Abstract
The use of light-emitting diode (LED) technology represents a promising approach to improve plant growth and metabolic activities. The aim of this study was to investigate the effect of different light spectra: red (656 nm), blue (450 nm), red/blue (3:1), and white (peak at 449 nm) on biochemical properties, photosynthesis and gene expression in two lettuce cultivars (Lollo Rossa and Lollo Bionda) grown under different methods of nutrient solution replacement in hydroponics. Complete replacement and EC-based replacement of nutrient solution increased content of proline and soluble sugars and activity of antioxidant enzymes (CAT, GPX and SOD) under the red/blue LED and red LED light treatments in both cultivars. In addition, the red/blue and the monochromatic red light increased the soluble protein content and the antioxidant activity in the Lollo Rosa cultivar under the replacement method according to the needs of the plant. An increase in flavonoid content in the EC-based method in the Lollo Rosa variety treated with a combination of red and blue light was also observed. The red/blue light had the greatest induction effect on anthocyanin content, expression of the UFGT, CHS, and Rubisco small subunit genes, and the net photosynthetic rate. Data presented here will directly contribute to the development of nutrient solution and LED spectrum management strategies to significantly improve plant growth and metabolism, while avoiding water and nutrient waste, and environmental pollution.
Subject terms: Biochemistry, Physiology, Plant sciences
Introduction
Today, water is a basic human need, and a valuable natural resource1. Agriculture, as the largest consumer of water, is the most affected during drought disasters2. In this situation, scientists are looking for solutions to reduce water consumption in agriculture. One of them is hydroponic cultivation of crops, and the floating raft system is one of the hydroponic systems for the production of vegetable crops, especially lettuce. In addition to better water use efficiency, hydroponic systems, when operated in a controlled environment, can provide high quality and quantity vegetable production3.
However, limited hydroponic cultivation in some countries is due to several reasons, including the high costs of initial tests to determine the amount of elements, EC, pH, the need for specialised labour, and new products and appropriate techniques to control pests and diseases4. Maintaining the correct nutrient ratio and electrical conductivity of the nutrient solution at different growth stages is essential for achieving high quality production. The rate of absorption of different ions by the root system is not equal, and elements such as ammonium, nitrate and potassium are absorbed rapidly, while calcium, magnesium and sulphate ions are absorbed slowly and therefore accumulate in solution5,6. Some researchers recommend determining the concentration of each element during the period of nutrient solution use, and, depending on the amounts absorbed, adding a replenishment solution containing all or some of the elements depleted from the nutrient solution5. Ding et al.7 reported that too high and too low EC decreased the soluble sugar content of pakchoi plant (Brassica campestris L. ssp. Chinensis), while showing a significant increase in antioxidant enzyme activities. In another study, higher antioxidant content and antioxidant capacity were observed in lettuce even when a concentration of 1/4 of the nutrient solution was applied8.
Numerous horticultural crops have been grown in hydroponics in closed growth chambers equipped with novel light source such as light-emitting diodes (LED), referred to as Plant Factory with Artificial Light (PFAL)9,10. Manipulations in the LED light environment, such as establishing specific light modes by integrating spectral quality and intensity, can increase crop productivity. It has been reported that different red/blue LED light ratios result in a species-dependent response in growth parameters such as fresh and dry matter and leaf optical indices such as chlorophyll, flavonoid, anthocyanin and carotenoid content11. As pointed out by Rehman et al.12, irradiation with red light decreased the proline content and the activities of superoxide dismutase and peroxidase in ramie plant (Boehmeria nivea L), whereas blue light increased the activities of these enzymes. Recently, red LED light has been reported to increase total phenolic content and radical scavenging activity in Rubus hongnoensis, while plants treated with white LED light were characterized by higher superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX) activities13.
Anthocyanins belong to the flavonoids and play an important role in plant adaption to environmental stress14. Anthocyanins are synthesized in the endoplasmic reticulum and transported to accumulate in the vacuoles of a wide range of cells and tissues in both the vegetative and reproductive organs of plants15. The synthesis of anthocyanins in plants is controlled by structural genes and can be divided into four stages16,17, with CHS and UFGT playing a critical role in this metabolic pathway. Shoji et al.18 isolated the genes CHS, F3H, DFR, ANS and UFGT from red lettuce and showed that treatment of red lettuce with blue light spectrum enhanced anthocyanin biosynthesis, with the CHS and UFGT genes being most associated with anthocyanin accumulation.
Several traits affecting photosynthesis are influenced by light quality, with blue and red light playing the most important role19. Hogewoning et al.20 reported that increasing blue light increased stomatal conductance (gs) and the photosynthetic rate (PN) in cucumber plants. The increase in photosynthesis under the influence of blue light treatment also include effects of a higher chlorophyll a/b ratio21 and a higher content of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco)22. Although reducing blue light during the growth phase of lettuce decreased the photosynthetic rate more than red light, applying more than 10% blue light to lettuce plants increased the photosynthetic rate23. Roni et al.24 found that Eustoma plants grown under blue light had higher PN, gs and the transpiration rate (E).
Here, an investigation into the effect of different LED spectra and different methods of nutrient solution replacement on biochemical properties, photosynthesis and expression of the Rubisco and anthocyanin biosynthesis pathway genes in two lettuce cultivars was described. The aim of the study was to determine which model of nutrient solution management best contributes to the improvement of these characteristics and allows the avoidance of stress imposed by excessive ion accumulation. The hypothesis was tested that the use of different light spectra may not only improve plant performance but also reduce the negative effect of stress. Most experiments reported so far are limited to the effect of some light spectra on plant growth and development, with fewer studies examining biochemical traits in plants.
Materials and methods
Plant material and growth conditions
This experiment was conducted in the plant experiment factory of Vali-e-Asr University in 2020. Seeds of two lettuce varieties (Lollo Rossa and Lollo Bionda) obtained from Sepahan Rooyesh Isfahan and Rijk Zwaan Co. were planted in a seed tray filled with perlite medium. After the four-leaf stage, the seedlings were planted in small plastic pots with perlite medium. These small pots were placed in the holes of the floating systems. After transferring the plants to the floating systems, the Resh nutrient solution formulated for lettuce25 (EC: 1.2 ds m−1, pH: 6.5) was used. The floating system consisted of 24 square plastic containers measuring 25 × 30 × 30 cm, each floating on a styrofoam measuring 25 × 30 × 30 cm, and four plants planted in each container. All culture containers were connected to the air pump (HAILA, model: ACO-388 D) through holes and the nutrient solution was continuously aerated. Then, 21-day-old seedlings were transferred into holes of Styrofoam. After transplanting, the seedlings were irrigated with a modified Resh nutrient solution containing: 5 mM KNO3, 5 mM Ca(NO3)2, 2 mM MgSO4, 1 mM KH2PO4, 7 μM MnCl2, 0.7 μM ZnSO4, 0.8 μM CuSO4, 0.8 μM Na2MoO4, 25 μM Fe-EDDHA, and 2 μM H3BO3. The nutrient solution (pH 6.7 ± 0.1, EC 2.1) was renewed every week. Deionised water was used for nutrient preparation. After reaching the four-leaf stage, the lettuce seedlings were supplied with nutrient solutions or the following 40 days, replaced in three different ways (complete replacement, partial replacement based on the EC value and partial replacement according to the needs of the plant).
In the complete replacement, the nutrient solution was replaced weekly. In the partial replacement according to EC, the EC value of the nutrient solution was adjusted to 1.2 dS m−1 by adding predetermined amounts of potassium sulphate, calcium nitrate, magnesium sulphate, potassium dihydrogen phosphate and the half-strength Resh micronutrient solution every 48 h. The replacement of the nutrient solution according to the needs of the plants based on previous reports on tomato26 and lettuce and spinach plants27 cultivated in a closed hydroponic system. In this method (developed by Roosta), potassium nitrate was used at the same concentration as in the original solution, but the addition of calcium nitrate, magnesium sulphate and potassium dihydrogen phosphate was reduced by three quarters and the microelements (iron, zinc, copper and manganese) were reduced by half and added every two days based on the volume of water added to the plastic containers with the plants. For example, if the lettuce plant absorbed one litre of the solution in two days, one litre of distilled water was first added to the plant container to compensate.
The amount of concentrated solution of different fertilisers needed for this volume was determined on the basis of the original formula, and concentrated solutions in the ratio given above were used instead of the complete solutions. The reason for this was to prevent the accumulation of elements such as magnesium, calcium, phosphorus, and even microelements in the solution and to avoid their toxicity to the plant, as their absorption rate is lower than that of potassium and nitrate. During the experiment, the concentrations of nitrogen (Kjeldahl), calcium and magnesium (by titration), potassium and sodium (flame photometer), phosphorus (spectrophotometer), iron, manganese, copper and zinc (atomic absorption)28 in the nutrient solution were determined weekly. Plants were grown at a fully controlled temperature of 25/15 (day/night) and a relative humidity of 50 ± 10%.
