ABSTRACT
Background and Aims:
The bilateral superficial cervical plexus block (BSCPB) is efficacious for post-operative analgesia in thyroid surgeries. We assessed the analgesic efficacy of dexmedetomidine and dexamethasone administered as adjuvants with 0.25 percent ropivacaine in BSCPB for thyroidectomy under general anaesthesia with regard to the duration of analgesia, total amount of rescue analgesic requirement, changes in intra- and post-operative haemodynamic parameters, VAS scores, and adverse events, if any.
Methods:
A prospective double-blind trial was planned with 80 adults undergoing thyroidectomy, randomized into two equal groups and given BSCPB with 20 ml 0.25% ropivacaine with adjuvants as either dexmedetomidine 50mg (group A) or dexamethasone 4mg (group B), 10 ml on each side, after the induction of general anaesthesia. Post-operative pain was monitored using the visual analog scale and the duration of analgesia was measured by time to first rescue analgesia. Post-operative haemodynamics and any adverse events were recorded.
Results:
The mean duration of analgesia was slightly prolonged in group A but statistically non-significant as compared to group B (1037 ± 97 vs. 1004 ± 122 minutes; P0.18). The post-operative median VAS scores and vital parameters were relatively comparable for both groups (P > 0.05) for the first 24h. There was a significant reduction in the incidence of PONV (P < 0.05) in group B.
Conclusion:
Although dexamethasone offers a slight advantage of decreased incidence of PONV, BSCPB using ropivacaine with either dexmedetomidine or dexamethasone as an adjuvant imparted adequate analgesia with stable haemodynamics and may be used as a pre-emptive analgesic technique in thyroid surgeries.
Key words: Analgesia, cervical plexus block, dexamethasone, dexmedetomidine, ropivacaine, thyroidectomy
INTRODUCTION
Thyroidectomy is associated with moderate incisional pain, burning sensation, uneasiness in swallowing and post-operative nausea vomiting (PONV) attributable to endotracheal intubation, tissue dissection, and hyperextension during surgery.[1,2] The bilateral superficial cervical plexus block (BSCPB) provides appreciable analgesia for thyroidectomy.
Local anaesthetics, when used alone, offer a short duration of analgesia. Therefore, various adjuvants have been studied, including dexmedetomidine, an a2 agonist, which provides stable haemodynamics, and prolonged analgesia with minimal side effects. Dexamethasone, a corticosteroid, is a recent addition to the list with promising results in BSCPB.[3]
We investigated the effects of dexmedetomidine and dexamethasone as adjuvants with ropivacaine on the duration of analgesia as a primary outcome and visual analog scale (VAS) score, rescue analgesic requirement, haemodynamic parameters, PONV, and adverse events as secondary outcomes in BSCPB for thyroidectomy under general anaesthesia.
METHODS
After the institutional ethical committee’s approval and registration with the Clinical Trials Registry of India, this prospective double-blind comparison study was carried out at a tertiary care facility for a period of eight months. All patients signed an informed consent form after a complete explanation about the study protocol, anaesthetic technique, merits and demerits of the procedure, and perioperative course of anaesthesia. The study was conducted on 80 American Society of Anaesthesiologists (ASA) physical status I and II euthyroid patients, aged 16 to 60 years, of either sex, undergoing elective thyroid surgeries to receive BSCPB after induction of general anaesthesia. Patients refusing to be a part of the study or those with severe respiratory, cardiac, or renal disorders, infection at the injection site, allergy to drugs used, co-morbidities like diabetes mellitus or chronic hypertension, and history of coagulation disorders were excluded.
The results from a randomized control study by Banu et al.[4] with an expected mean difference in duration of analgesia between the two groups being 13 minutes and a standard deviation of 16 from previous studies were used to compute the sample size. We calculated 36 subjects for each group using the standard formula, to have a power of 80% with a type a error of <0.05. Therefore, 40 subjects were taken in each group to compensate for dropouts.
