Abstract
Objective
We aimed to compare visual and anatomical outcome in subretinal aflibercept vs. intravitreal aflibercept in the context of Pars Plana Vitrectomy (PPV), pneumatic displacement with subretinal air and subretinal tPA in patients with naïve submacular haemorrhage (SMH) secondary to neovascular age-related macular degeneration (nAMD).
Design
Retrospective interventional cohort study.
Participants
80 patients treated with subretinal aflibercept vs. intravitreal aflibercept in the context of PPV, subretinal air and subretinal tPA in patients with SMH secondary to naïve nAMD.
Methods
Records were reviewed. Best corrected visual acuity (BCVA), central subfoveal thickness (CST), and intraocular pressure (IOP) were recorded at baseline and 24 months after treatment.
Main outcome measures
BCVA, CST, and number of anti VEGF treatment over follow-up period.
Results
The average duration from onset of symptoms to surgery was 1.26 days (range 0–3 days). Based on review of OCT images, SMH was subretinal in all 80 patients (100%), and sub-RPE in 29 patients (36.3%). Forty-one patients (51.25%) were treated with subretinal aflibercept (“subretinal group”), and 39 patients (48.75%) were treated with intravitreal aflibercept injections (“intravitreal group”). The groups were well balanced for age and gender p = 0.6588, and p = 0.263, respectively). Both groups showed statistically significant improvement in BCVA and CST (for all groups: p < 0.001). The mean number of anti VEGF given during follow-up period was statistically significantly lower in the “subretinal group” (p < 0.0001).
Conclusion
This study shows better management of the CNV, with a statistically significant lower need for anti-VEGF injections when treated with subretinal aflibercept compared to intravitreal application.
Subject terms: Surgery, Eye diseases
Introduction
Subretinal haemorrhage is an uncommon and serious complication of neovascular age-related macular degeneration (nAMD), which might lead to permanent visual impairment and vision loss due to atrophic retinal changes [1, 2]. Animal studies demonstrate that haemorrhage in the subretinal space results in severe damage to photoreceptors and the outer nuclear layer within 24 hours [3]. Furthermore, it has been shown that further degeneration of photoreceptors and retinal pigment epithelium (RPE) occurs at 7 days [1].
Several surgical techniques have been proposed to displace submacular haemorrhage (SMH) with variable success.
To improve the outcome, vitreoretinal surgery has been attempted to drain the subretinal blood. However, in the 20 G era functional results were impacted by high rates of complications [4, 5]. Surgical removal of subretinal clots required a large retinotomy (approximately 310 mm) before microneedles (38 G/41 G) were available to approach the subretinal space, thereby increasing the risk for retinal detachment and proliferative vitreoretinopathy [6].
The combined technique of intravitreal tissue plasminogen activator (tPA) and gas injection induces lysis of the clot by tPA and subsequent mechanical displacement of the liquefied blood by the gas bubble [7]. This less invasive outpatient procedure of intravitreal tPA and expansile gas to displace SMH pneumatically was described with initial encouraging results, but its usefulness was limited to small or thin SMH [8].
Subretinal tPA has been used to liquefy the SMH and facilitate aspiration [6]. Clot lysis with subretinal tPA might avoid the need to create large retinotomies needed to introduce needles to remove the thrombus, but may still require two retinotomies and evacuation of blood fragments is difficult with flattening of the retina [9].
Early reports used subretinal tPA and perfluorocarbon liquid to facilitate aspiration through a small retinotomy [10, 11]. However, this technique has fallen out of favour because of dismal postoperative visual acuity (VA) and high rates of retinal detachment.
The combination of pars plana vitrectomy (PPV), subretinal tPA, and pneumatic displacement of SMH was proposed with variable rates of success [12, 13].
Injection of subretinal air in combination with subretinal tPA as a way to manage subretinal haemorrhage was described first by Martel and Mahmoud [14]. Addition of subretinal air enhances the buoyant force exerted on the haemorrhage and allows for more consistent and further displacement of haemorrhage from the macula. After the original report, interventional case series in Japan and India showed favourable outcomes, including further displacement of the haemorrhage, fewer postoperative complications, and earlier VA improvement [15].
A recent comparative interventional case series with a large cohort of patients used PPV and subretinal tPA with postoperative intravitreal antivascular endothelial growth factor (VEGF), on the hypothesis that concurrent postoperative use of anti-VEGF may prevent regression of treatment effect [16]. This technique relies on the forces influencing SMH mobility as described by Martel, Mahmoud and Stopa et al. [14, 17].
