Abstract
Introduction
Recurrent Clostridioides difficile infection (rCDI) is common and associated with considerable clinical and economic consequences. REBYOTA™ (fecal microbiota, live-jslm [FMBL]) is a microbiota-based live biotherapeutic approved for the prevention of rCDI following antibiotic treatment for rCDI. We sought to evaluate cost-effectiveness of FMBL compared to standard of care (SOC) from a US third-party payer perspective among patients with one or more (≥ 1) recurrences.
Methods
A Markov model with a lifetime time horizon was developed. The model population included adult patients who had ≥ 1 recurrence after a primary CDI episode and had completed ≥ 1 round of antibiotics, or had ≥ 2 severe CDI episodes resulting in hospitalization within the last year. The model consisted of six health states with an 8-week model cycle: rCDI, absence of CDI after recurrence, colectomy, ileostomy, ileostomy reversal, and death. Drug costs and rCDI-related medical costs were estimated in 2022 US dollars and discounted at 3% annually. Deterministic sensitivity analyses were performed.
Results
Compared to SOC, FMBL at $9000/course resulted in an incremental cost-effectiveness ratio (ICER) of $18,727 per quality-adjusted life year (QALY) gained. The incremental cost was $5336 (FMBL $79,236, SOC $73,900) and the incremental effectiveness was 0.285 QALYs (FMBL 10.346, SOC 10.061). The cumulative drug acquisition and administration costs for the FMBL and SOC arms were $24,245 and $16,876, while rCDI-related medical costs for FMBL and SOC were $54,991 and $57,024, respectively. The ICER in the subgroup of patients at first recurrence was $13,727 per QALY gained. FMBL remained cost-effective across all sensitivity analyses.
Conclusions
FMBL was found to be cost-effective compared to SOC for the prevention of rCDI with more benefits among patients at first recurrence, with an ICER far below the payer ICER threshold of $100,000. Patients treated with FMBL experienced higher total QALYs and reduced healthcare resource utilization, including reduced hospitalizations.
Keywords: Recurrent Clostridioides difficile infection, REBYOTA, FMBL, Live fecal microbiota, Cost-effectiveness analysis, Economic modeling
Key Summary Points
| Why carry out this study? |
| Clostridioides difficile infection (CDI) is the most commonly isolated pathogen in patients with healthcare-associated infection in the USA. Many patients with CDI experience high recurrence rates. It is estimated that up to 35% of all patients with a primary CDI episode experience recurrent CDI (rCDI), and up to 65% of patients experience more recurrences after the first recurrence. |
| Treatments for primary CDI include oral antibiotics vancomycin or fidaxomicin. REBYOTA™ (fecal microbiota, live-jslm [FMBL]) has recently been approved for preventing recurrence of CDI following antibiotic treatment. |
| This study evaluated the cost-effectiveness of FMBL for the prevention of rCDI from a US third-party payer perspective. |
| What was learned from this study? |
| The effectiveness of FMBL in preventing recurrences compared to standard of care (SOC, no treatment to prevent recurrence following antibiotic treatment for rCDI, i.e., rCDI diarrhea being under control) led to improved quality of life, decreased healthcare resource utilization, and lower medical costs. |
| Compared to SOC, FMBL had an incremental cost-effectiveness ratio of $18,727 per quality-adjusted life year (QALY) gained, well below the cost-effectiveness threshold of $100,000 per QALY gained. |
| Furthermore, FMBL was more cost-effective when estimated in a subgroup of patients at first recurrence, suggesting potentially more benefits of treating patients early with FMBL to prevent subsequent recurrences. |
Introduction
Clostridioides difficile infection (CDI) is the most commonly isolated pathogen in patients with healthcare-associated infection in the USA [1]. Recurrent CDI (rCDI) is commonplace among patients with CDI. Current clinical treatment guidelines recommend antibiotics vancomycin or fidaxomicin for rCDI [2]. A real-world US claims study found that oral vancomycin was the most commonly used antibiotic for rCDI, with 55% of patients receiving it for their first recurrence, 56% for second recurrence, and 60% for third recurrence [3]. Despite the currently available treatments, rCDI remains common. Up to 35% of patients with a primary CDI episode experience recurrence(s) and up to 65% of patients who develop rCDI go on to have more recurrences [1, 4, 5].
REBYOTA™ (fecal microbiota, live-jslm [FMBL]) is a rectally administered suspension and is the first microbiota-based live biotherapeutic for the prevention of rCDI following antibiotic treatment for rCDI approved by the US Food and Drug Administration (FDA) [6]. Phase 3 clinical trial (PUNCH CD3 NCT03244644) has demonstrated superiority of FMBL in preventing rCDI, compared with standard of care (SOC), defined as no treatment to prevent recurrence following antibiotic treatment for rCDI (i.e., rCDI diarrhea being under control) in adult patients with rCDI [6]. The open-label phase 2 trial (NCT02589847) further suggested the durable treatment effect of FMBL up to 24 months [7]. Given the approval of FMBL for rCDI prevention, we evaluated cost-effectiveness of FMBL compared to SOC for prevention of rCDI from a US third-party payer perspective.
Methods
Model Overview
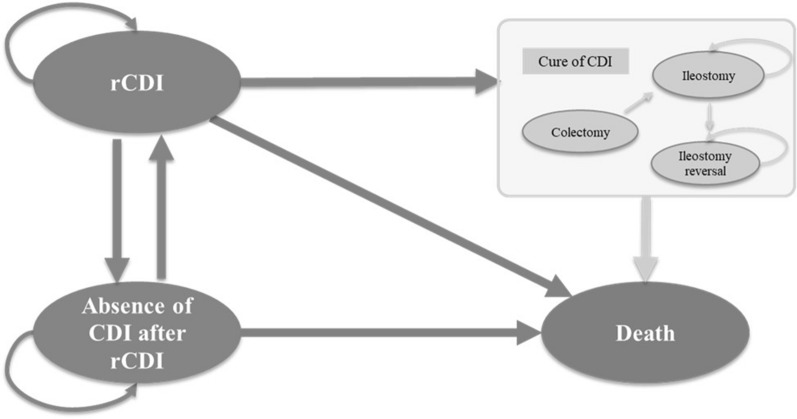
A Markov model was developed in Microsoft Excel® (Redmond, WA) to examine cost-effectiveness of FMBL to prevent rCDI vs. SOC from the perspective of a US third-party healthcare payer. In our model, six mutually exclusive health states were included: rCDI (starting state), absence of CDI after rCDI, colectomy (tunnel state to ileostomy), ileostomy, ileostomy reversal, and death (Fig. 1). All patients start in the rCDI state and receive FMBL or no treatment to prevent recurrence after antibiotic treatment for rCDI (i.e., rCDI diarrhea being under control). Successfully treated patients transition to the state of absence of CDI after rCDI. Patients in the absence of CDI after rCDI state can recur and move back to the rCDI state. Patients with rCDI who do not respond to FMBL (or SOC) can receive subsequent treatment with antibiotics (i.e., vancomycin taper-pulse or fidaxomicin), require colectomy, or die. Patients who undergo colectomy stay in the colectomy state for one model cycle and then transition to the post-colectomy state (i.e., ileostomy and/or ileostomy reversal) or death. After colectomy, patients are assumed to be cured of rCDI but are subject to death.