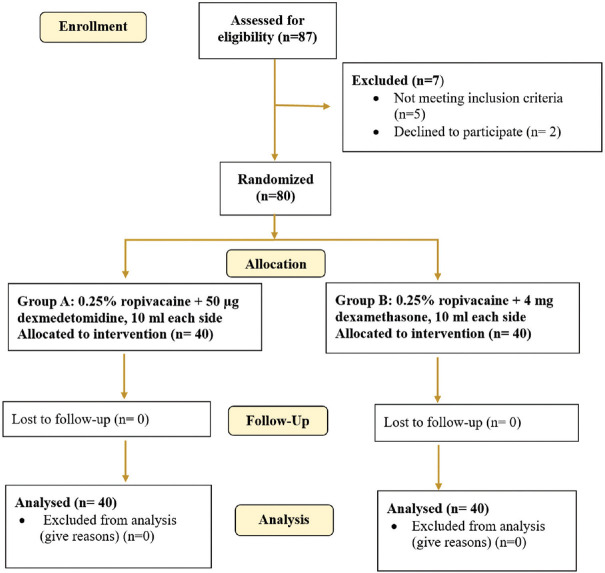
A total of 87 cases were evaluated for eligibility, with seven cases being rejected due to the exclusion criteria. Forty patients were randomly assigned to each group (A and B) using computer-generated random number tables [Figure 1].
Figure 1.
Consort flow diagram
Group A (n = 40): Ropivacaine 0.5% 10 ml + 0.9% normal saline 9 ml + 50 mg Dexmedetomidine made upto 1 ml (total volume 20 ml)
Group B (n = 40): Ropivacaine 0.5% 10 ml + 0.9% normal saline 9 ml + Dexamethasone 1 ml (total volume 20 ml)
To reduce subjective and objective bias, the study was double-blinded so that the anaesthesiologist doing the procedure, the patient, and the monitoring personnel were not aware of group allocation. A syringe containing the drugs ropivacaine along with dexmedetomidine or dexamethasone as per required concentrations was prepared by an anaesthetist not engaged in the application of block, post-operative monitoring of the patient, or evaluation of the results. The syringe was labelled with a number, and a record of the syringe’s contents was preserved in a notebook that was only revealed once the post-operative monitoring had ended.
All patients in this trial underwent a thorough pre-anaesthetic evaluation taking into consideration a detailed history, general physical and systemic examination, airway assessment, and routine investigations along with thyroid function tests and indirect laryngoscopy to confirm vocal cord mobility.
All standard ASA monitors (pulse oximeter, ECG, non-invasive blood pressure, temperature) were connected and baseline vitals were recorded. An intravenous cannula was secured. The patients were given intravenous midazolam 0.02 mg/kg and fentanyl 2mg/kg as premedication along with preoxygenation with 100% oxygen. Propofol 2 mg/kg and vecuronium 0.1 mg/kg were used to induce general anaesthesia, which was followed by tracheal intubation. Anaesthesia was maintained with inhalational agent sevoflurane (MAC 0.7–1.3) in a mixture of O2:N2O in a ratio of 50:50.
A BSCPB was performed after this under all aseptic precautions, prior to the start of surgery. The patient’s head was positioned away from the side to be blocked. With the anaesthesiologist standing at the head end of the patient, ipsilateral mastoid and C-6 transverse processes were identified as landmarks for the block. A 24-gauge, 1.5 inch needle was introduced along the posterior border of the sternocleidomastoid muscle, in the middle, between the landmarks of mastoid process and transverse process of C6. Next, 5 ml of anaesthetic was administered subcutaneously and horizontally, with the remaining 5 ml injected in a ‘fan’ pattern cephalad toward the tragus of the ipsilateral ear and caudad toward the sternal notch. An identical approach was used to perform the block on the contralateral side, and the time of the block was recorded.