The safety and the benefit of combined subretinal intervention, i.e. anti-VEGF with tPA and air displacement, has been demonstrated [15, 18, 19]. The equally early subretinal injection of anti-VEGF agent might be beneficial and even have a synergistic action in prevention of CNV progression or recurrence.
To the best of our knowledge, we present the first study that compares subretinal aflibercept vs. intravitreal aflibercept in the context of PPV, pneumatic displacement with subretinal air and subretinal tPA in patients with naïve submacular haemorrhage secondary to nAMD.
Methods
A retrospective consecutive chart review was approved by the Buenos Aires Ethics Committee for the collection and analysis of data. The research adhered to the tenets of the Declaration of Helsinki. All data discussed in this study were fully anonymized before they were accessed. There was no need for informed consent.
Patient records from January 1st 2015 to July 31st 2019 were reviewed for cases of SMH due to naïve nAMD treated by PPV with subretinal tPA with concomitant subretinal or intravitreal aflibercept injection. During this period of time, the treatment protocol changed from intravitreal to subretinal anti-VEGF injection.
Study Participants
Study inclusion criteria included: (1) age 55 years or older; (2) SMH due to naïve nAMD; (3) onset of vision loss within 3 days before surgery; (4) SMH classified as small, i.e. not extending beyond the arcades; (5) PPV with subretinal tPA, subretinal air, and concomitant subretinal or intravitreal aflibercept administration; (6) 24 months of follow-up after surgery.
Exclusion criteria were (1) another concomitant ocular disease that causes CNV; (2) any previous treatment with ocular or systemic anti-VEGF agents; (3) treatment with anticoagulants or antiplatelets, such as aspirin, warfarin, clopidogrel, etc.; (4) large SMH, i.e. haemorrhage reaching the arcades.
Consecutive patient records were reviewed for demographic and clinical data; best-corrected visual acuity (BCVA) intraocular pressure (IOP), and OCT data before surgery and after 24 months; the time between vision loss and surgery (in days); surgery details (intravitreal vs. subretinal aflibercept injection); intra- and post-operative complications; anti-VEGF injections over 24 months follow-up (which were administered on a pro-re nata regiment, i.e. patients were re-injected in case of signs of exudation on the OCT and/or retinal haemorrhage and/or decrease in vision attributable to exudative of AMD); cataract surgery over 24 months follow-up.
Surgical Procedure
All patients underwent standard 3-port PPV for pneumatic displacement with subretinal injection of tPA. Following vitrectomy, tPA (25 mcg/0.1 mL) was injected into the subretinal space utilising a 38-gauge subretinal needle. A subretinal bleb was created in order to bathe the entire clot and to allow for optimal displacement of the haemorrhage.
In the “subretinal group”, aflibercept (2 mg/0.05 mL) was injected subretinally together with tPA. Following tPA injection, air was injected in the subretinal space in both groups, and an air-fluid exchange was performed. In the “intravitreal group”, aflibercept (2 mg/0.05 mL) was injected intravitreally at the end of the surgery. Following initial supine positioning, patients were instructed to maintain an upright posture (Fig. 1 and Fig. 2).
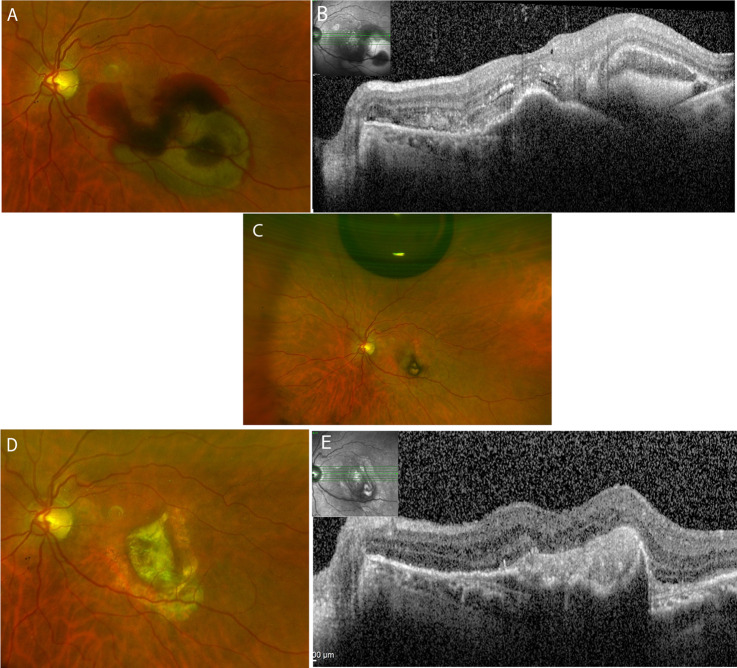
Fig. 1. Colour fundus and OCT over the follow-up of 24 months in a patient intreated with subretinal aflibercept during the surgery.
