylane2 is manufactured by Non-Animal Stabilized Hyaluronic Acid (NASHA ™) technology and featured as a gel-form filler unlike the other three HA products [15]. The main process of NASHA™ technology is to suspend HA cross-linked BDDE in a physiological solution, thereby forming an entangled matrix. Afterwards, the cross-linked HA matrix was sieved and then passed through cleaver filters of different diameters to produce gel particle sizes adapted to the clinical indications of the final product. During the process, solid HA “pearls” were dispersed in a non-crosslinked HA solution that acted as a vehicle. Therefore, the microscopic particles of Restylane2 gel are relatively palpable compared with the other three granular-form gels. The results of HE staining (Fig. 3) and the particle size test demonstrated that the Restylane2 particles seemed obviously and non-viscously and were measured as the largest among the four gels. The elastic modulus (G′) of Restylane2 was tested higher, indicating that the higher gel strength consisted of the superposition of elastic potential energy of the gel particle (Table 2). When the elastic modulus is higher, the product has a higher viscosity modulus and shear viscosity. All these properties would provide enough capability of deformation resistance that was beneficial to energy storage and rebound after shear stress. In addition, Restylane2 presented a lower swelling degree (Table 2), suggesting that the cross-linking density of the gel is higher. The maximum swelling of gel depended on the cross-linking density of the polymer network because the cross-links kept the polymer chains together and limited the possibility of the chains to separate apart. The more cross-links there were, the tighter the chains were kept together, and the more limited the flexibility of the chains was, reducing the swelling capacity. The analysis of testing physicochemical properties demonstrated that Restylane2 has a higher resistance to deformation (G′), which has been proven to be proportional to the value of cross-linking density [18]. Furthermore, Restylane2 also performed well in extrusion force, due to the suitable particle size of the gel and the lubricating action of the addition of non-cross-linked gel.
Juvéderm3 is the representative of granular-form gel and uses a technology called “Hylacross™ technology” [16]. The patented Hylacross™ technology incorporated high-molecular-weight HA and low-molecular-weight HA, improving the cross-linking efficiency of HA. The mixture of two HA of different molecular weight can roughly distinguish large and small particles (Table 1). Such features can be identified from the results of the H&E histological section as well (Fig. 3), indicating that particles of irregular shapes and different sizes existed in the same slide, and the accumulation between particles were more closely and interlocked with each other. Although the swelling degree of Juvéderm3 was significantly higher than Restylane2 (Table 1), current data showed that the cross-linking degree of Juvéderm3 was 5%-10% and Restylane2 was below 2% [16]. These results were contrary to the previous conclusions that the degree of swelling was inversely proportional to the degree of cross-linking. This might be explained by the fact that Restylane2 and Juvéderm3 belonged to different categories, namely gel-form and granular-form gel, and comparison across categories with different physicochemical properties might be unjustified. The desired effect of the gel injected into the body depended on the lifting capacity of the gel [19]. A strong gel should provide the force needed to lift the tissue and resist subsequent deformation to achieve the desired correction. It has been suggested that the lifting capacity of a granular-form gel was affected not only by the superposition of the particles’ elastic potential energy but also by the cohesion of the filler. G′ and cohesion showed a positive correlation with the overall lift capacity when fillers of similar composition or cross-linking technology were grouped. As the most typical HA filler product of granulated gel, G′ and G″ of Juvéderm3 were relatively lower than Cryohyaluron (Table 2), but the cohesive of Juvéderm3 was the highest in the three samples of a granular-form gel, demonstrating a better extent of integration intradermally [18].