Haemodynamic parameters including heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), SpO2, and respiratory gas (end-tidal CO2 and MAC) monitoring were done continually and recorded at 5-minute intervals for the first 15 minutes, then every 15 minutes until the surgery was completed. The depth of neuromuscular blockade was quantified with a train of four (TOF) and kept under a count of two with vecuronium. Sevoflurane and N2O were stopped at the completion of the surgery. The neuromuscular block was reversed with intravenous neostigmine 0.5 mg/kg and glycopyrrolate10 mg/kg. Upon clinical observation and neuromuscular monitoring with a TOF ratio >0.9, the patient was extubated following complete recovery.
The success of the block was verified post-operatively using a pin-prick test over the cervical plexus innervated sites. Analgesia was assessed by VAS score and vitals were monitored 2-hourly until the patient demanded rescue analgesia, upto 24 hours. Any occurrence of any side effects/complications was also noted down. Post-operatively, if the patient demanded a rescue analgesic or if VAS >3 was noted, whichever was earlier, then injection diclofenac 75mg or tablet diclofenac 50 mg was given and the time to the first rescue analgesia was recorded as the duration of analgesia. If pain persisted after receiving diclofenac, morphine 0.1 mg/Kg was kept in reserve as a rescue analgesic.
Categorical and nominal variables were expressed numerically and analysed using the Chi-square test or the Fischer exact test, as appropriate. Continuous variables were computed as mean with standard deviation and were analysed using an unpaired Student’s t test for comparison between the two groups. The data were analysed using the SPSS statistical software package for Windows, version 21 (SPSS inc., Chicago, IL, USA). Probability was deemed significant, if less than 0.05.
RESULTS
Both the groups were comparable in terms of age, gender, weight, ASA status, and mean duration of surgery and anaesthesia [Table 1].
Table 1.
Demographic characteristics of the patient and comparison of mean duration of analgesia, and total diclofenac consumption
| Parameter | Group A | Group B | P |
|---|---|---|---|
| Age (Years) Mean±SD | 36.37±11.64 | 36.85±12.83 | 0.60 |
| Sex (Male/Female) | 2:38 | 3:37 | 1.00 |
| Weight (Kg) Mean±SD | 59.30±7.85 | 58.35±7.71 | 0.59 |
| ASA status (Grade I/II) | 32:8 | 33:7 | 1.00 |
| Mean duration of surgery (min) | 97.55±30.76 | 94.80±29.23 | 0.69 |
| Mean duration of anaesthesia (min) | 109.25±27.30 | 108.68±28.62 | 0.93 |
| Mean duration of analgesia (min) | 1037±97 | 1004±122 | 0.18 |
| Mean total dose of rescue analgesic-diclofenac (mg) | 53.13±19.34 | 52.12±19.31 | 0.81 |
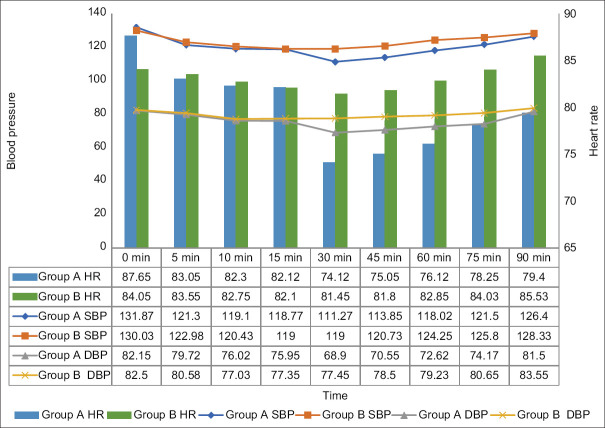
Haemodynamic parameters were recorded intraoperatively at specific times [Figure 2]. Over the course of 30 to 75 minutes, intraoperative mean SBP, DBP, and HR were significantly lower in group A (P < 0.05) than in group B. For the rest of the time, the parameters were comparable between the two groups (P > 0.05).
