Abstract
Schistosomiasis is a tropical parasitic disease, in which the major clinical manifestation includes hepatosplenomegaly, portal hypertension, and organs fibrosis. Clinically, treatment of schistosomiasis involves the use of praziquantel (PZQ) and supportive care, which does not improve the patient’s outcome as liver injuries persist. Here, we report for the first time the effect of N-acetyl-L-cysteine (NAC) and/or praziquantel (PQZ) on S. mansoni, hepatic granuloma, serum markers for liver function and oxidative damage in acute schistosomiasis. Infected mice were divided into control, NAC, PZQ and NAC+PZQ groups and uninfected into control and NAC groups. After infection, NAC (200 mg/kg/day) was administrated until the 60th day and PZQ (100 mg/kg/day) from the 45th to the 49th day, both orally. On day 61, the mice were euthanized for serum markers for liver function. Worms were recovered, fragments of intestine employed to ascertain the oviposition pattern, and the liver was used for histopathological analysis, histomorphometry, egg and granuloma counting and oxidative stress marker assays. NAC reduced the burden of worms and eggs and increased the dead eggs in intestinal tissue. NAC+PZQ brought about reduction in granulomatous infiltration and NAC and/or PZQ reduced levels of ALT, AST, and alkaline phosphatase and increased albumin. NAC, PZQ or NAC+PZQ reduced levels of the superoxide anion, lipid peroxidation and protein carbonyl and increased sulfhydryl groups. The reduction in parasitological parameters, granulomatous inflammation and oxy-redox imbalance suggests NAC acts as a adjuvant in treatment of acute experimental schistosomiasis.
Keywords: Schistosoma mansoni, Schistosomiasis granuloma, Liver, Oxidative stress, N-acetyl-L-cysteine, Drug association
Introduction
Schistosomiasis is an important neglected tropical disease that is associated with severe and irreversible liver damage and high mortality in hundreds of millions of people around the world (World Health Organization 2023). The presence of Schistosoma mansoni eggs in hepatic tissue triggers a granulomatous inflammatory process and causes imbalance of antioxidant defenses induced by soluble egg antigens (SEA) (Abdallahi et al. 1999; Seif El-Din et al. 2011). The activation of T-cell-dependent responses and macrophages (Kupffer cells) brings about an increase in reactive oxygen species (ROS), such as the superoxide anion. Moreover, eosinophil, which is one of the components of a Schistosoma-induced hepatic granuloma, causes an increase in other reactive species, such as the hydroxyl radical and peroxynitrite (Abdallahi et al. 1999; Fan et al. 2007). The intracellular redox status imbalance affects the signaling pathways responsible for cell proliferation, immune responses, and gene expression and plays an important role in the pathogenesis of schistosomiasis mansoni resulting in alterations in serum aminotransferase activity and total protein concentration, portal hypertension, hepatosplenomegaly and hepatic fibrosis (Seif El-Din et al. 2011; Mata-Santos et al. 2014).
One strategy for restricting hepatic tissue damage would be to restore the redox balance. Treatment with antioxidants can prolong the initiation phase or inhibit the propagation phase of reactive oxygen and nitrogen species, contribute to the prevention of oxidative damage and maintain immunologic functions capable of neutralizing S. mansoni antigens (Abdallahi et al. 1999). N-acetyl-L-cysteine (NAC) (Fig. 1A) is widely known for its mucolytic properties in the treatment of various respiratory diseases. In this context, NAC is a thiol-containing compound that has been used for over 30 years as the antidote for acetaminophen-induced liver injury and acute liver failure and is well-tolerated and safe for treatment of various medical conditions, especially liver diseases (Ntamo et al. 2021). In humans and experimental animals, NAC has shown important hepatoprotective effects attributed to its antioxidant and anti-inflammatory properties by directly inhibiting the deleterious effects of oxidation, preventing the activation of lipid peroxidation, elimination and degradation of free radicals and as a cofactor of an antioxidant enzymatic system (Licks et al. 2014; Rushworth and Megson 2014; Ntamo et al. 2021).
Fig. 1.
Chemical structure of N-acetyl-L-cysteine (A) and praziquantel (B)
Treatment of schistosomiasis relies solely on praziquantel (PZQ) (Fig. 1B), since vaccination is not yet available. PZQ does not prevent re-infection, has no prophylactic effect, is inactive against already installed granulomatous lesions and its main target is the adult worm, since the immature stages are less susceptible (El-Lakkany et al. 2001). Dependence on a single drug is a source of concern and is inadvisable for any infectious disease, but it is particularly troubling with a schistosomiasis, owing to its high prevalence and incidence. This encouraged us research and develop novel drugs for the prevention of S. mansoni infection and cure of schistosomiasis.
Knowing that schistosomiasis mansoni is a severe infection that compromises the tissue and function hepatic, NAC is a drug with broad hepatoprotective properties on inflammation and oxidative stress and that PZQ has no action on histopathological lesions already installed in the liver, and the present study investigated the action of NAC and/or PZQ in experimental acute mansonic schistosomiasis. Parasitological parameters (worm burden, number of eggs in hepatic tissue and pattern of oviposition) were measured, and histopathological and histomorphometric changes in hepatic granulomas, weight development (body and liver), serum markers of hepatic function (aminotransferase, alkaline phosphatase and albumin) and oxidative damage (superoxide anion, lipid peroxidation, protein carbonyls and sulfhydryl groups) were examined.
Materials and methods
Animals, experimental procedure and ethical considerations
Thirty-day-old Swiss Webster female mice (n = 48) weighing 30 ± 2 g received water and food ad libitum and were housed in collective cages under a light/dark cycle of 12 h in a room with a temperature of 22 ± 2 °C. Thirty-two mice were percutaneously infected with 50 cercariae (BH strain) of S. mansoni, and sixteen mice were not infected (Olivier end Stirewalt 1952). This strain is maintained in the sector of parasitic diseases and experimental schistosomiasis of the Laboratory of Instituto Keizo Asami (iLIKA) and in Department of Tropical Medicine of the Federal University of Pernambuco (UFPE) (Recife, PE, Brazil). All experimental procedures involving animals were performed in accordance with the Brazilian College of Standards for Ethics in Animal Experimentation (COBEA) and approved by the Animal Experiments Ethics Committee (CEUA) UFPE, process number 23076.020127/2010-47.