LED tubes and the light treatments
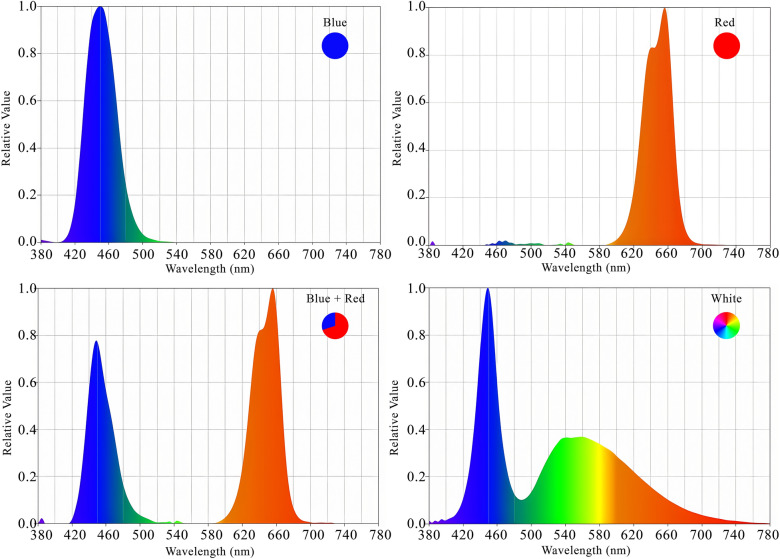
Lettuce plants were grown under 24 W LED lamps (Parto Roshd Novin Company, Iran) with different spectral ranges: red (R, with peak at 656 nm), red/blue (3:1; R:B, with peak 656 nm), blue (B, with peak at 450 nm) and white (W, with peak at 449 nm). The photosynthetic photon flux density (PPFD) was 215 ± 5 µmol m−2 s−1 in all treatments (Fig. 1 and Table 1). The photoperiod of 16/8 h (day/night) was maintained. The LED light systems were placed 30 cm above each individual plant.
Figure 1.
Relative distribution of different LED spectra (red, red/blue 3:1, blue and white) used during the experiment.
Table 1.
Characteristics of LEDs used in this experiment.
| Manufacture company | Efficiency | N. of LEDs | Light coverage area | Power consumption | Lens type | Input Voltage | DC Voltage | Output Current | Output Frequency |
|---|---|---|---|---|---|---|---|---|---|
| Iran Grow Light | 90% | 24 | 40 cm × 100 cm | 24 × 3W | 90 ° | AC100-260 V | 54–84 V | 600 mA ± 5% | 50/60 Hz |
Proline measurement
Ninhydrin reagent and the method developed by Paquin and Lechasseur29 were used to measure proline. Half a gram of fully developed leaves was homogenised in 5 mL of 95% ethanol in a Chinese mortar and the resulting solution was transferred into a Falcon tube. The solution was centrifuged at 3500 rpm for 10 min and the resulting supernatant was used for spectrophotometric (model T80 UV/VIS Spectrometer PG Instruments Ltd) determination of proline at a wavelength of 515 nm. Standards were prepared with L-proline at: 0, 31.25, 62.5, 125, 250 and 500 (mg L−1) concentrations.
Total soluble sugars
Soluble sugars were measured according to the method of Irigoyen et al.30. For this purpose, 0.1 mL of the extract prepared in ethanol (alcoholic extract for proline determination) was mixed with 3 mL of freshly prepared anthrone (200 mg anthrone in 100 mL 72% sulphuric acid) and then incubated in a hot water bath for 10 min. After cooling, the absorbance of the samples was measured at a wavelength of 625 nm.
Soluble proteins
The method of Bradford et al.31 was used to measure the proteins. 0.1 mL of protein extract and 5 mL of the Bradford reagent were added to the test tubes, vortexed briefly and the absorbance read at a wavelength of 595 nm using bovine serum albumin as a standard.
Determination of anthocyanins
The content of anthocyanins was measured according to the method of Nogués and Baker32. One gram of fresh leaf tissue was homogenised in 10 mL of acidic methanol and centrifuged at 3500 rpm. Absorbance was measured in the samples at 530 and 657 nm.
Total phenolic compounds
The method of Matta et al.33 was used to measure the total phenolic compound content. In this method, 0.1 g of leaf tissue was boiled in 10 mL of 80% ethanol and the sample was centrifuged at 3500 rpm. Finally, ithe content was determined with a spectrophotometer at a wavelength of 640 nm. The standard curve was prepared catechol.
DPPH radical scavenging activity
The measurement of radical scavenging activity in the plant tissue extracts was performed according to the method of Barros et al.34. The reduction of DPPH radicals was determined by measuring the absorbance at 517 nm. The radical scavenging activity was calculated as a percentage of DPPH discolouration using the following equation:
where A0 is the absorbance of the DPPH solution and A1 is the absorbance of the sample.
Antioxidant enzyme activity
Preparation of the extract
Half a gram of the fresh leaf tissue was homogenised in three to five millilitres of ice-cold 50 mM potassium phosphate sample buffer (pH 7.2). The homogenate was immediately centrifuged at 4000 rpm for 20–30 min, at four degrees Celsius. The resulting supernatant was collected and stored at − 20 °C until further analysis.
Peroxidase (POD) activity assay
The enzymatic activity of POD (EC 1.11.1.7) was determined by the oxidation rate of guaiacol to tetraguaiacol according to Plewa et al.35. In this method, three millilitres of the reaction mixture contained 2.77 mL of 50 mM potassium phosphate buffer (pH 7.0), 100 µL of 1% hydrogen peroxide, 100 µL of 4% guaiacol, and 30 µL of the enzyme extract. The POD activity was measured following the increase in absorbance at 470 nm for 3 min with an extinction coefficient of 25.5 mM cm−1.
Catalase (CAT) activity assay
CAT (EC 1.11.1.6) activity was measured as previously described by Dhindsa and Motowe36. The reaction mixture contained 10 mM H2O2 in 50 mM potassium phosphate buffer (pH 7.0) and 200 µL enzyme extract in a total volume of three millilitres. The decomposition of hydrogen peroxide was monitored spectrophotometrically by the decrease in absorbance at 240 nm for 3 min (extinction coefficient 28 mM cm−1) and expressed per mg of protein.
Superoxide dismutase (SOD) activity assay
The method of Blanchamp and Fridovitch37 was used to measure SOD (EC 1.15.1.1) activity. This measurement was based on the ability of the enzyme to stop the photochemical reduction of nitroblue tetrazolium (NBT) by superoxide radicals in the presence of riboflavin in the light. Fifty microlitres of the extract was mixed with one millilitre of the SOD reaction solution, containing 800 µL 50 mM potassium phosphate buffer (pH 7.8), 50 µL 75 μM NBT, 50 µL 13 mM L-methionine, 50 µL 0.1 mM EDTA, and 50 µL 2 μM riboflavin. The absorbance of thesamples was measured at a wavelength of 560 nm.
Leaf gas exchange
Photosynthetic parameters were measured using an Infra-Red Gas Analyser (LCi Ultra Compact, ADC BioScientific Ltd, Herts, UK). One fully expanded, intact leaf was selected from each replicate and placed in a leaf cuvette of the instrument. After 6 min to achieve steady-state conditions, net photosynthetic rate (PN; μmol m−2 mol−1), transpiration rate (E; mol H2O m−2 s−1), stomatal conductance (gs; mol m−2 s−1) and intercellular CO2 concentration (Ci; μmol CO2 mol−1) were recorded38.
Analysis of the Rubisco and anthocyanin pathway gene expression
Total RNA was extracted in triplicate from leaves four weeks after transplanting and treatment with LED spectra. Leaf samples were immediately frozen in liquid nitrogen and stored at − 80 °C until further use. Samples were ground in liquid nitrogen with a pestle and mortar and RNA was extracted using Column RNA isolation kit (DENAzist Asia, Ferdowsi University of Mashhad, Mashhad, Iran). The preparation of the stock and working solution of primers (oligonucleotides) was done according to the manufacturer’s guidelines (DENAzist Asia). cDNA was synthesised using the RevertAid first strand cDNA synthesis kit (Thermo Scientific™, Waltham, MA USA). Any DNA contamination was removed using DNaseI Digestion Set (Sigma-Aldrich). The purity and quantity of RNA was measured spectrophotometrically (Nanodrop™ Microvolume UV-Vis Spectrophotometer, Thermo Scientific™) and assessed by electrophoresis on a 1% (w/v) agarose gel using 200 ng of RNA. The A260/A280 and A260/A230 ratios measured in the samples were higher than 1.8. Real-time quantitative PCR (qRT-PCR, Thermo Fisher, USA) was performed to analyse the expression levels of the Rubisco small subunit gene (rbcS) and anthocyanin biosynthesis genes in the leaf tissue. Gene-specific primers were designed using Primer-BLAST (NCBI, https://www.ncbi.nlm.nih.gov/tools/primer-blast/) (Table 2). qRT-PCR was performed in triplicate using SYBR Green 2X Real-Time PCR MasterMix including SYBR Green 1, Low ROX (BioFACTTM, Korea). Actin (AB359898) was used as an internal control. The 2−ΔΔCT method was used to calculate expression levels as previously described39.
Table 2.