Figure 2.
Intraoperative vital monitoring (HR, SBP, DBP)
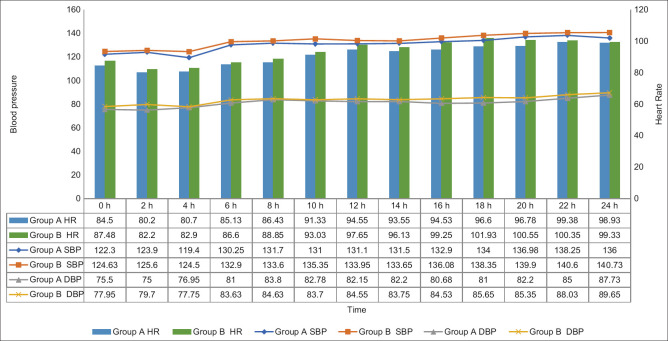
Post-operatively, haemodynamic parameters [Figure 3] were recorded at predefined time intervals. Throughout the post-operative period, the mean SBP, DBP, and HR were marginally lower in group A than in group B but statistically significant only at 16 and 18 hours (P < 0.05).
Figure 3.
Post-operative vital monitoring (HR, SBP, DBP)
Median VAS score was comparable [Figure 4] for the entire post-operative period in groups A and B (P > 0.05). The mean duration of analgesia [Table 1] was slightly longer in group A than in group B, but the difference was statistically insignificant when tested with an unpaired Student’s t test (P0.18). The mean total dose of diclofenac used as a rescue analgesic for both groups was also comparable (P0.81). Morphine was not used for any patient, thereby asserting the role of BSCPB in sparing the use of opioids post-operatively.
Figure 4.
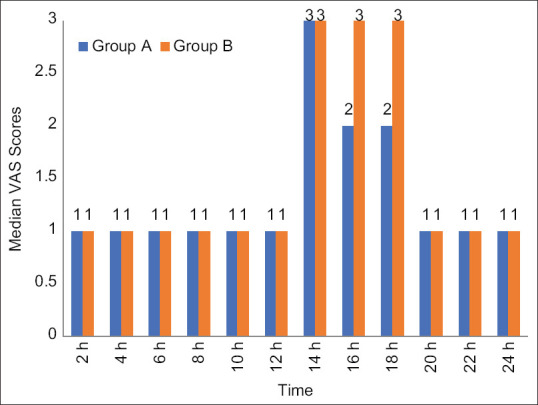
Comparison of post-operative VAS score among study groups
The incidence of hypotension was observed in both groups (22.5% of patients each), but it was statistically non-significant (P0.79). The incidence of nausea (group A: B; 27.5%:7.50%) and vomiting (group A: B; 20%:2.5%) was observed to be significantly lower in group B than in group A (P0.04 and P0.03, respectively). No incidence of intravenous injection, Horner’s syndrome, post-operative respiratory distress, or local anaesthetic toxicity was observed in any of the groups [Figure 5].
Figure 5.
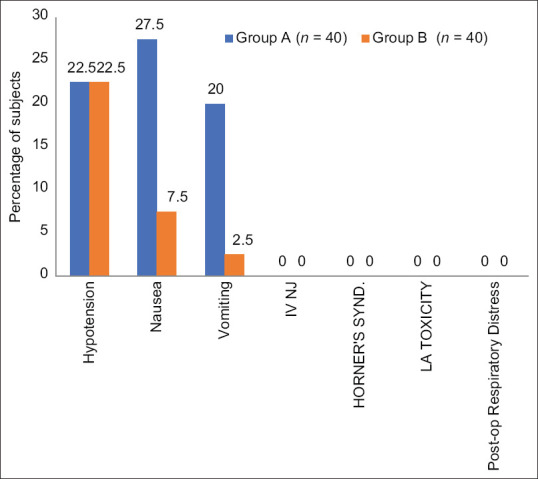
Frequency of adverse events among study groups
DISCUSSION
Several publications state that patients report a VAS score of post-thyroidectomy pain as 6.9 ± 1.7 with 90% of these patients requiring narcotic analgesics, particularly on the first day of surgery.[5-7] BSCPBs are remarkably safe and confer apposite analgesia after thyroidectomy and thereby have an opioid-sparing role in post-operative pain management. Our study aimed to examine the efficacy of dexmedetomidine and dexamethasone as adjuvants to ropivacaine for BSCPB in providing post-surgical pain relief.