Fig. 1.
Model schematic. CDI C. difficile infection, rCDI recurrent C. difficile infection
An 8-week cycle length was used to reflect the primary assessment for time to recurrence in the FMBL clinical trials [6]. This model structure is consistent with prior cost-effectiveness analysis (CEA) models of rCDI and captures recurring disease over a long-term horizon [8, 9]. The CEA model adopted a lifetime time horizon (with patients’ life years [LYs] from the model entry at the age of 60.1 years old [the mean age of PUNCH-CD3 trial population] to death) to comprehensively capture differences in costs and health effects across treatments. Outcomes included LYs, quality-adjusted LYs (QALYs), and total and disaggregated costs (inflated to 2022 US dollars [USD]). Incremental cost-effectiveness ratios (ICERs) were calculated in terms of incremental cost per QALY gained and incremental cost per LY gained.
Target Population
The overall population for the economic evaluation included adult patients who had at least one recurrence after a primary episode of CDI and had completed at least one round of oral antibiotic therapy, or had at least two episodes of severe CDI resulting in hospitalization within the last year, as in the FMBL PUNCH CD3 trial [6]. Consistent with the trial population, patients entering the model had a mean age of 60.1 years old and the proportion female was 69.1% [6].
FMBL also demonstrated effectiveness in reducing rCDI as early as the first recurrence in the clinical trial. Therefore, a subgroup analysis of adult patients who had their first recurrence after a primary episode of CDI was performed to evaluate the cost-effectiveness of early treatment with FMBL.
Intervention and Comparators
The intervention was one rectally administered FMBL suspension. The comparator was SOC, defined as no treatment to prevent recurrence following antibiotic treatment for rCDI (i.e., rCDI diarrhea being under control), as proxied by the PUNCH CD3 trial placebo arm. In the model, patients treated with FMBL or SOC could then receive subsequent treatment with antibiotics if they had rCDI. The proportion of patients receiving subsequent antibiotics in each treatment arm was based on the response rates reported in the PUNCH CD3 trial and PUNCH open label study (OLS), as shown in Table 1. The subsequent antibiotics were a composite of orally administered vancomycin taper-pulse and fidaxomicin with treatment utilization weights informed by antibiotics used at screening in the PUNCH CD3 trial, as detailed in Table 2.
Table 1.
Clinical and utility inputs
| Base case value | Sensitivity low | Sensitivity high | Sources | Original value as reporteda | |
|---|---|---|---|---|---|
| Clinical inputs | |||||
| FMBL-specific | |||||
| Among patients with ≥ 1 rCDI | |||||
| Treatment success (%) 8 weeks | 70.6% | 63.7% | 76.8% | PUNCH CD3 [6] | 70.6% |
| Sustained response per 8 weeks (%) between 8 weeks and 6 monthsb | 96.4% | N/A | N/A |
PUNCH CD3 (8 weeks and 6 months) [6] PUNCH CD Open Label (12 and 24 months) [10] |
92.1% |
| Sustained response per 8 weeks (%) between 6 and 12 monthsb | 98.5% | N/A | N/A | 95.3% | |
| Sustained response per 8 weeks (%) between 12 and 24 monthsb | 98.4% | N/A | N/A | 90.0% | |
| Among patients at first recurrence | |||||
| Treatment success (%) 8 weeks | 81.0% | N/A | N/A | PUNCH CD3 [6] | 81.0% |
| Sustained response per 8 weeks (%) between 8 weeks and 6 monthsb | 95.6% | N/A | N/A |
PUNCH CD3 (8 weeks and 6 months) [6] Assumption |
90.5% |
| Sustained response per 8 weeks (%) between 6 and 12 monthsb | 98.5% | N/A | N/A | 95.3% | |
| Sustained response per 8 weeks (%) between 12 and 24 monthsb | 98.4% | N/A | N/A | 90.0% | |
| SOC-specific | |||||
| Among patients with ≥ 1 rCDI | |||||
| Treatment success (%) 8 weeks | 57.5% | 48.4% | 68.2% | PUNCH CD3 [6] | 57.5% |
| Sustained response per 8 weeks (%) between 8 weeks and 6 monthsb | 95.7% | N/A | N/A |
PUNCH CD3 (8 weeks and 6 months) [6] PUNCH CD Open Label (12 and 24 months) [10] |
90.6% |
| Sustained response per 8 weeks (%) between 6 and 12 monthsb | 93.4% | N/A | N/A | 80.0% | |
| Sustained response per 8 weeks (%) between 12 and 24 monthsb | 93.3% | N/A | N/A | 63.6% | |
| Among patients at first recurrence | |||||
| Treatment success (%) 8 weeks | 60.0% | N/A | N/A | PUNCH CD3 [6] | 60.0% |
| Sustained response per 8 weeks (%) between 8 weeks and 6 monthsb | 93.0% | N/A | N/A |
PUNCH CD3 (8 weeks and 6 months) [6] Assumption |
85.0% |
| Sustained response per 8 weeks (%) between 6 and 12 monthsb | 93.4% | N/A | N/A | 80.0% | |
| Sustained response per 8 weeks (%) between 12 and 24 monthsb | 93.3% | N/A | N/A | 63.6% | |
| Subsequent antibiotic treatment, applied if patients experienced rCDI after entering the model | |||||
| Treatment success (%) 8 weeks | 54.3% | N/A | N/A | PUNCH CD3 [6] | 54.3%c |
| rCDI-related surgery rates | |||||
| Colectomy | 1.3% | N/A | N/A | Feuerstadt 2020 [11] | 7.3% (over 12 months) |
| Ileostomy reversal | 40.6% | N/A | N/A | Neal 2011 [12] | 79.0% (over 6 months) |
| Utility inputs | |||||
| Utility by health state | |||||
| Absence of CDI | 0.88 | 0.66 | 1.00 | Rajasingham 2020 [13] | – |
| rCDI | 0.42 | 0.32 | 0.53 | Wilcox 2017 [14] | – |
| Colectomy | 0.54 | 0.40 | 0.67 | Bartsch 2012 [15] | – |
| Ileostomy | 0.70 | 0.53 | 0.88 | Bartsch 2012 [15] | – |
| Ileostomy reversal | 0.86 | 0.65 | 1.00 | Rajasingham 2020 [13] | – |
| Mortality | |||||
| rCDI-related mortality | 1.8% | 0.6% | 3.2% | Olsen 2020 [16] | 10.9% (over 1 year) |
| Colectomy-related mortality | 55.5% | 43.6% | 66.1% | Peprah et al. 2019 [17] | 35.2% (over 30 days) |
Parameters were varied on the basis of clinical input from key opinion leaders, 95% confidence intervals in the case of efficacy inputs in the sensitivity analysis
CDI C. difficile infection, FMBL fecal microbiota, live-jslm, N/A not applicable, rCDI recurrent C. difficile infection, SOC standard of care
aInputs were converted to per 8-week cycle where applicable. Rates were transformed using the following formula 1 − e[ln(1 − original rate)/(time frame in weeks/8)]
bSustained response rates were reported among responders at the previous time point
cThe treatment success rate of subsequent antibiotic treatment was calculated among patients with at least three previous CDI episodes in the PUNCH CD3 trial
Table 2.