A Submacular haemorrhage at the day of the surgery. B OCT displaying subretinal haemorrhage and pigment epithelial detachment. C Colour fundus on day 7 after the surgery showing an intravitreal air bubble and resolution of the haemorrhage. D 24 months after the surgery, a macular scar with surrounding atrophy is seen. E The OCT displays the scarring without CNV activity.
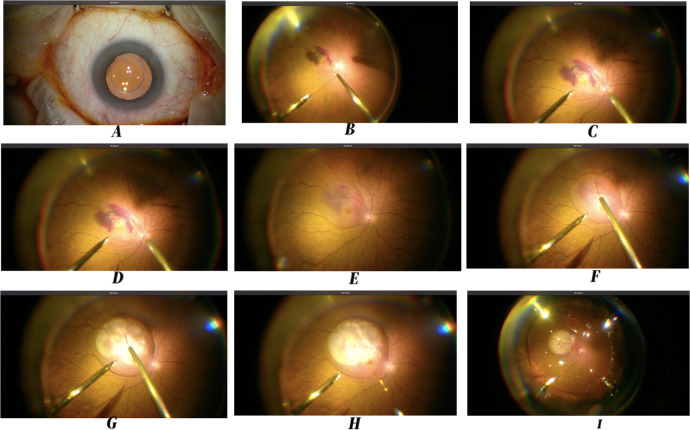
Fig. 2. Step by Step Surgery, including subretinal aflibercept injection.
A Betadine 5% Solution. B Central and peripheral vitrectomy. C Approaching subretinal space with 23–38 G cannula. D Entrance to the subretinal space with 23–38G cannula. E Subretinal tPA and aflibercept injected. F The clot has been dissolved by the tPA. G, H Air is injected into the subretinal space to displace the clot. I Active air-fluid exchange. Wrapping up the surgery with the eye filled up with air to displace the subretinal clot.
During the follow-up period after the surgical procedure, patients were treated based on a pro-re nata regiment: In case of increased central macular thickness due to intra- and/or subretinal fluid seen on OCT, new retinal haemorrhage on fundus exam, or decrease in vision which could be attributed to the CNV activity, patients received an intravitreal aflibercept injection 2 mg/0.05 ml.
OCT analysis
Prior to and 24 months after surgery, all included subjects underwent spectral domain-OCT scans (Spectralis; Heidelberg Engineering, Heidelberg, Germany). Retinal thickness was analysed using the retinal thickness map analysis protocol with nine Early Treatment Diabetic Retinopathy Study (ETDRS) subfields. Central foveal subfield thickness (CST) was defined as average retinal thickness of the circular area with 1 mm diameter around the foveal centre and recorded at baseline and 24 months after surgery. Qualitative OCT analysis included the presence of subretinal and sub-RPE haemorrhage, graded by 2 masked assessors (MI, JQ).
Outcome measures
Main outcome measures were the change of BCVA and CST at 24 months after the procedure and the number of anti-VEGF injections over the follow-up period in each group. Displacement of the haemorrhage was assessed within the first week after the intervention.
Statistical analysis
The demographics and clinical characteristics are described using mean ± standard deviation (numerical variables) and percent (categorical variables). Changes in VA and CST from baseline were tested by Wilcoxon sign-rank test. Univariate analysis for outcome measures was done using Mann-Whitney test (for numerical variables) and Chi Squared Test (for categorical variables) at baseline and after 24 months. A p value < 0.05 was considered significant. Statistical analysis was performed by the Stata 14.0 (StataCorp, Texas, USA).