Experimental groups and treatment protocol
The mice were divided into six groups (n = 8), and there was no statistical difference (p > 0.05) in body weight (29.63 ± 1.21) between the formed groups, according to the cercarial exposure (infected and uninfected) and treatment regimen adopted, as follows:
Uninfected groups treated with vehicle only (saline solution) or NAC
Infected groups treated with vehicle only, NAC, PZQ or NAC+PZQ.
NAC (Sigma-Aldrich Chemical, St. Louis, MO, USA) was diluted in saline solution and administered orally by gavage (200 mg/kg/day), as previously described, (Jones et al. 1994; Fan et al. 2007, 2011; Aires et al. 2012; Ntamo et al. 2021) from immediately after infection until the 60th day. PZQ (Sigma-Aldrich Chemical, St. Louis, MO, USA) was suspended in Cremophor (2%), diluted in saline solution and administered orally by gavage at a curative dose of 100 mg/kg/day, from the 45th until the 49th day after infection, as previously described (Aires et al. 2012). Infected and uninfected animals without NAC and/or PZQ treatment received the same amount of vehicle and were subjected to the same conditions. Sixty-one days after infection, all mice were euthanized under anesthesia.
Hepatic function serum markers and relative liver weight
Immediately after anesthesia, the mice were weighed, and the blood collected by cardiac puncture and of the serum was obtained (centrifugation at 400×g for 15 min at 4 °C). Levels of alanine aminotransferase (ALT), aspartate aminotransaminase (AST), albumin (ALB) and alkaline phosphatase (AP) were measured using a TARGA device (Random Generation Advanced Technology Analysis). The liver of each mouse was excised immediately after perfusion and weighed. The relative liver weight is calculated as follows:
Parasitological parameters
Recovery of worms
Hepatic and mesenteric vessels were perfused with sterile saline (0.9% NaCl w/v) for recovery, counting and classification of worms (couples or males), as described by Smithers and Terry (1965). The percentage reduction in the number of worms after treatment is calculated as follows: % reduction = C − V/C × 100, where C = the mean number of parasites recovered from infected untreated animals and V = the mean number of parasites recovered from treated animals (Tendler et al. 1968).
Eggs count in hepatic tissue
To estimate the number of eggs per gram of hepatic tissue, a samples of tissue was removed from the central remaining part of the right lobe of each animal and processed separately by way of digestion technique in potassium hydroxide (KOH 4%), as described by Cheever (1968). The results were expressed in the number of eggs per gram of tissue and the percentage reduction in eggs accumulated in the liver tissue.
Oogram pattern
Three fragments of the small intestine (3 cm each) were cut longitudinally, rinsed in saline, softly dried on filter paper, and then compressed between two glass slides to obtain the thin preparation. Fragments were examined under microscope (10×), and the stage of each egg was recorded. One hundred eggs were counted in each fragments and classified according to their developmental stage and maturation of S. mansoni eggs (Pellegrino et al. 1962).
Measurement and histopathological analysis of hepatic granulomas
Samples of liver were removed, fixed in 10% buffered formaldehyde, and processed for inclusion in paraffin blocks to obtain histological Sections (5 µm), which were stained with hematoxylin and eosin and examined histologically using light microscopy. Twenty random fields per animal were used to count the average number of granulomas, using Formula I, each field measuring 12.234 μm2. Twenty granulomas containing a single central egg were randomly used to determine the average diameter, using Formula II. The radii of the granulomas as well as their diameters were measured and used to calculate the volume of granulomas according to Formula III.
| I |
| II |
| III |
Images were obtained using an optical microscope (Leica®) connected to a digital camera (Nikon®) and a computer system (Motic Images Plus 2.0 MLTM). All analyses were performed by two different pathologists.
Sample preparation for oxidative stress in the homogenate of liver tissue
To remove the excess of blood, livers from uninfected and infected mice were perfused with sterile saline (0.9% NaCl w/v). Each mice’s liver was homogenized using a homogenizer Potter–Elvehjem in a cold buffer containing 50-mM TRIS and 1-mM EDTA (pH 7.4), with the addition of 1-mM sodium orthovanadate and 200-μg/mL phenylmethanesulfonyl (PMSF) fluoride at 0–4 °C. Then, the homogenate was centrifuged at 1.180×g for 10 min at 4 °C, the resulting supernatant was carefully removed, and protein content was determined spectrophotometrically according to Lowry assay (Sargent 1987). The homogenates of liver tissue (0.3 mg/mL) was used to measure the total superoxide dismutase (SOD) activity, thiobarbituric acid reactive species (TBARS), protein carbonyl content and total sulfhydryl (SH) groups. All measurements were performed in triplicate for every sample in two independent trials.
Total superoxide dismutase (SOD) activity
Total SOD activity was performed according to the method of Misra and Fridovich (1972). In brief, 300 μg of protein from the homogenate was added to sodium carbonate buffer (0.05%, pH 10.2, 0.1 mmol/L EDTA), and the reaction was started with the addition of 150-mM epinephrine (0.05% acetic acid). SOD activity at 30 °C was measured by the kinetics of inhibition of epinephrine auto-oxidation inhibition for 1.5 min at 480 nm. The results were expressed in U/mg protein.
Thiobarbituric acid reactive species (TBARS)
Lipid peroxidation was quantified by the production of malondialdehyde (MDA) in reaction with thiobarbituric acid (TBA, C4H4N2O2S), according to the method of Draper and Hadley (1990). The reaction was developed by the addition of an aliquot of the homogenate of tissue (0.3 mg/mL) with trichloroacetic acid (30%, w/v) and tris–HCl (10 mM) at 30 °C, pH 7.4, followed by mixing and centrifugation at 2.500 xg for 10 min. Supernatant was transferred to another tube and added 0.8% TBA (w/v) following by incubated at 100 °C for 15 min. Upon cooling, the mixture was vortexed for 1 min, centrifuged for 15 min at 4000×g and the TBARS determined spectrophotometrically at 535 nm. Protein content was assessed using the Lowry assay (Sargent 1987), and the results were expressed as nmol/mg of protein.