Cost inputs
| Base case value | Sensitivity low | Sensitivity high | Sources | |
|---|---|---|---|---|
| Drug acquisition costa | ||||
| Cost per unit (2022 USD) | ||||
| FMBL | $9000 | $6750 | $11,250 | Redbook [18] |
| SOC | $0 | N/A | N/A | Assumption |
| Subsequent antibiotic useb | ||||
| Vancomycin | $26.85 | $20.14 | $33.56 | Redbook [19] |
| Fidaxomicin | $194.14 | $145.61 | $242.68 | Redbook [19] |
| Strength per unit | ||||
| FMBL | 150 ml | N/A | N/A | PUNCH CD3 [6] |
| SOC | N/A | N/A | N/A | |
| Subsequent antibiotic useb | ||||
| Vancomycin | 125 mg | N/A | N/A | Redbook [19] |
| Fidaxomicin | 200 mg | N/A | N/A | Redbook [19] |
| Total units required per regimen | ||||
| FMBL | 1 | N/A | N/A | PUNCH CD3 [6] |
| SOC | N/A | N/A | N/A | Assumption |
| Subsequent antibiotic useb | ||||
| Vancomycin taper-pulse | 83 | N/A | N/A | IDSA 2021 guidelines [2] |
| Fidaxomicin | 20 | N/A | N/A | IDSA 2021 guidelines [2] |
| Treatment distribution among subsequent antibiotic users | ||||
| Vancomycin | 93.1% | N/A | N/A | PUNCH CD3 [6] |
| Fidaxomicin | 6.9% | N/A | N/A | PUNCH CD3 [6] |
| Total drug acquisition cost per regimen (2022 USD) | ||||
| FMBL | $9000 | $6750 | $11,250 | Calculation |
| SOC | $0 | N/A | N/A | |
| Subsequent antibiotic useb | $2342.36 | N/A | N/A | |
| Drug administration cost | ||||
| Unit cost per admin or pharmacy dispensing (2022 USD) | ||||
| FMBL | $113.85 | $85.39 | $142.31 | CMS physician fee schedule [20] |
| SOC | $0 | N/A | N/A | Assumption |
| rCDI-related medical cost | ||||
| Unit cost (2022 USD) | ||||
| Hospitalization per day | $2039.06 | $1529.30 | $2548.83 | HCUPnet [21] |
| ICU per day | $5232.00 | $3924.00 | $6540.00 | Halpern 2016 [22] |
| ED per visit | $1003.73 | $752.80 | $1254.66 | Nelson 2021 [23] |
| Post-acute care per day | $562.12 | $421.59 | $702.65 | Nelson 2021 [23] |
| Outpatient per visit | $208.67 | $156.50 | $260.84 | Optum360 National Fee Analyzer [24] |
| Stool test | $58.35 | $43.76 | $72.94 | Rodrigues 2017 [25] |
| Resource use per year | ||||
| Hospitalization stays | 1.60 | 1.20 | 2.00 | Rodrigues 2017 [25] |
| LOS per hospitalization | 15.80 days | N/A | N/A | Rodrigues 2017 [25] |
| ICU days | 0.18 | 0.14 | 0.23 | Rodrigues 2017 [25] |
| ED visits | 0.12 | 0.09 | 0.15 | Rodrigues 2017 [25] |
| Post-acute care days | 21.08 | 15.81 | 26.36 | Rodrigues 2017 [25]; Nelson 2021 [23] |
| Outpatient visits | 2.20 | 1.65 | 2.75 | Rodrigues 2017 [25] |
| Stool tests | 4.40 | 3.30 | 5.50 | Rodrigues 2017 [25] |
| Total rCDI-related medical costs (excluding colectomy-related cost and terminal care cost) | ||||
| Medical cost per year | $64,810.39 | N/A | N/A | Calculation |
| Medical cost per 8 weeksc | $9970.83 | N/A | N/A | Calculation |
| Colectomy-related costs | ||||
| Cost per event (2022 USD) | ||||
| Colectomy | $54,421.37 | $40,816.03 | $68,026.71 | Rodrigues 2017 [25] |
| Ileostomy reversal | $46,297.54 | $34,726.16 | $57,871.93 | Wilson 2013 [26] |
| Terminal care costs | ||||
| One-time cost (2022 USD) | ||||
| Terminal care | $53,332.75 | 39,999.56 | 66,665.94 | Byhoff 2017 [27] |
Cost parameters were varied by ± 25% in the sensitivity analysis
CMS Centers for Medicare & Medicaid Services, ED emergency department, FMBL fecal microbiota, live-jslm, HCUP Healthcare Cost and Utilization Project, ICU intensive care unit, LOS length of stay, N/A not applicable, rCDI recurrent C. difficile infection, SOC standard of care, USD United States dollar
aThe dosing schedules for subsequent antibiotics were based on IDSA 2021 guidelines (vancomycin taper-pulse over 6 weeks, fidaxomicin 10 days)
bDrug costs of subsequent antibiotics were estimated on the basis of the average wholesale acquisition cost of oral forms (tablet or solution) of vancomycin and fidaxomicin taken from Redbook
cThe total annual rCDI-related medical costs were calculated as the sum of the resource use per year multiplied by the unit cost across care settings and then were converted to cost per 8-week cycle (8 out of 52 weeks of the annual cost) and applied to patients that stayed in the rCDI health state per model cycle
Clinical Inputs
Transition probabilities between health states were based on the PUNCH CD3 trial, PUNCH OLS, and literature [6], as shown in Table 1. Treatment arm-specific transition probabilities between the absence of rCDI and rCDI health states were informed by the PUNCH CD3 trial and PUNCH OLS and were calculated separately for 0–8 weeks, 8 weeks–6 months, 6–12 months, and 12–24 months. All transition probabilities were converted to rates per 8 week to align with the model cycle. For patients with one or more recurrences, transition probabilities between the absence of rCDI and rCDI health states from 0 to 8 weeks were informed by the adjusted PUNCH CD3 trial treatment success rate at 8 weeks (FMBL 70.6%, placebo 57.5%) [28]. Transition probabilities from 8 weeks to 6 months were estimated from the sustained response rates from the PUNCH CD3 trial [6]. Transition probabilities from 6 to 12 months and from 12 to 24 months were estimated from the sustained response rates at 12 months and 24 months from the PUNCH CD OLS [10]. The transition probability for FMBL and SOC beyond 24 months was assumed to be the same as the sustained response rate for SOC between 12 and 24 months.