Results
This study included 80 eyes of 80 patients (mean age 80.3 ± 3.7 years, 50% female). All patients had treatment naïve nAMD as the underlying cause of SMH. No patient had glaucoma at baseline. The average duration from onset of symptoms to surgery was 1.26 days (range 0-3 days). Based on review of OCT images, SMH was subretinal in all 80 patients (100%), and sub-RPE in 29 patients (36.25%). In all patients, the SMH did not reach the arcades.
Forty-one patients (51.25%) were treated with subretinal aflibercept (“subretinal group”), and 39 patients (48.75%) were treated with intravitreal aflibercept injections (“intravitreal group”). Baseline characteristics of both groups are shown in Table 1. At baseline, the subretinal group presented statistically significant better VA and lower CST.
Table 1.
Baseline characteristics.
| “Subretinal” eyes (n = 41) | “Intravitreal”eyes (n = 39) | P value | |
|---|---|---|---|
| Age, years, mean ± SD | 80.5 ± 5.5 | 80.3 ± 3.7 | 0.6588 |
| Male, n (%) | 18 (43.90) | 22 (56.41) | 0.263 |
| CST at baseline, µm, mean ± SD | 648 ± 161 | 769 ± 98 | 0.0001 |
| Baseline VA, logMAR, mean ± SD | 0.651 ± 0.134 | 0.749 ± 0.085 | 0.0006 |
| Pseudophakia at baseline, n (%) | 41 (100) | 27 (69.23) | <0.001 |
| IOP at baseline, n | 15.41 ± 1.74 | 14.89 ± 2.39 | 0.3962 |
CST central subfield thickness, VA visual acuity.
All patients in the “subretinal” group were pseudophakic, while 12/39 patients in the “intravitreal” group were phakic. There was no difference in terms of anatomical displacement and visual outcomes between patients with and without sub-RPE haemorrhages. The follow-up post-surgery was 24 months in all cases. The outcomes are shown in Table 2.
Table 2.
Results at last follow up in aflibercept “subretinal group” and “intravitreal group”.
| “Subretinal eyes” (n = 41) | “Intravitreal eyes” (n = 39) | P value | |
|---|---|---|---|
| Anatomical outcome | |||
| CST, µm, mean ± SD | 253 ± 21 | 239 ± 25 | 0.0346 |
| CST change baseline-last FU, µm, mean ± SD | 395 ± 164 | 529 ± 106 | 0.0001 |
| Visual outcome | |||
| VA, logMAR, mean ± SD | 0.473 ± 0.100 | 0.687 ± 0.117 | 0.2561 |
| VA change baseline-last FU, logMAR, mean ± SD | 0.178 ± 0.099 | 0.061 ± 0.91 | <0.0001 |
| Additional interventions | |||
| Number of additional aflibercept injections during FU, mean ± SD | 6.2 ± 1.4 | 15.5 ± 1.9 | <0.0001 |
| Eyes that underwent cataract surgery within FU, n (% of phakic eyes) | 0 | 12 (30.74) | - |
| Eyes that needed IOP-lowering treatment within FU, n | 0 | 0 | - |
CST central subfield thickness, FU follow-up, VA visual acuity.
Anatomical outcome
In the “subretinal group”, CMT was 648 ± 161 µm at baseline and 253 ± 21 µm at 24 months (p < 0.001). In the “intravitreal group”, CMT was 769 ± 98 µm at baseline and 239 ± 25 µm at 24 months (p < 0.001). The difference in CMT decrease was significantly greater in the “intravitreal” group (Table 2).
Visual outcome
In the “subretinal group” baseline VA was 0.651 ± 0.134 logMAR and 0.473 ± 0.100 logMAR at 24 months (p < 0.001). In the “intravitreal group”, baseline VA was 0.749 ± 0.085 logMAR and 0.687 ± 0.117 logMAR at 24 months (p = 0.0001). The improvement in vision was significantly greater in the subretinal group (Table 2).
Anti-VEGF injections
All patients received at least one aflibercept injections during the 24-month follow-up.
Patients in the subretinal group received a mean of 6.2 ± 1.4 aflibercept injections during the 24 months follow-up, while patients in the intravitreal group needed 15.5 ± 1.9 injections (p < 0.0001). The subretinal group needed significantly less injections (Table 2).
