Abstract
Introduction
Hyperkalemia (HK) may result in disruptions of guidelines-concordant renin-angiotensin-aldosterone system inhibitors (RAASi), a standard of care in persons with chronic kidney disease (CKD). Such disruptions—dose reduction or discontinuation—diminish the benefits of RAASi, placing patients at risk of serious events and renal dysfunction. This real-world study evaluated RAASi modifications among patients who initiated sodium zirconium cyclosilicate (SZC) for HK.
Methods
Adults (≥ 18 years) initiating outpatient SZC (index date) while on RAASi were identified from a large US claims database (January 2018–June 2020). RAASi optimization (maintain same or up-titration of RAASi dosage), non-optimization (down-titration of RAASi dosage or discontinuation), and persistence were descriptively summarized following index. Predictors of RAASi optimization were assessed using multivariable logistic regression models. Analyses were conducted by subgroups, including patients without end-stage kidney disease (ESKD), with CKD, and with CKD + diabetes.
Results
A total of 589 patients initiated SZC during RAASi therapy (mean age 61.0 years, 65.2% male), and 82.7% patients (n = 487) kept RAASi after index (mean follow-up = 8.1 months). Most patients (77.4%) optimized RAASi therapy after initiating SZC; 69.6% maintained the same dosage while 7.8% had up-titrations. A similar rate of RAASi optimization was observed among subgroups without ESKD (78.4%), with CKD (78.9%), and with CKD + diabetes (78.1%). At 1-year post-index, 73.9% of all patients who optimized RAASi were still on therapy, while only 17.9% of patients who did not optimize therapy were still on a RAASi. Among all patients, predictors of RAASi optimization included fewer prior hospitalizations (odds ratio = 0.79, 95% CI [0.63–1.00]; p < 0.05) and fewer prior emergency department (ED) visits (0.78 [0.63–0.96]; p < 0.05).
Conclusion
Consistent with clinical trial findings, nearly 80% of patients who initiated SZC for HK optimized their RAASi therapy. Patients may require long-term SZC therapy to encourage continuation of RAASi therapy especially after inpatient and ED visits.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02518-w.
Keywords: Renin-angiotensin-aldosterone system inhibitors, Hyperkalemia, Sodium zirconium cyclosilicate, Chronic kidney disease, Modification, Optimization, Treatment persistence, Retrospective cohort study, Real-world evidence
Key Summary Points
| Why carry out this study? |
| Hyperkalemia (HK) may disrupt optimal use of renin-angiotensin-aldosterone system inhibitors (RAASi), thereby reducing their therapeutic benefits. |
| Since RAASi are a cornerstone therapy for chronic kidney disease (CKD) and other conditions such as heart failure and hypertension, it is important that patients can maintain their RAASi medications even after experiencing HK; that said, there are scant data beyond the clinical trial setting that characterizes RAASi modification after sodium zirconium cyclosilicate (SZC) treatment. |
| What did the study ask? |
| To address this knowledge gap, the authors sought to use administrative claims data to describe RAASi modification among patients treated with SZC to manage HK in the outpatient setting, including RAASi optimization (i.e., % of patients who maintained the same dosage or who had an up-titration), non-optimization (i.e., % of patients who discontinued or who had a down-titration), and persistence (i.e., duration of time on treatment). |
| What was learned from the study? |
| Consistent with clinical trial findings, this real-world study found that the majority of patients who initiated SZC for HK optimized their RAASi therapy (77.4%); similar results were observed among subgroups with different risk factors and underlying conditions, including those without end-stage renal disease, with CKD, and with CKD + diabetes. |
| Among the patients who optimized RAASi after SZC initiation, 73.9% were still on RAASi therapy at 1-year post-index (median time to discontinuation: not reached), whereas 17.9% of patients who did not optimize RAASi after SZC initiation were still on therapy at 1-year post-index (median 46.0 days to discontinuation). |
| Predictors of successful optimization of RAASi therapy at follow-up included fewer prior hospitalizations (odds ratio = 0.79, p < 0.05) and fewer prior emergency department (ED) visits (0.78; p < 0.05), indicating that patients may require long-term SZC therapy to encourage the use of ongoing RAASi therapy especially after hospitalization or ED visits. |
Introduction
Hyperkalemia (HK) is an electrolyte imbalance defined as a serum potassium level above the normal physiological range [1] with various thresholds used to indicate the degree of severity of HK [2]. Elevated levels of potassium may induce electrophysiological disturbances that can result in muscle weakness or paralysis and in cardiac arrhythmia [3]. The most serious clinical manifestations of HK are potentially life threatening and have been associated with increased all-cause and in-hospital mortality [4]. Since potassium is primarily eliminated via the kidneys, the risk of HK is increased among patients with renal dysfunction and/or metabolic disorders (e.g., diabetes) [1]. Indeed, HK is an established complication of reduced renal function in patients suffering from either chronic kidney disease (CKD) or acute renal failure [3]. The prevalence of HK in the USA in 2014 was 1.6%, and the prevalence of HK among patients with stage 3 CKD in the US was estimated to be 5% [4].
For patients with CKD or heart failure (HF), the current standard of care includes treatment with renin-angiotensin-aldosterone system inhibitors (RAASi) [5]. In particular, the KDIGO 2022 Diabetes in CKD and 2021 Clinical Practice Guidelines recommend treatment with RAASi such as angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) [5, 6] as they have been found to slow progression to end-stage kidney disease (ESKD) and reduce mortality [7–11]. However, the use of various RAASi such as ACEi, ARB, mineralocorticoid receptor antagonists (MRA), aldosterone receptor antagonists, angiotensin receptor neprilysin inhibitors (ARNi), and direct renin inhibitors (DRIs) has been shown to increase both the frequency and severity of HK [12]. Conversely, HK may disrupt optimal RAASi use, thereby diminishing its therapeutic benefits and placing patients at greater risk of cardiovascular events, renal dysfunction, or death [13, 14].