Specific primers selected for small subunit Rubisco gene and anthocyanin biosynthesis pathway genes by Real-time PCR analysis.
| Gene name | Accession number | Product length | Name | Primer sequence (5ʹ–3ʹ) |
|---|---|---|---|---|
| Rubisco | AF162210.1 | 835 bp | small subunit-For | TCTAACGTCGCTTTCCCAGT |
| small subunit-Rev | GTCGGACAATGGTGGTAGGT | |||
| Actin | AB359898 | 113 bp | LsACT-F01 | TGGTAGGTATGGGCCAGAAA |
| LsACT-R01 | GTCATCCCAGTTGCTCACAA | |||
| CHS | AB525909 | 169 bp | LsCHS-F02 | GGAGGTGGGGCTAACTTTTC |
| LsCHS-R02 | GAGCTCCACCTGGTCCAATA | |||
| UFGT | AB525911 | 203 bp | LsUFGT-F02 | AAGAGACCAGAACCCCGTTT |
| LsUFGT-R02 | AGCTCCAATGCTCTCCGATA |
Experimental design and data analysis
The experiment was a completely randomised design with three factors in three replicates in factorial form and two plants of each variety in a plastic container. SAS software version 9.4 was used to analyse all data (SA stitute, Cary, NC, USA). All data were statistically analysed using the two-way ANOVA. When analysis of variance indicated significant treatment effects, significant mean differences (P<0.05) were calculated with the Duncan Multiple Range Test. Range tests identify homogeneous subsets of means that do not differ from each other. A correlation plot was drawn with Origin Pro software version 2021. The graphs were made using Excel 2013 (Microsoft, Redmont, WA, USA). The results were expressed as mean values and their standard errors (SE) using MS Excel software.
Statement of compliance
The authors confirm that all the experimental research, including the collection of plant material, complied with relevant institutional, national, and international guidelines and legislation.
Results
Proline content
The results showed that the proline concentration in the lettuce leaves was affected by the variety, the light quality and the method used to replace the nutrient solution, as well as by the interaction of these factors (Table 3). The combination of red and blue LED light increased the proline content in both lettuce cultivars grown under the complete nutrient solution replacement and the method based on the EC value compared to the control treatment (white light spectra and complete nutrient solution replacement). The proline content was also elevated in Lollo rossa and Lollo Bionda in response to the red and red/blue spectra, respectively, fed according to the needs of the plants (Table 4).
Table 3.
The effect of different light qualities and three replacement methods of nutrient solution on physiological characteristics of two varieties of lettuce in floating hydroponics.
| Source of Variation | Df | Proline | Soluble sugar | Soluble protein | Phenolic compounds |
|---|---|---|---|---|---|
| Variety (V) | 1 | 0.044* | 3.04** | 0.21ns | 0.0001ns |
| Light (L) | 3 | 0.55** | 12.6** | 0.95** | 10.9** |
| Nutrient solution (N) | 2 | 0.05** | 0.07ns | 0.02ns | 1.20** |
| V*L | 3 | 0.26** | 13.4** | 0.76** | 4.33** |
| V*N | 2 | 0.36** | 16.3** | 1.29** | 12.24** |
| L*N | 6 | 0.54** | 7.49** | 0.56** | 8.25** |
| V*L*N | 6 | 0.22** | 4.51** | 0.25* | 4.48** |
| Experimental Error | 48 | 0.008 | 0.52 | 0.09 | 0.019 |
| Coefficient Variance (%) | – | 4.3 | 4.04 | 6.62 | 1.62 |
ns, * and ** indicate non-significance, significant at 5% and 1% probability level (Duncan’s Test), respectively.
Table 4.
The interactive effects of lettuce variety, light quality and three replacement methods of nutrient solution on the proline and total soluble sugar content in lettuce plants.
| Variety | Light | Proline (mg g−1 FW) | Total soluble sugars (mg g−1 FW) | ||||
|---|---|---|---|---|---|---|---|
| Complete replacement | Based on EC | Based on plant needs | Complete replacement | Based on EC | Based on plant needs | ||
| Lollo Rossa | White | 2.39cde | 2.54b | 2.15fgh | 18.01d-g | 16.68hi | 15.01j |
| Blue | 2.12gh | 1.91ijk | 1.74klm | 17.67e-h | 17.57fgh | 15.8ij | |
| Red/Blue | 2.88a | 2.58b | 1.88i-l | 20.03ab | 21.09a | 19.09bcd | |
| Red | 1.57n | 2.09gh | 2.29ef | 18.98b-e | 21.2a | 15.39ij | |
| Lollo Bionda | White | 2.10gh | 1.65mn | 2hi | 16.6hi | 18.35c-f | 16.7ghi |
| Blue | 2.33e | 2.36de | 1.92ij | 19.44bc | 19.33bcd | 16.5hi | |
| Red/Blue | 2.63b | 2.51bcd | 2.22efg | 17.51fgh | 17.4fgh | 18.4c-f | |
| Red | 1.83jkl | 2.26efg | 1.72lmn | 17.49fgh | 18.56c-f | 15.7ij | |
Values are means ± SE of three replicates. Different letters in each column show significant differences at P ≤ 0.05 (Duncan’s Test).
Total soluble sugars
Soluble sugar content in the lettuce plants was influenced by lettuce variety and light quality, interactions between variety and light quality, light quality and nutrient replacement methods, and interactions between these factors (Table 3). In the Lollo Rossa cultivar, the use of red/blue and monochromatic red light in the EC-based replacement method and red/blue LED light in the complete replacement had the most pronounced effect on soluble sugar content, with an increase of up to 18% observed in compared to the control plants. In Lollo Bionda, however, the application of blue light increased the amount of total soluble sugars by 17 and 16% in plants grown under the complete replacement and the replacement method based on EC, respectively. When the same plants were supplemented with nutrients according to the needs of plants, the combination of red and blue light increased the soluble sugar content by 11% (Table 4).
Soluble protein
The results showed that soluble protein concentration was influenced by the effects of light quality, the interaction between variety and light quality, the interaction between light quality and replacement methods of nutrient solution and the interaction of these three factors (Table 3). The red/blue and red LED light increased the amount of soluble protein in the Lollo Rossa variety by 41.2 and 31.5%, respectively, in the replacement method based on the EC value and by 31.3 and 27.1%, respectively, in the complete replacement method (Table 5). Moreover, the application of the red/blue and white light spectrum had a positive effect on the protein content when this variety was grown according to the needs of the plant. At the same time, the use of the blue light spectrum and both the EC-based replacement and the complete replacement method and the combination of red and blue LED light and replacement method based on the needs of the plant resulted in up to 23% increase of the total protein content in the leaf tissue of Lollo Bionda (Table 5).
Table 5.
The interactive effects of lettuce variety, light quality and three replacement methods of nutrient solution on soluble protein and phenol compounds in lettuce plants.
| Variety | Light | Soluble protein (mg g−1 FW) | Phenolic compounds (mg g−1 FW) | ||||
|---|---|---|---|---|---|---|---|
| Complete replacement | Based on EC | Based on plant needs | Complete replacement | Based on EC | Based on plant needs | ||
| Lollo Rossa | White | 4.02g | 4.48d-g | 4.84b-f | 8.47jk | 9.91e | 7.62l |
| Blue | 4.49d-g | 4.73c-f | 4.26efg | 8.75i | 7.87l | 6.55n | |
| Red/Blue | 5.28ab | 5.68a | 4.85b-e | 7.67l | 11.37a | 11.01b | |
| Red | 5.11abc | 5.29ab | 4.04g | 5.90o | 8.52ijk | 10.24d | |
| Lollo Bionda | White | 4.23fg | 4.93bcd | 4.24efg | 10.50c | 6.49n | 8.66ij |
| Blue | 4.94bcd | 5.20abc | 4.43d-g | 10.17d | 9.15h | 7.86l | |
| Red/Blue | 4.7c-f | 4.68c-f | 4.97bcd | 9.45fg | 9.62f | 9.32gh | |
| Red | 4.44d-g | 4.71c-f | 4.47d-g | 7.21m | 8.39k | 7.08n | |
Values are means ± SE of three replicates. Different letters in each column show significant differences at P ≤ 0.05 (Duncan’s Test).
Phenolic compounds
Based on the results, it was found that the concentration of phenolic compounds was affected by light quality, nutrient solution replacement method, interaction between variety and light quality, interaction between variety and nutrient solution replacement method, interaction between light and nutrient solution replacement method, and the interaction of these three factors (Table 3). As shown, the Lollo Rossa variety treated with red/blue LED light and supplied with nutrients based on both the EC value and the plant requirement had 34.2 and 30% higher phenolic compounds content, respectively, than the control plants (Table 5). In plants fed according to plant requirement, the monochromatic red light also showed a positive effect on this parameter, with an increase of 21%. In contrast, in the Lollo Bionda, variety the use of white and blue LED light resulted in increased concentrations of total phenolic compounds when the nutrient solution was completely replaced (Table 5).