Protein carbonyl content
This assay measures protein carbonyls, an indicator of protein oxidation, using 2,4-dinitrophenylhydrazine (DNPH), as previously described Levine et al. (1990). Proteins were precipitated by the addition of an aliquot of the homogenate (0.3 mg/mL) mixed with trichloroacetic acid (TCA, CCl3COOH, 30% (w/v)), and the mix was then centrifuged for 14 min at 1180×g. The pellet was re-suspended in 10-mM DNPH and immediately incubated under agitation for 15 min in a dark room. Thereafter, the samples were centrifuged and washed thrice with ethyl/acetate buffer. The final pellet re-suspended in 6-M guanidine hydrochloride was incubated for 30 min in a water bath (37 °C), and the carbonyl content was determined by measuring the absorbance at 370 nm using a molar absorption coefficient of 22 × 103 M−1 cm−1 22,000 M−1 cm−1. Equal amounts of protein samples without DNPH were used as controls. Values were expressed as nmol (mg protein)−1, and total protein content was measured using the Lowry assay (Sargent 1987).
Total sulfhydryls groups
The analysis of total sulfhydryls groups was determined using Ellman’s reagent, 5,5′-dithio-bis-(2-nitrobenzoic acid) ((DTNB, which in turn become oxidized (disulfide), generating a yellow derivative). The aliquot of the homogenate (300 µg of protein) was incubated phosphate buffer saline (PBS, pH 7.4) containing 50-mM tris base; 1-mM EDTA; 2-mM PMSF, 10-mM sodium orthovanadate, followed by incubation with 10-mM 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) for 30 min at room temperature under protected from light. The samples were measured at 412 nm as described by Ellman (1959).
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Differences between groups were determined using ANOVA followed by the Tukey test, and the D’Agostino and Pearson tests were used to test for normality. Analysis was performed using GraphPad PRISM® 4.03 software, and values of p < 0.05 were considered statistically significant.
Results
Effect of NAC and/or PZQ on S. mansoni
Administration of NAC from the first to the 60th day after infection reduced the adult worm burden by 31.31% and the number of female worms by 26.25%. The number of eggs per gram of hepatic tissue decreased 42.67% in the NAC group, 73.84% in the PZQ group and 81.81% in the NAC+PZQ group (Table 1). In the NAC and control groups, intestinal tissue samples contained eggs in mature and immature phases. However, treatment with NAC increased the number of dead eggs. The dose of PZQ achieved 100% therapeutic efficacy, since no worms were recovered, and intestinal tissue samples showed fewer mature eggs, an absence of immature eggs and increased numbers of dead eggs (Table 1).
Table 1.
Effects of N-acetyl-L-cysteine (NAC) and/or praziquantel (PZQ) on worm burden, number of eggs/g in hepatic tissue and pattern of oviposition in mice infected with S. mansoni
| Experimental groups | Worm burden | Number of eggs/g tissue | % Of eggs per developmental stage | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Reduction (%) | Female | Reduction (%) | Hepatic × 103 | Reduction (%) | Immature | Mature | Dead | |
| Control | 24.5 ± 4.97 | – | 10.17 ± 2.31 | – | 10.17 ± 2.22 | – | 56.1 ± 1.73 | 36.4 ± 1.95 | 7.5 ± 0.85 |
| NAC | 16.83 ± 3.54* | 31.31 | 7.5 ± 1.97* | 26.25 | 5.83 ± 1.47** | 42.67 | 48.7 ± 2.1 | 40.9 ± 1.52 | 10.4 ± 2.0* |
| PZQ | 0.0 ± 0.0** | 100.0 | 0.0 ± 0.0** | 100.0 | 2.66 ± 1.03** | 73.84 | 0.0 ± 0.0 | 13.7 ± 1.33* | 86.30 ± 1.33* |
| NAC + PZQ | 0.0 ± 0.0** | 100.0 | 0.0 ± 0.0** | 100.0 | 1.85 ± 0.89** | 81.81 | 0.0 ± 0.0 | 8.50 ± 2.76* | 91.5 ± 2.76* |
NAC (200 mg/kg/day from the first until the 60th day after infection), PZQ (100 mg/kg/day from the 45th to the 49th day after infection)
All animals were euthanized in 61st day after beginning of the experiment. Values expressed as average ± pattern deviation of eight mice
*P < 0.01 significant difference from the control group
**P < 0.001 significant difference in the control group
Effect of NAC and/or PZQ on the body weight, hepatomegaly and schistosomotic granuloma
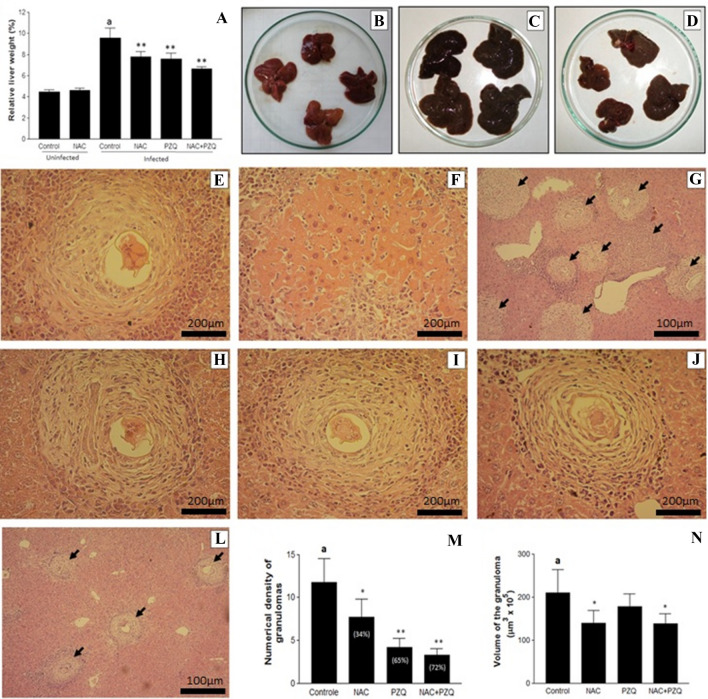
Uninfected animals treated with NAC or free of therapeutic intervention (control) showed similarity (p > 0.05) in total body weight, with values of 43.10 ± 1.62 g and 42.67 ± 2.20 g and in liver weight of 1.93 ± 0.03 g and 1.91 ± 0.02 g, respectively. Both groups also showed no difference in the relative percentage of liver/body weight (Fig. 2A and B) and exhibited histological sections of hepatic tissue within the anatomical norms, free of congestion, hemorrhage, vacuolar degeneration of hepatocytes, focal necrosis and leukocyte infiltration.