Novel anti-HK therapies (also known as novel oral potassium binders or gastrointestinal cation exchanger therapies), such as patiromer or sodium zirconium cyclosilicate (SZC), may be able to facilitate the use of RAASi and have recent guideline support from KDIGO 2021 and 2022 guidelines [5, 6] as well as a class 2b recommendation from ACC/AHA/HFSA guidelines in 2022 [15]. Notably, SZC, a selective potassium binder approved by the US Food and Drug Administration for the treatment of HK in 2018 [16], has been shown to promote RAASi persistence (i.e., continuing the treatment for the prescribed duration) and optimization (i.e., maintained or increased dose) in clinical trials [17].
Since RAASi are important treatments for CKD and other conditions such as HF and hypertension [5], it is important that patients with HK are able to maintain their RAASi medications even after experiencing HK. However, real-world data characterizing RAASi modifications including RAASi optimization after SZC treatment are limited. To address this knowledge gap, the present study aimed to describe RAASi modifications among patients treated with SZC to manage HK in the outpatient setting while the patients were on baseline RAASi therapy.
Methods
Data Source
Data were obtained from two large, closed medical and pharmacy insurance claims datasets from HealthVerity in the US between January 2018 and June 2020. The authors received permission to access and use the data from HealthVerity. The datasets contained information from over 115 million patients. The data were used in accordance with the Helsinki Declaration as revised in 2013 and de-identified in accordance with the Health Insurance Portability and Affordability Act of 1996. As this study utilized only de-identified data, no institutional review board waiver of informed consent approval or exemption was required, as per article 45 §CFR 164.514I.
Study Design and Sample Selection
OPTIMIZE I (Adoption of sodium zirconium cyclosilicate to utilize RAASi) was a retrospective, observational, noncomparative, and descriptive cohort study of adult patients with HK taking a RAASi who initiated SZC in an outpatient setting. The index date was the date of outpatient SZC therapy initiation, where the index RAASi was the RAASi being prescribed on the index date. The baseline period was the 6-month period prior to the index date. The follow-up period included the time post-index date until RAASi discontinuation, end of continuous enrollment, end of data availability, or death, whichever occurred first. All patients had at least 90 days of continuous pharmacy enrollment after the index date. Only patients who received at least one dose of SZC were included. However, there were no requirements regarding SZC use after the index dose; thus, patients who discontinued SZC were also included.
The study population included patients treated with SZC in the outpatient setting while on baseline RAASi treatment with at least 7 days of supply of overlap between SZC days’ supply and RAASi days’ supply. All patients were dispensed RAASi treatment during the baseline period. Eligible patients were aged 18 years or older on index with continuous pharmacy and medical enrollment during the 6 months prior to index date as well as continuous pharmacy enrollment at least 90 days after index RAASi days of supply. In this study, it was assumed that patients would only be taking SZC to treat hyperkalemia as this is the only indication in the label. Therefore, neither a diagnosis of HK nor a cut-off for serum potassium levels was required to determine patient eligibility. Analyses were conducted among all patients and in the following subgroups: patients without ESKD, patients with CKD, and patients with CKD + diabetes (either type I or type II diabetes). Furthermore, RAASi modifications were also examined among patients with HF, with hypertension, and by CKD stage.
Study Measurements and Outcomes
Patient demographics, clinical characteristics, treatment history, and healthcare resource utilization (HRU) were assessed during the baseline period. RAASi modifications and persistence were assessed during the follow-up period. Modifications in the dosing of RAASi included optimization and non-optimization. RAASi optimization was defined as maintenance of the same dose of RAASi or an up-titration of the RAASi dosage. Same dose of RAASi was the same average daily dose of the new prescription fill immediately post-index. Up-titration was defined as any increase in the average daily dose of the new prescription fill immediately post-index compared to the index RAASi or any new RAASi prescription (e.g., change in specific RAASi prescribed). Non-optimized RAASi was defined as down-titration of the RAASi dosage or discontinuation. Down-titration was defined as any decrease in the average daily dose of the new prescription fill immediately post-index compared to the index RAASi. Discontinuation was defined as no new RAASi prescription fill within 90 days of the end of days’ supply for the index RAASi. To avoid double counting of patients across different categories of RAASi modifications, patients were only classified to one category of RAASi modification following the hierarchy (highest to lowest order): (1) increased dose, (2) maintained same dose with no increased dose, (3) decreased dose with no increased/maintained dose, and (4) discontinued RAASi.
As an additional analysis, modifications in RAASi use were also dichotomized into kept RAASi vs. discontinued RAASi. Kept RAASi was defined as a new or refilled RAASi prescription fill, while discontinued RAASi was defined as no new or refilled RAASi prescription fill.
Statistical Analyses
Patient baseline characteristics were summarized descriptively using means and standard deviations (SDs) for continuous variables and counts and percentages for categorical variables. Characteristics were compared between patients who optimized RAASi and patients who did not optimize RAASi using Wilcoxon rank-sum tests for continuous variables and chi-square tests for categorical variables. Predictors of RAASi optimization were assessed using a multivariable logistic regression model and were reported as odds ratios (ORs) and 95% confidence intervals (CIs). Kaplan-Meier analyses were used to assess RAASi persistence, namely the median time from the index date to RAASi discontinuation. For all analyses, p value < 0.05 was considered statistically significant. All analyses were conducted using R 3.6.3.
Results
Overall, 589 patients treated with SZC met the study eligibility criteria (Fig. 1). Among this study population, 472 patients did not have ESKD, 398 had CKD, and 311 had CKD + diabetes. The mean duration of follow-up time in the full sample was 244.5 days (SD: 98.9 days, median: 229.0 days). The mean post-index duration of RAASi therapy was 210.4 days (SD: 126.5 days, median: 208.0 days), and the mean post-index duration of SZC therapy was 88.1 days (SD: 80.8 days, median: 58.0 days).
Fig. 1.