At the end of the follow-up period the disease activity was as follows: In the “subretinal group”, 36/41 patients had a stable CNV and did not need further intravitreal injection, 3/41 had a scar and 2/41 had retinal fluid which required intravitreal injection. In the “intravitreal group“, 3/39 patients had stable CNV and did not need further intravitreal injections, 4/39 had a scar and 32/39 had retinal fluid which required intravitreal injection.
Safety profile
All patients who were phakic at baseline (n = 12 in the “intravitreal group”) underwent cataract extraction during the follow-up period and were pseudophakic at the 24-month visit. No patient experienced increased intraocular pressure or developed postsurgical complication such us macular hole. No additional complications were noted in the subretinal group.
Discussion
To the best of our knowledge, we present the first study that shows better VEGF suppression with lower need for anti-VEGF injections in the 24months follow-up period after subretinal aflibercept vs. intravitreal aflibercept in the context of PPV, pneumatic displacement with subretinal air and subretinal tPA in patients with naïve submacular haemorrhage secondary to neovascular AMD.
We hypothesise that the better VEGF suppression after subretinal injection and less injections needed according with the PRN regiment protocol might be due to two reasons: First, by injecting aflibercept subretinally, the neovascular membrane is targeted at its actual site. VEGF is strongly expressed in the vascular endothelial cells and the RPE cells in the CNV membrane [20]. Second, pharmakokinetics might also play a role, i.e. the concentration of subretinal aflibercept in our study was 2 mg/0.05 mL. This concentration is far greater that the same amount of drug injected in the vitreal cavity of 4cc. This novel technique has not been standardised and the optimal concentration for subretinal application is still unknown. However, the safety of subretinal anti-VEGF injection using the “intravitreal” dosage in co-application with subretinal tPA has been shown to be safe and did not cause reduction of ERG amplitudes in a preclinical study [21]. Of note, tPA has been shown to degrade aflibercept in vitro [22]. We hypothesise, that in our study the drug worked directly without any mediators, as it was injected at the site of the pathology. The results of the ongoing TIGER study will shed more light on the synergy of subretinal tPA with intravitreal aflibercept [23]. Previous reports have showed the benefits of subretinal anti-VEGF in the management of subretinal haemorrhage from macular neovascularization [24, 25].
We believe that injection of the drug directly at the site of the CNV, and the supramaximal dose effect of aflibercept at the subretinal site, each on its one, and both together, might have impacted positively in the better control of the disease [26].
In the current study, we did not find any difference in terms of anatomical displacement and visual outcomes between patients with and without sub-RPE haemorrhages. We hypothesise that this is related with timing between diagnosis and treatment. Early intervention with subretinal tPA injection has been shown to be beneficial and induces liquefication of the clot and resolution of the sub-RPE component [27]. Sub RPE clot had worse prognosis than subretinal in some series of cases. A Series by Sacu et al. showed better functional results for patients who underwent early surgical intervention with tPA injection compared with anti-VEGF monotherapy.(6 days to 11.25 days from symptom onset to surgery) [28]. Our results are in accordance with some papers published showing that the faster the treatment is applied, the better the result will be in both groups, sub-RPE and subretinal. The limit of 1.1 ± 1.2 days from symptoms to treatment has been described to reach no differences neither in functional nor in anatomical results in these two groups: Sub-RPE clots and subretinal blood [29]. The presence of haemorrhage in the subretinal space results in severe damage to photoreceptors and the outer nuclear layer within 24 h and further degeneration of photoreceptors and RPE at 7 days [29].
The benefit of adding anti-VEGF to the initial procedure in cases of SMH has been shown in 2008 by Meyer et al. [30]. On the same page, we strongly believe that our early and consecutive approach of adding subretinal aflibercept to pneumatic displacement with subretinal tPA may minimise the scaffold for scar formation and might explain the favourable outcome with our novel triple treatment.
This manuscript shows that by using the subretinal aflibercept approach, the need and the burden of intravitreal anti-VEGF injections during 24 months after the surgery are reduced, when patients are treated with a Pro Re Nata regiment. One advantage of our study is that all patients had treatment naïve nAMD and presented a homogenous population regarding the neovascular membrane. On the other side, it has been shown previously that the half-life of anti VEGF agents is reduced after removal of the vitreous [30, 31]. Besides, an advantage of the subretinal co-application is to avoid the intravitreal injection at the end of the surgery and to spare another scleral intervention with possible complications, such as vitreous haemorrhage.