The demographic profile, i.e. age, gender, weight, and ASA physical status between the two groups of our study, was comparable and provided us with a uniform platform to evenly compare the results obtained. The gender distribution reflected a higher number of females likely because pathologies of the thyroid are more prevalent in females than in males which may be ascribed to autoimmunity, oestrogen dominance, nutritional deficiencies, and more likelihood to get medical attention in cases of thyroid enlargement for aesthetic appeasement.
The study done by Andrieu et al.[8] and Goulart et al.[9] corroborated the rationale for choosing 0.25% concentration who found that increasing the concentration of ropivacaine to 0.5% or 0.75% failed to enhance the duration of analgesia, implying that the risk of a higher total dose of local anaesthetic can be avoided. A study conducted by Ökmen and Ökmen[10] gauged the influence of the volume of the drug irrespective of its concentration and concluded that the block was more efficacious when the total volume of the study drug 0.25% bupivacaine was higher (20 ml vs 10 ml). Elbahrawy et al.[3] demonstrated that even with ultrasound guidance, where the spread and deposition of the study drug were clearly visualized, at least 20 ml of the study drug was required.
Intraoperatively, the mean SBP, DBP, and HR were comparable for both groups A and B in the initial 15-minute duration of surgery. A steady decline in all three parameters was observed in the initial part of intraoperative monitoring in both groups A and B, possibly due to the synergistic effect of drugs used for induction of anaesthesia, namely injection midazolam, fentanyl, propofol, and vecuronium. From 30 minutes to 75 minutes of surgery, there was a statistically significant difference in all SBP, DBP, and HR, with vitals being lower in group A than in group B. Aliste et al.,[11] Hassan et al.,[12] and Albrecht et al.[13] also reported a similar decrease with dexmedetomidine while comparing it with dexamethasone as an adjuvant for various blocks.
The post-operative SBP, DBP, and HR were found to be statistically non-significant (P > 0.05) for the initial 14 hours eliciting comparable haemodynamic characteristics of both dexmedetomidine and dexamethasone as adjuvants to ropivacaine in BSCPB. As the analgesic effect of the block began to wear off, there was a statistically significant difference in vital parameters at 16 and 18 hours. A PRISMA-compliant systematic review and meta-analysis conducted by Xiong et al.[14] comparing the effects of using dexmedetomidine and dexamethasone as adjuvants for peripheral nerve blocks also reported no significant difference in the haemodynamics with respect to HR and blood pressure in the post-operative period, with both the adjuvants.
The median VAS scores were comparable for the entire duration of 24 hours of the post-operative period (P > 0.05) which correlates with the comparable duration of analgesia seen in both group A and group B. This is also reflected in the study by Gao et al.[15] where they compared the efficacy of dexmedetomidine and dexamethasone as adjuvants for erector spinae plane block and observed comparable VAS scores.
The mean duration of analgesia in group A (1037 ± 97 min) as compared to group B (1004 ± 122 min) was slightly prolonged but the difference was statistically non-significant (P0.18). Therefore, both dexmedetomidine and dexamethasone offered a comparable duration of analgesia, when used as an adjuvant with ropivacaine in BSCPB.