As another granular-form filler, Ifresh was more prominent in the market and featured as a kind of small molecule gel. As listed in Tables 1 and 2, the particle size of Ifresh was smaller than Juvéderm3 and the distribution of particle size was relatively narrow. Several physicochemical indexes of G′, G″ and swelling degree were obtained, and it had been suggested that the above-mentioned properties of Ifresh gel stayed at a similar level as Juvéderm3. However, the cohesion of Ifresh was significantly lower than the group of Juvéderm3. Lower cohesion of Ifresh reflected the adhesion between cross-linked HA domains with weak interactions, which might be resulted from the smaller particle size and the narrow distribution range of Ifresh. The cohesion of the gel affected the satisfaction of clinical indications of HA fillers. The HA fillers with high cohesivity were more suitable for bulk facial volumization, whereas the HA fillers with low cohesivity were easier to mold tending to form thin and even layers in the dermis [18]. Moreover, Ifresh had no non-crosslinked gel components added as a lubricating agent, and its extrusion force was the highest among the four products.
The dermal HA fillers named Cryohyaluron were manufactured using the patented Cryogel™ technology. This technology relies on the cross-linking reaction carried out in sub-zero temperatures, leading to a product with internal macroporous architecture within the cross-linking gel through the generation and dissolving of the ice crystal. This process is commonly applied in tissue engineering to prepare the scaffolds which are conducive to cell growth and attachment [19]. As it was previously discussed, the HA fillers migration and degradation would result in progressive volume loss and shape deformity in the dermis after injection. Subsequent fibroblasts were activated and stimulated to produce new collagen surrounding the HA fillers [27]. The ideal dermal HA fillers were supposed to promote oxygen, nutrient passage, and cell migration to support new tissue growth. Surprisingly, the macroporous structure and the internal thick wall of the gel caused by Cryogel™ technology were likely be able to meet these criteria. In terms of appearances under SEM imaging from the slides of transversal surface (Fig. 1), Cryohyaluron consisted of a stable thick poring system which was not significantly changed over time in the experimental environment. Cryohyaluron was also categorized as a granular-form fillers product and presented a relatively lower swelling degree than Ifresh and Juvéderm3, assuming a higher cross-linking degree. Unlikely the particle size and distribution of Juvéderm3 and Ifresh, Cryohyaluron presented medium particle size but narrow particle size distribution. In the evaluation of the rheological properties of granular-form fillers tested in this study, the G′ and G″ of Cryohyaluron gels were significantly higher than the Juvéderm3 and Ifresh. However, the cohesion of Cryohyaluron was tested at a high level comparable to Juvéderm3. No addition of lubricant agent (non-crosslinked gel) was found but Cryohyaluron still maintained the fitness of extrusion, which was another advantage of Cryohyaluron.
This study selected four typical dermal fillers for analysis. Restylane2, a dispersive gel product with large particles, had higher G′ and firmness, which is suitable for deeper injection sites and provides a greater degree of correction. This filler presents a higher elasticity response to compression and lower cohesivity, contributing to the uniform spreading of filler, formation of thin sheeting, and molding [18]. Hence, Restylane2 is more suitable for injection in the nasal tip, chin, and apple muscle. A high cohesiveness granular-form gel product with both large and small particles named Juvéderm3, has a lower G′ (like the other two fillers), softer texture, and less elasticity, which indicates that it is more suitable for superficial planes such as correction of fine lines, forehead, or skin folds. Juvéderm3 with both large and small particles can fit the dermal space more closely. A low cohesiveness granular-form gel product with small particles named Ifresh, is of medium cohesivity and keeps a balance between vertical projection and relatively easy moldability, and the smaller particles fit better in the fine and superficial skin but may degrade faster. Therefore, it should be treated as a short-time filling or detailed filling. A high cohesiveness granular-form gel product with specific characteristics of thick-walled pore structure named Cryohyaluron, has a higher cohesivity and helps the filler maintain vertical projection while the soft tissue is subjected to vertical stress, which is more suitable for large facial plumpness. Meanwhile, the medium size of particles and special structure may provide enough space for tissue regeneration to prolong the stability of the filler. The function of Cryohyaluron is easier to be fit, bringing more convenience to the clinicians.