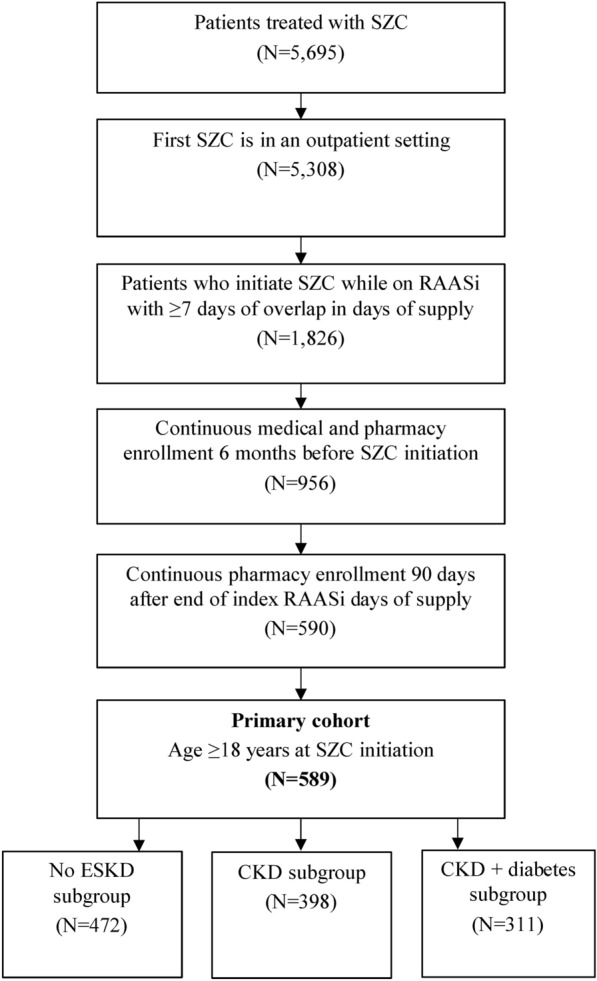
Selection of patients initiating SZC while taking a RAASi therapy. CKD chronic kidney disease; ESKD end-stage kidney disease; RAASi renin-angiotensin-aldosterone system inhibitor; SZC sodium zirconium cyclosilicate
RAASi Optimization
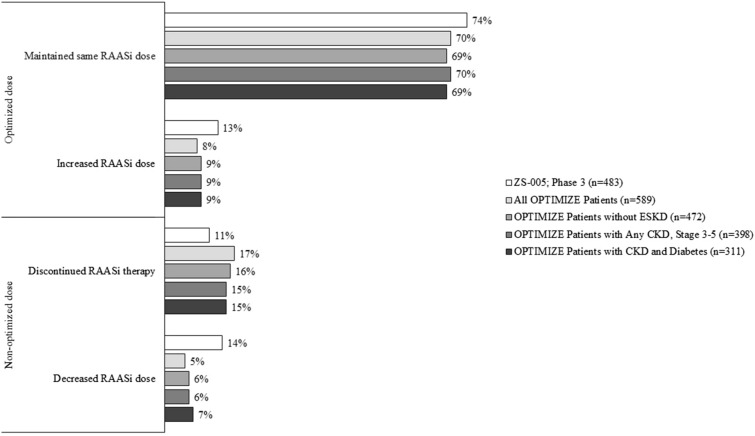
Among the overall sample, most patients (456/589 [77.4%]) optimized RAASi therapy after initiating SZC (Fig. 2). In particular, most patients (69.6%; 410/589) maintained the same dosage and 7.8% (46/589) had up-titrations. Fewer than a quarter of patients did not optimize RAASi therapy after initiating SZC (22.6%). Specifically, some patients (17.3%) discontinued RAASi (102/589) while fewer (5.3%) had down-titrations (31/589).
Fig. 2.
RAASi modifications after SZC initiation. Note: For the ZS-005 trial, patients were counted more than once if they required more than one RAASi adjustment so the total may exceed 100%. Rounding may cause differences of 1% or less among OPTIMIZE patient percentages. CKD chronic kidney disease; ESKD end-stage kidney disease; RAASi renin-angiotensin-aldosterone system inhibitors; SZC sodium zirconium cyclosilicate
The subgroup analysis of RAASi optimization yielded similar results for patients without ESKD, with CKD, and with CKD + diabetes. Specifically, the proportion of patients that optimized RAASi after initiating SZC was 78.4% in the subgroup without ESKD, 78.9% in the subgroup with CKD, and 78.1% in the subgroup with CKD + diabetes. RAASi modifications after SZC initiation among additional subgroups of individual stages of CKD, including patients with HF and hypertension, are presented in Supplementary Materials Table 1.
In the sensitivity analysis dichotomizing modifications in RAASi use (i.e., kept vs. discontinued), 82.7% kept RAASi therapy and 17.3% discontinued RAASi therapy after index. Among the patients that kept RAASi therapy, the mean post-index RAASi duration was 246.2 days (8.1 months, median 233.0 days).
Baseline Characteristics
Demographics, clinical characteristics, treatment history, and HRU at baseline are presented in Table 1. Demographics were similar between patients who optimized and did not optimize RAASi. In particular, the mean age was 61.0 years old, and most were male (65.2%). There was a mix of insurance types with patients who optimized RAASi vs. patients who did not optimize RAASi having a numerically higher proportion of patients with commercial insurance (48.0% vs. 41.4%) and lower proportion of patients with Medicare Advantage (27.6% vs. 38.3%, p = 0.06). Most patients on SZC had a HK diagnosis code during baseline (61.0%), with a numerically higher proportion of patients not optimizing RAASi having a diagnosis during baseline than patients who optimized RAASi (68.4% vs. 58.8%, p = 0.06). The most common comorbidities were hypertension (91.3%), diabetes (70.1%), and stage 3–5 CKD (67.6%). Additionally, 19.9% of patients had ESKD at baseline.
Table 1.