For the subgroup of patients at first recurrence, transition probabilities between the absence of rCDI and rCDI health states were informed by the adjusted analysis of the PUNCH CD3 trial data among the subgroup of patients at first recurrence. In a post hoc analysis of the modified intention-to-treat (mITT) population enrolled after exactly one CDI recurrence (86/262 patients [32.8%]), FMBL demonstrated a 21% absolute risk reduction and a 52.5% relative risk reduction of recurrence in comparison to placebo by week 8. Treatment success was achieved by 81% of FMBL-treated patients compared to 60% of placebo-treated patients at week 8. This analysis adjusted for differences in known risk factors for recurrence, including age, gender, antibiotics use, and proton pump inhibitor (PPI) use between the FMBL and placebo arms (Ferring Data on File 2022). Transition probabilities from 0 to 8 weeks were informed by the 8-week treatment success (FMBL 81.0%, placebo 60.0%) and the 8 weeks–6 months were informed by the sustained response at 6 months (FMBL 90.5%, placebo 85.0%) converted to an 8-week rate (FMBL 95.6%, placebo 93.0%) based on subgroup analyses of the PUNCH CD3 trial data among patients at first recurrence [6]. As a result of lack of data, transition probability beyond 6 months was assumed to be the same as the overall population with more than one rCDI.
Transition probabilities to the surgery states (colectomy, ileostomy, ileostomy reversal) were informed by literature and converted to 8-week cycle rates [11, 12]. Within each 8-week model cycle, a health state-specific probability of death was applied based on the literature. The natural mortality rate of the target population was informed by a combination of age- and sex-specific mortality, based on the US life tables from the National Center for Health Statistics, and was multiplied by the standard mortality ratio for the rCDI population compared to the general US population [29]. Additionally, rCDI-related and surgery-related mortality rates were extracted from the literature and converted to an 8-week cycle rate and applied to the transition from rCDI or colectomy to death [16, 17].
Utility Inputs
Utility values were applied to specific health states, independent of treatment arm. As shown in Table 1, utilities were estimated using data from the literature [13–15]. The model did not include adverse events (AEs) because while patients experienced a higher incidence of mild gastrointestinal events in the FMBL vs. SOC arm, the moderate and severe AEs were comparable between the two treatment arms [28]. As a result, AE-related disutility was not considered in our model because of the anticipated minimal impact, if any.
Cost Inputs
The model considered costs of initial and subsequent treatments, rCDI-related medical care, and terminal care, detailed in Table 2, and were discounted at 3% annually as recommended by the Institute for Clinical and Economic Review [30]. The price of FMBL was set at $9000 per course. The drug costs for subsequent oral antibiotic treatments were estimated to be $2342 per treatment regimen based on the average IBM Micromedex Red Book wholesale acquisition cost (WAC) prices [19] and dosing schedules taken from the PUNCH CD3 trial and clinical guidelines [2, 6]. Drug administration costs were based on the Centers for Medicare and Medicaid Services (CMS) physician fee schedule from 2022 [20]. rCDI-related medical costs, including hospitalizations, intensive care unit (ICU) stays, emergency department (ED) visits, post-acute care (defined as a stay in skilled nursing facility, inpatient rehabilitation facility, or long-term acute care hospital or services provided by a home health agency), outpatient visits, and stool tests, as detailed in Table 2, were based on the literature [22, 23, 25, 26, 31], the Optum360 National Fee Analyzer [24], and the Healthcare Cost and Utilization Project (HCUP) [21]. The frequency of healthcare resource utilization (HRU) for rCDI was informed by the literature [25]. Rodrigues et al. 2017 was selected to inform HRU because it provided recent real-world data of rCDI-related HRU among patients with one or more recurrences. Publications focusing on patients with one recurrence (likely less severe patients) were not considered appropriate for this study because of the focus of the patient population. In addition, publications reporting all-cause HRU among patients with at least one rCDI were not used because rCDI-related HRU cannot be distinguished from the reported all-cause HRU. The annual medical cost was calculated as the sum of the resource use per year as reported in the literature multiplied by the unit cost for each medical cost component and was then transformed into a cost per 8 weeks (estimated as 8 out of 52 weeks of the annual medical cost). The 8-week medical cost was then applied to patients who stayed in rCDI health state per 8-week model cycle. Cumulatively, the total rCDI-related medical cost for a patient was proportional to the time the patient spent in rCDI health state (i.e., number of 8-week model cycles in rCDI health state) over the model time horizon. Colectomy-related cost was applied at the time of an event (i.e., colectomy and ileostomy reversal). Terminal care cost was one-time cost applied upon death. All costs were inflated to 2022 USD using the US Bureau of Economic Analysis Personal Consumption Expenditure (PCE) Index for health care services [32].