H&E staining results (Fig. 3) revealed that in the space between larger particles of HA (e.g., Restylane2), the distinction of cells was scattered in the gap varies and was unable to penetrate gels, as shown by VG/CI staining (Fig. 4). The clear boundaries between the gels and cells identified the presence of an "inert physical barrier" and, to some extent, these barriers might obstruct the metabolism laterally. In contrast, the surroundings around small particles present a better phenomenon (Figs. 3, 6A).The large particles internal the gel and the high rheological properties endow the Restylane2 with excellent support. However, these large-size particles have a significant impact on the metabolism of the local tissue surrounding the gel, which altered in response to the narrow channels between the gel particles in cellular activity. Juvéderm3 presented gel integrity with the high cohesiveness and superior support. Both large and small particles could be clearly seen and uniformly mixed in the gel. The large particles among them still showed the inert filling features that unable to support the transport of nutrients and removing wastes. On the contrary, the cells around the small particles exhibited good biological behavior. Therefore, the rational matching of large and small particles provides the Juvéderm3 with the capability of support and excellent biological performance. The characteristics of Ifresh is that small-size particles, moderate cohesiveness, good integrity, lower viscoelasticity and the superior cellular activity located the surrounding tissues. Nevertheless, the degradation rate and maintenance time of Ifresh are rapid and short probably because of the extended superficial area of the small particles in the gel. Surprisingly, from the result showed in Col3/Col1 (Fig. 6B), the ratio of Col3/Col1 decreased over time until W26 when the minimum value was reached except for Cryohyaluron. Under the stimulation of HA and the injection, the fibers might have, to some extent, grown, and most of them grew in the space between the gels. It was also found that the green fibers expressed in the gels might be influenced by the special structure of Cryohyaluron. Epithelial migration and proliferation were determined by the production of collagen. Col3 was a provisional collagen produced in the early phases of healing, while Col1 was formed during the later phases. The biological properties of Cryohyaluron involving the cell behavior located in the local tissues exhibited outstanding. The reason may be that the specific macroporous structure in the gel could benefit for the nutrients delivering and removing the waste. In addition, owning large particles and high rheological properties make the gel maintain in longer period.
For improving the properties of fillers, the design of the internal structure of particles may become the focus of consideration, such as loading growth factors or drugs through microspheres [28] and freeze cross-linking [29]. The intended clinical application of each filler depends on the rheological property of its manufacturing process. Whether manufacturers want to have higher elasticity (G′) or higher viscosity, they can confer firmness and resistance to applied force [6, 30]. During or after implantation, a certain degree of cohesion can be treated as a prerequisite to maintaining filler integrity. Therefore, the maintenance time is attributed to its cross-linking, cohesion, and total HA concentration [31]. After reaching a certain degree of cross-linking, the water retention capacity first increases and then decreases. Low cross-linking agent and control of production environment (microorganism) contribute to improving the biocompatibility of gels as well.
In conclusion, the rheological properties and particle size of Hyaluronic acid dermal fillers are directly related to the in vivo performance of the filler. The higher rheological properties and the larger particles of the gel, the longer period the gel maintains and the better performance the gel has in vivo. The low rheological properties and small-size particles cause the gel lacking of support and extended superficial area, which will lead to the HA dermal fillers losing their role at an early stage. However, when the particle size in the gel is too large, it will engender the barrier between cells resulting in changes of cellular act in local tissues, showing the abnormal in shape and structure of skin tissue. Therefore, it is necessary to make the filler has both great support and biocompatibility through the rational matching of large and small particles. The gel characterized by macroporous structure shows a definite advantage in this respect, which can not only bring good supporting through large particles and high rheological properties, but also provide local well-organized biological activity due to the perforated structure inside.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Youth Fund of the Second Hospital of Tianjin Medical University (Award number 2022ydey18).
Author contribution
ZW (M.D.): Investigation, Formal analysis, Data Curation, Writing—Original Draft. HS (M.D.): Conceptualization, Validation, Investigation, Writing—Original Draft. DS (M.D.): Conceptualization, Project administration. PY (M.D.): Validation, Resources, Data Curation. FW (M.D.): Resources, Visualization. ZY (M.D.): Resources, Data Curation. YJ (M.D.): Writing—Review and Editing, Funding acquisition. PC (Ph.D.): Project administration, Supervision P.S.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
All animal surgical experiments were performed under the approval by the Institutional Animal Care and Use Committee of Yi Shengyuan Gene Technology (Tianjin) Co., Ltd. (Approval No. YSY-DWLL-2022102).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Weiwei Zhou and Shuai Hou contribute equally to the article as co-first author.