Baseline characteristics of patients initiating SZC
| All patients (N = 589) | Optimized RAASia (N = 456) | Did not optimize RAASib (N = 133) | p value | |
|---|---|---|---|---|
| Demographics | ||||
| Age on index date (years), mean ± SD | 61.02 ± 12.97 | 60.82 ± 12.81 | 61.70 ± 13.53 | 0.45 |
| Female gender, n (%) | 205 (34.8%) | 165 (36.2%) | 40 (30.1%) | 0.23 |
| Claims payer, n (%) | 0.06 | |||
| Commercial or unknown | 274 (46.5%) | 219 (48.0%) | 55 (41.4%) | |
| Medicaid | 138 (23.4%) | 111 (24.3%) | 27 (20.3%) | |
| Medicare advantage | 177 (30.1%) | 126 (27.6%) | 51 (38.3%) | |
| Region, n (%) | 0.71 | |||
| Midwest | 81 (13.8%) | 63 (13.8%) | 18 (13.5%) | |
| Northeast | 133 (22.6%) | 101 (22.1%) | 32 (24.1%) | |
| South | 214 (36.3%) | 170 (37.3%) | 44 (33.1%) | |
| West | 156 (26.5%) | 117 (25.7%) | 39 (29.3%) | |
| Other | 5 (0.8%) | 5 (1.1%) | 0 (0.0%) | |
| HK | ||||
| HK diagnosis during baseline, n (%) | 359 (61.0%) | 268 (58.8%) | 91 (68.4%) | 0.06 |
| Comorbidities, n (%) | ||||
| CKD stages 3–5 | 398 (67.6%) | 314 (68.9%) | 84 (63.2%) | 0.26 |
| ESKDc | 117 (19.9%) | 86 (18.9%) | 31 (23.3%) | 0.31 |
| Congestive heart failure | 149 (25.3%) | 114 (25.0%) | 35 (26.3%) | 0.85 |
| Coronary artery disease | 154 (26.1%) | 112 (24.6%) | 42 (31.6%) | 0.13 |
| Diabetes | 413 (70.1%) | 316 (69.3%) | 97 (72.9%) | 0.49 |
| Hypertension | 538 (91.3%) | 416 (91.2%) | 122 (91.7%) | 1.00 |
| Prior treatment, n (%) | ||||
| HK treatments | ||||
| Patiromer | 106 (18.0%) | 85 (18.6%) | 21 (15.8%) | 0.53 |
| Sodium polystyrene sulfonate | 92 (15.6%) | 66 (14.5%) | 26 (19.5%) | 0.20 |
| Loop diuretics | 250 (42.4%) | 185 (40.6%) | 65 (48.9%) | 0.11 |
| Thiazides and thiazide-like diuretics | 96 (16.3%) | 75 (16.4%) | 21 (15.8%) | 0.96 |
| Other treatments | ||||
| Beta blockers | 367 (62.3%) | 284 (62.3%) | 83 (62.4%) | 1.00 |
| NSAIDs | 51 (8.7%) | 40 (8.8%) | 11 (8.3%) | 1.00 |
| All-cause HRU | ||||
| Any inpatient stays, n (%) | 153 (26.0%) | 106 (23.2%) | 47 (35.3%) | < 0.01 |
| Number of inpatient stays, mean ± SD | 0.44 ± 0.93 | 0.38 ± 0.83 | 0.65 ± 1.19 | < 0.01 |
| Any ED visits, n (%) | 178 (30.2%) | 122 (26.8%) | 56 (42.1%) | < 0.01 |
| Number of ED visits, mean ± SD | 0.49 ± 1.00 | 0.41 ± 0.95 | 0.74 ± 1.13 | < 0.001 |
| Any outpatient visits, n (%) | 545 (92.5%) | 425 (93.2%) | 120 (90.2%) | 0.34 |
| Number of outpatient visits, mean ± SD | 14.66 ± 16.68 | 13.86 ± 15.15 | 17.41 ± 20.94 | < 0.05 |
Baseline was the 6-month period prior to the initiation of SZC
CKD chronic kidney disease; ED emergency department; ESKD end-stage kidney disease; HK hyperkalemia; HRU healthcare resource utilization; NSAID non-steroidal anti-inflammatory drug; SD standard deviation; SZC sodium zirconium cyclosilicate
aOptimized RAASi included patients with the same dose or with an up-titration
bNon-optimized RAASi included patients who discontinued or with a down-titration
cESKD was identified using ICD-10 diagnosis codes for ESKD or an ICD-10 diagnosis code for CKD stage 5 + a procedure code for dialysis
During the baseline period prior to SZC initiation, the most common HK treatments were loop diuretics (42.4%), patiromer (18.0%), and thiazide and thiazide-like diuretics (16.3%). Many patients were also taking beta blockers (62.3%) during the baseline period. While outpatient visits were common among the overall population at baseline (92.5% of patients had at least one), they were more frequent among patients who did not optimize RAASi compared to those who did optimize RAASi (mean of 17.4 vs. 13.9 outpatient visits, respectively; p < 0.05). Patients who did not optimize RAASi were more likely to have an emergency department (ED) visit (42.1% vs. 26.8%, p < 0.01) or inpatient hospital stay (35.3% vs. 23.2%, p < 0.01) than patients who optimized RAASi. Compared to patients who optimized RAASi, patients who did not optimize RAASi had a higher mean number of ED visits (0.74 vs. 0.41, p < 0.001) and inpatient stays (0.65 vs. 0.38, p < 0.01) during baseline. HK-related HRU was also common, with almost half of patients having an HK-related outpatient visit during baseline (48.6%). Patients who did not optimize RAASi were more likely than patients who optimized RAASi to have an HK-related ED visit (12.0% vs. 6.4%, p < 0.05) or inpatient stay (24.1% vs. 11.6%, p < 0.001) at baseline. Baseline characteristics for the subgroups were similar to those of the overall group (Supplementary Materials Tables 2–4).