Sensitivity Analyses
Deterministic sensitivity analyses (DSAs) were conducted to examine the influence of specific inputs and assumptions. Parameters such as efficacy, annual HRU and unit costs, and utility were varied one at a time for sensitivity analyses. Additional sensitivity analyses were conducted including restricting the target population to Medicare and commercial populations, varying the time horizon, and altering assumptions around sustained response. The high and low inputs for sensitivity analyses are outlined in Tables 1 and 2. Variability in primary clinical inputs was informed by the 95% confidence interval (CI) reported in the clinical trials. In the absence of data on the variability around costs and utility inputs, the high and low inputs were assumed to be plus or minus 25% of the base case value.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Base-Case Results
The cost-effectiveness results among patients with one of more rCDI is summarized in Table 3. Treatment with FMBL resulted in higher costs and improved quality of life compared to SOC. The incremental cost was $5336, with a total cost for FMBL at $79,236 and SOC at $73,900. The incremental effectiveness with FMBL was 0.285 QALYs, with the total QALYs over the lifetime time horizon being 10.346 for FMBL and 10.061 for SOC. In terms of LYs, the incremental effectiveness with FMBL was 0.264 LYs, with the total LYs over the lifetime horizon being 12.504 for FMBL and 12.240 for SOC. The resulting ICERs were $18,727 per QALY gained and $20,186 per LY gained for FMBL vs. SOC. The increase in cost due to the cumulative higher drug acquisition and administration costs (total costs $24,245 and $16,876 for the FMBL and SOC arms respectively) was slightly offset by the lower direct medical costs (direct rCDI-related costs $54,991 and $57,024 for the FMBL and the SOC arms, respectively).
Table 3.
Cost-effectiveness results among patients with ≥ 1 rCDI
| Outcomes | FMBL | SOC |
|---|---|---|
| Cost (2022 USD) | ||
| Total costs | $79,236 | $73,900 |
| Drug acquisition and administration costs | $24,245 | $16,876 |
| Medical costs | $54,991 | $57,024 |
| Effectiveness | ||
| Total QALYs | 10.346 | 10.061 |
| Total LYs | 12.504 | 12.240 |
| Incremental outcome of FMBL vs. SOC | ||
| Incremental costs | $5336 | |
| Incremental QALYs | 0.285 | |
| Incremental LYs | 0.264 | |
| ICER of FMBL vs. SOC (2022 USD) | ||
| Incremental cost per QALY gained | $18,727 | |
| Incremental cost per LY gained | $20,186 | |
ICER results are subject to rounding errors. Precise values of costs and effectiveness with more decimals were used in the model calculation
FMBL fecal microbiota, live-jslm, ICER incremental cost-effectiveness ratio, LY life year, QALY quality-adjusted life year, SOC standard of care, USD United States dollar
For the first recurrence subgroup, the resulting ICERs were $13,727 per QALY gained and $14,781 per LY gained for FMBL vs. SOC. The cost-effectiveness results for this subgroup are summarized in Table 4.
Table 4.
Cost-effectiveness results among patients at first recurrence
| Outcomes | FMBL | SOC |
|---|---|---|
| Cost (2022 USD) | ||
| Total costs | $78,607 | $73,969 |
| Drug acquisition and administration costs | $23,956 | $16,908 |
| Medical costs | $54,652 | $57,061 |
| Effectiveness | ||
| Total QALYs | 10.394 | 10.056 |
| Total LYs | 12.549 | 12.235 |
| Incremental outcome of FMBL vs. SOC | ||
| Incremental costs | $4638 | |
| Incremental QALYs | 0.338 | |
| Incremental LYs | 0.314 | |
| ICER of FMBL vs. SOC (2022 USD) | ||
| Incremental cost per QALY gained | $13,727 | |
| Incremental cost per LY gained | $14,781 | |
ICER results are subject to rounding errors. Precise values of costs and effectiveness with more decimals were used in the model calculation
FMBL fecal microbiota, live-jslm, ICER incremental cost-effectiveness ratio, LY life year, QALY quality-adjusted life year, SOC standard of care, USD United States dollar
Sensitivity Analyses
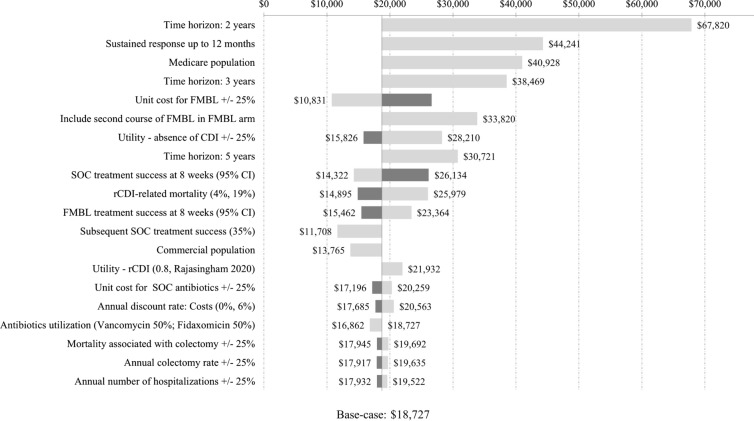
The results of one-way sensitivity and scenario analyses comparing FMBL vs. SOC among patients with one or more rCDI are shown as a tornado diagram in Fig. 2. The diagram sorts the sensitivity analyses from the widest to narrowest range of impact on the ICER for FMBL vs. SOC. Across the sensitivity analyses, the ICER ranged from $10,831 per QALY to $67,820 per QALY gained. For HRU/cost/utility-related inputs varied in the sensitivity analyses (± 25%), the most impactful model drivers included the unit cost of FMBL (increase $26,624/QALY, decrease $10,831/QALY), utility value for the absence of CDI health state (increase $28,210/QALY, decrease $15,826/QALY), and SOC treatment success at 8 weeks (increase $26,134/QALY, decrease $14,322/QALY).
Fig. 2.
Tornado diagram based on DSA/scenario analyses among patients with one or more rCDI.  Decrease in input value;
Decrease in input value;  Increase in input value. CI confidence interval, CDI C. difficile infection, DSA deterministic sensitivity analyses, FMBL fecal microbiota, live-jslm, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life year, rCDI recurrent C. difficile infection, SOC standard of care
Increase in input value. CI confidence interval, CDI C. difficile infection, DSA deterministic sensitivity analyses, FMBL fecal microbiota, live-jslm, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life year, rCDI recurrent C. difficile infection, SOC standard of care
For other varied parameters, the most impactful inputs included shortening the time horizon to 2 years and 3 years (ICER $67,820/QALY and $38,469/QALY, respectively), considering sustained response up to 12 months, instead of 24 months in base case ($44,241/QALY), Medicare population with a mean age of 78.1 years vs. the trial-based population with mean age of 60.1 years in base case ($40,928/QALY) and the inclusion of a second course of FMBL among patients treated with FMBL who experienced a recurrence ($33,820/QALY).