Jie Yang and Cheng Peng contribute equally as corresponding author.
Contributor Information
Jie Yang, Email: yangjie0325@163.com.
Cheng Peng, Email: peng_cheng2013@163.com.
References
- 1.Iannitti T, Morales-Medina JC, Coacci A, Palmieri B. Experimental and clinical efficacy of two hyaluronic acid-based compounds of different cross-linkage and composition in the rejuvenation of the skin. Pharm Res. 2016;33:2879–2890. doi: 10.1007/s11095-014-1354-y. [DOI] [PubMed] [Google Scholar]
- 2.La Gatta A, Schiraldi C, Zaccaria G, Cassuto D. Hyaluronan dermal fillers: efforts towards a wider biophysical characterization and the correlation of the biophysical parameters to the clinical outcome. Clin Cosmet Investig Dermatol. 2020;13:87–97. doi: 10.2147/CCID.S220227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beasley KL, Weiss MA, Weiss RA. Hyaluronic acid fillers: a comprehensive review. Facial Plast Surg. 2009;25:86–94. doi: 10.1055/s-0029-1220647. [DOI] [PubMed] [Google Scholar]
- 4.American Society of Plastic Surgeons . 2013 plastic surgery statistic report. Arlington Heights: ASPS National Clearinghouse of Plastic Surgery Procedural Statistics; 2013. [Google Scholar]
- 5.Wu DC, Karnik J, Margarella T, Nguyen VL, Calame A, Goldman MP. Evaluation of the in vivo effects of various laser, light, or ultrasound modalities on human skin treated with a collagen and polymethylmethacrylate microsphere dermal filler product. Lasers Surg Med. 2016;48:811–9. [DOI] [PubMed]
- 6.Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132:5S–21S. doi: 10.1097/PRS.0b013e31829d1d40. [DOI] [PubMed] [Google Scholar]
- 7.Trombino S, Servidio C, Curcio F, Cassano R. Strategies for hyaluronic acid-based hydrogel design in drug delivery. Pharmaceutics. 2019;11:407. doi: 10.3390/pharmaceutics11080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Preston BN, Davies M, Ogston AG. The composition and physicochemical properties of hyaluronic acids prepared from ox synovial fluid and from a case of mesothelioma. Biochem J. 1965;96:449–471. doi: 10.1042/bj0960449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kablik J, Monheit GD, Yu LP, Chang G, Gershkovich J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35:302–312. doi: 10.1111/j.1524-4725.2008.01046.x. [DOI] [PubMed] [Google Scholar]
- 10.Stocks D, Sundaram H, Michaels J, Durrani MJ, Wortzman MS, Nelson DB. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol. 2011;10:974–980. [PubMed] [Google Scholar]
- 11.La Gatta A, Salzillo R, Catalano C, Pirozzi AVA, D'Agostino A, Bedini E, et al. Hyaluronan-based hydrogels via ether-crosslinking: is HA molecular weight an effective means to tune gel performance? Int J Biol Macromol. 2020;144:94–101. [DOI] [PubMed]
- 12.Edsman K, Nord NI, Ohrlund A, Lärkner H, Kenne AH. Gel properties of hyaluronic acid dermal fillers. Dermatol Surg. 2012;38:1170–1179. doi: 10.1111/j.1524-4725.2012.02472.x. [DOI] [PubMed] [Google Scholar]
- 13.Flynn TC, Sarazin D, Bezzola A, Terrani C, Micheels P. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637–43. [DOI] [PubMed]
- 14.Choi SC, Yoo MA, Lee SY, Lee HJ, Son DH, Jung J. Modulation of biomechanical properties of hyaluronic acid hydrogels by crosslinking agents. J Biomed Mater Res A. 2015;103:3072–3080. doi: 10.1002/jbm.a.35437. [DOI] [PubMed] [Google Scholar]