Overview of RAASi at Index and RAASi Persistence
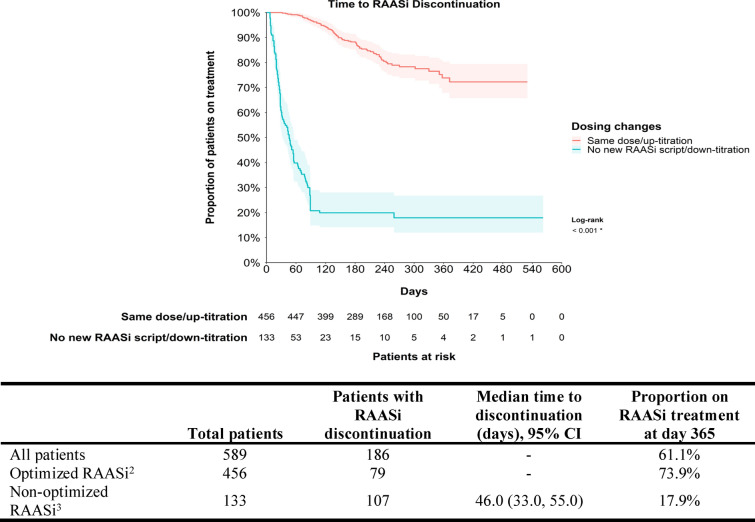
The most common RAASi agents used at index were similar between patients who optimized and did not optimize RAASi, with the most common being ACEi (optimized RAASi: 50.9%, non-optimized RAASi: 51.9%) and ARBs (42.8%, 40.6%; Table 2). Most patients were using monotherapy (91.7%). During follow-up, the median time to discontinuation was not reached for the overall sample, with 61.1% of patients still on a RAASi at 1 year (Fig. 3). In the sample that optimized RAASi, 73.9% were still on a RAASi at 1 year (median time to discontinuation: NR [not reached]). In the sample that did not optimize RAASi, only 17.9% of patients were still on a RAASi at 1 year (median time to discontinuation: 46.0 days). The RAASi used at initiation of SZC among patients without ESKD, with CKD, and with CKD + diabetes are presented in Supplementary Materials Table 5.
Table 2.
RAASi therapy at initiation of SZC
| All patients (N = 589) | Optimized RAASia (N = 456) | Did not optimize RAASib (N = 133) | p value | |
|---|---|---|---|---|
| RASi | 556 (94.4%) | 430 (94.3%) | 126 (94.7%) | 1.00 |
| ACEi | 301 (51.1%) | 232 (50.9%) | 69 (51.9%) | 0.92 |
| ARB | 249 (42.3%) | 195 (42.8%) | 54 (40.6%) | 0.73 |
| ARNI | 19 (3.2%) | 15 (3.3%) | 4 (3.0%) | 1.00 |
| DRI | 1 (0.2%) | 1 (0.2%) | 0 (0.0%) | 1.00 |
| MRA | 70 (11.9%) | 56 (12.3%) | 14 (10.5%) | 0.69 |
Patients could be taking monotherapy or combination therapy so the percentages may not add to 100%
ACEi angiotensin-converting enzyme inhibitors; ARB angiotensin receptor blocker; ARNI angiotensin receptor neprilysin inhibitor; DRI direct renin inhibitor; MRA mineralocorticoid receptor antagonist, RAASi renin-angiotensin-aldosterone system inhibitor; RASi renin-angiotensin system inhibitor
aOptimized RAASi included patients with the same dose or with an up-titration
bNon-optimized RAASi included patients who discontinued or with a down-titration
Fig. 3.
Median time from SZC initiation to RAASi discontinuation1. CI confidence interval; RAASi renin-angiotensin-aldosterone system inhibitor; SZC sodium zirconium cyclosilicate. 1Discontinuation was defined as the first gap > 90 days between the end of one record and the beginning of the next. 2Optimized RAASi included patients with the same dose or with an up-titration. 3Non-optimized RAASi included patients who discontinued or with a down-titration
Multivariable Model
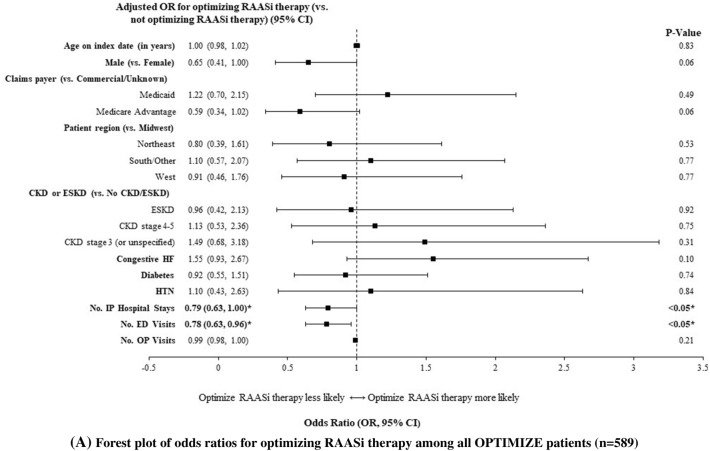
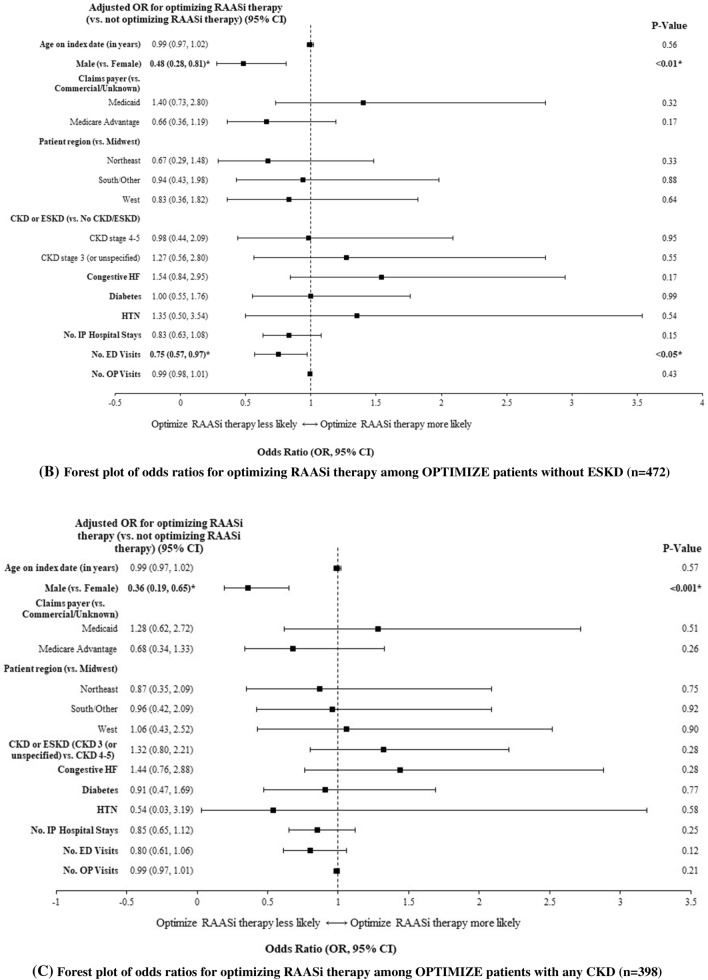
Predictors of successful optimization of RAASi therapy included fewer prior hospitalizations (OR = 0.79, 95% CI [0.63–1.00]; p < 0.05) and fewer prior ED visits (0.78 [0.63–0.96]; p < 0.05; Fig. 4). Similar findings were noted for the sensitivity definition, as predictors of having kept RAASi prescription fill included fewer prior hospitalizations (OR = 0.77, 95% CI [0.60–0.98]; p < 0.05) and fewer prior ED visits (0.78 [0.63–0.97]; p < 0.05). Among patients without ESKD, predictors of successful optimization of RAASi therapy included fewer prior ED visits (OR = 0.75, 95% CI: [0.57–0.97], p < 0.05) and female sex (0.48 [0.28–0.81], p < 0.01). Among patients with CKD, the only predictor of successful optimization of RAASi therapy was female sex (0.36 [0.19–0.65], p < 0.001).