Discussion
Despite current antibiotic treatments, patients with rCDI experience a disproportionately higher economic burden due to elevated use of healthcare resources, including increased hospitalization and post-acute care, and more costly surgeries compared to patients with CDI who have not experienced a recurrence, let alone a higher mortality [23, 33, 34]. FMBL is the first in class microbiota-based live biotherapeutic which demonstrated efficacy in the prevention of rCDI following antibiotic treatment in clinical trials [6]. In the PUNCH studies, the treatment effect of FMBL in preventing rCDI was estimated to be 13.1% (70.6% with FMBL vs. 57.5% with placebo) at 8 weeks [6]. A sustained response was seen in over 90% of FMBL-treated patients at 6, 12, and 24 months [10]. To fully understand the clinical and economic values of FMBL in clinical practice, we evaluated the cost-effectiveness of FMBL compared to SOC from a US third-party payer perspective among patients with at least one recurrence. Other treatments (e.g., fecal microbiota transplant [FMT], bezlotoxumab) that are not FDA-approved or have limited use in real-world practice were not considered in our analysis [3, 35]. FMT is not approved by the FDA for the prevention of rCDI while bezlotoxumab is indicated for use in conjunction with antibiotic therapy to reduce rCDI, rather than after antibiotic therapy, and for patients with congestive heart failure, bezlotoxumab can only be used when benefit outweighs the risks. Further, recent real-world studies revealed that FMT and bezlotoxumab were used infrequently (only 8.5% of episodes were treated with bezlotoxumab or FMT for preventing rCDI) and bezlotoxumab was used mostly in immunosuppressed patients [35].
The findings from the CEA model demonstrated that FMBL was cost-effective for the prevention of rCDI vs. SOC. Based on the US Institute for Clinical and Economic Review’s recommended health–benefit price benchmark of $100,000 per QALY gained [30], the base case ICER of $18,727 per QALY is well below that threshold, suggesting that FMBL is a highly cost-effective treatment for rCDI compared to SOC. The treatment was even more cost-effective at $13,727 per QALY gained when estimated among the subgroup of patients at first recurrence, suggesting that FMBL would be beneficial for treating patients as early as after their first recurrence. When varying other input parameters and assumptions to account for uncertainty in the model, all results remain cost-effective based on the health–benefit price benchmark threshold of $100,000 per QALY.
Several things should be noted when interpreting the results of the CEA. Utilities were applied by health state and FMBL-treated patients were more likely to remain in the absence of rCDI state, which was associated with a higher/better utility and thus led to higher QALYs compared to SOC. Similarly, medical costs were considered by health state, and the higher effectiveness of FMBL in preventing recurrences led to a lower probability of patients transitioning to more costly health states, such as rCDI and the surgery-related states. The cost-effectiveness results were most sensitive to changes in the duration of sustained response, the model time horizon, Medicare population, and the inclusion of a second course of FMBL in the FMBL arm. FMBL remained cost-effective compared to SOC even when restricting to a shorter period of sustained response up to 12 months instead of 24 months. The 24-month response values were used in the base case as this fully captured FMBL’s efficacy over a longer duration of time as evidenced in the FMBL OLS trial. Shortening the time horizon to 2 or 3 years increased the ICER, due to the high initial drug costs of FMBL acquisition and administration. Similarly, the inclusion of an additional course of FMBL in the FMBL arm upon recurrence resulted in a higher ICER. Variations in other parameters such as health state utility values, rCDI-related mortality rate, and treatment success at 8 weeks in the SOC and FMBL treatment arms resulted in smaller variations in the ICER.
The findings of our study vary from previous CEA analyses assessing the value of treatments for rCDI. The incremental effectiveness of FMBL vs. SOC by 0.281 QALYs in our CEA was higher compared to previous CEA models of antibiotics, fecal microbiota transplant (FMT) and bezlotoxumab for treating or preventing rCDI. It is worth noting that the incremental effectiveness from previous studies may not be directly comparable to our study given the differences in model design (e.g., time horizon, comparators, target population). For example, Lam et al. (2018) compared fidaxomicin and bezlotoxumab + vancomycin with vancomycin alone, which resulted in incremental QALYs of 0.0027 and 0.0020 over 1-year time horizon, respectively [36]. Rajasingham et al. (2020) and Aby et al. (2022) evaluated the treatment strategies recommended by the Infectious Diseases Society of America (IDSA)/the Society for Healthcare Epidemiology of America (SHEA) guidelines where the incremental effectiveness varied from 0.009 to 0.072 QALYs over a lifetime time horizon [13, 37]. However, Rajasingham et al. (2020) and Aby et al. (2022) compared treatment strategies sequentially from the least expensive to the most expensive rather than a common comparator. Prabhu et al. (2017) found bezlotoxumab was associated with 0.12 QALYs gain compared to with placebo in preventing rCDI over a lifetime time horizon, which was about half of the QALY gain of FMBL vs. SOC in our study [9]. One explanation for their lower QALY gain could be that the patient population in Prabhu et al. (2017) were older and in worse health condition, including patients aged 65 years and older, patients who were immunocompromised, and patients with a clinically severe CDI episode [9].
Overall, the ICER of $18,727 per QALY gained for FMBL vs. SOC in our study was lower or comparable to previous CEAs. In a 2018 systemic literature review of CEA models among patients with rCDI, ICERs of fidaxomicin vs. vancomycin ranged from $20,757 per QALY gained (2016 USD) to dominating vancomycin (i.e., both clinically superior and cost saving) [38]. In two recent US CEA studies, ICER of treatment strategies for the first and subsequent rCDI was $31,751 per QALY gained (2018 USD) when comparing fidaxomicin + FMT with vancomycin + vancomycin [13] and $27,135 per QALY gained (2020 USD) for FMT only vs. vancomycin only [37]. The ICER of bezlotoxumab vs. placebo was estimated to be $19,824 per QALY gained (2015 USD) in preventing rCDI [9], which was comparable to the ICER of FMBL vs. SOC in our study.