We strongly believe that the beneficial visual outcomes in our study are explained by the inclusion of small SMH and early intervention within 3 days from vision loss. Better initial VA has been shown to correlate with lesion size and the size of the haemorrhage [29]. Moreover, VA after treatment for SMH, was demonstrated to be better in patients with smaller haemorrhages and prompt surgery [32].
The limitations of the current study are related to the study population which included only patients with small haemorrhage who underwent surgery at a mean of 1.26 days. None of the patients were treated with anticoagulants or antiplatelet treatment. Angiography studies were not performed after the surgical intervention. During the 24-month follow-up period, patients were treated following a PRN regiment. When taking treatment decisions, physicians were not masked to the surgical procedure, and this might have caused bias.
In conclusion, we presented the first study that evaluated the efficacy and safety profile of sub-retinal compared to intravitreal aflibercept in the context of PPV, pneumatic displacement with subretinal air and subretinal tPA in patients with naïve SMH secondary to neovascular AMD. This study shows better management of the CNV, with statistically significant lower need for anti-VEGF injections over 24 months when treated with subretinal aflibercept compared to intravitreal application.
A randomised controlled trial is needed in order to establish the impact of pneumatic displacement with subretinal tPA and the current role of subretinal anti-VEGF application in the treatment of SMH surgery.
Data statement
All data generated or analysed during this study are included in this article or its supplementary material files. Further enquiries can be directed to the corresponding author.
Summary
What was known before
Intravitreal Aflibercept in Subretinal Haemorrhage due to naive neovascular AMD works well in order to ameliorate the condition.
What this study adds
This study shows better management of the CNV, with statistically significant lower need for anti-VEGF injections over 24 months when treated with subretinal aflibercept compared to intravitreal application.
Supplementary information
Acknowledgements
Javier Ignacio Melamud MD. Head of external Emergency room at the Medical intitute of investigation “ Alfredo Lanari “, University of Buenos Aires. General pactitiones and Legal medical specialst, Univesity of Buenos Aires, Argentina. Marina Koury MD. consultant in methodology, epidemiologic, and research from the teaching and investigation department at medical institute of investigation “Alfredo Lanari“, Univestity of Buenos Aires, Argentina.
Author contributions
MI, DJQ, MK—Substantial contributions to the conception or design of the work; the acquisition, analysis, and interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. JIM, LD, AB, AL, DZ—Substantial contributions to the conception or design of the work and interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submit- ted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethics
As this was a retrospective study, participants’ informed consent was not needed, in compliance with the Institutional review board (IRB) approval. This study protocol was reviewed and approved by the local IRB. The research adhered to the tenets of the Declaration of Helsinki.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-022-02222-z.
References
- 1.Toth CA, Morse LS, Hjelmeland LM, Landers MB. Fibrin directs early retinal damage after experimental subretinal hemorrhage. Arch. Ophthalmol. 1991;109:723-9. https://pubmed.ncbi.nlm.nih.gov/2025175/. Accessed 18 Dec 2021. [DOI] [PubMed]
- 2.Scupola A, Coscas G, Soubrane G, Balestrazzi E. Natural history of macular subretinal hemorrhage in age-related macular degeneration. Ophthalmologica. 1999;213:97–102. doi: 10.1159/000027400. [DOI] [PubMed] [Google Scholar]
- 3.Glatt H, Machemer R. Experimental subretinal hemorrhage in rabbits. Am J Ophthalmol. 1982;94:762–73. doi: 10.1016/0002-9394(82)90301-4. [DOI] [PubMed] [Google Scholar]