Fig. 4.
Predictors of RAASi optimization1 after initiation of SZC among A all patients, B patients without ESKD, and C patients with CKD2,3. A Forest plot of odds ratios for optimizing RAASi therapy among all OPTIMIZE patients (n = 589). B Forest plot of odds ratios for optimizing RAASi therapy among OPTIMIZE patients without ESKD (n = 472). C Forest plot of odds ratios for optimizing RAASi therapy among OPTIMIZE patients with any CKD (n = 398). CI confidence interval; CKD chronic kidney disease; ED emergency department; ESKD end-stage kidney disease; HF heart failure; HTN hypertension; IP inpatient; OP outpatient; OR odds ratio; RAASi renin-angiotensin-aldosterone system inhibitor; SZC sodium zirconium cyclosilicate. 1Optimized RAASi included patients with the same dose or with an up-titration. Non-optimized RAASi included patients who discontinued or with a down-titration. 2Significant ORs are shown in bold. 3The above models’ dependent variable is RAASi optimization (levels: “RAASi optimized” and “RAASi not optimized”), and the reference level is “RAASi not optimized”
Discussion
The clinical burden associated with non-optimal use of RAASi among patients with HK is known to be substantial [13]. While RAASi are important in the treatment of many conditions, including CKD and HF, fears of HK and other cardiovascular effects often lead to the underutilization of RAASi (discontinuation, dose down-titration, and treatment interruption). Indeed, HK has been shown to be one of the main reasons for RAASi discontinuation and RAASi underutilization [18, 19]. The underutilization of RAASi is in turn associated with worsened cardiovascular and renal disease burden and death among patients with CKD [13, 14]. Thus, it is important to consider therapeutic approaches that would enable patients with HK to optimize their RAASi use. This retrospective, observational, cohort study examined the impact of SZC initiation on RAASi modification among a large sample of real-world patients. Results indicate that most patients kept their RAASi therapy (83%) and were able to optimize their RAASi use (77%). Nearly three quarters of patients who optimized therapy were still on RAASi at 1-year follow-up (median time to discontinuation: NR), whereas less than a quarter of those who did not optimize were still on RAASi (median time to discontinuation: 46.0 days). Even among patients at increased risk of recurrent HK (e.g., those with CKD + diabetes), consistent levels of keeping and optimizing RAASi were observed. Moreover, with respect to keeping and optimizing of RAASi, a consistent pattern of results was also observed in subgroups of patients without ESKD with all stages of CKD and for each stage of CKD. Factors predictive of RAASi optimization among the overall population included fewer prior ED visits and fewer prior inpatient hospitalizations, while female sex was a significant predictor among patients without ESKD and patients with CKD. Based on the results of the present study, treatment with SZC may be an effective strategy for optimizing the use of RAASi among patients in routine clinical practice, which is critical to ensuring that patients receive the documented cardiorenal benefits of maintained therapy.
The present real-world findings corroborate those of clinical trials, which show a benefit of novel anti-hyperkalemia therapies on RAASi persistence and optimization. In a two-part, open label, Phase III trial (ZS-005) of patients treated with SZC for HK, 89% continued RAASi use and only 11% discontinued RAASi use while taking SZC [17]. Moreover, the ZS-005 found that among patients on RAASi at study baseline, 87% of patients initiating SZC therapy for HK management maintained or increased their RAASi dose, while 74% continued their RAASi and 13% increased their dose [17]. To date, one retrospective observational cohort study of US veterans with HK found that those who received continuous treatment with the potassium binder, patiromer, over 6 months had high rates (~ 80%) of RAASi therapy continuation [20], which aligns with the beneficial impact of novel potassium binder treatment on RAASi use in the present study.
Previously, other real-world studies have examined how HK events and the severity of HK might impact RAASi use. In a large, electronic health records study by Epstein et al. [21], RAASi modifications were measured following HK events that were classified as mild or moderate-severe. After a mild HK event, about half of patients kept RAASi, while 16% down-titrated and 22% discontinued treatment. After a moderate-severe HK event, only 41% of patients kept RAASi, while 21% down-titrated and 26% discontinued treatment. Furthermore, patients who down-titrated or discontinued RAASi had worse clinical outcomes than patients on maximum doses [21]. A recent cohort study of patients treated with RAASi by Johnson et al. [14] found that patients with HK had a higher risk of a composite endpoint of cardiovascular events, renal dysfunction, and all-cause mortality than patients without HK (63.9% vs. 37.2%, respectively). Patients with HK were also more likely to deintensify RAASi therapy than patients without HK (34.4% vs. 29.2%), which may have contributed to their higher incidence of multiple adverse outcomes. Consistent with this, the risk of the composite outcome was higher among patients who lowered or stopped their RAASi therapy than those who continued RAASi therapy. In another study by Jun et al. [22], 47% of patients taking a RAASi who had a HK event changed their RAASi treatment (discontinuation: 37% and dose reduction: 10%). These studies by Epstein et al. [21] and Jun et al. [22] underscore an unmet need for strategies to help facilitate RAASi use following HK. However, neither study assessed the impact of treatment with oral anti-HK therapy (e.g., patiromer, SZC) on RAASi persistence and optimization. By assessing the impact of SZC, our study provides supportive evidence of anti-HK therapy as a facilitator of RAASi use following an HK event. Further research is needed to confirm this, given that the present study did not include a comparison to a control group of patients without potassium binder therapy after experiencing hyperkalemia.