Though the literature reveals conflicting results while comparing the efficacy of both the adjuvants, most publications support the comparable results obtained for the duration of analgesia with either dexmedetomidine or dexamethasone as an adjuvant perineurally. A meta-analysis of 13 RCTs conducted by Xiong et al.[14] comparing the two drugs as perineural adjuvants on peripheral nerve block revealed that dexamethasone provided equivalent analgesic duration as compared to dexmedetomidine. Song et al.[16] had similar observations while conducting a meta-analysis of six RCTs.
Hassan et al.[12] in their study compared dexmedetomidine and dexamethasone as adjuvants to levobupivacaine for BSCPB and observed that dexmedetomidine added to levobupivacaine resulted in considerably longer analgesic duration than dexamethasone administered to the levobupivacaine group (232.34 versus 303.55 min; P < 0.05). Similar observations were reported in a study by Singla et al.[17] where the addition of dexmedetomidine to ropivacaine, as opposed to dexamethasone, prolonged the time to first rescue analgesic consumption in bilateral transversus abdominis plane block following caesarean delivery. Contrarily, it was seen in a systematic review and indirect meta-analysis done by Albrecht et al.[13] comparing both adjuvants as perineural adjuncts for supraclavicular brachial plexus block wherein after studying 50 different trials, they concluded that dexamethasone may be a preferable adjunct since it extends analgesic duration by a statistically significant 2.5 hours longer than dexmedetomidine without the risk of hypotension or sedation.
The mean total analgesic dose of tablet or injection of diclofenac, given in the first 24 hours in both groups, was comparable. It has been similarly observed in studies done by Elbahrawy et al.,[3] Banu et al.,[4] Hassan et al.,[12] and Santosh et al.[18] that the addition of either dexmedetomidine or dexamethasone as an adjunct to the LA in BSCPB results in a reduction of post-operative analgesic requirement.
Despite the fact that dexmedetomidine possesses central a2-mediated analgesic effects, an animal experiment by Brummett et al.[19] revealed that the drug’s effects were due to peripheral blockade of hyperpolarization-activated cation currents, not by its central or peripheral agonistic qualities. The research by Andersen et al.[20] further supports the idea of predominantly peripheral effects of perineural dexmedetomidine. Similarly, perineural dexamethasone extends the analgesic duration of peripheral nerve blocks by decreasing transmission in thin unmyelinated C-fibres, as well as having a local vasoconstrictive effect and anti-inflammatory properties.[16]
In our trial, the use of dexamethasone as an adjuvant resulted in a statistically significant decrease in the incidence of PONV when compared to dexmedetomidine. Previous research suggests that PONV is frequent with thyroid surgeries (~ 75%).[21] Despite the fact that no antiemetic was administered intraoperatively, the incidence of PONV was much lower in both groups.
The antiemetic action seen with the use of dexmedetomidine as an adjuvant may be ascribed to the modulation of neurotransmitters as well as an opioid- and anaesthetic-saving effect as the underlying mechanism.[22] Dexamethasone’s mechanism of action as an antiemetic drug is unknown, but numerous theories have been posited, such as depletion of g-aminobutyric acid stores, enhancement of the blood–brain barrier to emetogenic toxins, and suppression of central prostaglandins and serotonin.
When using the landmark technique for BSCPB with larger volumes of LA (10–15 ml), the likelihood of problems such as phrenic nerve block (manifesting as post-operative respiratory distress), hoarseness of voice, Horner’s syndrome, vertebral artery injury, and total spinal block increases.[23] In either of our two groups, however, no such occurrence was found.
This study makes no recommendations for patients under the age of 16 or over the age of 60, or for those with ASA physical category III or IV. With its ease of visualization of anatomical structures and relationships, as well as the spread of the local anaesthetic, ultrasound guidance is preferred over landmark technique, something that our study lacks. The effect of varied doses of the adjuvants dexmedetomidine and dexamethasone on the block characteristics is also a matter of further research.