Strengths and Limitations
This study is the first to evaluate the cost-effectiveness of FMBL compared to SOC for patients with rCDI in the USA. Prior studies have compared the cost-effectiveness of antibiotics and/or FMT in treating rCDI [13, 36–38], but antibiotics have proven to be ineffective in achieving a long-lasting cure for CDI [39], and FMT has not been approved by the FDA. Furthermore, the majority of prior studies evaluated treatments over abbreviated time horizons, whereas our model was conducted across a patient’s lifetime in order to capture all relevant difference in costs and benefits between the treatment arms that may occur beyond 1 year [13, 36, 37]. Further, a lifetime horizon was chosen as the International Society of Pharmacoeconomics and Outcomes Research recommends a sufficiently long time horizon to capture relative difference in outcomes across treatments [40]. In addition, Markov modeling, the approach used in this study, is a well-established modeling approach and has been commonly used in prior cost-effectiveness studies for rCDI treatments [13, 37]. We also included efficacy inputs directly from the PUNCH CD3 and PUNCH CD OLS trials [6]. Based on input from clinical experts, the model appropriately accounted for subsequent-line therapies by incorporating the drug acquisition and administration costs of these therapies, based on observed usage in the PUNCH CD3 trial.
A few limitations should be considered when interpreting the findings. Our model compared FMBL with SOC which was proxied by the placebo arm of the PUNCH CD3 trial following a course of antibiotic treatment. The trial data did not specify whether and to what extent a tapered/pulsed regimen may be used by a patient which may potentially bias findings against or for FMBL or placebo. However, given the randomization, the potential risk of the uneven distribution across the treatment arms is minimal. Additionally, efficacy results were based on the PUNCH CD3 and PUNCH CD OLS trials and only available up to 24 months because of the length of follow-up in the trials. Assumptions on long-term sustained response beyond 24 months were undertaken regarding the duration of treatment effects. Further, this study assumes that the efficacy seen in the trials would be transferrable to the effectiveness of a treatment observed in real-world practice, which could be revisited as real-world data on the use of FMBL become available. While sensitivity analyses tested variations in the assumptions around sustained response and the resulting ICERs remained cost-effective, additional studies examining long-term patient response beyond 24 months are warranted. Further research may consider using real-world data to confirm the findings when available. In addition, moderate and severe AEs were comparable between the two treatment groups [6] and are expected to have a minimal effect on the model results; therefore costs, and disutilities associated with AEs were not included in the model. Furthermore, this model was developed from a US payer perspective in general and did not differentiate the commercial payer from Medicare. Patients enrolled in a commercial health plan may have different HRU and costs from those enrolled in Medicare. Future studies can further evaluate the cost-effectiveness of FMBL among different types of payers. Lastly, while this study compared FMBL to SOC, future work could evaluate the cost-effectiveness of FMBL compared to other treatments for the prevention of rCDI.
Conclusion
FMBL is cost-effective compared to SOC at preventing rCDI with an ICER of $18,727 per QALY gained among patients with one or more rCDI, below the $100,000 threshold. The cost-effectiveness of FMBL is likely due to improved total QALYs, and reduced HRU, including lower costs of rehospitalization and/or subsequent treatments for rCDI. Compared to SOC, the higher drug costs associated with FMBL are partially offset by savings in medical costs. FMBL remains a cost-effective treatment strategy when alternative parameters and assumptions are tested. The cost-effectiveness findings support the use of FMBL for preventing CDI recurrences, with even more benefits among patients at first recurrence.
Acknowledgements
Funding
This study, including the journal fee and open access fee, was funded by Ferring Pharmaceuticals, Inc.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article. All authors have contributed significantly and are in agreement with the content of the manuscript.
Author Contributions
Model conceptualization and idea for this study: Amy Guo, Min Yang, Erin E. Cook, Wei Song, Thomas Lodise, and Markian Bochan; Model input collection and development: Amy Guo, Min Yang, Erin E. Cook, Wei Song, Danni Yang, Qingyuan Wang, and Angela Zhao; Model review: Amy Guo, Thomas Lodise, Markian Bochan; All authors contributed to the writing and reviewing of the manuscript and have approved the final manuscript.
Prior Presentation
An abstract reporting some of the data included in this manuscript was presented at the annual Academy of Managed Care Pharmacy 2023 conference in San Antonio, Texas.
Disclosures
Authors have a conflict of interest. Amy Guo is an employee of Ferring Pharmaceuticals, Inc. Min Yang, Erin Cook, Wei Song, Danni Yang, Qingyuan Wang, and Angela Zhao are employees of Analysis Group, which received funding from Ferring Pharmaceuticals, Inc. to conduct this study. Thomas Lodise and Markian Bochan received consulting fees from Ferring Pharmaceuticals, Inc. related to the submitted work.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson S, Lavergne V, Skinner AM, Gonzales-Luna AJ. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. IDSA Guidelines. 2021. [DOI] [PubMed]
- 3.Feuerstadt P, Boules M, Stong L, et al. Clinical complications in patients with primary and recurrent Clostridioides difficile infection: a real-world data analysis. SAGE Open Med. 2021;9:1–8. doi: 10.1177/2050312120986733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leong C, Zelenitsky S. Treatment strategies for recurrent Clostridium difficile infection. Can J Hosp Pharm. 2013;66(6):361. doi: 10.4212/cjhp.v66i6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl_2):S154–S161. doi: 10.1093/cid/cis462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khanna S, Assi M, Lee C, et al. Efficacy and safety of RBX2660 in PUNCH CD3, a phase III, randomized, double-blind, placebo-controlled trial with a Bayesian primary analysis for the prevention of recurrent Clostridioides difficile infection. Drugs. 2022;82(15):1527–1538. doi: 10.1007/s40265-022-01797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DE Orenstein R, Khanna S, et al. Durable reduction of Clostridioides difficile infection recurrence and microbiome restoration after treatment with RBX2660: results from an open-label phase 2 clinical trial. BMC Infect Dis. 2022;22(1):245. doi: 10.1186/s12879-022-07256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merlo G, Graves N, Brain D, Connelly LB. Economic evaluation of fecal microbiota transplantation for the treatment of recurrent Clostridium difficile infection in Australia. J Gastroenterol Hepatol. 2016;31:1927–1932. doi: 10.1111/jgh.13402. [DOI] [PubMed] [Google Scholar]