Antioxidant activity
DPPH radical scavenging activity was affected by light quality, method of nutrient solution replacement, interaction between variety and light quality, interaction between variety and nutrient solution replacement method, interaction between light and replacement method, and the interaction of these three factors (Table 6). It was found that combination of red and blue LED light increased antioxidant activity in leaf tissue by 30 and 11%, respectively, in Lollo Rossa plants grown under the replacement method based on plant needs and EC value compared to the control (Table 7). In Lollo Bionda, plants grown under the white LED light and complete nutrient solution replacement showed the highest DPPH radical scavenging activity in this cultivar, with two other replacement methods contributing to decrease in this trait (Table 7).
Table 6.
The effect of different qualities of light and three methods of replacement of nutrient solution on DPPH radical scavenging activity and enzymes activity of two varieties of lettuce in floating hydroponics.
| Source of variation | Df | DPPH radical scavenging activity | Guaiacol peroxidase enzyme activity | Catalase enzyme activity | Superoxide dismutase enzyme activity |
|---|---|---|---|---|---|
| Variety (V) | 1 | 0.0001ns | 2234** | 8.69* | 534** |
| Light (L) | 3 | 176** | 24,004** | 275** | 10,162** |
| Nutrient solution (N) | 2 | 26.5** | 2361** | 27.09** | 118ns |
| V*L | 3 | 194** | 6937** | 63.9** | 1990** |
| V*N | 2 | 142** | 20,389** | 154** | 7233** |
| L*N | 6 | 183** | 18,891** | 255** | 11,573** |
| V*L*N | 6 | 82.2** | 9500** | 135** | 2339** |
| Experimental Error | 48 | 1.91 | 80.2 | 1.46 | 52.8 |
| Coefficient Variance (%) | – | 3.28 | 3.28 | 4.0 | 3.06 |
ns, * and ** indicate non-significance, significant at 5% and 1% probability level (Duncan’s Test), respectively.
Table 7.
The interactive effects of lettuce variety, light quality and three replacement methods of nutrient solution on DPPH radical scavenging activity and guaiacol peroxidase enzyme activity in lettuce plants.
| Variety | Light | Antioxidant activity (% inhibition of DPPH radical) | Guaiacol peroxidase enzyme activity (unit enzyme activity ∙ mg−1 protein) | ||||
|---|---|---|---|---|---|---|---|
| Complete replacement | Based on EC | Based on plant needs | Complete replacement | Based on EC | Based on plant needs | ||
| Lollo Rossa | White | 44.1d | 51.6b | 39.7fgh | 276e | 329c | 235fg |
| Blue | 37.7ghi | 33.9j | 34.07j | 269e | 229g | 176j | |
| Red/Blue | 39.8fg | 49.03c | 57.4a | 387a | 377a | 209h | |
| Red | 30.66k | 44.3d | 44.1d | 321c | 247f | 149k | |
| Lollo Bionda | White | 52.9b | 33.7j | 37.3hi | 359b | 179j | 275e |
| Blue | 45.2d | 47.6c | 40.89f. | 350b | 305d | 229g | |
| Red/Blue | 40.7f. | 41.4ef | 48.5c | 289d | 323c | 331c | |
| Red | 37.4ghi | 43.7de | 36.8i | 211h | 282e | 194i | |
Values are means ± SE of three replicates. Different letters in each column show significant differences at P ≤ 0.05 (Duncan’s test).
Guaiacol peroxidase activity
The enzymatic activity of guaiacol peroxidase activity was affected by plant variety, light quality, method of nutrient solution replacement, and the interaction of these three factors (Table 6). The application of red/blue LED light in the complete replacement method and the EC-based replacement method resulted in a 40.2 and 36.5% increase in guaiacol peroxidase activity in Lollo Rossa, respectively (Table 7). In the Lollo Bionda cultivar, plants grown under the complete replacement method showed the highest enzyme activity when exposed to both white and blue LED light. For the other two replacement methods applied to Lollo Bionda, the combination of red and blue light spectra maintained higher activities of guaiacol peroxidase (Table 7).
Catalase activity
Results from ANOVA showed that catalase activity was affected by variety, light quality, nutrient solution replacement method, and the interaction of these three factors (Table 6). It was found that red/blue light increased catalase activity in the Lollo Rossa cultivar by 10.2 and 20% when plants were grown using the EC-based nutrient solution replacement method and the complete replacement method, respectively (Table 8). However, in Lollo Bionda variety, the plants fed with the complete replacement method under white LED light and with the EC-based replacement method under red/blue LED light showed the highest enzymatic activity (Table 8).
Table 8.
The interactive effects of lettuce variety, light quality and three replacement methods of nutrient solution on catalase enzyme activity and superoxide dismutase enzyme activity in lettuce plants.
| Variety | Light | Catalase enzyme activity (unit enzyme activity mg−1 protein) | Superoxide dismutase enzyme activity (unit enzyme activity mg−1 protein) | ||||
|---|---|---|---|---|---|---|---|
| Complete replacement | Based on EC | Based on plant needs | Complete replacement | Based on EC | Based on plant needs | ||
| Lollo Rossa | White | 35.2cde | 34.9cde | 31.6gh | 269e | 258ef | 215ij |
| Blue | 32.3fgh | 20.4k-n | 29.10i | 275ef | 206jk | 199kl | |
| Red/Blue | 38.8b | 42.2a | 21.8kl | 309b | 323a | 191l | |
| Red | 24.2j | 38.01b | 18.2n | 239gh | 247fg | 159n | |
| Lollo Bionda | White | 38.7b | 30.5hi | 19.13mn | 296c | 216ij | 167mn |
| Blue | 35.5cd | 22.2k | 34.8cde | 283d | 237gh | 219ij | |
| Red/Blue | 33.9def | 36.9bc | 32.0fgh | 270e | 282d | 226hi | |
| Red | 33.2efg | 20lmn | 21.2klm | 226hi | 209jk | 175m | |
Values are means ± SE of three replicates. Different letters in each column show significant differences at P ≤ 0.05 (Duncan’s test).
Superoxide dismutase activity
Superoxide dismutase activity was affected by variety, light quality, interaction of variety and light quality, interaction of variety and replacement method of nutrient solution, interaction between light and nutrient solution replacement method, and the interaction of these three factors (Table 6). The combination of red and blue LED light significantly induced superoxide dismutase activity in the leaves of Lollo Rossa—it was increased by 15 and 20% compared to the control conditions when the complete replacement method and the EC-based replacement method were used, respectively. In contrast, in the Lollo Bionda variety, the use of blue light in the complete replacement method and red/blue LED light in the EC-based replacement method resulted in the highest enzyme activity, which was close to the control level (Table 8).
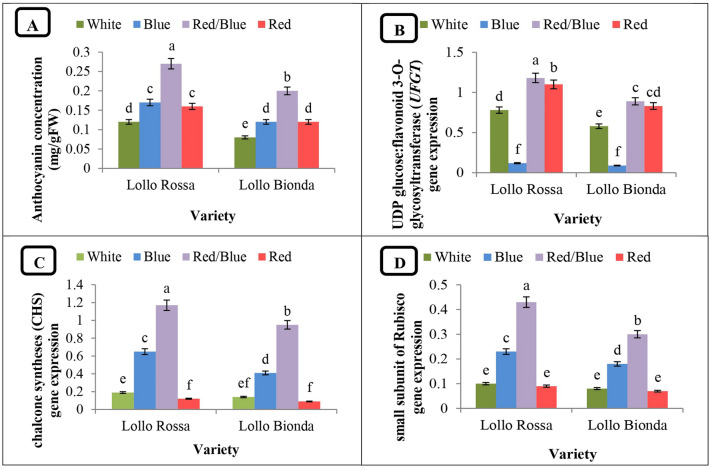
Anthocyanins concentration
Anthocyanin concentration was affected by variety, light quality and the interaction of variety and light quality (Table 9). It was found that irradiation with red/blue LED light resulted in a more than twofold increase of anthocyanin content in both varieties in compared to control plants, however, in Lollo Bionda this never reached the high levels characteristic of Lollo Rossa (Fig. 2). Importantly, the separate application of both monochromatic spectra was able to increase anthocyanin concentration compared to the control treatment (white light). However, in both varieties, plants treated with red and blue light did not differ significantly in anthocyanin content (Fig. 2A).
Table 9.
The effect of variety and LED light quality on anthocyanins concentration and the expression of genes of anthocyanin biosynthesis pathway and the small sub-section of Rubisco.
| Source of Variation | DF | Anthocyanins concentration | UFGT | CHS | Small subunit of rubisco |
|---|---|---|---|---|---|
| Variety (V) | 1 | 0.014** | 0.24** | 0.25** | 0.04** |
| Light (L) | 3 | 0.018** | 1.06** | 3.77** | 0.73** |
| L*V | 3 | 0.0003* | 0.02** | 0.07** | 0.01** |
| Experimental Error | 16 | 0.001 | 0.001 | 0.03 | 0.0003 |
| Coefficient Variance (%) | – | 5.36 | 5.36 | 4.26 | 6.19 |
* and ** indicate significance at the 5 and 1 percent probability level (Duncan’s test), respectively.
Figure 2.