- 4.Hanscom TA, Diddie KR. Early surgical drainage of macular subretinal hemorrhage. Arch Ophthalmol. 1987;105:1722–3. doi: 10.1001/archopht.1987.01060120120037. [DOI] [PubMed] [Google Scholar]
- 5.Vander JF, Federman JL, Greven C, Slusher MM, Gabel VP. Surgical removal of massive subretinal hemorrhage associated with age-related macular degeneration. Ophthalmology. 1991;98:23–27. doi: 10.1016/S0161-6420(91)32348-0. [DOI] [PubMed] [Google Scholar]
- 6.Toth CA, Benner JD, Hjelmeland LM, Landers MB, 3rd, Morse LS. Ultramicrosurgical removal of subretinal hemorrhage in cats. Am J Ophthalmol. 1992;113:175–82. doi: 10.1016/S0002-9394(14)71530-2. [DOI] [PubMed] [Google Scholar]
- 7.Chen CY, Hooper C, Chiu D, Chamberlain M, Karia N, Heriot WJ. Management of submacular hemorrhage with intravitreal injection of tissue plasminogen activator and expansile gas. Retina. 2007;27. https://journals.lww.com/retinajournal/Fulltext/2007/03000/MANAGEMENT_OF_SUBMACULAR_HEMORRHAGE_WITH.7.aspx. [DOI] [PubMed]
- 8.Tsai S-C, Lin J-M, Chen H-Y. Intravitreous recombinant tissue plasminogen activator and gas to treat submacular hemorrhage in age-related macular degeneration. Kaohsiung J Med Sci. 2003;19:608–15. doi: 10.1016/S1607-551X(09)70514-5. [DOI] [PubMed] [Google Scholar]
- 9.Moriarty AP, McAllister IL, Constable IJ. Initial clinical experience with tissue plasminogen activator (tPA) assisted removal of submacular haemorrhage. Eye. 1995;9:582–8. doi: 10.1038/eye.1995.144. [DOI] [PubMed] [Google Scholar]
- 10.Kamei M, Tano Y, Maeno T, Ikuno Y, Mitsuda H, Yuasa T. Surgical removal of submacular hemorrhage using tissue plasminogen activator and perfluorocarbon liquid. Am J Ophthalmol. 1996;121:267–75. doi: 10.1016/S0002-9394(14)70274-0. [DOI] [PubMed] [Google Scholar]
- 11.Claes C, Zivojnovic R. Efficacy of tissue plasminogen activator (t-PA) in subretinal hemorrhage removal. Bull Soc Belg Ophtalmol. 1996;261:115–8. [PubMed] [Google Scholar]
- 12.Haupert CL, McCuen BW, 2nd, Jaffe GJ, Steuer ER, Cox TA, Toth CA, et al. Pars plana vitrectomy, subretinal injection of tissue plasminogen activator, and fluid-gas exchange for displacement of thick submacular hemorrhage in age-related macular degeneration. Am J Ophthalmol. 2001;131:208–15. doi: 10.1016/S0002-9394(00)00734-0. [DOI] [PubMed] [Google Scholar]
- 13.Olivier S, Chow DR, Packo KH, MacCumber MW, Awh CC. Subretinal recombinant tissue plasminogen activator injection and pneumatic displacement of thick submacular hemorrhage in age-related macular degeneration. Ophthalmology. 2004;111:1201–8. doi: 10.1016/j.ophtha.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Martel JN, Mahmoud TH. Subretinal pneumatic displacement of subretinal hemorrhage. JAMA Ophthalmol. 2013;131:1632–5. doi: 10.1001/jamaophthalmol.2013.5464. [DOI] [PubMed] [Google Scholar]
- 15.Kadonosono K, Arakawa A, Yamane S, Inoue M, Yamakawa T, Uchio E, et al. Displacement of submacular hemorrhages in age-related macular degeneration with subretinal tissue plasminogen activator and air. Ophthalmology. 2015;122:123–8. doi: 10.1016/j.ophtha.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 16.Chang W, Garg SJ, Maturi R, Hsu J, Sivalingam A, Gupta SA, et al. Management of thick submacular hemorrhage with subretinal tissue plasminogen activator and pneumatic displacement for age-related macular degeneration. Am J Ophthalmol. 2014;157:1250–7. doi: 10.1016/j.ajo.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Stopa M, Lincoff A, Lincoff H. Analysis of forces acting upon submacular hemorrhage in pneumatic displacement. Retina. 2007;27:370–4. doi: 10.1097/IAE.0b013e3180439bc9. [DOI] [PubMed] [Google Scholar]
- 18.Treumer F, Roider J, Hillenkamp J. Long-term outcome of subretinal coapplication of rtPA and bevacizumab followed by repeated intravitreal anti-VEGF injections for neovascular AMD with submacular haemorrhage. Br J Ophthalmol. 2012;96:708–13. doi: 10.1136/bjophthalmol-2011-300655. [DOI] [PubMed] [Google Scholar]