Fig. 2.
A–N Effects of N-acetyl-L-cysteine (NAC, 200 mg/kg/day from the first until the 60th day after infection) and/or praziquantel (PZQ, 100 mg/kg/day from the 45th to the 49th day after infection) in uninfected mice and those infected by S. mansoni. A Relative percentage of liver weight. B, C and D Liver of uninfected animals treated with NAC, infected animals free of NAC and/or PZQ and infected animals treated with NAC+PZQ, respectively. E–L Photomicrographs of hepatic tissue of mice infected with S. mansoni. E (×400) and G (×100) (group control) and H (×400) (treated with NAC), showing intense infiltration of eosinophils and polymorphonuclear neutrophils. F (×400), central coagulative necrosis in the infected control group. I (×400) (treated with PZQ) and J (×400) and L (×100) (treated with NAC+PZQ) peripheral infiltration of eosinophils and polymorphonuclear neutrophils, few epithelioid histiocytes and fibroblasts surrounding the granuloma, most evident findings in the association of drugs. M and N numerical density and volume of granulomas, respectively. Results are expressed as mean of eight animals per group ± SD. aP < 0.001 difference between uninfected control group and infected control group. *P < 0.01 difference between infected control group and infected control group NAC, PZQ and NAC+PZQ. **P < 0.001 difference between infected control group and infected control group NAC, PZQ and NAC+PZQ
The treatment of infected animals with NAC+PQZ preserved the total body weight when compared to uninfected animals free of therapeutic intervention (42.41 ± 2.03 g vs 43.10 ± 1.62 g, respectively), more showed significant difference (p < 0.001) when compared to the infected control group (39.20 ± 1.54 g). In contrast, the total body weight of infected animals treated with NAC (37.83 ± 2.21 g) or PZQ (41.60 ± 2.72 g) showed no significant difference when compared to control infected. In addition, treatment with NAC (2.75 ± 0.11 g), PZQ (2.67 ± 0.05 g) or NAC+PZQ (2.51 ± 0.06 g) significantly reduced (p < 0.0001) the liver weight when compared to control infected (3.48 ± 0.12 g).
Infected groups treated with NAC and/or PZQ showed significant reduction in relative percentage of liver weight (Fig. 2A, C and D). The sections of infected mice liver, control and NAC, revealed a typical inflammatory granulomatous reaction in the hepatic parenchyma and medium and large portal tract with intense infiltration of eosinophils, polymorphonuclear neutrophils, in addition to a few macrophages and lymphocytes. Collagen fibers surrounding the degenerating egg were scarce, thin and irregular (Fig. 2E and H). On the other hand, focal areas of central coagulative necrosis, as well as cytoplasmic vacuolation and degeneration of hepatocytes, were found in infected animals free of NAC and/or PZQ (Fig. 2F). By contrast, the PZQ and NAC+PZQ groups exhibited inflammatory granulomatous reaction with less peripheral infiltration of eosinophils and polymorphonuclear neutrophils, in addition to a few epithelioid histiocytes and fibroblasts surrounding the granuloma, as is commonly found when drugs are used (Fig. 2I and J).
The density of granuloma in the infected groups, for both those untreated and those treated with NAC+PZQ, is shown in Fig. 2G and L. There was a reduction of 34.38%, 64.76% and 72.08% in the average number of hepatic granulomas in the NAC, PZQ and NAC+PZQ groups, respectively, compared to the infected control (Fig. 2M). The results also show NAC-dependent modulation reducing hepatic granuloma volume by ~ 40%, in the NAC and NAC+PZQ groups (Fig. 2N and E compared to H and J).
Serum markers of liver function and oxy-redox imbalance
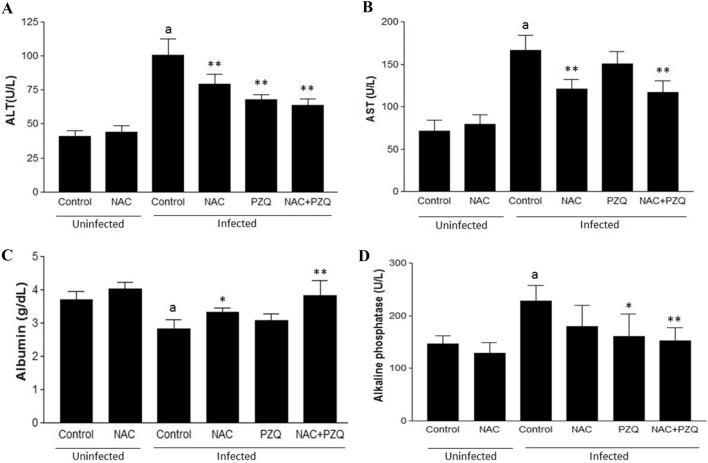
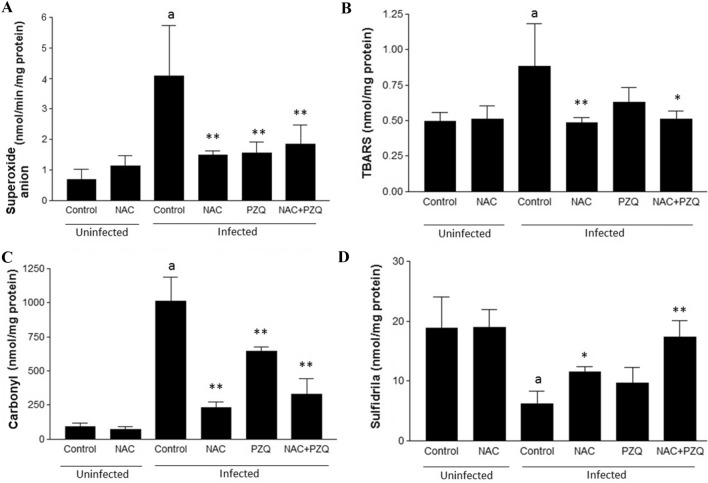
Figures 3 and 4 show the effect of experimental infection by S. mansoni on the serum markers of liver function and oxidative stress, respectively, and reveals favorable modulation of these markers for treatment with NAC and/or PZQ. Treatment with NAC and/or PZQ reduced levels of ALT, AST and ALP and increased ALB, compared to the infected control (Fig. 3A–D). NAC was able to reduce levels of the superoxide anion (67.66%), lipid peroxidation (44.84%) and protein carbonyls (76.99%) and increase that of sulfhydryl groups (46.61%), compared to infected control (Fig. 4A–D). During infection, treatment with NAC was similar to treatment with PZQ or NAC combined with PZQ (NAC + PZQ) in significantly reducing (p < 0.001) levels of superoxide anion and protein carbonyl, when compared to the infected control group (Fig. 4A and C). Treatment with PZQ was not significant in modulating of the lipid peroxidation and sulfhydryl groups, and NAC+PZQ was able to restore levels of sulfhydryl groups, when compared to the infected control group (Fig. 4D).