The interactive effect of lettuce variety and LED light quality on (A) anthocyanin concentration, (B) UDP glucose:flavonoid 3-O-glycosyltransferase (UFGT) gene expression, (C) chalcone synthase (CHS) gene expression and (D) Rubisco small subunit gene expression in lettuce plants.
UDP glucose: flavonoid 3-O-glycosyltransferase (UFGT) gene expression
The expression level of the UFGT gene was affected by variety, light spectrum and the interaction of lettuce variety and LED light quality (Table 9). It was found that treatment with the red/blue LED light and the red monochromatic LED light resulted in increased (by 50% and 40%, respectively) expression level of the UFGT gene in both lettuce cultivars compared to control plants, while the lowest UFGT expression was observed in lettuce plants grown under the blue spectrum (Fig. 2B).
Chalcone synthase (CHS) gene expression
Chalcone synthase gene expression was affected by lettuce variety, light quality and the interaction of these factors (Table 9). As shown, treatment of lettuce plants with the red/blue light spectrum and with monochromatic blue light in a significant increase in CHS expression compared with control plants—6.2- and 3.3-fold in Lollo Rossa and 6.2- and 2.7-fold in Lollo Bionda, respectively (Fig. 2C). The lowest gene expression was observed in the plants grown under monochromatic red light (Fig. 2C).
Small Rubisco subunit gene expression
Expression of the Rubisco small subunit gene was affected by lettuce variety, light quality, and the interaction of these factors (Table 9). During the experiment, the plants treated with the combination of red and blue LED light and monochromatic blue light showed the highest Rubisco gene expression (Fig. 2D). Lollo Rossa responded to the red/blue and blue lights with a 4.3- and 2.3-fold increase in Rubisco expression, respectively, while it was increased 3.7- and 2.2-fold in Lollo Bionada compared with control plants. The expression levels determined in the plants treated with monochromatic red light were significantly lower and close to the control values (Fig. 2D).
Net photosynthetic rate (PN)
The net photosynthetic rate was affected by the light spectrum and the interaction of light quality and nutrient solution replacement method (Table 10). The use of combined red and blue light in all three nutrient solution replacement methods resulted in a 30–45% increase in net photosynthesis of lettuce cultivars compared to control conditions. The other light spectra did not show statistically significant differences from each other, except for the red LED light treatment in plants fed according to the EC value (Fig. 3).
Table 10.
The effect of different light qualities and three replacement methods of nutrient solution on gas exchange characteristics of two varieties of lettuce in floating hydroponics.
| Source of Variation | Df | PN | Ci | E | gs |
|---|---|---|---|---|---|
| Variety (V) | 1 | 0.20ns | 826ns | 2.55ns | 0.007ns |
| Light (L) | 3 | 30.8* | 2305* | 2.6* | 0.025** |
| Nutrient solution (N) | 2 | 3.74ns | 710ns | 0.17ns | 0.002ns |
| V*L | 3 | 5.99ns | 672ns | 1.8ns | 0.034** |
| V*N | 2 | 17.2ns | 324ns | 9.1** | 0.034** |
| L*N | 6 | 50.1** | 2742** | 5.48** | 0.067** |
| V*L*N | 6 | 18.1ns | 966ns | 5.05** | 0.017** |
| Experimental Error | 48 | 10.35 | 700 | 0.80 | 0.002 |
| Coefficient Variance (%) | – | 4.59 | 7.98 | 20.8 | 23.5 |
ns, * and ** indicate non-significance, significant at 5% and 1% probability level (Duncan’s Test), respectively.
Figure 3.
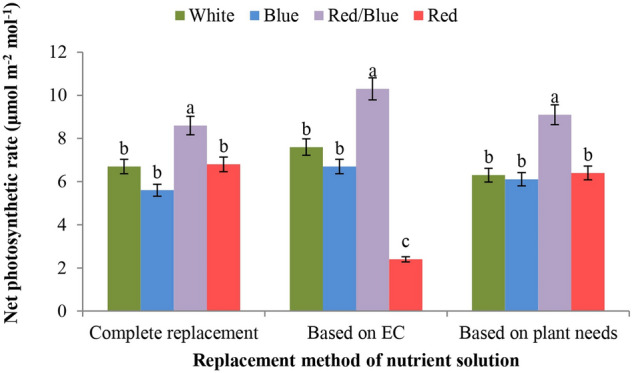
The interactive effect of nutrient solution replacement method and LED light quality on net photosynthetic rate in lettuce plants.
Intercellular CO2 concentration (Ci)
The Ci parameter was affected only by the light spectrum and the interaction of light and nutrient solution replacement method (Table 10). It was found that the plants grown under complete replacement and EC-based method exposed to monochromatic red and blue light, respectively, showed higher Ci than the plants exposed to white light (control). Other light spectra showed no statistically significant differences among all three replace methods (Fig. 4).
Figure 4.
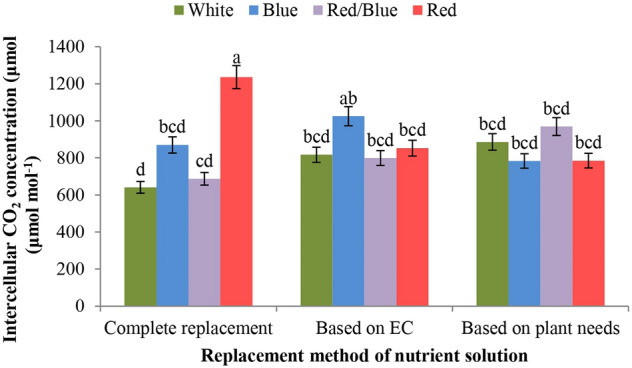
The interactive effect of nutrient solution replacement method and LED light quality on intercellular CO2 concentration in lettuce plants.
Transpiration rate (E)
Based on the results, it was found that transpiration rate was affected by light spectrum, interaction of light spectrum and replacement method of nutrient solution, interaction of variety and light spectrum, variety and replacement method of nutrient solution, light and replacement method of nutrient solution, and the interaction of these three factors (Table 10). The application of monochromatic blue and white light and the replacement method based on the needs of the plants in the Lollo Bionda cultivar increased the transpiration rate by 82.7 and 65.5%, respectively, in relation to the control. Overall, the transpiration rate was higher all light spectra applied to lettuce varieties fed based on the needs of plants in comparison to the control treatment (Table 11).
Table 11.
The interactive effects of lettuce variety, light quality and three replacement methods of nutrient solution on the transpiration rate and stomatal conductance in lettuce plants.
| Variety | Light | Transpiration rate (mol (H2O) m−2 s−1) (E) | Stomatal conductance (mol H2O m−2 s−1) (gs) | ||||
|---|---|---|---|---|---|---|---|
| Complete replacement | Based on EC | Based on plant needs | Complete replacement | Based on EC | Based on plant needs | ||
| Lollo Rossa | White | 3.08 efgh | 3.92 cdef | 5.46 abc | 0.12def | 0.18def | 0.21cde |
| Blue | 4.14 bcdef | 4.44 bcdef | 5.22 abcd | 0.16def | 0.32b | 0.33b | |
| Red/Blue | 2.91 fgh | 4.52 bcdef | 5.43 abc | 0.09f | 0.31b | 0.40ab | |
| Red | 4.52 bcdef | 3.96 cdef | 5.37 abc | 0.18def | 0.13def | 0.34ab | |
| Lollo Bionda | White | 3.54 defg | 4.01 cdef | 5.86 ab | 0.22cd | 0.15def | 0.40ab |
| Blue | 4.01 cdef | 1.87 h | 6.47 a | 0.113ef | 0.31b | 0.43a | |
| Red/Blue | 3.02 efgh | 1.94 gh | 4.70 bcde | 0.116def | 0.113ef | 0.21de | |
| Red | 3.57 defg | 4.15 bcdef | 4.77 abcde | 0.116def | 0.16def | 0.22cd | |
Values are means ± SE of three replicates. Different letters in each column show significant differences at P ≤ 0.05 (Duncan’s test).
Stomatal conductance (gs)
Stomatal conductance was affected by light spectrum, interaction of light and replacement method of nutrient solution, the interaction of variety and light spectrum, variety and replacement method of nutrient solution, light and replacement method of nutrient solution, and the effects of these three factor (Table 10). The use of the blue and white light spectrum under the replacement method based on plant needs resulted in the highest values of stomatal conductance in the Lollo Bionda cultivar (95.4 and 81.8% higher than the control, respectively). In the Lollo Rossa variety, the red/blue light was the most effective in inducing stomata opening. The stomatal conductance determined under all light spectra was higher in lettuce supplemented according to the needs of the plant than in the control (Table 11).
Discussion
Light is an essential environmental factor for plants playing an important role in regulating growth, morphology, and metabolism40. Proline is a compatible osmolyte component that is usually induced in response to osmotic imbalance in the cytoplasm of cells41. Under stress, plants need to maintain intracellular water potential to sustain turgescence and effectively absorb water, with proline facilitating water uptake and turgor pressure stability. Proline is also a highly effective osmoprotectant, improving protein stability and protecting membrane integrity42. In the present experiment, the lettuce varieties (Lollo Rossa and Lollo Bionda) treated with red/blue LED light and grown under the complete replacement and EC-based method had the highest proline concentration (Table 4), supporting the role of this osmolyte in preventing dehydration, reducing cellular damage, maintaining osmotic balance and stress tolerance to the plant43. The results obtained here are consistent with studies on the responses of a number of plant species to osmotic stress, including studies on model plant Arabidopsis thaliana41.