- 19.Avcı R, Yıldız AM, Çınar E, Yılmaz S, Küçükerdönmez C, Akalp FD, et al. Subretinal coapplication of tissue plasminogen activator and bevacizumab with concurrent pneumatic displacement for submacular hemorrhages secondary to neovascular age-related macular degeneration. Turk J Ophthalmol. 2021;51:38. doi: 10.4274/tjo.galenos.2020.72540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuoka M, Ogata N, Otsuji T, Nishimura T, Takahashi K, Matsumura M. Expression of pigment epithelium-derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol. 2004;88:809–15. doi: 10.1136/bjo.2003.032466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lüke M, Januschowski K, Warga M, Beutel J, Leitritz M, Gelisken F, et al. The retinal tolerance to bevacizumab in co-application with a recombinant tissue plasminogen activator. Br J Ophthalmol. 2007;91:1077–82. doi: 10.1136/bjo.2006.111260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klettner A, Grotelüschen S, Treumer F, Roider J, Hillenkamp J. Compatibility of recombinant tissue plasminogen activator (rtPA) and aflibercept or ranibizumab coapplied for neovascular age-related macular degeneration with submacular haemorrhage. Br J Ophthalmol. 2015;99:864–9. doi: 10.1136/bjophthalmol-2014-306454. [DOI] [PubMed] [Google Scholar]
- 23.Jackson TL, Bunce C, Desai R, Hillenkamp J, Lee CN, Lois N, et al. Vitrectomy, subretinal tissue plasminogen activator and Intravitreal Gas for submacular haemorrhage secondary to Exudative Age-Related macular degeneration (TIGER): study protocol for a phase 3, pan-European, two-group, non-commercial, active-control, observer-masked, superiority, randomised controlled surgical trial. Trials. 2022;23:99. doi: 10.1186/s13063-021-05966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gros-Otero J, Benítez-Herreros J, Beckford-Torngren C, Cámara-González C, Castro-Rebollo M. Management of subretinal hemorrhage with r-TPA, SF-6 and ranibizumab. Arch Soc Esp Oftalmol. 2010;85:114–7. doi: 10.1016/S0365-6691(10)70031-9. [DOI] [PubMed] [Google Scholar]
- 25.Chalam KV, Gasparian S. Successful delivery of subretinal aflibercept (new surgical technique) for the treatment of submacular hemorrhage in idiopathic polypoidal choroidal vasculopathy. J. Surg. Case Rep. 2021; 2021. https://pubmed.ncbi.nlm.nih.gov/34408845/ Accessed 9 Jul 2022. [DOI] [PMC free article] [PubMed]
- 26.Broadhead GK, Keenan TDL, Chew EY, Wiley HE, Cukras CA. Comparison of agents using higher dose anti-VEGF therapy for treatment-resistant neovascular age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022;260:2239–47. doi: 10.1007/s00417-021-05547-9. [DOI] [PubMed] [Google Scholar]
- 27.Sharma S, Kumar JB, Kim JE, Thordsen J, Dayani P, Ober M, et al. Pneumatic displacement of submacular hemorrhage with subretinal air and tissue plasminogen activator: initial united states experience. Ophthalmol Retin. 2018;2:180–6. doi: 10.1016/j.oret.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Sacu S, Stifter E, Vécsei-Marlovits PV, Michels S, Schütze C, Prünte C, et al. Management of extensive subfoveal haemorrhage secondary to neovascular age-related macular degeneration. Eye. 2009;23:1404–10. doi: 10.1038/eye.2008.267. [DOI] [PubMed] [Google Scholar]
- 29.de Silva SR, Bindra MS. Early treatment of acute submacular haemorrhage secondary to wet AMD using intravitreal tissue plasminogen activator, C3F8, and an anti-VEGF agent. Eye. 2016;30:952–7. doi: 10.1038/eye.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer CH, Scholl HP, Eter N, Helb H-M, Holz FG. Combined treatment of acute subretinal haemorrhages with intravitreal recombined tissue plasminogen activator, expansile gas and bevacizumab: a retrospective pilot study. Acta Ophthalmol. 2008;86:490–4. doi: 10.1111/j.1600-0420.2007.01125.x. [DOI] [PubMed] [Google Scholar]
- 31.Iglicki M, Busch C, Lanzetta P, Sarao V, Veritti D, Rassu N, et al. Vitrectomized vs non-vitrectomized eyes in DEX implant treatment for DMO-Is there any difference? the VITDEX study. Eye (Lond). 2022. 10.1038/s41433-022-01931-9. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 32.Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina. 1996;16:183–9. doi: 10.1097/00006982-199616030-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.