Fig. 3.
A–D Effects of N-acetyl-L-cysteine (NAC, 200 mg/kg/day from the first until the 60th day after infection) and/or praziquantel (PZQ, 100 mg/kg/day from the 45th to the 49th day after infection) on A Alanine aminotransferase (ALT), B aspartate aminotransferase (AST), C Albumin and D Alkaline phosphatase, in uninfected mice or those infected by S. mansoni. All animals were euthanized on the 61st day of the experiment. Results are expressed as means for eight animals per group ± SD. aP < 0.001 difference between uninfected control group and infected control group. *P < 0.01 difference between infected control group and infected control group NAC, PZQ and NAC + PZQ. **P < 0.001 difference between infected control group and infected control group NAC, PZQ and NAC+PZQ
Fig. 4.
A–D Effects of N-acetyl-L-cysteine (NAC, 200 mg/kg/day from the first until the 60th day after infection) and/or praziquantel (PZQ, 100 mg/kg/day from the 45th to the 49th day after infection) on A superoxide anion, B lipid peroxidation, C protein carbonyls and D sulfhydryl groups, in uninfected mice and those infected by S. mansoni. All animals were euthanized on the 61st day of the experiment. Results are expressed as means of eight animals per group ± SD. aP < 0.001 difference between uninfected control group and infected control group. *P < 0.01 difference between infected control group and infected control group NAC, PZQ and NAC+PZQ. **P < 0.001 difference between infected control group and infected control group NAC, PZQ and NAC+PZQ
Discussion
In addition to provide a cure, it is desirable that schistosomicidal drugs favorably modulate pathophysiological changes caused by the parasite. Redox imbalance is thus an important target, since it plays a key role in the pathogenesis of S. mansoni infection. In the present study, treatment with PZQ achieved 100% schistosomicidal effect. This eliminated the parasite load and the accumulation of eggs and antigens and established a pathophysiological model of already installed granulomas, as a way of measuring the antioxidant effect of NAC and/or as a drug adjuvant to PZQ. The results show that NAC, besides changing parasitological patterns, brought about a reduction in hepatic morbidity by reducing granulomatous lesions and restoring the redox balance in experimental acute schistosomiasis.
PZQ acts on Ca2+ homeostasis in the integument and is responsible for causing spasms, damage to the integument, and muscular paralysis, leading to the death of adult worms, since PZQ is less active against the juvenile stages of Schistosoma spp. (El-Lakkany et al. 2001; Dkhil et al. 2015; Araújo et al. 2019; 2020a; b). On the other hand, positive changes in parasitological parameters related to NAC have been documented in studies where the intervention was performed during immature phases of the worm (Seif El-Din 2006; Seif El-Din et al. 2010; Aires et al. 2012). In fact, even with chronic administration initiated after complete development of the adult worm, NAC showed no effect on the parasite load (Aires et al. 2012). However, when the intervention is started in the third week after infection (young worm stage), the parasite load was reduced by 22.7% (Seif El-Din et al. 2011). In prophylactic use, one week before cercarial exposure, NAC reduced the parasite load by up to 76%, as well as reducing the egg load and granuloma in liver tissue and increasing the percentage of eggs killed in intestinal tissue (Seif El-Din 2006; Seif El-Din et al. 2010). The data from the present study showed a reduction of 31.31% in the parasite burden.
So far, the mechanism by which NAC exerts its schistosomicidal effect is unclear. However, additional in vitro and in vivo studies are needed to clarify its mode of action against S. mansoni, including research against different stages of development, study of ultrastructural morphological changes by scanning electron microscopy and transmission and molecular evaluation, including proteomic analysis. However, in terms of immunity, the schistosome is most susceptible to immune elimination during migration and development in the skin and lungs (Mei and LoVerde 1997; Kwatia et al. 2000). Seif El-Din (2006; Seif El-Din et al. 2011) suggest that the use of NAC during S. mansoni infection can inactivate or reduce the activity of the schistosome’s peroxidase glutathione (SmGPx), which is a key enzyme in the worm’s survival mechanism. SmGPx protects S. mansoni by catalyzing the reduction in hydrogen peroxide (H2O2), protecting against damage to the membranes and DNA by reducing lipid peroxidation (Kwatia et al. 2000). Immunolocalization studies have demonstrated that SmGPX is localized in the tegument (host-parasite interface) of the adult but not in the immature stages (Mei and LoVerde 1997), and mice infected with S. mansoni and immunized with cytosolic superoxide dismutase (SmCT-SOD) and SmGPX have shown a reduction in the burden of worms. Besides antiparasitic drug candidates and vaccines, antioxidants are also being studied for the treatment of schistosomiasis (Simeonov et al. 2008; Huang et al. 2012; Carvalho-Queiroz et al. 2015).
Despite different mechanisms of action, NAC+PZQ brought about a reduction in the egg load in the hepatic tissue and increased the number of dead eggs in intestinal tissue. Yepes et al. (2015) highlight the importance of studies comparing drugs with different mechanisms of action on different stages of development of Schistosoma.