Soluble sugars play many important roles in the plant cell, including the functions of key components of energetic and biosynthetic metabolism. Importantly, however, they can also act as compatible osmolytes, restoring osmotic balance, and as protective macromolecules41. It has long been known that salinity and a high EC in the nutrient solution lead to a decrease in the content of soluble sugars in the tissues of leafy vegetables43. However, it has been shown that different light conditions can modulate the sugar content in plants by influencing the activity of enzymes of sucrose metabolism44. Here, the red/blue and the monochromatic red LED spectra in lettuce cultivars grown under the replacement method based on the EC values and the complete replacement resulted in a higher soluble sugar content (Table 4). In the studies by Ding et al.7 it was shown that an increase light intensity caused a decrease in soluble sugar content. In contrast, Chen et al.44 reported that the combination of red and blue light as well as monochromatic red light can increase the accumulation of soluble sugar in lettuce plants, which is consistent with the results of the present study (Table 4). Our results are further supported by the fact that the 70/30% and 80/20% red/blue combination and 100% red light were described as most effective in increasing soluble sugar content in lettuce plants45.
Abiotic stresses, such as salinity and an increased EC in the nutrient solution, can lead to decrease in soluble protein content. This loss may be due to an increase in the rate of protein degradation46. This is in agreement with our findings, where the increased accumulation of proline in plants grown under complete replacement and the EC-based method of nutrient solution was clearly correlated with the decreased soluble protein content. This is also confirmed by Liu et al.47 who indicated that high light intensity and both high and low EC can reduce soluble protein concentration in lettuce plants. However, it is important to note that the results of the present experiment showed that the red/blue and monochromatic red LED spectrum resulted in higher soluble protein content in lettuce varieties in plants cultivated under the complete nutrient solution replacement and replacement method based on EC, despite the increased proline accumulation (Table 5).
Phenolic compounds are among the most important antioxidants in plant cells whose production is stimulated under the influence of stress48,49. In the present experiment, the complete replacement of the nutrient solution in Lollo Bionda and the EC-based replacement method in Lollo Rossa had the highest effect on the total content of phenolic compounds under the combination of red and blue LED light (Table 5), which is in agreement with previous results in turnip plants where an increased EC level in the nutrient solution led to an increased content of phenolic and flavonoid compounds. The induction in the phenolic accumulation could possibly be explained by a modulation of the enzymatic activity of phenylalanine aminolyase—the first enzyme in the phenylpropanoid pathway of phenolic compounds synthesis, by the reduced water potential due to the high EC values in the nutrient solution7. It is well known that phenolic compounds can reduce cell membrane damage caused by oxidative stress due to their antioxidant potential in plants50. Previously, it was reported that blue and red LED spectra increase the content of flavonoids and phenolic compounds in cucumber and R. hongnoensis plants13,51.
Antioxidants in plants are important intracellular compounds functioning to prevent the activity of reactive oxygen species (ROS). Previous studies have reported that light quantity and quality can have strong effects on impaired stimulation of PSII or PSI, leading to energy imbalance between photosystems52 and increased ROS generation53. To scavenge ROS, plants induce antioxidant mechanisms by increasing the levels of non-enzymatic antioxidants and the activity of enzymes such as catalase, superoxide dismutase and peroxidase54. Antioxidant enzymes protect important cellular components, therefore assessing their activity is one of the most reliable ways to evaluate the stress resistance of plants55. Furthermore, measuring the antioxidant capacity of plants is of particular importance in the context of dietary values56. In the present experiment, it was shown that the complete replacement of the nutrient solution and the EC-based replacement method led to an increase in antioxidant enzymes in both lettuce varieties. This is consistent with previous founding in basil and saffron plants, where increasing the concentration of the Hoagland nutrient solution provoked an increase in both enzymatic and non-enzymatic antioxidants57,58. The high activity of antioxidant enzymes observed in treatments with high EC levels could be explained by the direct toxic effect of the minerals accumulating at high levels and by a negative effect on water uptake, provoking dehydration symptoms59. In the present study, data was also provided that the treatment of the plants with primarily red/blue LED light was able to induce the activity of the antioxidant enzymes and the radical scavenging activity in the cultivated lettuce varieties (Tables 7, 8). Consistent with previous findings48,50, there was evidence that the amount of phenolic compounds and antioxidant activity depended on the variety and plant species.
The accumulation of anthocyanins is regulated by transcription factors and stimulated by various environmental factors such as light, low temperatures, drought and salt stress60. Different light qualities and intensities are sensed by receptors or signalling factors to be transduced into the downstream transcription factors61. Next, enzymes encoded by structural genes (these genes are in turn regulated by transcription factors) synthesise metabolites in response to light. In the present study, the combined application of red LED and blue LED light significantly increased the anthocyanin content in both lettuce varieties compared to other light spectra used during the experiment (Fig. 2). At the same time, the use of the individual monochromatic spectra resulted in a higher anthocyanin concentration compared to the control values determined in the plants grown under the white LED light. However, the plants treated with red LED and blue LED light did not differ significantly in terms of anthocyanin content (Fig. 2). As expected, our results showed that the anthocyanin content was higher in the red-leaved lettuce variety (Lollo Rossa) than in the green-leaved variety (Lollo Bionda). These results are consistent with previous reports where the combination of red and blue LED light increased the accumulation of anthocyanins in the Batavia lettuce variety (Lactuca sativa cv. Batavia)62.
According to the data from this study, the red/blue LED light increased the expression of the UFGT gene in both lettuce cultivars compared to the control (white LED light; Fig. 3). It was also shown that in both lettuce cultivars, the highest expression of the CHS gene was observed under the red/blue LED light and the monochromatic blue light spectrum, which was clearly superior to the white LED light (Fig. 4). In general, the expression of the genes CHS and UFGT determined in this study was higher in the red-leaved lettuce variety (Lollo Rossa) than in the green-leaved variety (Lollo Bionda). This is in line with previous reports indicating that the expression of the genes CHS and UFGT in red-leaved lettuce is dependent on the ratio between red and blue light18. Recently, the combination of red and blue light was shown to stimulate the expression of genes involved in the biosynthesis of secondary metabolites such as anthocyanins and phenols in lettuce62. Since the use of monochromatic blue light increased the expression of the UFGT gene, while the expression of CHS increased under monochromatic red light (Figs. 3, 4), it can be concluded that the combination of red and blue light has a synergistic effect on the production of anthocyanins. More recently, it was pointed out that increasing the amount of blue light increases the anthocyanin content in purple pepper fruits via an increase in anthocyanin biosynthesis, whichis supported by a higher expression of the anthocyanin biosynthesis genes CaMYB, CaCHS, CaDFR, CaANS and CaUFGT63.
Stomata in plants are important channels for water and CO2 exchange, which is strictly regulated by light64. In this study, the red/blue LED light spectrum had a clear inductive effect on net photosynthesis under all three nutrient solution replacement methods (Fig. 3). At the same time, the lettuce plants treated with monochromatic red and monochromatic blue LED light, respectively, under the complete replacement method and the EC-based method showed the most pronounced effect on intercellular CO2 concentration (Fig. 4). The use of the blue light spectrum under the replacement method based on the needs of plant resulted in the highest level of stomatal conductance and transpiration rate in Lollo Bionda cultivar (Table 11), which is consistent with the role of blue light in the cryptochrome- and phototropin-mediated mechanism of stomata opening64,65. The control of gas exchange between the leaf and the atmosphere through stomata determines photosynthetic CO2 uptake for photosynthesis and transpiration, and thus plant productivity and water use efficiency. The balance between these two processes depends on the responses of stomata to environmental and internal stimuli and on the synchrony of stomata behaviour relative to the CO2 demand of the mesophyll65.
In the present study, irradiation of the investigated lettuce varieties with red/blue light and the monochromatic blue light resulted in increased expression of the Rubisco small subunit gene (RBCS) compared to the control treatment (white light; Fig. 2D). The combination of red and blue light is a factor that modulates the morphological and photosynthetic characteristics of plants through its effect on leaf anatomy, photosynthetic electron transport and expression of key Calvin cycle enzymes such as Rubisco, fructose-1,6-bisphosphatase (FBP) and glyceraldehyde phosphatase dehydrogenase (GAPDH)66. The results of this experiment are consistent with previous findings that the expression of the Rubisco genes RBCS and RBCL increased under red and blue LED light as opposed to white light67. More recently, white LED light was reported to increase the expression of the Rubisco activase (RCA) gene in Gerbera plants compared to the combination of red and blue light68. Since a negative correlation between the Rubisco activase and Rubisco levels has been reported, we speculate that RBCS expression may decrease in response to upregulated RCA69. The observed increase in Rubisco gene expression (Fig. 2D) with the concomitant increase in net photosynthesis (Fig. 3) due to the interaction of red and blue LED light reflects the ability of this light spectrum to enhance CO2 assimilation through increased Rubisco activity, resulting in increased biomass production and yields in plants fed according to the nutritional needs.