Based on our multivariable analysis, patients with more frequent all-cause hospitalizations or ED visits are less likely to optimize RAASi. Findings of the present study suggest that patients with more all-cause hospitalizations and ED visits were less likely to optimize RAASi therapy after a HK event. This indicates that all-cause hospitalizations and ED visits after a HK event may lead clinicians to interrupt RAASi treatment and additional follow-up care may be required to improve RAASi optimization and encourage ongoing RAASi therapy. In addition to all-cause hospitalizations or ED visits, our subgroup analysis found that female sex was a significant predictor of RAASi optimization among patients with CKD and without ESKD. However, further studies may be needed to confirm this association and determine whether it reflects a biological sex difference or a gender difference in healthcare-seeking behavior. Additionally, further studies are needed to help clarify and better inform the approach of clinicians to long-term SZC therapy among patients taking a RAASi.
The present study had notable strengths. First, this is one of the first real-world studies to examine the impact of SZC initiation on RAASi modifications, including optimization and non-optimization, among a broader group of patients beyond the clinical trial setting. Previously, most clinical trial data of SZC had included self-selected patients who chose to participate in relatively small studies. In contrast, the present study made use of a large, closed claims database, which provided a rich set of data on a large number of eligible patients. As a result, our study sample was representative of patients with HK initiating SZC in the outpatient setting in the US and covered multiple payer types including those with Medicaid coverage. Moreover, the use of a closed database allowed all encounters covered by the insurance provider to be seen in the database.
The results of our study should also be viewed in the context of certain limitations. First, visibility into RAASi persistence and dosing is limited in insurance claims data by prescription fills and no visibility into the actual amount of medication taken. In this study, a new prescription fill record was required to classify patients as optimizing or not optimizing RAASi. However, there may have been changes in dosing or discontinuation not visible in the claims data. Thus, further research with detailed records of dosing and discontinuation would be beneficial to validate the findings of this study. Although we did not examine the dosing of SZC, we would expect it to be similar to the dosing observed in the RECOGNIZE I study, which used the same dataset and a similar patient population [23]. Second, since the study population is required to have continuous enrollment in a healthcare plan, patients who were enrolled for less than required (e.g., due to a change in employment status) were not included. If those patients differ from the overall population of patients initiating SZC, the results may not be generalizable. However, given the relatively short continuous enrollment requirement in the present study, we do not expect that this affected the generalizability of our findings significantly. Third, not all variables of interest are included in the claims data or may not be well recorded in claims data. For example, reasons for changes in RAASi dosing or treatment are not included and hence not analyzed. Additionally, data collected during the baseline period did not include the day of SZC initiation, during which a HK diagnosis might have been recorded. Indeed, hyperkalemia is often underdiagnosed in insurance claim datasets; therefore, the requirement of hyperkalemia diagnosis in addition to SZC treatment may have been unnecessarily narrow for our study population. Since the insurance claims data we used had very limited availability of laboratory data, we also could not characterize serum potassium levels at baseline. Nonetheless, even in the absence of HK diagnosis codes and serum potassium levels, we assumed HK was present among patients receiving SZC, as there are no other known indications for this treatment. Furthermore, the absence of laboratory potassium values in claims data limited our ability to assess the impact of HK severity and recurrence of HK on RAASi modifications. In future studies, a data source rich in laboratory values would be required to characterize potassium levels before RAASi modification and examine the impact of potassium levels on RAASi modification thereafter.
Conclusion
In a pattern consistent with clinical trial results, this real-world, administrative claims-based study found that the majority of patients were able to maintain and even optimize RAASi therapy after SZC initiation. These benefits of SZC treatment were observed overall and across subgroups of patients without ESKD and with CKD. Even among patients at increased risk of recurrent HK (e.g., those with CKD + diabetes), high levels of keeping and optimizing RAASi were observed. Patients may require long-term SZC therapy to encourage the use of ongoing RAASi therapy, especially after hospitalization or ED visits.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This work, including the study, medical writing support, Rapid Service Fee, and Open Access Fee, was supported by AstraZeneca.
Medical Writing and Editorial Assistance
Medical writing and editorial assistance were provided by Mona Lisa Chanda, PhD, a professional medical writer employed by Analysis Group, Inc., a company that received support from AstraZeneca.
Author Contributions
All authors, Abiy Agiro, Alpesh N. Amin, Erin E. Cook, Fan Mu, Jingyi Chen, Pooja Desai, Yemmie Oluwatosin, and Charles V. Pollack, have made substantial contributions to the conception or design of the study, or the acquisition, analysis, or interpretation of data, drafting the manuscript, and revising it critically for important intellectual content, and have provided final approval of this version to be published and agree to be accountable for all aspects of the work.
Prior Presentation
Portions of this manuscript were previously presented at the American Heart Association (AHA) Virtual Meeting from November 13–15, 2021 (VP258), and American Society of Nephrology (ASN) Kidney Week (virtual) from November 2–7, 2021 (PO1133), and will be presented at the National Kidney Foundation 2023 Meeting on April 11–15, 2023, in Austin, TX.
Disclosures
Alpesh N. Amin reported serving as PI or co-I of clinical trials sponsored by NIH/NIAID, NeuroRx Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, Alexion, unrelated to the present study; speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achogen LaJolla, Ferring, Seres, Millenium, Spero, Eli Lilly, PeraHealth, HeartRite, Aseptiscope, Sprightly, unrelated to the present study. Fan Mu, Erin Cook, and Jingyi Chen are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to AstraZeneca, which funded the development and conduct of this study and manuscript. Abiy Agiro, Pooja Desai, and Yemmie Oluwatosin are employees of AstraZeneca. Charles Pollack is a consultant for AstraZeneca.