Some antioxidants have shown potential hepatoprotective properties in infection by S. mansoni. These include β-carotene (Seif El-Din 2006), curcumin (Allam 2009), pentoxifylline (El-Lakkany et al. 2001), artemether (Abdul-Ghani et al. 2011), silymarin (Mata-Santos et al. 2014) and resveratrol (Soliman et al. 2017). Here, NAC plus PZQ reduces the burden of eggs and the volume of granuloma in hepatic tissue, thus decreasing the number and the extent of granulomatous lesions and the development of hepatic fibrosis throughout the infection. Additionally, this therapeutic scheme produces more circumscribed granulomatous reactions, with a smaller population of inflammatory cells. Fan et al. (2007) reported that NAC favorably modulates the hepatic pathogenesis induced by S. japonica. In this study model, NAC decreases levels of NO (nitric oxide) and iNOS (nitric oxide synthase) and increases levels of GSH (glutathione). In S. mansoni infection, NAC reduces levels of IFN-γ, NO and peroxynitrite and increases IL-10, a combination of events associated with reduction in size of granuloma and decreased schistosomiasis mortality (Seif El-Din 2006; Herbert et al. 2008; Aires et al. 2012; Yepes et al. 2015).
Here, treatment with NAC and/or PZQ improves/preserves total body weight and reduces liver weight. Hepatomegaly was reduced by treatment with NAC and/or PZQ (Fig. 2A–D). Besides the favorable immune modulation, experimental studies involving hepatic diseases suggest that this reduction may be attributed to the antifibrotic activity of NAC (Pereira-Filho et al. 2008; Aires et al. 2012; Galicia-Moreno et al. 2012; Mata-Santos et al. 2014; Yepes et al. 2015), and NAC also improves hepatic congestion (Lee et al. 2012; Takhtfooladi et al. 2014) and portal hypertension (Licks et al. 2014).
As occurred in the present study, NAC is able to modulate favorably the serological markers of hepatic damage (ALT, AST, ALP and ALB) in hepatotoxicity induced by CCL4 (Cai et al. 2015), non-alcoholic fatty liver (El-Lakkany et al. 2016) and liver ischemia–reperfusion injury after 30% hepatectomy (Lee et al. 2012). S. mansoni infection can cause imbalance of oxidative factors by different mechanisms, including the deposition and accumulation of eggs in tissues, changes in vascular tone and soluble immune intermediates (Licks et al. 2014; Rushworth and Megson 2014).
In accordance, it reported change in oxidative markers of the superoxide anion, lipid peroxidation, protein carbonyls and sulfhydryl groups representing excessive production of oxidants. However, NAC decreased levels of the superoxide anion, TBARS and protein carbonyls and recovered the contents of sulfhydryl groups (Fig. 4). According to Seif El-Din (2006; Seif El-Din et al. 2011) and Fan et al. (2007, 2011) in schistosomiasis mansoni and schistosomiasis japonica, respectively, NAC-dependent reduction in the levels of superoxide anion can be attributed to the drug’s capacity to increase synthesis of superoxide dismutase (SOD), since SOD catalyzes the superoxide anion in H2O2. The H2O2 is converted to H2O and O2 by glutathione peroxidase and catalase. This reaction is important since H2O2 can react with fatty acids present in the cell membrane, increasing lipid peroxidation, commonly detected as TBARS and carbonyl groups, widely measured by thiobarbituric acid (TBA) reactive substances (TBARS) assay (Misra, and Fridovich 1972; Draper and Hadley 1990).
The production of TBARS and carbonyl groups is clearly related to fibrogenesis and collagen deposition in hepatic cells. Oliveira et al. (2013) emphasize that the increase in protein carbonyl is an important biomarker of oxidative damage in S. mansoni infection. Dalle-Donne et al. (2003) showed that protein carbonyl measurement has advantages as a biomarker of protein oxidation, because of rapid formation. Furthermore, it is known that highly sensitive assays for detection of protein carbonyls involve derivatization of the carbonyl group with 2,4-dinitrophenylhydrazine (DNPH) which results in the formation of 2,4-dinitrophenyl (DNP) hydrazine, a product resulting from its covalent reaction that has been widely used as an oxidative marker of proteins in numerous human pathologies. Corroborating with the method applied and established by Levine et al. (1990) in the determination of carbonyl content in oxidatively modified proteins.
Here, NAC was able to restore TBARS levels since its supplementation increases the synthesis of GSH through donation of the thiol grouping of cysteine found in the NAC molecule. This also suggests an increase in total thiol, as measured by sulfuric proteins. Rushworth and Megson (2014) emphasize that increasing intracellular thiol levels by the direct administration of GSH is not recommended, since GSH does not easily pass through the cell. Furthermore, direct administration of cysteine is not possible in clinical settings because of its limited bioavailability, toxicity and insolubility (Cacciatore et al. 2010). Therefore, there is a need for alternatives for inducing synthesis of glutathione, such as the supply of the precursor amino acid contained in NAC.
Conclusion
Co-administration of NAC provides protection in acute S. mansoni infection by reducing the parasitological parameters, limiting histopathological alterations and granulomatous inflammation and improving oxidative damage and serum markers of liver function. NAC is thus a promising adjuvant in treatment of this infection. Healthy mice and treated with NAC do not show changes in all the parameters investigated here.
Acknowledgements
This work received financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES—Grant Nº.001) and Fundação de Amparo a Ciência e Tecnologia do Estado de Pernambuco (FACEPE). H.D.A. Araújo thanks FACEPE for the Researcher Fixation Scholarship (BFP-0080-2.08/20).
Author contributions
AdLA and MCPdAA: Albuquerque designed the study protocol. AdLA, HDAdA, AMG, SBdA, RLdS, ZPdA, MBdSM, VMOS and MCPdAA: carried out the assays and/or were involved in the analysis and interpretation of all data. AdLA, HDAdA, VMOS and MCPdAA: contributed to drafting the manuscript and/or critically revising the paper and intellectual content. All authors read and approved the final manuscript.
Data availability
Data will be made available upon request.
Declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Abdallahi OM, Hanna S, De Reggi M, Gharib B. Visualization of oxygen radical production in mouse liver in response to infection with Schistosoma mansoni. Liver Int. 1999;19:495–500. doi: 10.1111/j.1478-3231.1999.tb00082.x. [DOI] [PubMed] [Google Scholar]
- Abdul-Ghani R, Loutfy N, Sheta M, Hassan A. Artemether shows promising female schistosomicidal and ovicidal effects on the Egyptian strain of Schistosoma mansoni after maturity of infection. Parasitol Res. 2011;108:1199–1205. doi: 10.1007/s00436-010-2163-9. [DOI] [PubMed] [Google Scholar]
- Aires AL, Albuquerque MCPA, Silva RA, Schirato GV, Pontes Filho NT, Araújo SB, Souza VM, Costa VM, Malagueño E. Immunohistopathological changes in murine Schistosomiasis mansoni under the influence of N-acetyl-L-cysteine. Parasitol Res. 2012;111:1569–1578. doi: 10.1007/s00436-012-2997-4. [DOI] [PubMed] [Google Scholar]
- Allam G. Immunomodulatory effects of curcumin treatment on murine schistosomiasis mansoni. Immunobiology. 2009;214:712–727. doi: 10.1016/j.imbio.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Araújo HDA, Aires AL, Soares CLR, Brito TGS, Nascimento WM, Martins MCB, Silva TG, Brayner FA, Alves LC, Silva NH, Albuquerque MCPA, Lima VLM. Usnic acid potassium salt from Cladonia substellata (Lichen): synthesis, cytotoxicity and in vitro anthelmintic activity and ultrastructural analysis against adult worms of Schistosoma mansoni. Acta Trop. 2019;192:1–10. doi: 10.1016/j.actatropica.2018.12.024. [DOI] [PubMed] [Google Scholar]
- Araújo HDA, Santos VHB, Brayner FA, Alves LC, Silva NH, Albuquerque MCPA, Aires AL, Lima VLM. In vitro activity of usnic acid potassium salt against different developmental stages of Schistosoma mansoni: an ultrastructural study. Acta Trop. 2020;201:1–11. doi: 10.1016/j.actatropica.2019.105159. [DOI] [PubMed] [Google Scholar]
- Araújo HDA, Silva NH, Albuquerque MCPA, Aires AL, Lima VLM. Potassium usnate, a water-soluble usnic acid salt, shows enhanced activity against Schistosoma mansoni in vitro. Exp Parasitol. 2020;208:1–5. doi: 10.1016/j.exppara.2019.107779. [DOI] [PubMed] [Google Scholar]
- Cacciatore I, Cornacchia C, Pinnen F, Mollica A, Di Stefano A. Prodrug approach for increasing cellular glutathione levels. Molecules. 2010;15:1242–1264. doi: 10.3390/molecules15031242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Lou Q, Wang F, Li E, Sun J, Fang H, Xi J, Ju L. N-acetylcysteine protects against liver injure induced by carbon tetrachloride via activation of the Nrf2/HO-1 pathway. Int J Clin Exp Pathol. 2015;8:8655–8662. [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Queiroz C, Nyakundi R, Ogongo P, Rikoi H, Egilmez NK, Farah IO, Kariuki TM, LoVerde PT. Protective potential of antioxidant enzymes as vaccines for schistosomiasis in a non-human primate model. Front Immunol. 2015;2:1–16. doi: 10.3389/fimmu.2015.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever AW. Conditions affecting the accuracy of potassium hydroxidedigestion techniques for counting Schistosoma mansoni eggs in tissues. Bull World Health Organ. 1968;39:328–331. [PMC free article] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Dkhil MA, Bauomy AA, Diab MS, Al-Quraishy S. Antioxidant and hepatoprotective role of gold nanoparticles against murine hepatic schistosomiasis. Int J Nanomedicine. 2015;16:7467–7475. doi: 10.2147/IJN.S97622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- El-Lakkany NM, El-Din SH, Sabra AA, Hammam OA. Pharmacodynamics of mefloquine and praziquantel combination therapy in mice harbouring juvenile and adult Schistosoma mansoni. Mem Inst Oswaldo Cruz. 2001;106:814–822. doi: 10.1590/S0074-02762011000700006. [DOI] [PubMed] [Google Scholar]
- El-Lakkany NM, Seif El-Din SH, Sabra AA, Hammam OA, Ebeid FA. Co-administration of metformin and N-acetylcysteine with dietary control improves the biochemical and histological manifestations in rats with non-alcoholic fatty liver. Res Pharm Sci. 2016;11:374–382. doi: 10.4103/1735-5362.192487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fan ZG, Zhang LM, Li KJ, Li W, Zhu PX, Yang G. Effect of N-acetylcysteine on the egg granuloma in hepatic tissue of mice with schistosomiasis japonica. Chin J Parasitol Parasit Dis. 2007;25:137–140. [PubMed] [Google Scholar]
- Fan ZG, Li KJ, Zhang LM. Effect of N-acetylcysteine on malondialdehyde and superoxide dismutase in hepatic tissue of mice with Schistosomiasis japonica. Chin J Parasitol Parasit Dis. 2011;29:457–460. [PubMed] [Google Scholar]
- Galicia-Moreno M, Favari L, Muriel P. Antifibrotic and antioxidant effects of N-acetylcysteine in an experimental cholestatic model. Eur J Gastroenterol Hepatol. 2012;24:179–185. doi: 10.1097/MEG.0b013e32834f3123. [DOI] [PubMed] [Google Scholar]
- Herbert DR, Orekov T, Perkins C, Finkelman FD. IL-10 and TGF-β redundantly protect against severe liver injury and mortality during acute schistosomiasis. J Immunol. 2008;181:7214–7220. doi: 10.4049/jimmunol.181.10.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HH, Rigouin C, Williams DL. The redox biology of schistosome parasites applications for drug development. Curr Pharm Des. 2012;18:3595–3611. doi: 10.2174/138161212801327220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AL, Bangash IH, Bouchier IA, Hayes PC. Portal and systemic haemodynamic action of N-acetylcysteine in patients with stable cirrhosis. Gut. 1994;35:1290–1293. doi: 10.1136/gut.35.9.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwatia MA, Botkin DJ, Williams DL. Molecular and enzymatic characterization of Schistosoma mansoni thioredoxin peroxidase. J Parasitol. 2000;86:908–915. doi: 10.1645/0022-3395(2000)086[0908:MAECOS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Silva SM, Simões MJ, Montero EF. Effect of N-acetylcysteine in liver ischemia-reperfusion injury after 30% hepatectomy in mice. Acta Cir Bras. 2012;27:346–349. doi: 10.1590/s0102-86502012000400011. [DOI] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Licks F, Marques C, Zetler C, Martins MI, Marroni CA, Marroni NP. Antioxidant effect of N-acetylcysteine on prehepatic portal hypertensive gastropathy in rats. Ann Hepatol. 2014;13:370–377. doi: 10.1016/S1665-2681(19)30867-1. [DOI] [PubMed] [Google Scholar]