Conclusions
In conclusion, it was shown that, in contrast to nutrient replacement based on plant needs, the methods of complete replacement and EC-based replacement increase the amount of compatible osmolytes due to stress conditions resulting from the accumulation of mineral elements in the nutrient solution. The combination of red and blue light was able to reduce the damage caused by osmotic stress by increasing the content of proline and soluble sugar and the activity of antioxidant enzymes. The increased expression of CHS and UFGT genes observed under combination of red and blue LED light resulted in increased accumulation of anthocyanins in the leaves of red-leaved lettuce cultivar Lollo Rossa. The combination of red and blue LED light and the replacement method based on the needs of the plants are recommended for lettuce cultivars because they have significant positive effects on the biochemical properties and gene expression in anthocyanin synthesis. At the same time, it can prevent the waste of water and nutrients and protect the environment.
Author contributions
Conceptualization: H.R.S., H.R.R. and P.S.; Methodology: H.R.S., H.R.R. and P.S.; Investigation: H.R.R.; Formal analysis: H.R.S., H.R.R., P.S., M.H. and K.H.M.; Data curation: H.R.S.; Writing—original draft preparation: H.R.S., H.R.R., P.S., M.H. and K.H.M; Writing—review and editing: H.R.R. and P.S. All authors have read and approved the final manuscript.
Funding
The authors are grateful to the Vali-e-Asr University of Rafsanjan for funding this study. The article processing charge is financed by Wroclaw University of Environmental and Life Sciences.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hamid Reza Roosta, Email: roosta_h@yahoo.com.
Piotr Stępień, Email: piotr.stepien@upwr.edu.pl.
References
- 1.Zhushi-Etemi F, et al. Correlation between physical and chemical parameters of water and biotic indices: The case study the White Drin River basin. Kosovo. J. Water Land Dev. 2020;46:229–241. [Google Scholar]
- 2.Łabędzki L, Bąk B. Impact of meteorological drought on crop water deficit and crop yield reduction in Polish agriculture. J. Water Land Dev. 2017;34:181–190. doi: 10.1515/jwld-2017-0052. [DOI] [Google Scholar]
- 3.Silva L, Gasca-Leyva E, Escalante E, Fitzsimmons KM, Lozano DV. Evaluation of biomass yield and water treatment in two aquaponic systems using the dynamic root floating technique (DRF) Sustainability. 2015;7:15384–15399. doi: 10.3390/su71115384. [DOI] [Google Scholar]
- 4.Domingues DS, Takahashi HW, Camara CA, Nixdorf SL. Automated system developed to control pH and concentration of nutrient solution evaluated in hydroponic lettuce production. Comput. Electron. Agric. 2012;84:53–61. doi: 10.1016/j.compag.2012.02.006. [DOI] [Google Scholar]
- 5.Bugbee, B. Nutrient Management in recirculating hydroponic culture. In Proceedings of the South Pacific Soilless Culture Conference (ed. Nichols, M.) Acta Hort64, 99–112 (2004).
- 6.Carmassi G, et al. Modeling salinity build-up in recirculating nutrient solution culture. J. Plant Nutr. 2005;28:431–445. doi: 10.1081/PLN-200049163. [DOI] [Google Scholar]
- 7.Ding X, et al. Electrical conductivity of nutrient solution influenced photosynthesis, quality, and antioxidant enzyme activity of pakchoi (Brassica campestris L. ssp. Chinensis) in a hydroponic system. PLoS ONE. 2018;13:e0202090. doi: 10.1371/journal.pone.0202090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song J, et al. Nutritional quality, mineral and antioxidant content in lettuce affected by interaction of light intensity and nutrient solution concentration. Sci. Rep. 2020;10:1–9. doi: 10.1038/s41598-020-59574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabzalian MR, et al. High performance of vegetables, flowers, and medicinal plants in a red-blue LED incubator for indoor plant production. Agron. Sustain. Dev. 2014;34:879–886. doi: 10.1007/s13593-014-0209-6. [DOI] [Google Scholar]
- 10.Zha L, Liu W. Effects of light quality, light intensity, and photoperiod on growth and yield of cherry radish grown under red plus blue LEDs. Hortic. Environ. Biotechnol. 2018;59:511–518. doi: 10.1007/s13580-018-0048-5. [DOI] [Google Scholar]
- 11.Brazaitytė A, et al. Effect of different ratios of blue and red led light on brassicaceae microgreens under a controlled environment. Plants. 2021;10:801. doi: 10.3390/plants10040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehman M, et al. Red light optimized physiological traits and enhanced the growth of ramie (Boehmeria nivea L.) Photosynthetica. 2020;58:922–931. doi: 10.32615/ps.2020.040. [DOI] [Google Scholar]
- 13.Oh HE, Yoon A, Park YG. Red Light enhances the antioxidant properties and growth of Rubus hongnoensis. Plants. 2021;10:2589. doi: 10.3390/plants10122589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Y, Ma X, Gao X, Wu W, Zhou B. Light induced regulation pathway of anthocyanin biosynthesis in plants. Int. J. Mol. Sci. 2021;22:11116. doi: 10.3390/ijms222011116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landi M, Tattini M, Gould KS. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015;119:4–17. doi: 10.1016/j.envexpbot.2015.05.012. [DOI] [Google Scholar]
- 16.Kitamura S, Shikazono N, Tanaka A. TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J. 2004;37:104–114. doi: 10.1046/j.1365-313X.2003.01943.x. [DOI] [PubMed] [Google Scholar]
- 17.Routaboul J-M, et al. Metabolite profiling and quantitative genetics of natural variation for flavonoids in Arabidopsis. J. Exp. Bot. 2012;63:3749–3764. doi: 10.1093/jxb/ers067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoji K, Goto E, Hashida S, Goto F, Yoshihara T. Effect of red light and blue light on the anthocyanin accumulation and expression of anthocyanin biosynthesis genes in red-leaf lettuce. J. Agric. Sci. Technol. 2010;22:107–113. doi: 10.2525/shita.22.107. [DOI] [Google Scholar]
- 19.Zheng L, Van Labeke MC. Long-term effects of red-and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant Sci. 2017;8:917. doi: 10.3389/fpls.2017.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogewoning SW, et al. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010;61:3107–3117. doi: 10.1093/jxb/erq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leong TY, Anderson JM. Effect of light quality on the composition and function of thylakoid membranes in Atriplex triangularis. Biochim. Biophys. Acta Bioenerg. 1984;766:533–541. doi: 10.1016/0005-2728(84)90111-7. [DOI] [Google Scholar]
- 22.Eskins K, Jiang CZ, Shibles R. Light-quality and irradiance effects on pigments, light-harvesting proteins and Rubisco activity in a chlorophyll and light-harvesting- deficient soybean mutant. Physiol Plant. 1991;83:47–53. doi: 10.1111/j.1399-3054.1991.tb01280.x. [DOI] [Google Scholar]
- 23.Son KH, Oh MM. Growth, photosynthetic and antioxidant parameters of two lettuce cultivars as affected by red, green, and blue light-emitting diodes. Hortic. Environ. Biotechnol. 2015;56:639–653. doi: 10.1007/s13580-015-1064-3. [DOI] [Google Scholar]
- 24.Roni MZK, Islam MS, Shimasaki K. Response of Eustoma leaf phenotype and photosynthetic performance to LED light quality. Horticulturae. 2017;3:50. doi: 10.3390/horticulturae3040050. [DOI] [Google Scholar]
- 25.Resh, H. M. Hydroponic Food Production: A Definitive Guidebook for The Advanced Home Gardener and The Commercial Hydroponic Grower (ed. Resh, H.M.), 560. (CRC Press, 2022).
- 26.Gent M, Short M. Managing a simple system to recycle nutrient solution to greenhouse tomato grown in rockwool. Acta Hortic. 2012;927:913–919. doi: 10.17660/ActaHortic.2012.927.112. [DOI] [Google Scholar]
- 27.Gent MP. Factors affecting relative growth rate of lettuce and spinach in hydroponics in a greenhouse. HortScience. 2017;52:1742–1747. doi: 10.21273/HORTSCI12477-17. [DOI] [Google Scholar]
- 28.Estefan, G. Methods of soil, plant, and water analysis: A manual for the West Asia and North Africa region: Third Edition. Beirut, Lebanon (International Center for Agricultural Research in the Dry Areas (ICARDA) (2013).