- 9.Prabhu VS, Dubberke ER, Dorr MB, et al. Cost-effectiveness of bezlotoxumab compared with placebo for the prevention of recurrent Clostridium difficile infection. Clin Infect Dis. 2018;66(3):355–362. doi: 10.1093/cid/cix809. [DOI] [PubMed] [Google Scholar]
- 10.Ferring. Durable reduction of Clostridioides difficile infection recurrence and microbiome restoration after treatment with RBX2660: results from an open-label phase 2 clinical trial [Ferring data on file]. 2021. [DOI] [PMC free article] [PubMed]
- 11.Feuerstadt P, Stong L, Dahdal DN, Sacks N, Lang K, Nelson WW. Healthcare resource utilization and direct medical costs associated with index and recurrent Clostridioides difficile infection: a real-world data analysis. J Med Econ. 2020;23(6):603–609. doi: 10.1080/13696998.2020.1724117. [DOI] [PubMed] [Google Scholar]
- 12.Neal MD, Alverdy JC, Hall DE, Simmons RL, Zuckerbraun BS. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Ann Surg. 2011;254(3):423–429. doi: 10.1097/SLA.0b013e31822ade48. [DOI] [PubMed] [Google Scholar]
- 13.Rajasingham R, Enns EA, Khoruts A, Vaughn BP. Cost-effectiveness of treatment regimens for Clostridioides difficile infection: an evaluation of the 2018 Infectious Diseases Society of America Guidelines. Clin Infect Dis. 2020;70(5):754–762. doi: 10.1093/cid/ciz318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilcox MH, Ahir H, Coia JE, et al. Impact of recurrent Clostridium difficile infection: hospitalization and patient quality of life. J Antimicrob Chemother. 2017;72(9):2647–2656. doi: 10.1093/jac/dkx174. [DOI] [PubMed] [Google Scholar]
- 15.Bartsch SM, Curry SR, Harrison LH, Lee BY. The potential economic value of screening hospital admissions for Clostridium difficile. Eur J Clin Microbiol Infect Dis. 2012;31(11):3163–3171. doi: 10.1007/s10096-012-1681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsen MA, Stwalley D, Demont C, Dubberke ER. Clostridium difficile infection increases acute and chronic morbidity and mortality. Infect Control Hosp Epidemiol. 2019;40(1):65–71. doi: 10.1017/ice.2018.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peprah D, Chiu AS, Jean RA, Pei KY. Comparison of outcomes between total abdominal and partial colectomy for the management of severe, complicated Clostridium difficile infection. J Am Coll Surg. 2019;228(6):925–930. doi: 10.1016/j.jamcollsurg.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.IBM Micromedex. Redbook. 2023.
- 19.IBM Micromedex. Redbook. 2022.
- 20.Centers for Medicare & Medicaid Services. Physician Fee Schedule. 2021.
- 21.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. 2019.
- 22.Halpern NA, Goldman DA, Tan KS, Pastores SM. Trends in critical care beds and use among population groups and Medicare and Medicaid beneficiaries in the United States: 2000–2010. Crit Care Med. 2016;44(8):1490. doi: 10.1097/CCM.0000000000001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson WW, Scott TA, Boules M, et al. Health care resource utilization and costs of recurrent Clostridioides difficile infection in the elderly: a real-world claims analysis. J Manag Care Spec Pharm. 2021;27(7):828–838. doi: 10.18553/jmcp.2021.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Optum360. National Fee Analyzer. 2020.
- 25.Rodrigues R, Barber GE, Ananthakrishnan AN. A comprehensive study of costs associated with recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2017;38(2):196–202. doi: 10.1017/ice.2016.246. [DOI] [PubMed] [Google Scholar]
- 26.Wilson M, Hollenbeak C, Stewart D. Impact of Clostridium difficile colitis following closure of a diverting loop ileostomy: results of a matched cohort study. Colorectal Dis. 2013;15(8):974–981. doi: 10.1111/codi.12128. [DOI] [PubMed] [Google Scholar]
- 27.Byhoff E, Harris JA, Langa KM, Iwashyna TJ. Racial and ethnic differences in end-of-life medicare expenditures. J Am Geriatr Soc. 2016;64(9):1789–1797. doi: 10.1111/jgs.14263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.FDA. FDA review of effectiveness and safety fecal microbiota, live (RBX2660). 2022.
- 29.National Center for Health Statistics. United States life tables, 2017. June 24, 2019. [PubMed]
- 30.ICER. 2020–2023 Value Assessment Framework. Institute for Clinical and Economic Review; 2020.
- 31.Paoli CJ, Reynolds MA, Sinha M, Gitlin M, Crouser E. Epidemiology and costs of sepsis in the United States—an analysis based on timing of diagnosis and severity level. Crit Care Med. 2018;46(12):1889. doi: 10.1097/CCM.0000000000003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.US Bureau of Economic Analysis. Price indexes for personal consumption expenditures by major type of product and by major function. 2022.
- 33.Amin ANW, Dreyfus J, Wong AC, et al. Mortality, healthcare resource utilization, and cost among Medicare beneficiaries with Clostridioides difficile infection with and without sepsis. Ther Adv Infect Dis. 2022 doi: 10.1177/20499361221095679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang D, Prabhu VS, Marcella SW. Attributable healthcare resource utilization and costs for patients with primary and recurrent Clostridium difficile infection in the United States. Clin Infect Dis. 2018;66(9):1326–1332. doi: 10.1093/cid/cix1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de la Villa S, Herrero S, Muñoz P, et al., editors. Real-world use of bezlotoxumab and fecal microbiota transplantation for the treatment of Clostridioides difficile infection. Open Forum Infect Dis. 2023;10(2):ofad028. [DOI] [PMC free article] [PubMed]
- 36.Lam SW, Neuner EA, Fraser TG, Delgado D, Chalfin DB. Cost-effectiveness of three different strategies for the treatment of first recurrent Clostridium difficile infection diagnosed in a community setting. Infect Control Hosp Epidemiol. 2018;39:924–930. doi: 10.1017/ice.2018.139. [DOI] [PubMed] [Google Scholar]
- 37.Aby ES, Vaughn BP, Enns EA, Rajasingham R. Cost-effectiveness of fecal microbiota transplantation for first recurrent Clostridioides difficile infection. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le PNV, Mullen PD, Deshpande A. Cost-effectiveness of competing treatment strategies for Clostridium difficile infection: a systematic review. Infect Control Hosp Epidemiol. 2018;39(4):412–424. doi: 10.1017/ice.2017.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrosillo N, Granata G, Cataldo MA. Novel antimicrobials for the treatment of Clostridium difficile infection. Front Med. 2018 doi: 10.3389/fmed.2018.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caro JJBA, Siebert U, et al. Modeling good research practices—overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Value Health. 2012;15(5):796–803. doi: 10.1016/j.jval.2012.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.