- Mata-Santos HA, Dutra FF, Rocha CC, Lino FG, Xavier FR, Chinalia LA, Hossy BH, Castelo-Branco MT, Teodoro AJ, Paiva CN, Pyrrho AS. Silymarin reduces profibrogenic cytokines and reverses hepatic fibrosis in chronic murine schistosomiasis. Antimicrob Agents Chemother. 2014;58:2076–2083. doi: 10.1128/AAC.01936-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei H, LoVerde PT. Schistosoma mansoni: the developmental regulation and immunolocalization of antioxidant enzymes. Exp Parasitol. 1997;86:69–78. doi: 10.1006/expr.1997.4150. [DOI] [PubMed] [Google Scholar]
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. doi: 10.1016/S0021-9258(19)45228-9. [DOI] [PubMed] [Google Scholar]
- Ntamo Y, Ziqubu K, Chellan N, Nkambule BB, Nyambuya TM, Mazibuko-Mbeje SE, Gabuza KB, Marcheggiani F, Tiano L, Dludla PV. Drug-induced liver injury: clinical evidence of N-acetyl cysteine protective effects. Oxid Med Cell Longev. 2021;2021:1–12. doi: 10.1155/2021/3320325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RB, Senger MR, Vasques LM, Gasparotto J, Santos JP, Pasquali MA, Moreira JC, Silva FP, Jr, Gelain DP. Schistosoma mansoni infection causes oxidative stress and alters receptor for advanced glycation endproduct (RAGE) and tau levels in multiple organs in mice. Int J Parasitol. 2013;43:371–379. doi: 10.1016/j.ijpara.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Olivier L, Stirewalt MA. An efficient method for exposure of mice to cercariae of Schistosoma mansoni. J Parasitol. 1952;38:19–23. doi: 10.2307/3274166. [DOI] [PubMed] [Google Scholar]
- Pellegrino J, Oliveira CA, Faria J, Cunha AS. New approach to the screeningof drugs in experimental Schistosomiasis mansoni in mice. Am J Trop Med Hyg. 1962;11:201–215. doi: 10.4269/ajtmh.1962.11.201. [DOI] [PubMed] [Google Scholar]
- Pereira-Filho G, Ferreira C, Schwengber A, Marroni C, Zettler C, Marroni N. Role of N-acetylcysteine on fibrosis and oxidative stress in cirrhotic rats. Arq Gastroenterol. 2008;45:156–162. doi: 10.1590/s0004-28032008000200013. [DOI] [PubMed] [Google Scholar]
- Rushworth GF, Megson IL. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther. 2014;141:150–159. doi: 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Sargent MG. Fiftyfold amplification of the Lowry protein assay. Anal Bioch. 1987;163:476–481. doi: 10.1016/0003-2697(87)90251-x. [DOI] [PubMed] [Google Scholar]
- Seif El-Din SH. Protective effects of β-carotene; N-acetyl-L-cysteine with and without praziquantel treatment in Schistosoma mansoni-infected mice. Egypt J Schistosomiasis Infect Endem Dis. 2006;28:67–90. [Google Scholar]
- Seif El-Din SH, El-Lakkany NM, Hagag HA, Ebeid FA. Role of B-carotene and N-acetyl-L-cysteine with and without praziquantel treatment in modulating Schistosoma mansoni-induced genotoxic effects on albino mice. Res J Medicine and Med Sci. 2010;5:8–17. [Google Scholar]
- Seif El-Din SH, Al-Hroob AM, Ebeid FA. Schistosoma mansoni: N-acetylcysteine downregulates oxidative stress and enhances the antischistosomal activity of artemether in mice. Exp Parasitol. 2011;128:230–235. doi: 10.1016/j.exppara.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Simeonov A, Jadhav A, Sayed AA, Wang Y, Nelson ME, Thomas CJ, Inglese J, Williams DL, Austin CP. Quantitative high throughput screen identifies inhibitors of the Schistosoma mansoni redox cascade. PLoS Negl Trop Dis. 2008;2:1–10. doi: 10.1371/journal.pntd.0000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithers SR, Terry RJ. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965;55:695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- Soliman RH, Ismail OA, Badr MS, Nasr SM. Resveratrol ameliorates oxidative stress and organ dysfunction in Schistosoma mansoni infectedmice. Exp Parasitol. 2017;174:52–58. doi: 10.1016/j.exppara.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Takhtfooladi MA, Jahanshahi G, Jahanshahi A, Sotoudeh A, Amlashi OS, Allahverdi A. Effects of N-acetylcysteine on liver remote injury after skeletal muscle ischemia reperfusion in rats. Turk J Gastroenterol. 2014;25:43–47. doi: 10.5152/tjg.2014.6008. [DOI] [PubMed] [Google Scholar]
- Tendler M, Pinto RM, Lima AO, Gebara G, Katz N. Schistosoma mansoni: vaccination with adult worm antigen. Int J Parasitol. 1968;16:347–352. doi: 10.1016/0020-7519(86)90113-x. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2023) Schistosomiasis. Fact sheet detail. http://www.who.int/news-room/fact-sheets/detail/schistosomiasis. Accessed 7 Feb 2023
- Yepes E, Varela-M RE, López-Abán J, Rojas-Caraballo J, Muro A, Mollinedo F. Inhibition of granulomatous inflammation and prophylactic treatment of schistosomiasis with a combination of edelfosine and praziquantel. PLoS Negl Trop Dis. 2015;9:1–22. doi: 10.1371/journal.pntd.0003893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon request.