- 29.Paquin R, Lechasseur P. Observations sur une méthode de dosage de la proline libre dans les extraits de plantes. Can. J. Bot. 1979;57:1851–1854. doi: 10.1139/b79-233. [DOI] [Google Scholar]
- 30.Irigoyen J, Einerich D, Sánchez-Díaz M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992;84:55–60. doi: 10.1111/j.1399-3054.1992.tb08764.x. [DOI] [Google Scholar]
- 31.Bradford N. A rapid and sensitive method for the quantitation microgram quantities of a protein isolated from red cell membranes. Anal. Biochem. 1976;72:e254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 32.Nogués S, Baker NR. Effects of drought on photosynthesis in Mediterranean plants grown under enhanced UV-B radiation. J. Exp. Bot. 2000;51:1309–1317. doi: 10.1093/jxb/51.348.1309. [DOI] [PubMed] [Google Scholar]
- 33.Matta A, Gentile I, Giai I. Effect of mixed inoculations with Fusarium oxysporum f. sp. lycopersici and F. oxysporum f. sp. dianthi on the phenols content of tomato plants. Neth. J. Plant Pathol. 1970;76:144–146. doi: 10.1007/BF01974321. [DOI] [Google Scholar]
- 34.Barros, L., Baptista, P. & Isabel CFR Ferreira. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food Chem. Toxicol.45, 1731–1737 (2007). [DOI] [PubMed]
- 35.Plewa MJ, Smith SR, Wagner ED. Diethyldithiocarbamate suppresses the plant activation of aromatic amines into mutagens by inhibiting tobacco cell peroxidase. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1991;247:57–64. doi: 10.1016/0027-5107(91)90033-K. [DOI] [PubMed] [Google Scholar]
- 36.Dhindsa RS, Matowe W. Drought tolerance in two mosses: Correlated with enzymatic defence against lipid peroxidation. J. Exp. Bot. 1981;32:79–91. doi: 10.1093/jxb/32.1.79. [DOI] [Google Scholar]
- 37.Blanchamp C, Fridovich I. Superoxide dismutage: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 38.Afridi M, et al. Effect of light emitting diode (LED) on the growth and photosynthetic characteristics of Brassica Juncea. J. Bio-mol. Sci. 2020;7:01–09. [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Samuolienė G, et al. The physiological response of lettuce to red and blue light dynamics over different photoperiods. Front. Plant Sci. 2021;11:610174. doi: 10.3389/fpls.2020.610174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurrieri L, Merico M, Trost P, Forlani G, Sparla F. Impact of drought on soluble sugars and free proline content in selected Arabidopsis mutants. Biology. 2020;9:367. doi: 10.3390/biology9110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hosseinifard M, et al. Contribution of exogenous proline to abiotic stresses tolerance in plants: A review. Int. J. Mol. Sci. 2022;23:5186. doi: 10.3390/ijms23095186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh P, et al. Salt stress resilience in plants mediated through osmolyte accumulation and its crosstalk mechanism with phytohormones. Front. Plant Sci. 2022;13:1–21. doi: 10.3389/fpls.2022.1006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X, Wang L, Li T, Yang Q, Guo W. Sugar accumulation and growth of lettuce exposed to different lighting modes of red and blue LED light. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-43498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X-L, Li Y-L, Wang L-C, Guo W-Z. Red and blue wavelengths affect the morphology, energy use efficiency and nutritional content of lettuce (Lactuca sativa L.) Sci. Rep. 2021;11:8374. doi: 10.1038/s41598-021-87911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogbaga CC, Stepien P, Johnson GN. Sorghum (Sorghum bicolor) varieties adopt strongly contrasting strategies in response to drought. Physiol. Plant. 2014;152:389–401. doi: 10.1111/ppl.12196. [DOI] [PubMed] [Google Scholar]
- 47.Liu W, Zha L, Zhang Y. Growth and nutrient element content of hydroponic lettuce are modified by LED continuous lighting of different intensities and spectral qualities. Agronomy. 2020;10:1678. doi: 10.3390/agronomy10111678. [DOI] [Google Scholar]
- 48.Gan Y, Azrina A. Antioxidant properties of selected varieties of lettuce (Lactuca sativa L.) commercially available in Malaysia. Int. Food Res. J. 2016;23:2357–2362. [Google Scholar]
- 49.Sgherri C, et al. Elevated CO2 and salinity are responsible for phenolics-enrichment in two differently pigmented lettuces. Plant Physiol. Biochem. 2017;115:269–278. doi: 10.1016/j.plaphy.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Sardar H, et al. Enhancement of salinity stress tolerance in lettuce (Lactuca sativa L.) via foliar application of nitric oxide. Plants. 2023;12(5):115. doi: 10.3390/plants12051115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palma CFF, et al. Metabolic changes in cucumber leaves are enhanced by blue light but differentially affected by UV interactions with light signalling pathways in the visible spectrum. Plant Sci. 2022;321:111326. doi: 10.1016/j.plantsci.2022.111326. [DOI] [PubMed] [Google Scholar]
- 52.Schöttler MA, Tóth SZ, Boulouis A, Kahlau S. Photosynthetic complex stoichiometry dynamics in higher plants: Biogenesis, function, and turnover of ATP synthase and the cytochrome b 6 f complex. J. Exp. Bot. 2015;66:2373–2400. doi: 10.1093/jxb/eru495. [DOI] [PubMed] [Google Scholar]
- 53.Tikkanen M, Aro EM. Integrative regulatory network of plant thylakoid energy transduction. Trends Plant Sci. 2014;19:10–17. doi: 10.1016/j.tplants.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Nandini Y, Samir S. Reactive oxygen species, oxidative stress and ROS scavenging system in plants. J. Chem. Pharm. Res. 2016;8:595–604. [Google Scholar]
- 55.Ding X, et al. Exogenous glutathione improves high root-zone temperature tolerance by modulating photosynthesis, antioxidant and osmolytes systems in cucumber seedlings. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akram NA, et al. Foliage application and seed priming with nitric oxide causes mitigation of salinity-induced metabolic adversaries in broccoli (Brassica oleracea L.) plants. Acta Physiol. Plant. 2020;42:1–9. doi: 10.1007/s11738-020-03140-x. [DOI] [Google Scholar]
- 57.Jadczak D, Bojko K, Kaymakanova M, Berova M. Salinity-induced changes in the antioxidant status of common basil plants (Ocimum basilicum L.) grown under controlled conditions. Horticulturae. 2022;8:775. doi: 10.3390/horticulturae8090775. [DOI] [Google Scholar]
- 58.Dewir YH, Alsadon A. Effects of nutrient solution electrical conductivity on the leaf gas exchange, biochemical stress markers, growth, stigma yield, and daughter corm yield of saffron in a plant factory. Horticulturae. 2022;8:673. doi: 10.3390/horticulturae8080673. [DOI] [Google Scholar]
- 59.Samarakoon UC, Weerasinghe PA, Weerakkody WAP. Effect of electrical conductivity (EC) of the nutrient solution on nutrient uptake, growth and yield of leaf lettuce (Lactuca sativa L.) in stationary culture. Trop. Agric. Res. 2006;18:13–21. [Google Scholar]
- 60.Chen Z, Soltis DE. Evolution of environmental stress responses in plants. Plant Cell Environ. 2020;43:2827–2831. doi: 10.1111/pce.13922. [DOI] [PubMed] [Google Scholar]
- 61.Kong SG, Okajima K. Diverse photoreceptors and light responses in plants. J. Plant Res. 2016;129:111–114. doi: 10.1007/s10265-016-0792-5. [DOI] [PubMed] [Google Scholar]
- 62.Sng BJR, et al. Combination of red and blue light induces anthocyanin and other secondary metabolite biosynthesis pathways in an age-dependent manner in Batavia lettuce. Plant Sci. 2021;310:110977. doi: 10.1016/j.plantsci.2021.110977. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y, et al. Blue light increases anthocyanin content and delays fruit ripening in purple pepper fruit. Postharvest Biol. Technol. 2022;192:112024. doi: 10.1016/j.postharvbio.2022.112024. [DOI] [Google Scholar]
- 64.Zhang Q, Tang W, Xiong Z, Peng S, Li Y. Stomatal conductance in rice leaves and panicles responds differently to abscisic acid and soil drought. J. Exp. Bot. 2022;74:1551–1563. doi: 10.1093/jxb/erac496. [DOI] [PubMed] [Google Scholar]
- 65.Lawson T, Blatt MR. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014;164:1556–1570. doi: 10.1104/pp.114.237107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y, et al. Effects of red and blue light on leaf anatomy, CO 2 assimilation and the photosynthetic electron transport capacity of sweet pepper (Capsicum annuum L.) seedlings. BMC Plant Biol. 2020;20:1–16. doi: 10.1186/s12870-020-02523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Su N, Wu Q, Shen Z, Xia K, Cui J. Effects of light quality on the chloroplastic ultrastructure and photosynthetic characteristics of cucumber seedlings. Plant Growth Regul. 2014;73:227–235. doi: 10.1007/s10725-013-9883-7. [DOI] [Google Scholar]
- 68.Hung NK, Trang HTH, Ha CH, Ngoc PB. Effects of blue and red LED lights on rubisco activase gene expression, CO2 fixation efficiency and plant morphology of Gerbera jamesonii. Vietnam J. Biotechnol. 2022;20:467–477. doi: 10.15625/1811-4989/16043. [DOI] [Google Scholar]
- 69.Suganami M, Suzuki Y, Sato T, Makino A. Relationship between Rubisco activase and Rubisco contents in transgenic rice plants with overproduced or decreased Rubisco content. Soil Sci. Plant Nutr. 2018;64:352–359. doi: 10.1080/00380768.2018.1433455. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.