CONCLUSION
Although dexamethasone offers the advantage of decreased incidence of PONV, BSCPB using ropivacaine with either dexmedetomidine or dexamethasone as an adjuvant imparts adequate analgesia with a comparable duration, rescue analgesic dose consumption, VAS scores, and stable haemodynamics, without potential side effects or complications and may be used as a pre-emptive analgesic technique in thyroid surgeries.
Financial support and sponsorship
Department of Anaesthesia, J. L. N. Medical College, Ajmer.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Shih ML, Duh QY, Hsieh CB, Liu YC, Lu CH, Wong CS, et al. Bilateral superficial cervical plexus block combined with general anaesthesia administered in thyroid operations. World JSurg. 2010;34:2338–43. doi: 10.1007/s00268-010-0698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajwa SJ, Sehgal V. Anaesthesia and thyroid surgery:The never ending challenges. Indian J Endocrinol Metab. 2013;17:228–34. doi: 10.4103/2230-8210.109671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elbahrawy K, El-Deeb A. Superficial cervical plexus block in thyroid surgery and the effect of adding dexamethasone:A randomized, double-blinded study. Res Opin Anaesthesia Intensive Care. 2018;5:98. [Google Scholar]
- 4.Banu P, Paul S, Naser SM, Bhattacharjee DP, Neogi M. A randomized double-blind placebo-controlled clinical trial to assess the efficacy of dexamethasone to provide postoperative analgesia after cervical plexus block in patients undergoing elective thyroid surgery. Ann Int Med Den Res. 2019;5:AN39–43. [Google Scholar]
- 5.Aweke Z, Sahile WA, Abiy S, Ayalew N. Effectiveness of bilateral superficial cervical plexus block as part of postoperative analgesia for patients undergoing thyroidectomy in empress Zewditu Memorial Hospital, Addis Ababa, Ethiopia. AnaesthesiolResPractice. 2018;2018 doi: 10.1155/2018/6107674. doi:10.1155/2018/6107674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karakış A, Tapar H, Özsoy Z, Suren M, Dogru S, Karaman T, et al. Perioperative analgesic efficacy of bilateral superficial cervical plexus block in patients undergoing thyroidectomy:A randomized controlled trial. Rev Bras Anaestesiol. 2019;69:455–60. doi: 10.1016/j.bjane.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gozal Y, Shapira S, Gozal D, Magora F. Bupivacaine wound infiltration in thyroid surgery reduces postoperative pain and opioid demand. Acta Anaesthesiol Scand. 1994;38:813–5. doi: 10.1111/j.1399-6576.1994.tb04010.x. [DOI] [PubMed] [Google Scholar]
- 8.Andrieu G, Amrouni H, Robin E, Carnaille B, Wattier JM, Pattou F, et al. Analgesic efficacy of bilateral superficial cervical plexus block administered before thyroid surgery under general anaesthesia. Br J Anaesth. 2007;99:561–6. doi: 10.1093/bja/aem230. [DOI] [PubMed] [Google Scholar]
- 9.Goulart TF, Araujo-Filho VJ, Cernea CR, Matos LL. Superficial cervical plexus blockade improves pain control after thyroidectomy:A randomized controlled trial. Clinics. 2019;74:e605. doi: 10.6061/clinics/2019/e605. doi:10.6061/clinics/2019/e605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ökmen K, Ökmen BM. Efficacy of different doses of superficial cervical plexus block on pain after thyroid surgery. J Clin Anal Med. 2017;8:496–500. [Google Scholar]
- 11.Aliste J, Layera S, Bravo D, Fernández D, Jara Á, García A, et al. Randomized comparison between perineural dexamethasone and dexmedetomidine for ultrasound-guided infraclavicular block. Reg Anaesth Pain Med. 2019;44:911–6. doi: 10.1136/rapm-2019-100680. [DOI] [PubMed] [Google Scholar]