Compliance with Ethics Guidelines
The authors received permission to access and use the data from HealthVerity. The data were used in accordance with the Helsinki Declaration as revised in 2013 and de-identified in accordance with the Health Insurance Portability and Affordability Act of 1996. As this study utilized only de-identified data, no institutional review board waiver of informed consent approval or exemption was required, as per article 45 §CFR 164.514I.
Data Availability
The data that support the findings of this study are not available from the authors but are available with permission from HealthVerity. Restrictions apply to the availability of these data, which were used under license for this study.
Contributor Information
Abiy Agiro, Email: abiy.agiro@astrazeneca.com.
Amin AN, Email: anamin@hs.uci.edu.
Erin E. Cook, Email: Erin.cook@analysisgroup.com
Fan Mu, Email: fan.mu@analysisgroup.com.
Jingyi Chen, Email: Jingyi.chen@analysisgroup.com.
Pooja Desai, Email: pooja.desai@astrazeneca.com.
Yemmie Oluwatosin, Email: yosolajes@gmail.com.
Charles V. Pollack, Jr., Email: cvpollack@gmail.com
References
- 1.Theisen-Toupal J. Hypokalemia and hyperkalemia. Issue Hosp Med Clin. 2014;4(1):34. doi: 10.1016/j.ehmc.2014.09.008. [DOI] [Google Scholar]
- 2.Tran HA. Extreme hyperkalemia. South Med J. 2005;98(7):729–732. doi: 10.1097/01.smj.0000149407.51134.77. [DOI] [PubMed] [Google Scholar]
- 3.Youn JH, McDonough AA. Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol. 2009;71:381–401. doi: 10.1146/annurev.physiol.010908.163241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betts KA, Woolley JM, Mu F, McDonald E, Tang W, Wu EQ. The prevalence of hyperkalemia in the United States. Curr Med Res Opin. 2018;34(6):971–978. doi: 10.1080/03007995.2018.1433141. [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes Blood Pressure Work G KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3):S1–S87. doi: 10.1016/j.kint.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes Blood Pressure Work G KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102(5S):S1–S127. doi: 10.1016/j.kint.2022.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 8.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 9.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997;349(9069):1857–1863. doi: 10.1016/S0140-6736(96)11445-8. [DOI] [PubMed] [Google Scholar]
- 10.Ruggenenti P, Perna A, Gherardi G, Gaspari F, Benini R, Remuzzi G. Renal function and requirement for dialysis in chronic nephropathy patients on long-term ramipril: REIN follow-up trial. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Ramipril Efficacy in Nephropathy. Lancet. 1998;352(9136):1252–1256. doi: 10.1016/s0140-6736(98)04433-x. [DOI] [PubMed] [Google Scholar]
- 11.Ruggenenti P, Perna A, Benini R, et al. In chronic nephropathies prolonged ACE inhibition can induce remission: dynamics of time-dependent changes in GFR. Investigators of the GISEN Group. Gruppo Italiano Studi Epidemiologici in Nefrologia. J Am Soc Nephrol. 1999;10(5):997–1006. doi: 10.1681/ASN.V105997. [DOI] [PubMed] [Google Scholar]
- 12.Tamargo J, Caballero R, Delpon E. New therapeutic approaches for the treatment of hyperkalemia in patients treated with renin-angiotensin-aldosterone system inhibitors. Cardiovasc Drugs Ther. 2018;32(1):99–119. doi: 10.1007/s10557-017-6767-5. [DOI] [PubMed] [Google Scholar]
- 13.Santoro A, Perrone V, Giacomini E, Sangiorgi D, Alessandrini D, Degli EL. Association between hyperkalemia, RAASi non-adherence and outcomes in chronic kidney disease. J Nephrol. 2022;35(2):463–472. doi: 10.1007/s40620-021-01070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson M, Morrison FJ, McMahon G, Su M, Turchin A. Outcomes in patients with cardiometabolic disease who develop hyperkalemia while treated with a renin-angiotensin-aldosterone system inhibitor. Am Heart J. 2023;258:49–59. doi: 10.1016/j.ahj.2023.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–e1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 16.LOKELMA™. Label and Prescribing Information. 2018.
- 17.Spinowitz BS, Fishbane S, Pergola PE, et al. Sodium zirconium cyclosilicate among individuals with hyperkalemia: a 12-month phase 3 study. Clin J Am Soc Nephrol. 2019;14(6):798–809. doi: 10.2215/CJN.12651018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yildirim T, Arici M, Piskinpasa S, et al. Major barriers against renin-angiotensin-aldosterone system blocker use in chronic kidney disease stages 3–5 in clinical practice: a safety concern? Ren Fail. 2012;34(9):1095–1099. doi: 10.3109/0886022X.2012.717478. [DOI] [PubMed] [Google Scholar]
- 19.Shirazian S, Grant CD, Mujeeb S, et al. Underprescription of renin-angiotensin system blockers in moderate to severe chronic kidney disease. Am J Med Sci. 2015;349(6):510–515. doi: 10.1097/MAJ.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 20.Kovesdy CP, Gosmanova EO, Woods SD, et al. Real-world management of hyperkalemia with patiromer among United States Veterans. Postgrad Med. 2020;132(2):176–183. doi: 10.1080/00325481.2019.1706920. [DOI] [PubMed] [Google Scholar]
- 21.Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21(11 Suppl):S212–S220. [PubMed] [Google Scholar]
- 22.Jun M, Jardine MJ, Perkovic V, et al. Hyperkalemia and renin-angiotensin aldosterone system inhibitor therapy in chronic kidney disease: a general practice-based, observational study. PLoS ONE. 2019;14(3):e0213192. doi: 10.1371/journal.pone.0213192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollack CV, Jr, Agiro A, Mu F, et al. Impact on hospitalizations of long-term versus short-term therapy with sodium zirconium cyclosilicate during routine outpatient care of patients with hyperkalemia: the recognize I study. Expert Rev Pharmacoecon Outcomes Res. 2023;23(2):241–250. doi: 10.1080/14737167.2023.2161514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not available from the authors but are available with permission from HealthVerity. Restrictions apply to the availability of these data, which were used under license for this study.