- 15.Verpaele A, Strand A. Restylane SubQ, a non-animal stabilized hyaluronic acid gel for soft tissue augmentation of the mid- and lower face. Aesthet Surg J. 2006;26:S10–S17. doi: 10.1016/j.asj.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Bogdan Allemann I, Baumann L. Hyaluronic acid gel (Juvéderm) preparations in the treatment of facial wrinkles and folds. Clin Interv Aging. 2008;3:629–634. doi: 10.2147/CIA.S3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prager W, Wissmueller E, Havermann I, Bee EK, Howell DJ, Zschocke I, et al. A prospective, split-face, randomized, comparative study of safety and 12-month longevity of three formulations of hyaluronic acid dermal filler for treatment of nasolabial folds. Dermatol Surg. 2012;38:1143–50. [DOI] [PubMed]
- 18.Pierre S, Liew S, Bernardin A. Basics of dermal filler rheology. Dermatol Surg. 2015;41:S120–S126. doi: 10.1097/DSS.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 19.Hee CK, Shumate GT, Narurkar V, Bernardin A, Messina DJ. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol Surg. 2015;41:S373–S381. doi: 10.1097/DSS.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 20.La Gatta A, Salzillo R, Catalano C, D'Agostino A, Pirozzi AVA, De Rosa M, et al. Hyaluronan-based hydrogels as dermal fillers: the biophysical properties that translate into a “volumetric” effect. PLoS One. 2019;14:e0218287. [DOI] [PMC free article] [PubMed]
- 21.Edsman KLM, Öhrlund Å. Cohesion of hyaluronic acid fillers: correlation between cohesion and other physicochemical properties. Dermatol Surg. 2018;44:557–562. doi: 10.1097/DSS.0000000000001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romagnoli M, Belmontesi M. Hyaluronic acid-based fillers: theory and practice. Clin Dermatol. 2008;26:123–159. doi: 10.1016/j.clindermatol.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Edsman KL, Wiebensjö ÅM, Risberg AM, Öhrlund JÅ. Is there a method that can measure cohesivity? Cohesion by sensory evaluation compared with other test methods. Dermatol Surg. 2015;41:S365–S372. doi: 10.1097/DSS.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 24.Tran C, Carraux P, Micheels P, Kaya G, Salomon D. In vivo bio-integration of three hyaluronic acid fillers in human skin: a histological study. Dermatology. 2014;228:47–54. [DOI] [PubMed]
- 25.Ministry of health of the People's Republic of China, GB/T 16175-2008, Biological evaluation test methods of medical organic silicon materials. Chinese Standard Press, Beijing
- 26.Junqueira LC, Carneiro J. Histologia Básica. 11. Rio de Janeiro: Guanabara Koogan; 2008. [Google Scholar]
- 27.Falcone SJ, Berg RA. Crosslinked hyaluronic acid dermal fillers: a comparison of rheological properties. J Biomed Mater Res A. 2008;87:264–271. doi: 10.1002/jbm.a.31675. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton M, Harrington S, Dhar P, Stehno-Bittel L. Hyaluronic acid hydrogel microspheres for slow release stem cell delivery. ACS Biomater Sci Eng. 2021;7:3754–3763. doi: 10.1021/acsbiomaterials.1c00658. [DOI] [PubMed] [Google Scholar]
- 29.Cheng L, Ji K, Shih TY, Haddad A, Giatsidis G, Mooney DJ. Injectable shape-memorizing three-dimensional hyaluronic acid cryogels for skin sculpting and soft tissue reconstruction. Tissue Eng Part A. 2017;23:243–251. doi: 10.1089/ten.tea.2016.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundaram H, Voigts B, Beer K, Meland M. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxylapatite and hyaluronic acid. Dermatol Surg. 2010;36:1859–1865. doi: 10.1111/j.1524-4725.2010.01743.x. [DOI] [PubMed] [Google Scholar]
- 31.Borrell M, Leslie DB, Tezel A. Lift capabilities of hyaluronic acid fillers. J Cosmet Laser Ther. 2011;13:21–27. doi: 10.3109/14764172.2011.552609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.