- 12.Hassan AH, Amer IA, Abdelkareem AM. Comparative study between dexmedetomidine versus dexamethasone as adjuvants to levobupivacaine for cervical plexus block in patients undergoing thyroid operation. Prospective-randomized clinical trial. Egypt J Hosp Med. 2021;84:1638–43. [Google Scholar]
- 13.Albrecht E, Vorobeichik L, Jacot-Guillarmod A, Fournier N, Abdallah FW. Dexamethasone is superior to dexmedetomidine as a perineural adjunct for supraclavicular brachial plexus block:Systematic review and indirect meta-analysis. Anaesth Analg. 2019;128:543–54. doi: 10.1213/ANE.0000000000003860. [DOI] [PubMed] [Google Scholar]
- 14.Xiong C, Han CP, Zhao D, Tang ZH, Zhang YF, Wang J. Comparing the effects of dexmedetomidine and dexamethasone as perineural adjuvantst on peripheral nerve block:A PRISMA-compliant systematic review and meta-analysis. Medicine. 2021;100:e27064. doi: 10.1097/MD.0000000000027064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Z, Xiao Y, Wang Q, Li Y. Comparison of dexmedetomidine and dexamethasone as adjuvant for ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery:A randomized, double-blind, placebo-controlled trial. AnnTranslMed. 2019;7:668. doi: 10.21037/atm.2019.10.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song ZG, Pang SY, Wang GY, Zhang Z. Comparison of postoperative analgesic effects in response to either dexamethasone or dexmedetomidine as local anaesthetic adjuvants:A systematic review and meta-analysis of randomized controlled trials. J Anaesth. 2021;35:270–87. doi: 10.1007/s00540-021-02895-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singla N, Garg K, Jain R, Malhotra A, Singh MR, Grewal A. Analgesic efficacy of dexamethasone versus dexmedetomidine as an adjuvant to ropivacaine in ultrasound-guided transversus abdominis plane block for post-operative pain relief in caesarean section:A prospective randomized controlled study. Indian J Anaesth. 2021;65:S121–6. doi: 10.4103/ija.IJA_228_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santosh BS, Mehandale SG. Does Dexmedetomidine improve analgesia of superficial cervical plexus block for thyroid surgery? Indian J Anaesth. 2016;60:34–8. doi: 10.4103/0019-5049.174797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation Current. Anesthesiology. 2011;115:836–43. doi: 10.1097/ALN.0b013e318221fcc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen JH, Grevstad U, Siegel H, Dahl JB, Mathiesen O, Jæger P. Does dexmedetomidine have a perineural mechanism of action when used as an adjuvant to ropivacaine?A paired, blinded, randomized trial in healthy volunteers. Anesthesiology. 2017;126:66–73. doi: 10.1097/ALN.0000000000001429. [DOI] [PubMed] [Google Scholar]
- 21.Cai HD, Lin CZ, Yu CX, Lin XZ. Bilateral superficial cervical plexus block reduces postoperative nausea and vomiting and early postoperative pain after thyroidectomy. J Int Med Res. 2012;40:1390–8. doi: 10.1177/147323001204000417. [DOI] [PubMed] [Google Scholar]
- 22.Liang X, Zhou M, Feng JJ, Wu L, Fang SP, Ge XY, et al. Efficacy of dexmedetomidine on postoperative nausea and vomiting:A meta-analysis of randomized controlled trials. IntJClin ExpMed. 2015;8:8450–71. [PMC free article] [PubMed] [Google Scholar]
- 23.Deepika V, Ahuja V, Thapa D, Gombar S, Gupta N. Evaluation of analgesic efficacy of superficial cervical plexus block in patients undergoing modified radical mastoidectomy:A randomised controlled trial. Indian J Anaesth. 2021;65:S115–20. doi: 10.4103/ija.ija_339_21. [DOI] [PMC free article] [PubMed] [Google Scholar]